Abstract
Context
Saikosaponin D (SSD) is a commonly prescribed agent against inflammatory diseases in Asian countries. However, the anti-allergic inflammatory effect of SSD in allergic rhinitis (AR) model is not well known.
Objective
We investigated the anti-allergic and anti-inflammatory effects of SSD on the ovalbumin (OVA)-induced AR model.
Materials and method
BALB/c mice were divided into the control, OVA, OVA + SSD, and OVA + dexamethasone (Dex) groups. AR was established by intraperitoneal injection with OVA adsorbed to aluminum hydroxide, and intranasal challenge with OVA. Thereafter, the mice were treated with 10 mg/kg BW (Body weight) of OVA + SSD and 2.5 mg/kg BW of Dex orally for 11 days before being challenged. Subsequently, the mice were challenged with OVA 1 h after SSD or Dex treatment. The Control group was treated with saline only.
Results
The addition of 10 mg/kg BW of OVA + SSD significantly ameliorated the nasal symptoms including sneezing and rubbing from 30 ± 5.2 times in OVA group to 20 ± 5.8 times. Moreover, OVA + SSD group decreased the production of TNF-α, IL-4, IL-5, IL-17, GATA-3 and RORγ about 1.2–1.4-fold compared to the OVA-induced AR mice near to 2.5 mg/kg BW of Dex levels. Meanwhile OVA + SSD group slightly increased the levels of INF-γ, IL-12 and T-bet about 1.8–2.0-fold compared to the OVA group near to control group. Notably, OVA + SSD group also reduced the levels of OVA-specific IgE and IgG1 about 0.5–2.5-fold compared OVA group but increased the levels of IgG2a in serum. The results were analyzed using Graph Pad Prism software (v5.0, La Jolla, CA, USA).
Conclusion
SSD may represent an alternative therapeutic approach for the treatment of patients with AR through the regulation of transcription factors T-bet, GATA-3, and RORγ in inflammatory cells.
Keywords: SSD, Anti-allergic inflammatory effect, Cytokines, Th1, Th2, Th17, OVA-Specific IgE
Graphical abstract
1. Introduction
Allergic rhinitis (AR) is an allergic inflammation of the nasal airways and is characterized by sneezing, rhinorrhea, itching, and nasal congestion. These clinical conditions are widely known to be induced by inflammatory mediators, including histamine, leukotrienes, and inflammatory cytokines, which are secreted by eosinophils [1]. The prevalence of AR has progressively increased and has conferred a substantial economic burden associated with AR management [2]. AR is one of the world's most common chronic illnesses among children and young adults, and its prevalence is estimated to range from approximately 10-50% [3]. Anti-allergic and anti-histamine agents like intranasal steroids are commonly used in the treatment of allergic rhinitis; however, their effects are transient as they are only able to suppress the symptoms of the inflammatory response [4]. Therefore, it is important to develop novel therapeutic strategies that are effective for the treatment of AR [5]. Effective therapeutic strategies would aim to either downregulate Th2 or Th17 cytokines or to downregulate the transcription factors that inhibit their production. RORγ is a splice variant of ROR, which has been identified as an essential transcription factor during Th17 cellular differentiation [6].
Patients with AR show an inflammatory immunoglobulin E (IgE)-mediated response characterized by the Th2 immunological pattern together with mast cells, goblet cells, and eosinophil activation, as well as the release of inflammatory mediators, such as interleukin (IL)-4 and IL-5, against allergen exposure [7]. To date, the discovery of Th17 cells has introduced complexity into the existing Th1/Th2 balance paradigm and expanded our understanding of the pathogenesis of AR [8]. Th17 cells are newly emerged immune/inflammatory cell subsets, which are now widely believed to be critical for the regulation of various chronic immune diseases [8]. Th17 cells enhance eosinophilic airway inflammation, which is mediated by Th2 cells [9]. Nuclear factor-kappa B (NF-κB) is a multicellular transcription factor, and it plays an important role in inflammatory and immune responses by regulating the gene expression of immune and inflammation-related cytokines and inflammatory mediators, which is a vital role in the initiation and perpetuation of allergic inflammation [10,11]. The transcription factor T-bet drives Th1 differentiation, while the transcription factor GATA binding protein 3 (GATA-3) drives Th2 differentiation.
Natural products are regarded as abundant sources of novel drug candidates, and their pharmacological usefulness has been proven through decades of research [12]. In addition, drugs derived from natural products are known to induce fewer side effects than many synthetic drugs [13]. The traditional Chinese medicine Bupleurum falcatum L that has been widely used in China, and saikosaponins are the major bioactive compounds of Bupleurum falcatum L. Saikosaponins, saikosaponin A (SSA) and it's epimer saikosaponin d (SSD) are a pair of isomers. SSD is one of the triterpenoid saponins derived from Bupleurum falcatum L. (Apiaceae), its chemical structure is similar to that of steroid hormones and is the most functional monomer in the Bupleurum extract [14,15]. Bupleurum falcatum is known as “Bei Chaihu” in China and has been one of the most important traditional herbs for more than a thousand years. SSD is soluble in methanol, ethanol and slightly soluble in water. Previous study showed that it has anti-inflammatory, antioxidant, hepato-protective, antipyretic, antifibrotic, analgesic, and immunomodulatory effects [16]. Several studies have shown that SSD possesses multiple pharmacological activities, including anti-inflammation,antiviral and anticancer activities [17]. Other researchers have found that SSD exhibited an anti-proliferative effect on the activated T lymphocyte via suppression of the NF-κB, NF-AT and AP-1 signaling pathways [15]. In animal experiments, SSD significantly reduced the oxidative stress injury and decreased the apoptosis rate in lung tissue [17]. According to our previous study, SSAis the most important active saikosaponins also and provides significant inhibitory effect on allergic inflammation of nasal mucosa in OVA-induced AR mice [18]. However, the mechanism underlying the anti-allergic effect of SSD on allergic rhinitis remains unclear. In the current study, we explore the protective effects and therapeutic efficacy of SSD on an OVA-induced AR mice model, which potentially involves the regulation of the Th1/Th2 and Th17 cellular responses.
2. Materials and methods
2.1. Reagents
OVA (grade VI), and SSD were purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA). Aluminum hydroxide adjuvant was purchased from Pierce (Thermo Scientific, Rockford, MD, USA) and Dex was purchased from Innovative Research of America (Toledo, OH, USA).
2.2. Animals
Five-week-old male BALB/c mice, weighing approximately 20 g were purchased from Damool Science (Dae-jeon, Korea). These mice were housed 5 per cage in a laminar air-conditioned room maintained at a temperature of 23 ± 2 °C and a relative humidity of 55 ± 10% with a 12 h light/dark cycle. All animal experiments were performed in compliance with the guidelines on the care and use of laboratory animals and were approved by the Animal Research Committee of Jeonbuk National University (CBNU 2019-071).
2.3. Induction of AR mice model and treatment
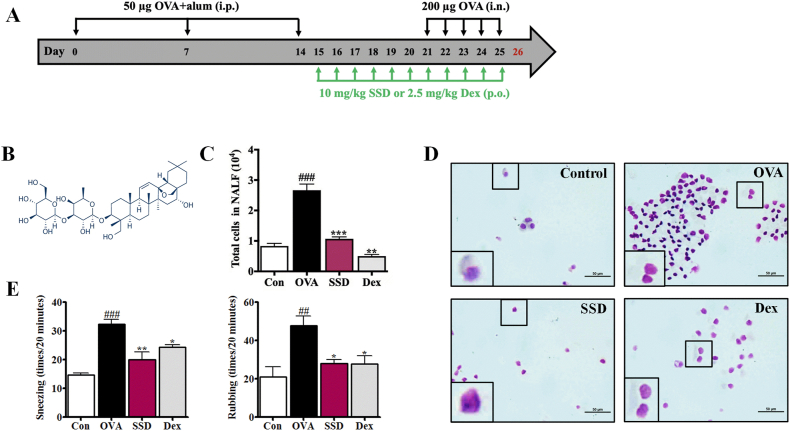
An OVA-induced AR mice models was established as described previously [18]. Briefly, mice were randomly divided into four groups (n = 6 per group): the control, OVA, OVA+10 mg/kg of OVA + SSD, and OVA + dexamethasone (Dex, 2.5 mg/kg). The schedule for the OVA-induced AR animal model and treatment is summarized in Fig. 1A. Briefly, mice were sensitized by intraperitoneal injection of 50 μg of OVA (Grade V, Sigma, St. Louis, MO, USA) emulsified in 1 mg of aluminum hydroxide in a total volume of 200 μL on days 0, 7, and 14. After one week of the last sensitization (days 21–25), mice were treated with an intranasal instillation of OVA (10 mg/mL, 20 μL/nostril). From day 15, mice received SSD (10 mg/kg body weight, SSD chemical structure shown in Fig. 1B) and Dex (2.5 mg/kg body weight, Innovative Research of America, Toledo, OH, USA) dissolved in saline in a total volume of 200 μL and administered by oral gavage 1 h before every OVA treatment, every day for 11 consecutive days. While mice from the Control and OVA groups received equal volumes of saline from the 15th day to the 25th day once a day. Mice of the control group were treated with saline but without sensitization and OVA treatment. Mice were sacrificed 24 h after the last OVA treatment on day 26 to investigate the anti-inflammatory effect of SSD on OVA-induced AR mice.
Fig. 1.
Experimental protocol for AR, nasal symptoms, and inflammation of OVA-induced AR mice. (A) Experimental protocol. (B) Chemical structure of saikosaponin D (SSD). (C) Total cell numbers were counted using a hemocytometer. (D) Cytospin cell preparations were made by NALF and stained with Diff-Quik. Scale bar = 100 μm. The frequencies of total rubbing and sneezing numbers for 5 days were evaluated by counting that occurred in the 20-min period after OVA treatment without anesthesia. (E) The frequencies of total rubbing and sneezing numbers for 5 days were evaluated by counting that occurred in the 20-min period after OVA treatment without anesthesia. #P < 0.05, ##P < 0.01, ###P < 0.001, significant differences of the control group. Values are presented as the mean ± SD (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant differences of the OVA group.
2.4. Analysis of nasal lavage fluid (NALF) and lung homogenates
Twenty-four hours after the last OVA challenge, the mice were sacrificed. The trachea was partially resected, a catheter was inserted from the trachea into the nasopharynx, and the nasal cavity was gently perfused with 1 mL of saline from the choana to the nostril. The NALF was centrifuged at 10,000 g for 10 min, and the supernatants were stored at −80 °C. The total NALF cells were counted using the double-blind counting system with a hemocytometer. Differential cell counts were determined with cytospin cell preparations, which were made by transferring NALF onto clean glass slides using a cytospin device (Centrifuge 5403, Eppendorf, Hamburg, Germany) for 10 min and staining with Diff-Quik (Sysmex Co., Kobe, Japan), according to the manufacturer's instructions. Lung tissues were homogenized in saline to a concentration of 100 mg/mL with the complete, Mini, EDTA-free Protease Inhibitor (Roche Applied Science) and the debris-free supernatant was used for cytokine measurement.
2.5. Evaluation of nasal symptoms
Nasal symptoms were evaluated by counting the frequencies of nasal rubbing and sneezing that occurred in the 20 min observation period and then counted by blinded observers to evaluate early allergic responses [18].
2.6. Histological examination
After performing the NALF, the heads of the mice were removed and then fixed in 10% neutral buffered formalin for 3 days and then decalcified in an ethylenediamine triacetic acid decalcifying solution for 5 days. The head tissues were dehydrated with ethyl alcohol and xylene and embedded in paraffin. The lung lobes were removed for histological examination and were fixed in 10% paraformaldehyde for 3 days. The nasal and lung tissues were coronally sectioned into 5-μm slices for histological assessment. Subsequently, the number of inflammatory cells was counted, and the epithelial damage was evaluated on randomly selected high-power fields at 400 × magnification.
Hematoxylin and eosin (HE, Sigma Aldrich, St. Louis, MO, USA) staining was used to assess the general morphological structure of the nasal and lung tissues.
Periodic acid-Schiff (PAS, Sigma, St. Louis, MO, USA) staining was used for goblet cell hyperplasia.
Giemsa staining (Sigma, St. Louis, MO, USA) was used for mast cell numbers in nasal mucosa.
Masson's trichrome staining was used to reveal the sub-epithelial deposition of collagen in the lung tissue.
2.7. Measurement of Th1/Th2/Th17 cytokines and related transcription factor levels and Th1/Th2 balance
In the Th1 response, the levels of anti-inflammatory cytokines (Th1-associated cytokine), such as IFN-γ and IL-12 levels, and the relative transcription factor T-bet in NALF and lung homogenates were assayed using enzyme-linked immunosorbent assay kits (ELISA, R&D Systems, Minneapolis, MN, USA), following the manufacturer's instructions. Briefly, the supernatant and standard solution were transferred to a 96-well plate pre-coated with monoclonal antibodies against each target cytokine and incubated at room temperature for 2 h. After washing with the washing buffer in the kit, horseradish peroxidase binding secondary antibody was added to each well and incubation was continued at room temperature for 2 h. After the secondary antibody was removed and the wells were thoroughly washed, the enzymatic reaction was carried out by adding the substrate solution, and samples were incubated in the dark for 30 min. The reaction was terminated by addition of the stop solution and measured the absorbance at 450 nm in a microplate reader (Molecular Devices, Inc., CA, USA).
In the Th2 response, we examined the secretion of the inflammatory cytokines (Th2-associated cytokine) IL-4, and IL-5 and relative transcription factor GATA-3 in NALF and lung homogenates were measured using ELISA (R&D Systems, Minneapolis, MN, USA), following manufacturer's instructions.
In the Th17 response, Th17-associated cytokines, IL-17, and relative transcription factor RORγ in NALF and lung homogenates were measured using ELISA (R&D Systems, Minneapolis, MN, USA), following manufacturer's instructions.
The imbalance in the Th1/Th2 ratio is associated with many disease states. The balance of Th1/Th2 and Th1/Th17 were measured the levels of cytokines INF-γ and IL-12, IL-4 and IL-13 and Th17 in NALF and lung homogenates. The ratio of IFN-γ/IL-4 levels in the serum is used to determine the balance between Th1 and Th2 immune responses, and the ratio of IFN-γ/IL-17 levels in the serum is used to determine the Th1/Th17 ratio.
2.8. Measurement of OVA-specific immunoglobulins
The blood specimens were collected from the retro-orbital plexus and centrifuged at 1,000 g for 10 min at 4 °C (Centrifuge 5403; Eppendorf, Hamburg, Germany) to obtain the serum. Then, the concentrations of OVA-specific IgE, OVA-specific IgG1, and OVA-specific IgG2α levels were measured in each group using ELISA (R&D Systems, Minneapolis, MN, USA), following manufacturer's instructions.
2.9. Measurement of the NF-kB signaling pathway
The lung homogenates were centrifuged at 1,000 g for 15 min at 4 °C. The supernatants of NF-кB p65 and P– NF-кB, both in NALF and lung homogenates, were obtained for analysis. And NF-κB nuclear translocations were performed by immunohistochemical staining. Immunohistochemical staining readily provided a quantitative measure of NF-κB expression in nasal mucosa.
2.10. Statistical analysis
The results were analyzed using Graph Pad Prism software (v5.0, La Jolla, CA, USA). The data were presented as mean ± SEMs. The statistical significance of the differences among the groups was performed using one-way analysis of variance (ANOVA), followed by the Tukey's test. Statistical significance was defined at the 95% confidence level (P < 0.05).
3. Results
3.1. Inhibitory effects of SSD on the infiltration of inflammatory cells in the NALF of OVA-induced AR mice
To evaluate the underlying mechanism of SSD on the level of inflammatory cells in OVA-induced AR mice, the total number of cells and the differential inflammatory cells in NALF were counted. OVA-induced AR mice exhibited a more significant increase in total cells and inflammatory cells of NALF than mice of the control group (Fig. 1C and D). In contrast, oral administration of 10 mg/kg/day SSD and 2.5 mg/kg/day Dex subsequently reduced the total cell number and the infiltration of eosinophils, epithelial cells, and other inflammatory cells in NALF (Fig. 1C and D).
3.2. Inhibitory effects of SSD on clinical symptoms of OVA-induced AR mice
In animal models, early allergic responses have been quantitated by the nasal rubbing and sneezing score. To examine the regulatory effect of SSD in the OVA-induced AR mice model, after 400 μg of OVA intranasal treatment, we measured the frequency of nasal sneezing and the rubbing behaviors were recorded during a 20- min period by blinded observers on days 22–26. The OVA-induced AR mice showed a significant increase in sneezing and nasal rubbing as compared to the control group (Fig. 1E). However, oral administration of 10 mg/kg/day OVA + SSD revealed a tendency to decrease the sneezing and nasal rubbing (Fig. 1E). SSD treatments considerably improved nasal symptoms compared to those of OVA-induced AR mice. These results suggest that treatment with SSD may ameliorate the symptoms associated with AR.
3.3. Inhibitory effects of SSD on the histopathological changes of nasal mucosal tissues and lungs of OVA-induced AR mice
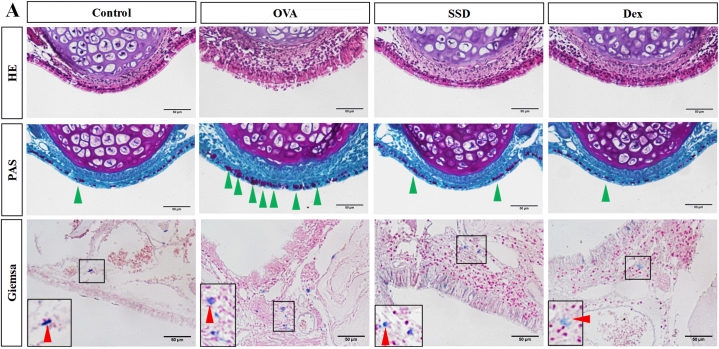
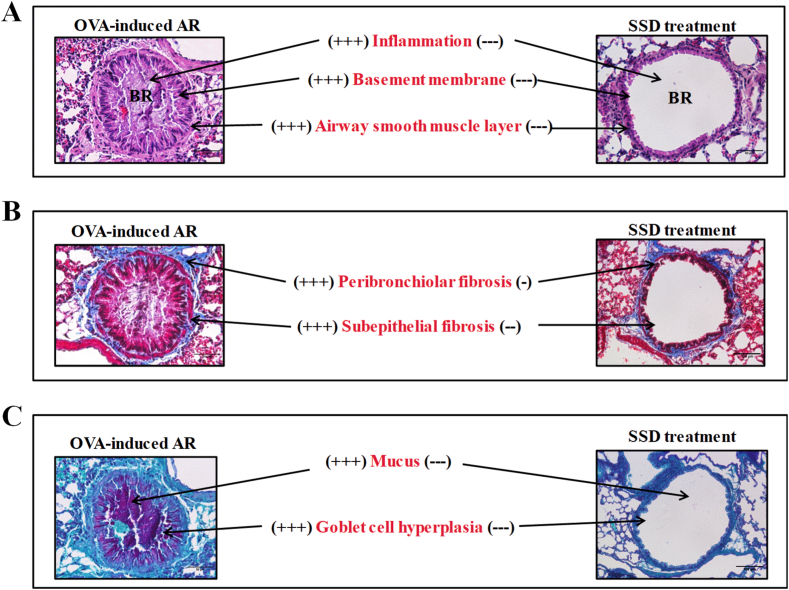
To evaluate the effects of SSD administration on the infiltration of inflammatory cells in nasal mucosal tissues and lung tissues, we next examined the histological changes of OVA-induced AR mice with HE, PAS, Giemsa, Sirius Red and Masson's trichrome. As shown in Fig. 2, OVA treatment caused remarkable histological alterations, such as epithelial disruption and edema. Infiltration of mast cells, as well as goblet cell hyperplasia, was significantly increased compared to the control group. (Fig. 2). The mucosal thickness of the nasal septum was significantly higher in OVA-induced AR mice than in control group. However, SSD treatment notably mitigated epithelial disruption and inhibited the infiltration of mast cells, and the hyperplasia of goblet cells. The histological changes in lung tissues in OVA-induced AR mice were then evaluated, and the level of general pathological alterations was examined. Histological observations revealed that there was a more severe infiltration of inflammatory cells and airway epithelial thickening in the peribronchial and perivascular regions in the control group. While SSD treatment showed a significantly reduced infiltration (Fig. 3A). Representative images of lung tissues are shown in Fig. 3B and C, OVA-induced AR exhibited a significant increase goblet cell hyperplasia and mucus overproduction, accompanied by the collagen deposition and tissue fibrosis compared with control group. However, treatment with SSD notably downregulated the goblet cell hyperplasia and tissue fibrosis. These results suggest that SSD may alleviate allergic responses in OVA-treated group by attenuating the inflammatory cells infiltration both in the upper and lower airway.
Fig. 2.
Effect of SSD on histological changes in the nasal tissues of OVA-induced AR mice. (A) The general histology and epithelium thickness of the nasal mucosa, HE staining; Goblet cells hyperplasia, PAS staining; Mast cells immigration to the epithelium, Giemsa staining; The green arrow indicates the goblet cell. Red arrow indicates the mast cells. Scale bar = 50 μm in the nasal tissues. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Effect of SSAD on histological changes in lung tissues of OVA-induced AR mice. Lung tissues from each group were stained with HE. Goblet cell hyperplasia, PAS staining; Collagen deposition, Masson's Trichrome staining. Scale bar = 100 μm in the lungs.
3.4. Inhibitory effects of SSD on the serum levels of OVA-specific IgE, IgG1, and IgG2a of OVA-induced AR mice
To investigate the therapeutic impact of SSD on OVA-specific immune responses, we determined the serum levels of OVA-specific immunoglobulin in OVA-induced AR mice. As shown in Fig. 4, both levels of OVA-specific IgE and IgG1 in OVA-induced AR mice were markedly increased. However, treatment with SSD significantly inhibited OVA-specific IgE and IgG1 production (Fig. 4A and B). Moreover, the level of OVA-specific IgG2a was slightly up-regulated by SSD treatment compared with OVA-induced AR mice (Fig. 4C). These results indicate that SSD might have anti-allergic activity via suppression of allergic mediator production.
Fig. 4.
Effect of SSD on the level of OVA-specific IgE, IgG1, and IgG2a expression in OVA-induced AR mice. The serum levels of OVA-specific IgE (A), IgG1 (B), and IgG2a (C). Values are presented as the mean ± SD (n = 6 per group). #P < 0.05, ##P < 0.01, ###P < 0.001, significant differences of the control group. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences of the OVA group.
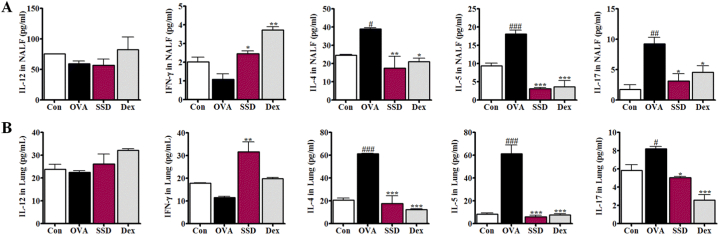
3.5. Effects of SSD on the levels of Th1, Th2, and Th17 cytokines and Th1/Th2 Balance in NALF and lung homogenates of OVA-induced AR mice
To further evaluate the effect of SSD on anti-allergic responses in the OVA-induced AR mice model, the Th1/Th2/Th17 levels of secreted cytokines in NALF and lung homogenates were assessed. First, we examined the secretion of the Th2-associated cytokines, including IL-4, and IL-5, using ELISA. OVA-induced AR mice showed increased levels of IL-4, and IL-5 in NALF compared to control group; however, SSD treatment significantly decreased the cytokine levels (Fig. 5A). Moreover, the anti-inflammatory effect of Th1-associated cytokines, such as IFN-γ and IL-12, were measured using ELISA. The OVA-induced release of IFN-γ was significantly increased by SSD and Dex treatment (Fig. 5A), while the production of IL-12 did not show any significant difference (Fig. 5A). Th17-associated cytokine IL-17 was increased in the AR group (Fig. 5A). In contrast, SSD treatment significantly diminished IL-17 production in the NALF and Dex groups (Fig. 5A).
Fig. 5.
Effect of SSD on Th1/Th2/Th17 cytokines in OVA-induced AR mice. The levels of (A), Th1 cytokines, IL-12, and IFN- γ; Th2 cytokines, IL-5and IL-13; and Th17 cytokines, IL-17, were determined in NALF and (B) lung homogenates in OVA-induced AR mice. Values are presented as the mean ± SD (n = 6 per group). #P < 0.05, ##P < 0.01, ###P < 0.001, significant differences of the control group. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences of the OVA group.
Since the administration of SSD suppressed the levels of inflammatory and pro-inflammatory cytokines in NALF, we investigated the possibility that IL-4, IL-5 and IL-17 production was down-regulated in the lung tissue and up-regulated the production of IFN-γ. As expected, SSD treatment dramatically decreased IL-4, IL-5 and IL-17 levels in lung homogenates and increased IFN-γ compared to the OVA group (Fig. 5 B). These results were consistent with the reduced infiltration of inflammatory cells both in NALF and lung tissues of SSD treated mice via the modulation of the Th1/Th2/Th17 cytokines.
To further clarify the Th1/Th2 and Th1/Th17 balance of AR, the ratio between the INF-γ and IL-12 and the IL-4 and IL-13 levels were calculated. The Th1/Th2 and Th1/Th17 ratio were significantly higher in SSD and Dex treatment compared to OVA control group (Fig. 6A). Based on these findings, the importance of the Th1/Th2/Th17 paradigm cannot be ignored when investigating alternating therapeutic strategies for treating AR.
Fig. 6.
Effect of SSD on Th1/Th2/Th17 cytokines related transcription factor levels in OVA-induced AR mice. (A) The balance of Th1/Th2 and Th1/Th17 were measured both in NALF and lung homogenates. (B) Analysis of T-bet, GATA-3, and RORγ expression levels in lung homogenates. Values are presented as the mean ± SD (n = 6 per group). #P < 0.05, significant differences of the control group. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences of the OVA group.
3.6. Effect of SSD on the levels of Th1-, Th2-, and Th17-related transcription factors T-bet, GATA-3, and RORγ in the lung homogenates of OVA-induced AR mice
It was hypothesized that the mechanisms underlying the anti-allergic effects of SSD involve the regulation of transcription factors, including T-bet, GATA-3, and RORγ, during Th1, Th2, and Th17 cellular differentiation. The expression of the specific transcription factors, T-bet, GATA-3, and RORγ in the nucleus, was used to identify whether Th1, Th2, Th17 cells, respectively, were altered in the lung tissue following SSD and Dex administration. The production of GATA-3 in the OVA-induced AR mice increased significantly (Fig. 6B). In contrast, the T-bet expression levels in the OVA-induced AR mice were lower than those in the OVA + SSD and Dex groups. The expression of GATA-3 expression was decreased in the OVA + SSD group compared to the control group. There were no significant differences in the expression levels of T-bet between the OVA and OVA + SSD groups. Similar effects were observed in the Th17 transcription factor RORγ. The expression of RORγ appeared to be up-regulated in OVA-induced AR mice. Conversely, treatment with SSD and Dex ameliorated this effect.
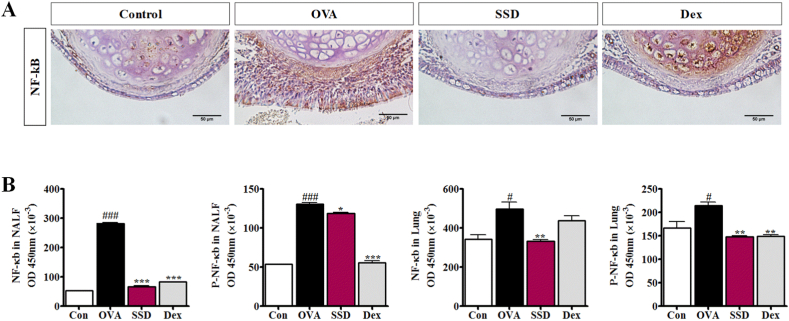
3.7. Inhibitory effect of SSD on the activation of NF-κB signaling in nasal tissue, lung homogenates and NALF of OVA-induced AR mice
To further explore the roles of the NF-κB signaling pathway with SSD treatment, consistent with immunohistochemistry was conducted to ascertain the expression of NF-κB in nasal tissue. Obviously, NF-κB-stained cells were observed in the OVA-induced AR mice. However, the SSD treatment showed a higher prevalence of stained cells in nasal tissues (Fig. 7A). Next, we assess the effect of SSD on the signaling pathway, NF-κB and P–NF-κB, both in NALF and lung homogenates were analyzed by ELISA. The results showed that in OVA-induced AR mice, the levels of NF-κB in NALF and lung homogenates were significantly increased as compared to control group; however, their levels were significantly decreased by SSD treatment (Fig. 7B). In addition, as a marker of NF-κB activation, degradation levels of P–NF-κB in NALF and lung homogenates were examined. OVA-induced AR mice showed an increased level of P–NF-κB degradation in NALF and lung homogenates as compared to control group. Moreover, we found that SSD treatment down-regulated the levels of P–NF-κB in NALF and lung homogenates as compared to OVA-induced AR. These findings indicate the potential role of the NF-κB pathway in the suppression of inflammatory mediators by SSD in OVA-induced AR.
Fig. 7.
Effect of SSD on NF-кB p65 and P–NF-κB signaling pathways in OVA-induced AR mice. (A) Detection of NF-κB and P–NF-κB in NALF and (B) lung homogenates. Values are presented as the mean ± SD (n = 6 per group). #P < 0.05, ###P < 0.001, significant differences of the control group. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences of the OVA group.
4. Discussion
The allergic symptoms of AR include sneezing, rubbing, rhinorrhea, lacrimation, and nasal congestion, which mainly originate from mast cells and other inflammatory cells [19]. These symptoms are presumed to be triggered by various mediators such as cytokines (IL-4, TNF-α), prostaglandins, NO from mast cells, and other inflammatory cells [20]. In the present study, we investigated the effects of SSD treatment on the nasal mucosa in an AR mouse model. We demonstrated that SSD treatment was associated with significantly decreased clinical symptoms as well as an increase in the number of disrupted epithelial cells in the NALF, serum OVA-specific immunoglobulin levels, Th2, and Th17 cytokines levels. Moreover, SSD treatment inhibited the infiltration of mast cells, goblet cells, and epithelial cells in the nasal mucosa. In addition, goblet cell hyperplasia and collagen deposition were also alleviated in lung tissue. The down-regulatory effect of transcription factor GATA-3, RORγ expression, and the up-regulation of T-bet expression were observed in the OVA + SSD group. We further demonstrated that SSD was able to suppress the release of allergic mediators by blocking the NF-κB signaling pathway.
GATA-3 is a transcription factor that is specifically expressed in Th2 cells, whereas T-bet is a transcription factor specifically expressed in Th1 cells. GATA-3 and T-bet have been reported to modulate gene expression during T cell differentiation, thus serving a critical role in the development of Th1 and Th2 lineages [21,22]. The present study revealed that in OVA-induced AR mice, GATA-3 expression was markedly up-regulated in lung tissue compared to control group. Notably, GATA-3 expression levels were markedly decreased following treatment with SSD. Conversely, T-bet levels were up-regulated following SSD administration; however, there was no significant difference compared to OVA-induced AR mice. The present findings demonstrate that the expression of GATA-3 and T-bet may be altered during allergic immune responses and may be implicated in the regulation of Th1/Th2 differentiation. Following the retroviral vector-mediated transduction of RORγ into naive T cells, Th17 cell development was enhanced, therefore suggesting that RORγ may be essential for Th17 cellular proliferation [23]. The present study revealed that the protein expression of RORγ was down-regulated following SSD administration.
AR is one of the most common chronic conditions in the adult and pediatric population, affecting 10–30% of adults and 20–40% of children [24]. Therefore, the development of new and promising therapeutic options for the treatment of AR is required. In the present study, we demonstrated that OVA treatment of AR mice induced nasal airway remodeling compared to control group via increased epithelial disruption, mucosa edema, and mast cell infiltration, goblet cell hyperplasia in the nasal mucosa, OVA-specific immunoglobulins, Th2, and Th17 polarization, and NF-κB signaling pathway activation in nasal epithelial cells. In the present study, we assessed the anti-allergic and anti-inflammatory activities of SSD in the OVA-induced AR mice to elucidate the effects of SSD on inflammation-related NF-κB pathways. Here, we demonstrate for the first time, that SSD, which is a commonly prescribed agent against inflammatory diseases, significantly attenuated allergic responses.
SSD is considered one of the major active components isolated and identified from the herb [25]. Protection against CCl4-induced inflammation and fibro-genesis by SSD was correlated with the downregulation of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and the up-regulation of the anti-inflammatory cytokine, IL-10 [26]. A previous report suggested that IL-17 has the potential to promote airway inflammatory cell infiltration [27]. The pathogenesis of AR is characterized by the secretion of many cytokines and immune cells. IL-4 expression is related to the differentiation of Th2 lymphocytes, the synthesis of IgE, and the hypersecretion of mucus [1]. The biologic effects of IL-5 are best characterized for eosinophils [28]. Previous study showed that SSA was effective SSA provides significant inhibitory effect on allergic inflammation of nasal mucosa in OVA-induced AR mice through the regulation of Th2 and Th17 responses and the inhibition of IL-6/STAT3/ROR-γt and NF-κB pathway [18]. In this study, we demonstrated that SSD diminished Th2 cytokine levels, including IL-4, and IL-5, as well as Th17 cytokine IL-17 expression levels in NALF and lung homogenates by inhibiting NF-κB activation.
IκB is rapidly removed by IKKα/β and activated NF-κB is released and then translocated to the nucleus, where it activates the transcription of target genes [29]. The effect of SSD treatment supported the hypothesis that the expression of these pro-inflammatory cytokines is NF-κB dependent. These results revealed that SSD suppressed NF-κB and P–NF-κB activation. In addition, the NF-κB dependent increase in the levels of inflammatory cytokines was markedly inhibited. Therefore, we postulate that SSD has an anti-inflammatory effect, which acts through the inhibition of NF-κB activation.
The OVA sensitization and treatment of mice led to an increase in the levels of antigen-specific immunoglobulins in serum and the infiltration of inflammatory cells in the epithelium and sub-epithelium of the nasal mucosa [30]. IgE is a pleiotropic molecule that acts as a key inflammatory molecule in the Th2-inflammatory cascade as well as a modulatory molecule of innate and adaptive immune systems (Oettgen HC. 2016). IL-4 is a Th2 cytokine, which plays an important role in promoting B-cell proliferation and stimulating B cells to secrete IgE (Iwaszko et al., 2021). Allergen-specific IgE antibodies can be detected in serum for extended periods of time after prolonged periods with allergen exposure. Our study revealed that SSD remarkably inhibited OVA-specific IgE and IgG1 levels and increased the production of IgG2a in OVA-induced mice compared with OVA group. These results demonstrated that SSD displays an anti-allergic activity against allergic responses.
Mast cells and eosinophils regulate local immune and inflammatory responses, and their accumulation in blood and tissue is associated with several inflammatory diseases [31]. Our results demonstrated that SSD inhibited the infiltration of mast cells, and goblet cell hyperplasia into the nasal tissues of OVA-induced AR mice. Therefore, we postulate that SSD undergoes anti-allergic and anti-inflammatory reactions through the inhibition of inflammatory cell infiltration, of both the nasal and lung tissues.
5. Conclusion
Our findings provide in vivo evidence that the anti-allergic and anti-inflammatory agent SSD significantly diminishes allergic reactions by alleviating the symptoms of OVA-induced AR, by mediating the Th1/Th2 cell balance, via regulation of the T-bet, GATA-3, and RORγ expression levels. Therefore, SSD may represent an alternative therapeutic approach for the treatment of patients with AR.
Author contributions
OHC conceived and designed the experiments. CHP and TTB performed the experiments. CHP and CHS analyzed and interpreted the data. SCZ contributed reagents, materials, analysis tools or data. SCZ and QOHC wrote the paper. All authors discussed the results and reviewed the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Korea Food Research Institute (grant number E0170400-04) and the Fund of BK21FOUR 21st Century of Medical Science Creative Human Resource Development Center.
Contributor Information
Chun Hua Piaoa, Email: chpiao@qud.edu.com.
Ok Hee Chai, Email: okchai1004@jbnu.ac.kr.
References
- 1.Pawankar R., Mori S., Ozu C., Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy. 2011;1:157–167. doi: 10.5415/apallergy.2011.1.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S.I., Lee I.H., Kim M., Ryu G., Kang S.Y., Kim M.A., Lee S.M., Kim H.J., Park D.Y., Lee Y.J., Kim D.K., Kim S.W., Kim D.H., Jun Y.J., Park S.C., Kim B.S., Chung S.J., Lee H.J., Kim H.B., Choi J.H., Choi G.S., Yang H.J. KAAACI allergic rhinitis guidelines: Part 1. Update in pharmacotherapy. Allergy Asthma Immunol. Res. 2023;15:19–31. doi: 10.4168/aair.2023.15.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellanti J.A., Wallerstedt D.B. Allergic rhinitis update: epidemiology and natural history. Allergy Asthma Proc. 2000;21:367–370. doi: 10.2500/108854100778249088. [DOI] [PubMed] [Google Scholar]
- 4.Adamia N., Jorjoliani L., Khachapuridze D., Katamadze N., Chkuaseli N. Georgian Med News; 2015. Allergic Diseases and Asthma in Adolescents; pp. 58–62. [PubMed] [Google Scholar]
- 5.Mowen K.A., Glimcher L.H. Signaling pathways in Th2 development. Immunol. Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D.H., Zhang J.Z.H. Uniform J-shifting approach for calculating reaction rate constant. J. Chem. Phys. 1999;110:7622–7626. [Google Scholar]
- 7.Chen Z.J., Lin F., Gao Y.Y., Li Z.Y., Zhang J., Xing Y., Deng Z.H., Yao Z.J., Tsun A., Li B. FOXP3 and ROR gamma t: transcriptional regulation of Treg and Th17. Int. Immunopharm. 2011;11:536–542. doi: 10.1016/j.intimp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Halwani R., Al-Muhsen S., Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013;143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- 9.Wakashin H., Hirose K., Maezawa Y., Kagami S., Suto A., Watanabe N., Saito Y., Hatano M., Tokuhisa T., Iwakura Y., Puccetti P., Iwamoto I., Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 10.Wan F., Lenardo M.J. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A., Takada Y., Boriek A.M., Aggarwal B.B. Nuclear factor-kappa B: its role in health and disease. J. Mol. Med. Jmm. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 12.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 13.Mathur S., Hoskins C. Drug development: lessons from nature. Biomed. Rep. 2017;6:612–614. doi: 10.3892/br.2017.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C.N., Yuan Z.G., Zhang X.L., Yan R., Zhao Y.Q., Liao M., Chen J.X. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-kappaB signaling pathway. Int. Immunopharm. 2012;14:121–126. doi: 10.1016/j.intimp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Wong V.K., Zhang M.M., Zhou H., Lam K.Y., Chan P.L., Law C.K., Yue P.Y., Liu L. Saikosaponin-d enhances the anticancer potency of TNF-alpha via overcoming its undesirable response of activating NF-kappa B signalling in cancer cells. Evid. Bas. Compl. Alter. Med. 2013;2013 doi: 10.1155/2013/745295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bui T.T., Piao C.H., Hyeon E., Fan Y., Choi D.W., Jung S.Y., Jang B.H., Shin H.S., Song C.H., Chai O.H. Preventive effect of Bupleurum chinense on nasal inflammation via suppressing T helper type 2, eosinophil and mast cell activation. Am. J. Chin. Med. 2019;47:405–421. doi: 10.1142/S0192415X19500204. [DOI] [PubMed] [Google Scholar]
- 17.Wang H.W., Liu M., Zhong T.D., Fang X.M. Saikosaponin-d attenuates ventilator-induced lung injury in rats. Int. J. Clin. Exp. Med. 2015;8:15137–15145. [PMC free article] [PubMed] [Google Scholar]
- 18.Piao C.H., Song C.H., Lee E.J., Chai O.H. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-gammat/STAT3/IL-17/NF-kappaB pathway in OVA-induced allergic rhinitis. Chem. Biol. Interact. 2020;315 doi: 10.1016/j.cbi.2019.108874. [DOI] [PubMed] [Google Scholar]
- 19.Mandhane S.N., Shah J.H., Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int. Immunopharm. 2011;11:1646–1662. doi: 10.1016/j.intimp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Fu L.J., Dai Y., Wang Z.T., Zhang M. Inhibition of experimental allergic rhinitis by the n-butanol fraction from the anomalous fruits of Gleditsia sinensis. Biol. Pharm. Bull. 2003;26:974–977. doi: 10.1248/bpb.26.974. [DOI] [PubMed] [Google Scholar]
- 21.Han L.N., Guo S.L., Li T.L., Ding G.L., Zhang Y.J., Ma J.L. Effect of immune modulation therapy on cardiac function and T-bet/GATA-3 gene expression in aging male patients with chronic cardiac insufficiency. Immunotherapy. 2013;5:143–153. doi: 10.2217/imt.12.139. [DOI] [PubMed] [Google Scholar]
- 22.Ku C.J., Lim K.C., Kalantry S., Maillard I., Engel J.D., Hosoya T. A monoallelic-to-biallelic T-cell transcriptional switch regulates GATA3 abundance. Genes Dev. 2015;29:1930–1941. doi: 10.1101/gad.265025.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer E.O., Blaiss M.S., Derebery M.J., Mahr T.A., Gordon B.R., Sheth K.K., Simmons A.L., Wingertzahn M.A., Boyle J.M. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J. Allergy Clin. Immunol. 2009;124:S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Song R., Tian J.X., Tian Y., Liu G.Q., Zhang Z.J. Analysis of saikosaponins in rat plasma by anionic adducts-based liquid chromatography tandem mass spectrometry method. Biomed. Chromatogr. 2012;26:808–815. doi: 10.1002/bmc.1734. [DOI] [PubMed] [Google Scholar]
- 26.Wu S.J., Tam K.W., Tsai Y.H., Chang C.C., Chao J.C. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am. J. Chin. Med. 2010;38:99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- 27.Molet S., Hamid Q., Davoine F., Nutku E., Taha R., Page N., Olivenstein R., Elias J., Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 28.Foster P.S., Hogan S.P., Ramsay A.J., Matthaei K.I., Young I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 30.Bahekar P.C., Shah J.H., Ayer U.B., Mandhane S.N., Thennati R. Validation of Guinea pig model of allergic rhinitis by oral and topical drugs. Int. Immunopharm. 2008;8:1540–1551. doi: 10.1016/j.intimp.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Tian B.P., Xia L.X., Bao Z.Q., Zhang H., Xu Z.W., Mao Y.Y., Cao C., Che L.Q., Liu J.K., Li W., Chen Z.H., Ying S., Shen H.H. Bcl-2 inhibitors reduce steroid-insensitive airway inflammation. J. Allergy Clin. Immunol. 2017;140:418–430. doi: 10.1016/j.jaci.2016.11.027. [DOI] [PubMed] [Google Scholar]