Abstract
Objective:
To describe the prevalence of infertility and infertility treatment-seeking among people enrolled in the Sickle Cell Disease Implementation Consortium (SCDIC) registry and identify sociodemographic and clinical correlates of infertility.
Design:
Cross-sectional.
Participants:
The study population included 2108 women and men (≥18 years of age) enrolled in the SCDIC registry who completed the fertility questionnaire.
Results:
All participants who completed the infertility-specific questions were included in the analysis (1,224 females; 884 males). Of these, 16.9% of males and 23.7% of females reported infertility, in contrast to rates in the general population (12% of males; 11% of females). Only 22.8% of this subgroup had sought a fertility consultation; of these, 41% received infertility testing, and 58% received advice, yet only a few received specific treatment: ovulation medication (19.1%), fallopian tubal surgery (4.8%), other female treatment (17.5%), varicocelectomy (8.1%), or other male treatment (10.8%). Increasing age, employment status, and interaction between gender and single marital status are associated with reported infertility. We did not observe differences between groups relative to SCD genotype, a broad category of self-reported hydroxyurea use any time during life, type of medical insurance, income, or education.
Conclusion:
To our knowledge, this is the first study to examine self-reported identification of and treatment for infertility among a large sample of people with sickle cell disease. These findings suggest that (a) infertility occurs at a higher rate but fertility care treatment-seeking is less frequent than in the general public; and (b) sociodemographic and clinical differences between individuals who report experiencing infertility and those who do not did not emerge in this study.
Keywords: male infertility, female infertility, sickle cell disease, infertility treatment
Introduction
Sickle cell disease (SCD) is an inherited hemoglobinopathy and one of the most common monogenetic disorders globally1. SCD is a multisystem disease characterized by sickle-shaped erythrocytes causing vaso-occlusive disease, vasculopathy, and systemic inflammation. Persons with SCD experience progressive multi-organ damage throughout their lifespan, starting in childhood2. Episodes of sickling are unpredictable and frequently occur, over time leading to hemolytic anemia, parenchymal injury, and eventually, chronic organ damage3. In the past, children with SCD died before reaching adulthood, but now, with the use of early interventions and close monitoring, they have a 98% overall survival rate to adulthood4, resulting in increasing numbers of adults with SCD participating in family-planning decisions.
Both women and men can experience challenges to family building, including infertility, defined as the inability to achieve a clinical pregnancy following 12 months of regular, unprotected sexual intercourse5. Diminished ovarian reserve (DOR), in which there is a low oocyte supply, is a well-known risk factor for infertility6. Among females with SCD, data have identified an increased risk for diminished ovarian reserve compared to age-matched controls7–9. Specifically, Garba et al found that after controlling for age and body mass index, ovarian reserve as measured by serum Anti-Mullerian Hormone, was lower among those with HbSS versus HbAA10. Treatment for SCD may also impact fertility among women, with data to support that younger women ages 10 to 24 years of age treated with hydroxyurea had higher rates of DOR11. Among males with SCD, evidence indicates that factors contributing to infertility include delayed puberty, low testosterone, and sperm abnormalities12 due to testicular dysfunction13. Up to 24% of males with SCD develop hypogonadism, characterized by low testosterone production, infertility, erectile dysfunction, and poor libido14. Sickled erythrocytes can cause infarction and associated end-organ damage, such as testicular dysfunction and abnormalities in the accessory sex organs, including the seminal vesicles and the prostate gland, leading to significant decreases in ejaculate volume15. Approximately 40% of males with SCD suffer from painful priapism caused by the obstruction of venous outflow from the penis. Available studies suggest an association between hydroxyurea, a common and standard treatment for those with SCD, and reductions in overall sperm counts and motility, and increase in abnormal sperm morphology16,17.
We are unaware of published reports regarding infertility prevalence and related treatment seeking among females and males with SCD. A fuller understanding of infertility and SCD in the United States may contribute to more patient-centered, robust fertility care for persons with SCD who seek genetic parenthood; therefore, to address this gap in the literature, we examined sociodemographic and treatment-seeking decisions related to infertility care among women and men living with SCD and currently enrolled in the Sickle Cell Disease Implementation Consortium (SCDIC) registry. The aims of this study were to (1) describe the prevalence of female and male factor infertility in persons with sickle cell disease in the SCDIC registry; 2) Describe infertility treatment seeking among females and males with infertility who are enrolled in the SCDIC registry; and 3) Determine if there are differences between the fertile and infertile group based on sociodemographic and clinical characteristics.
Materials and Methods
Design and Study Population
This study used a cross-sectional study design. The study population included all 2,440 women and men enrolled in the patient registry of the SCDIC, a consortium funded by the National Heart Lung and Blood Institute and comprised of eight comprehensive sickle cell disease centers and one data coordinating center in the United States. The consortium’s primary goal is the improvement of care for people with SCD18; its recruitment strategy and data collection details can be found in previously published reports18,19. Recruitment for this study began in October 2017 with a minimum enrollment goal of 300 participants into the registry at each site (2,400 total) and occurred in outpatient clinics (e.g., sickle cell and primary care clinics), hospital inpatient settings, SCD support group meetings, conferences, and other platforms at the discretion of the centers and as their local IRB approval permitted.
Ethical Approval
Each of the eight SCDIC study sites sought local ethical approval prior to recruitment and data collection. Written informed consent was obtained before enrollment in the study. IRB approval was also obtained for this analysis of existing medical record data.
Data Collection and Measures
Consortium data were collected using self-report surveys, medical records, and laboratory abstraction forms. Full details regarding the data collection process and full content of patient surveys can be found in published reports20. Both female and male participants completed all data collection forms, which contained some infertility questions specific to the participant’s biological sex. Of the 2,440 persons in the consortium, 2,374 completed pregnancy forms; of these, 2,315 responded to the infertility-specific questions (Figure 1). Women and men completed the infertility-specific surveys regardless of where they were in their family-building process. Infertility was defined as failure to conceive despite twelve or more months of regular unprotected intercourse, consistent with the World Health Organization and American Society of Reproductive Medicine definition of infertility5. Therefore, female participants were asked:
Has there ever been a time in your life when you didn’t become pregnant despite 12 or more months of regular unprotected intercourse?
Did you ever go to a doctor or other medical care provider to talk about ways to help you have a baby?
Which of the services did you have to help you have a baby? Check all the apply. (Advice; Infertility testing; Drugs to improve ovulation; Surgery to correct blocked tubes; Artificial insemination; Other types of medical help)
Has a doctor or other medical care provider ever told you that you had fibroid tumors or myomas in your uterus?
Has a doctor or other medical care provider ever told you that you had endometriosis?
FIGURE 1.
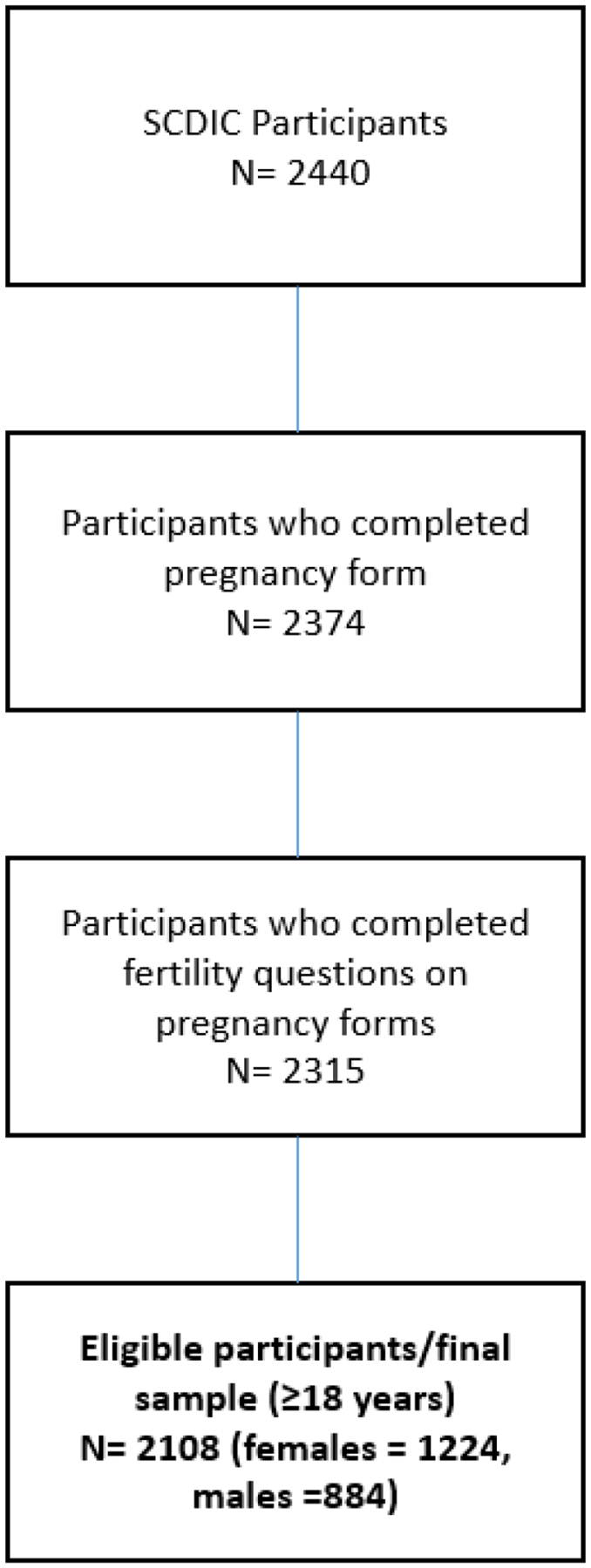
Eligibility for final sample
Men were asked the following:
Have you ever had a painful continuous erection, which is also called priapism?
Has there ever been a time in your life during which you weren’t able to get your partner pregnant despite 12 or more months of regular unprotected intercourse?
Did you ever go to a doctor or other medical care provider to talk about ways to help you father a baby?
Which of the following services did you have to help you father a baby? Check all that apply. (Advice; Infertility testing; Surgery to reverse a vasectomy; Treatment for varicocele; Other types of medical help)
When you went for medical help to father a baby, were you ever told that you had any of the following male infertility problems? Check all that apply. (Sperm or semen problems; Varicocele; Other; None of the above)
In addition to collecting self-report demographic information, participants were also asked if they had ‘ever’ taken hydroxyurea, and if they were ‘currently’ taking hydroxyurea. Site research completed staff medical records, laboratory abstraction forms included SCD genotype and history of blood transfusions. Data for the medical and laboratory forms were abstracted from participants’ electronic health records, entered into a REDCap database, assessed for completeness and accuracy by the data coordinating center, and returned to each site for correction.
Data analysis
Baseline characteristics and distributions of risk factors were analyzed as frequencies and percentages for categorical variables and median and interquartile ranges (IQR) or mean and SD for continuous variables. Categorical variables were analyzed using chi-square or Fisher’s exact test for sparse tables. Continuous variables were compared using t-test or Mann-Whitney U test, as appropriate. Two-sided p-values < .05 were considered statistically significant.
Modeling Strategy. The logistic regression model was developed in 2 phases. Initially, univariate analysis was used as a first screening step to analyze the association between infertility and sociodemographic and clinical variables hypothesized to be related to this outcome. These variables were selected based on subject matter expertise and prior scientific literature. Next, all variables considered to be biologically and socially influential factors were used in a multivariable regression model. These variables included age group (18–29, 30–34, 35+ years of age), gender, SCD genotype, history of hydroxyurea use (at any point in their lives), income, marital status, education, and employment status. Then, a backward elimination logistic regression with an exit criterion of p < .05 was used to identify statistically significant covariates and derive a more parsimonious final model. Interactions between selected predictors were included in the initial model, but the only significant interaction found was between gender and marital status. Model fitting also included a comparison of the full and reduced models to provide tests of improvement in fit assessed by the Schwarz Criterion and Akaike Information Criterion. Odds ratios (OR) and corresponding 95% confidence intervals were obtained for variables remaining in the final model. All analyses were conducted in SAS Version 9.421.
Results
The most up-to-date SCDIC Registry data were used to run these models, which included 2,440 SCDIC participants. We excluded participants <18 years of age to avoid confounding marital status, working status, and the highest level of educational attainment. This resulted in the total number of individuals (n = 2,108) included in this analysis (Figure 1).
Table 1 reports demographics, genotypes, and hydroxyurea use within the sample. Mean age was 29.2 years, and the majority were Black (95.6%) and never married (77.3%). Most participants (62.8%) reported Medicare/Medicaid/Military as their primary insurer. Most participants (73.4%) reported having the Hb SS genotype, and 73.8% reported taking or having taken hydroxyurea.
TABLE 1.
Sample Demographic and baseline characteristics by gender
| Characteristic | Females (n = 1,224) | Males (n= 884) | Total (n = 2108) |
|---|---|---|---|
| Age at enrollment | |||
| Mean (SD) years | 29.4 (7.1) | 28.9 | 29.2 (7.2) |
| Median (Q1–Q3) | 29 (24–35) | 28 (23–24) | 28 (23–24) |
| Range (min-max) | (18–45) | (18–45) | (18–45) |
| Age groups, n, % | |||
| 18–29 | 665 (54.3%) | 510 (57.7%) | 1175 (55.7%) |
| 30–34 | 243 (19.9%) | 163 (18.4%) | 406 (19.3%) |
| 35+ | 316 (25.8%) | 211 (23.9%) | 527 (25.0%) |
| Race, n (%) | |||
| American Indian/Alaska Native | 7 (0.6%) | 4 (0.5%) | 11 (0.5%) |
| Asian | 2 (0.2%) | 2 (0.2%) | 4 (0.2%) |
| Black | 1148 (96.2%) | 814 (94.8%) | 1962 (95.6%) |
| Multi-racial | 31 (2.6%) | 35 (4.1%) | 66 (3.2%) |
| White | 5 (0.4%) | 4 (0.5%) | 9 (0.4%) |
| Missing (no response) * | 31 (2.5%) | 25 (2.8%) | 56 (2.7%) |
| Ethnicity | |||
| Hispanic/Latino | 59 (5.0%) | 46 (5.3%) | 105 (5.1%) |
| Missing (no response) * | 33 (2.7%) | 13 (1.5%) | 46 (2.2%) |
| Marital status | |||
| 1. Never married | 905 (77.7%) | 637 (76.7%) | 1542 (77.3%) |
| 2. Married/Living as married | 184 (15.8%) | 137 (16.5%) | 321 (16.1%) |
| 3. Divorced/separated/widowed | 75 (6.4%) | 56 (6.7%) | 131 (6.6%) |
| Missing (no response) * | 60 (4.9%) | 54 (6.1%) | 114 (5.4%) |
| Education | |||
| Less than High School | 12 (1.0%) | 14 (1.6%) | 26 (1.2%) |
| Some high school | 93 (7.7%) | 89 (10.2%) | 182 (8.7%) |
| High school graduate or GED equivalent | 306 (25.3%) | 311 (35.6%) | 617 (29.6%) |
| Some college or vocational training | 440 (36.3%) | 295 (33.8%) | 735 (35.3%) |
| College graduate | 360 (29.7%) | 164 (18.8%) | 524 (25.1%) |
| Missing (no response) * | 13 (1.1%) | 11 (1.2%) | 24 (1.1%) |
| Employment | |||
| Working now | 466 (38.8%) | 345 (40.1%) | 811 (39.3%) |
| Not employed by choice | 227 (18.9%) | 138 (16.0%) | 365 (17.7%) |
| Not employed, other | 509 (42.3%) | 378 (43.9%) | 887 (43.0%) |
| Missing (no response) * | 22 (1.8%) | 23 (2.6%) | 45 (2.1%) |
| Income | |||
| $25,000 and under | 626 (56.5%) | 388 (50.8%) | 1014 (54.2%) |
| $25,001 – $50,000 | 240 (21.7%) | 180 (23.6%) | 420 (22.4%) |
| $50,001 – $75,000 | 105 (9.5%) | 100 (13.1%) | 205 (11.0%) |
| $75,001 – $100,000 | 54 (4.9%) | 47 (6.2%) | 101 (5.4%) |
| >$100,000 | 82 (7.4%) | 49 (6.4%) | 131 (7.0%) |
| Missing (no response) * | 117 (9.6%) | 120 (13.6%) | 237 (11.2%) |
| Medical Insurance | |||
| None | 143 (11.7%) | 90 (10.2%) | 233 (11.1%) |
| Medicare, Medicaid or military health plan | 773 (63.3%) | 548 (62.1%) | 1321 (62.8%) |
| Private | 305 (25.0%) | 245 (27.7%) | 550 (26.1%) |
| Missing (no response) * | 3 (0.2%) | 1 (0.1%) | 4 (0.2%) |
| SCD genotype | |||
| Hb SS or Hb B0 | 876 (72.3%) | 660 (74.9%) | 1536 (73.4%) |
| Hb SC disease | 256 (21.1%) | 178 (20.2%) | 434 (20.7%) |
| Hb B+ | 67 (5.5%) | 34 (3.9%) | 101 (4.8%) |
| Other | 13 (1.1%) | 9 (1.0%) | 22 (1.1%) |
| Missing * | 12 (1.0%) | 3 (0.3%) | 15 (0.7%) |
| Hydroxyurea use, ever | 874 (72.1%) | 670 (76.2%) | 1544 (73.8%) |
| Missing * | 11 (0.9%) | 5 (0.6%) | 16 (0.8%) |
| Hydroxyurea use, current | 526 (43.9%) | 465 (53.3%) | 991 (47.9%) |
| Missing * | 25 (2.0%) | 12 (1.4%) | 37 (1.8%) |
| History of blood transfusions | 976 (64.8%) | 251 (64.7%) | 1227 (64.7%) |
| Missing * | 162 (9.7%) | 51 (11.6%) | 213 (10.1%) |
Patients with missing data are not included in calculations of percentages unless otherwise specified
Number missing is included for informative purposes only, Percent of missing values computed as proportion of eligible population (n = 2,108)
Of our study participants, 20.9% (n = 441; 23.9% of females and 16.9% of males), reported infertility, the reasons for which are provided in Figure 2. Only 22.8% (n = 100/441) of the participants who reported infertility had sought an infertility consultation; of these, 21.7% (n = 63) were female and 25% (n = 37) were male. Of the 100 participants who had sought a consultation, most had received advice (58% [n = 58 total]; 61.9% [n = 39] female; 51.4% [n = 19] male); only 41% (n = 41) had received infertility testing (23.8% [n = 15] women; 70.3% [n = 26] males); and very few had received specific treatment: ovulation medication (19.1% [n = 12]), fallopian tubal surgery (4.8% [n = 3]), IUI (1.6% [n = 1], other female treatment (17.5% [n = 11]), surgery to repair a varicocele (8.1% [n = 3]), and other male treatment (10.8% [n = 4]).
FIGURE 2.
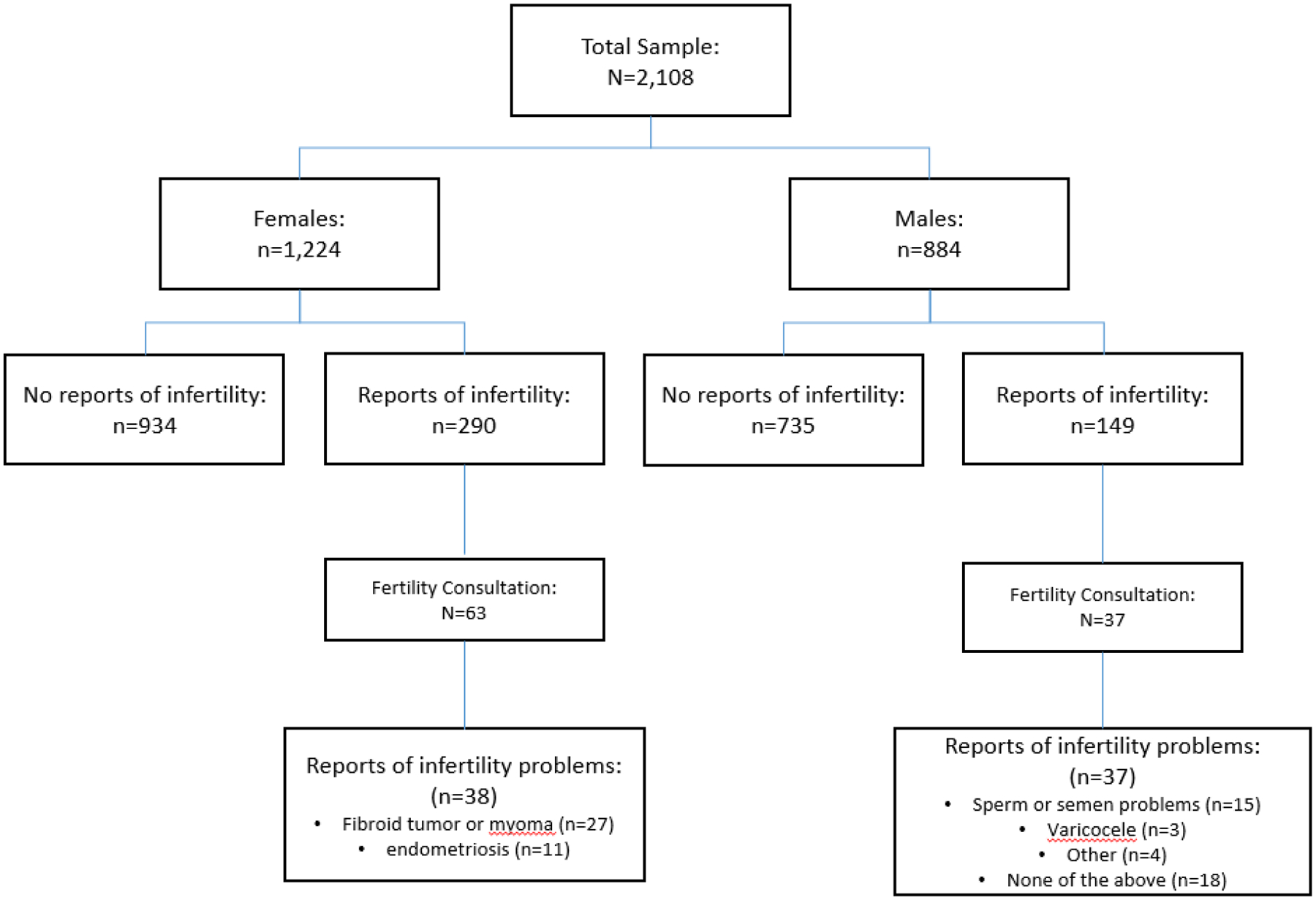
Prevalence & Diagnosis of Female & Male Factor Infertility
In a comparison of those with infertility (n = 441) and without infertility (n = 1,667) (See Table 2), the multivariable model identified increasing age (p < 0.0001), employment status (p = 0.007), and interaction between gender and marital status (p = 0.01) as significantly associated with infertility. Compared to individuals 18–29 years of age, individuals 30–34 years of age had more than twice the odds (OR = 2.15, 95% CI [1.62–2.87])) of infertility, and those 35+ years had a 1.66 (95% CI [1.25–2.2) increase in odds for infertility. Unemployed individuals had higher odds of infertility (OR 1.31, 95% CI [1.03–1.67]) compared to those employed, while there was no statistically significant difference in the odds of infertility between the not employed by choice and the employed groups. Marital status was associated with infertility among males with males who had never been married having lower odds of reporting infertility compared to their married counterparts (OR = 0.36, .024–0.85, while for women, there was no statistically significant difference in the odds of infertility for those who were never married or were separated/divorces/widowed compared to their married counterparts (OR = 0.84, 95% CI [0.59–1.22] and OR = 1.38, CI (0.77–2.46, respectively). We did not observe differences between group with hydroxyurea use nor sickle cell genotype. In the full model including these 2 variables, odds ratios of infertility were not significantly different for patients with any history of hydroxyurea during lifetime (OR (95% CI)= 1.3 (0.9–1.7)), or for Hb SC or other genotypes compared with Hb SS (OR (95% CI)=0.9 (0.7–1.3) and OR (95% CI)= 1.4 (0.8–2.2), respectively.
TABLE 2.
Final multivariable logistic regression model for infertility
| Results of final multivariable logistic regression model for infertility | ||
|---|---|---|
| Variables | Overall type III p-value | OR (95% CI) |
| Age | < 0.0001 | |
| 18–29 | Ref | |
| 30–34 | 2.15 (1.62–2.87) ** | |
| 35+ | 1.66 (1.25–2.2) ** | |
| Employment | 0.007 | |
| Working now | Ref | |
| Not employed by choice | 0.79 (0.54–1.12) | |
| Not employed, other | 1.31 (1.03–1.67) * | |
| gender*marital status § | 0.01 | |
| Male | ||
| Married/Living as married | Ref | |
| Never married | 0.36 (0.24–0.58) ** | |
| Divorced/separated/widowed | 0.68 (0.33–1.34) | |
| Female | ||
| Married/Living as married | Ref | |
| Never married | 0.84 (0.59–1.22) | |
| Divorced/separated/widowed | 1.38 (0.77–2.46) | |
| * Defined as a “yes” response to question “[has there] ever been a time in your life during which you didn’t become pregnant/weren’t able to get your partner pregnant despite 12 or more months of regular unprotected intercourse?” | ||
p ≤ .001,
p < 0.05
Marital status and gender were added in the regression also as main effects. ORs are not reported separately for these variables as they depend on the level of the interacting variable
Discussion
This study sought to increase understanding of infertility trends, treatment seeking, and possible sociodemographic and clinical characteristics in people with SCD and infertility challenges. The incidence of infertility reported by participants with SCD in our study (20.9% collectively, and by sex, 16.9% of males and 23.9% of females) was higher than national trends (about 12% of males and 11% of females)22. This is the largest cohort assessing infertility among men and women with SCD, and our findings about the incidence of infertility are consistent with other data, particularly concerning males with SCD12. Infertility in males with SCD has previously received more attention in the literature than infertility in females, which might be attributed to data suggesting that infertility among males with SCD has multiple causes, including hypogonadism, sperm abnormalities, and erectile dysfunction due to priapism23. However, there is recent attention on females with SCD and infertility. Evidence suggests that features specific to SCD, including chronic inflammation, oxidative stress, transfusion-related hemochromatosis, and ischemia injury to the ovaries increase the risk for inability to conceive24. Much of what is known about incidence of infertility has been derived from using the number of reported pregnancies during reproductive years as a surrogate for fertility. Studies comparing the pregnancy rates of people with and without SCD have surmised that women with SCD experience a lower number of pregnancies because fertility is reduced in women with SCD; however, this is an imprecise approach25. A recent report examining reproductive outcomes of a large cohort of patients with SCD found that about a quarter of their adult female sample reported a pregnancy but much fewer among men26. It is important to note that this study did not examine intent for pregnancy or infertility was not collected, however the low rate of pregnancies is consistent with our findings. Premature ovarian insufficiency in women with SCD has historically been estimated by observational studies of the total rate of pregnancy, which is lower than that of the general population, but these data are insufficient as a basis for causal conclusions. In a previously report utilizing the same dataset as ours, hydroxyurea was found to be associated with miscarriage26, however, it should be noted that repeat pregnancy loss (two or more miscarriages) denotes infertility, thus additional inquiry about pregnancy success over time for patients being treated with hydroxyurea is needed. Social influence on pregnancy seeking further complicates the use of pregnancy to infer fecundity. Research indicates that women with SCD are influenced by factors such as fear of pregnancy complications, increased pain during the pregnancy and birth, and overall lack of information from healthcare providers on reproductive planning27.
Although our results demonstrated an increased risk of infertility with increasing age to the early thirties, the risk was not linear with the oldest age group. It is possible that individuals with infertility were underrepresented in the oldest age group. This may in part have been due to SCD treatment advances not being available to older women during their earlier reproductive years28, hence not actively pursuing family building at the same rate as younger women. Also, this may be due to premature mortality in the population. A recent study by researchers from the Centers for Disease Control indicates that the median age at death of persons with SCD has increased from 28 years to 43 years29; however, a generally increased risk for infertility in persons with SCD remains30.
In the US, about half of women between 15 and 44 years of age seek advice for infertility concerns31. In our study, only 22.5% of participants with infertility had sought an infertility consultation for advice, and 41% of these had received an infertility evaluation, a percentage significantly lower than in the general population. The American Society for Reproductive Medicine advocates for systematic and cost-effective infertility assessment for females32 and accurate identification of male infertility and associated health conditions33. In most instances, private health insurance covers infertility evaluation, yet few states cover infertility diagnostic services (GA, HI, MA, MI, MN, NH, NM and NY) in at least one of their Medicaid plans (many of which limit coverage of infertility testing)34 35. Most likely, lack of coverage for infertility testing and treatment is a major barrier to patients with SCD; our findings have important policy implications for Medicaid plans nationally. It is important to note that only 26.5% of our participants had private insurance coverage; the majority (63.2%) reported Medicaid/Medicare as their insurance provider. Of the 100 participants in our study who reported infertility, very few had received medical help in conceiving. Most (58) had received advice, and ovulation medication was the treatment most often reported (n = 12). One participant reported having had intrauterine insemination, and none reported in-vitro fertilization (IVF). According to a recent Kaiser Family Foundation report, women from racial and ethnic minorities utilize infertility services less than non-Hispanic White women: only 13% of non-Hispanic White women and 7% of non-Hispanic Black women nationally seek medical help to get pregnant35. While we recognize that SCD can affect people of all races, the majority in the U.S. are black, as reflected in our study’s sample.
Given these challenges, comprehensively addressing inadequate access to care is essential. Although state efforts are slowly addressing access for the privately insured, legislative efforts on the federal level requiring private insurers to cover infertility services have made little forward progress. For example, the Access to Infertility Treatment and Care Act (HR 2803 and S 1461) would require the provision of infertility treatment by all health plans offered on group and individual markets (including Medicaid, EHBP, TRICARE, VA) but has never made it out of committee35. Additional efforts are needed to increase awareness about and access to fertility services for people with SCD. Most young adults with SCD have expressed a desire for future biological children, yet most receive little if any information about fertility from their healthcare provider36. In one study, less than half of the participants with SCD knew that SCD could have a negative impact on their future fertility36. Further, people with SCD and Medicaid may not realize that specific testing and treatments that impact fertility may exist for conditions related to their disease. However, their policy does not state this directly35.
We examined whether there were differences based on sociodemographic and clinical characteristics between the fertile and infertile participants in our study and found that neither self reported hydroxyurea used ever during their lifetime nor sickle cell genotype were associated with infertility incidence. It is important to note that some current SCD treatments can have a significant impact on fertility status37. Patients with SCD who receive chronic transfusions often develop iron overload/buildup in the hypothalamus, pituitary, and ovaries7. Disruption of the hypothalamic-pituitary-gonadal axis in both sexes can cause hormonal changes leading to gonadal failure. Hydroxyurea, which is frequently used during the reproductive years, inhibits DNA synthesis in actively dividing cells, including gametes. Recent evidence found hydroxyurea is associated with diminished ovarian reserve7,11,38. A recent report of a small cohort of 33 young females with SCD on hydroxyurea looked at those who “ever took” hydroxyurea, and “currently exposed” to therapy, and found that 24% of participants had diminished ovarian reserve, suggesting hydroxyurea may be an infertility risk factor, however needs additional study. In males, the use of hydroxyurea has been associated with higher numbers of abnormal sperm, lower sperm count, and decreased fertility39 with one study finding higher rates of oligospermia and azoospermia among men with SCD who are being treated with hydroxyurea13. Of note, there is some evidence of the protective nature of transfusions on sperm in the setting of pre-pubertal hydroxyurea use7,40. The meaning of our findings relative to hydroxyurea use is unclear given the variable used to measure hydrxyurea use was self reported use “ever during their lifetime”. Some studies have associated the SCD genotype, specifically HbSS, with adverse birth outcomes26,41; however, to our knowledge, there are no other studies that have examined genotype differences in those with infertility. Our data did not uncover an association between infertility and genotype, however further verification is needed.
Fertility planning for people with SCD is very recently gaining attention and comprehensive recommendations for those seeking to build their families are available42. All women with SCD would benefit from a consultation with a reproductive endocrinologist to discuss fertility preservation and surrogacy options23,24.
Our study has several strengths, including a robust sample size sampled via a consortium with eight geographically diverse settings in the United States. This is the first large sample including data prospectively collected directly from men and women with questions about infertility and treatment seeking patterns. Women and men enrolled in the consortium continue to be followed through the prospective registry, allowing future inquiries to extend our study aims.
Limitations
Our study also has several limitations. First, as all participants with SCD were recruited from SCD specialty settings, findings may not be representative of people without access to SCD specialists; however, we believe our findings may overestimate treatment seeking because care providers in specialized centers typically refer women to gynecologists/reproductive endocrinologists, which is likely not the case for patients with SCD seen in non-specialty centers. Second, our results are not generalizable to patients with low English language literacy or who are non-English speaking. Third, infertility was self-reported. Although the definition of infertility was provided at the time of data collection, self-identification was not verified by a medical provider or laboratory markers of fertility potential (e.g., AMH and FSH for ovarian reserve and sperm count). These findings should be interpreted with caution because the mean age of participants was approximately 28 years, and the self-report infertility status, and participants who were fertile may not have tried to conceive at the time of the survey. It is important to note that our participants were asked about the inability to become pregnant after one year of unprotected intercourse, which is the definition of infertility for otherwise healthy people under 35 years of age, however, the time reduces to 6 months for those 35 years of age and older5. This was not reflected in our study and thus may have affected the results, and it should also be noted that we currently do not know the age threshold for infertility for people with SCD given the global acceleration of age. Importantly, there are limitations to the data related to HU use. The data was self reported use ever during their lifetime. We were unable to assess whether participants were actually ever taking HU on a consistent basis during the time of pregnancy or pregnancy attempts. Laboratory data verifying hydroxyurea use, and a control group, would be required to assess causality. Finally, this study is limited by its use of a cross-sectional design. The timing of participants’ completion of the study surveys and the abstraction of their medical records may not fully reflect their experience of infertility during or later than their family-building phase.
Conclusion and Future Directions
To our knowledge, this is the first study to examine the prevalence of and treatment seeking for infertility among a large sample of women and men with SCD. These findings suggest that (a) infertility occurs at a higher rate in this population than in the general public, (b) infertility care treatment seeking in this population is less frequent than in the general public, and (c) only increased age and unemployment were associated with a higher likelihood of reporting infertility; no clinical differences were observed. It is important to continue to pursue knowledge about infertility in populations of people with SCD and advocate for fair and equitable access to related testing and treatment, regardless of location, financial status, or insurance provider.
Funding statement:
The SCD Implementation Consortium was supported by US Federal Government cooperative agreements 3U01HL133964-04S1, U24HL133948, U01HL133964, U01HL133990, U01HL133996, U01HL133994, U01HL133997, U01HL134004, U01HL134007, and U01HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD).
Abbreviations key:
- SCD
Sickle cell disease
- SCDIC
Sickle Cell Disease Implementation Consortium
- OR
Odds ratio
- IVF
In-vitro fertilization
Footnotes
Conflict of Interest Disclosure Statement:
Eleanor Stevenson: none
Paula Tanabe: Consultant: CSL Behring
Mitchell Knisely: none
Rita Masese: none
Dominique Bulgin: Consultant for RTI International, PhenX Toolkit
Liliana Preiss: none
Jane S. Hankins: none
Allison A. King: none
Victor Gordeuk: none
Nirmish Shah: Research: GBT; Consultation: GBT, Novartis, Emmaus Pharmaceuticals, Agios; Speaker: GBT, Novartis, Emaus Pharmaceuticals, Alexion.
Ethics Statement: Each of the eight SCDIC study sites sought local ethical approval prior to recruitment and data collection. Written informed consent was obtained before enrollment in the study. IRB approval was also obtained for this analysis of existing medical record data.
- The subjects in this trial have not concomitantly been involved in other randomized trials.
- Data regarding any of the subjects in the study has not been previously published.
- Data will be made available to the editors of the journal for review or query upon request.
Data Availability Statement:
We have ethical restrictions about openly releasing the data set to the public as the nature of the data set would result in loss of participant anonymity. The ethical restrictions were imposed by the Sickle Cell Disease Implementation Consortium (SCDIC). However, data set requests can be made to SCDIC and their data coordinating center at RTI international. Requests will be reviewed by the SCDIC Publications Committee. Data set requests can be sent to SCDIC at scdic-publications-subcommittee@rtiresearch.org or +1 301-230-4674.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet Lond Engl. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X [DOI] [PubMed] [Google Scholar]
- 2.Azar S, Wong TE. Sickle Cell Disease: A Brief Update. Med Clin North Am. 2017;101(2):375–393. doi: 10.1016/j.mcna.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet Lond Engl. 2017;390(10091):311–323. doi: 10.1016/S0140-6736(17)30193-9 [DOI] [PubMed] [Google Scholar]
- 4.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zegers-Hochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Tremellen K, Savulescu J. Ovarian reserve screening: a scientific and ethical analysis. Hum Reprod Oxf Engl. 2014;29(12):2606–2614. doi: 10.1093/humrep/deu265 [DOI] [PubMed] [Google Scholar]
- 7.Pecker LH, Hussain S, Mahesh J, Varadhan R, Christianson MS, Lanzkron S. Diminished ovarian reserve in young women with sickle cell anemia. Blood. 2022;139(7):1111–1115. doi: 10.1182/blood.2021012756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopeika J, Oyewo A, Punnialingam S, et al. Ovarian reserve in women with sickle cell disease. PloS One. 2019;14(2):e0213024. doi: 10.1371/journal.pone.0213024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y, Li-Ling J, Xiong D, Wei J, Zhong T, Tan H. Age-related decline in the expression of GDF9 and BMP15 genes in follicle fluid and granulosa cells derived from poor ovarian responders. J Ovarian Res. 2021;14(1):1. doi: 10.1186/s13048-020-00757-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garba SR, Makwe CC, Osunkalu VO, et al. Ovarian reserve in nigerian women with sickle cell anaemia: a cross- sectional study. J Ovarian Res. 2021;14(1):174. doi: 10.1186/s13048-021-00927-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elchuri SV, Williamson RS, Clark Brown R, et al. The effects of hydroxyurea and bone marrow transplant on Anti-Müllerian hormone (AMH) levels in females with sickle cell anemia. Blood Cells Mol Dis. 2015;55(1):56–61. doi: 10.1016/j.bcmd.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Huang AW, Muneyyirci-Delale O. Reproductive endocrine issues in men with sickle cell anemia. Andrology. 2017;5(4):679–690. doi: 10.1111/andr.12370 [DOI] [PubMed] [Google Scholar]
- 13.Sahoo LK, Kullu BK, Patel S, et al. Study of Seminal Fluid Parameters and Fertility of Male Sickle Cell Disease Patients and Potential Impact of Hydroxyurea Treatment. J Assoc Physicians India. 2017;65(6):22–25. [PubMed] [Google Scholar]
- 14.Taddesse A, Woldie IL, Khana P, et al. Hypogonadism in patients with sickle cell disease: central or peripheral? Acta Haematol. 2012;128(2):65–68. doi: 10.1159/000337344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agbaraji VO, Scott RB, Leto S, Kingslow LW. Fertility studies in sickle cell disease: semen analysis in adult male patients. Int J Fertil. 1988;33(5):347–352. [PubMed] [Google Scholar]
- 16.Berthaut I, Guignedoux G, Kirsch-Noir F, et al. Influence of sickle cell disease and treatment with hydroxyurea on sperm parameters and fertility of human males. Haematologica. 2008;93(7):988–993. doi: 10.3324/haematol.11515 [DOI] [PubMed] [Google Scholar]
- 17.Grigg A Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J. 2007;37(3):190–192. doi: 10.1111/j.1445-5994.2006.01290.x [DOI] [PubMed] [Google Scholar]
- 18.DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93(12):E391–E395. doi: 10.1002/ajh.25282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masese RV, DeMartino T, Bonnabeau E, et al. Effective Recruitment Strategies for a Sickle Cell Patient Registry Across Sites from the Sickle Cell Disease Implementation Consortium (SCDIC). J Immigr Minor Health. 2021;23(4):725–732. doi: 10.1007/s10903-020-01102-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glassberg JA, Linton EA, Burson K, et al. Publication of data collection forms from NHLBI funded sickle cell disease implementation consortium (SCDIC) registry. Orphanet J Rare Dis. 2020;15(1):178. doi: 10.1186/s13023-020-01457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute Inc. SAS® 9.4 Statements: Reference. SAS Institute; 2013. [Google Scholar]
- 22.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Rep. 2013;(67):1–18, 1 p following 19. [PubMed] [Google Scholar]
- 23.Smith-Whitley K Reproductive issues in sickle cell disease. Blood. 2014;124(24):3538–3543. doi: 10.1182/blood-2014-07-577619 [DOI] [PubMed] [Google Scholar]
- 24.Ghafuri DL, Stimpson SJ, Day ME, James A, DeBaun MR, Sharma D. Fertility challenges for women with sickle cell disease. Expert Rev Hematol. 2017;10(10):891–901. doi: 10.1080/17474086.2017.1367279 [DOI] [PubMed] [Google Scholar]
- 25.Eissa AA, Tuck SM, Rantell K, Stott D. Trends in family planning and counselling for women with sickle cell disease in the UK over two decades. J Fam Plann Reprod Health Care. 2015;41(2):96–101. doi: 10.1136/jfprhc-2013-100763 [DOI] [PubMed] [Google Scholar]
- 26.Kroner BL, Hankins JS, Pugh N, et al. Pregnancy outcomes with hydroxyurea use in women with sickle cell disease. Am J Hematol. 2022;97(5):603–612. doi: 10.1002/ajh.26495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedrosa EN, Corrêa MSM, Ferreira ALCG, Sousa CE da S, Silva RA da, Souza AI. Contraception and reproductive planning from the perspective of women with sickle cell disease. Rev Gaucha Enferm. 2021;42:e20200109. doi: 10.1590/1983-1447.2021.20200109 [DOI] [PubMed] [Google Scholar]
- 28.Egesa WI, Nakalema G, Waibi WM, et al. Sickle Cell Disease in Children and Adolescents: A Review of the Historical, Clinical, and Public Health Perspective of Sub-Saharan Africa and Beyond. Int J Pediatr. 2022;2022:3885979. doi: 10.1155/2022/3885979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzkron S, Carroll CP, Haywood C. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep Wash DC 1974. 2013;128(2):110–116. doi: 10.1177/003335491312800206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne AB, Mehal JM, Chapman C, et al. Trends in Sickle Cell Disease-Related Mortality in the United States, 1979 to 2017. Ann Emerg Med. 2020;76(3S):S28–S36. doi: 10.1016/j.annemergmed.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Key Statistics from the National Survey of Family Growth. Published online 2017. https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertilityservices
- 32.Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org, Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. 2021;116(5):1255–1265. doi: 10.1016/j.fertnstert.2021.08.038 [DOI] [PubMed] [Google Scholar]
- 33.Schlegel PN, Sigman M, Collura B, et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J Urol. 2021;205(1):36–43. doi: 10.1097/JU.0000000000001521 [DOI] [PubMed] [Google Scholar]
- 34.Pecker L, Oteng-Ntim E, Nero A, et al. Expecting more: the case for incorporating fertility services into comprehensive sickle cell disease care. Lancet Haematol. 2023;S2352–3026(22)00353–2. doi: 10.1016/S2352-3026(22)00353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser Family Foundation. Coverage and Use of Fertility Services in the U.S Published online 2020. https://www.kff.org/womens-health-policy/issue-brief/coverage-and-use-of-fertility-services-in-the-u-s/
- 36.Nahata L, Caltabellotta NM, Ball K, O’Brien SH, Creary SE. Desire for parenthood and reproductive health knowledge in adolescents and young adults with sickle cell disease and their caregivers. Pediatr Blood Cancer. 2018;65(2). doi: 10.1002/pbc.26829 [DOI] [PubMed] [Google Scholar]
- 37.Mishkin AD, Mapara MY, Barhaghi M, Reshef R. Fertility Concerns and Access to Care for Stem Cell Transplantation Candidates with Sickle Cell Disease. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2020;26(8):e192–e197. doi: 10.1016/j.bbmt.2020.03.025 [DOI] [PubMed] [Google Scholar]
- 38.Pecker LH, Hussain S, Christianson MS, Lanzkron S. Hydroxycarbamide exposure and ovarian reserve in women with sickle cell disease in the Multicenter Study of Hydroxycarbamide. Br J Haematol. 2020;191(5):880–887. doi: 10.1111/bjh.16976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBaun MR. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: a review. Expert Rev Hematol. 2014;7(6):767–773. doi: 10.1586/17474086.2014.959922 [DOI] [PubMed] [Google Scholar]
- 40.Joseph L, Jean C, Manceau S, et al. Effect of hydroxyurea exposure before puberty on sperm parameters in males with sickle cell disease. Blood. 2021;137(6):826–829. doi: 10.1182/blood.2020006270 [DOI] [PubMed] [Google Scholar]
- 41.Oakley LL, Mitchell S, von Rege I, et al. Perinatal outcomes in women with sickle cell disease: a matched cohort study from London, UK. Br J Haematol. 2022;196(4):1069–1075. doi: 10.1111/bjh.17983 [DOI] [PubMed] [Google Scholar]
- 42.Pecker LH, Kuo KHM. Go the Distance: Reproductive Health Care for People with Sickle Cell Disease. Hematol Oncol Clin North Am. 2022;36(6):1255–1270. doi: 10.1016/j.hoc.2022.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have ethical restrictions about openly releasing the data set to the public as the nature of the data set would result in loss of participant anonymity. The ethical restrictions were imposed by the Sickle Cell Disease Implementation Consortium (SCDIC). However, data set requests can be made to SCDIC and their data coordinating center at RTI international. Requests will be reviewed by the SCDIC Publications Committee. Data set requests can be sent to SCDIC at scdic-publications-subcommittee@rtiresearch.org or +1 301-230-4674.


