Abstract
Tropheryma whipplei (TW) is the root cause of Whipple’s disease (WD), a rare infectious illness leading to multi-organ impairment. A prominent feature of WD is acute pneumonia, which can be exceedingly challenging to diagnose clinically due to the pathogen’s surreptitious nature. However and significantly, with the advent of metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid (BALF), it offers clinicians a potent tool at their disposal to detect TW infections. The present study conducted a retrospective analysis of clinical data gleaned from five patients in Hunan Province in China. Findings in this study demonstrated the potential of BALF-mNGS in diagnosing pneumonia caused by TW infection.
Keywords: Tropheryma whipplei, Metagenomic next-generation sequencing, Bronchoalveolar lavage fluid, Pneumonia
1. Introduction
Whipple's disease (WD) is an exceedingly rare and highly intricate systemic infectious disorder that is caused by infection with Tropheryma whipplei (TW), which can perniciously impact multiple physiological systems of the human body. The incidence of WD is approximately 1:1,000,000 [1]. It predominately afflicts the articular, alimentary, cardiovascular, and neurological systems of the affected individuals. TW infection can engender chronic infection (systemic/classical WD or local infection), acute infection (largely gastroenteritis, pneumonia), and asymptomatic carriers. WD shows inordinately intricate clinical presentations owing to multifaceted involvement of multiple physiological systems [2]. Consequently, there may be high risks of missed diagnosis and misdiagnose, leading to inappropriate treatment and deleterious outcomes [3]. Patients with pulmonary infection provoked by TW may present with high fever, prolonged cough illness, unexplained weight loss, and other nondescript manifestations; respiratory failure and even death may occur in severe cases [4]. At present, there exist only a meager number of reported cases of pneumonia caused by TW infection, and its pathophysiology remains a nebulous enigma [5].
Accordingly, this study retrospectively collected the medical records of 5 patients with pneumonia caused by TW infection from three different medical institutions in Hunan Province, with follow-up of patient prognosis simultaneously. A comprehensive analysis was carried out on the clinical symptoms, diagnostic testing outcomes and therapeutic interventions of these patients. Further extensive review of pertinent literature was performed to summarize this disease. The express purpose of this study was to provide clinicians with some much-needed reference regarding the diagnostic and therapeutic management of similar patients in the future.
2. Research methods
The subjects of study were 5 patients who were admitted across three hospitals in Hunan Province of China and subsequently diagnosed with pneumonia caused by TW infection by the expert team (experts in respiratory medicine, infectious diseases, and radiology) between April 2021 and September 2022. Among these patients, 3 cases were admitted to the Xiangtan Central Hospital, 1 case to the Xiangtan First People’s Hospital, and the remaining patient to the Huaihua Central Hospital. Each patient was admitted to the hospital with various respiratory-related symptoms. Upon admission, an assortment of routine laboratory tests and chest CT scans were conducted in addition to a fiberoptic bronchoscopy procedure. During the bronchoscopy procedure, samples of the diseased lung subsegment were collected in strict adherence to clinical operation standards and asepsis principles. Specifically, bronchoalveolar lavage fluid (BALF) samples were collected and subsequently sent for testing to the Vision Medicals Co., Ltd., Guangzhou, China. Instructions for metagenomic next-generation sequencing (mNGS) and bioinformatics analysis refer to Supplementary Material 1.
2.1. Ethical clearance and informed consent
The attainment of ethical clearance and informed consent is a crucial component in medical research, and as such, it is imperative to adhere to strict protocols to ensure the degree of compliance. To that end, this study has been approved by the Medical Ethics Committees of Xiangtan Central Hospital (Approval No. 2022-10-002), the First People's Hospital of Huaihua (Approval No. HHSYY-EC-202210-CI), and the First People's Hospital of Xiangtan City affiliated to Nanhua University (Approval No. 2022121301). The enrolled patients with competence to give informed consent or the patient's authorized person agreed to describe the situation and report it publicly. The performance of this study also followed related international and privacy protocols.
3. Results
3.1. Clinical characteristics
The baseline characteristics of the enrolled 5 patients are summarized in Table 1. Of the 5 patients, there were 3 males and 2 females, with an average age of 53 years (range: 29–80 years). Body mass index (BMI) values of these patients were within the normal range. Two patients had chronic onset of respiratory-related symptoms, with the course of disease ranging from 2 months to 1 year. Meanwhile, 3 patients had acute onset with a clinical course of 2–5 days. The included patients showed diverse main clinical symptoms, with cough being present in all patients, expectoration in 60%, fever in 20%, shortness of breath in 40%, and hemoptysis in 20% of all patients. Interestingly, there were no symptoms such as weight loss, diarrhea, abdominal pain, joint pain, or headache.
Table 1.
Baseline characteristics of patients with pneumonia caused by TW infection.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (Years) | 29 | 49 | 57 | 80 | 66 |
| Sex | Male | Female | Male | Female | Male |
| Clinical symptoms | Shortness of breath, and cough | Cough, and phlegm | Cough, phlegm, and fever | Shortness of breath, cough, and phlegm | Cough, and hemoptysis |
| Comorbid Conditions | OSA, and fatty liver | None | None | CHD, CSI, ANEFH, AFHR, OP, and HT | MDS, DM, and HT |
| Smoking history | Yes | None | Yes | None | Yes |
OSA: Obstructive sleep apnea; AIDS: Acquired immunodeficiency syndrome; CHD: Coronary heart disease; CSI: Coronary stent implantation; ANEFH: Avascular necrosis of femoral head; OP: Osteoporosis; AFHR: Artificial femoral head replacement; HT: Hypertension; MDS: Myelodysplastic syndrome; and DM: Diabetes.
Significant differences were also observed in the results of routine laboratory examination, with increase detected in erythrocyte sedimentation rate (80%), hypersensitive C reactive protein (60%), serum lactate dehydrogenase (60%), and platelet (50%). Conversely, decreased trends were found in blood oxygen partial pressure (40%) and hemoglobin (40%). No abnormality was reported in white blood cell (WBC), procalcitonin, and serum albumin. The results of laboratory examination, electrocardiogram, echocardiography, and bronchoscopy of the patients are shown in Table 2.
Table 2.
Clinical characteristics of patients with pneumonia caused by TW infection.
| Variable | Normal range | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Mean ± SD |
|---|---|---|---|---|---|---|---|
| WBC (10^9/L) | 3.97–9.15 | 5.57 | 7.95 | 10.58 | 9.64 | 3.43 | 7.43 ± 2.94 |
| PLT (10^9/L) | 85–303 | 373 | 383 | 268 | 379 | 71 | 294.8 ± 133.97 |
| HB (g/L) | 120–160 | 93 | 129 | 123 | 115 | 67 | 105.4 ± 25.43 |
| ESR (mm/h) | 0–15 | 45 | 10 | 52 | 140 | 60 | 61.4 ± 47.90 |
| hsCRP (mg/L) | 0–3 | 39.76 | 1.07 | 2.42 | 185.84 | 38.73 | 53.56 ± 76.29 |
| LDH (IU/L) | 114–240 | 275 | 228 | 269 | 363.5 | 193 | 265.7 ± 63.97 |
| ALB (g/L) | 35–55 | 41.3 | 42.4 | 40.5 | 30.5 | 34.9 | 37.92 ± 5.06 |
| PaO₂ (mmHg) | 80–100 | 72 | 92 | 83 | 75 | No done | 80.5 ± 8.96 |
| 12-lead ECG | Normal | Normal | Normal | Crbbb | Normal | ||
| ECHO | Normal | Normal | Normal | No done | LAE, AS widening | ||
| Bronchoscopy | Bronchial inflammation and hemorrhage | Bronchial inflammation | Bronchial inflammation | Bronchial inflammation | Bronchial inflammation | ||
| BALF-LCT | Epithelial cells 40% neutrophils 30% lymphocytes 30% No Cancer cells |
macrophages 90% lymphocytes 10% Epithelial cells very few No Cancer cells |
inflammatory cells (unclassified) few No Cancer cells |
Epithelial cells 20% neutrophils 20% lymphocytes 60% No Cancer cells |
Epithelial cells 30% neutrophils 30% lymphocytes 40% No Cancer cells |
WBC: White blood cell; HB: Hemoglobin; PLT: Platelets; ESR: erythrocyte sedimentation rate; hsCRP: Hypersensitive C reactive protein; LDH: Lactate dehydrogenase; ALB: Serum albumin; PaO₂: Partial pressure of blood oxygen; ECG: electrocardiogram; ECHO: Echocardiography; BALF: Bronchoalveolar lavage fluid; LCT: liquid-based cytology; Crbbb: Complete right bundle branch block; LAE: Left atrial enlargement, and AS: ascending aorta.
3.2. Chest CT scan
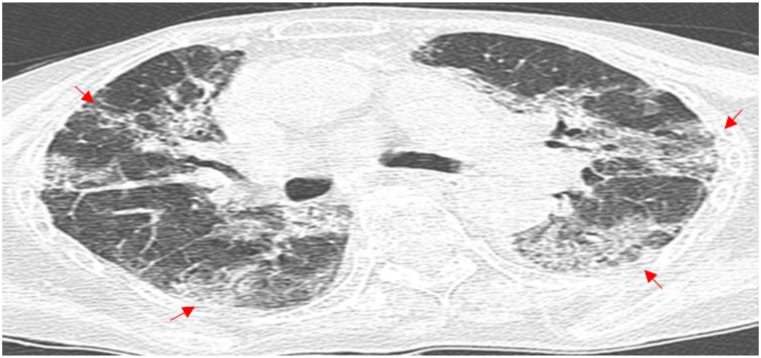
The results of CT scan showed abnormalities in all the 5 patients. Table 3 includes the main features of chest CT. These abnormalities were classified according to their morphological features. To be specific, Patient 1 showed diffuse ground glass opacity (GGO), and multiple exudative lesions in both lungs; Patient 2 had a nodule in the middle of the right lung and few exudative lesions in both lungs; Patient 3 had cavity lesions in the upper lobe of the left lung; Patient 4 exhibited GGO, and multiple exudative lesions in both lungs; While Patient 5 had consolidation in the right upper lung, bronchial inflation sign, multiple exudative lesions in both lungs, pleural effusion bilaterally, and pericardial thickening.
Table 3.
Chest CT of patients with pneumonia caused by TW infection.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| GGO | ✓ | ✓ | ✓ | ||
| Consolidation | ✓ | ||||
| Diffuse lesion | ✓ | ✓ | |||
| Exudative lesion | ✓ | ✓ | ✓ | ✓ | |
| Cavity lesion | ✓ | ||||
| Nodule | ✓ | ||||
| Pleural effusion | ✓ |
GGO: Ground glass opacity. The tick indicates the presence of imaging feature(s).
3.3. mNGS
Finally, all 5 patients underwent mNGS of BALF samples (Table 4), which revealed even more complexity. Patient 1 was detected with TW (sequence number 1480, relative abundance of 12.46%), Streptococcus pneumoniae (sequence number 4919, relative abundance of 1.45%), and Haemophilus influenzae (sequence number 1253, relative abundance of <0.01%). Patient 2 was detected with TW (sequence number 1640, relative abundance of 64.36%), Haemophilus parainfluenzae (sequence number 60, relative abundance of 0.91%), and Haemophilus influenzae (sequence number 7, relative abundance of <0.01%). Patient 3 was detected with TW (sequence number 1340, relative abundance of 51.06%), Haemophilus haemolyticus (sequence number 73, relative abundance of 1.13%), and Candida albicans (sequence number 9, relative abundance of <0.01%). Patient 4 was detected with TW (sequence number 13452, relative abundance of 81.60%), Klebsiella pneumoniae (sequence number 2165, relative abundance of 5.3%), and Moraxella catarrhalis (sequence number 35, relative abundance of 0.1%). Besides, Patient 5 was detected with TW (sequence number 16951, relative abundance of 2.1%), Klebsiella aerogenes (sequence number 304, relative abundance of <0.1%), and Candida albicans (sequence number 35, relative abundance of <0.1%).
Table 4.
The results of mNGS in BALF.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Pathogen | TW | TW | TW | TW | TW |
| Sequence number | 1480 | 1640 | 1340 | 13,452 | 16,951 |
| Relative abundance | 12.46% | 64.36% | 51.06% | 81.60% | 2.1% |
| Pathogen | Streptococcus pneumonia | Haemophilus parainfluenzae | Haemophilus hemolyticus | Klebsiella pneumoniae | Klebsiella aerogenes |
| Sequence number | 4919 | 60 | 73 | 2165 | 304 |
| Relative abundance | 1.45% | 0.91% | 1.13% | 5.3% | <0.1% |
| Pathogen | Haemophilus influenza | Haemophilus influenza | Candida albicans | Moraxella catarrhalis | Candida albicans |
| Sequence number | 1253 | 7 | 9 | 35 | 35 |
| Relative abundance | <0.01% | <0.01% | <0.01% | 0.1% | <0.1% |
TW: Tropheryma whipplei.
Sequence number: The number of specific sequences that are unique to a genus or species of microorganisms in a high-throughput sequencing sequence.
Relative abundance: Pathogens are classified according to bacteria, fungi, viruses and parasites, and relative abundance is the relative proportion of the genome of the pathogen in the corresponding classification.
3.4. Treatment and prognosis
All the 5 patients were clinically diagnosed with pneumonia caused by TW infection after detailed discussions with infectious disease experts, respiratory medicine experts, and radiology experts.
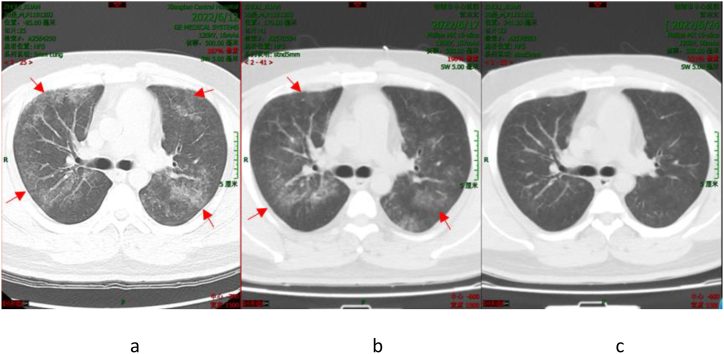
Patient 1 was initially treated empirically with moxifloxacin for 4 days after admission. However unfortunately, the patient showed no improvement in the condition. Consequently, corresponding treatment plan was modified to meropenem combined with doxycycline by the attending physician based on expert opinion. Re-examination of chest CT (Fig. 1(a–c)) revealed significant improvement in lung lesions. Upon discharge, Patient 1 was advised to take doxycycline for an extended period; and the patient coughs occasionally at present.
Fig. 1.
Chest CT Scan of Patient 1 (The dates from left to right: June 12, 2022 (a); June 17, 2022 (b); and June 24, 2022 (c)). The arrowhead shows the focus.
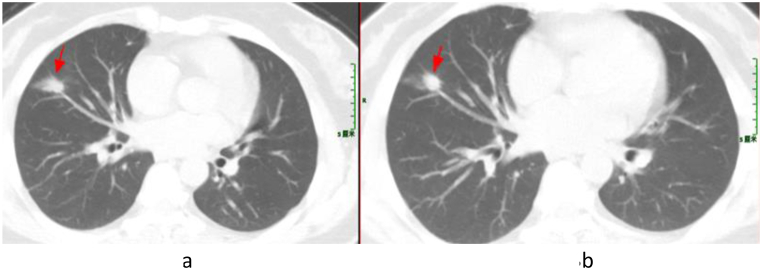
Patient 2, upon admission, was initially treated empirically with a combined medication of piperacillin-tazobactam and moxifloxacin. Despite the confirmed diagnosis of pneumonia caused by TW infection based on expert group’s discussion, re-examination of chest CT demonstrated improved condition in lung lesions (Fig. 2(a and b)). But the patient declined to accept the standard treatment for WD, and was discharged. At present, the patient still has occasional cough and expectoration.
Fig. 2.
Chest CT Scan of Patient 2 (The dates from left to right are August 22, 2022 (a) and September 2, 2022 (b)). The arrowhead shows the focus.
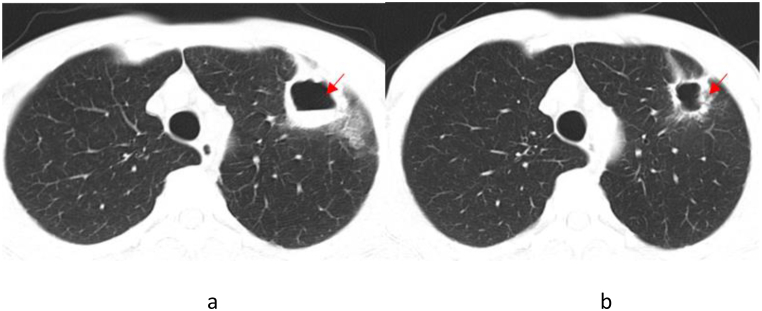
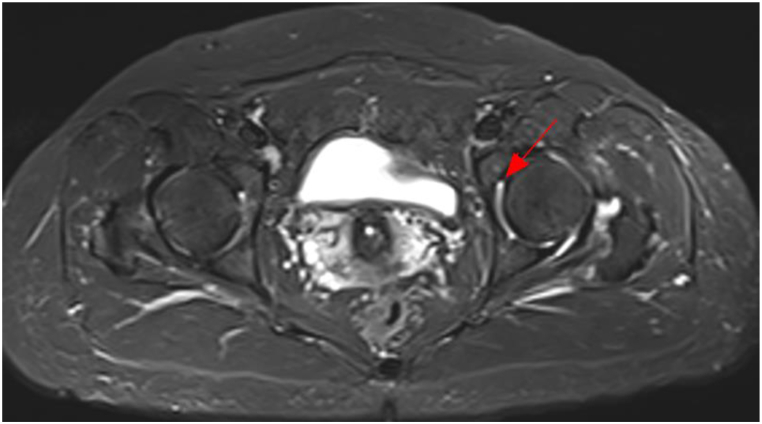
Following a diagnosis of pneumonia caused by TW infection, a treatment plan consisting of ceftriaxone followed by compound sulfamethoxazole was formulated for Patient 3. However, the patient refused intravenous ceftriaxone and was discharged from the hospital. Subsequently, Patient 3 changed to take TMP-SMZ orally. After twenty days, the patient developed gastrointestinal reactions, and discontinued taking the medicine independently. Despite no significant change (Fig. 3(a and b)) indicated by chest CT scan one year later, Patient 3 experienced intermittent cough, recurrent joint pain and diarrhea, and reported joint pain on September 7, 2022. MRI examination of both hips revealed a small amount of fluid in the left hip joint of this patient (Fig. 4).
Fig. 3.
Chest CT Scan of Patient 3 (The dates from left to right are April 16, 2021 (a) and May 21, 2022 (b)). The arrowhead shows the focus.
Fig. 4.
MRI of both hips of Patient 3. The arrowhead shows the focus.
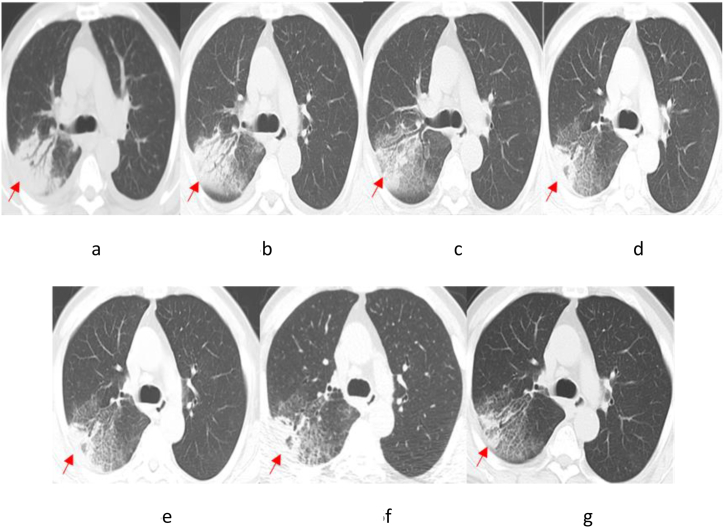
Patient 4 had an underlying condition of coronary atherosclerotic heart disease. Following admission, Patient 4 was treated with moxifloxacin for 2 days, yet without any improvement in the condition. Then, the patient was given an altered therapy of piperacillin-tazobactam combined with levofloxacin for two days. Unfortunately, the patient suddenly developed acute Myocardial infarction and died. After death, the patient was diagnosed with pneumonia caused by TW infection following a discussion by an expert group (Chest CT results in Fig. 5). Patient 5 had underlying conditions of MDS and diabetes. After admission, Patient 5 was initially treated with moxifloxacin for 2 days and subsequently administered piperacillin for 15 days, yet without significant improvement in lung lesions. Subsequently, the treatment plan was modified by the attending physician to long-term treatment with levofloxacin combined with doxycycline, resulting in a gradual improvement in the lung lesions (Fig. 6(a–g)). After a retrospective discussion by the expert group, the patient was confirmed with pneumonia caused by TW infection, and was recommended to be hospitalized to receive the standard treatment plan for WD. However, the patient declined hospitalization, still has a paroxysmal cough at present.
Fig. 5.
Chest CT Scan of Patient 4 (The date is June 6, 2022). The arrowhead shows the focus.
Fig. 6.
Chest CT Scan of Patient 5 (First line: the dates from left to right: July 15, 2022 (a); July 25, 2022 (b); August 1, 2022 (c); and August 23, 2022 (d). Last line: the dates from left to right: September 4, 2022 (e); October 12, 2022 (f); and November 9, 2022 (g)). The arrowhead shows the focus.
4. Discussion
Pneumonia, a frequently encountered clinical disorder, presents a significant challenge in both its diagnosis and treatment, particularly when it is induced by infection with rare or unfamiliar pathogens [3]. Following infection with TW, a majority of individuals exhibit either asymptomatic or self-limiting infection through the development of protective humoral and cellular immunity, while only a small part of cases present with WD [6]. Typical WD manifestations include joint disease/arthritis, weight loss, abdominal pain, and diarrhea [7].
It is currently evident that TW can cause acute pneumonia. In 2007, a study in the United States reported the detection of TW in children with interstitial lung disease through rRNA sequencing [8]. Some researchers employed 16SrDNA and specific quantitative polymerase chain reaction (PCR) to identify TW in the intensive care unit, and about 3% of the bronchoalveolar lavage fluid contained TW DNA via testing the BALF of pneumonia patients [9].
There have been reports strongly supporting the role of TW as a causative agent of community-acquired pneumonia [3]. The researchers also found TW in the saliva of asymptomatic individuals [9], and TW can be inhaled from the oral flora [10], potentially leading to community-acquired and aspiration pneumonia [11]. Respiratory involvement in TW infection is uncommon in clinical practice, with respiratory tract infection reported in only 13–14% of cases [12,13], but pleural effusion and/or pulmonary hypertension are more common clinically [14]. In our study, shortness of breath and cough were primary symptoms of the admitted patients, which were uncommon in prior reports. However, no distinct symptoms could be used for reference to distinguish from pneumonia caused by other pathogenic bacteria. In addition, similar to previous research [15], there was no significant difference in incidence between males and females. Most of our patients were young and middle-aged. However, further research is necessary to explore the age group with high prevalence of pneumonia caused by TW infection.
The incidence of WD among immunocompromised populations is an ongoing and controversial debate. It has been reported that the incidence of TW infection in HIV patients is notably higher than that in non-HIV patients [16]. While a large-sample study revealed no correlation between the immune status of patients and the occurrence of TW infection [17]. Of the 5 patients in our study, only one patient had diabetes, one patient exhibited symptoms of severe mixed sleep apnea hypoventilation syndrome, which may lead to immune dysfunction and, consequently, infection with TW. The remaining patients did not have any diseases associated with impaired immunity, such as HIV, diabetes, kidney transplantation, etc. Thus, clinicians must remain vigilant regarding the atypical manifestations of the disease and the diversity of patients, both of which are essential for improving our diagnostic success rate and optimizing our treatment methods.
Previous research has indicated that routine laboratory tests for patients with WD frequently yield non-specific results, including anemia (60–76%), hypoalbuminemia (50%), and elevated ESR or C-reactive protein levels (84%) [3]. Our findings were in line with these previous studies. However, TW infection often led to increased serum lactate dehydrogenase (LDH) levels. LDH is a critical enzyme in the glycolysis pathway, which is widely present in myocardial, liver, kidney, and lung tissues, with concentrations in these tissues being significantly higher than those found in serum [18]. When ischemia and hypoxic necrosis occur in these tissues, a large quantity of LDH may be released to induce a marked rise in serum LDH levels. In our study, except for Patient 3, who had symptoms of myocardial injury, no apparent abnormalities were found in the myocardium, liver, or kidneys of other patients. Therefore, it was speculated that the rise in LDH levels may be associated with the damage to lung tissue induced by TW. Monitoring LDH levels may assist the assessment of patients' condition and prediction of prognosis.
Chest CT scan of the enrolled patients exhibited a diverse range of findings, including the presence of lung cavities and air-fluid levels that closely resembled lung abscesses. Besides, the scan also indicated the existence of GGO, pulmonary nodules, pleural thickening, and exudative lesions. These findings, however, make it incredibly arduous to distinguish pneumonia caused by other pathogens, which is also a common observation in other similar studies [11]. It is crucial to note that pneumonia caused by TW infection is a highly elusive disease that is often misdiagnosed or even overlooked. The diagnosis of WD primarily relies on PAS staining of duodenal/jejunal biopsy specimens, PCR of TW, and immunohistochemical examination of affected organs [19]. Nonetheless, duodenal/jejunal biopsy specimen sampling or TW culture is quite challenging for patients without gastrointestinal symptoms.
Notably, recent research has shown high similarity of the genome features of Whipple strains from different countries [20]. Compared with traditional bacterial culture, mNGS of BALF has been demonstrated to have the capability to efficiently screen TW, thereby providing a more comprehensive view of airway bacterial infections [21,22]. Moreover, mNGS offers more clinical advantages and is capable of detecting rare microorganisms such as TW [11,22]. Therefore, in case of unknown cause of pneumonia, especially for patients with extrapulmonary manifestations, it is critical for healthcare professionals to consider mNGS as early as possible [23]. It may expedite the process of clinical assessment and benefit the identification of potential pathogens inducing pneumonia, leading to improved diagnostic success rate, particularly for rare microorganisms such as TW [3].
A multitude of pharmaceutical remedies readily are available to treat TW infection [24]. These pharmacological agents include, but are not limited to, penicillin, streptomycin, tetracycline, ceftriaxone, meropenem, trimethoprim, doxycycline, and hydroxychloroquine [16]. At present, the recommended therapeutic schedule for WD involves the administration of either ceftriaxone (a singular dosage of 2 g/day) or meropenem (three dosages of 1 g/day) for a duration of 14 days, followed by the oral administration of TMP-SMZ for 12 months. In case of intolerance towards ceftriaxone during treatment, meropenem may be prescribed as a replacement; conversely, doxycycline may be utilized in the event that a patient demonstrates intolerance towards TMP-SMZ [24,25].
Notably, the treatment of WD in patients with respiratory symptoms as the principal complaint remains in its incipient stages, and the treatment plans implemented by clinicians are far from standardized [5]. In our study, Patient 1 was subjected to a rigorous and intensive analysis via mNGS, and the subsequent results of this analysis were utilized to tailor the treatment regime to more effectively target the underlying pathogen. Ultimately, remarkable improvement was achieved in the condition of Patient 1. Conversely, Patient 2 demonstrated notable improvement after receiving empirical treatment with piperacillin-tazobactam. Despite our provision of a standardized treatment plan, Patient 2 adamantly refused to follow the scheme. Similarly, Patient 3 refused the administration of intravenous ceftriaxone on account of personal reasons, and instead received TMP-SMZ. Upon discontinuation of treatment, the patient evinced the reappearance of joint pain and sporadic diarrhea. Notably, one year subsequent to the cessation of treatment, re-examination of chest CT scans in this patient indicated no appreciable improvement in lung lesions, while hip MRI indicated the presence of effusion in the left hip joint. Our supposition is that this poor prognosis can be attributed to the patient's failure to receive an effective and standardized course of anti-TW infection treatment. This failure resulted in the persistence of lung lesions, which in turn accelerated the involvement of extrapulmonary organs such as the gastrointestinal tract and bones and joints, leading to recurrent joint pain and intermittent diarrhea. Tragically, Patient 4 died suddenly before receiving proper and standardized treatment. Patient 5 did not undergo the standard WD treatment regime due to the inadequate experience and training of the attending physician in the diagnosis and treatment of pneumonia caused by TW infection.
This disheartening state of affairs, described in our study, highlights the need for clinicians to deepen their understanding of the intricacies and complexities of rare diseases such as pneumonia caused by TW infection. To this end, it is essential that expert consensus and clinical guidelines should be developed to strengthen the learning and education of clinicians. Moreover, the diagnosis and treatment of rare diseases necessitate the participation and input of multidisciplinary teams. Finally and importantly, piperacillin-tazobactam may hold great promise as an efficacious pharmacological agent in the treatment of TW infection. However, further studies and investigations are needed to validate and substantiate this promising possibility.
This paper still has some limitations. It must be noted that TW was never detected as the sole agent by BALF-mNGS, pneumonia might also be the consequence of the coinfection. This fact could potentially interfere with the interpretation of chest CT scans and consequently, affect the success rate of subsequent treatments. Nevertheless, after a careful comparison of relevant data of pathogens, it can be found that TW had the highest sequence number and relative abundance, and was, therefore, most likely to be the pathogenic bacteria. Regrettably, due to poor patient compliance and the stringent medical ethics requirements that were put in place, this study failed to conduct pathological examinations to provide a more powerful explanation as to why TW is a pathogenic bacterium. However, it should be emphasized that each patient included in this study was clinically diagnosed as having pneumonia caused by TW infection after intense and rigorous discussions by experts in respiratory medicine, infectious diseases, and radiology. Just as important, all WD patients in this study did not receive standard treatment (intravenous ceftriaxone or meropenem followed by TMP-SMZ or doxycycline). The primary reasons were poor patient compliance and insufficient awareness of the diagnosis and treatment of the disease by doctors. This, in turn, limits the comparison of our findings with other case reports or studies. It is imperative to note that the use of TMP-SMZ or doxycycline as monotherapy must be avoided to avert future treatment failure. It is expected that through further continuous follow-up of patients, it may be possible to learn more about the development and prognosis of the proposed disease.
5. Conclusion
Pneumonia of unknown etiology shall be dealt cautiously by clinicians. mNGS sequencing using BALF is an excellent diagnostic method that can identify rare microbial infections, including TW. Early mNGS sequencing of BALF may significantly benefit patients with pneumonia of unknown etiology by expediting patient management and adjusting treatment accordingly.
Author contribution statement
Zhixiong Fang; Wei Tang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qiong Liu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hongyin Yu; Min Zou; Haiming Zhang; Haiyan Xue: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Yi Pei: Performed the experiments.
Sha Lin: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Jingwen Ai; Jun Chen: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to the patients for their cooperation in our investigation and to the Vision Medicals Co., Ltd., Guangzhou, China for support in our work.The work was supported by Hunan Province Science and Technology Major project (grant number: 20200855).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17132.
Contributor Information
Zhixiong Fang, Email: fzx8214907@sina.com.
Qiong Liu, Email: 18673052611@163.com.
Wei Tang, Email: 1310733799@qq.com.
Hongyin Yu, Email: yhy19959@163.com.
Min Zou, Email: minzou15@126.com.
Haiming Zhang, Email: 157134449@qq.com.
Haiyan Xue, Email: 237140766@qq.com.
Sha Lin, Email: 11938987@qq.com.
Yi Pei, Email: 1113284116@qq.com.
Jingwen Ai, Email: jingwenai1990@sina.com.
Jun Chen, Email: docter1793@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Obst W., von Arnim U., Malfertheiner P. Whipple's disease. Viszeralmedizin. 2014;30(3):167–172. doi: 10.1159/000363781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Compain C., et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltim.) 2013;92(6):324–330. doi: 10.1097/MD.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenollar F., et al. First isolation of Tropheryma whipplei from bronchoalveolar fluid and clinical implications. J. Infect. 2012;65(3):275–278. doi: 10.1016/j.jinf.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Lagier J.C., et al. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltim.) 2010;89(5):337–345. doi: 10.1097/MD.0b013e3181f204a8. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., et al. Severe pneumonia caused by infection with Tropheryma whipplei complicated with acinetobacter baumannii infection: a case report involving a young woman. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.729595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenollar F., Perreal C., Raoult D. Tropheryma whipplei natural resistance to trimethoprim and sulphonamides in vitro. Int. J. Antimicrob. Agents. 2014;43(4):388–390. doi: 10.1016/j.ijantimicag.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Duss F.R., et al. Whipple disease: a 15-year retrospective study on 36 patients with positive polymerase chain reaction for Tropheryma whipplei. Clin. Microbiol. Infect. 2021;27(6) doi: 10.1016/j.cmi.2020.08.036. 910.e9-910.e13. [DOI] [PubMed] [Google Scholar]
- 8.Harris J.K., et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousbia S., et al. Tropheryma whipplei in patients with pneumonia. Emerg. Infect. Dis. 2010;16(2):258–263. doi: 10.3201/eid1602.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinetti M., et al. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple's disease. Gastroenterology. 2009;136(7):2289–2294. doi: 10.1053/j.gastro.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Lin M., et al. Tropheryma whipplei detection by metagenomic next-generation sequencing in bronchoalveolar lavage fluid: a cross-sectional study. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.961297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Scola B., et al. Epidemiologic implications of the first isolation and cultivation of Tropheryma whipplei from a saliva sample. Ann. Intern. Med. 2011;154(6):443–444. doi: 10.7326/0003-4819-154-6-201103150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Fenollar F., et al. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: usefulness of saliva and stool specimens for first-line screening. Clin. Infect. Dis. 2008;47(5):659–667. doi: 10.1086/590559. [DOI] [PubMed] [Google Scholar]
- 14.Bousbia S., Papazian L., Auffray J.P., Fenollar F., Martin C., Li W. Tropheryma whipplei in patients with pneumonia. Emerg. Infect. Dis. 2010;16(2):258–263. doi: 10.3201/eid1602.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., et al. Genotyping reveals a wide heterogeneity of Tropheryma whipplei. Microbiology (Read.) 2008;154(Pt 2):521–527. doi: 10.1099/mic.0.2007/011668-0. [DOI] [PubMed] [Google Scholar]
- 16.Lozupone C., et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am. J. Respir. Crit. Care Med. 2013;187(10):1110–1117. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagier J.C., et al. Tropheryma whipplei DNA in bronchoalveolar lavage samples: a case control study. Clin. Microbiol. Infect. 2016;22(10):875–879. doi: 10.1016/j.cmi.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Li H., Xing B. [Prognostic value of lactate dehydrogenase in septic shock patients] Chin. Emerg. Med. 2019;39(3):211–215. [Google Scholar]
- 19.El-Abassi R., et al. Whipple’s disease. J. Neurol. Sci. 2017;377:197–206. doi: 10.1016/j.jns.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Lv Z., et al. Genomic characterization of two metagenome-assembled genomes of Tropheryma whipplei from China. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.947486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marth T. Systematic review: Whipple's disease (Tropheryma whipplei infection) and its unmasking by tumour necrosis factor inhibitors. Aliment. Pharmacol. Ther. 2015;41(8):709–724. doi: 10.1111/apt.13140. [DOI] [PubMed] [Google Scholar]
- 22.Li W., et al. Severe pneumonia in adults caused by Tropheryma whipplei and Candida sp. infection: a 2019 case series. BMC Pulm. Med. 2021;21(1):29. doi: 10.1186/s12890-020-01384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W.M., Xu L. Pulmonary parenchymal involvement caused by Tropheryma whipplei. Open Med. (Wars) 2021;16(1):843–846. doi: 10.1515/med-2021-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolmans R.A., et al. Clinical manifestations, treatment, and diagnosis of Tropheryma whipplei infections. Clin. Microbiol. Rev. 2017;30(2):529–555. doi: 10.1128/CMR.00033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feurle G.E., et al. Intravenous ceftriaxone, followed by 12 or three months of oral treatment with trimethoprim-sulfamethoxazole in Whipple's disease. J. Infect. 2013;66(3):263–270. doi: 10.1016/j.jinf.2012.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.