Abstract
Successful management of pain during endodontic treatment is essential for both patients and dentists. Achieving adequate pulp anesthesia in mandibular molars is a significant concern for patients with irreversible pulpitis during endodontic treatment. The increased sensitization of nociceptors due to inflammation decreases the success of inferior alveolar nerve block (IANB). The main focus is on reducing inflammation before delivery of local anesthesia to increase the success of anesthetic drugs. This umbrella review aimed to revise, qualify and summarize the existing body of evidence on the effect of premedication on IANB in patients with irreversible pulpitis. A literature search was conducted using electronic databases (PubMed, Scopus, the Web of Science, and the Cochrane Library) with no date restriction until September 2021 to identify the relevant studies. All the cross-references of the selected studies and grey literature were also screened. Four systematic reviews assessing the effect of premedication on the success of IANB were selected. A conclusion was drawn that premedication with >400 mg of ibuprofen can positively affect the success of IANB.
Keywords: Mandibular nerve, pulpitis, systematic review
INTRODUCTION
A systematic review is a method of study that uses scientific ways to identify and collect quantitative or quantitative findings of all studies related to a question at the highest level required in health research. This type of study helps review evidence, develop guidelines, inform policies, and assess the cost-effectiveness of interventions.[1,2] Over the past decade, systematic reviews have grown as critical tools for promoting evidence-based health care and as a type of high-level, low-cost research. However, some found that the quality of the articles was generally poor as a result of the reports and methodological shortcomings.[3,4] This field has both opportunities and risks: In particular, it creates an environment where researchers can make the best decision based on accurate, concise, credible, and understandable evidence. However, variations in empirical quality and validity can lead to biases or inaccuracies that affect the validity of findings.[1]
Successful management of pain during endodontic treatment is essential for both patients and dentists.[5] Achieving adequate pulp anesthesia is a significant concern for patients with irreversible pulpitis during endodontic treatment.[6] Practitioners commonly use the inferior alveolar nerve block (IANB) technique to achieve pulp anesthesia in mandibular teeth. Researchers observed that the IANB failure rate was between 43% and 83% in patients with irreversible pulpitis.[7,8,9] The researchers have mainly related IANB failure in teeth with irreversible pulpitis to inflammation in the pulp.[10] Inflammation occurs through the production of prostaglandins, which are involved in causing and enhancing pain, from arachidonic acid in cell membranes by cyclooxygenase enzymes.[10,11] The increased sensitization of nociceptors due to inflammation decreases the success of IANB.[10,11,12] The main focus is on reducing inflammation before delivery of local anesthesia to increase the success of anesthetic drugs. Inflammation is responsible for inappropriate anesthesia because inflammation mediators can stimulate pain fibers even at very low thresholds. It has been suggested that reducing prostaglandin levels may increase the local anesthetic effects.[13] As a result, several authors have made efforts to prescribe the best drug or combination of medicines before endodontic therapy to reduce inflammation and reduce the mediators that are the leading cause of painful symptoms. However, while some drugs are promising, there is no clinical consensus among authors on the subject through systematic reviews.
With the increase in the number of available systematic reviews, a logical and appropriate next step is to review the existing systematic reviews, allowing the findings of independent studies to be compared and contrasted, thus providing the best evidence needed for practitioners. A review of systematic reviews is carried out under several different names in the scientific literature, including umbrella reviews, an overview of reviews, summaries of systematic reviews, and synthesis of reviews. Irrespective of their name, these reviews have a defining feature in common: A systematic review is a principal and often sole “study type” that is considered for inclusion.[14,15,16] The principal reason for conducting an umbrella review is summarizing the evidence from multiple research syntheses.[15] The conduct of an umbrella review may also offer a means for a rapid review of the evidence to address broad and high-quality information about a topic.[16] Umbrella reviews are conducted to comprehensively examine a given topic's available body of knowledge and compare and contrast published systematic reviews’ results.[14] Thus, this umbrella review aimed to revise, qualify and summarize the existing body of evidence on the effect of premedication on IANB in patients diagnosed with irreversible pulpitis in molar teeth.
MATERIALS AND METHODS
This umbrella review was undertook following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and its protocol is registered in in PROSPERO (CRD42021286004).[17]
Review question
The review question was made based on the patient, intervention, comparison, outcome framework.
Does premedication (I) improve the success rate of IANB (O) compared to placebo (C) in adult patients (P) diagnosed with irreversible pulpitis in molar teeth?
Sources and search-line
PubMed (MEDLINE), Scopus, the Web of Science, and the Cochrane Library of systematic reviews were reviewed up to September 15th, 2021, Considering MeSH terms and synonyms related to IANB (“IANB” OR “mandibular nerve block” OR “IANB”) and related to premedication (“Oral premedication” OR “premedication”) and related to systematic reviews (“systematic review” OR “meta-analysis”) adapted for each database, with no date restrictions, only records published in English were selected. We performed a hand search in the reference lists of selected records. OpenGrey, ProQuest, and WorldCat were searched to obtain grey literature and unpublished reviews.
Eligibility criteria
The included records had to report the anesthetic solution used and the premedication drug to identify any association. The included studies had to clarify the method of checking the proper anesthesia (no response to electric pulp tester (EPT), no pain feeling during access cavity preparation or instrumentation). We excluded network meta-analysis (due to different methodology), literature reviews, critical reviews, letters to the editor, case reports or case series, and observational or clinical studies.
Study selection
Two reviewers (S. B. and N. M) independently evaluated the titles and abstracts of all records. Next full-text copies from studies that met the inclusion criteria or for which there were insufficient data available to make a clear decision possible were retrieved. Two reviewers resolved any disagreements through consensus or discussion with a third expert reviewer (A. K.).
Data extraction
Details of each study (first author's name, date of search, and date of publication), study methods (method of analysis, methodological quality assessment tool (s) used), results (number of studies included, meta-analysis), and study conclusions were extracted by two reviewers (S. B. and N. M.) independently. Any disagreements between authors were resolved through a third author (A. K). When necessary, we contacted corresponding authors to obtain missing (or not specified) data from the included studies via E-mail.
Risk of bias (methodological quality assessment)
AMSTAR 2 approach was used to evaluate the methodological quality of retrieved systematic reviews.[1,2] Two reviewers (S. B. and N. M.) independently discussed the AMSTAR 2 [Supplementary Table 1] criteria and this instrument's application in the selected studies, defining each parameter of analysis. Any disagreement was resolved by consultation with a third reviewer (A. K.). AMSTAR 2 includes a 16-item checklist covering all of the steps taken during a systematic review and meta-analysis. The following seven domains can critically affect the conclusions (items 2, 7, 9, 11, 13, 14 and 15).[1] Based on critical and noncritical domains, AMSTAR 2 calculates the degree of confidence in the results of a review as either critically low, low, moderate, or high.[1]
Supplementary Table 1.
AMSTAR 2 checklist
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | ||
|---|---|---|
| For Yes: | Optional (recommended) | |
| • Population | • Timeframe for follow-up | □ Yes |
| • Intervention | □ No | |
| • Comparator group | ||
| • Outcome | ||
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | ||
|
| ||
| For Partial Yes: | For Yes: | |
| • The authors state that they had a written protocol or guide that included ALL the following: | • As for partial yes, plus the protocol should be registered and should also have specified: | □ Yes |
| • review question (s) | • a meta-analysis/synthesis plan, if appropriate, and | □ Partial Yes |
| • a search strategy | • a plan for investigating causes of heterogeneity | □ No |
| • inclusion/exclusion criteria | • justification for any deviations from the protocol | |
| • a risk of bias assessment | ||
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | ||
|
| ||
| For Yes, the review should satisfy ONE of the following: | ||
| • Explanation for including only RCTs | □ Yes | |
| • OR Explanation for including only NRSI | □ No | |
| • OR Explanation for including both RCTs and NRSI | ||
| 4. Did the review authors use a comprehensive literature search strategy? | ||
|
| ||
| For Partial Yes (all the following): | For Yes, should also have (all the following): • searched the reference lists/bibliographies of included studies |
Yes |
| • searched at least 2 databases (relevant to research question) • provided key word and/or search strategy |
□ Partial Yes | |
| • justified publication restrictions (e.g. language) | □ □ No | |
| 5. Did the review authors perform study selection in duplicate? | ||
|
| ||
| For Yes, either ONE of the following: | ||
| • at least two reviewers independently agreed on selection of eligible studies and achieved consensus on which studies to include | Yes | |
| • OR two reviewers selected a sample of eligible studies and achieved good agreement (at least 80 percent), with the remainder selected by one reviewer. | No | |
| 6.Did the review authors perform data extraction in duplicate? | ||
|
| ||
| For Yes, either ONE of the following: | ||
| • at least two reviewers achieved consensus on which data to extract from included studies | Yes | |
| • OR two reviewers extracted data from a sample of eligible studies and achieved good agreement (at least 80 percent), with the remainder extracted by one reviewer. | No | |
| 7.Did the review authors provide a list of excluded studies and justify the exclusions? | ||
|
| ||
| For Partial Yes: | For Yes, must also have: | |
| • provided a list of all potentially relevant studies that were read | • Justified the exclusion from the review of each potentially relevant | Yes Partial Yes |
| • in full-text form but excluded from the review | study | No |
| 8.Did the review authors describe the included studies in adequate detail? | ||
|
| ||
| For Partial Yes (ALL the following): | For Yes, should also have ALL the following: | |
| • described populations | • described population in detail | Yes |
| • described interventions | • described intervention in detail (including doses where | Partial Yes |
| • described comparators | relevant) | No |
| • described outcomes | • described comparator in detail (including doses where relevant) | |
| • described research designs | • described study’s setting | |
| • timeframe for follow-up | ||
| 9.Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? | ||
|
| ||
| RCTs | For Yes, must also have assessed RoB from: | |
| For Partial Yes, must have assessed RoB from • unconcealed allocation, and • lack of blinding of patients and assessors when assessing outcomes (unnecessary for objective outcomes such as all- cause mortality) |
• allocation sequence that was not truly random, and • selection of the reported result from among multiple measurements or analyses of a specified outcome |
Yes Partial Yes No Includes only NRSI |
| NRSI For Partial Yes, must have assessed RoB: from confounding, and from selection bias | For Yes, must also have assessed RoB: • methods used to ascertain exposures and outcomes, and • selection of the reported result from among multiple measurements or analyses of a specified outcome |
Yes Partial Yes No Includes only RCTs |
| 10. Did the review authors report on the source of funding for the studies included in the review | ||
|
| ||
| For Yes | □ Yes | |
| • Must have reported on the sources of funding for individual studies includedthe review. Note: Reporting that the reviewers looked for this information but it was not reported by study authors also qualifies | □ No | |
| 11. If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | ||
|
| ||
| RCTs | ||
| For Yes: | Yes | |
| • The authors justified combining the data in a meta-analysis | No No meta-analysis conducted | |
| • AND they used an appropriate weighted technique to combine study results and adjusted for heterogeneity if present. | ||
| • AND investigated the causes of any heterogeneity | ||
| For NRSI | ||
| For Yes: | Yes | |
| • The authors justified combining the data in a meta-analysis | No | |
| • AND they used an appropriate weighted technique to combine study results, adjusting for heterogeneity if present | No meta-analysis conducted | |
| • AND they statistically combined effect estimates from NRSI that were adjusted for confounding, rather than combining raw data, or justified combining raw data when adjusted effect estimates were not available | ||
| • AND they reported separate summary estimates for RCTs and NRSI separately when both were included in the review | ||
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | ||
|
| ||
| For Yes: | ||
| • included only low risk of bias RCTs | Yes | |
| • OR, if the pooled estimate was based on RCTs and/or NRSI at variable RoB, the authors performed analyses to investigate possible impact of RoB on summary estimates of effect. | No No meta-analysis conducted | |
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | ||
|
| ||
| For Yes: | ||
| • included only low risk of bias RCTs | Yes | |
| • OR, if RCTs with moderate or high RoB, or NRSI were included the review provided a discussion of the likely impact of RoB on the results | No | |
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | ||
|
| ||
| For Yes: | ||
| • There was no significant heterogeneity in the results | Yes | |
| • OR if heterogeneity was present the authors performed an investigation of sources of any heterogeneity in the results and discussed the impact of this on the results of the review | No | |
| 15. If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | ||
|
| ||
| For Yes: • performed graphical or statistical tests for publication bias and discussed the likelihood and magnitude of impact of publication bias |
Yes No No meta-analysis conducted | |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | ||
|
| ||
| For Yes: | ||
| • The authors reported no competing interests OR | Yes | |
| • The authors described their funding sources and how they managed potential conflicts of interest | No | |
Choice of the best body of evidence
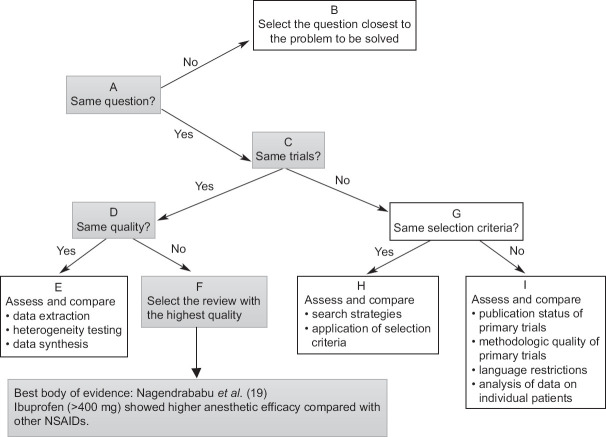
When a premedication was addressed by more than one systematic review with discordance, the Jadad decision algorithm was applied to select the systematic review that provided the best body of evidence according to the currently available studies. The Jadad decision algorithm is designed as an adjunct decision tool to help decision-makers interpret and choose among discordant systematic reviews.[18] It is a sequence of reasoning (comprising questions on the methodology of the studies) used when two or more systematic reviews had discordant conclusions about the same exposure. This decision (choice of the study, or studies, that preset the best methodology and, consequently, the best evidence) is based on differences in the study question, trials included, type of study method selected, quality of assessments, criteria for the selection of primary studies, data extraction methods, data combinations, statistical analysis methods, search strategies, and study selection.
RESULTS
Literature search
Relevant systematic reviews were identified and selected [Figure 1]. The initial search resulted in 29 reviews, and of these, 9 were removed as they were duplicates. Following title and abstract screening, a total of 16 studies were excluded, because they did not satisfy the inclusion criteria with 4 studies being shortlisted for full-text retrieval. After reading the full text, all 4 systematic reviews were selected for this umbrella review (Nagendrababu et al.,[19] de Geus et al.,[20] Shirvani et al.[21] and Karapina-Kazandag et al.[22]).
Figure 1.
Flow diagram of study selection process.
Characteristics of studies included in the systematic review
Out of 4 selected systematic reviews, three performed meta-analysis,[19,20,21] and the other one was a systematic review without meta-analysis.[22] The number of randomized clinical trials included in the systematic reviews ranged between 7 and 35 [Table 1]. The number of databases searched for results in studies ranged from 2 to 7, and only one of the reviews searched the grey literature.[20] At least two researchers performed data extraction and evaluation of the risk bias of all studies.
Table 1.
Summary of the included systematic reviews
| Author (year) | Search period | Databases searched | Number of studies | Number of patients | Tool used for quality assessment | Method of analysis | Findings |
|---|---|---|---|---|---|---|---|
| Shirvani (2017) | Up to March 2015 | Cochrane Databases for Systematic Review, Pub Med, Science Direct, Scopus, and Google Scholar | 16 | 1900 | Cochrane collaboration risk of bias tool | MA | Indomethacin, meloxicam, piroxicam, diclofenac potassium, acetaminophen+opioid |
| Nagendrababu (2018) | Up to September 2017 | PubMed, EBSCOhost, and Scopus | 13 | 1174 | Cochrane collaboration risk of bias tool | MA | Ibuprofen (>400 mg) |
| De Gues (2019) | Up to August 2017 | PubMed, Scopus, Web of Science, LILACS, BBO, Cochrane Library, SIGLE, and grey literature | 7 | NR | Cochrane collaboration risk of bias tool | MA | Ibuprofen |
| Karapinar-Kazandag (2019) | Up to April 2018 | Cochrane Library database and PubMed | 35 | NR | Cochrane collaboration risk of bias tool | SR | Ibuprofen, ketamine (oral administration) |
MA: Meta-analysis, SR: Systematic review, NR: Not reported
The anesthetic solution used in most studies to anesthetize the inferior alveolar nerve was 2% lidocaine with 1:100,000 epinephrine. Nagendrababu et al.[19] reported using ibuprofen at a dose of more than 400 mg to be the most effective premedication for increasing the success of anesthesia. In this study, 50 mg diclofenac and 10 mg ketorolac were following ibuprofen.
De Geus et al.'s study,[20] which focused solely on ibuprofen premedication, concluded that taking a single dose 1 h before treatment increased success by 79% compared with placebo and reduced pain intensity (success rate was almost 20% in placebo group). Shirvani et al.[21] reported that the administration of preemptive analgesics can induce superior intraoperative analgesia for patients with irreversible pulpitis. However, strategies such as co-administration of certain types of analgesics and anesthetic solution might be predictors of treatment effect. They also reported that there was no association between different timing and dosage of analgesics and treatment effect.
Karapinar-Kazandag et al.[22] reported that Ibuprofen and some other NSAIDs appear to be premedications that may contribute to the overall success of IANB rather than Acetaminophen. They also concluded that oral administration of ketamine can be used to reduce the number of cartridges used for IANB in patients with irreversible pulpitis and postoperative pain was significantly lower.
Risk of bias (quality assessment)
Excellent inter-examiner reliability at the risk of bias screening was recorded (kappa score = 0.91; 95% confidence interval: 0.89–0.92). Overall, two were rated as “high quality”[19,20] and two as “low quality”[21,22] [Table 2]. We found major concerns regarding methodological quality on the:
Table 2.
Risk of bias of systematic reviews based on AMSTAR 2 checklist
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Review quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shirvani (2017) | Y | PY | Y | PY | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Low |
| Nagendrababu (2018) | Y | PY | Y | PY | Y | Y | Y | PY | PY | N | Y | Y | Y | Y | Y | Y | High |
| De Gues (2019) | Y | Y | Y | PY | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | High |
| Karapinar-Kazandag (2019) | Y | Y | Y | PY | Y | Y | Y | Y | Y | N | 0 | 0 | N | N | 0 | Y | Low |
Y: Yes, PY: Partial yes, N: No, 0: Not indicated in this type of study
The literature search strategy as no study fully satisfied the AMSTAR 2 criteria
Declaration of funding sources as none of the selected studies reported this item.
Choice of the best body of evidence
Of the four studies that evaluated the association between oral premedication and anesthetic success in mandibular molars with irreversible pulpitis, three considered the same question (one only comparing ibuprofen with placebo)[19,21,22] (question A). The remaining three studies included the same trials (question C) but one study[19] had higher quality based on AMSTAR 2 checklist (question D). So base on JADAD algorithm the study performed by Nagendrababu et al.[19] can be categorized as the best body of evidence [Figure 2].
Figure 2.
Selecting the best body of evidence based on JADAD algorithm.
DISCUSSION
This umbrella review attempted to identify the best premedication drug for successful local anesthesia in the mandible in adult patients with irreversible pulpitis requiring root canal treatment. Inability to achieve pulpal anesthesia during root canal treatment can increase fear and anxiety in patients. Thus, patient management will be more challenging, prolonging the duration of the appointments and creating concern in the mind of patients about physician competence.[23] In an umbrella review, the results of several systematic reviews are summarized in an overview before combining the data to integrate all relevant information. The goal is to make it more straightforward, reduce uncertainty about decisions, identify gaps in knowledge, and provide a reference publication that contains essential information on the subject.[24,25] Therefore, an umbrella review offers the highest level of scientific evidence and is a benchmark for clinical decision-making.
Consequently, we used an umbrella review approach in this study to provide specific and clear recommendations to clinicians on what premedication they should use in addition to local anesthesia for their adult patients presenting with irreversible pulpitis and needing root canal treatment. The authors of the current umbrella review had planned to perform a meta-analysis if the primary outcome of the four included studies revealed a disagreement. However, the systematic review with the best body of evidence[19] as well as the other systematic reviews[20,21,22] concluded that premedication with 400 mg of ibuprofen significantly improved the success of IANB. As a consequence of the consistent conclusions, we considered that there was no need for a meta-analysis.
Quality of systematic reviews
The quality of two individual systematic reviews included in this umbrella review was categorized as “high.”[19,20] The quality of the other two was classified as “low”[21,22] when using the AMSTAR 2 tool. AMSTAR has been reported to provide reasonable evidence of validity and reliability and help the reader evaluate the crucial components that a systematic review should include to interpret the results and implications correctly.[2] AMSTAR 2 has 11 aspects which include a priori design, study selection, and data extraction process, literature search, publication status, study list, characteristics of the included studies, scientific quality of included and evaluated studies, scientific quality of included studies used in formulating conclusions, methods of combining findings, publication bias, and conflict of interest.[2] AMSTAR's high score for a systematic review does not necessarily mean that the initial randomized clinical trials they included were of high quality. However, it is crucial to perform a qualitative evaluation of the randomized clinical trials included in a systematic review to evaluate the quality of the evidence obtained from a subsequent meta-analysis.
Strengths
We conducted the current umbrella review with a strong methodology because it used three electronic databases to search for and identify relevant systematic reviews. Two independent reviewers participated in the selection of the systematic review and data extraction. This accurate method improves the quality of the review process. Umbrella review only included systematic reviews that included randomized clinical trials to provide the highest level of evidence. In addition, registering a priori protocol in the PROSPERO database improves the methodological quality and reporting of the review, increases transparency, reduces the potential for bias, and helps prevent unwanted duplication of studies.
Limitations
The heterogeneity between the randomized clinical trials included in the systematic reviews is widely one of the limitations of this umbrella review. Study heterogeneity included geographical location, sample size, operator experience, criteria for detecting irreversible pulpitis, the volume of anesthetic solution, vasoconstrictor concentration, and injection rate. Systematic reviews published in languages other than English have been omitted, creating a degree of selection bias.
Inconsistencies at the initial research-level complicate the interpretation in this umbrella review because the outcome criterion used to evaluate the efficacy of premedication alongside local anesthetic solutions in randomized clinical trials varied between studies. In some clinical trials, the efficacy of local anesthesia was assessed by a pulp sensitivity test (cold test or EPT). In contrast, in others, efficacy was requested by the patient to show discomfort/pain using the Visual Analogue Scale during the access cavity preparation or pulp removal. This variation in outcome measures causes uncertainty and confusion in the subsequent systematic review, so physicians are unsure of the best premedication and anesthetic solution to use in the root canal treatment.
Concluding remarks
There is ample evidence that premedication with ibuprofen at a dose of >400 mg is associated with a higher success rate of local anesthesia following IANBs
There are limited studies to suggest the use of opioids in patients with irreversible pulpitis undergoing root canal treatment
Acetaminophen appears to be an alternative in patients who are not allowed to use NSAIDs (patients allergic to aspirin-like drugs), but it is not as effective as ibuprofen in enhancing the success of IANB
Ibuprofen at a dose of <400 mg has no significant effect on the success of IANB.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Footnotes
To cite this tool: Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008.]
REFERENCES
- 1.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Mrkobrada M, Thiessen-Philbrook H, Haynes RB, Iansavichus AV, Rehman F, Garg AX. Need for quality improvement in renal systematic reviews. Clin J Am Soc Nephrol. 2008;3:1102–14. doi: 10.2215/CJN.04401007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: A cross-sectional study. PLoS Med. 2016;13:e1002028. doi: 10.1371/journal.pmed.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton RE, Reader A, Nusstein JM. Local anesthesia. In: Torabinejad M, Walton RE, editors. Endodontics, Principles and Practice. 4th ed. St. Louis, MO: Elsevier; 2008. pp. 129–47. [Google Scholar]
- 6.Drum M, Reader A, Nusstein J, Fowler S. Successful pulpal anesthesia for symptomatic irreversible pulpitis. J Am Dent Assoc. 2017;148:267–71. doi: 10.1016/j.adaj.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Nusstein J, Reader A, Nist R, Beck M, Meyers WJ. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1: 100,000 epinephrine in irreversible pulpitis. J Endod. 1998;24:487–91. doi: 10.1016/S0099-2399(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 8.Reisman D, Reader A, Nist R, Beck M, Weaver J. Anesthetic efficacy of the supplemental intraosseous injection of 3% mepivacaine in irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:676–82. doi: 10.1016/s1079-2104(97)90372-3. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy S, Reader A, Nusstein J, Beck M, Weaver J. The significance of needle deflection in success of the inferior alveolar nerve block in patients with irreversible pulpitis. J Endod. 2003;29:630–3. doi: 10.1097/00004770-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Henry MA, Hargreaves KM. Peripheral mechanisms of odontogenic pain. Dent Clin North Am. 2007;51:19–44, v. doi: 10.1016/j.cden.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–31. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves KM, Keiser K. Local anesthetic failure in endodontics. Endod Topics. 2002;1:26–39. [Google Scholar]
- 13.Saha SG, Jain S, Dubey S, Kala S, Misuriya A, Kataria D. Effect of oral premedication on the efficacy of inferior alveolar nerve block in patients with symptomatic irreversible pulpitis: A prospective, double-blind, randomized controlled clinical trial. J Clin Diagn Res. 2016;10:ZC25–9. doi: 10.7860/JCDR/2016/16873.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartling L, Chisholm A, Thomson D, Dryden DM. A descriptive analysis of overviews of reviews published between 2000 and 2011. PLoS One. 2012;7:e49667. doi: 10.1371/journal.pone.0049667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker LA, Oxman AD. Overviews of reviews. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). Ch. 22. Chichester (UK): The Cochrane Collaboration; 2011. [Google Scholar]
- 16.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. CMAJ. 1997;156:1411–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Nagendrababu V, Pulikkotil SJ, Veettil SK, Teerawattanapong N, Setzer FC. Effect of nonsteroidal anti-inflammatory drug as an oral premedication on the anesthetic success of inferior alveolar nerve block in treatment of irreversible pulpitis: A systematic review with meta-analysis and trial sequential analysis. J Endod. 2018;44:914–22.e2. doi: 10.1016/j.joen.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 20.de Geus JL, Wambier LM, Boing TF, Loguercio AD, Reis A. Effect of ibuprofen on the efficacy of inferior alveolar nerve block in patients with irreversible pulpitis: A meta-analysis. Aust Endod J. 2019;45:246–58. doi: 10.1111/aej.12306. [DOI] [PubMed] [Google Scholar]
- 21.Shirvani A, Shamszadeh S, Eghbal MJ, Marvasti LA, Asgary S. Effect of preoperative oral analgesics on pulpal anesthesia in patients with irreversible pulpitis-a systematic review and meta-analysis. Clin Oral Investig. 2017;21:43–52. doi: 10.1007/s00784-016-1974-1. [DOI] [PubMed] [Google Scholar]
- 22.Karapinar-Kazandag M, Tanalp J, Ersev H. Effect of premedication on the success of inferior alveolar nerve block in patients with irreversible pulpitis: A systematic review of the literature. Biomed Res Int 2019. 2019:6587429. doi: 10.1155/2019/6587429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung J, McDonagh M, Sedgley CM. Does articaine provide an advantage over lidocaine in patients with symptomatic irreversible pulpitis? A systematic review and meta-analysis. J Endod. 2015;41:1784–94. doi: 10.1016/j.joen.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Silva V, Grande AJ, Martimbianco AL, Riera R, Carvalho AP. Overview of systematic reviews – A new type of study: Part I: Why and for whom? Sao Paulo Med J. 2012;130:398–404. doi: 10.1590/S1516-31802012000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva V, Grande AJ, Carvalho AP, Martimbianco AL, Riera R. Overview of systematic reviews – A new type of study.Part II. Sao Paulo Med J. 2015;133:206–17. doi: 10.1590/1516-3180.2013.8150015. [DOI] [PMC free article] [PubMed] [Google Scholar]