Abstract
Innate immunity and the actions of type I and III interferons (IFNs) are essential for protection from SARS-CoV-2 and COVID-19. Each are induced in response to infection and serve to restrict viral replication and spread while directing the polarization and modulation of the adaptive immune response. Owing to the distribution of their specific receptors, type I and III IFN respectively impart systemic and local actions. Therapeutic IFN has been administered to combat COVID-19 but with differential outcomes when given early or late in infection. In this perspective, we sort out the role of innate immunity and complex actions of IFNs in the context of SARS-CoV-2 infection and COVID-19. We conclude that IFNs are a beneficial component of innate immunity that has mediated natural clearance of infection in over 700 million people. Therapeutic induction of innate immunity and use of IFN should be featured in strategies to treat acute SARS-CoV-2 infection in people at risk for severe COVID-19.
Introduction
Nearly 700 million people worldwide have been infected with the severe acute respiratory coronavirus type 2 (SARS-CoV-2), the cause of coronavirus infectious disease 2019 (COVID-19) (https://covid19.who.int/). 6.8 million people are known to have died from infection. Vaccination for SARS-CoV-2 clearly protects against COVID-19 severity, wherein humoral immunity is the major biomarker and mediator of disease protection1. Importantly, prior to vaccination and in nonvaccinated persons today, the great majority of people infected with SARS-CoV-2 recovered following asymptomatic infection or having only acute mild to moderate disease, with recovery occurring prior to the onset or peak of humoral immunity2,3. The kinetics of infection with recovery in these COVID-19 cases, and that many cases are asymptomatic, underscore a major role for innate immunity in early control of SARS-CoV-2 infection.
Innate immune control of SARS-CoV-2.
Innate immunity provides our first line of defense against virus infection and is triggered in the infected cell through the actions of pathogen recognition receptors (PRRs) that recognize and bind specific pathogen-associated molecular patterns (PAMPs) within viral products to signal downstream innate immune activation4. In lung epithelial cells, the melanoma differentiation antigen 5 (MDA5) protein, a member of the RIG-I-like receptor (RLR) family of PRRs, recognizes SARS-CoV-2 likely through binding of viral RNA PAMPs, and signals through the RLR pathway for induction of innate immunity5–7. Additional innate immune programs, possibly directed by toll-like receptors (TLRs) or other PRRs, may also contribute to innate immune activation. Innate immune activation occurs rapidly following infection in a host cell. This process first directs the rapid expression of virus-stimulated genes (VSGs) triggered by interferon-regulatory factor (IRF)3, NF-κB, and other transcription factors activated by PRR signaling8. SARS-CoV-2 infected patients with rare inborn errors leading to TLR3 and TLR7 deficiencies have resulted in severe COVID-19 outcomes9–11. Further polymorphisms in transcription factors including IRF7 and IRF9 result in severe COVID-1911. These observations, underscore the role of PRRs and innate immune signaling and response in early control of SARS-CoV-2. Among the VSGs are types I and III interferons (IFN) whose production and secretion lead to IFN signaling in the infected cell and neighboring cells to induce a major second wave of gene expression by directing the induction of hundreds of interferon-stimulated genes (ISGs) and a tissue-wide and even systemic antiviral state (Figure 1). While VSG products provide a initial wave of gene expression for first-line defense to limit local virus replication and spread, while ISGs expand and diversify this response to systemically mediate antiviral defenses12. VSG and ISG products also include specific chemokines and cytokines that serve to program and polarize the adaptive immune response for effective immunity and viral clearance. Cytokine and chemokine products such as interleukin (IL)-6, tumor necrosis factor (TNF) α, and CXCL10 are also implicated in the pathogenic cytokine storm underlying severe COVID-19 outcomes13,14, so the innate immune response must be fine-tuned to direct recovery versus disease15,16. Moreover, viral proteins that accumulate in the infected cell during the course of SARS-CoV-2 replication can attenuate innate immunity [described below and reviewed elsewhere17], thus marking the rapid onset and actions of innate immunity as key features in controlling infection and protecting against development and severity of COVID-19.
Figure 1.

SARS-CoV-2 induction of innate immune activation and IFNs. SAR-CoV-2 infection and cell entry results in viral RNA deposition in the cell cytosol to initiate viral protein synthesis and viral RNA replication. Left: MDA5 first senses viral products, likely viral RNA components, and undergoes signaling activation involving interaction with the MAVS adaptor protein. SARS-CoV-2 might also be sensed through other pathogen recognition receptors including specific Toll-like receptors. Signaling mediates downstream activation and nuclear accumulation of IRF3, NF-KB, and other transcription factors that direct the expression of virus-stimulated genes (VSGs), many with antiviral and immune-modulatory activity. Right: Type I and III IFNs are cytokine VSG products and are secreted from the infected cell and signal via distinct receptors engaging the Jak-STAT pathway leading to interferon stimulated gene (ISG) expression. Hundreds of ISGs are induced in lung epithelial cells. ISG actions amplify and diversify the innate immune response, with the responses to type I and type III IFNs being temporally distinct and respectively partitioned by systemic versus compartmentalized (type III IFN) receptor expression distribution. Specific ISGs mediate antiviral actions against SARS-CoV-2. IFN therapy strategies leverage these actions and the immunomodulatory activities of ISGs for the treatment of infection and COVID-19.
Contrasting roles of type I and III IFNs in SARS-CoV-2 clearance.
While type I and III IFNs are induced through the same PRRs, their transcriptional response kinetics are distinct and should be expected to impart temporally distinct antiviral actions against SARS-CoV-2. Type I IFNs induce ISG expression early following receptor signaling, while type III IFN-induced ISGs are comparatively delayed, but their expression is sustained for a longer period18,19. While both IFNs activate the Jak-STAT signaling cascade to induce ISGs (see Figure 1), type I IFNs alone can also activate STAT1 homodimers to induce inflammatory signals, thus driving a different composition of ISGs18. Further, type III IFN receptor expression is restricted to epithelial layers at the mucosal surfaces, compared to the ubiquitous expression of type I IFN receptor. These differences impart a division of labor, with type I IFNs playing a dominant role in inducing broad tissue and systemic responses and type III IFNs activating innate antiviral responses at the mucosal tissues.
Primary infection of nasal and lung epithelium marks SARS-CoV-2 as a mucosal virus20. As type III IFN responses are mainly confined to surfaces of the mucosal epithelium, this IFN is critical to control infection by mucosal viruses including norovirus21–23, respiratory syncytial virus, influenza A virus (IAV)24 and SARS-CoV-225. The SARS-CoV-2/COVID-19 pandemic has drawn new attention to the ongoing debate on IFNs as therapeutic agents, particularly the role of type III IFN in the lung. Severe COVID-19 patients have high levels of circulating IFNs, thereby associating IFNs with a worse prognosis of the disease26 (Figure 2). To address the mechanisms of bad outcomes due to high, chronic levels of IFNs in vivo, studies have evaluated mucosal integrity in mouse models in the context of high dose IAV or treatment with poly I:C (a double-stranded RNA PAMP commonly used to induce IFN expression) to model SARS-CoV-2 infection27–29. The induced and chronic IFNs delayed epithelial repair of damage caused by virus infection or PAMP-induced inflammatory response in the mucosa. By contrast, pre-infection or early treatment of mice with type III IFN was protective against disease following virus challenge. Thus, location, timing, and duration of IFN exposure determines the protective or detrimental effects of IFN actions. More recent studies have clarified the role of type III IFN using mouse-adapted strains of SARS-CoV-2 in the mouse model of infection. Here, type III IFN protects mice challenged with SARS-CoV-2 alpha and omicron variants, protecting against virus-induced inflammation and tissue damage in both the upper and lower airways25. Early type III IFN intranasal administration to mice can also provide protection from morbidity and mortality otherwise caused by SARS-CoV-2, indicating that the timing of IFN production and signaling are critical for protection against disease25. These outcomes align with previous studies from us and others showing that type III IFN induces tissue-specific immunity without causing damaging inflammation, as type III IFN does not recruit immune cells to the site of infection18,30,31.
Figure 2.
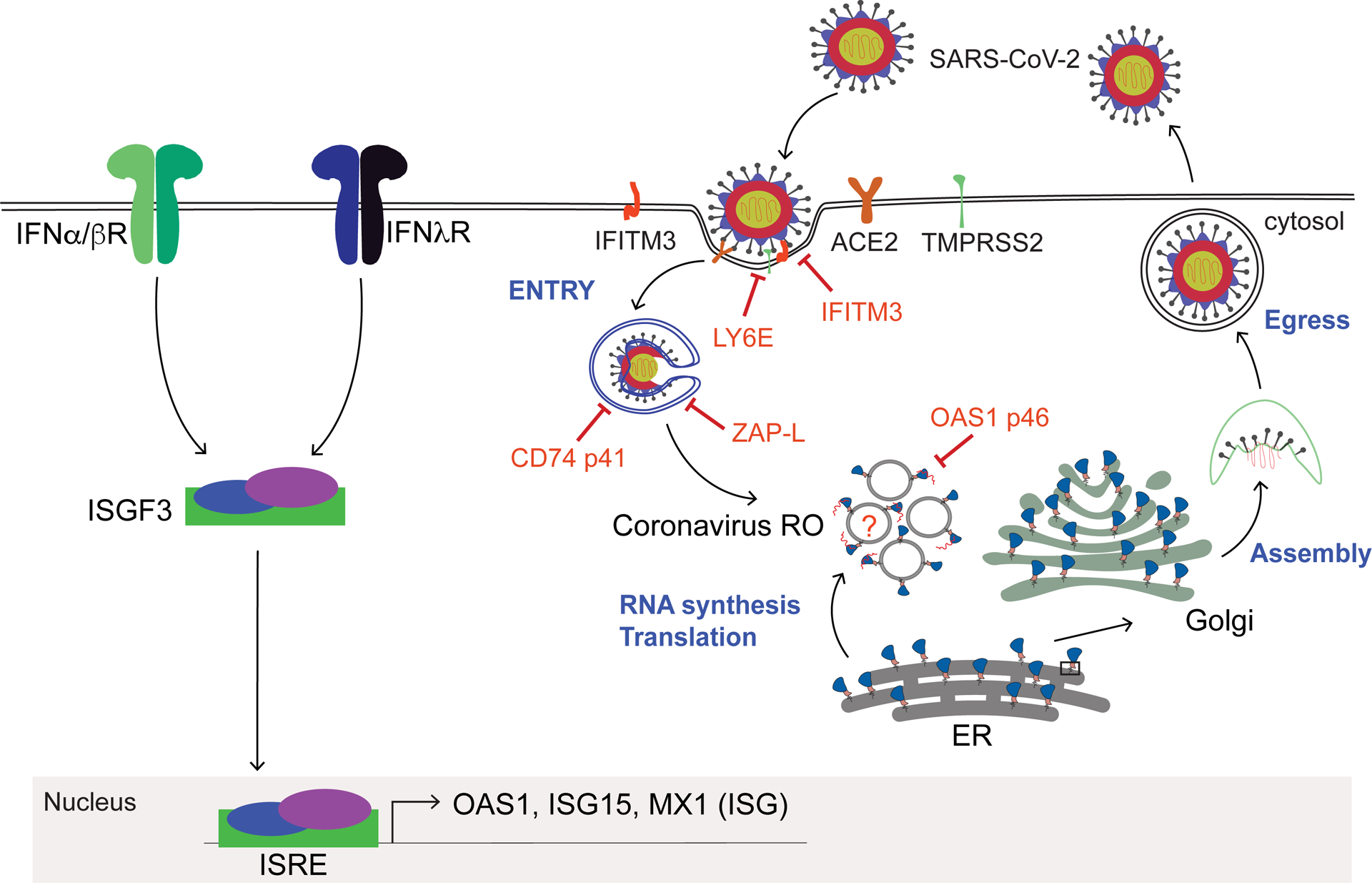
IFN actions against SARS-CoV-2 mediated by select ISGs. Cell infection progression of SARS-CoV-2 is depicted. In the context of IFN production or treatment type I and III IFNs signal through the Jak-STAT pathway and induce the assembly, nuclear accumulation, and activity of IFN-stimulated gene factor 3 (ISGF3) transcription factor that binds to the interferon-stimulated response element (ISRE) promoter region of ISGs, inducing their expression. Hundreds of ISGs, including OAS1, ISG15, MX1, are induced by IFNs. Major ISGs that suppress/restrict SARS-CoV-2 replication at specific steps in the viral life cycle are shown.
There are several contrasting studies regarding the role of type I IFNs in the outcome of experimental SARS-CoV-2 infection. Considering the analyses of SARS-CoV-2 mouse models of infection utilizing the transgenic expression of human angiotensin-converting enzyme (ACE)2 in mice, some studies show that the response to IFN links with control of infection in this model25,32, while others document enhanced lung inflammation caused by type I IFN linked with lack of virus clearance33. These contrasting outcomes can be due to differences in virus challenge dose and route of challenge, and should consider the impact of tissue distribution of transgenic human ACE2 expression, which is aberrantly expressed in a range of tissues in this model34. However, we note that previous studies of the MERS-CoV have shown that IFNs can mediate protective actions in vivo35. These observations warrant an in-depth biological characterization of IFN actions against SARS-CoV-2, taking routes of infection, dose, cell-type, and location-specific depletion of IFN receptors into consideration. While more studies are needed to characterize the role of type I IFNs in the context of SARS-CoV-2, it is clear from other virus infections that they are critical to early virus control and initiating/modulating adaptive immune responses36. We propose that type I and III IFNs play a collaborative but non-redundant role in protecting against SARS-CoV-2 and COVID-19.
Specific ISGs restricting SARS-CoV-2.
IFNs mediate their antiviral actions through the function of ISGs, including genes that are differentially expressed by types I and III IFNs37. Most ISGs described in the literature are those whose expression is induced in response to IFN but it should be noted that there are many ISGs whose expression is reduced or downregulated” from pre-IFN baseline levels12,38. While induced ISGs can impact viral replication directly, the reduced expression ISGs include genes encoding factors that regulate metabolic and cell growth control to confer restriction of virus infection38 but the spectrum of the ISGs impacting SARS-CoV-2 is not defined. The antiviral response linked with ISGs is designed to affect every step of the virus life cycle, with specific ISGs thus far shown to restrict SARS-CoV-2 entry or viral RNA replication but restriction factors affecting SARS-CoV-2 egress have not been identified (Figure 2). Some specific ISGs restricting SARS-CoV-2 are featured below.
SARS-CoV-2 can enter the cell through two main mechanisms, ACE2 mediated endosomal entry or TMPRSS2-mediated cell-surface entry via membrane fusion. The major mode of SARS-CoV-2 entry is through TMPRSS2-mediated activation/cleavage of Spike to an S2 cleavage product facilitating binding to Ace239. Among ISG products, LY6E was found to restrict S-protein mediated membrane entry40,41. The effect of LY6E diverges beyond SARS-CoV-2, as mice lacking LY6E were protected against another coronavirus – the mouse hepatitis virus (MHV). This observation is intriguing, given LY6E has proviral functions in the context of flaviviruses, IAV, and HIV42–44. Dissecting the underlying molecular mechanisms of how LY6E mediates its activity to restrict coronaviruses will shed light on these seemingly opposing virus-specific functions.
A transposon screen to identify restriction factors of the ebola virus identified a specific isoform of CD74 that importantly also inhibits the cleavage of the SARS-CoV-2 S protein, disrupting its processing in the endosome45. The p41 isoform of CD74 inhibited viral entry by blocking cathepsin-mediated processing of the ebola virus glycoprotein. Remarkably, CD74 p41 can also block the endosomal entry pathway of coronaviruses, including SARS-CoV-2, through actions induced by CIITA, an ISG and transcription co-factor of MHC expression and upstream regulator of CD7445. Thus, CIITA and CD74 impart an IFN-induced pathway of ISG actions against SARS-CoV-2 entry. Moreover, multiple studies have implicated IFN-induced transmembrane protein 3 (IFITM3) in SARS-CoV-2 entry restriction46,47. IFITM3, a well-studied restriction factor against IAV, HIV, and West Nile virus, inhibits the fusion of viral and cellular membranes to preclude virus entry into target cells48. In the case of SARS-CoV-2, the IFITM3 protein binds to the virus , blocking viral entry. A single-nucleotide polymorphism of IFITM3 is linked to increased susceptibility to SARS-CoV-249, with the potential of utilizing this SNP to identify individuals who are more likely to be susceptible to the virus.
OAS1 is one of the major SARS-CoV-2 restriction factors that could partially explain the heterogeneity in innate mediated control of SARS-CoV-2 infection in susceptible people. A 46 kDa splice-isoform of oligoadenylate synthetases (OAS)1, called as OAS1p46 or prenylated OAS1, displayed robust antiviral activity against SARS-CoV-2. OAS proteins are IFN-inducible innate immune proteins that sense and bind to viral RNA and activate a ribonuclease RNaseL to control virus replication50–52, 53. The antiviral activity of OAS1p46 against SARS-CoV-2 is dependent on prenylation of OAS1p46. Prenylated OAS1 traffics to the Golgi including possibly the SARS-CoV-2 double membrane replication organelle (Figure 2)54,55. Interestingly, the human OAS1 gene produces two major isoforms, OAS1p42 or OAS1p46 (prenylated isoform), and their expression is controlled by a single nucleotide polymorphism in the sixth exon splice site acceptor (A/G; rs10774671) of the OAS1 gene. The G allele generates the OAS1p46 isoform, and the A allele generates OAS1p42. Both OAS1p42 and OAS1p46 are expressed if an individual carries A & G alleles. Individuals who carry homozygous alleles naturally lack either OAS1p42 (G/G) or OAS1p46 (A/A) isoforms, while heterozygotes express both isoforms. In the context of COVID-19, A/A homozygous patients who are OAS1p46-deficient progress to severe COVID-19, requiring mechanical ventilation or leading to death55. Recent studies have replicated these virological and genetic findings, implicating prenylated OAS1 as a central restriction factor of SARS-CoV-2 whose loss of function links with progression to severe COVID-1956,57. While genetic variation in OAS1 has emerged as a significant disease predictor in multiple COVID-19 patient cohorts, it is still puzzling that the odds ratio linked to this SNP is not higher, which suggests that there are other variables affecting the function of prenylated OAS1 against SARS-CoV-2. As early innate immune-mediated control of SARS-CoV-2 is critical in preventing the infected individual from progressing to severe COVID-19, we presume that prenylated OAS1 expression is directly affected by the level of IFNs induced and hence the actions of other ISGs during actute SARS-CoV-2 infection. We propose that the SNPs in genes operating in the IFN signaling pathway58 or IFN autoantibodies59–61 (see below) could differentially influence the protective effects of prenylated OAS1. These observations underscore the significance of prenylated OAS1 in restricting SARS-CoV-2.
Zinc finger antiviral protein (ZAP) is another ISG that restricts SARS-CoV-2. The Zap gene produces multiple splice isoforms, with predominant expression of long (ZAP-L) and short (ZAP-S) isoforms. ZAP-L is constitutively expressed, while ZAP-S is an ISG. SARS-CoV-2 studies have implicated both isoforms as playing a role in virus restriction, with each mediating distinct mechanisms of action against the virus54. Additionally, another study shows that ZAP-L mediates virus restriction through RNAse activity, requiring KHYHN and TRIM25 as accessory factors62, with ZAP potently restricting SARS-CoV-2. This work also shows that ZAP-L enforces constitutive antiviral activity, with ZAP-S directing IFN-induced antiviral actions. Another study implicates ZAP-S in restricting SARS-CoV-2 by interfering with ribosomal frameshifting, thereby inhibiting viral replication63. Thus, ZAP isoforms mediate basal and IFN-induced antiviral actions against SARS-CoV-2 (Figure 2).
While the afore described ISGs are shown to have defined functions to mediate IFN antiviral actions against SARS-CoV-2, the spectrum of antiviral ISGs that restrict SARS-CoV2 is yet to be identified. CRISPR and mass-spectrometry screens have revealed several host factors that directly or indirectly interact with SARS-CoV-2 products or whose expression levels impact viral production64–73. Defined host factors from these studies are shown to impact SARS-CoV-2 processes of viral entry, replication, and virion morphogenesis, and to regulate organelle modifications during infection, thus impacting the cellular homeostasis. We note that several of these factors could have also pro-viral functions, direct or indirect linkage with ISGs and IFN actions, and could represent novel antiviral programs of innate immunity. Important next steps from these multiple studies are to validate the “hits” from each screen to identify the gene, protein, and signaling networks that confer mechanism of action to regulate SARS-CoV-2 infection outcome.
Perturbations of interferon signaling and implications to SARS-CoV-2 control.
Type I IFNs signal through IFNα/βreceptors (IFNAR), which are expressed on nucleated cells. Humans express 14 subtypes of type I IFNs with varying affinity to the IFN receptors36. Only IFNα and IFNβ are produced in the lung during SARS-CoV-2 infection. Although type I IFNs induce an early but transient innate antiviral program, they are also critical for promoting the adaptive immune response. For example, IFNs activate antigen-presenting cells and cooperate with other cytokines to polarize T cells, mediate induction of the immuno-proteasome, and enhanced expression of major histocompatibility (MHC) molecules on the surface of cells to facilitate antigen presentation and T-cell mediated killing of infected cells, respectively74 . In virus infection, the absence of IFN is catastrophic for the host cell and organism36. As such, IFNAR-deficient mice exhibit increased susceptibility and tissue pathology compared to wild-type mice when infected with mouse-adapted SARS-CoV-275. Moreover, recent studies uncovered autoantibodies against type I IFNs in people, with an implication that the autoantibodies will block IFNs from binding the IFN receptor and activate downstream signaling76,77 (see Figure 1). These important studies show that 10% of severe COVID-19 patients carry IFN autoantibodies that attenuate IFN signaling and ISG expression78. Notably, the prevalence of IFN autoantibodies is higher in older individuals, and SARS-CoV-2 patients harboring such autoantibodies progress to severe COVID-19 or mortality77. These findings reveal an important role for IFN actions in recovery from SARS-CoV-2 infection and mitigation of COVID-19, and are broadly relevant to other virus infections. Importantly, the presence of type III IFN autoantibodies in patients is not as prevalent as for type I IFN autoantibodies. If this differential observation holds up in multiple cohorts, this finding could have significant biological implications, including the use of recombinant type III IFNs as therapy against SARS-CoV-2 infections. Human genetic variations also have a significant biological effect on IFN signaling and COVID-19 severity. Recent genome-wide association studies revealed polymorphisms in IFNAR2 and TYK2 genes that affect COVID-19 severity58. While further studies are needed to decipher the mechanisms of action underlying disease susceptibility in this genetic context, human variations in the IFN signaling pathway explain some of the heterogeneity in the outcome of acute SARS-CoV-2 infection and clearly implicate IFN as crucial for innate immune defense and protection from COVID-19.
IFN evasion by SARS-CoV-2.
Like other pathogenic viruses, SARS-CoV-2 encodes proteins that can suppress IFN induction and actions This topic has been reviewed extensively (see17 for example) but without context of the temporal nature of innate immune activation and the SARS-CoV-2 replication cycle. We should make it clear that for any virus, initial infection launches an arms race for the host to induce innate immune defenses and IFNs to suppress virus replication and spread wherein the virus must strike with countermeasures to evade and/or actively suppress innate immune and IFN actions of the host cell in order to survive, make more virions, and spread to new hosts36. Disease is a side effect of these collective host and virus actions. SARS-CoV-2 encodes proteins reported to suppress PRR signaling, block protein nuclear import that impacts the actions of activated IRF3 and STAT proteins, and to suppress specific ISG functions17. Many studies that have described innate immune and IFN evasion actions of SARS-CoV-2 to date have relied on cell culture models of viral protein over-expression conducted without context of actual SARS-CoV-2 infection and/or without consideration of host and virus response kinetics, including the kinetics of VSG and ISG expression and function. Studies to model infection in relevant cells of the upper and lower airway mucosa and across specific tissues of infection spread are required. Additionally, studies to document effects of wild type and mutant SARS-CoV-2 engineered with specific protein-expression/functional deletions, as well as across contemporary viral variants are needed. Such studies are essential to ascertain the true actions of viral proteins in evasion from innate immune and IFN defenses given that these innate defenses facilitate viral control and recovery from infection in most people exposed to SARS-CoV-2 even in the era of widespread vaccination. It is also important to note that as new SARS-CoV-2 variants continue to emerge and escape vaccine immunity79 innate immune and IFN defenses remain our foundational immune defense against new virus exposure and infection, linking with hybrid immunity to suppress infection and progression to COVID-1980,81. Continued research to define the virus/host interactions of innate immune and IFN control of infection is needed to ascertain the spectrum of host genes that serve to innately protect us against SARS-CoV-2 infection and disease and to understand how these processes regulate the contemporary status of hybrid immunity to infection. Such efforts should reveal the spectrum VSG and ISG targets and their upstream regulatory processes for consideration in strategies of host-directed therapeutics to suppress SARS-CoV-2 and enhance hybrid immunity.
IFN treatment and outcomes.
The clinical use of IFNs against SARS-CoV-2 has been met with skepticism due to the history of varied outcomes while treating cancer, autoimmunity, and other virus infections. A major barrier to the clinical use of type I IFN are the side effects that include flu-like symptoms, anemia, and depression82,83. Multiple randomized controlled clinical trials have now evaluated IFN therapy for improving outcomes in SARS-CoV-2 infection [summarized in84,85]. These include studies of systemic or aerosol/inhaled administration of IFN, which it is important to note was typically administered late in infection to hospitalized patients with severe COVID-19. In clinical trials of IFNα treatment, there was no significant difference in mortality from placebo control across COVID-19 cohorts, but treatment was indeed linked with a significant increase in the number of patients who improved enough to be discharged from the hospital86. Moreover, the administration of IFNβ did not increase the survival of severe COVID-19 cases among hospitalized patients but it did impact the disease to reduce the need for intensive care85. In a departure from treating hospitalized patients with IFN, two clinical trials of an early, systemic single administration of pegylated (long-lived) type III IFN evaluated efficacy for reducing acute phase virus and suppressing disease onset among outpatients. In one study, the cohorts included outpatients with laboratory-confirmed SARS-CoV-2 within seven days of symptom onset, and asymptomatic outpatients testing positive or within 7 days of study87. Type III IFN administration was found to increase the number of outpatients with viral clearance by day seven, including individuals with highest initial viral load. Thus, early administration of type III IFN is beneficial against SARS-CoV-2. Similar observations were recorded in another study where pegylated IFN were given to mostly vaccinated although infected with SARS-CoV-2 outpatients88. All IFNs were shown to be safe and tolerable across these studies, with some adverse events reported. Taken together, these clinical studies show that late administration of IFN does not impact survival in cases of severe COVID-19 but does significantly link with clinical case improvement that can reduce hospital stay or entry into intensive care units. Most importantly, early administration of IFN (shown by type III IFN) during acute infection clearly presents the opportunity to reduce viral load and hence virus shedding and transmission of SARS-CoV-2, outcomes that mitigate disease progression and interpersonal spreading of the virus.
Conclusions.
IFNs are critical components of innate immune defense and immune programming against virus infection. The actions of IFNs are mediated through hundreds of ISGs, the function of most of which are not defined. A growing list of VSGs and ISGs are now known to mediate antiviral actions that suppress specific stages of SARS-CoV-2 infection/replication but continued work in this area is sorely needed. IFNs are among the VSGs that are induced during acute virus infection and are indeed produced in response to SARS-CoV-2 infection despite viral counter measures to suppress IFN production from infected cells. Most people infected with SARS-CoV-2 have little to moderate disease and recover from infection owing to the antiviral actions the innate immune response and IFNs. While IFN levels in the blood are linked with inflammatory disease and worse COVID-19 in hospitalized patients, one cannot attribute COVID-19 severity to IFNs among the complex inflammatory response underlying COVID-19. In our opinion, IFN remains a viable therapeutic for the early treatment of acute SARS-CoV-2 infection. Placement of aerosol or systemic IFN treatments into clinical strategies aimed at protecting people at high risk for severe COVID-19, with IFN treatment administration occurring as soon as possible after testing virus-positive, would induce an antiviral response to reduce the duration of viral shedding, suppress viral load, and suppress or mitigate disease. Finally, research to define innate immune and IFN actions in the growing context global/continued vaccination, emergence of vaccine-escape viral variants, and hybrid immunity is needed to define therapeutic strategies for control of SARS-CoV-2 infection and to inform preparation for future epidemics and pandemics from emerging viruses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Agyapon-Ntra K, and McSharry PE (2023). A global analysis of the effectiveness of policy responses to COVID-19. Sci Rep 13, 5629. 10.1038/s41598-023-31709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drain PK, Dalmat RR, Hao L, Bemer MJ, Budiawan E, Morton JF, Ireton RC, Hsiang TY, Marfatia Z, Prabhu R, et al. (2023). Duration of viral infectiousness and correlation with symptoms and diagnostic testing in non-hospitalized adults during acute SARS-CoV-2 infection: A longitudinal cohort study. J Clin Virol 161, 105420. 10.1016/j.jcv.2023.105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, Narasimhan H, Zhu B, and Sun J (2023). Host Recovery from Respiratory Viral Infection. Annu Rev Immunol. 10.1146/annurev-immunol-101921-040450. [DOI] [PubMed] [Google Scholar]
- 4.Chow KT, Gale M Jr., and Loo YM (2018). RIG-I and Other RNA Sensors in Antiviral Immunity. Annu Rev Immunol 36, 667–694. 10.1146/annurev-immunol-042617-053309. [DOI] [PubMed] [Google Scholar]
- 5.Rebendenne A, Valadao ALC, Tauziet M, Maarifi G, Bonaventure B, McKellar J, Planes R, Nisole S, Arnaud-Arnould M, Moncorge O, and Goujon C (2021). SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J Virol 95. 10.1128/JVI.02415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin X, Riva L, Pu Y, Martin-Sancho L, Kanamune J, Yamamoto Y, Sakai K, Gotoh S, Miorin L, De Jesus PD, et al. (2021). MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep 34, 108628. 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehwinkel J, and Gack MU (2020). RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol 20, 537–551. 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen J, VanScoy S, Cheng TF, Gomez D, and Reich NC (2008). IRF-3-dependent and augmented target genes during viral infection. Genes Immun 9, 168–175. 10.1038/sj.gene.6364449. [DOI] [PubMed] [Google Scholar]
- 9.Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, Zhang P, Meertens L, Bolze A, Materna M, et al. (2021). X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol 6. 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallerini C, Daga S, Mantovani S, Benetti E, Picchiotti N, Francisci D, Paciosi F, Schiaroli E, Baldassarri M, Fava F, et al. (2021). Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife 10. 10.7554/eLife.67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, et al. (2020). Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370. 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green R, Ireton RC, and Gale M Jr. (2018). Interferon-stimulated genes: new platforms and computational approaches. Mamm Genome 29, 593–602. 10.1007/s00335-018-9755-6. [DOI] [PubMed] [Google Scholar]
- 13.Lore NI, De Lorenzo R, Rancoita PMV, Cugnata F, Agresti A, Benedetti F, Bianchi E, Bonini C, Capobianco A, Conte C, et al. (2021). CXCL10 levels at hospital admission predict COVID-19 outcome: hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Mol Med 27, 129. 10.1186/s10020-021-00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, et al. (2020). An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26, 1636–1643. 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed D, Al-Daraawi M, and Cassol E (2023). Innate sensing and cellular metabolism: role in fine tuning antiviral immune responses. J Leukoc Biol 113, 164–190. 10.1093/jleuko/qiac011. [DOI] [PubMed] [Google Scholar]
- 16.Anaeigoudari A, Mollaei HR, Arababadi MK, and Nosratabadi R (2021). Severe Acute Respiratory Syndrome Coronavirus 2: The Role of the Main Components of the Innate Immune System. Inflammation 44, 2151–2169. 10.1007/s10753-021-01519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minkoff JM, and tenOever B (2023). Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol 21, 178–194. 10.1038/s41579-022-00839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forero A, Ozarkar S, Li H, Lee CH, Hemann EA, Nadjsombati MS, Hendricks MR, So L, Green R, Roy CN, et al. (2019). Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 51, 451–464 e456. 10.1016/j.immuni.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowling JW, and Forero A (2022). Beyond Good and Evil: Molecular Mechanisms of Type I and III IFN Functions. J Immunol 208, 247–256. 10.4049/jimmunol.2100707. [DOI] [PubMed] [Google Scholar]
- 20.Russell MW, and Mestecky J (2022). Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol 13, 957107. 10.3389/fimmu.2022.957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldridge MT, Lee S, Brown JJ, McAllister N, Urbanek K, Dermody TS, Nice TJ, and Virgin HW (2017). Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus. J Virol 91. 10.1128/JVI.02079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, and Virgin HW (2015). Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347, 266–269. 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, and Virgin HW (2015). Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273. 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemann EA, Green R, Turnbull JB, Langlois RA, Savan R, and Gale M Jr. (2019). Interferon-lambda modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol 20, 1035–1045. 10.1038/s41590-019-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong Z, Karl CE, Halfmann PJ, Kawaoka Y, Winkler ES, Keeler SP, Holtzman J, Yu J, and Diamond MS (2022). Nasally delivered interferon-lambda protects mice against infection by SARS-CoV-2 variants including Omicron. Cell Rep 39, 110799. 10.1016/j.celrep.2022.110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva RP, Goncalves JIB, Zanin RF, Schuch FB, and de Souza APD (2021). Circulating Type I Interferon Levels and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front Immunol 12, 657363. 10.3389/fimmu.2021.657363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendricks MR, and Savan R (2020). Interferon-lambda at the Center of the Storm. Immunity 53, 245–247. 10.1016/j.immuni.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Major J, Crotta S, Llorian M, McCabe TM, Gad HH, Priestnall SL, Hartmann R, and Wack A (2020). Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369, 712–717. 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broggi A, Ghosh S, Sposito B, Spreafico R, Balzarini F, Lo Cascio A, Clementi N, De Santis M, Mancini N, Granucci F, and Zanoni I (2020). Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 369, 706–712. 10.1126/science.abc3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henden AS, Koyama M, Robb RJ, Forero A, Kuns RD, Chang K, Ensbey KS, Varelias A, Kazakoff SH, Waddell N, et al. (2021). IFN-lambda therapy prevents severe gastrointestinal graft-versus-host disease. Blood 138, 722–737. 10.1182/blood.2020006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casazza RL, and Lazear HM (2019). Why Is IFN-lambda Less Inflammatory? One IRF Decides. Immunity 51, 415–417. 10.1016/j.immuni.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Sohn SY, Hearing J, Mugavero J, Kirillov V, Gorbunova E, Helminiak L, Mishra S, Mackow E, Hearing P, Reich NC, and Kim HK (2021). Interferon-Lambda Intranasal Protection and Differential Sex Pathology in a Murine Model of SARS-CoV-2 Infection. mBio 12, e0275621. 10.1128/mBio.02756-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, Alfajaro MM, Wei J, Dong H, Homer RJ, et al. (2020). Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med 217. 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Cao L, Xu W, Xu T, Zheng B, Ji Y, Huang S, Liu L, Du J, Peng H, et al. (2022). Comparison of model-specific histopathology in mouse models of COVID-19. J Med Virol 94, 3605–3612. 10.1002/jmv.27747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijkman R, Verma AK, Selvaraj M, Ghimire R, Gad HH, Hartmann R, More S, Perlman S, Thiel V, and Channappanavar R (2022). Effective Interferon Lambda Treatment Regimen To Control Lethal MERS-CoV Infection in Mice. J Virol 96, e0036422. 10.1128/jvi.00364-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galligan CL, Murooka TT, Rahbar R, Baig E, Majchrzak-Kita B, and Fish EN (2006). Interferons and viruses: signaling for supremacy. Immunol Res 35, 27–40. 10.1385/IR:35:1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang BX, and Fish EN (2012). The yin and yang of viruses and interferons. Trends Immunol 33, 190–197. 10.1016/j.it.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loo YM, and Gale M Jr. (2007). Viral regulation and evasion of the host response. Curr Top Microbiol Immunol 316, 295–313. 10.1007/978-3-540-71329-6_14. [DOI] [PubMed] [Google Scholar]
- 39.Jackson CB, Farzan M, Chen B, and Choe H (2022). Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23, 3–20. 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaender S, Mar KB, Michailidis E, Kratzel A, Boys IN, V’Kovski P, Fan W, Kelly JN, Hirt D, Ebert N, et al. (2020). LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat Microbiol 5, 1330–1339. 10.1038/s41564-020-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Zheng S, Chen D, Zheng M, Li X, Li G, Lin H, Chang J, Zeng H, and Guo JT (2020). LY6E Restricts Entry of Human Coronaviruses, Including Currently Pandemic SARS-CoV-2. J Virol 94. 10.1128/JVI.00562-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Liang C, and Liu SL (2017). Interferon-inducible LY6E Protein Promotes HIV-1 Infection. J Biol Chem 292, 4674–4685. 10.1074/jbc.M116.755819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mar KB, Rinkenberger NR, Boys IN, Eitson JL, McDougal MB, Richardson RB, and Schoggins JW (2018). LY6E mediates an evolutionarily conserved enhancement of virus infection by targeting a late entry step. Nat Commun 9, 3603. 10.1038/s41467-018-06000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lesage S, Chazal M, Beauclair G, Batalie D, Cerboni S, Couderc E, Lescure A, Del Nery E, Tangy F, Martin A, et al. (2022). Discovery of Genes that Modulate Flavivirus Replication in an Interferon-Dependent Manner. J Mol Biol 434, 167277. 10.1016/j.jmb.2021.167277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruchez A, Sha K, Johnson J, Chen L, Stefani C, McConnell H, Gaucherand L, Prins R, Matreyek KA, Hume AJ, et al. (2020). MHC class II transactivator CIITA induces cell resistance to Ebola virus and SARS-like coronaviruses. Science 370, 241–247. 10.1126/science.abb3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenney AD, Zani A, Kawahara J, Eddy AC, Wang XL, Mahesh KC, Lu M, Thomas J, Kohlmeier JE, Suthar MS, et al. (2023). Interferon-induced transmembrane protein 3 (IFITM3) limits lethality of SARS-CoV-2 in mice. EMBO Rep 24, e56660. 10.15252/embr.202256660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu F, Wang G, Zhao F, Huang Y, Fan Z, Mei S, Xie Y, Wei L, Hu Y, Wang C, et al. (2022). IFITM3 Inhibits SARS-CoV-2 Infection and Is Associated with COVID-19 Susceptibility. Viruses 14. 10.3390/v14112553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jimenez-Munguia I, Beaven AH, Blank PS, Sodt AJ, and Zimmerberg J (2022). Interferon-induced transmembrane protein 3 (IFITM3) and its antiviral activity. Curr Opin Struct Biol 77, 102467. 10.1016/j.sbi.2022.102467. [DOI] [PubMed] [Google Scholar]
- 49.Gholami M, Sakhaee F, Sotoodehnejadnematalahi F, Zamani MS, Ahmadi I, Anvari E, and Fateh A (2022). Increased risk of COVID-19 mortality rate in IFITM3 rs6598045 G allele carriers infected by SARS-CoV-2 delta variant. Hum Genomics 16, 60. 10.1186/s40246-022-00434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, and Hartmann R (2011). The Oligoadenylate Synthetase Family: An Ancient Protein Family with Multiple Antiviral Activities. Journal of Interferon & Cytokine Research 31, 41–47. 10.1089/jir.2010.0107. [DOI] [PubMed] [Google Scholar]
- 51.Morin B, Rabah N, Boretto-Soler J, Tolou H, Alvarez K, and Canard B (2010). High yield synthesis, purification and characterisation of the RNase L activators 5′-triphosphate 2′–5′-oligoadenylates. Antiviral Research 87, 345–352. 10.1016/j.antiviral.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarti A, Jha BK, and Silverman RH (2011). New Insights into the Role of RNase L in Innate Immunity. Journal of Interferon & Cytokine Research 31, 49–57. 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drappier M, and Michiels T (2015). Inhibition of the OAS/RNase L pathway by viruses. Current Opinion in Virology 15, 19–26. 10.1016/j.coviro.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwerk J, Soveg FW, Ryan AP, Thomas KR, Hatfield LD, Ozarkar S, Forero A, Kell AM, Roby JA, So L, et al. (2019). RNA-binding protein isoforms ZAP-S and ZAP-L have distinct antiviral and immune resolution functions. Nat Immunol 20, 1610–1620. 10.1038/s41590-019-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soveg FW, Schwerk J, Gokhale NS, Cerosaletti K, Smith JR, Pairo-Castineira E, Kell AM, Forero A, Zaver SA, Esser-Nobis K, et al. (2021). Endomembrane targeting of human OAS1 p46 augments antiviral activity. Elife 10. 10.7554/eLife.71047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wickenhagen A, Sugrue E, Lytras S, Kuchi S, Noerenberg M, Turnbull ML, Loney C, Herder V, Allan J, Jarmson I, et al. (2021). A prenylated dsRNA sensor protects against severe COVID-19. Science 374, eabj3624. 10.1126/science.abj3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huffman JE, Butler-Laporte G, Khan A, Pairo-Castineira E, Drivas TG, Peloso GM, Nakanishi T, Initiative C-HG, Ganna A, Verma A, et al. (2022). Multi-ancestry fine mapping implicates OAS1 splicing in risk of severe COVID-19. Nat Genet 54, 125–127. 10.1038/s41588-021-00996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman MH, Russell CD, et al. (2021). Genetic mechanisms of critical illness in COVID-19. Nature 591, 92–98. 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q, Pizzorno A, Miorin L, Bastard P, Gervais A, Le Voyer T, Bizien L, Manry J, Rosain J, Philippot Q, et al. (2022). Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J Exp Med 219. 10.1084/jem.20220514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastard P, Vazquez S, Liu J, Laurie MT, Wang CY, Gervais A, Le Voyer T, Bizien L, Zamecnik C, Philippot Q, et al. (2022). Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-Abs neutralizing type I IFNs. Sci Immunol, eabp8966. 10.1126/sciimmunol.abp8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manry J, Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Michailidis E, Hoffmann HH, Eto S, Garcia-Prat M, et al. (2022). The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc Natl Acad Sci U S A 119, e2200413119. 10.1073/pnas.2200413119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nchioua R, Kmiec D, Muller JA, Conzelmann C, Gross R, Swanson CM, Neil SJD, Stenger S, Sauter D, Munch J, et al. (2020). SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. mBio 11. 10.1128/mBio.01930-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmer MM, Kibe A, Rand U, Pekarek L, Ye L, Buck S, Smyth RP, Cicin-Sain L, and Caliskan N (2021). The short isoform of the host antiviral protein ZAP acts as an inhibitor of SARS-CoV-2 programmed ribosomal frameshifting. Nat Commun 12, 7193. 10.1038/s41467-021-27431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song J, Chow RD, Pena-Hernandez MA, Zhang L, Loeb SA, So EY, Liang OD, Ren P, Chen S, Wilen CB, and Lee S (2022). LRRC15 inhibits SARS-CoV-2 cellular entry in trans. PLoS Biol 20, e3001805. 10.1371/journal.pbio.3001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kratzel A, Kelly JN, V’Kovski P, Portmann J, Bruggemann Y, Todt D, Ebert N, Shrestha N, Plattet P, Staab-Weijnitz CA, et al. (2021). A genome-wide CRISPR screen identifies interactors of the autophagy pathway as conserved coronavirus targets. PLoS Biol 19, e3001490. 10.1371/journal.pbio.3001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, Feng F, Hu G, Wang Y, Yu Y, Zhu Y, Xu W, Cai X, Sun Z, Han W, et al. (2021). A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat Commun 12, 961. 10.1038/s41467-021-21213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mac Kain A, Maarifi G, Aicher SM, Arhel N, Baidaliuk A, Munier S, Donati F, Vallet T, Tran QD, Hardy A, et al. (2022). Identification of DAXX as a restriction factor of SARS-CoV-2 through a CRISPR/Cas9 screen. Nat Commun 13, 2442. 10.1038/s41467-022-30134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Israeli M, Finkel Y, Yahalom-Ronen Y, Paran N, Chitlaru T, Israeli O, Cohen-Gihon I, Aftalion M, Falach R, Rotem S, et al. (2022). Genome-wide CRISPR screens identify GATA6 as a proviral host factor for SARS-CoV-2 via modulation of ACE2. Nat Commun 13, 2237. 10.1038/s41467-022-29896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ugalde AP, Bretones G, Rodriguez D, Quesada V, Llorente F, Fernandez-Delgado R, Jimenez-Clavero MA, Vazquez J, Calvo E, Tamargo-Gomez I, et al. (2022). Autophagy-linked plasma and lysosomal membrane protein PLAC8 is a key host factor for SARS-CoV-2 entry into human cells. EMBO J 41, e110727. 10.15252/embj.2022110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann HH, Sanchez-Rivera FJ, Schneider WM, Luna JM, Soto-Feliciano YM, Ashbrook AW, Le Pen J, Leal AA, Ricardo-Lax I, Michailidis E, et al. (2021). Functional interrogation of a SARS-CoV-2 host protein interactome identifies unique and shared coronavirus host factors. Cell Host Microbe 29, 267–280 e265. 10.1016/j.chom.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei J, Alfajaro MM, DeWeirdt PC, Hanna RE, Lu-Culligan WJ, Cai WL, Strine MS, Zhang SM, Graziano VR, Schmitz CO, et al. (2021). Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell 184, 76–91 e13. 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang R, Simoneau CR, Kulsuptrakul J, Bouhaddou M, Travisano KA, Hayashi JM, Carlson-Stevermer J, Zengel JR, Richards CM, Fozouni P, et al. (2021). Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 184, 106–119 e114. 10.1016/j.cell.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniloski Z, Jordan TX, Wessels HH, Hoagland DA, Kasela S, Legut M, Maniatis S, Mimitou EP, Lu L, Geller E, et al. (2021). Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 184, 92–105 e116. 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crouse J, Kalinke U, and Oxenius A (2015). Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 15, 231–242. 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 75.Leist SR, Dinnon KH 3rd, Schafer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, et al. (2020). A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 183, 1070–1085 e1012. 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez J, Mommert M, Mouton W, Pizzorno A, Brengel-Pesce K, Mezidi M, Villard M, Lina B, Richard JC, Fassier JB, et al. (2021). Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J Exp Med 218. 10.1084/jem.20211211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Beziat V, et al. (2020). Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370. 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solanich X, Rigo-Bonnin R, Gumucio VD, Bastard P, Rosain J, Philippot Q, Perez-Fernandez XL, Fuset-Cabanes MP, Gordillo-Benitez MA, Suarez-Cuartin G, et al. (2021). Pre-existing Autoantibodies Neutralizing High Concentrations of Type I Interferons in Almost 10% of COVID-19 Patients Admitted to Intensive Care in Barcelona. J Clin Immunol 41, 1733–1744. 10.1007/s10875-021-01136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hao L, Hsiang TY, Dalmat RR, Ireton R, Morton JF, Stokes C, Netland J, Hale M, Thouvenel C, Wald A, et al. (2023). Dynamics of SARS-CoV-2 VOC Neutralization and Novel mAb Reveal Protection against Omicron. Viruses 15. 10.3390/v15020530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown B, Ojha V, Fricke I, Al-Sheboul SA, Imarogbe C, Gravier T, Green M, Peterson L, Koutsaroff IP, Demir A, et al. (2023). Innate and Adaptive Immunity during SARS-CoV-2 Infection: Biomolecular Cellular Markers and Mechanisms. Vaccines (Basel) 11. 10.3390/vaccines11020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinardi JR, and Srivastava A (2023). Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination-Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 11. 10.3390/biomedicines11020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai JY, Ho JX, Kow ASF, Liang G, Tham CL, Ho YC, and Lee MT (2023). Interferon therapy and its association with depressive disorders - A review. Front Immunol 14, 1048592. 10.3389/fimmu.2023.1048592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang LW, Liranzo M, and Bergfeld WF (1995). Cutaneous side effects associated with interferon-alpha therapy: a review. Cutis 56, 144. [PubMed] [Google Scholar]
- 84.Ryoo S, Koh DH, Yu SY, Choi M, Huh K, Yeom JS, and Heo JY (2023). Clinical efficacy and safety of interferon (Type I and Type III) therapy in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. PLoS One 18, e0272826. 10.1371/journal.pone.0272826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen WC, Hsu CK, Chen CY, Lai CC, Hung SH, and Lin WT (2022). Clinical efficacy and safety of interferon-beta-containing regimens in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther 20, 741–747. 10.1080/14787210.2022.2004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchynskyi M, Kamyshna I, Lyubomirskaya K, Moshynets O, Kobyliak N, Oksenych V, and Kamyshnyi A (2023). Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: A meta-analysis. Front Immunol 14, 1069894. 10.3389/fimmu.2023.1069894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, Borgia SM, Boggild AK, Powis J, McCready J, et al. (2021). Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 9, 498–510. 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reis G, Moreira Silva EAS, Medeiros Silva DC, Thabane L, Campos VHS, Ferreira TS, Santos CVQ, Nogueira AMR, Almeida A, Savassi LCM, et al. (2023). Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med 388, 518–528. 10.1056/NEJMoa2209760. [DOI] [PMC free article] [PubMed] [Google Scholar]


