ABSTRACT
This study investigated the underlying mechanism of miR-18a-5p regulating the proliferation, invasion, and metastasis of nasopharyngeal carcinoma (NPC) cells in vitro and in vivo to indicate the pathogenesis of NPC. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was utilized to determine miR-18a-5p expression level in NPC tissues and cell lines. Besides, 2,5-diphenyl-2 H-tetrazolium bromide (MTT) and colony formation assays were employed to detect the effect of miR-18a-5p expression level on NPC cell proliferation. Wound healing and Transwell assays were utilized to detect the effect of miR-18a-5p on NPC cell invasion and migration. The expression levels of epithelial-mesenchymal transition (EMT)-related proteins (Vimentin, N-cadherin, and E-cadherin) were identified by Western blot assay. After collecting exosomes from CNE-2 cells, it was found that exosomal miR-18a-5p secreted from NPC cells promoted NPC cell proliferation, migration, invasion, and EMT, whereas inhibition of miR-18a-5p expression level led to the opposite results. The dual-luciferase reporter assay showed that BTG anti-proliferation factor 3 (BTG3) was the target gene of miR-18a-5p, and BTG3 could overturn the effect of miR-18a-5p on NPC cells. Xenograft mouse model of NPC nude mice showed that miR-18a-5p promoted NPC growth and metastasis in vivo. This study revealed that exosomal miR-18a-5p derived from NPC cells promoted angiogenesis via targeting BTG3 and activating the Wnt/β-catenin signaling pathway.
KEYWORDS: BTG3, EMT, Nasopharyngeal carcinoma, Metastasis, MiR-18a-5p, Wnt/β-catenin signaling pathway
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor that arises from the nasopharyngeal cavity’s top and lateral wall, and its incidence ranks first among head and neck malignant tumors. The incidence of NPC has shown to be associated with age, geographical characteristics, and gender, primarily in Southeast Asia and North Africa. NPC patients’ peak age is in the range of 50–60 years old, and its incidence rate in men is three times higher than that in women [1,2]. Recently, radiotherapy and adjuvant chemotherapy have been the primary NPC treatment. The majority of NPC patients are well controlled with modern therapeutic approaches, while the high drug resistance and the metastatic tendency of NPC patients remain clinical challenges [2,3]. Therefore, a detailed analysis of the invasion and migration mechanisms of NPC is essential to search for novel molecular targets, enhance the efficacy of clinical treatment, and improve the patients’ prognosis.
MicroRNA (miRNA) is a single-strand non-coding RNA that comprises 20–24nt, and it can be widely found in eukaryotes. MiRNAs are highly conservative in evolution; they can reportedly bind to the 3´-untranslated region (3´-UTR) of the target gene, inhibit mRNA translation, and promote mRNA degradation, thereby regulating cell differentiation, proliferation, and apoptosis [4–7]. MiRNAs can play an essential role in the occurrence and development of NPC. For instance, miR-506-3p can suppress LHX2 expression and inhibit Wnt/β-catenin signal transduction, thereby inhibiting growth and metastasis of NPC tumors [8]. MiR-203a-3p inhibits growth and metastasis of NPC tumors via targeting LASP1, and miR-129-5p inhibits lymphangiogenesis and lymph node metastasis of NPC via reducing ZIC2 expression level, thereby potentially inhibiting the Hedgehog signaling pathway [9]. These studies have provided a theoretical basis for the investigation of the molecular mechanism of miRNA in NPC. As an essential miRNA, miR-18a-5p plays a crucial regulatory role in different types of cancer. Some studies have found that miR-18a-5p expression level is upregulated in renal cell carcinoma cell lines and tissues and the upregulation of miR-18a-5p expression level can promote renal cancer cell proliferation, invasion, and migration, while inhibit renal cancer cell apoptosis [10]. The miR-18a-5p expression level is significantly upregulated in non-small cell lung cancer (NSCLC) cell lines and tissues, and it can target interferon regulatory factor 2 (IRF2) to promote NSCLC tumorigenesis [11]. In addition, other studies have demonstrated that miR-18a-5p plays an inhibitory function in breast cancer [12] and prostate cancer [13]. Moreover, miR-18a-5p is highly expressed in NPC tissues and cells, which has an essential clinical value in diagnosing NPC [14]. These results indicate that miR-18a-5p plays a vital role in NPC occurrence, proliferation, and development, while the biological function of miR-18a-5p in NPC has not yet been investigated.
Recent research has shown that tumor cells could regulate the tumor microenvironment by secreting nano-vesicles called exosomes, with the size of about 30–100 nm in diameter [15]. Exosomes, which mainly function by passing on their contents to receptor cells, are membranous vesicles formed and secreted by living cells. They contain several bioactive components, such as miRNAs, mRNAs, proteins, lipids, etc., and they have distinct bioactive functions in physiological and pathological conditions [16,17]. To date, numerous studies have concentrated on investigating the effects of tumor-derived exosomes on angiogenesis [18,19]. It was confirmed that tumor-derived exosomes are engaged in tumor microenvironment remodeling, and they release different bioactive components acting on the surface receptors of endothelial cells, leading to angiogenesis and promoting tumor migration.
Therefore, the present study aimed to assess the underlying mechanism of miR-18a-5p regulating the proliferation, invasion, and metastasis of NPC cells in vitro and in vivo, in order to explore the pathogenesis of NPC and provide novel ideas for the future diagnosis and therapy of NPC.
Materials and Methods
Cell lines and patients
NPC cell lines (6-10B and CNE2) and human normal nasopharyngeal cell line NP69 were purchased from the American Type Culture Collection (Manassas, VA, USA). Cell lines were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Waltham, MA, USA) at 37°C and 5% CO2.
Samples from NPC patients and their adjacent tissues were collected from those who were admitted to the Affiliated Ganzhou Hospital of Nanchang University (Nanchang, China). All patients were pathologically confirmed as NPC. The present study has been approved by the Ethics Committee of the Affiliated Ganzhou Hospital of Nanchang University (Approval No. …). All study participants provided written informed consent before enrollment. Samples were collected postoperatively and subsequently kept at −80°C until further analysis.
Cell transfection
The miR-18a-5p inhibitor, NC inhibitor, miR-18a-5p mimics, NC mimics, ago-miR-NC, and ago-miR-18a-5p were purchased from RiboBio Co., Ltd. (Guangzhou, China). Small interference RNA (siRNA) targeting BTG anti-proliferation factor 3 (BTG3) was synthesized by RiboBio Co., Ltd. Lipofectamine® 3000 reagent (Invitrogen) was used for transfection of NPC cells following the manufacturer’s protocol. The siRNA sequences utilized in this experiment were summarized as follows: siBTG3#1 5’-3’ GCTGAGAAATTGACCCTAATA and siBTG3#2 5’-3’ GCACCTTTGAGAATTTACTTT.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from NPC cells and tissues. Then, cDNA was synthesized using a reverse transcription kit (Takara, Tokyo, Japan). RT-qPCR was performed using the ABI7500 instrument (Thermo Fisher Scientific, Waltham, MA, USA). BTG3 expression level was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal reference, and miR-18a-5p expression level was normalized to that of U6 as an internal control. The relative gene expression was compared using the 2−ΔΔCt method. The primers used for RT-qPCR were summarized as follows: miR-18a-5p, forward 5’-TAAGGTGCATCTAGTGCAGATAG-3’, reverse 5’-CTCAACTGGTGTCGTGGA-3’; BTG3, forward 5’-ATGAAATTGCTGCCGTTGTCT-3’, reverse 5’-GCCTGTCCTTTCGATGGTTTT-3’; pri-miR-18a, forward 5’-TTAATACGACTCACTATAGGGCTGTTCTAAGGTGCATCTAGTGC-3’, reverse 5’-TGCCAGAAGGAGCACTTAGGGC-3’; GAPDH, forward 5’-CCTCTGACTTCAACAGCGAC-3’, reverse 5’-TCCTCTTGTGCTCTTGCTGG-3’.
Western blotting
At 48 h after transfection, total proteins were extracted with radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Shanghai China). After that, 10 μL of each sample was boiled for 10 min at 95°C, and the samples were electrophoresed on sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 100 V. Proteins were then transferred onto nitrocellulose membrane at 100 mA for 120 min, blocked with 5% skimmed milk for 1 h, and incubated overnight at 4°C with the corresponding primary antibodies. On the next day, the membrane was washed with 1× TBST solution (Beyotime Institute of Biotechnology) at room temperature, and it was then incubated with secondary horseradish peroxidase-labeled goat anti-rabbit IgG antibody at room temperature for 90 min. The protein bands were visualized using the ECL kit (Beyotime Institute of Biotechnology), and protein imprinting was observed. The primary antibodies against Bcl-2 (#3498; Cell Signaling Technology (CST), Danvers, MA, USA), Bax (#2772; CST), c-caspase 3 (#9661; CST), t-caspase 3 (#14220; CST), c-caspase 9 (#7237; CST), t-caspase 9 (#9508; CST), E-cadherin (#8834; CST), N-cadherin (#4061; CST), Vimentin (#12826; CST), BTG3 (#ab92309, Abcam, Cambridge, UK), CD81 (#56039; CST), CD63 (#ab134045; Abcam), TSG101 (#ab125011; Abcam), Wnt1 (#ab15251; Abcam), Wnt3 (#ab172612; Abcam), active-β-catenin (#4270; CST), p-GSK3β (#ab75814; Abcam), GSK3β (#ab32391; Abcam), and GAPDH (#5174; CST) were used. The experiment was repeated three times.
Cell proliferation and colony formation assays
Cell proliferation was assessed by the 2,5-diphenyl-2 H-tetrazolium bromide (MTT) method, and the cells (5 × 103 cells per 100 μL) were seeded into a 96-well plate. After cell culturing with the indicated reagent (CHIR990213 µM) for different time points, cell proliferation was evaluated using MTT solution (Beyotime Institute of Biotechnology) according to the manufacturer’s protocol. A microplate reader was utilized to measure the absorbance at 490 nm wavelength.
Colony formation experiment was performed as follows: 1.5 × 103 cells/plate were cultured and incubated in the presence of 5% CO2 at 37°C for 14 days. Afterwards, the culture medium was discarded, and the cells were twice washed with phosphate-buffered saline (PBS). Next, the cells were stained with 0.5% crystal violet at room temperature for 15 min. Then, the cells were rinsed with water. Colonies comprised more than 50 cells were counted under a light microscope.
Wound healing assay
Cells (5 × 106) were seeded into a 6-well plate until they reached the confluence of 80%. After that, 200 μL pipette was used to gently scratch the monolayer through the center of the well. The lower chamber was twice washed with a medium. After adding the fresh medium, cells were cultured for additional 24 h, and cell migration was assessed at 0 and 24 h under a microscope.
Transwell invasion and migration assays
Transwell invasion assay was conducted using a 24-well Transwell chamber (8 μm aperture; Corning Inc., Corning, NY, USA). In this experiment, 2 × 104 cells were seeded into the upper chamber, and coated with Matrigel matrix. The lower chamber was then filled with a Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. After culturing at 37°C for 48 h, the Transwell was twice washed with PBS, and 5% glutaraldehyde was added and kept at 4°C for 30 min. The upper chamber was removed, and the invasive cells were imaged and counted under a microscope. In the migration assay, no matrix was added to the upper chamber, and the remaining processes were carried out similar to those in the Transwell invasion assay.
Dual-luciferase reporter assay
The interaction between miR-18a-5p and BTG3 3´UTR was identified using psiCHECK luciferase reporter vector (Promega, Madison, WI, USA) containing the constructed wild-type (wt) and mutant (mut) 3´UTRs of BTG3. Next, CNE2 and 6-10B cells were seeded into 48-well plates and cultured for 24 h. Afterwards, psiCHECKwt/mut and miR-18a-5p/NC plasmids were co-transfected into the cells. Finally, luciferase activity was measured by luciferase assay reagent.
MiRNA expression profiles and clinical data of NPC
The EBI ArrayExpress and Gene Expression Omnibus (GEO) databases were used in the miRNA search, and cohorts with miRNA expression and clinical survival data were then selected. Finally, GSE36682 dataset with involvement of at least 50 NPC patients was selected for analysis. The miRNA profiling platform GSE36682 was GPL15311 (with 1,004 miRNA probes).
Identification of differentially expressed miRnas
The expression values of different miRNAs were 0 in GSE70970 dataset, indicating severe degradation. Therefore, only 10% of the expressed miRNAs in the samples were maintained, and 337 out of 734 miRNA probes from GSE70970 dataset remained. Besides, GSE32960 and GSE70970 datasets were merged through specific miRNA ID, and the batch effects were removed using the “ComBat” method [20]. Finally, 309 common miRNAs were maintained for further differential expression analysis and survival analysis. The miRbase database [21] was used to normalize miRNAs from each platform. The “limma” R package [22] was utilized to identify differentially expressed miRNAs according to the following criteria: (1) adjusted P < 0.05 and (2) absolute fold-change (FC) > 1.5 between cancer and normal tissues.
The predictive efficiency of miR-18a-5p expression level
The predictive efficiency of miR-18a-5p expression level was evaluated using the univariate Cox regression model and the log-rank test. Three penalized Cox proportional hazard regression models were utilized to predict the overall survival rate. Ten-fold cross-validation was used to determine the fittest penalty parameters. All models were fitted using the “glmnet” R package [23]. Time-dependent receiver operating characteristic (ROC) curves [24], the area under the ROC curve (AUC), and the concordance index (C-index) were utilized to evaluate the predictive efficiency of the prognostic models, which could be estimated using the “timeROC” [24] and “pec” [25] R packages.
MiRNA-targeted gene prediction
The miRTarBase was used to obtain the putative targets of human miRNAs [26], in which the miRNA-target interactions (MTIs) were confirmed experimentally by Western blotting, dual-luciferase reporter assay, and microarray experiments. The putative targets of miR-18a-5p were predicted using the “‘MirTarget’” prediction algorithm in the miRDB [27].
Expression correlation analysis between miRNAs and their target genes was applied to select more reliable MTIs using the GSE118721 dataset and to narrow down the list of putative target genes of miRNAs [28], which provided miRNA and mRNA expression profiles for seven NPC specimens and four normal nasopharyngeal mucosal specimens. If the target genes of miRNAs were differentially expressed with opposite changing direction and their negative correlation was significant (P < 0.05), the MTI would be considered reliable, and the target genes would be utilized for further analysis.
Gene set enrichment analysis (GSEA)
The “clusterProfiler” R package [29] was used to perform Gene Ontology (GO) and KEGG pathway enrichment analyses. P-values <0.01 obtained by the BH method were considered statistically significant.
In vivo analysis
Male nude mice (5-week-old) were purchased from Experimental Animal Science and Technology Center of Jiangxi University of Traditional Chinese Medicine (Nanchang, China) and maintained under aseptic conditions (12 h-light:12 h-dark cycles, 25°C, 60% ultraviolet (UV), 70% humidity). Moreover, CNE1 cells were injected into feet of mice. When the tumor volume reached 60 mm3, miR-18a-5p inhibitor (10 nmol/50 mL), miR-NC inhibitor, and PBS were injected twice a week, and intratumoral therapy was performed eight times. Tumor volume was measured as follows: volume (cm3) = (length × width2)/2. After 4 weeks, mice were euthanized, and the tumor weight was measured and photographed. Every procedure was approved by the Animal Care and Use Committee of the Affiliated Ganzhou Hospital of Nanchang University.
Immunohistochemistry
Xenograft tumors from mice were fixed in 4% paraformaldehyde solution and maintained in a refrigerator at 4°C. Specimens were dehydrated in different concentrations of ethanol and xylene; then, the samples were embedded into paraffin and sliced for further immunochemical staining. Samples were sealed with xylene transparent and neutral gum, covered with glass slides, and air-dried for further examination.
Statistical analysis
Data were statistically analyzed using SPSS 22.0 software (IBM, Armonk, NY, USA) and were expressed as mean ± standard deviation. The comparison between the two groups was conducted using t-test, and *, # indicated P < 0.05.
Results
MiR-18a-5p was functionally enriched in NPC cells and tissues and was associated with metastasis
The enriched miRNAs from clinical characteristics of 312 NPC patients in the GSE32906 microarray dataset were functionally investigated. Heatmap results showed that miR-18a-5p was significantly enriched in the advanced stage of NPC compared with the primary stage (Figure 1a). It was also found to be significantly enriched in the advanced stages of NPC (I-IV) and remarkably expressed in the metastatic phase (Figure 1b). The GSE36682 dataset, containing 62 NPC cases and 6 healthy cases, was employed, and it was found that miR-18a-5p was highly expressed in metastatic cases than that in non-metastatic cases (Figure 1c). Subsequently, the time-dependent ROC curve analysis was performed to evaluate the predictive capacity of miR-18a-5p. As shown in Figure 1d, the AUC values of the signature of miR-18a-5p in 1- and 5-year overall survival (OS) of NPC patients were 0.778 and 0.837, respectively, in the training dataset. The predictive performance of miR-18a-5p signature for OS in NPC patients was further evaluated using Kaplan-Meier survival curves in the GSE36682 dataset. The results revealed that the high-risk patients identified by the miR-18a-5p signature had worse survival probability (P < 0.001) (Figure 1e). These findings indicated that the miR-18a-5p signature constructed in the present study was accurate and stable in predicting the OS of NPC patients. Clinical samples collected from 15 NPC patients and 10 healthy participants showed that miR-18a-5p was significantly expressed in NPC tissues compared with that in normal tissues (Figure 1f). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay confirmed the enrichment of miR-18a-5p in NPC cells versus that in their normal counterparts (Figure 1g). Moreover, miR-18a-5p was found to be significantly expressed in NPC cells (CNE2 and 6-10B) compared with that in normal nasopharyngeal epithelial cell line NP69. Notably, miR-18a-5p was significantly expressed in CNE2 cells versus that in 6-10B cells (Figure 1h). The above-mentioned results demonstrated that miR-18a-5p might be a vital regulator of NPC progression.
Figure 1.
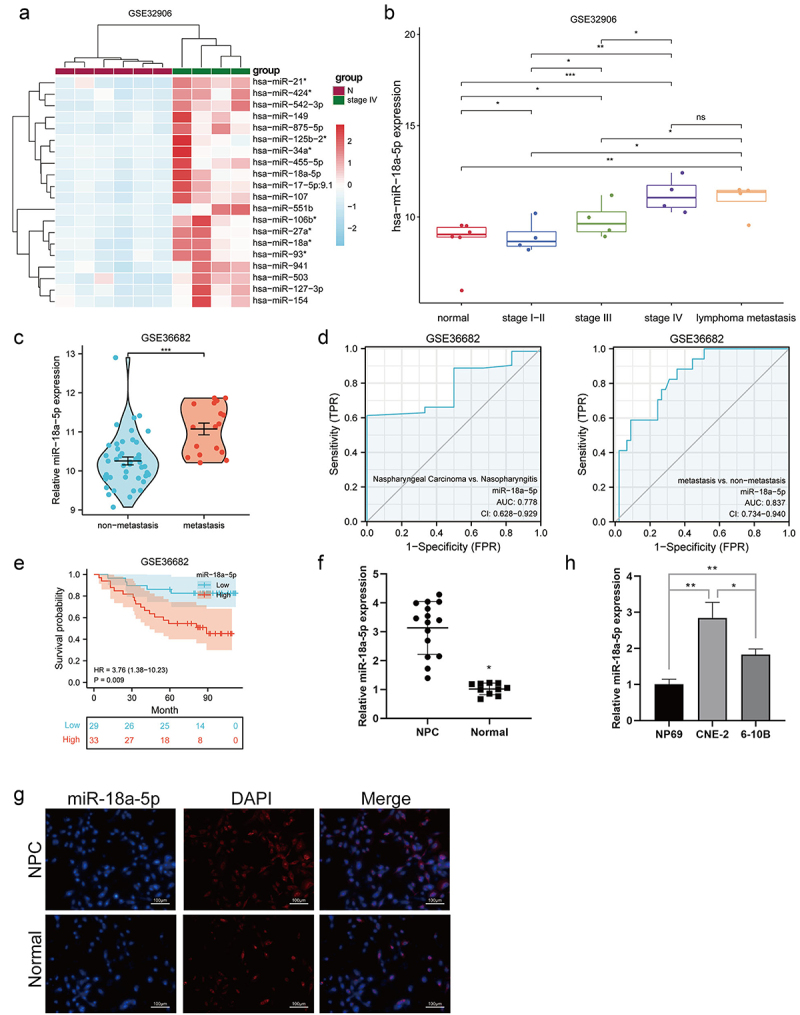
a. The heat map illustrating the upregulated expression levels of top 20 miRNA target genes in GSE32906 dataset. b. The miR-18a-5p expression level in GSE32906 dataset. c. The miR-18a-5p expression level in GSE36682 dataset. d. The ROC curve of miR-18a-5p expression level in GSE36682 dataset. e. The predictive analysis of miR-18a-5p expression level in GSE36682 dataset. f. The miR-18a-5p expression level in tissues was detected by RT-Qpcr. g. The FISH was employed to examine the miR-18a-5p expression level in tissues. h. RT-Qpcr detected the miR-18a-5p expression level in NPC cell lines.
MiR-18a-5p promoted proliferation, invasion, migration, and epithelial-mesenchymal transition (EMT) of NPC cells
In order to investigate the influence of miR-18a-5p on the proliferation of NPC cell lines, miR-18a-5p mimics, miR-18a-5p inhibitors, and their corresponding vectors were transfected into 6-10B and CNE1 cell lines (Figure 2a and Supplementary Figure S1A). The results of MTT assay revealed that miR-18a-5p mimic dramatically promoted the proliferation of NPC cells, whereas miR-18a-5p inhibitor showed the opposite effect (Figure 2b and Supplementary Figure S1B). The results of Western blotting indicated that miR-18a-5p inhibition suppressed the expression level of Bcl-2 protein, while promoted the expression levels of Bax and cleaved caspase-3 proteins; on the contrary, miR-18a-5p mimic exhibited the opposite effect (Figure 2c). Furthermore, miR-18a-5p promoted the migration and invasion of NPC cells, whereas miR-18a-5p inhibition led to the opposite result, as shown by wound healing and Transwell assays (Figures 2d–e and Supplementary Figures S1C-D). Moreover, the effect of miR-18a-5p on the EMT of NPC cells was confirmed, and the results showed that miR-18a-5p overexpression increased the expression levels of N-cadherin and Vimentin proteins, while decreased the expression level of E-cadherin protein (Figure 2f). The above-mentioned results indicated that miR-18a-5p overexpression could accelerate NPC cell proliferation, migration, invasion, and EMT, whereas miR-18a-5p inhibition led to the opposite effect.
Figure 2.

The miR-18a-5p was silenced in CNE-2p cells and overexpressed in 6-10B cells. a. The detection of miR-18a-5p expression level by RT-Qpcr. b. The cell proliferation was determined by the MTT assay. c. Expression levels of Bcl-2, Bax, cleaved caspase-3, total caspase-3, cleaved caspase-9, and total caspase-9 proteins were detected by Western blotting. d. The migratory ability was evaluated by wound healing assay. e. The invasive ability was assessed by Transwell assay. f. Western blotting was used to determine the expression levels of E-cadherin, N-cadherin, and Vimentin.
MiR-18a-5p target BTG3 in NPC cells
It was attempted to identify the potential target of miR-18a-5p in NPC cell lines. Bioinformatics analysis showed that BTG3 could be a potential target of miR-18a-5p (Figure 3a). BTG3 expression level was significantly elevated in normal tissues compared with that in NPC tissues (Figure 3b) and in NPC cell lines (Figure 3c). Importantly, the correlation analysis between the expression levels of BTG3 and miR-18a-5p revealed that there was an inverse correlation between these expression levels (Figure 3d). The inhibitory effect of miR-18a-5p on BTG3 expression level was assessed. The results indicated that miR-18a-5p inhibition promoted BTG3 expression level, while miR-18a-5p mimic was resulted in the opposite effect (Figures 3e, f). Mutant BTG3, where the binding site with miR-18a-5p-mutated was constructed, is shown in Figure 3g. The results of dual-luciferase activity assay indicated that miR-18a-5p inhibitor promoted the activity of wild-type BTG3, while no change was found in the case of mutant-BTG3 (Figure 3h). RNA-immunoprecipitation analysis revealed that miR-18a-5p could bind to BTG3 (Figure 3i). These results concluded that miR-18a-5p could bind and regulate BTG3 expression level in NPC cells.
Figure 3.
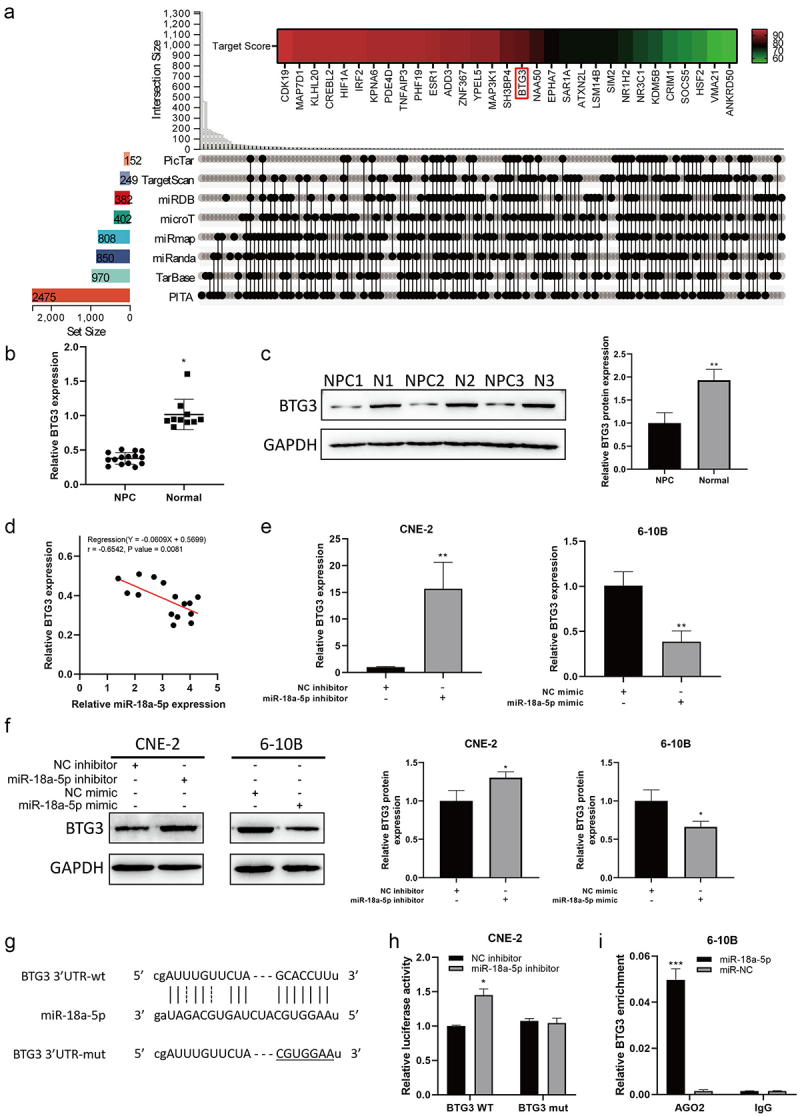
a. Prediction of miR-18a-5p target genes. b. The BTG3 mRNA expression level in tissues was determined by RT-Qpcr. c. The BTG3 protein expression in tissues was identified by Western blotting. d. The miR-18a-5p expression level showed a negative correlation with BTG3 expression level in NPC tissue. e. The BTG3 mRNA expression level was detected by RT-Qpcr after transfection with a miR-18a-5p inhibitor in CNE-2 cells and transfection with a miR-18a-5p mimic in 6-10B cells. f. The protein expression of BTG3 was detected by Western blotting after transfection with a miR-18a-5p inhibitor in CNE-2 cells and transfection with a miR-18a-5p mimic in 6-10B cells. g. The prediction of binding site of BTG3 and miR-18a-5p. h. The miR-18a-5p activity was evaluated by dual-luciferase reporter assay after silencing of BTG3 in CNE-2 cells. i. The miR-18a-5p was over-expressed in 6-10B cells, and AGO2 antibody was then used to examine the enrichment of BTG3 by RIP assay.
BTG3 inhibited the proliferation, migration, invasion, and EMT of NPC cells
As previously reported, low BTG3 expression level was correlated with poor prognosis of NPC patients [30]. Nevertheless, the role of BTG3 in NPC has still remained elusive. Therefore, the effects of BTG3 on NPC cells were assessed in the present study. For this purpose, siRNA constructs targeting BTG3 were synthesized, and it was found that construct number 2 exhibited the most significant inhibitory effect on BTG3 (Figure 4a). It was also revealed that silencing of BTG3 promoted CNE2 cell proliferation, while inhibiting miR-18a-5p expression level suppressed CNE2 cell proliferation; the combination of silencing of BTG3 and miR-18a-5p inhibition induced no change in the cell proliferation compared with the control group (Figure 4b). The Western blotting confirmed that silencing of BTG3 promoted the expression level of Bcl-2 protein and inhibited the expression levels of Bax and cleaved caspase-3 and caspase-9, while the combination of miR-18a-5p inhibition and silencing of BTG3 partially restored the expression levels of Bcl-2, Bax, caspase-3, and caspase-9 (Figure 4c). These results indicated the vitality of BTG3 in NPC cell proliferation. The wound healing, cell invasion, and migration assays also exhibited similar results. Silencing of BTG3 promoted wound healing, invasion, and migration of CNE2 cells, while miR-18a-5p inhibition suppressed these effects, and the combination of silencing of BTG3 and miR-18a-5p inhibition exhibited no changes in wound healing, invasive, and migratory capacities of CNE2 cells (Figures 4d, e). Further analysis was conducted to examine the effects of BTG3 on the EMT of NPC cells. The results indicated that silencing of BTG3 inhibited E-cadherin expression level, while promoted the expression levels of N-cadherin and vimentin; on the other hand, the combination of silencing of BTG3 and miR-18a-5p inhibition partially restored the expression levels of E-cadherin, N-cadherin, and vimentin to normal levels compared with those in the NC group (Figure 4f). Collectively, BTG3 showed to play an important role in the proliferation, invasion, migration, and EMT of NPC cells. Besides, there was a correlation between BTG3 expression level and miR-18a-5p expression level in NPC cells.
Figure 4.

a. The BTG3 expression level was detected by RT-Qpcr and Western blotting after silencing of BTG3 in CNE-2 cells. b. The proliferation ability was detected by MTT assay after silencing of miR-18a-5p or BTG3 in CNE-2 cells. c. The expression levels of Bcl-2, Bax, cleaved caspase-3, total caspase-3, cleaved caspase-9, and total caspase-9 proteins were identified by Western blotting after silencing of miR-18a-5p or BTG3 in CNE-2 cells. d. The migratory ability was detected by scratch wound healing assay after silencing of miR-18a-5p or BTG3 in CNE-2 cells. e. The invasive ability was detected by Transwell assay after silencing of miR-18a-5p or BTG3 in CNE-2 cells. f. The expression levels of E-cadherin, N-cadherin, and Vimentin proteins were determined by Western blotting after silencing of miR-18a-5p or BTG3 in CNE-2 cells.
MiR-18a-5p was highly expressed in NPC exosomes
Exosomes are diminutive membrane vesicles secreted by the most of cells in the human body, and they are broadly distributed in various body fluids. They are functionally active in distinct physio-pathological processes by carrying and transmitting vital signaling molecules, such as mRNAs, miRNAs, lipid, proteins, etc. Exosomal miRNAs are vitally engaged in tumorigenesis and the progression of NPC. They are extensively involved in NPC cell proliferation, invasion, migration, neovascularization, radiotherapy resistance, and tumor immune microenvironment regulation via intercellular communication and control of gene expression [31]. In the present study, the exosomes were isolated from the NPC cells and normal cells (Figures 5a–b). The exosomal isolation was confirmed by Western blotting and RT-qPCR; the exosomal markers (CD81, CD63, and TSG101) were found to be highly expressed in the isolated exosomes compared with those in normal cells (Figure 5c). These results indicated the successful isolation of the NPC exosomes. The exosomal expression of miR-18a-5p was confirmed when 6-10B exomes were isolated and incubated with anti-CD81 antibody, and miR-18a-5p expression level was then assessed and found to be highly expressed in the exosome group versus that in the PBS group; besides, the pri-miR-18a expression level did not change in all groups, indicating that miR-18a-5p was primarily expressed in the NPC exosomes (Figures 5d,e). Notably, miR-18a-5p inhibitor drastically restrained the delivery of exosomal miR-18a-5p to NPC cells, while miR-18a-5p mimic significantly promoted miR-18a-5p expression level in exosomes and transferred to the NPC cells; on the other hand, there was no change in the pri-miR-18a expression level among all the groups Figure 5f. These results demonstrated that miR-18a-5p was mainly secreted and expressed by the NPC exosomes.
Figure 5.

The miR-18a-p functions as a signaling molecule between tumor cells. a. The exosomes were isolated from NPC cell lines, and their morphological features were then observed under TEM. b. The diameters of isolated exosomes. c. the expression levels of CD9, CD63, and TSG101 in NPC cell lines and their exosomes were detected by Western blotting. d. The miR-18a-5p expression level in NPC exosomes was examined using RT-Qpcr. e. After incubating 6-10B cells with CNE-2-derived exosome, the expression levels of miR-18a-5p and pri-miR-18a in 6-10B cells were measured by RT-Qpcr.. f. Exosomes were collected from CNE-2 cells, which were transfected with miR-18a-5p mimics or inhibitors. Exosomes were co-cultured with 6-10B cells. The expression levels of miR-18a-5p and pri-miR-18a in 6-10B cells were identified by RT-Qpcr..
Exosomal miR-18a-5p promoted proliferation, invasion, migration, and EMT of NPC cells
Next, the effect of exosomal miR-18a-5p on the growth of NPC cells was investigated, exosomes isolated from NPC cells, which were transfected with miR-18a-5p mimics or inhibitor, were treated with 6-10B cells. The results of MTT assay showed that exosomal miR-18a-5p mimics remarkably promoted the proliferation of 6-10B cells compared with the NC group, while transfection with miR-18a-5p inhibitor led to the opposite effect (Figure 6a). Western blotting revealed that the expression level of Bcl-2 protein was lower in NPC cells treated with exosomes isolated from miR-18a-5p inhibitor-transfected NPC cells than that in the control group, while the expression levels of Bax and cleaved caspase-3 proteins were upregulated; on the contrary, exosomal miR-18a-5p exhibited the opposite effect (Figure 6b). Taken together, the exosomal miR-18a-5p could be involved in the regulation of NPC cell proliferation. The results of the wound healing and transwell assays showed that exosomal miR-18a-5p mimic promoted NPC cell migration and invasion, whereas exosomal miR-18a-5p inhibition led to the opposite result (Figures 6c,d). Moreover, the expression levels of N-cadherin and vimentin proteins were elevated, and the expression level of E-cadherin protein was reduced after exosomal miR-18a-5p overexpression, whereas exosomal miR-18a-5p inhibition led to the opposite result (Figure 6e). The above-mentioned results indicated that exosomal miR-18a-5p overexpression could promote NPC cell proliferation, migration, invasion, and EMT, whereas exosomal miR-18a-5p inhibition led to the opposite effect.
Figure 6.
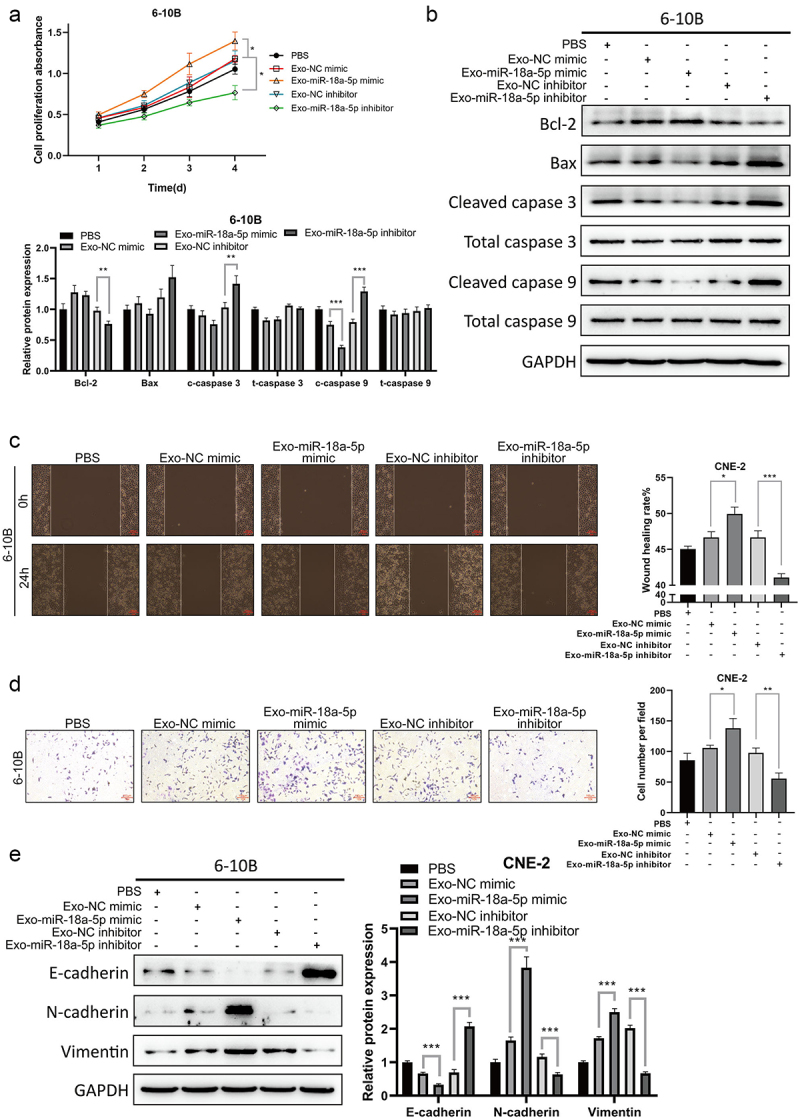
Exosomes were extracted from CNE-2 cells transfected with miR-18a-5p mimic or inhibitor. 6-10B cells were co-cultured with exosomes or PBS. a. 6-10B cell proliferation ability was detected by the CCK-8 assay. b. The expression levels of Bcl-2, Bax, cleaved caspase-3, total caspase-3, cleaved caspase-9, and total caspase-9 proteins were determined by Western blotting. c. The migratory ability was evaluated by wound healing assay. d. The invasive ability was assessed by Transwell assay. e. The expression levels of E-cadherin, N-cadherin, and Vimentin proteins were examined by Western blotting.
Exosomal miR-18a-5p promoted functions via targeting BTG3 and activating the Wnt/β-catenin signaling pathway
Exosomes might be involved in exchanges of genetic information between stromal cells and tumor cells and promote tumor growth, invasion, migration, and distant metastasis. Additionally, the exosomes produced by NPC cells after being infected with the Epstein-Barr (EB) virus contain viral microRNAs that activate Wnt, JNK, PI3K/Akt, mitogen-activated protein kinase (MAPK), and nuclear factor-κB (NF-κB) signaling pathways [32]. In the present study, the underlying mechanism of miR-18a-5p and its subsequent signaling pathway in NPC cells was explored. Western blotting showed that the expression levels of BTG3 and GSK3β were downregulated in 6-10B cells and isolated exosomes; however, the expression levels of Wnt1, Wnt3, β-catenin, and pGSK3β were upregulated (Figures 7a,b). The inhibition of exosomal miR-18a-5p promoted the expression levels of BTG3 and GSK3β proteins, while reduced the expression levels of Wnt1, Wnt3, β-catenin, and pGSK3β proteins. On the contrary, exosomal miR-18a-5p mimic exhibited the opposite effect (Figure 7c). In NPC cells (CNE2 and 6-10B), BTG3 overexpression downregulated the expression levels of Wnt1, Wnt3, β-catenin, and pGSK3β, while silencing of BTG3 exhibited the opposite effect (Figure 7d). On the other hand, the combination of BTG3 knockdown and miR-18a-5p inhibition partially restored the expression levels of Wnt1, Wnt3, β-catenin, and pGSK3β compared with those in the NC group (Figure 7e). Furthermore, in vivo assay confirmed that inhibition of GSK3β with a GSK3β inhibitor (CHIR99021) could block miR-18a-5p mimics-induced promotion of NPC cell proliferation, migration, and invasion (Supplementary Figure S2). These results indicated that miR-18a-5p could target BTG3 protein and modulate NPC cell growth via the Wnt/β-catenin signaling pathway.
Figure 7.
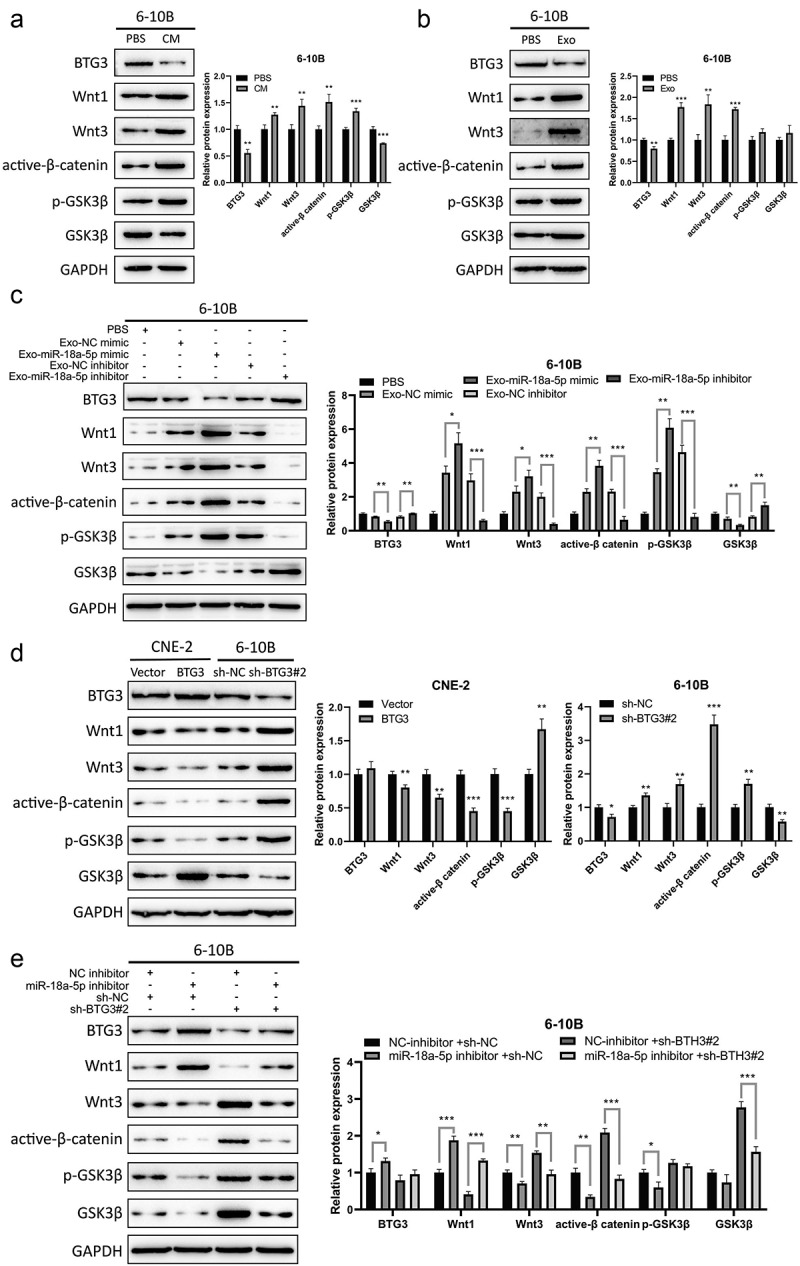
a. The expression levels of BTG3, Wnt1, Wnt3, active-β-catenin, p-GSK3β, and GSK3β in 6-10B cells, which were co-cultured with CNE-2 CM, were determined by Western blotting. b. The expression levels of BTG3, Wnt1, Wnt3, active-β-catenin, p-GSK3β, and GSK3β proteins in 6-10B cells, which were co-cultured with CNE-2-derived exosomes, were examined by Western blotting. c. Exosomes were extracted from CNE-2 cells transfected with miR-18a-5p mimic or inhibitor. 6-10B cells were co-cultured with exosomes. d. The expression levels of BTG3, Wnt1, Wnt3, active-β-catenin, p-GSK3β, and GSK3β proteins in BTG3-overexpression CNE-2 and BTG3-knockdown 6-10B cells were measured by Western blotting. E. The exosomes derived from CNE-2 cell, transfected with miR-18a-5p inhibitor, were used to incubate with 6-10B cells, and BTG3 was then knocked down in 6-10B cells. The expression levels of BTG3, Wnt1, Wnt3, active-β-catenin, p-GSK3β, and GSK3β proteins in 6-10B cells were identified by Western blotting.
Inhibition of exosomal miR-18a-5p suppressed tumor growth in vivo
After assessment of the vital role of miR-18a-5p in the NPC cell lines, the results were confirmed in vivo. Xenograft tumors have been found to be significantly smaller in size when exosomal miR-18a-5p was inhibited than those in the control group (Figure 8a). The volume and weight of tumors were assessed, and it was found that volume and weight of tumors were significantly reduced in the case of inhibiting exosomal miR-18a-5p, while they rapidly increased in the Exo-NC group compared with those in the PBS group (Figure 8b). Histochemical analysis confirmed that the inhibition of exosomal miR-18a-5p reduced the number of nodes in NPC tumor tissues, indicating that miR-18a-5p inhibition could attenuate NPC tumor growth (Figure 8c). The expression levels of miR-18a-5p and BTG3 in NPC tumor xenografts were determined, and it was revealed that the inhibition of exosomal miR-18a-5p significantly promoted BTG3 expression level (Figure 8d). The expression levels of EMT markers (E-cadherin and vimentin) were examined in NPC tumor specimens, which were found to be downregulated, while BTG3 expression level was upregulated after inhibiting exosomal miR-18a-5p (Figure 8e). These results demonstrated that exosomal miR-18a-5p could play a vital role in NPC tumor growth, and it could be involved in the progression of EMT of NPC tumors via downregulating BTG3 expression level.
Figure 8.
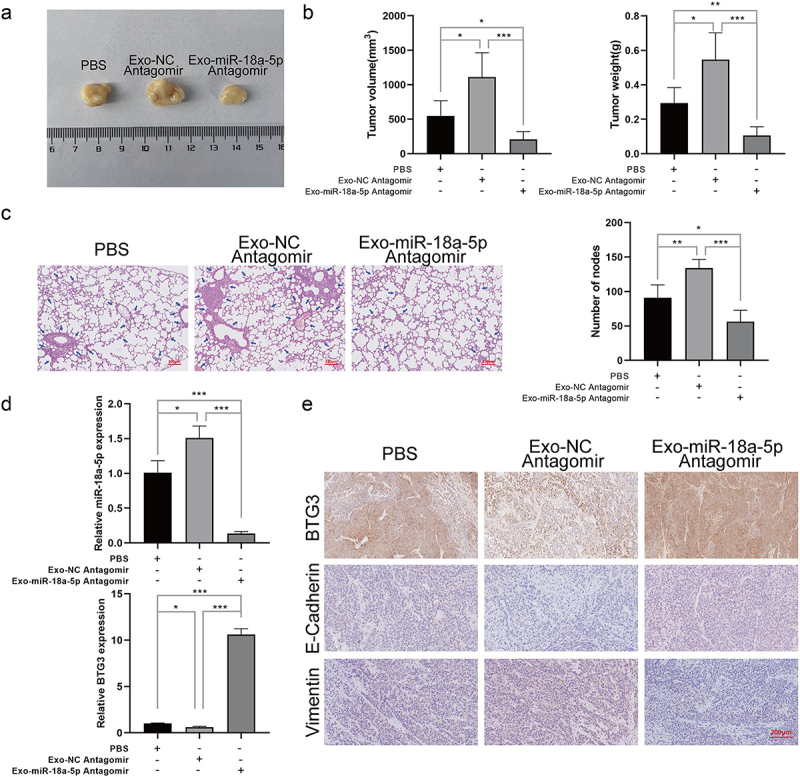
6-10B cells were co-cultured with exosomes derived from silenced miR-18a-5pin CNE-2 cells. The transplanted models were established by subcutaneous injection of 6-10B cells into mice. The pulmonary metastasis models were established by injection of 6-10B cells into tail veins of mice. a. Representative image of transplanted tumors. b. Measurement of volume and weight of the transplanted tumors. c. The number of pulmonary metastases was determined by H&E staining. d. The expression levels of miR-18a-5p and BTG3 were detected using RT-qPCR. e. The expression levels of BTG3, E-cadherin, and vimentin in the transplanted tumors were determined by immunohistochemistry.
Discussion
It is broadly accepted that NPC is a malignant epithelial tumor associated with EB virus infection [1]. NPC is endemic with a high prevalence in Asia and North Africa, while its incidence increases worldwide, and it is a severe public health concern [33]. The exact etiology of NPC remains elusive, while risk factors include EB virus infection, dietary habits, genetic predispositions, and environmental factors [34,35]. Exosomes have shown to play a vital role in the development and progression of NPC [32]. Therefore, identifying novel targets and viable biomarkers for the diagnosis and prognosis of NPC is of great importance.
The functions of miRNAs are mainly influenced by the expression levels of their primary target genes. Some miRNAs promote tumorigenesis differently or suppress it in different cells. Therefore, classifying miRNAs as oncogenes or tumor suppressors depends largely on the type of cells. Typically, miRNAs target multiple mRNAs concurrently and are involved in complex interactions that control the transcriptome. MiRNAs are mainly dysregulated in cancer cells and function distinctly as oncogenes or tumor suppressors. MiRNAs were reported to be aberrantly expressed in cancer cells, and they exerted pivotal effects by altering the expression levels of their specific mRNA targets [36]. Tumor suppressor genes are typically inhibited directly by miRNAs, and such miRNAs are considered as onco-miRNAs. For instance, cell cycle inhibitor p21 can be directly targeted by miR-663 in NPC, promoting NPC cell proliferation and tumorigenesis [37]. An onco-miRNA, miR-125b, was significantly upregulated in NPC tissue compared with that healthy nasopharyngeal mucosa, and it was correlated with poor survival outcomes; furthermore, high miR-125b expression level was considered as an independent predictor for shorter survival of NPC patients [38]. The same report found that miR-125b promoted NPC cell proliferation and inhibited apoptosis. The tumor necrosis factor-alpha-induced protein 3 (eTNFAIP3) gene is a direct target of miR-125b, acting as a tumor suppressor in NPC, and it mediates NPC tumorigenesis via activating the nuclear factor-κB (NF-κB) signaling pathway. In addition, a recent study (preprint) has shown that miR-18a-5p was involved in breast cancer progression by targeting SREBP1. The present study revealed that miR-18a-5p was significantly upregulated in NPC cells and tissues, and it was also associated with metastasis. Taken together, these findings demonstrated that miR-18a-5p is an onco-miRNA target tumor suppressor gene, leading to NPC initiation.
BTG3 is a tumor suppressor gene that has been reported to be targeted by the trans-activated mIR106A-5p and suppresses macroautophagy/autophagy via the MAPK signaling pathway in NPC [30]. Consistently, in the present study, it was further confirmed that miR-18a-5p could target BTG3, which could be involved in NPC progression.
In cancer, exosomes may exchange genetic information between stromal cells and tumor cells and promote angiogenesis and cancer cell growth, invasion, migration, and distant metastasis [39–42]. Different types of exosomes have been isolated from the serum of NPC patients, such as pure NPC-derived exosomes and EBV-related exosomes [43]. These exosomes contain distinct contents and thus play different roles in tumor-host crosstalk, and they modulate angiogenesis, immune response, cell proliferation, cell-to-cell communication, and tumor invasion [44,45]. For instance, miRNAs derived from NPC-isolated exosomes regulate the expression levels of genes involved in cell proliferation, differentiation, and stress response [46]. In NPC, exosomal miR-9 regulates PDK/AKT signaling pathway and inhibits angiogenesis [47]. Moreover, exosomal miR-24-3p serves as a potential biomarker for NPC prognosis [46]. Therefore, exosomal miRNAs are involved in the initiation and progression of diverse types of cancer, including NPC, and they can be biomarkers for NPC patients. The results of the present study indicated that miR-18a-5p was primarily expressed and secreted by exosomes in NPC cells, and its inhibition could hinder NPC cell growth.
Aberrant activation of the Wingless-type (Wnt) signaling pathway plays a critical role in the oncogenesis of various types of human cancer, and aberrant Wnt signaling is a joint event in NPC carcinogenesis [48]. In the present study, it was found that inhibiting exosomal miR-18a-5p could promote BTG3 expression level, while suppress the expression levels of Wnt1, Wnt3, β-catenin, and pGSK3β proteins.
Altogether, the results of the present study indicated that miR-18a-5p could be a potential marker for NPC; it was secreted and expressed in the exosomes of NPC, and its inhibition could hinder NPC progression. Besides, miR-18a-5p could target the tumor suppressor gene BTG3 and attenuate its function via targeting the Wnt/β-catenin signaling pathway. This study contains several limitations, such as a small sample size, indicating the necessity of further large-scale research on NPC patients to support the finding of this study with even more solid proof; the interaction between miR-18a-5p and BTG3 shall be validated using different assays. Moreover, in vitro findings shall be confirmed in vivo. In conclusion, this study presented a novel mechanism of NPC progression, and the results may provide a reliable basis for developing more efficient therapeutics for NPC.
Supplementary Material
Funding Statement
This study was supported by the Jiangxi Provincial Key R&D Program General Project (Grant No. 20203BBGL73204)
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2023.2216508.
References
- [1].Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. [DOI] [PubMed] [Google Scholar]
- [2].Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. [DOI] [PubMed] [Google Scholar]
- [3].Zucchetto A, Taborelli M, Bosetti C, et al. Metabolic disorders and the risk of nasopharyngeal carcinoma: a case–control study in Italy. Eur J Cancer Prev. 2018;27(2):180–183. [DOI] [PubMed] [Google Scholar]
- [4].Domingues C, Serambeque BP, Laranjo Cândido MS, et al. Epithelial-mesenchymal transition and microRnas: challenges and future perspectives in oral cancer. Head Neck. 2018;40(10):2304–2313. [DOI] [PubMed] [Google Scholar]
- [5].Li M, Huo X, Davuljigari CB, et al. MicroRNAs and their role in environmental chemical carcinogenesis. Environ Geochem Health. 2019;41(1):225–247. [DOI] [PubMed] [Google Scholar]
- [6].Summerer I, Niyazi M, Unger K, et al. Changes in circulating microRnas after radiochemotherapy in head and neck cancer patients. Radiat Oncol. 2013;8(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zheng H, Liu JY, Song FJ, et al. Advances in circulating microRnas as diagnostic and prognostic markers for ovarian cancer. Cancer Biol Med. 2013;10(3):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Menon H, Chen D, Ramapriyan R, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer. 2019;7(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yu D, Han GH, Zhao X, et al. MicroRNA-129-5p suppresses nasopharyngeal carcinoma lymphangiogenesis and lymph node metastasis by targeting ZIC2. Cell Oncol. 2020;43(2):249–261. [DOI] [PubMed] [Google Scholar]
- [10].Zhou L, Li Z, Pan X, et al. Identification of miR-18a-5p as an oncogene and prognostic biomarker in RCC. Am J Transl Res. 2018;10(6):1874–1886. [PMC free article] [PubMed] [Google Scholar]
- [11].Tsao SW, Tsang CM, Lo KW.. Epstein–Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang N, Zhang H, Liu Y, et al. SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death Differ. 2019;26(5):843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang G, Han G, Zhang X, et al. Long non-coding RNA FENDRR reduces prostate cancer malignancy by competitively binding miR-18a-5p with RUNX1. Biomarkers. 2018;23:435–445. [DOI] [PubMed] [Google Scholar]
- [14].Wang H, Wei X, Wu B, et al. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res. 2019;11:3351–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu X, Odenthal M, Fries JW. Exosomes as miRNA Carriers: formation–Function–Future. Int J Mol Sci. 2016;17(12):2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hou J, Wang F, Liu X, et al. Tumor-derived exosomes enhance invasion and metastasis of salivary adenoid cystic carcinoma cells. J Oral Pathol Med. 2018;47(2):144–151. [DOI] [PubMed] [Google Scholar]
- [19].Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017;13(28):2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kozomara A, Birgaoanu M, Griffiths-Jones S. miRbase: from microRNA sequences to function. Nucleic Acids Res. 2019;47:D155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1. [PMC free article] [PubMed] [Google Scholar]
- [24].Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chou C-H, Shrestha S, Yang C-D, et al. miRtarbase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang X. Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics. 2016;32:1316–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin C, Zong J, Lin W, et al. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. Journal Of Experimental & Clinical Cancer Research. 2018;37:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu Q, Zhang Q, Gu M, et al. Mir106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy. 2021;17:1667–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liao C, Liu H, Luo X. The emerging roles of exosomal miRnas in nasopharyngeal carcinoma. Am J Cancer Res. 2021;11(6):2508–2520. [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Y, Xia L, Lin J, et al. Exosomes in Nasopharyngeal Carcinoma. J Cancer. 2018;9(5):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].BBY M, Hui EP, Chan ATC. Investigational drugs for nasopharyngeal carcinoma. Expert Opin Investig Drugs. 2017;26(6):677–685. [DOI] [PubMed] [Google Scholar]
- [34].Liao Q, Zeng Z, Guo X, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33:2098–2109. [DOI] [PubMed] [Google Scholar]
- [35].Lung ML, Cheung AK, Ko JM, et al. The interplay of host genetic factors and Epstein-Barr virus in the development of nasopharyngeal carcinoma. Chin J Cancer. 2014;33(11):556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100(6):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yi C, Wang Q, Wang L, et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene. 2012;31(41):4421–4433. [DOI] [PubMed] [Google Scholar]
- [38].Zheng Z, Qu JQ, Yi HM, et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway. Cell Death Dis. 2017;8(6):e2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bastos N, Ruivo CF, da Silva S, et al. Exosomes in cancer: use them or target them? Semin Cell Dev Biol. 2018;78:13–21. [DOI] [PubMed] [Google Scholar]
- [40].Liu H, Wang J, Chen Y, et al. NPC-EXs Alleviate Endothelial Oxidative Stress and Dysfunction through the miR-210 Downstream Nox2 and VEGFR2 Pathways. Oxid Med Cell Longev. 2017;2017:9397631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shao Y, Shen Y, Chen T, et al. The functions and clinical applications of tumor-derived exosomes. Oncotarget. 2016;7:60736–60751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Z, Chen JQ, Liu JL, et al. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med. 2016;14(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harmati M, Tarnai Z, Decsi G, et al. Stressors alter intercellular communication and exosome profile of nasopharyngeal carcinoma cells. J Oral Pathol Med. 2017;46(4):259–266. [DOI] [PubMed] [Google Scholar]
- [44].Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].D’Asti E, Chennakrishnaiah S, Lee TH, et al. Extracellular Vesicles in Brain Tumor Progression. Cell Mol Neurobiol. 2016;36(3):383–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ye SB, Zhang H, Cai TT, et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240(3):329–340. [DOI] [PubMed] [Google Scholar]
- [47].Lu J, Liu QH, Wang F, et al. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2018;37(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lin YC, You L, Xu Z, et al. Wnt signaling activation and WIF-1 silencing in nasopharyngeal cancer cell lines. Biochem Biophys Res Commun. 2006;341(2):635–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


