Abstract
Background.
Nicotine is commonly co-used with other psychostimulants. These high co-use rates have prompted much research on interactions between nicotine and psychostimulant drugs. These studies range from examination of illicitly used psychostimulants such as cocaine and methamphetamine to prescription psychostimulants used to treat attention deficit hyperactivity disorder (ADHD) such as methylphenidate (Ritalin™) and d-amphetamine (active ingredient of Adderall™). However, previous reviews largely focus on nicotine interactions with illicitly used psychostimulants with sparse mention of prescription psychostimulants. The currently available epidemiological and laboratory research, however, suggests high co-use between nicotine and prescription psychostimulants, and that these drugs interact to modulate use liability of either drug. The present review synthesizes epidemiological and experimental human and pre-clinical research assessing the behavioral and neuropharmacological interactions between nicotine and prescription psychostimulants that may contribute to high nicotine-prescription psychostimulant co-use.
Methods.
We searched databases for literature investigating acute and chronic nicotine and prescription psychostimulant interactions. Inclusion criteria were that participants/subjects had to experience nicotine and a prescription psychostimulant compound at least once in the study, in addition to assessment of their interaction.
Results & Conclusions.
Nicotine clearly interacts with d-amphetamine and methylphenidate in a variety of behavioral tasks and neurochemical assays assessing co-use liability across preclinical, clinical, and epidemiological research. The currently available research suggests research gaps examining these interactions in women/female rodents, in consideration of ADHD symptoms, and how prescription psychostimulant exposure influences later nicotine-related outcomes. Nicotine has been less widely studied with alternative ADHD pharmacotherapy bupropion, but we also discuss this research.
Keywords: Prescription psychostimulants, d-amphetamine, methylphenidate, bupropion, nicotine, smoking, vaping, ADHD
1. Introduction
1.1. Background
Nicotine is the primary addictive chemical in cigarettes and e-cigarettes (Benowitz, 2010). Nicotine-containing products are one of the most highly used substances in the United States. Roughly 21% of people aged 12 or older used nicotine-containing products in 2020, including cigarettes, cigars, smokeless or pipe tobacco, and e-cigarettes (Substance Abuse and Mental Health Services Administration [SAMHSA], 2023). The use of nicotine products creates a significant public health burden, causing increased rates of asthma, cancer, and heart disease. These smoking-related illnesses lead to 480,000 deaths per year in the United States alone.
The use of nicotine products is also associated with increased rates of substance use disorders and increased rates of other substance use more generally (Crummy et al., 2020; Lyzwinski and Eisenberg, 2022). Nicotine use is particularly comorbid with the use of other psychostimulants; roughly 70 to 95% of individuals who use psychostimulants also use nicotine (Brecht et al., 2007; Weinberger and Sofuoglu, 2009). High rates of co-use between nicotine and other psychostimulants have prompted extensive research on their interactions. Studies range from examination of illicitly used psychostimulants such as cocaine and methamphetamine (e.g., Alajaji et al., 2016; Harmony et al., 2020) to prescription psychostimulant drugs used in the treatment of attention deficit hyperactivity disorder such as d-amphetamine (Dexedrine™; Adams et al., 2013; Henningfield and Griffiths, 1981), l-amphetamine (Vyvanse ™; Kollins et al., 2014), a mix of d- and l-amphetamines (Adderall ™; Gehricke et al., 2011), or methylphenidate (Ritalin ™; Shanks et al., 2015).
Despite the body of research on nicotine interactions with both illicit and prescription psychostimulants, previous reviews have largely focused on the interaction between nicotine and illicitly used psychostimulants such as methamphetamine or cocaine. Surprisingly, there has been little to no mention of nicotine-prescription psychostimulant compound interactions (e.g., Crummy et al., 2020; Cross et al., 2017; Kohut, 2017). The lack of reviews in this area may be due to the common misconception that prescription psychostimulants do not hold significant use liability (Weyandt and Bjorn, 2018), and as such, presumed low co-use liability. However, even when examining smoking rates among people using or misusing prescription psychostimulants specifically, smoking rates are higher among those who use or misuse prescription psychostimulants than among those who do not (Barrett et al., 2006; Compton et al., 2018; Silveira et al., 2018).
Over 50% of people misusing prescription psychostimulants also consume nicotine-containing products (Compton et al., 2018; Silveira et al., 2018). Further, in the general population, a 2006 survey identified that 67% of people who used amphetamine and 56.8% of people that used methylphenidate reported co-using with nicotine in the same poly-substance use session (i.e., where someone uses multiple drugs in tandem). An estimated 84% of these individuals increased their smoking while using amphetamine and 56% reported doing so while using methylphenidate (Barrett et al., 2006). Individuals with ADHD have a roughly 2 to 3x higher smoking rate than the general population (van Amsterdam et al., 2018), likely producing high lifetime rates of co-use between nicotine and prescription psychostimulant treatments for ADHD. Indeed, evidence suggests prior stimulant treatment for ADHD increases the likelihood of future substance use, including nicotine (Kristin and Bradley, 2009; McCabe et al., 2016). Additionally, because nicotine users have a 3 to 4 times higher probability of being diagnosed with a substance use disorder (Chou et al., 2016), nicotine use represents an important risk factor for escalation to any problematic substance use and therefore should be examined for its interactions with any commonly misused substance. The examination of prescription drugs of this nature is particularly important given their widespread use for ADHD treatment.
Basic and preclinical research, in general, corroborate the observations from the correlational and epidemiological studies we just described. For example, in rodents and humans, pre-exposure to nicotine can modulate behavioral and neurochemical systems in response to later administered prescription psychostimulant compounds (Cortright et al., 2012; Santos et al., 2009; Zakiniaeiz et al., 2019). Acutely co-administered nicotine and d-amphetamine or methylphenidate also interact in behavioral tasks assessing use liability such as increasing smoking behavior in humans and enhancing self-administration, locomotor activity, or place conditioning in rodents (Jutkiewicz et al., 2008; Kim et al., 2011; Wooters et al., 2008). Given the evidence we just described, it is surprising that reviews have not incorporated the vast majority of research with nicotine and prescription psychostimulant compounds.
A substantive literature review that focuses on prescription psychostimulant interactions with nicotine is also needed given the differentiation between use trajectories of prescription psychostimulants and illicitly used psychostimulants that may warrant different research questions. For example, psychostimulants such as methamphetamine or cocaine are not typically used until late adolescence or adulthood (Alcover and Thompson, 2020). In contrast, prescription drugs for ADHD can be prescribed as early as age six (Heal et al., 2013), in addition to their frequent recreational use starting in late adolescence or adulthood (Compton et al., 2018). Because the average age of smoking onset is 15 years-old (Barrington-Trimis et al., 2020; United States Surgeon General, 2014), individuals are likely co-exposed to nicotine and prescription psychostimulants in a variety of temporal patterns. That is, individuals may smoke before ever taking prescription psychostimulants or vice versa. As such, understanding the effects of nicotine on the behavioral effects of prescription psychostimulant compounds is just as important as understanding the effects of prescription psychostimulant exposure on nicotine-related outcomes.
The point made in the previous paragraph is even more salient given that prior basic research in mice and rats has repeatedly suggested that different neurobiological and behavioral effects are observed with different exposure patterns (Kandel and Kandel, 2014; McNealy et al., 2022). For example, nicotine exposure before cocaine exposure enhanced the addiction-related behavioral and neurobiological effects of cocaine, whereas cocaine exposure before nicotine did not enhance addiction-related effects of nicotine. These behavioral changes were parallel with epidemiological patterns showing that over 95% of people began smoking before ever using cocaine (Levine et al., 2011). In contrast, when we examined the effects of order of nicotine and d-amphetamine exposure, nicotine exposure before d-amphetamine did not enhance the behavioral effects of d-amphetamine, while d-amphetamine before nicotine did enhance the behavioral effects of nicotine (McNealy et al., 2022).
Many prior reviews of nicotine-psychostimulant interactions have primarily considered nicotine pre-exposure given the evidence suggesting it as a “molecular gateway” to substance use (Cross et al., 2017; Ren and Lotfipour, 2019). However, the above research suggests that not all stimulants are created equal. Further, early epidemiological and basic research of this kind did not include prescription psychostimulant compounds, nor amphetamines of any kind. Thus, there is increasing importance in understanding the interaction between nicotine and prescription-psychostimulants on use/co-use liability measures in a variety of exposure patterns. This understanding would allow researchers to determine how these different exposure patterns disparately impact measures of use and misuse liability. A critical first step in this understanding is synthesizing the available research to determine our current gaps in knowledge.
At present, we have a collection of exemplary reviews that synthesize the wide variety of interactions between nicotine and other commonly misused substances. However, these tend to include little to no mention of nicotine interactions with prescription psychostimulant compounds (Cross et al., 2017; Crummy et al., 2020; Kohut, 2017). In the current review, we will remediate this gap. To do so, we will summarize epidemiological and experimental human and pre-clinical research assessing the acute or chronic interactions between nicotine and prescription psychostimulants used in the treatment of ADHD, as well as disclose existing gaps in our current knowledge in this area of study. We define chronic administration as the persistent impacts of prior or concurrent repeated drug exposure (such as a 5-day pre-exposure regimen in rats or long-term stimulant administration consistent with ADHD pharmacotherapy for humans). In contrast, acute administration refers to the effects of a substance when administered in close proximity to the test, such as a pre-session administration of amphetamine before measuring nicotine intake. This review will specifically focus on nicotine interactions with amphetamine and methylphenidate. Nicotine has been less widely studied with alternative ADHD pharmacotherapy bupropion, but we will also include a discussion of this research. All nicotine doses are reported as freebase and prescription psychostimulant compounds are reported as salt weight per field standard. Note that we acknowledge that methamphetamine can be prescribed for ADHD in limited cases; however, currently available epidemiological, clinical, and basic research still focuses on illicit use. Further, methamphetamine interactions with nicotine are well-reviewed elsewhere (see Cross et al., 2017; Crummy et al., 2020; Kohut, 2017) and are thus not a focus of the present review. We further note that nicotine when consumed via smoking or vaping, is delivered with a myriad of other pharmacologically active constituents that could impact use and co-use liability of nicotine (Harris et al., 2019; Hoffman and Evans, 2013; Majdi et al., 2019). However, the research explicitly examining the role of these constituents in nicotine interactions with prescription psychostimulants does not exist. Thus, our silence reflects the lack of research and not the potential importance of these constituents.
1.2. Methods
We searched PubMed, Google Scholar, and ResearchRabbit for available literature investigating acute and chronic nicotine and prescription psychostimulant interactions. Search terms included nicotine-related terms, such as “nicotine,” “tobacco,” “cigarettes,” “vaping,” and “smoking,” as well as prescription psychostimulant-related terms. These included the prescription name, such as “Ritalin,” or “Adderall,” but also the active ingredient, such as “methylphenidate,” and “amphetamine.” Criteria for inclusion of each publication included that the participants/subjects had to experience nicotine and a prescription psychostimulant compound at least once in the study. Even when both were administered, studies also had to take at least one measure of their interaction. For example, studies examining only the separate effects of nicotine and the prescription stimulant or that were not properly controlled to assess the interaction were not included in this review. Additionally, studies that examined methamphetamine rather than d- or l-amphetamine were excluded for being outside the scope of the present review. We conducted searches during multiple timeframes beginning in January 2021, with the final search conducted in March 2023. During the search process, a provisional eligibility was determined by reading the abstract, and the total count of publications deemed eligible was 135 papers. After detailed reading and synthesis of the literature, a total of 97 studies were included in the current review.
1.3. Behavioral Mechanisms of Use or Co-Use Liability
A large majority of the literature reviewed herein reflects preclinical research with rats or mice. The different tasks employed to study drug interactions are thought to represent distinct behavioral mechanisms of use liability. To increase accessibility of this review, we start with a summary of the main experimental tasks used in this area of research – self-administration, reward-enhancement, place conditioning, intracranial self-stimulation (ICSS), drug discrimination, and locomotor sensitization.
Intravenous self-administration procedures are generally the gold-standard for determining whether a drug can serve as a reinforcer and thus hold misuse potential (Kuhn et al., 2019). In these experiments, the animal is implanted with a jugular catheter. Subsequently, the animal is attached to a drug pump via a tube within a chamber (see Figure 1a), sometimes more colloquially referred to as a “Skinner box.” In the chamber, there are generally two available operant devices (e.g., levers or nose-poke holes). One device is designated as the active operant for which responding on the programmed schedule of reinforcement produces an intravenous drug infusion. Brief stimuli such as light or audiovisual presentations typically occur following the infusion to signal a timeout where the animal cannot earn additional infusions. The second operant device is designated as inactive. Responding on the inactive device has no programmed consequence. A drug is interpreted to have reinforcing effects if response levels for drug infusions are significantly greater than saline infusions or active operant responding is greater than inactive operant responding.
Figure 1.
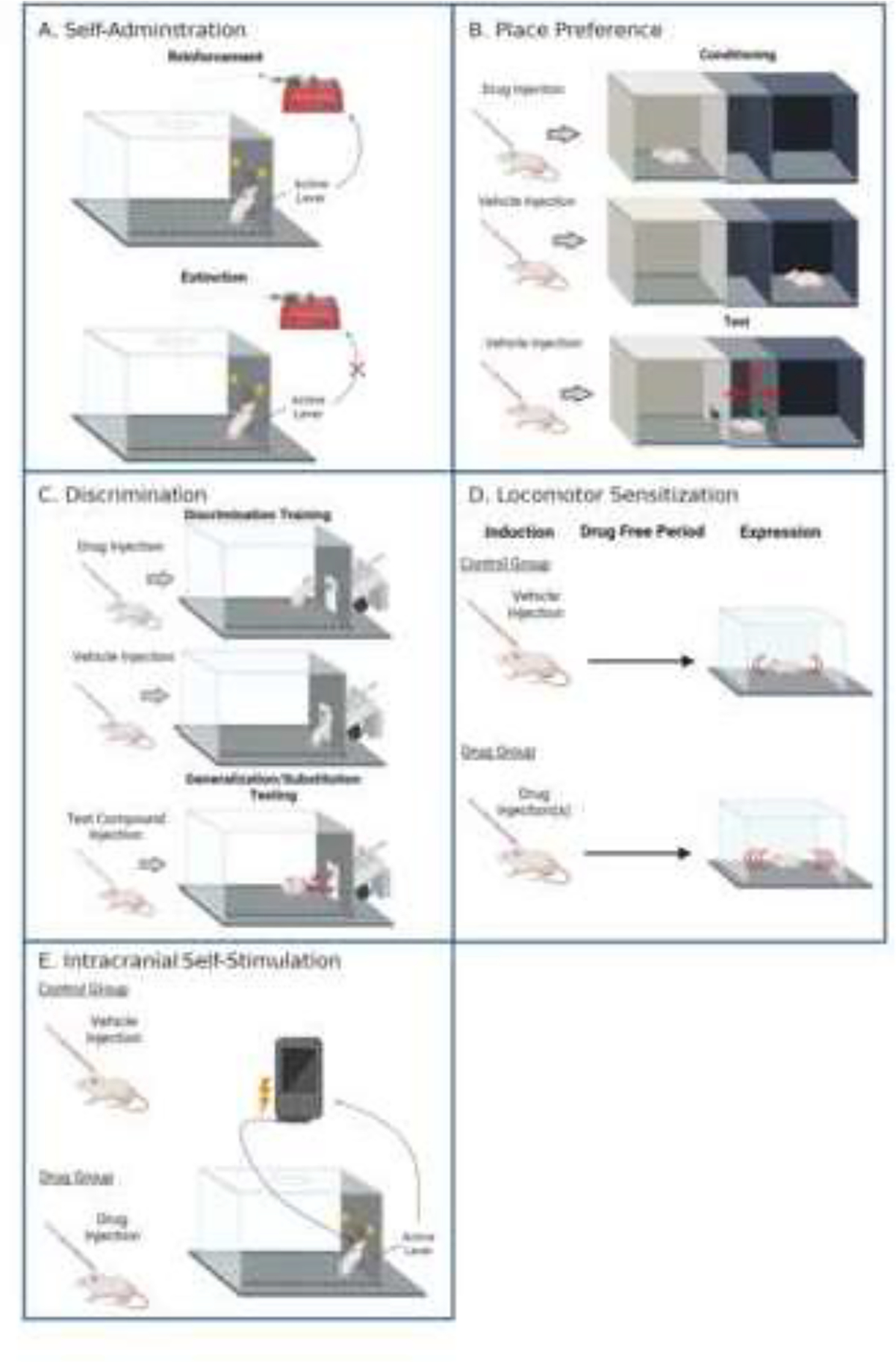
Preclinical Use Liability Tasks. Diagram of select preclinical behavioral tasks. Created with BioRender.com.
Some self-administration studies also examine extinction of drug-maintained responding to measure the degree to which drug-seeking behavior persists even when the drug is no longer available (Charntikov et al., 2017; Maher et al., 2021; Swalve et al., 2015). The extinction phase is often followed by a reinstatement session, where these data together are often used to provide an animal model of relapse after an abstinence period (Epstein et al., 2006; Knackstedt et al., 2010; Reichel and Bevins, 2009). While many experimental variations exist to examine extinction and reinstatement of drug-seeking, in the present report we specifically discuss procedures used for extinction before a drug-primed reinstatement test given our focus on drug interactions. In this variation, extinction sessions typically take the form of the same schedule of reinforcement and timeout stimuli used in the self-administration phase being associated with responding on the active operant, but the drug infusion withheld. The inactive operant still produces no consequence. For drug-primed reinstatement, the experimenter administers a low dose of either the initial self-administration drug or a test drug of interest to the animal before an extinction session. The reintroduction of this drug stimulus often induces a relatively specific increase in active operant responding toward pre-extinction self-administration levels (cf. Epstein et al., 2006; Harmony et al., 2020; Swalve et al., 2015).
Psychostimulants such as nicotine and amphetamine, in addition to their primary reinforcing effects, can also enhance the rewarding value of other environmental stimuli or outcomes (Barrett and Bevins, 2012; Winterbauer and Balleine, 2007). Reward-enhancement can be measured by examining operant response rates for weakly reinforcing visual stimuli. In this case, the animal is injected with a drug or vehicle control, then placed in a conditioning chamber (much like the one in Figure 1c) and allowed to respond for a weak to moderate reinforcer such as low concentration sucrose (Barret and Bevins, 2013) or a brief light presentation (Barrett and Bevins, 2012; McNealy et al., 2021; Raiff and Dallery, 2008). In these studies, nicotine and other psychostimulants produce roughly two-fold increases in responding for visual stimuli (Barrett and Bevins, 2012; Winterbauer and Balleine, 2007). Reward-enhancement is thought to be a particularly important for use liability of nicotine given its role in nicotine intake. That is, nicotine is not readily self-administered when available on its own. However, when nicotine is delivered with weak reinforcers, self-administration is dramatically increased (Caggiula et al., 2009, 2002; Chaudhri et al., 2005). In turn, any prescription psychostimulant compound that augments the reward-enhancing effects of nicotine would be particularly relevant as a potential mechanism of nicotine-psychostimulant co-use.
Place conditioning, sometimes referred to as “conditioned place preference,” is a measure of the conditioned rewarding effects of a drug. In the conditioning phase, there are intermixed paired and unpaired days. On paired days, the animal is injected with the drug and confined to one of two distinct contexts. On unpaired days, the animal is injected with vehicle control and confined to the other context (see Figure 1b). As such, one context comes into association with the drug while the other context does not. In the most common variation of this task, the two contexts are separated by a divider during training. After repeated pairings of the drug with a context, the rodent is tested for which context they prefer. For this preference test, the contexts are no longer divided and the animal is placed between them and allowed to roam freely. More time in the drug-associated context would be interpreted as the drug having conditioned rewarding effects or the paired-context evoked anticipatory approach towards drug-associated stimuli. If the organism spends less time in the drug-associated context than the unpaired context then the drug is said to have conditioned aversive effects (Bardo and Bevins, 2000; White, 1989).
Like self-administration, extinction and reinstatement of the drug-context association is sometimes conducted following the place conditioning test. This task provides a model of relapse in relation to the drug’s conditioned rewarding effects (e.g., Biala and Budzynska, 2008). Extinction is either conducted by repeated sessions where the rat or mouse is allowed to roam drug-free in the entire place conditioning apparatus (Biala and Budzynska, 2008) or sessions where the animal is placed in the previously drug-paired context in the absence of the initial training drug (McKendrick and Graziane, 2020). Drug-primed reinstatement can similarly be conducted by administering the training drug or a low dose of an alternate test compound (Biala and Budzynska, 2008; McKendrick and Graziane, 2020).
Drug discrimination is often utilized as a preclinical analogue to measure the subjective or interoceptive (internal) stimulus effects of a drug (McMahon, 2015). As an example, consider the jitters after consuming too much of a caffeinated beverage or the sleepiness following an allergy medicine with sedative side effects. The most common variant of the drug discrimination task consists of two phases – training and substitution testing. During training, there are intermixed sessions where an animal (rats, mice, or primate) is injected with either drug or vehicle and subsequently placed into an experimental chamber. The chamber is equipped with two response options (e.g., left and right lever; see Figure 1c). On days where the drug is injected, responding on only one of the levers or operant devices is reinforced; responding on the opposite lever is reinforced on saline days. The ability to discriminate the drug from saline is measured by the distribution of responding on the lever appropriate to the injection. Namely, discrimination between the drug and vehicle is said to have occurred when a greater proportion of responses occur on the injection-appropriate lever.
Once the discrimination has been acquired, animals move to substitution testing. In this phase, the animal is tested with various compounds to determine the degree to which the compounds substitute for the training drug. The general consensus for no, partial, and full substitution for the training drug stimulus is as follows: No substitution is declared if the test compound produce responding on the drug-appropriate operant comparable to saline. If the test drug produces responding on the drug-appropriate operant to a greater degree than saline but to a lesser degree than the training drug, then partial substitution is often declared. If the test drug engenders responding on the drug-appropriate operant to the same degree as the training drug, full substitution is interpreted. Partial or full substitution is generally taken as evidence that the drugs have similar or identical interoceptive stimulus effects, respectively (McMahon, 2015; Stolerman, 2002; Young, 2009).
Locomotor sensitization, or increases in drug-evoked activity following prior drug exposure, is thought to reflect potentiation of addiction-related neurochemical pathways (Kuhn et al., 2019). Most common variations of this experimental task involve a single or repeated non-contingent injection of a drug or saline over one or several days. Locomotor activity is measured following drug administration (see Figure 1d). This phase can be referred to as “induction” of sensitization. The later “expression” phase is where sensitization can be observed. The expression phase occurs after a withdrawal period that can range from one day to several months. During the expression phase, the animals are tested with a drug challenge to determine whether activity has increased as a function of the prior drug exposure (DiFranza and Wellman, 2007). A significant increase in locomotor activation evoked by the injected drug in the previously drug exposed animals relative to the saline animals represents locomotor sensitization.
The rewarding effects of drugs in rodents can also be measured via intracranial self-stimulation (ICSS; Negus and Miller, 2014; see Figure 1e). An electrode is first implanted into a brain reward center such as the medial forebrain bundle (see Figure 1e). This behavioral task consists of acute administration of a drug or saline prior to placement in an ICSS chamber. The threshold for which animals will respond for this brain stimulation reinforcer is determined using a discrete trial operant task. For each trial, a noncontingent electrical impulse is delivered followed by a waiting period. The trial is complete once the animal either emits a response or the waiting period has elapsed. Trials continue with successively increasing magnitudes of electrical impulses until the threshold for electrical stimulation, or the highest electrical impulse that produces reinforcement, is determined. Drug challenges can then be administered before ICSS sessions to determine whether the threshold for ICSS reinforcement is increased or decreased, which indicates a decrease or increase in drug reward, respectively (Negus and Miller, 2014).
2. Amphetamine and Nicotine
2.1. Primary Reinforcement
There has been a wealth of human research examining the effects of d-amphetamine administration on smoking-related outcomes (see Table 1). Among these outcome measures, acute d-amphetamine administration has increased responding on a progressive ratio schedule maintained by cigarette puffs (Sigmon et al., 2003), the number of cigarettes smoked during an ad libitum session (Chait and Griffiths, 1983; Cousins et al., 2001; Henningfield and Griffiths, 1981; Tidey et al., 2000), cue- and abstinence-induced smoking craving (Alsene et al., 2005), and number of choices made for cigarettes over money in a discrete-trial choice task (Tidey et al., 2000). These findings suggest a clear enhancement of nicotine intake by acute d-amphetamine. However, one study using chronically administered amphetamine, similar to the long-term administration associated with the treatment of ADHD, found a decreased smoking rate and enjoyment (Low et al., 1984). No other experimental or clinical study has explicitly examined the effects of d-amphetamine on smoking cessation outcomes. This gap is surprising given the body of smoking cessation research examining prescription psychostimulants methylphenidate and bupropion (discussed in detail later). That being said, amphetamine-containing drugs such as Adderall™ are perceived to, and may very well have, greater misuse liability effects than methylphenidate, particularly in individuals without ADHD who smoke (Kollins, 2003).
Table 1.
Table of experimental studies examining smoking behavior following acute or chronic amphetamine or methylphenidate administration.
| Exposure Drug, Dose, and Type | Participants/Subjects | Primary Question and Outcome(s) | Main Finding | Ref. |
|---|---|---|---|---|
|
| ||||
| Acute pre-session d-amphetamine at 0, 5, 10, or 15 mg/kg | People who smoke N = 18; 7M, 11F | Responding on PR schedule for cigarettes or money | d-Amphetamine increased breakpoints for smoking for 56% of subjects, breakpoint increases were dependent on reporting greater amphetamine subjective effects. | Sigmon et al., 2003 |
| Acute pre-session d-amphetamine at 25 mg | People who smoke, N = 6; 3M, 3F | Ad libitum smoking following drug administration Subjective ratings of craving and sensory aspects of smoking. Number of cigarettes during ad libitum smoking. | d-Amphetamine increased ad libitum smoking and decreased time between cigarettes. | Chait and Griffiths, 1983 |
| Acute pre-session d-amphetamine at 10 or 20 mg. | People who smoke, N = 10 (6M, 4F) | 10 and 20 mg amphetamine increased smoking. Neither drug altered ratings of smoking craving. | Cousins et al., 2001 | |
| Acute pre-session placebo or d-amphetamine at 5, 15, and 25 mg (within-subjects). | People who smoke, N = 8; 3M, 5F | Ad libitum smoking, smoking satisfaction post smoking session. | Dose-dependent increases in smoking, burned more grams of tobacco, and spent more time smoking. Participants reported cigarettes tasting better and greater smoking satisfaction. | Henningfield and Griffiths, 1981 |
| Acute pre-session d-amphetamine at placebo 7.5 or 15 mg/70kg. | People who smoke, N = 13; 9M, 4F | Choices during a discrete-trial choice procedure between cigarettes or money and smoking subjective effects. | Dose-dependent increases for smoking choices over money, such that higher doses increased smoking choices more. | Tidey et al., 2000 |
| One-week of chronic administration of placebo, 5 or 7.5 mg d-amphetamine (within-subjects). | People who smoke, N = 17; gender NS. n = 17 light smokers, n = 6 heavy smoker. | Smoking enjoyment and tobacco smoked over one week period of drug treatment. | Chronic amphetamine treatment decreased smoking enjoyment across the sample. However, smoking was only reduced in heavy smokers but not light smokers. | Low et al., 1984 |
| Placebo (n = 15) chronic l-amphetamine at 30, 50, and 70mg (7–14 days/dose within-subjects, n = 17) w/NRT | People with ADHD who smoke, N = 32; 20M, 12 F | Smoking cessation measured in cigarettes per day | NRT reduced smoking, but no smoking cessation outcomes were altered by l-amphetamine. | Kollins et al., 2014 |
| 5 injections of saline or 0.4 mg/kg nicotine base injection, over 15 day period. Exposure either in home cage, IVSA chamber, explicitly paired with IVSA chamber, or explicitly unpaired. | Adult male Long Evans rats (N = 63). | Effects of nicotine exposure and type on 0.01 mg/kg/infusion amphetamine IVSA on PR Schedule, extinction, and amphetamine-primed reinstatement. | Nicotine enhanced amphetamine IVSA and reinstatement of amphetamine-seeking only when nicotine exposure occurred in IVSA chamber in IVSA chamber and paired rats, but not for unpaired rats or home cage rats. Regardless of exposure type, prior nicotine exposure halted extinction. | Cortright et al., 2012 |
| 7-days of saline or 0.04 mg/kg nicotine during adolescence. | Male Sprague-Dawley rats (N = 24) raised in enriched (n =12) or isolated (n=12) conditions. | Interaction between environmental conditions and adolescent drug exposure on amphetamine IVSA. | Adolescent nicotine exposure only increased amphetamine IVSA in rats that were reared in socially isolated conditions. | Stairs et al., 2017 |
| Acute pre-session administration of placebo, 5, 10, 20, or 40 mg methylphenidate (within-subjects) | People who smoke (N = 10); 5M, 5F | Dose-dependent effects of methylphenidate on cigarettes smoked, number of puffs, and CO levels. | Methylphenidate dose-dependently increased all smoking-related outcomes, such that higher doses produced larger magnitude increases in smoking outcomes. | Rush et al., 2005 |
| Acute pre-session administration of placebo or 10, 20, or 40 mg methylphenidate (within-subjects). | People with ADHD who smoke (N=9); 4M, 5F | Dose-dependent effects of methylphenidate on cigarettes smoked, number of puffs, and CO levels. | Methylphenidate dose-dependently increased all smoking-related outcomes in people with ADHD who smoke, higher doses produced larger increases in smoking outcomes. | Vansickel et al., 2011 |
| Acute pre-session administration of placebo, immediate (7.5–30 mg), or sustained-release methylphenidate (18, 36, or 72 mg) | People who smoke (N = 8), 3M, 5F | Dose-and release rate-dependent effects of methylphenidate on cigarettes smoked, number of puffs, and CO levels. | Methylphenidate dose-dependently increased smoking-outcomes, but there was no effect of release formulation on smoking outcomes. | Vansickel et al., 2009 |
| Acute pre-session administration of placebo, methylphenidate (10, 20, 40 mg) within-subjects. | People who smoke (N=12); 6M, 6F. | Cigarettes smoked, number of puffs, and CO levels. | Methylphenidate dose-dependently increased all smoking variables. | Vansickel et al., 2007 |
| Acute pre-session methylphenidate (0, 10, 20, or 40 mg) within-subjects. | People who smoke (N = 11); 6M, 5F. | Dose-dependent effects of methylphenidate on number of choices made for smoking a cigarette versus $0.25. | Methylphenidate dose-dependently increased the number of cigarette choices over money. | Stoops et al., 2011 |
| 11 weeks of Osmotic Release (OROS) methylphenidate or placebo (dose NS). Delivered with 21mg nicotine patch starting Week 4. | People with ADHD who are trying to quit smoking (N = 253). | Effects of methylphenidate administration on smoking abstinence. | Methylphenidate increased smoking abstinence rates. | Covey et al., 2010 |
| Chronic placebo (n = 128) or OROS methylphenidate (n = 127) administration (dose NS) with 21mg nicotine patch. | Secondary analysis of people who smoke (N = 255). | Smoking abstinence during one month methylphenidate discontinuation follow-up and interaction with ADHD symptom severity. | Individuals that previously took methylphenidate had greater abstinence even after methylphenidate was discontinued, but only for people high severity ADHD symptoms. | Luo et al., 2019 |
| Chronic placebo (n = 127) or 72 mg/day OROS methylphenidate (n = 128) with 2img nicotine patch for 11 weeks. | People trying to quit smoking (N = 255). | Prolonged smoking abstinence in response to drug treatment group and interaction with ADHD symptom severity. | 70% of patients with higher severity ADHD who took OROS-methylphenidate had prolonged abstinence compared to 37% of placebo. Individuals with low severity ADHD taking methylphenidate exhibited 30% abstinence compared to 61% taking placebo. | Nunes et al., 2013 |
| Chronic placebo (n = 127) or 72 mg/day OROS methylphenidate (n = 128) with 21mg nicotine patch for 11 weeks. | People trying to quit smoking (N = 255). | Prolonged smoking abstinence in response to drug treatment group. | No effect of methylphenidate on abstinence during methylphenidate treatment period, but methylphenidate group had higher abstinence post-treatment than placebo. | Winhusen et al., 2010 |
| Chronic placebo (n =40)or 54 mg/day OROS methylphenidate treatment for 8 weeks. | People trying to quit smoking (N = 80). | Nicotine withdrawal, point-prevalent smoking at end of medication phase. | No effect of methylphenidate on smoking abstinence or withdrawal symptom relief. | Hurt et al., 2011 |
| Chronic methylphenidate (30 mg) over 5 days following abrupt cessation | People who currently smoke that have previously attempted to quit (N = 19) | Tobacco withdrawal, ease of quit attempt | Tobacco withdrawal increased when methylphenidate was administered, but 76% of the sample said methylphenidate quit attempt was easier than prior quit attempts. | Robinson et al., 1995 |
| Exp 1: Acute presession methylphenidate at 0, 1.25, 2.5, 5, and 10 mg/kg (within-subjects) Exp 2: Chronic presession methylphenidate at 0 or 2.5 mg/kg (between-subjects) | Male Sprague-Dawley rats (N = 28; n = 14 per exp) | Exp 1: Effects of acute doses of methylphenidate on 0.01 (n = 7) or 0.03 (n =7) mg/kg/inf nicotine IVSA. Exp 2: Effects of repeated 0 (n = 6) or 2.5 mg/kg (n = 8) methylphenidate on 0.03 mg/kg/inf nicotine IVSA. | Methylphenidate increased nicotine IVSA in both experiments. 2.5 and 5 mg/kg increased 0.03 mg/kg/inf nicotine IVSA in Exp 1. Only 1.25 mg/kg methylphenidate increased 0.01 mg/kg/inf nicotine IVSA in Exp 1. Tolerance to the effects of 2.5 mg/kg methylphenidate on nicotine IVSA with repeated exposure (Exp 2) not observed. |
Wooters et al., 2008 |
However, one study examined l-amphetamine, the less potent amphetamine isomer and the main active component of Vyvanse™ and one of the active components of Adderall™, as a smoking cessation aid. In this study, l-amphetamine did not alter smoking behavior (Kollins et al., 2014). No other study described in the present review examined l-amphetamine, so hereafter d-amphetamine and amphetamine will be used interchangeably.
Interestingly, self-administration research in non-human animals seems to have had a different focus. We could not find self-administration studies that examined the impact of amphetamine pre-exposure on later nicotine intake. Rather, the limited extant preclinical literature has only examined the impact of nicotine exposure on later d-amphetamine self-administration (Cortright et al., 2012; Stairs et al., 2017). Cortright and colleagues found that nicotine exposure in adult rats enhanced d-amphetamine self-administration and amphetamine-primed reinstatement only when prior nicotine exposure occurred in the self-administration chamber. Equal nicotine exposure in the home cage had no effect on amphetamine self-administration (Cortright et al., 2012). This pattern of results might suggest that the neurochemical effects from prior nicotine exposure were not driving enhanced amphetamine intake. Rather, nicotine’s association with the drug-taking environment was required to enhance amphetamine-taking and seeking. Interestingly, another study found that home cage nicotine exposure during the rat adolescent period enhanced d-amphetamine self-administration in adulthood. This study by Stairs and colleagues (2017), however, only found enhanced d-amphetamine self-administration as a function of nicotine exposure in rats that were housed in isolation, but not in socially-housed rats (Stairs et al., 2017). Notably, Cortright and colleagues utilized individually housed rats, akin to the isolation group of Stairs and colleagues. The discrepancy in findings despite similar housing conditions might suggest that home cage nicotine exposure must occur in adolescence to exert effects on later d-amphetamine self-administration.
2.2. Reward-enhancement
As we mentioned earlier, psychostimulants such as nicotine and amphetamine, in addition to their primary reinforcing effects, can also enhance the rewarding effects of other environmental stimuli or outcomes (Barrett and Bevins, 2012; Winterbauer and Balleine, 2007). We have conducted two studies in our laboratory examining the chronic and acute interactions between nicotine and d-amphetamine on reward-enhancement. Both studies found that acutely co-administered nicotine and d-amphetamine interacted to additively enhance responding maintained by a visual stimulus reinforcer (McNealy et al., 2022, 2021). Our later study examined the impact of drug exposure order. Nicotine-evoked increases in responding for a weak visual stimulus reinforcer were enhanced by prior amphetamine exposure. However, the opposite was not found – prior nicotine exposure did not change the reward-enhancing effects of d-amphetamine (McNealy et al., 2022). Following these exposure order manipulations, we found that the female rats that experienced nicotine exposure before amphetamine exposure exhibited greater interactive effects of nicotine and d-amphetamine on reward-enhancement than vice versa, while amphetamine before nicotine experience enhanced the interaction for males (McNealy et al., 2022).
2.3. Conditioned and General Drug Reward
The available research examining how nicotine and amphetamine interact in terms of conditioned drug reward is also sparse. One study exposed mice to nicotine or saline during adolescence and tested them for the presence of a place preference to an amphetamine-associated context in adulthood. Mice exposed to nicotine exhibited a larger magnitude preference for the amphetamine-associated context in adulthood (Alajaji et al., 2016). We could interpret this outcome as nicotine exposure strengthening amphetamine-context associations later in life. Drug-context associations in humans are powerful modulators of drug taking and seeking such that drug-associated contexts can instigate relapse (Crombag et al., 2008). While further examination is needed with contingent drug experiments, nicotine enhancement of later amphetamine-context associations might lead to more tenacious and perseverant amphetamine-seeking.
Another study using adult rats found that an acute amphetamine-priming injection did not reinstate a previously established and extinguished nicotine-associated context preference (Biala and Budzynska, 2008). This finding may tell us that chronic drug exposure or exposure during adolescence is necessary to augment conditioned drug reward. Alternatively, this finding could tell us that while drug exposure augments place preference expression of other drugs, it cannot reinvigorate a previously extinguished conditioned place preference. As we mentioned earlier, ICSS measures more general drug reward by determining the degree to which test injections modulate the reinforcement threshold of an electrical impulse into a brain reward center. One study using ICSS in rats found that a low dose of d-amphetamine (0.06 mg/kg) decreased ICSS thresholds relative to nicotine alone (Huston-Lyons et al., 1993). That is, acute d-amphetamine increased the rewarding effects of nicotine.
2.4. Stimulus Effects
Drug discrimination studies examining nicotine substitution for the amphetamine stimulus by-in-large have found that nicotine partially substituted for amphetamine (Bardo et al., 1997; Cunningham et al., 2006; Li and McMillan, 2003; Palmatier et al., 2005; Quarta et al., 2009). Likewise, studies examining amphetamine substitution for a nicotine stimulus have largely found that amphetamine partially substituted for nicotine (Besheer et al., 2004; Chance et al., 1977; Chandler and Stolerman, 1997; Mansbach et al., 1998; Palmatier et al., 2005; Quarta et al., 2009; Varvel et al., 1999). Two studies found that nicotine and amphetamine fully substituted for each other Stolerman, 1989). Although the minority, there are a few studies that have reported nicotine not having substituted for amphetamine (Ho and Huang, 1975; Schechter and Rosecrans, 1973).
Drug discrimination studies can also assess the degree to which a compound interacts with the training drug to enhance stimulus effects during the substitution testing phase. One such study trained rats to discriminate between 0.6 mg/kg amphetamine and saline. Co-administration of nicotine enhanced substitution by lower doses of amphetamine. While low dose amphetamine only partially substituted for the 0.6 mg/kg amphetamine dose, co-administration of these low doses with nicotine led to full substitution of the amphetamine stimulus (Reavill and Stolerman, 1987).
2.5. Aversive Effects
Amphetamine or nicotine administration is generally found to produce anxiety-like effects in rodents (Biala and Kruk, 2008; Caldarone et al., 2008; Lin et al., 1999; Rauhut, 2019). Two studies with rats found that repeated exposure to amphetamine produced tolerance to the later anxiogenic effects of amphetamine or nicotine administration as measured by performance on an elevated plus-maze task (Biala et al., 2009; Biala and Kruk, 2008). Aversive experiences with nicotine may reduce the likelihood of people becoming dependent on nicotine (for a review, see Fowler and Kenny [2014]). Although this area of research is extremely limited, perhaps amphetamine exposure could mitigate the aversive effects of nicotine, thereby increasing the likelihood for persistent nicotine use. Indeed, amphetamine decreased alcohol withdrawal-like symptoms in a rodent model (Popkin et al., 2018). Of course, further preclinical and human research is required, but these findings may suggest careful consideration of amelioration of nicotine withdrawal symptoms by amphetamine as a potential co-use mechanism.
2.6. Locomotor Sensitization
Studies that exposed rats to nicotine in adolescence have generally observed locomotor cross-sensitization from nicotine to amphetamine under a variety of circumstances (Collins et al., 2004; Santos et al., 2009; Adams et al., 2013). Adams and colleagues (2013) identified that one week of 0.4 mg/kg nicotine exposure produced cross-sensitization to 0.5 and 1.0 mg/kg amphetamine in socially isolated rats. However, 0.4 mg/kg nicotine only produced cross-sensitization to 1.0 mg/kg amphetamine in environmentally enriched rats. These results follow the same pattern as those described in Section 2.1 identifying enhancement of amphetamine self-administration by prior nicotine exposure in isolated but not enriched rats (Stairs et al., 2017). Perhaps the protective effects of environmental enrichment on neurochemical sensitization by nicotine prevents enhancement of future amphetamine self-administration. Locomotor sensitization may also be sensitive to age and sex. For example, cross-sensitization to amphetamine was only observed in male rats that were exposed during the early adolescent period and not at all for adult males or any age of exposure group for females. These cross-sensitization findings were identical whether rats were tested in adolescence or adulthood (Collins et al., 2004).
Santos and colleagues (2009) were the only authors to examine both the effect of nicotine cross-sensitization to amphetamine and amphetamine cross-sensitization to nicotine. Interestingly, amphetamine pre-exposure in adolescence produced cross-sensitization to nicotine, whether rats were tested for nicotine-evoked locomotor activity in adolescence or adulthood. In contrast, adolescent nicotine pre-exposure produced cross-sensitization to amphetamine only when tested in adulthood (Santos et al., 2009).
Notably, a few studies have reported no effect of prior nicotine exposure on amphetamine-evoked locomotor activity (Biala and Weglinska, 2004; Bruijnzeel et al., 2011; Edwards et al., 2014). In each of these cases, the nicotine exposure protocol diverged from the previously mentioned studies. For two of these studies, the injected nicotine dose was much lower than previously shown to produce cross-sensitization (Biala and Weglinska, 2004; Edwards et al., 2014). Further, Edwards and colleagues implemented a two-day exposure protocol that was much shorter than other studies (Edwards et al., 2014). Lastly, the blood levels of nicotine achieved in the experiment by Bruijnzeel and colleagues were far lower than previous studies. This is likely attributed to the use of tobacco smoke exposure versus injected nicotine (Bruijnzeel et al., 2011). Notably, the inhalation route of exposure used by Bruijnzeel and colleagues also exposed animals to tobacco constituents that are not present when administering injected nicotine. Perhaps these constituents play an important role in modulating nicotine-amphetamine interactions. In summary, exposure dose, length of treatment, and route of administration appear important to consider when examining locomotor cross-sensitization to amphetamine. Ultimately, these factors may provide clues for how results relate to human co-use liability. For example, smoking or vaping may only increase vulnerability to amphetamine use via sensitization of addiction-related pathways later in life when nicotine exposure is frequent, in high concentrations, and/or over a long period of time.
2.7. Neurochemical Interactions
Only a few studies have directly assessed the interaction between amphetamine and nicotine on neurochemical systems, largely focusing on acetylcholine and dopamine. The primary receptor systems of interest concerning nicotine and amphetamine are nicotinic acetylcholine (nAChRs) and dopamine receptors, respectively. Nicotine works as an agonist at nAChRs, binding to and activating these receptors to produce downstream dopamine release (Tiwari et al., 2020). In contrast, amphetamine works by targeting the dopamine transporter, thereby increasing dopamine levels by transporter-mediated exchange (Rudnick and Clark, 1993). Glutamatergic systems have also been implicated in nicotine and amphetamine use liability behaviors (Castillo-Rolón et al., 2021; D’Souza and Markou, 2013; Palmatier et al., 2008). Glutamate plays an important role in learning and memory more generally (Zhou and Danbolt, 2014), but also as it relates to drug use behaviors (D’Souza and Markou, 2013). Nicotinic, dopaminergic, and glutamatergic systems are all composed of multiple receptor subtypes that are potentially responsible for different behavioral outcomes.
These neurochemical systems can be probed through multiple neuroscientific methods. One study examining dopamine overflow (measure of dopamine levels) via microdialysis found that nicotine pre-exposure did not alter amphetamine-evoked dopamine overflow (Birrell and Balfour, 1998). Another study found that acute nicotine administered between two and four hours before d-amphetamine heightened amphetamine-evoked dopamine overflow. Acute amphetamine administration also heightened nicotine-evoked dopamine overflow (Jutkiewicz et al., 2008; Kim et al., 2011).
Much of the research examining nicotine-amphetamine interactions has used pharmacological antagonism. Dihydro-β-erythroidine hydrobromide (DHβE) is a nAChR antagonist with selectivity for α4 and β2 receptor subunits. Many use liability effects of nicotine are attributable to α4β2-containing nAChRs (Rahman, 2011). Thus, probing these receptor subtypes is of particular interest when examining mechanisms of co-use liability. MK-801 is a noncompetitive glutamatergic N-Methyl-D-aspartate (NMDA) receptor antagonist (Song et al., 2018). Pretreatment with either DHβE or MK-801 reduced the effect of acute nicotine on amphetamine-evoked dopamine overflow suggesting a role for α4β2-containing nAChRs and NMDA glutamate receptors (Kim et al., 2011). The role of glutamate receptors in nicotine-induced enhancement of amphetamine-evoked dopamine overflow is consistent with later work examining MK-801 interactions with mecamylamine. Mecamylamine is a general nicotinic receptor antagonist that does not necessarily favor any specific receptor subtype (Crooks et al., 2014). Mecamylamine blocked locomotor sensitization to amphetamine when co-administered with MK-801, but neither did so by themselves (Degoulet et al., 2013). Further, DHβE or varenicline, a partial agonist at α4β2-containing nAChRs that competes with activation of these receptors by nicotine, administered before nicotine blocked later locomotor sensitization evoked by an amphetamine challenge (Kim et al., 2011). The summarized research suggests that both nAChRs and glutamate receptor systems underly behavioral sensitization to amphetamine.
One study in humans further supports nicotine and amphetamine interacting neurochemically. Participants that currently smoked at the time of study showed lower baseline dopamine availability on positron emission tomography (PET) scans than controls that did not currently smoke, and females that currently smoked exhibited lower amphetamine-induced dopamine release than males who smoked or female controls that did not smoke (Zakiniaeiz et al., 2019). This sex-specific alteration in the dopamine system by smoking may lead to greater quantities of dopamine-increasing drugs being required to produce positive drug effects in women. Considering that nicotine and d-amphetamine combined increase dopamine levels more so than either alone, this may lead to greater co-use of nicotine and d-amphetamine amongst women. As we mention in the closing sections of this review, we do not yet have enough data in women or female animals to assess this conclusion.
2.8. Conclusions
Synthesizing the findings in this section, we might conclude that d-amphetamine only increases nicotine intake or misuse liability when taken acutely, such as in the case of occasional or recreational amphetamine use. While more research is needed, the single study that used chronic administration of d-amphetamine found decreases in smoking, suggesting those who use amphetamines chronically, such as in the treatment of ADHD, may not be at a greater risk for increased nicotine use. A topic of great interest would be whether previously non-medicated ADHD patients are at greater risk after the start of pharmacotherapy with d-amphetamine.
It is difficult to synthesize self-administration findings from human and preclinical studies given that all preclinical studies examine the impact of nicotine pre-exposure on later amphetamine self-administration. The discrepant findings between the two preclinical studies suggest that neurochemical alterations by nicotine exposure alone play a larger role in increased amphetamine taking only during the sensitive developmental period of adolescence (for a review of developmental nicotine exposure effects, see Ren and Lotfipour [2019]). In contrast, learning or environmental factors may be more important for enhanced co-use vulnerability in adulthood. Taken together, the research described throughout this section supports the existence of acute and long-term interactions of nicotine and amphetamine on primary reinforcement and nicotine intake.
The finding that amphetamine exposure enhanced the reward-enhancing effects of nicotine, but not vice versa (McNealy et al., 2022), suggests that considering the variety of potential nicotine and d-amphetamine use patterns is of clear import when designing preclinical studies. While not a traditional reward-enhancement study, somewhat parallel findings in humans have found that d-amphetamine enhances the sensory aspects of smoking, specifically augmenting cigarette taste (Henningfield and Griffiths, 1981). That finding, and reward-enhancement findings more generally, suggests that consideration of how amphetamine impacts stimuli that commonly co-occur with nicotine (e.g., the smell, taste, or sight of cigarettes or vapes) may be beneficial for uncovering mechanisms of enhanced use liability. This consideration is especially urgent given the advent of vapes or electronic cigarettes that come in myriad flavors with an entirely new nicotine use experience.
Overlapping stimulus effects may be one explanation for intake enhancement. As mentioned earlier, we can think of drug discrimination studies in rodents as a preclinical analogue to human studies examining the subjective effects of drugs (McMahon, 2015; Young, 2009). Considering the numerous studies we described that identified partial and full cross-substitution, nicotine and amphetamine clearly share some stimulus effects. Indeed, nicotine and amphetamine have been found to produce similar subjective effects in humans such as cognitive enhancement (Sofuoglu, 2010; Thornton et al., 1996). Indeed, some research suggests high rates of smoking among individuals with ADHD is due to the similar cognitive enhancement and ADHD symptom reduction produced by nicotine and prescription stimulants (otherwise termed as the “self-medication hypothesis”; Gehricke et al., 2006; van Amsterdam et al., 2018; Vansickel et al., 2007). From the reviewed literature, nicotine and d-amphetamine clearly share some stimulus effects that could promote amphetamine use following nicotine use or vice versa. Further, the findings suggest that subjective effect enhancement is a potential mechanism for high rates of acute co-use between nicotine and amphetamine that should be further explored.
The review of amphetamine and nicotine interaction research described herein illustrated several gaps in this literature. As noted earlier in Section 2.1 and discussed in detail later, there is a surprising lack of preclinical research empirically examining the effects of amphetamine pre-exposure on nicotine-related outcomes. In fact, only two of the above cited preclinical studies assess this pattern of drug administration. Further, given the divergent findings between adult and adolescent drug exposure observed in preclinical studies, and that prescription psychostimulants are prescribed as early as age 6, there is a great lack of research examining possible divergent impacts of adolescent or adult exposure to nicotine or amphetamine on later co-use liability outcomes. Research examining general and conditioned drug reward interactions between nicotine and d-amphetamine, and how they may alter each other’s aversive drug effects is also sparse – with the limited research in each area pointing towards import of these factors.
3. Methylphenidate and Nicotine
3.1. Primary Reinforcement
Like amphetamine, acutely administered methylphenidate before experimental sessions has consistently enhanced smoking-related outcomes (see Table 1). These outcome measures include increases in cigarette puffs per session, total cigarettes smoked, and choice for cigarette puffs over money (Rush et al., 2005; Vansickel et al., 2011, 2009, 2007; Stoops et al., 2011). Currently, the only preclinical study we could identify examining the effect of pre-session methylphenidate on nicotine self-administration mirrored these findings. That is, rats dose-dependently increased nicotine self-administration when acutely administered a range of methylphenidate doses (1.25 to 5 mg/kg; Wooters et al. [2008]).
Acute methylphenidate administration also enhanced the subjective effects of smoking in humans such as ratings of enjoyment, craving, pleasure, and stimulation (Rush et al., 2005; Vansickel et al., 2009, 2007). Acute methylphenidate administration at therapeutic doses also increased smoking in participants with ADHD that were previously not medicated for their ADHD (Vansickel et al., 2011). These findings suggest that acute methylphenidate can exacerbate nicotine use liability in both ADHD and non-ADHD individuals. In contrast to studies examining acute methylphenidate administration, studies examining the effect of chronic methylphenidate exposure on future nicotine consumption, and vice versa, in humans are mixed. On one hand, a longitudinal retrospective study conducted on individuals who were diagnosed with ADHD as children found no differences in smoking between methylphenidate-treated and non-methylphenidate treated groups (Huss et al., 2008). Similarly, in a general population sample, researchers found no relationship between smoking and methylphenidate misuse (Fleary et al., 2011).
On the other hand, several studies support methylphenidate enhancing smoking or vice versa. Students who smoked cigarettes or experimented with tobacco products were more likely to misuse methylphenidate (Shillington et al., 2006). In a study by Bron and colleagues, researchers placed methylphenidate-naïve participants with ADHD that currently smoked at the time of the study, participants that had previously smoked, and participants that had never smoked on a three-month methylphenidate treatment regimen. Participants that reported currently smoking increased tobacco use by 40 to 50% and nearly 30% of participants that had previously smoked reinitiated smoking. Notably, none of the participants that had never smoked started smoking, nor did they report nicotine craving (Bron et al., 2013). These increased nicotine consumption findings are consistent with smoking self-administration studies with ADHD patients described earlier in this review (e.g., Vansickel et al., 2011). Notably, the study Bron and colleagues was a prospective cohort study and as such, there is no placebo control group. No comparable study with randomized control trial (RCT) has been conducted to our knowledge.
The findings we just described are discordant with research on the potential efficacy of methylphenidate as a smoking cessation aid. Double-blinded clinical trials examining methylphenidate as a cessation aid in individuals with ADHD that smoke found that methylphenidate increased smoking abstinence relative to placebo (Covey et al., 2010; Luo et al., 2019; Nunes et al., 2013; Winhusen et al., 2010). Interestingly, the magnitude of this reduction was greater in individuals with more severe ADHD symptoms (Nunes et al., 2013). Studies in individuals without ADHD that smoke have consistently found no differences in smoking abstinence rates following chronic methylphenidate treatment (Hurt et al., 2011; Robinson et al., 1995). Methylphenidate did provide enhanced withdrawal relief compared to previous quit attempts in people without ADHD that smoke. However, this withdrawal relief was not commensurate with improved abstinence rates (Robinson et al., 1995).
3.2. Conditioned Reward
Research examining methylphenidate-nicotine interactions on tasks other than self-administration are limited but may point to other potential mechanisms for enhanced co-use liability. In the single place conditioning study examining the interaction of methylphenidate and nicotine, adolescent nicotine exposure in rats decreased the later conditioned rewarding effects of methylphenidate in adulthood (Nolley and Kelley, 2007).
3.3. Stimulus Effects
Research examining the degree to which methylphenidate and nicotine share stimulus effects is also sparse. In a drug discrimination experiment by Reichel et al. (2007), a moderate dose of methylphenidate (10 mg/kg) partially substituted for the 0.2 mg/kg training dose of nicotine. In contrast, a later study by Wooters and colleagues (2008) found that a wide range of methylphenidate doses (0, 1.25, 2.5, 5, and 10 mg/kg) did not substitute for a 0.3 mg/kg nicotine stimulus. Despite this lack of substitution, that same study found that methylphenidate co-administration with nicotine during substitution testing enhanced the degree to which 0.056 mg/kg nicotine substituted for the 0.3 mg/kg nicotine stimulus. Interestingly, neither methylphenidate nor that low dose of nicotine substituted for the training dose of nicotine when tested alone (Wooters et al., 2008).
3.4. Locomotor Sensitization
Studies examining locomotor cross-sensitization between nicotine and methylphenidate have reported mixed results. When adult rats were exposed to nicotine or saline and tested for methylphenidate-evoked locomotor activity after a washout period, nicotine exposed rats exhibited higher methylphenidate-evoked locomotor activity than saline exposed controls (Wooters et al., 2008). In contrast, locomotor cross-sensitization to nicotine was not observed when adult rats were pre-exposed to methylphenidate (Justo et al., 2010). These findings are in line with findings earlier in this review (Section 3.1) indicating that previous nicotine exposure appears to exacerbate methylphenidate misuse-liability outcomes (Shillington et al., 2006).
3.5. Neurochemical Interactions
Neurochemically, the interaction of nicotine and methylphenidate has only been explicitly examined in relation to dopaminergic systems. A study in rats found that acutely co-administered 0.4 mg/kg nicotine and high dose methylphenidate (10 mg/kg) interacted synergistically to increase synaptic dopamine concentrations in the nucleus accumbens (Gerasimov et al., 2000). Interestingly, in that same study, low dose methylphenidate (5 mg/kg) only additively enhanced dopamine concentrations when co-administered with nicotine. These findings were attributed to methylphenidate dose-dependent alterations in dopamine transporter occupancy augmenting nicotine-evoked dopamine transmission.
Wheeler and colleagues (2013) examined mRNA levels of dopamine receptor subtypes in the ventral striatum of the rat brain following adolescent exposure to 2 mg/kg/day nicotine, 3 mg/kg/day methylphenidate, or co-administered nicotine and methylphenidate at these doses. Rats that had combined nicotine and methylphenidate exposure during adolescence exhibited higher locomotor activity (and thus greater tolerance to initial locomotor suppressant effects of nicotine) during adulthood after a long period with no drug exposure. These behavioral effects were accompanied with higher dopamine D3 receptor levels in the nucleus accumbens core and increased D1 receptor sensitivity in the nucleus accumbens shell during adulthood. Different dopamine receptor subtypes are thought to be responsible for different stimulant actions, such that dopamine D1 and D2 receptors are likely responsible for locomotor or general activating effects of stimulants, while D3 receptors may act to produce tolerance to the behavioral effects of stimulants (Richtand, 2006). Both subtypes represent key factors of import to stimulant use or co-use liability, such that the general activating effects of stimulants may promote their use while behavioral tolerance allows an organism to consume higher drug concentrations. Thus, upregulation of both receptor subtypes suggests enhanced stimulant efficacy and tolerance as potential neural mechanisms of nicotine-methylphenidate co-use liability.
3.6. Conclusions
Compared to amphetamine, the literature investigating nicotine and methylphenidate interactions is sparse. With these findings, we suggest that methylphenidate may not necessarily confer additional risk for onset of nicotine use. Rather, methylphenidate may increase or reinvigorate smoking if there was an established smoking history prior to methylphenidate treatment in individuals with or without ADHD. Consistent with this idea, based on drug discrimination studies, methylphenidate and nicotine share similar stimulus effects and can potentiate each other’s stimulus effects. Perhaps experience with the stimulus effects of nicotine are required for the like-stimulus effects of methylphenidate to reinstate or enhance smoking.
In the same vein, the fact that methylphenidate pre-exposure did not alter nicotine-evoked locomotor activation, but nicotine pre-exposure did augment methylphenidate-evoked locomotor activity suggests that the order of drug exposure may be relevant for alteration of neurochemical pathways. Importantly, these locomotor findings come from separate empirical studies (Justo et al., 2010; Wooters et al., 2008). Further research examining patterns of drug exposure with methylphenidate and nicotine is important to corroborate this conclusion.
Another interesting emergence from this report is that methylphenidate only acts as a cessation aid in individuals with ADHD. This suggests that ADHD symptom relief underlies the observed efficacy of methylphenidate as a cessation aid and that nicotine serves to reduce ADHD symptoms (e.g., self-medication; van Amsterdam et al., 2018). This conclusion reconciles the above findings that when people who smoke do not have ADHD or when those treated with methylphenidate have never smoked, methylphenidate does not alter smoking behavior. However, why acute methylphenidate increases smoking in people who smoke both with and without ADHD is unclear. Perhaps the onset of pharmacotherapy, where acute drug effects are observed before chronic effects of long-term treatment, may confer additional nicotine use vulnerability in people with ADHD that smoke. This possibility should be carefully considered in future research.
The summarized neurochemical studies suggest that nicotine and methylphenidate have a combinatory effect on dopamine concentrations (Gerasimov et al., 2000) and receptors in the nucleus accumbens (Wheeler et al., 2013). Further that these neurochemical alterations have downstream influences on behavior that may be important for later co-use liability. A neglected but promising area of future research is the examination of nicotine and methylphenidate interactions as it relates to nicotinic receptors. Like amphetamine, methylphenidate behavioral sensitization is thought to be at least in part controlled by nAChRs. That is, treatment with various nAChR antagonists attenuates development of behavioral sensitization to the locomotor activating and stereotypy-inducing effects of methylphenidate (Wooters and Bardo, 2009). Thus, methylphenidate-evoked behavioral and neurochemical sensitization would likely also be altered by nAChR agonists such as nicotine. Knowledge of receptor mechanisms involved in methylphenidate-nicotine co-use liability may provide insights into effective treatments for individuals exhibiting problematic poly-substance use involving these drugs.
Given the sparse methylphenidate and nicotine interaction research, there are numerous gaps that emerge from the present review in addition to those already mentioned in this section. First, there is a surprising lack of preclinical research examining how methylphenidate alters nicotine self-administration and vice versa, with only a single study (Wooters et al., 2008). Additional research examining chronic exposure paradigms consistent with treatment for ADHD may be of interest. While two studies examined conditioned drug reward interactions between methylphenidate and nicotine, no published study has examined drug reward or reward-enhancement interactions. Such a gap may prompt ICSS studies to determine how prior or acute methylphenidate exposure alters the reward threshold decreasing effects of nicotine and vice versa. Like nicotine, methylphenidate also decreases ICSS reward-thresholds (Ide et al., 2018), so nicotine and methylphenidate would likely interact to alter drug reward.
4. Bupropion and Nicotine
Bupropion is also known as prescription psychostimulant Zyban™ and Wellbutrin™. In addition to bupropion’s anti-depressant effects (Patel et al., 2016), bupropion is used for smoking cessation (Richmond and Zwar, 2003) and as an off-label ADHD medication (Paterson, 2009). Bupropion and its role in smoking cessation has been reviewed extensively elsewhere (for reviews, see Lindson et al., 2019; Mooney and Sofuoglu, 2006; Patel et al., 2016; Paterson, 2009; Richmond and Zwar, 2003; Wilkes, 2008). Thus, we point the reader to these reviews and will only discuss this literature here as it directly relates to the primary goal of this review.
4.1. Primary Reinforcement
Briefly, cessation and abstinence studies in humans have found that bupropion acts as an effective smoking cessation aid in both individuals with or without ADHD (Hayford et al., 1999; Hurt et al., 1997; Durcan et al., 2002; Gonzales et al., 2002; Shiffman et al., 2000; Verbeeck et al., 2017). Bupropion appears to do so, at least in part, by mitigating adverse effects typically induced by nicotine abstinence. For example, bupropion has been found to reduce cigarette craving (Durcan et al., 2002), ameliorate abstinence-related cognitive deficits (Perkins et al., 2013), and blunt negative affect during abstinence and reported satisfaction of cigarettes during a smoking relapse (West et al., 2008). In preclinical studies, bupropion pre-treatment also attenuated a conditioned place aversion to a context associated with mecamylamine-induced nicotine withdrawal (Malin et al., 2006) suggesting that aversive effects of nicotine withdrawal were also reduced by bupropion in rats. Chronic bupropion also diminished nicotine withdrawal associated increases in ICSS reward thresholds in rats. Further, bupropion attenuated physical signs of nicotine withdrawal such as anhedonia, chewing behavior, and headshakes (Paterson et al., 2007).
Bupropion has also increased nicotine intake under some circumstances. One study found that acute bupropion increased the number of cigarettes smoked ad libitum compared to placebo (Cousins et al., 2001). Conflicting with the above described human findings, daily pre-session administration of 30 mg/kg bupropion in adult rats tend to increase nicotine self-administration (Shoaib et al., 2003; Stairs and Dworkin, 2008). Notably, Shoaib and colleagues found that while bupropion tended to increase overall self-administration at 0.01 and 0.09 mg/kg/infusion nicotine, the increase was only significant for rats in the high dose nicotine group (Shoaib et al., 2003). Additionally, low doses of acutely administered bupropion (9 and 15 mg/kg) have either increased (Rauhut et al., 2003) or left nicotine self-administration unchanged (10 mg/kg; Stairs and Dworkin, 2008).
In contrast, high doses (30–78 mg/kg) tended to decrease nicotine self-administration (Bruijnzeel and Markou, 2003; Kazan and Charntikov, 2019; Rauhut et al., 2003). Kazan and Charntikov (2019) found that if baseline nicotine consumption was higher, the magnitude of the bupropion-evoked decrease in nicotine self-administration was greater. In contrast to the prior studies, Stairs and Dworkin (2008) found that 56 mg/kg bupropion did not significantly decrease nicotine self-administration. However, this dose did produce a non-significant decrease in nicotine self-administration consistent with the aforementioned prior high dose bupropion studies. Interestingly, this study also found that bupropion decreased food-maintained responding in food restricted rats (thus baseline response rates were high) but increased food-maintained responding in satiated rats where baseline response rates were lower and more comparable to nicotine-maintained responding (Stairs and Dworkin, 2008). Thus, the baseline-dependent effects of bupropion on nicotine self-administration reported in Kazan and Charntikov (2019) could be due to general rate-dependent effects of bupropion on operant responding.
Conflicting results have also been identified with short versus long access self-administration tasks. While all self-administration studies we have reviewed so far were “short-access,” or standard one-two hour sessions, there are also “long-access” variations where sessions are six or more hours. Long-access sessions are thought to lead to faster development of dependence-like behaviors in rodents that may better simulate some aspects of human drug use (Allain and Samaha, 2019; Knackstedt et al., 2010). Unlike the short access studies previously described, moderate to high doses of bupropion (30 to 60 mg/kg) increased nicotine self-administration in a long-access self-administration study (Kazan et al., 2020). Considering that long-access variations may be more commensurate with the human experience (e.g., having access to cigarettes or vapes during most or all of the day), exploring the root of the discrepancy between short and long-access paradigms may be of interest for determining mechanisms of potential nicotine-prescription psychostimulant co-use liability.
4.2. Drug Reward
A moderate to high dose of bupropion alone (up to 60 mg/kg) decreased ICSS thresholds indicating an increase in nicotine reward. However, a low dose of bupropion (5 mg/kg) completely blocked nicotine’s ability to reduce reward thresholds in rats – a pattern denoting reduction of nicotine reward (Cryan et al., 2003). Perhaps the reduced rewarding effects of nicotine drive enhanced nicotine taking by requiring more nicotine to achieve the same reinforcing effects. However, this finding is not in line with a study by Paterson and colleagues that examined the effects of 30 or 60 mg/kg/day bupropion administered via osmotic mini pump on nicotine self-administration and ICSS. Bupropion did not change nicotine self-administration in this study, but did attenuate the ICSS-threshold reducing effects of self-administered nicotine (Paterson et al., 2008). It appears that bupropion altered the rewarding effects of nicotine without producing comparable alterations in nicotine intake. This pattern of results suggests mechanisms other than bupropion-evoked alteration of nicotine reward are involved in changes in nicotine intake.
4.3. Stimulus Effects
One study found that a range of bupropion doses (1, 3, 10, and 30 mg/kg) did not substitute for a low training dose of nicotine (0.2 mg/kg; Shoaib et al., 2003). In other studies, 20 to 30 mg/kg bupropion fully (Bevins et al., 2006; Charntikov et al., 2014; Wiley et al., 2002; Wilkinson et al., 2010) or partially substituted (Besheer et al., 2004; Wilkinson et al., 2010; Young and Glennon, 2002) for a 0.4 to 0.6 mg/kg nicotine stimulus, suggesting that higher doses of bupropion exhibited similar stimulus effects to more moderate-to-high nicotine doses. Indeed, in a study where a low dose of 10 mg/kg bupropion was used as the training drug, a high dose of 30 mg/kg bupropion produced less bupropion-appropriate conditioned behavior during substitution testing than the 10 mg/kg training dose (Wilkinson et al., 2009). Likewise, when rats were trained to discriminate 0.2 mg/kg nicotine from saline and later tested for nicotine substitution, 0.3 mg/kg reduced nicotine-appropriate responding relative to 0.2 mg/kg nicotine (Reichel et al., 2007). That is to say, a high dose of bupropion may have qualitatively different stimulus effects than lower doses, and likewise for high and low doses of nicotine. These qualitative differences in stimulus effects may explain the divergent patterns of cross-substitution at high versus low drug doses described earlier.
Few studies have examined nicotine substitution for bupropion in drug discrimination tasks. Wilkinson and colleagues trained rats to discriminate between saline and 10 mg/kg bupropion. During substitution testing, a very low nicotine dose (0.05 mg/kg) substituted fully for 10 mg/kg bupropion. That is, 0.05 mg/kg nicotine produced bupropion-appropriate responding similar to that produced by the original bupropion training dose (Wilkinson et al., 2009). In a recent drug-drug discrimination study in our laboratory, rats were trained to discriminate between 0.4 mg/kg nicotine and 10 or 20 mg/kg bupropion. This study by Moran and colleagues (2022) found that rats quickly and robustly discriminated between nicotine and 10 mg/kg bupropion. Rats also acquired the discrimination between nicotine and 20 mg/kg bupropion stimulus, but much more slowly and less robustly (Moran et al., 2022), which suggests that nicotine and 20 mg/kg bupropion exhibit more similar stimulus effects than nicotine and a lower 10 mg/kg bupropion dose.
When the interaction between bupropion and nicotine has been examined in substitution tests, low bupropion doses (0.1 to 10 mg/kg) consistently did not alter or block nicotine-appropriate responding (Shoaib et al., 2003; Wiley et al., 2002; Young and Glennon, 2002). Thus, the fact that bupropion increased nicotine self-administration in rodents under some circumstances may not be due to alteration of the stimulus effects of nicotine.
4.4. Locomotor Studies
A study by Wilkinson and colleagues (2006) found that chronic pre-exposure to 0.4 mg/kg nicotine enhanced the locomotor stimulatory effects of 30 mg/kg, but not 20 mg/kg bupropion in locomotor chambers (Wilkinson et al., 2006). Acute bupropion treatment also enhanced nicotine-evoked locomotor activity testing (Slemmer et al., 2000). In a later study, 0.4 mg/kg nicotine delivered 20 minutes following an acute 30 mg/kg bupropion injection enhanced bupropion-evoked locomotor activity (Sidhpura et al., 2007).
4.5. Neurochemical Interactions
Neurochemically, a large body of research suggests that bupropion serves as a noncompetitive nAChR antagonist at α4β2, α3β2, and α7 subtypes, in addition to its action as a dopamine and norepinephrine reuptake inhibitor (Arias, 2009; Slemmer et al., 2000). 86Rb+ efflux is an in vitro measure of activity through nAChR channels that can be used to identify substances acting at nAChRs; efflux was increased by nAChRs agonists and decreased by antagonists (Kassner et al., 2022). In one study, rats that were administered 30 mg/kg bupropion or vehicle were sacrificed and synaptosomes (isolated synaptic terminals) from the frontal cortex, hippocampus, striatum, and thalamus were isolated. Synaptosomes from rats that were administered bupropion showed greater in vitro nicotine-evoked 86Rb+ efflux, suggesting an nAChR agonist effect of bupropion (Vann et al., 2006).
Bupropion treatment reduced nicotine-evoked dopamine release in in-vitro striatal brain slices, suggesting nAChR antagonist effects (Sidhpura et al., 2007). In vivo, acutely administered nicotine enhanced bupropion-evoked dopamine overflow in an additive fashion (Sidhpura et al., 2007). In rats that underwent abstinence-related nicotine withdrawal, bupropion enhanced dopamine overflow during the withdrawal period as measured via microdialysis in the nucleus accumbens shell. However, bupropion also did so in saline-exposed controls, so this effect of bupropion was unrelated to its interaction with nicotine (Paterson et al., 2007). Even non-specific enhancement of dopamine levels could potentially be responsible for the attenuating effects of bupropion on typical anhedonia and general adverse effects associated with nicotine withdrawal mentioned earlier in this review.
4.6. Conclusions
The research reviewed above (Section 4) suggests that the moderate efficacy of bupropion as a cessation aid may be rooted in amelioration of the adverse effects of nicotine withdrawal. This occurs in both human and animal models, such that bupropion blunts physical withdrawal symptoms during abstinence in rodents and cognitive deficits associated with nicotine abstinence. While chronic bupropion administration in humans appears to decrease nicotine intake consistent with its use as a smoking cessation drug, acute bupropion increased human smoking. This finding appears consistent with patterns observed in the Amphetamine and Nicotine and Methylphenidate and Nicotine sections of this report; thus, like other prescription psychostimulants reviewed here, bupropion appears to increase nicotine use liability under acute circumstances and mitigate nicotine use liability under chronic conditions. The root of this discrepancy, to our knowledge, has not been explored.
In preclinical studies, bupropion exhibits a different pattern of mixed effects on nicotine self-administration. Kazan and colleagues (2020) identified that high-dose bupropion increased nicotine self-administration in their long-access study, while short-access studies reviewed here consistently found high dose bupropion to decrease nicotine self-administration. To our knowledge, there is only one long-access study examining the impacts of prescription psychostimulants on nicotine self-administration. This gap may highlight an interesting area for future research, particularly considering our earlier point that long-access self-administration may better parallel the human condition (cf. Allain and Samaha, 2019; Knackstedt et al., 2010).
Bupropion impacts on nicotine self-administration are also mixed by dose, such that low bupropion doses appear to increase nicotine self-administration and vice versa for high doses. The cause of this bidirectional dose-dependency is unclear. One potential reason is divergent impacts of high and low dose bupropion on nicotine reward, where one ICSS study found that high doses of bupropion heightened nicotine reward, where low doses reduced nicotine reward (Cryan et al., 2003). That reduction in nicotine reward could be responsible for increasing nicotine intake by requiring more drug to produce the same rewarding effect. Notably, this was conflicting with one study examining continuous bupropion administration where 30 to 60 mg/kg/day blunted nicotine reward without altering nicotine intake (Paterson et al., 2008). Studies exploring the root of the discrepancy between continuously infused and acute bupropion may be informative for determining effective formulations for cessation.
Some research reviewed here may also suggest that the divergent stimulus effects of low and high dose bupropion may play a role in dose-dependent alterations in nicotine self-administration. That is, high doses of bupropion may better substitute for nicotine thus blunting nicotine intake, while low doses do not substitute as well.
The vast majority of research examining bupropion and nicotine interactions has focused on nicotine intake in the form of cessation or self-administration studies, stimulus effect substitution, and adverse effects of nicotine withdrawal. Some notable gaps that we have not yet mentioned exist in exploration of how nicotine and bupropion interact to alter conditioned reward and the ability of nicotine and bupropion to alter the reinforcing efficacy of other non-drug rewards. For example, how bupropion alters the ability of nicotine to produce a preference to a nicotine-associated context might be of interest. Likewise, considering the import of reward-enhancement in nicotine self-administration, understanding how bupropion alters this important use liability effect of nicotine could be of import for understanding bupropion alterations in nicotine intake.
5. Putting it All Together: Concluding Remarks and Future Research
While we have reflected on gaps and future directions in the previous sections, some themes that emerge throughout the body of this review warrant further discussion. Amphetamine and methylphenidate clearly impact nicotine use liability across an array of doses and use liability tasks. However, research with bupropion is mixed with low to moderate doses increasing nicotine use liability on a variety of tasks, and high doses not changing or decreasing use liability.
This review reveals a great lack of research examining female rats, such that only ~10% of the rodent studies reviewed here included females. An issue that is all-to-common with virtually every misused substance (Bevins and Charntikov, 2015; Killien et al., 2000). This gap in research leaves us knowing very little in how nicotine interacts with psychostimulants in women or female animals, which is particularly troubling given enhanced nicotine use vulnerability in women (Greaves and Hemsing, 2009; Ortner et al., 2002) and consistently higher rates of amphetamine and other psychostimulant addiction amongst women (Rungnirundorn et al., 2017). The human research in this report generally included men and women, but few were powered to robustly assess sex differences. Of the few preclinical studies that did examine sex as a biological variable, all examined amphetamine or bupropion. Highlighting our concern, each of the preclinical studies powered to assess sex differences found greater effects in women or female rodents (Collins et al., 2004; Íbias and Nazarian, 2020; McNealy et al., 2022, 2021; Zakiniaeiz et al., 2019). This work is consistent with females and human women being more sensitive to most behavioral effects of psychostimulants (Becker and Koob, 2016; Camp and Robinson, 1988; Milesi-Hallé et al., 2007). No preclinical study mentioned here examining methylphenidate utilized female rats. The literature being devoid of studies examining sex or gender differences leaves limitless possibilities for future research. For example, how nicotine might impact amphetamine self-administration or place conditioning differentially between the sexes would be of interest and of importance for ADHD-pharmacotherapy guidance.
Across all the prescription psychostimulants reviewed herein aside from methylphenidate, consideration of ADHD diagnosis or symptoms in relation to observed increases in smoking is lacking. In fact, most human research in this area lists any psychiatric diagnosis, including ADHD, as exclusionary criterion (e.g., Alsene et al., 2005; Henningfield and Griffiths, 1981). It is well documented that prescription stimulants evoke lower misuse liability and produce different drug effects in ADHD and non-ADHD individuals (Lakhan and Kirchgessner, 2012). Further, individuals with ADHD smoke at 2 to 3 times higher rates than individuals without ADHD suggesting altered nicotine use liability at baseline (van Amsterdam et al., 2018). Preclinical researchers examining nicotine-psychostimulant interactions could take advantage of robust animal models of ADHD such as the spontaneous hypertensive rat (Cho et al., 2014; Sanabria and Killeen, 2008). Using such models could account for ADHD-like symptoms in animal research to determine whether co-use liability effects of nicotine and prescription treatments for ADHD vary by the presence or severity of ADHD-like symptoms.
Another interesting discrepancy revealed by this review was the opposing findings regarding order of drug exposure between methylphenidate and amphetamine. Even with the limited research, interaction research with methylphenidate and nicotine are generally in accordance with “gateway” models of substance use suggesting that nicotine produces neurobiological alterations that enhance vulnerability for future substance use (Levine et al., 2011). That is, methylphenidate did not enhance smoking unless there was an established smoking history (Bron et al., 2013) – nicotine had to be experienced before methylphenidate. Further, on a number of preclinical behavioral tasks, nicotine use liability measures were not increased with prior exposure to methylphenidate (e.g., Justo et al., 2010). In contrast, methylphenidate use liability measures were increased by prior exposure to nicotine (e.g., Wooters et al., 2008). The alignment of these findings with prior cocaine research (discussed in the Introduction) makes sense considering the foundational exposure pattern research with nicotine has largely focused on cocaine (Kandel and Kandel, 2014; Levine et al., 2011). Cocaine and methylphenidate share a mechanism of action as dopamine transporter inhibitors, which then causes an increase in extracellular dopamine (Rudnick and Clark, 1993). Thus, we may expect similar findings between methylphenidate and cocaine. For readers interested in making comparisons between illicit and prescription psychostimulants, we point them towards the existing body of reviews describing the interaction of nicotine with illicit psychostimulants (Crummy et al., 2020; Cross et al., 2017; Kohut, 2017).
Future research may also consider exploring pharmacokinetic effects when examining nicotine interactions with psychostimulants. All animal research in the present report used injected/intravenous prescription psychostimulant compounds and all but one study used injected/intravenous nicotine (cf. Bruijnzeel et al., 2011). Further, all human research used oral formulations for psychostimulant administration. However, individuals who misuse amphetamine, methylphenidate, and bupropion also consume these drugs via insufflation or injection (Farquhar et al., 2002; Lewis et al., 2014). Pharmacokinetic changes as a result of these divergent routes of administration almost certainly influence subjective drug effects (e.g., Lile et al., 2011). Thus, perhaps results would diverge from those synthesized here with differing routes of administration.
The research with amphetamine discussed in this report suggests an opposite predominate tested pattern of exposure. Only two papers summarized in this review examined the effects of amphetamine exposure on nicotine-related outcomes, both of which found effects divergent from nicotine pre-exposure (McNealy et al., 2022; Santos et al., 2009). The original research characterizing drug use trajectories did not examine any type of amphetamine in neither pre-clinical or epidemiological studies examining patterns of drug use and concordant use liability effects (Degenhardt et al., 2010; Kandel and Kandel, 2015). Further, amphetamine’s mechanism of action is disparate from that of cocaine and methylphenidate. Amphetamine works by binding to the dopamine transporter, thereby increasing dopamine by transporter-mediated exchange (Rudnick and Clark, 1993). We suggest that this review reveals that research design has been biased in the direction of nicotine pre-exposure, perhaps due to prior research supposing nicotine as an antecedent to other psychostimulant use. Thus, there is a lack of research assessing amphetamine pre-exposure effects. This bias is unfortunate given that amphetamine-containing drugs can be, and often are, prescribed as early as age six (Heal et al., 2013). Future research should focus on testing how amphetamine-containing drugs, and prescription psychostimulant drugs more generally, fit within current gateway models of substance use. This includes epidemiological studies examine progression of substance use, in addition to preclinical research varying order of drug exposure or correcting the current research bias by examining amphetamine or methylphenidate’s effects on nicotine-related outcomes.
Future research may also consider exploring atomoxetine interactions with nicotine. Atomoxetine is the active component of Strattera™, a nonstimulant alternative to other ADHD pharmacotherapies typically prescribed to individuals with ADHD who have comorbid mental or physical health conditions that contraindicate stimulant use (e.g., insomnia, tic disorders; Yu et al., 2016). Unlike amphetamine and methylphenidate, atomoxetine administration does not increase smoking behavior (Vansickel et al., 2007). In fact, evidence from animal studies suggests that atomoxetine may attenuate the effects of nicotine (Reichel et al., 2007; Gould et al., 2005, Davis and Gould, 2007). Due to the contrasting effects of atomoxetine with bupropion, amphetamine, and methylphenidate, expanding the small literature examining nicotine and atomoxetine interactions may help in providing clinical guidance for treatment of ADHD in people who smoke and increase the understanding on the mechanisms of these differences between substances.
Highlights.
Nicotine exhibits high co-use liability with amphetamine and methylphenidate
High dose bupropion may decrease nicotine use liability while low doses decrease
The limited research with atomoxetine suggests attenuation of nicotine’s effects
Scant research examines prescription psychostimulant-nicotine interactions in females
Drug exposure patterns and ADHD symptoms are relevant factors for co-use liability
6. Acknowledgements
RAB was partially supported by the National Institutes of Health [R01-DA034389] and [GM130461] while preparing this review. KM was fully supported by [R01-DA034389] while preparing this review.
Role of Funding Source:
Nothing declared.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Adams E, Klug J, Quast M, Stairs DJ, 2013. Effects of environmental enrichment on nicotine-induced sensitization and cross-sensitization to d-amphetamine in rats. Drug Alcohol Depend. 129, 247–253. 10.1016/j.drugalcdep.2013.02.019 [DOI] [PubMed] [Google Scholar]
- Alajaji M, Lazenka M. f., Kota D, Wise LE, Younis RM, Carroll FI, Levine A, Selley DE, Sim-Selley LJ, Damaj MI, 2016. Early adolescent nicotine exposure affects later-life cocaine reward in mice. Neuropharmacology 105, 308–317. 10.1016/j.neuropharm.2016.01.032 [DOI] [PubMed] [Google Scholar]
- Alcover KC, Thompson CL, 2020. Patterns of mean age at drug use initiation among adolescents and emerging adults, 2004–2017. JAMA Pediatr. 174, 725–727. 10.1001/jamapediatrics.2019.6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Samaha A-N, 2019. Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length. Addict. Biol. 24, 641–651. 10.1111/adb.12629 [DOI] [PubMed] [Google Scholar]
- Alsene KM, Mahler SV, de Wit H, 2005. Effects of d-amphetamine and smoking abstinence on cue-induced cigarette craving. Exp. Clin. Psychopharmacol. 13, 209–218. 10.1037/1064-1297.13.3.209 [DOI] [PubMed] [Google Scholar]
- Arias HR, 2009. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int. J. Biochem. Cell Biol. 41, 2098–2108. 10.1016/j.biocel.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA, 2000. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 153, 31–43. 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA, Klebaur JE, Crooks PA, Dwoskin LP, 1997. (Ϫ)-Nornicotine partially substitutes for (ϩ)-amphetamine in a drug discrimination paradigm in rats. Pharmacol. Biochem. Behav. 58, 1083–1087. [DOI] [PubMed] [Google Scholar]
- Barret ST, Bevins RA, 2013. Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol. Biochem. Behav. 114–115, 9–15. 10.1016/j.pbb.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO, 2006. Patterns of simultaneous polysubstance use in drug using university students. Hum. Psychopharmacol. Clin. Exp. 21, 255–263. 10.1002/hup.766 [DOI] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA, 2012. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand: Behav. Pharmacol. 23, 781–789. 10.1097/FBP.0b013e32835a38d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Braymiller JL, Unger JB, McConnell R, Stokes A, Leventhal AM, Sargent JD, Samet JM, Goodwin RD, 2020. Trends in the age of cigarette smoking initiation among young adults in the US from 2002 to 2018. JAMA Netw. Open 3, e2019022. 10.1001/jamanetworkopen.2020.19022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: Focus on addiction. Pharmacol. Rev. 68, 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, 2010. Nicotine Addiction. N. Engl. J. Med. 362, 2295–2303. 10.1056/NEJMra0809890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA, 2004. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl.) 172, 108–117. 10.1007/s00213-003-1621-9 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Charntikov S, 2015. We know very little about the subjective effects of drugs in females. ACS Chem. Neurosci. 6, 359–361. 10.1021/acschemneuro.5b00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM, 2006. Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology (Berl.) 184, 470–481. 10.1007/s00213-005-0079-3 [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B, 2008. Calcium-dependent mechanisms of the reinstatement of nicotine-conditioned place preference by drug priming in rats. Pharmacol. Biochem. Behav. 89, 116–125. 10.1016/j.pbb.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Biala G, Kruk M, 2008. Calcium channel antagonists suppress cross-tolerance to the anxiogenic effects of d-amphetamine and nicotine in the mouse elevated plus maze test. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 54–61. 10.1016/j.pnpbp.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Biala G, Kruk M, Budzynska B, 2009. Effects of the cannabinoid receptor ligands on anxiety-related effects of d-amphetamine and nicotine in the mouse elevated plus maze test. J. Physiol. Pharmacol. 60, 113–122. [PubMed] [Google Scholar]
- Biala G, Weglinska B, 2004. Calcium channel antagonists attenuate cross-sensitization to the rewarding and/or locomotor effects of nicotine, morphine and MK-801. J. Pharm. Pharmacol. 56, 1021–1028. 10.1211/0022357043888 [DOI] [PubMed] [Google Scholar]
- Birrell CE, Balfour DJK, 1998. The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to D - amphetamine. Psychopharmacology (Berl.) 140, 142–149. 10.1007/s002130050751 [DOI] [PubMed] [Google Scholar]
- Brecht M-L, Greenwell L, Anglin MD, 2007. Substance use pathways to methamphetamine use among treated users. Addict. Behav. 32, 24–38. 10.1016/j.addbeh.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Bron TI, Bijlenga D, Kasander MV, Spuijbroek AT, Beekman ATF, Kooij JJS, 2013. Long-term relationship between methylphenidate and tobacco consumption and nicotine craving in adults with ADHD in a prospective cohort study. Eur. Neuropsychopharmacol. 23, 542–554. 10.1016/j.euroneuro.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A, 2003. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50, 20–28. 10.1002/syn.10242 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Rodrick G, Singh RP, Derendorf H, Bauzo RM, 2011. Repeated pre-exposure to tobacco smoke potentiates subsequent locomotor responses to nicotine and tobacco smoke but not amphetamine in adult rats. Pharmacol. Biochem. Behav. 100, 109–118. 10.1016/j.pbb.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF, 2009. Chapter 6: The role of nicotine in smoking: A dual-reinforcement model. Neb. Symp. Motiv. Neb. Symp. Motiv. 55, 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF, 2002. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl.) 163, 230–237. 10.1007/s00213-002-1156-5 [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR, 2008. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci. Lett. 439, 187–191. 10.1016/j.neulet.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Robinson TE, 1988. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic d-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav. Brain Res. 30, 55–68. 10.1016/0166-4328(88)90008-3 [DOI] [PubMed] [Google Scholar]
- Castillo-Rolón D, Ramírez-Sánchez E, Arenas-López G, Garduño J, Hernández-González O, Mihailescu S, Hernández-López S, 2021. Nicotine increases spontaneous glutamate release in the rostromedial tegmental nucleus. Front. Neurosci. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Griffiths RR, 1983. Effects of caffeine on cigarette smoking and subjective response. Clin. Pharmacol. Ther. 34, 612–622. 10.1038/clpt.1983.223 [DOI] [PubMed] [Google Scholar]
- Chance WT, Murfin D, Krynock GM, Rosecrans JA, 1977. A description of the nicotine stimulus and tests of its generalization to amphetamine. Psychopharmacology (Berl.) 55, 19–26. 10.1007/BF00432812 [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP, 1997. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl.) 129, 257–264. 10.1007/s002130050188 [DOI] [PubMed] [Google Scholar]
- Charntikov S, deWit NR, Bevins RA, 2014. Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion. Neuropharmacology 86, 181–191. 10.1016/j.neuropharm.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Li M, Bevins RA, 2017. Double dissociation of the anterior and posterior dorsomedial caudate-putamen in the acquisition and expression of associative learning with the nicotine stimulus. Neuropharmacology 121, 111–119. 10.1016/j.neuropharm.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA, 2005. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl.) 180, 258–266. 10.1007/s00213-005-2152-3 [DOI] [PubMed] [Google Scholar]
- Cho HS, Baek DJ, Baek SS, 2014. Effect of exercise on hyperactivity, impulsivity and dopamine D2 receptor expression in the substantia nigra and striatum of spontaneous hypertensive rats. J. Exerc. Nutr. Biochem. 18, 379–384. 10.5717/jenb.2014.18.4.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SP, Goldstein RB, Smith SM, Huang B, Ruan WJ, Zhang H, Jung J, Saha TD, Pickering RP, Grant BF, 2016. The epidemiology of DSM-5 nicotine use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. J. Clin. Psychiatry 77, 0–0. 10.4088/JCP.15m10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P, 2002. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing e¡ects of nicotine in rats. Behav. Pharmacol. 13, 451–463. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S, 2004. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev. Brain Res. 153, 175–187. 10.1016/j.devbrainres.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Compton WM, Han B, Blanco C, Johnson K, Jones CM, 2018. Prevalence and Correlates of Prescription Stimulant Use, Misuse, Use Disorders, and Motivations for Misuse Among Adults in the United States. Am. J. Psychiatry 175, 741–755. 10.1176/appi.ajp.2018.17091048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P, 2012. Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology 37, 2277–2284. 10.1038/npp.2012.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H, 2001. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl.) 157, 243–253. 10.1007/s002130100802 [DOI] [PubMed] [Google Scholar]
- Covey LS, Hu M-C, Winhusen T, Weissman J, Berlin I, Nunes EV, 2010. OROS-methylphenidate or placebo for adult smokers with attention deficit hyperactivity disorder: Racial/ethnic differences. Drug Alcohol Depend. 110, 156–159. 10.1016/j.drugalcdep.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y, 2008. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. B Biol. Sci. 363, 3233–3243. 10.1098/rstb.2008.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Bardo MT, Dwoskin LP, 2014. Chapter Thirteen - Nicotinic Receptor Antagonists as Treatments for Nicotine Abuse, in: Dwoskin LP (Ed.), Advances in Pharmacology, Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Academic Press, pp. 513–551. 10.1016/B978-0-12-420118-7.00013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Lotfipour S, Leslie FM, 2017. Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am. J. Drug Alcohol Abuse 43, 171–185. 10.1080/00952990.2016.1209512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crummy EA, O’Neal TJ, Baskin BM, Ferguson SM, 2020. One is not enough: Understanding and modeling polysubstance use. Front. Neurosci. 14. 10.3389/fnins.2020.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A, 2003. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl.) 168, 347–358. 10.1007/s00213-003-1445-7 [DOI] [PubMed] [Google Scholar]
- Cunningham C, Polston J, Jany J, Segert I, Miller D, 2006. Interaction of lobeline and nicotinic receptor ligands with the discriminative stimulus properties of cocaine and amphetamine. Drug Alcohol Depend. 84, 211–222. 10.1016/j.drugalcdep.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ, 2007. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology 32, 2011–2019. 10.1038/sj.npp.1301315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Dierker L, Chiu WT, Medina-Mora ME, Neumark Y, Sampson N, Alonso J…, 2010. Evaluating the drug use “gateway” theory using cross-national data: Consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 108, 84–97. 10.1016/j.drugalcdep.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoulet M, Rostain J-C, Abraini JH, David HN, 2013. Short-term development of behavioral sensitization to amphetamine requires N-methyl-D-aspartate- and nicotinic-dependent mechanisms in the nucleus accumbens: Nucleus accumbens and sensitization development. Addict. Biol. 18, 417–424. 10.1111/j.1369-1600.2010.00297.x [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ, 2007. Sensitization to nicotine: How the animal literature might inform future human research. Nicotine Tob. Res. 9, 9–20. 10.1080/14622200601078277 [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A, 2013. The “stop” and “go” of nicotine dependence: Role of GABA and glutamate. Cold Spring Harb. Perspect. Med. 3, a012146. 10.1101/cshperspect.a012146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, Rigotti N, Sachs DPL, 2002. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin. Ther. 24, 540–551. 10.1016/S0149-2918(02)85130-X [DOI] [PubMed] [Google Scholar]
- Edwards AW, Konz N, Hirsch Z, Weedon J, Dow-Edwards DL, 2014. Single trial nicotine conditioned place preference in pre-adolescent male and female rats. Pharmacol. Biochem. Behav. 125, 1–7. 10.1016/j.pbb.2014.07.016 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y, 2006. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl.) 189, 1–16. 10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar S, Fawcett P, Fountain J, 2002. Illicit intravenous use of methylphenidate (ritalin) tablets: A review of four cases. Aust. Emerg. Nurs. J. 5, 25–29. 10.1016/S1328-2743(02)80016-5 [DOI] [Google Scholar]
- Fleary SA, Heffer RW, McKyer ELJ, 2011. Dispositional, ecological and biological influences on adolescent tranquilizer, Ritalin, and narcotics misuse. J. Adolesc. 34, 653–663. 10.1016/j.adolescence.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2014. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76, 10.1016/j.neuropharm.2013.09.008. 10.1016/j.neuropharm.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke J, Whalen C, Jamner L, Wigal T, Steinhoff K, 2006. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine Tob. Res. 8, 37–47. 10.1080/14622200500431619 [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL, 2000. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor 6. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rukstalis M, Lewis MC, 2005. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neurosci. Lett. 377, 85–90. 10.1016/j.neulet.2004.11.073 [DOI] [PubMed] [Google Scholar]
- Greaves L, Hemsing N, 2009. Women and tobacco control policies: Social-structural and psychosocial contributions to vulnerability to tobacco use and exposure. Drug Alcohol Depend. 104, S121–S130. 10.1016/j.drugalcdep.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Harmony ZR, Alderson EM, Garcia-Carachure I, Bituin LD, Crawford CA, 2020. Effects of nicotine exposure on oral methamphetamine self-administration, extinction, and drug-primed reinstatement in adolescent male and female rats. Drug Alcohol Depend. 209, 107927. 10.1016/j.drugalcdep.2020.107927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Muelken P, Swain Y, Palumbo M, Jain V, Goniewicz ML, Stepanov I, LeSage MG, 2019. Non-nicotine constituents in e-cigarette aerosol extract attenuate nicotine’s aversive effects in adolescent rats. Drug Alcohol Depend. 203, 51–60. 10.1016/j.drugalcdep.2019.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, Glover ED, Sachs DPL, Hurt RD, 1999. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br. J. Psychiatry 174, 173–178. 10.1192/bjp.174.2.173 [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ, 2013. Amphetamine, past and present – a pharmacological and clinical perspective. J. Psychopharmacol. (Oxf.) 27, 479–496. 10.1177/0269881113482532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR, 1981. Cigarette smoking and subjective response: effects of d-amphetamine. Clin. Pharmacol. Ther. 30, 497–505. 10.1038/clpt.1981.194 [DOI] [PubMed] [Google Scholar]
- Ho BT, Huang J-T, 1975. Role of dopamine in d-amphetamine-induced discriminative responding. Pharmacol. Biochem. Behav. 3, 1085–1092. 10.1016/0091-3057(75)90021-0 [DOI] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE, 2013. Abuse potential of non-nicotine tobacco smoke components: Acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob. Res. 15, 622–632. 10.1093/ntr/nts192 [DOI] [PubMed] [Google Scholar]
- Hurt RD, Ebbert JO, Croghan IT, Schroeder DR, Sood A, Hays JT, 2011. Methylphenidate for treating tobacco dependence in non-attention deficit hyperactivity disorder smokers: A pilot randomized placebo-controlled trial. J. Negat. Results Biomed. 10, 1. 10.1186/1477-5751-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Sachs DPL, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM, 1997. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 337, 1195–1202. 10.1056/NEJM199710233371703 [DOI] [PubMed] [Google Scholar]
- Huss M, Poustka F, Lehmkuhl G, Lehmkuhl U, 2008. No increase in long-term risk for nicotine use disorders after treatment with methylphenidate in children with attention-deficit/hyperactivity disorder (ADHD): evidence from a non-randomised retrospective study. J. Neural Transm. 115, 335–339. 10.1007/s00702-008-0872-3 [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Sarkar M, Kornetsky C, 1993. Nicotine and brain-stimulation reward: Interactions with morphine, amphetamine and pimozide. Pharmacol. Biochem. Behav. 46, 453–457. 10.1016/0091-3057(93)90378-7 [DOI] [PubMed] [Google Scholar]
- Íbias J, Nazarian A, 2020. Sex differences in nicotine-induced impulsivity and its reversal with bupropion in rats. J. Psychopharmacol. (Oxf.) 34, 1382–1392. 10.1177/0269881120937543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Ikekubo Y, Hua J, Takamatsu Y, Uhl GR, Sora I, Ikeda K, 2018. Reward-enhancing effect of methylphenidate is abolished in dopamine transporter knockout mice: A model of attention-deficit/hyperactivity disorder. Neuropsychopharmacol. Rep. 38, 149–153. 10.1002/npr2.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB, 1999. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N. Engl. J. Med. 340, 685–691. 10.1056/NEJM199903043400903 [DOI] [PubMed] [Google Scholar]
- Justo CC, Carneiro-de-Oliveira PE, DeLucia R, Aizenstein ML, Planeta CS, 2010. Repeated exposure of adolescent rats to oral methylphenidate does not induce behavioral sensitization or cross-sensitization to nicotine. Braz. J. Med. Biol. Res. 43, 651–656. 10.1590/S0100-879X2010007500042 [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME, 2008. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology (Berl.) 200, 93–103. 10.1007/s00213-008-1159-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D, Kandel E, 2015. The Gateway Hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatr. 104, 130–137. 10.1111/apa.12851 [DOI] [PubMed] [Google Scholar]
- Kandel ER, Kandel DB, 2014. A molecular basis for nicotine as a gateway drug. N. Engl. J. Med. 371, 932–943. 10.1056/NEJMsa1405092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner M, Eaton JB, Tang N, Petit JL, Meurice N, Yin HH, Whiteaker P, 2022. High-throughput cell-based assays for identifying antagonists of multiple smoking-associated human nicotinic acetylcholine receptor subtypes. SLAS Discov. 27, 68–76. 10.1016/j.slasd.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan T, Charntikov S, 2019. Individual differences in responding to bupropion or varenicline in a preclinical model of nicotine self-administration vary according to individual demand for nicotine. Neuropharmacology 148, 139–150. 10.1016/j.neuropharm.2018.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan T, Robison CL, Cova N, Madore VM, Charntikov S, 2020. Assessment of individual differences in response to acute bupropion or varenicline treatment using a long-access nicotine self-administration model and behavioral economics in female rats. Behav. Brain Res. 385, 112558. 10.1016/j.bbr.2020.112558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killien M, Bigby JA, Champion V, Fernandez-Repollet E, Jackson RD, Kagawa-Singer M, Kidd K, Naughton MJ, Prout M, 2000. Involving minority and underrepresented women in clinical trials: The National Centers of Excellence in Women’s Health. J. Womens Health Gend. Based Med. 9, 1061–1070. 10.1089/152460900445974 [DOI] [PubMed] [Google Scholar]
- Kim MN, Jutkiewicz EM, Zhang M, Gnegy ME, 2011. The sensitizing effect of acute nicotine on amphetamine-stimulated behavior and dopamine efflux requires activation of β2 subunit-containing nicotinic acetylcholine receptors and glutamate N-methyl-D-aspartate receptors. Neuropharmacology 60, 1126–1134. 10.1016/j.neuropharm.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW, 2010. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J. Neurosci. 30, 7984–7992. 10.1523/JNEUROSCI.1244-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, 2017. Interactions between nicotine and drugs of abuse: a review of preclinical findings. Am. J. Drug Alcohol Abuse 43, 155–170. 10.1080/00952990.2016.1209513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, 2003. Comparing the abuse potential of methylphenidate versus other stimulants: A review of available evidence and relevance to the ADHD patient. J Clin Psychiatry 64, 14–18. [PubMed] [Google Scholar]
- Kollins SH, English JS, Itchon-Ramos N, Chrisman AK, Dew R, O’Brien B, McClernon FJ, 2014. A pilot study of lis-dexamfetamine dimesylate (LDX/SPD489) to facilitate smoking cessation in nicotine-dependent adults with ADHD. J. Atten. Disord. 18, 158–168. 10.1177/1087054712440320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristin EB, Bradley HS, 2009. Illicit methylphenidate use: A review of prevalence, availability, pharmacology, and consequences. Curr. Drug Abuse Rev. 2, 157–176. [DOI] [PubMed] [Google Scholar]
- Kuhn BN, Kalivas PW, Bobadilla A-C, 2019. Understanding addiction using animal models. Front. Behav. Neurosci. 13. 10.3389/fnbeh.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, 2012. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2, 661–677. 10.1002/brb3.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER, 2011. Molecular mechanism for a gateway drug: Epigenetic changes initiated by nicotine primed gene expression by cocaine. Sci. Transl. Med. 3, 107ra109. 10.1126/scitranslmed.3003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JC, Sutter ME, Albertson TE, Owen KP, Ford JB, 2014. An 11-year review of bupropion insufflation exposures in adults reported to the California Poison Control System. Clin. Toxicol. Phila. Pa 52, 969–972. 10.3109/15563650.2014.969372 [DOI] [PubMed] [Google Scholar]
- Li M, McMillan DE, 2003. Retention of sequential drug discriminations under fixed-interval schedules for long time periods without training. Eur. J. Pharmacol. 476, 79–85. 10.1016/S0014-2999(03)02150-2 [DOI] [PubMed] [Google Scholar]
- Lile JA, Babalonis S, Emurian C, Martin CA, Wermeling DP, Kelly TH, 2011. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. J. Clin. Pharmacol. 51, 888–898. 10.1177/0091270010375956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HQ, Burden PM, Christie MJ, Johnston GAR, 1999. The anxiogenic-like and anxiolytic-like effects of mdma on mice in the elevated plus-maze: A comparison with amphetamine. Pharmacol. Biochem. Behav. 62, 403–408. 10.1016/S0091-3057(98)00191-9 [DOI] [PubMed] [Google Scholar]
- Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P, 2019. Smoking reduction interventions for smoking cessation. Cochrane Database Syst. Rev. 9, CD013183. 10.1002/14651858.CD013183.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low RB, Jones M, Carter B, Cadoret RJ, 1984. The effect of d-amphetamine and ephedrine on smoking attitude and behavior. Addict. Behav. 9, 335–345. 10.1016/0306-4603(84)90032-7 [DOI] [PubMed] [Google Scholar]
- Luo SX, Covey LS, Hu M, Winhusen TM, Nunes EV, 2019. Differential posttreatment outcomes of methylphenidate for smoking cessation for individuals with ADHD. Am. J. Addict. 28, 497–502. 10.1111/ajad.12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzwinski LN, Eisenberg MJ, 2022. E-cigarette Polysubstance Vaping in Youth: Public Health Concerns, in: Patel VB, Preedy VR (Eds.), Handbook of Substance Misuse and Addictions. Springer International Publishing, Cham, pp. 1–21. 10.1007/978-3-030-67928-6_38-1 [DOI] [Google Scholar]
- Maher EE, Overby PF, Bull AH, Beckmann JS, Leyrer-Jackson JM, Koebele SV, Bimonte-Nelson HA, Gipson CD, 2021. Natural and synthetic estrogens specifically alter nicotine demand and cue-induced nicotine seeking in female rats. Neuropharmacology 108756. 10.1016/j.neuropharm.2021.108756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdi A, Kamari F, Sadigh-Eteghad S, Gjedde A, 2019. Molecular Insights Into Memory-Enhancing Metabolites of Nicotine in Brain: A Systematic Review. Front. Neurosci. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA, 2006. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl.) 184, 494–503. 10.1007/s00213-005-0135-z [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Freedland CS, 1998. The role of monoamine neurotransmitter systems in the nicotine discriminative stimulus. Drug Alcohol Depend. 52, 125–134. 10.1016/S0376-8716(98)00085-4 [DOI] [PubMed] [Google Scholar]
- McCabe SE, Dickinson K, West BT, Wilens TE, 2016. Age of onset, duration, and type of medication therapy for attention-deficit/hyperactivity disorder (ADHD) and substance use during adolescence: A multi-cohort national study. J. Am. Acad. Child Adolesc. Psychiatry 55, 479–486. 10.1016/j.jaac.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick G, Graziane NM, 2020. Drug-induced conditioned place preference and its practical use in substance use disorder research. Front. Behav. Neurosci. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, 2015. The rise (and fall?) of drug discrimination research. Drug Alcohol Depend. 151, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy KR, Houser SD, Barrett ST, Bevins RA, 2022. Investigating sex differences and the effect of drug exposure order in the sensory reward-enhancing effects of nicotine and d-amphetamine alone and in combination. Neuropharmacology 108845. 10.1016/j.neuropharm.2021.108845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy KR, Ramsay ME, Barrett ST, Bevins RA, 2021. Reward-enhancing effects of d-amphetamine and its interactions with nicotine were greater in female rats and persisted across schedules of reinforcement. Behav. Pharmacol. 32, 435–447. 10.1097/FBP.0000000000000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM, 2007. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 86, 140–149. 10.1016/j.pbb.2006.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Sofuoglu M, 2006. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev. Neurother. 6, 965–981. 10.1586/14737175.6.7.965 [DOI] [PubMed] [Google Scholar]
- Moran AE, Huynh YW, Finkner AP, Selleck C, Thompson A, Barrett ST, Bevins RA, 2022. Understanding the stimulus effects of nicotine and bupropion in a drug-drug discriminated goal-tracking task. Psychopharmacology (Berl.). 10.1007/s00213-022-06072-1 [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL, 2014. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol. Rev. 66, 869–917. 10.1124/pr.112.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolley EP, Kelley BM, 2007. Adolescent reward system perseveration due to nicotine: Studies with methylphenidate. Neurotoxicol. Teratol. 29, 47–56. 10.1016/j.ntt.2006.09.026 [DOI] [PubMed] [Google Scholar]
- Nunes EV, Covey LS, Brigham G, Hu M-C, Levin FR, Somoza EC, Winhusen TM, 2013. treating nicotine dependence by targeting attention-deficit/hyperactivity disorder (ADHD) with OROS methylphenidate: The role of baseline ADHD severity and treatment response. J. Clin. Psychiatry 74, 983–990. 10.4088/JCP.12m08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner R, Schindler SD, Kraigher D, Mendelsohn A, Fischer G, 2002. Women addicted to nicotine. Arch. Womens Ment. Health 4, 103–109. 10.1007/s007370200008 [DOI] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF, 2008. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 33, 2139–2147. 10.1038/sj.npp.1301623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA, 2005. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology 30, 731–741. 10.1038/sj.npp.1300629 [DOI] [PubMed] [Google Scholar]
- Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK, 2016. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther. Adv. Psychopharmacol. 6, 99–144. 10.1177/2045125316629071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson N, Balfour D, Markou A, 2008. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob. Res. 10, 995–1008. 10.1080/14622200802097571 [DOI] [PubMed] [Google Scholar]
- Paterson NE, 2009. Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: Recent preclinical and clinical insights. Eur. J. Pharmacol. 603, 1–11. 10.1016/j.ejphar.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A, 2007. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell: Chronic bupropion, nicotine withdrawal and dopamine. Eur. J. Neurosci. 25, 3099–3108. 10.1111/j.1460-9568.2007.05546.x [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Gur RC, Lerman C, 2013. Effects of bupropion on cognitive performance during initial tobacco abstinence. Drug Alcohol Depend. 133, 283–286. 10.1016/j.drugalcdep.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin S, Nanchanatt A, Mauterer MI, Rhoads DE, 2018. Co-administration of amphetamine with alcohol results in decreased alcohol withdrawal severity in adolescent rats. Behav. Pharmacol. 29, 547–550. 10.1097/FBP.0000000000000405 [DOI] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP, 2009. Drug discrimination and neurochemical studies in α7 null mutant mice: tests for the role of nicotinic α7 receptors in dopamine release. Psychopharmacology (Berl.) 203, 399–410. 10.1007/s00213-008-1281-x [DOI] [PubMed] [Google Scholar]
- Rahman S, 2011. Chapter 8 - Brain Nicotinic Receptors as Emerging Targets for Drug Addiction: Neurobiology to Translational Research, in: Rahman S (Ed.), Progress in Molecular Biology and Translational Science, The Brain as a Drug Target. Academic Press, pp. 349–365. 10.1016/B978-0-12-385506-0.00008-9 [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J, 2008. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology (Berl.) 201, 305–314. 10.1007/s00213-008-1282-9 [DOI] [PubMed] [Google Scholar]
- Rauhut AS, 2019. Voluntary exercise ameliorates anxiogenic effects of acute methamphetamine exposure in Swiss-Webster mice. Pharmacol. Rep. PR 71, 1020–1024. 10.1016/j.pharep.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT, 2003. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl.) 169, 1–9. 10.1007/s00213-003-1450-x [DOI] [PubMed] [Google Scholar]
- Reavill C, Stolerman IP, 1987. Interaction of nicotine with dopaminergic mechanisms assessed through drug discrimination and rotational behaviour in rats. J. Psychopharmacol. (Oxf.) 1, 264–273. 10.1177/026988118700100408 [DOI] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA, 2009. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr. Drug Abuse Rev. 2, 184–194. 10.2174/1874473710902020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA, 2007. Nicotine as a conditioned stimulus: Impact of attention-deficit/hyperactivity disorder medications. Exp. Clin. Psychopharmacol. 15, 501–509. 10.1037/1064-1297.15.5.501 [DOI] [PubMed] [Google Scholar]
- Ren M, Lotfipour S, 2019. Nicotine gateway effects on adolescent substance use. West. J. Emerg. Med. 20, 696–709. 10.5811/westjem.2019.7.41661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Zwar N, 2003. Review of bupropion for smoking cessation. Drug Alcohol Rev. 22, 203–220. 10.1080/09595230100100642 [DOI] [PubMed] [Google Scholar]
- Richtand NM, 2006. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology 31, 2368–2375. 10.1038/sj.npp.1301163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Anastasio D, Little JM, Sigmon JL, Pettice YJ, Norton HJ, 1995. Ritalin for nicotine withdrawal: Nesbitt’s Paradox revisited. Addict Behav 20, 481–490. 10.1016/0306-4603(95)00009-2 [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J, 1993. From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. Biochim. Biophys. Acta BBA - Bioenerg. 1144, 249–263. 10.1016/0005-2728(93)90109-S [DOI] [PubMed] [Google Scholar]
- Rungnirundorn T, Verachai V, Gelernter J, Malison RT, Kalayasiri R, 2017. Sex differences in methamphetamine use and dependence in a Thai treatment center. J. Addict. Med. 11, 19–27. 10.1097/ADM.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PEA, 2005. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl.) 181, 781–789. 10.1007/s00213-005-0021-8 [DOI] [PubMed] [Google Scholar]
- Sanabria F, Killeen PR, 2008. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav. Brain Funct. 4, 7. 10.1186/1744-9081-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GC, Marin MT, Cruz FC, DeLucia R, Planeta CS, 2009. Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict. Biol. 14, 270–275. 10.1111/j.1369-1600.2009.00153.x [DOI] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA, 1973. D-amphetamine as a discriminative cue: Drugs with similar stimulus properties. Eur. J. Pharmacol. 21, 212–216. 10.1016/0014-2999(73)90228-8 [DOI] [PubMed] [Google Scholar]
- Shanks RA, Ross JM, Doyle HH, Helton AK, Picou BN, Schulz J, Tavares C, Bryant S, Dawson BL, Lloyd SA, 2015. Adolescent exposure to cocaine, amphetamine, and methylphenidate cross-sensitizes adults to methamphetamine with drug- and sex-specific effects. Behav. Brain Res. 281, 116–124. 10.1016/j.bbr.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J, 2000. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl.) 148, 33–40. 10.1007/s002130050022 [DOI] [PubMed] [Google Scholar]
- Shillington AM, Reed MB, Lange JE, Clapp JD, Henry S, 2006. College undergraduate ritalin abusers in southwestern california: protective and risk factors. J. Drug Issues 36, 999–1014. 10.1177/002204260603600411 [DOI] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S, 2003. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology (Berl.) 165, 405–412. 10.1007/s00213-002-1277-x [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Redfern P, Wonnacott S, 2007. Comparison of the effects of bupropion on nicotinic receptor-evoked [(3)H]dopamine release from rat striatal synaptosomes and slices. Eur. J. Pharmacol. 567, 102–109. 10.1016/j.ejphar.2007.03.052 [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST, 2003. Acute effects of d-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl.) 167, 393–402. 10.1007/s00213-003-1416-z [DOI] [PubMed] [Google Scholar]
- Silveira ML, Conway KP, Green VR, Kasza KA, Sargent JD, Borek N, Stanton C A… Longitudinal associations between youth tobacco and substance use in waves 1 and 2 of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend. 191, 25–36. 10.1016/j.drugalcdep.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI, 2000. Bupropion Is a Nicotinic Antagonist. J. Pharmacol. Exp. Ther. 295, 321–327. [PubMed] [Google Scholar]
- Sofuoglu M, 2010. Cognitive enhancement as a pharmacotherapy target for stimulant addiction: Cognitive enhancement for stimulant addiction. Addiction 105, 38–48. 10.1111/j.1360-0443.2009.02791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Jensen MØ, Jogini V, Stein RA, Lee C-H, Mchaourab HS, Shaw DE, Gouaux E, 2018. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature 556, 515–519. 10.1038/s41586-018-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs D, Dworkin S, 2008. Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats. Pharmacol. Biochem. Behav. 90, 701–711. 10.1016/j.pbb.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Ewin SE, Kangiser MM, Pfaff MN, 2017. Effects of environmental enrichment on d-amphetamine self-administration following nicotine exposure. Exp. Clin. Psychopharmacol. 25, 393–401. 10.1037/pha0000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, 2002. Drug stimulus generalization and Gossop’s ‘Web of Dependence.’ Addiction 97, 152–154. 10.1046/j.1360-0443.2002.00009.x [DOI] [PubMed] [Google Scholar]
- Stolerman IP, 1989. Discriminative stimulus effects of nicotine in rats trained under different schedules of reinforcement. Psychopharmacology (Berl.) 97, 131–138. 10.1007/BF00443427 [DOI] [PubMed] [Google Scholar]
- Stoops WW, Poole MM, Vansickel AR, Hays KA, Glaser PEA, Rush CR, 2011. Methylphenidate increases choice of cigarettes over money. Nicotine Tob. Res. 13, 29–33. 10.1093/ntr/ntq198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [SAMHSA], 2023. 2021 NSDUH Annual National Report. [Google Scholar]
- Swalve N, Pittenger ST, Bevins RA, Li M, 2015. Behavioral effects of phencyclidine on nicotine self-administration and reinstatement in the presence or absence of a visual stimulus in rats. Psychopharmacology (Berl.) 232, 2877–2887. 10.1007/s00213-015-3923-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JC, Dawe S, Lee C, Capstick C, Corr PJ, Cotter P, Frangou S, Gray NS, Russell MAH, Gray JA, 1996. Effects of nicotine and amphetamine on latent inhibition in human subjects. Psychopharmacology (Berl.) 127, 164–173. 10.1007/BF02805990 [DOI] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST, 2000. d-Amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl.) 153, 85–92. 10.1007/s002130000600 [DOI] [PubMed] [Google Scholar]
- Tiwari RK, Sharma V, Pandey RK, Shukla SS, 2020. Nicotine addiction: Neurobiology and mechanism. J. Pharmacopuncture 23, 1–7. 10.3831/KPI.2020.23.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Surgeon General, 2014. 2014 Surgeon General’s Report: The Health Consequences of Smoking--50 Years of Progress. [Google Scholar]
- van Amsterdam J, van der Velde B, Schulte M, van den Brink W, 2018. Causal factors of increased smoking in ADHD: A systematic review. Subst. Use Misuse 53, 432–445. 10.1080/10826084.2017.1334066 [DOI] [PubMed] [Google Scholar]
- Vann RE, Rosecrans JA, James JR, Philibin SD, Robinson SE, 2006. Neurochemical and behavioral effects of bupropion and mecamylamine in the presence of nicotine. Brain Res. 1117, 18–24. 10.1016/j.brainres.2006.07.110 [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Poole MM, Stoops WW, Hays KE, Upchurch MB, Glaser PEA, Rush CR, 2009. Stimulant-induced changes in smoking and caloric intake: Influence of rate of onset. Pharmacol. Biochem. Behav. 92, 597–602. 10.1016/j.pbb.2009.02.012 [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PEA, Poole MM, Rush CR, 2011. Methylphenidate increases cigarette smoking in participants with ADHD. Psychopharmacology (Berl.) 218, 381–390. 10.1007/s00213-011-2328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PEA, Rush CR, 2007. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology (Berl.) 193, 305–313. 10.1007/s00213-007-0786-z [DOI] [PubMed] [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD, 1999. Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol. Biochem. Behav. 63, 27–32. 10.1016/S0091-3057(98)00262-7 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M, 2009. The impact of cigarette smoking on stimulant addiction. Am. J. Drug Alcohol Abuse 35, 12–17. 10.1080/00952990802326280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG, 2008. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl.) 197, 371–377. 10.1007/s00213-007-1041-3 [DOI] [PubMed] [Google Scholar]
- Weyandt LL, Bjorn S, 2018. Issues pertaining to misuse of ADHD prescription medications. [Google Scholar]
- Wheeler TL, Smith LN, Bachus SE, McDonald CG, Fryxell KJ, Smith RF, 2013. Low-dose adolescent nicotine and methylphenidate have additive effects on adult behavior and neurochemistry. Pharmacol. Biochem. Behav. 103, 723–734. 10.1016/j.pbb.2012.12.005 [DOI] [PubMed] [Google Scholar]
- White NM, 1989. Reward or reinforcement: What’s the difference? Neurosci. Biobehav. Rev., The Neural Basis of Reward and Reinforcement: A Conference in Honour of Peter M. Milner 13, 181–186. 10.1016/S0149-7634(89)80028-4 [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Martin BR, Damaj MI, 2002. Nicotine-like discriminative stimulus effects of bupropion in rats. Exp. Clin. Psychopharmacol. 10, 129–135. 10.1037/1064-1297.10.2.129 [DOI] [PubMed] [Google Scholar]
- Wilkes S, 2008. The use of bupropion SR in cigarette smoking cessation. Int. J. Chron. Obstruct. Pulmon. Dis. 3, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Carroll F, Bevins R, 2010. An investigation of bupropion substitution for the interoceptive stimulus effects of nicotine. J. Psychopharmacol. (Oxf.) 24, 817–828. 10.1177/0269881109102518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Palmatier M, Bevins R, 2006. Preexposure to nicotine alters the subsequent locomotor stimulant effects of bupropion in rats. Nicotine Tob. Res. 8, 141–146. 10.1080/14622200500484642 [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Li C, Bevins RA, 2009. Pavlovian drug discrimination with bupropion as a feature positive occasion setter: substitution by methamphetamine and nicotine, but not cocaine. Addict. Biol. 14, 165–173. 10.1111/j.1369-1600.2008.00141.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, Croghan IT, Adler LA, Weiss RD, Leimberger JD, Lewis DF, Dorer EM, 2010. Impact of attention-deficit/hyperactivity disorder (adhd) treatment on smoking cessation intervention in adhd smokers: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 71, 1680–1688. 10.4088/JCP.09m05089gry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Balleine BW, 2007. The influence of amphetamine on sensory and conditioned reinforcement: Evidence for the re-selection hypothesis of dopamine function. Front. Integr. Neurosci. 1. 10.3389/neuro.07.009.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, 2009. Nicotinic receptors differentially modulate the induction and expression of behavioral sensitization to methylphenidate in rats. Psychopharmacology (Berl.) 204, 551–562. 10.1007/s00213-009-1487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters Thomas E., Neugebauer NM, Rush CR, Bardo MT, 2008. Methylphenidate enhances the abuse-related behavioral effects of nicotine in rats: Intravenous self-administration, drug discrimination, and locomotor cross-sensitization. Neuropsychopharmacology 33, 1137–1148. 10.1038/sj.npp.1301477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, 2009. Drug Discrimination, in: Buccafusco JJ (Ed.), Methods of Behavior Analysis in Neuroscience, Frontiers in Neuroscience. CRC Press/Taylor & Francis, Boca Raton (FL). [PubMed] [Google Scholar]
- Young R, Glennon RA, 2002. Nicotine and bupropion share a similar discriminative stimulus effect. Eur. J. Pharmacol. 6. [DOI] [PubMed] [Google Scholar]
- Yu G, Li G-F, Markowitz JS, 2016. Atomoxetine: A review of its pharmacokinetics and pharmacogenomics relative to drug disposition. J. Child Adolesc. Psychopharmacol. 26, 314–326. 10.1089/cap.2015.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Hillmer AT, Matuskey D, Nabulsi N, Ropchan J, Mazure CM, Picciotto MR, Huang Y, McKee SA, Morris ED, Cosgrove KP, 2019. Sex differences in amphetamine-induced dopamine release in the dorsolateral prefrontal cortex of tobacco smokers. Neuropsychopharmacology 44, 2205–2211. 10.1038/s41386-019-0456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC, 2014. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 121, 799–817. 10.1007/s00702-014-1180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


