Abstract
Background
interleukin 23 (IL-23) is an important factor involved in the survival and proliferation of T helper 17 cells (Th17), known for their implication in multiple sclerosis (MS). By contrast, IL-27 regulates and modulates the function of T lymphocytes, in particular as a suppressor of Th17 differentiation. The aims of the study were i) to test the association of cytokines with the clinical and genetic characteristics in each of the multiple sclerosis groups (CIS - clinically isolated syndrome, RRMS - relapsing-remitting MS and SPMS – Secondary progressive MS) and ii) to evaluate the association between serum levels of IL-23 and IL-27 with T4730C (IL-27), A964G (IL-27) and R381Q (IL-23) gene polymorphisms in RRMS patients.
Methods
Blood samples were obtained from 82 patients diagnosed with MS under treatment with glatiramer acetate (GA), interferon beta (IFN) 1 A and 1 B. IL-23 and IL-27 serum concentrations were measured by enzyme-linked immunosorbant assay (ELISA). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used in order to determine the genotypes for R381Q (IL-23) polymorphisms, T4730C (IL-27) and A964G (IL-27).
Results
Patients with SPMS, RRMS and CIS respectively differed significantly regarding age distribution (p = 0.003) but the studied MS groups were similar regarding age at disease onset (p = 0.528) and treatment type (p = 0.479). A significant increase of mean serum IL-27 was noticed in cases with early onset (age at disease onset <28 years) of RRMS (mean difference: 4.2 pg/ml, 95% CI: 0.8–5.3 pg/ml), compared to cases with later onset of RRMS (age at disease onset ≥28 years). RRMS patients with wild GG genotype of R381Q (IL-23) showed a significant increase of mean serum IL-23 than patients with variant AG genotype (mean difference: 115.1 pg/ml, 95% CI: 8.6–221.6 pg/ml). A trend for a higher increase in means of serum IL-23 (p = 0.086) was observed in RRMS patients carriers of AA genotype of A964G (IL-27) polymorphism in comparison with patients with AG or GG genotypes. We found no significant monotonic correlation of IL-27, IL-23 serum levels with age at disease onset (years) and duration of disease (p > 0.05) in the CIS and SPMS group respectively but a significant correlation between IL-23 and the duration of disease-modifying treatment was noticed only in the SPMS group.
Conclusions
The results of the current study suggest an association between IL-23 levels and the R381Q gene polymorphism and also a relationship between IL-27 serum levels and early age at disease onset in RRMS patients.
Keywords: Interleukin-23, Interleukin-27, Polymorphism, Multiple sclerosis, Glatiramer acetate, Inteferon beta
Highlights
-
•
IL-23 and IL-27 contribute to MS pathology.
-
•
IL-23 R381Q polymorphism might be associated with IL-23 level in MS.
-
•
IL-27 A964G polymorphism in RRMS might be correlated with higher levels of serum IL-23.
-
•
Higher serum levels of IL-27 were found in cases with early MS onset.
1. Introduction
Multiple sclerosis (MS) is a complex autoimmune disorder of the central nervous system (CNS) characterized by myelin loss, in which the pathogenic process evolves in two distinct phases represented by neuroinflammation and neurodegeneration [1]. The fluctuating course of the disease individualizes MS from other inflammatory diseases of the CNS. In the first phase of the disease, the episodes of neurological and radiological worsening alternate with variable degrees of recovery, known as relapsing remitting stage. In the evolution of the disease, simultaneously with the inflammation resolution, a chronic progressive process of neurodegeneration begins, delineating the secondary progressive stage [1].
MS affects 3 million people worldwide with an estimated prevalence of 142 cases per 100.000 people in western Europe and 34.8 cases per 100.000 people in Romania [2,3].
The etiology of MS is not entirely understood, but environmental and genetic factors acting on a dysregulated adaptive immune system were shown to be the ground for MS development [4]. Cytokines have key roles in the immunopathogenic process of MS, displaying both pro-inflammatory or anti-inflammatory properties in a disease stage dependent manner, enhancing tissues destruction or promoting function restoration [5]. Over time, experimental studies investigating MS pathogenesis, showed that T cells and B cells activated in the peripheral lymph nodes, migrate through the brain blood barrier (BBB) in the CNS, where they reactivate and differentiate into effector cells [6]. Through cytokines production, T cells mediate myelin destruction and attract other immune cells from the peripheral blood [6]. From the differentiated T cells subsets, Th1 and Th17 are recognized for their pathogenic role [6].
IL-23, a defender against extracellular bacteria, is part of IL-12 family and is produced by dendritic cells and macrophages in peripheral tissues: skin, lungs and intestinal mucosa [7,8]. IL-23 is a heterodimeric cytokine composed of p19 and p40 (shared with IL-12) polypeptidic chains [9]. IL-23 plays a major role in chronic inflammation, which is a common feature of all autoimmune diseases, acting through distinct pathways. The first one consists of the activation of Th17 cells and the second one consists of the stimulation of IL-17 secretion by other immune cells [10].
IL-23 acts as an inducer and stabilizer of Th17 and recruiter of neutrophils and monocytes in MS and its corresponding animal models [11,12]. Studies evaluating the role of IL-23 in MS pathogenesis, revealed that the exposure of naïve CD4+ T cells to IL-23 is mandatory, in order to obtain a complete differentiation into fully pathogenic Th17 lymphocytes, together with the presence of antigenic stimulation, transforming growth factor-β (TGF-β) and IL-6 [13,14]. The population of Th17 lymphocytes exposed only to TGF-β and IL-6 were found less immunologically reactive than the ones exposed to IL-23 [14].
IL-23 receptor (IL-23 R) is expressed on the surface of natural killer, dendritic cells, macrophages and activated T cells, particularly on the Th17 subtype [9]. IL-23 attached to IL23-R stimulates the transcription of pro-inflammatory cytokine genes, such as IL-17 [15]. R381Q (rs11209026) is a single nucleotide polymorphism (SNP) consisting in the substitution of guanine (G) by adenine (A) in nucleotide 1142 of the IL-23 R gene (G1142A) [16]. This genetic variation is followed by functional consequences, resulting into an amino acid substitution at residue 381 in the final protein product, leading to arginine (Arg) into glutamine (Gln) modification (Arg381 Gln- R381Q) [16]. Studies showed that the amino acid replacement of Arg with Gln changes the communication pathways of IL-23 [17,18].
IL-23 was evaluated in MS, for its implication in the inflammatory process of the optic nerve and the CNS, attracting into the CNS the myelin specific T cells and stimulating their survival in the brain parenchyma [[19], [20], [21]].
IL-27 a member of IL-12 family, has distinct roles in shaping T lymphocytes activity [22,23]. IL-27 has a heterodimeric structure, consisting of Epstein–Barr-induced gene 3 product (EBI3) and IL-27p28 [24]. The receptor for IL-27 is expressed on the surface of microglia, NK, plasma cells, endothelial cells and placental trophoblasts emphasizing the importance of IL-27 in balancing the immune status of the brain and uterus [25]. IL-27 has different roles in acute and chronic inflammation [26]. IL-27 behaves as a downregulater in chronic inflammation through IL-10 production, in order to prevent organs dysfunction and tissues injuries [26]. IL-27 stimulates the production of T regulatory cells, it inhibits Th2 and Th17 responses, reducing the severity of the autoimmune processes [27].
Evaluating the levels of IL-27 in postmortem studies of patients with MS, higher levels were found in demyelinating plaques comparing with controls brains [23]. The production of IL-27 b y astrocytes and microglial cells promotes remyelination and enhances neurotrophic factor and nerve growth factor production [22,28].
The gene encoding IL-27 was found on chromosome 16p1 [29]. Several SNPs were found in IL-27 gene. One of the SNPs studied in MS and other autoimmune diseases is A964G (rs153109), with functional consequences. A964G is located in close proximity to the transcription locus of IL-27 gene and consists of the replacement of adenine (A) with guanine (G) in nucleotide 964, giving rise to a new binding site in the gene promoter, which influences the expression pattern [30,31]. The localization of A964G in close proximity to other functional epigenetic sites, which influence the transcriptional activity of the promoter and enhancer and controls the protein expression and transcription, raised interest in evaluating this genetic variation in different types of digestive and gynecological neoplasms [32].
T4730C (rs181206, Leu119Pro) is another IL-27 SNP, studied in Behcet's disease (BD), ulcerative colitis (UC) and rheumatoid arthritis (RA) [31,33,34]. T4730C is a missense SNP, found in exon 4 of IL-27 gene, consisting of the substitution of thimine (T) with cytosine (C) in nucleotide 4730, leading to the replacement in position 119, of the amino acid leucine with proline (Leu119Pro) [[35], [36], [37]].
Considering the crucial role of IL-23 and IL-27 in modulating the activity of Th17 cells, we determined the concentration of this cytokines in our sample of MS patients under treatment with interferon and glatiramer acetate, aiming: i) to test the association between the cytokines (IL-23 and IL-27) values and the clinical characteristics in each of the multiple sclerosis groups (CIS, RRMS and SPMS group) and ii) to evaluate the association between IL-23 and IL-27 serum levels and T4730C (IL-27), A964G (IL-27) and R381Q (IL-23) gene polymorphisms in RRMS patients.
2. Materials and methods
In our study, between March 2019–January 2020, a non-probabilistic sample of eighty two patients with MS were selected and included: 6 patients with CIS, 67 patients with RRMS and 9 patients with SPMS.
The patients were recruited in Cluj-Napoca, from the National Program of Multiple Sclerosis in the Neurology Clinic. Multiple sclerosis diagnosis was formulated according to 2017 McDonald criteria [38]. The information was obtained from neurological examinations and personal interviews. Data about imaging investigations were obtained from the patients records.
The inclusion criteria were: patients with the diagnosis of MS, under IFN or GA treatment for MS. We chose to include only patients who started injectable disease-modifying treatments (DMTs), given that oral DMTs were available only after 2017 in Romania.
The exclusion criteria were: other immunomodulatory therapies recently used, other autoimmune diseases such as SLE (systemic lupus erythematosus), RA (rheumatoid arthritis), ankylosing spondylitis (AS), inflammatory bowel diseases (IBD), psoriasis, type 1 diabetes mellitus, use of cortisone in the last month, clinical relapse at the time of evaluation.
Ethics statement: The study was performed in conformity with the principles of the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca (Protocol Code 31, February 25, 2019). All patients were informed about the aim of the study and signed an informed content.
2.1. Methods
2.1.1. Serum IL-23 and IL-27 determination
We determined human IL-23 and IL-27 concentrations in serum, using micro enzyme-linked immunosorbant assay (ELISA) plates pre-coated with an antibody specific to human IL-23 and IL-27 (Elabscience Biotechnology Inc., Houston, TX, USA) according to the manufacturer's protocol (IL-23- Catalog No:E-EL-H0107, detection range: 39.06–2500 pg/ml; IL-27- Catalog No: E-EL-H2338, detection range: 31.25–2000 pg/ml).
Samples were added to the micro ELISA plate wells pre-coated with an antibody specific to Human IL-23 and IL-27 respectively, and combined with the specific antibody. For each probe, a biotinylated detection antibody specific for Human IL-23 and IL-27 respectively and Avidin-Horseradish Peroxidase (HRP) conjugate were added. We added the substrate solution to each well. The enzyme-substrate reaction was stopped by the addition of stop solution, the color turning yellow. For both IL-23 and IL-27, we measured spectrophotometrically the optical density (OD) at a wavelength of 450 nm ± 2 nm using a microplate reader (Absorbance Microplate Reader Sunrise Tecan; Tecan Group Ltd., Männedorf, Switzerland) and Biochrom Asys Atlantis Microplate Washer (Biochrom Ltd. Cambridge, UK). The OD value was proportional to the concentration of Human IL-23 and IL-27, respectively. Both the serum IL-23 and IL-27 concentrations were measured by comparing the OD of the samples to the standard curve (Sensitivity (IL-23) = 23.44 pg/ml, Sensitivity (IL-27) = 18.75 pg/ml).
2.1.2. T4730C (IL-27), A964G (IL-27) and R381Q (IL-23) identification
In order to identify the genotypes for T4730C (IL-27), A964G (IL-27) and R381Q (IL-23) polymorphisms, we used DNA extraction from blood leukocytes using a Zymoresearch kit (Quick DNAMiniprep, Kit-Zymo Research Corporation, Freiburg, Germany), followed by PCR- RFLP analysis using specific primers from Eurogentec (Kaneka Eurogentec S.A. Biologics Division, LIEGE, Belgium), and FuaI, XhoI and Hpy188i restriction enzymes from New England Biolabs (New England Biolabs UK, Ltd, Hitchin, UK). The methods were presented by Mohamadi et al. Anber et al. and Mosayebian et al. [[39], [40], [41]] and optimized in our laboratory [42].
2.2. Statistical analysis
Demographic variables were summarized by arithmetic mean and standard deviation (SD) or frequencies (%). Clinical variables with departures from Gaussian distribution were summarized by median with interquartile interval IQR = [25th percentile; 75th percentile]. Comparison of demographic and clinical characteristics between CIS, RRMS and SPMS groups was performed using the Chi-squared (χ2), Fisher's exact tests, one-way ANOVA or Kruskal-Wallis test. Parametric and nonparametric pairwise multiple-comparison procedures as Turkey HSD and Dunn's test were applied when the overall estimated significance level (p-value) for ANOVA or Kruskal-Wallis tests reached the statistical threshold.
Distributions of serum cytokines followed Gaussian distributions in RRMS patients but not in SPMS patients or CIS patients. Assessment of univariate normality was done using several methods, such as descriptive statistics, quantile-quantile (Q-Q) plot and Shapiro-Wilk test with Holm correction for multiple comparisons.
The associations of serum cytokines with clinical variables and gene polymorphisms were tested using Student-t test with equal variances, Welch Two Sample t-test or ANOVA test.
Because of the small sample size of CIS group (n = 6) and SPMS group (n = 9) and small frequencies (<2) of certain variant genotypes, the associations between serum cytokines and studied SNPs was not tested in CIS and SPMS groups.
The departure from Hardy–Weinberg Equilibrium (HWE) for studied SNPs was tested using the exact Chi-square test from “genetics” R package [43].
All statistical analyses were performed in R software, version 4.1.3[44].
3. Results
3.1. Description of the studied groups
The demographic and clinical characteristics of the studied clinical forms of MS are described in Table 1. SPMS, RRMS patients and CIS patients differed significantly in age distribution, SPMS patients presenting increased age (age range: 35 − 64 years, median age: 51) when compared with RRMS patients (age range: 25 − 67 years, median: 43) and CIS patients (age range: 22− 45 years, median: 32). In the CIS group 4 patients were men (66.7%) and 2 patients were women (33.3%). In the RRMS group 57 patients were women (85.1%) and 10 patients were men (10.9%) and in the SPMS group 6 patients were men (66.7%) and 3 patients were women (33.3%). Concerning the presence of the personal history of autoimmune diseases, only four (6%) patients in the RRMS group presented personal history of autoimmune disease.
Table 1.
Demographic and clinical characteristics of the CIS, RRMS and SPMS patients.
| Variables | CIS patients (n1 = 6) | RRMS patients (n2 = 67) | SPMS patients (n3 = 9) | p-value |
|---|---|---|---|---|
| Age (years), mean ± SD | 33.0 ± 7.8 | 43.4 ± 10.2 | 51.6 ± 9.2 | 0.003* |
| Gender, n (%) | 0.0003* | |||
| Male | 4 (66.7) | 10 (14.9) | 6 (66.7) | |
| Female | 2 (33.3) | 57 (85.1) | 3 (33.3) | |
| Age at disease onset (years), median [IQR] | 28 [27; 30.5] | 28 [23.5; 39.5] | 34 [32.0; 41.0] | 0.528 |
| Duration of disease (years), median [IQR] | 2 [1.25; 2.75] | 10 [7.5; 15.5] | 13 [11.0; 24.0] | 0.001* |
| Lesion location on MRI | 0.874 | |||
| Supratentorial or infratentorial region | 2 (33.3) | 14 (20.9) | 4 (44.4) | |
| Both regions | 1 (16.7) | 23 (34.3) | 2 (22.2) | |
| supratentorial + infratentorial + MS | 2 (33.3) | 21 (31.3) | 2 (22.2) | |
| supratentorial + MS | 1 (16.7) | 9 (13.4) | 1 (11.1) | |
| EDSS Score at admission | 0 [0, 0.75] | 2 [1; 3] | 6 [4; 6.5] | <0.0001* |
| Disease-modifying treatment, n (%) | 0.479 | |||
| Interferon-beta | 2 (33.3) | 33 (49.3) | 6 (66.7) | |
| Glatiramer acetate | 4 (66.7) | 34 (50.7) | 3 (33.3) | |
| Duration of disease-modifying treatment (months), median [IQR] | 16 [8.3; 24.5] | 72 [30.0; 120.0] | 156 [132.0; 156.0] | 0.001* |
CIS-clinically isolated syndrome; RRMS-relapsing remitting multiple sclerosis; SPMS-secondary progressive multiple sclerosis; MRI-magnetic resonance imaging; MS- multiple sclerosis; SD = standard deviation; IQR = [Q1, Q3] where Q1 = Quartile1; Q3 = Quartile 3; n (%) = absolute frequencies (% percentages estimated from the size of group); p-values obtained from ANOVA, Kruskal-Wallis test or Fisher's exact test; * significant results: p-value <0.05.
We found no significant differences concerning age at disease onset between the studied clinical forms of MS (p = 0.528). However, RRMS patients significantly differed from SPMS patients in EDSS scores (adjusted p-value = 0.00004). The frequency distribution of MS treatment was similar in the studied groups (p = 0.479) with significant differences regarding the duration of treatment between RRMS patients and SPMS patients (adjusted p-value = 0.0161) (Table 1).
3.2. Association analysis between serum levels of IL-23 and IL-27 and clinical characteristics in RRMS patients
Correlation analysis highlighted a significant monotonic relationship between the IL-27 and patients'age values and a tendency toward statistical significance regarding the relationships between the IL-27 and patients'age at disease onset (Table 2).
Table 2.
Correlations between IL-23 and IL-27 values and the clinical characteristics of RRMS patients.
| Variables |
IL-23 (pg./ml) |
IL-27(pg./ml) |
|---|---|---|
| Correlation Coefficient ρ (p-value) | Correlation Coefficient ρ (p-value) | |
| Age (years) | 0.04 (0.758) | −0.31 (0.012*) |
| Age at disease onset (years) | −0.08 (0.529) | −0.20 (0.097) |
| Duration of disease (years) | 0.09 (0.475) | −0.12 (0.328) |
| EDDS (points) | 0.04 (0.729) | −0.08 (0.500) |
| Duration of Disease-modifying treatment (months) | −0.01 (0.910) | −0.06 (0.647) |
| Number of relapses under treatment | −0.03 (0.809) | 0.02 (0.859) |
When quantitative clinical characteristics were dichotomized, bivariate analysis did not reveal significant relationships between the IL-23 serum levels and gender, age at disease onset (<28 years vs. ≥ 28 years), degree of disability (EDSS >2 vs. EDSS ≤2) and duration of disease-treatment (≥72 months vs. < 72 months) (Table 3). Patients with early onset of RRMS (age at disease onset <28 years) had significantly higher mean serum IL-27 levels as compared with patients with later onset of RRMS (age at disease onset ≥28 years) (21.7 ± 5.3 pg/ml vs. 19.0 ± 4.4 pg/ml, p = 0.026).
Table 3.
Serum levels of IL-23 and IL-27 in relation to the clinical characteristics of RRMS patients.
| Variables | Groups | Mean ± SD or Median [IQR] | p-value |
|---|---|---|---|
| IL-23 (pg./ml) | |||
| Gender | Female (n = 57) | 178.8 ± 93.8 | 0.342 |
| Male (n = 10) | 209.2 ± 86.5 | ||
| Age at disease onset(a | <28 years (n = 33) | 192.7 ± 80.2 | 0.421 |
| ≥28 years (n = 34) | 174.3 ± 103.8 | ||
| Duration of disease (b | ≤10 years (n = 34) | 183.6 ± 110.5 | 0.979 |
| >10 years (n = 33) | 183.1 ± 71.7 | ||
| EDSS (c | ≤2 (n = 38) | 172.3 ± 100.8 | 0.257 |
| >2 (n = 29) | 197.7 ± 80.4 | ||
| Number of relapses under treatment (d | <2 (n = 42) | 186.0 ± 95.0 | 0.763 |
| ≥2 (n = 25) | 178.9 ± 90.5 | ||
| Duration of Disease-modifying treatment (e | <72 months | 194.2 ± 102.7 | 0.404 |
| ≥72 months | 175.1 ± 84.8 | ||
| Disease-modifying treatment | Interferon-beta (n = 33) | 183.3 ± 108.8 | 0.996 |
| Glatiramer acetate (n = 34) | 183.4 ± 75.6 | ||
| IL-27(pg./ml) | |||
| Gender | Female (n = 57) | 19.9 ± 4.9 | 0.139 |
| Male (n = 10) | 22.5 ± 5.2 | ||
| Age at disease onset (a | <28 years (n = 33) | 21.7 ± 5.3 | 0.026* |
| ≥28 years (n = 34) | 19.0 ± 4.4 | ||
| Duration of disease (b | ≤10 years (n = 34) | 19.9 ± 3.9 | 0.554 |
| >10 years (n = 33) | 20.6 ± 5.6 | ||
| EDSS (c | ≤2 (n = 38) | 20.1 [16.6; 23.8] | 0.672 |
| >2 (n = 29) | 19.2 [16.5; 22.5] | ||
| Number of relapses under treatment (d | <2 (n = 42) | 20.2 ± 5.0 | 0.764 |
| ≥2 (n = 25) | 20.6 ± 4.9 | ||
| Duration of disease-modifying treatment (e | <72 months | 20.3 ± 4.5 | 0.922 |
| ≥72 months | 20.4 ± 5.3 | ||
| Disease-modifying treatment | Interferon-beta (n = 33) | 21.4 ± 5.4 | |
| Glatiramer acetate (n = 34) | 19.3 ± 4.4 | 0.079 | |
IQR = [Q1, Q3] where Q1 = Quartile1; Q3 = Quartile 3; n = number of cases; p-values were obtained from Student-t test with equal variances or Welch Two Sample t-test; (a the median value was equal to 28 years; (b the median value was equal to 10 years;(c the median value was 2 points; (d the median value was equal to one relapse; (e the median value was equal to 72 months; *significant result: p < 0.05.
3.3. Correlation analysis between serum levels of IL-23 and IL-27 and clinical characteristics in CIS and SPMS patients
We found no significant monotonic correlation between IL-27 and values of age at disease onset, duration of disease and duration of disease-modifying treatment in the CIS and SPMS group respectively (Table 4). We also noticed a positive correlation of IL-27 with patients ‘age values (ρ = 0.75, p = 0.021) and a positive correlation between IL-23 and duration of disease-modifying treatment (ρ = 0.70, p = 0.037) in the SPMS group (Table 4).
Table 4.
Correlations between IL-23 and IL-27 values and the clinical characteristics of CIS and SPMS patients.
| Variables |
IL-23 (pg./ml) |
IL-27(pg./ml) |
|---|---|---|
| Correlation Coefficient ρ (p-value) | Correlation Coefficient ρ (p-value) | |
| CIS group (n = 6) | ||
| Age (years) | −0.35 (0.499) | 0.29 (0.577) |
| Age at disease onset (years) | 0.06 (0.913) | 0.09 (0.869) |
| Duration of disease (years) | −0.20 (0.699) | 0.06 (0.913) |
| EDDS (points) | 0.78 (0.069) | −0.78 (0.069) |
| Duration of Disease-modifying treatment (months) | −0.54 (0.297) | 0.43 (0.419) |
| Number of relapses under treatment | NA | NA |
| SPMS group (n = 9) | ||
| Age (years) | −0.22 (0.581) | 0.75 (0.021*) |
| Age at disease onset (years) | −0.48 (0.192) | 0.34 (0.368) |
| Duration of disease (years) | 0.34 (0.366) | 0.06 (0.881) |
| EDDS (points) | −0.04 (0.729) | −0.08 (0.500) |
| Duration of Disease-modifying treatment (months) | 0.70 (0.037*) | −0.17 (0.653) |
| Number of relapses under treatment | −0.27 (0.478) | 0.14 (0.724) |
*significant result: p < 0.05; ρ = Spearman correlation coefficient; NA = not available (no CIS patients with relapses under treatment).
3.4. Association analysis between serum levels of IL-23 and IL-27 and studied SNPs in RRMS patients
The genotype distributions of studied SNPs in RRMS cases did not reveal any significant deviation from the Hardy–Weinberg equilibrium (p = 0.368 for T4730C (IL-27), p = 0.630 for A964G (IL-27) and p = 1.00 for R381Q (IL-23)).
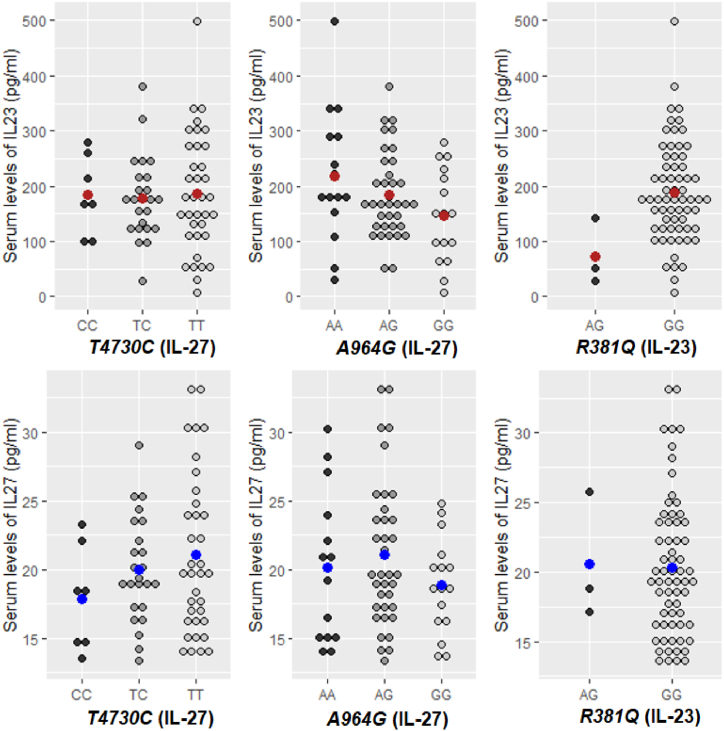
In order to identify the associations of IL-23 and IL-27 with studied SNPs, the serum cytokines levels were compared across the genotypes (Fig. 1). In the dominant model, significantly higher IL-23 levels (p = 0.035) were observed in RRMS patients carriers of the GG (R381Q-IL-23) genotype (188.5 ± 91.1 pg/ml) as compared with carriers of the AG (73.4 ± 59.9 pg/ml) genotype. In the codominant model, higher IL-23 levels were observed in patients carriers of the AA (A964G-IL-23) genotype (218.9 ± 121.1 pg/ml) as compared with those who were carriers of the AG (185.3 ± 77.7 pg/ml) and GG (145.6 ± 85.2 pg/ml) genotypes (Table 5).
Fig. 1.
Distributions of serum levels of IL-23 and IL-27 in different genotypes groups in RRMS patients. Note. The dot plots represent measured values for serum IL-23 and IL-27 in each genotype for RRMS patients. Red and blue points represent the mean values of cytokines. The first row represents the level of serum IL-23 in the studied genotypes and the second row represents the serum level of IL-27 in each studied genotype. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 5.
Serum levels of IL-23 and IL-27 in relation to IL-27 and IL-23 gene polymorphisms of RRMS patients.
| SNPs | Genetic model | Genotypes | Mean ± SD | p-value |
|---|---|---|---|---|
| IL-23 (pg./ml) | ||||
| T4730C (IL-27) | Codominant | TT (n = 36) | 186.5 ± 107.5 | 0.950 |
| TC (n = 24) | 178.5 ± 75.5 | |||
| CC (n = 7) | 183.8 ± 71.5 | |||
| Dominant | TT (n = 36) | 186.5 ± 107.5 | 0.762 | |
| TC + CC (n = 31) | 179.7 ± 73.5 | |||
| A964G (IL-27) | Codominant | AA (n = 15) | 218.9 ± 121.1 | 0.086 |
| AG (n = 36) | 185.3 ± 77.7 | |||
| GG (n = 16) | 145.6 ± 85.2 | |||
| Dominant | AA (n = 15) | 218.9 ± 121.1 | 0.184 | |
| AG + GG (n = 52) | 173.1 ± 81.4 | |||
| R381Q (IL-23) | Codominant | GG (n = 64) | 188.5 ± 91.1 | 0.035* |
| AG (n = 3) | 73.4 ± 59.9 | |||
| IL-27 (pg./ml) | ||||
| T4730C (IL-27) | Codominant | TT (n = 36) | 21.1.5 ± 5.7 | 0.934 |
| TC (n = 24) | 20.0 ± 3.8 | |||
| CC (n = 7) | 17.9 ± 3.8 | |||
| Dominant | TT (n = 36) | 21.1 ± 5.7 | 0.198 | |
| TC + CC (n = 31) | 19.5 ± 3.9 | |||
| A964G (IL-27) | Codominant | AA (n = 15) | 20.2 ± 5.4 | 0.314 |
| AG (n = 36) | 21.1 ± 5.3 | |||
| GG (n = 16) | 18.8 ± 3.5 | |||
| Dominant | AA (n = 15) | 20.2 ± 5.4 | 0.875 | |
| AG + GG (n = 52) | 20.4 ± 4.9 | |||
| R381Q (IL-23) | Codominant | GG (n = 64) | 20.3 ± 5.0 | 0.940 |
| AG (n = 3) | 20.6 ± 4.6 | |||
SD = sample standard deviation; p-values were obtained from Student-t test with equal variances or Welch Two Sample t-test or ANOVA.
4. Discussion
In MS, the consolidation of the inflammatory process and also the resolution of this process is dependent upon cytokines activation.
Based on the importance of both IL-27 and IL-23 and their receptors in the inflammatory processes in patients with MS, as well as on the recent findings that IL-23 R may be a genetic risk factor for autoimmune diseases [45], we studied IL-27 and IL-23 R SNPs and their serological levels in MS.
Di Meglio et al. (2013), Pidasheva et al. (2011) and Sarin et al. (2011) showed a defective IL-23 signal in cells from individuals carrying the protective A381 allele, causing a selective decreasing of IL-17 production, by reducing STAT3 phosphorylation, offering protection against psoriasis, Crohn's disease (CD) and AS [[45], [46], [47]]. The same results were reported by Deveci et al. (2019), demonstrating a significantly higher presentation of R381Q among healthy controls comparing with patients, suggesting a protective effect of the A381 allele from immune-mediated chronic inflammation [48]. We obtained preliminary results in two anterior pilot studies in which we analyzed the possible role of T4730C (IL-27), A964G (IL-27), R381Q (IL-23) gene polymorphisms and the susceptibility to MS [42,49].
In the current study, we found that RRMS patients with wild (GG) genotype of R381Q (IL-23) showed a significantly higher mean serum IL-23 than patients with variant (AG) genotype, suggesting the possible association of this genetic variation with the serum IL-23 concentration (difference in means: 115.1 pg/ml, 95% CI for difference: 8.6–221.6 pg/ml). The gender, age of onset, the duration of treatment followed, the EDSS score, the number of relapses and the disease duration were not significantly associated with the variation of IL-23 in each of the studied MS groups.
A double-blinded placebo-controlled study conducted by Vollmer et al. (2011) showed a lower rate of relapses in patients with active MS treated with IL-23 monoclonal antibodies [50]. In Guillain Barre syndrome (GBS), a peripheral nervous system demyelinating disorder, Peng et al. (2018) have demonstrated that IL-23 shows pro-inflammatory effects at the early stage of GBS [51]. IL-23 was shown to promote the progression of the disease, with higher levels of IL-23 in older individuals, suggesting that, inflammation may be more harmful with the increasing age [51].
The disease duration is also important in the evolution of MS, but in our study we did not find any significant association between IL-23 levels and disease duration in any of the clinical forms of the disease. The following clinical factors were described as poor prognostic factors in MS: primary progressive MS form, a high relapsing rate, a shorter period of time between the first and the second relapse, brainstem, cerebellar and spinal symptoms at the onset, poor recovery from the first relapse, a higher EDSS score at diagnosis, a polysymptomatic onset and early cognitive deficits [[52], [53], [54]]. In our study, we did not find significant association of IL-23 levels with gender, age of MS onset, the number of relapses under treatment in none of clinical forms of MS and a positive correlation between IL-23 levels and the duration of disease-modifying treatment being found in SPMS patients. In contrast with IL-23, which stimulates the traffic of myelin specific T lymphocytes in the CNS and favours an inflammatory environment through IL-17 production, IL-27 prevents autoimmunity maintaining an immunotolerogenic state through its broad immunoregulatory roles [19,20,55]. IL-27 was proposed as a possible therapeutic agent for some autoimmune diseases, due to its implication in the production of T regulatory cells and suppression of Th17 cells, through IL-10 and programmed death ligand 1 (PD-L1) secretion and IL-6 and TGF-β [56].
Lalive et al. (2017) had a different approach regarding IL-27 involvement in MS, evaluating its CSF levels and showing higher levels of IL-27 in patients, compared to controls, showing astrocytes producing IL-27 in the active demyelinating lesions of MS patients [57]. The same study showed a negative correlation between a higher IL-27 level in the CSF and a higher EDSS score.
Our results showed that in RRMS, patients'age is significantly negatively correlated with IL-27 levels (ρ = -0.31, p = 0.012). Also, a significant increase of mean serum IL-27 was noticed in cases with early MS onset (age at disease onset <28 years) (difference in means: 2.7 pg/ml, 95% CI for difference: 0.3–5.0 pg/ml) of RRMS compared to cases with later onset of RRMS (age at disease onset ≥28 years), We observed that in RRMS, age at disease onset tend to be negatively correlated with IL-27 levels (ρ = -0.2, p = 0.097). The finding that age at onset is an important predictor for disability was demonstrated by previous reports [58,59]. Our finding supports the “treat early concept” presented by Jokubaitis et al. (2015), Kavaliunas et al. (2016) and Zarei et al. (2019), who emphasized the urge to initiate treatment in MS in the first 12 months after de diagnosis, in order to control the worsening of disability and the relapse activity [[60], [61], [62]]. Regarding the SPMS group, patients'age is positively correlated with IL-27 levels. There was no significant correlation between age, age at disease onset, duration of disease (years) and IL-23 levels in both CIS and SPMS groups.
Prosperini et al. (2021) also demonstrated that age at treatment start and presence of spinal cord lesions were the main factors associated with progression independent of relapses activity (PIRA) in apparently stable patients [63]. Further studies are needed in order to understand how IL-27 levels fluctuate in MS progression. The gender, the EDSS score, the number of relapses, and the disease duration were not significantly associated with the variation of IL-27 serum levels in our study. In RRMS group, there is a tendency toward statistical significance regarding the relationship between the type of treatment and the IL-27 level.
Moreover, we found no significant differences concerning the serum levels of IL-27 in relation to IL-27 SNPs of RRMS patients, but we observed a trend for statistical significance (p = 0.086) concerning the relationship between serum levels of IL-23 and A964G-IL27 gene polymorphism, a higher increase of the means of serum IL-23 in RRMS patients carriers of AA (A964G-IL27) genotype in comparison with patients with AG or GG genotypes.
To our knowledge, for Romania, this is the first study evaluating the variation of IL-27 and IL-23 in relation to DMT's characteristics stratified by three stages of MS (CIS, RRMS and SPMS), but extensive studies are required in order to confirm these results. Also, this is the first study comparing the serological level of IL-23 and IL-27 and different polymorphisms located in their genes in RRMS patients treated with injectable DMTs.
Despite evaluating for the first time in the Romanian population the relation between SNPs and serological levels of IL-27 and IL-23 in MS patients under IFN-β and GA therapy, our study has some limitations. One of the main limitations is related to the sample size of CIS group (n = 6) and SPMS group (n = 9) which may not be large enough: i) to depict a definite cytokines profile in each of MS clinical forms and ii) to identify the potential effect of MS type in the variation of cytokines levels controlling for other known covariates. Second limitation is related to the cross-sectional study design, a future longitudinal observational study being required to draw a definite conclusion between serological levels of IL-23 and IL-27 and their usage as potential markers to follow MS evolution and the patients’ response to treatment. Another limitation can be related to the lack of evaluating patients with MS following other oral treatments (Teriflunomide, Dymethil fumarate, Fingolimodum) in order to compare treatment responses in patients with different therapies and the lack of comparative evaluation of IL-23 and IL-27 in the serum and in the CSF. A larger cohort of patients is required for a more precise conclusion and evaluation, and other ethnic groups could be further explored.
Our results add new insight on the IL-23 and IL-27 involvement in MS and support the important roles of these cytokines in autoimmunity, but further investigations are still required to understand the context-dependent inflammatory activities of both interleukins. The complex functions of both IL-27 and IL-23 should be further studied in order to find the right modality to use them as therapeutic tools or evolution biomarkers.
The adequate therapeutic intervention at the beginning of the inflammatory stage, in the so called “window of opportunity”, prevents disease progression and improves the quality of patients lives.
5. Conclusions
Our study revealed a significant relationship between IL-27 serum levels and early disease onset in RRMS patients with higher serum levels of IL-27 in patients with an earlier age of disease onset (age at disease onset <28 years). We also found a significant difference in IL-23 serum levels in RRMS patients carrying AG and GG genotypes of R381Q polymorphism,. The disease duration, number of relapses under treatment were not significantly associated with the serum levels of IL-27 and IL-23 in none of clinical forms of MS, a positive correlation between IL-23 serum levels and disease treatment duration being found only in SPMS patients but further studies should confirm this result on a larger sample size.
Funding
“IULIU HAȚIEGANU UNIVERSITY OF MEDICINE AND PHARMACY” founded this research-grant number: 1529/27 doctoral research grant.”
Institutional review board statement
The study was performed in conformity with the principles of the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca. All patients were informed about the aim of the study and signed an informed content.
Informed consent statement
An informed consent was signed by all the patients and all the healthy volunteers, who were informed about the aim of the study.
Author contribution statement
Ioana Simina Barac: Performed the experiments; Wrote the paper.
Vitalie Vacaras, M. D; Dafin Fior Muresanu, Professor: Contributed reagents, materials, analysis tools or data.
Mihaela Iancu: Analyzed and interpreted the data; Wrote the paper.
Lucia Maria Procopciuc, Professor: Conceived and designed the experiments; Wrote the paper.
Data availability statement
The raw data involved in this study can be obtained upon reasonable request addressed to Lucia M. Procopciuc (luciamariaprocopciuc@yahoo.com) and Ioana S. Barac (siminabarac@gmail.com).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The Authors are thankful to all the participants.
Contributor Information
Vitalie Văcăraș, Email: vitalievacaras.umf@gmail.com.
Mihaela Iancu, Email: miancu@umfcluj.ro.
References
- 1.TaŞKapilioĞLu Öz. Recent advances in the treatment for multiple sclerosis; current new drugs specific for multiple sclerosis. Noro Psikiyatr. Ars. 2018;55:S15–S20. doi: 10.29399/npa.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Brain Council (EBC) Rethinking MS in Europe. https://www.braincouncil.eu/projects/rethinkingms/
- 3.Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., Wallin M., Helme A., Angood Napier C., Rijke N., Baneke P. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS. Mult. Scler. J. 2020;26:1816–1821. doi: 10.1177/1352458520970841. third edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar B., Khalifehzadeh-Esfahani Z., Seyfizadeh N., Rezaei Danbaran G., Hemmatzadeh M., Mohammadi H. The role of immune regulatory molecules in multiple sclerosis. J. Neuroimmunol. 2019;337 doi: 10.1016/j.jneuroim.2019.577061. [DOI] [PubMed] [Google Scholar]
- 5.Egwuagu C.E., Larkin J., III Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAK-STAT. 2013;2 doi: 10.4161/jkst.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maglione A., Rolla S., De Mercanti S.F., Cutrupi S., Clerico M. The adaptive immune system in multiple sclerosis: an estrogen-mediated point of view. Cells. 2019;8 doi: 10.3390/cells8101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toru Yago H.Y., Nanke Yuki, Kawamoto Manabu, Kobashigawa Tsuyoshi, Kotake S. IL-23 and Th17 disease in inflammatory arthritis. J. Clin. Med. 2017;6:81. doi: 10.3390/jcm6090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenzie B.S., Kastelein R.A., Cua D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Lewis D B.S. fifth ed. Elsevier Ltd; 2019. Clinical Immunology. [DOI] [Google Scholar]
- 10.Alsheikh M.M., El-Shafey A.M., Gawish H.H., El-Desoky E.T. Serum interleukin-23 level in rheumatoid arthritis patients: relation to disease activity and severity, Egypt. Rheumatology. 2019;41:99–103. doi: 10.1016/j.ejr.2018.07.001. [DOI] [Google Scholar]
- 11.Lee P.W., Smith A.J., Yang Y., Selhorst A.J., Liu Y., Racke M.K., Lovett-Racke A.E. IL-23R-activated STAT3/STAT4 is essential for Th1/Th17-mediated CNS autoimmunity. JCI Insight. 2017 doi: 10.1172/jci.insight.91663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahmasebinia F., Pourgholaminejad A. The role of Th17 cells in auto-inflammatory neurological disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2017 doi: 10.1016/j.pnpbp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Zúñiga L.A., Jain R., Haines C., Cua D.J. Th17 cell development: from the cradle to the grave. Immunol. Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 14.Floss D.M., Schröder J., Franke M., Scheller J. Insights into IL-23 biology: from structure to function. Cytokine Growth Factor Rev. 2015;26:569–578. doi: 10.1016/j.cytogfr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Sivanesan D., Beauchamp C., Quinou C., Lee J., Lesage S., Chemtob S., Rioux J.D., Michnick S.W. IL23R (Interleukin 23 Receptor) variants protective against inflammatory bowel diseases (IBD) display loss of function due to impaired protein stability and intracellular trafficking. J. Biol. Chem. 2016;291:8673–8685. doi: 10.1074/jbc.M116.715870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu R.Y., Brazaitis J., Gallagher G. The human IL-23 receptor rs11209026 A allele promotes the expression of a soluble IL-23r–encoding mRNA species. J. Immunol. 2015;194:1062–1068. doi: 10.4049/jimmunol.1401850. [DOI] [PubMed] [Google Scholar]
- 17.Oliver J., Rueda B., López-Nevot M.A., Gómez-García M., Martín J. Replication of an association between IL23R gene polymorphism with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2007;5:977–981. doi: 10.1016/j.cgh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Sáfrány E., Pazár B., Csöngei V., Járomi L., Polgár N., Sipeky C., Horváth I.F., Zeher M., Poór G., Melegh B. Variants of the IL23R gene are associated with ankylosing spondylitis but not with sjögren syndrome in Hungarian population samples. Scand. J. Immunol. 2009 doi: 10.1111/j.1365-3083.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- 19.Gyülvészi G., Haak S., Becher B. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur. J. Immunol. 2009;39:1864–1869. doi: 10.1002/eji.200939305. [DOI] [PubMed] [Google Scholar]
- 20.Brucklacher-Waldert V., Stuerner K., Kolster M., Wolthausen J., Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 21.Hasheminia S.J., Tolouei S., Zarkesh-Esfahani S.H., Shaygannejad V., Shirzad H.A., Torabi R., Hashem Zadeh Chaloshtory M. Cytokines gene expression in newly diagnosed multiple sclerosis patients. J. Allergy. Asthma. Immunol. 2015;14:208–216. https://ijaai.tums.ac.ir/index.php/ijaai/article/view/393 Iran. [PubMed] [Google Scholar]
- 22.Tüzün E. Immunopathological factors associated with disability in multiple sclerosis. Noro Psikiyatr. Ars. 2018;55:S26–S30. doi: 10.29399/npa.23303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sénécal V., Deblois G., Beauseigle D., Schneider R., Brandenburg J., Newcombe J., Moore C.S., Prat A., Antel J., Arbour N. Production of IL-27 in multiple sclerosis lesions by astrocytes and myeloid cells: modulation of local immune responses. Glia. 2016;64:553–569. doi: 10.1002/glia.22948. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski M., Wandtke T. first ed. Springer International Publishing; Cham: 2016. Interleukin-27: Biological Properties and Clinical Application. [DOI] [Google Scholar]
- 25.Tait Wojno E.D., Hunter C.A., Stumhofer J.S. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50:851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosmann M., Ward P.A. Modulation of inflammation by interleukin-27. J. Leukoc. Biol. 2013;94:1159–1165. doi: 10.1189/jlb.0213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbi M., Carbotti G., Ferrini S. Mediators Inflamm; 2017. Dual Roles of IL-27 in Cancer Biology and Immunotherapy; p. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garces K., Carmy T., Illiano P., Brambilla R., Hackam A.S. Increased neuroprotective microglia and photoreceptor survival in the retina from a peptide inhibitor of myeloid differentiation factor 88 (MyD88) J. Mol. Neurosci. 2020;70:968–980. doi: 10.1007/s12031-020-01503-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B., Zhang P., Tang T., Liao H., Zhang K., Pu Y., Chen P., Song Y., Zhang L. Polymorphisms and plasma levels of IL-27: impact on genetic susceptibility and clinical outcome of bladder cancer. BMC Cancer. 2015;15:1–10. doi: 10.1186/s12885-015-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahantigh D., Ghazaey Zidanloo S., Forghani F., Doroudian M. IL-27 variants might be genetic risk factors for preeclampsia: based on genetic polymorphisms, haplotypes and in silico approach. Mol. Biol. Rep. 2020;47:7929–7940. doi: 10.1007/s11033-020-05871-z. [DOI] [PubMed] [Google Scholar]
- 31.He J., Zhang Q., Zhang W., Chen F., Zhao T., Lin Y., Li J., Liu Y., Liu Y., Shao Y. The interleukin-27 -964A>G polymorphism enhances sepsis-induced inflammatory responses and confers susceptibility to the development of sepsis. Crit. Care. 2018;22:248. doi: 10.1186/s13054-018-2180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghavami A., Fathpour G., Amirghofran Z. Association of IL-27 rs153109 and rs17855750 polymorphisms with risk and response to therapy in acute lymphoblastic leukemia. Pathol. Oncol. Res. 2018;24:653–662. doi: 10.1007/s12253-017-0295-2. [DOI] [PubMed] [Google Scholar]
- 33.Gholijani N., Daryabor G., Kalantar K., Yazdani M.R., Shenavandeh S., Zahed M., Jafarpour Z., Malekmakan M.R., Amirghofran Z. Interleukin-27 gene variant rs153109 is associated with enhanced cytokine serum levels and susceptibility to Behçet’s disease in the Iranian population. Eur. Cytokine Netw. 2020;31:140–146. doi: 10.1684/ecn.2020.0458. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto-Furusho J.K., Posadas-Sánchez R., Alvarez-León E., Vargas-Alarcón G. Protective role of Interleukin 27 (IL-27) gene polymorphisms in patients with ulcerative colitis. Immunol. Lett. 2016;172:79–83. doi: 10.1016/j.imlet.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Chae S.-C., Li C.-S., Kim K.M., Yang J.Y., Zhang Q., Lee Y.-C., Yang Y.-S., Chung H.-T. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J. Hum. Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 36.Citations - Homo sapiens - GRCh37 archive browser 100. http://grch37.ensembl.org/Homo_sapiens/Variation/Citations?db=core rs181206 (SNP) g=ENSG00000197272;r=16:28510683-28518155;t=ENST00000356897;v=rs181206;vdb=variation;vf=309953932.
- 37.Si F., Wu Y., Wang X., Gao F., Yang D., Liu R., Yi Q. The relationship between Interleukin-27 gene polymorphisms and Kawasaki disease in a population of Chinese children. Cardiol. Young. 2018;28:1123–1128. doi: 10.1017/S1047951118000914. [DOI] [PubMed] [Google Scholar]
- 38.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 39.Anber N., El Sayed Zaki M., El Wassefy M. Interleukin-27 levels and its polymorphism in hepatocellular carcinoma associated with hepatitis C, nov. Res. Microbiol. J. 2020;4:613–623. doi: 10.21608/nrmj.2020.73434. [DOI] [Google Scholar]
- 40.Mosayebian A., Hakemi M.G., Meshkat R., Ghasemi R., Ahmad H.K., Samadi M. Association between interleukin-23 receptor R381Q gene polymorphism and asthma, Iran. J. Allergy, Asthma Immunol. 2015;14:386–391. [PubMed] [Google Scholar]
- 41.Anaraki Mohammadi S., Mansouri R., Shahi A., Akhlaghi M., Dashti N., Aslani S., Mansouri M., Poursani S., Mahmoudi M. IL27 gene single nucleotide polymorphisms confer susceptibility to rheumatoid arthritis in Iranian population. Meta Gene. 2018;18:149–152. doi: 10.1016/j.mgene.2018.09.002. [DOI] [Google Scholar]
- 42.Barac I.S., Iancu M., Văcăraș V., Cozma A., Negrean V., Sâmpelean D., Mureșanu D.F., Procopciuc L.M. Potential contribution of IL-27 and IL-23 gene polymorphisms to multiple sclerosis susceptibility: an association analysis at genotype and haplotype level. J. Clin. Med. 2021;11:37. doi: 10.3390/jcm11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leisch F., Man M. 2021. Package “Genetics” Title Population Genetics.https://cran.r-project.org/package=genetics [Google Scholar]
- 44.Wilson A., Norden N., R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A Language and Environment for Statistical Computing.http://www.r-project.org/ [Google Scholar]
- 45.Sarin R., Wu X., Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9560–9565. doi: 10.1073/pnas.1017854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Meglio P., Villanova F., Napolitano L., Tosi I., Terranova Barberio M., Mak R.K., Nutland S., Smith C.H., Barker J.N.W.N., Todd J.A., Nestle F.O. The IL23R A/Gln 381 allele promotes IL-23 unresponsiveness in human memory T-helper 17 cells and impairs Th17 responses in psoriasis patients. J. Invest. Dermatol. 2013;133:2381–2389. doi: 10.1038/jid.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pidasheva S., Trifari S., Phillips A., Hackney J.A., Ma Y., Smith A., Sohn S.J., Spits H., Little R.D., Behrens T.W., Honigberg L., Ghilardi N., Clark H.F. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deveci H., Turk A.C., Ozmen Z.C., Demir A.K., Coskun S.U.S. Biological and genetic evaluation of IL-23/IL-17 pathway in ankylosing spondylitis patients. Cent. Eur. J. Immunol. 2019;44:433–439. doi: 10.5114/ceji.2019.92805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barac I.S., Vacaras V., Cozma A., Valeanu M., Decea N., Muresanu D.F., Procopciuc L.M. Il27 t4730c polymorphism and serology in multiple sclerosis: a pilot study. In Vivo. 2021;35:2845–2853. doi: 10.21873/INVIVO.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollmer T.L., Wynn D.R., Alam M.S., Valdes J. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Mult. Scler. 2011;17:181–191. doi: 10.1177/1352458510384496. [DOI] [PubMed] [Google Scholar]
- 51.Peng J., Zhang H., Liu P., Chen M., Xue B., Wang R., Shou J., Qian J., Zhao Z., Xing Y., Liu H. IL-23 and IL-27 levels in serum are associated with the process and the recovery of guillain-barré syndrome. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-21025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotstein D., Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat. Rev. Neurol. 2019;15:287–300. doi: 10.1038/S41582-019-0170-8. [DOI] [PubMed] [Google Scholar]
- 53.Crielaard L., Kavaliunas A., Ramanujam R., Olsson T., Hillert J., Stridh P., Kockum I., Manouchehrinia A. Factors associated with and long-term outcome of benign multiple sclerosis: a nationwide cohort study. J. Neurol. Neurosurg. Psychiatry. 2019;90:761–767. doi: 10.1136/jnnp-2018-319913. [DOI] [PubMed] [Google Scholar]
- 54.Ayrignac X., Bigaut K., Pelletier J., de Seze J., Demortiere S., Collongues N., Maarouf A., Pinna F., Aouinti S., Carra Dallière C., Kremer L., Charif M., Picot M.C., Labauge P. First line treatment failure: predictive factors in a cohort of 863 Relapsing Remitting MS patients. Mult. Scler. Relat. Disord. 2021;48 doi: 10.1016/J.MSARD.2020.102686. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H., Hunter C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 56.Gaber W., Sayed S., Rady H.M., Mohey A.M. Interleukin-27 and its relation to disease parameters in SLE patients, Egypt. Rheumatology. 2012;34:99–105. doi: 10.1016/j.ejr.2012.04.002. [DOI] [Google Scholar]
- 57.Lalive P.H., Kreutzfeldt M., Devergne O., Metz I., Bruck W., Merkler D., Pot C. Increased interleukin-27 cytokine expression in the central nervous system of multiple sclerosis patients. J. Neuroinflammation. 2017;14:144. doi: 10.1186/s12974-017-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Confavreux C., Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129:595–605. doi: 10.1093/BRAIN/AWH714. [DOI] [PubMed] [Google Scholar]
- 59.Langer-Gould A., Popat R.A., Huang S.M., Cobb K., Fontoura P., Gould M.K., Nelson L.M. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch. Neurol. 2006;63:1686–1691. doi: 10.1001/ARCHNEUR.63.12.1686. [DOI] [PubMed] [Google Scholar]
- 60.Jokubaitis V.G., Spelman T., Kalincik T., Izquierdo G., Grand'Maison F., Duquette P., Girard M., Lugaresi A., Grammond P., Hupperts R., Cabrera-Gomez J., Oreja-Guevara C., Boz C., Giuliani G., Fernández-Bolaños R., Iuliano G., Lechner-Scott J., Verheul F., van Pesch V., Petkovska-Boskova T., Fiol M., Moore F., Cristiano E., Alroughani R., Bergamaschi R., Barnett M., Slee M., Vella N., Herbert J., Shaw C., Saladino M.L., Amato M.P., Liew D., Paolicelli D., Butzkueven H., Trojano M. Predictors of disability worsening in clinically isolated syndrome. Ann. Clin. Transl. Neurol. 2015;2:479–491. doi: 10.1002/acn3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarei S., Maldonado I., Franqui-Dominguez L., Rubi C., Rosa Y.T., Diaz-Marty C., Coronado G., Rivera Nieves M.C., Akhlaghipour G., Chinea A. Impact of delayed treatment on exacerbations of multiple sclerosis among Puerto Rican patients. Surg. Neurol. Int. 2019;10:1–15. doi: 10.25259/SNI_252_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kavaliunas A., Manouchehrinia A., Stawiarz L., Ramanujam R., Agholme J., Hedström A.K., Beiki O., Glaser A., Hillert J. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult. Scler. 2017;23:1233–1240. doi: 10.1177/1352458516675039. [DOI] [PubMed] [Google Scholar]
- 63.Prosperini L., Ruggieri S., Haggiag S., Tortorella C., Pozzilli C., Gasperini C. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurol. Neuroimmunol. Neuroinflammation. 2021;8 doi: 10.1212/NXI.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data involved in this study can be obtained upon reasonable request addressed to Lucia M. Procopciuc (luciamariaprocopciuc@yahoo.com) and Ioana S. Barac (siminabarac@gmail.com).