Abstract
Wireworms and white grubs are destructive underground pests in maize fields in China. Cyantraniliprole has good control effect on coleoptera pests. Here, we evaluated the toxicity of cyantraniliprole to the second instar larvae of Anomala corpulenta Motschulsky and third-instar of larvae of Pleonomus canaliculatus Faldermann and the effects of sublethal concentrations on the activity of antioxidant and detoxification enzymes. We also explored the efficacy of cyantraniliprole on underground pests under indoor and field conditions. The LC50 of cyantraniliprole for the third instar larvae of P. canaliculatus was 23.3712 mg/L, and that for the second instar larvae of A. corpulenta was 5.9715 mg/L. Cyantraniliprole can activate the activity of superoxide dismutase (SOD), peroxidase (POD), and glutathione S-transferase (GST) to different degrees at a sublethal dose. According to the pot experiment and the control efficacy test in the field, the indoor control effect of cyantraniliprole seed treatment on P. canaliculatus and white grubs was approximately 80%, and the maximum increase in yield achieved through cyantraniliprole application was approximately 15% in the field efficacy test. Cyantraniliprole has a strong control effect on wireworms and white grubs, so it can be used to treat seeds to control underground pests in maize fields.
Keywords: Cyantraniliprole, Wireworms, White grubs, Seed treatment, Underground pest control
1. Introduction
Maize (Zea mays L.) is an important food and feed crop in China [1]. The total planting area and output of maize in China are second only to rice and wheat, which together are the world's three major food crops [2]. In recent years, the consumption of irrigation water and the planting area of maize have increased. This, coupled with the warming of the climate and changes in the farming system (e.g. zero tillage technology, straw counters, field rotation mode), creates highly suitable conditions for the survival of underground pests, increases the degree of crop damage, and reduces crop yield and quality space [3,4]. Common underground pests include wireworms, white grubs, Agrotis ipsilon Hufnagel, and mole crickets [[5], [6], [7]]. White grubs are larvae in the family Scarabaeidae and the order Coleoptera [8]. They are some of the most widely distributed and most harmful underground pests. There are more than 10 species of white grubs that damage crops in China, and the dominant species include Holotrichia oblita Faldermann, Holotrichia parallela Motschulsky, and Anomala corpulenta Motschulsky [9]. White grubs have a global distribution, and they mainly induce damage to crops, flowers, and trees and consume underground roots and seeds [10]. Wireworms (Coleoptera: Elateroidea) are also globally distributed underground pests that can damage maize, wheat, and other crops as well as forests and pastures [11]. In the larval stage, wireworms live in the soil. They feed on newly sown seeds, preventing them from germinating, as well as the roots and underground stems of plants, which causes the seedlings to wither and die [12]. There are a variety of wireworm species known to damage crops, and the main species in China include Pleonomus canaliculatus Faldermann, Agriotes fuscicollis Miwa, and Selatosomus latus Fabricius [13].
The main insecticides used to control wireworms and white grubs include neonicotinoids, organophosphates, and pyrethroids [[14], [15], [16]]. However, the widespread use of pesticides in recent years has led to several problems, such as the evolution of resistance to traditional insecticides in insects as well as environmental contamination [[17], [18], [19]]. Underground pest control mainly included seed treatment, soil treatment, direct spraying and other application methods. Seed treatment has the advantages of less environmental pollution, reducing the amount of application. There is thus an urgent need to identify highly effective and less toxic insecticides that could be used to control underground pests. Cyantraniliprole is a new diamide insecticide developed by DuPont (Wilmington, USA) from chlorantraniliprole that mainly acts on the ryanodine receptor of insects [20]. Cyantraniliprole can be used to control lepidopteran, homopteran, coleopteran, and dipteran pests through a variety of application methods [21,22]. Cyantraniliprole has been applied in seed treatments and soil mixture treatments to control the underground pests of food crops [23,24]. Syngenta registered cyantraniliprole as a flowable concentrate for seed treatment (FS) in 2020 for the control of A. ipsilon in maize fields. Therefore, cyantraniliprole could be used to control underground pests in maize fields.
The aim of this experiment was to characterize the control effect of cyantraniliprole seed treatment on pests in maize fields. Two underground pests, white grubs and wireworms, which are common and seriously harmful in maize fields, were selected for study. Two years of field and laboratory efficacy tests were conducted, including safety tests of cyantraniliprole maize seed treatment, as well as acute toxicity and sublethal physiological and biochemical tests on wireworms and white grubs. Finally, this study provides data support and theoretical guidance for pesticide screening for underground pest control under maize fields, and ultimately ensures the safety of corn production and improves the yield and quality of maize.
2. Materials and methods
2.1. Chemicals, soils, and maize seeds
Cyantraniliprole (94% purity) was obtained from Dupont Agrochemical Co. Ltd. (Shanghai, China), and 19% cyantraniliprole suspending agent (SC) was purchased from FMC Corporation. 600 g/L imidacloprid flowable concentrate for seed coating (FS) was provided by Bayer CropScience LP (Monheim, Germany). The indoor pot soil of maize was collected from the experimental field of Huang-Huai-Hai Regional Maize Technology Innovation Center (36°11′41″N, 117°07′7″E; 151 masl). Sandy loam soil (0–20 cm depth) that had not been previously treated with pesticides was collected, and impurities were removed. After air-drying, the soil was passed through a 60-mesh sieve and mixed with quartz sand at a 3:1 ratio for subsequent use.
The maize seeds of the ‘Denghai 605' (DH605) hybrid line were provided by Shandong Denghai Seeds Co, Ltd. Before the experiment, maize seeds with full grains and uniform sizes were coated with different concentrations of insecticides. Diluted insecticide was poured with water into a plastic bag containing 1 kg of maize seeds (minsecticide: mseed = 3:100) to coat the seeds. The bags were inflated and shaken by hand for 3 min until the pesticide evenly covered the surface of maize seeds. Then poured out, and the maize seeds (covered) were placed in the shade to dry.
2.2. Insect breeding
Larvae of Pleonomus canaliculatus were originally collected from a maize field in Ningyang City (35.76° N, 116.80° E), Shandong Province, China in 2018. Larvae of Anomala corpulenta were obtained from a peanut field in Nanyang City, Henan Province (33.27° N, 113.00° E). Larvae were kept in a feeding box (55 cm × 35 cm × 25 cm); the soil moisture was 15–18%, and the soil depth was 15 cm. P. canaliculatus was reared at 20 ± 1 °C and 40–50% relative humidity (RH) in the absence of light. A. corpulenta was reared at 25 ± 1 °C and 60–80% RH in the absence of light in indoor simulated field conditions. P. canaliculatus was fed wheat, and A. corpulenta was fed potato tubers.
2.3. Cyantraniliprole concentrations
A stock solution of cyantraniliprole (2000 mg/L) was prepared by dissolving 0.2128 g of cyantraniliprole in 1 L of acetone, and the stock solution was diluted with 0.05% Triton X-100 aqueous solution to a series of test concentrations. The control consisted of 0.05% Triton X-100 aqueous solution. The diluent of the stock solution was used for acute toxicity tests and subacute physiological and biochemical tests. The doses of subacute physiological and biochemical tests were LC5, LC15, LC25, and LC45. In the indoor safety tests, the doses were set according to the Guidelines for the Crop Safety Evaluation of Pesticides NY/T 1965.3–2013 [25]. The experimental doses were 1, 1.5, 2, and 2.5 times the maximum field dose, which were 4, 6, 8, and 10 g a.i./kg seed, respectively. The treatment without pesticide was the control. The concentrations of pesticides in the indoor and field control experiments were 1, 2, 3, and 4 g a.i./kg seed of cyantraniliprole (19% SC), and 4 g a.i./kg seed of imidacloprid (600 g/L FS) in 2019 and 3 and 4 g a.i./kg seed in 2020. Uncoated maize was used as a control.
2.4. Bioassay
The indoor toxicity of cyantraniliprole to the third instar larvae of P. canaliculatus was determined by the immersion method, following the Pesticides Guidelines for Laboratory Bioactivity Tests NY/T1154.6–2006 [26] with modifications according to Li et al. [27]. The method of Li et al. was used for the second instar larvae of A. corpulenta [28]. The test larvaes with uniform size were selected and put into the insect impregnator, impregnated in the liquid medicine for 30 s, then put on the filter paper to crawl and dry on their own, then put into the sterilized glass insect tube with a diameter of 1.8 cm and a height of 8 cm, and feed the test insects with newly germinated wheat seeds. There were 4 replicates per concentration and 20 larvae per replicate. After 3 and 5 days of treatment, the death of the larvae was examined, and larvae that could not crawl after being touched on the back with tweezers were considered dead. Dead insects were counted, and the mortality rate and LC50 were calculated.
2.5. Enzyme assay
To prepare the enzyme solution, three of the test insects was placed in a pre-cooled glass homogenizer with 5 mL of 0.1 mol/L phosphate buffer (pH = 7.8) and ground in an ice bath. The extract was then placed in a 2 mL centrifuge tube for centrifugation at 15,000 rpm/min at 4 °C for 20 min; the supernatant was subsequently used as the enzyme source. Each treatment was set to three repetitions.
According to the procedure of Song et al. [29], superoxide dismutase (SOD) activity was assessed by measuring the amount required to induce reduced nitrogen blue tetrazole. The absorbance of each treatment was measured at 560 nm using a microplate reader (EPOCH2). Peroxidase (POD) enzyme activity was determined following the method of Simon et al. [30] using the Peroxidase kit (POD-1-Y) of Suzhou Keming Biotechnology Co, Ltd. The absorbance was measured at 470 nm at 1 and 2 min with a microplate reader. Glutathione S-transferase (GST) activity was determined following the method of Oppenoorth et al. [31] using the glutathione S-transferase (GST) kit (GST-1-W) from Suzhou Keming Biotechnology Co, Ltd. Reagents were added per the instructions, and the absorbance values were measured at 340 nm at 10 and 310 s using a microplate reader.
2.6. Phytotoxicity test
The sand culture method [32] was used to evaluate the effect of cyantraniliprole on seed germination. Washed and high-temperature sterilized quartz sand was placed into a plastic crisper (40 cm × 20 cm × 15 cm) with an appropriate amount of sterilized and deionized water and pressed flat. Evenly coated maize seeds were then placed on the surface of quartz sand with the endosperm facing upward; 30 seeds were sown per box, and the seeds were covered by 1 cm of quartz sand and pressed flat. Each treatment consisted of four boxes with a total of 120 granules. Each box was placed in an artificial climate incubator at temperatures of 15 °C, 20 °C, and 25 °C. The germination potential of maize was measured on the 4th day after sowing, and the germination rate, plant height, and root length of maize were measured on the 7th day after sowing.
The soil culture method was used to assess the effect of cyantraniliprole on the growth of maize seedlings. The prepared soil was placed into a plastic flowerpot with a diameter of 25 cm. Five seeds were sown per pot; there were 20 seeds per replicate, and four replicates per treatment. Each pot was placed in a controlled greenhouse at 15 °C, 20 °C, and 25 °C. The plant height, root length, root number, aboveground fresh weight, and underground fresh weight of maize were measured at the third leaf stage.
2.7. Determination of the control effect of cyantraniliprole on P. canaliculatus and A. corpulenta under indoor potted culture
This experiment was conducted in the sunroom of Shandong Agricultural University. The prepared soil was placed into a plastic flowerpot with a diameter of 25 cm and a height of 30 cm, and each pot was sown with five seeds. The test conditions under normal watering management were as follows: temperature, 25 ± 1 °C; RH, 70–80%, and light: dark photoperiod, 14 h light:10 h dark. At 5 and 15 days after sowing, the second instar larvae of A. corpulenta and P. canaliculatus were placed five larvae in each pot. Each replicate had six pots, and each treatment had four replicates. Five days later, the number of dead A. corpulenta and P. canaliculatus was determined, and the mortality and control effect were calculated by Abbott (925).
2.8. Control effect of cyantraniliprole on underground pests under field conditions
The effectiveness of cyantraniliprole for controlling underground pests in the field was assessed through field experiments conducted on June 17, 2019 and June 14, 2020 in Tai'a City, Ningyang County, Shandong Province. At the field site, a perennial wheat-maize, single-crop rotation is employed, various types of weeds are present, and underground pests are abundant. The main underground pests at this site are wireworms and white grubs. A handheld single-seed seeder was used for seeding; the spacing between plants was 20 cm, and the spacing between rows was 30 cm. There were four replicates (each 30 m2) per treatment. A randomized block design was used, and protection lines were set between the plots. On the 3rd, 7th, 14th, and 21st days after emergence, the number of damaged plants was determined from 30 randomly selected maize plants from each plot. A damaged plant was defined as one where the roots or stems have been eaten or the plant has withered. The rate of damaged plants and the control effect were then calculated. The yield of the plot was measured, and the increase in the yield associated with cyantraniliprole application was calculated at harvest.
2.9. Data analysis
Probit statistical analysis was carried out in SPSS 20.0 (Standard Version 20.0, SPSS Inc.) to obtain the regression equation of toxicity. Enzyme activities were expressed as the mean ± standard deviation (SD) (n = 3) by LSD and calculated using SPSS software and Origin 2019 (P < 0.05). Tukey HSD test were used to evaluate the results of the maize safety test and underground pest control effect (P < 0.05), SPSS 20.0 was used to conduct analysis the data were presented as mean ± SD (n = 4).
3. Results
3.1. Toxicity of cyantraniliprole to P. canaliculatus and A. corpulenta
Cyantraniliprole is highly toxic to the third instar larvae of P. canaliculatus and the second instar larvae of A. corpulenta (Table 1). At the 3rd day of treatment, the LC50 of the third instar larvae of P. canaliculatus was 36.6035 mg/L, and at the 5th day of treatment, the LC50 was 23.3712 mg/L. The LC50 for the second instar larvae of A. corpulenta was 13.7839 mg/L at the 3rd day of treatment and 5.9715 mg/L at the 5th day of treatment.
Table 1.
Toxicity of cyantraniliprole to the third instar Pleonomus canaliculatus and 2nd-instar of Anomala corpulenta (Immersion method).
| Insecticide | Name of pests | Treatment time/d | Toxicity equation | LC50 mg/L | 95% Confidence interval/mg/L | LC90 mg/L | 95% Confidence interval/mg/L | Correlation coefficient |
|---|---|---|---|---|---|---|---|---|
| Cyantraniliprole | 3rd-instar of Pleonomus canaliculatus | 3 | y = 1.2915x+2.9807 | 36.6035 | 35.0893–38.1794 | 359.5744 | 317.8723–406.7473 | 0.9776 |
| 5 | y = 1.5992x+2.8114 | 23.3712 | 20.4048–26.7684 | 147.9534 | 111.0939–197.0423 | 0.9859 | ||
| 2nd-instar of Anomala corpulenta | 3 | y=1.3593x+3.4512 | 13.7839 | 12.5074–15.1906 | 120.8235 | 96.2223–151.71460 | 0.9864 | |
| 5 | y=1.4523x+3.8729 | 5.9715 | 5.3016–6.7263 | 45.5534 | 39.9579–51.9325 | 0.9907 |
LC50: Lethal medium concentration.
3.2. Effect of cyantraniliprole on the activity of antioxidant and detoxification enzymes
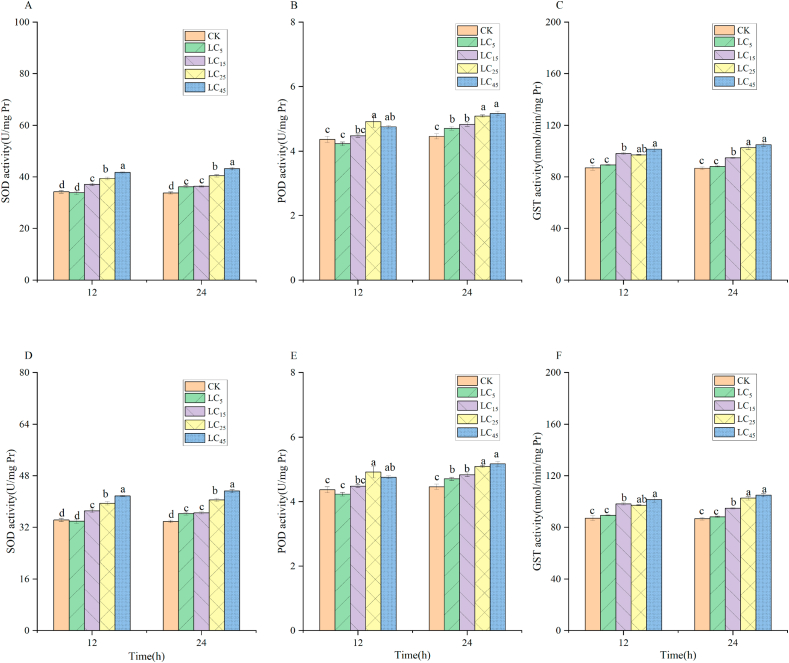
Fig. 1 (A,B,C) shows the effects of sublethal cyantraniliprole concentrations on the activity of SOD, POD, and GST in P. canaliculatus. Cyantraniliprole can significantly increase the activity of the three enzymes. At 12 h, there was no significant difference in the activity of the three enzymes between the LC5 dose and control. At the LC15, LC25, and LC45 doses, the activity of all three enzymes was enhanced throughout the treatment period. The effect of cyantraniliprole on the activity of the three enzymes in P. canaliculatus was enhanced as the concentration increased (LC15, LC25, and LC45 doses). Fig. 1 (D,E,F) shows the effects of sublethal cyantraniliprole concentrations on the activity of SOD, POD, and GST in the third instar larvae of A. corpulenta. The effects of cyantraniliprole on the activity of the three enzymes in the third instar larvae of A. corpulenta during the entire treatment period were similar to those observed in P. canaliculatus. The dose of cyantraniliprole affected the activity of the three enzymes during the entire treatment period.
Fig. 1.
Activity of the protective enzymes, (A,D) superoxide dismutase (SOD) and (B,E) peroxidase (POD), and the detoxification enzyme (C,F) glutathione S-transferase (GST) in the third instar larvae of P. canaliculatus and second instar larvae of A. corpulenta (mean ± SD, n = 3) after exposure to low lethal concentrations of cyantraniliprole. Bars with the same lowercase letters indicate no significant differences (LSD test, P < 0.05, n = 3). (CK: 0.05% Triton X-100 aqueous solution, LC5: 5% Lethal concentration, LC15: 15% Lethal concentration, LC25: 25% Lethal concentration, LC45: 45% Lethal concentration).
3.3. Phytotoxicity test of cyantraniliprole seed treatment
The indoor sand culture test (Table 2) showed that the cyantraniliprole seed treatment had no significant effect on the germination potential and germination rate of maize under each dose under all temperatures tested (15 °C, 20 °C, and 25 °C), and no delayed germination was observed compared with the control. Cyantraniliprole seed treatment at a dose of 4–8 g a.i./kg seed significantly increased the plant height and root length of maize compared with the control, and this treatment had no adverse effect on the germination and growth of maize. The indoor soil culture test (Table 3) revealed that cyantraniliprole seed treatment had no adverse effects on plant height, root length, root number, and other indexes, but its promoting effects on plant traits varied. Compared with the control, plant height, root length, root number, aboveground fresh weight, and underground fresh weight were significantly increased by the 4–8 g a.i./kg seed doses.
Table 2.
Effect of 19% cyantraniliprole SC seed treatment on seed germination of maize at 15 °C, 20 °C and 25 °C (Indoor sand culture) (n = 4).
| Temperature °C | Dose g a.i./kg seed | Germination energy % | Germination rate % | Stem height cm | Root length cm |
|---|---|---|---|---|---|
| 15 | 4 | 55.56 ± 2.94a | 91.67 ± 2.2a | 5.25 ± 0.15c | 6 ± 0.29 ab |
| 6 | 50 ± 3.33a | 90.83 ± 2.2a | 5.5 ± 0.06b | 6.1 ± 0.24 ab | |
| 8 | 52.22 ± 2.22a | 93.33±3a | 5.87 ± 0.21a | 6.35 ± 0.16a | |
| 10 | 51.11 ± 4.44a | 91.67 ± 3.63a | 5.19 ± 0.05c | 5.92 ± 0.16 ab | |
| CK | 54.44 ± 4.44a | 92.5 ± 1.44a | 5.27 ± 0.12c | 5.66 ± 0.19b | |
| 20 | 4 | 73.33 ± 8.39a | 95 ± 1.44a | 7.82 ± 0.07b | 8.3 ± 0.12b |
| 6 | 74.44 ± 2.94a | 93.33 ± 2.2a | 8.1 ± 0.08a | 8.48 ± 0.2b | |
| 8 | 70 ± 3.33a | 94.17 ± 0.83a | 8.12 ± 0.1a | 9.05 ± 0.24a | |
| 10 | 72.22 ± 4.84a | 92.5 ± 1.44a | 7.7 ± 0.15bc | 8.52 ± 0.09b | |
| CK | 72.22 ± 2.94a | 94.17 ± 2.2a | 7.57 ± 0.12c | 7.65 ± 0.27c | |
| 25 | 4 | 91.11 ± 2.94a | 94.17 ± 0.83a | 9.45 ± 0.05b | 11.5 ± 0.21 ab |
| 6 | 88.89 ± 2.94a | 93.33 ± 2.2a | 9.78 ± 0.11a | 11.93 ± 0.17a | |
| 8 | 93.33 ± 3.85a | 95 ± 2.89a | 9.74 ± 0.1a | 12.02 ± 0.24a | |
| 10 | 92.22 ± 2.22a | 95.83±3a | 9.36 ± 0.07b | 11.6 ± 0.13 ab | |
| CK | 90 ± 1.92a | 95.83 ± 2.5a | 9.09 ± 0.16c | 11.1 ± 0.18b |
SC: Suspension concentrate.
Different letters: Significant difference in p < 0.05. There are significant differences among different concentrations at the same temperature.
Table 3.
Effect of 19% cyantraniliprole SC seed treatment on the growth of maize seedlings at 15 °C, 20 °C and 25 °C (Indoor soil culture) (n = 4).
| Temperature/°C | Dose g a.i./kg seed | Stem height cm | Root length cm | Root number | Ground fresh weight g | Underground fresh weight g |
|---|---|---|---|---|---|---|
| 15 | 4 | 18.68 ± 0.25a | 17.14 ± 0.29 ab | 4.04 ± 0.19bc | 1.26 ± 0.13b | 1.15 ± 0.06 ab |
| 6 | 18.36 ± 0.27a | 17.01 ± 0.37 ab | 4.77 ± 0.25a | 1.32 ± 0.04 ab | 1.23 ± 0.07a | |
| 8 | 18.75 ± 0.25a | 17.64 ± 0.26a | 4.8 ± 0.35a | 1.42 ± 0.1a | 1.22 ± 0.05a | |
| 10 | 18.03 ± 0.38a | 17.06 ± 0.2 ab | 4.34 ± 0.11 ab | 1.26 ± 0.08b | 1.13 ± 0.1 ab | |
| CK | 17.7 ± 0.28b | 16.44 ± 0.22b | 3.58 ± 0.09c | 1.2 ± 0.08b | 1.04 ± 0.15b | |
| 20 | 4 | 20.83 ± 0.26 ab | 21.62 ± 0.26a | 5.09 ± 0.31 ab | 1.42 ± 0.05a | 1.34 ± 0.11 ab |
| 6 | 21.88 ± 0.24a | 21.72 ± 0.34a | 4.77 ± 0.18 ab | 1.45 ± 0.06a | 1.45 ± 0.07a | |
| 8 | 21.91 ± 0.35a | 21.44 ± 0.49 ab | 5.17 ± 0.04a | 1.5 ± 0.14a | 1.42 ± 0.12a | |
| 10 | 21.23 ± 0.32a | 21.33 ± 0.35 ab | 4.82 ± 0.27 ab | 1.4 ± 0.06a | 1.4 ± 0.1a | |
| CK | 20.03 ± 0.37b | 20.35 ± 0.15b | 4.4 ± 0.31b | 1.26 ± 0.08b | 1.24 ± 0.07b | |
| 25 | 4 | 23.38 ± 0.56 ab | 21.52 ± 0.25b | 5.31 ± 0.15 ab | 1.45 ± 0.05b | 1.45 ± 0.08 ab |
| 6 | 24.35 ± 0.22a | 21.42 ± 0.18b | 5.55 ± 0.19a | 1.53 ± 0.04a | 1.49 ± 0.09 ab | |
| 8 | 24.75 ± 0.23a | 22.21 ± 0.19a | 5.73 ± 0.18a | 1.47 ± 0.05a | 1.53 ± 0.04a | |
| 10 | 23.62 ± 0.27 ab | 20.98 ± 0.27b | 5.43 ± 0.12a | 1.49 ± 0.08a | 1.48 ± 0.18 ab | |
| CK | 22.76 ± 0.39b | 20.88 ± 0.25b | 4.89 ± 0.13b | 1.32 ± 0.01b | 1.34 ± 0.06b |
3.4. Control effect of cyantraniliprole on P. canaliculatus and A. corpulenta under indoor potted culture
After cyantraniliprole seed treatment at doses of 1, 2, 3, and 4 g a.i./kg seed, the indoor potted maize was infested for 5 days, and the control effects on the P. canaliculatus were 75.66%, 80.92%, 85.53%, and 89.47%. The control pesticide imidacloprid at a dose of 4 g a.i./kg seed had a control effect of 90.13%. After 15 days of indoor potting, the control effects at doses of 1, 2, 3, and 4 g a.i./kg were 64.71%, 72.55%, 75.82%, and 83.66%, respectively. The control effect of imidacloprid was 81.7% at a dose of 4 g a.i./kg seed (Table 4). Under cyantraniliprole seed treatment at 1, 2, 3, and 4 g a.i./kg 5 days after indoor potting, the control effects on the second instar larvae of A. corpulenta were 66.15%, 79.82%, 87.24%, and 92.76%, respectively. The control effect of imidacloprid was 88.79% at a dose of 4 g a.i./kg seed. After 15 days of indoor pot planting, the control effects at doses of 1, 2, 3, and 4 g a.i./kg were 57.44%, 69.37%, 79.94%, and 83.25%, respectively, and the control effect of imidacloprid was 86.07%. The control effect increased as the seed dressing dose increased.
Table 4.
Indoor control efficacy of cyantraniliprole seed treatment on third instar Pleonomus canaliculatus and 2nd-instar of Anomala corpulenta (n = 4).
| Insecticide | Name of pests | Dose g a.i./kg seed | 5th day after sowing |
15th day after sowing |
||
|---|---|---|---|---|---|---|
| 5th day after Inoculation pest |
5th day after Inoculation pest |
|||||
| Mortality rate/% | Control efficiency/% | Mortality rate/% | Control efficiency/% | |||
| 19% Cyantraniliprole SC | 3rd-instar Pleonomus canaliculatus | 1 | 68.92 ± 1.95c | 66.15 ± 1.92c | 60.59 ± 0.46b | 57.44 ± 1.89c |
| 2 | 81.47 ± 1.56b | 79.82 ± 1.92b | 70.7 ± 0.36 ab | 69.37 ± 2.26b | ||
| 3 | 88.28 ± 1.25 ab | 87.24 ± 1.21 ab | 79.87 ± 0.64a | 79.94 ± 2.85a | ||
| 4 | 92.76 ± 1.99a | 92.12 ± 2.02a | 84.31 ± 0.37a | 83.25 ± 1.25a | ||
| 600 g/L Imidacloprid FS | 4 | 89.71 ± 2.41a | 88.79 ± 2.49a | 86.8 ± 0.2a | 86.07 ± 0.87a | |
| CK | – | 7.93 ± 1.35d | – | 7.31 ± 0.1c | – | |
| 19% Cyantraniliprole SC | 2nd-instar of Anomala corpulenta | 1 | 76.88 ± 2.13c | 75.66 ± 2.25c | 66.25 ± 0.72d | 64.71 ± 0.75d |
| 2 | 81.88 ± 1.88b | 80.92 ± 1.97b | 73.75 ± 2.17c | 72.55 ± 2.26c | ||
| 3 | 86.25 ± 1.61 ab | 85.53 ± 1.7 ab | 76.88 ± 2.13bc | 75.82 ± 2.23bc | ||
| 4 | 90 ± 1.02a | 89.47 ± 1.08a | 84.38 ± 2.37a | 83.66 ± 2.47a | ||
| 600 g/L Imidacloprid FS | 4 | 90.63 ± 1.2a | 90.13 ± 1.26a | 82.5 ± 2.7 ab | 81.7 ± 2.82 ab | |
| CK | – | 5 ± 1.02d | – | 4.38 ± 1.19e | – | |
FS:Flowable concentrate for seed treatment.
Different letters: Significant difference in p < 0.05. There are significant differences among different concentrations at the same temperature.
3.5. Analysis of the control effect in the field
We conducted control experiments on underground pests in maize fields in Deshi Village, Gangcheng Town, Ningyang County, Tai'a City, Shandong Province on June 17, 2019 and June 14, 2020. The results of the field control experiments in 2019 (Table 5) indicate that cyantraniliprole seed treatment at doses of 3 and 4 g a.i./kg seed had the optimal control effect at 7–14 days after emergence (71.64–71.93% and 79.1–80.7%, respectively). The control effect reached 67.12–73.97% 21 days after emergence, which is equivalent to the control agent (imidacloprid). The yield under the cyantraniliprole seed treatment was increased 12.00–14.70% at doses of 3 and 4 g a.i./kg seed compared with the control (Table 6). The results of the comprehensive field control experiment in 2020 (Table 5) showed that the control effect of cyantraniliprole seed treatment at 3 and 4 g a.i./kg seed was highest at 7–14 days after emergence (69.46–71.48% and 75.41–76.25%, respectively). The control effect was still 67.69–74.7% 21 days after emergence, which is equivalent to imidacloprid. The yield under the cyantraniliprole seed treatment was increased 13.23–18.14% at doses of 3 and 4 g a.i./kg seed compared with the control (Table 6). In general, the control effect of cyantraniliprole seed treatment on underground pests at 3 and 4 g a.i./kg seed doses had the same control effect as imidacloprid.
Table 5.
Effect of cyantraniliprole seed treatment on integrated pest control in maize field (2019 and 2020) (n = 4).
| Years | Insecticide | Dose (g a.i./kg seed) | 3rd day after emergence |
7th day after emergence |
14th day after emergence |
21st day after emergence |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Damagerate/% | Control efficacy/% | Damagerate/% | Control efficacy/% | Damagerate/% | Control efficacy/% | Damagerate/% | Control efficacy/% | |||
| 2019 | 19% Cyantraniliprole SC | 1 | 11.88 ± 1.2b | 26.92 ± 2.36c | 16.88 ± 0.63b | 52.63 ± 1.76c | 19.38 ± 0.63b | 53.73 ± 1.51d | 23.12 ± 0.62b | 49.31 ± 1.36d |
| 2 | 9.38 ± 1.19bc | 42.31 ± 4.37bc | 15 ± 1.44b | 57.89 ± 4.05c | 16.88 ± 1.19c | 59.7 ± 2.85c | 20.62 ± 0.63c | 54.79 ± 1.37c | ||
| 3 | 6.25 ± 0.72cd | 61.54 ± 1.44 ab | 10 ± 1.02c | 71.93 ± 2.87b | 11.88 ± 0.62d | 71.64 ± 1.49b | 15 ± 1.02d | 67.12 ± 2.23b | ||
| 4 | 5 ± 1.02d | 69.23 ± 1.28a | 6.88 ± 0.63d | 80.7 ± 1.75 ab | 8.75 ± 1.25e | 79.1 ± 1.72a | 11.87 ± 0.62e | 73.97 ± 1.37a | ||
| 600 g/L Imidacloprid FS | 4 | 4.38 ± 1.1d | 73.08 ± 4.37a | 6.25 ± 0.72d | 82.45 ± 2.02a | 9.38 ± 1.23e | 77.61 ± 1.68 ab | 12.5 ± 1.14e | 72.6 ± 2.24a | |
| CK | – | 16.25 ± 0.72a | – | 35.63 ± 0.63a | – | 41.87 ± 0.36a | – | 45.63 ± 0.79a | – | |
| 2020 | 19% Cyantraniliprole SC | 1 | 11.45 ± 0.69 ab | 20.69 ± 5.18b | 14.31 ± 0.65b | 52.89 ± 1.63e | 18.13 ± 1.02b | 54 ± 1.34c | 21.88 ± 1.18b | 50.7 ± 1.43d |
| 2 | 9.96 ± 1.15b | 31.03 ± 7.21b | 12.45 ± 0.94bc | 59.08±2de | 14.38 ± 0.93c | 63.57 ± 1.3b | 19.38 ± 0.59b | 56.35 ± 0.8d | ||
| 3 | 5.39 ± 1.08c | 62.07 ± 6.12a | 9.28 ± 0.84de | 69.46 ± 1.8bc | 11.25 ± 0.56d | 71.48 ± 0.99a | 14.38 ± 0.8cd | 67.69 ± 1.29bc | ||
| 4 | 3.87 ± 1.05c | 72.41 ± 4.5a | 7.5 ± 0.43ef | 75.41 ± 0.96 ab | 9.38 ± 0.64d | 76.25 ±1.24a | 11.25 ± 0.74e | 74.7 ± 1.06a | ||
| 600 g/L Imidacloprid FS | 3 | 6.39 ± 0.75c | 55.17 ± 4.64a | 11.22 ± 0.61cd | 63.16 ± 1.19cd | 11.25 ± 0.96d | 71.48 ± 1.51a | 15.63 ± 0.45c | 64.81 ± 0.69c | |
| 4 | 4.46 ± 0.88c | 68.96 ± 4.64a | 6.46 ± 0.57f | 78.8 ± 1.02a | 10 ± 0.31d | 74.6 ± 0.53a | 11.88 ± 0.97de | 73.28 ± 1.21 ab | ||
| CK | – | 14.37 ± 1.03a | – | 30.49 ± 0.49a | – | 39.38 ± 0.64a | – | 44.38 ± 0.8a | – | |
Table 6.
Effect of cyantraniliprole seed treatment on maize yield (2019 and 2020) (n = 4).
| Years | Insecticide | Dose g a.i./kg seed | Diameter transversacm | Panicle length cm | Grain amount per head grain | Kernel rows line | Thousandkernel weight g | Grain yield per plot kg/30 m2 | Stimulation ratio % |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 19%Cyantraniliprole | 1 | 4.77 ± 0.11a | 17.66 ± 0.22 ab | 35.22 ± 0.06c | 15.53 ± 0.23a | 353.83 ± 2.92a | 21.42 ± 0.35c | 6.31 ± 1.51d |
| 2 | 4.8 ± 0.05a | 18.23 ± 0.78a | 37.72 ± 0.35a | 15.33 ± 0.38 ab | 351.3 ± 10.8a | 22.29 ± 0.28b | 10.61 ± 1.2c | ||
| SC | 3 | 4.86 ± 0.06a | 18.07 ± 0.32a | 37.74 ± 0.77a | 15.53 ± 0.23a | 350.63 ± 2.05a | 22.57 ± 0.31 ab | 12.00 ± 1.33bc | |
| 4 | 4.8 ± 0.02a | 18.52 ± 0.31a | 38.05 ± 0.60a | 15.55 ± 0.81a | 353.83 ± 8.18a | 23.11 ± 0.25a | 14.70 ± 1.09a | ||
| 600 g/L Imidacloprid FS | 4 | 4.81 ± 0.02a | 18.63 ± 0.53a | 37.27 ± 0.12 ab | 16.00 ± 0.40a | 351.8 ± 7.56a | 22.81 ± 0.26 ab | 13.18 ± 1.14 ab | |
| CK | – | 4.63 ± 0.1b | 17.01 ± 0.35b | 35.26 ± 0.40c | 14.89 ± 0.47b | 347.71 ± 4.96a | 20.15 ± 0.56d | – | |
| 2020 | 19%Cyantraniliprole SC | 1 | 4.99 ± 0.05a | 19.92 ± 0.06a | 34.43 ± 0.14a | 16.47 ± 0.17a | 380 ± 3.33b | 23.79 ± 0.41c | 8.85 ± 1.71d |
| 2 | 4.89 ± 0.06a | 19.41 ± 0.08a | 35.1 ± 0.18a | 16.9 ± 0.13a | 376.7 ± 1.09c | 24.68 ± 0.25 ab | 12.97 ± 1.12c | ||
| 3 | 4.86 ± 0.04 ab | 20.11 ± 0.61a | 34.9 ± 0.19a | 16.9 ± 0.16a | 379.97 ± 3.06b | 24.74 ± 0.31 ab | 13.23 ± 1.18bc | ||
| 4 | 4.96 ± 0.03a | 20.09 ± 0.57a | 35.43 ± 0.22a | 16.66 ± 0.31a | 395.43 ± 0.67a | 25.81 ± 0.48a | 18.14 ± 1.56a | ||
| 600 g/L Imidacloprid FS | 3 | 4.93 ± 0.04a | 19.92 ± 0.38a | 34.66 ± 0.64a | 17.13 ± 0.31a | 372.63 ± 2.44c | 24.43 ± 0.82 ab | 11.78 ± 1.47c | |
| 4 | 4.96 ± 0.04a | 19.71 ± 0.21a | 34.8 ± 0.18a | 17.33 ± 0.3a | 380.67 ± 2.26b | 25.35 ± 0.48a | 16.05 ± 1.26 ab | ||
| CK | – | 4.81 ± 0.06b | 17.67 ± 0.07b | 32.56 ± 0.47b | 16.9 ± 0.13a | 359.67 ± 1.12d | 21.83 ± 0.21d | – |
4. Discussion
4.1. Toxicity of cyantraniliprole to insect pests in maize fields
Laboratory bioassay experiments showed that cyantraniliprole had high activity against both the third instar larvae of P. canaliculatus and the second instar larvae of A. corpulenta. The LC50 for the third instar larva of P. canaliculatus was 23.3712 mg/L (5 d), and the LC50 for the second instar larva of the A. corpulenta was 5.9715 mg/L (5 d). Insecticides currently registered for the control of maize field pests mainly include neonicotinoids, pyrethroids, carbamates, and organophosphates [[14], [15], [16]]. The toxicity of cyantraniliprole to the second instar larvae of A. corpulenta and P. canaliculatus was superior to that of traditional pesticides. For example, the LC50 of imidacloprid for the second instar larvae of A. corpulenta was 9.27 mg/L, and the LC50 of chlorantraniliprole for the third instar larvae of P. canaliculatus was 8.4748 mg/L [33]. The LC50 of chlorpyrifos for P. canaliculatus is 30.22 mg/L, and that of lambda-cyhalothrin is 33.2111 mg/L [34]. Underground pests have evolved resistance to pesticides because of their long-term use, and the use of highly toxic and high-residue pesticides is now prohibited. Although few studies have evaluated the ability of cyantraniliprole to control underground pests, chlorantraniliprole has begun to be widely used for the control of underground pests [34,35]. The results of toxicity tests showed that cyantraniliprole could be used to control the insect pests in maize fields.
4.2. Sublethal effects of cyantraniliprole on pests in maize fields
Low doses of insecticides do not kill insects but can disrupt the activity of protective and detoxifying enzymes [36,37]. SOD and POD are important enzymes in insect bodies that can protect insects from free radical attack [38,39]. In this experiment, cyantraniliprole at LC15, LC25, and LC45 doses significantly increased the activity of SOD and POD of the two pests compared with the control, which was consistent with the results of Zhang et al. [39]. Therefore, insecticides may disrupt metabolic homeostasis by activating or inhibiting the activity of SOD and POD, eventually leading to the inhibition of growth and insect death. Detoxification enzymes are the main enzymes used to metabolize toxic substances in insects [37]. GST is a crucial detoxifying enzyme in organisms that can remove lipid peroxides in the body to protect against oxidative damage [40]. In this experiment, the activity of GST was significantly increased throughout the treatment period compared with the control except at the LC5 dose. The results of this study are consistent with those of He et al. [34] and Devorshak et al. [41].The increase in GST activity indicated that cyantraniliprole is toxic to pests.
4.3. Evaluation of cyantraniliprole seed treatment on the control of pests in maize fields
In this study, the safety test demonstrated that cyantraniliprole seed treatment at 1, 1.5, 2, and 2.5 times the maximum field dose was safe for maize and enhanced plant height and root length. The indoor pot experiment showed that cyantraniliprole had a strong control effect on second instar larvae of A. corpulenta and third instar larvae of P. canaliculatus. Chlorantraniliprole is a diamide insecticide that has been used as a seed treatment to control lepidopteran and coleopteran pests. Chlorantraniliprole can control Heliothis virescens Fabricius larvae through the treatment of tobacco seeds [42], and research has shown that chlorantraniliprole seed treatment can control Lissorhoptrus oryzophilus Kuschel in rice fields [43]. Chlorantraniliprole is currently registered in China and can be used to control underground pests in maize fields. Cyantraniliprole, which is similar to chlorantraniliprole, was first registered by Syngenta on April 16, 2020 as a seed treatment suspension for the control of A. ipsilon in maize fields. Cyantraniliprole is a part of a new generation of diamide insecticides that is mainly used to control lepidopteran, hemipteran, and coleopteran pests [24,44]. Previous studies have shown that cyantraniliprole and its degradation products last longer in soil [45]. The underground pests we studied are all coleopterans. The control effect of cyantraniliprole seed treatment at doses of 3 and 4 g a.i./kg seed in the field in 2019 and 2020 could reach approximately 80%, which can significantly alleviate the reduction in maize yield caused by insect pests by approximately 15%. Overall, our findings indicate that cyantraniliprole can be used to treat maize seeds to control underground pests in maize fields.
Cyantraniliprole seed treatment can promote the growth and development of maize. It can effectively control the damage caused by pests in maize fields, reduce the damage caused by underground pests to maize in the early stage of growth, and significantly alleviate the reduction in production caused by underground pests to maize.
Author contribution statement
Zhihua Qiao: Conceived and designed the experiments; Performed the experiments.
Peiyao Li, Xiangfeng Yao; Xiangdong Li: Analyzed and interpreted the data; Wrote the paper.
Shiang Sun and Fengwen Zhang: Performed the experiments and Contributed reagents, materials, analysis tools or data.
Xingyin Jiang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
The authors do not have permission to share data.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study received funding from the National Key Research and Development Project (2018YFD0200604), National Modern Agricultural Technology & Industry System (SDAIT-02-10), the Key R & D Program of Shandong Province (2022CXGC010607).
Contributor Information
Fengwen Zhang, Email: 864038633@qq.com.
Xingyin Jiang, Email: xyjiang@sdau.edu.cn.
References
- 1.Kvaternjak I., Kisi I., Birkás M. Yields and yield components of maize (Zea Mays L.) and soybean (Glycine max) as affected by different tillage methods. Ekologia (Bratisl.)/Ecology (Bratisl.) 2015;34:371–379. [Google Scholar]
- 2.Zhao J.R., Wang R.H., Chen Y.C. China Agriculture Press; 2012. Corn Production Technology. [Google Scholar]
- 3.Zhang M.C., Yin J., Li K.B., Cao Y. Research progress on the occurrences of white grub and its control. China Plant Protection. 2014;34:20–28. [Google Scholar]
- 4.Lu J.J., Dong J.M., Ren M.F., Li X., Wu Y.P., Li D.Q., Ma E.B. Migration patterns of subterranean pest insects in the soil of winter wheat-summer corn rotation fields in Linfen, Shanxi. Acta Entomol. Sin. 2017;60:1046–1059. (in Chinese) [Google Scholar]
- 5.Zhang Z., Xu C., Ding J., Zhao Y.H., Mu W. Cyantraniliprole seed treatment efficiency against Agrotis ipsilon (Lepidoptera: noctuidae) and residue concentrations in corn plants and soil. Pest Manag. Sci. 2018;75:1464–1472. doi: 10.1002/ps.5269. [DOI] [PubMed] [Google Scholar]
- 6.Jia Z.C., Fang H., Jiang L. Morphological description of the white grub melolontha incana (Coleoptera: Scarabaeidae: melolonthinae: melolonthini) Microsc. Res. Tech. 2020;84(5):921–928. doi: 10.1002/jemt.23653. [DOI] [PubMed] [Google Scholar]
- 7.Razinger J., Praprotnik E., Schroers H.J. Bioaugmentation of entomopathogenic fungi for sustainable Agriotes larvae (wireworms) management in maize. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.535005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragón-García A., Morón M.A., Rodríguez-Velázquez S.Y. Description of the larvae of three species of Macrodactylus Dejean (Coleoptera: Scarabaeidae: Melolonthinae) from Mexico, with notes on the reproductive behavior of Macrodactylus ocreatus Bates. Coleopt. Bull. 2010;64:193–200. [Google Scholar]
- 9.Antonio L.G., Ortega-Arenas L.D., Agustín A.G. White grub species (Coleoptera: scarabaeoidea) associated to corn in a home, Sinaloa, México. Agrociencia. 2020;46:307–320. [Google Scholar]
- 10.Wang S.Z., Liu S.T., Duan A.J. Efficacy and virulence determination of different agents on Anomala corpulenta Motschulsky and instar larvae indoor. Journal of Shanxi Agricultural Sciences. 2014;42:603–605. (in Chinese) [Google Scholar]
- 11.Andrews K.R., Gerritsen A., Rashed A. Wireworm (Coleoptera: elateridae) genomic analysis reveals putative cryptic species, population structure, and adaptation to pest control. Communications Biology. 2020;3:489. doi: 10.1038/s42003-020-01169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J.X. China Agriculture Press; Beijing: 2009. Agricultural Entomology. [Google Scholar]
- 13.Zhang Z.Q., Zhang X.F., Zhao Y.H., Liu F., Mu W. Efficacy of insecticidal seed treatments against the wireworm Pleonomus canaliculatus (Coleoptera: elateridae) in China. Crop Protect. 2017;92:134–142. [Google Scholar]
- 14.Herk W.G., Vernon R.S., McGinnis S. Response of the dusky wireworm, Agriotes obscurus (Coleoptera: elateridae), to residual levels of bifenthrin in field soil. J. Pest. Sci. 2013;86:125–136. [Google Scholar]
- 15.Esser A.D., Milosavljević I., Crowder D.W. Effects of neonicotinoids and crop rotation for managing wireworms in wheat crops. J. Econ. Entomol. 2015;108:1786–1794. doi: 10.1093/jee/tov160. [DOI] [PubMed] [Google Scholar]
- 16.Mosavljević I., Esser A.D., Murphy K.M., Crowder D.W. Effects of imidacloprid seed treatments on crop yields and economic returns of cereal crops. Crop Protect. 2019;119:166–171. [Google Scholar]
- 17.Bishop C.A., Woundneh M.B., Maisonneuve F., Common J., Elliott J.E., Moran A.J. Determination of neonicotinoids and butenolide residues in avian and insect pollinators and their ambient environment in Western Canada (2017, 2018) Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.139386. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q., Yang Y., Zhong Y., Lao Z., O'Neill P., Hong D., Zhang K., Zhao S. Synthesis, insecticidal activity, resistance, photodegradation and toxicity of pyrethroids (A review) Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126779. [DOI] [PubMed] [Google Scholar]
- 19.Poomagal S., Sujatha R., Kumar P.S., Vo D.V. A fuzzy cognitive map approach to predict the hazardous effects of malathion to environment (air, water and soil) Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.127926. [DOI] [PubMed] [Google Scholar]
- 20.Sparks T.C., Nauen R. IRAC: mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Wen Z., Li K., Xu W., Liang N., Yu X., Li C., Chu D., Guo L. Cytochrome P450 gene, CYP6CX3, is involved in the resistance to cyantraniliprole in Bemisia tabaci. J. Agric. Food Chem. 2022;70(39):12398–12407. doi: 10.1021/acs.jafc.2c04699. [DOI] [PubMed] [Google Scholar]
- 22.Grout T.G., Stephen P.R., Rison J.-L. Cyantraniliprole can replace malathion in baits for Ceratitis capitata (Diptera: tephritidae) Crop Protect. 2018;112:304–312. [Google Scholar]
- 23.Zhang Z., Xu C., Ding J., Zhao Y.H., Mu W. Cyantraniliprole seed treatment efficiency against Agrotis ipsilon (Lepidoptera: noctuidae) and residue concentrations in corn plants and soil. Pest Manag. Sci. 2018;75:1464–1472. doi: 10.1002/ps.5269. [DOI] [PubMed] [Google Scholar]
- 24.Pes M.P., Melo A.A., Stacke R.S., Zanella R., Perini C.R., Silva F.M.A., Carús G.J.V. Translocation of chlorantraniliprole and cyantraniliprole applied to corn as seed treatment and foliar spraying to control Spodoptera frugiperda (Lepidoptera: noctuidae) PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0229151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NY/T . 1965. Guidelines for Crop Safety Evaluation of Pesticides Part 3: Laboratory Test for Crop Safety Evaluation of Seed Treatment Agents. [Google Scholar]
- 26.NY/T1154.6-2006 . 2006. Pesticide Guidelines for Laboratory Bioactivity Tests Part 6: the Immersion Test for Insecticide Activity. [Google Scholar]
- 27.Li Y.F., Dang Z.H., Gao Z.L. A bioassay suitable for screening pesticides to control the wireworm Agriotes spp. Chinese Journal of Applied Entomology. 2014;51:1356–1361. [Google Scholar]
- 28.Li Y.F., Dang Z.H., Gao Z.L. China Society of Plant Protection. Food Security and Plant Protection Technology Innovation. 2009. Three methods were used to determine the toxicity of several insecticides to Holotrichia oblita Fald; pp. 683–685. [Google Scholar]
- 29.Song Y., Zhu L.S., Wang J., Wang J.H., Liu W., Xie H. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol. Biochem. 2009;41:905–909. [Google Scholar]
- 30.Simon L.M., Fatrai Z., Jonas D.E., Matkovics B. Study of peroxide metabolism enzymes during the development of Phaseolus vulgaris. Biochem. Physiol. Pflanz. (BPP) 1974;166:387–392. [Google Scholar]
- 31.Oppenoorth F.J., Pas L.J.T.V.D., Houx N.W.H. Glutathiones-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic. Biochem. Physiol. 1979;11:176–188. [Google Scholar]
- 32.Sun G., Yang X.W., Tian X.H., Li S.X. Response of different maize of variou susceptibility to zinc application under sand culture conditions. J. Northwest For. Univ. 2010;38:101–108+116. (in Chinese) [Google Scholar]
- 33.He F.L., Qiao Z.H., Yao X.F., Yu H.Y., Sun S.A., Li X.D., Zhang J.W., Jiang X.Y. Co-toxicity of different insecticides against Anomala corpulenta and screening of synergistic agents mixed with different insecticides. Plant Prot. 2020;46:228–233+257. [Google Scholar]
- 34.He F.L., Sun S., Tan H. Chlorantraniliprole against the black cutworm Agrotis ipsilon (Lepidoptera: noctuidae): from biochemical/physiological to demographic responses. Sci. Rep. 2019;9:28–34. doi: 10.1038/s41598-019-46915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X.F., Jiang X.Y., He F.L., Liu Y., Li X.D., Zhang J.W. Chlorantraniliprole and bifenthrin compound seed treatment on peanut underground and control effect of aboveground pests. Agrochemicals. 2020;59:150–153+156. (in Chinese) [Google Scholar]
- 36.Ramsey J.S. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol. Biol. 2010;19:155–164. doi: 10.1111/j.1365-2583.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y.H., Wang Q.H., Ding J.F., Wang Y., Zhang Z.Q., Liu F., Mu W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: sciaridae) Pestic. Biochem. Physiol. 2018;148:93–102. doi: 10.1016/j.pestbp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Huang Q.C., Liu M.H., Feng J., Liu Y. Effect of dietary benzoxadiazole on larval development, cuticle enzyme and antioxidant defense system in housefly (Musca domestica L.) Pestic. Biochem. Physiol. 2008;90:119–125. [Google Scholar]
- 39.Zhang Y.N., He P., Xue J.P., Guo Q., Zhu X.Y., Fang L.P., Li J.B. Insecticidal activities and biochemical properties of Pinellia ternata, extracts against the beet armyworm Spodoptera exigua. J. Asia Pac. Entomol. 2017;20:469–476. [Google Scholar]
- 40.Pickett C.B., Lu A.Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu. Rev. Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- 41.Devorshak C., Roe R.M. Te role of esterases in insecticide resistance. Rev. Toxicol. 1998;2:501. [Google Scholar]
- 42.Burrack H.J., Chapman A.V. Evaluation of biweekly pesticide applications of new insecticides for tobacco budworm (Heliothis virescens (Fabricius)) management in tobacco (Nicotiana tabacum L.) seed production. Crop Protect. 2013;45:117–123. [Google Scholar]
- 43.Hummel N.A., Mészáros A. Evaluation of seed treatment insecticides for management of the rice water weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae), in commercial rice fields in Louisiana. Crop Protect. 2014;65:37–42. [Google Scholar]
- 44.Guo L., Li C., Coupland G., Li P., Chu D. Up-regulation of calmodulin involved in the stress response to cyantraniliprole in the whitefly, Bemisia tabaci (Hemiptera: aleyrodidae) Insect Sci. 2020;12887 doi: 10.1111/1744-7917.12887. [DOI] [PubMed] [Google Scholar]
- 45.Kumar N., Gupta S. Persistence and degradation of cyantraniliprole in soil under the influence of varying light sources, temperatures, moisture regimes and carbon dioxide levels. J. Environ. Sci. Health - Part B Pesticides, Food Contam. Agric. Wastes. 2020:1–9. doi: 10.1080/03601234.2020.1808416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.