Abstract
The endothelial cells (ECs) make up the inner lining of blood vessels, acting as a barrier separating the blood and the tissues in several organs. ECs maintain endothelium integrity by controlling the constriction and relaxation of the vasculature, blood fluidity, adhesion, and migration. These actions of ECs are efficiently coordinated via an intricate signaling network connecting receptors, and a wide range of cellular macromolecules. ECs are naturally quiescent i.e.; they are not stimulated and do not proliferate. Upon infection or disease, ECs become activated, and this alteration is pivotal in the pathogenesis of a spectrum of human neurological, cardiovascular, diabetic, cancerous, and viral diseases. Considering the central position that ECs play in disease pathogenesis, therapeutic options have been targeted at improving ECs integrity, assembly, functioning, and health. The dietary intake of flavonoids present in citrus fruits has been associated with a reduced risk of endothelium dysfunction. Naringenin (NGN) and Naringin (NAR), major flavonoids in grapefruit, tomatoes, and oranges possess anti-inflammatory, antioxidant properties, and cell survival potentials, which improve the health of the vascular endothelium. In this review, we provide a comprehensive summary and present the advances in understanding of the mechanisms through which NGN and NAR modulate the biomarkers of vascular dysfunction and protect the endothelium against unresolved inflammation, oxidative stress, atherosclerosis, and angiogenesis. We also provide perspectives and suggest further studies that will help assess the efficacy of citrus flavonoids in the therapeutics of human vascular diseases.
Keywords: Endothelial dysfunction, Naringin, Naringenin, Citrus flavonoids, Microvasculature, Therapeutics
Highlights
-
•
Endothelial cell dysfunction is a common denominator in several diseases.
-
•
Mechanisms of Endothelial cell dysfunction are associated with inflammation, procoagulants, and vasoconstricting factors.
-
•
Naringenin and Naringin attenuate inflammation, reduce oxidative stress, and improve the permeability of ECs.
-
•
Naringenin and Naringin improve complications of vascular diseases and maintain the integrity of healthy endothelial cells.
-
•
Naringenin and Naringin are promising therapeutic agents for the treatment of diseases associated with the vasculature.
Abbreviations
- ACE
Angiotensin Converting Enzyme
- AJs
Adherent Junctions
- ATH
Atherosclerosis
- BBB
Blood-brain Barrier
- BDNF
Brain-Derived Neurotrophic Factor
- BMP
Bone Morphogenesis Protein
- CNS
Central Nervous System
- COVID 19
Corona Virus Disease 2019
- CRP
C-reactive Proteins
- CVD
Cardiovascular disease
- DAMP
Danger Associated Molecular Patterns
- ECM
Extracellular Matrix
- ECs
Endothelial cells
- ERRs
Estrogen Related Receptors
- FIK-1
Fetal Liver Kinase-1
- GFAP
Glial Fibrillary Acid Protein
- HUVECS
Human Umbilical Vein Endothelial Cells
- ICAM-1
Intercellular Adhesion Molecule-1
- ICH
Intracerebral Hemorrhage
- IFN
Interferons
- IGF
Insulin-like Growth Factor
- IJs
Intracellular Junctions
- IL-1
Interleukin 1
- iNOS
Inducible Nitric oxide Synthase
- IRS-1
Insulin Receptor Substrate-1
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- JAMs
Junction Adhesion Molecules
- KDR
Kinase insert Domain-containing Receptor
- MAPK
Mitogen Activated Kinase
- MCPs
Monocyte Chemoattract Proteins
- MMP
Matrix Metalloproteinases
- NAR
Naringin
- NF-KB
Nuclear factor kappa B
- NGN
Naringenin
- NLRs
Nucleotide-binding Oligomerization Domain-like Receptors
- NO
Nitric oxide
- NO-CGMP
Nitric Oxide-Cyclic Guanosine Monophosphate
- Nrf2
Nuclear erythroid-related factor 2
- ox-LDL
Oxidized low-density lipoprotein
- PARP
Poly-ADP ribose polymerase.
- pCAM
Promoter Cell Adhesion Molecules
- PI3K
Phosphatidylinositol 3-kinases
- PKB
Protein Kinase B
- PKC
Protein kinase C
- RIG-1
Retinoid acid-inducible Gene 1
- RLRs
RIG-1-like Receptors
- ROS
Reactive Oxygen Species
- SARS-CoV-2
Severe Acute Respiratory Syndrome - Corona Virus 2
- SMC
Smooth Muscle Cell
- TGF-β
Transforming Growth Factor-beta
- TJs
Tight Junctions
- TLRs
Toll-like Receptors
- TMPRSS 2
Transmembrane protease serine 2
- TNF-
Tumor Necrosis Factor-alpha
- VCAM-1
Vascular Cell adhesion Molecules-1
- VEGF
Vascular Endothelial Growth Factor
- VSMCs
Vascular Smooth Muscule Cells
- YAP
Yes Associated Protein
1. Introduction
1.1. Endothelial cell biology and function
The pioneering discovery of endothelial cells (ECs) dates back to the Seventeenth century when Marcello Malpighi demonstrated the existence of capillaries in frog lungs. During this period, the advent of microscopy and injection techniques aided the discovery of the epithelium in the vessel lumen [1]. The term “epithelium” was introduced by Gustav Jacob Henle in the mid-19th century [2], and thereafter, there arose discussions as to capillaries possessing walls with nuclei. Up till the 20th century, the endothelium was thought of as a cellophane paper with no specific function other than selective permeability to water and small molecules [3] or a single layer of endothelial cells that permits small molecules through the walls of blood vessels [4]. To date, ECs are being used for the in vitro studies of metabolism and disease [5,6].
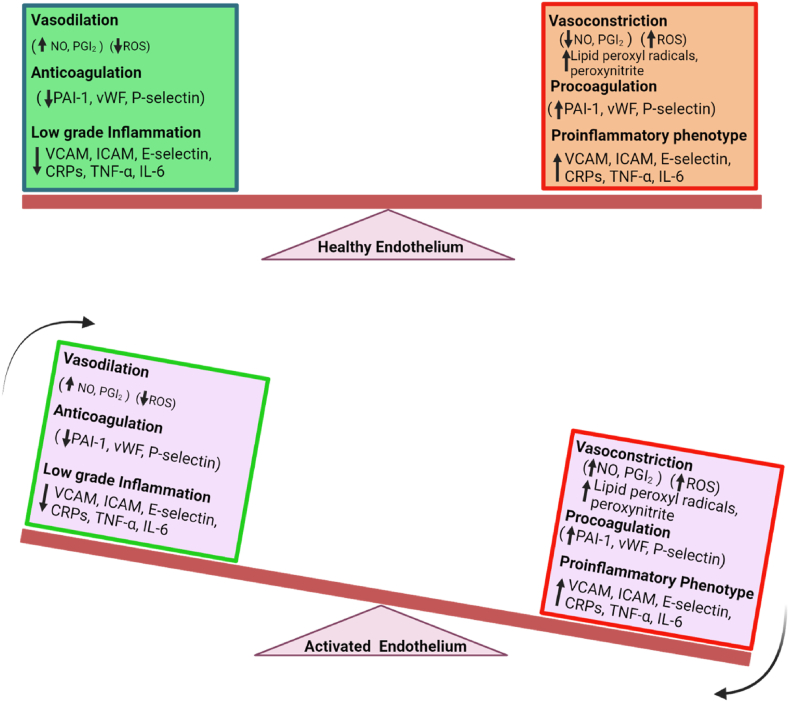
The vascular endothelium is a monolayer of ECs that lines the circulatory system i.e., the capillaries and veins with varying volumes and surface area. Endothelial cells perform multiple unique functions including the maintenance of vascular homeostasis, fluid filtration associated with the renal glomeruli, prevention of thrombosis and its complications, recruitment of immunological molecules, trafficking of hormones, and many others [7]. Of particular importance is the ability of endothelial cells to act as both sensors and effectors; properties that enable them to mediate homeostasis [3]. The effector functions of ECs are made possible via membrane-spanning proteins such as receptors, cell-cell adhering proteins, cell-matrix, hormones, and other metabolites [8]. Being the inner lining of blood vessels, endothelial cells play a significant role in regulating the flow of blood via the presentation of antithrombotic surfaces and facilitating the smooth transit of cellular contents. Moreover, under quiescent conditions, ECs regulate the uptake of vasoactive substances that mediate vascular permeability, trafficking, and coagulation [[9], [10], [11], [12]]. ECs also maintain the balance between vasodilatory and vasoconstricting factors (Nitric oxide (NO), prostacyclin, thromboxane) to maintain vascular smooth muscle tone. Further, ECs secrete a number of intracellular growth-enhancing and adhesion proteins and immune molecules such as Bone morphogenesis protein (BMP), Interleukins (IL-1 alpha and beta, IL-6, frizzled receptor protein, Insulin-like growth factor (IGF), von Willebrand factor and Placenta growth factor [11,[13], [14], [15], [16]]. Inflammatory mediators such as IL-6, Intercellular adhesion molecule 1 (ICAM-1), Vascular cell adhesion molecule 1 (VCAM-1), and C-reactive proteins (CRP) are conventionally used as endothelial dysfunction biomarkers in Diabetes, and Cardiovascular diseases [17]. During periods of infection, inflammation, hyperglycemia, or hypertension, ECs become increasingly permeable. This is due to oxidative stress, dysregulated expression of procoagulants, cell adhesion molecules, nitric oxide, and the patrolling of monocytes and leukocytes to infection sites (Fig. 1; [18,19]).
Fig. 1.
Balance between a healthy and a dysfunctional endothelium. Equilibrium shift to the left characterizes a quiescent state and functional endothelium while the shift to the right indicates the effectual weight of vasoconstriction (↓NO, PGI2), oxidative stress (↑ROS), proinflammatory cytokines (TNF-α, IL-6, MCP-1) and adhesion molecules (↑ICAM, VCAM, E-selectin) as well as procoagulants (↑PAI-1, vWF, P-selectin), which characterize endothelial dysfunction. During infection or in a disease state, the balance is tipped downward, and the endothelium is activated and dysfunctional. This leads to systemic inflammation, oxidative damage, impaired vasculature, and increased permeability and ultimately accelerates disease progression.
1.1.1. Endothelial cell heterogeneity and phenotypes
The ECs in the blood or lymphatic vessels primarily control vascular tone and permeability, recruitment of blood and immune cells, and migration of smooth muscle cells. To carry out these functions, ECs adopt a heterogeneous phenotype, determined early during the cell developmental stages of differentiation into target tissues [20,21]. Under different conditions whether pathological or physiological, ECs can modify the structure of large and small vessels into distinct phenotypes that assume different roles depending on their localization [22]. For instance, ECs stimulate tip cells and activate other cell types to modulate signaling, which guides the sprouting of developing vessels [23]. Consequently, tip cells initiate the repression of notch signaling and upregulate the vascular endothelial growth factor gene (Flk-1) [23]. In tumorigenic cells, ECs express the Vascular Endothelial Growth Factor (VEGF), a pro-angiogenic factor that promotes abnormal proliferation of blood vessels, leading to angiogenesis. The structure, function, and mechanisms of ECs heterogeneity have been extensively documented [21,[24], [25], [26]], and this knowledge is particularly essential for understanding how bioactive compounds modulate site-specific ECs in health and disease.
1.1.2. Endothelial barriers, intercellular junctions, and endothelial cell permeability
Under normal conditions, along the vasculature, ECs align with a tightly arranged functional barrier, made up of intact cell-to-cell junctions [27,28]. The integrity of this vascular barrier of ECs is supported by the function of intercellular junction molecules (IJs). IJs are transmembrane proteins that activate homophilic interactions, and ultimately form a “zipper-like” structure along the border of the cell [29,30]. When disrupted or structurally modified, IJs lead to inflammatory responses and pathological processes which can exacerbate ECs, and lead to cellular leakage and permeability. Known mediators of vascular permeability are inflammatory factors such as Tumor necrosis factor (TNF), protein kinase C (PKC), and thrombin to mention a few [31]. The IJs are of two subtypes; tight junctions (TJs) and adherent junctions (AJs) [32,33]. TJs, located around the intercellular cleft between close cells, act as barriers against solutes [34]. Proteins in the TJ include occludins, claudins, and Junction adhesion molecules (JAMs). Claudins are tetraspanins that connect directly with cytoskeletons. Claudin isotypes 1 and 5 have been implicated in the regulation of the permeability of TJs in human cells [33]. Occludins represent an integral TJ protein that forms complexes end-to-end with claudins and are strategic in providing barrier functions to the ECs via the seal from its N-terminal domain [35]. AJs on the other hand are composed of cadherins, which are transmembrane proteins that mediate cell-to-cell adhesion via interactions with Vascular Endothelial (VE)-cadherin molecules present on the surface of an adjacent cell. Gavard, [36] suggested that these VE-cadherin contacts between ECs are necessary for the maintenance of ECs integrity and functionality of the ECs barrier.
1.1.3. Endothelial cells as conditional innate immune cells
ECs and other structural cells including epithelial cells, stroma cells, and smooth muscle cells among others are involved in innate and adaptive immune functions [[37], [38], [39], [40], [41], [42]]. ECs could be considered innate immune cells [11]. By their location, ECs act as sentinel cells, being in the first line of exposure to foreign microbial contents in circulation [9]. Asides from this, ECs have (Danger Associated Molecular Patterns (DAMP) sensors expressing Toll-Like Receptors (TLRs) at different levels depending on the severity of infection or inflammation [43,44]. ECs also express other receptors such as Nucleotide-binding oligomerization domain-like receptors (NLRs), chemokine receptors, Retinoic acid-inducible gene 1 (RIG-1)-like receptors (RLRs), and secrete pro-inflammatory cytokines (IL-8) in response to stimulation by foreign microbes [45,46]. These properties of ECs show that ECs respond to infections and inflammation and thus enhance or suppress immune functioning depending on their site-specific cytokine profiles [9]. For Instance, ECs use their pathogen recognition receptors (PRRs) to detect foreign or inflammatory stimuli and in response, express pathogen recognition molecules such as MHC II, which is presented to immune cells [11]. Other responses of ECs may include the expression of adhesion molecules which facilitates leukocyte transmigration to underlying tissues or a direct secretion of inflammatory mediators, cytokines, and chemokines [9,11,47,48].
ECs like toll-like receptors are involved in pathogen detection via interactions between the PRRs and DAMPs [49], ECs specifically limit pathogen replication via the transcriptional activation of immune molecules such as type 1 Interferons IFN-gamma [50], and also observed in the tryptophan degradation in Toxoplasma gondii infections [51]. Since ECs are conditional immune cells, in many physiological and pathological conditions [11], they can be necessary targets for anti-inflammatory and immunomodulatory agents.
2. Naringenin and naringin as functional polyphenolic compounds
Bioactive compounds have become an emerging and significant aspect of pharmaceutics and the nutrition-based therapeutic industry, owing to their nutritional and medicinal aspects. A growing body of evidence indicates the consumption of polyphenolic compounds as remedies for several diseases via metabolic regulation, modulation of cell proliferation, and amelioration of chronic diseases [52,53]. Structurally, polyphenols vary; from containing multiple phenol moieties to existing in polymerized forms. Polyphenols can be simple phenols (some are not), flavonoids, and non-flavonoids. Flavonoids can also be subclassified into flavanols (catechins), flavonols (Kaempferol and quercetin), flavones (apigenin, luteolin, tangeritin) and flavanones (naringenin, and hesperidin) [54,55].
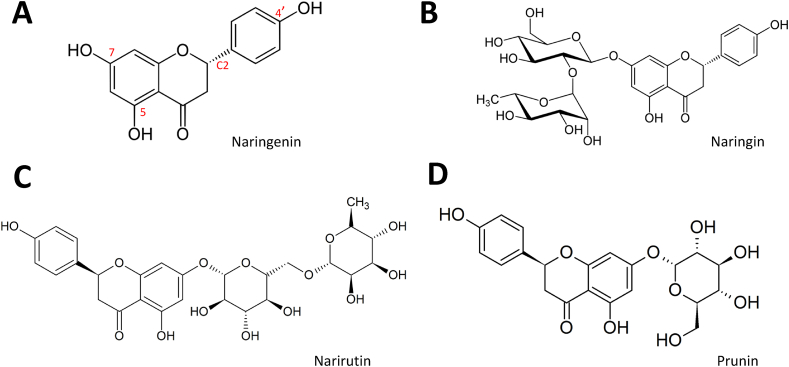
Naringin (NAR; 4′,5,7-trihydroxyflavanone-7-rhamnoglucoside) and Naringenin (NGN; 4′,5,7-trihydroxyflavonone; Fig. 3A and B) are naturally occurring flavanones found in different ratios in citrus fruits, grapes, vegetables and tomatoes [[56], [57], [58], [59]].
Fig. 3.
Structure of Naringenin and its analogs. (A) Naringenin indicates free hydroxyl groups at the 5, 7, and 4′positions as well as a chiral center at C2. The substitution of OH at position 7 for 2-O-α l-rhamnosyl-D-glucoside gives Naringin (B) and for 6-O-α l-rhamnosyl-D-glucoside forms Narirutin (C). Prunin or Floribundoside is produced by substitutions at positions 5 and 7 of the Naringenin structure with glucose (D).
NAR is metabolized by the hydrolytic action of the liver enzyme naringinase to produce NGN and rhamnose [60,61]. Therefore, NGN exists naturally as an aglycone without the “7-O-glucoside” moiety present in NAR (glycosylated form). NAR is responsible for the sour and bitter taste of citrus fruits and is the pharmacological ingredient responsible for the effects of grapefruit in most clinical studies [[62], [63], [64]].
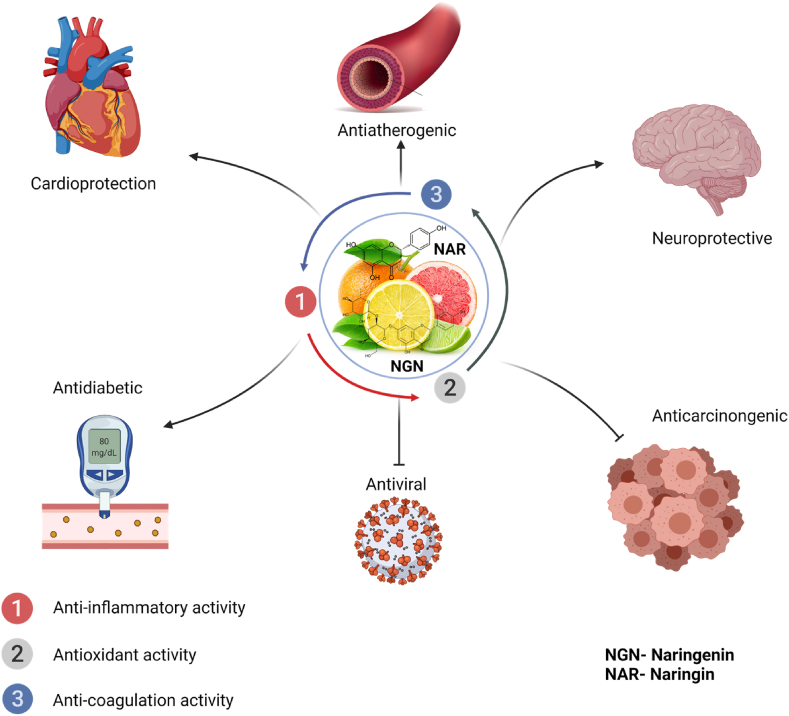
NAR has been reported to possess anti-atherosclerotic, anti-inflammatory, and anti-oxidant properties ([59,65]; Fig. 2). It has also been reported that NGN can protect against cardiovascular disease, particularly in vulnerable patients [66]. NAR also reduces the expression of signaling molecules such as IL-6, IL-8, inducible nitric oxide synthase (iNOS), and nuclear erythroid-related factor 2 (Nrf2) associated with lung injury in LPS-treated mice [67]. NAR similarly improved endothelial function through the production of NO via acetylcholine-mediated mechanisms, and the downregulation of gluconeogenesis enzyme expression in diabetic rats [68]. In addition to NAR’s activities, NGN possesses antifibrogenic, and anticancer properties (Fig. 2). These properties are mediated via a variety of signal transduction cascades including the inhibition of mitogen-activated protein kinase (MAPK), Transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF). Further, NGN has been reported to possess antiviral activity against flaviviruses [69], and hepatitis C virus [70], and more recently against the COVID-19 virus [71,72]. NGN & NAR act by targeting different proteins in metabolism and signaling [73], and as well as stimulating protective activities against metabolic syndromes and cancer in vitro and in vivo [74]. Although NGN & NAR have a low bioavailability, they are traditionally used in treatment therapies against disease states [75]. These activities may be correlated to the action of NGN on endothelial cell function. Since the alteration in endothelial cell integrity is a mandatory common denominator in metabolic syndromes, cancer and stroke, this review focuses on the biological activities and molecular mechanisms of action of endothelial cell protection by NGN & NAR.
Fig. 2.
Pharmacological Roles of Naringin and Naringenin. NGN and NAR protect against the onset and severity of many human diseases via their antioxidant and anti-inflammatory activities, inhibition of adhesion molecules, and enhancement of vascular smooth muscle relaxation in the endothelial cells. In (A), Cardioprotective through NO-signaling effects on the smooth muscle cells (B), antiatherogenic by reducing the feasibility of thrombosis and thrombocytopenia (C), neuroprotective by maintaining the integrity of the blood-brain barrier (D), antidiabetic via the attenuation of glucose-related inflammation and oxidative damage (E), antiviral by the stimulation of anti-inflammatory molecules, inhibition of spike protein interactions (F), anti-cancer through the inhibition of VEGF, and stimulation of apoptosis.
2.1. Biochemistry of naringenin and naringin: structure-function relationship
Structurally, like every other flavonoid, NGN contains atoms arranged in three rings: two benzene rings linked by a 3-carbon O-heterocycle. At the 5th, 7th, and 4′ positions are hydroxyl groups that can be substituted by other functional groups to produce structural analogs of NGN [76]. NGN can exist as NAR, Narirutin, and prunin/floribundoside, ([77]; Fig. 3). NGN also possesses a stereogenic chiral center at position C2, which makes it possible to form enantiomers; NGN enantiomers were found present in natural sources of NGN [78]. The differences in the biological activities of NGN and NAR may be due to the differences in their structures. The hydroxyl substituents and heme oxygenase moieties on NGN and NAR structures help to scavenge free radicals, chelate metal ions, induce antioxidant enzyme systems and inhibit ROS generating oxidases [79,80]. However, NAR has less potency caused by the steric hindrance of some of its functional scavenging groups [68].
NAR when ingested undergoes biotransformation into NGN via the action of α rhamnosidase and β-glucosidase enzymes. NGN has a low oral bioavailability; [62] the hydrolysis of NAR to NGN occurs just before absorption, and NGN is less soluble largely due to its largely hydrophobic structure [81]. On the other hand, NGN (the aglycone) is highly lipophilic and absorbed via passive diffusion by the epithelial cells of the small intestine and then moves into the general circulation and back to the intestinal lumen through ATP-mediated multidrug resistance proteins and P-glycoproteins [82].
An important factor determining the rate of absorption of these flavonoids is glycosylation. For instance, Naringenin -7- rhamnoglucoside (narirutin) and naringenin-7-O-glucoside (prunin) have sugar moieties that make them hydrophilic (Fig. 3C and D). However, they do not diffuse passively across the cell membranes [83]. The sugar moieties at the site of absorption of the glycosides may determine the rate of bioavailability. The flavonoid monoglucosides are estimated several folds higher in bioavailability than the rutinosides and are readily absorbed in the small intestine. Moreover, rutinosides can be metabolized on reaching the colon when exposed to residing bacteria via the action of a-rhamnosidases [84]. Taken together, understanding the differences in structure and function of these flavonoids is strategic to unraveling how they may facilitate endothelial cell survival and improve the functioning of the endothelium. The protective role of NGN and NAR in several disease states including but not limited to their role in endothelial dysfunction are documented [62,68,85,86].
3. Naringin and Naringenin prevent endothelial dysfunction in cardiovascular diseases
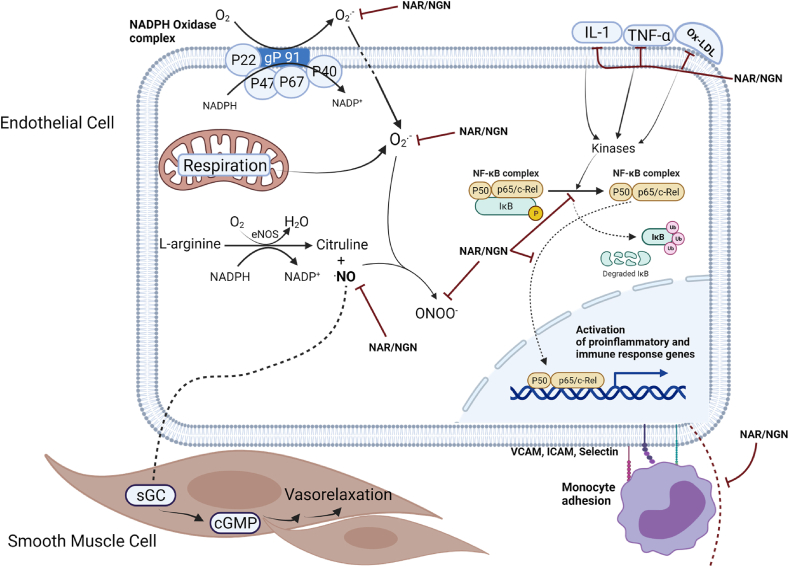
Endothelial dysfunction is associated with high expression of adhesion molecules and the promotion of inflammation, which modulates integrin receptors, causes platelet degranulation, and allows for growing mass of platelet aggregates [87,88] This platelet-endothelium interaction and NO-mediated endothelium-dependent vascular homeostasis has been implicated in atherosclerosis and cardiovascular diseases [17]. Atherosclerosis is characterized by abnormal accumulation and deposition of fibrous tissue and lipids or cholesterol in the innermost layer of the arteries [89]. Accumulation of fibrous or lipid materials causes the narrowing and partial blockage of arteries and thus restricting blood flow. Irregular blood flow leads to myocardial infarction and ischemic heart failure. While atherosclerosis may be caused by various factors such as diabetes, smoking, and hyperlipidemia, a dysfunctional endothelium and hypertension increase the risk of atherosclerotic plaques in the cardiovascular system [89]. There is a recent focus on developing treatments for atherosclerosis by the administration of statins, blood thinners, and cholesterol reduction medications, however, these treatments are associated with toxic side effects when taken long-term. A safe, non-toxic, and efficient therapeutic agent is needed to treat atherosclerosis. Natural functional compounds, phytochemicals, and polyphenols have been shown to exhibit anti-atherosclerotic properties [90,91]. NGN and NAR are examples of these compounds. They modulate heart endothelial cells and smooth muscle cells for vasorelaxation and permissive free blood flow (Fig. 4).
Fig. 4.
Naringenin and Naringin improve Cardiac Endothelial Cell functioning. NGN and NAR promote the scavenging of ROS and free radicals generated from NADPH oxidase and the respiratory activity of the mitochondria. They also inhibit inflammatory cytokine action and attenuate inflammation via the inhibition of NFκB and regulation of leukocyte adhesion. NGN and NAR prevent the formation of toxic peroxynitrite from NO and facilitate endothelial cell vasodilation and smooth blood flow through the heart vessels.
NAR has been reported to play a significant role in the modulation of endothelial cells in animal studies. For instance, reduced vascular fatty streak arrangement and macrophage infiltration were observed in cholesterol-fed rabbits, that had a daily intake of 500 mg/kg NAR supplements. NAR exhibited anti-atherogenic activity via the reduction of ICAM-1 expression in high cholesterol-fed rabbits [92]. When combined, NGN and NAR reduced the expression of aortic VCAM-1 and prevented atherosclerosis in diet-induced hypercholesterolemia in mice [93]. Further, NGN inhibited left ventricular hypertrophy via the downregulation of the Angiotensin-converting enzyme in hypertensive rats [94]. YAP-Yes associated protein is important in endothelial cell activation and vascular inflammation [95]; NAR prevented ox-LDL-induced endothelial apoptosis and injury in human umbilical vein endothelial cells (HUVECs) via the downregulation of the Hippo-YAP pathway [96]. Naringin reduced dysfunctional endothelium in a rat model fed 100 mg/kg per day of fructose. Increased aortic expression of and phosphorylated levels of eNOS were similar features of this treatment [97]. More recently, NAR has been shown to preserve the endothelium by ameliorating pulmonary endothelial permeability and attenuating inflammation in a LPS/cigarette smoke-induced mice [98]. NGN acts via the AMPK/Sirt1 pathway to reduce ROS levels and increase NO bioavailability against homocysteine induced endothelial dysfunction in HUVECs [99]. NGN suppresses the action of inflammatory cytokines and improves endothelial dysfunction via the modulation of the NO-cGMP pathway in a rat model of hypertensive oculopathy [100]. While many researchers have investigated the protective effects of NGN and NAR on CVDs or CVD-related conditions [93,101,102], only a few studies have evaluated direct effects on the Vascular Smooth Muscle Cells (VSMCs) and the ECs. In vivo studies are warranted.
4. Naringenin and naringin protect brain vascular endothelium and prevent cerebral damage
Dysfunctional endothelium in the brain is tightly linked to the disruption and permeability of the blood-brain barrier (BBB), a common dysfunction found in neurodegenerative diseases [103], including cerebral small vessel diseases [104] The brain endothelial cells, form the tight BBB property and are linked together by a complex of transmembrane and inter-endothelial proteins. This barrier selectively regulates in identity and amounts, the proteins that reach the brain parenchyma [105]. TJs and JAMs, that seal up inter-endothelial cell spaces and maintain the polarization of brain endothelial cells via regulated exchange of transporters [106], also make up the brain endothelium defense. The ability of NGN and NAR to modulate these proteins would demonstrate a possible protective or detrimental effect on neurological diseases. Many studies show the effects of Naringin and Naringenin on Alzheimer’s disease, Parkinson’s disease, Multiple Sclerosis, neurotoxicity, neurobehavioural manifestation, and neuroinflammation [57,[107], [108], [109], [110]]. However, not many have used in vitro endothelial cell systems and there is little evidence of in vivo studies. There is evidence for the involvement of NGN & NAR on endothelial cells of the Blood-brain barrier [111,112], intracerebral hemorrhage [113], Stroke [114,115], and cerebral infarction [116,117].
Youdim et al. [111] demonstrated the uptake of NGN and Hesperetin in vitro using different mouse brain endothelial cells compared to other flavonoids that were exposed to the cells. The uptake of NGN at a peak concentration of 30 μM indicated the ability of NGN to cross the blood-brain barrier and interact with the protein contents of the BBB, leading to the activation of signaling and anti-inflammatory processes. Intracerebral hemorrhage (ICH), the rupture of blood vessels in the brain parenchyma leads to the formation of blood clots (hematoma). The consequent release of thrombin stimulates the permeability of inflammatory cells, cytokines, and chemokines, to damage the brain tissue [118]. NAR attenuated TNF-α and MMPs compared to control groups in a study carried out by Singh and colleagues on collagenase-induced intracerebral hemorrhage in rat brains [113]. Similarly, NAR was found to confer Cerebro-protective activity against cerebral infarction in ischemia-reperfusion injured rats. Reduced leukocyte infiltration and attenuated inflammation were observed in the same experimental animals [116]. NGN & NAR have neurotherapeutic potential in the treatment of neurological complications arising from a stroke. NGN & NAR have been shown to improve recovery via enhancing the expression of Brain-Derived Neurotrophic Factor (BDNF) and VEGF following spinal cord injury in rats [119]. NAR inhibited the activation of MAPK signaling pathway to reduce oxidative stress, apoptosis and ameliorate BBB damage in a rat model of subarachnoid hemorrhage [120]. NAR also alleviated locomotor impairments and protected against BBB disruption by downregulating MMPs and inducing Glial Fibrillary Acid Protein (GFAP) in 3-nitropropionic acid-induced neurodegeneration in rats [121]. Of particular importance is the fact that NGN and NAR reduce pro-inflammatory activities in and around the brain [[122], [123], [124]]. This does not necessarily indicate neuromodulation to the microvessel endothelial cells, since other cell types and neurons also secret pro-inflammatory mediators and cytokines [125]. There is need for more conclusive evidences using brain endothelial cell cultures and in vivo studies in mice, to elucidate the neuropharmacological mechanisms by which NGN and NAR protect the brain.
5. Naringenin and naringin preserve the vascular endothelium in diabetes
Dysfunctional endothelium results from the inability of the endothelial cells to regulate events in the vascular wall, leading to a shift in homeostatic balance towards pro-inflammatory, prothrombotic, vasoconstrictive, and atherogenic activities [126,127]. Diabetes is a metabolic disease as well as a disease of vascular homeostasis due to its effects on several vascular beds and alteration of the vascular tone. The vascular complications associated with diabetes link it with the pathogenesis of cardiovascular diseases [128,129]. The intercellular milieu in diabetes triggers a cycle of activities that take place within the vascular wall, including oxidative stress, inflammation, and impaired fibrinolytic functions. These events lead to thrombosis, vasoconstriction, and impaired endothelial cell functioning in both the early and late stages of the disease [130,131]. It is not yet clear how there might be a differential impact of type 1 and type 2 diabetes on endothelial cell dysfunction; some lines of evidence and reports believe that impaired endothelial-dependent vasodilation links both type 1 and type 2 diabetes [132,133], however, a later study showed that endothelial dysfunction pathogenesis differs between type 1 and type 2 diabetes [134]. Since there are multifactorial causes of endothelial dysfunction in diabetic patients, the manifestation of multiple homeostatic imbalances makes it challenging to delineate the above relationship. Hyperglycemia, oxidative stress, proinflammatory activity, and insulin resistance are specific systems that impact the endothelial cell functioning in diabetes [128]. In this section, we highlight studies that report the beneficial effects and possible mechanisms of NAR and NGN on vascular diabetic disease.
5.1. Hyperglycemia
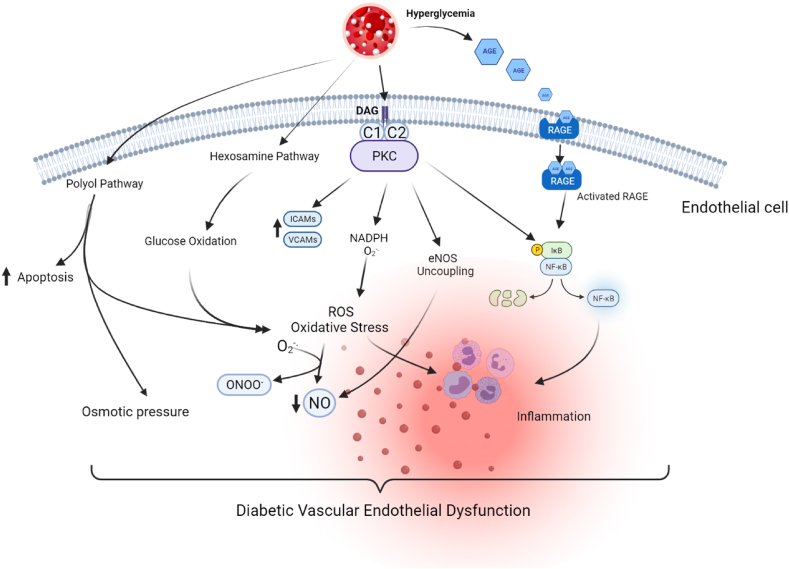
Hyperglycemia, known to be the hallmark of diabetes mellitus, has been shown to impair endothelial cell function and disruption of vascular beds in human subjects and animal studies especially when prolonged [[135], [136], [137]]. Hyperglycemia causes vascular dysfunction via the following mechanisms (Fig. 5) – the activation of Protein Kinase C (PKC) pathway, the increased affinity and shunting of glucose through the activation of hexosamine and polyol pathways, the increased expression of advanced glycation end products (AGEs) and their receptors (RAGE-Receptors for Advanced Glycation End Products), leading to intracellular and extracellular modification of proteins of the extracellular matrix [133,138]. The results of these mechanisms are alterations in gene expression, generation of oxidative stress, increased apoptosis, and permeability of the vascular endothelium ([135,139]; Fig. 5). NGN and NAR have been shown to modulate the homeostatic shift of hyperglycemia in diabetes in animal models. Among many studies, NGN alleviated vascular dysfunction in Streptozotocin-induced diabetic rats as well as in palmitic acid HUVECs [77,140]. NGN also reduced blood glucose levels, insulin levels and regulates the cell adhesion molecule (ICAM) and oxidative stress associated with hyperglycemia in type 2 diabetic rats [141,142]. NGN was reported to reduce serum glucose levels, oxidative stress markers, production of NO, and proinflammatory cytokines in STZ-induced diabetic rats [143]. Similarly, NGN exerted antihyperglycemic and antioxidant effects in STZ-nicotinamide-induced diabetic rats [144]. Further, NGN reduced PKC activity and reduced the expression of the pro-inflammatory transcription factor, nuclear factor κB (NF-κB) p65 expression in the kidney of diabetic mice [145]. NAR protected against hyperglycemia induced myocardial fibrotic lesions via the upregulation of PKC and P38 in male spraque-dawley rats [146]; Similarly, NAR improved endothelial cell damage caused by high glucose through the activation of antioxidant status and downregulation of CX3Cl [147]. Through the modulation of the leptin-JAK2/STAT3 pathway, NAR ameliorates hyperglycemia-induced injuries in H9C2 cardiomyocytes [148]. There is not much evidence for the role of NAR in hyperglycemia, but NGN possesses signaling modulating roles that make it potentially useful in the management of diabetes.
Fig. 5.
Hyperglycemia-induced Endothelial Dysfunction. High levels of glucose and fatty acids activate the polyol pathway, hexosamine, and glucose autooxidation pathway, PKC activation, and increase the levels of advanced glycation end products, stimulating the RAGE and its downstream pathways. Hyperglycemia activates PKC via DAG, leading to the upregulation of cell adhesion molecules (VCAMs and ICAMs), generation of ROS by NADPH oxidase, activation of the pro-inflammatory transcription factor NFkB and the uncoupling of endothelial nitric oxide synthase. The RAGE mediated pathway, glucose oxidation, hexosamine pathway, and polyol pathway all generate oxidative stress, leading to excessive ROS levels in the cell. NO reacts with superoxide anion, to form deleterious molecule peroxynitrite which is largely indicated in endothelial cell dysfunction. ROS also stimulates NFkB to express pro-inflammatory genes leading to systemic inflammation. These events lead to activated endothelium and vascular dysfunction in diabetes.
5.2. Oxidative stress
Oxidative stress is a unifying mechanism involved in endothelial dysfunction. That is, most other mechanisms that cause endothelial cell dysfunction are directly or indirectly related to ROS generation and oxidative stress-related damages. Nitric oxide metabolism and signaling are central hallmarks of endothelium dysfunction. Oxidative stress diminishes the availability of NO via the alteration of eNOS enzyme activity or the formation of peroxy radicals with superoxide anion. The peroxyl radical (ONOO-) is capable of binding cellular macromolecules and causing alteration in cellular functioning [149]. ROS generating systems in the vasculature include the xanthine oxidases (XOs), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, cytochrome P450, and lipoxygenase systems as well as the mitochondria [128,138]. These ROS sources have been characterized in type 2 diabetes and implicated in impaired endothelium-dependent vasodilation associated with diabetes mellitus [150].
Indeed, several studies have reported the antioxidative role of NGN & NAR in various treatment models. In diabetes-related oxidative stress conditions, NGN and NAR suppress oxidative damage via their free radical scavenging property, reducing the possibility of lipid peroxidation and endothelial cell damage. NAR supplementation to diabetic rats elevated antioxidant enzymes levels, inhibited xanthine oxidases – partial sources of superoxide anions and scavenged ROS including hydroxyl radicals [68].
5.3. Chronic inflammation
Chronic inflammation also contributes to the compromised integrity of the vascular walls in diabetes mellitus. Following the discovery of elevated levels of pro-inflammatory cytokines in diabetic and obese patients, a compromised EC phenotype that leads to vascular leakage and damage was observed [140,151]. Many of the inflammatory cytokines and chemokines (TNF-α, ILs, CCL2, CCL5), transcription factors (NFκB), and cell adhesion molecules (ICAM, VCAM) found in the diabetic milieu are presented in Table 1. These classes of molecules act via oxidative stress-related activation and increased expression of one another, which eventually leads to increased cell permeability and impaired vascular homeostasis. NGN ameliorated renal impairments in streptozotocin-induced diabetes in rats via the downregulation of IL-1 and proinflammatory TGF-beta 1 [140]. Also, in a dose-dependent manner, NGN reduced the expression and protein levels of TNF-alpha, (IL)-1β, and MCP-1 in diabetic mice [145]. NAR reduced the expression of the CX3CL1 and ROS, and additionally reduced NO levels in HUVECs treated with high glucose [147]. More recent evidences of NGN on attenuating chronic inflammation include; its suppression of oxidative stress, apoptosis and neuropathic inflammation in STZ-induced diabetic mice and rats via the modulation of Nrf2 and NFkB pathways [152,153], and the improval of renal damage by the induction of cell proliferation and hypertrophy in cloned rat kidney (NRK-52E) cells [154]. NGN activates the eicosatetraenoic acid pathway to reduce inflammation in diabetic nephropathy [154]. Further, NGN suppressed NFkB activation, improved glucose tolerance, and increased the expression of antioxidant enzymes mRNA in gestational diabetes mellitus ([155]; Fig. 5). Lastly, NGN suppresses the infiltration of immune cells, neutrophils and macrophages in obese mice [156].
Table 1.
Inflammatory mediators of endothelium-derived microvascular complications in diabetes.
| S/N | Mediator | Key Players | Cellular Role References |
|---|---|---|---|
| 1. | Inflammatory Cytokines | Interleukins; IL-1, IL-6, IL-8, TNF-α, CRP, IL-22 |
Secreted by immune cells and upregulate inflammatory reactions by synthesis of secondary mediators, more [234,235] pro-inflammatory cytokines, and the attraction of immune cells. |
| 2. | Adhesion Molecules | Selectins, Integrins, Immunoglobulins ICAM-1, VCAM-1 |
Enhance leukocyte adhesion and [236] recruitment of inflammatory cells via chemokines. |
| 3. | Local Inflammation | iNOS forming NO COX forming prostaglandins |
Prostaglandins lead to acute inflammatory [237,238] responses in inflamed tissue. |
| 4. | Transcription factors | Nuclear Factor kappa B (NFκB) | Redox-sensitive transcription factor, which [239] translocates to the nucleus upon inflammatory insult to induce the expression of more inflammatory cytokines. |
| 5. | Chemokines/Chemoattractants | CCL-2 (MCP-1), CX3CL1, CCL5, CCL7 | Attracts immune cells to the sites of [[240], [241], [242]] inflammation and facilitates the formation of atherosclerotic plaques. |
| 6. | Toll-like Receptors | TLR2 and TLR4 | Mediates immune response during [243,244] infection or injury to the ECs. |
5.4. Insulin resistance
Insulin resistance is described as the inability or reduced sensitivity of insulin to facilitate the uptake of glucose into the body organs including the liver, adipose tissue, and the skeletal muscle [157]. Insulin resistance impacts the endothelium in the diabetic milieu via notable pathways related to insulin signaling. Insulin signaling involves the PI3–K pathway and MAPK pathway, which regulate gene expression, cell proliferation, and differentiation [138,158]. Insulin generally promotes the production of NO via the PI3–K pathway and the production of endothelin-1 via the MAPK pathway. However, in conditions of diabetes or insulin resistance, this pathway is halted and impaired, leading to endothelial dysfunction [159].
In the condition of insulin resistance, there is excess production of FFAs as well as the increased generation of ROS and the consequent activation of PKC. In addition, increased inflammatory cytokines have also been associated with insulin resistance [160]. All these events foster inflammatory processes, vascular permeability, and endothelial dysfunction. NAR has been shown to ameliorate hyperinsulinemia, insulin resistance, and hyperglycemia-mediated cytokine levels in streptozotocin-induced diabetic rats [161]. NAR also suppressed glycolytic enzymes, glucokinase, and phosphoenolpyruvate carboxykinase in diabetic mice [162]. 200 mg/kg of NAR mediated the phosphorylation and activation of insulin receptor substrate-1 (IRS-1) and blocking of Insulin-MAPK signaling pathways [163,164]. NGN has been shown to improve insulin sensitivity, attenuate inflammation and reduce the oxidative stress burdens in human in vitro models including high glucose HepG2 cells and human models of gestational diabetes mellitus [155,165,166]. The mechanisms for this is believed to be via the activation and phosphorylation of AMPK [165,166].
Taken together, NGN & NAR preserve the vascular endothelium in diabetes by controlling the mentioned signaling and inflammatory molecules and therefore represent an important class of compounds to be considered in the treatment and management of diabetes mellitus.
6. Naringenin and naringin protect endothelial cell in cancer
An activated endothelium indicates the morphological changes in endothelial cells that lead to the modulation of cell surface molecules. Many of the endothelial cell vasoconstriction, and vasodilation factors including cell adhesion molecules, and cytokines, all conspire to cause local inflammation [167]. For instance, Angiopoietin-2, one of the growth factors involved in angiogenic signaling promotes apoptosis, inflammation, disruption of vascularization, and barrier function [168]. This protein is released upon endothelial cell activation [169]. EC dysfunction is intricately linked to cancer, and while activation of ECs presents a good motive, it can increase the susceptibility of ECs to dysfunctional processes implicated in cancer [170,171]. Dysfunctional ECs involved in cancer progression are activated by growth factors, cytokines, hormones, oxidative stress, estrogen-related receptors (ERRs), and PI3K/Akt pathway amongst others. In understanding the molecular basis of endothelial dysfunction in cancer, much attention has been drawn to the signaling of cellular protein players, vascular endothelial growth factor (VEGF), JAK/STAT, ERRs, and NF-κB [[172], [173], [174], [175]]. The signaling pathways of these protein players are target points for cancer chemoprevention, immunotherapy, and nutrition-based therapy.
NGN has been shown to possess anti-cancer properties, inhibiting inflammation, tumor migration, and progression in B16F10 mouse and SK-MEL-28 human melanoma cells [176]. Similarly, NAR was reported to significantly inhibit tumor growth and reduce TNF-α, and IL-6 in a rat model of carcinosarcoma [177]. Over the years, VEGF signaling necessary for angiogenesis has been explored as a target for a novel therapeutic agent [175,178]; an increase in VEGF mRNA expression occurs as a result of hypoxia, cytokines induction, the action of tumor suppressor genes, and the Estrogen Related Receptor (ERR) [179,180]. In vitro studies reveal that NGN induces apoptosis in estrogen-dependent breast cancer cell lines, human hepatoma, and colon adenocarcinoma [181,182]. In glioma-induced rats, NGN administration reduces the expression of PI3–K and PKB which ultimately contributes to cell proliferation and migration [183]. NAR suppressed VEGF and the activation of VEGFR, inhibited tumor growth and angiogenesis in a xenograft mouse model of glioblastoma and human umbilical vascular endothelial cells [184]. NGN blocks voltage-gated sodium channels to inhibit metastasis in malignant rat prostate tumor (MAT-Lylu) cells [185], strengthen immunity against tumorigenesis by induction and activation of cytotoxic T cells and macrophages in primary tumor specimens from human patients and other murine models [186]. NGN similarly promotes anti-angiogenesis and evokes a positive immune response in human breast cancer and endothelial cells via the modulation of JAK2/STAT3, and ERRalpha/VEGF/KDR signaling pathways [187,188] Further, NGN causes significant changes in the cell cycle by reducing the expression of cyclin-dependent kinase 4 and cyclin D1 found in the G0/G1 phase of the cell cycle [189]. This was concomitant with the activation of caspase 3 via the intrinsic pathway of apoptosis in human hepatocellular carcinoma HepG2 and epidermoid carcinoma A431 cell lines [189,190]. Recently, the use of nanoparticle phytotherapy: Curcumin-Naringenin magnetic nanoparticles induces apoptosis and inhibits cell proliferation in MCF-7 human breast cancer cell lines [191]. With the evidences presented above, NGN stimulates apoptosis in oncogenic phenotypes of ECs and this highlights the potential role of the citrus flavonoid in the prevention or treatment of cancer. The effect of the racemic mixture, R, and the S enantiomers, of NGN, was also studied in caco2 cells. At 10 μg/mL, both S and R enantiomers of NGN reduce the expression of miR-17-3p and miR-25-5p thus impeding human colorectal adenocarcinoma cells, but at a high concentration of 100 μg/mL a lower activity was recorded [192]. Many of the broad anticancer effects of NGN and NAR have been recently reviewed [193]. However, not many studies outline the effects of these flavanones on the endothelium integrity and angiogenesis in Cancer. More studies are warranted.
7. Naringenin and naringin mechanisms of endothelium protection via cellular targets
Aside from the antioxidant and anti-inflammatory roles of NAR & NGN on endothelial cell function, they act on biological targets such as the sarcolemma and mitochondrial potassium channels, SIRT1 enzyme, and transcription factors. In cardiomyocytes, the mitochondria are the main source of ATP and the loss of mitochondria membrane homeostasis is a major cause of cell death in CVDs. Therefore, during ischemia/reperfusion injury, the regulation of potassium channels is important to preserving the function of cardiac cells [194]. Many studies have elucidated the role of important large-conductance calcium and voltage-activated potassium channels (BKca) in cardiovascular diseases and have presented the channels as therapeutic targets for cardioprotection [[194], [195], [196], [197], [198]]. More recently, BKca channels have been discovered as targets of flavonoids such as naringenin, quercetin, luteolin, and others [199], enhancing cardioprotection. This implies that natural plant flavonoids possess ionic mechanisms for modulating the potassium channels. For instance, Micromolar concentrations of NGN, activated mitoBKca channel, increased the consumption of oxygen and decreased membrane potential in both human endothelial cell line EA.hy926 and dermal fibroblasts [200,201]. This activity was dependent on calcium levels. NGN also mediated the activation of ATP-sensitive potassium channels in both cell and mitochondrial membranes following ischemic reperfusion injury [85]. In another study, NGN significantly inhibited Ca2+ overload and contractility in isolated cardiomyocytes [202]. NAR attenuated cardiac injury and increased cell viability in the cardiomyocytes of type 2 diabetic rats [203], through its activity on calcium and potassium channels. NAR also promotes mild depolarization of the membrane potential and reduces calcium overload by inhibiting L-type calcium channels and activating the expression of mitochondria potassium channels, thus reducing apoptosis and promoting cell viability [202,204].
Sirtuin 1 (SIRT1), a member of the family of sirtuins, expressed ubiquitously in endothelial cells has been shown to play protective roles on the vasculature via its interaction with endothelial nitric oxide synthase [205]. As a result, sirtuins have been molecular targets for therapeutics in endothelial cell functioning, arterial wall modeling, and vascular aging [206]. Testai et al. demonstrated the effect of naringenin on SIRT1 activation. Naringenin, which has certain structural similarities with a known SIRT1 activator enhanced the expression of SIRT1 enzyme at 100 mg/kg/day in mice of 6 months old. Although the treatment was chronic, spanning a duration of 6 months, the resultant effect was a reduction in ROS levels and inflammatory cytokines in the myocardium [207].
A challenge associated with the therapeutic potential of NAR and NGN is its ADMET properties. Sequel to oral consumption, only about 15% of NGN is bioavailable in the gut, and this impacts the efficacy of these flavonoids in vivo [208]. NAR has a bioavailability score of 0.17 with three and four violations of Lipinski and Ghose’s rule of drug-likeness respectively. However, lead optimization can be performed on these flavonoids to make them druggable and optimize their potential in pharmacotherapy. NGN passes the Lipinski and Ghose rule for drug-likeness and had a bioavailability score of 0.55 [209].
8. Pre-clinical data and clinical trials for naringenin and naringin
For some time, the consumption of citrus fruits like orange juice, grape juice and lemons has been connected to cardioprotective and neuroprotective events. In some instances, the intake of grapefruit or one glass of orange juice demonstrated lower risks of stroke, cardiovascular disease and obesity [[210], [211], [212], [213], [214]]. However, there are very few studies using purified flavonoids such as naringenin and naringin. The most recent and accessible clinical study was a single ascending dose randomized controlled trial carried out within 2018–2020 (NCT03582553).
The study involved 18 subjects including White, Black or African Americans, and Asians with a mean age of 38 years. Four doses of a citrus sinensis extract of naringenin (150 mg, 300 mg, 600 mg, and 900 mg) were administered and the serum concentrations of naringenin were measured before and over 24 h after ingestion [215]. The results indicated that there were no serious adverse effects and no cases of mortality. However, mild adverse effects such as headache, sinus congestion, drowsiness, abdominal pain, itching, acne, and a cyst on the foot were recorded. While the study was focused on the safety and pharmacokinetic properties of Naringenin, it still demonstrated the need for more pharmacokinetic studies using the purified flavonoids, and not naringenin as a little percentage of a whole citrus juice. Moreover, there exist other clinical studies seeking to evaluate the effect of using whole orange juices or citrus fruit juice on metabolic and cardiovascular diseases (NCT03527277-still recruiting; NCT01963416 – completed).
A recent study by Bai et al. investigated the pharmacokinetics and metabolism of NAR and NGN in rats, dogs, and humans upon naringin administration. According to this group, NGN demonstrated linear pharmacokinetics in humans as opposed to the nonlinear trend observed in rats and dogs when NAR was administered. NAR also had a reduced “area under the curve” and a prolonged pharmacological profile in humans compared to the other non-clinical species [216].
Also, following a single oral dose of NAR (42 mg/kg), Zeng and his colleagues used a rapid-resolution liquid chromatography tandem triple quadrupole mass spectrometry method to determine the amounts of NAR and NGN in the plasma, urine, feces, and tissue homogenate of aged rats (20 months old) [217]. They observed a marked distribution of NAR and NGN in the gastrointestinal tract and other organs; however, absorption and distribution of the flavonoids were affected likely due to the age of the rats. There were no traces of unaltered NAR, but abundant traces of NGN in the GIT and the liver, indicating metabolism by phase II enzymes in these organs. O-disulphate and glucuronide conjugates were identified in urine and fecal samples [217].
In the bid to enhance the solubility and bioavailability of NGN, Xu, and colleagues [218], prepared naringenin-nicotinamide cocrystals NAR-NCT (50 mg of NAR and 45 mg of NCT; 1:2) and administered the combination orally to 18 rats. Samples were collected from the rats at 0.05,0.083,0.167,0.333,0.5,1,2,4,8,10,12, and 24 h following administration. The results demonstrated increased bioavailability, increased half-life, quick absorption, and slow elimination of NAR, suggesting that the cocrystal complexes could improve NAR activity and protective effects.
While most of the studies were performed in rats and animals, the results may not necessarily be concordant in humans. There still exist obvious pharmacokinetic limits on NAR and NGN effects on chronic diseases including the dysfunctions of the vasculature.
9. Perspectives and conclusion
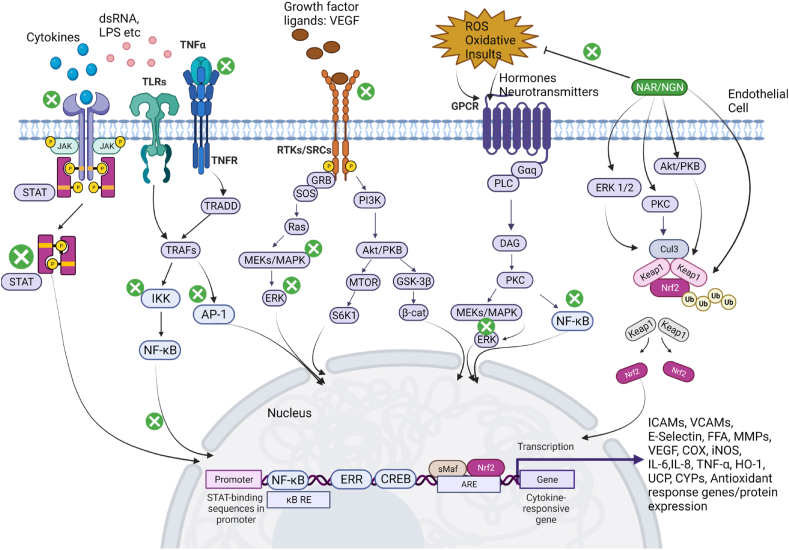
In this review, we summarized the possible mechanisms of endothelial cell function, activation, and dysfunction in various diseases. As the vascular endothelium is the major connecting bridge between the blood circulation and tissues, mechanisms exploring the impact of a dysfunctional endothelium in neurological diseases, diabetes, and cardiovascular diseases open therapeutic windows. ECs in various cell types more commonly have a central mechanism in disease especially as it relates to vascular permeability. From all indications, inflammatory processes impact the physiological functions of ECs and modulate several signaling pathways in the endothelial cells leading to activation or dysfunctional endothelium. As reported by many studies cited in this review, more careful control of endothelial inflammation, including the regulation of leukocyte-mediated infiltration and the production of pro-inflammatory cytokines can attenuate the pathological effects present in several vascular diseases. Targeting the multiple signaling pathways activated in disease states either directly or indirectly could substantially improve EC functioning by mobilizing the ECs to a quiescent state. NGN & NAR, major citrus flavonoids discussed in this review have been well documented for various beneficial effects on the vascular endothelium, including antioxidant, anti-inflammatory, anticancer, antidiabetic, neuroprotective, and cardioprotective effects. They also have wide availability and a history of use. As a result, they are promising candidates for the treatment of many diseases. We have summarized the modulation of NGN & NAR, showing its multi-therapeutic modulation of intracellular signaling molecules necessary for the preservation and survival of ECs. (Fig. 6). However, the low yield and high purification costs still request alternative methods for NGN production. Bacteria E. coli and yeast (S. cerevisiae) have now been shown to produce pure NGN [219,220], yet, more strategies are warranted. A major challenge in clinical trials of citrus flavonoids generally has been their bioavailability, with an estimated peak plasma concentration of 6 h after consumption; concentrations being in the micromolar range [221]. The reasons presented for this are the differences in flavonoid types found in various citrus fruits, the relative solubility of the flavanones in the fruit juice considering the various techniques of preparation, and inter-individual variability of the gut flora metabolism [222]. A more productive approach to enhancing the bioavailability of citrus flavonoids will be to evaluate the role of the human intestinal microbiota on its metabolism [223]. Alternatively, increasing the amount of NGN at 1 μM per 7 mL of orange juice can as well improve the bioavailability of NGN; about 22% of phenolic acids and phase II metabolism products were detected in a clinical trial study [221]. In another clinical trial study examining the role of NAR on endothelial function, 480 μM/340 mL of grapefruit per day provided beneficial effects on arterial stiffness in menopausal women [224]. Seeing the effects of NGN and NAR on EC and smooth muscle cell functioning aiding the prevention of atherogenesis and improving cardiovascular health, a rising concern is whether the effects of the supplementation of these flavonoids will lead to persistent productive effects even after the supplementation is stopped or citrus fruits are not taken [93]. It will be important to establish a correlation between the daily withdrawal of NGN/NAR and the integrity of the healthy or diseased vascular endothelium. Although NGN/NAR may possess significant disease alleviating properties alone, their combination with other citrus flavonoids such as hesperetin, hesperidin, and quercetin or with essential trace elements such as Cu [225], may present more effective therapeutic candidates. This will allow for the improvement of endothelial cell function via a synergistic multi-mechanistic modulation of altered signaling pathways in ECs during infection or disease.
Fig. 6.
Chemo-modulative properties of Naringin and Naringenin; signaling mechanisms on the vascular endothelium. The activation of the vascular endothelium in disease states entails ROS generation, the binding of cytokines, immune cells, growth factors, and disease-causing agents (such as viruses and bacteria), to the membrane of endothelial cells. These agents bind upon transmembrane receptors which activate the downstream signaling pathways as shown above. NAR and NGN when administered or ingested are metabolized and inhibit the activity of ILRs, TNFRs, RTKs, TLRs, and other intracellular kinases and transcription factors (denoted by the circular green inhibition sign). This regulates the expression of proinflammatory genes and pro-oxidant genes. Importantly, NAR and NGN modulate redox-sensitive kinases ERKs, PKC, and Akt/PKB, which activate transcription factor Nrf2. Nrf2 translocates to the nucleus where it binds the Nrf2-ARE and expresses anti-inflammatory proteins and antioxidant enzymes necessary for cell survival. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Endothelial cell inflammation seems more like the “double-crosser” in most diseases associated with the vascular endothelium. For instance, severe forms of COVID-19 are characterized by unresolved inflammation associated with the epithelial and endothelial cells of the lungs. Non-specific targeting (excluding the endothelial cells) of inflammation may be less effective in the therapeutics of Diabetes, CVDs, and COVID-19. Therefore, as Jin et al. [226] opined, there may be much more to the role of endothelial cell inflammation in the pathology of neurological and infectious diseases. Further studies are needed to potentially show a causal relationship between NGN & NAR and the attenuation of unresolved inflammation in the endothelial cells and not merely speculative correlations.
An emerging aspect of EC dysfunction is the involvement of extracellular vesicles (EVs) in disease pathologies. EVs, emerging from different cell types are a means of communication and transfer of contents between cells. EVs have been shown to play a role in EC activation via the stimulation of vascular inflammation and upregulation of cell adhesion molecules [227]. EVs derived from monocytes, leukocytes and platelets increase proangiogenic, proinflammatory, and atherothrombotic stimuli, in the ECs and activate them. This is due to the ability of the EVs to bind the endothelium via the interaction with membrane-bound integrins [227], and possibly tetraspanins. Interestingly, plant polyphenols interfere with the biogenesis pathways of EVs and can modify EV content and release [228]. Studies on how citrus flavonoids impact EV release from ECs would improve our knowledge of the effects of NGN and NAR, especially in vascular endothelial dysfunctions. This approach may also unravel the flavonoid-mediated transmission of infectious particles by EVs from cell to cell. Indeed, both polyphenols and EVs can be used as therapeutic tools in the management and treatment of several diseases. Yet, it is plausible that the packaging and release of EVs may be modulated by NGN which would likely have beneficial implications on the potency of EVs in activating ECs.
Finally, NGN and NAR have additional protective effects on the vascular endothelium in degenerative eye diseases [229], ulcer and ulcerative lesions [230,231], and viral diseases, including COVID-19 [72], hepatitis [232], and HIV [233]. Research areas left to be explored for the role of NGN and NAR include genetic diseases such as Lesch Nyhan syndrome, sickle cell anemia, fragile x mental retardation, and others. Altogether, NGN & NAR have the potential to be involved in the management and treatment of human severe diseases associated with vasculature.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This article received no specific funding or grant.
Data availability statement
This manuscript is a review manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgment
The authors would like to thank Dr. Micheal Amoa-Bosempem (University of Tennessee, Knoxville), Dr. Babajide Ojo (Stanford University), and Sanmi Alake (Oklahoma State University), for proofreading and giving valuable comments on the manuscript. The figures in this manuscript were created with Biorender.com.
References
- 1.Mezzogiorno A., Mezzogiorno V. Marcello Malpighi (1628-1694) Am. J. Nephrol. 1997;17:269–273. doi: 10.1159/000169112. [DOI] [PubMed] [Google Scholar]
- 2.KinneSaffran E., Kinne R.K.H. Jacob Henle: the kidney and beyond. Am. J. Nephrol. 1994;14:355–360. doi: 10.1159/000168747. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., et al. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pate M., Damarla V., Chi D.S., Negi S., Krishnaswamy G. 2010. Endothelial Cell Biology; pp. 109–130. [DOI] [PubMed] [Google Scholar]
- 5.Oosterhoff L.A., Kruitwagen H.S., Spee B., van Steenbeek F.G. Isolation and culture of primary endothelial cells from canine arteries and veins. J. Vis. Exp. 2016 doi: 10.3791/54786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachman R.L., Jaffe E.A. Endothelial cell culture: beginnings of modern vascular biology. J. Clin. Invest. 2004;114:1037–1040. doi: 10.1172/JCI23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand M.J., Gutterman D.D. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20:239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berra Romani R., Raqeeb A., Laforenza U., Scaffino M.F., Moccia F., Avelino-Cruz J.E., et al. Cardiac microvascular endothelial cells express a functional Ca2+-sensing receptor. J. Vasc. Res. 2009;46:73–82. doi: 10.1159/000140677. [DOI] [PubMed] [Google Scholar]
- 9.Mai J., Virtue A., Shen J., Wang H., Yang X.-F. An evolving new paradigm: endothelial cells – conditional innate immune cells. J. Hematol. Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson S.M. Endothelial mitochondria and heart disease. Cardiovasc. Res. 2010;88:58–66. doi: 10.1093/cvr/cvq195. [DOI] [PubMed] [Google Scholar]
- 11.Shao Y., Saredy J., Yang W.Y., Sun Y., Lu Y., Saaoud F., et al. Vascular endothelial cells and innate immunity. Arterioscler. Thromb. Vasc. Biol. 2020:E138–E152. doi: 10.1161/ATVBAHA.120.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q., Zheng M., Betancourt C.E., Liu L., Sitikov A., Sladojevic N., et al. Increase in blood-brain barrier (BBB) permeability is regulated by MMP3 via the ERK signaling pathway. Oxid. Med. Cell. Longev. 2021;2021:1–14. doi: 10.1155/2021/6655122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetterman J.L., Weisbrod R.M., Feng B., Bastin R., Tuttle S.T., Holbrook M., et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler. Thromb. Vasc. Biol. 2018;38:1607–1615. doi: 10.1161/ATVBAHA.118.311156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Wang D.W., Chen Y., Chen C., Guo J., Zhang S., et al. Genome-wide association and functional studies identify SCML4 and THSD7A as novel susceptibility genes for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2018;38:964–975. doi: 10.1161/ATVBAHA.117.310594. [DOI] [PubMed] [Google Scholar]
- 15.Garshick M.S., Barrett T.J., Wechter T., Azarchi S., Scher J.U., Neimann A., et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler. Thromb. Vasc. Biol. 2019;39:787–798. doi: 10.1161/ATVBAHA.118.312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas I., Panicker S.R., Cai X., Mehta-D’souza P., Rezaie A.R. Inorganic polyphosphate amplifies high mobility group box 1–mediated von Willebrand factor release and platelet string formation on endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2018;38:1868–1877. doi: 10.1161/ATVBAHA.118.311165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsalve B., Concha-Meyer A., Palomo I., Fuentes E. Mechanisms of endothelial protection by natural bioactive compounds from fruit and vegetables. An. Acad. Bras. Cienc. 2017;89:615–633. doi: 10.1590/0001-3765201720160509. [DOI] [PubMed] [Google Scholar]
- 18.Jones P.D., Kaiser M.A., Ghaderi Najafabadi M., Koplev S., Zhao Y., Douglas G., et al. JCAD , a gene at the 10p11 coronary artery disease locus, regulates Hippo signaling in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2018;38:1711–1722. doi: 10.1161/ATVBAHA.118.310976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catar R., Chen L., Zhao H., Wu D., Kamhieh-Milz J., Lücht C., et al. Native and oxidized low-density lipoproteins increase the expression of the LDL receptor and the LOX-1 receptor, respectively, in arterial endothelial cells. Cells. 2022;11:204. doi: 10.3390/cells11020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N.M., Minamisawa S. InTech; 2017. Unique Phenotypes of Endothelial Cells in Developing Arteries: A Lesson from the Ductus Arteriosus. (Physiol. Pathol. Angiogenes. - Signal. Mech. Target. Ther.). [DOI] [Google Scholar]
- 21.Gifre-Renom L., Daems M., Luttun A., Jones E.A.V. Organ-specific endothelial cell differentiation and impact of microenvironmental cues on endothelial heterogeneity. Int. J. Mol. Sci. 2022;23:1477. doi: 10.3390/ijms23031477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennigs J.K., Matuszcak C., Trepel M., Körbelin J. Vascular endothelial cells: heterogeneity and targeting approaches. Cells. 2021;10:2712. doi: 10.3390/cells10102712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miloudi K., Oubaha M., Ménard C., Dejda A., Guber V., Cagnone G., et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc. Natl. Acad. Sci. USA. 2019;116:4538–4547. doi: 10.1073/pnas.1814711116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aird W.C. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:1–14. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jambusaria A., Hong Z., Zhang L., Srivastava S., Jana A., Toth P.T., et al. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 2020;9:1–32. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mojiri A., Alavi P., Lorenzana Carrillo M.A., Nakhaei-Nejad M., Sergi C.M., Thebaud B., et al. Endothelial cells of different organs exhibit heterogeneity in von Willebrand factor expression in response to hypoxia. Atherosclerosis. 2019;282:1–10. doi: 10.1016/j.atherosclerosis.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Gavard J., Gutkind J.S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 28.Lee W.L., Slutsky A.S. Sepsis and endothelial permeability. N. Engl. J. Med. 2010;363:689–691. doi: 10.1056/nejmcibr1007320. [DOI] [PubMed] [Google Scholar]
- 29.González-Mariscal L., Tapia R., Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta, Biomembr. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta, Biomembr. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Fan J., Ray P., Lu Y., Kaur G., Schwarz J.J., Wan L.Q. Cell chirality regulates intercellular junctions and endothelial permeability. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Endothelial barrier and its abnormalities in cardiovascular disease. Front. Physiol. 2015;6:1–11. doi: 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards M., Nwadozi E., Pal S., Martinsson P., Kaakinen M., Gloger M., et al. Claudin5 protects the peripheral endothelial barrier in an organ and vessel-type-specific manner. Elife. 2022;11 doi: 10.7554/eLife.78517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runkle E.A., Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannotta M., Trani M., Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Gavard J. Endothelial permeability and VE-cadherin. Cell Adhes. Migrat. 2014;8:158–164. doi: 10.4161/cam.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 38.Jain A., Pasare C. Innate control of adaptive immunity: beyond the three-signal paradigm. J. Immunol. 2017;198:3791–3800. doi: 10.4049/jimmunol.1602000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowarski R., Jackson R., Flavell R.A. The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell. 2017;168:362–375. doi: 10.1016/j.cell.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Ren J., Zhou T., Sekhar Pilli V.S., Phan N., Wang Q., Gupta K., et al. Novel paracrine functions of smooth muscle cells in supporting endothelial regeneration following arterial injury. Circ. Res. 2019;124:1253–1265. doi: 10.1161/CIRCRESAHA.118.314567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Z., Liu F., Blair R., Wang C., Yang H., Mudd J., et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021;11:8076–8091. doi: 10.7150/thno.61810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mussbacher M., Schossleitner K., Kral-Pointner J.B., Salzmann M., Schrammel A., Schmid J.A. More than just a monolayer: the multifaceted role of endothelial cells in the pathophysiology of atherosclerosis. Curr. Atherosclerosis Rep. 2022;24:483–492. doi: 10.1007/s11883-022-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput C., Sander L.E., Suttorp N., Opitz B. NOD-like receptors in lung diseases. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Wang L., Fang P., Sun Y., Jiang X., Wang H., et al. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J. Biol. Chem. 2018;293:11033–11045. doi: 10.1074/jbc.RA118.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Opitz B., Forster S., Hocke A.C., Maass M., Schmeck B., Hippenstiel S., et al. Nod1-Mediated endothelial cell activation by chlamydophila pneumoniae. Circ. Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 46.Xu S., Liu Z., Huang Y., Le K., Tang F., Huang H., et al. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-κB activation. Transl. Res. 2012;160:114–124. doi: 10.1016/j.trsl.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Yu M., Ma X., Jiang D., Wang L., Zhan Q., Zhao J. CXC chemokine ligand 5 (CXCL5) disrupted the permeability of human brain microvascular endothelial cells via regulating p38 signal. Microbiol. Immunol. 2021;65:40–47. doi: 10.1111/1348-0421.12854. [DOI] [PubMed] [Google Scholar]
- 48.Al-Soudi A., Kaaij M.H., Tas S.W. Endothelial cells: from innocent bystanders to active participants in immune responses. Autoimmun. Rev. 2017;16:951–962. doi: 10.1016/j.autrev.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Hamzeh-Cognasse H., Berthelot P., Tardy B., Pozzetto B., Bourlet T., Laradi S., et al. Platelet toll-like receptors are crucial sensors of infectious danger moieties. Platelets. 2018;29:533–540. doi: 10.1080/09537104.2018.1445842. [DOI] [PubMed] [Google Scholar]
- 50.Le K.T.T., Chu X., Jaeger M., Plantinga J.A., Matzaraki V., Withoff S., et al. Leukocyte-released mediators in response to both bacterial and fungal infections trigger IFN pathways, independent of IL-1 and TNF-α, in endothelial cells. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lio C.-W.J., McDonald B., Takahashi M., Dhanwani R., Sharma N., Huang J., et al. cGAS-STING signaling regulates initial innate control of cytomegalovirus infection. J. Virol. 2016;90:7789–7797. doi: 10.1128/JVI.01040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Testai L., Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9:1–13. doi: 10.3390/nu9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasmi A., Mujawdiya P.K., Noor S., Lysiuk R., Darmohray R., Piscopo S., et al. Polyphenols in metabolic diseases. Molecules. 2022;27:6280. doi: 10.3390/molecules27196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niewiadomska J., Gajek-Marecka A., Gajek J., Noszczyk-Nowak A. Biological potential of polyphenols in the context of metabolic syndrome: an analysis of studies on animal models. Biology (Basel) 2022;11:559. doi: 10.3390/biology11040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva LCRC e, David J.M., Borges R. dos SQ., Ferreira S.L.C., David J.P., Reis PS dos, et al. Determination of flavanones in orange juices obtained from different sources by HPLC/DAD. J. Anal. Methods Chem. 2014;2014:1–5. doi: 10.1155/2014/296838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan H.M., Elnagar M.R., Abdelrazik E., Mahdi M.R., Hamza E., Elattar E.M., et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer’s disease through modulation of autophagy, oxidative stress and tau expression: an experimental study. Front. Neuroanat. 2022;16 doi: 10.3389/fnana.2022.1012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elsawy H., Alzahrani A.M., Alfwuaires M., Abdel-Moneim A.M., Khalil M. Beneficial role of naringin against methotrexate-induced injury to rat testes: biochemical and ultrastructural analyses. Redox Rep. 2022;27:158–166. doi: 10.1080/13510002.2022.2101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salehi B., Fokou P., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng X., Zheng Y., He Y., Zhang J., Peng W., Su W. Microbial metabolism of naringin and the impact on antioxidant capacity. Nutrients. 2022;14:3765. doi: 10.3390/nu14183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stabrauskiene J., Kopustinskiene D.M., Lazauskas R., Bernatoniene J. Naringin and naringenin: their mechanisms of action and the potential anticancer activities. Biomedicines. 2022;10:1686. doi: 10.3390/biomedicines10071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bacanlı M., Başaran A.A., Başaran N. Elsevier; 2018. The major flavonoid of grapefruit: naringin; pp. 37–44. (Polyphenols Prev. Treat. Hum. Dis.). [DOI] [Google Scholar]
- 63.Bailey D.G., Dresser G.K., Leake B.F., Kim R.B. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin. Pharmacol. Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 64.Sutanto H., Hertanto D.M., Susilo H., Wungu C.D.K. Grapefruit flavonoid naringenin sex-dependently modulates action potential in an in silico human ventricular cardiomyocyte model. Antioxidants. 2022;11:1672. doi: 10.3390/antiox11091672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abedi F., Razavi B.M., Hosseinzadeh H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phyther. Res. 2020;34:729–741. doi: 10.1002/ptr.6573. [DOI] [PubMed] [Google Scholar]
- 66.Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:1–18. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Wu H., Nie Y., Chen J., Su W., Li P. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int. Immunopharm. 2011;11:1606–1612. doi: 10.1016/j.intimp.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Alam M.A., Subhan N., Rahman M.M., Uddin S.J., Reza H.M., Sarker S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014;5:404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frabasile S., Koishi A.C., Kuczera D., Silveira G.F., Verri W.A., Duarte dos Santos C.N., et al. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017;7 doi: 10.1038/srep41864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldwasser J., Cohen P.Y., Lin W., Kitsberg D., Balaguer P., Polyak S.J., et al. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J. Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tutunchi H., Naeini F., Ostadrahimi A., Hosseinzadeh‐Attar M.J. Naringenin, a flavanone with antiviral and anti‐inflammatory effects: a promising treatment strategy against <scp>COVID</scp> ‐19. Phyther. Res. 2020;34:3137–3147. doi: 10.1002/ptr.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agrawal P.K., Agrawal C., Blunden G. Naringenin as a possible candidate against SARS-CoV-2 infection and in the pathogenesis of COVID-19. Nat. Prod. Commun. 2021;16 doi: 10.1177/1934578X211066723. [DOI] [Google Scholar]
- 73.Hernández-Aquino E., Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J. Gastroenterol. 2018;24:1679–1707. doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tripoli E., Guardia M La, Giammanco S., Majo D Di, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 75.Guo X., Li K., Guo A., Li E. Intestinal absorption and distribution of naringin, hesperidin, and their metabolites in mice. J. Funct.Foods. 2020;74 doi: 10.1016/j.jff.2020.104158. [DOI] [Google Scholar]
- 76.Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004;24:851–874. doi: 10.1016/j.nutres.2004.07.005. [DOI] [Google Scholar]
- 77.Karim N., Jia Z., Zheng X., Cui S., Chen W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018;79:35–54. doi: 10.1016/j.tifs.2018.06.012. [DOI] [Google Scholar]
- 78.Yáñez J.A., Remsberg C.M., Miranda N.D., Vega-Villa K.R., Andrews P.K., Davies N.M. Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 2008;29:63–82. doi: 10.1002/bdd.588. [DOI] [PubMed] [Google Scholar]
- 79.Gelen V., Yıldırım S., Şengül E., Çınar A., Çelebi F., Küçükkalem M., et al. Naringin attenuates oxidative stress, inflammation, apoptosis, and oxidative DNA damage in acrylamide-induced nephrotoxicity in rats. Asian Pac. J. Trop. Biomed. 2022;12:223. doi: 10.4103/2221-1691.343390. [DOI] [Google Scholar]
- 80.Yadav B., Vishwakarma V., Kumar S., Aggarwal N.K., Gupta R., Yadav A. Ameliorative role of naringenin against lead‐induced genetic damage and oxidative stress in cultured human lymphocytes. J. Biochem. Mol. Toxicol. 2022;36 doi: 10.1002/jbt.23036. [DOI] [PubMed] [Google Scholar]
- 81.Shulman M., Cohen M., Soto-Gutierrez A., Yagi H., Wang H., Goldwasser J., et al. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chabane M.N., Ahmad A Al, Peluso J., Muller C.D., Ubeaud-Séquier G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2010;61:1473–1483. doi: 10.1211/jpp.61.11.0006. [DOI] [PubMed] [Google Scholar]
- 83.Németh K., Plumb G.W., Berrin J.G., Juge N., Jacob R., Naim H.Y., et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 84.Hernández-Aquino E., Muriel P. Naringenin and the liver. Liver Pathophysiol. Ther. Antioxidants. 2017:633–651. doi: 10.1016/B978-0-12-804274-8.00046-1. [DOI] [Google Scholar]
- 85.Chen R., Qi Q.-L., Wang M.-T., Li Q.-Y. Therapeutic potential of naringin: an overview. Pharm. Biol. 2016;54:3203–3210. doi: 10.1080/13880209.2016.1216131. [DOI] [PubMed] [Google Scholar]
- 86.Lin Z., Wang G., Gu W., Zhao S., Shen Z., Liu W., et al. Exploration of the protective mechanism of naringin in the acetaminophen-induced hepatic injury by metabolomics. Oxid. Med. Cell. Longev. 2022;2022:1–17. doi: 10.1155/2022/7138194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Won T., Wood M.K., Hughes D.M., Talor M.V., Ma Z., Schneider J., et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2022.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossouw T.M., Anderson R., Manga P., Feldman C. Emerging role of platelet-endothelium interactions in the pathogenesis of severe SARS-CoV-2 infection-associated myocardial injury. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.776861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022;23:73. doi: 10.31083/j.rcm2302073. [DOI] [PubMed] [Google Scholar]
- 90.Lee S.E., Park Y.S. The emerging roles of antioxidant enzymes by dietary phytochemicals in vascular diseases. Life. 2021;11:199. doi: 10.3390/life11030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ito F. Polyphenols can potentially prevent atherosclerosis and cardiovascular disease by modulating macrophage cholesterol metabolism. Curr. Mol. Pharmacol. 2020;14:175–190. doi: 10.2174/1874467213666200320153410. [DOI] [PubMed] [Google Scholar]
- 92.Lee C.-H., Jeong T.-S., Choi Y.-K., Hyun B.-H., Oh G.-T., Kim E.-H., et al. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001;284:681–688. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- 93.Chanet A., Milenkovic D., Deval C., Potier M., Constans J., Mazur A., et al. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012;23:469–477. doi: 10.1016/j.jnutbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 94.Gao Y., Wang Z., Zhang Y., Liu Y., Wang S., Sun W., et al. Naringenin inhibits NG-nitro-L-arginine methyl ester-induced hypertensive left ventricular hypertrophy by decreasing angiotensin-converting enzyme 1 expression. Exp. Ther. Med. 2018 doi: 10.3892/etm.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lv Y., Kim K., Sheng Y., Cho J., Qian Z., Zhao Y.-Y., et al. YAP controls endothelial activation and vascular inflammation through TRAF6. Circ. Res. 2018;123:43–56. doi: 10.1161/CIRCRESAHA.118.313143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao H., Liu M., Liu H., Suo R., Lu C. Naringin protects endothelial cells from apoptosis and inflammation by regulating the Hippo-YAP Pathway. Biosci. Rep. 2020;40 doi: 10.1042/BSR20193431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Malakul W., Pengnet S., Kumchoom C., Tunsophon S. Naringin ameliorates endothelial dysfunction in fructose-fed rats. Exp. Ther. Med. 2018 doi: 10.3892/etm.2018.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H., Zhou X., Zhong Y., Ji L., Yu W., Fang J., et al. Naringin suppressed airway inflammation and ameliorated pulmonary endothelial hyperpermeability by upregulating Aquaporin1 in lipopolysaccharide/cigarette smoke-induced mice. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.113035. [DOI] [PubMed] [Google Scholar]
- 99.Li H., Liu L., Cao Z., Li W., Liu R., Chen Y., et al. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKα/Sirt1 pathway. J. Adv. Res. 2021;34:137–147. doi: 10.1016/j.jare.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akintunde J.K., Akintola T.E., Hammed M.O., Amoo C.O., Adegoke A.M., Ajisafe L.O. Naringin protects against Bisphenol-A induced oculopathy as implication of cataract in hypertensive rat model. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110043. [DOI] [PubMed] [Google Scholar]
- 101.Feng J., Luo J., Deng L., Zhong Y., Wen X., Cai Y., et al. Naringenin-induced HO-1 ameliorates high glucose or free fatty acids-associated apoptosis via PI3K and JNK/Nrf2 pathways in human umbilical vein endothelial cells. Int. Immunopharm. 2019;75 doi: 10.1016/j.intimp.2019.105769. [DOI] [PubMed] [Google Scholar]
- 102.Heidary Moghaddam R., Samimi Z., Moradi S.Z., Little P.J., Xu S., Farzaei M.H. Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173535. [DOI] [PubMed] [Google Scholar]
- 103.Sashindranath M., Nandurkar H.H. Endothelial dysfunction in the brain. Stroke. 2021;52:1895–1904. doi: 10.1161/STROKEAHA.120.032711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quick S., Moss J., Rajani R.M., Williams A. A vessel for change: endothelial dysfunction in cerebral small vessel disease. Trends Neurosci. 2021;44:289–305. doi: 10.1016/j.tins.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Jana A., Wang X., Leasure J.W., Magana L., Wang L., Kim Y.-M., et al. Increased Type I interferon signaling and brain endothelial barrier dysfunction in an experimental model of Alzheimer’s disease. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 107.Nouri Fakhri, El-Senduny, Sanadgol, Abd-ElGhani, Farzaei, et al. On the neuroprotective effects of naringenin: pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules. 2019;9:690. doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmed S., Khan H., Aschner M., Hasan M.M., Hassan S.T.S. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110646. [DOI] [PubMed] [Google Scholar]
- 109.Ben-Azu B., Nwoke E.E., Aderibigbe A.O., Omogbiya I.A., Ajayi A.M., Olonode E.T., et al. Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed. Pharmacother. 2019;109:536–546. doi: 10.1016/j.biopha.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 110.Thayumanavan G., Jeyabalan S., Fuloria S., Sekar M., Ravi M., Selvaraj L.K., et al. Silibinin and naringenin against bisphenol A-induced neurotoxicity in zebrafish model—potential flavonoid molecules for new drug design, development, and therapy for neurological disorders. Molecules. 2022;27:2572. doi: 10.3390/molecules27082572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Youdim K.A., Dobbie M.S., Kuhnle G., Proteggente A.R., Abbott N.J., Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J. Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 112.Niu X., Sang H., Wang J. Naringenin attenuates experimental autoimmune encephalomyelitis by protecting the intact of blood-brain barrier and controlling inflammatory cell migration. J. Nutr. Biochem. 2021;89 doi: 10.1016/j.jnutbio.2020.108560. [DOI] [PubMed] [Google Scholar]
- 113.Singh N., Bansal Y., Bhandari R., Marwaha L., Singh R., Chopra K., et al. Naringin reverses neurobehavioral and biochemical alterations in intracerebroventricular collagenase-induced intracerebral hemorrhage in rats. Pharmacology. 2017;100:172–187. doi: 10.1159/000453580. [DOI] [PubMed] [Google Scholar]
- 114.Bai X., Zhang X., Chen L., Zhang J., Zhang L., Zhao X., et al. Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem. Res. 2014;39:1405–1415. doi: 10.1007/s11064-014-1326-y. [DOI] [PubMed] [Google Scholar]
- 115.Cui Y., Zhao W., Wang X., Chen M., Zhang L., Liu Z. Protective effects of Naringin in a rat model of spinal cord ischemia–reperfusion injury. Trop. J. Pharmaceut. Res. 2017;16:649. doi: 10.4314/tjpr.v16i3.21. [DOI] [Google Scholar]
- 116.Prabhakar O. Naringenin attenuates cerebral ischemia-reperfusion injury through inhibiting oxidative stress and inflammation in diabetic rats. Res. J. Pharm. Technol. 2021:3751–3756. doi: 10.52711/0974-360X.2021.00649. [DOI] [Google Scholar]
- 117.Wang L., Zhang Z., Wang H. Naringin attenuates cerebral ischemia-reperfusion injury in rats by inhibiting endoplasmic reticulum stress. Transl. Neurosci. 2021;12:190–197. doi: 10.1515/tnsci-2020-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao X., Aronowski J. Nrf2 to pre-condition the brain against injury caused by Products of hemolysis after ICH. Transl. Stroke Res. 2013;4:71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rong W., Wang J., Liu X., Jiang L., Wei F., Hu X., et al. Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem. Res. 2012;37:1615–1623. doi: 10.1007/s11064-012-0756-7. [DOI] [PubMed] [Google Scholar]
- 120.Han Y., Su J., Liu X., Zhao Y., Wang C., Li X. Naringin alleviates early brain injury after experimental subarachnoid hemorrhage by reducing oxidative stress and inhibiting apoptosis. Brain Res. Bull. 2017;133:42–50. doi: 10.1016/j.brainresbull.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 121.Gopinath K., Sudhandiran G. Protective effect of naringin on 3-nitropropionic acid-induced neurodegeneration through the modulation of matrix metalloproteinases and glial fibrillary acidic protein. Can. J. Physiol. Pharmacol. 2016;94:65–71. doi: 10.1139/cjpp-2015-0035. [DOI] [PubMed] [Google Scholar]
- 122.Wu L.-H., Lin C., Lin H.-Y., Liu Y.-S., Wu C.Y.-J., Tsai C.-F., et al. Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol. Neurobiol. 2016;53:1080–1091. doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- 123.Zhang B., Wei Y.-Z., Wang G.-Q., Li D.-D., Shi J.-S., Zhang F. Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell. Neurosci. 2019;12 doi: 10.3389/fncel.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long J., Chen J., Liao Y., Zhou Y., Liang B., Zhou Y. Naringin provides neuroprotection in CCL2-induced cognition impairment by attenuating neuronal apoptosis in the hippocampus. Behav. Brain Funct. 2020;16:4. doi: 10.1186/s12993-020-00166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Galic M.A., Riazi K., Pittman Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang R., Niu S., Rong Z., Li F., Ni L., Di X., et al. A potential target for diabetic vascular damage: high glucose-induced monocyte extracellular vesicles impair endothelial cells by delivering miR-142-5p. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang M., Li Y., Li S., Lv J. Endothelial dysfunction and diabetic cardiomyopathy. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.851941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sena C.M., Pereira A.M., Seiça R. Endothelial dysfunction — a major mediator of diabetic vascular disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 129.Cai Z., Yuan S., Zhong Y., Deng L., Li J., Tan X., et al. Amelioration of endothelial dysfunction in diabetes: role of takeda G protein–coupled receptor 5. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.637051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Basha B., Samuel S.M., Triggle C.R., Ding H. Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress? Exp. Diabetes Res. 2012;2012:1–14. doi: 10.1155/2012/481840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi Y., Vanhoutte P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes. 2017;9:434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 132.Johnstone M.T., Creager S.J., Scales K.M., Cusco J.A., Lee B.K., Creager M.A. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.CIR.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 133.Roberts A.C., Porter K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes Vasc. Dis. Res. 2013;10:472–482. doi: 10.1177/1479164113500680. [DOI] [PubMed] [Google Scholar]
- 134.Singh D.K., Winocour P., Farrington K. Review: endothelial cell dysfunction, medial arterial calcification and osteoprotegerin in diabetes. Br. J. Diabetes Vasc. Dis. 2010;10:71–77. doi: 10.1177/1474651409355453. [DOI] [Google Scholar]
- 135.Grassi D., Desideri G., Necozione S., Ruggieri F., Blumberg J.B., Stornello M., et al. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension. 2012;60:827–832. doi: 10.1161/HYPERTENSIONAHA.112.193995. [DOI] [PubMed] [Google Scholar]
- 136.Gero D. InTech; 2018. Hyperglycemia-induced endothelial dysfunction. (Endothel. Dysfunct. - Old Concepts New Challenges). [DOI] [Google Scholar]
- 137.Dhananjayan R., Koundinya K.S.S., Malati T., Kutala V.K. Endothelial dysfunction in type 2 diabetes mellitus. Indian J. Clin. Biochem. 2016;31:372–379. doi: 10.1007/s12291-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaur R., Kaur M., Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018;17:121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goldin A., Beckman J.A., Schmidt A.M., Creager M.A. Advanced glycation end products. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 140.Roy S., Ahmed F., Banerjee S., Saha U. Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-β1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events. Pharm. Biol. 2016;54:1616–1627. doi: 10.3109/13880209.2015.1110599. [DOI] [PubMed] [Google Scholar]
- 141.Ren B., Qin W., Wu F., Wang S., Pan C., Wang L., et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 142.Zaidun N.H., Sahema Z.C.T., Mardiana A.A., Santhana R.L., Latiff A.A., Syed Ahmad Fuad S.B. Effects of naringenin on vascular changes in prolonged hyperglycaemia in fructose-STZ diabetic rat model. Drug Discov. Ther. 2019;13:212–221. doi: 10.5582/ddt.2019.01034. [DOI] [PubMed] [Google Scholar]
- 143.Al-Rejaie S.S., Aleisa A.M., Abuohashish H.M., Parmar M.Y., Ola M.S., Al-Hosaini A.A., et al. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol. Res. 2015;37:924–933. doi: 10.1179/1743132815Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 144.Annadurai T., Muralidharan A.R., Joseph T., Hsu M.J., Thomas P.A., Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012;68:307–318. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- 145.Tsai S.-J., Huang C.-S., Mong M.-C., Kam W.-Y., Huang H.-Y., Yin M.-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012;60:514–521. doi: 10.1021/jf203259h. [DOI] [PubMed] [Google Scholar]
- 146.Adebiyi O.A., Adebiyi O.O., Owira P.M.O. Naringin reduces hyperglycemia-induced cardiac fibrosis by relieving oxidative stress. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li G., Xu Y., Sheng X., Liu H., Guo J., Wang J., et al. Naringin protects against high glucose-induced human endothelial cell injury via antioxidation and CX3CL1 downregulation. Cell. Physiol. Biochem. 2017;42:2540–2551. doi: 10.1159/000480215. [DOI] [PubMed] [Google Scholar]
- 148.Luo C., Ke X., Xiong S., Sun Y., Xu Q., Zhang W., et al. Naringin attenuates high glucose-induced injuries and inflammation by modulating the leptin-JAK2/STAT3 pathway in H9c2 cardiac cells. Arch. Med. Sci. 2019;17:1145–1157. doi: 10.5114/aoms.2019.84854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harrison D., Griendling K.K., Landmesser U., Hornig B., Drexler H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003;91:7–11. doi: 10.1016/S0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 151.Vozarova B., Weyer C., Hanson K., Tataranni P.A., Bogardus C., Pratley R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 152.He Y., Wang S., Sun H., Li Y., Feng J. Naringenin ameliorates myocardial injury in STZ-induced diabetic mice by reducing oxidative stress, inflammation and apoptosis via regulating the Nrf2 and NF-κB signaling pathways. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.946766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singh P., Bansal S., Kuhad A., Kumar A., Chopra K. Naringenin ameliorates diabetic neuropathic pain by modulation of oxidative-nitrosative stress, cytokines and MMP-9 levels. Food Funct. 2020;11:4548–4560. doi: 10.1039/C9FO00881K. [DOI] [PubMed] [Google Scholar]
- 154.Ding S., Qiu H., Huang J., Chen R., Zhang J., Huang B., et al. Activation of 20-HETE/PPARs involved in reno-therapeutic effect of naringenin on diabetic nephropathy. Chem. Biol. Interact. 2019;307:116–124. doi: 10.1016/j.cbi.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 155.Nguyen‐Ngo C., Willcox J.C., Lappas M. Anti‐diabetic, anti‐inflammatory, and anti‐oxidant effects of naringenin in an in vitro human model and an in vivo murine model of gestational diabetes mellitus. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201900224. [DOI] [PubMed] [Google Scholar]
- 156.Tsuhako R., Yoshida H., Sugita C., Kurokawa M. Naringenin suppresses neutrophil infiltration into adipose tissue in high-fat diet-induced obese mice. J. Nat. Med. 2020;74:229–237. doi: 10.1007/s11418-019-01332-5. [DOI] [PubMed] [Google Scholar]
- 157.Fu J., Yu M.G., Li Q., Park K., King G.L. Insulin’s actions on vascular tissues: physiological effects and pathophysiological contributions to vascular complications of diabetes. Mol. Metabol. 2021;52 doi: 10.1016/j.molmet.2021.101236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y., Yang S., Zhang M., Wang Z., He X., Hou Y., et al. Glycyrrhetinic acid improves insulin-response pathway by regulating the balance between the ras/MAPK and PI3K/Akt pathways. Nutrients. 2019;11:604. doi: 10.3390/nu11030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wen X., Zhang B., Wu B., Xiao H., Li Z., Li R., et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct. Targeted Ther. 2022;7:298. doi: 10.1038/s41392-022-01149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Natali A., Toschi E., Baldeweg S., Ciociaro D., Favilla S., Sacca L., et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 161.Mahmoud A.M., Ashour M.B., Abdel-Moneim A., Ahmed O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabet. Complicat. 2012;26:483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 162.Jung U.J., Lee M.-K., Jeong K.-S., Choi M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 163.Pu P., Gao D.-M., Mohamed S., Chen J., Zhang J., Zhou X.-Y., et al. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012;518:61–70. doi: 10.1016/j.abb.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 164.Qi Z., Xu Y., Liang Z., Li S., Wang J., Wei Y., et al. Naringin ameliorates cognitive deficits via oxidative stress, proinflammatory factors and the PPARγ signaling pathway in a type 2 diabetic rat model. Mol. Med. Rep. 2015;12:7093–7101. doi: 10.3892/mmr.2015.4232. [DOI] [PubMed] [Google Scholar]
- 165.Li S., Zhang Y., Sun Y., Zhang G., Bai J., Guo J., et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr. Diabetes. 2019;9:28. doi: 10.1038/s41387-019-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dayarathne L.A., Ranaweera S.S., Natraj P., Rajan P., Lee Y.J., Han C.-H. The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells. J. Vet. Sci. 2021:22. doi: 10.4142/jvs.2021.22.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Klein D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Akwii R.G., Sajib M.S., Zahra F.T., Mikelis C.M. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8:471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee W.L., Liles W.C. Endothelial activation, dysfunction and permeability during severe infections. Curr. Opin. Hematol. 2011;18:191–196. doi: 10.1097/MOH.0b013e328345a3d1. [DOI] [PubMed] [Google Scholar]
- 170.Franses J.W., Drosu N.C., Gibson W.J., Chitalia V.C., Edelman E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer. 2013;133:1334–1344. doi: 10.1002/ijc.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rahbari N.N., Kedrin D., Incio J., Liu H., Ho W.W., Nia H.T., et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf5219. 360ra135-360ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Memariani Z., Abbas S.Q., ul Hassan S.S., Ahmadi A., Chabra A. Naringin and naringeninin as anticancer agents and adjuvants in cancer combination therapy; efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.105264. [DOI] [PubMed] [Google Scholar]
- 173.Franses J.W. 2011. Regulatory Roles of Endothelial Cells in Cancer. [Google Scholar]
- 174.Franses J.W., Drosu N.C., Gibson W.J., Chitalia V.C., Edelman E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer. 2013;133:1334–1344. doi: 10.1002/ijc.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li Q., Wang Y., Zhang L., Chen L., Du Y., Ye T., et al. Naringenin exerts anti-angiogenic effects in human endothelial cells: involvement of ERRα/VEGF/KDR signaling pathway. Fitoterapia. 2016;111:78–86. doi: 10.1016/j.fitote.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 176.Choi J., Lee D.-H., Jang H., Park S.-Y., Seol J.-W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020;17:3049–3057. doi: 10.7150/ijms.44804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Camargo C.A., Gomes-Marcondes M.C.C., Wutzki N.C., Aoyama H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012;32:129–133. [PubMed] [Google Scholar]
- 178.Fontanella C., Ongaro E., Bolzonello S., Guardascione M., Fasola G., Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann. Transl. Med. 2014;2 doi: 10.3978/j.issn.2305-5839.2014.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matsumoto K., Ema M. Roles of VEGF-A signalling in development, regeneration, and tumours. J. Biochem. 2014;156:1–10. doi: 10.1093/jb/mvu031. [DOI] [PubMed] [Google Scholar]
- 180.Stein R.A., Gaillard S., McDonnell D.P. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009;114:106–112. doi: 10.1016/j.jsbmb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bulzomi P., Bolli A., Galluzzo P., Acconcia F., Ascenzi P., Marino M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life. 2012;64:690–696. doi: 10.1002/iub.1049. [DOI] [PubMed] [Google Scholar]
- 182.Totta P., Acconcia F., Leone S., Cardillo I., Marino M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor α and β signalling. IUBMB Life. 2004;56:491–499. doi: 10.1080/15216540400010792. [DOI] [PubMed] [Google Scholar]
- 183.Sabarinathan D., Vanisree A.J. Plausible role of naringenin against cerebrally implanted C6 glioma cells in rats. Mol. Cell. Biochem. 2013;375:171–178. doi: 10.1007/s11010-012-1539-9. [DOI] [PubMed] [Google Scholar]
- 184.Aroui S., Fetoui H., Kenani A. Natural dietary compound naringin inhibits glioblastoma cancer neoangiogenesis. BMC Pharmacol. Toxicol. 2020;21:46. doi: 10.1186/s40360-020-00426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gumushan Aktas H., Akgun T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed. Pharmacother. 2018;106:770–775. doi: 10.1016/j.biopha.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 186.Kawaguchi S., Kawahara K., Fujiwara Y., Ohnishi K., Pan C., Yano H., et al. Naringenin potentiates anti-tumor immunity against oral cancer by inducing lymph node CD169-positive macrophage activation and cytotoxic T cell infiltration. Cancer Immunol. Immunother. 2022;71:2127–2139. doi: 10.1007/s00262-022-03149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Q., Wang Y., Zhang L., Chen L., Du Y., Ye T., et al. Naringenin exerts anti-angiogenic effects in human endothelial cells: involvement of ERRα/VEGF/KDR signaling pathway. Fitoterapia. 2016;111:78–86. doi: 10.1016/j.fitote.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 188.Noori S., Nourbakhsh M., Imani H., Deravi N., Salehi N., Abdolvahabi Z. Naringenin and cryptotanshinone shift the immune response towards Th1 and modulate T regulatory cells via JAK2/STAT3 pathway in breast cancer. BMC Complement. Med. Ther. 2022;22:145. doi: 10.1186/s12906-022-03625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Arul D., Subramanian P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013;19:763–770. doi: 10.1007/s12253-013-9641-1. [DOI] [PubMed] [Google Scholar]
- 190.Ahamad M.S., Siddiqui S., Jafri A., Ahmad S., Afzal M., Arshad M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Askar M.A., El Shawi O.E., Abou zaid O.A.R., Mansour N.A., Hanafy A.M. Breast cancer suppression by curcumin-naringenin-magnetic-nano-particles: in vitro and in vivo studies. Tumor Biol. 2021;43:225–247. doi: 10.3233/TUB-211506. [DOI] [PubMed] [Google Scholar]
- 192.Curti V., Di Lorenzo A., Rossi D., Martino E., Capelli E., Collina S., et al. Enantioselective modulatory effects of naringenin enantiomers on the expression levels of miR-17-3p involved in endogenous antioxidant defenses. Nutrients. 2017;9 doi: 10.3390/nu9030215. [DOI] [Google Scholar]
- 193.Memariani Z., Abbas S.Q., ul Hassan S.S., Ahmadi A., Chabra A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021;171 doi: 10.1016/j.phrs.2020.105264. [DOI] [PubMed] [Google Scholar]
- 194.Kulawiak B., Bednarczyk P., Szewczyk A. Multidimensional regulation of cardiac mitochondrial potassium channels. Cells. 2021;10:1554. doi: 10.3390/cells10061554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Szteyn K., Singh H. BKCa channels as targets for cardioprotection. Antioxidants. 2020;9:760. doi: 10.3390/antiox9080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goswami S.K., Ponnalagu D., Hussain A.T., Shah K., Karekar P., Gururaja Rao S., et al. Expression and activation of BKCa channels in mice protects against ischemia-reperfusion injury of isolated hearts by modulating mitochondrial function. Front. Cardiovasc. Med. 2019;5 doi: 10.3389/fcvm.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Frankenreiter S., Bednarczyk P., Kniess A., Bork N.I., Straubinger J., Koprowski P., et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation. 2017;136:2337–2355. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 198.Brenner R., Peréz G.J., Bonev A.D., Eckman D.M., Kosek J.C., Wiler S.W., et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 199.Kampa R.P., Sęk A., Bednarczyk P., Szewczyk A., Calderone V., Testai L. Flavonoids as new regulators of mitochondrial potassium channels: contribution to cardioprotection. J. Pharm. Pharmacol. 2023;75:466–481. doi: 10.1093/jpp/rgac093. [DOI] [PubMed] [Google Scholar]
- 200.Kicinska A., Kampa R.P., Daniluk J., Sek A., Jarmuszkiewicz W., Szewczyk A., et al. Regulation of the mitochondrial BKCa channel by the citrus flavonoid naringenin as a potential means of preventing cell damage. Molecules. 2020;25:3010. doi: 10.3390/molecules25133010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kampa R.P., Kicinska A., Jarmuszkiewicz W., Pasikowska‐Piwko M., Dolegowska B., Debowska R., et al. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Dermatol. 2019;28:543–550. doi: 10.1111/exd.13903. [DOI] [PubMed] [Google Scholar]
- 202.Yang Y., Qi J., Zhang M., Chen P., Liu Y., Sun X., et al. The cardioprotective effects and mechanisms of naringenin in myocardial ischemia based on network pharmacology and experiment verification. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.954555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Uryash A., Mijares A., Flores V., Adams J.A., Lopez J.R. Effects of naringin on cardiomyocytes from a rodent model of type 2 diabetes. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.719268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Flori L., Albanese L., Calderone V., Meneguzzo F., Pagliaro M., Ciriminna R., et al. Cardioprotective effects of grapefruit IntegroPectin extracted via hydrodynamic cavitation from by-products of citrus fruits industry: role of mitochondrial potassium channels. Foods. 2022;11:2799. doi: 10.3390/foods11182799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bai B., Wang Y. 2013. Methods to Investigate the Role of SIRT1 in Endothelial Senescence; pp. 327–339. [DOI] [PubMed] [Google Scholar]
- 206.Man A.W.C., Li H., Xia N. The role of Sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Testai L., Piragine E., Piano I., Flori L., Da Pozzo E., Miragliotta V., et al. The citrus flavonoid naringenin protects the myocardium from ageing-dependent dysfunction: potential role of SIRT1. Oxid. Med. Cell. Longev. 2020;2020:1–15. doi: 10.1155/2020/4650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Alotaibi B.S., Ijaz M., Buabeid M., Kharaba Z.J., Yaseen H.S., Murtaza G. Therapeutic effects and safe uses of plant-derived polyphenolic compounds in cardiovascular diseases: a review. Drug Des. Dev. Ther. 2021;15:4713–4732. doi: 10.2147/DDDT.S327238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7 doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Reshef N., Hayari Y., Goren C., Boaz M., Madar Z., Knobler H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005;18:1360–1363. doi: 10.1016/j.amjhyper.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 211.Krga I., Corral-Jara K.F., Barber-Chamoux N., Dubray C., Morand C., Milenkovic D. Grapefruit juice flavanones modulate the expression of genes regulating inflammation, cell interactions and vascular function in peripheral blood mononuclear cells of postmenopausal women. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.907595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.D’Elia L., Dinu M., Sofi F., Volpe M., Strazzullo P., Bordoni A., et al. 100% Fruit juice intake and cardiovascular risk: a systematic review and meta-analysis of prospective and randomised controlled studies. Eur. J. Nutr. 2021;60:2449–2467. doi: 10.1007/s00394-020-02426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Constans J., Bennetau-Pelissero C., Martin J.-F., Rock E., Mazur A., Bedel A., et al. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin. Nutr. 2015;34:1093–1100. doi: 10.1016/j.clnu.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 214.Aschoff J.K., Riedl K.M., Cooperstone J.L., Högel J., Bosy-Westphal A., Schwartz S.J., et al. Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: a randomized cross-over study. Mol. Nutr. Food Res. 2016;60:2602–2610. doi: 10.1002/mnfr.201600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rebello C.J., Beyl R.A., Lertora J.J.L., Greenway F.L., Ravussin E., Ribnicky D.M., et al. Safety and pharmacokinetics of naringenin: a randomized, controlled, single‐ascending‐dose clinical trial. Diabetes Obes. Metabol. 2020;22:91–98. doi: 10.1111/dom.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bai Y., Peng W., Yang C., Zou W., Liu M., Wu H., et al. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zeng X., Su W., Zheng Y., He Y., He Y., Rao H., et al. Pharmacokinetics, tissue distribution, metabolism, and excretion of naringin in aged rats. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu D., Zhang G.-Q., Zhang T.-T., Jin B., Ma C. Pharmacokinetic comparisons of naringenin and naringenin-nicotinamide cocrystal in rats by LC-MS/MS. J. Anal. Methods Chem. 2020;2020:1–10. doi: 10.1155/2020/8364218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mark R., Lyu X., Ng K.R., Chen W.N. Gene source screening as a tool for naringenin production in engineered Saccharomyces cerevisiae. ACS Omega. 2019;4:12872–12879. doi: 10.1021/acsomega.9b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levisson M., Araya-Cloutier C., de Bruijn W.J.C., van der Heide M., Salvador López J.M., Daran J.-M., et al. Toward developing a yeast cell factory for the production of prenylated flavonoids. J. Agric. Food Chem. 2019;67:13478–13486. doi: 10.1021/acs.jafc.9b01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pereira-Caro G., Polyviou T., Ludwig I.A., Nastase A.-M., Moreno-Rojas J.M., Garcia A.L., et al. Bioavailability of orange juice (poly)phenols: the impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017 doi: 10.3945/ajcn.116.149898. [DOI] [PubMed] [Google Scholar]
- 222.Testai L., Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9:502. doi: 10.3390/nu9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Habauzit V., Verny M.-A., Milenkovic D., Barber-Chamoux N., Mazur A., Dubray C., et al. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: a randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2015;102:66–74. doi: 10.3945/ajcn.114.104646. [DOI] [PubMed] [Google Scholar]
- 225.Pereira R., Andrades N., Paulino N., Sawaya A., Eberlin M., Marcucci M., et al. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules. 2007;12:1352–1366. doi: 10.3390/12071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Targeted Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oggero S., Austin-Williams S., Norling L.V. The contrasting role of extracellular vesicles in vascular inflammation and tissue repair. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Soleti R., Andriantsitohaina R., Martinez M.C. Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch. Biochem. Biophys. 2018;644:57–63. doi: 10.1016/j.abb.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 229.Li Q., Liu S., Yang G., Li M., Qiao P., Xue Q. Naringenin inhibits autophagy and epithelial‐mesenchymal transition of human lens epithelial cells by regulating the Smad2/3 pathway. Drug Dev. Res. 2021 doi: 10.1002/ddr.21868. ddr. [DOI] [PubMed] [Google Scholar]
- 230.Li W.-S., Lin S.-C., Chu C.-H., Chang Y.-K., Zhang X., Lin C.-C., et al. The gastroprotective effect of naringenin against ethanol-induced gastric ulcers in mice through inhibiting oxidative and inflammatory responses. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cao H., Liu J., Shen P., Cai J., Han Y., Zhu K., et al. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J. Agric. Food Chem. 2018;66:13133–13140. doi: 10.1021/acs.jafc.8b03942. [DOI] [PubMed] [Google Scholar]
- 232.Jia B., Yu D., Yu G., Cheng Y., Wang Y., Yi X., et al. Naringenin improve hepatitis C virus infection induced insulin resistance by increase PTEN expression via p53-dependent manner. Biomed. Pharmacother. 2018;103:746–754. doi: 10.1016/j.biopha.2018.04.110. [DOI] [PubMed] [Google Scholar]
- 233.Nzuza S., Zondi S., Owira P.M.O. Naringin prevents HIV-1 protease inhibitors-induced metabolic complications in vivo. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee J., Lee S., Zhang H., Hill M.A., Zhang C., Park Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tryggestad J.B., Shah R.D., Braffett B.H., Bacha F., Gidding S.S., Gubitosi‐Klug R.A., et al. Circulating adhesion molecules and associations with <scp>HbA1c</scp> , hypertension, nephropathy, and retinopathy in the Treatment Options for type 2 Diabetes in Adolescent and Youth study. Pediatr. Diabetes. 2020;21:923–931. doi: 10.1111/pedi.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qi J., Wu Q., Cheng Q., Chen X., Zhu M., Miao C. High glucose induces endothelial COX2 and iNOS expression via inhibition of monomethyltransferase SETD8 expression. J. Diabetes Res. 2020;2020:1–10. doi: 10.1155/2020/2308520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Suganya N., Bhakkiyalakshmi E., Sarada D.V.L., Ramkumar K.M. Reversibility of endothelial dysfunction in diabetes: role of polyphenols. Br. J. Nutr. 2016;116:223–246. doi: 10.1017/S0007114516001884. [DOI] [PubMed] [Google Scholar]
- 239.Suryavanshi S.V., Kulkarni Y.A. NF-κβ: a potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rathjen T., Kunkemoeller B., Cederquist C.T., Wang X., Lockhart S.M., Patti J.C., et al. Endothelial cell insulin signaling regulates CXCR4 (C-X-C motif chemokine receptor 4) and limits leukocyte adhesion to endothelium. Arterioscler. Thromb. Vasc. Biol. 2022;42 doi: 10.1161/ATVBAHA.122.317476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pan X., Kaminga A.C., Kinra S., Wen S.W., Liu H., Tan X., et al. Chemokines in type 1 diabetes mellitus. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.690082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chang T.-T., Chen C., Chen J.-W. CCL7 as a novel inflammatory mediator in cardiovascular disease, diabetes mellitus, and kidney disease. Cardiovasc. Diabetol. 2022;21:185. doi: 10.1186/s12933-022-01626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang J., Liu Z. Mechanistic pathogenesis of endothelial dysfunction in diabetic nephropathy and retinopathy. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nedosugova L.V., Markina Y.V., Bochkareva L.A., Kuzina I.A., Petunina N.A., Yudina I.Y., et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines. 2022;10:1168. doi: 10.3390/biomedicines10051168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript is a review manuscript.