Abstract
Chronic liver disease is a significant public health issue that can lead to considerable morbidity and mortality, imposing an enormous burden on healthcare resources. Understanding the mechanisms underlying chronic liver disease pathogenesis and developing effective treatment strategies are urgently needed. In this regard, the activation of liver resident macrophages, namely Kupffer cells, plays a vital role in liver inflammation and fibrosis. Macrophages display remarkable plasticity and can polarize into different phenotypes according to diverse microenvironmental stimuli. The polarization of macrophages into M1 pro-inflammatory or M2 anti-inflammatory phenotypes is regulated by complex signaling pathways such as the PI3K/Akt pathway. This review focuses on investigating the potential of using plant chemicals targeting the PI3K/Akt pathway for treating chronic liver disease while elucidating the polarization mechanism of macrophages under different microenvironments. Studies have demonstrated that inhibiting M1-type macrophage polarization or promoting M2-type polarization can effectively combat chronic liver diseases such as alcoholic liver disease, non-alcoholic fatty liver disease, and liver fibrosis. The PI3K/Akt pathway acts as a pivotal modulator of macrophage survival, migration, proliferation, and their responses to metabolism and inflammatory signals. Activating the PI3K/Akt pathway induces anti-inflammatory cytokine expression, resulting in the promotion of M2-like phenotype to facilitate tissue repair and resolution of inflammation. Conversely, inhibiting PI3K/Akt signaling could enhance the M1-like phenotype, which exacerbates liver damage. Targeting the PI3K/Akt pathway has tremendous potential as a therapeutic strategy for regulating macrophage polarization and activity to treat chronic liver diseases with plant chemicals, providing new avenues for liver disease treatment.
Keywords: Chronic liver disease, Macrophage polarization, PI3K/Akt signaling pathway, Therapeutic strategy
1. Introduction
Chronic liver disease (CLD), which includes chronic viral hepatitis, alcoholic liver disease, nonalcoholic steatohepatitis, cirrhosis, liver cancer, and other conditions, is a major global health issue [1]. More than 1.5 billion people globally suffer from CLD, which results in approximately 2 million deaths each year: 1 million from complications of cirrhosis and 1 million from viral hepatitis and hepatocellular carcinoma (HCC) [2,3]. There are multiple causes of CLD, with the primary causes being chronic hepatitis B virus, chronic hepatitis C virus, non-alcoholic fatty liver disease, and alcoholic liver disease. The most common reasons are viral hepatitis and non-alcoholic fatty liver disease [4]. In the past 20 years, the incidence rate of CLD has increased rapidly, not only becoming one of the main causes of premature death but also a global health concern [5]. The Global Burden of Liver Disease report in 2023 indicates that every 25 deaths globally involves one related to liver disease. As the 11th leading cause of death, liver disease disproportionately impacts the 25–49 age group, which represents the segment with the most potential workforce productivity. In 2020, 1.1 million people died from diseases related to hepatitis B and C, yet there are minimal resources globally to control and eliminate viral hepatitis [6]. HCC has a high mortality rate, being the fourth leading cause of cancer-associated deaths. Cirrhosis is an important risk factor for HCC, representing the 15th leading life years lost due to disability worldwide. Furthermore, patients with cirrhosis require enormous healthcare costs [6,7]. Compared to other chronic diseases, most CLDs can be prevented, treated, and/or cured if diagnosed early [8]. Thus, understanding the mechanisms underlying CLD pathogenesis and developing effective treatment strategies are urgently needed. Macrophages are the first line of defense against infections [9]. Under physiological conditions, they are present in almost all tissues and play a crucial role in immunity, repair, and maintenance of tissue homeostasis [10]. Depending on their location in the body, macrophages have different names, such as alveolar macrophages, kupffer cells (KCs) in the liver, microglia in the central nervous system, etc. [11,12]. Liver macrophages include both resident KCs and recruited macrophages derived from monocytes. KCs are the largest population of macrophages residing around the sinusoids of the liver, with high phagocytic activity and functions in clearing apoptotic cells and particulate matter in the portal circulation and microbes. Studies have shown that liver macrophages play an important role in maintaining liver homeostasis and are closely associated with various CLDs [13,14]. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway is one of the most important intracellular signaling pathways [15]. It plays a crucial role in regulating macrophage survival, migration, proliferation, and orchestrating responses to various metabolic and inflammatory signals [16]. The PI3K/Akt pathway is also a focus of research for many diseases, including renal cell carcinoma [17], metastatic prostate cancer [18], metastatic urothelial carcinoma [19], and others. In this review, we summarize the interplay between macrophage polarization, the PI3K/Akt signaling pathway, and CLDs, as well as potential therapies for CLD by controlling macrophage polarization through the PI3K/Akt pathway.
2. Macrophage polarization in chronic liver disease
2.1. Macrophage polarization
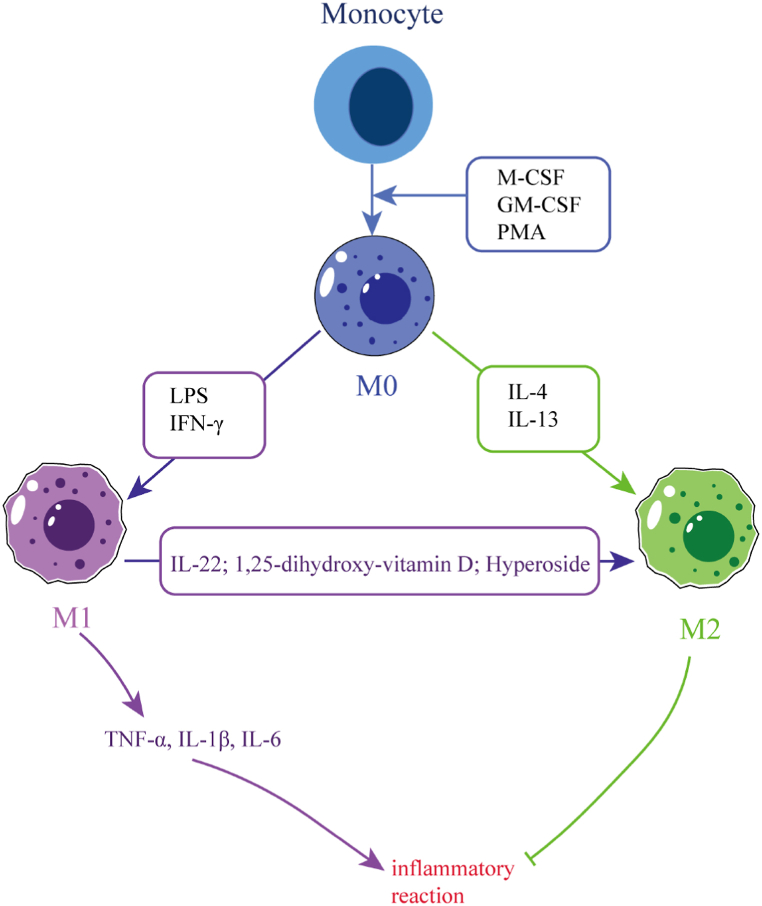
Macrophage polarization refers to the activation of macrophages under the stimulation of pathogenic microorganisms, inflammatory reactions, cytokines or some physical and chemical factors. Macrophages differentiate into different phenotypes based on the prevailing conditions and changes in the microenvironment (as shown in Fig. 1). These differentiated macrophages possess unique properties that enable them to effectively combat pathogens or repair damage caused by inflammation [20]. There are three ways to control macrophage polarization. The first way involves using epigenetics and cell survival mechanisms to prolong or shorten the time for macrophage development and survival. The second way is by manipulating the normal tissue microenvironment. Finally, the third way is by being influenced by exogenous factors, such as cytokine release due to inflammation [21,22]. Macrophages possess a high degree of plasticity and can be polarized into two main phenotypes, namely classically activated (M1) and alternatively activated (M2) macrophages [23]. M1 polarization is characterized by the promotion of inflammatory responses. This type of polarization is driven by factors such as granulocyte-macrophage colony-stimulating factor, lipopolysaccharide (LPS), or other pathogen-associated molecular patterns [24]. The secretion of cytokines such as interleukin-1β(IL-1β), tumor necrosis factor-a (TNF-a), and interleukin-12 (IL-12) by M1 macrophages boosts pro-inflammatory T helper type 1 (Th1) responses. Furthermore, chemokines such as CXC chemokine ligand 9 (CXCL9) and CXC chemokine ligand 10 (CXCL10) are secreted by these macrophages to enhance the recruitment of Th1 cells to inflammatory sites [25]. Interleukin-4 (IL-4) and interleukin-13 (IL-13) can activate M2 macrophages [26]. These types of macrophages are involved in anti-inflammation, immune regulation, tissue remodeling, parasitic infection prevention, and participate in processes such as angiogenesis, immune regulation, and tumorigenesis [27]. M2 macrophages produce anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Based on various activation stimuli, M2 macrophages can be further subdivided into M2a, M2b, and M2c subtypes. The most immunosuppressive of these subsets is M2c [28,29]. M2-polarized macrophages typically emerge after M1 polarization as a means to restore homeostasis when an infection or inflammation is severe enough to affect an organ. Such macrophages function to counterbalance the inflammatory response and promote tissue repair [30].
Fig. 1.
Macrophage polarization. This figure illustrates the process of macrophage polarization. Monocytes mature into macrophages under certain stimulation, which can be further differentiated into M1 or M2 types based on the changes in the microenvironment. M1 macrophages secrete pro-inflammatory cytokines and promote inflammatory responses, whereas M2 macrophages have the opposite effect. Additionally, M1 macrophages can also be polarized into M2 macrophages under certain factors.→ indicates promotion, and ⊣ indicates inhibition. The abbreviations used in the figure are: GM-CSF (Granulocyte-macrophage Colony Stimulating Factor), IFNγ (Interferon-γ), LPS (Lipopolysaccharide), TNF-α (Tumor Necrosis Factor-α), M-CSF (Macrophage colony-stimulating factor), IL-1β (interleukin-1β), PAM (phorbol ester), IL-4 (Interleukin-4), IL-22 (Interleukin-22), and IL-13 (Interleukin-13).
2.2. Macrophage polarization and chronic liver disease
Alcoholic liver disease (ALD) is a leading cause of CLD worldwide, responsible for 0.9% of all deaths globally [31,32]. It occurs due to prolonged and excessive alcohol consumption and is characterized by the activation of KCs and recruitment of inflammatory monocytes/macrophages [33,34]. KCs in ALD patients exhibit increased levels of reticulon 4B (Nogo-B), which correlates with disease severity. Nogo-B has been found to promote M1 polarization of KCs in ethanol-fed mice and inhibit M2 polarization, thereby aggravating liver injury in human and mouse ALD [35]. Similarly, inhibiting M1 polarization while promoting M2 polarization could be a potential therapeutic strategy for ALD. For instance, studies have found that inulin can reduce the inflammatory response of ALD in female C57BL/6j mice by inhibiting M1 macrophage polarization while activating M2 macrophages via the production of short-chain fatty acids [36,37]. Through this intervention, the proportion of liver M1 macrophages was reduced, and that of M2 macrophages was increased, ultimately leading to a reduction in inflammation in mice with ALD.
Nonalcoholic steatohepatitis (NASH) is commonly defined as the advanced stage of nonalcoholic fatty liver disease (NAFLD), characterized by the accumulation of fat, inflammation, and progressive fibrosis in the liver [38,39]. NASH often lacks apparent clinical symptoms in the early stages. However, if left untreated, it can progress to cirrhosis, end-stage liver disease, or require liver transplantation over time [40]. Studies have revealed that the overexpression of M1 markers in the liver is associated with increased inflammation in methionine-choline-deficient (MCD)-induced NASH C57BL/6 mice [41]. Therefore, targeting the inhibition of M1 macrophage polarization might represent an effective approach for treating NASH. In wild-type mice, growth hormone secretagogue receptor 1a (GHSR1a) was detected in KCs. Ghrelin, a 28-amino acid gastric hormone, has been found to inhibit M1 polarization of KCs mediated by GHSR1a and subsequently block the progression of lipopolysaccharide-induced NASH [42]. Furthermore, inhibiting M1 macrophage polarization or activating M2 macrophage polarization can both be beneficial for treating NASH. Tong et al. demonstrated that curcumin can inhibit the activation of M1 macrophages both in vitro and in vivo, reduce the expression of IL-1β and TNF-α, thus reducing liver damage and inflammatory response in NASH [43]. Similarly, honokiol (HNK) reduced the expression of M1 marker genes (such as TNFα and monocyte chemoattractant protein-1 (MCP-1) and increased the expression of M2 marker genes (such as IL-10 and IL-13) in the livers of mice fed with a high cholesterol and high-fat (CL) diet. HNK alleviates CL diet-induced NASH by activating peroxisome proliferator-activated receptor γ (PPARγ) to regulate macrophage polarization towards the M2 phenotype. Moreover, it was observed that treatment with 10 μM HNK induced M2 macrophage polarization in mouse peritoneal cells, RAW264.7 cells, and ANA-1 cells [44].
Hepatic fibrosis is an excessive healing response to prolonged liver injury in CLD. The progressive accumulation of fibrotic tissue can lead to cirrhosis, which can further develop into HCC in severe cases and even result in liver failure in some patients with liver fibrosis. Hepatic macrophages have been found to play a crucial role in initiating inflammatory responses to liver injury, promoting fibrosis progression, and the formation of fibrosis [45]. Su et al. discovered that the level of interleukin-22 (IL-22) was positively correlated with the number of M2-KCs during liver fibrosis development. IL-22 can regulate the signal transducer and activator of transcription 3 (STAT3)/extracellular signal-regulated kinase (Erk)/Akt pathways to increase the ratio of M2/M1 KCs, thus slowing down the progression of liver fibrosis [46]. Similarly, inhibiting M1 macrophage polarization can also play a role in the treatment of liver fibrosis. Xu et al. found that Yiguanjian (YGJ) has an anti-hepatic fibrosis effect. In rats with liver fibrosis induced by 2-acetylaminofluorene (2-AAF)/carbon tetrachloride (CCl4) and RAW264.7 cells, YGJ treatment significantly decreased the expression levels of signal transducer and activator of transcription 1 (STAT1), interferon regulatory factor 3 (IRF3), interferon regulatory factor 5 (IRF5), and suppressor of cytokine signaling 3 (SOCS3) in M1 macrophages [47].
Liver cirrhosis is a chronic disease caused by liver inflammation and fibrosis. It has been revealed that advanced fibrosis and even cirrhosis can be reversed by treating the underlying cause, with a liver biopsy being the gold standard for diagnosing cirrhosis [48]. Liver cirrhosis is closely related to KCs polarization. Zhang et al. discovered that angiopoietin-like 4 (ANGPTL4), an important regulator of KCs, can regulate KCs polarization and hepatic stellate cell activation, thereby reducing hepatitis B virus-induced cirrhosis in mice. The mechanism may be related to the activation of Toll-like receptor 4 (TLR4)/nuclear factor kappaB (NF-κB) signaling pathway [49]. Relevant studies have found that inhibiting M1 macrophages and promoting M2 macrophages can also play a role in the treatment of cirrhosis. YGJ can improve the therapeutic effect of fetal liver stem/progenitor cells (FLSPCs) on 2-AAF/CCl4-induced liver cirrhosis in rats by regulating macrophage activation (significantly reducing TNF-α and CD68 expression levels, while significantly increasing CD163 expression levels). Moreover, the results of in vitro experiments were consistent with those of in vivo experiments [50].
HCC is the most common primary liver cancer and is considered to be the leading cause of death in patients with cirrhosis [51]. Studies have discovered that M2 KCs polarization is the primary cause of HCC in Akt (myr-Akt) and NRas (NRas-V12) oncogenes (Akt/Ras) mice. This phenotype can be recapitulated in vivo by hydrodynamically transfecting activated forms of Akt/Ras into the mouse liver. MicroRNA-206 drives the M1 polarization of KCs, which promotes the recruitment of CD8+ T cells and prevents HCC [52]. Furthermore, HCC has been found to be closely associated with both M1 and M2 macrophages [53,54]. Lu et al. discovered that cantharidin (NCTD) may play an anti-HCC role by regulating macrophage polarization through miR-214. The addition of NCTD-treated RAW264.7 or tumor-associated macrophages (TAMs) enhanced M1 polarization by increasing microRNA-214 (miR-214) expression [55]. Min et al. found that astragaloside IV (AS-IV) reduced the expression of macrophage markers CD206, CD209 and TGF-β. AS-IV may inhibit macrophage M2 polarization through the TLR4/NF-κB/STAT3 signaling pathway and inhibit the proliferation, invasion, and migration of HCC [59]. The above passage illustrates that CLD is closely related to macrophage polarization. Table 1 summarizes the therapeutic effects of traditional Chinese medicine targeting macrophage polarization in the treatment of CLD.
Table 1.
Overview of Chinese medicine targeting macrophage polarization in chronic liver disease.
| Disease | Chinese medicine and active ingredients | In vitro/in vivo | Mechanisms | M1 | M2 | Reference |
|---|---|---|---|---|---|---|
| Alcoholic liver disease | Inulin | In vitro and in vivo | Single-chain fatty acids↑ | ↓ | ↑ | [37] |
| Cannabinoid CB2 Receptors | In vitro and in vivo | HO-1↑ | ↓ | ↑ | [36] | |
| Nonalcoholic steatohepatitis | Honokiol | In vivo | PPAR γ↑ | - | ↑ | [44] |
| Nobiletin | In vitro and in vivo | KLF4↑ | ↓ | ↑ | [56] | |
| Jiangzhi Granule | In vivo | TNF/NFκB signaling↓ | ↓ | – | [57] | |
| Myricetin | In vitro and in vivo | TREM-1-TLR2/4-MyD88 signaling molecules↓ | ↓ | ↑ | [58] | |
| Curcumin | In vitro and in vivo | IL-1βand TNF-α↓ | ↓ | – | [43] | |
| Non-alcoholic fatty liver disease | artemether | In vitro and in vivo | TGF-β/SMAD pathway↓ | – | ↑ | [60] |
| Hyperoside | In vivo | Nr4A1↑ | – | ↑ | [61] | |
| DWJ504 | In vivo | TLR4↓ | ↓ | ↑ | [62] | |
| Liver fibrosis | Yiguanjian decoction | In vivo | Non-canonical Wnt signaling pathway↓ | ↓ | - | [47] |
| Resveratrol | In vitro and in vivo | IL-10↑ | ↓ | ↑ | [63] | |
| Curcumin | In vitro | ERK1/2 and p38 pathways↑ | ↓ | – | [64] | |
| Liver cirrhosis | Yiguanjian decoction | In vitro and in vivo | Fetal liver stem/progenitor cell↑ | ↓ | ↑ | [50] |
| Hepatocellular carcinoma | Cantharidin | In vitro and in vivo | miR - 214↑ | ↑ | - | [55] |
| Astragaloside IV | In vitro | TLR4/NF-κB/STAT3 signaling pathway↓ | - | ↓ | [59] | |
| Astragalus polysacharin | In vitro | – | ↑ | ↓ | [65] | |
| Ganoderma lucidum polysaccharid | In vitro | MAPK/NF-κB signaling pathway↑ | ↑ | – | [66] | |
| Emodin | In vitro and in vivo | miR-26/TGF-β1/Akt axis↑ insulin-like growth factor-1 secretion↓ | ↓ | ↑ | [67] |
Abbreviation: HO-1, Heme oxygenase-1; PPARγ, peroxisome proliferator-activated receptor γ; KLF4, Kruppel like factor 4; TNF/NFκB, tumor necrosis factor/nuclear factor kappa-B; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; DWJ504, extract of Opuntia ficus indica seed; Nr4A1, the orphan nuclear hormone receptor nuclear receptor subfamily 4, group a, member 1; TLR4, Toll-like Receptor 4; IL-10, interleukin-10; ERK1/2, extracellular signal-regulated kinases 1/2; STAT3, signal transducer and activator of transcription3; MAPK, mitogen activated protein kinase; TGF-β, transforming growth factor-β1; Akt, protein kinase B.
The symbol ↑ represents an increase, while the symbol ↓ represents a decrease.
3. PI3K/Akt signaling pathway in chronic liver disease
3.1. PI3K/Akt signaling pathway
The PI3K/Akt pathway is a crucial signaling pathway that controls cellular processes such as cell division, autophagy, survival and differentiation [68]. Recent research has identified the role of this pathway in regulating macrophage survival, migration, proliferation and response to various metabolic and inflammatory signals [16]. The primary proteins involved in the PI3K/Akt pathway are phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) [15]. Based on their primary structure, regulation and lipid substrate specificity, the PI3K family is divided into three categories: class I, class II and class III. Class I includes two subgroups, IA and IB, which are coupled to different receptors. Class II catalyzes the conversion of PI to PI(3)P and PI(3,4)P2 while class III is composed of a regulatory and catalytic subunit heterodimer [69]. Akt is the primary downstream target of PI3K and belongs to the AGC kinase family of serine/threonine kinases that regulate cell cycle, transcription, translation, apoptosis, and differentiation. There are three subtypes of Akt: Akt1, Akt2, and Akt3. Akt1 is present in all tissues and regulates multiple cellular processes, Akt2 is mostly found in muscle tissue and adipocytes, and Akt3 is mostly found in the brain [68,70]. Activation of receptor tyrosine kinases and growth factors initiates the formation of heterodimers of class Ia lipid kinases composed of catalytic and regulatory subunits, which generate transient lipid second messengers PIP2 and PIP3. The latter activates T308 and S473 residues of Akt. T308 phosphorylation is both necessary and sufficient for inducing Akt signaling, while S473 phosphorylation is required for maximum kinase activation. However, phosphatase and tensin homolog (PTEN) normally quickly metabolizes PIP3 by removing the 30-phosphate from it, terminating PI3K signaling [16,71]. There are two main mechanisms that inhibit the PI3K/Akt pathway. Firstly, PTEN converts PI (3,4,5) P3 to phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), thus removing it from the pathway. Secondly, Akt inactivation is primarily accomplished by the PH domain leucine-rich repeat protein phosphatase (PHLPP) family, which inhibits Akt signaling by directly dephosphorylating Akt [72].
The PI3K/Akt pathway is activated by TLR4 and other pathogen recognition receptors, cytokines and chemokines, and Fc receptors to regulate downstream signals that control cytokine production [[73], [74], [75]]. IL-1 receptor-associated kinase M (IRAK-M) is a negative regulator of Toll-like receptor (TLR) signaling and plays a crucial role in maintaining the homeostasis of innate immunity [76]. It has been discovered that the PI3K/Akt1 axis up-regulates IRAK-M and suppresses TLR4 signaling by deactivating tumor necrosis factor receptor-associated factor 6 (TRAF6) [16]. Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) has been found to inhibit the M2 polarization of TAMs through the PI3K/Akt pathway, reversing the process of endometrial carcinoma (EC) cells inducing naive macrophages (M0) to differentiate into M2 macrophages [77]. Conversely, Zhang et al. found that alpha-fetoprotein (AFP) promotes the polarization of macrophages to M2 macrophages and inhibits the phagocytosis of M1 macrophages to liver cancer cells, possibly by activating the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway [78]. However, a single PI3K and Akt subtype can also cause M1 or M2 macrophage polarization. Inhibition of PI3K enhances NF-κB activation and inducible nitric oxide synthase (iNOS) synthesis, promoting M1 macrophage polarization. Inhibition of PI3K negative regulators leads to decreased secretion of pro-inflammatory cytokines in macrophages, inducing the synthesis of M2 macrophage surface markers and changing macrophage phenotype to M2 type [16]. Additionally, studies have shown that Akt1 and Akt2 have different effects on macrophage polarization. The absence of Akt1 promotes M2 macrophage polarization, while the absence of Akt2 promotes M1 macrophage polarization [71].
3.2. PI3K/Akt signaling pathway and chronic liver disease
An increasing number of studies have found that the PI3K/Akt signaling pathway is closely related to CLD, such as ALD, liver fibrosis, HCC, and more [79,80]. Therefore, targeting the PI3K/Akt pathway may be an effective way to treat CLD. Wu et al. showed that quercetin could reduce liver fibrosis in bile duct ligation or CCl4 mice by inhibiting autophagy through the transforming growth factor-β1 (TGF-β1)/Smads signaling pathway and activating the PI3K/Akt signaling pathway, regulating matrix metalloproteinase (MMPs)-9 and tissue inhibitor of metalloproteinase (TIMP)-1, and preventing liver fibrosis [81]. Similarly, dihydroartemisinin has been found to prevent liver fibrosis in bile duct ligated rats by inhibiting the PI3K/Akt pathway, inducing caspase-related mitochondrial apoptosis in hepatic stellate cells (HSCs), and giving cytotoxic effects [82]. Additionally, dihydroartemisinin has also demonstrated potential in treating ALD and inhibiting the activation of endoplasmic reticulum stress-c-Jun N -terminal binase (JNK)/C/EBP homologous protein (CHOP)--mitochondrial cascade in ethanol-treated hepatocytes, showing promise for reducing hepatocyte lipid apoptosis by regulating the PI3K/Akt pathway [83]. In terms of treating HCC, targeting the PI3K/Akt pathway has demonstrated effectiveness. Sun et al. found that the matrine derivative WM622 (a matrine derivatives named WM622) exhibited anti-proliferative properties by inducing apoptosis and G0/G1 phase cell cycle arrest in vitro and inhibiting tumor growth in vivo via the PI3K/Akt pathway [84]. Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease with an unknown cause. It has been discovered that cellular communication network factor 1 (CCN1) can induce the production of interleukin 6 (IL-6) through the a6b1/PI3K/Akt/NF-κB pathway, leading to an increased inflammatory response in autoimmune hepatitis [85]. Consequently, inhibiting this pathway may prevent further development of AIH. In animal experiments, Wang et al. discovered that celastrol significantly reduced AIH by inhibiting the PI3K/Akt pathway [86]. Similarly, scoparone has also been found to inhibit the activation of PI3K/Akt/mTOR pathway in mice with nonalcoholic steatohepatitis, improving liver inflammation and autophagy. Furthermore, it enhances autophagic flux and regulates autophagy by inhibiting reactive oxygen species (ROS)/P38/nuclear factor erythroid-2 related factor 2 (Nrf2) axis and PI3K/Akt/mTOR pathway in LPS-induced macrophages, thereby inhibiting inflammatory response [87]. Therefore, modulation of the PI3K/Akt signaling pathway could potentially serve as an effective therapeutic strategy for the management of chronic liver disease (CLD) [[88], [89], [90], [91]] (Fig. 2).
Fig. 2.
Medications targeting the PI3K/Akt signaling pathway are used for treating CLD. Green and purple arrows indicate promotion of liver fibrosis and nonalcoholic fatty liver disease, respectively, while blue and orange arrows represent inhibition of HCC development (Fig. 2). The abbreviations used in the figure are: HCC (hepatocellular carcinoma), TDF (Tenofovir disoproxil fumarate), PARP (poly ADP-ribose polymerase), Bcl-xl (B-cell lymphoma-extra large), ROS (reactive oxygen species), LC3I (microtubule-associated protein light chain 3I), LC3II (microtubule-associated protein light chain 3II), HO-1 (heme oxygenase-1), GCLC (glutamate-l-cysteine ligase catalytic subunit), Nrf2 (Nuclear factor erythroid-2-related factor 2), EADM (Epirubicin), Tan I (Tanshinone I), HIF-1α (hypoxia-inducible factor-1α), Rg3 (ginsenoside Rg3), and SFN (sorafenib). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. PI3K/Akt signaling pathway regulates macrophage polarization in chronic liver disease
Recent studies have shown that the PI3K/Akt pathway also plays a crucial role in innate immunity and macrophage activation [92,93]. Fibrosis-induced M1 and M2 macrophage markers increased, indicating that both M1 and M2 macrophages were triggered by CCl4 treatment. Conversely, regression of hepatic fibrosis led to a decrease in M1 and M2 hepatic macrophages. These findings further demonstrate the significant impact of activated hepatic macrophages on hepatic fibrosis development and recovery [94,95]. PTEN, the primary negative regulator of the PI3K/Akt pathway, is an upstream regulator of hepatic macrophage polarization and function. PTEN collaborates with PI3K/Akt/signal transducer and activator of transcription 6 (STAT6) to regulate IL-4-induced murine M2 macrophages [94]. Chen et al. found that triggering receptor-1 expressed on low-expressed myeloid cells may regulate the transformation of M2 macrophages to M1 macrophages through the PI3K/Akt pathway, leading to inhibited migration and invasion of HepG2 and MHCC97H cells when the signaling pathway was blocked [96]. P. amarus has been found to inhibit Myeloid differentiation factor SS (MyD88)-dependent signaling pathways and suppress inflammatory responses in LPS-induced U937 (ATCC ® CRL-1593.2) macrophages through its ethanol extract, which may be related to its inhibitory effects on pro-inflammatory mediators. Thus, the ethanol extract of P. amarus has promising anti-inflammatory activities by blocking the NF-κB, mitogen activated protein kinase (MAPK), and PI3K-Akt signaling pathways [97]. Song et al. found that Ganoderma lucidum spore polysaccharide can promote the polarization of primary macrophages to M1 type, up-regulate the expression of cytokines such as TNF-α, IL-1β, IL-6 and transforming growth factor-β1 (TGF-β1), block H22 tumor cells in G2/M phase, and activate PI3K/Akt pathway to affect mitochondrial apoptosis pathway and promote tumor cell apoptosis [98]. Acetaminophen-induced liver injury is the most common medicine-induced liver injury in CLD patients [99]. Studies have found that styrene maleic acid copolymer (SMA) micelle encapsulating CO releasing molecule (SMA/CORM2) regulates macrophage reprogramming to an M2 phenotype by inhibiting HIF-1α and activating the PI3K/Akt/mTOR pathway, promoting hepatocyte proliferation, and aiding hepatic protection against inflammatory damage [100]. Interleukin-33 (IL-33) enhances M2 polarization via the PI3K/Akt pathway while activating AMP-activated protein kinase (AMPK)α/mTOR signaling pathways to promote protective hepatocyte autophagy in male C57BL/6 N mice with Acetaminophen (APAP)-induced liver injury [101]. Hyperglycemia promotes liver macrophage pro-inflammatory responses through the AMPK/PI3K/Akt-mediated oxidative stress pathway, aggravating APAP-induced acute liver injury [102]. The codelivery of HBx-siRNA and Plasmid Encoding IL-12 (siRNA/pIL-12) exhibits good potential in preventing viral hepatitis type B (HBV)-induced HCC by inhibiting the PI3K/Akt and ERK pathways. Transfecting siRNA/pIL-12 complexes mediates pIL-12 in immune cells (J774A.1 macrophages), successfully regulating the immune response and increasing cytokine secretion [103]. In conclusion, regulating macrophage polarization through the PI3K/Akt signaling pathway may be an effective approach to treating CLD (Table 2).
Table 2.
Overview of the research on regulating macrophage polarization through PI3K/Akt signaling pathway in the treatment of chronic liver disease.
| Disease | Regulation Factor | Research objects | The administration of modeling and dose | Duration | Macrophage polarization | Mechanisms | Reference |
|---|---|---|---|---|---|---|---|
| Liver fibrosis | ↓PTEN | Mice and cell | CCL4(0.02 mL/g/mouse),; LPS(1000 ng/mL), IL-4 (20 ng/mL) | 8weeks; 24 h | M2↑ | p-Akt↑, p-STAT6↑ | [94] |
| Drug-induced liver injury | SMA/CORM2 | Mice and cell | APAP (300 mg/kg) | – | M1↓M2↑ | p-Pi3k↑, p-Akt↑, p-mTOR↑, PCNA↑ | [100] |
| Viral hepatitis type B | siRNA/pIL-12@lipo | Cell | – | – | Mø→M1 | p-PI3K↓, p-Akt↓, p-ERK↓, Bcl-2↓, p53↑ | [103] |
| HCC | ↓TREM1 | Cell and patients and specimens | – | – | M2→M1 | p-PI3K/PI3K↓, p-Akt/Akt↓, p-mTOR/mTOR↓ | [96] |
| GLSP | Mouse and cell | GLSP (800,400,200 μg/mL) | 24 h | Mø→M1 | PI3K↓, p-Akt↓, BAX↑, BCC-2↓, CASD-9↑ | [98] | |
| AFP | Cell | LPS(50 ng/mL)+IFN-γ(20 ng/mL); IL-4 (20 ng/mL) +IL-13 (20 ng/mL) | 24 h; 72 h | M1phagocytic ability↓ | PI3K/Akt↑ | [78] |
Abbreviation: PTEN, phosphatase and tensin homolog; CCl4, carbon tetrachloride; LPS, lipopolysaccharide; IL-4, Interleukin-4; KCs, Kupffer cells; SMA/CORM2, styrene maleic acid copolymer (SMA) micelle encapsulating CO releasing molecule; siRNA/pIL-12@lipo, Codelivery of HBx-siRNA and Plasmid Encoding IL-12; HCC, Hepatocellular carcinoma; TREM1, Triggering receptor expressed on myeloid cells 1; THP-1, The human leukemia monocytic cell line; Mø, primary macrophage; GLSP, Ganoderma lucidum Spore Polysaccharide; AFP: alpha-fetoprotein.
The symbols ↑, ↓, and → represent an increase, decrease, and or polarization, respectively.
5. Conclusions and perspectives
Macrophages can polarize into M1 or M2 types under different microenvironmental stimuli, and regulating this polarization through the PI3K/Akt signaling pathway is a new avenue for treating chronic liver disease. While inhibiting M1 polarization or promoting M2 polarization has shown promise in treating various chronic liver diseases, additional research is needed to determine the best methods for targeting and administering therapies, appropriate dosages, and potential technical challenges. Further exploration of cellular and molecular mechanisms through advanced technologies such as gene editing, proteomics, metabolomics, single-cell RNA sequencing, and bioinformatics may lead to the development of more personalized and precise treatment strategies for chronic liver diseases.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Xiaotao Jia was supported by The Shaanxi Province Research and Development Program project, PR China {2023-YBSF-394}
Yanfang Pan was supported by The National Science Foundation of China {81703842}, Traditional Chinese Medicine Scientific Research Projects of Shaanxi Province, PR China {SZY-KJCYC-2023-031}.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.De Muynck K., Vanderborght B., Van Vlierberghe H., Devisscher L. The gut-liver Axis in chronic liver disease: a macrophage perspective. Cells. 2021;10(11):2959–2992. doi: 10.3390/cells10112959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghadimi M., Habibabadi R.R., Hazhirkarzar B., Shaghaghi M., Ameli S., Khoshpouri P., Ghasabeh M.A., Gurakar A., Pawlik T.M., Kamel I.R. Advances in imaging of diffuse parenchymal liver disease. J. Clin. Gastroenterol. 2020;54(8):682–695. doi: 10.1097/MCG.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 3.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72(5):1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 5.Moura Cunha G., Navin P.J., Fowler K.J., Venkatesh S.K., Ehman R.L., Sirlin C.B. Quantitative magnetic resonance imaging for chronic liver disease. Br. J. Radiol. 2021;94(1121):20201377–20201389. doi: 10.1259/bjr.20201377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J. Hepatol.. S0168-8278(23) (2023) 00194-00271. (in press). [DOI] [PubMed]
- 7.GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5(3):245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcellin P., Kutala B.K. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl 1):2–6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- 9.Locati M., Curtale G., Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentek R., Molawi K., Sieweke M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014;262(1):56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front. Immunol. 2014;5:514–535. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazankov K., Jørgensen S.M.D., Thomsen K.L., Møller H.J., Vilstrup H., George J., Schuppan D., Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16(3):145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 14.Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59(5):2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 15.Noorolyai S., Shajari N., Baghbani E., Sadreddini S., Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 16.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198(3):1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 17.Aurilio G., Santoni M., Massari F., Cimadamore A., Rizzo A., Mollica V., Verri E., Battelli N., Montironi R. Metabolomic profiling in renal cell carcinoma patients: news and views. Cancers. 2021;13(20):5229–5239. doi: 10.3390/cancers13205229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollica V., Marchetti A., Rosellini M., Nuvola G., Rizzo A., Santoni M., Cimadamore A., Montironi R., Massari F. An insight on novel molecular pathways in metastatic prostate cancer: a focus on DDR, MSI and AKT. Int. J. Mol. Sci. 2021;22(24):13519–13538. doi: 10.3390/ijms222413519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollica V., Maggio I., Lopez-Beltran A., Montironi R., Cimadamore A., Cheng L., Rizzo A., Giunchi F., Schiavina R., Fiorentino M., Brunocilla E., Massari F. Combination therapy in advanced urothelial cancer: the role of PARP, HER-2 and mTOR inhibitors. Expert Rev. Anticancer Ther. 2020;20(9):755–763. doi: 10.1080/14737140.2020.1807334. [DOI] [PubMed] [Google Scholar]
- 20.Artyomov M.N., Sergushichev A., Schilling J.D. Integrating immunometabolism and macrophage diversity. Semin. Immunol. 2016;28(5):417–424. doi: 10.1016/j.smim.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 22.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., Cline G.W., Phillips A.J., Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Msheik Z., El Massry M., Rovini A., Billet F., Desmoulière A. The macrophage: a key player in the pathophysiology of peripheral neuropathies. J. Neuroinflammation. 2022;19(1):97–114. doi: 10.1186/s12974-022-02454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasiliadou I., Holen I. The role of macrophages in bone metastasis. J Bone Oncol. 2013;2(4):158–166. doi: 10.1016/j.jbo.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81(5):1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 26.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 28.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 29.Wang C., Ma C., Gong L., Guo Y., Fu K., Zhang Y., Zhou H., Li Y. Macrophage polarization and its role in liver disease. Front. Immunol. 2021;12:803037–803061. doi: 10.3389/fimmu.2021.803037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahl E.C., Haschak M.J., Popovic B., Brown B.N. Macrophages in the aging liver and age-related liver disease. Front. Immunol. 2018;9:2795–2807. doi: 10.3389/fimmu.2018.02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am. J. Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha B., Tornai D., Kodys K., Adejumo A., Lowe P., McClain C., Mitchell M., McCullough A., Dasarathy S., Kroll-Desrosiers A., Barton B., Radaeva S., Szabo G. Biomarkers of macrophage activation and immune danger signals predict clinical outcomes in alcoholic hepatitis. Hepatology. 2019;70(4):1134–1149. doi: 10.1002/hep.30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salama R.M., Abbas S.S., Darwish S.F., Sallam A.A., Elmongy N.F., El Wakeel S.A. Regulation of NOX/p38 MAPK/PPARα pathways and miR-155 expression by boswellic acids reduces hepatic injury in experimentally-induced alcoholic liver disease mouse model: novel mechanistic insight. Arch Pharm. Res. 2023;46(4):323–338. doi: 10.1007/s12272-023-01441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha B., Momen-Heravi F., Furi I., Kodys K., Catalano D., Gangopadhyay A., Haraszti R., Satishchandran A., Iracheta-Vellve A., Adejumo A., Shaffer S.A., Szabo G. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018;67(5):1986–2000. doi: 10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J.K., Shao M., Kim M.Y., Baik S.K., Cho M.Y., Utsumi T., Satoh A., Ouyang X., Chung C., Iwakiri Y. An endoplasmic reticulum protein, Nogo-B, facilitates alcoholic liver disease through regulation of kupffer cell polarization. Hepatology. 2017;65(5):1720–1734. doi: 10.1002/hep.29051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louvet A., Teixeira-Clerc F., Chobert M.N., Deveaux V., Pavoine C., Zimmer A., Pecker F., Mallat A., Lotersztajn S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54(4):1217–1226. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z., Zhang X., Zhu L., Yang X., He F., Wang T., Bao T., Lu H., Wang H., Yang S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharm. 2020;78:106062–106073. doi: 10.1016/j.intimp.2019.106062. [DOI] [PubMed] [Google Scholar]
- 38.Cai B., Dongiovanni P., Corey K.E., Wang X., Shmarakov I.O., Zheng Z., Kasikara C., Davra V., Meroni M., Chung R.T., Rothlin C.V., Schwabe R.F., Blaner W.S., Birge R.B., Valenti L., Tabas I. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metabol. 2020;31(2):406–421.e7. doi: 10.1016/j.cmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni Y., Zhuge F., Nagashimada M., Ota T. Novel action of carotenoids on non-alcoholic fatty liver disease: macrophage polarization and liver homeostasis. Nutrients. 2016;8(7):391–406. doi: 10.3390/nu8070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 41.Maina V., Sutti S., Locatelli I., Vidali M., Mombello C., Bozzola C., Albano E. Bias in macrophage activation pattern influences non-alcoholic steatohepatitis (NASH) in mice. Clin. Sci. 2012;122(11):545–553. doi: 10.1042/CS20110366. [DOI] [PubMed] [Google Scholar]
- 42.Yin Y., Wang Q., Qi M., Zhang C., Li Z., Zhang W. Ghrelin ameliorates nonalcoholic steatohepatitis induced by chronic low-grade inflammation via blockade of Kupffer cell M1 polarization. J. Cell. Physiol. 2021;236(7):5121–5133. doi: 10.1002/jcp.30218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong C., Wu H., Gu D., Li Y., Fan Y., Zeng J., Ding W. Effect of curcumin on the non-alcoholic steatohepatitis via inhibiting the M1 polarization of macrophages. Hum. Exp. Toxicol. 2021;40(12_suppl):S310–S317. doi: 10.1177/09603271211038741. [DOI] [PubMed] [Google Scholar]
- 44.Zhong X., Liu H. Honokiol attenuates diet-induced non-alcoholic steatohepatitis by regulating macrophage polarization through activating peroxisome proliferator-activated receptor γ. J. Gastroenterol. Hepatol. 2018;33(2):524–532. doi: 10.1111/jgh.13853. [DOI] [PubMed] [Google Scholar]
- 45.Cheng D., Chai J., Wang H., Fu L., Peng S., Ni X. Hepatic macrophages: key players in the development and progression of liver fibrosis. Liver Int. 2021;41(10):2279–2294. doi: 10.1111/liv.14940. [DOI] [PubMed] [Google Scholar]
- 46.Su S.B., Qin S.Y., Xian X.L., Huang F.F., Huang Q.L., ZhangDi H.J., Jiang H.X. Interleukin-22 regulating Kupffer cell polarization through STAT3/Erk/Akt crosstalk pathways to extenuate liver fibrosis. Life Sci. 2021;264:118677–118706. doi: 10.1016/j.lfs.2020.118677. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y., Xu W., Liu W., Chen G., Jiang S., Chen J., Jian X., Zhang H., Liu P., Mu Y. Yiguanjian decoction inhibits macrophage M1 polarization and attenuates hepatic fibrosis induced by CCl4/2-AAF. Pharm. Biol. 2021;59(1):1150–1160. doi: 10.1080/13880209.2021.1961820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson R., Williams D.M. Cirrhosis. Med. Clin. North Am. 2022;106(3):437–446. doi: 10.1016/j.mcna.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Yuan S., Zhang X., Zhang T., Meng C., Zhuang K., Dang S. ANGPTL4 regulates CD163 expression and Kuppfer cell polarization induced cirrhosis via TLR4/NF-κB pathway. Exp. Cell Res. 2021;405(2):112706–112712. doi: 10.1016/j.yexcr.2021.112706. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y., Fan W.W., Xu W., Jiang S.L., Chen G.F., Liu C., Chen J.M., Zhang H., Liu P., Mu Y.P. Yiguanjian decoction enhances fetal liver stem/progenitor cell-mediated repair of liver cirrhosis through regulation of macrophage activation state. World J. Gastroenterol. 2018;24(42):4759–4772. doi: 10.3748/wjg.v24.i42.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 52.Liu N., Wang X., Steer C.J., Song G. MicroRNA-206 promotes the recruitment of CD8+ T cells by driving M1 polarisation of Kupffer cells. Gut. 2022;71(8):1642–1655. doi: 10.1136/gutjnl-2021-324170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022;56:102463–102477. doi: 10.1016/j.redox.2022.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y.L., Li Q., Yang X.M., Fang F., Li J., Wang Y.H., Yang Q., Zhu L., Nie H.Z., Zhang X.L., Feng M.X., Jiang S.H., Tian G.A., Hu L.P., Lee H.Y., Lee S.J., Xia Q., Zhang Z.G. SPON2 promotes M1-like macrophage recruitment and inhibits hepatocellular carcinoma metastasis by distinct integrin-rho GTPase-hippo pathways. Cancer Res. 2018;78(9):2305–2317. doi: 10.1158/0008-5472.CAN-17-2867. [DOI] [PubMed] [Google Scholar]
- 55.Lu S., Gao Y., Huang X., Wang X. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int. J. Biol. Sci. 2014;10(4):415–425. doi: 10.7150/ijbs.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S.W., Lan T., Sheng H., Zheng F., Lei M.K., Wang L.X., Chen H.F., Xu C.Y., Zhang F. Nobiletin alleviates non-alcoholic steatohepatitis in MCD-induced mice by regulating macrophage polarization. Front. Physiol. 2021;12:687744–687755. doi: 10.3389/fphys.2021.687744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou W., Zhu Z., Xiao X., Li C., Zhang L., Dang Y., Ge G., Ji G., Zhu M., Xu H. Jiangzhi Granule attenuates non-alcoholic steatohepatitis by suppressing TNF/NFκB signaling pathway-a study based on network pharmacology. Biomed. Pharmacother. 2021;143:112181–112192. doi: 10.1016/j.biopha.2021.112181. [DOI] [PubMed] [Google Scholar]
- 58.Yao Q., Li S., Li X., Wang F., Tu C. Myricetin modulates macrophage polarization and mitigates liver inflammation and fibrosis in a murine model of nonalcoholic steatohepatitis. Front. Med. 2020;7:71–86. doi: 10.3389/fmed.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min L., Wang H., Qi H. Astragaloside IV inhibits the progression of liver cancer by modulating macrophage polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am. J. Transl. Res. 2022;14(3):1551–1566. [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J., He X., Huang X., Zhang F., Ren X., Asakiya C., Li Y., Huang K. Artemether ameliorates non-alcoholic steatohepatitis by repressing lipogenesis, inflammation, and fibrosis in mice. Front. Pharmacol. 2022;13:851342–851356. doi: 10.3389/fphar.2022.851342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun B., Zhang R., Liang Z., Fan A., Kang D. Hyperoside attenuates non-alcoholic fatty liver disease through targeting Nr4A1 in macrophages. Int. Immunopharm. 2021;94:107438–107447. doi: 10.1016/j.intimp.2021.107438. [DOI] [PubMed] [Google Scholar]
- 62.Kang J.W., Shin J.K., Koh E.J., Ryu H., Kim H.J., Lee S.M. Opuntia ficus-indica seed attenuates hepatic steatosis and promotes M2 macrophage polarization in high-fat diet-fed mice. Nutr. Res. 2016;36(4):369–379. doi: 10.1016/j.nutres.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Yu B., Qin S.Y., Hu B.L., Qin Q.Y., Jiang H.X., Luo W. Resveratrol improves CCL4-induced liver fibrosis in mouse by upregulating endogenous IL-10 to reprogramme macrophages phenotype from M(LPS) to M(IL-4) Biomed. Pharmacother. 2019;117:109110–109118. doi: 10.1016/j.biopha.2019.109110. [DOI] [PubMed] [Google Scholar]
- 64.Zhao X.A., Chen G., Liu Y., Chen Y., Wu H., Xiong Y., Wang G., Jia B., Li Y., Xia J., Wang J., Yan X., Zhang Z., Huang R., Wu C. Curcumin reduces Ly6C monocyte infiltration to protect against liver fibrosis by inhibiting Kupffer cells activation to reduce chemokines secretion. Biomed. Pharmacother. 2018;106:868–878. doi: 10.1016/j.biopha.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 65.Astragalus polysacharin inhibits hepatocellular carcinoma-like phenotypes in a murine HCC model through repression of M2 polarization of tumour-associated macrophages. Pharm. Biol. 2021;59(1):1533–1539. doi: 10.1080/13880209.2021.1991384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li G.L., Tang J.F., Tan W.L., Zhang T., Zeng D., Zhao S., Ran J.H., Li J., Wang Y.P., Chen D.L. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food Funct. 2023;14(7):3155–3168. doi: 10.1039/d2fo02191a. [DOI] [PubMed] [Google Scholar]
- 67.Yin J., Zhao X., Chen X., Shen G. Emodin suppresses hepatocellular carcinoma growth by regulating macrophage polarization via microRNA-26a/transforming growth factor beta 1/protein kinase B. Bioengineered. 2022;13(4):9548–9563. doi: 10.1080/21655979.2022.2061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jafari M., Ghadami E., Dadkhah T., Akhavan-Niaki H. PI3k/AKT signaling pathway: erythropoiesis and beyond. J. Cell. Physiol. 2019;234(3):2373–2385. doi: 10.1002/jcp.27262. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y., Wang B.C., Xiao Y. PI3K: a potential therapeutic target for cancer. J. Cell. Physiol. 2012;227(7):2818–2821. doi: 10.1002/jcp.23038. [DOI] [PubMed] [Google Scholar]
- 70.Slattery M.L., Mullany L.E., Sakoda L.C., Wolff R.K., Stevens J.R., Samowitz W.S., Herrick J.S. The PI3K/AKT signaling pathway: associations of miRNAs with dysregulated gene expression in colorectal cancer. Mol. Carcinog. 2018;57(2):243–261. doi: 10.1002/mc.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linton M.F., Moslehi J.J., Babaev V.R. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int. J. Mol. Sci. 2019;20(11):2703–2716. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pompura S.L., Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018:1–12. doi: 10.1002/JLB.2MIR0817-349R. [DOI] [PubMed] [Google Scholar]
- 73.Polumuri S.K., Toshchakov V.Y., Vogel S.N. Role of phosphatidylinositol-3 kinase in transcriptional regulation of TLR-induced IL-12 and IL-10 by Fc gamma receptor ligation in murine macrophages. J. Immunol. 2007;179(1):236–246. doi: 10.4049/jimmunol.179.1.236. [DOI] [PubMed] [Google Scholar]
- 74.Troutman T.D., Bazan J.F., Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11(19):3559–3567. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.López-Peláez M., Soria-Castro I., Boscá L., Fernández M., Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: implications for NO synthase 2 expression. Eur. J. Immunol. 2011;41(6):1733–1741. doi: 10.1002/eji.201041101. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi K., Hernandez L.D., Galán J.E., Janeway C.A., Jr., Medzhitov R., Flavell R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 77.Gao J., Suo S., Li J., Wang C., Deng R., Hu Y., Zhang C. IGFBP-rP1 affects the proliferation, apoptosis and macrophage polarization of endometrial cancer cells by regulating the PI3K/AKT pathway. Exp. Ther. Med. 2023;25(4):169–177. doi: 10.3892/etm.2023.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M., Liu K., Zhang Q., Xu J., Liu J., Lin H., Lin B., Zhu M., Li M. Alpha fetoprotein promotes polarization of macrophages towards M2-like phenotype and inhibits macrophages to phagocytize hepatoma cells. Front. Immunol. 2023;14:1081572–1081588. doi: 10.3389/fimmu.2023.1081572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X., Hu Y., Li C., Chen J., Liu X., Shen Y., Xu Y., Chen W., Xu X. Overexpression of YEATS2 remodels the extracellular matrix to promote hepatocellular carcinoma progression via the PI3K/AKT pathway. Cancers. 2023;15(6):1850–1862. doi: 10.3390/cancers15061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng X., Han Z.X., Su Z.J., Zhang F.L., Li B.P., Jiang Z.R., Tang L., Yang J.S. Network pharmacology-based exploration on the intervention of Qinghao Biejia decoction on the inflammation-carcinoma transformation process of chronic liver disease via MAPK and PI3k/AKT pathway. BioMed Res. Int. 2022;2022:9202128–9202150. doi: 10.1155/2022/9202128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu L., Zhang Q., Mo W., Feng J., Li S., Li J., Liu T., Xu S., Wang W., Lu X., Yu Q., Chen K., Xia Y., Lu J., Xu L., Zhou Y., Fan X., Guo C. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci. Rep. 2017;7(1):9289–9301. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Q., Chen L., Wu X., Zhang F., Jin H., Lu C., Shao J., Kong D., Wu L., Zheng S. Dihydroartemisinin prevents liver fibrosis in bile duct ligated rats by inducing hepatic stellate cell apoptosis through modulating the PI3K/Akt pathway. IUBMB Life. 2016;68(3):220–231. doi: 10.1002/iub.1478. [DOI] [PubMed] [Google Scholar]
- 83.Chen X., Bian M., Zhang C., Kai J., Yao Z., Jin H., Lu C., Shao J., Chen A., Zhang F., Zheng S. Dihydroartemisinin inhibits ER stress-mediated mitochondrial pathway to attenuate hepatocyte lipoapoptosis via blocking the activation of the PI3K/Akt pathway. Biomed. Pharmacother. 2018;97:975–984. doi: 10.1016/j.biopha.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Sun X., Zhuo X.B., Hu Y.P., Zheng X., Zhao Q.J. A novel matrine derivative WM622 inhibits hepatocellular carcinoma by inhibiting PI3K/AKT signaling pathways. Mol. Cell. Biochem. 2018;449(1–2):47–54. doi: 10.1007/s11010-018-3341-9. [DOI] [PubMed] [Google Scholar]
- 85.Jiang R., Tang J., Zhang X., He Y., Yu Z., Chen S., Xia J., Lin J., Ou Q. CCN1 promotes inflammation by inducing IL-6 production via α6β1/PI3K/Akt/NF-κB pathway in autoimmune hepatitis. Front. Immunol. 2022;13:810671–810682. doi: 10.3389/fimmu.2022.810671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang S., Huang Z., Lei Y., Han X., Tian D., Gong J., Liu M. Celastrol alleviates autoimmune hepatitis through the PI3K/AKT signaling pathway based on network pharmacology and experiments. Front. Pharmacol. 2022;13:816350–816362. doi: 10.3389/fphar.2022.816350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu B., Deng X., Jiang Q., Li G., Zhang J., Zhang N., Xin S., Xu K. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed. Pharmacother. 2020;125:109895–109908. doi: 10.1016/j.biopha.2020.109895. [DOI] [PubMed] [Google Scholar]
- 88.Lee S.W., Kim S.M., Hur W., Kang B.Y., Lee H.L., Nam H., Yoo S.H., Sung P.S., Kwon J.H., Jang J.W., Kim S.J., Yoon S.K. Tenofovir disoproxil fumarate directly ameliorates liver fibrosis by inducing hepatic stellate cell apoptosis via downregulation of PI3K/Akt/mTOR signaling pathway. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0261067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei Q., Ren Y., Zheng X., Yang S., Lu T., Ji H., Hua H., Shan K. Ginsenoside Rg3 and sorafenib combination therapy relieves the hepatocellular carcinomaprogression through regulating the HK2-mediated glycolysis and PI3K/Akt signaling pathway. Bioengineered. 2022;13(5):13919–13928. doi: 10.1080/21655979.2022.2074616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J., Wang T., Liu P., Yang F., Wang X., Zheng W., Sun W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12(9):3898–3918. doi: 10.1039/d0fo02736g. [DOI] [PubMed] [Google Scholar]
- 91.Zhao J., Lin E., Cai C., Zhang M., Li D., Cai S., Zeng G., Yin Z., Wang B., Li P., Hong X., Chen J., Zou B., Li J. Combined treatment of tanshinone I and epirubicin revealed enhanced inhibition of hepatocellular carcinoma by targeting PI3K/AKT/HIF-1α. Drug Des. Dev. Ther. 2022;16:3197–3213. doi: 10.2147/DDDT.S360691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guerau-de-Arellano M., Piedra-Quintero Z.L., Tsichlis P.N. Akt isoforms in the immune system. Front. Immunol. 2022;13:990874–990884. doi: 10.3389/fimmu.2022.990874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ni B., Huang G., Yang R., Wang Z., Song H., Li K., Zhang Y., Wu K., Shi G., Wang X., Shen J., Liu Y. The short isoform of MS4A7 is a novel player in glioblastoma microenvironment, M2 macrophage polarization, and tumor progression. J. Neuroinflammation. 2023;20(1):80–95. doi: 10.1186/s12974-023-02766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng Y., Tian Y., Xia J., Wu X., Yang Y., Li X., Huang C., Meng X., Ma T., Li J. The role of PTEN in regulation of hepatic macrophages activation and function in progression and reversal of liver fibrosis. Toxicol. Appl. Pharmacol. 2017;317:51–62. doi: 10.1016/j.taap.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Fabre T., Barron A.M.S., Christensen S.M., Asano S., Bound K., Lech M.P., Wadsworth M.H., 2nd, Chen X., Wang C., Wang J., McMahon J., Schlerman F., White A., Kravarik K.M., Fisher A.J., Borthwick L.A., Hart K.M., Henderson N.C., Wynn T.A., Dower K. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci. Immunol. 2023;8(82):eadd8945–eadd8960. doi: 10.1126/sciimmunol.add8945. [DOI] [PubMed] [Google Scholar]
- 96.Chen M., Lai R., Lin X., Chen W., Wu H., Zheng Q. Downregulation of triggering receptor expressed on myeloid cells 1 inhibits invasion and migration of liver cancer cells by mediating macrophage polarization. Oncol. Rep. 2021;45(4):37–48. doi: 10.3892/or.2021.7988. [DOI] [PubMed] [Google Scholar]
- 97.Harikrishnan H., Jantan I., Haque M.A., Kumolosasi E. Anti-inflammatory effects of Phyllanthus amarus Schum. & Thonn. through inhibition of NF-κB, MAPK, and PI3K-Akt signaling pathways in LPS-induced human macrophages. BMC Compl. Alternative Med. 2018;18(1):224–236. doi: 10.1186/s12906-018-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song M., Li Z.H., Gu H.S., Tang R.Y., Zhang R., Zhu Y.L., Liu J.L., Zhang J.J., Wang L.Y. Ganoderma lucidum spore polysaccharide inhibits the growth of hepatocellular carcinoma cells by altering macrophage polarity and induction of apoptosis. J. Immunol. Res. 2021;2021:6696606–6696619. doi: 10.1155/2021/6696606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoppmann N.A., Gray M.E., McGuire B.M. Drug-induced liver injury in the setting of chronic liver disease. Clin. Liver Dis. 2020;24(1):89–106. doi: 10.1016/j.cld.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 100.Song B., Zhang C., Hu W., Guo C., Xia Z., Hu W., Qin M., Jiang W., Lv J., Xu D., Zhang S., Fang J. Nano-designed carbon monoxide donor SMA/CORM2 exhibits protective effect against acetaminophen induced liver injury through macrophage reprograming and promoting liver regeneration. J. Contr. Release. 2021;331:350–363. doi: 10.1016/j.jconrel.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 101.Wang Z., Wu L., Pan B., Chen Y., Zhang T., Tang N. Interleukin 33 mediates hepatocyte autophagy and innate immune response in the early phase of acetaminophen-induced acute liver injury. Toxicology. 2021;456:152788–152799. doi: 10.1016/j.tox.2021.152788. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q., Wei S., Zhou H., Shen G., Gan X., Zhou S., Qiu J., Shi C., Lu L. Hyperglycemia exacerbates acetaminophen-induced acute liver injury by promoting liver-resident macrophage proinflammatory response via AMPK/PI3K/AKT-mediated oxidative stress. Cell Death Dis. 2019;5:119–130. doi: 10.1038/s41420-019-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mu Y., Ren X.H., Han D., Guan Y.Y., Liu P.L., Cheng S.X., Liu H. Codelivery of HBx-siRNA and Plasmid encoding IL-12 for inhibition of hepatitis B virus and reactivation of antiviral immunity. Pharmaceutics. 2022;14(7):1439–1453. doi: 10.3390/pharmaceutics14071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.