Abstract
Background
Electrospinning is an effective method for producing high-quality biopolymer nanofibers, such as cellulose and chitosan. Cellulose nanofibers have excellent mechanical properties and biocompatibility, making them a promising material for tissue engineering. Chitosan nanofibers are biodegradable, biocompatible, and antimicrobial, making them ideal for biomedical applications. The electrospinning parameters, including solution concentration, power supply voltage, orifice diameter, temperature, humidity, and flow rate, play a crucial role in determining the nanofiber diameter, morphology, and mechanical properties, as well as their suitability for various applications.
Objective
This systematic review aims to synthesize and evaluate the current evidence on the influence of electrospinning parameters on the production and properties of cellulose and chitosan nanofibers.
Methods
A comprehensive search of electronic databases was conducted to identify relevant studies. The inclusion criteria were studies that investigated the effect of electrospinning parameters on cellulose and chitosan nanofibers.
Results
It was found that for cellulose, the average fiber diameter increased with increasing each of solution concentration, power supply voltage, orifice diameter, temperature, and humidity. Contrary to tip - collector distance and some optimal points in temperature, where average fiber diameter decreased. For chitosan, the change in voltage and tip to collector distance did not alter the average fiber diameter except for some readings of voltage, which behaved differently. On the other hand, the average fiber diameter increased with increasing flow rate.
Conclusion
The review highlights the importance of considering electrospinning parameters in the production of high-quality biopolymer nanofibers and provides insights into the optimization of these parameters for specific applications. This review also highlights the need for further research to better understand the underlying mechanisms of electrospinning and to optimize the process to produce biopolymer nanofibers with improved properties.
Keywords: Nanofibers, Electrospinning, Cellulose, Chitosan, Nanocellulose, Membrane
1. Introduction
Biopolymers have received much interest in recent years due to their lower environmental impact compared to the use of fossil fuels and other pollutants, which have a direct harmful impact on the environmental system’s stability [[1], [2], [3]]. They’re utilized in a variety of applications, including medicine, food packaging, and cosmetics, where they may substitute any petroleum-based substance. Polynucleotides, polypeptides, and polysaccharides are three different types of biopolymers. Cellulose is a polysaccharide-type biopolymer that is extremely crystalline and extensively abundant biopolymer on the terrestrial/aquatic environments, since it can be found in all plant/algal cells (a part of its cell wall composition) [4]. Because it is so readily available in nature, it has been widely exploited in the field of electrospinning. However, it is limited by its inability to dissolve in common solvents such as water (although some experiments have shown that it can slightly dissolve in water); moreover, it cannot melt, making it processable only in solution form [5,6]. Cellulose may also be modified by introducing hydroxyl groups on the cellulose backbone, and the resulting derivatives (acetate, hydroxyl propyl, and so on) can be utilized to make nanofibers [[7], [51]]. Both cellulose and its derivatives are important raw materials in the electrospinning process, where the resulting fibers made their way into a variety of biomedical applications, igniting the interest in cellulose and its derivatives.
Chitosan is another significant biopolymer that is also of polysaccharide nature. It is biodiverse, biocompatible, and biodegradable [8]. Chitosan is made by treating chitin shells of shrimps and other crustaceans with an alkaline substance such as sodium hydroxide. It is considered the second most abundant biopolymer after cellulose. Chitosan is insoluble in water, alkali, and mineral acidic systems. It is, however, soluble in organic acids. The study and development of these materials at the nanoscale is one of the current fastest developing scientific fields due to its enormous potential for developing novel resulting derivatives with advanced applications [9,10]. Many diverse sciences and engineering disciplines, such as electronics, material science, and polymer engineering, have been significantly influenced by nanotechnology. Nanofibers are used in Nano-catalysis, tissue scaffolds, protective clothing, filtration, and optical electronics, among other things, due to their large surface area and porosity [[11], [12], [52]]. Electrospinning is a viable technique for producing nanofibers. It has gotten a lot of attention in the last years, not only because of its versatility in spinning a wide range of polymeric fibers, high specific surface area, ease of surface functionality, and interfibrous pore sizes [13], but also because of its consistency in creating fibers using polymer melts or solution of both natural and synthetic polymers [[14], [15], [16]]. The electrospinning technique produces electrically charged jets from a polymer solution or melts using a high-voltage electric field. The evaporation of the solvent causes the solution or melt to dry, resulting in nanofibers [13]. A positively charged collector, which might be a flat surface or a revolving drum, attracts the highly charged fibers [9,17]. Fiber is subjected to a collection of tensile, gravitational, aerodynamic, rheological, and inertial forces in traditional spinning procedures. Tensile force induced by Electric field created axial direction of polymeric flow, yielding spinning operation [9].
The aim of this systematic review was, therefore, to study the influence of electrospinning parameters on biopolymers nanofibers, with emphasis on cellulose & chitosan.
-
a)
What’s the effect of material parameters on the output chitosan and cellulose nanofibers resulting from the electrospinning process?
-
b)
What’s the effect of machine parameters on the output chitosan and cellulose nanofibers resulting from the electrospinning process?
-
c)
What’s the effect of ambient parameters on the output chitosan and cellulose nanofibers resulting from the electrospinning process?
2. Methods
2.1. Review protocol
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used to identify eligibility and selecting of articles for this review.
2.2. Search strategy
The literature search started from September 1st, 2022, to February 5th, 2023, with the use of electronic databases like Google Scholar, Science Direct, Web of Sciences, Scopus, PubMed, and official websites of different organizations and universities. Published articles written in English language were considered without restriction to year of publication. Electrospinning, Biopolymers Nanofibers, Cellulose & Chitosan, Electrospinning Parameters, Cellulose Nanofibers, Chitosan Nanofibers were the search terms used. These terms were used in an advanced PubMed search to widen the search that included all fields [All fields] in record. Furthermore, Boolean operators (AND, OR) were appropriately employed for identifying research. For all the papers included in this review, the searching words that is used for advanced search was as: “Electrospinning” OR “Biopolymers Nanofibers” [All fields] OR “Nanofibers” [All fields] OR “Electrospinning Parameters” [All fields] AND “Cellulose & Chitosan” [All fields].
2.3. Eligibility criteria
2.3.1. Inclusion criteria
All prospective and retrospective observational studies (cross-sectional, case controls, and cohort) articles conducted in any field of Cellulose & Chitosan and written only in English language were included if they reported the influence of electrospinning parameters on biopolymers nanofibers.
2.3.2. Exclusion criteria
Those studies did not report our variables of interest or comprised incomplete information (parameters, method of preparation, and findings). Articles where the full text cannot be accessed were excluded from this systematic review.
2.3.3. Evaluation of articles quality
The quality of each article was evaluated by using a 14-points checklist. ‘High quality’ for an article with score > and = 70%. A score of 69 to 51% considered as “moderate quality”, less than or equal to 50% were considered “Poor quality”. Two authors scored each article individually and the mean value of the results was used. No article got excluded due to inferior quality, as all articles scored more than 50%.
2.4. Data extraction and analysis
Using Microsoft Excel 2019, authors created a data extraction tool to collect all the needed data from selected articles. Data related to characteristics of the articles such Parameter, Material, Methods and Preparations, Results, Findings were extracted. Solution concentration and applied volt were also extracted. The data extraction was carried out by two authors independently. When disagreement was encountered by the two authors, a third author was delegated to extract the data. Microsoft Excel 2019 was used to analyze the Effect of the parameters and the results were depicted in charts and tables.
2.5. Risk of bias in individual studies
This study design was made to determine the risk of bias of individual. Validity of the data were assured for each outcome by identifying the methods and analyses that were employed to assess the outcomes. Both study and outcome level information were used in synthesis.
2.6. Summary measures and synthesis of results
As the effectiveness was presented in percentage without always mentioning the means and confidence intervals, the principal summary measures could not be estimated.
2.7. Risk of bias across studies
After assessing the individual study, the bias across the studies was evaluated. The risks of bias included studies from publication. Selection and reporting are described in the discussion section of the review.
3. Results
3.1. Characteristics of the studies included
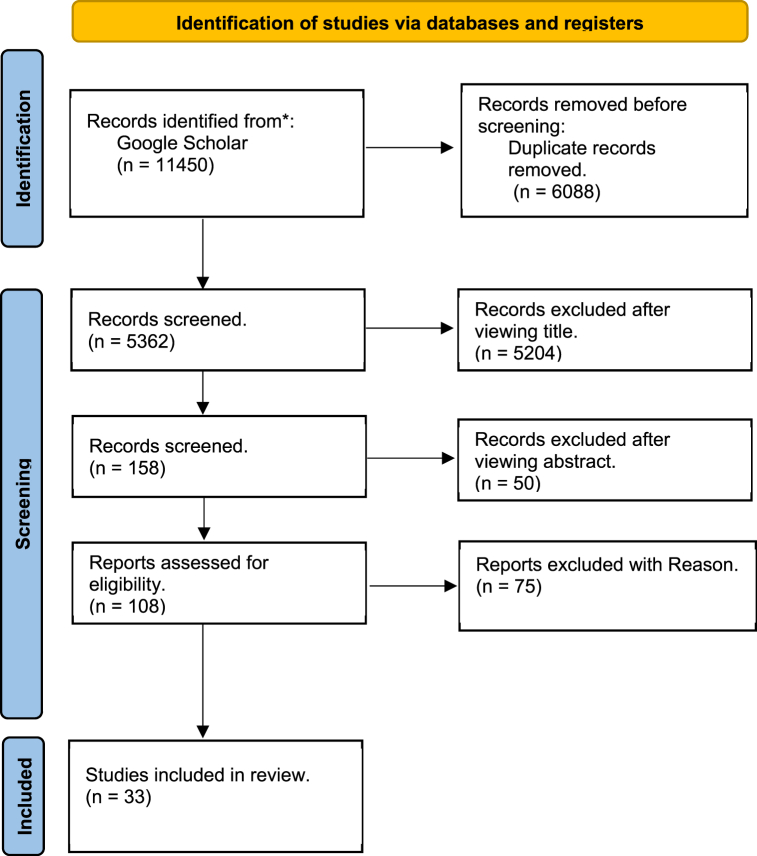
During our advanced research, we identified 11,450 articles, and we used the PRISMA flow diagram method (Fig. 1) to demonstrate our search procedure. After removing duplicates, we screened 5362 articles for eligibility. Finally, we found 33 studies that met the eligibility criteria and were included in this review.
Fig. 1.
Flow diagram of articles searching, screening and selection process for our systematic review.
3.2. Principles of electrospinning method
The electrospinning technique was initially developed by Rayleigh in 1897 and presented in detail by Zeleny in early 1900 [18]. It is now seen as a critical scientific and commercial enterprise with worldwide economic advantages [19]. Electrospinning has grown exponentially and now became the preferred technique among others to produce nanofibers, because of its simplicity in usage, & cost effectiveness [18]. The electrospinning process starts when electric charges passes through the metallic needle and into the polymer solution. As a result of the charges induction on the polymer droplet, instability is produced within the polymer droplet. At the same time, reciprocal charge repulsion creates a force that resists surface tension (only when the polymer solution has sufficient cohesive force a stable charge jet can be produced) [19]. The spherical droplets deform and take on a conical shape (Taylor cone). As the electric field increases, internal and external charge forces cause the liquid jet to whip in the direction of the collector during the operation [21]. This whipping motion causes the polymer chains in the solution to stretch and glide past each other, resulting in ultrafine nanofibers [19,22,23]. They are collected on the metallic collector, which is held at an optimal distance. The most frequent way to collect electrospinning nanofibers is in the form of randomly oriented or parallel-aligned mats. When a basic static collecting surface is utilized, randomly oriented fiber mats emerge. While the parallel-aligned fiber mats are collected with a spinning mandrel [19,24]. Nanofibers can also be collected in a linear orientation over an air gap between two parallel plates, fibers align perpendicular to the plates and extend across them when an electric field is created between the two parallel plates. It extends the scope of electrospinning’s practical applications because this technique can gather individual nanofibers [24].
3.3. Effects of parameters on electrospinning
The electrospinning process is determined by several parameters. They are divided into three categories: machine parameters, material parameters, and ambient parameters. Applied electric field, distance between needle and collector, flow rate, and needle diameter are machine parameters. Solvent type, polymer concentration, viscosity, and material conductivity are material parameters. Relative humidity and temperature are ambient parameters. All these parameters have an impact on the production of smooth, bead-free electrospinning fibers, therefore, it is important to fully understand the impacts of all of these key parameters to obtain a better knowledge of the electrospinning process and production of polymeric nanofibers [19,25].
4. Discussion
4.1. Material parameters (effects of polymer concentration and solution viscosity)
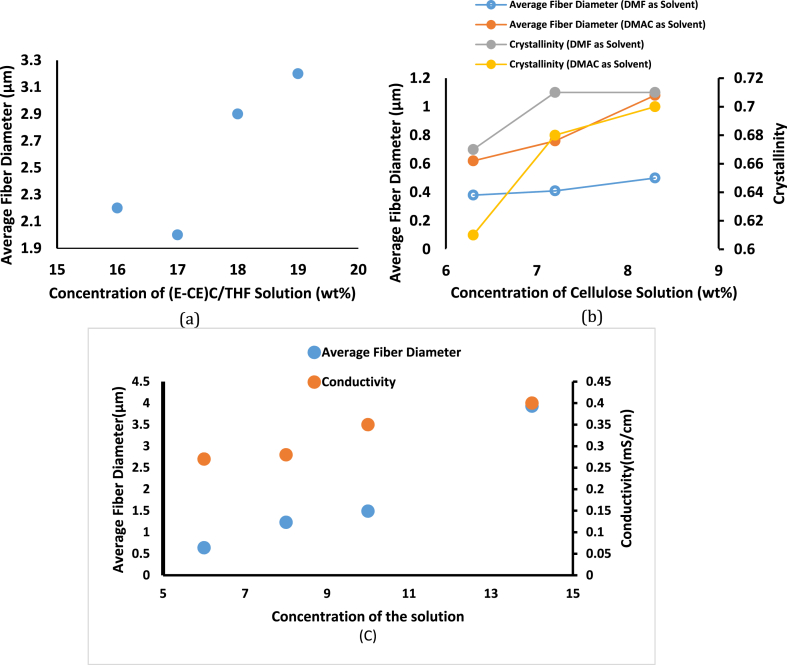
The electrospinning method is based on the phenomenon of a charged jet extending uniaxially. Changing the concentration of the polymeric solution has a tremendous impact on the stretching of the charged jet. For example, when the concentration of the polymeric solution is low, the applied electric field and surface tension cause the entangled polymer chains to break up before reaching the collector. Beads or beaded nanofibers are formed because of these fragments; therefore, at very low concentrations, the average fiber diameter is high until the critical value of concentration, as explained in Table 1 [22,27,49]. On the other hand, the viscosity of the polymeric solution increases as the concentration of the solution increases, and the number of macromolecular chains in the solution increases, as does the macromolecular chain entanglement. The surface tension of the solution levels up with increasing concentration. Because the repulsion among the charges in the jet must be higher than the surface tension when the jet splits, increasing the surface tension can also prevent the charged solution jet from splitting. Therefore, as the concentration of the solution increases, the average diameter of the fibers boosts up considerably, and the diameter distribution widens, see Fig. 2(a–c) [26,27]. Moreover, increasing the concentration above a critical value (the concentration at which beadless homogeneous nanofibers are formed) obstructs the flow of the solution through the needle tip (the polymer solution dries at the tip of the metallic needle and blocks it), resulting in defective or beaded nanofibers [19,22,49] It is also noted that there is a difference in values when using different solvents, as explained in Table 1. There are considerable differences in the average and standard deviation of fiber diameter depending on the co-solvent types. In comparison to the fiber web prepared with DMAc, the average and standard deviation of fiber diameter in the fiber web prepared with DMF were significantly lower, as shown in Table 1 [26]. The difference in charge-induced partial polarity between the two co-solvents might explain the effect of the co-solvent type. DMF has a higher partial polarity by electrical charge for spinning than DMAc. During the whipping process, the solution containing DMF would have a higher probability of being properly stretched. Better whipping resulted in finer, more uniform fibers. Crystallinity was higher in the fiber produced with higher co-solvent content [26]. The co-solvent type impacted the degree of crystallinity. The DMF electrospinning fiber has higher crystallinity than the DMAc electrospinning fiber, see Fig. 2(b) [26]. The diffusion rate of the co-solvent type is responsible for this outcome.
Table 1.
Effect of material parameters (concentration, viscosity, Type of Solvent, surface tension, and Conductivity) on the average fiber diameter.
| Material parameters | |||||
|---|---|---|---|---|---|
| Parameter | Material | Methods and Preparations | Results | Findings | Citation |
| Cellulose |
|
|
|
[26] | |
| Concentration, Viscosity and Type of Solvent | (E-CE)C |
|
|
|
[27] |
| (CMC) & (PEO) |
|
|
|
[28] | |
| Chitosan & PVA |
|
|
|
[29] | |
| Chitosan |
|
|
|
[30] | |
| Chitosan & PVA |
|
|
|
[31] | |
| Chitosan |
|
|
|
[32] | |
| CA |
|
|
|
[33] | |
| Cellulose |
|
|
|
[34] | |
| CA & CB |
|
|
|
[35] | |
| Chitosan |
|
|
|
[36] | |
| (PLA/CMC/GO-f-COOH) |
|
|
|
[37] | |
| Chitosan & PEO |
|
|
|
[38] | |
| Chitosan & PEO |
|
|
|
[39] | |
| Surface Tension | (E-CE)C |
|
|
|
[27] |
| H-Chitosan |
|
|
|
[40] | |
|
Conductivity Chitosan & PEO |
|
|
|
[41] | |
Fig. 2.
Effect of solution concentration for (a) (E-CE)C/THF solution on Average Fiber Diameter, for (b) Cellulose solution on Average Fiber Diameter and Crystallinity and (c) chitosan/chloroform solution on Average Fiber Diameter and Conductivity [26,27].
4.2. Machine parameters
4.2.1. Effect of applied voltage
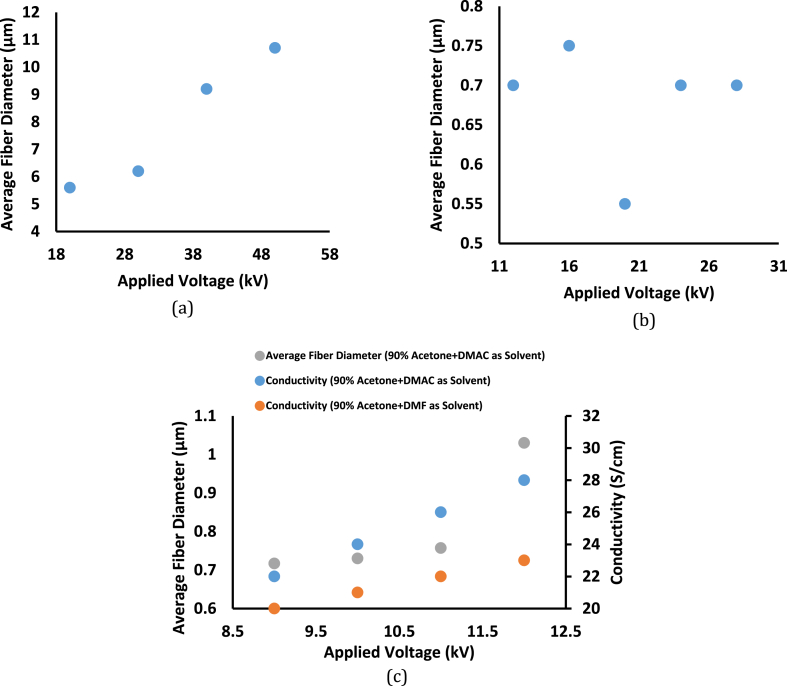
Fiber diameter increases with the increase of the applied voltage, as depicted in Fig. 3(a–c) [27,42]. The decrease in the size of the Taylor cone and rise in the jet velocity for the same flow rate is related to the increased diameter and formation of beads or beaded nanofibers with an increase in the applied voltage [19,25,50]. With decreasing electrostatic field voltage, the average diameter of the fiber decreases, and the dispersion of diameters narrows. The influence of the electrostatic field on the charged solution jet decreases as the voltage of the field decreases, and the jet’s flight speed decreases, consequently the time it takes to go from the anode to the collector increases. The charged jet’s ability to divide and elongate increases, making it easier to create thin fibers with a narrow diameter distribution [27,42,50]. While for chitosan, voltage doesn’t have a significant effect on the fiber diameter, see Fig. 3(b) [43]. Some parameters, such as the mass of polymer fed out from the needle’s tip, the elongation level of a jet caused by an electrical force, and the shape of a jet may be affected by the applied voltage (a single or multiple jets). The resulting diameter of electrospinning fibers may be controlled by a balance of several parameters, as explained in Table 2 [43]. For any type of solvent, conductivity increases by increasing applied voltage, due to the increase of electrostatic forces on the jet (Fig. 3(c)) [42].
Fig. 3.
Effect of Applied Voltage (a) for 17 wt% of (E-CE)C/THF Solution on Average Fiber Diameter, (b) for 8% of Chitosan Collagen dissolved in HFIP/TFA on Average Fiber Diameter and (c) for (Cellulose Acetate:DMAC or DMF) (1:9 wt/wt) on Conductivity and Average Fiber Diameter [27,42].
Table 2.
Effect of machine parameters (applied voltage, flow rate, TCD, and Orifice Diameter) on the average fiber diameter.
| Machine parameters | |||||
|---|---|---|---|---|---|
| Parameter | Material | Methods and Preparations | Results | Findings | Citation |
| Applied Voltage | (E-CE)C |
|
|
|
[27] |
| CA & EC |
|
|
|
[42] | |
| Chitosan-collagen |
|
|
|
[43] | |
| Chitosan-Collagen & PEO |
|
|
|
[44] | |
| CA |
|
|
|
[45] | |
| Flow Rate | Chitosan-collagen |
|
|
|
[43] |
| (E-CE)C |
|
|
|
[27] | |
| Tip to Collector Distance | Chitosan-collagen |
|
|
|
[43] |
| Chitosan & gelatin |
|
|
|
[46] | |
| Orifice Diameter | (E-CE)C |
|
|
|
[27] |
4.2.2. Effect of flow rate
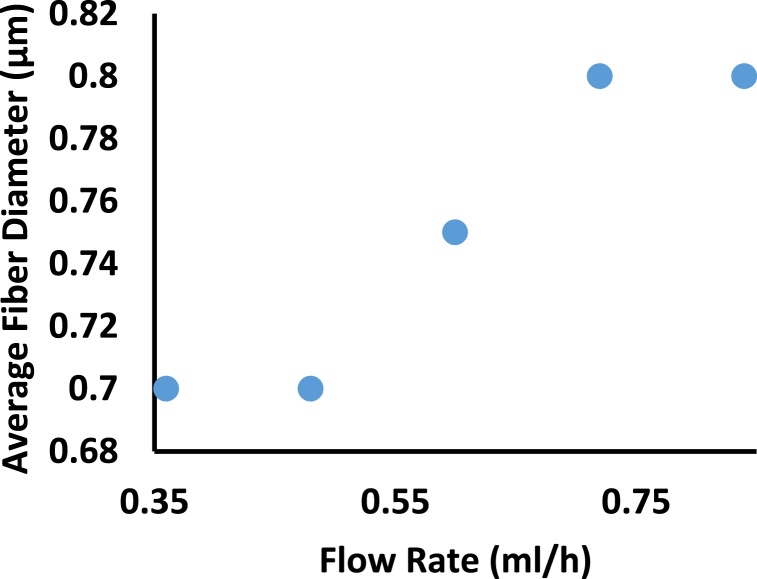
Increasing the flow rate above a critical threshold causes an increase in fiber diameter, as in Fig. 4 [43] and bead formation (Table 2) and this happens because the nanofiber jet is not completely dried during its journey between the needle tip and the metallic collector, as the pumped solution has no time to dry out and form intact fiber [43]. To maintain a balance, it is preferable to minimize the flow rate. This allows the formation of a stable jet cone and on sometimes a receded jet. Receded jet exits from the needle’s inside without forming a droplet or cone. These jets are not stable, and they are regularly replaced by cone jets during the electrospinning process [19,49].
Fig. 4.
Effect of Flow Rate on Average Fiber Diameter for 8% of Chitosan Collagen dissolved in HFIP/TFA [43].
4.2.3. Effect of needle to collector distance
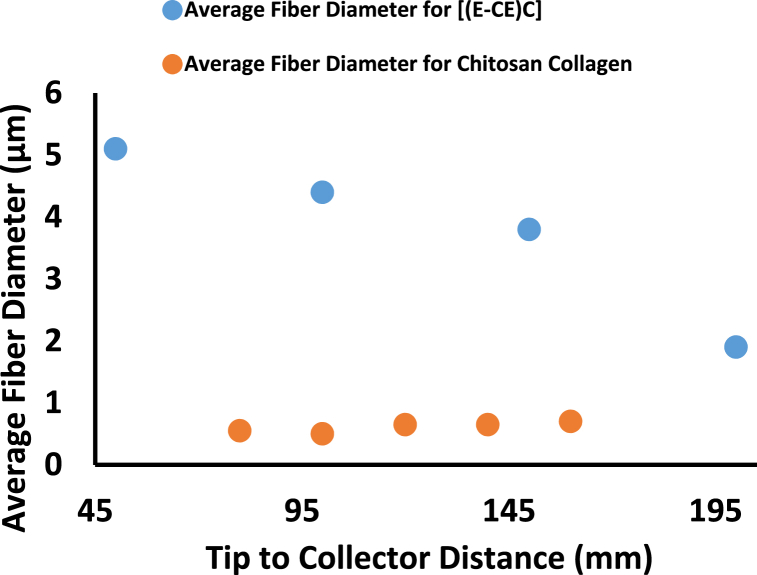
The needle to collector distance affects deposition time, evaporation rate, and whipping or instability interval so, the nanofiber morphology depends on it. To create smooth and uniform electrospinning nanofibers, a critical distance must be maintained, as explained in Table 2, and any alterations on either side of the critical distance will influence the morphology of the nanofibers [19,25,49,50]. The average fiber diameter decreases by increasing TCD (see Fig. 5) [27]. Extreme reduction of collecting distance leads to higher jet stretching and elongation, resulting in lower fiber diameters [27,43]. The jet may not have enough time to dry if the collecting distance is too short, resulting in a non-uniform fiber sample. Suitable flight duration to allow the solvents to evaporate is a critical requirement for the polymer solution jet. The fiber can be obtained within the acceptable collecting distance. Collecting distance may affect some factors such as the evaporation of the solvent, electric field strength, and so forth, as explained in Table 2.
Fig. 5.
Effect of TCD on Average Fiber Diameter for 17% of [(E-CE)C]/THF Solution and 8% of Chitosan Collagen dissolved in HFIP/TFA [27].
4.2.4. Effect of orifice diameter
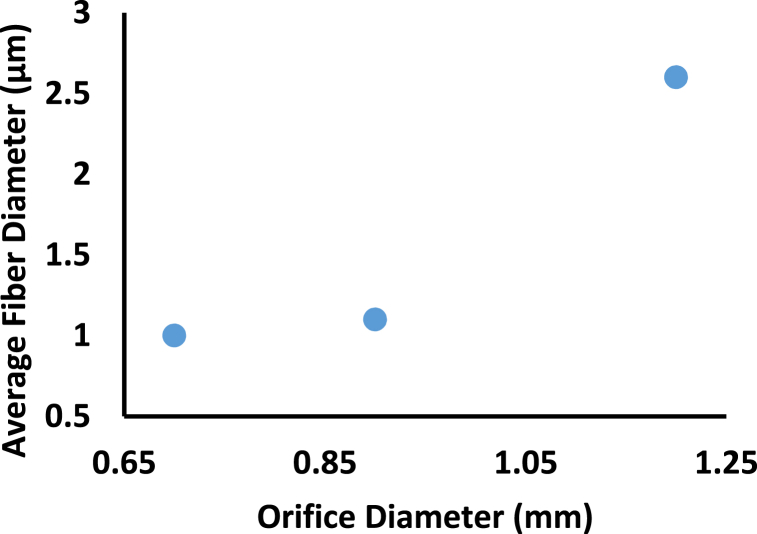
The average fiber diameter increases due to the increase in the orifice diameter, see Fig. 6 [27]. As the radius of the droplet decreases, the surface tension of the droplet increases which, in turn, reduces both the initial acceleration and average velocity. So, it takes a long time for a jet to travel from the anode to the collector. The charged solution jet has a higher chance to split and elongate. Using a narrow aperture, tiny diameter fibers with a limited dispersion may be produced [27].
Fig. 6.
Effect of orifice diameter on average fiber diameter for 17% of [(E-CE)C]/THF solution [27].
4.3. Ambient parameters (effect of humidity and temperature)
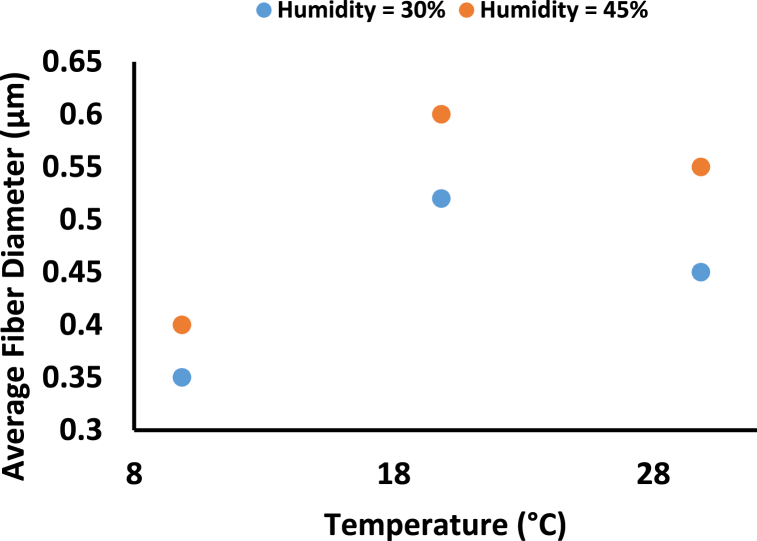
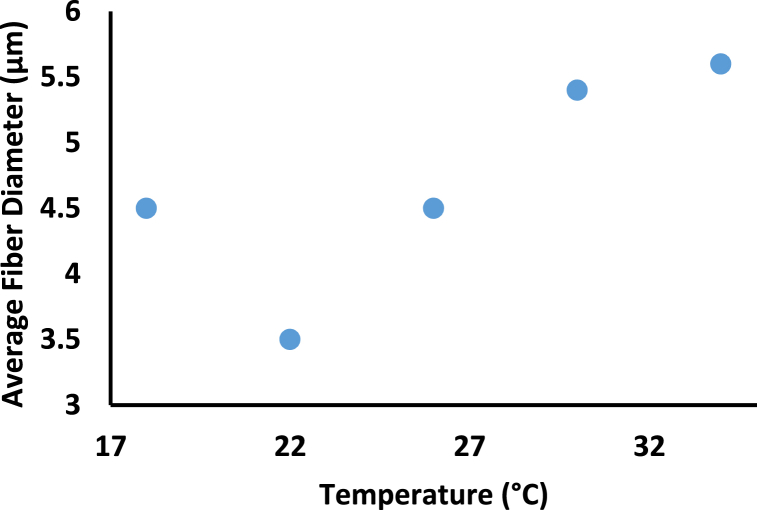
Environmental (ambient) variables such as relative humidity and temperature, in addition to machine and material characteristics, have recently been shown to impact the diameter and shape of nanofibers. The average diameter increased by increasing humidity, see Fig. 7 [47]. Precipitation will result in the formation of non-woven fibers. Humidity leads to a variation in the diameter of the nanofibers caused by the varying solidification of the charged jet and, thus, leads to forming bead fibers for individual polymers and almost no electrospinning for the blends [19]. An Increase in the spinning temperature results in an increase in the fiber diameter (Fig. 8) [27] and wider diameter distribution. When the solution temperature becomes higher, the diameter of the fibers also increases, as explained in Table 3 and diameter distribution widens [27]. There is an optimal value of spinning temperature at 22 °C that has a minimum average fiber diameter (see Fig. 8) [27]. A low spinning temperature leads to a low solvent evaporation speed, and the solvent cannot be entirely volatilized when the charged solution jets reach the collector. Agglutination of solution jets on the collector can result in increased fiber diameter and a broader diameter dispersion. Because of the rapid evaporation of the surface solvents at higher temperatures, the charged solution jets have less time to divide and elongate throughout their flight. At the optimal spinning temperature, which was 22 °C in this case, the solution jets solidify by solvent volatilization when they arrive at the collector, and the jets have enough time to split throughout their flight to the collector.
Fig. 7.
Effect of Humidity on Average Fiber Diameter for 17% Cellulose Acetate dissolved in (2:1 v/v) aceton:DMAC [47].
Fig. 8.
Effect of Temperature on Average Fiber Diameter for 17% [(E-CE)C]/THF solution [27].
Table 3.
Effect of Ambient parameters (humidity and temperature) on the average fiber diameter.
| Ambient parameters | |||||
|---|---|---|---|---|---|
| Parameter | Material | Methods and Preparations | Results | Findings | Citation |
| (E-CE)C |
|
|
|
[27] | |
| Humidity and Temperature | CA |
|
|
|
[47] |
| Chitosan |
|
|
|
[48] | |
5. Conclusion
Electrospinning is a reliable and widely used method that has been implemented in various fields. Cellulose and chitosan nanofibers are particularly popular due to their numerous applications. Cellulose nanofibers, for instance, find use in tissue engineering and medical implants, while chitosan nanofibers are used for water purification and air filters. To improve the characteristics of electrospinning nanofibers, extensive research has been conducted to explore the impact of various parameters such as concentration, viscosity, type of solvent, surface tension, conductivity, applied voltage, flow rate, TCD, orifice diameter, humidity, and temperature. Based on the findings, it is concluded for:
Cellulose:
-
•
The average fiber diameter increased gradually with the increase in solution concentration.
-
•
With the increase of the applied voltage, the average fiber diameter increased.
-
•
Due to the increase in TCD, the average fiber diameter decreased gradually.
-
•
The average fiber diameter increased with the increase of the orifice diameter.
-
•
With the increase in spinning and solution temperature, the average fiber diameter gradually increased.
-
•
the average fiber diameter increased with the increase in humidity.
Chitosan:
-
•
The average fiber diameter was almost constant with the increase in the applied voltage.
-
•
The average diameter gradually increased with the increase of the flow rate.
-
•
The average fiber diameter is nearly fixed by the increase of the TCD.
According to previous findings, it was discovered that to produce fine fibers from cellulose and chitosan, optimized monitoring of the different parameters (material, machine, and ambient parameters) should be performed, and this will be accomplished through the study of changing these parameters all at once rather than individually.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgement
Not applicable.
Abbreviations
- IL
Ionic liquid
- [C2min][OAc]
1-ethyl-3-methylimidazolium acetate
- DMF
Dimethylformamide
- DMAc
Dimethylacetamide
- TCD
Tip to collector distance
- (E-CE)C
Ethyl– cyanoethyl cellulose
- Mn
Number average molecular weight
- THF
Tetrahydrofuran
- CMC
Carboxy-methyl cellulose sodium salt
- PEO
Polyethylene oxide
- MW
Molecular weight
- DS
Degree of substitution
- Mv
viscosity average molecular weight
- PVA
Poly(vinyl alcohol)
- FA
Formic acid
- DWD
Distilled water
- DCMD
Dichloromethane
- CA
Cellulose Acetate
- EC
Ethyl cellulose
- H-chitosan
Hexanoyl chitosan
- HFIP
1,1,1,3,3,3- hexafluoroisopropanol
- RH
Relative humidity
- BMIMAc
1-butyl-3-methyl-imidazolium acetate
- DMAA
N,N-dimethylacetamide
- PEG
Polyethylene glycol
- CB
Carbon Black
- TFA
Trifluoroacetic acid
- PLA
Polylactic acid
- CMC
Carboxyl functionalized graphene oxide
- GO
Graphene oxide
References
- 1.Ibrahim H., Mehanny S., Darwish L., Farag M. A comparative study on the mechanical and biodegradation characteristics of starch-based composites reinforced with different lignocellulosic fibers. J. Polym. Environ. 2018;26:2434–2447. doi: 10.1007/s10924-017-1143-x. [DOI] [Google Scholar]
- 2.Fathy S., Mehanny A. 2012. Fabrication and Characterization of Starch Based Sugar Cane Bagasse Fibers Fabrication and Characterization of Starch Based Sugar Cane Bagasse Fibers. [Google Scholar]
- 3.Mehanny S., Ibrahim H., Darwish L., Farag M., El-Habbak A.-H.M., El-Kashif E. In: Effect of Environmental Conditions on Date Palm Fiber Composites. Midani M., Saba N., Alothman O.Y., editors. Springer Singapore; Singapore: 2020. pp. 287–320. (Date Palm Fiber Composites: Processing, Properties and Applications). [DOI] [Google Scholar]
- 4.Mehanny S., Abu-El Magd E.E., Ibrahim M., Farag M., Gil-San-Millan R., Navarro J., el Habbak A.E.H., El-Kashif E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021;10:526–537. doi: 10.1016/j.jmrt.2020.12.027. [DOI] [Google Scholar]
- 5.Ammar Z., Ibrahim H., Adly M., Sarris I., Mehanny S. Influence of natural fiber content on the frictional material of brake pads—a review. J. Compos. Sci. 2023;7:72. doi: 10.3390/jcs7020072. [DOI] [Google Scholar]
- 6.El-Moayed M.H., Kühn J., Lee S.-H., Farag M., Mehanny S. IntechOpen; 2022. Potential of Lignin Valorization with Emphasis on Bioepoxy Production. (Lignin - Chemistry, Structure, and Application [Working Title]). [DOI] [Google Scholar]
- 7.Hassan M.K., El-sherbiny A., Mehanny S. 2021. Current Status of Cellulosic and Nanocellulosic Materials for Oil Spill Cleanup. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim M.M., Moustafa H., Rahman E.N.A.E.L., Mehanny S., Hemida M.H., El-Kashif E. Reinforcement of starch based biodegradable composite using nile rose residues. J. Mater. Res. Technol. 2020;9:6160–6171. doi: 10.1016/j.jmrt.2020.04.018. [DOI] [Google Scholar]
- 9.Subbiah T., Bhat G.S., Tock R.W., Parameswaran S., Ramkumar S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005;96:557–569. doi: 10.1002/app.21481. [DOI] [Google Scholar]
- 10.Mehanny S., Magd E.E.A.-E., Sorbara S., Navarro J., Gil-San-Millan R. Spanish poplar biomass as a precursor for nanocellulose extraction. Appl. Sci. 2021;11 doi: 10.3390/app11156863. [DOI] [Google Scholar]
- 11.Lyddy R. Nanotechnology. Inf. Resour. Toxicol. 2009:321–328. doi: 10.1016/B978-0-12-373593-5.00036-7. [DOI] [Google Scholar]
- 12.Khare S., Williams K., Gokulan K. second ed. 2014. Nanotechnology, Encyclopedia of Food Microbiology; pp. 893–900. [DOI] [Google Scholar]
- 13.el Fawal G.F. Polymer nanofibers electrospinning: a review, Egypt. J. Chem. 2020;63:1279–1303. doi: 10.21608/ejchem.2019.14837.1898. [DOI] [Google Scholar]
- 14.Rezaei F., de Geyter N., Morent R. 2016. Pre-electrospinning Polymer Solution Treatment by Atmospheric-Pressure Argon Plasma Jet; p. 1. (2016 IEEE International Conference on Plasma Science (ICOPS)). [DOI] [Google Scholar]
- 15.Raghavan P., Lim D.H., Ahn J.H., Nah C., Sherrington D.C., Ryu H.S., Ahn H.J. Electrospun polymer nanofibers: the booming cutting edge technology. React. Funct. Polym. 2012;72:915–930. doi: 10.1016/j.reactfunctpolym.2012.08.018. [DOI] [Google Scholar]
- 16.Topuz F., Uyar T. Electrospinning of nanocomposite nanofibers from cyclodextrin and laponite. Compos. Commun. 2019;12:33–38. doi: 10.1016/J.COCO.2018.12.002. [DOI] [Google Scholar]
- 17.Reneker D.H., Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. doi: 10.1088/0957-4484/7/3/009. [DOI] [Google Scholar]
- 18.Bhardwaj N., Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Haider A., Haider S., Kang I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018;11:1165–1188. doi: 10.1016/j.arabjc.2015.11.015. [DOI] [Google Scholar]
- 21.Cavo M., Serio F., Kale N.R., D’Amone E., Gigli G., del Mercato L.L. Electrospun nanofibers in cancer research: from engineering of: from vitro 3D cancer models to therapy. Biomater. Sci. 2020;8:4887–4905. doi: 10.1039/d0bm00390e. [DOI] [PubMed] [Google Scholar]
- 22.Haider S., Al-Zeghayer Y., Ahmed Ali F.A., Haider A., Mahmood A., Al-Masry W.A., Imran M., Aijaz M.O. Highly aligned narrow diameter chitosan electrospun nanofibers. J. Polym. Res. 2013;20 doi: 10.1007/s10965-013-0105-9. [DOI] [Google Scholar]
- 23.Bae H.S., Haider A., Selim K.M.K., Kang D.Y., Kim E.J., Kang I.K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 2013;20 doi: 10.1007/s10965-013-0158-9. [DOI] [Google Scholar]
- 24.Beachley V., Wen X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C. 2009;29:663–668. doi: 10.1016/j.msec.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslan E., Almeida H., Al-Deyab S., El-Newehy M., Bartolo H., Bártolo P.J. 2021. The Electrospinning Process. [DOI] [Google Scholar]
- 26.Ahn Y., Hu D.H., Hong J.H., Lee S.H., Kim H.J., Kim H. Effect of co-solvent on the spinnability and properties of electrospun cellulose nanofiber. Carbohydr. Polym. 2012;89:340–345. doi: 10.1016/j.carbpol.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S., Wu X., Wang L., Huang Y. 2003. Electrospinning of Ethyl – Cyanoethyl Cellulose/Tetrahydrofuran Solutions. [Google Scholar]
- 28.Audrey Frenot, Henriksson Walenius Maria, Pernilla Walkenstrom. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2006 doi: 10.1002/app. [DOI] [Google Scholar]
- 29.Ohkawa K., Cha D., Kim H., Nishida A., Yamamoto H. Electrospinning of chitosan. Macromol. Rapid Commun. 2004;25:1600–1605. doi: 10.1002/marc.200400253. [DOI] [Google Scholar]
- 30.Sencadas V., Correia D.M., Areias A., Botelho G., Fonseca A.M., Neves I.C., Gomez Ribelles J.L., Lanceros Mendez S. Determination of the parameters affecting electrospun chitosan fiber size distribution and morphology. Carbohydr. Polym. 2012;87:1295–1301. doi: 10.1016/j.carbpol.2011.09.017. [DOI] [Google Scholar]
- 31.Jamnongkan T., Wattanakornsiri A., Pansila P.P., Migliaresi C., Kaewpirom S. Effect of poly(vinyl alcohol)/chitosan ratio on electrospun-nanofiber morphologies. Adv. Mater. Res. 2012;463–464:734–738. doi: 10.4028/www.scientific.net/AMR.463-464.734. [DOI] [Google Scholar]
- 32.Geng X., Kwon O.H., Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26:5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 33.Han S.O., Youk J.H., Min K.D., Kang Y.O., Park W.H. Electrospinning of cellulose acetate nanofibers using a mixed solvent of acetic acid/water: effects of solvent composition on the fiber diameter. Mater. Lett. 2008;62:759–762. doi: 10.1016/j.matlet.2007.06.059. [DOI] [Google Scholar]
- 34.Ciuzas D., Krugly E., Sriubaite S., Pauliukaityte I., Baniukaitiene O., Bulota M., Martuzevicius D. Electrospun cellulose fibers from ionic liquid: practical implications toward robust morphology. J. Appl. Polym. Sci. 2022;139 doi: 10.1002/app.51525. [DOI] [Google Scholar]
- 35.Elmaghraby N.A., Omer A.M., Kenawy E.R., Gaber M., el Nemr A. Electrospun composites nanofibers from cellulose acetate/carbon black as efficient adsorbents for heavy and light machine oil from aquatic environment. J. Iran. Chem. Soc. 2022;19:3013–3027. doi: 10.1007/s13738-022-02510-1. [DOI] [Google Scholar]
- 36.Totito T.C., Laatikainen K., Bode-Aluko C., Pereao O., Petrik L. Fabrication and characterization of electrospun waste polyethylene terephthalate blended with chitosan: a potential single-use material. Polymers. 2023;15 doi: 10.3390/polym15020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Arjan W.S. Self-assembled nanofibrous membranes by electrospinning as efficient dye photocatalysts for wastewater treatment. Polymers. 2023;15 doi: 10.3390/polym15020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abd El Hady W.E., Soliman O.A.E.A., el Sabbagh H.M., Mohamed E.A. Glutaraldehyde-crosslinked chitosan-polyethylene oxide nanofibers as a potential gastroretentive delivery system of nizatidine for augmented gastroprotective activity. Drug Deliv. 2021;28:1795–1809. doi: 10.1080/10717544.2021.1971796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dodero A., Brunengo E., Alloisio M., Sionkowska A., Vicini S., Castellano M. Chitosan-based electrospun membranes: effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020;235 doi: 10.1016/j.carbpol.2020.115976. [DOI] [PubMed] [Google Scholar]
- 40.Neamnark A., Rujiravanit R., Supaphol P. Electrospinning of hexanoyl chitosan. Carbohydr. Polym. 2006;66:298–305. doi: 10.1016/j.carbpol.2006.03.015. [DOI] [Google Scholar]
- 41.Duan B., Dong C., Yuan X. Polymer Edition Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide) J. Biomater. Sci. 2012:37–41. doi: 10.1163/156856204774196171. [DOI] [PubMed] [Google Scholar]
- 42.Um-I-Zahra S., Li H., Zhu L. Effect of different parameters on the fabrication of sustained release cellulose acetate and ethyl cellulose polymer blends. Cellul. Chem. Technol. 2017;51:899–909. [Google Scholar]
- 43.Erukhimovich I., de la Cruz M.O. 2004. Phase Equilibria and Charge Fractionation in Polydisperse Polyelectrolyte Solutions; pp. 1949–1955. [DOI] [Google Scholar]
- 44.Amiri N., Moradi A., Tabasi S.A.S., Movaffagh J. Modeling and process optimization of electrospinning of chitosan-collagen nanofiber by response surface methodology. Mater. Res. Express. 2018;5 doi: 10.1088/2053-1591/aaba1d. [DOI] [Google Scholar]
- 45.Angel N., Guo L., Yan F., Wang H., Kong L. Effect of processing parameters on the electrospinning of cellulose acetate studied by response surface methodology. J Agric Food Res. 2020;2 doi: 10.1016/j.jafr.2019.100015. [DOI] [Google Scholar]
- 46.O.A. Journal, Biointerface Research in Applied Chemistry. 2020;10:5556–5563. [Google Scholar]
- 47.De Vrieze S., Van Camp T., Nelvig A., Hagström B., Westbroek P., De Clerck K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009;44:1357–1362. doi: 10.1007/s10853-008-3010-6. [DOI] [Google Scholar]
- 48.Thi T., Quyen B., Van Toan P., Ho M.H., Thien D.V.H. Temperature e ff ects on electrospun chitosan nano fi bers. Green Process. Synth. 2020;9:488–495. [Google Scholar]
- 49.Pillay V., Dott C., Choonara Y.E., Tyagi C., Tomar L., Kumar P., du Toit L.C., Ndesendo V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013;2013 doi: 10.1155/2013/789289. [DOI] [Google Scholar]
- 50.Long Y.Z., Yan X., Wang X.X., Zhang J., Yu M. 2019. Electrospinning: the setup and procedure; pp. 21–52. (Electrospinning: Nanofabrication and Applications). [DOI] [Google Scholar]
- 51.Elsayed Hamdy, Mahmoud Farag, Hassan Megahed, Sherif Mehanny. ASME International Mechanical Engineering Congress and Exposition. vol. 45196. American Society of Mechanical Engineers; 2012. Influence of flax fibers on properties of starch-based composites; pp. 1397–1408. [DOI] [Google Scholar]
- 52.Hemida Mohamed H., Moustafa Hesham, Mehanny Sherif, Morsy Mohamed, Dufresne Alain, Abd EL Rahman Eid N., Ibrahim M.M. Cellulose nanocrystals from agricultural residues (Eichhornia crassipes): extraction and characterization. Heliyon. 2023 doi: 10.1016/j.heliyon.2023.e16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.