Abstract
Objective
To systematically review and summarize the existing evidence related to the influence of the menstrual cycle (MC) and hormonal contraceptive (HC) use on O2max in physically active women.
Methods
This systematic review and meta-analysis conforms to the PRISMA statement guidelines. Four (sub-)meta-analyses were performed. Two focused on longitudinal studies examining the same women several times to compare the O2max during the different menstrual phases or oral contraceptive (OC) use and withdrawal. Two meta-analyses examined if there is a difference in O2max between OC users and normally menstruating women by analyzing cross-sectional studies assigning physically active women to one of these two groups as well as intervention-based studies (cross-over studies, randomized controlled trials considering only the data of the intervention group) comparing women intra-individually with and without OCs.
Results
Nine of the included studies (107 women) evaluated the influence of the MC, five studies (69 women) the impact of OCs on O2max, and six studies investigated both topics (88 women). A mean difference of O2max −0.03 ml/kg/min (95%CI –1.06 to 1.01) between the early follicular and luteal menstrual phase was observed. Between the active and inactive phases of OCs, a mean difference of −0.11 ml/kg/min (95%CI –2.32 to 2.10) was found. The inter-individual comparison of naturally menstruating women and OC users showed a mean difference in O2max of 0.23 ml/kg/min (95% CI –2.33 to 2.79) in favor of OC use. The intra-individual comparison of the same women showed a mean decrease in O2max of −0.84 ml/kg/min (95% CI –2.38 to 0.70) after a new start with OCs.
Conclusions
Our meta-analyses showed no effects of the MC or the OCs on O2max. More high-quality studies are needed determining the MC phases more precisely, including OCs with the current standard formulations and comparing the influence of different progestins.
Keywords: Menstrual cycle, Hormonal contraceptive, Cardiorespiratory fitness, Physically active women
1. Introduction
Several studies have been conducted to investigate the effect of the menstrual cycle (MC) or hormonal contraceptives (HC) on exercise performance. A group of researchers summarized these studies in two systematic reviews and meta-analyses [1,2]. These analyses indicated that exercise performance might be slightly reduced during the early follicular phase of the MC and that the use of oral contraceptives (OC) might result in an inferior exercise performance compared to naturally menstruating women. Due to the trivial effect size, the large between-study variation, and the number of poor-quality studies included in these meta-analyses, general guidelines on exercise performance could not be deducted [1,2]. Another systematic review included studies on the effect of the MC and OCs on responses to resistance training [3]. The review article reported conflicting results, with studies often limited by small sample sizes and methodological issues, but do appear to suggest that female hormones may affect the resistance training response [3].
It should be noted that there are some limitations within studies in sport and exercise science with women as participants [4]. Due to a lack of agreement on the terminology and methodological approaches, MC phases were not uniformly defined and examined, OCs with variable dosages of ethinyl estradiol (EE) and different kinds of progestin were included and compared. Furthermore, exercise performance was measured using different parameters and participants ranged from sedentary to physically active to elite athletes [1,2,4].
The current systematic review and meta-analysis investigates the influence of endogenous and exogenous female steroid hormones on cardiorespiratory fitness. In addition to the reproductive system, these hormones have numerous physiological effects that could affect the cardiovascular system, respiratory function, thermoregulation, and substrate metabolism [[5], [6], [7], [8]]. For example, progesterone augments body temperature and appears to stimulate minute ventilation [5,6], whereas estrogen seems to enhance muscular glycogen storage and increase lipid availability and utilization [7,8]. An understanding of this influence is important in terms of maximizing health benefits [9] and could also be relevant to optimizing training and competition practices [10].
Hence, previous reviews on the topic [1,2] have considered females in general with conflicting or trivial results, the present one focuses on physically active women in order to minimize the risk of potential changes in the outcome measures of the performance test due to the training effect, to reduce a large variability in the measures and to increase the sensitivity to detect smaller changes [11]. Furthermore, the current systematic review and meta-analysis is focused on a specific outcome, which is cardiorespiratory fitness (operationalized by the maximum oxygen uptake [O2max]). O2max is an objective and standardized parameter that directly measures cardiorespiratory fitness. It represents the “gold standard” for assessing exercise capacity [9] and is one of the most important physiological determinants of endurance performance [12]. Specifically, O2max is a parameter to evaluate the integrated functioning of the pulmonary, cardiovascular, and muscle systems to uptake, transport, and utilize O2 predominantly in the contracting muscle mitochondria [13].
Thus, the aim of this review and meta-analysis was to determine the influence of different MC phases as well as the use of HCs on O2max in physically active women.
2. Material and methods
This systematic review and meta-analysis is based on the PRISMA statement guidelines [14] and was conducted using a registered protocol (PROSPERO CRD42021291213).
2.1. Study inclusion and exclusion criteria
We defined the eligibility criteria using the PICOS model as follows:
Population: healthy, physically active women. A population was defined as “physically active” if the mean relative O2max was equal to or greater than 40 ml/min/kg [15].
Intervention: the influence of the cyclic hormone changes of endogenous hormones throughout the MC in eumenorrheic women and the influence of exogenous hormones in women taking HCs on cardiorespiratory fitness. Eumenorrhea was defined as an MC ranging between 21 and 35 days in length. All forms of HCs were considered: OC, vaginal ring, patch, progestin-only contraceptive (pill, implant, intrauterine device [IUD])
Comparator and study design: Four comparison/(sub-)meta-analyses were performed. Two focused on longitudinal studies examining the same women several times in order to compare the O2max during the different menstrual phases or during OC use and withdrawal. The other two meta-analyses assessed a potential difference in O2max between OC users and eumenorrheic women by analyzing cross-sectional studies assigning physically active women to one of these two groups (inter-individual comparison) as well as intervention-based studies (randomized-controlled trials [RCT], cross-over studies) comparing the same women with and without HCs (intra-individual comparison).
Outcome: cardiorespiratory fitness measured by relative O2max assessed in ml/kg/min.
2.2. Search strategy
The electronic search was conducted on PubMed, Web of Science, Embase, Cochrane Library, and Google Scholar [16]. The Google Scholar search was limited to the first 300 articles [17]. The search strategy was based on combinations of “menstrual cycle” or “hormonal contraceptives” and “exercise performance” terms and was designed and supported by a clinical librarian. The following search terms and their combinations were used: (‘oral contraceptive’ OR ‘levonorgestrel’ OR ‘ethinyl estradiol’ OR ‘contraceptive’) OR (‘menstrual cycle’ OR ‘menstrual phase’, OR ‘follicular phase’, OR ‘luteal phase’) AND (‘aerobic’, OR ‘aerobic power’, OR ‘aerobic capacity’, OR ‘endurance’, OR ‘endurance capacity’, OR ‘anaerobic’, OR ‘anaerobic capacity’ OR ‘aerobic threshold’ OR ‘oxygen consumption’ OR ‘oxygen uptake’ OR ‘VO2peak’ OR ‘VO2max’). The electronic database search was conducted by two reviewers (C.B., MJ.S.) on November 2nd, 2021. No language or date restrictions were applied.
2.3. Data selection and extraction
All duplicates were removed by the Endnote “find duplicates” function. Two independent reviewers (C.B., LF.S.) screened the titles and abstracts as well as, in a second stage, the full articles with the Rayyan QCRI app [18]. Only published full-text articles were taken into consideration. Any disagreement about the inclusion of studies was resolved by consensus or a third reviewer (MJ.S.) where necessary.
Two reviewers (C.B., LF.S.) independently extracted data using a predesigned data collection form that captured information on study design, sample size, performance test, methods of MC verification, definition of test phases, formulation and application period of the used OC and outcome (relative O2max, assessed in ml/kg/min). If studies just published the absolute O2max value (assessed in l/min), authors were contacted in order to obtain the relative O2max value. Thus, one of six contacted authors [19] provided us with unpublished information, the other five studies were excluded.
2.4. Quality assessment
The methodological quality of the included articles was assessed with the Newcastle-Ottawa Scale (NOS) [20] for the non-randomized studies and with the Cochrane Collaboration's tool for assessing risk of bias (RoB 2) [21] for the RCTs.
NOS assesses the quality of the articles in three domains of selection, comparability and exposure. An adapted version of the NOS for case-control studies was used for the cross-sectional studies. An adapted version of NOS for cohort studies was applied for the longitudinal studies examining the same women at different phases of the MC, during the inactive/active OC phase or with/without OCs. In both adapted versions, specific quality characteristics relevant to the conclusion of the current meta-analysis were included. Studies that received a score of eight to nine stars were judged to be at low risk of bias, studies that scored seven stars were considered at medium risk, and studies that scored less than six stars were considered at high risk of bias.
With the RoB 2 tool, studies are judged to be at low, some concern or high risk of bias on the basis of criteria evaluating the randomization process, deviation from the intended interventions, missing outcome data, measurement of outcome and selection of the reported results [21].
Two reviewers (C.B., LF.S.) performed the assessment independently. Scores were compared, and any inter-reviewer disagreements were resolved by consensus or by a third reviewer (MJ.S.) where necessary.
2.5. Data analysis
Most of the included studies differentiated solely the follicular and the luteal phase of the MC. The follicular phase should be divided into an early follicular (low progesterone, low estrogen) and late follicular (low progesterone, high estrogen) phase [22]. Based on serum hormone analysis, the “follicular” phase was interpreted as the “early follicular” phase of the MC in the included studies. Only two [23,24] studies correctly examined three phases and made a serum measurement of estrogen and progesterone to verify them. Therefore, we could just examine the early follicular and the luteal phase of the MC.
Just two RCTs [25,26] met the inclusion criteria for the intra-individual comparison of women with and without OCs, whereas one of them did not publish the results of the placebo group [25]. Since OC use causes alterations in the pattern of the normal MC and bleeding, performing true double-blind studies is highly challenging. In the study of Lebrun et al. [26], approximately half of the participants were aware that they were taking OCs or the placebo. Therefore, we merged the data of the intervention group (OC use) of the RCTs with the data of studies analyzed women prior to and after OC treatment in a cross-over design.
Some studies examined O2peak instead of O2max [19,27,28]. These studies were included in the current meta-analysis using O2peak as an indicator for O2max based on the suggestion that the O2peak attained on a maximum-effort incremental test in subjects exercising to the limit of tolerance is likely to be a valid index of O2max [29].
Depending on the range of mean O2max and the number of subjects included, the highly trained women with a O2max > 53 ml/kg/min [15] would be considered separately because, especially for this population, even a small difference could be relevant.
For each of the four meta-analyses (see chapter 2.1.), a random-effect model with the REML (restricted maximum likelihood) estimation method was applied. Between-study heterogeneity was determined using I2. Effect estimates for O2max were provided per study and as mean differences with corresponding 95% confidence intervals and visualized using forest plots. Given the small sample size of several included studies, the robustness of the estimates was assessed by sensitivity analyses utilizing fixed-effects inverse-variance models. Analyses were undertaken using Stata (Version 16.1, StataCorp, College Station, Texas, USA).
3. Results
3.1. Literature search
The literature search and selection of studies are presented in Fig. 1.
Fig. 1.
PRISMA flow diagram presenting screening process and search results.
3.2. Study characteristics
Details of the included studies are shown in Table 1, Table 2, Table 3, Table 4. A total of 20 studies were included in this systematic review and meta-analysis. Nine of the 20 studies examined the influence of the MC on O2max (including 107 women) [19,23,24,[30], [31], [32], [33], [34], [35]], five studies examined the influence of OCs on O2max (including 69 women) [26,27,[36], [37], [38]] and six assessed both topics (including 88 women) [11,25,28,[39], [40], [41]].
Table 1.
Longitudinal studies, examining the effect of menstrual cycle phases on O2max.
| Author(s), year | n | Performance test | Method of MC phase verification | Phase of MC |
Results |
Risk of bias (NOS) | ||
|---|---|---|---|---|---|---|---|---|
| Definition of the different MC phases | Serum estradiol (pmol/L) | Serum progesterone (nmol/L) | O2max (ml/kg/min) | |||||
| Beidleman et al., 1999 [][30] | 8 | treadmill | calendar-based counting method, urinary LH, serum hormone analysis | EF (day 3–6) | 143.2 +/− 80.8 | 1.5 +/− 0.6 | 46.8 +/− 4.0 | moderate |
| L (6–9 days after LH surge) | 411.2 +/− 139.5 | 42.7 +/− 28.1 | 46.3 +/− 5.6 | |||||
| Bemben et al., 1995 [][31] | 5 | treadmill | calendar-based counting method, basal body temperature, serum hormone analysis | EF (day 2–5) | not examined | 1.4 +/− 0.2 | 42.0 +/− 7.6 | high |
| LF (day 12–15) | not examined | 1.3 +/− 0.2 | 42.1 +/− 7.8 | |||||
| L (day 20–23) | not examined | 21.2 +/− 13.9 | 43.6 +/− 6.5 | |||||
| Bryner et al., 1996 [25] | 10 | treadmill | calendar-based counting method (ovulation = cycle length minus 12–14 days), transabdominal ultrasound to confirm ovulation | F (day of ovulation divided by two) | not examined | not examined | 41.6 +/− 12.1 | high |
| L (6–8 days after ovulation) | not examined | not examined | 39.7 +/− 11.4 | |||||
| Casazza et al., 2002 [28] | 6 | cycle ergometer | calendar-based counting method, urinary LH, serum hormone analysis | F (day 4–8) | 125.2 +/− 38.9 | 1.2 +/− 0.1 | 42.3 +/− 8.1a | moderate |
| L (day 17–25) | 271.3 +/− 56.2 | 33.7 +/− 7.6 | 42.6 +/− 7.8a | |||||
| Dean et al., 2003 [23] | 8 | cycle ergometer | calendar-based counting method, basal body temperature, serum hormone analysis | EF (day 3 +/− 1.6) | 121.2 +/− 38.5 | 2.5 +/− 0.8 | 43.0 +/− 6.5 | low |
| LF (day 10 +/− 2.6) | 433.2 +/− 244.1 | 4.1 +/− 2.8 | 42.7 +/− 6.5 | |||||
| L (day 21 +/− 1.8) | 422.2 +/− 88.2 | 37.2 +/− 25.2 | 42.5 +/− 5.1 | |||||
| De Souza et al., 1990 [32] | 8 | treadmill | calendar-based counting method, urinary LH, serum hormone analysis | EF (day 2–4) | 135.4 +/− 71.3 | not examined | 53.1 +/− 4.5 | moderate |
| L (6–8 days after ovulation) | 554.9 +/− 258.2 | not examined | 53.7 +/− 3.8 | |||||
| Frandsen et al., 2020 [24] | 19 | cycle ergometer | calendar-based counting method, serum hormone analysis | EF (tested at 25% of the MC length) | 155 +/− 68.5 | 0.8 +/− 0.3 | 43.9 +/− 5.71 | moderate |
| LF (tested at 40–45% of the MC length) | 574 +/− 437.8 | 0.9 +/− 0.5 | 43.9 +/− 5.4 | |||||
| L (tested at 75% of the MC length) | 581 +/− 438.8 | 37.6 +/− 24.1 | 43.3 +/− 5.6 | |||||
| Goldsmith & Glaister, 2020 [33] | 10 | treadmill | calendar-based counting method, serum hormone analysis | EF (day 2 +/− 2) | not examined | 3.7 +/− 4.3 | 58.2 +/− 4.2 | moderate |
| LF (4 +/− 2 days prior to ovulation) | not examined | 1.5 +/− 1.4 | 58.4 +/− 4.7 | |||||
| L (2 +/− 1 days from the progesterone peak) | not examined | 32.7 +/− 30.8 | 59.7 +/− 4.7 | |||||
| Gorden et al., 2018 [40] | 10 | cycle ergometer | calendar-based counting method, salivary hormone analysis | menstruation (day 1–5, interpreted as EF) | not examined | not examined | 41.6 +/− 3.7 | high |
| LF (day 9–11) | not examined | not examined | 44.1 +/− 3.9 | |||||
| L (day 19–20) | not examined | not examined | 42.6 +/− 2.9 | |||||
| Lebrun et al., 1995 [34] | 16 | treadmill | calendar-based counting method, serum hormone analysis | EF (day 3–8) | 141.4 +/− 63.2 | 1.2 +/− 0.4 | 53.7 +/− 3.6 | moderate |
| L (4–9 days after ovulation) | 461.4 +/− 147.6 | 40.6 +/− 14.8 | 52.8 +/− 3.2 | |||||
| Mattu et al., 2019 [11] | 15 | cycle ergometer | calendar-based counting method, urinary LH | F (day 5–10) | not examined | not examined | 47.1 +/− 4.1 | high |
| L (day 19–24) | not examined | not examined | 46.0 +/− 3.9 | |||||
| Nakamura & Nose-Ogura 2021 [39] | 10 | cycle ergometer | calendar-based counting method, serum hormone analysis | EF (day 4–8) | 249.7 +/− 185.8 | 1.0 +/− 1.3 | 41.8 +/− 6.1 | moderate |
| L (5–15 days after ovulation) | 397.2 +/− 208.5 | 22.9 +/− 16.9 | 41.9 +/− 5.3 | |||||
| Redman et al., 2003[19] | 14 | cycle ergometer | calendar-based counting method, urinary LH, serum hormone analysis | F (day 5–7) | 131.7 +/− 130.6 | 1.7 +/− 0.8 | 42.1 +/− 8.4a | low |
| L (day 21–23) | 348.0 +/− 230.5 | 26.8 +/− 20.1 | 44.3 +/− 6.2a | |||||
| Smekal et al., 2007 [35] | 19 | cycle ergometer | calendar-based counting method, basal body temperature, serum hormone analysis | F (day 9 +/− 1) | 203.3 +/− 108.3 | 2.3 +/− 0.6 | 43.2 +/− 5.1 | high |
| L (day 25 +/− 2) | 456.3 +/− 187.9 | 27.4 +/− 13.8 | 43.5 +/− 5.1 | |||||
| Vaiksaar et al., 2011 [41] | 8a | rowing ergometer | calendar-based counting method, serum hormone analysis | F (day 8 +/− 3) | 176.8 +/− 51.9 | 1.9 +/− 0.5 | 49.0 +/− 6.6 | moderate |
| L (day 20 +/− 2) | 481.4 +/− 131.0 | 38.9 +/− 11.0 | 50.6 +/− 7.1 | |||||
| 7b | F (day 8 +/− 3) | 163.4 +/− 98.2 | 1.3 +/− 0.7 | 45.2 +/− 9.4 | ||||
| L (day 20 +/− 2) | 517.7 +/− 21.7 | 30.1 +/− 0.7 | 45.4 +/− 4.1 | |||||
Note. Values are reported as means +/− SD. Vaiksaar et al. [41] distinguished between competitive cyclic athletes (a) and recreationally trained cyclic athletes (b).
EF: early follicular phase, F: follicular phase, L: luteal phase, LF: late follicular phase, LH: luteinizing hormone, MC: menstrual cycle, n: number of participants, NOS: Newcastle-Ottawa Quality Assessment Scale.
O2peak was measured.
Table 2.
Cross-sectional studies, examining the inter-individual difference between oral contraceptive users and naturally menstruating women.
| Author(s), year | n | Performance test | Oral contraceptive | EE | Progestin | Duration of use | Test phase | Results |
Risk of bias (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| O2max (ml/kg/min) | |||||||||
| Gordon et al., 2018 [40] | 16 | cycle ergometer | mono-phasic | 30 mcg 150 mcg |
levonorgestrel | >3 months | OC users (n = 6): day 19–20, interpreted as active-pill phase | 44.3 +/− 3.6 | high |
| NCOC users (n = 10): menstruation, interpreted as F | 41.6 +/− 3.7 | ||||||||
| Mattu et al., 2019 [11] | 30 | cycle ergometer | mono-phasic | 20–35 mcg | levonorgestrel (n = 10) desogestrel (n = 5) | >6 months | OC users (n = 15): active-pill phase | 45.2 +/− 4.3 | moderate |
| NOC users (n = 15): F (day 5–10) | 47.1 +/− 4.1 | ||||||||
| Quinn et al., 2018 [27] | 16 | cycle ergometer | mono-phasic | not specified | >12 months | OC users (n = 8): active-pill phase | 44.5 +/− 4.2a | high | |
| NOC users (n = 15): F (day 4–10) | 43.5 +/− 5.2a | ||||||||
| Vaiksaar et al., 2011 [41] | 16 | rowing ergometer | mono-phasic | 20 mcg | gestodene 75 mcg | >3 months | OC users (n = 9): day 20 +/− 2, interpreted as active-pill phase | 44.5 +/− 5.2 | moderate |
| NOC users (n = 7): F (day 8 +/−3) | 45.2 +/− 9.4 |
Note. Values are reported as means +/− SD.
OC: combined oral contraceptive, EE: Ethinyl estradiol, F: follicular phase, n: number of participants, NOC: non-combined oral contraceptive, NOS: Newcastle-Ottawa Quality Assessment Scale.
O2peak was measured.
Table 3.
Longitudinal studies, examining the effect of the active/inactive phase of the oral contraceptive cycle on O2max.
| Author(s), year | n | Performance test | Oral contraceptive | EE | Progestin | Duration of OC use | Test phase | Results |
Risk of bias (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| O2max (ml/kg/min) | |||||||||
| Gordon et al., 2018[40] | 6 | cycle ergometer | mono-phasic | 30 mcg | levonorgestrel 150 mcg | >3 months | day 1–3, interpreted as inactive-pill phase | 44.9 +/− 5.0 | high |
| day 19–20, interpreted as active-pill phase | 44.3 +/− 3.6 | ||||||||
| Mattu et al., 2019 [11] | 15 | cycle ergometer | mono-phasic | 20–35 mcg | levonorgestrel (n = 10) desogestrel (n = 5) | >6 months | inactive-pill phase | 45.5 +/− 5.3 | moderate |
| active-pill phase | 45.2 +/− 4.3 | ||||||||
| Nakamura & Nose-Ogura, 2021 [39] | 10 | cycle ergometer | mono-phasic | 20 mcg | norethisterone 1 mg | 3 months | inactive-pill phase | 40.7 +/− 5.3 | low |
| active-pill phase | 41.1 +/− 5.3 | ||||||||
| Vaiksaar et al., 2011 [36] | 8 | rowing ergometer | mono-phasic | 20 mcg | 75 mcg gestodene | >3 months | active-pill phase | 45.9 +/− 5.7 | low |
| inactive-pill phase | 44.3 +/−5.5 |
Note. Values are reported as means +/− SD.
OC: oral contraceptive, EE: Ethinyl estradiol, n: number of participants, NOS: Newcastle-Ottawa Quality Assessment Scale.
Table 4.
Intervention studies, examining the intra-individual effect of O2max after initiating oral contraceptive.
| Author(s), year, | n | Performance test | Oral contraceptive | EE | Progestin | Duration of OC use | Test phase | Results |
Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| O2max (ml/kg/min) | |||||||||
| Bryner et al., 1996[25] | 10 | treadmill | mono-phasic | 35 mcg | norethisterone 1 mg | first intake cycle | without OC: F (day of ovulation divided by two) | 41.6 +/− 12.1 | high (RoB2) |
| with OC: third week (interpreted as active-pill phase) | 41.0 +/− 12.4 | ||||||||
| Casazza et al., 2002 [28] | 6 | cycle ergometer | triphasic | 35 mcg | norgestimatep2p0.18 mg−0.25 mg | 4 months | without OC: F (day 4–8) | 42.3 +/− 8.1a | moderate (NOS) |
| with OC: active-pill phase | 36.9 +/− 5.4a | ||||||||
| Lebrun et al., 2003[26] | 7 | treadmill | triphasic | 35 mcg | norethisterone 0.5 mg–1.0 mg | 2 months | without OC: F (day 3–8) | 54.7 +/− 4.3 | some concerns (RoB2) |
| with OC: active-pill phase | 52.0 +/− 4.2 | ||||||||
| Nakamura & Nose-Ogura, 2021 [39] | 10 | cycle ergometer | mono-phasic | 20 mcg | norethisterone 1 mg | 3 months | without OC: F (day 4–8) | 41.8 +/− 6.1 | moderate (NOS) |
| with OC: active-pill phase | 41.1 +/− 5.3 | ||||||||
| Notelovitz et al., 1987 [38] | 6 | treadmill | mono-phasic | 35 mcg | norethisteronep2p0.4 mg | 6 months | without OC (phase not specified) | 41.2 +/− 11.8 | moderate (NOS) |
| with OC (phase not specified) | 38.4 +/− 9.4 | ||||||||
| Rickenlund et al., 2004 [37] | 13a | treadmill | mono-phasic | 30 mcg | levonorgestrel 150 mcg | 10 months | without OC: F (day 1–5) | 55.3 +/− 4.4 | high (NOS) |
| with OC: active-pill phase | 55.6 +/− 3.1 | ||||||||
| 12b | without OC: F (day 1–5) | 41.9 +/− 3.3 | |||||||
| with OC: active-pill phase | 41.7 +/− 3.1 |
Note. Values are reported as means +/− SD. Rickenlund et al. [37] distinguished between athletes (a) and sedentary controls (b).
OC: oral contraceptive, EE: Ethinyl estradiol, F: follicular phase, n: number of participants, NOS: Newcastle-Ottawa Quality Assessment Scale, RoB2: Cochrane Collaboration's tool for assessing risk of bias.
O2peak was measured.
All 15 studies examining the influence of the MC phases on O2max used a calendar-based counting method to identify the different phases of the MC. To estimate the day of ovulation, two studies included daily recordings of basal body temperature [23,31], four studies used an ovulation prediction kit identifying the urinary LH surge [11,19,28,30] and one study performed a transabdominal ultrasound to confirm that ovulation did occur [25]. Ten studies measured serum concentrations of estrogen and progesterone [19,23,24,28,30,32,34,35,39,41], two studies serum progesterone only [31,33], one study conducted a salivary hormone analysis [40], and in two studies no hormones were measured [11,25].
Most studies analyzed the effect of monophasic pills on O2max [11,25,27,[36], [37], [38], [39], [40], [41]], two studies the effect of triphasic pills [26,28]. The utilized pills contained a total of five different progestins (levonorgestrel, desogestrel, norethisterone, gestodene, and norgestimate). The dosing of EE varied between 20 and 35 mcg per pill. None of the included studies analyzed progestin-only pills.
3.3. Quality
The quality classifications are presented in Table 1, Table 2, Table 3, Table 4
The studies were considered at high (34.5%), moderate (51.7%) to low (13.8%) risk of bias as described in the methodology section.
In 80% of the included studies [11,19,23,24,[26], [27], [28]], [[32], [33], [34], [35], [36], [37], [38], [39],41], two identical performance tests were conducted, also considering testing at the same time of day as well as controlling diet and activities on the days before the test. In 90% [11,19,23,24,[26], [27], [28]], [[32], [33], [34], [35], [36], [37], [38], [39],41], care was taken to include women with similar fitness levels. Of the studies examining the same women several times, just 22% [19,32,33,40] made one familiarization trial prior to the test phase. Hence, to minimize test order effects the test conditions were randomized or the order of testing was counterbalanced in 80% of the longitudinal studies examining the influence of the different menstrual/OC phases on O2max [19,24,25,30,31,[34], [35], [36],39].
The primary sources of bias for studies considered at high or moderate risk were insufficient precision in the determination of the different menstrual phases and inaccurate consideration of the different properties of OCs. In the adapted version of NOS, trials were considered if the three phases of MC (early follicular, late follicular and luteal phase) were correctly examined and a verification of the MC phase was performed by measuring serum estrogen and progesterone concentrations at the time of testing [22] which was the case in two studies only [23,24]. In two of the four cross-sectional studies [11,27], not all participants received an OC with the same progestin. In 50% of the cross-sectional [11,27] as well as the intervention-based studies [25,37,38] examining a possible inter-/, respectively intra-individual influence of OCs on O2max, it remains unclear whether the women were in the active or the pill-free interval at the moment of the investigation. Furthermore, two [25,26] of the six intervention-based studies chose an interval shorter than three months between new start with OCs and test phase [25,26]. Common side effects of OCs are generally self-limiting and improve with the duration of use [42]. Therefore, testing after at least three or even six pill cycles seems more reasonable to ensure that any short-term effects are also long-term effects and vice-versa [10].
3.4. Effect of the menstrual cycle phases on O2max
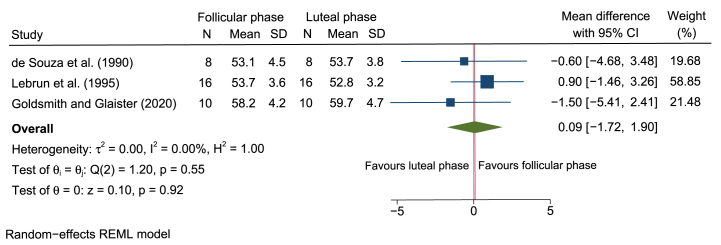
A total of 15 studies (including 173 women) investigated the effect of the MC phase on O2max[[11], [19], [23], [24], [25], [28], [30], [31], [32], [33], [34], [35], [37], [39], [40], [41]] . Our meta-analysis found a mean decrease in O2max of −0.03 ml/kg/min (95% CI –1.06 to 1.01; Fig. 2) during the early follicular compared with the luteal phase of the MC. If the highly trained women with a O2max > 53 ml/kg/min [15] were considered separately, the results did not change substantially (0.09 ml/kg/min (95% CI –1.72 to 1.90; Fig. 3).
Fig. 2.
Forest plot of studies examining the effect of the early follicular and the luteal phases of the menstrual cycle on O2max in physically active women. Mean difference represents O2max assessed in ml/kg/min. Note: Vaiksaar et al. [41]distinguished between competitive cyclic athletes (a) and recreationally trained cyclic athletes (b).
Fig. 3.
Forest plot of studies examining the effect of the early follicular and the luteal phases of the menstrual cycle on O2max in highly trained women with a O2max > 53 ml/kg/min. Mean difference represents O2max assessed in ml/kg/min.
3.5. Effect of oral contraceptives on O2max
Four longitudinal studies (including 39 women) [11,36,39,40] analyzed the influence of different OC phases on O2max. The current meta-analysis showed a mean decrease in O2max of −0.11 ml/kg/min (95% CI –2.32 to 2.10; Fig. 4) during the inactive compared with the active OC phases.
Fig. 4.
Forest plot of studies examining the effect of the inactive and the active phase of the oral contraceptive cycle on O2max in physically active women. Mean difference represents O2max assessed in ml/kg/min.
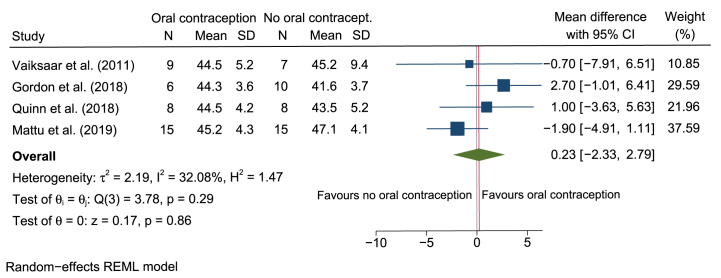
Four cross sectional studies [11,27,40,41] analyzed the potential inter-individual difference in O2max between OC users and eumenorrheic women (including 78 women). A mean difference in O2max of 0.23 ml/kg/min (95% CI –2.33 to 2.79; Fig. 5) use was found in favor of OC use.
Fig. 5.
Forest plot of cross-sectional studies examining the inter-individual difference in O2max between OC users and non-OC users in physically active women. Mean difference represents O2max assessed in ml/kg/min.
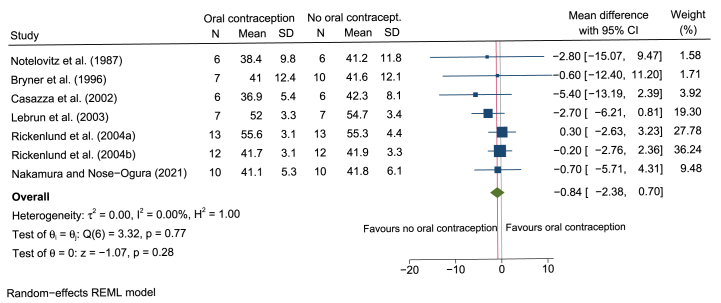
Six studies were intervention-based studies (including 71 women) examining a possible intra-individual difference in O2max comparing the same women without and with OCs. Four studies analyzed women prior to and after 3–10 months of OC treatment in a cross-over design (including 47 women) [28,[37], [38], [39]]. Two studies were randomized double-blind placebo-controlled (including 24 women) [25,26]. While Bryner et al. [25] observed no difference in O2max, Lebrun et al. [26] showed a decrease of O2max in the OC group (−2.7 ml/kg/h), whereas O2max in the placebo group slightly increased (+0.8 ml/kg/min) over the same time period. Overall, the meta-analysis combining the data of the cross-over studies and of the intervention groups of the RCT showed a mean intra-individual decrease in O2max of −0.84 ml/kg/min (95% CI –2.38 to 0.70; Fig. 6) after new start with OCs.
Fig. 6.
Forest plot of interventional-based studies examining the intra-individual difference in O2max prior und after OC treatment. Mean difference represent O2max assessed in ml/kg/min. Note: Rickenlund et al.[37]distinguished between athletes (a) and sedentary controls (b).
3.6. Sensitivity analysis
The robustness of the estimations of the meta-analyses was confirmed by sensitivity analyses utilizing fixed-effects inverse-variance models. Since heterogeneity between studies was relatively low with regard to the outcomes of interest (mostly even I2 = 0, except I2 = 32% for the assessment of the OC effect in the cross-sectional studies), the fixed-effects models provided very similar, often even identical estimates. The results were persistent and robust when the available absolute values of O2max (l/min) were analyzed.
4. Discussion
4.1. Main implications
Our meta-analyses did not find changes in O2max between the early follicular and the luteal phases of the MC or during the active and inactive pill phases in physically active women using an OC. Furthermore, no difference in O2max was found in the inter-individual comparison between OC users and eumenorrheic women as well as in the intra-individual comparison of the same women without and with OCs.
The results of the current meta-analysis are not in line with two meta-analyses published in 2020 investigating women regardless of their training level and an unspecific dependent variable (i.e. exercise performance) [1,2]. They concluded that exercise performance is slightly reduced during the early follicular phase of the MC compared to all other phases and that the use of OCs might result in a slightly reduced exercise performance compared to naturally menstruating women. The results of the current meta-analysis do not confirm this observation in regard of a change in O2max. Endogenous hormonal fluctuations between the early follicular and luteal phase of MC or exogenous hormone application may have a different effect in a homogenous population of physically active women.
In line with the results of the previously mentioned meta-analysis [1] no change in O2max during the inactive compared to the active pill phase was observed. This could be due to a slow elimination of EE from the bloodstream and affected organs, only resulting in little changes in endogenous concentration of estradiol and progesterone within the seven days of OC free interval [43].
The only accurate randomized double-blind placebo-controlled study examining a potential intra-individual difference in O2max with and without OCs [26] showed that the use of a triphasic OCs resulted in a mean decrease of −2.7 ml/kg/h compared with a +0.8 ml/kg/min improvement with placebo. However, the small number of participants (n = 14) limits the validity of this result and could also not be strengthened by our meta-analysis. Moreover, the studies investigating the effect of the OCs and MC phases on O2max or exercise performance have several deficits. Thus, there is insufficient data to discourage a woman from OCs due to potentially adverse effects on physical potential. The magnitude of the effect of endogenous and exogenous hormones on physical performance may vary substantially between subjects and be important on an individual basis. If a possible effect on physical performance in women is of interest, individualised recommendations should be made. In particular, the large variability in the type and severity of symptoms experienced during the MC as well as the possible negative and positive effects of OCs and other HCs should be considered.
4.2. Strengths and limitations
The available studies analyzing the possible effect of endogenous and exogenous female steroid hormones on exercise performance are very heterogenous and the outcome varies considerably across studies. In order to provide a meaningful conclusion, we have narrowed the sample selection and the dependent variable. With the selection of the studies, we limited the qualitative heterogeneity so that little statistical heterogeneity was observed within our meta-analyses, as shown by the small I2 values (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6).
We only examined physically active women in order to minimize the risk of potential changes in the outcome measures of the performance test due to the training effect. Physically active women can push their performance limits more consistently and tend to show less variation in their test performance within themselves. A change in exercise performance in less active women might be more strongly affected by the ongoing training.
As a limitation, we have to mention that we considered the mean O2max value of the study population as inclusion criteria and not the individual values of the participants. Therefore, it is possible that women with a lower value were also included. However, 85% of the included studies considered the training history of the women for inclusion and described them as “moderately” [31], “recreationally” [27], “habitually” [23,28], “highly” [11], “physically” [30,40] active/trained [33], as “exercising women” [38], or “athletes” [26,32,34,36,37,39,41].
As the dependent variable, we chose O2max, which is widely used as an indicator of cardiorespiratory fitness and reflects endurance capacity in exercise performance [9]. However, other parameters should be taken into account in the evaluation of cardiorespiratory fitness and endurance performance to be able to assess a possible influence conclusively. For example, the simultaneous measurement of minute ventilation (E) and carbon dioxide production (CO2) by cardiopulmonary exercise testing allows for the more comprehensive assessment of other clinically significant variables like the E/ CO2 slope, which is a key indicator of ventilatory efficiency [9]. Submaximal exercise tests can also provide valuable information but are less precise than peak exercise testing in quantitating cardiorespiratory fitness [9]. In elite female athletes, even very small effects due to OC of MC can be meaningful [10], thus competition performances should be examined.
Furthermore, caution needs to be applied when interpreting the findings of the current data on the potential impact of the phases of the MC and the use of OCs on cardiorespiratory fitness and physical performance in general [4]. Overall, 86% of the studies included in this meta-analysis were classified as at moderate to high risk of bias, partially due to methodological heterogeneity in the determination of the different MC phases or insufficient accuracy in consideration of different characteristics of the OCs used in the trials. Thus, the current meta-analysis could not make any statement regarding the late follicular estrogen peak because most of the available studies did not consider this phase. In studies examining the influence of OCs on O2max, it has to be considered that most of the OCs used did not correspond to current standard formulations.
4.3. Recommendation for future research
4.3.1. More attention to highly trained women
Further research focusing on well-trained female athletes is needed. It is remarkable that the studies including highly trained women with a O2max > 53 ml/kg/min are the most precise with the smallest standard deviation [[32], [33], [34]]. Thus, with athletes more sensitive analysis could be performed. Furthermore, for female athletes even a small change in O2max could be relevant to the optimization of training practices and competition. For illustration, a study examining endurance athletes participating in Olympic Games/World Championships detected a difference in O2max of 3.2 ml/kg/min between female medalists and non-medalists in cross-country skiing [44].
The mean O2max of women aged 20–29 years is 37.6±10.2 ml/kg/min and 30.9±8.0 ml/kg/min for ages 30–39 years, respectively [45]. We examined physically active women with a O2max equal to or greater than 40 ml/min/kg, while highly endurance trained women have a O2max > 53 ml/kg/min [15]. While just three studies [[32], [33], [34]] were found investigating this population, our results cannot be generalized to female athletes, which is why additional studies are needed.
4.3.2. Standardization of menstrual cycle research
There is a need for agreement on the terminology and methodological approaches within exercise science with women as participants to improve the quality of future research [4]. It is hypothesized that methodical differences play a major role in the contradictory results of recent evidence on exercise performance and the MC [22]. In order to provide a foundation for future high-quality MC research, a combination of three methods to verify MC phases is recommended: the calendar-based counting method combined with urinary luteinizing hormone surge testing as well as the measurement of serum estrogen and progesterone concentrations. Measurement of serum estrogen and progesterone in a resting state prior to the performance test is also important since potential effects of the MC on exercise performance are expected to be a consequence of the female steroid hormone levels [22].
4.3.3. Consideration of the different hormonal contraceptive formulations
With regard to studies investigating the influence of OCs on exercise performances, further research with the current standard formulations (20–30 mcg EE) is needed. Four intervention-based studies utilized an OC with a dosage of 35 mcg EE [25,26,28,38]. Interestingly, three of them [26,28,38] observed a possible adverse effect of the OC due to O2max, while those studies using a dosage of 20–30 mcg EE showed no effect [37,39]. Furthermore, the varying dose and type of progestin in OC formulations confer distinct pharmacokinetic properties, potentially resulting in substantially different physiological effects [46]. Different properties of synthetic progestins were not considered in any study. Estranes and gonanes are related to testosterone and are associated with more androgenic side effects including also altered carbohydrate and lipid metabolism [42]. The 19-norpregnanes, including nestorone, nomogestrol acetate and trimegestone are related to progesterone with a high specificity to bind the progesterone receptors. They have no to little interaction with other steroid receptors. Newer progestins, like dienogest and drospirenone have partial antiandrogenic effects, while drospirenone offers additional anti-mineralocorticoid properties [47].
Moreover, research on the potential effect of the use of continuous cycle OCs as well as progestin-only pills and other HC on exercise performance is needed. Despite an extensive search strategy, no studies evaluating the effect on O2max were identified including HCs that did not qualify as OCs.
5. Conclusions
Our meta-analyses could not demonstrate any relevant effects of the MC, considering the early follicular and luteal phase, or the OCs on O2max.
Due to the methodical issues, there is a lack of reliable, evidence-based data on the potential impact of the MC and the use of OCs on O2max and physical performance in general. There is a need for agreement on the terminology and methodological approaches within research in exercise science with women as participants. More high-quality studies with larger sample sizes focusing on well-trained women are needed. Further research should address the late follicular estrogen peak, and the different properties of OCs should be considered more accurately. Studies of OCs with the current standard formulations and studies comparing the influence of different progestins on cardiorespiratory fitness are needed, as well as studies on the use of continuous cycle OCs and progestin-only contraceptives.
Author contribution statement
Lea Franziska Schumpf, Christian Braun, Adriana Peric, Michael Johannes Schmid, Dirk Lehnick, Corina Christmann-Schmid, Christine Brambs: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding statement
No source of funding was used to assist in preparing this article.
Attestation statements
Data regarding this meta-analysis has not been previously published. Data will be made available to the editors of the journal for review or query upon request.
Registration
Prospero CRD42021291213. Date of registration: December 14, 2021.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Daniela Maurer (Information Specialist/Clinical Librarian) for her assistance with development of the search strategy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17049.
Contributor Information
Lea Franziska Schumpf, Email: lea.schumpf@luks.ch.
Christian Braun, Email: christian.braun@luks.ch.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Elliott-Sale K.J., McNulty K.L., Ansdell P., Goodall S., Hick K.M., Thomas K., et al. The effects of oral contraceptives on exercise performance in women: a systematic review and meta-analysis. Sports Med. 2020;50:1785–1812. doi: 10.1007/s40279-020-01317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNulty K.L., Elliott-Sale K., Dolan E., Swinton P.A., Ansdell P., Goodall S., et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50:1813–1827. doi: 10.1007/s40279-020-01319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson B., Almarjawi A., Sculley D., Janse de Jonge X. The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med. 2020;50:171–185. doi: 10.1007/s40279-019-01219-1. [DOI] [PubMed] [Google Scholar]
- 4.Elliott-Sale K.J., Minahan C.L., Janse de Jonge X.A.K., Ackerman K.E., Sipilä S., et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021;51:843–861. doi: 10.1007/s40279-021-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dombovy M.L., Bonekat H.W., Williams T.J., Staats B.A. Exercise performance and ventilatory response in the menstrual cycle. Med. Sci. Sports Exerc. 1987;19:111–117. [PubMed] [Google Scholar]
- 6.Schoene R.B., Robertson H.T., Pierson D.J., Peterson A.P. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981;50:1300–1305. doi: 10.1152/jappl.1981.50.6.1300. [DOI] [PubMed] [Google Scholar]
- 7.Campbell S.E., Angus D.J., Febbraio M.A. Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am. J. Physiol. Endocrinol. Metab. 2001;281:E817–E825. doi: 10.1152/ajpendo.2001.281.4.E817. [DOI] [PubMed] [Google Scholar]
- 8.Zderic T.W., Coggan A.R., Ruby B.C. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J. Appl. Physiol. 2001;90:447–453. doi: 10.1152/jappl.2001.90.2.447. [DOI] [PubMed] [Google Scholar]
- 9.Ross R., Blair S.N., Arena R., Church A.S., Despreés J.P., Frankling B.A., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American heart association. Circulation. 2016;134:e653–e699. doi: 10.1161/cir.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 10.Burrows M., Peters C.E. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007;37:557–574. doi: 10.2165/00007256-200737070-00001. [DOI] [PubMed] [Google Scholar]
- 11.Mattu A.T., Iannetta D., Maclnnis M.J., Doyle-Baker P.K., Murias J.M. Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand. J. Med. Sci. Sports. 2020;30:472–484. doi: 10.1111/sms.13590. [DOI] [PubMed] [Google Scholar]
- 12.Mäestu J., Jürimäe J., Jürimäe T. Monitoring of performance and training in rowing. Sports Med. 2005;35:597–617. doi: 10.2165/00007256-200535070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Poole D.C., Jones A.M. Measurement of the maximum oxygen uptake VO2max: VO2peak is no longer acceptable. J. Appl. Physiol. 2017;122:997–1002. doi: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decroix L., de Pauw K., Forster C., Meeusen R. Guidelines to classify female subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2016;11:204–213. doi: 10.1123/ijspp.2015-0153. [DOI] [PubMed] [Google Scholar]
- 16.Muka T., Glisic M., Milic J., Verhoog S., Bohlius J., Bramer W., et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020;35:49–60. doi: 10.1007/s10654-019-00576-5. [DOI] [PubMed] [Google Scholar]
- 17.Bramer W.M., Rethlefsen M., Kleijnen J., Franco O.H. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst. Rev. 2017;6:245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan - a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redman L.M., Scroop G.C., Norman R.J. Impact of menstrual cycle phase on the exercise status of young, sedentary women. Eur. J. Appl. Physiol. 2003;90:505–513. doi: 10.1007/s00421-003-0889-0. [DOI] [PubMed] [Google Scholar]
- 20.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 21.Higgins J.P.T., Gotzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janse de Jonge X., Thompson B., Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med. Sci. Sports Exerc. 2019;51:2610–2617. doi: 10.1249/mss.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 23.Dean T.M., Perreault L., Mazzeo R.S., Horton T.J. No effect of menstrual cycle phase on lactate threshold. J. Appl. Physiol. 2003;95:2537–2543. doi: 10.1152/japplphysiol.00672.2003. [DOI] [PubMed] [Google Scholar]
- 24.Frandsen J., Pistoljevic N., Quesada J.P., Amaro-Gahete F.J., Ritz C., Larsen S., et al. Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test. J. Appl. Physiol. 2020;128:681–687. doi: 10.1152/japplphysiol.00774.2019. [DOI] [PubMed] [Google Scholar]
- 25.Bryner R.W., Toffle R.C., Ullrich I.H., Yeater R.A. Effect of low dose oral contraceptives on exercise performance. Br. J. Sports Med. 1996;30:36–40. doi: 10.1136/bjsm.30.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebrun C.M., Petit M.A., McKenzie D.C., Taunton J.E., Prior J.C. Decreased maximal aerobic capacity with use of a triphasic oral contraceptive in highly active women: a randomised controlled trial. Br. J. Sports Med. 2003;37:315–320. doi: 10.1136/bjsm.37.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn K.M., Billaut F., Bulmer A.C., Minahan C.L. Cerebral oxygenation declines but does not impair peak oxygen uptake during incremental cycling in women using oral contraceptives. Eur. J. Appl. Physiol. 2018;118:2417–2427. doi: 10.1007/s00421-018-3968-y. [DOI] [PubMed] [Google Scholar]
- 28.Casazza G.A., Suh S.H., Miller B.F., Navazio F.M., Brooks G.A. Effects of oral contraceptives on peak exercise capacity. J. Appl. Physiol. 2002;93:1698–1702. doi: 10.1152/japplphysiol.00622.2002. [DOI] [PubMed] [Google Scholar]
- 29.Day J.R., Rossiter H.B., Coats E.M., Skasick A., Whipp B.J. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. J. Appl. Physiol. 2003;95:1901–1907. doi: 10.1152/japplphysiol.00024.2003. [DOI] [PubMed] [Google Scholar]
- 30.Beidleman B.A., Rock P.B., Muza S.R., Fulco C.S., Forte V.A., Jr., Cymerman A. Exercise VE and physical performance at altitude are not affected by menstrual cycle phase. J. Appl. Physiol. 1999;86:1519–1526. doi: 10.1152/jappl.1999.86.5.1519. [DOI] [PubMed] [Google Scholar]
- 31.Bemben D.A., Salm P.C., Salm A.J. Ventilatory and blood lactate responses to maximal treadmill exercise during the menstrual cycle. J. Sports Med. Phys. Fit. 1995;35:257–262. [PubMed] [Google Scholar]
- 32.De Souza M.J., Maguire M.S., Rubin K.R., Maresh C.M. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med. Sci. Sports Exerc. 1990;22:575–580. doi: 10.1249/00005768-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith E., Glaister M. The effect of the menstrual cycle on running economy. J. Sports Med. Phys. Fit. 2020;60:610–617. doi: 10.23736/s0022-4707.20.10229-9. [DOI] [PubMed] [Google Scholar]
- 34.Lebrun C.M., McKenzie D.C., Taunton J.E. Effects of menstrual cycle phase on athletic performance. Med. Sci. Sports Exerc. 1995;27:437–444. [PubMed] [Google Scholar]
- 35.Smekal G., von Duvillard S.P., Frigo P., Tegelhofer T., Pokan R., Hofman P., Tschan H., et al. Menstrual cycle: no effect on exercise cardiorespiratory variables or blood lactate concentration. Med. Sci. Sports Exerc. 2007;39:1098–1106. doi: 10.1249/mss.0b013e31805371e7. [DOI] [PubMed] [Google Scholar]
- 36.Vaiksaar S., Jürimae J., Maestu J., Purge P., Kalytka S., Shakhlina L., et al. Phase of oral contraceptive cycle and endurance capacity of rowers. Percept. Mot. Skills. 2011;113:764–772. doi: 10.2466/05.06.PMS.113.6.764-772. [DOI] [PubMed] [Google Scholar]
- 37.Rickenlund A., Carlström K., Ekblom B., Brismar T.B., von Schoultz B., Hirschberg A.L. Effects of oral contraceptives on body composition and physical performance in female athletes. J. Clin. Endocrinol. Metab. 2004;89:4364–4370. doi: 10.1210/jc.2003-031334. [DOI] [PubMed] [Google Scholar]
- 38.Notelovitz M., Zauner C., McKenzie L., Suggs Y., Fields C., Kitchens C. The effect of low-dose oral contraceptives on cardiorespiratory function, coagulation, and lipids in exercising young women: a preliminary report. Am. J. Obstet. Gynecol. 1987;156:591–598. doi: 10.1016/0002-9378(87)90059-7. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M., Nose-Ogura S. Effect of administration of monophasic oral contraceptive on the body composition and aerobic and anaerobic capacities of female athletes. J. Obstet. Gynaecol. Res. 2021;47:792–799. doi: 10.1111/jog.14613. [DOI] [PubMed] [Google Scholar]
- 40.Gordon D., Scruton A., Barnes R., Baker J., Prado L., Merzbach V. The effects of menstrual cycle phase on the incidence of plateau at VO2max and associated cardiorespiratory dynamics. Clin. Physiol. Funct. Imag. 2018;38:689–698. doi: 10.1111/cpf.12469. [DOI] [PubMed] [Google Scholar]
- 41.Vaiksaar S., Jürimae J., Maestu J., Purge P., Kalytka, Shakhlina L., et al. No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J. Strength Condit Res. 2011;25:1571–1578. doi: 10.1519/JSC.0b013e3181df7fd2. [DOI] [PubMed] [Google Scholar]
- 42.Dragoman M.V. The combined oral contraceptive pill -- recent developments, risks and benefits. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28:825–834. doi: 10.1016/j.bpobgyn.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Elliott K.J., Cable N.T., Reily T. Does oral contraceptive use affect maximum force production in women? Br. J. Sports Med. 2005;39:15–19. doi: 10.1136/bjsm.2003.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tønnessen E., Haugen T.A., Hem E., Leirstein S., Seiler S. Maximal aerobic capacity in the winter-Olympics endurance disciplines: Olympic-medal benchmarks for the time period 1990-2013. Int. J. Sports Physiol. Perform. 2015;10:835–839. doi: 10.1123/ijspp.2014-0431. [DOI] [PubMed] [Google Scholar]
- 45.Kaminsky L.A., Arena R., Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin. Proc. 2015;90:1515–1523. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhl H. Pharmacokinetics of the newer progestogens and the controversy about gestodene. Contraception. 1997;56:401–403. doi: 10.1016/s0010-7824(97)00189-3. [DOI] [PubMed] [Google Scholar]
- 47.Sitruk-Ware R., Nath A. The use of newer progestins for contraception. Contraception. 2010;82:410–417. doi: 10.1016/j.contraception.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.