Abstract
An alternate medium consisting of sugarcane juice (SJ) (Saccharum spp.) and chicken feather peptone (CFP) was employed for microbial synthesis of levan. SJ has considerable amounts of vital minerals, vitamins, and amino acids in addition to its major constituent, sucrose. Meanwhile, CFP is also a rich source of essential nutrients such as amino acids, micro and macro elements. Amino acids present in SJ and CFP, such as glutamic acid, arginine, aspartic acid, asparagine and elements such as Ca, Mg favoured the cell growth and levan production. In this present work, levan was produced using Bacillus subtilis MTCC 441 in five different media, namely, sucrose along with defined nutrients (M1), Sugarcane Juice without nutrients (M2), SJ with defined nutrients (M3), SJ along with chicken feather peptone (M4) and sucrose without nutrient (M5). Alternative nutrient medium using SJ and CFP (M4) showed a promising levan yield of 0.32 ± 0.01 g of levan/g of sucrose consumed, which is 64% of the theoretical levan yield possible. Levan produced was characterized using Nuclear Magnetic Resonance (NMR) and Gel Permeation Chromatography (GPC). There is a change in low molecular weight fractions of levan obtained from SJ and CFP medium compared to the defined medium. Produced levan from the composite medium exhibited strong antioxidant activity and was biocompatible when tested against endothelial cells. The substrate cost was 20% lower than the cost of defined medium. Thus, a composite medium made of SJ and CFP can serve as an alternate low-cost medium for microbial fermentation.
Keywords: Biocompatible, Antioxidant activity, Chicken feather peptone, Fermentation, Levan, Microbial exopolysaccharide, Sugarcane juice
Graphical abstract
Highlights
-
•
Nutrients in nascent sugarcane juice (SJ) support Bacillus subtilis growth and metabolism.
-
•
Value addition to chicken feather peptone (CFP), a poultry industry waste, is demonstrated.
-
•
64% theoretical yield obtained with the composite medium.
-
•
Levan produced exhibited antioxidant activity and was biocompatible.
1. Introduction
Sugarcane juice (Saccharum spp.) (SJ) is a rich source of sucrose that contains several amino acids, minerals and trace elements [1]. Sugarcane is produced in millions of tonnes every year, mainly in countries such as India, Brazil, China, etc. [2]. India is the world's second-largest producer of sugarcane (Sugar and sugarcane policy, GOI, 2022). It is a reasonably affordable and renewable feedstock which can be used in fermentation industries. SJ can be extracted from sugarcane by simple mechanical treatment [3]. Short shelf-life due to microbial and enzyme degradation, it is necessary that the juice is used immediately [4].
Levan is a unique microbial exo-polysaccharide having a wide range of industrial applications [5,6]. Levan is a water-soluble biopolymer, and it is composed of repeating monomeric units of fructose with d-glucosyl residue as a terminal group. It is soluble in water and insoluble in organic solvents such as acetone, ethanol, isopropanol, methanol, etc. Levan has desirable properties such as biodegradability, biocompatibility, flexibility, antioxidant, anti-inflammatory, etc. [7]. Both plants and microorganisms are known to produce levan. Typically, microbial levan has comparatively high molecular weight (Degree of polymerization (DP) > 103 to 104) [8] than plant-produced levan (DP < 102) [9].
Levan is commercially produced from refined sugar (sucrose). Levan biosynthesis is a two-stage process, and it is catalyzed by the enzyme levansucrase. The initial stage involves the hydrolysis of sucrose, and the next stage involves the polymerization of fructose (transfructosylation reaction). Microbial levan synthesis is mainly affected by factors such as microorganism species, media composition, and process conditions (pH, temperature, incubation time, etc.). Particularly, the cost of media significantly contributes to the product cost. About 35–60% of the production cost is typically due to medium cost [10]. Thus, using an alternative media, that is cheap, renewable, and capable of producing high yields will enhance the overall economy of the process [11].
Considering this need, significant research efforts have been taken to identify suitable alternate carbon sources for levan production in recent years. Beet molasses, coconut inflorescence sap, date syrup, starch molasses, sugarcane molasses, and sugarcane syrup [12,13,14,15,16,17] have been used as alternate production media in the past. However, it has been reported that levan yields obtained from these mediums were lower than those obtained from sucrose. The researchers indicated that further optimization of the media composition is necessary to achieve comparable yield. It is well recognized that besides a carbon source, nitrogen source (yeast extract, bacterial peptone, chicken feather peptone, etc.) also plays a crucial role in microbial fermentation and exopolysaccharide production [14,18,19]. However, research on alternative fermentation media sources that consist of carbon, nitrogen, and other micro- and macro-nutrients is inadequate in the literature.
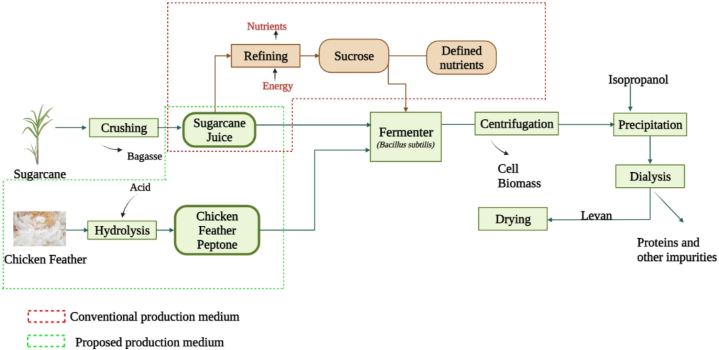
The use of unrefined SJ can save resources as well as energy. The present work hypothesizes that SJ combined with CFP has the potential to be used as an alternate low-cost media for the production of levan (Fig. 1). Commercial sucrose has a purity of almost 99.7% wherein the native nutrient contents are removed sugarcane juice refining. Substituting raw SJ instead of refined sucrose in the fermentation medium have two major benefits (i) loss of nutrients from sugarcane juice during refining process is prevented and (ii) the cost of refining sucrose is saved. On the other hand, CFP is rich in nitrogen, micronutrients, and macronutrients which are important for microbial growth as well as the synthesis of products. Annually, the poultry industry generates millions of tons of chicken feathers as solid waste. The principal constituent of chicken feathers is protein (Keratin) [20]. Previous studies demonstrate that chicken feathers could be used as raw material for the preparation of peptone. Thus the obtained CFP could be used as an organic nitrogen source for various microbial fermentation [[19], [20], [21], [22]] [[19], [20], [21], [22]] [[19], [20], [21], [22]]. To the best of our knowledge, the mixture of SJ and CFP has not been investigated as an alternative cost-effective medium for levan production. Hence, the first objective of the present work is to produce levan from a composite medium prepared using SJ & CFP using Bacillus subtilis MTCC 441. The second objective of this study is to evaluate the antioxidant property and cytotoxic activity of the levan so produced.
Fig. 1.
A schematic comparison of levan production process flow from conventional medium and proposed composite medium.
2. Materials and methods
2.1. Materials and microorganism
The sugarcane (Saccharum spp.) juice (SJ) was obtained from a local juice shop near Thanjavur, Tamilnadu, India. Isopropanol (IPA) was procured from M/s Molychem., India. All other chemicals were procured from M/s Himedia, India. All chemical ingredients were analytical grade (>99% purity). Chicken feather peptone (CFP) was produced using the method outlined in Ref. [19]. The microbial strain used in this study Bacillus subtilis MTCC441 was obtained from a culture bank (Institute of Microbial Technology (IMT), India). A glycerol stock was prepared and stored at – 80 °C until further use.
2.2. Preparation of sugarcane juice for fermentation
Collected SJ was centrifuged at 8000 RPM for 10 min to remove any suspended particles. The supernatant collected was filtered using filter paper. The initial sugar concentration of this filtrate was determined using High-Performance Liquid Chromatography (HPLC) and it was diluted to an initial concentration of 100 g/L before fermentation.
2.3. Levan production
In this present work, levan was produced using Bacillus subtilis MTCC 441 in five different media; namely, sucrose along with defined nutrients (DN) (M1), Sugarcane Juice without nutrients (M2), SJ with defined nutrients (M3), SJ along with chicken feather peptone (M4) and sucrose without nutrient (M5) and the results were compared. The defined nutrients (DN) include the following ingredients (in g/L): Yeast extract- 2.0, (NH4)2SO4– 3.0, KH2PO4 – 1.0, MgSO4 .7H2O – 0.6, MnSO4 - 0.2.
For each experiment, 100 mL of culture media was prepared in an Erlenmeyer's flask (250 mL) with an inoculum concentration of 10% (v/v) with an optical density (OD) of 0.6–0.7. Before autoclaving, pH of the medium was adjusted to 7. The culture was incubated for 20 h at 150 rpm and 37 °C in an orbital shaker [23].
2.4. Recovery of levan
Post-fermentation, the culture was collected and centrifuged at 8000 rpm at room temperature for 10 min. Biomass was recovered as a pellet. The mass of biomass produced was obtained by drying the pellet at 60 °C until constant weight [23]. The pH of the supernatant was changed to 9.0 and then the polymer from the supernatant was recovered by adding 3 vol of ice-cold IPA per unit volume of supernatant. The precipitate was removed by centrifuging the mixture at 8000 rpm for 10 min. Levan pellet was dried at 45 °C overnight and weighed. Further, levan was purified by re-precipitating twice using IPA. Then, the pellet was dissolved in hot water and dialyzed (molecular weight cut of 14,000 Da) for 3 days by changing the water twice a day. The levan was lyophilized and used for further studies.
2.5. Analytical methods
1H NMR and 13C NMR samples were prepared using D2O and analyzed in Bruker 400 MHz instrument at room temperature. The molecular weight of the produced levan was measured by gel permeation chromatography using Ultrahydrogel 1000 column. The mobile phase, sodium nitrate solution (0.1 M), was used at a 0.6 mL/min flow rate. The sugar was estimated using Hiplex Ca (8 μm, 7.7 × 300 mm) analytical column with water as mobile phase (flow rate 0.6 mL/min). The elemental composition of SJ and CFP analyzed using Inductive Coupled Plasma Mass Spectrometry (ICP- MS) (AOAC, 2019).
2.6. Antioxidant activity
The antioxidant activity of obtained levan was estimated in-vitro by 1,1- Diphenyl-2-picrylhydrazyl (DPPH) assay and superoxide anion radical assay as described by Wang et al. (2020). Briefly, 2 mL of levan solutions of different concentrations (0–15 mg/mL) were mixed with 2 mL DPPH (0.2 mM) ethanolic solution. The mixtures were incubated at room temperature in the dark for 30min. After incubation, the UV–Visible absorbance was measured at 517 nm. Ascorbic acid was taken as the positive control. Superoxide anion scavenging experiment, 1 mL of levan solutions of different concentrations (0–15 mg/mL) was mixed with 3 mL Tris-HCL buffer solution (50 mM, pH 8.2) and incubated at 25 °C for 20 min. After incubation, 0.3 mL of pyrogallol (30 mM) and kept at 25 °C for 5 min. Then, 1 mL HCL was added to stop the reaction. After termination, the absorbance was measured using UV–visible spectrophotometer at 325 nm.
2.7. Cytotoxicity analysis
The cytotoxicity assay was performed in a 96-well plate (coated with 0.5% gelatine), HUVEC cells were seeded at an approximate density of 1 × 104/well. After incubating overnight, they were treated with levan at defined concentrations (100, 250, 500, 750 and 1000 μg/mL) for 24 h. Cells were treated with N-acetyl cysteine (NAC - 1000 μg/mL) as a positive control. Later, the cells were washed using 1X PBS and incubated with 1 g/L MTT. After incubating at 37 °C for 4 h, formazan crystals were dissolved with DMSO, and then the absorbance was measured at 570 nm in a microplate reader (Synergy H1).
2.8. Statistical analysis
All the experiments were performed and reported in triplicate. The average mean values were reported along with the standard deviation values. One-way ANOVA and Tukey's tests were used to assess the data's significance and compare the means (α < 0.05). Graph-pad prism software is used for statistical analysis.
3. Results and discussion
3.1. Effect of SJ on levan production
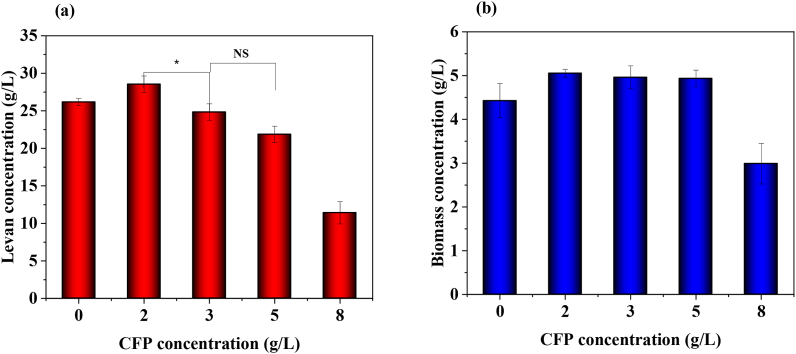
Sucrose is the main constituent of SJ. Apart from sucrose, SJ also contains amino acids, micro and macro elements that are required for the growth and metabolism of B. subtilis [24]. The estimated sucrose concentration in the raw SJ used in this study was 185.0 ± 1.0 g/L. It was suitably diluted to a concentration of 100 g/L using de-ionized water to maintain a constant initial concentration of 100 g/L in all experiment. On replacing yeast extract 2 g/L with an alternative nitrogen source of CFP at different concentrations was studied for levan production. To determine the optimum CFP concentration, experiments were initially conducted with different CFP concentration (0, 2, 3, 5, 8 g/L). The product was recovered as described in section 2.4, and the results are shown in Fig. 2. From the figure, it can be seen that biomass and levan production increased with increase in CFP concentration till 2 g/L CFP and the maximum levan yield of 28 ± 1.09 g/L was obtained at this CFP concentration. However, at higher dosing of CFP, levan production exhibited a declining trend, and this decline may be attributed to the presence of inhibitory ions. Thus, CFP concentration of 2 g/L was considered as optimum for further experiments.
Fig. 2.
Effect of CFP concentration on levan production (a) Levan concentration (g/L) and (b) Biomass concentration (g/L).
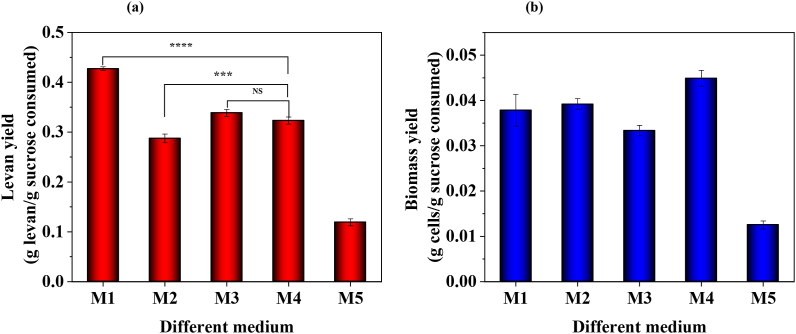
Fig. 3A shows levan and Fig. 3B shows biomass yield obtained from five different media formulations listed in Table 1. Based on the levan yield obtained, the media were ranked as M1 > (M3, M4) > M2 > M5. The maximum theoretical yield of levan that can be obtained from sucrose is 0.50 g/g sucrose. The yield obtained from pure sucrose (M5) as a carbon source resulted in a minimum yield of 0.12 g levan/g sucrose consumed, as expected, due to the absence of essential nutrients required for growth and metabolism. In comparison, the yield obtained from the raw SJ (M2) was 1.4 times higher (0.29 g levan/g sucrose consumed) indicating that the nutrients present in the raw SJ aided the levan production. Meanwhile, the levan yield obtained from M4 (SJ & CFP) was 0.32 ± 0.01 g/g sucrose consumed (which is approximately 64% of the theoretical yield), and the yield obtained from M3 (SJ + defined nutrients) was 0.34 ± 0.01 g/g sucrose consumed (approximately 68% of theoretical yield). The difference in the yield obtained from M3 and M4 was not statistically significant.
Fig. 3.
Levan production using Bacillus subtilis MTCC 441 with different media compositions (a) levan yield and (b) biomass yield.
Table 1.
Compositions of different production medium media used in this study.
| Media label | Sucrose (g/L) | Sugarcane Juice (g/L) | Chicken feather peptone (g/L) | Micronutrients (g/L) |
|---|---|---|---|---|
| M1 | 100 | – | – | Yeast extract- 2.0 (NH4)2SO4– 3.0 KH2PO4 – 1.0 MgSO4 .7H2O – 0.6 MnSO4 - 0.2 |
| M2 | – | 100 | – | – |
| M3 | – | 100 | – | Yeast extract- 2.0 (NH4)2SO4– 3.0 KH2PO4 – 1.0 MgSO4 .7H2O – 0.6 MnSO4 - 0.2 |
| M4 | – | 100 | 2 | – |
| M5 | 100 | – | – | – |
This suggests that the CFP can act as an effective substitute for defined nutrients when used along with a suitable carbon source. Levan yield obtained from the defined medium (M1) consists of refined sugar and defined nutrients hence resulted in higher yield when compared with other fermentation media investigated in this study. Though the yields obtained with SJ & DN (M3) and SJ & CFP (M4) were marginally (about 20%) lower than that obtained with commercial medium (M1), because of their low cost, SJ and CFP can reduce the cost of production media significantly and improve profit margin.
Biomass production results shown in Fig. 3B suggest that there is no significant change in the biomass yield attained with M1 and M2. Meanwhile, biomass yield was maximum with M4 might be due to the presence of essential amino acids that enhanced the growth of Bacillus subtilis and minimum with M5 might be due to the absence of essential nitrogen, micro and macro nutrients needed for the growth of Bacillus subtilis (Fig. 3B). The results further affirm that the composite medium containing SJ and CFP can serve as a low-cost alternate substrate for levan synthesis.
Carbon sources, nitrogen sources, and micro- & macro-nutrients influence the growth of microorganisms and metabolite production patterns. Previous investigations show that the levan produced from B. subtilis requires amino acids such as arginine, glutamic acid, asparagine and aspartic acid for the catalysis and polymerase activity of levansucrase [25]. SJ is rich in alanine, glycine, cysteine, and glutamic acid which are important for the growth and metabolism of B. subtilis [26]. Similarly, it is reported that micronutrients such as calcium might play an important role in levansucrase activity in gram-positive bacteria such as Lactobacillus reuteri, Lactobacillus johnsonii, Bacillus spp [27,28].
Cultures supplied with calcium ions showed increased levansucrase activity which was later attributed to the calcium ion binding site in the levansucrase enzyme [29,30]. It is also postulated that the micronutrients such as calcium, iron, manganese, and magnesium present in the SJ and CFP increase levansucrase activity [29,30]. The levansucrase enzyme catalyzes the hydrolysis of sucrose and assists in the transfructosylation process for levan synthesis [7]. The result from the elemental composition analysis of SJ and CFP used in the present study is shown in Table 2. Both SJ and CFP contain calcium, magnesium, iron, and manganese, specifically needed for levansucrase activity. The observations in this study are consistent with the data obtained using alternative low-cost carbon and nitrogen sources reported in previous literature (Table 3). Results from the literature show that a lower yield of 0.20–0.25 g levan/g sucrose was obtained with alternative complex carbon sources such as sugarcane molasses and sugar beet molasses. It may be attributed to the presence of inhibitors in these waste streams [31,32]. A previous study from the literature also suggests that feeding organic nitrogen sources such as peptone and yeast extract to the production medium improved both microbial growth and metabolite synthesis [21].
Table 2.
Elemental composition of SJ and CFP.
| Elemental composition | ||
|---|---|---|
| Elements | CFP (mg/kg) | SJ (mg/kg) |
| Ca | 171.16 | 90.75 |
| K | 495.50 | 979.44 |
| Mg | 209.97 | 378.53 |
| Cu | 5.16 | 0.22 |
| Zn | 104.38 | 2.16 |
| Mn | 12.22 | 4.26 |
| Fe | 370.11 | 36.96 |
| S | 4532.41 | ND |
| P | 38.88 | 122.02 |
Table 3.
Comparison of different alternative substrates for levan production.
| Microorganism | Sucrose Based medium | Alternative complex medium | Product yield (g levan/g substrate) (levan concentration in g/L) |
References | |
|---|---|---|---|---|---|
| From sucrose based medium | From alternate substrate | ||||
| Bacillus subtilis MTCC 441 | 100 g/L | SJ (100 g/L) + CFP (Nitrogen source) | 0.39 (39 g/L) | 0.32 (32 g/L) | Present study |
| Bacillus lentus V8 strain | 250 g/L | Sugarcane molasses (250 g/L) | 0.23 (57.5 g/L) | 0.20 (50 g/L) | [31] |
| Leuconostoc citreum BD1707 | Tomato juice + sucrose (150 g/L) | 0.19 (28.5 g/L) | [33] | ||
| Bacillus licheniformis NS032 | 200 g/L | Sugar beet molasses (140 g/L)+ Sucrose (60 g/L) | 0.26 (52 g/L) | 0.27 (54 g/L) | [32] |
| Bacillus polymyxa (NRRL-18475) | 250 g/L | Sugarcane molasses (250 g/L) | 0.14 (35 g/L) | 0.03 (7.5 g/L) | [14] |
| Sugarcane syrup (250 g/L) | N.A. | 0.08 (20 g/L) | |||
| Halomonas sp. AAD6 | Pretreated Sugar beet molasses (30 g/L) | N.A. | 0.41 (12.3 g/L) | [13] | |
| Starch molasses (30 g/L) | N.A. | 0.15 (4.5 g/L) | |||
| Microbacterium levaniformans | 200 g/L | Date syrup (250 g/L) | 0.25 (50 g/L) | 0.04 (10 g/L) | [12] |
| Zymomonas mobilis | 250 g/L | Sugarcane molasses (250 g/L) | 0.09 (22.5 g/L) | 0.01 (2.5 g/L) | [15] |
| Sugarcane syrup (250 g/L) | N.A. | 0.06 (15 g/L) | |||
Note: N.A. Not available.
As stated already, the low yield of levan from SJ (M2) and sucrose (M5) might be due to the absence of nitrogen and other micro-nutrients [14]. Hence, the addition of CFP to the SJ-based production medium might have favoured levan production. The results obtained from Fig. 3A support our hypothesis that the vitamins, amino acids, minerals, and salts present in SJ and CFP can support microbial growth and extracellular polysaccharide synthesis in B. subtilis MTCC 441. We have also identified that adding both SJ and CFP in fermentation media favors levan production, which could reduce the production cost compared to fermentation media supplied with pure sucrose and defined nutrients.
3.2. Characterization of levan
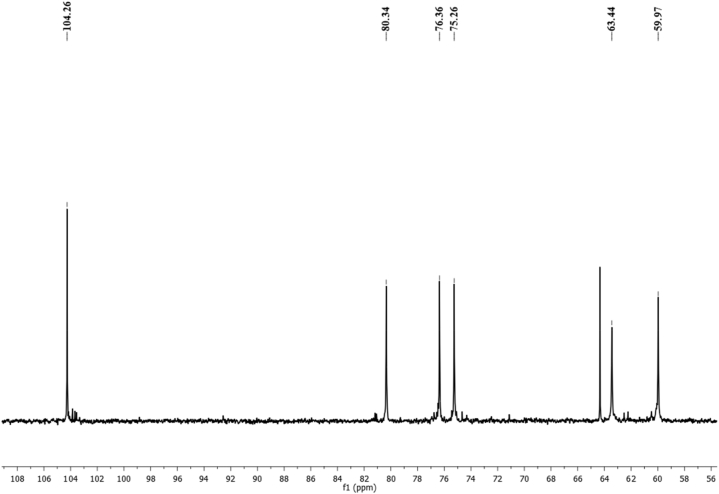
The structure of levan produced using SJ and CFP (M4) medium by B. subtilis MTCC 441 was confirmed by 13C NMR (Fig. 4). The observed chemical shifts in the six-carbon resonance (59.97, 104.26, 76.36, 75.26, 80.34, and 63.44 ppm) were the peaks of C1–C6 atoms of the levan structure which are almost identical with peak positions reported previously [23,34,35] The chemical shifts of 104.17 ppm (C-2) and 63.44 ppm (C-6) correspond to the presence of β-(2–6) - linkages of levan [23,36].
Fig. 4.
13C NMR spectra of levan synthesized using M4.
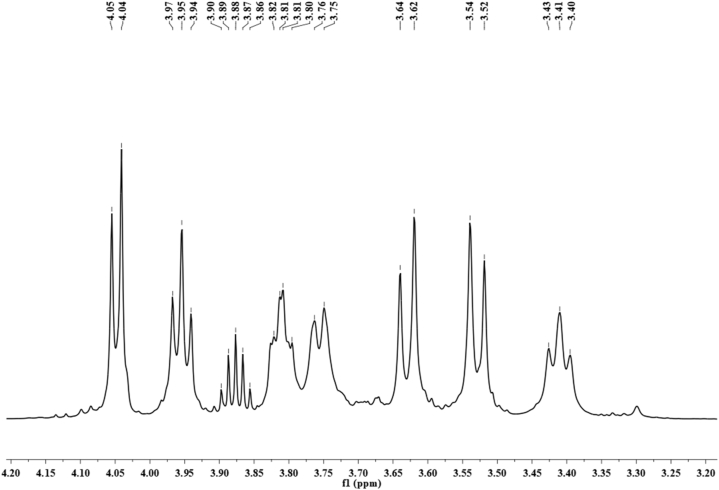
The 1H- NMR spectrum of the synthesized polymer from SJ and CFP (M4) represents the chemical shift (proton) signals corresponding to the structure of the fructan backbone (Fig. 5). The signals in the range of 3.45 ppm–4.05 ppm are similar to those reported in the previous literature [23,36].
Fig. 5.
1H NMR spectra of levan synthesized using M4.
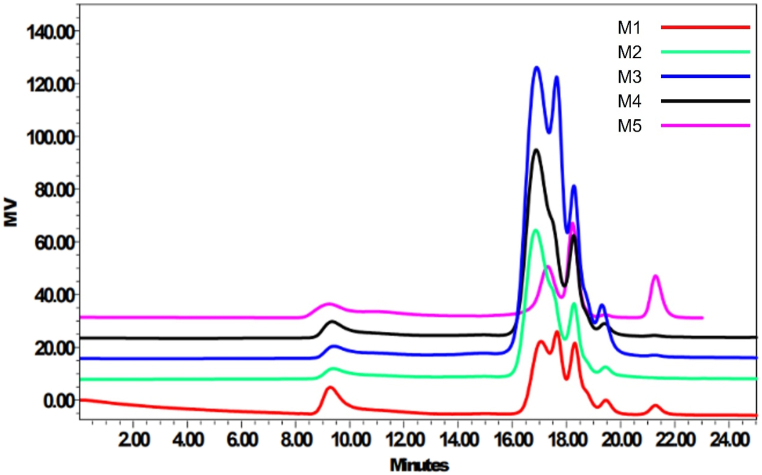
The GPC results of obtained polymer are shown in Fig. 6. The molecular weight distribution of the levan produced using a defined and complex substrate (M1-M4) showed an identical pattern as listed in Table 4. Gram-positive bacteria like Bacillus species exhibit both processive and non-processive mechanisms that produce levan [37,38]. Likewise, levan produced by B. subtilis MTCC 441 from various substrates also expressed two major fractions, one with a high molecular weight (2700–2800 kDa) and a low molecular weight (1.7–11.0 kDa). The degree of polymerization of levan was around 15937 for high molecular weight fraction synthesized by media M1. The results from this study are comparable with the previously published results from the literature [23,29].
Fig. 6.
Gel permeation chromatographs of produced levan from different alternative nutrients.
Table 4.
Molecular weight distribution of levan samples from different media.
| Medium | F1 (kDa) | DP | F2 (kDa) | DP | F3 (kDa) | DP | F4 (kDa) | DP | F5 (kDa) | DP | F6 (kDa) | DP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 2868.7 | 15937 | 9.6 | 53 | N.A. | N.A. | 6.3 | 35.0 | 3.9 | 22.0 | 1.7 | 9.0 |
| M2 | 2796.1 | 15534 | N.A. | N.A. | 11.1 | 61.0 | N.A. | N.A. | 4.0 | 22.0 | 1.7 | 9.0 |
| M3 | 2729.4 | 15163 | N.A. | N.A. | 10.8 | 60.0 | 6.3 | 35.0 | 4.0 | 22.0 | 1.9 | 11.0 |
| M4 | 2831.2 | 15729 | N.A. | N.A. | 11.0 | 61.0 | N.A. | N.A. | 4.0 | 22.0 | 1.7 | 9.0 |
| M5 | 3120.8 | 17338 | 8.1 | 45 | N.A. | N.A. | N.A. | N.A. | 4.1 | 22.0 | 1.7 | 9.0 |
Note: F1, F2, F3, …F6 = Fraction 1, Fraction 2, Fraction 3, …Fraction 6; DP = Degree of polymerization; N.A. = No Fraction Available.
3.3. Antioxidant activity
3.3.1. DPPH assay
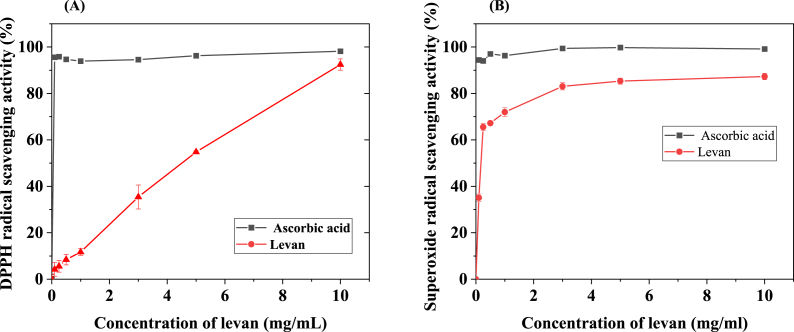
Levan produced from various sources is reported to exhibit antioxidant activity [16,39]. The antioxidant activity of levan produced from B. subtilis MTCC441 was investigated using DPPH assay and the results are shown in Fig. 7A. The antioxidant activity of levan had shown an increasing trend with increasing concentration of levan. The maximum scavenging activity (93.68%) was achieved at a 10 mg/mL levan concentration. In comparison, Ascorbic acid (positive control) has shown >95% activity in the complete range.
Fig. 7.
(A) DPPH and (B) Superoxide radical scavenging activity of levan from Bacillus subtilis MTCC441.
3.3.2. Superoxide anion assay
Superoxide anion is a free radical precursor. They can seriously destroy proteins, DNA, lipids, and other biomolecules by encouraging its conversion to the potentially dangerous hydroxyl radical, hypochlorite anion, and hydrogen peroxide [40]. In this study, superoxide anion radicals are generated in a pyrogallol system, and the decrease in absorbance at 325 nm with levan indicates the consumption of superoxide anion in the reaction mixture, resulting in a concentration-dependent increase in superoxide scavenging activity, as shown in Fig. 7B. Levan with an increasing concentration showed higher scavenging activity of 86.25% at 10 mg/mL whereas positive control of vitamin C showed 99.13% at 10 mg/mL. These results show that the levan produced from B. subtilis MTCC441 can efficiently be used as a strong antioxidant in pharmaceutical and biomedical applications.
3.4. Effect of levan on HUVECs viability
The cytotoxicity of produced levan using SJ and CFP was examined using MTT assay. As shown in Fig. 8, levan of different concentrations (100, 250,500,750 and 1000 μg/mL) did not alter the cell viability of HUVECs in 24 h. NAC of 1000 μM was used as positive control significantly increased the cell viability (α < 0.05). These results indicate that the produced levan using SJ and CFP does not show any inhibitory effect on the cell lines. Hence, the levan from SJ and CFP is biocompatible and can be utilized for biomedical applications such as carrier vehicles for drugs, etc.
Fig. 8.
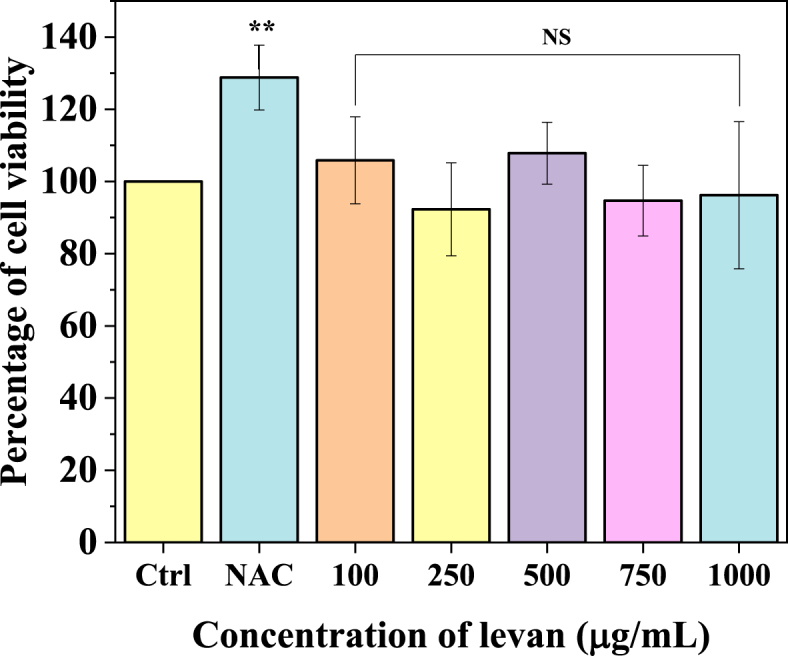
Effect of levan produced using SJ and CFP on HUVEC viability. [Cells were treated with different concentrations of levan for 24 h, and cell viability was measured using MTT assay. The experiments were conducted in triplicates and the values were represented as mean ± SEM. N-acetyl cysteine (NAC - 1000 μg/mL) was used as a positive control].
3.5. Substrate cost comparison
The cost of substrates required for producing 1 kg of levan was calculated based on the market rate of the nutrients for all the five media used in the study and the comparison is shown in Table 5. It is evident from the table that the cost of medium M4 is the minimum among all five media compared, and it was 20% lower compared to the cost of defined medium M1. However, the overall economic benefit of using the alternate composite medium consisting of SJ and CFP in commercial production can be ascertained only after considering the implications on the downstream processing side.
Table 5.
Substrate cost comparison for the production of 1000 kg levan.
| Raw materials | Cost (INR/kg)a | Medium (in kg) |
Cost of the medium (In INR) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | ||
| Sucrose | 45 | 2325.58 | 0 | 0 | 0 | 8333.33 | 104651 | 0 | 0 | 0 | 375000 |
| Sugarcane Juice | 5.6 | 0.00 | 19158.62 | 16341.18 | 17362.50 | 0 | 0 | 107288 | 91510 | 97230 | 0 |
| Yeast extract | 290 | 46.51 | 0 | 58.82 | 0 | 0 | 13488 | 0 | 17059 | 0 | 0 |
| Ammonium Sulphate | 10 | 69.77 | 0 | 88.24 | 0 | 0 | 698 | 0 | 882 | 0 | 0 |
| Potassium di hydrogen phosphate | 140 | 23.26 | 0 | 29.41 | 0 | 0 | 3256 | 0 | 4118 | 0 | 0 |
| Magnesium sulphate heptahydrate | 9.5 | 13.95 | 0 | 17.65 | 0 | 0 | 133 | 0 | 167 | 0 | 0 |
| Manganese sulphate | 45 | 4.65 | 0 | 5.88 | 0 | 0 | 209 | 0 | 265 | 0 | 0 |
| Chicken Feather Peptone | 26 | 0 | 0 | 0 | 6.25 | 0 | 0 | 0 | 0 | 162.5 | 0 |
| Yield (kg) | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | |
| Specific Yield (g/g of sucrose) | 0.43 | 0.290 | 0.340 | 0.320 | 0.12 | ||||||
| Total raw material cost | 122435 | 107288 | 114002 | 97393 | 375000 | ||||||
| Raw material cost per kg of levan | 122.43 | 107.29 | 114.00 | 97.39 | 375.00 | ||||||
* From 1 kg of sugarcane, 0.750 kg juice containing 0.135 kg sucrose is obtained.
For calculating the media cost, the prevailing commercial price were taken for all chemicals. The data was collected from www.indiamart.com on Dec 15, 2022. Cost of Chicken feather peptone was calculated based on the chemicals consumed for the preparation of CFP [19].
4. Conclusion
The study focused on levan production, a high-value microbial exo-polysaccharide, through microbial fermentation of a composite medium made of SJ, an agro-industry raw material for sucrose, and CFP a poultry industry by-product. Levan was produced using an alternate composite medium consisting of sugarcane juice and chicken feather peptone. Under optimized conditions, a levan yield of 0.32 ± 0.01 g/g of sucrose consumed was obtained. Though the yield was 20% lower, using this alternate composite medium would result in 7% decrease in raw material cost. The use of SJ as an alternative to refined sucrose could save a lot of processing steps and hence improve the overall economics of the fermentation process. In addition, using CFP as a nutrient source could also be beneficial in reducing upstream (fermentation) cost during levan production. Levan produced using SJ and CFP showed strong antioxidant activity and levan produced using alternative media is bio-compatible and can be used for food and biomedical applications.
Author contribution statement
Bhuvaneshwari Veerapandian: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Saravanan Ramiah Shanmugam, Ponnusami Venkatachalam: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Subramaniyasharma Sivaraman: Conceived and designed the experiments; Analyzed and interpreted the data.
Malinee Sriariyanun, Sugumaran Karuppiah: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the Department of Science & Technology, India, for support through the FIST programme (Grant: SR/FST/ETI-331/2013). Dr. Saravanan Ramiah Shanmugam sincerely thanks DST for support through INSPIRE grant (File No: DST/INSPIRE/04/2017/002528). The first author Mrs. Bhuvaneshwari Veerapandian thank CSIR for SRF fellowship award (Direct SRF/09/1095(0061)/2020 EMR-I).
References
- 1.Jiang W., Zhao J., Wang Z., Yang S.T. Stable high-titer n-butanol production from sucrose and sugarcane juice by Clostridium acetobutylicum JB200 in repeated batch fermentations. Bioresour. Technol. 2014;163:172–179. doi: 10.1016/j.biortech.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan N., Kumar D., Singh P., Patel S. Status of sugarcane juice preservation processes and technologies : a review. Int. J. Chem. Stud. 2019;7:2720–2728. [Google Scholar]
- 3.Ali S.E., El Gedaily R.A., Mocan A., Farag M.A., El-Seedi H.R. Profiling metabolites and biological activities of sugarcane (saccharum officinarum linn.) juice and its product molasses via a multiplex metabolomics approach. Molecules. 2019;24 doi: 10.3390/molecules24050934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geremias-Andrade I.M., Rocheto A.C., Gallo F.A., Petrus R.R. The shelf life of standardized sugarcane juice stored under refrigeration. Food Sci. Technol. 2020;40:95–101. doi: 10.1590/fst.33918. [DOI] [Google Scholar]
- 5.Pantelić I., Lukić M., Gojgić-Cvijović G., Jakovljević D., Nikolić I., Lunter D.J., Daniels R., Savić S. Bacillus licheniformis levan as a functional biopolymer in topical drug dosage forms: from basic colloidal considerations to actual pharmaceutical application. Eur. J. Pharmaceut. Sci. 2020;142 doi: 10.1016/j.ejps.2019.105109. [DOI] [PubMed] [Google Scholar]
- 6.Lončarević B., Lješević M., Marković M., Anđelković I., Gojgić-Cvijović G., Jakovljević D., Beškoski V. Microbial levan and pullulan as potential protective agents for reducing adverse effects of copper on Daphnia magna and Vibrio fischeri. Ecotoxicol. Environ. Saf. 2019;181:187–193. doi: 10.1016/j.ecoenv.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Srikanth R., Reddy C.H.S.S.S., Siddartha G., Ramaiah M.J., Uppuluri K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015;120:102–114. doi: 10.1016/j.carbpol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Nasir A., Ahmad W., Sattar F., Ashfaq I., Lindemann S.R., Chen M.-H., Van den Ende W., Ӧner E.T., Kirtel O., Khaliq S., Ghauri M.A., Anwar M.A. Production of a high molecular weight levan by Bacillus paralicheniformis, an industrially and agriculturally important isolate from the buffalo grass rhizosphere. Antonie Leeuwenhoek. 2022;115:1101–1112. doi: 10.1007/s10482-022-01760-6. [DOI] [PubMed] [Google Scholar]
- 9.Matsuhira H., Tamura K., Tamagake H., Sato Y. High production of plant type levan in sugar beet transformed with timothy (Phleum pratense) 6-SFT genes. J. Biotechnol. 2014;192:215–222. doi: 10.1016/j.jbiotec.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Stanbury J., Peter F., Whitaker Allan, Hall Stephen. Vasa; 2003. Principle of Fermentation Technology; p. 367.http://medcontent.metapress.com/index/A65RM03P4874243N.pdf [Google Scholar]
- 11.Yezza A., Tyagi R.D., Valéro J.R., Surampalli R.Y. Bioconversion of industrial wastewater and wastewater sludge into Bacillus thuringiensis based biopesticides in pilot fermentor. Bioresour. Technol. 2006;97:1850–1857. doi: 10.1016/j.biortech.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Moosavi-Nasab M., Layegh B., Aminlari L., Hashemi M.B. Microbial production of levan using date syrup and investigation of its properties. Int. J. Nutr. Food Eng. World Acad. Sci. Eng. Technol. 2010;44:1248–1254. http://waset.org/publications/2614/microbial-production-of-levan-using-date-syrup-and-investigation-of-its-properties [Google Scholar]
- 13.Küçükaşik F., Kazak H., Güney D., Finore I., Poli A., Yenigün O., Nicolaus B., Öner E.T. Molasses as fermentation substrate for levan production by Halomonas sp. Appl. Microbiol. Biotechnol. 2011;89:1729–1740. doi: 10.1007/s00253-010-3055-8. [DOI] [PubMed] [Google Scholar]
- 14.Han Y.W., Watson M.A. Production of microbial levan from sucrose, sugarcane juice and beet molasses. J. Ind. Microbiol. 1992;9:257–260. doi: 10.1007/BF01569633. [DOI] [Google Scholar]
- 15.de Oliveira M.R., da Silva R.S.S.F., Buzato J.B., Celligoi M.A.P.C. Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochem. Eng. J. 2007;37:177–183. doi: 10.1016/j.bej.2007.04.009. [DOI] [Google Scholar]
- 16.Mummaleti G., Sarma C., Kalakandan S.K., Gazula H., Sivanandham V., Anandharaj A. Characterization of levan produced from coconut inflorescence sap using Bacillus subtilis and its application as a sweetener. LWT--Food Sci. Technol. 2022;154 doi: 10.1016/j.lwt.2021.112697. [DOI] [Google Scholar]
- 17.Mummaleti G., Sarma C., Kalakandan S.K., Rawson A., Anandharaj A. Optimization and extraction of edible microbial polysaccharide from Fresh Coconut Inflorescence Sap: an alternative substrate. LWT--Food Sci. Technol. 2021;138 doi: 10.1016/j.lwt.2020.110619. [DOI] [Google Scholar]
- 18.Ragab T.I.M., Malek R.A., Elsehemy I.A., Farag M.M.S., Salama B.M., Abd El-Baseer M.A., Gamal-Eldeen A.M., El Enshasy H.A., Esawy M.A. Scaling up of levan yield in Bacillus subtilis M and cytotoxicity study on levan and its derivatives. J. Biosci. Bioeng. 2019;127:655–662. doi: 10.1016/j.jbiosc.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Veerapandian B., Shanmugam S.R., Varadhan S., Sarwareddy K.K., Mani K.P., Ponnusami V. Levan production from sucrose using chicken feather peptone as a low cost supplemental nutrient source. Carbohydr. Polym. 2020;227 doi: 10.1016/j.carbpol.2019.115361. [DOI] [PubMed] [Google Scholar]
- 20.Ozdal M., Başaran Kurbanoglu E. Use of chicken feather peptone and sugar beet molasses as low cost substrates for xanthan production by xanthomonas campestris MO-03. Fermentation. 2019;5:9. doi: 10.3390/fermentation5010009. [DOI] [Google Scholar]
- 21.Ozdal M., Kurbanoglu E.B. Valorisation of chicken feathers for xanthan gum production using Xanthomonas campestris MO-03. J. Genet. Eng. Biotechnol. 2018;16:259–263. doi: 10.1016/j.jgeb.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdal M., Kurbanoglu E.B. Citric acid production by Aspergillus Niger from agro-industrial by-products: molasses and chicken feather peptone. Waste Biomass Valoriz. 2019;10:631–640. doi: 10.1007/s12649-018-0240-y. [DOI] [Google Scholar]
- 23.Chidambaram J.S.C.A., Veerapandian B., Sarwareddy K.K., Mani K.P., Shanmugam S.R., Venkatachalam P. Studies on solvent precipitation of levan synthesized using Bacillus subtilis MTCC 441. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jegatheesan V., Shu L., Keir G., Phong D.D. Evaluating membrane technology for clarification of sugarcane juice. Rev. Environ. Sci. Biotechnol. 2012;11:109–124. doi: 10.1007/s11157-012-9271-1. [DOI] [Google Scholar]
- 25.Homann A., Biedendieck R., Götze S., Jahn D., Seibel J. Insights into polymer versus oligosaccharide synthesis: mutagenesis and mechanistic studies of a novel levansucrase from Bacillus megaterium. Biochem. J. 2007;407:189–198. doi: 10.1042/BJ20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majumdar S.K., Bose S.K. Utilization of amino acids by Bacillus subtilis during growth and antibiotic production. BBA - Biochim. Biophys. Acta. 1958;29:509–513. doi: 10.1016/0006-3002(58)90006-4. [DOI] [PubMed] [Google Scholar]
- 27.van Hijum S.A.F.T., Szalowska E., van der Maarel M.J.E.C., Dijkhuizen L. Biochemical and molecular characterization of a levansucrase from Lactobacillus reuteri. Microbiology. 2004;150:621–630. doi: 10.1099/mic.0.26671-0. [DOI] [PubMed] [Google Scholar]
- 28.Szwengiel A., Wiesner M. Effect of metal ions on levan synthesis efficiency and its parameters by levansucrase from Bacillus subtilis. Int. J. Biol. Macromol. 2019;128:237–243. doi: 10.1016/j.ijbiomac.2019.01.155. [DOI] [PubMed] [Google Scholar]
- 29.Shih I.L., Chen L.D., Wu J.Y. Levan production using Bacillus subtilis natto cells immobilized on alginate. Carbohydr. Polym. 2010;82:111–117. doi: 10.1016/j.carbpol.2010.04.030. [DOI] [Google Scholar]
- 30.Hou Y., Huang F., Yang H., Cong H., Zhang X., Xie X., Yang H., Tong Q., Luo N., Zhu P., Meng J. Factors affecting the production and molecular weight of levan in enzymatic synthesis by recombinant Bacillus subtilis levansucrase SacB-T305A. Polym. Int. 2021;70:185–192. doi: 10.1002/pi.6112. [DOI] [Google Scholar]
- 31.Abou-taleb K., Abdel-Monem M., Yassin M., Draz A. Production, purification and characterization of levan polymer from Bacillus lentus V8 strain. Br. Microbiol. Res. J. 2015;5:22–32. doi: 10.9734/bmrj/2015/12448. [DOI] [Google Scholar]
- 32.Gojgic-Cvijovic G.D., Jakovljevic D.M., Loncarevic B.D., Todorovic N.M., Pergal M.V., Ciric J., Loos K., Beskoski V.P., Vrvic M.M. Production of levan by Bacillus licheniformis NS032 in sugar beet molasses-based medium. Int. J. Biol. Macromol. 2019;121:142–151. doi: 10.1016/j.ijbiomac.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Han J., Xu X., Gao C., Liu Z. Levan-producing leuconostoc citreum strain BD1707 and its growth in tomato juice supplemented with sucrose jin, Appl. Environ. Microbiol. 2016;82:1383–1390. doi: 10.1128/AEM.02944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih I.L., Chen L.D., Wang T.C., Wu J.Y., Liaw K.S. Tandem production of levan and ethanol by microbial fermentation. Green Chem. 2010;12:1242–1247. doi: 10.1039/b924765c. [DOI] [Google Scholar]
- 35.Ahmad W., Nasir A., Sattar F., Ashfaq I., Chen M.H., Hayat A., ur Rehman M., Zhao S., Khaliq S., Ghauri M.A., Anwar M.A. Production of bimodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022;67:21–31. doi: 10.1007/s12223-021-00913-w. [DOI] [PubMed] [Google Scholar]
- 36.Nasir D.Q., Wahyuningrum D., Hertadi R. Screening and characterization of levan secreted by halophilic bacterium of halomonas and chromohalobacter genuses originated from bledug Kuwu mud crater. Procedia Chem. 2015;16:272–278. doi: 10.1016/J.PROCHE.2015.12.050. [DOI] [Google Scholar]
- 37.Raga-carbajal E., López-munguía A., Alvarez L., Olvera C. 2018. Understanding the Transfer Reaction Network behind the Non-processive Synthesis of Low Molecular Weight Levan Catalyzed by Bacillus Subtilis Levansucrase; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz-Soto M.E., Porras-Domínguez J.R., Seibel J., López-Munguía A.L.M. A close look at the structural features and reaction conditions that modulate the synthesis of low and high molecular weight fructans by levansucrases. Carbohydr. Polym. 2019;219:130–142. doi: 10.1016/j.carbpol.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Pei F., Ma Y., Chen X., Liu H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol. 2020;161:1181–1188. doi: 10.1016/j.ijbiomac.2020.06.140. [DOI] [PubMed] [Google Scholar]
- 40.Parwani L., Bhatnagar M., Bhatnagar A., Sharma V. Reactive oxygen species control by plant biopolymers intended to be used in wound dressings. Int. J. Pharm. Pharmaceut. Sci. 2012;4:506–510. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.