Abstract
Hypoxia-inducible factor-1 (HIF-1), a heterodimeric transcription factor composed of the α and β subunits, regulates cellular adaptive responses to hypoxia. Macrophages, which are derived from monocytes, function as antigen-presenting cells that activate various immune responses. HIF-1α regulates the immune response, viability, migration, phenotypic plasticity, and metabolism of macrophages. Specifically, macrophage-derived HIF-1α can prevent excessive pro-inflammatory responses by attenuating the transcriptional activity of nuclear factor-kappa B in vivo and in vitro. HIF-1α modulates macrophage migration by inducing the release of various chemokines and providing necessary energy. HIF-1α promotes macrophage M1 polarization by targeting glucose metabolism. Additionally, HIF-1α induces the upregulation of glycolysis-related enzymes and intermediates of the tricarboxylic acid cycle and pentose phosphate pathway. HIF-1α promotes macrophage apoptosis, necroptosis and reduces autophagy. The current review highlights the mechanisms associated with the regulation of HIF-1α stabilization in macrophages as well as the role of HIF-1α in modulating the physiological functions of macrophages.
Keywords: HIF-1α, Stability, Macrophage, Biology, Diseases
Graphical abstract
1. Introduction
Hypoxia-inducible factor-1α (HIF-1α), a subunit of the heterodimeric transcription factor HIF-1, is specifically stabilized and induced by hypoxia [[1], [2], [3], [4], [5], [6], [7]]. HIF-1α contributes to the regulation of cellular metabolism and the modulation of immune responses in macrophages [8]. For instance, in obesity, expression of the HIF-1α mRNA and protein is increased in macrophages, combined with upregulation of glycolysis and enhanced release of inflammatory cytokines [9]. HIF-1α expression is upregulated in macrophages through activation of the nuclear factor-kappa B (NF-κB) pathway [10]. In lipopolysaccharide (LPS)-stimulated mouse macrophages, HIF-1α not only promoted NF-κB-mediated expression of proinflammatory cytokines but also enhanced the host defense response [11]. HIF-1α activity regulates macrophage immune responses via releasing pro-inflammatory cytokines and antimicrobial peptides, enhancing phagocytosis and nitric oxide (NO) production, inhibiting apoptosis, and facilitating the re-distribution of intracellular oxygen and the inhibition of prolyl hydroxylase domain (PHD) activity, which further improves HIF-1α protein stability and promotes phagocytic activity [12]. Therefore, HIF-1α plays a critical role in macrophage cellular metabolism and immune responses.
HIF-1α is also critical for macrophage recruitment and migration. Notably, a specific knockout of HIF-1α in myeloid lineage cells impairs their migration and normal function [13]. In severe hypoxia, HIF-1α upregulates the expression of pyruvate dehydrogenase kinase isozyme 1 (PDK1) to reprogram glucose metabolism and enhances the migration potential of macrophages [14,15]. Moreover, HIF-1α contributes to macrophage plasticity; during Th1 cytokine-induced macrophage polarization, HIF-1α promotes M1 phenotypic switching by binding inducible nitric oxidase synthase (iNOS) to maintain NO homeostasis during inflammation [12,16,17]. These findings indicate that HIF-1α contributes to macrophage polarity and behavior.
Studies on macrophage-derived HIF-1α have revealed the importance of HIF-1α-regulated processes in macrophages and highlighted the potential of HIF-1α as a possible therapeutic target for different macrophage-related diseases. In this context, by conducting a PubMed search with “HIF-1α” and “macrophage”, the current review discusses key advances in knowledge of the roles of HIF-1α as an important regulator of macrophage function, both in terms of underlying mechanisms and disease implications. Additionally, we review current literature on the roles of HIF-1α in modulating the behavior, function, polarity, and physiological processes of macrophages, as well as its regulatory effects in macrophage-associated diseases.
2. Regulation of HIF-1α stability
Hypoxia inducible factor-1 (HIF-1) is a basic loop–helix–loop protein comprising a constitutively expressed beta subunit (HIF-1β), known as the aryl hydrocarbon receptor nuclear transporter, and an oxygen-regulated alpha subunit (HIF-1α) [18]. HIF-1α or HIF-2α heterodimerizes with HIF-1β, which binds to hypoxia response elements (HREs) within the promoter region of target genes, thereby regulating their expression [19]. Other proteins interacting with the HIF-1 complex act as coactivators to transcriptionally regulate the target genes [20] with myriad functions, such as controlling metabolic reprogramming and supporting organ development and physiological adaptation [21].
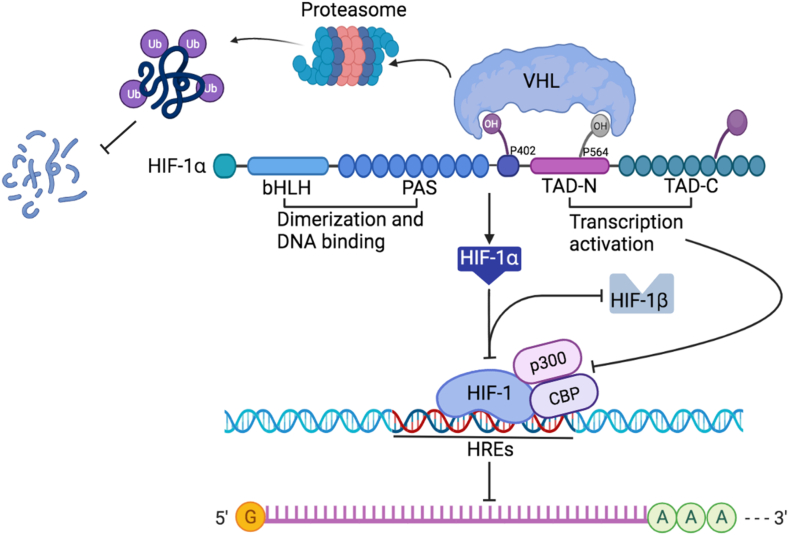
HIF-1α stability is regulated by the hydroxylation of its proline residues via PHDs (Fig. 1). Under normoxic conditions, activated PHD hydroxylates HIF-1α at its oxygen-dependent degradation domain (ODDD), facilitating its association with the von hippel-lindau (VHL) protein E3 ligase complex, subsequently resulting in HIF-1α degradation via the ubiquitin–proteasome pathway [22,23]. Conversely, under hypoxic conditions, PHD-dependent HIF-1α hydroxylation is inhibited, resulting in enhanced protein stability, nuclear translocation, and accumulation of HIF-1α. Notably, factor-inhibiting hypoxia-inducible factor (FIH)-mediated HIF-1α and HIF-2α hydroxylation at asparagine residues attenuates the interaction between HIF-1α and its transcriptional co-activators, such as cAMP-response element binding protein (CREB) binding protein (CBP) and p300, thereby preventing efficient formation of transcriptional complexes [21].
Fig. 1.
Structure and regulation of hypoxia-inducible factor-1α (HIF-1α) stability. The stability of HIF-1α is regulated via proline hydroxylation modulated by prolyl hydroxylase domains (PHDs). Activated PHDs hydroxylate HIF-1α at its oxygen dependent degradation domain (ODDD), triggering its association with von hippel-lindau (VHL) protein E3 ligase complex and leading to ubiquitin–proteasome pathway-dependent degradation. Hypoxia increases protein stability of HIF-1α and promotes its nuclear translocation and accumulation. HIF-1α associates with transcriptional co-activators, such as cAMP-response element binding protein (CREB) binding protein (CBP) and p300; the efficient transcriptional complexes form in hypoxia response elements (HREs) to regulate gene expression.
The stability of HIF-1α in immune cells can be regulated in an oxygen-independent manner. Oxidation of low-density lipoprotein in macrophages leads to HIF-1α accumulation through reactive oxygen species (ROS) [11]. Additionally, bacteria induce HIF-1α expression in macrophages cultured under normoxic conditions [24]. Specifically, LPS—the major cell membrane component of Gram-negative bacteria—induces transcription and mRNA translation of HIF-1α in macrophages, resulting in HIF-1α protein accumulation, which differs from hypoxia-induced HIF-1α protein stabilization [25]. Hypoxia upregulates HIF-1α protein levels through inhibiting PHD-dependent hydroxylation and the rapid ubiquitination and proteasome degradation of HIF-1α protein. Moreover, HIF-1α expression can be upregulated via the mammalian target of rapamycin (mTOR) [26], signal transducer and activator of transcription 3 (STAT3), and NF-κB-dependent pathways [27,28]. NF-κB is required for enhanced HIF-1α transcription in macrophages induced by bacteria.
3. HIF-1α regulates macrophage functions and physiological processes
3.1. HIF-1α regulates immune responses in macrophages
Hypoxia is closely associated with inflammation and inflammation-related diseases [10]. Under hypoxic and inflammatory conditions, HIF-1α and NF-κB are intricately associated as they share stimuli, regulatory factors, and target genes. Hypoxia activates HIF-1α and NF-κB in an inhibitor kappa B kinase β (IKKβ)- and transforming growth factor-β-activated kinase1 (TAK1)-dependent manner. Moreover, given that NF-κB regulates HIF-1α levels and activity within normoxic and hypoxic conditions, the associated pathways are evolutionarily conserved [29]. Under inflammatory conditions, HIF-1α can attenuate the transcriptional activity of NF-κB in vivo and in vitro to prevent excessive and damaging pro-inflammatory responses [30,31].
HIF-1α also interacts with NF-κB in murine macrophages. HIF-1α expression is attenuated by IKKβ knockdown in macrophages following exposure to bacterial infection and hypoxia [11,12]. In LPS-stimulated murine macrophages, HIF-1α promotes NF-κB-mediated pro-inflammatory cytokine expression. The microenvironment at the infection site maintains HIF-1α protein stability by reducing hydroxylase activity. Additionally, the direct contact between murine macrophages and pathogens causes upregulation of HIF-1α expression via NF-κB pathway activation. That is, activated NF-κB induces HIF-1α transcription, thereby enhancing the host defense response. Subsequently, HIF-1α activity promotes the immune response of macrophages, including the release of pro-inflammatory cytokines and antimicrobial peptides, promotion of phagocytosis, NO production, and re-distribution of intracellular oxygen, as well as inhibition of apoptosis and PHD activity, effectively improving HIF-1α protein stability and promoting phagocyte activation [12]. Interestingly, VHL protein-deficient macrophages can kill bacteria more efficiently than wild-type macrophages due to enhanced HIF-1α protein stability [24].
Rheumatoid arthritis is an autoimmune disease characterized by persistent synovitis that can lead to joint destruction and disability [32]. Increased macrophage infiltration into the synovium is an early hallmark of the active synovitis [33]. Activation of the HIF-1α/STAT3/NOD-like receptor protein 3 (NLRP3) pathway aggravates rheumatoid arthritis by promoting the inflammatory macrophage phenotype. Moreover, the HIF-1α/STAT3/NLRP3 axis connects metabolic reprogramming and inflammatory activation of macrophages under hypoxia. Suppression of the HIF-1α/STAT3/NLRP3 pathway abolishes interleukin (IL)-1β secretion by macrophages, alleviating rheumatoid arthritis progression [34].
In summary, HIF-1α can regulate cellular immunity and inflammation, primarily through the NF-κB pathway, to maintain a normal host immune response to pathogens. However, HIF-1α also contributes to immune diseases by promoting macrophage inflammation.
3.2. HIF-1α regulates macrophage migration
HIF-1α activity is also critical for macrophage adhesion and recruitment. That is, specific knockout of HIF-1α in myeloid lineage cells does not impair monocyte and macrophage development but impairs their migration and normal functioning [13]. In fact, specific knockout of HIF-1α in mice bone marrow increases host susceptibility to several bacterial infections [13].
HIF-1α can target aerobic glycolytic metabolism to regulate macrophage migration to the site of inflammation. During the migration of monocyte-derived macrophages from blood vessels to the inflammatory area, the oxygen concentration within the microenvironment around the macrophages gradually decreases, leading to the activation of aerobic glycolysis [35], which increases the migratory potential of macrophages. HIF-1α is a potent activator of glycolysis, and macrophage migration capacity in HIF-1α knockout mice is significantly inhibited in hypoxia [36]. That is, glycolysis induced by the HIF-1α-PDK1 axis affects the migration capacity of macrophages; the oxygen utilization rate of monocyte-derived macrophages gradually decreases during their migration to the site of inflammation. In severe hypoxia, HIF-1α-mediated upregulation of PDK1 prevents pyruvate from entering the Krebs cycle to regulate glucose oxidation. Hypoxia-induced glucose metabolism reprogramming contributes to enhanced migration potential of macrophages [14]. Monocyte chemotactic protein-1 (MCP-1)-induced migration is reduced, and intracellular adenosine triphosphate (ATP) levels are decreased within the peritoneal macrophages of bone marrow-specific HIF-1α knockout mice [37]. In contrast, macrophages overexpressing active HIF-1α exhibit increased expression of netrin-1 and unc-5 netrin receptor b (Unc5b), as well as decreased migration ability, suggesting that hypoxia and HIF-1α-regulated netrin-1/Unc5b maintain inflammation by inhibiting migration and promoting the survival of focal macrophages [38]. Hence, HIF-1α maintains the migration of macrophages into inflammatory tissues by providing the necessary energy, whereas HIF-1α deficiency can impair the migration capacity of macrophages.
3.3. HIF-1α regulates macrophage polarization
Macrophages are highly plastic cells that rapidly switch their phenotype in response to microenvironmental signals [15]. Macrophage pattern recognition receptors (PRRs) or cytokine receptors are stimulated by the toll-like receptor 4 (TLR4) ligand LPS, Th1 factor, or tumor necrosis factor α (TNF-α), thereby initiating signaling cascades and promoting macrophage polarization to a pro-inflammatory M1 phenotype. However, when stimulated by Th2 cytokines, such as IL-4 or IL-13, macrophages can polarize toward the M2 phenotype (immunomodulatory type, alternative activation phenotype), which can be further subdivided into M2a, M2b, M2c, M2d, etc.
HIF is involved in macrophage plasticity. However, HIF-1α and HIF-2α regulate macrophage polarization in opposite ways [17]. Although Th1 cytokines induce M1-type polarization and upregulate HIF-1α protein level in mouse bone marrow-derived macrophages (BMDMs), IL-4-induced M2-type polarization leads to HIF-2α protein accumulation [17].
M1/M2 macrophage polarization plays an important role in the development of acute lung injury. Dysfunctional inflammatory responses, the release of high levels of inflammatory factors and oxidative stress-associated damage promote acute lung injury progression [39]. The activation of HIF-1α/pyruvate kinase M2 (PKM2) axis promotes macrophage M1 polarization and the release of IL-6 and TNF-α, contributing to lung injury. By inhibiting the HIF-1α/PKM2 axis, LPS-induced acute lung injury can be alleviated by suppressing macrophage M1 polarization and reducing inflammation [40,41].
Atherosclerosis is a chronic inflammatory disease that involves the recruitment of monocyte-derived macrophages to accumulated lipoproteins that ultimately become foam cells [42]. Analyzing the progression of atherosclerosis in animal models (Fig. 2) has revealed that the abundance of macrophages, as well as their inflammatory phenotype, influences plaque progression and regression. The hypoxia status of mouse atherosclerotic plaques adversely impacts macrophages, substantially reverses hypoxia-induced sterol accumulation, and reduces cholesterol efflux in vitro by reducing HIF-1α expression [43]. Inflammatory activation (LPS and IFN-γ) promotes polarization toward the M1 phenotype and alters the mitochondrial function in macrophages from oxidative phosphorylation to reactive oxygen species production, thus, promoting necrotic core formation in atherosclerotic lesions. Moreover, within inflammatory bone marrow–derived macrophages, deletion of HIF-1α increases oxidative phosphorylation, ATP levels, and the expression of genes encoding mitochondrial proteins, while reducing reactive oxygen species production and necroptosis. Additionally, a new mechanism has been reported that involves activation of HIF-1α in inflammatory macrophages, leading to an increase in necrotic plaque formation through microRNA-210-mediated ATP consumption, thus increasing atherosclerosis [44].
Fig. 2.
HIF-1α regulates atherosclerosis by targeting macrophage function. Activation of HIF-1α in inflammatory macrophages can increase the necrotic plaque area through miRNA-210 and miRNA-383-mediated adenosine triphosphate (ATP) exhaustion, thus promoting atherosclerosis progression. In contrast, HIF-1α induces netrin-1 and unc-5 netrin receptor b (Unc5b) expression in hypoxia and decreases macrophage migration, thereby alleviating atherosclerosis.
These studies demonstrate that hypoxia can serve as a key driver of macrophage recruitment and polarization. In particular, HIF-1α promotes macrophage M1 polarization by targeting glucose metabolism. Consequently, M1 polarization can contribute to atherosclerosis/acute lung injury progression.
3.4. HIF-1α regulates macrophage metabolism
HIF-1α is a predominant regulator of macrophage glycolysis (Fig. 3). LPS—an TLR4 agonist—stabilizes HIF-1α in macrophages [45]. mTOR participates in the activation of HIF-1α following LPS treatment. mTOR can transcriptionally upregulate HIF-1α expression [46] and can associate with HIF-1α to promote its activity [47]. Moreover, leucine influx mediated by the solute carrier family 7 member 5 (SLC7A5) can induce activation of human monocyte/macrophage glycolysis through mTOR complex 1 (mTORC1) [48]. HIF-1α heterodimerizes with HIF-1β to bind to HREs of glycolytic gene promoters (such as glucose transporters), thus, promoting the expression of target genes and upregulating aerobic glycolysis [[49], [50], [51]]. Additionally, HIF-1α-induced upregulation of glycolysis-related enzymes and TCA cycle intermediates can improve the stability of HIF-1α, forming a positive feedback loop that promotes HIF-1α accumulation.
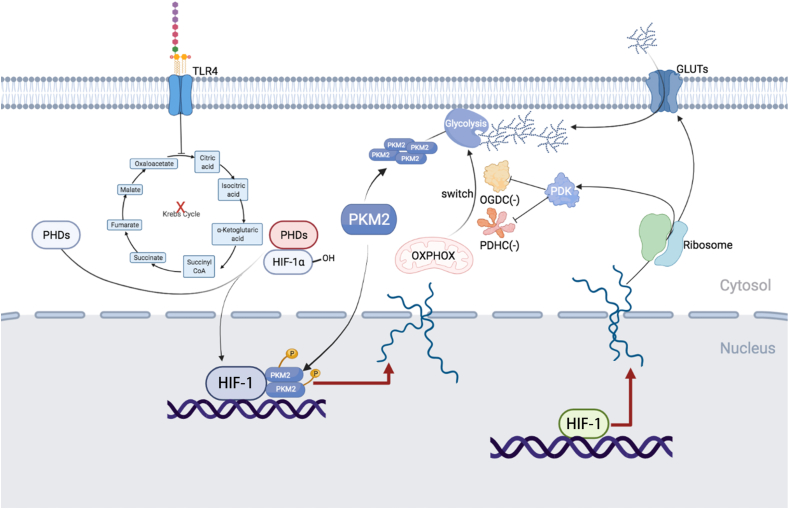
Fig. 3.
HIF-1α participates in glucose metabolic reprogramming. HIF-1α induces glycolytic gene expression to promote glucose uptake, glycolysis, and tricarboxylic acid cycle (TCA cycle), thereby enhancing HIF-1α stability. Pyruvate dehydrogenase kinase isozyme 1 (PDK1) inhibits pyruvate dehydrogenase complex (PDHC) and oxoglutarate dehydrogenase complex (OGDC), thereby promoting switching from mitochondrial oxidative phosphorylation to aerobic glycolysis. Lipopolysaccharide (LPS), an inflammation inducer, inhibits the TCA cycle and induces succinic acid, fumaric acid, citric acid, and itaconic acid accumulation, resulting in stabilization of HIF-1α. The tetramer pyruvate kinase M2 (PKM2) is primarily cytoplasmic and exhibits high pyruvate kinase activity, which can catalyze phosphoenolpyruvate to generate pyruvate. Dimeric PKM2 can translocate into the nucleus with high protein kinase activity, acting as a transcription factor to regulate downstream transcription. The toll-like receptor 4 (TLR4) signaling pathway stimuli, such as LPS, promote phosphorylation of PKM2 to maintain the monomer/dimer state. PKM2 monomer or dimer can form a complex with HIF-1α in the nucleus and significantly increase the transcriptional activity of HIF-1α.
Pyruvate kinase (PK) is a key pacemaker enzyme in the last step of the glycolytic pathway and comprises two isoforms: pyruvate kinase M1 (PKM1) and PKM2. PKM2 has three states: monomer, dimer, and tetramer; the tetramer exists primarily in the cytoplasm and exhibits high pyruvate kinase activity, which can catalyze phosphoenolpyruvate (PEP) to generate pyruvate. In contrast, the dimer PKM2 can translocate into the nucleus with high protein kinase activity, acting as a transcription factor to regulate downstream transcription [52]. TLR4 signaling pathway stimuli, such as LPS, promote phosphorylation of PKM2 to maintain the monomer/dimer state, which form a complex with HIF-1α in the nucleus to significantly increase the transcriptional activity of HIF-1α, thereby enhancing the expression of glycolytic enzymes [53,54], which suggest a positive feedforward control loop between PKM2 and the HIF-1α signaling pathway [55]. Moreover, deoxyelephantopin (DET) attenuates LPS-induced IL-1β and high-mobility group box 1 (HMGB1) release in vitro and in vivo and protects mice against lethal endotoxemia via impairing association between PKM2 and HIF-1α [56]. Moreover, dimerized nuclear PKM2 promotes the nuclear localization of STAT3 and directly phosphorylates STAT3 at tyrosine 705 to activate STAT3 [57], thereby inducing HIF-1α and glycolytic enzyme expression [58,59]. These studies suggest a PKM2-STAT3-HIF-1α positive feedback loop in macrophages.
In addition, succinic acid, fumaric acid, citric acid, and itaconic acid accumulate following interruption of the TCA cycle induced by LPS stimulation, which can inhibit HIF-1α degradation by directly inhibiting PHDs and promoting ROS production [[58], [59], [60], [61]]. Therefore, the stabilization of HIF-1α in activated macrophages and the induction of aerobic glycolysis reinforce each other in a positive feedback loop, rapidly promoting aerobic glycolysis in macrophages.
Adipose tissue macrophage inflammation is associated with metabolic inflammation and metabolism complications [62]. In the context of obesity, elevated lactate production in adipose cells is a critical signal that promotes adipose tissue macrophage polarization to an inflammatory phenotype. That is, lactate competes with α-ketoglutaric acid by directly binding to the catalytic domain of PHD2 to stabilize HIF-1α and promote adipose tissue macrophage polarization to an inflammatory phenotype [63]. Lactate-induced upregulation of IL-1β levels is eliminated in PHD2-deficient macrophages, revealing a positive correlation between lactate level and local inflammatory characteristics. Adipocyte-derived lactate regulates the proinflammatory microenvironment in fat by influencing the inflammatory phenotype of macrophages through PHD2-induced HIF-1α stabilization. In addition, macrophage-derived HIF-1α functions as a hub connecting abnormal energy metabolism and chronic inflammation [63].
In LPS- and interferon gamma (IFN-γ)-stimulated macrophages the HIF-1α level is dynamic, which was modulated by dynamic changes in levels of succinate and itaconate. That is, LPS and IFN-γ significantly increases levels of succinate and itaconate early (6–24 h), which inhibit PHD activity and subsequent HIF-1α degradation to stabilize HIF-1α. A steady increase in HIF-1α promotes transcription of downstream pyruvate dehydrogenase kinase 3 (PDK3) and inhibition of pyruvate dehydrogenase complex (PDHC), which promotes the transition of mitochondrial oxidative respiration to glycolysis. In addition, the reduction of PDHC and oxoglutarate dehydrogenase complex (OGDC) E2 subunit lipidation can inhibit PDHC and OGDC. In the late stimulation phase (48–72 h), significant inhibition of PDHC results in decreased production of glucose-derived acetyl-CoA, citric acid, and itaconic acid, and inhibition of OGDC, which decreases production of succinyl-CoA and succinic acid. A sharp drop in the abundance of these metabolites returns HIF-1α levels to normal. Consequently, metabolic flux through the TCA cycle in macrophages is dynamically reprogrammed. These changes in key metabolite levels correlate with changes in HIF-1α protein. This flux modulation in macrophages mediated by HIF-1α leads to profound and dynamic shifts in important metabolites, which correlate strongly with the subsequent production of pro-inflammatory cytokines TNF-α and IL6 and HIF-1α protein levels. These findings elucidate the highly dynamic metabolic reprogramming in macrophages in response to LPS and IFN-γ stimulation, and highlight the important temporal association between metabolism and the inflammatory state [61].
Since HIF-1α is the main regulator of macrophage glycolysis, its overexpression leads to an increase in glycolysis and intermediates of the pentose phosphate pathway [64]. Moreover, palmitic acid exposure promotes glycolytic metabolism by upregulating HIF-1α expression [65], while the gaseous signaling molecule hydrogen sulfide (H2S) [66], metal Co2+ [67], chitin [68], metallothionein 3 [69], G-toxin [70] and sedum extract [71] inhibit macrophage glycolysis and inflammatory pathways by targeting HIF-1α. Additionally, an increase in adipose tissue and local hypoxia causes an increase in the mRNA and protein expression levels of HIF-1α in macrophages and the promotion of glycolysis- and inflammatory-related cytokine release [9]. These studies indicate that the mTOR–HIF–1α axis plays an important role in modulating glucose metabolism reprogramming in macrophages, and its diverse downstream target genes are critical for regulating glucose metabolic reprogramming of activated macrophages [46,47].
3.5. HIF-1α regulates macrophage viability
Macrophage apoptosis, necrosis, and reduced efferocytosis and autophagy play important roles in increasing the size of the necrotic core area of atherosclerotic plaques. The necrotic core area of atherosclerotic plaques in myeloid HIF-1α knockout mice was smaller compared with the WT mice. And the proportion of early apoptotic cells in the primary macrophages from myeloid HIF-1α knockout mice was lower than that from WT mice. The apoptosis of HIF-1α knockout BMDMs was also alleviated in vivo and in vitro [72]. This study demonstrates that HIF-1α promotes macrophage apoptosis and play proatherogenic role by necrotic core formation.
HIF-1α promotes macrophage necroptosis. In atherosclerosis models, RIP3-mediated macrophage necrosis in macrophage HIF-1α knockout mice was less than that from WT mice. Activation of HIF-1α in inflammatory BMDM could reduce oxidative phosphorylation and ATP, but increase ROS and necroptosis. HIF-1α is closely related to microRNA (miRNA). In particular, HIF-1α upregulates miR-210 and downregulates miR-383 in diseased macrophages and inflammatory BMDMs, respectively. MiR-383 affects the DNA damage repair pathway in BMDMs by targeting poly(ADP-ribose)-glycosyl hydrolase (PARg), thereby reducing energy consumption and necroptosis but increasing ATP levels and cell survival, ultimately reducing the atherosclerosis sclerotic plaque area and necrotic core formation [44].
HIF-1α reduces macrophage autophagy. Atherosclerotic lesions are characterized by inhibition of autophagy and activation of ferroptosis in macrophages. Oxidized low-density lipoprotein (ox-LDL) induces foam cell formation of THP-1 macrophages, autophagy dysfunction, and ferroptosis development. Upregulated expression of HIF-1α has been reported in ox-LDL-induced atherosclerotic lesions and THP-1 macrophages. However, the HIF-1α inhibitor PX-478 restores autophagy function, depresses ferroptosis, and reduces lipid accumulation in ox-LDL-induced THP-1 macrophages. PX-478 treatment also down-regulates HIF-1α expression and reduces atherosclerotic plaques in a murine model [73].
4. Conclusion
Substantial efforts have been undertaken over the past decades to elucidate the role of HIF-1α in regulating the biological functions of macrophages. Data have shown that macrophage-derived HIF-1α stabilization occurs under hypoxic conditions, thereby regulating macrophage immune responses, migration, polarization, metabolism, and viability. Moreover, the roles of HIF-1α in macrophage-associated diseases, particularly obesity and atherosclerosis, have been determined.
HIF-1α stability is regulated by the hydroxylation of proline residues via PHDs. Under normoxia, activated PHD hydroxylates and subsequently degrades HIF-1α via the ubiquitin–proteasome pathway. However, PHD-dependent HIF-1α hydroxylation is inhibited under hypoxia, resulting in enhanced protein stability, nuclear translocation, and accumulation of HIF-1α [22,23]. The transcription and protein translation of HIF-1α in macrophages are induced by LPS stimulation under aerobic conditions. Moreover, HIF-1α expression can be upregulated via the mTOR, STAT3, and NF-κB pathways [27,28].
In LPS-stimulated mouse macrophages, HIF-1α promotes NF-κB-mediated pro-inflammatory cytokine expression. When mouse macrophages come in direct contact with the pathogens, HIF-1α expression is upregulated via the activation of NF-κB pathway. Activated NF-κB further induces the transcription of HIF-1α, thereby enhancing the host defense response. Moreover, specific knockout of HIF-1α in myeloid lineage cells impairs their migration and normal function [13]. HIF-1α could target aerobic glycolytic metabolism to regulate macrophage migration to the inflammatory area. Additionally, in macrophage glycolysis, HIF-1α binds to the HREs of glycolytic enzyme-related gene promoters and upregulates the expression of target genes, including the glucose transporter 1.
Although an increasing number of studies have focused on HIF-1α and its potential role in macrophages, other HIF subunits, such as HIF-2α, also coordinate with HIF-1α to regulate macrophage polarization. However, the regulatory roles of HIF-2α in macrophage biology have been largely unexplored and warrant further investigation. Moreover, given that hypoxia signaling serves as a direct link between HIF-1α subunits and macrophage-related diseases, the use of HIF-1α-stabilizing or -inhibiting drugs might represent a promising therapeutic approach for such diseases. Therefore, future studies should aim to elucidate the therapeutic potential of HIF-1α in various disease models.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82070445, 81921001 and 82270428), and Beijing Natural Science Foundation (7222188).
References
- 1.Jiang B.H., Semenza G.L., Bauer C., Marti H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 3.Cockman M.E., Masson N., Mole D.R., Jaakkola P., Chang G.W., Clifford S.C., et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von hippel-lindau tumor suppressor protein. J. Biol. Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 4.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., et al. HIFalpha targeted for vhl-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., et al. Targeting of HIF-alpha to the von hippel-lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 6.Jewell U.R., Kvietikova I., Scheid A., Bauer C., Wenger R.H., Gassmann M. Induction of HIF-1alpha in response to hypoxia is instantaneous. Faseb. J. 2001;15:1312–1314. [PubMed] [Google Scholar]
- 7.Bex C., Knauth K., Dambacher S., Buchberger A. A yeast two-hybrid system reconstituting substrate recognition of the von Hippel-Lindau tumor suppressor protein. Nucleic Acids Res. 2007;35:e142. doi: 10.1093/nar/gkm932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran S.E., O'Neill L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutens L., Hooiveld G.J., Dhingra S., Cramer R.A., Netea M.G., Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biddlestone J., Bandarra D., Rocha S. The role of hypoxia in inflammatory disease (review) Int. J. Mol. Med. 2015;35:859–869. doi: 10.3892/ijmm.2015.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V., et al. NF-KappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nizet V., Johnson R.S. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer T., Yamanishi Y., Clausen B.E., Förster I., Pawlinski R., Mackman N., et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semba H., Takeda N., Isagawa T., Sugiura Y., Honda K., Wake M., et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016;7 doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrado C., Fontana S. Hypoxia and HIF signaling: one axis with divergent effects. Int. J. Mol. Sci. 2020;21:5611. doi: 10.3390/ijms21165611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda N., O'Dea E.L., Doedens A., Kim J.W., Weidemann A., Stockmann C., et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for no homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenza G.L. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 19.Wenger R.H., Stiehl D.P., Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci. STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 20.Arany Z., Huang L.E., Eckner R., Bhattacharya S., Jiang C., Goldberg M.A., et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 22.Ohh M., Park C.W., Ivan M., Hoffman M.A., Kim T.Y., Huang L.E., et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 23.Metzen E., Zhou J., Jelkmann W., Fandrey J., Brüne B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol. Biol. Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyssonnaux C., Datta V., Cramer T., Doedens A., Theodorakis E.A., Gallo R.L., et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blouin C.C., Pagé E.L., Soucy G.M., Richard D.E. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H., Makino Y., Okamoto K., Poellinger L., Ohnuma K., Morimoto C., et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J. Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 27.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., et al. Control of t(h)17/t(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moniz S., Biddlestone J., Rocha S. Grow: the HIF system, energy homeostasis and the cell cycle. Histol. Histopathol. 2014;29:589–600. doi: 10.14670/HH-29.10.589. [DOI] [PubMed] [Google Scholar]
- 29.van Uden P., Kenneth N.S., Webster R., Müller H.A., Mudie S., Rocha S. Evolutionary conserved regulation of HIF-1β by NF-κB. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandarra D., Biddlestone J., Mudie S., Müller H.A., Rocha S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis. Model Mech. 2015;8:169–181. doi: 10.1242/dmm.017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue X., Ramakrishnan S., Anderson E., Taylor M., Zimmermann E.M., Spence J.R., et al. Endothelial pas domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherer H.U., Häupl T., Burmester G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 33.Udalova I.A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 34.Wu D., Xu J., Jiao W., Liu L., Yu J., Zhang M., et al. Suppression of macrophage activation by sodium danshensu via HIF-1α/STAT3/NLRP3 pathway ameliorated collagen-induced arthritis in mice. Molecules. 2023;28 doi: 10.3390/molecules28041551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doedens A.L., Stockmann C., Rubinstein M.P., Liao D., Zhang N., DeNardo D.G., et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marelli-Berg F.M., Jangani M. Metabolic regulation of leukocyte motility and migration. J. Leukoc. Biol. 2018;104:285–293. doi: 10.1002/JLB.1MR1117-472R. [DOI] [PubMed] [Google Scholar]
- 37.Kojima H., Tokunou T., Takahara Y., Sunagawa K., Hirooka Y., Ichiki T., et al. Hypoxia-inducible factor-1 α deletion in myeloid lineage attenuates hypoxia-induced pulmonary hypertension. Phys. Rep. 2019;7 doi: 10.14814/phy2.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramkhelawon B., Yang Y., van Gils J.M., Hewing B., Rayner K.J., Parathath S., et al. Hypoxia induces netrin-1 and unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler. Thromb. Vasc. Biol. 2013;33:1180–1188. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain N., Moeller J., Vogel V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 2019;21:267–297. doi: 10.1146/annurev-bioeng-062117-121224. [DOI] [PubMed] [Google Scholar]
- 40.He S., Fan C., Ji Y., Su Q., Zhao F., Xie C., et al. Senp3 facilitates M1 macrophage polarization via the HIF-1α/PKM2 axis in lipopolysaccharide-induced acute lung injury. Innate Immun. 2023 doi: 10.1177/17534259231166212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X., Tang J., Shuai W., Meng J., Feng J., Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020;69:883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randolph G.J. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parathath S., Mick S.L., Feig J.E., Joaquin V., Grauer L., Habiel D.M., et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karshovska E., Wei Y., Subramanian P., Mohibullah R., Geißler C., Baatsch I., et al. HIF-1α (hypoxia-inducible factor-1α) promotes macrophage necroptosis by regulating miR-210 and miR-383. Arterioscler. Thromb. Vasc. Biol. 2020;40:583–596. doi: 10.1161/ATVBAHA.119.313290. [DOI] [PubMed] [Google Scholar]
- 45.Stothers C.L., Luan L., Fensterheim B.A., Bohannon J.K. Hypoxia-inducible factor-1α regulation of myeloid cells. J. Mol. Med. (Berl.) 2018;96:1293–1306. doi: 10.1007/s00109-018-1710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo Y., Iadevaia V., Yao Z., Kelly I., Cosulich S., Guichard S., et al. Stable isotope-labelling analysis of the impact of inhibition of the mammalian target of rapamycin on protein synthesis. Biochem. J. 2012;444:141–151. doi: 10.1042/BJ20112107. [DOI] [PubMed] [Google Scholar]
- 47.Land S.C., Tee A.R. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mtor signaling motif. J. Biol. Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 48.Yoon B.R., Oh Y.J., Kang S.W., Lee E.B., Lee W.W. Role of SLC7A5 in metabolic reprogramming of human monocyte/macrophage immune responses. Front. Immunol. 2018;9:53. doi: 10.3389/fimmu.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Pore N., Behrooz A., Ismail-Beigi F., Maity A. Regulation of GLUT1 mRNA by hypoxia-inducible factor-1. Interaction between h-RAS and hypoxia. J. Biol. Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 50.Semenza G.L., Nejfelt M.K., Chi S.M., Antonarakis S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mole D.R., Blancher C., Copley R.R., Pollard P.J., Gleadle J.M., Ragoussis J., et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palsson-McDermott E.M., Dyck L., Zasłona Z., Menon D., McGettrick A.F., Mills K.H.G., et al. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-l1 in immune cells and tumors. Front. Immunol. 2017;8:1300. doi: 10.3389/fimmu.2017.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo W., Semenza G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metabol. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo W., Semenza G.L. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2011;2:551–556. doi: 10.18632/oncotarget.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo W., Hu H., Chang R., Zhong J., Knabel M., O'Meally R., et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan L., Hu L., Zhang L., Xu H., Chen Y., Bian Q., et al. Deoxyelephantopin decreases the release of inflammatory cytokines in macrophage associated with attenuation of aerobic glycolysis via modulation of PKM2. Int. Immunopharm. 2020;79 doi: 10.1016/j.intimp.2019.106048. [DOI] [PubMed] [Google Scholar]
- 57.Yang P., Li Z., Fu R., Wu H., Li Z. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell. Signal. 2014;26:1853–1862. doi: 10.1016/j.cellsig.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Demaria M., Poli V. PKM2, STAT3 and HIF-1α: the warburg's vicious circle. JAK-STAT. 2012;1:194–196. doi: 10.4161/jkst.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demaria M., Giorgi C., Lebiedzinska M., Esposito G., D'Angeli L., Bartoli A., et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2010;2:823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight M., Stanley S. HIF-1α as a central mediator of cellular resistance to intracellular pathogens. Curr. Opin. Immunol. 2019;60:111–116. doi: 10.1016/j.coi.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seim G.L., Britt E.C., John S.V., Yeo F.J., Johnson A.R., Eisenstein R.S., et al. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat Metab. 2019;1:731–742. doi: 10.1038/s42255-019-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng T., Zhao X., Gu P., Yang W., Wang C., Guo Q., et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 2022;13:5208. doi: 10.1038/s41467-022-32871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T., Liu H., Lian G., Zhang S.Y., Wang X., Jiang C. HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma M., Boytard L., Hadi T., Koelwyn G., Simon R., Ouimet M., et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci. Rep. 2020;10:5555. doi: 10.1038/s41598-020-62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahman M.A., Cumming B.M., Addicott K.W., Pacl H.T., Russell S.L., Nargan K., et al. Hydrogen sulfide dysregulates the immune response by suppressing central carbon metabolism to promote tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2020;117:6663–6674. doi: 10.1073/pnas.1919211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salloum Z., Lehoux E.A., Harper M.E., Catelas I. Effects of cobalt and chromium ions on glycolytic flux and the stabilization of hypoxia-inducible factor-1α in macrophages in vitro. J. Orthop. Res. 2021;39:112–120. doi: 10.1002/jor.24758. [DOI] [PubMed] [Google Scholar]
- 68.Mian W., Zhang M., Ma Y., Liu F., Chen S., Lu J., et al. Chaetocin attenuates gout in mice through inhibiting HIF-1α and NLRP3 inflammasome-dependent IL-1β secretion in macrophages. Arch. Biochem. Biophys. 2019;670:94–103. doi: 10.1016/j.abb.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Chowdhury D., Alrefai H., Landero Figueroa J.A., Candor K., Porollo A., Fecher R., et al. Metallothionein 3 controls the phenotype and metabolic programming of alternatively activated macrophages. Cell Rep. 2019;27:3873–3886.e3877. doi: 10.1016/j.celrep.2019.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao C., Lin S., Liu S., Jin P., Lu W., Li N., et al. HIF1α-induced glycolysis in macrophage is essential for the protective effect of ouabain during endotoxemia. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7136585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu H., Cheng S., Wu C., Zheng S., Hong W., Liu L., et al. Sedum sarmentosum bunge extract alleviates inflammation and kidney injury via inhibition of M1-macrophage polarization. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152976. [DOI] [PubMed] [Google Scholar]
- 72.Aarup A., Pedersen T.X., Junker N., Christoffersen C., Bartels E.D., Madsen M., et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:1782–1790. doi: 10.1161/ATVBAHA.116.307830. [DOI] [PubMed] [Google Scholar]
- 73.Hu G., Yuan Z., Wang J. Autophagy inhibition and ferroptosis activation during atherosclerosis: hypoxia-inducible factor 1α inhibitor PX-478 alleviates atherosclerosis by inducing autophagy and suppressing ferroptosis in macrophages. Biomed. Pharmacother. 2023;161 doi: 10.1016/j.biopha.2023.114333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.