ABSTRACT
MasR is a critical element in the RAS accessory pathway that protects the heart against myocardial infarction, ischemia-reperfusion injury, and pathological remodeling by counteracting the effects of AT1R. This receptor is mainly stimulated by Ang 1–7, which is a bioactive metabolite of the angiotensin produced by ACE2. MasR activation attenuates ischemia-related myocardial damage by facilitating vasorelaxation, improving cell metabolism, reducing inflammation and oxidative stress, inhibiting thrombosis, and stabilizing atherosclerotic plaque. It also prevents pathological cardiac remodeling by suppressing hypertrophy- and fibrosis-inducing signals. In addition, the potential of MasR in lowering blood pressure, improving blood glucose and lipid profiles, and weight loss has made it effective in modulating risk factors for coronary artery disease including hypertension, diabetes, dyslipidemia, and obesity. Considering these properties, the administration of MasR agonists offers a promising approach to the prevention and treatment of ischemic heart disease.
Abbreviations: Acetylcholine (Ach); AMP-activated protein kinase (AMPK); Angiotensin (Ang); Angiotensin receptor (ATR); Angiotensin receptor blocker (ARB); Angiotensin-converting enzyme (ACE); Angiotensin-converting enzyme inhibitor (ACEI); Anti-PRD1-BF1-RIZ1 homologous domain containing 16 (PRDM16); bradykinin (BK); Calcineurin (CaN); cAMP-response element binding protein (CREB); Catalase (CAT); C-C Motif Chemokine Ligand 2 (CCL2); Chloride channel 3 (CIC3); c-Jun N-terminal kinases (JNK); Cluster of differentiation 36 (CD36); Cocaine- and amphetamine-regulated transcript (CART); Connective tissue growth factor (CTGF); Coronary artery disease (CAD); Creatine phosphokinase (CPK); C-X-C motif chemokine ligand 10 (CXCL10); Cystic fibrosis transmembrane conductance regulator (CFTR); Endothelial nitric oxide synthase (eNOS); Extracellular signal-regulated kinase 1/2 (ERK 1/2); Fatty acid transport protein (FATP); Fibroblast growth factor 21 (FGF21); Forkhead box protein O1 (FoxO1); Glucokinase (Gk); Glucose transporter (GLUT); Glycogen synthase kinase 3β (GSK3β); High density lipoprotein (HDL); High sensitive C-reactive protein (hs-CRP); Inositol trisphosphate (IP3); Interleukin (IL); Ischemic heart disease (IHD); Janus kinase (JAK); Kruppel-like factor 4 (KLF4); Lactate dehydrogenase (LDH); Left ventricular end-diastolic pressure (LVEDP); Left ventricular end-systolic pressure (LVESP); Lipoprotein lipase (LPL); L-NG-Nitro arginine methyl ester (L-NAME); Low density lipoprotein (LDL); Mammalian target of rapamycin (mTOR); Mas-related G protein-coupled receptors (Mrgpr); Matrix metalloproteinase (MMP); MAPK phosphatase-1 (MKP-1); Mitogen-activated protein kinase (MAPK); Monocyte chemoattractant protein-1 (MCP-1); NADPH oxidase (NOX); Neuropeptide FF (NPFF); Neutral endopeptidase (NEP); Nitric oxide (NO); Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB); Nuclear-factor of activated T-cells (NFAT); Pancreatic and duodenal homeobox 1 (Pdx1); Peroxisome proliferator- activated receptor γ (PPARγ); Phosphoinositide 3-kinases (PI3k); Phospholipase C (PLC); Prepro-orexin (PPO); Prolyl-endopeptidase (PEP); Prostacyclin (PGI2); Protein kinase B (Akt); Reactive oxygen species (ROS); Renin-angiotensin system (RAS); Rho-associated protein kinase (ROCK); Serum amyloid A (SAA); Signal transducer and activator of transcription (STAT); Sirtuin 1 (Sirt1); Slit guidance ligand 3 (Slit3); Smooth muscle 22α (SM22α); Sterol regulatory element-binding protein 1 (SREBP-1c); Stromal-derived factor-1a (SDF); Superoxide dismutase (SOD); Thiobarbituric acid reactive substances (TBARS); Tissue factor (TF); Toll-like receptor 4 (TLR4); Transforming growth factor β1 (TGF-β1); Tumor necrosis factor α (TNF-α); Uncoupling protein 1 (UCP1); Ventrolateral medulla (VLM)
KEYWORDS: MasR, Ang 1-7, Ischemic heart disease, Atherosclerosis, Diabetes, Obesity
1. Introduction
Ischemic heart disease (IHD) leads to more deaths and disabilities and incurs greater economic costs than any other illness. The most common cause of myocardial ischemia is atherosclerosis of coronary arteries, of which hypertension, diabetes, dyslipidemia, and obesity are major risk factors [1].
One mechanism involved in the pathophysiology of coronary artery disease (CAD) is RAS over-activity, and its suppression is the basis for the development of the widely used angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) [2]. ARBs seem to cause fewer side effects than ACEIs. The identification of RAS sub-pathways in recent years has revealed several important aspects of this neurohormonal system. Angiotensin 1–7 (Ang 1–7)/Mas receptor (MasR), as an accessory pathway in RAS, attenuates the deleterious effects of classical Ang II/angiotensin 1 receptor (AT1R) signaling. Since one of the main consequences of Ang II/AT1R activity is endothelium dysfunction and progression of cardiac fibrosis and hypertrophy, the anti-hypertension and anti-remodeling properties of Ang 1–7/MasR have been investigated. Other effects such as anti-inflammatory, anti-thrombosis, and stabilization of atherosclerosis plaques have been reported for MasR, which can be useful in treating acute coronary syndrome [3–6]. In addition, it has been proposed that stimulation of this receptor can reduce blood glucose, improve the lipid profile, and induce weight loss, which is associated with the mitigation of cardiovascular complications [7,8].
This review summarizes recent knowledge about the function and mechanism of MasR and issues related to its association with IHD and coronary atherosclerosis risk factors.
2. MasR
MasR belongs to the family of Mas-related G protein-coupled receptors (Mrgpr) [9]. In humans, the genes encoding for this receptor are located on chromosome 6 [10]. The highest expression of MasR is in the brain and testis, with lower levels detected in other organs [11]. The heart, kidneys, liver, and skeletal muscle are other organs containing MasR, which is located primarily in the endothelium of vessels in these organs [12].
Because MasR was isolated from the DNA of a human epidermoid carcinoma cell line, it was originally thought to be a proto-oncogene [9]. However, subsequent experiments showed that it is not amplified in primary tumors and can transform cells only when artificially overexpressed [13]. The antitumor potential of MasR is currently being investigated [14].
The early attempts to clarify the function of MasR showed that it induces an inward current in response to Ang I, II, and III. Based on these results, it was assumed that MasR was an angiotensin receptor [15]. However, the inward current in Mas-mRNA-injected cells is not inhibited by angiotensin antagonists [15]. In addition, cloning of the AT1R did not support the original hypothesis of Ang II being a ligand for MasR [16]. Identification of Ang 1–7 as a MasR agonist and investigation of the interaction between MasR and AT1R supported the conclusion that MasR is not an Ang II receptor but modulates the activity of Ang II [17,18].
2.1. MasR signaling pathway
A well-known effect of MasR stimulation is increased nitric oxide (NO) release and the main vasodilatory property of MasR depends on this molecule [19]. Amplification of the phosphoinositide 3-kinases (PI3k)/protein kinase B (Akt) cascade under the influence of this receptor activates endothelial nitric oxide synthase (eNOS) by phosphorylation at Ser1177 and dephosphorylation at Thr495 [20]. MasR also suppresses c-Src-dependent signaling via enhancement of SHP-2 tyrosine phosphatase activity, so the activity of NADPH oxidase (NOX) is attenuated and the bioavailability of NO is promoted [21].
Other intracellular routes targeted by MasR in regulating cellular metabolism include PI3K/Akt and AMP-activated protein kinase (AMPK)/forkhead box protein O1 (FoxO1) signaling, respectively, through increasing glycogen synthase kinase 3β (GSK3β) phosphorylation and accelerating peroxisome proliferator-activated receptor γ (PPARγ) expression [22–25]. The antiproliferative potential of MasR is more strongly related to the inhibition of mitogen-activated protein kinase (MAPK) activity, which has an essential role in regulating apoptosis and cell cycle arrest [26,27]. MasR also reduces the production of inflammatory cytokines and pro-fibrotic mediators by down-regulation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) [28–32].
MasR has the potential to enhance Ca2+ influx by triggering phospholipase C (PLC) and accumulation of inositol trisphosphate (IP3) [33–35]. However, this mechanism is not exerted by all its ligands. The phenomenon that different ligands activate diverse signaling pathways is functional selectivity or biased signaling [36,37].
2.2. MasR agonist
Ang 1–7, angioprotectin, and neuropeptide FF (NPFF) are physiological ligands of MasR [38–40]. Ang 1–7 is a bioactive metabolite in the renin-angiotensin system (RAS), which is formed from Ang I by endopeptidases, including neutral endopeptidase (NEP) and prolyl-endopeptidase (PEP), or indirectly through angiotensin-converting enzyme 2 (ACE2)-mediated production of Ang 1–9 [41–44]. Interestingly, the administration of angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) drugs can elevate the plasma level of Ang 1–7 [45–48]. Angioprotectin is also an octapeptide produced from Ang II, enzymatically by substituting Pro1 and Glu2 instead of Asp1 and Arg. Its affinity for MasR is higher than Ang 1–7 [40]. NPFF is a member of the RFamide peptide family that is characterized as an opioid-related neuromodulator. Although NPFF has its own specific receptor, it can also stimulate MasR [49,50]. Unlike the other two ligands, this neuropeptide is capable of applying PLC/Ca2+ mechanism [51].
Several synthetic agonists for the MasR have been investigated. AVE 0991, 1-ethyl-3-[3-[4-[(5-formyl-4-methoxy-2-phenylimidazol-1-yl) methyl] phenyl]-5-(2-methylpropyl) thiophen-2-yl] sulfonylurea, was the first physiologically tolerable compound to be reported that mimicked the Ang 1–7 effects in vessels, kidneys and the heart [52]. AVE 0991 and Ang 1–7 competed for binding to the membrane of bovine endothelial cells with IC50 values of 21 ± 35 and 220 ± 280 nmol/L, respectively. AVE 0991 induced the release of NO at a rate at least five times higher than Ang 1–7. Most of the effects of AVE 0991 disappeared after blocking MasR by A-779 or inhibition of eNOS by L-NG-Nitro arginine methyl ester (L-NAME) [53]. It was observed, however, that antagonizing AT2R by PD 123,177 in endothelial cells reduced the effects of AVE 0991, which was probably due to the functional or physical (heterodimers) interactions between MasR and AT2R [54].
CGEN 856S is a novel MasR agonist. CGEN 856S induces aorta ring dilatation through a NO-dependent pathway. Treatment of hypertensive rats with CGEN 856S lowered blood pressure and was cardioprotective [55]. In addition, the administration of CGEN 856S in rats receiving isoproterenol attenuated hypertrophic and fibrotic effects. It also significantly reduced the infarct size in rats undergoing coronary ligation [56].
AR 234,960 is another agonist of MasR. Although several studies have indicated the anti-fibrotic property of stimulating MasR by AR 234,960, AR 234,960 increased both mRNA and protein levels of connective tissue growth factor (CTGF) via extracellular signal-regulated kinase 1/2 (ERK 1/2) signaling in adult human cardiac fibroblasts, and these effects were attenuated by the MasR inverse agonist AR 244,555 [57].
3. MasR and Ischemic heart disease
The beneficial effect of MasR agonists on the progression of IHD has been investigated in several in vivo and in vitro models. The mechanisms involved in the pathophysiology of IHD in the different phases of this disease as mediated through the MasR signaling pathway are discussed below.
3.1. Myocardial infarction
Most of the drugs currently used to treat IHD have anti-ischemic properties that help balance the oxygen supply demands of the heart [58,59].
Almeida et al. (2000) and Kozlovski et al. (2007) both reported that Ang 1–7 injection into isolated animal hearts increased coronary flow in a dose-dependent manner. This coronary vasodilation effect was exerted through endogenous bradykinin (BK) and endothelial B2 receptor-dependent NO release [60,61]. In another study, the effect of Ang 1–7 on the dilation of heart vessels was reported, but this effect was significantly blunted in hypertrophic hearts, more than likely due to the negative effect of AT1R on MasR signaling because the administration of losartan restored Ang 1–7-induced vasorelaxation [62].
Short-term infusion of Ang 1–7 or AVE 0991 in rats enhanced the hypotensive effect of acetylcholine, which can potentially attenuate myocardial oxygen demand by reducing the afterload of the heart. This mechanism was more than likely related to the improvement of endothelium function by the facilitation of NO release. The use of L-NAME as a NOS inhibitor reversed this effect [63]. However, Ang 1–7 also affected the vasomotor center in the midbrain. Micro-injection of Ang 1–7 into the vasodepressor area of the caudal ventrolateral medulla (VLM) produced a dose-dependent decrease in mean arterial pressure, whereas it had the opposite effect in the rostral VLM. Ang 1–7 probably acted directly as a neurotransmitter in the synapse or as a neuromodulator for Ang II in the nucleus of the solitary tract [64].
Antithrombotic and atherosclerotic plaque stabilizing drugs are another part of the overall therapeutic plan for patients with myocardial infarction [58,59].
Fang et al. (2013 and 2015) reported that prolongation of bleeding time following the deletion of the bradykinin B2 receptor or inhibition of this signaling in prekallikrein deficiency resulted from compensatory MasR overactivity. B2 receptor deletion in mice activated ACE to metabolize the accumulated BK, and in parallel, the stimulation of ACE with increased levels of Ang 1–7 occurred. Although prekallikrein deficiency did not increase the Ang 1–7 level, it facilitated the expression of MasR. In both models, increasing the production of NO and prostacyclin (PGI2) elevated vasculoprotective transcription factors such as sirtuin 1 (Sirt1) and Kruppel-like factor 4 (KLF4). Sirt1 indirectly down-regulates tissue factor (TF) expression by inactivating a coactivator for NF-κB, and KLF4 affected TF expression by influencing eNO and thrombomodulin. The risk of thrombosis was decreased by changes in the coagulation factors [65–68].
Several studies in ApoE -/- mice have reported that treatment with MasR agonists attenuated plaque vulnerability. Ang 1–7 and AVE 0991 inhibited the proliferation and migration of vascular smooth muscle cells by suppressing ERK/p38 and Janus kinase (JAK)/signal transducer and activators of the transcription (STAT) cascade. They also enhanced the expression of smooth muscle 22α (SM22α) and decreased the activity of matrix metalloproteinase (MMP), which in turn modulated vascular smooth muscle cell phenotype and elevated plaque collagen content resulting in the atherosclerotic plaque became more stable but still depending on the local pattern of shear stress forces. MasR stimulation was effective in stabilizing atherosclerotic plaques by reducing neutrophil and macrophage infiltration. This mechanism was related to the alleviation of perivascular inflammation due to decreased expression of chemokines such as interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), C-C Motif Chemokine Ligand 2 (CCL2), and C-X-C motif chemokine ligand 10 (CXCL10) in perivascular adipose tissue [69–72].
3.2. Ischemia-reperfusion cardiac injury
The most effective method in saving the lives of patients with CAD is revascularization [73]. A related concern is myocardial ischemia-reperfusion injury (IRI) caused by the accumulation of reactive oxygen species (ROS) and the triggering of the inflammatory response [74].
The work of Pachauri et al. (2017) showed that Ang 1–7 administration as a preconditioning strategy in IRI-challenged rat hearts significantly decreased lactate dehydrogenase (LDH) and creatine phosphokinase (CPK), and limited the infarct size. By stimulating MasR, Ang 1–7 inhibited the NOX enzyme, increased the level of NADPH, and activated MAPK phosphatase-1 (MKP-1). As a result of the suppression of MAPK signaling, mitochondrial integrity and function improved and the progression of cell damage was prevented. Also, by inducing the production of antioxidants such as superoxide dismutase (SOD) and catalase (CAT), the expression of cleaved caspase-3 and apoptosis was reduced [75]. Recently, the use of Ang 1–5, an Ang 1–7 metabolite, has been shown to produce similar cardioprotective effects against IRI through the stimulation of MasR [76]. Pretreatment with AVE 0991 alleviated IRI-induced myocardial necrosis in rats. The group receiving the drug was about 20% better in terms of myocardial infarct size-to-area at-risk ratio than the control group. However, the combination of AVE 0991 with either captopril or losartan did not make a significant difference in the efficacy of AVE 0991 [77].
3.3. Post-infarction cardiac remodeling and heart failure
One of the most important processes after myocardial infarction, which leads to heart failure, is cardiac remodeling. The basis of cardiac remodeling is ventricular dysfunction due to the deposition of fibrotic tissue and pathological hypertrophy [78].
Ang 1–7 infusion after myocardial infarction prevented the development of heart failure in rats undergoing coronary ligation, which was illustrated by a 40% reduction in left ventricular end-diastolic pressure (LVEDP) [79]. In another study that used Ang II, a major suspect in the pathophysiology of cardiac remodeling, Ang 1–7 significantly alleviated myocyte hypertrophy and interstitial fibrosis [80]. MasR agonists act through activation of NO/cGMP and GSK3β suppression of Ang II-induced hypertrophic signals such as calcineurin (CaN)/nuclear-factor activated T-cells (NFAT) and transforming growth factor β1 (TGF-β1)/Smad2 [81,82]. Also, down-regulation of the TGF-β1/Smad2 axis and decreased expression of the inflammatory mediator TNF-α attenuated collagen synthesis [83].
Another beneficial effect of MasR agonist that can alleviate the symptoms of heart failure, especially in the case of reduced ejection fraction, is the strengthening of myocardial contractility [84].
Mechanistically, MasR stimulation modified the effect of acetylcholine (Ach) in reducing left ventricular end-systolic pressure (LVESP) and dP/dt. It also prevented an ACh-induced decrease in maximal contraction and relaxation velocity and shortening in cardiomyocytes [85]. In addition, the role of MasR signaling in the regulation of cellular metabolism also affected the function of the heart muscle. This receptor facilitated the translocation of glucose transporter 4 (GLUT4) and the cluster of differentiation 36 (CD36) by maintaining Akt activity without changing serum insulin levels. Following the increase in glucose and lipid uptake capacity, the ATP content and energy efficiency of the myocardium improved [86].
4. MasR and coronary atherosclerosis risk factors
Toton-Zuranska et al. (2010) reported that AVE 0991 administration could ameliorate atherosclerosis progression in ApoE -/- mice [87]. The results of another study showed that MasR deficiency augmented AngII-induced atherosclerosis in experimental models through mechanisms related to increased oxidative stress, inflammation, and apoptosis [88]. In a comparison between Ang 1–7 and losartan, both showed similar effects in reducing atherosclerosis plaque formation but the drug combination was more effective than either drug alone [89].
Clinical studies have confirmed the protective effects of the ACE2/Ang 1–7/MasR axis in coronary atherosclerosis. The Ang II/Ang 1–7 ratio measurement in patients with known or suspected CAD indicated that the critical CAD group had a higher Ang II/Ang 1–7 ratio than the normal and noncritical CAD group [90]. Although no difference was reported in another study in Ang 1–7 levels between CHD and non-CHD groups, it was observed in that study that circulating ACE2 levels were significantly increased in the female CHD group but not in the males. ACE2 may participate as a compensatory mechanism in CHD [91].
Mechanistically, Ang 1–7 appears to prevent endothelium dysfunction and the development of atherosclerosis by increasing NO bioavailability [92]. Ang 1–7 analogs have been reported to modulate the function of innate immune cells by down-regulating inflammatory factors such as IL-6, IL-12, serum amyloid A (SAA), and monocyte chemoattractant protein-1 (MCP-1) [93]. Inhibiting the expression of co-stimulatory molecules including CD86, CD80, and CD40 on dendritic cells and macrophages led to a decrease in the activity of CD4+ T cells and reduced chronic inflammation as an underlying factor to promote atherosclerosis [94].
The protective effect of MasR against dyslipidemia also played a key role in reducing lipid deposition on the arterial walls. Results obtained from these studies suggested that MasR deficiency is associated with an increase in the serum levels of triglycerides and cholesterol, and a decrease in high-density lipoproteins (HDL). A weakening of the sensitivity to insulin and leptin was the primary mechanism involved in the occurrence of this adverse outcome [95,96]. An oral formulation of Ang 1–7 significantly improved the lipid profile in mice fed a high-fat diet. By stimulating MasR in hepatocytes, this molecule inhibited the presence of fatty acid transport protein (FATP) and reduced fatty acid uptake for storage in the liver. It also down-regulated the expression of genes related to de novo fatty acid synthesis, including PPARγ and sterol regulatory element-binding protein 1 (SREBP-1c). In addition, the effect of Ang 1–7 on adipocytes led to the inhibition of lipogenesis and lipoprotein lipase (LPL) activity [97,98].
Hypertension, diabetes mellitus, and obesity are major risk factors for atherosclerosis. There is an overlap between the incidence and complications of these disorders, so together they are referred to as metabolic syndrome. Hypertension through the initiation of mechanical injury in the endothelium layer can lead to an increase in vascular permeability to certain substances including low-density lipoprotein (LDL) and the formation of atherosclerotic plaque. Diabetes and obesity are associated with chronic systemic inflammation that disrupts normal vascular function. Hyperglycemia and dyslipidemia are also related to oxidative damage, which makes the vessels susceptible to LDL deposition [99–101]. Numerous studies have evaluated the potential of MasR agonists in ameliorating these risk factors for atherosclerosis, as discussed below.
4.1. Hypertension
In a study using renal artery clamp mice, MasR knockout accelerated the rise in blood pressure and its final value was significantly increased [102]. Consistent with this result, MasR and ACE2 deficiency exacerbated Ang II-induced hypertension and its complications in an animal model [103].
Some evidence also supported the hypothesis that Ang 1–7/MasR plays a role in regulating blood pressure. Regarding the cause of lower sensitivity to the hypertensive effects of Ang II or a high salt diet in females compared to males, the higher level of Ang 1–7 and its receptor in the kidney cortex has been suggested to lead to this difference [104,105]. In addition, some of the antihypertensive effects of ACEI and ARB drugs were due to increased levels of Ang 1–7. The renin inhibitor combined with AVE 0991 was synergistic [106–109]. Recently, it has been proposed that two endogenous biomolecules, miR-27a and fibroblast growth factor 21 (FGF21), are involved in blood pressure regulation and act by changing the activity of MasR [110–112]. Interestingly, the beneficial effects of exercise in lowering blood pressure and mitigating the cardiac complications of hypertension have been reported to be partly related to balancing the activity of Ang II and Ang 1–7 [113,114].
Intravenous administration of Ang 1–7 was effective in attenuating renovascular and salt-induced hypertension by restoring vasodilatation and reducing oxidative stress [115–117]. In a clinical study, Ang 1–7 infusion in patients with essential hypertension led to an increase in arterial flow in a dose-dependent manner [118]. The main mechanism involved in the occurrence of these properties was the stimulation of MasR, which promoted the bioavailability of NO and preserved the response to vasodilators such as BK and Ach [119].
Ang 1–7 injection into the hypothalamic paraventricular nucleus also contributed to lowering blood pressure by modulating sympathetic activity [120]. Under the influence of Ang 1–7, the increase in the level of excitatory neurotransmitters such as norepinephrine and glutamate was prevented, and instead, the release of inhibitory neurotransmitters such as GABA was facilitated. Following this alternation, the production of NO, an inhibitor of the sympathetic nervous system, was enhanced [121]. Modulating the expression of pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, and MCP-1, and increasing the production of gp91phox and SOD in the paraventricular nuclei attenuated the sympathetic outflow [122]. Finally, by adjusting the function of baroreflex in the kidneys, the mean arterial pressure decreased [123–126].
Activation of the Ang 1–7/MasR axis alleviated hypertension-related organ damage specifically in the heart [127]. Cardiac hypertrophy and fibrosis were restricted both dependently and independently of blood pressure reduction [127–129]. Downstream pathways of MasR suppressed oxidative stress and inflammatory cell infiltration in the myocardium by counteracting the effects of AT1R. As a result, apoptosis and collagen deposition were reduced and tissue repair was improved [130–132].
The antihypertensive effects of Ang 1–7 have been reported to be negligible in at least one study. Although Ang 1–7 initially reduced heart rate, possibly by improving cardiac baroreflex control, the final effect on reducing cardiac output and total vascular resistance were not significant [133]. These results have been challenged based on the observation of an increase in the plasma level of Ang 1–7 in spontaneous hypertension. Rather, this biomolecule caused kidney dysfunction and increased blood pressure by facilitating sodium reabsorption and reducing diuresis [134–137]. Overall, it is not clear whether increased Ang 1–7 plays a positive role in hypertension or is simply a compensatory response to vasoconstriction or a reduction in its clearance [138–140].
4.2. Diabetes mellitus
It has been proposed that the activation of MasR is effective in lowering blood glucose and relieving the complications of diabetes by increasing insulin sensitivity, promoting insulin secretion, and improving the survival of pancreatic cells [141]. Some of the hypoglycemic effects of pioglitazone and some of the cardioprotective properties of losartan and captopril in diabetes depend on this receptor and even synergize with Ang 1–7 [142,143].
The administration of cyclic Ang 1–7, an ACE-resistant analog, in type I and II diabetic mouse models increased insulin levels and decreased blood glucose and HbA1C levels compared to control mice [7]. Regarding a correlation of MasR activity with the severity of cardiovascular complications of diabetes, a high Ang II/Ang 1–7 ratio has been associated with a reduction in the left ventricular ejection fraction, the development of vascular media thickening, and an increase in high sensitive C-reactive protein (hs-CRP) [144–146]. Both Ang 1–7 and AVE 0991 administration significantly reversed these effects [143,147].
Stimulation of MasR with Ang1–7 up-regulated pancreatic and duodenal homeobox 1 (Pdx1). This transcription factor, insulin promoter factor 1, increased the production of insulin in the pancreas and accelerated glycogenesis in the liver by triggering the expression of GLUT2 and glucokinase (Gk) [148]. Another mechanism that mediates the relationship between MasR and insulin level is the abundance of intracellular cAMP and the activation of cAMP response element binding protein (CREB). This nuclear protein enhanced glucose-stimulated insulin secretion by facilitating the expression of cystic fibrosis transmembrane conductance regulator (CFTR) [149]. MasR was effective in improving insulin levels by preventing mitochondrial dysfunction and apoptosis in pancreatic β-cells by inhibiting c-Jun N-terminal kinases (JNK) [150]. Furthermore, MasR activated PI3K/Akt, the mammalian target of the rapamycin (mTOR) signaling pathway. As a result, glucose uptake in insulin-responsive tissues was potentiated by increasing the phosphorylation of GSK3β and increasing the presence of GLUT4 [151].
Following the lowering of blood glucose by downstream pathways of MasR, the over-expression of chloride channel 3 (CIC3) was modulated. CIC3 plays an important role in hyperglycemia-induced apoptosis by activating ERK 1/2 and p38-MAPK, stimulating NOX, and increasing ROS generation. Its inhibition leads to the reduction of intracellular superoxide and the relief of cardiomyocyte and vascular endothelial oxidative damage [152,153]. In addition, the effect of MasR on improving the mobilization and vasoreparative functions of progenitor cells is involved in the alleviation of diabetes-related vascular injury. Ang 1–7/MasR increased Slit guidance ligand 3 (Slit3) levels in the bone marrow and by activating Rho-associated protein kinase (ROCK) in progenitor cells sensitized them to stromal-derived factor-1a (SDF)-induced migration to promote vascular repair. The ACE2/Ang 1–7/MasR axis contributed to protecting vasodilatory function by reducing NOX activity and increasing NO persistence [154–157].
4.3. Obesity
A study comparing transgenic rats overexpressing Ang 1–7 with a control group in terms of diet-induced weight gain showed that MasR activation protected against the development of obesity. Enhancement of Ang 1–7/MasR signaling prevented obesity by increasing energy expenditure when rats were regularly fed a chow diet. This treatment also significantly limited weight gain by reducing energy intake and improving feeding behavior when they were fed a cafeteria diet [158]. Recently, clinical studies have suggested that an imbalance between Ang II and Ang 1–7 is associated with obesity, confirming the role of MasR in body weight control [159,160].
Ang 1–7/MasR has been shown to diminish fat accumulation by two mechanisms, first by inducing lipid catabolism and secondly by reducing caloric intake [8,158,161,162]. Lipid catabolism results from both the proliferation of brown adipocytes, which was marked by prohibitin expression, and the increase of lipolysis-related enzymes in brown adipocytes. Ang 1–7 stimulated AMPK and mTOR activity by preserving insulin sensitivity, improving leptin response, and suppressing the resistin/toll-like receptor 4 (TLR4) axis. The up-regulation of the anti-PRD1-BF1-RIZ1 homologous domain containing 16 (PRDM16) then activated the hormone-sensitive lipase and inhibited perilipin. Finally, by triggering the uncoupling protein 1 (UCP1), β-oxidation was accelerated in brown adipose tissue [8,158,161]. The decrease in caloric intake under the influence of Ang 1–7 also involved the enhancement of insulin and leptin signaling. Insulin and leptin induced the release of dopamine and weaken the hypothalamic-pituitary-adrenal axis. As a result, decreasing the level of orexigenic peptide prepro-orexin (PPO) and increasing the level of anorexic peptide cocaine- and amphetamine-regulated transcript (CART) reduced caloric intake by inducing food preference from chocolate/cookie bars to chow [158,162].
MasR activation is useful in mitigating endothelium dysfunction caused by obesity., Ang 1–7 infusion in obese patients not only improved insulin-stimulated vasodilation but also blunted endothelin-1-dependent vasoconstrictor tone [163]. This vasoprotective effect was mediated by down-regulating the expression of NOX subunits (p22phox and p47phox) and plasma thiobarbituric acid reactive substances (TBARS), which led to an abundance of NO [164]. Despite these beneficial properties, Ang1–7 administration in an experimental rat model of obesity did not affect their cardiac performance [165].
5. Conclusion and future prospects
MasR activity plays a key role in the pathophysiology of the cardiovascular system by increasing the bioavailability of NO through inhibition of NOX activity and stimulation of eNOS. By this action, MasR can alter endothelium dysfunction and facilitate vasorelaxation by preserving coronary flow. The triggering of MasR involves increasing the levels of NO and PGI2 and is associated with limiting TF expression, thereby reducing the risk of thrombosis. MasR stabilizes atherosclerotic plaques by inhibiting the proliferation and migration of vascular smooth muscle cells, reducing the infiltration of macrophages, and increasing the content of collagen. Following the activation of MasR, the down-regulation of NF-κB reduces the expression of inflammatory cytokines, accelerates the production of antioxidant factors, and ultimately attenuates oxidative stress. The suppression of the signaling pathways related to mTOR, ERK 1/2, NFAT, and TGF-β1 by this receptor prevents or at least reduces the development of cardiac hypertrophy and fibrosis. It also acts as a positive inotrope by modulating the cardiomyocyte’s response to Ach and preventing the reduction of maximal contraction and relaxation velocity and shortening in them. In addition, the downstream pathways of MasR, including PI3K/Akt/GSK3β and AMPK/FoxO1/PPARγ, contribute to the improvement of cellular metabolism and increased myocardial contractility by promoting glucose and fatty acid uptake capacity (Figure 1).
Figure 1.
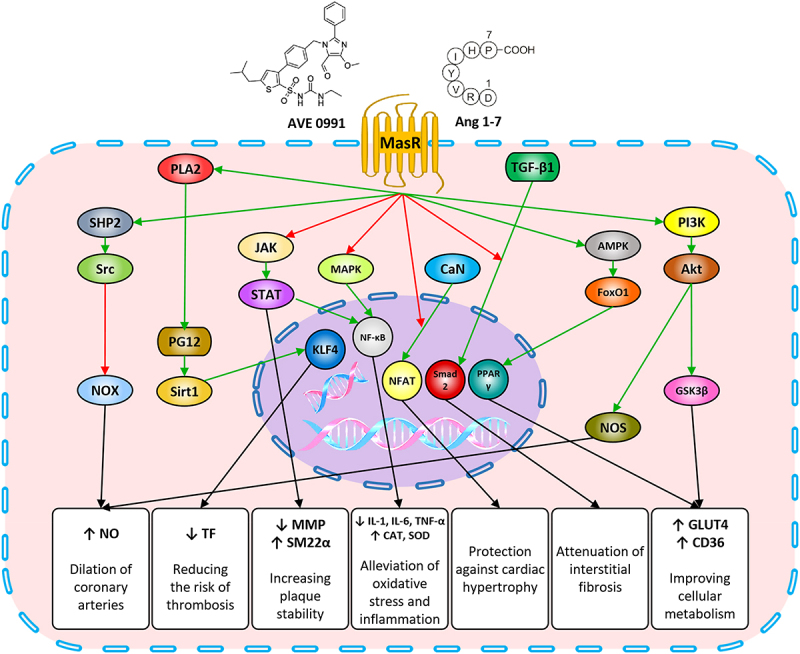
MasR-induced intracellular signaling pathways and their effects on ischemic heart disease.
Note: MasR through the SHP2 and Akt stimulation, respectively, activates the NOS and inhibits the NOX, resulted in an increase in NO levels, induction of vasorelaxation, and increased coronary flow. NO and PGI increased by this receptor reinforce the Sirt1/KLF4, which reduces TF expression and tendency to thrombosis. MasR by suppressing JAK/STAT signaling pathway, reducing the activity of MMP enzymes, instead increases the level of SM22α expression, which results in inhibition of proliferation and migration of vascular smooth muscle cells, increased collagen content and ultimately plaque stabilization. NF-κB down-regulation by this receptor reduces inflammatory cytokines, increased production of antioxidant proteins and ultimately attenuates oxidative stress. MasR protects myocardial hypertrophy and interstitial fibrosis by inhibiting CaN/NFAT and TGF-β1/Smad2 signals. The trigger of PI3K/Akt/GSK3β signaling pathway by this receptor also increases the presence of GLUT4 and CD36, which improves the uptake of energy substrate and cell metabolism in cardiomyocytes
The association of MasR activity with the proper function of vascular endothelium through the regulation of NO release and inflammatory cytokines production is the mechanism by which MasR directly prevents or reduces the formation of atherosclerotic plaques. Indirectly, MasR limits this process by modifying major risk factors such as hypertension, dyslipidemia, diabetes, and obesity. MasR lowers blood pressure through NO-dependent vasodilatation and balancing the neurotransmitters of the sympathetic system. It reduces the blood glucose level by stimulating insulin secretion-inducing enzymes such as Pdx1 and CREB/CFTR and maintains insulin sensitivity by activating the GSK3β/GLUT4 pathway. Improved response to insulin and leptin under the influence of this receptor protects against weight gain both by facilitating lipolysis dependent on AMPK/PRDM16/UCP1 and by adjusting caloric intake regulatory peptides including PPO and CART. The inhibitory effect of MasR on the expression of genes related to fatty acid synthesis, especially SREBP-1c, can lead to the improvement of the lipid profile (Figure 2) (Table 1).
Figure 2.
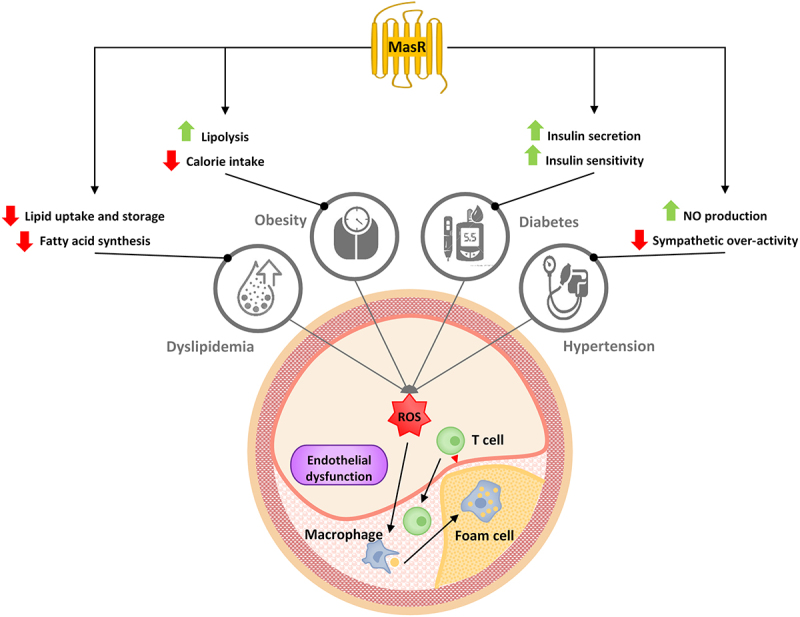
Relationship between MasR and coronary atherosclerosis risk factor.
Note: LDL infiltration into the vessel wall, along with the induction of oxidative stress and the release of inflammatory mediators that can be exacerbated by hypertension, diabetes, and obesity lead to the formation of atherosclerosis plaque. MasR stimulation not only improves lipid profiles by reducing uptake, storage and synthesis of fatty acid, but also modifies other risk factors. Increased bioavailability and weakening sympathetic signals due to the activation of MasR reduces blood pressure. The downstream pathways of this receptor increase insulin secretion and sensitivity to it. In addition, induction of lipolysis and reduced calorie intake by MasR results in weight loss
Table 1.
A summary of the beneficial role of MasR in relation to coronary atherosclerosis risk factors.
| Risk factor | MasR agonist or antagonist (Dose/Route/Duration) | Study design | Results | Reference | |
|---|---|---|---|---|---|
| Hypertension | Ang 1–7 | 1 to 100 nmol.kg−1/IV/before and during the first and third weeks after onset of renovascular hypertension | Dogs | ↑vasodilator component of the blood pressure response; ↓hypertensive dogs fed the NOS inhibitor; synergistic effect with endothelium-derived relaxing factors to buffer the increase in vascular resistance produced by chronic renal ischemia | (119) |
| AVE-0991 Ang 1–7 |
10−7 M/PO in the drinking water/for the last 3 days 29 μM·kg−1·h−1/IV/continuous infusion for the last 3 days |
Sprague-Dawley rats | no significant effect on body weight or arterial blood pressure; restore endothelium-dependent vascular relaxation; ↑superoxide levels in rats fed a high-salt diet, but the expression of Cu/Zn SOD and Mn SOD enzyme proteins in the vessel wall was unaffected; ↓vascular oxidant stress and enhancing NO availability | (120) | |
| Ang 1–7 | 576 μg·kg−1·day−1/IV/infused via a mini-osmotic pump for 4 weeks | Sprague-Dawley rats | ↓hypertension and cardiac hypertrophy (only in trained 2K1C rats but not in sedentary 2K1C rats); ↓myocyte diameter and cardiac fibrosis | (121) | |
| Ang 1–7 | 10−10, 10−9, or 10−8 M/min/IA/ infused for 5 minutes |
Human (Clinical trial) |
↑forearm blood flow response in a dose-dependent manner; vasodilatory effect that is independent of NOS | (122) | |
| Ang 1–7 | 10−6 M/in tissue bath | Sprague-Dawley rats | ↑endothelium-dependent and NO-mediated relaxation; unmasked vasodilator responses to bradykinin; ↑ BK and Ach responses; restoring endothelial function impaired by elevated dietary salt intake | (123) | |
| Ang 1–7 | 50–250 ng/ intra-hypothalamic/stabilization period of 30 min |
Wistar Kyoto rats Spontaneously hypertensive rats |
not the change basal MAP; co-administration of Ang-(1–7) with Ang II did not affect the pressor response; not participate in the hypothalamic blood pressure regulation pleiotropic effects on blood pressure regulation (high dose of the heptapeptide produced a pressor response by ↑activation of AT1 receptors and lower doses of Ang-(1–7) with Ang II ↓the pressor response to the octapeptide) |
(124) | |
| Ang 1–7 | 200 ng.h−1/ intra-cerebroventricular/ for 14 days |
Sprague-Dawley rats | improvement of baroreflex control of HR; normalized mRNA expression of collagen type I in the left ventricle; balance of cardiac autonomic tone; ↓ MAP; no effect on circulating or cardiac changes in angiotensin levels | (125) | |
| Ang 1–7 | 1.8 μg.h−1/ intra-cerebroventricular/for 6 weeks |
Dahl salt-sensitive rats | ↑NO level; ↓sympathetic activity; ↓blood pressure; ↓cardiac hypertrophy; regulates neurotransmitter levels; ↑expression of GABA and GAD67; expression of norepinephrine, glutamate and tyrosine hydroxylase | (126) | |
| A-779 | 3 nM/ intra-paraventricular |
Sprague-Dawley rats | ↑ MAP; ↑renal sympathetic nerve activity (RSNA); ↑plasma norepinephrine; ↑level of IL-1β, IL-6 and TNF-α; ↑MCP-1, gp91phox expression and superoxide production; ↑oxidative stress | (127) | |
| AVE-0991 | 20 mg/kg/day/PO/ for 5 weeks |
Spontaneously hypertensive rats | ↓heart rate and blood pressure variability (HRV, BPV); ↓spontaneous baroreceptor sensitivity (BRS); ↓baroreflex fluctuations; influence on the circadian rhythm | (128) | |
| Ang 1–7 | 1.8 μg.h−1/ intra-cerebroventricular/ for 4 weeks |
Sprague-Dawley rats | ↓expression of several renin-angiotensin system components, estrogen receptors and an NADPH oxidase; ↓Aldo/NaCl pressor effect | (131) | |
| Ang 1–7 AVE-0991 |
24 μg.kg−1.h−1/IP/ for 4 weeks |
Wistar-Kyoto rats Spontaneously hypertensive rats |
↓MAP; ↓proteinuria; ↓abnormal vascular responsiveness to endothelin-1; recovery of cardiac function; ↓development of severe hypertension and end-organ damage; ↓cardiac ischemia | (132) | |
| Ang 1–7 | 100 ng.kg−1.min−1/implanted mini-osmotic pump/for 4 weeks | Sprague-Dawley rats | ↓collagen deposition effects; prevents cardiac fibrosis and cardiac remodeling independent of blood pressure or cardiac hypertrophy; not significantly ↓blood pressure | (133) | |
| Ang 1–7 | 24 μg.kg−1.h−1/IP/ for 14 days |
Wistar-Kyoto rats Spontaneously hypertensive rats |
no effects on blood pressure; down-regulated cardiac Mas mRNA and renal Mas mRNA; ↓Cardiac and renal ACE2 mRNA; not change renal ACE2 | (134) | |
| A-779 | 1 mg.day−1/implanted SC/ for 4 weeks |
Sprague-Dawley rats | ↑cardiac inflammation and fibrosis; ↑apoptotic responses; no effects on cardiac function; MasR expressions in the hypertensive heart and kidney are not regulated by circulating AngII levels | (135) | |
| AVE-0991 | 20 mg.kg−1.day−1/PO/ for 4 weeks |
C57BL/6J mice | ↓mean myocyte diameter; ↓gene expression of the hypertrophic markers; ↓expression of NOX 2 and NOX; ↓oxidative stress; ↓cardiac hypertrophy; improve heart function; ↓left ventricular weight and left ventricular end-diastolic diameter | (136) | |
| Ang 1–7 | 576 μg·kg−1·day−1 /IP/ for 4 weeks |
Sprague-Dawley rats | not modify the increase in blood pressure; ↓development of myocardial fibrosis and hypertrophy; ↓myocardial inflammatory cell infiltration and tyrosine hydroxylase expression | (137) | |
| Dyslipidemia | Ang 1–7 | 0.1 mg.kg−1/PO/ for 4 weeks |
FVB/N mice | ↓body weight and food intake; ↓blood parameters such as total cholesterol, triglyceride, alanine transaminases, and aspartate transaminases; ↓proinflammatory profile such as expression of IL-6 and TNF-α; ↓acetyl-CoA carboxylase, PPARγ, and SREBP-1c mRNA expression | (100) |
| Transgenic rats overexpressing Ang 1–7 | ↓ LPL expression; ↑ PPARγ expression; ↓adiposity index and lipogenesis independent of the stimulatory effect of insulin; ↓concentration of triacylglycerol in the liver; ↑activity of cytosolic lipases; ↓fatty acid uptake from the adipose tissue | (101) | |||
| Diabetes mellitus | Ang 1–7 | 300 μg·kg−1·day−1/SC/ for 8 weeks |
Wistar rats | ↓body weight; ↓blood glucose levels; ↓fasting serum Ang II levels; ↑fasting serum insulin levels and facilitated insulin production; ↓homeostasis model assessment of insulin resistance (HOMA-IR); ↓ iNOS, caspase-3, caspase-9, caspase-8, Bax and reduction of Bcl-2; ↑improvement of insulin resistance, insulin secretion, and pancreatic cell survival | (147) |
| Ang 1–7 | 50 μg·kg−1·day−1/SC/ for 48 days |
C57BL/6J mice | ↑insulin level; ↓blood glucose levels; ↓glycated hemoglobin levels; improves oral glucose tolerance; no significantly weight loss | (8) | |
| Ang 1–7 | 576 μg·kg−1·day−1/IP/ for 4 weeks |
Wistar rats | ↓abnormal vascular reactivity to endothelin-1 and cardiac dysfunction; improvement in cardiac recovery or vascular reactivity; ↓NOX activity and end-organ damage | (148) | |
| AVE0991 | 20 mg·kg−1·day−1/PO/ for 5 weeks |
Sprague-Dawley rats | no effect on any of the investigated hemodynamic parameters under normoglycemic conditions; ↓cardiac function; normalization of blood pressure and contractility parameters; protective effect on the diabetic heart | (153) | |
| Ang 1–7 | Wistar rats | ↓fasting blood glucose; ↓serum angiotensin II level; ↓HOMA-IR value; ↑serum insulin level; ↑insulin secretion; ↓insulin resistance and islet fibrosis; upregulation of Pdx1, Glut2 and Gk expressions | (154) | ||
| Ang 1–7 | 1–10 µM/for 24 h | RINm5F cell | ↑ CREB activation; ↑intracellular cAMP; ↑CFTR expression; ↑glucose-stimulated insulin secretion | (155) | |
| Ang 1–7 | Wistar rats | ↓cleaved caspase 3 levels in pancreas; ↓expression of JNK, Bax, and Bcl2 genes; ↑islet function and histopathology; reversed high glucose (HG)-induced mitochondrial apoptosis augments; improved the islet β-cells apoptosis by JNK-mediated mitochondrial dysfunction | (156) | ||
| Ang 1–7 | 30 µg.kg−1/PO/ on days 28 to 36 then 100 µg.kg−1/ on days 57 to 72 |
Tet29 rats | ↑improved glucose uptake; ↓blood glucose levels; ↑improved insulin sensitivity; ↓ plasma insulin; ↓diabetic nephropathy | (157) | |
| Ang 1–7 | 1 µM.L−1/for 24 h | H9c2 cell culture | ↓overexpression levels of leptin; ↓p-p38 MAPK and ERK1/2, but not p-c-Jun N-terminal kinase; ↑cell viability; ↓apoptotic rate; ↓ROS production; ↑mitochondrial membrane potential | (158) | |
| Ang 1–7 | bEnd3 cell culture | ↓HG-induced endothelial injury through downregulating CIC-3; ↓productions of ROS and cytokine such as IL-1β, IL-8, IL-6, NF-κB and TNF-α; ↓ NO level | (159) | ||
| Ang 1–7 | 1, 10, 100 and 1000 pM | Wistar rats | not potentiate bradykinin-induce vasodilation in diabetic rats; ↑vasodilatory effect; restoring effect via mechanism involving membrane hyperpolarization but not NO release | (160) | |
| Ang 1–7 | 576 μg·kg−1·day−1/IP/ for 4 weeks |
Wistar Kyoto rats Spontaneously hypertensive rats |
↓renal NADPH oxidase activity; ↓renal vascular dysfunction; ↓renal catalase, and PPAR-γ levels; ↓degree of proteinuria and hyperglycemia, but had little or modest effect on reducing mean arterial pressure; ↑renal vascular responsiveness to endothelin-1 | (163) | |
| Obesity | Transgenic rats overexpressing Ang 1–7 | regulated food intake and body weight; ↓weight after AT1 receptor blockade; ↑ insulin response to OGTT and ↓insulin resistance; ↓energy intake; remained responsive to leptin | (164) | ||
| Ang 1–7 | 0.54 mg kg−1 day−1/implanted SC/for 28 days | C57BL/6J mice | ↓body weight and lipid accumulation; ↑thermogenesis; ↓impaired glucose homeostasis; ↑expression of UCP1, PRDM16, and prohibitin; ↑AMPK and phosphorylation of mTOR; preserved insulin signaling concomitant with phosphorylation of hormone-sensitive lipase; ↓expression of perilipin; improved metabolic profile | (7) | |
| Ang 1–7 | 100 μg.kg−1/PO/ for 8 weeks |
Sprague-Dawley rats | ↓body weight and abdominal fat-mass; ↑glucose tolerance; ↑insulin-sensitivity; ↓plasma-insulin levels; ↓circulating lipid levels (cholesterol, HDL and triglycerides); ↓expression of resistin, TLR4, ACE and ↑ACE2 expression in liver; ↓phosphorylation of MAPK and NF-κB expression; ↓expression of IL-6 and TNF-α | (168) | |
| Ang 1–7 | 100 μg/locally in the striatum through the micro-dialysis probe/ for 8 weeks |
Sprague-Dawley rats | ↑extracellular dopamine and GABA but had no effect on glutamate release; ↑GABA release by the NOS inhibitor L-NAME | (167) | |
| Ang 1–7 | 10 nM.min−1/IA/ infused for 5 minutes |
Human (Clinical trial) |
↑unstimulated forearm flow; ↑improve insulin-stimulated endothelium-dependent vasodilation; ↓endothelin-1–dependent vasoconstrictor tone | (169) | |
| Ang 1–7 | 0.4 μg.kg−1.min−1/SC/ for 4 weeks |
C57BL/6J mice | improves Ang II-induced vascular dysfunctions; ↑endothelial-dependent vasodilatation by increased Ach-induced relaxation; ↑aortic expression of NAD(P)H oxidase subunits (p22phox and p47phox) and plasma TBARS; not affect arterial pressure and heart rate; not normalize the altered contractions | (170) | |
| Transgenic rats overexpressing Ang 1–7 | ↓body weight, ↓heart weight to femur length ratio | (171) | |||
These results suggest a promising therapeutic potential of MasR agonists in the management of IHD and coronary atherosclerosis risk factors. However, there are significant translational gaps in the implementation of pre-clinical knowledge into the clinic. Thus, more research is needed to better understand the impact of the efficacy of MasR agonists in ameliorating ischemic cardiovascular diseases.
Acknowledgements
The authors thank Mashhad University of Medical Sciences for their support.
Funding Statement
The authors reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].King MW, Bambharoliya T, Ramakrishna H, et al. Epidemiology and risk factors. Coronary artery disease and the evolution of angioplasty devices. Cham: Springer International Publishing; 2020. pp. 1–2. [Google Scholar]
- [2].Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89(2):3–9. doi: 10.1016/S0002-9149(01)02321-9 [DOI] [PubMed] [Google Scholar]
- [3].Ferreira AJ, Santos RA. Cardiovascular actions of angiotensin-(1-7). Braz J Med Biol Res = Rev Bras Pesqui Med Biol. 2005;38(4):499–507. doi: 10.1590/S0100-879X2005000400003 [DOI] [PubMed] [Google Scholar]
- [4].Santos RA, Frézard F, Ferreira AJ. Angiotensin-(1-7): blood, heart, and blood vessels. Current Medicinal Chemistry Cardiovascular And Hematological Agents. 2005;3:383–391. doi: 10.2174/156801605774322373 [DOI] [PubMed] [Google Scholar]
- [5].Zhang F, Liu J, Li SF, et al. Angiotensin-(1-7): new perspectives in atherosclerosis treatment. J Geriatr Cardiol. 2015;12(6):676–682. doi: 10.11909/j.issn.1671-5411.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iwata M, Cowling RT, Yeo SJ, et al. Targeting the ACE2-ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol. 2011;51:542–547. doi: 10.1016/j.yjmcc.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kuipers A, Moll GN, Wagner E, et al. Efficacy of lanthionine-stabilized angiotensin-(1-7) in type I and type II diabetes mouse models. Peptides. 2019;112:78–84. doi: 10.1016/j.peptides.2018.10.015 [DOI] [PubMed] [Google Scholar]
- [8].Morimoto H, Mori J, Nakajima H, et al. Angiotensin 1-7 stimulates brown adipose tissue and reduces diet-induced obesity. Am J Physiol Endocrinol Metab. 2018;314:E131–e8. doi: 10.1152/ajpendo.00192.2017 [DOI] [PubMed] [Google Scholar]
- [9].Young D, Waitches G, Birchmeier C, et al. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3 [DOI] [PubMed] [Google Scholar]
- [10].Aly TA, Baschal EE, Jahromi MM, et al. Analysis of single nucleotide polymorphisms identifies major type 1A diabetes locus telomeric of the major histocompatibility complex. Diabetes. 2008;57(3):770–776. doi: 10.2337/db07-0900 [DOI] [PubMed] [Google Scholar]
- [11].Metzger R, Bader M, Ludwig T, et al. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett. 1995;357(1):27–32. doi: 10.1016/0014-5793(94)01292-9 [DOI] [PubMed] [Google Scholar]
- [12].Alenina N, Xu P, Rentzsch B, et al. Genetically altered animal models for mas and angiotensin-(1-7). Exp Physiol. 2008;93(5):528–537. doi: 10.1113/expphysiol.2007.040345 [DOI] [PubMed] [Google Scholar]
- [13].van ‘t Veer LJ, van der Feltz MJ, van den Berg-Bakker CA, et al. Activation of the mas oncogene involves coupling to human alphoid sequences. Oncogene. 1993;8:2673–2681. [PubMed] [Google Scholar]
- [14].Gallagher PE, Cook K, Soto-Pantoja D, et al. Angiotensin peptides and lung cancer. Curr Cancer Drug Targets. 2011;11(4):394–404. doi: 10.2174/156800911795538048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jackson TR, Blair LA, Marshall J, et al. The mas oncogene encodes an angiotensin receptor. Nature. 1988;335(6189):437–440. doi: 10.1038/335437a0 [DOI] [PubMed] [Google Scholar]
- [16].Murphy TJ, Alexander RW, Griendling KK, et al. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351(6323):233–236. DOI: 10.1038/351233a0 [DOI] [PubMed] [Google Scholar]
- [17].Santos EL, Reis RI, Silva RG, et al. Functional rescue of a defective angiotensin II AT1 receptor mutant by the mas protooncogene. Regul Pept. 2007;141:159–167. doi: 10.1016/j.regpep.2006.12.030 [DOI] [PubMed] [Google Scholar]
- [18].Kostenis E, Milligan G, Christopoulos A, et al. G-protein-coupled receptor mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D [DOI] [PubMed] [Google Scholar]
- [19].Heitsch H, Brovkovych S, Malinski T, et al. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.HYP.37.1.72 [DOI] [PubMed] [Google Scholar]
- [20].Sampaio WO, Souza dos Santos RA, Faria-Silva R, et al. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f [DOI] [PubMed] [Google Scholar]
- [21].Sampaio WO, Henrique de Castro C, Santos RA, et al. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848 [DOI] [PubMed] [Google Scholar]
- [22].Muñoz MC, Giani JF, Dominici FP. Angiotensin-(1-7) stimulates the phosphorylation of akt in rat extracardiac tissues in vivo via receptor mas. Regul Pept. 2010;161(1–3):1–7. doi: 10.1016/j.regpep.2010.02.001 [DOI] [PubMed] [Google Scholar]
- [23].Mario EG, Santos SH, Ferreira AV, et al. Angiotensin-(1-7) mas-receptor deficiency decreases peroxisome proliferator-activated receptor gamma expression in adipocytes. Peptides. 2012;33:174–177. doi: 10.1016/j.peptides.2011.11.014 [DOI] [PubMed] [Google Scholar]
- [24].Molaei A, Molaei E, Sadeghnia H, et al. LKB1: an emerging therapeutic target for cardiovascular diseases. Life Sci. 2022;306:120844. doi: 10.1016/j.lfs.2022.120844 [DOI] [PubMed] [Google Scholar]
- [25].Yarmohammadi F, Hayes AW, Karimi G. Targeting pPARs signaling pathways in cardiotoxicity by natural compounds. Cardiovasc Toxicol. 2022;22(4):281–291. doi: 10.1007/s12012-021-09715-5 [DOI] [PubMed] [Google Scholar]
- [26].Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7). Hypertension. 2003;42(4):574–579. doi: 10.1161/01.HYP.0000090322.55782.30 [DOI] [PubMed] [Google Scholar]
- [27].Tian J, Zhang L, Zhou Y, et al. Angiotensin-(1-7) attenuates damage to podocytes induced by preeclamptic serum through MAPK pathways. Int J Mol Med. 2014;34(4):1057–1064. doi: 10.3892/ijmm.2014.1870 [DOI] [PubMed] [Google Scholar]
- [28].Esteban V, Heringer-Walther S, Sterner-Kock A, et al. Angiotensin-(1-7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS One. 2009;4:e5406. doi: 10.1371/journal.pone.0005406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giani JF, Muñoz MC, Pons RA, et al. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F272–82. doi: 10.1152/ajprenal.00278.2010 [DOI] [PubMed] [Google Scholar]
- [30].Li Y, Zeng Z, Li Y, et al. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock. 2015;43(4):395–404. doi: 10.1097/SHK.0000000000000302 [DOI] [PubMed] [Google Scholar]
- [31].Meng Y, Li T, Zhou GS, et al. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/rho kinase pathway. Antioxid Redox Signaling. 2015;22:241–258. doi: 10.1089/ars.2013.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Molaei E, Molaei A, Hayes AW, et al. Resolvin D1, therapeutic target in acute respiratory distress syndrome. Eur J Pharmacol. 2021;911:174527. doi: 10.1016/j.ejphar.2021.174527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dias-Peixoto MF, Santos RA, Gomes ER, et al. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52:542–548. doi: 10.1161/HYPERTENSIONAHA.108.114280 [DOI] [PubMed] [Google Scholar]
- [34].Shemesh R, Toporik A, Levine Z, et al. Discovery and validation of novel peptide agonists for G-protein-coupled receptors. J Biol Chem. 2008;283(50):34643–34649. doi: 10.1074/jbc.M805181200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gomes ER, Santos RA, Guatimosim S. Angiotensin-(1-7)-mediated signaling in cardiomyocytes. Int J Hypertens. 2012;2012:493129. doi: 10.1155/2012/493129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Urban JD, Clarke WP, von Zastrow M, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463 [DOI] [PubMed] [Google Scholar]
- [37].Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12(3):205–216. doi: 10.1038/nrd3954 [DOI] [PubMed] [Google Scholar]
- [38].Dong X, Han S, Zylka MJ, et al. A diverse family of gPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106(5):619–632. doi: 10.1016/S0092-8674(01)00483-4 [DOI] [PubMed] [Google Scholar]
- [39].Santos RA, E Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci, USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jankowski V, Tölle M, Santos RA, et al. Angioprotectin: an angiotensin II-like peptide causing vasodilatory effects. Faseb J. 2011;25:2987–2995. doi: 10.1096/fj.11-185470 [DOI] [PubMed] [Google Scholar]
- [41].Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.RES.87.5.e1 [DOI] [PubMed] [Google Scholar]
- [42].Rice GI, Thomas DA, Grant PJ, et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(1):45–51. doi: 10.1042/BJ20040634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan F, Morris PW, Skidgel RA, et al. Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). similarity to both serine carboxypeptidase and prolylendopeptidase families. J Biol Chem. 1993;268(22):16631–16638. doi: 10.1016/S0021-9258(19)85465-0 [DOI] [PubMed] [Google Scholar]
- [44].Chappell MC, Pirro NT, Sykes A, et al. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension (Dallas, Tex: 1979). 1998;31:362–367. doi: 10.1161/01.HYP.31.1.362 [DOI] [PubMed] [Google Scholar]
- [45].Kohara K, Brosnihan KB, Chappell MC, et al. Angiotensin-(1-7). A member of circulating angiotensin peptides. Hypertension. 1991;17(2):131–138. doi: 10.1161/01.HYP.17.2.131 [DOI] [PubMed] [Google Scholar]
- [46].Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23(4):439–449. doi: 10.1161/01.HYP.23.4.439 [DOI] [PubMed] [Google Scholar]
- [47].Iyer SN, Chappell MC, Averill DB, et al. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension (Dallas, Tex: 1979). 1998;31:699–705. doi: 10.1161/01.HYP.31.2.699 [DOI] [PubMed] [Google Scholar]
- [48].Yamada K, Iyer SN, Chappell MC, et al. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept. 1999;80(1–2):57–66. doi: 10.1016/S0167-0115(99)00005-1 [DOI] [PubMed] [Google Scholar]
- [49].Perry SJ, Yi-Kung Huang E, Cronk D, et al. A human gene encoding morphine modulating peptides related to NPFF and fMRFamide. FEBS Lett. 1997;409(3):426–430. doi: 10.1016/S0014-5793(97)00557-7 [DOI] [PubMed] [Google Scholar]
- [50].Lee M-G, Dong X, Liu Q, et al. Agonists of the MAS-related gene (mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol. 2008;180(4):2251–2255. doi: 10.4049/jimmunol.180.4.2251 [DOI] [PubMed] [Google Scholar]
- [51].Tirupula KC, Desnoyer R, Speth RC, et al. Atypical signaling and functional desensitization response of MAS receptor to peptide ligands. PLoS One. 2014;9(7):e103520. doi: 10.1371/journal.pone.0103520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Derdau V, Oekonomopulos R, Schubert G. 14 C-Labeled and large-scale synthesis of the angiotensin-(1−7)-receptor agonist AVE 0991 by cross-coupling reactions. J Org Chem. 2003;68:5168–5173. doi: 10.1021/jo034372c [DOI] [PubMed] [Google Scholar]
- [53].Wiemer G, Dobrucki LW, Louka FR, et al. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40:847–852. doi: 10.1161/01.HYP.0000037979.53963.8F [DOI] [PubMed] [Google Scholar]
- [54].Castro CH, Santos RA, Ferreira AJ, et al. Evidence for a functional interaction of the angiotensin-(1-7) receptor mas with AT1 and AT2 receptors in the mouse heart. Hypertension (Dallas, Tex: 1979). 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a [DOI] [PubMed] [Google Scholar]
- [55].Savergnini SQ, Beiman M, Lautner RQ, et al. Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension. 2010;56(1):112–120. doi: 10.1161/HYPERTENSIONAHA.110.152942 [DOI] [PubMed] [Google Scholar]
- [56].Savergnini SQ, Ianzer D, Carvalho MB, et al. The novel mas agonist, CGEN-856S, attenuates isoproterenol-induced cardiac remodeling and myocardial infarction injury in rats. PLoS One. 2013;8:e57757. doi: 10.1371/journal.pone.0057757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chatterjee A, Barnard J, Moravec C, et al. Connective tissue growth factor dependent collagen gene expression induced by MAS agonist AR234960 in human cardiac fibroblasts. PLoS One. 2017;12(12):e0190217. doi: 10.1371/journal.pone.0190217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- [59].Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non–ST-Segment elevation myocardial infarction: executive summary and recommendations. Circulation. 2000;102(10):1193–1209. doi: 10.1161/01.CIR.102.10.1193 [DOI] [PubMed] [Google Scholar]
- [60].Almeida AP, Frábregas BC, Madureira MM, et al. Angiotensin-(1-7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J Med Biol Res = Rev Bras Pesqui Med Biol. 2000;33(6):709–713. doi: 10.1590/S0100-879X2000000600012 [DOI] [PubMed] [Google Scholar]
- [61].Kozlovski VI, Lomnicka M, Fedorowicz A, et al. On the mechanism of coronary vasodilation induced by angiotensin-(1-7) in the isolated guinea pig heart. Basic Clin Pharmacol Toxicol. 2007;100:361–365. doi: 10.1111/j.1742-7843.2007.00057.x [DOI] [PubMed] [Google Scholar]
- [62].Souza ÁP, Sobrinho DB, Almeida JF, et al. Angiotensin II type 1 receptor blockade restores angiotensin-(1-7)-induced coronary vasodilation in hypertrophic rat hearts. Clin Sci (Lond). 2013;125:449–459. doi: 10.1042/CS20120519 [DOI] [PubMed] [Google Scholar]
- [63].Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1-7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension. 2005;46:948–952. doi: 10.1161/01.HYP.0000174594.17052.33 [DOI] [PubMed] [Google Scholar]
- [64].Silva LC, Fontes MA, Campagnole-Santos MJ, et al. Cardiovascular effects produced by micro-injection of angiotensin-(1-7) on vasopressor and vasodepressor sites of the ventrolateral medulla. Brain Res. 1993;613:321–325. doi: 10.1016/0006-8993(93)90920-I [DOI] [PubMed] [Google Scholar]
- [65].Fang C, Stavrou E, Schmaier AA, et al. Angiotensin 1-7 and mas decrease thrombosis in bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023–3032. doi: 10.1182/blood-2012-09-459156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stavrou EX, Fang C, Merkulova A, et al. Reduced thrombosis in klkb1-/- mice is mediated by increased mas receptor, prostacyclin, sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125:710–719. doi: 10.1182/blood-2014-01-550285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2017;90:386–392. doi: 10.1016/j.biopha.2017.03.056 [DOI] [PubMed] [Google Scholar]
- [68].Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic retinopathy. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2018;97:190–194. doi: 10.1016/j.biopha.2017.10.075 [DOI] [PubMed] [Google Scholar]
- [69].Yang JM, Dong M, Meng X, et al. Angiotensin-(1-7) dose-dependently inhibits atherosclerotic lesion formation and enhances plaque stability by targeting vascular cells. Arteriosclerosis Thrombosis Vasc Biol. 2013;33:1978–1985. doi: 10.1161/ATVBAHA.113.301320 [DOI] [PubMed] [Google Scholar]
- [70].Fraga-Silva RA, Savergnini SQ, Montecucco F, et al. Treatment with angiotensin-(1–7) reduces inflammation in carotid atherosclerotic plaques. Thromb Haemost. 2014;111:736–747. doi: 10.1160/TH13-06-0448 [DOI] [PubMed] [Google Scholar]
- [71].Yang J, Yang X, Meng X, et al. Endogenous activated angiotensin-(1-7) plays a protective effect against atherosclerotic plaques unstability in high fat diet fed ApoE knockout mice. Int J Cardiol. 2015;184:645–652. doi: 10.1016/j.ijcard.2015.03.059 [DOI] [PubMed] [Google Scholar]
- [72].Skiba DS, Nosalski R, Mikolajczyk TP, et al. Anti-atherosclerotic effect of the angiotensin 1-7 mimetic AVE0991 is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis. Br J Pharmacol. 2017;174(22):4055–4069. doi: 10.1111/bph.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001039 [DOI] [PubMed] [Google Scholar]
- [74].Verma S, Fedak PWM, Weisel RD, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105(20):2332–2336. doi: 10.1161/01.CIR.0000016602.96363.36 [DOI] [PubMed] [Google Scholar]
- [75].Pachauri P, Garabadu D, Goyal A, et al. Angiotensin (1-7) facilitates cardioprotection of ischemic preconditioning on ischemia-reperfusion-challenged rat heart. Mol Cell Biochem. 2017;430:99–113. doi: 10.1007/s11010-017-2958-4 [DOI] [PubMed] [Google Scholar]
- [76].Park BM, Li W, Kim SH. Cardio-protective effects of angiotensin-(1-5) via mas receptor in rats against ischemic-perfusion injury. Peptides. 2021;139:170516. doi: 10.1016/j.peptides.2021.170516 [DOI] [PubMed] [Google Scholar]
- [77].Ozhan O, Parlakpinar H, Acet A. Comparison of the effects of losartan, captopril, angiotensin II type 2 receptor agonist compound 21, and MAS receptor agonist AVE 0991 on myocardial ischemia–reperfusion necrosis in rats. Fundam Clin Pharmacol. 2021;35:669–680. doi: 10.1111/fcp.12599 [DOI] [PubMed] [Google Scholar]
- [78].Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. 2017;19(8):71. doi: 10.1007/s11886-017-0876-4 [DOI] [PubMed] [Google Scholar]
- [79].Loot AE, Roks AJ, Henning RH, et al. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.CIR.0000013847.07035.B9 [DOI] [PubMed] [Google Scholar]
- [80].Grobe JL, Mecca AP, Lingis M, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol. 2007;292:H736–42. doi: 10.1152/ajpheart.00937.2006 [DOI] [PubMed] [Google Scholar]
- [81].Gomes ER, Lara AA, Almeida PW, et al. Angiotensin-(1-7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3‘,5’-cyclic monophosphate-dependent pathway. Hypertension (Dallas, Tex: 1979). 2010;55:153–160. doi: 10.1161/HYPERTENSIONAHA.109.143255 [DOI] [PubMed] [Google Scholar]
- [82].He JG, Chen SL, Huang YY, et al. The nonpeptide AVE0991 attenuates myocardial hypertrophy as induced by angiotensin II through downregulation of transforming growth factor-β1/Smad2 expression. Heart Vessels. 2010;25:438–443. doi: 10.1007/s00380-009-1213-7 [DOI] [PubMed] [Google Scholar]
- [83].Zeng WT, Chen WY, Leng XY, et al. Impairment of cardiac function and remodeling induced by myocardial infarction in rats are attenuated by the nonpeptide angiotensin-(1-7) analog AVE 0991. Cardiovasc Ther. 2012;30(3):152–161. doi: 10.1111/j.1755-5922.2010.00255.x [DOI] [PubMed] [Google Scholar]
- [84].Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- [85].Pontes CNR, Scalzo S, Jesus ICG, et al. Angiotensin-(1−7) attenuates the negative inotropic response to acetylcholine in the heart. Peptides. 2022;158:170862. doi: 10.1016/j.peptides.2022.170862 [DOI] [PubMed] [Google Scholar]
- [86].Monteiro BL, Santos RAS, Mario EG, et al. Genetic deletion of mas receptor in FVB/N mice impairs cardiac use of glucose and lipids. Peptides. 2022;151:170764. doi: 10.1016/j.peptides.2022.170764 [DOI] [PubMed] [Google Scholar]
- [87].Toton-Zuranska J, Gajda M, Pyka-Fosciak G, et al. AVE 0991-angiotensin-(1-7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol. 2010;61(2):181–183. [PubMed] [Google Scholar]
- [88].Stegbauer J, Thatcher SE, Yang G, et al. Mas receptor deficiency augments angiotensin II-induced atherosclerosis and aortic aneurysm ruptures in hypercholesterolemic male mice. J Vascular Surg. 2019;70(5):1658–68.e1. doi: 10.1016/j.jvs.2018.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yang J, Sun Y, Dong M, et al. Comparison of angiotensin-(1-7), losartan and their combination on atherosclerotic plaque formation in apolipoprotein E knockout mice. Atherosclerosis. 2015;240:544–549. doi: 10.1016/j.atherosclerosis.2015.02.055 [DOI] [PubMed] [Google Scholar]
- [90].Li W, Li J, Hao P, et al. Imbalance between angiotensin II and angiotensin-(1–7) in human coronary atherosclerosis. J Renin Angiotensin Aldosterone Syst. 2016;17:147032031665961. doi: 10.1177/1470320316659618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhou X, Zhang P, Liang T, et al. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS axis and coronary heart disease. Heart Vessels. 2020;35:153–161. doi: 10.1007/s00380-019-01478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yang G, Istas G, Höges S, et al. Angiotensin-(1-7)-induced mas receptor activation attenuates atherosclerosis through a nitric oxide-dependent mechanism in apolipoproteinE-KO mice. Pflugers Arch - Eur J Physiol. 2018;470:661–667. doi: 10.1007/s00424-018-2108-1 [DOI] [PubMed] [Google Scholar]
- [93].Jawien J, Toton-Zuranska J, Kus K, et al. The effect of AVE 0991, nebivolol and doxycycline on inflammatory mediators in an apoE-knockout mouse model of atherosclerosis. Med Sci Monit. 2012;18(10):BR389–BR393. doi: 10.12659/MSM.883478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1-7) receptor mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol. 2012;63(1):77–85. [PubMed] [Google Scholar]
- [95].Santos SH, Fernandes LR, Mario EG, et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340–347. doi: 10.2337/db07-0953 [DOI] [PubMed] [Google Scholar]
- [96].Silva AR, Aguilar EC, Alvarez-Leite JI, et al. Mas receptor deficiency is associated with worsening of lipid profile and severe hepatic steatosis in ApoE-knockout mice. Am J Physiol Regulatory Integr Comp Physiol. 2013;305:R1323–30. doi: 10.1152/ajpregu.00249.2013 [DOI] [PubMed] [Google Scholar]
- [97].Feltenberger JD, Andrade JM, Paraíso A, et al. Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension (Dallas, Tex: 1979). 2013;62:324–330. doi: 10.1161/HYPERTENSIONAHA.111.00919 [DOI] [PubMed] [Google Scholar]
- [98].Moreira CCL, Lourenço FC, Mario ÉG, et al. Long-term effects of angiotensin-(1-7) on lipid metabolism in the adipose tissue and liver. Peptides. 2017;92:16–22. doi: 10.1016/j.peptides.2017.04.004 [DOI] [PubMed] [Google Scholar]
- [99].Blessey R. Epidemiology, risk factors, and pathophysiology of ischemic heart disease. Phys Ther. 1985;65(12):1796–1805. doi: 10.1093/ptj/65.12.1796 [DOI] [PubMed] [Google Scholar]
- [100].Campbell CY, Nasir K, Blumenthal RS. Metabolic syndrome, subclinical coronary atherosclerosis, and cardiovascular risk. Am Heart Hosp J. 2005;3(2):105–110. doi: 10.1111/j.1541-9215.2005.04441.x [DOI] [PubMed] [Google Scholar]
- [101].Bayturan O, Tuzcu EM, Lavoie A, et al. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch Internal Med. 2010;170:478–484. doi: 10.1001/archinternmed.2009.551 [DOI] [PubMed] [Google Scholar]
- [102].Rakušan D, Bürgelová M, Vaněčková I, et al. Knockout of angiotensin 1-7 receptor mas worsens the course of two-kidney, one-clip goldblatt hypertension: roles of nitric oxide deficiency and enhanced vascular responsiveness to angiotensin II. Kidney Blood Pressure Res. 2010;33:476–488. doi: 10.1159/000320689 [DOI] [PubMed] [Google Scholar]
- [103].Ni J, Yang F, Huang XR, et al. Dual deficiency of angiotensin-converting enzyme-2 and mas receptor enhances angiotensin II-induced hypertension and hypertensive nephropathy. J Cell Mol Med. 2020;24(22):13093–13103. doi: 10.1111/jcmm.15914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Eatman D, Wang M, Socci RR, et al. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1-7). Peptides. 2001;22(6):927–933. doi: 10.1016/S0196-9781(01)00404-1 [DOI] [PubMed] [Google Scholar]
- [105].Sullivan JC, Bhatia K, Yamamoto T, et al. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Benter IF, Yousif MH, Al-Saleh FM, et al. Angiotensin-(1-7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J Cardiovasc Pharmacol. 2011;57:559–567. doi: 10.1097/FJC.0b013e31821324b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Castro-Moreno P, Pardo JP, Hernández-Muñoz R, et al. Captopril avoids hypertension, the increase in plasma angiotensin II but increases angiotensin 1-7 and angiotensin II-induced perfusion pressure in isolated kidney in SHR. Auton Autacoid Pharmacol. 2012;32(3–4):61–69. doi: 10.1111/aap.12001 [DOI] [PubMed] [Google Scholar]
- [108].Singh Y, Singh K, Sharma PL. Effect of combination of renin inhibitor and mas-receptor agonist in DOCA-salt-induced hypertension in rats. Mol Cell Biochem. 2013;373:189–194. doi: 10.1007/s11010-012-1489-2 [DOI] [PubMed] [Google Scholar]
- [109].Savoia C, Arrabito E, Parente R, et al. Mas receptor activation contributes to the improvement of nitric oxide bioavailability and vascular remodeling during chronic AT1R (angiotensin type-1 receptor) blockade in experimental hypertension. Hypertension. 2020;76(6):1753–1761. doi: 10.1161/HYPERTENSIONAHA.120.15527 [DOI] [PubMed] [Google Scholar]
- [110].Zou X, Wang J, Chen C, et al. Secreted monocyte miR-27a causes hypertension by reducing mas receptor expression and function in the artery. J Am Soc Hypertens. 2016;10 Suppl 1:e3. doi: 10.1016/j.jash.2016.06.010 [DOI] [PubMed] [Google Scholar]
- [111].Pan X, Shao Y, Wu F, et al. FGF21 prevents angiotensin II-Induced hypertension and vascular dysfunction by activation of aCE2/Angiotensin-(1-7) axis in mice. Cell Metabolism. 2018;27:1323–37.e5. doi: 10.1016/j.cmet.2018.04.002 [DOI] [PubMed] [Google Scholar]
- [112].Zou X, Wang J, Chen C, et al. Secreted monocyte miR-27a, via mesenteric arterial mas receptor-Enos pathway, causes hypertension. Am J Hypertens. 2020;33(1):31–42. doi: 10.1093/ajh/hpz112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Peng WW, Hong L, Liu GY, et al. Prehypertension exercise training attenuates hypertension and cardiac hypertrophy accompanied by temporal changes in the levels of angiotensin II and angiotensin (1-7). Hypertens Res. 2019;42(11):1745–1756. doi: 10.1038/s41440-019-0297-4 [DOI] [PubMed] [Google Scholar]
- [114].Raquel HA, Manica LA, Ceroni A, et al. Exercise training improves cardiovascular control in sinoaortic denervated SHR by reducing the elevated angiotensin II and augmenting angiotensin-(1-7) availability within autonomic and neuroendocrine PVN nuclei. Peptides. 2022;153:170798. doi: 10.1016/j.peptides.2022.170798 [DOI] [PubMed] [Google Scholar]
- [115].Nakamoto H, Ferrario CM, Fuller SB, et al. Angiotensin-(1-7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25(4):796–802. doi: 10.1161/01.HYP.25.4.796 [DOI] [PubMed] [Google Scholar]
- [116].Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol. 2011;301:H1341–52. doi: 10.1152/ajpheart.00202.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shah A, Oh YB, Lee SH, et al. Angiotensin-(1-7) attenuates hypertension in exercise-trained renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;302:H2372–80. doi: 10.1152/ajpheart.00846.2011 [DOI] [PubMed] [Google Scholar]
- [118].Sasaki S, Higashi Y, Nakagawa K, et al. Effects of angiotensin-(1-7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension. 2001;38(1):90–94. doi: 10.1161/01.HYP.38.1.90 [DOI] [PubMed] [Google Scholar]
- [119].Raffai G, Lombard JH. Angiotensin-(1-7) selectively induces relaxation and modulates endothelium-dependent dilation in mesenteric arteries of salt-fed rats. J Vasc Res. 2016;53(1–2):105–118. doi: 10.1159/000448714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Guimaraes PS, Santiago NM, Xavier CH, et al. Chronic infusion of angiotensin-(1-7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am J Physiol Heart Circ Physiol. 2012;303:H393–400. doi: 10.1152/ajpheart.00075.2012 [DOI] [PubMed] [Google Scholar]
- [121].Liang B, Zhao YN, Wang X, et al. Angiotensin-(1-7) attenuates hypertension and cardiac hypertrophy via modulation of nitric oxide and neurotransmitter levels in the paraventricular nucleus in salt-sensitive hypertensive rats. RSC Adv. 2018;8(16):8779–8786. doi: 10.1039/C7RA09136B [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yu XJ, Miao YW, Li HB, et al. Blockade of endogenous angiotensin-(1–7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neurosci Bull. 2019;35(1):47–56. doi: 10.1007/s12264-018-0297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wessel N, Malberg H, Heringer-Walther S, et al. The angiotensin-(1-7) receptor agonist AVE0991 dominates the circadian rhythm and baroreflex in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2007;49(2):67–73. doi: 10.1097/FJC.0b013e31802cffe9 [DOI] [PubMed] [Google Scholar]
- [124].Nautiyal M, Shaltout HA, de Lima DC, et al. Response to angiotensin-(1-7) and bradykinin in baroreceptor reflex sensitivity in hypertension. Hypertension. 2013;61(2):e20. doi: 10.1161/HYPERTENSIONAHA.111.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tsuda K. Angiotensin-(1-7) and bradykinin in baroreceptor reflex sensitivity in hypertension. Hypertension. 2013;61(2):e19. doi: 10.1161/HYPERTENSIONAHA.111.00523 [DOI] [PubMed] [Google Scholar]
- [126].Xue B, Zhang Z, Johnson RF, et al. Central endogenous angiotensin-(1-7) protects against aldosterone/NaCl-induced hypertension in female rats. Am J Physiol Heart Circ Physiol. 2013;305:H699–705. doi: 10.1152/ajpheart.00193.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Benter IF, Yousif MH, Anim JT, et al. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–91. doi: 10.1152/ajpheart.00632.2005 [DOI] [PubMed] [Google Scholar]
- [128].Grobe JL, Mecca AP, Mao H, et al. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–23. doi: 10.1152/ajpheart.01170.2005 [DOI] [PubMed] [Google Scholar]
- [129].Tan Z, Wu J, Ma H. Regulation of angiotensin-converting enzyme 2 and mas receptor by ang-(1–7) in heart and kidney of spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst. 2011;12(4):413–419. doi: 10.1177/1470320311402109 [DOI] [PubMed] [Google Scholar]
- [130].Meng W, Zhao W, Zhao T, et al. Autocrine and paracrine function of angiotensin 1-7 in tissue repair during hypertension. Am J Hypertens. 2014;27(6):775–782. doi: 10.1093/ajh/hpt270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ma Y, Huang H, Jiang J, et al. AVE 0991 attenuates cardiac hypertrophy through reducing oxidative stress. Biochem Biophys Res Commun. 2016;474(4):621–625. doi: 10.1016/j.bbrc.2015.09.050 [DOI] [PubMed] [Google Scholar]
- [132].Castoldi G, Carletti R, Ippolito S, et al. Angiotensin type 2 and mas receptor activation prevents myocardial fibrosis and hypertrophy through the reduction of inflammatory cell infiltration and local sympathetic activity in angiotensin II-Dependent hypertension. Int J Mol Sci. 2021;22:22. doi: 10.3390/ijms222413678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Stoyell-Conti FF, Chabbra A, Puthentharayil J, et al. Chronic administration of pharmacological doses of angiotensin 1-7 and iodoangiotensin 1-7 has minimal effects on blood pressure, heart rate, and cognitive function of spontaneously hypertensive rats. Physiological Reports. 2021;9(7):e14812. doi: 10.14814/phy2.14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kohara K, Brosnihan KB, Ferrario CM. Angiotensin(1-7) in the spontaneously hypertensive rat. Peptides. 1993;14:883–891. doi: 10.1016/0196-9781(93)90063-M [DOI] [PubMed] [Google Scholar]
- [135].Santos RA, Simões e Silva AC, Magaldi AJ, et al. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension (Dallas, Tex: 1979). 1996;27:875–884. doi: 10.1161/01.HYP.27.4.875 [DOI] [PubMed] [Google Scholar]
- [136].Simões e Silva AC, Bello AP, Baracho NC, et al. Diuresis and natriuresis produced by long term administration of a selective angiotensin-(1-7) antagonist in normotensive and hypertensive rats. Regul Pept. 1998;74:177–184. doi: 10.1016/S0167-0115(98)00038-X [DOI] [PubMed] [Google Scholar]
- [137].Simões ESAC, Diniz JS, Regueira Filho A, et al. The renin angiotensin system in childhood hypertension: selective increase of angiotensin-(1-7) in essential hypertension. J Paediatr. 2004;145:93–98. doi: 10.1016/j.jpeds.2004.03.055 [DOI] [PubMed] [Google Scholar]
- [138].Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000;91:45–62. doi: 10.1016/S0167-0115(00)00138-5 [DOI] [PubMed] [Google Scholar]
- [139].Souza Dos Santos RA, Passaglio KT, Pesquero JB, et al. Interactions between angiotensin-(1-7), kinins, and angiotensin II in kidney and blood vessels. Hypertension. 2001;38:660–664. doi: 10.1161/01.HYP.38.3.660 [DOI] [PubMed] [Google Scholar]
- [140].Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocrine Reviews. 2003;24(3):261–271. doi: 10.1210/er.2003-0001 [DOI] [PubMed] [Google Scholar]
- [141].He J, Yang Z, Yang H, et al. Regulation of insulin sensitivity, insulin production, and pancreatic β cell survival by angiotensin-(1-7) in a rat model of streptozotocin-induced diabetes mellitus. Peptides. 2015;64:49–54. doi: 10.1016/j.peptides.2014.12.012 [DOI] [PubMed] [Google Scholar]
- [142].Ahmed YM, Abdelgawad MA, Shalaby K, et al. Pioglitazone synthetic analogue ameliorates streptozotocin-induced diabetes mellitus through modulation of ACE 2/angiotensin 1–7 via PI3K/AKT/mTOR signaling pathway. Pharmaceuticals (Basel). 2022;15(3):15. doi: 10.3390/ph15030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yousif MH, Dhaunsi GS, Makki BM, et al. Characterization of angiotensin-(1-7) effects on the cardiovascular system in an experimental model of type-1 diabetes. Pharmacol Res. 2012;66:269–275. doi: 10.1016/j.phrs.2012.05.001 [DOI] [PubMed] [Google Scholar]
- [144].Hao PP, Chen YG, Liu YP, et al. Association of plasma angiotensin-(1-7) level and left ventricular function in patients with type 2 diabetes mellitus. PLoS One. 2013;8:e62788. doi: 10.1371/journal.pone.0062788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Srivastava P, Badhwar S, Chandran DS, et al. Imbalance between angiotensin II - Angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab Syndr. 2019;13:2061–2068. doi: 10.1016/j.dsx.2019.04.042 [DOI] [PubMed] [Google Scholar]
- [146].Srivastava P, Badhwar S, Chandran DS, et al. Improvement in angiotensin 1-7 precedes and correlates with improvement in arterial stiffness and endothelial function following renin-angiotensin system inhibition in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab Syndr. 2020;14:1253–1263. doi: 10.1016/j.dsx.2020.06.043 [DOI] [PubMed] [Google Scholar]
- [147].Ebermann L, Spillmann F, Sidiropoulos M, et al. The angiotensin-(1-7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol. 2008;590:276–280. doi: 10.1016/j.ejphar.2008.05.024 [DOI] [PubMed] [Google Scholar]
- [148].Li J, Zhu R, Liu Y, et al. Angiotensin-(1-7) improves islet function in a rat model of streptozotocin- induced diabetes mellitus by up-regulating the expression of pdx1/glut2. Endocrine, Metabolic & Immune Disorders Drug Targets. 2021;21(1):156–162. doi: 10.2174/1871530320666200717161538 [DOI] [PubMed] [Google Scholar]
- [149].Zhang XL, Zhao X, Wu Y, et al. Angiotensin(1–7) activates MAS-1 and upregulates CFTR to promote insulin secretion in pancreatic β-cells: the association with type 2 diabetes. Endocr Connect. 2022;11:11. doi: 10.1530/EC-21-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liu J, Li X, Wang X, et al. Angiotensin(1-7) improves islet function in diabetes through reducing jNK/Caspase-3 signaling. hormone and metabolic research = hormon- und stoffwechselforschung = hormones et metabolisme. 2022;54:250–258. doi: 10.1055/a-1796-9286. [DOI] [PubMed] [Google Scholar]
- [151].Santos SH, Giani JF, Burghi V, et al. Oral administration of angiotensin-(1–7) ameliorates type 2 diabetes in rats. J Mol Med. 2014;92:255–265. doi: 10.1007/s00109-013-1087-0 [DOI] [PubMed] [Google Scholar]
- [152].Lei Y, Xu Q, Zeng B, et al. Angiotensin-(1-7) protects cardiomyocytes against high glucose-induced injuries through inhibiting reactive oxygen species-activated leptin-p38 mitogen-activated protein kinase/extracellular signal-regulated protein kinase 1/2 pathways, but not the leptin-c-jun N-terminal kinase pathway in vitro. J Diabetes Investig. 2017;8(4):434–445. DOI: 10.1111/jdi.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cheng F, Liu J, Guo Z, et al. Angiotensin-(1-7) ameliorates high glucose-induced vascular endothelial injury through suppressing chloride channel 3. Bioengineered. 2022;13(2):4100–4111. doi: 10.1080/21655979.2021.1997695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Oliveira MA, Carvalho MH, Nigro D, et al. Angiotensin-(1-7) and bradykinin interaction in diabetes mellitus: in vivo study. Peptides. 2002;23:1449–1455. doi: 10.1016/S0196-9781(02)00080-3 [DOI] [PubMed] [Google Scholar]
- [155].Oliveira MA, Carvalho MH, Nigro D, et al. Elevated glucose blocks angiotensin-(1-7) and bradykinin interaction: the role of cyclooxygenase products. Peptides. 2003;24:449–454. doi: 10.1016/S0196-9781(03)00061-5 [DOI] [PubMed] [Google Scholar]
- [156].Rastelli VM, Oliveira MA, dos Santos R, et al. Lack of potentiation of bradykinin by angiotensin-(1-7) in a type 2 diabetes model: role of insulin. Peptides. 2007;28:1040–1049. doi: 10.1016/j.peptides.2007.02.006 [DOI] [PubMed] [Google Scholar]
- [157].Dhaunsi GS, Yousif MH, Akhtar S, et al. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol. 2010;638:108–114. doi: 10.1016/j.ejphar.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Schuchard J, Winkler M, Stölting I, et al. Lack of weight gain after angiotensin AT1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(1-7)/Mas-dependent pathway. Br J Pharmacol. 2015;172(15):3764–3778. doi: 10.1111/bph.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].South AM, Nixon PA, Chappell MC, et al. Obesity is associated with higher blood pressure and higher levels of angiotensin II but lower angiotensin-(1-7) in adolescents born preterm. J Paediatr. 2019;205:55–60.e1. doi: 10.1016/j.jpeds.2018.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nozato Y, Yamamoto K. Angiotensin-(1–7) as a biomarker of childhood obesity: is there a causal relationship? Hypertens Res. 2021;44(9):1233–1235. doi: 10.1038/s41440-021-00684-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Santos SH, Andrade JM, Fernandes LR, et al. Oral angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides. 2013;46:47–52. doi: 10.1016/j.peptides.2013.05.010 [DOI] [PubMed] [Google Scholar]
- [162].Stragier B, Hristova I, Sarre S, et al. In vivo characterization of the angiotensin-(1-7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. Eur J Neurosci. 2005;22:658–664. doi: 10.1111/j.1460-9568.2005.04188.x [DOI] [PubMed] [Google Scholar]
- [163].Schinzari F, Tesauro M, Veneziani A, et al. Favorable vascular actions of angiotensin-(1-7) in human obesity. Hypertension (Dallas, Tex: 1979). 2018;71:185–191. doi: 10.1161/HYPERTENSIONAHA.117.10280 [DOI] [PubMed] [Google Scholar]
- [164].Beyer AM, Guo DF, Rahmouni K. Prolonged treatment with angiotensin 1-7 improves endothelial function in diet-induced obesity. J Hypertens. 2013;31:730–738. doi: 10.1097/HJH.0b013e32835ecbe5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Blanke K, Schlegel F, Raasch W, et al. Effect of angiotensin(1-7) on heart function in an experimental rat model of obesity. Front Physiol. 2015;6:392. doi: 10.3389/fphys.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]


