Abstract
Background
Although several studies have found that the serum lipid profile may be correlated with hepatocellular carcinoma (HCC), the causal relationships between the serum lipid profile and HCC have not been determined due to potential confounder. Here, Mendelian randomization (MR) analysis was performed to identify the relationship between the serum lipid profile and HCC in the East Asian population.
Method
Our study made a MR analysis with the validation of two data sets. We obtained genome-wide association study (GWAS) data related to the serum lipid profile from Asian Genetic Epidemiology Network (AGEN). Then, the data from a recent large GWAS of the East Asian ancestry in Japan (BioBank Japan, BBJ) were extracted. Summary-level statistical data for HCC were obtained from a large GWAS of the East Asian ancestry in Japan. Univariable MR analysis were performed to identify whether the genetic evidence of serum lipid profile was significantly associated with HCC risk. Multivariable MR analysis was conducted to estimate the independent effects of exposures on HCC.
Results
Univariable and multivariable MR analyses indicated that the serum lipid profile was not a risk factor for HCC incidence in either data set based on the East Asian population. Multivariable MR analysis revealed that the hazard ratios of the probability of HCC in AGEN were 1.134 (95% confidence interval (CI), 0.903–1.424) for TG, 1.010 (95% CI: 0.824–1.237) for HDL-C, 0.974 (95% CI: 0.746–1.271) for TC, 0.918 (95% CI: 0.734–1.147) for LDL-C, while the results in BBJ were also non-significant: 1.111 (95% CI: 0.869–1.419) for TG, 0.957 (95% CI: 0.790–1.158) for HDL-C, 0.917 (95% CI: 0.643–1.308) for TC, 0.932 (95% CI: 0.699–1.243) for LDL-C.
Conclusion
Our MR study with the validation of two data sets found no strong evidence to support causal associations between the serum lipid profile and HCC risk.
Keywords: Mendelian randomization, Serum lipid profile, HCC, Causal relationship
1. Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide [[1], [2], [3], [4]]. In recent decades, considerable progress has been made in the study of the epidemiology, risk factors, molecular characteristics and pathogenesis of HCC. Epidemiological and experimental studies have identified several major risk factors that associated with hepatocarcinogenesis, including chronic hepatitis B/C, type 2 diabetes mellitus (T2DM) and cirrhosis. Targeting these risk factors, therapeutic measures such as direct antivirals, and the use of metformin, are associated with risk reduction of HCC, and can even delay the postoperative recurrence of HCC [3,[5], [6], [7], [8]]. Identifying new risk factors and taking appropriate treatment measures will contribute to improving HCC patients prognosis.
Lipid metabolism plays a vital role in the stability of the cell membrane and energy supply, thereby impacting cell growth and proliferation by regulating multiple signaling pathways. Lipids and cholesterol can be exploited by most types of cancer to meet their increased energy demands. Cancer cells even have some characteristics of fat cells, storing excess lipids as droplets of fat that provide energy for cancer cells to expand and metastasize [[9], [10], [11], [12]]. Some studies have been explored to determine the underlying association between the serum lipid profile and HCC. One study showed that the mean serum total lipoprotein cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) levels in HCC patients were lower than those in controls, while another study reported that the HCC group had significantly lower serum TC, TG, LDL-C, and high-density lipoprotein cholesterol (HDL-C) levels than the non-HCC group [13,14]. Although some studies have found that a lower lipid profile is positively correlated with HCC, the causality cannot be determined through these observational studies. For example, low serum lipid profiles could be a manifestation of HCC cachexia, and the consumption of cholesterol is higher in HCC tissues than in nontumor tissues. Second, preclinical HCC might itself reduce the serum cholesterol [15,16], which by activating the receptor activity of LDL-C in HCC cells. Due to the methodological limitations of traditional observational studies these associations may be biased, including confounding and measurement error. Clarifying the relationship between the serum lipid profile and HCC is beneficial for our understanding of the mechanism of HCC and for the potential development of prevention policies.
Mendelian randomization (MR) is a new statistical method originally applied in economics. As an emerging statistical analysis method, MR is used for causal reasoning of risk factors and diseases in epidemiology. In MR, SNPs were used as an instrumental variable to reveal the causality of the association between exposure and outcome, so the analysis results were not affected by potential confounding factors. These advantages make MR an important approach for determining the causal association of modified risk factors with diseases. MR can be used to analyze whether the biomarker contributes to the incidence and development of disease or the observed association is confounded by unrecognized exogenous factors or reverse causation and has been widely used in various fields, including finding risk factors for diseases.
Here, an MR study was performed to validate and identify the risk factors for HCC in the East Asian population by enrolling a series of definite or unclear HCC risk factors. An important aim of this MR study is to clarify the causal relationship between the serum lipid profile and HCC in the East Asian population.
2. Methods
2.1. Summarized statistics of risk factors from genome-wide association study (GWAS) in Asian Genetic Epidemiology Network (AGEN)
We obtained the GWAS summary data of HDL-C, LDL-C, TG and TC from the Asian Genetic Epidemiology Network (AGEN), (https://blog.nus.edu.sg/agen/). AGEN is a consortium of genetic epidemiology studies of several diseases including T2DM and cardiovascular disease (CVD) based on East Asian populations. The study included 4 lipid traits: HDL-C, LDL-C, TC, and TG. The samples contained 34,421 East Asians. Within each study, residuals of lipid traits were adjusted by specific covariates including age, sex, body mass index (BMI) in a linear regression model (Table 1).
Table 1.
The description of summary statistics of AGEN.
| Ethnicity | Age |
BMI |
HDL-C |
LDL-C |
TC |
TG |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| (SD) | (SD) | NO. | Mean (SD) | NO. | Mean (SD) | NO. | Mean (SD) | NO. | Mean (SD) | |
| F | - | - | 110.37 | 102.51 | 67.06 | 137.68 | ||||
| R2 | - | - | 5.74 | 2.68 | 15.73 | 7.35 | ||||
| Japanese | 65.8 (8.0) | 23.4 (3.2) | 1283 | 59.6 (16.9) | 1282 | 122.9 (30.9) | 1290 | 205.9 (34.7) | 1290 | 118.3 (64.2) |
| Filipino | 48.4 (6.1) | 24.3 (4.4) | 1790 | 41.0 (10.4) | 1759 | 119.8 (33.6) | 1781 | 186.8 (39.3) | 1781 | 130.9 (83.7) |
| Chinese | 38.7 (9.5) | 23.4 (3.2) | 1859 | 51.0 (11.3) | 1859 | 95.1 (27.4) | 1859 | 170.7 (33.7) | 1859 | 123.5 (78.2) |
| Korean | 52.2 (8.9) | 24.6 (3.1) | 8801 | 44.7 (10.1) | 8543 | 115.7 (32.1) | 8801 | 191.4 (35.9) | 8801 | 162.4 (104.5) |
| Korean | 41.3 (8.5) | 23.7 (3.1) | 959 | 54.2 (12.9) | 960 | 109.8 (29.5) | 959 | 185.2 (32.5) | 959 | 117.6 (89.5) |
| Chinese | 49.9 (8.5) | 23.3 (3.4) | 2014 | 51.3 (12.8) | 1032 | 100.6 (31.9) | 1032 | 173.3 (38.5) | 1064 | 129.0 (143.5) |
| Chinese | 56.6 (8.9) | 23.4 (3.5) | 1375 | 51.6 (15.3) | 1375 | 134.5 (33.3) | 1375 | 219.5 (38.1) | – | – |
| Malay | 58.5 (11.2) | 26.2 (5.2) | 2076 | 52.5 (12.8) | 2076 | 139.9 (38.3) | 2077 | 220.4 (43.8) | – | – |
| Chinese | 46.2 (9.9) | 22.8 (3.5) | 879 | 53.7 (14.1) | 879 | 120.7 (30.8) | 879 | 199.4 (33.4) | 879 | 117.0 (83.6) |
| Chinese | 47.5 (11.0) | 22.5 (3.9) | 1035 | 59.1 (14.1) | 1035 | 121.2 (31.8) | 1035 | 201.6 (35.7) | 1035 | 107.5 (65.5) |
| Chinese | 48.2 (12.4) | 23.6 (3.6) | 292 | 54.5 (12.8) | 292 | 122.7 (33.6) | 292 | 202.7 (37.9) | 292 | 128.4 (74.5) |
| Chinese | 57.4 (9.4) | 24.4 (3.4) | 1817 | 50.0 (12.9) | 445 | 114.7 (34.8) | 445 | 193.5 (41.7) | 461 | 154.2 (113.6) |
| Chinese | 51.0 (17.8) | 23.8 (3.5) | 989 | 49.5 (12.8) | 989 | 90.7 (28.2) | 989 | 189.6 (38.9) | – | – |
Notes: R2 is the phenotype variance explained by genetic; F is the F statistics. NO. Is sample number.
2.2. Summarized statistics of risk factors from a genome-wide association study (GWAS) in BioBank Japan (BBJ)
The GWAS summary statistics of the serum lipid profile were extracted from Masahiro Kanai's study [17], which tested 5,961,600 autosomal variants and 147,353 X-chromosome variants for association with 58 traits in 162,255 East Asian ancestry Japanese individuals and identified 1407 trait-associated loci (P < 5.0 × 10−8), 679 of which were novel. The GWAS summary statistics of the serum lipid profile in our study included four phenotypes: TC, HDL-C, LDL-C and TG. For TC GWAS, the participants are 128,305 Japanese individuals. The GWAS summary statistics of HDL-C and LDL-C comprised 70,657 and 72,866 Japanese individuals, respectively. For TG GWAS, the study included 105,597 Japanese individuals (Table 2).
Table 2.
The description of summary statistics of BBJ.
| Exposure | Mean Age | Sample Size | R2 (%) | F | PMID | Sex (%) |
|---|---|---|---|---|---|---|
| TC | 63.1 ± 12.9 | 128,305 | 5.85 | 78.29 | 29,403,010 | Male (55.0) Female (45.0) |
| HDL-C | 63.7 ± 12.1 | 70,657 | 10.70 | 128.58 | 29,403,010 | Male (57.4) Female (42.6) |
| LDL-C | 63.9 ± 12.0 | 72,866 | 3.71 | 65.01 | 29,403,010 | Male (57.2) Female (42.8) |
| TG | 63.6 ± 12.5 | 105,597 | 16.82 | 178.14 | 29,403,010 | Male (55.9) Female (44.1) |
Notes: R2 is the phenotype variance explained by genetics. F is the F statistics.
2.3. Extraction of SNPs associated with HCC
Summary-level statistical data for HCC were also obtained from a large GWAS of the East Asian ancestry in Japan. This study included 212,453 Japanese people and performed genetic variance analysis (GWAS) for 42 diseases, elucidating the biological characteristics of polygenic diseases in East Asian populations [18]. In this study, they adjusted for covariates that included age, sex, and the first five principal components.
2.4. Mendelian randomization design and instrumental variables selection
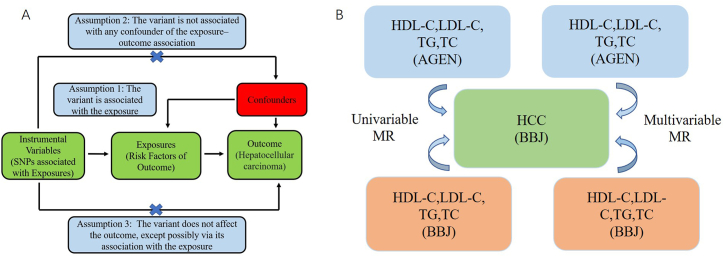
Mendelian randomized analysis is used to screen eligible SNPs from public large-scale GWAS data as IVs to detect causality between exposure and outcome [19]. The IV must satisfy the fundamental conditions as follows: I. The IVs are associated with the exposures. II. IVs are not associated with outcome by means other than exposures. III. IVs cannot directly affect outcome, if only through exposure (Fig. 1A). SNPS significantly correlated with circulating blood lipids were selected from the GWAS data, and the significance level was P < 5 × 10−8. The minor allele frequency of the SNP was >0.01. Meeting the linkage disequilibrium (LD) of a given genomic region is a condition that SNPs as IVs in MR analysis must meet, and SNPs (selection threshold: r2 < 0.001, kb > 10,000) with palindromic structure need to be removed. The power of the SNP used as IV was assessed using the F-statistic: F statistic (F = bet2/se2). SNPs with an F-statistic less than 10 were determined to be weak instruments, and we exclude them (Fig. 1B). Besides, SNPs related gene names are listed in table S1 (AGEN) and table S2 (BBJ).
Fig. 1.
The basic assumptions of Mendelian randomization (A) and the main design of this study (B).
2.5. Mendelian randomization analysis and sensitivity test
Inverse variance weighted (IVW) is the main method conducted in MR analysis of GWAS data. The supplementary analysis methods to the IVW method were MR‒Egger and weighted-median (WM) methods [20]. For univariable MR, IVW, MR-Egger and WM were used to estimate the effect of the serum lipid profile on HCC. For multivariable MR, the IVW module was used to identify the relationship between the serum lipid profile and HCC. If any outliers existed, we would remove the SNPs with horizontal pleiotropy and retested the causal relationship between the serum lipid profile and HCC. To assess pleiotropy, we performed the intercept test of MR-Egger. To detected the heterogeneity, Cochran's Q test in IVW and MR Egger model were performed [21]. Moreover, an online application were used to test the statistical power of this study (https://cnsgenomics.shinyapps.io/mRnd/) [22]. The and support data is provided in this Github repository (https://github.com/pgqpgq/MR-HCC.).
We used the Clump_data command in the “TwoSampleMR” package to select the independent SNPs as IVs for risk factors. The R packages “Two Sample MR” and “Mendelian randomization” were used to identify the relationship between exposures and outcome in univariable MR analysis [23,24]. MR and MR-PRESSO detection were conducted by the R package “multivariable Mendelian randomization” (“MVMR”) [24,25].
3. Result
Among the exposures of both data sets in our study, the genetically-predicted lipid profile might not associate with the risk of HCC. This study provides novel insight into the risk factors for HCC in an East-Asian ancestry population.
3.1. Univariable MR analysis of exposures on HCC risks in AGEN
Although some previous conventional studies have shown a positive association between serum lipid levels and HCC risk, our MR analysis showed no strong evidence to support a causal relationship between circulating lipid levels and HCC risk in East Asian populations. In AGEN, the hazard ratios of the probability of HCC were 1.134 (95% confidence interval (CI), 0.938–1.371, P = 0.193) for TGs, 1.043 (95% CI: 0.886–1.228, P = 0.613) for HDL-C, 0.948 (95% CI: 0.765–1.175, P = 0.627) for TC, 0.911 (95% CI: 0.762–1.089, P = 0.308) for LDL-C (Table 3).
Table 3.
The MR estimates, test of heterogeneity and test of pleiotropy of exposures on HCC based on AGEN.
| Exposure | NSNP | MR methodology | Effect Estimates HCC |
Test of heterogeneity |
Test of pleiotropy |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%LCI | 95%UCI | P value | Cochrane Q test | Phetero- geneity | MR Egger intercept | Ppleio- tropy | |||
| TG | 8 | IVW | 1.134 | 0.938 | 1.371 | 0.193 | 21.467 | 0.003 | ||
| MR Egger | 0.852 | 0.435 | 1.669 | 0.657 | 18.600 | 0.004 | 0.047 | 0.373 | ||
| Weighted median | 0.972 | 0.771 | 1.224 | 0.808 | ||||||
| TC | 16 | IVW | 0.948 | 0.765 | 1.175 | 0.627 | 23.283 | 0.078 | ||
| MR Egger | 0.879 | 0.489 | 1.582 | 0.674 | 23.149 | 0.058 | 0.008 | 0.780 | ||
| Weighted median | 1.064 | 0.773 | 1.466 | 0.703 | ||||||
| HDL-C | 16 | IVW | 1.043 | 0.886 | 1.228 | 0.613 | 12.477 | 0.643 | ||
| MR Egger | 1.079 | 0.764 | 1.524 | 0.672 | 12.429 | 0.572 | −0.005 | 0.829 | ||
| Weighted median | 1.047 | 0.835 | 1.314 | 0.689 | ||||||
| LDL-C | 13 | IVW | 0.911 | 0.762 | 1.089 | 0.308 | 17.837 | 0.121 | ||
| MR Egger | 0.891 | 0.636 | 1.249 | 0.403 | 17.786 | 0.087 | 0.004 | 0.862 | ||
| Weighted median | 0.975 | 0.772 | 1.232 | 0.813 | ||||||
Notes: NSNP is the number of single nucleotide polymorphism; The odds ratio (OR) is per 1 SD increase; 95%LCI is the lower limit of 95% confidence interval; 95%UCI is the upper limit of 95% confidence interval; P value is the p-value of OR; Pheterogeneity is the p-value of Cochrane's Q value in heterogeneity test; Ppleiotropy is the p-value of MR-Egger intercept.
3.2. Multivariable MR analysis of exposures on HCC risks in AGEN
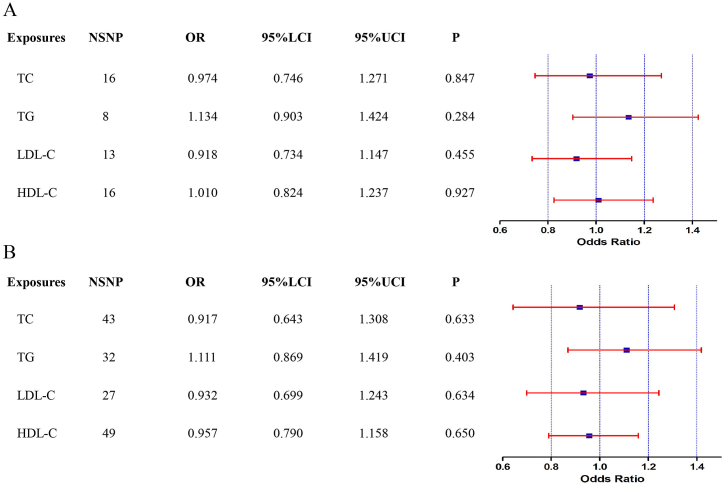
Among the four traits of the serum lipid profile, we did not observe that the serum lipid profile had a causal effect on HCC occurrence when using SNP associated exposures. Multivariable MR analysis revealed that the hazard ratios of the probability of HCC in AGEN were 1.134 (95% CI: 0.903–1.424, P = 0.284) for TG, 1.010 (95% CI: 0.824–1.237, P = 0.927) for HDL-C, 0.974 (95% CI: 0.746–1.271, P = 0.847) for TC, 0.918 (95% CI: 0.734–1.147, P = 0.455) for LDL-C (Fig. 2A).
Fig. 2.
The forest plot of multivariable Mendelian randomization results. OR is the odds ratio; 95% LCI is the lower limit of the 95% confidence interval; 95% UCI is the upper limit of the 95% confidence interval. A (Forest plot of multivariable MR results in AGEN), B (Forest plot of multivariable MR results in BBJ).
3.3. Univariable MR analysis of exposures on HCC risks in BBJ
To further verify the reliability of our results, we extracted lipid-related GWAS data from another published large-scale serum lipid profile GWAS data and confirmed that serum lipid profiles at genetic predictive levels were not associated with HCC. We identified that TG, HDL-C, TC, and LDL-C were not risk factors for HCC. The hazard ratios of the probability of HCC were 1.114 (95% CI, 0.911–1.362, P = 0.292) for TG, 0.950 (95% CI: 0.817–1.111, P = 0.519) for HDL-C, 0.903 (95% CI: 0.680–1.200, P = 0.482) for TC, 0.917 (95% CI: 0.729–1.155, P = 0.462) for LDL-C (Table 4).
Table 4.
The MR estimates, test of heterogeneity and test of pleiotropy of exposures on HCC based on BBJ.
| Exposure | NSNP | MR methodology | Effect Estimates HCC |
Test of heterogeneity |
Test of pleiotropy |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%LCI | 95%UCI | P value | Cochrane Q test | Phetero- geneity | MR Egger intercept | Ppleio- tropy | |||
| TG | 32 | IVW | 1.114 | 0.911 | 1.362 | 0.292 | 59.760 | 0.001 | ||
| MR Egger | 1.014 | 0.649 | 1.584 | 0.952 | 59.198 | 0.001 | 0.008 | 0.597 | ||
| Weighted median | 1.059 | 0.758 | 1.479 | 0.738 | ||||||
| TC | 43 | IVW | 0.903 | 0.680 | 1.200 | 0.482 | 61.154 | 0.028 | ||
| MR Egger | 1.012 | 0.415 | 2.465 | 0.980 | 61.044 | 0.023 | 0.019 | 0.788 | ||
| Weighted median | 0.740 | 0.473 | 1.158 | 0.188 | ||||||
| HDL-C | 49 | IVW | 0.950 | 0.817 | 1.111 | 0.519 | 58.691 | 0.122 | ||
| MR Egger | 1.053 | 0.781 | 1.419 | 0.736 | 47 | 0.118 | −0.006 | 0.600 | ||
| Weighted median | 0.984 | 0.759 | 1.276 | 0.904 | ||||||
| LDL-C | 27 | IVW | 0.917 | 0.729 | 1.155 | 0.462 | 31.142 | 0.223 | ||
| MR Egger | 1.050 | 0.702 | 1.570 | 0.816 | 30.276 | 0.214 | −0.012 | 0.406 | ||
| Weighted median | 1.075 | 0.747 | 1.549 | 0.666 | ||||||
Notes: NSNP is the number of single nucleotide polymorphism; The odds ratio (OR) is per 1 SD increase; 95%LCI is the lower limit of 95% confidence interval; 95%UCI is the upper limit of 95% confidence interval; P value is the p-value of OR; Pheterogeneity is the p-value of Cochrane's Q value in heterogeneity test; Ppleiotropy is the p-value of MR-Egger intercept.
3.4. Multivariable MR analysis of exposures on HCC risks in BBJ
Multivariable MR analysis supports the notion that a lower serum lipid profile might not be responsible for the reported association between a lower serum lipid profile and HCC. Multivariable MR analysis revealed that the hazard ratios of the probability of HCC in BBJ were nonsignificant: 1.111 (95% CI: 0.869–1.419, P = 0.403) for TG, 0.957 (95% CI: 0.790–1.158, P = 0.650) for HDL-C, 0.917 (95% CI: 0.643–1.308, P = 0.633) for TC, and 0.932 (95% CI:0.699–1.243, P = 0.634) for LDL-C (Fig. 2B). The effects between SNP associated exposures and outcome were visualized by R software 4.1.1.
3.5. Sensitivity analysis
The heterogeneity and pleiotropy of the results tested in our study. The MR-Egger intercept represented the average level of pleiotropy of all SNP-associated exposure [26]. We did not identify significant horizontal pleiotropic effects in the MR Egger test (all P values greater than 0.1). According to Cochran's Q test in the IVW model and MR Egger model, there was heterogeneity of TG (P < 0.05) in both data sets.
The statistical power of these exposures was 100%.
4. Discussion
As an important part of lipid traits, serum lipid cholesterol plays a vital role in cell structure and energy supply. In addition to maintaining the stability of the cell membrane and energy supply, lipid metabolism can impact the physiological activities of cancer cells. As the liver is a crucial organ responsible for lipid metabolism which is altered in the early stage of HCC, lipid metabolism might play an important role in HCC development [27,28]. For instance, various metabolites of cholesterol are positively correlated with the growth and metastasis of cancers including HCC [[29], [30], [31], [32], [33]]. On the other hand, several studies have shown that cholesterol has some tumour suppressant activity [34,35]. The relationship between the serum lipid profile and HCC has not yet been elucidated [9,36]. Recently, a study implied that a lower serum lipid profile, including TC, LDL-C, TG and HDL-C, could increase the risk of HCC in the Korean population [13]. Another study showed that the serum TC, LDL-C, and TG levels in HCC patients were lower than those in the healthy population [14]. An MR study reported that lower HDL-C was a risk factor for HCC, but this study was based on a mixed-ancestry population [37]. In addition, total cholesterol, high density cholesterol and low-density cholesterol levels were shown to be significantly lower in Chinese HCC patients than in healthy controls [38].
Our study utilized the serum lipid profile GWAS data from AGEN to analyze the causality between the serum lipid profile and HCC. To complete our MR analysis, another publicly available large-scale GWAS data (BBJ) of the serum lipid profile was also used to validate the causality. Our results indicated that there was no causal relationship between the serum lipid profile and HCC in the East Asian population based on both two data sets. Our multivariate MR Analysis showed that increased HDL-C levels in the AGEN database appeared to increase HCC risk, but elevated HDL-C levels in patients with BBJ were associated with reduced HCC risk. This may be due to differences in several SNPs associated with HDL-C in the two data sets. For example, the top 5 SNPs with the largest effect value of HDL-C in AGEN are rs3741297, rs1883025, rs7165077, rs429358 and rs72654473. On the other hand, the top 5 SNPs with the largest effect value of HDL-C in BBJ are rs1883025, rs429358, rs76083992, rs7165077, rs72654473. The possibility of false negative results should be low in our study because of rigorous instrumental variable selections, sensitivity tests procedures and MR sensitivity tests.
Our study has several limitations that need to be addressed. First, several SNPs included in our study may be associated with obesity and T2DM [39], which may lead to horizontal pleiotropy. Second, although a large number of individuals were enrolled, the number of cases for each lipid trait and HCC was still relatively limited, which led to the potential underestimated effect of lipid profiles on HCC. Third, the negative association between the serum lipid profile and HCC may be attributed to the low proportion of variance which was explained by the instrumental variable in our MR analysis. Finally, our MR analysis was performed in two databases from East-Asian populations, so our results should be further validated in other ethnicities.
Although there is no correlation between HCC risk and TG according to our MR the impact of the serum lipid profile on HCC development might be smaller than we thought. There was an overestimation of the association between the serum lipid profile and HCC in the conventional regression analyses, possibly due to uncontrolled confounding by common risk factors or reverse causation. However, controlling serum lipids is certainly important for the prevention of HCC, because serum lipids and HCC share numerous established modifiable risk factors such as obesity and T2DM [40].
5. Conclusion
In conclusion, our MR results suggested that there is little evidence to support the genetic role of the serum lipid profile in HCC development in the East Asian population. Future studies are needed to extend the population and verify our results.
Author contribution statement
All authors listed have substantial contributed to the development and the writing of this article.
Guo-Qiang Pan: Conceived and designed the study; Performed the MR analysis; Wrote the paper.
Yan Jiao: Performed the MR analysis; Analysed and interpreted the data.
Guang-Xiao Meng: Analysed and interpreted the data; Contributed GWAS data.
Zhao-Ru Dong; Tao Li: Conceived and designed the MR study; Contributed GWAS data, analysis data; Wrote the paper.
Data availability statement
GWAS Catalog: https://www.ebi.ac.uk/gwas/
Open GWAS: IEU OpenGWAS project (mrcieu.ac.uk).
Funding information
This work was supported by the National Natural Science Foundation of China (82172647, 82073200, 81874178), the Taishan Scholars Program for Experts of Shandong Province (tstp20221158), Funds for Independent Cultivation of Innovative Team from Universities in Jinan (2020GXRC023), Shandong Provincial Natural Science Foundation (ZR2021ZD26, ZR2021MH194) and China Postdoctoral Science Foundation (2020M682192, 2022T150385).
Ethics statement
The GWAS summary statistics data used in our study were publicly available, which obtained informed consent from all participating studies by following the protocols approved by their respective institutional review boards. No separate ethical approval was required for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The authors thank all investigators and participants from BBJ (Biobank-Japan) for sharing genetic association estimates for HCC and serum lipid profile. Besides, we would like to thank all investigators contributing to GWAS of Asian Genetic Epidemiology Network (AGEN).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17126.
Contributor Information
Zhao-Ru Dong, Email: dongzhaoru0911@163.com.
Tao Li, Email: litao7706@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Fattovich G., Stroffolini T., Zagni I., et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004 Nov;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Dong Z.R., Cai J.B., Shi G.M., et al. Oncogenic miR-93-5p/Gal-9 axis drives CD8 (+) T-cell inactivation and is a therapeutic target for hepatocellular carcinoma immunotherapy. Cancer Lett. 2023 Jun 28;564 doi: 10.1016/j.canlet.2023.216186. [DOI] [PubMed] [Google Scholar]
- 3.Chen J., Ding C., Chen Y., et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett. 2021 Apr 1;502:154–165. doi: 10.1016/j.canlet.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Pan G.Q., Yang C.C., Shang X.L., et al. The causal relationship between white blood cell counts and hepatocellular carcinoma: a Mendelian randomization study. Eur. J. Med. Res. 2022 Dec 6;27(1):278. doi: 10.1186/s40001-022-00900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Wang H., Xiao H. Metformin actions on the liver: protection mechanisms emerging in hepatocytes and immune cells against NASH-related HCC. Int. J. Mol. Sci. 2021 May 9;22(9) doi: 10.3390/ijms22095016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng C.H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018 Nov;38(11):2018–2027. doi: 10.1111/liv.13872. [DOI] [PubMed] [Google Scholar]
- 7.Hall Z., Chiarugi D., Charidemou E., et al. Lipid remodeling in hepatocyte proliferation and hepatocellular carcinoma. Hepatology. 2021 Mar;73(3):1028–1044. doi: 10.1002/hep.31391. [DOI] [PubMed] [Google Scholar]
- 8.Wu S., Ye S., Lin X., et al. Small hepatitis B virus surface antigen promotes malignant progression of hepatocellular carcinoma via endoplasmic reticulum stress-induced FGF19/JAK2/STAT3 signaling. Cancer Lett. 2021 Feb 28;499:175–187. doi: 10.1016/j.canlet.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Patel K.K., Kashfi K. Lipoproteins and cancer: the role of HDL-C, LDL-C, and cholesterol-lowering drugs. Biochem. Pharmacol. 2022 Feb;196 doi: 10.1016/j.bcp.2021.114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long J., Zhang C.J., Zhu N., et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8(5):778–791. [PMC free article] [PubMed] [Google Scholar]
- 11.Huang B., Song B.L., Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020 Feb;2(2):132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuzu O.F., Noory M.A., Robertson G.P. The role of cholesterol in cancer. Cancer Res. 2016 Apr 15;76(8):2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho Y., Cho E.J., Yoo J.J., et al. Association between lipid profiles and the incidence of hepatocellular carcinoma: a nationwide population-based study. Cancers. 2021 Mar 30;13(7) doi: 10.3390/cancers13071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motta M., Giugno I., Ruello P., et al. Lipoprotein (a) behaviour in patients with hepatocellular carcinoma. Minerva Med. 2001 Oct;92(5):301–305. [PubMed] [Google Scholar]
- 15.Alsabti E.A. Serum lipids in hepatoma. Oncology. 1979;36(1):11–14. doi: 10.1159/000225310. [DOI] [PubMed] [Google Scholar]
- 16.Ahaneku J.E., Taylor G.O., Olubuyide I.O., et al. Abnormal lipid and lipoprotein patterns in liver cirrhosis with and without hepatocellular carcinoma. J. Pakistan Med. Assoc. 1992 Nov;42(11):260–263. [PubMed] [Google Scholar]
- 17.Kanai M., Akiyama M., Takahashi A., et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 2018 Mar;50(3):390–400. doi: 10.1038/s41588-018-0047-6. [DOI] [PubMed] [Google Scholar]
- 18.Ishigaki K., Akiyama M., Kanai M., et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat. Genet. 2020 Jul;52(7):669–679. doi: 10.1038/s41588-020-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014 Sep 15;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013 Nov;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017 May;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brion M.J., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013 Oct;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemani G., Zheng J., Elsworth B., et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018 May 30:7. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yavorska O.O., Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017 Dec 1;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson E., Spiller W., Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021 Nov 10;40(25):5434–5452. doi: 10.1002/sim.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemani G., Bowden J., Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018 Aug 1;27(R2):R195–r208. doi: 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berndt N., Eckstein J., Heucke N., et al. Characterization of lipid and lipid droplet metabolism in human HCC. Cells. 2019 May 27;8(5) doi: 10.3390/cells8050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao X., Cheung K.S., Peng C., et al. Steatosis, HBV-related HCC, cirrhosis, and HBsAg seroclearance: a systematic review and meta-analysis. Hepatology. 2022 Sep 15;77(5):1735–1745. doi: 10.1002/hep.32792. [DOI] [PubMed] [Google Scholar]
- 29.Montero J., Morales A., Llacuna L., et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008 Jul 1;68(13):5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 30.Ribas V., García-Ruiz C., Fernández-Checa J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016 Dec;5(1):22. doi: 10.1186/s40169-016-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facciorusso A., Abd El Aziz M.A., Singh S., et al. Statin use decreases the incidence of hepatocellular carcinoma: an updated meta-analysis. Cancers. 2020 Apr 3;12(4) doi: 10.3390/cancers12040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam M.M., Poly T.N., Walther B.A., et al. Statin use and the risk of hepatocellular carcinoma: a meta-analysis of observational studies. Cancers. 2020 Mar 13;12(3) doi: 10.3390/cancers12030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai S.W., Liao K.F., Lai H.C., et al. Statin use and risk of hepatocellular carcinoma. Eur. J. Epidemiol. 2013 Jun;28(6):485–492. doi: 10.1007/s10654-013-9806-y. [DOI] [PubMed] [Google Scholar]
- 34.de Medina P., Paillasse M.R., Segala G., et al. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat. Commun. 2013;4:1840. doi: 10.1038/ncomms2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Medina P., Paillasse M.R., Segala G., et al. Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc. Natl. Acad. Sci. U. S. A. 2010 Jul 27;107(30):13520–13525. doi: 10.1073/pnas.1002922107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgquist S., Butt T., Almgren P., et al. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer. 2016 Jun 1;138(11):2648–2656. doi: 10.1002/ijc.30013. [DOI] [PubMed] [Google Scholar]
- 37.Yang C., Tian G., Mi J., et al. Causal relevance of circulating high-density lipoprotein cholesterol with cancer: a Mendelian randomization meta-analysis. Sci. Rep. 2015 Mar 30;5:9495. doi: 10.1038/srep09495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Cao X., Liu B., et al. The alterations of cholesterol, HDL-cholesterol and LDL-cholesterol in Chinese with hepatocellular carcinoma: a cross-sectional study. Asian J. Surg. 2019 Oct;42(10):938–939. doi: 10.1016/j.asjsur.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Aron-Wisnewsky J., Warmbrunn M.V., Nieuwdorp M., et al. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 2021 Jan;160(2):573–599. doi: 10.1053/j.gastro.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 40.Klop B., Elte J.W., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013 Apr 12;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GWAS Catalog: https://www.ebi.ac.uk/gwas/
Open GWAS: IEU OpenGWAS project (mrcieu.ac.uk).