Abstract
Objective
Zishen Yutai Pill (ZYP), containing 15 Chinese traditional medicine, is a safe and well quality-controlled TCM preparation with promising effects in many fields of reproduction. The current study was designed to investigate the therapeutic effects of ZYP on sperm quality and testis in varicocele (VC) rats.
Materials and methods
Male Wistar rats were randomly divided into four groups (n = 6), i.e., a sham group, a VC group, and VC groups treated with different dose of ZYP (1575 and 3150 mg/kg/d, respectively). The experimental VC model was established by partial ligation of left renal vein. Six weeks after model establishment, ZYP was orally administered once a day for the next 6 weeks. Parameters relating to testis and sperm quality were assessed. Hematoxylin–eosin staining was used to showed testicular tissue damage in experimental VC rats. Expressions of proteins relating to NLRP3 inflammasome pathways were determined using Western blot (WB). The mRNA expressions of relating genes were determined using quantitative real-time PCR (qRT-PCR) analysis.
Results
ZYP could significantly improve sperm motility and decrease sperm DNA fragmentation index in VC rats (P < 0.05). Hematoxylin-eosin (HE) staining showed that ZYP could alleviate testicular tissue damage caused by experimental varicocele in rats. Compared to the VC model, expressions of NLRP3, ASC, and caspase-1 in rats treated with ZYP were significantly down-regulated, as validated by both qRT-PCR and WB analysis (P < 0.05).
Conclusions
In brief, ZYP could improve sperm DNA integrity by inhibiting the NLRP3 inflammasome pathway and alleviating the chronic inflammation of testicular tissue induced by experimental varicocele in rats.
Keywords: Traditional Chinese medicine, Zishen Yutai Pill, Varicocele, Chronic inflammation, NLRP3
Abbreviation
- ANOVA
Analysis of variance
- ART
Assisted reproduction technology
- ASC
Apoptosis-associated speck-like protein
- CAD
Charged aerosol detector
- DFI
DNA fragmentation index
- ELISA
Enzyme-linked immunosorbent assay
- HE
Hematoxylin–eosin
- IL-1β
Interleukin-1 beta
- NLRP3
Nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domains-containing protein 3
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SD
Standard deviation
- TCM
Traditional Chinese medicine
- UPLC
Ultrahigh performance liquid chromatography
- VC
varicocele
- WB
Western blot
- ZYP
Zishen Yutai Pill
- LZYP
Low Zishen Yutai Pill
- HZYP
High Zishen Yutai Pill
1. Introduction
Varicocele, characterized by dilated veins of the pampiniform plexus in the scrotum, is generally acknowledged as the most common but curable cause of male infertility. The prevalence of varicocele is approximately 15% among the male population [1]. In 90% of patients with varicoceles, pathological changes occur on the left renal vein due to the anatomical differences in the spermatic venous drainage between the left and right sides [2]. Various hypotheses were established explaining the connection between varicocele and male infertility. Mechanisms involved in these hypotheses mainly include testicular hypoxia, germ cell apoptosis, oxidative stress, toxic metabolite reflux, hypertension in spermatic veins, elevated testicular temperature, etc [3]. However, the real pathological process of varicocele remained perplexed.
It is generally accepted that varicocelectomy, both microsurgical and non-microsurgical, can significantly improve semen parameters [4]. Nowadays, the optimal technique for performing varicocelectomy is a microsurgical approach due to its reduced complication rates and improved pregnancy outcomes. However, the cost of surgical methods may not be affordable for all patients. And for patients who suffered from subclinical varicocele, varicocelectomy may not be clinically effective [5]. Thus, the development of medical treatment for varicocele is still demanding. At present, there are several kinds of medicines used during the treatment of varicocele, including selective estrogen receptor modulators, antioxidants, etc [6,7]. In addition, more and more people have turned their attention to herbal medicine, seeking safer and more cost-effective complementary medicine for varicocele, like Aescin (extract of Aesculus turbinata BLUME), Daflon (extract of Citrus aurantium L. mainly containing flavonoids), alpha-lipoic acid (a short-chain fatty acid widely distributed in the plant kingdom), resveratrol (a stilbene with widespread biological activities), etc [[8], [9], [10]].
Traditional Chinese medicine (TCM) possesses a rich resource of data in the treatment of both varicocele and male infertility. The basic theory for the treatment of varicocele and male infertility in TCM is to tonify the kidney, or “Zishen” [11]. Zishen Yutai Pill (ZYP) is a TCM preparation mainly used in the fields of reproduction, including prevention of miscarriage, improvement of pregnancy outcomes during assisted reproduction technology (ART) [12]. Previous reports had validated the safety of this preparation [13]. The detailed information of this preparation is shown in Table 1.
Table 1.
Formula of Zishen Yutai Pill, origins of natural medicines and the amounts of crude drugs administered in daily dosage.
| Natural medicine | Origin of natural medicine | Amounts of crude drugs administered in daily dosage/g |
|---|---|---|
| Cuscutae Semen | Ripe dried seed of Cuscuta Chinensis Lam. | 9.60 |
| Ginseng Radix et Rhizoma | Dried root and rhizome of Panax ginseng C. A. Mey. | 0.60 |
| Dipsaci Radix | Dried root of Dipsacus asper Wall. ex DC. | 5.76 |
| Taxilli Herba | Dried leafy stem and branch of Taxillus chinensis (DC.) Danser | 5.76 |
| Eucommiae Cortex | Dried bark of Eucommia ulmoides Oilv. | 3.48 |
| Morindae Officinalis Radix | Dried root of Marinda officinalis How | 2.28 |
| Cervi Cornu Degelatinatum | Residue after water extraction of ossified antler of Cervus nippon Temminck | 1.68 |
| Codonopsis Radix | Dried root of Codonopsis pilosula (Franch.) Nannf. | 6.96 |
| Atractylodis Macrocephalae Rhizoma | Dried rhizome of Atractylodes macrocephala Koidz. | 2.88 |
| Asini Corii Colla | Solid glue prepared by stewing and concentrating from the hide of Equus asinus L. | 0.36 |
| Lycii Fructus | Dried ripe fruit of Lycium barbarum L. | 2.28 |
| Rehmanniae Radix Praeparata | Steamed and dried root of Rehmannia glutinosa (Gaertn.) DC. | 5.76 |
| Polygoni Multiflori Radix Praeparata | Steamed and dried root of Polygonum multiflorum Thunb. | 2.88 |
| Artemisiae Argyi Folium | Dried leaf of Artemisia argyi Lévl. et Vant. | 1.68 |
| Amomi Fructus | Dried fruit of Amomum villosum Lour. | 0.84 |
| Sum the dosages of crude drugs | 52.80 |
Note: According to the instruction, ZYP is given at a dose of 15 g per day for human.
The metabonomic study suggested that metabolites changed after administration of ZYP may enroll in biological processes, including up-regulation of PI3K/Akt pathway, down-regulation of both oxidative stress and inflammation. These biological activations indicate that ZYP may possess the potentials in the treatment of varicocele [14]. Several herbal medicines in ZYP are also widely applied in the treatment of varicocele, including Cuscutae Semen, Ginseng Radix et Rhizoma, Dipsaci Radix, Eucommiae Cortex, Morindae Officinalis Radix, Lycii Fructus, Polygoni Multiflori Radix Praeparata, etc [15,16]. It is well acknowledged that varicocele may lead to changes in the immune microenvironment of the testis. According to previous research, the inflammasome pathway will be activated upon the exposure to cellular damage [17]. The nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domains-containing protein 3 (NLRP3) inflammasome is a key mediator during the process, leading to the apoptosis, pyroptosis and generation of inflammatory cytokines [18]. In testis, the abnormal activation of NLRP3 can lead to dysregulation of immune surveillance and presumably, causing male infertility [19]. In addition, a previous report had also demonstrated that elevated levels of pro-inflammatory molecules were observed in the plasma or semen of infertile patients with varicocele [20,21]. Thus, this study explored the mechanisms of ZYP on varicocele from the perspective of anti-inflammation.
In the present study, varicocele model was established in rats. The curative effects of ZYP were assessed with regards to the sperm quality and testis functions in varicocele model. In addition, mechanisms of ZYP were explored mainly through the NLRP3 inflammasome pathway.
2. Material and method
2.1. Zishen Yutai Pill and its chemical profiles
The production of ZYP complies with the legislations of China's Drug Administration (Approval No. Z44020008) and Good Manufacture Practice (GMP). ZYP was obtained from Guangzhou Baiyunshan Zhongyi Pharmaceutical Co. Ltd. (Guangzhou, China). The batch number used in the current research was No. 20130301. Voucher specimens of ZYP were deposited at the Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Chemical profiles and quantitative determination were shown in Fig. 1 according to methods previously established, using ultrahigh performance liquid chromatography equipped with charged aerosol detector (UPLC-CAD) [22]. A detailed method of UPLC-CAD analysis was reported in Supplementary Material.
Fig. 1.
Fingerprint chromatogram of three batches of ZYP in UPLC-CAD. Fourteen peaks were confirmed as followed, chlorogenic acid (1), sweroside (2), loganin (3), astragaline (4), ginsenoside Rg1 (5), ginsenoside Re (6), Akebia saponin D (7), ginsenoside Rb1 (8), ginsenoside Rc (9), ginsenoside Rb2 (10), ginsenoside Rd (11), chrysophanol (12), physion (13) and β-carotene (14).
2.2. Animals, establishment of varicocele model, and administration of drugs
Wistar rats (180–200 g, 6–8 weeks, male) were obtained from Hubei Provincial Center for Disease Control and Prevention. The whole process of animal experiments was conducted in Laboratory Animal Center, Tongji Medical College. The study protocol was approved by the Reproductive Medicine Institutional Review Board of Tongji Medical College, Huazhong University of Science and Technology. All animal procedures were approved by the Animal Care and Use Committee of the Huazhong University of Science and Technology.
Sixty rats were randomly divided into two groups, i.e., a sham group (6 rats), and varicocele model group (54 rats). The varicocele model was established by the partial ligation of the left kidney vein as previously described [23]. Six weeks after the operation, the rats were anesthetized with chloral hydrate and checked the left spermatic vein and left kidney to determine whether the experimental varicocele model was established successfully.
Six rats underwent the same procedures with the experimental groups, but without ligation, being the sham group. Eighteen rats were randomly divided into three groups (six in each group), i.e., a varicocele model group, and two varicocele model groups treated with 1575 and 3150 mg/kg/day ZYP. The daily dose of ZYP for humans is 15 g/d (also called 1-fold clinical dose). Thus, the dose equivalent to 1-fold clinical dose for rats is 1575 mg/kg/d, as confirmed by previous report [12]. In the current study, two levels of dose were set accordingly, that is, low dose group (1575 mg/kg/d) and high dose group (3150 mg/kg/d). Before intragastric administration, ZYP was powdered and suspended to the required concentration with saline. Both the sham group and the varicocele group were administered with saline daily for 6 weeks. During the experiment, the solution was intragastrically given at 2 ml, once a day.
After injection of chloral hydrate, blood samples were then collected from the heart under anesthesia. Rats were sacrificed after blood collection. The blood samples were stored at 4 °C for 30 min then the serum was obtained after centrifugation. Finally, the serum was stored at −80 °C until further analysis.
2.3. Epididymal sperm count and motility analysis
According to previous studies, the cauda epididymis of rats was taken for the study of sperm motility and sperm count [24]. The left cauda epididymis was dissected, placed in 2 ml of 37 °C preheated saline and cut into small pieces. To let the sperm swim out, we placed the EP tube into a 37 °C incubator at 5% CO2 atmosphere for 20 min. Finally, the sperm count in the EP tube was measured with the hemocytometer and the sperm reserve of caudal epididymal was determined according to the method previously mentioned [25]. The motility assessment was expressed as percentage motile forms.
2.4. Sperm chromatin structure assay
Epididymal sperm DNA fragmentation index (DFI) was analyzed using SCSA (sperm chromatin structure assay) as previously described [26]. The spermatozoa were stained with acridine orange (AO), and the samples were immediately analyzed with flow cytometry. Red and green fluorescence detected by a 515–530 and 630–650 nm band-pass filter was shown when AO combined with single- and double-stranded DNA, respectively. A total of 5000 events were analyzed for each sample. The raw data were analyzed using the FlowJo 10.0 software (Leonard Herzenberg Software House, Stanford University, USA). The mean DFI relating to the susceptibility of sperm DNA fragmentation was assessed.
2.5. Hematoxylin-eosin (HE) staining
After the sacrifice of animals, left testis was dissected, washed with cold saline and then fixed in 10% formalin for 24 h. The testis was embedded in paraffin. The cross sections (5 μm thick) were placed on slides, stained with HE. Pathological changes in testis were observed under a microscope.
2.6. Enzyme-linked immunosorbent assay (ELISA)
IL-1β levels were detected using an ELISA Kit from R&D Systems (USA) according to the manufacturer's instructions.
2.7. Quantitative real-time PCR analysis (RT-qPCR)
Total RNA was harvested from the testis tissue by using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. cDNA was obtained by carrying out experiments according to the Prime Script™ RT reagent Kit's instructions. Using the Life Cycle Realtime PCR (Applied Biosystems, USA), we performed PCR in 20 μL of reaction mixture, including 1 μL of cDNA and 4 μL of water, the forward primers and the reverse primers both are 0.5 μL, and 10 μL of SYBR Green PCR MasterMix (Takara, Japan). The reaction system steps were as follows: 95 °C for 30 s and amplification over 35 cycles at 95 °C for 5 s and 60 °C for 20 s. Each sample was loaded in duplicate. The primer sequences of related genes are shown in Supplementary Material.
2.8. Western blot analysis
A Tissue Protein Extraction kit (Thermo Fisher Scientific, USA) was used to extract the total proteins of the left testis and enhanced BCA protein assay was used to measure the protein concentration. Equivalent amounts of protein were separated by 10% SDS-PAGE and then transferred to NC membrane. Then the NC membranes were incubated in 2% fat-free milk containing primary antibodies against NLRP3 (1:1000, Novus, USA), ASC (1:1000, Novus, USA), caspase-1 (1:1000, Novus, USA), IL-1β (1:1000, R&D Systems, USA), and GAPDH (1:1000, Novus, USA) at 4 °C overnight. The NC membranes were washed thrice, 15 min each time and then the secondary antibody was incubated at room temperature for 2 h and then washed thrice, 15 min each time. Finally, the experimental results were observed under a fluorescence imaging system.
2.9. Statistical analysis
The continuous variables were expressed as mean ± standard deviation (SD). Normal distribution was examined using Shapiro-Wilk test. For non-normalized distribution variables, the difference among groups was analyzed by one-way ANOVA, followed by a post hoc Bonferroni test for between-group analysis. For non-normalized distribution variables, Kruskal Wallis H test were used in comparison among groups. SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) was used to analyze all data, with P < 0.05 as statistically significant.
2.10. Ethics statement
The study protocol was approved by the Reproductive Medicine Institutional Review Board of Tongji Medical College, Huazhong University of Science and Technology with an IACUC approval (No. 2018-S808) on September 23, 2018. All animal procedures were approved by the Animal Care and Use Committee of the Huazhong University of Science and Technology, and conformed to the Regulations of Hubei Province on The Management of Experimental Animals.
3. Results
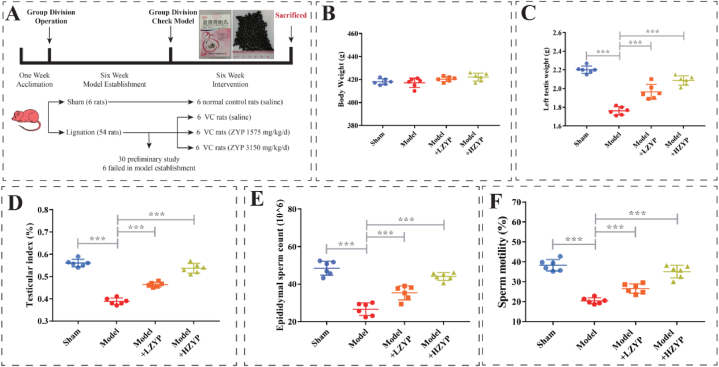
3.1. Effects of ZYP on testicular and semen parameters with varicocele
No significant difference was observed with regard to bodyweight among the four groups (Fig. 2A). The left testicular index and the left testis weight were significantly increased in the ZYP groups compared with the model group (P < 0.01, Fig. 2B and C). Epididymal sperm counts and motility in the model group were significantly lower than those in the sham group (P < 0.01; Fig. 2D and E, respectively), but both parameters were significantly higher in the LZYP and HZYP groups compared with the model group (P < 0.01; Fig. 2D and E, respectively). The epididymal sperm count and motility of HZYP groups were significantly elevated compared with that of LZYP groups (P < 0.01).
Fig. 2.
Experimental design and effects of ZYP on body weight, testis, and semen parameters in rats. (A) Flowchart of animal experiment, (B) body weight, (C) testis weight, (D) testis index, (E) epididymal sperm count, (F) sperm mobility. The data are presented as mean ± SD (n = 6). The statistical significance level was set at *P < 0.05, **P < 0.01, ***P < 0.001. LZYP: low ZYP (1575 mg/kg/d). HZYP: high ZYP (3150 mg/kg/d).
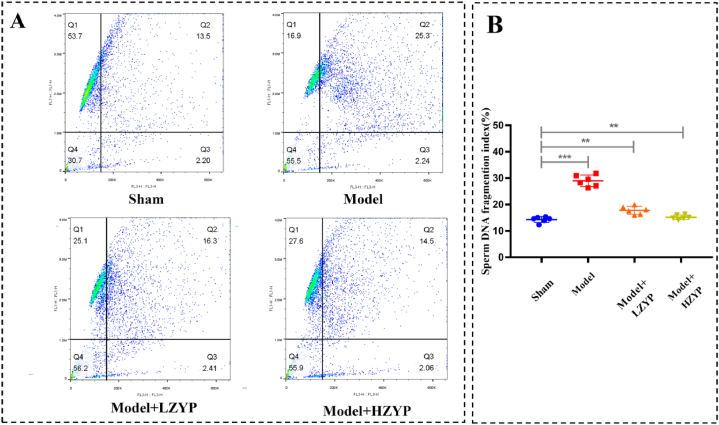
3.2. ZYP significantly reduced sperm DFI in varicocele rats
Representative graphs of sperm chromatin structure assay were shown in Fig. 3A. RAs shown in Fig. 3B, the DFI of cauda epididymis sperm changed significantly after partial ligation of the left renal vein. The model group showed significant increase regarding epididymal sperm DFI (P < 0.01) compared with the model group (P < 0.01). And the LZYP and HZYP groups showed significantly decreased epididymal sperm DFI (P < 0.01) compared with the sham group.
Fig. 3.
Effect of ZYP on epididymis sperm DFI. (A) Representative graph of sperm chromatin structure assay, (B) sperm DFI fragmentation index. The data are presented as mean ± SD (n = 6). The statistical significance level was set at *P < 0.05, **P < 0.01, ***P < 0.001. DFI: DNA fragmentation index. LZYP: low ZYP (1575 mg/kg/d). HZYP: high ZYP (3150 mg/kg/d).
3.3. ZYP improved testicular morphology in varicocele rats
The result of H&E staining showed that varicocele destroyed the testicular structure. The rats in the varicocele model group appeared as thinner and disorganized seminiferous tubules in comparison with the sham group (Fig. 4A). The sham group values of mean seminiferous tubule diameters (MSTD, 297.7 ± 11.3 μm vs 268.0 ± 13.2 μm, P < 0.01) and mean seminiferous epithelial thickness (MSET, 81.4 ± 7.3 μm vs 56.8 ± 6.5 μm, P < 0.01) were significantly reduced compared with varicocele model group. The LZYP and HZYP groups showed overt repairing effects, as thicker MSTD and MSET spermatogenic epithelium and more germ cells and mature sperms were observed compared with the varicocele model group (Fig. 4B–C). The testicular morphology of HZYP groups recovered slightly than that of LZYP groups but without significant difference. Nevertheless, the structure of the testis did not completely recover to the normal level.
Fig. 4.
Changes in the testis morphology. (A) HE staining of the testis. (B) Mean seminiferous tubule diameter (MSTD) in different groups. (C) Mean seminiferous germinal epithelial thickness (MSED) in different groups. In Fig. 4A, the red arrows showed thinning of spermatogenic epithelium. The black arrow showed the thickened basement membrane. The red box showed a reduced sperm count and atrophy of some seminiferous tubules. Scale bar 100 μm, 50 μm and 25 μm respectively. LZYP: low ZYP (1575 mg/kg/d). HZYP: high ZYP (3150 mg/kg/d); MSTD, mean seminiferous tubule diameters; MSET, mean seminiferous epithelial thickness. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Effects of ZYP on the mRNA expressions of NLRP3, ASC, and caspase-1 in the testis of rats, and IL-1β in serum
To study the changes of the local immune microenvironment of testicular, the relative mRNA levels of inflammasomes, NLRP3 (Fig. 5A), ASC (Fig. 5B), and caspase-1 (Fig. 5C) in the left testis tissue were quantified by qPCR and the expression of IL-1β (Fig. 5D) was determined by ELISA. The levels of IL-β and the mRNA levels of NLRP3, ASC, and caspase-1 in the varicocele model group were higher than those in the sham group (P < 0.05). The levels of these inflammation-related substances in the ZYP groups were lower than those in the varicocele model group (P < 0.05). This finding indicates that experimental varicocele can increase the expression of NLRP3, ASC, caspase-1, and IL-β in the left testis, whereas ZYP can reduce the levels of these substances.
Fig. 5.
Effects of ZYP on the mRNA expressions of NLRP3, ASC, and caspase-1 in the testis of rats, and the level of IL-1β in serum. NLRP3 (A), ASC (B), caspase-1 (C) in the testis of rats, and the level of IL-1β (D) in serum. The data are presented as mean ± SD (n = 3 for NLRP3, ASC, and caspase-1; n = 6 for IL-1β). The statistical significance level was set at *P < 0.05, **P < 0.01, ***P < 0.001. LZYP: low ZYP (1575 mg/kg/d). HZYP: high ZYP (3150 mg/kg/d).
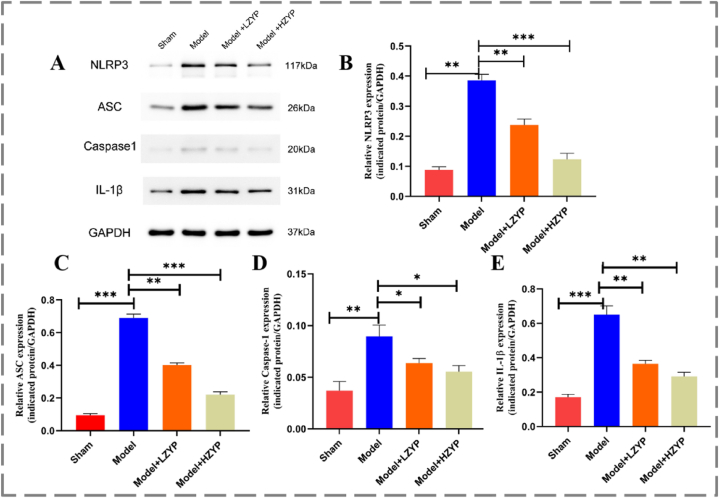
3.5. Effects of ZYP on the protein expressions of NLRP3, ASC, and caspase-1 in the testis of rats
Similarly, the proteins expression of NLRP3 pathway were tested by Western blot (Fig. 6A), and the expression levels of NLRP3 (Fig. 6B), ASC (Fig. 6C), Caspase-1 (Fig. 6D), and IL-1β (Fig. 6E) in the varicocele model group were significantly higher than those in the sham group. The levels of these inflammation-related substances in the ZYP groups were lower than those in the varicocele model group (P < 0.05). HZYP groups are more effective than LZYP. This finding indicates that experimental varicocele can increase the expression of NLRP3, ASC, caspase-1, and IL-β in the left testis, whereas ZYP can reduce the levels of these substances.
Fig. 6.
Effects of ZYP on the protein expressions of NLRP3, ASC, and caspase-1 in the testis of rats. (A) Representative radiographs. (B–E) Quantitative analysis of Western blot assessed with relative intensity (fold difference of each protein to β-actin). NLRP3 (B), ASC (C), caspase-1 (D) and IL-1β (E). The data are presented as mean ± SD (n = 3). The statistical significance level was set at *P < 0.05, **P < 0.01, ***P < 0.001. LZYP: low ZYP (1575 mg/kg/d). HZYP: high ZYP (3150 mg/kg/d).
4. Discussion
Varicocele is a common disease, affecting male fertility and thus hindering patients in achieving their fatherhood. To date, in clinical practice, many methods can be used to treat male infertility caused by varicocele, including surgery, drug intervention, and lifestyle management [27]. However, due to the lack of understanding of the mechanics of varicocele, the effects of drug intervention remain debatable. Selective estrogen receptor modulators (SERMs), including clomiphene or tamoxifen, are recommended in some guidelines for the treatment of varicocele. The use of SERMs may exhibit beneficial effects in pregnancy outcomes, but the effects of drugs in this category remained inconclusive [28]. Studies up-to-date hypothesized that ionic imbalance, hypoxia, change of blood flow status could finally develop into elevated of oxidative stress in varicoceles patients. In testis, abnormality of spermatogenesis and damage of sperms would also occur thereafter, being the cause of oligozoospermia or azoospermia [29]. In clinical practice, anti-oxidants are also used in the treatment of varicocele, including kallikrein, l-carnitine, anthocyanin, silymarin, chrysin, selenium, resveratrol etc [30]. But the current study is still far from sufficient to support the use of these chemicals in varicocele treatment [7].
Various herbal medicines in ZYP are studied in the treatment of male infertility and varicocele. Ginseng (Ginseng Radix et Rhizoma) is a typical case [31]. In both clinical study and animal models, Ginseng exbibit promising effects in male infertility individuals with varicocele. Other medicinal materials in ZYP also exhibit curative effects in male infertility and varicoceles. Both Semen Cuscutae and Fructus Lycii could alleviate spermatogenic dysfunction through the regulation PI3K/Akt/Bcl-2 pathway, inhibiting testicular cell apoptosis [32]. Extracts of Morinda Officinalis F. C. How and chemicals in Eucommiae Cortex could prevent testis impairment in rodent models [33]. The evidences listed above provides the rationale for the possible use of ZYP in the treatment of varicocele.
In this study, the effects of ZYP on sperm quality in the varicocele model were assessed. The results of HE staining, sperm count, and motility analysis indicated that ZYP could not only promote spermatogenesis but also decrease testicular tissue damage. It has been well acknowledged that sperm DFI could be used as a quantitative indicator in the assessment of sperm DNA damage, and sperm DNA damage is an important factor in the cause of male infertility [34,35]. An elevated level of sperm DFI was observed in the model group compared with that of the sham group, thus contributing to the decrease in sperm motility. After administration of ZYP for six weeks, sperm DFI underwent a significant decrease.
Although the pathological process of varicocele remained unexplained, it is believed that varicocele could induce abnormal inflammatory responses in testis tissues, thus casting harm on male reproduction function [36]. The varicocele condition causes lack of blood supply, elevation of temperature and accumulation of metabonomic byproducts. Changes in the environment of the testis can lead to increase of reactive oxygen species (ROS) and reactive nitrogen species (RNS), casting damage to the germinal cells [30]. Apoptosis is increased in germinal and sperm cells, with elevation of inflammatory cytokines upregulated. In addition, endocrine system dysfunction also takes place under varicocele condition, with decrease of testosterone and increase of follicle-stimulating hormone and luteinizing hormone [37]. In all, spermatogenesis and spermiogenesis processes are greatly impaired.
Therefore, the effects of ZYP on testicular inflammation in varicocele rats were explored. Our results showed that six weeks after the partial ligation of the left renal vein, the levels of NLRP inflammasome components, including NLRP3, ASC, and caspase-1, were upregulated. ZYP administration for the next six weeks could significantly reduce the expressions of these genes as proved by qRT-PCR analysis. WB analysis also confirmed such results. Furthermore, the serum level of IL-1β was also significantly reduced. Studies have shown that once NLRP3 is activated, levels of caspase-1 are increased thereafter, converting the pro-interleukin-1β to its active form [38]. The NLRP3 inflammasome activation can trigger the cytokine cascade. Prospective cohort studies had indicated that patients with varicocele had elevated seminal level of IL-1β, caspase-1, ASC [39]. The deterioration of inflammation environment can then further develop into sperm DNA fragmentation, and mitochondrial and motility dysfunction, and eventually impairing the reproductive performance [40]. Metabonomic study of ZYP implied the potential anti-inflammatory effects of ZYP. Our results suggest that ZYP exerts anti-inflammatory effects by reducing NLRP3 gene expression. It could be hypothesized that ZYP could as an NLRP3 inhibitor, suppressing the IL-1β-dependent inflammatory reaction of testicles in varicocele rats. Through this mechanism, ZYP can significantly alleviate the semen quality of patients with varicocele and reduce the sperm DFI in varicocele rats.
Our chemical profiles indicate the presence of 14 compounds in ZYP, including phenylpropanoids, flavonoids, iridoids, triterpenoids, anthraquinones, etc. Interestingly enough, some chemicals also possess curative effects on the reduction of inflammasome through various pathways. Ginsenosides, the principal components of Ginseng Radix et Rhizoma, exert beneficial effects on male infertility in various aspects, including improvements in sperm quality, protection against testicular tissue damage, regulation of reproductive hormones, etc [[41], [42], [43]]. In addition, ginseng and ginsenosides enriched fractions were also used in clinical practice for the treatment of varicocele [31,44]. Chemicals, including chlorogenic acid, sweroside, loganin, Akebia saponin D, chrysophanol, physion, all exhibited in vivo and in vitro anti-inflammation activity through the NLRP3 inflammasome pathway [33,[45], [46], [47]].
5. Conclusions
ZYP can improve sperm DNA integrity by inhibiting the NLRP3 inflammasome pathway and alleviating the chronic inflammation of testicular tissue induced by experimental varicocele in rats. In general, our results indicated that ZYP may be an effective therapeutic option in patients with varicocele by decreasing inflammatory response by inhibiting the NLRP3/ASC/caspase-1 pathways. Further studies are required to determine if ZYP improves fertility outcomes in patients with varicocele.
Author contribution statement
Meilin Peng, Wei Wang: Analyzed and interpreted the data; Wrote the paper.
Wei Zhu, Yang Bai: Performed the experiments.
Na Ning, Qiuling Huang, Xiufei Pang, Jiewen Zhou: Contributed reagents, materials, analysis tools or data.
Huiping Zhang, Kai Zhao: Conceived and designed the experiments.
Funding statement
Prof Kai Zhao was supported by Guangdong Secondary Development Project of Famous Chinese Patent Medicine [20174002], National Health and Family Planning Commission's Male Reproductive and Genetic Key Laboratory [KF201803].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17161.
Contributor Information
Meilin Peng, Email: pml@hust.edu.cn.
Wei Wang, Email: wangei220509@163.com.
Huiping Zhang, Email: zhpmed@126.com.
Kai Zhao, Email: kai_zhao@hust.edu.cn.
Supplementary materials
1. Analysis of Zishen Yutai Pill using UHPLC-CAD.
2. PCR primer sequences.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alsaikhan B., Alrabeeah K., Delouya G., Zini A. Epidemiology of varicocele. Asian J. Androl. 2016;18(2):179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen C.F.S., Østergren P., Dupree J.M., Ohl D.A., Sønksen J., Fode M. Varicocele and male infertility. Nat. Rev. Urol. 2017;14(9):523–533. doi: 10.1038/nrurol.2017.98. [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Sun Y., Wang L., Xu C., Yang Q., Liu B., Liu Z. Hypoxia-induced apoptosis in the bilateral testes of rats with left-sided varicocele: a new way to think about the varicocele. J. Androl. 2010;31(3):299–305. doi: 10.2164/jandrol.108.007153. [DOI] [PubMed] [Google Scholar]
- 4.Silay M.S., Hoen L., Quadackaers J., Undre S., Bogaert G., Dogan H.S., Kocvara R., Nijman R.J.M., Radmayr C., Tekgul S., et al. Treatment of varicocele in children and adolescents: a systematic review and meta-analysis from the European association of urology/European society for paediatric urology guidelines panel. Eur. Urol. 2019;75(3):448–461. doi: 10.1016/j.eururo.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel P.N., Sigman M., Collura B., De Jonge C.J., Eisenberg M.L., Lamb D.J., Mulhall J.P., Niederberger C., Sandlow J.I., Sokol R.Z., et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline PART II. J. Urol. 2021;205(1):44–51. doi: 10.1097/JU.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 6.Hughes E.G., Grantmyre J., Zini A. An integrated approach to male-factor subfertility: bridging the gap between fertility specialists trained in urology and gynaecology. J. Obstet. Gynaecol. Can. 2015;37(3):258–265. doi: 10.1016/S1701-2163(15)30312-1. [DOI] [PubMed] [Google Scholar]
- 7.Garg H., Kumar R. An update on the role of medical treatment including antioxidant therapy in varicocele. Asian J. Androl. 2016;18(2):222–228. doi: 10.4103/1008-682X.171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y., Zhao L., Yan F., Xia X., Xu D., Cui X. Escin improves sperm quality in male patients with varicocele-associated infertility. Phytomedicine. 2010;17(3–4):192–196. doi: 10.1016/j.phymed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Abbasi B., Molavi N., Tavalaee M., Abbasi H., Nasr-Esfahani M.H. Alpha-lipoic acid improves sperm motility in infertile men after varicocelectomy: a triple-blind randomized controlled trial. Reprod. Biomed. Online. 2020;41(6):1084–1091. doi: 10.1016/j.rbmo.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Shaygannia E., Nasr-Esfahani M.H., Sotoodehnejadnematalahi F., Parivar K. Is ferroptosis involved in ROS-induced testicular lesions in a varicocele rat model? Basic Clin. Androl. 2021;31(1):10. doi: 10.1186/s12610-021-00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Yang J., Kong T., Wang C., Ning P., Chen X., Li X., Jia Y., Chen X., Liu S., et al. Traditional Chinese medicine syndrome elements of male infertility revealed by latent tree model analysis. J. Tradit. Chin. Med. 2018;38(6):926–935. [PubMed] [Google Scholar]
- 12.Gao Q., Han L., Li X., Cai X. Traditional Chinese medicine, the Zishen Yutai Pill, ameliorates precocious endometrial maturation induced by controlled ovarian hyperstimulation and improves uterine receptivity via upregulation of HOXA10. Evid. Based Compl. Alternat. Med. 2015;2015 doi: 10.1155/2015/317586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing X., Deng X., Shi J., Zhang M., Sun G., Tang S., Huang Q., Sun X. The chronic hepatotoxicity assessment of the herbal formula Zishen Yutai Pill. Regul. Toxicol. Pharmacol. 2017;83:81–88. doi: 10.1016/j.yrtph.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Li L., Ning N., Wei J.-A., Huang Q.-L., Lu Y., Pang X.-F., Wu J.-J., Zhou J.-B., Zhou J.-W., Luo G.-A., et al. Metabonomics study on the infertility treated with Zishen Yutai Pills combined with fertilization-embryo transfer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.686133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong G., Wang S., Feng Q., Qiu M. A systematic review of efficacy and safety of traditional Chinese herbal medicine for the treatment of male infertility. Pract. J. Clin. Med. 2017;14(6):28–36. [Google Scholar]
- 16.Jiang D., Coscione A., Li L., Zeng B.-Y. Effect of Chinese herbal medicine on male infertility. Int. Rev. Neurobiol. 2017;135:297–311. doi: 10.1016/bs.irn.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Antonuccio P., Micali A.G., Romeo C., Freni J., Vermiglio G., Puzzolo D., Squadrito F., Irrera N., Marini H.R., Rana R.A., et al. NLRP3 inflammasome: a new pharmacological target for reducing testicular damage associated with varicocele. Int. J. Mol. Sci. 2021;22(3) doi: 10.3390/ijms22031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavalaee M., Rahmani M., Drevet J.R., Nasr-Esfahani M.H. The NLRP3 inflammasome: molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic Clin Androl. 2022;32(1):8. doi: 10.1186/s12610-022-00157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walenta L., Schmid N., Ju Schwarzer, Köhn F.-M., Urbanski H.F., Behr R., Strauss L., Poutanen M., Mayerhofer A. NLRP3 in somatic non-immune cells of rodent and primate testes. Reproduction. 2018;156(3):231–238. doi: 10.1530/REP-18-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagheri V., Hassanshahi G., Zeinali M., Abedinzadeh M., Khorramdelazad H. Elevated levels of S100A12 in the seminal plasma of infertile men with varicocele. Int. Urol. Nephrol. 2016;48(3):343–347. doi: 10.1007/s11255-015-1188-5. [DOI] [PubMed] [Google Scholar]
- 21.Baazm M., Ghafarizadeh A.A., Noshad Kamran A.R., Beyer C., Zendedel A. Presence of the NLRP3 inflammasome components in semen of varicocele patients. Int J Fertil Steril. 2020;14(1):46–50. doi: 10.22074/ijfs.2020.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J., Lei T., Wu S., Li H., Deng Y., Lin R., Ning N., Geng C., Wang S., Wu X., et al. Development of a comprehensive method combining UHPLC-CAD fingerprint, multi-components quantitative analysis for quality evaluation of Zishen Yutai Pills: a step towards quality control of Chinese patent medicine. J. Pharm. Biomed. Anal. 2020;191 doi: 10.1016/j.jpba.2020.113570. [DOI] [PubMed] [Google Scholar]
- 23.Katz M.J., Najari B.B., Li P.S., Goldstein M. The role of animal models in the study of varicocele. Transl. Androl. Urol. 2014;3(1):59–63. doi: 10.3978/j.issn.2223-4683.2014.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaalan M.F., Ramadan B.K., H Abd Elwahab A. Ameliorative effect of taurine-chloramine in azathioprine-induced testicular damage; a deeper insight into the mechanism of protection. BMC Compl. Alternative Med. 2018;18(1):255. doi: 10.1186/s12906-018-2272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amann R.P., Almquist J.O. Reproductive capacity of dairy bulls. VI. Effect of unilateral vasectomy and ejaculation frequency on sperm reserves; aspects of epididymal physiology. J. Reprod. Fertil. 1962;3:260–268. doi: 10.1530/jrf.0.0030260. [DOI] [PubMed] [Google Scholar]
- 26.Evenson D.P., Larson K.L., Jost L.K. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 27.Deng C., Shang X., Jiang H., Dai Y., Dai J., Li H., Hong K., Sun X., Zhou H., Zhang Y., et al. Diagnoses and treatments of varicocele: an experts consensus. Natl. J. Androl. 2015;21(11):1035–1042. [Google Scholar]
- 28.Comhaire F. Clinical andrology: from evidence-base to ethics. The 'E' quintet in clinical andrology. Hum. Reprod. 2000;15(10):2067–2071. doi: 10.1093/humrep/15.10.2067. [DOI] [PubMed] [Google Scholar]
- 29.Albano Nogueira G.A.K., Maciel Junior V.L., Minas A., Antoniassi M.P. Characterization of varicocele-induced animal models: potential role of inflammasome complex in the varicocele pathophysiology. J. Reprod. Immunol. 2022;149 doi: 10.1016/j.jri.2021.103442. [DOI] [PubMed] [Google Scholar]
- 30.Razi M., Tavalaee M., Sarrafzadeh-Rezaei F., Moazamian A., Gharagozloo P., Drevet J.R., Nasr-Eshafani M.-H. Varicocoele and oxidative stress: new perspectives from animal and human studies. Andrology. 2021;9(2):546–558. doi: 10.1111/andr.12940. [DOI] [PubMed] [Google Scholar]
- 31.Park H.J., Choe S., Park N.C. Effects of Korean red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chin. J. Integr. Med. 2016;22(7):490–495. doi: 10.1007/s11655-015-2139-9. [DOI] [PubMed] [Google Scholar]
- 32.Guan S., Zhu Y., Wang J., Dong L., Zhao Q., Wang L., Wang B., Li H. A combination of Semen Cuscutae and Fructus Lycii improves testicular cell proliferation and inhibits their apoptosis in rats with spermatogenic dysfunction by regulating the SCF/c-kit--PI3K--Bcl-2 pathway. J. Ethnopharmacol. 2020;251 doi: 10.1016/j.jep.2019.112525. [DOI] [PubMed] [Google Scholar]
- 33.Selim N.M., Elgazar A.A., Abdel-Hamid N.M., El-Magd M.R.A., Yasri A., Hefnawy H.M.E., Sobeh M. Chrysophanol, physcion, hesperidin and curcumin modulate the gene expression of pro-inflammatory mediators induced by LPS in HepG2: in silico and molecular studies. Antioxidants. 2019;8(9) doi: 10.3390/antiox8090371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal A., Said T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update. 2003;9(4):331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 35.Wu H., Sun L., Wen Y., Liu Y., Yu J., Mao F., Wang Y., Tong C., Guo X., Hu Z., et al. Major spliceosome defects cause male infertility and are associated with nonobstructive azoospermia in humans. Proc. Natl. Acad. Sci. U.S.A. 2016;113(15):4134–4139. doi: 10.1073/pnas.1513682113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habibi B., Seifi B., Mougahi S.M.H.N., Ojaghi M., Sadeghipour H.R. Increases in interleukin-6 and interferon-gamma levels is progressive in immature rats with varicocele. Ir. J. Med. Sci. 2015;184(2):531–537. doi: 10.1007/s11845-014-1167-3. [DOI] [PubMed] [Google Scholar]
- 37.Razi M., Malekinejad H. Varicocele-induced infertility in animal models. Int. J. Fertil. Steril. 2015;9(2):141–149. doi: 10.22074/ijfs.2015.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masumoto J. ["The inflammasomes"] Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(5):346–354. doi: 10.2177/jsci.34.346. [DOI] [PubMed] [Google Scholar]
- 39.Camargo M., Ibrahim E., Intasqui P., Belardin L.B., Antoniassi M.P., Lynne C.M., Brackett N.L., Bertolla R.P. Seminal inflammasome activity in the adult varicocele. Hum. Fertil. 2022;25(3):548–556. doi: 10.1080/14647273.2020.1870756. [DOI] [PubMed] [Google Scholar]
- 40.Poli G., Fabi C., Sugoni C., Bellet M.M., Costantini C., Luca G., Brancorsini S. The role of NLRP3 inflammasome activation and oxidative stress in varicocele-mediated male hypofertility. Int. J. Mol. Sci. 2022;23(9) doi: 10.3390/ijms23095233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X., Qu Z., Qian H., Li Z., Sun X., Zhao X., Li H. Ginsenoside Rg1 ameliorates reproductive function injury in C57BL/6J mice induced by di-N-butyl-phthalate. Environ. Toxicol. 2021;36(5):789–799. doi: 10.1002/tox.23081. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., Zhou Q.-M., Li X.-D., Xie Y., Duan X., Min F.-L., Liu B., Yuan Z.-G. Ginsenoside R(e) increases fertile and asthenozoospermic infertile human sperm motility by induction of nitric oxide synthase. Arch Pharm. Res. (Seoul) 2006;29(2):145–151. doi: 10.1007/BF02974276. [DOI] [PubMed] [Google Scholar]
- 43.Ji M., Minami N., Yamada M., Imai H. Effect of protopanaxatriol saponin on spermatogenic stem cell survival in busulfan-treated male mice. Reprod. Med. Biol. 2007;6(2) doi: 10.1111/j.1447-0578.2007.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvati G., Genovesi G., Marcellini L., Paolini P., De Nuccio I., Pepe M., Re M. Effects of Panax Ginseng C.A. Meyer saponins on male fertility. Panminerva Med. 1996;38(4):249–254. [PubMed] [Google Scholar]
- 45.Zeng J., Zhang D., Wan X., Bai Y., Yuan C., Wang T., Yuan D., Zhang C., Liu C. Chlorogenic acid suppresses miR-155 and ameliorates ulcerative colitis through the NF-κB/NLRP3 inflammasome pathway. Mol. Nutr. Food Res. 2020 doi: 10.1002/mnfr.202000452. [DOI] [PubMed] [Google Scholar]
- 46.Choi N., Yang G., Jang J.H., Kang H.C., Cho Y.-Y., Lee H.S., Lee J.Y. Loganin alleviates gout inflammation by suppressing NLRP3 inflammasome activation and mitochondrial damage. Molecules. 2021;26(4) doi: 10.3390/molecules26041071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang N., Zhang X., Liu X., Wang H., Xue J., Yu J., Kang N., Wang X. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/370530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.