Abstract
Thermal springs are the sites where the water temperature lies above ambient temperature. They are widely used for power generation, hot water spas, balneotherapy, agriculture, laundering, and aquaculture. In Nepal, many thermal springs are reported but scientific understanding on water quality and hydrogeochemistry of the springs is very limited. In this study, a total of 28 physico-chemical parameters were measured in water samples collected from 12 thermal springs from Gandaki Province, Nepal. Correlation matrix and multivariate statistical analysis such as principal component analysis (PCA) and cluster analysis were used to understand the water quality and hydrogeochemistry of the hot water springs. The pH, temperature, electrical conductivity, total dissolved solids, and turbidity in the hot water springs ranged from 7.3 to 8.8, 31.6–64.3 °C, 206–16270 μS/cm, 115–6637 mg/L, and 0.21–63.7 NTU; respectively. The dominance order of major anions and cations were: Cl− > HCO3− > SO42− > NO3− > F− and Na+ > Ca2+ > K+ > Mg2+; respectively. Comparison of the water quality parameters with the WHO and National Water Quality Standards suggested that the majority of the parameters were within the safe limit. Out of 9 heavy metals and trace elements analyzed Zn2+, Ni2+, Cr3+, Cd+2, Hg (total), and Pb2+ were found below the safe limit but Fe (total), As (total) and Cu (total) were found higher than the WHO safe limit in total of 3, 5 and 1 sampling sites; respectively. The water quality index (WQI), sodium absorption ratio (SAR), and magnesium hazard (MH) ratio in the hot water springs ranged from 40.9 to 573, 2.2–49.3, 7.1–70.8; respectively. The result of PCA analysis showed that four principal components are required to explain hydrogeochemistry. Cluster analysis suggested that the sampling sites can be grouped into three distinct clusters based on total dissolved solids. Interestingly, the classification of hydrochemical facies using a Piper diagram suggested that 7 out of 12 thermal springs have Na–Cl type water. Finally, a perspective on the suitability of the hot springs for hot water spas and balneotherapy and policy recommendation is provided.
Keywords: Hydrogeochemistry, Hot springs, Water quality, Heavy metals, Multivariate analysis
1. Introduction
Water is one of the most important resources for all living organisms. In different geographical regions, water resources having diverse physicochemical properties are reported to exist [[1], [2], [3], [4], [5], [6], [7], [8]]. The hot or thermal springs are groundwater resources having temperature significantly higher than the mean annual air temperature of that area. The elevated temperature of the thermal springs could arise due to geothermal energy, exothermic chemical reactions, and radioactive disintegration. The geothermal cycles could involve percolation of meteoritic water, heating of the water due to high temperature curst, water drainage along fault zones, and appearance to the surface [9]. Like other water resources, physiochemical parameters such as pH, temperature, electrical conductivity (EC), total dissolved solids (TDS), various ions, and heavy metals affect the quality of thermal spring. Water at elevated temperatures can interact more with the underlying rocks, minerals and adjoining environment. Depending on geography and geology of the springs, the higher temperature can result in an elevated level of dissolved solids, various minerals, and gases [[10], [11], [12]].
Warm water contains less dissolved oxygen making the existence of living species very limited. However, such water can have several applications, such as power generation, balneotherapy, agriculture, laundering, aquaculture, and many more [[13], [14], [15]]. Balneotherapy is a traditional medicine technique in which hot water bath is used for the treatment as well as cure of disease. The presence of minerals, salt, and major ions including sulfate (SO42−) make the hot springs suitable for several therapeutic applications, such as skin therapy, bone healing, gastrological diseases, and soothing body ache [13,[16], [17], [18]]. In a clinical study it is reported that low salt hot water helps to cure atopic dermatitis [17].
Literature studies on thermal springs in other countries report significant variation in the major water quality parameters [2,3,16,[19], [20], [21], [22]]. Statistical tools such as principal component analysis (PCA) and cluster analysis are often used to find the interrelation between variables, their spatial and temporal dynamics, and explain the hydrogeochemistry [21,[23], [24], [25]]. As compared to other water sources, water quality parameters such as electrical conductivity (EC), total dissolved solids (TDS), major anions and cations, and trace elements are found at elevated levels. This variation can be attributed to differences in geochemistry, water temperature, and anthropogenic factors.
A study on three hot springs in Sikkim, India [20] reported that temperature, pH, TDS, EC, alkalinity, Cl−, F−, NO3−, and SO42− in the range of 45–70 °C, 7.4–8, 398–612 mg/L, 240–1180 μmhos, 78–459 mg/L, 12–103 mg/L, 1.6–5.0 mg/L, 0.05–3.4 mg/L, and 3.6–20.7 mg/L; respectively. The concentration of trace elements such as Zn2+, Cd2+, Pb2+, Se2+, Cu, and As were below the WHO guideline for drinking and bathing purposes. Another study, reported the selected physical‒chemical parameters of 22 non‒volcanic hot springs of West Malaysia [21]. The temperature, pH, Cl−, F−, CO32−, HCO3−, and SO42− content ranged from 41 to 99 °C, 5.5–9, 1.1–16800 mg/L, 2.5–12 mg/L, 2–16 mg/L, 10–282 mg/L, 1–1550 mg/L, respectively. The cations Na+, K+, Ca2+, Mg2+, and Fe in mg/L were found as 32–8500, 1.5–277, 1–418, 0.1–1360, <0.1–43; respectively. A study on 4 thermal springs of Shigar Valley Pakistan [22] reported temperature, pH, TDS, hardness, turbidity, and alkalinity of the spring water in the range of 40–42 °C, 7.2–7.8, 300–310 mg/L, 278–285 mg/L, <5 NTU, and 250–260 mg/L; respectively. The Na+, K+, Ca2+, Mg2+ metals and anions Cl−, F−, HCO3−, SO42− were found below 100 mg/L. The heavy metal and trace elements Zn2+, Cu, Mn2+, As, and Fe were in safe levels.
Hot water springs are locally called Tatopani Kunda in Nepal. Around thirty five major hot springs have been identified in different parts of Nepal and the majority of them are located in the Gandaki Province of western Nepal [10,26]. Nevertheless, the studies were primarily confined to a few parameters without exploring the potential association with water quality and hydrogeochemistry [27]. The variation in geographical, geological and other factors can cause significant variation in the physical and chemical parameters of hot springs. It is also important to assess the water quality parameters for safe and sustainable use of the water resources. The novelty of this research lies in its investigation into the water quality and hydrogeochemistry of socially and culturally important hot water springs located at different topographical segments of the Himalayas.
In this study, several physical and chemical parameters of water samples collected from 12 hot springs of Gandki Province, Nepal were measured. The water quality parameters were compared with the WHO and Nepal Drinking Water Quality Standard (NDWQS). Water quality indices were also calculated using the experimental parameters. Different statistical methods, such as correlation matrix, cluster analysis, and principal components analysis were performed to find a link between the parameters and understand the water quality and hydrogeochemistry. Finally our perspective on the suitability of the hot springs for hot water spas and balneotherapy, and policy recommendation is provided.
2. Materials and methods
2.1. Study area
This study was carried out in the Gandaki River Basin (GRB), one of the three major glacier-fed river basins in Nepal, is located in the Central Himalaya and between longitudes 82.88° to 85.81°E and latitudes 27.32° to 29.33°N (Fig. 1). The mean air temperature and annual discharge of the GRB is 17.7 °C and 1753 m3 s−1, respectively. Besides, 80% of the annual precipitation is concentrated during the monsoon season from June to September in the study region.
Fig. 1.
Location map of the study area.
In Nepal, about 35 thermal water springs are reported to exist across the country [10,26]; and few of them are not easily accessible. Among them, 12 thermal water samples (S1–S12) were selected from the Lamgung, Manang, Kaski, and Myagdi districts of Gandaki Province, Nepal (Fig. 1 and Table 1). The sites were selected based on their accessibility and variability on altitude (∼900–2700 masl; Table 1) and underlying geology. The majority of hot water springs selected here are being used for water bathing and therapy. These sites also lie close to major trekking routes/tourist destinations. Most of the selected sites lie near the residential area i.e. within a few km of the human settlements.
Table 1.
Information of the sampling sites.
| Sample ID | Location | Latitude (N) | Longitude (E) | Altitude (m) | Approx. flow rate (L/sec) |
|---|---|---|---|---|---|
| S1 | Bahun danda, Lamjung | 28.339258 | 84.397786 | 995 | 1.5 |
| S2 | Jagat, Lamjung | 28.413584 | 84.406597 | 1319 | 3.7 |
| S3 | Chame, Manang | 28.55217 | 84.241702 | 2699 | 0.8 |
| S4 | Bhurjungkhola, Kaski | 28.36106 | 83.960363 | 1261 | 0.1 |
| S5 | Annapurna 1, Kaski | 28.421764 | 83.820621 | 2005 | 0.3 |
| S6 | Annapurna 2, Myagdi | 28.459395 | 83.627178 | 1127 | 0.2 |
| S7 | Ratopani, Myagdi | 28.478222 | 83.641139 | 1164 | 2.0 |
| S8 | Bhurung, Myagdi | 28.496267 | 83.654278 | 1258 | 0.6 |
| S9 | Naarchhyang, Myagdi | 28.499314 | 83.654466 | 1252 | 4.0 |
| S10 | Raghuganga-cheu, Myagdi | 28.453195 | 83.513988 | 1431 | 0.5 |
| S11 | Raghuganga-kuna, Myagdi | 28.453195 | 83.513988 | 1431 | 0.5 |
| S12 | Singa, Myagdi | 28.367592 | 83.502616 | 931 | 0.4 |
The discharge rate of the springs is seasonal dependent and in the month of March (2021) it was found to range 0.1–4.0 L/s (Table 1). The discharge rate was measured using passive sampling method; in which time taken to fill the fixed volume of the container was recorded. The spring opening roughly ranged 1–10 cm in diameter. The water from the springs was found to form a small pond(s) or reservoir(s) nearby. The water is widely used for hot water spas, balneotherapy and also for irrigation.
The thermal springs lie close to/or at the well-defined geographical fault zone. Perrier et al. suggested that the hot springs are the exit points of meteoric water recharged from Tibetan plateau and High Himalaya regions [28]. The geothermal circulation of hot water springs in the Nepal Himalayas could involve the percolation of meteoritic water to great depth, water heating due to high temperature of the curst, drainage along the Main Central Thrust (MCT) zone, and final appearance to the surface as hot water spring [9].
The hydrogeochemistry of hot water springs is influenced by the chemical composition of the rocks surrounding them. Overall, the study region is characterized by the green schist-grade to lower amphibolite-grade of meta-sedimentary rocks ranging in age from Proterozoic to Cenozoic. Precisely, the siltstones and carbonaceous schist rock deposits are predominant in the region, which is characterized by the dominance of carbonate-rich mineralogical composition along with sulfur and chloride contents [27]. Specifically, two of the study sites are located in Lamjung and Kaski districts i.e., S1 and S4, respectively. These sites are reported to have slightly different underlying geological formation. For instance, the underlying rocks in S1 is characterized by garnet and kyanite deposits which may have formed by multiple metamorphisms and dynamic crystallization of minerals [29]; whereas the rocks at S4 exhibit a composition consisting of sediments rich in detritus, along with limestone, sandstone, and shale [30].
2.2. Sampling method
Considering the availability, potential use, climatic and lithological conditions, the sampling campaigns were conducted in pre-monsoon season i.e., March 2021. Twelve water samples (S1–S12) were collected from the respective thermal springs (Fig. 1). The samples were collected in 1 L high density polyethylene (HDPE) bottles. Sampling bottles were washed with distilled water and then rinsed three times with the sample water at the study sites.
2.3. Data collection
The physical parameters temperature, pH, turbidity, EC, and TDS were measured onsite. Alkalinity, total hardness, Ca and Mg hardness, and the ions F−, SO42−, Cl−, NO3−, PO43−, Na+, and K+ were measured in the laboratory within a week.
Alkalinity, hardness, and Cl− were determined by titrimetric methods, SO42− by turbidity measurement method, and Ca2+ by EDTA titrimetric methods (APHA 3500-Ca D). The anions NO3−, F− and PO43− were measured by spectrophotometric methods (APHA 4500-NO3 B, 4500-F D, and 4500-P E). Na+ and K+ were determined using a flame photometer (Fischer Scientific, Jenway PFP7) following standard test methods (APHA 3500-Na and APHA 3500-K) and Mg2+, Zn2+, Cu, and Fe were measured using an atomic absorption spectrometer following standard operating procedure. All chemicals used in the measurements were of analytical grade.
For the determination of trace elements, samples were preserved by adding few drops of ultrapure nitric acid to make pH < 2 and stored at 4 °C. The trace elements As, Ni, Cr, Cd, Pb, and Hg were analyzed at the Department of Food Technology and Quality Control Laboratory, Nepal using Inductively Coupled Plasma Mass Spectrometry (Agilent, ICP‒MS, 7900) following standard operating conditions. Before measurement, all the hot spring water samples were filtered through 0.45 μm syringe filter (Polypropylene, Sigma Aldrich) to remove the suspended particles. After filtration, the samples had no turbidity, so digestion was not required. Five calibration standards (0.5 ppb, 5 ppb, 20 ppb and 100 ppb) were prepared for each element by diluting a certified reference standard (NIST, 10 ppm, periodic mix). To check the drift stability of the ICP-MS system, 50 ppb of internal standard of Sc, Ge, and Rh was added in blanks, standards, and spring water samples. Additionally, to check the matrix effect all water samples were spiked with 2.5 and 20.00 ppb of the trace elements investigated. The spiking recovery data for internal standards and trace elements and as measured data sets for all the physico-chemical parameters are reported in supporting information (Tables S1, S2, and S3).
2.4. Water quality indices
Water quality index (WQI) for drinking purpose was calculated using literature reported methods [31]. The relative weight of each parameter (Wi) was calculated as:
| (1) |
where wi is the weight of each parameters and n is the total number parameters included.
Quality rating qi for each parameter was calculated as:
| (2) |
where Ci is the concentration of each parameter (mg/L) in the water samples and Si is WHO standard values (mg/L).
Finally, water quality index (WQI) was calculated as:
| (3) |
The WQI values of <50, 50–100, 100–200, 200–300, >300 is classified as excellent, good, poor, very poor, and unsuitable water for drinking [31].
The suitability of the spring water for irrigation purpose was evaluated by calculating the sodium absorption ratio (SAR) and magnesium hazard (MH). SAR was calculated using Equation (4):
| (4) |
SAR value of <10, 10–26, and >26 indicates that the water is good, moderately suitable, and unsuitable for irrigation [25,32].
Magnesium hazard (MH) was calculated using Equation (5):
| (5) |
If MH > 50, the water is considered unsuitable for irrigation [25,32].
2.5. Statistical analysis
To find the link between different water parameters, descriptive statistical analysis, such as minimum, maximum, mean and standard deviation and other statistical analyses like correlation, cluster analysis, and principal component analysis/factor analysis (PCA/FA) were performed using SPSS version 22.0 software. Kaiser-Meyer-Olkin (KMO) tests were performed to check the appropriateness of the data set for PCA/FA. For PCA/FA, the Varimax rotation was performed in normalized data sets and significant principal components (PCs) that contribute to the water quality and hydrogeochemistry were identified [33].
A brief flowchart that highlights conceptual framework of this work is provided in Fig. 2.
Fig. 2.
A flow chart for the methodological design used in this work.
3. Results
3.1. General hydrogeochemistry
The individual data sets for all the spring water samples along with WHO and Nepal Drinking Water Quality Standard (NDWQS) values are provided in Tables S2 and S3. The data were analyzed to get major descriptive statistical parameters such as minimum, maximum, and mean values with standard deviations (Table 2). For easy comparison, data are also reported as Box plot (Fig. 3).
Table 2.
Descriptive statistics of hydrogeochemical variables of the geothermal springs of Gandaki Province, Nepal.
| Parameters | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Tem. | 31.6 | 64.3 | 50.0 | 9.97 |
| pH | 7.30 | 8.80 | 7.93 | 0.420 |
| EC | 206 | 16,270 | 3902 | 4956 |
| TDS | 115 | 6637 | 1540 | 1900 |
| Turb. | 0.21 | 63.7 | 7.82 | 18.04 |
| Ca2+ | 8.00 | 364 | 88.3 | 103 |
| Mg2+ | 1.90 | 121 | 27 | 33.5 |
| K+ | 2.80 | 137 | 33.3 | 39.0 |
| Na+ | 7.90 | 370 | 146 | 124 |
| Cl− | 28.2 | 3964 | 642 | 1106 |
| NO3− | 0.100 | 2.00 | 0.590 | 0.680 |
| SO42- | 0.000 | 24.9 | 6.98 | 8.30 |
| F− | 0.000 | 2.60 | 1.22 | 0.91 |
| HCO3− | 61.33 | 490 | 184 | 142 |
| HN | 60.00 | 1417 | 333 | 388 |
| Fe | 0.030 | 5.75 | 0.710 | 1.63 |
| As | 0.001 | 0.140 | 0.020 | 0.040 |
Tem.: Temperature, Turb.: Turbidity, HN: Hardness. Units mg/L except pH, EC (μS/cm), Tem. (°C), and Turb. (NTU).
Fig. 3.
Box-and-Whisker plot for the quality parameters of the thermal springs. For better display, X-axis is plotted in Log10 scale. Tem.: temperature, EC: electrical conductivity, TDS: total dissolved solids, and TH: total hardness. All units in mg/L except pH, EC (μS/cm), and Tem. (°C).
For hydrogeochemical variables, there were distinct characteristics among the different springs. The highest water temperature of 64.3 °C was reported in sample S9. pH was found to be slightly alkaline throughout the assessment. In the study area, pH varied from 7.30 to 8.80 (7.93 ± 0.42).
The pH was highest in the S12 which could be due to the carbonate rock outcrop in the region (Table S2). EC and TDS indicate the salinity character of water in thermal springs, which also implies the high concentrations of any salts or ions dissolved in water. The values of EC ranged from 206 to 16,270 μS/cm (3902 ± 4956).
The TDS varied from 115 to 6637 mg/L (1540 ± 1900). The highest value of TDS was recorded in S1. Turbidity in the study area was in the range of 0.210–63.7 NTU (7.82 ± 18.0). The turbidity value was found higher than the WHO guideline of 1 NTU in six samples only (i.e. S1, S2, S3, S4, S6, and S7).
The Na+ was the most dominant cation and it varied from 7.90 to 370 mg/L (146 ± 124.4). The dominance order of major cations was found to be Na+ > Ca2+ > K+ > Mg2+. Wide variation in concentration of Na+, Ca2+, and Mg2+ was observed among the sampling sites as evidenced by high standard deviation and individual data sets are provided in Table S3. Ca2+ was found to be higher than WHO drinking quality water guidelines in three samples (S1, S5, and S9) Na+ in seven (S1–S4, S8, S9, and S12), and Mg2+ in 2 (S1 and S4), and K+ in one (S1) samples.
We also measured major anions Cl−, HCO3−, SO42−, NO3−, F−, and PO43− in the spring water samples (Table 2 and S2). Cl− was the most dominant anion in the study springs whereas PO43− was below the detection limit in all the samples. The concentrations of Cl− ranged from 28.2 to 3964 mg/L (642 mg/L ± 1106). In the sample analyzed Cl− was found below the WHO limit in five samples (S5, S6, S7, S10, and S11), F− also in five samples (S5, S7, S9, S10, and S11), and the rest of the ions were below the WHO limit in all the samples.
The mean concentrations of Fe and As were found to be 0.710 and 0.020 mg/L; respectively. In all samples Fe was found to be lower than WHO guideline (Table S3) and As was found to be higher than WHO guideline only in 5 samples (S3, S6, S7, S10, and S11). We also analyzed Ni2+, Cr3+, Cd2+, Hg, and Pb2+ in all the samples (Table S3). Interestingly, in all the samples these elements were found lower than their respective WHO safe limit for drinking water.
3.2. Water quality indices
To assess the water quality, water quality index (WQI), sodium absorption ratio (SAR), and magnesium hazard (MH) ratio were calculated using Equations (1), (2), (3), (4), (5). The input parameters for WQI calculation are provided in Table 3.
Table 3.
Parameters used in calculation of water quality index.
| Parameters | WHO values | Weightage (wi) | Relative weightage (Wi) |
|---|---|---|---|
| TDS (mg/L) | 1000a | 4 | 0.0800 |
| pH | 7 | 4 | 0.0800 |
| EC (μS/cm) | 1500a | 4 | 0.0800 |
| TH (mg/L) | 200 | 2 | 0.0400 |
| Turbidity (NTU) | 1 | 1 | 0.0200 |
| HCO3−(mg/L) | 300 | 3 | 0.0600 |
| Cl−(mg/L) | 250 | 3 | 0.0600 |
| SO42−(mg/L) | 200 | 4 | 0.0800 |
| NO3−(mg/L) | 50 | 5 | 0.1000 |
| F−(mg/L) | 1 | 4 | 0.0800 |
| Na+(mg/L) | 200 | 2 | 0.0400 |
| K+(mg/L) | 100 | 1 | 0.0200 |
| Ca2+(mg/L) | 100 | 2 | 0.0400 |
| Mg2+(mg/L) | 50 | 2 | 0.0400 |
| Fetotal(mg/L) | 0.3 | 4 | 0.0800 |
| Astotal(mg/L) | 0.01 | 5 | 0.1000 |
| Σwi | 50 |
WHO has no strict values for these parameters.
The WQI, SAR, and MH ratio for the water samples ranged from 40.9 to 573, 2.2–49.3, and 7.1–70.8; respectively (Table 4).
Table 4.
Water quality indices of different samples.
| Sample | WQI | SAR | MH |
|---|---|---|---|
| S1 | 573 | 23.7 | 25.0 |
| S2 | 97.8 | 28.3 | 18.1 |
| S3 | 125.3 | 23.5 | 7.1 |
| S4 | 195.8 | 37.2 | 47.0 |
| S5 | 40.9 | 5.1 | 20.6 |
| S6 | 61.1 | 18.6 | 8.4 |
| S7 | 177.5 | 7.0 | 25.2 |
| S8 | 74.2 | 18.0 | 18.7 |
| S9 | 129.8 | 29.4 | 16.0 |
| S10 | 45.7 | 2.2 | 21.8 |
| S11 | 48.7 | 3.1 | 31.3 |
| S12 | 81.9 | 49.3 | 70.8 |
4. Discussion
4.1. General hydrochemistry
Nepali Himalaya has carbonate dominated underlying lithology [[34], [35], [36]]. Due to this factor, the pH of water remains more or less neutral even though other parameters fluctuate (Table 2). The marked variation in EC values with high SD indicates the nature and sources of chemicals in the study springs are not uniform. EC values had a direct relation with dissolved solids and the high EC values signify a high level of anthropogenic signatures [37]. Weathering of carbonate and silicate dominated underlying lithology, and disintegration of salts results in elevated EC values, whereas high a dilution factor lowers its value. As expected, spatial fluctuations in the TDS values resemble those of EC, is consistent with the previous studies from the Himalayas [30,38].
High values of TDS in the hot water springs could be due to the high rock-water interactions aided by high temperature [23,38]. Also, the high standard deviation in TDS signifies the distinctly different nature of thermal springs in the study regions. The moderately high turbidity in some of the samples (S1 and S4) could be due to colloidal particles of iron and calcium or other suspended particles from multiple sources.
The dominance order of major anions was found to be Cl− > HCO3− > SO42− > NO3− > F−. Interestingly, the dominance order is contrary to the previous studies in river water systems from the same segments Himalayas; where the dominance of HCO3− was observed [35,39]. The dominance of Cl− in the hot water springs could be attributed to different geogenic process including chloride dominant mineralogical compositions and elevated temperature [21,40,41]. Among the major cations, Na+ was the predominant cation in the springs but Ca2+ was found to be dominant ion in the river water system of the same study area [35,39]. The dominance of Na+ in this study could be attributed due to high temperature of the thermal springs [21,23]. The relatively low values of Na+ and K+ in S7, S10 and S11 could be due to the influence of the dilution factor due to freshwater input from the diverse sources [41].
In all the thermal springs explored, the concentration of toxic trace elements (Pb, Cd, and Hg) is relatively low (Tables S2 and S3). At neural pH, relatively low leaching of potentially toxic trace elements takes place. Only one sample (S7) showed a significantly elevated level of As (∼140 ppb) but in the rest of the samples As was found at or near WHO safe limit. Elevated levels of As (i.e. >10 ppb as recommend by WHO safe limit) in S3, S6, S7, S10, and S11 sites could be due to 1) enhanced interaction and longer residence time with arsenic ores at elevated temperature [23], 2) pedogenesis, 3) anthropogenic sources. The studied sites are located in the young mountain land topography, so pedogenesis could be a likely cause.
Physcio-chemical characterization and hydrochemistry of hot water springs from other regions of the globe is reported in several studies. There are a few physico-chemical parameters reported for the hot water springs from different physiographic regions of Nepal other than Gandaki Province [27]. Nevertheless, understanding on the interrelation between chemical variables and underlying lithology remains unexplored.
The hot water springs explored here are easily accessible and located near several notable attractions such as the renowned Annapurna trekking route, and a few highest mountains in the world having elevations >8000 m (e.g., Annapurna, Manaslu, and Dhaulagiri). These locations are globally famous for mountaineering, trekking, and hiking. Additionally, the springs also lie close to Muktinath; a world-famous religious site. Considering the importance of the region, the physicochemical parameters of the thermal springs of this study are compared to those found elsewhere (Table 5). From the study, it is evident that the hot water springs have moderate temperatures, neutral to moderately alkaline pH, relatively low to moderate concentrations of potentially toxic trace elements, and moderate levels of fluoride and sulfur. These indicators could suggest low dermal adsorption or inhalation hazards during hot water spas or bathing. It can be suggested that the thermal springs could be recommended as safe for hot water spas and balneotherapy.
Table 5.
Physico-chemical parameters of hot water springs reported in this work and other parts of the world.
| Parameters | Chutrun, Pakistan [22] | Chilime, Rasuwa, Nepal [10] | Sikkim India [20] | Tibet, China [24] | West Malaysia [21] | Limpopo, South Africa [42] | Oregon, USA [40] | Bolivian Altiplano [23] | This work |
|---|---|---|---|---|---|---|---|---|---|
| Tem. | 40.0–42.0 | 48.0 | 45.0–70.0 | 104.8–159.3 | 41.0–99.0 | 26–67.5 | 16.0–86.0 | 40–75 | 31.6–64.3 |
| pH | 7.21–7.80 | 7.00 | 7.40–8.00 | 8.30–9.60 | 5.5–9.0 | 7.35–9.70 | 6.90–9.30 | 6.33–8.29 | 7.30–8.80 |
| TDS | 300–310 | 166 | 112–608 | – | – | 104.7–1385 | 288–2570 | – | 115–6637 |
| EC | – | – | 238–1180 | 2340–4060 | – | – | 228–5140 | 4270–30400 | 206–16270 |
| Na+ | 12.0–18.0 | 7.35 | – | 1.40–709 | 32.5–8500 | 10.5–156 | 63–810 | 339–12866 | 7.90–370 |
| K+ | 3.80–4.10 | 8.10 | – | 0.800–135 | 1.50–277 | 0.99–4.25 | 0.300–54 | 14.2–328 | 2.80–137 |
| Ca2+ | 80.0–82.0 | 25.8 | 7.00–61.0 | 2.10–11.8 | 1.50–418 | 1.31–13.7 | 0.310–101 | 9.7–200 | 8.00–364 |
| Mg2+ | 20.0 | 20.8 | 2.00–25.0 | 0.200–1.03 | 0.100–1360 | 0.000–27.6 | 0.010–1.31 | 2.4–196 | 1.90–121 |
| As | – | – | 0.002–0.006 | 0.02–5.70 | – | – | – | 0.008–0.065 | 0.001–0.140 |
| Cl− | 9.60–12.0 | 10.0 | 12.0–109 | 503.5–1020 | 1.10–16800 | 19.4–535 | 0.69–1310 | 177–21022 | 28.2–3964 |
| F− | 3.40–3.90 | – | 1.60–5.80 | 18.5–19.6 | 3.30–12.0 | 0.180–6.50 | 0.690–7.10 | – | 0.000–2.60 |
| NO3− | – | – | 0.050–3.50 | – | – | 0.000–19.9 | – | <0.1–55 | 0.00–2.60 |
| SO42- | 80.0–85.0 | 6.00 | 3.40–21.2 | 27–60.3 | 0.900–1550 | 2.98–226 | 1.67–145 | 8–746 | 0.000–24.9 |
| HCO3− | 260–282 | 180 | – | 151–363 | 10.0–282 | – | – | 429–1892 | 61.3–490 |
Tem.: Temperature; Units mg/L except pH, EC (μS/cm), Tem. (°C).
The hot water springs are human-reachable and lie close to: 1) world-famous Annapurna trekking route, 2) Annapurna, Manaslu and Dhaulagiri; three major Himalayas of Nepal with elevation >8000 m, 3) Muktinath; a world-famous religious site. In these respects, both national and international tourists can entertain hot water spas besides engaging in other activities.
4.2. Water quality indices
WQI, SAR, and MH are frequently used indices to assess water quality [25,32,32,39]. The WQI values of <50, 50–100, 100–200, 200–300, >300 is classified as excellent, good, poor, very poor, and unsuitable water for drinking; respectively. In this study, WQI of the thermal springs ranged from 40.9 to 573 (Table 4). WQI values of <50 was found only in three springs (S5, S10, and S11) suggesting that the springs have excellent water quality. The springs S2, S6, S8, and S12 have WQI in the range of 50–100 indicating good water quality. The remaining 5 springs have WQI > 100 suggesting poor water quality.
The SAR for the water samples ranged from 2.2 to 49.3 (Table 4). SAR of <10, 10–26, and >26 indicates that the water is good, moderately suitable, and unsuitable for irrigation. The high SAR water can have permeability issues due to shrinkage and swelling of clayey soils and formation of alkali rich soil [25,32]. SAR values of <10 was found in four springs (S5, S7, S10, and S11) suggesting that the water is good for irrigation. The springs S1, S3, S6, S8, and S12 have SAR in the range of 10–26 indicating the water is moderately suitable for irrigation. The remaining springs (S2, S9, and S12) have SAR > 26 suggesting the water is not fit for irrigation purpose. Similarly, MH > 50, suggests that the water is unsuitable for irrigation [25,32]. In the present study, MH > 50 was found only in S12. Thus, on the basis of this index, all water samples except S12 is suitable for irrigation (Table 4).
4.3. Spearman’s correlation
The correlation matrix is a commonly used statistical tool to find the relationship between two hydrochemical variables for predicting their association [35]. The correlation matrix of this investigation is provided in Table 6. A strong correlation between EC and TDS (r = 0.99) might be attributed to ions that contribute both to TDS and EC [35]. Both Ca2+ and Mg2+ indicate a positive association with EC and TDS signifying their major role in the elevated values of the later variables. Similarly, other major ions such as K+, Na+, Cl−, SO42−, and HCO3− could also contribute to the elevated concentrations of TDS and EC, so all these ions show a positive correlation with TDS and EC (Table 6).
Table 6.
Correlation matrix hydrochemical variables of the geothermal springs of Gandaki Province, Nepal.
| Tem. | pH | EC | TDS | Turbidity | Ca2+ | Mg2+ | K+ | Na+ | Cl− | NO3− | SO42- | HCO3− | Hardness | Fe | As | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. | 1.00 | |||||||||||||||
| pH | 0.34 | 1.00 | ||||||||||||||
| EC | 0.04 | −0.43 | 1.00 | |||||||||||||
| TDS | 0.02 | −0.38 | 0.99b | 1.00 | ||||||||||||
| Turb. | −0.37 | 0.04 | 0.43 | 0.39 | 1.00 | |||||||||||
| Ca2+ | 0.09 | −0.74b | 0.66a | 0.68a | 0.09 | 1.00 | ||||||||||
| Mg2+ | −0.27 | −0.51 | 0.73b | 0.75b | 0.14 | 0.64a | 1.00 | |||||||||
| K+ | 0.05 | −0.36 | 0.95b | 0.97b | 0.33 | 0.71b | 0.79b | 1.00 | ||||||||
| Na+ | −0.05 | −0.31 | 0.97b | 0.97b | 0.44 | 0.55 | 0.72b | 0.94b | 1.00 | |||||||
| Cl− | −0.23 | −0.19 | 0.87b | 0.86b | 0.73b | 0.47 | 0.56 | 0.83b | 0.87b | 1.00 | ||||||
| NO3− | −0.44 | −0.19 | 0.36 | 0.31 | 0.82b | 0.22 | 0.27 | 0.27 | 0.33 | 0.59a | 1.00 | |||||
| SO42− | −0.12 | −0.52 | 0.85b | 0.87b | 0.14 | 0.73b | 0.87b | 0.89b | 0.82b | 0.67a | 0.22 | 1.00 | ||||
| HCO3− | −0.05 | −0.12 | 0.63a | 0.66a | −0.01 | 0.34 | 0.78b | 0.67a | 0.64a | 0.45 | 0.08 | 0.83b | 1.00 | |||
| HN | −0.07 | −0.71b | 0.76b | 0.78b | 0.10 | 0.91b | 0.87b | 0.81b | 0.67a | 0.55 | 0.27 | 0.87b | 0.59a | 1.00 | ||
| Fe | −0.50 | −0.50 | 0.34 | 0.29 | 0.45 | 0.30 | 0.58a | 0.29 | 0.32 | 0.30 | 0.62a | 0.29 | 0.13 | 0.47 | 1.00 | |
| As | 0.33 | 0.47 | −0.70b | −0.70b | 0.00 | −0.45 | −0.79b | −0.69a | −0.70b | −0.51 | 0.04 | −0.83b | −0.76b | −0.66a | −0.27 | 1.00 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
As expected, the major cations such as Ca2+ and Mg2+ have a significant positive correlation with hardness indicating that they were the major contributors to the hardness of the water. Most of the measured cations and anions showed positive associations with each other indicating that the ions could be derived from the same source; i.e. interaction of hot water with underlying rocks. However, arsenic showed a negative association with the majority of the ions except for NO3−. This suggests that As could have originated by different process such as pedogenesis and anthropogenic sources.
4.4. Principal component analysis
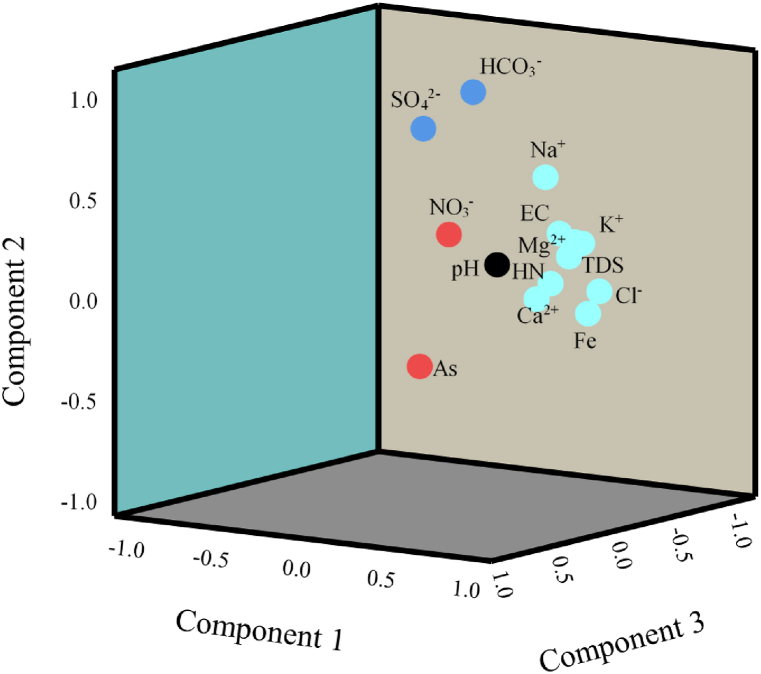
Principal component analysis (PCA) is one of the commonly used statistical methods to reduce the dimensionally and complexity of large data sets [43,44]. In this study, PCA was carried out on 14 hydrochemical variables (Table 7). The elements Ni, Cr, Cd, Pb, Zn, Cu, and Hg were at or below the detection limit. So, these elements were excluded in PCA analysis. The varimax rotation was carried out to recognize increased principal components of hydrochemical significance. The similar approach based on PCA has been used to identify the main components in hydrogeochemistry [35,44]. The KMO and Bartlett's results were obtained as 0.74 and 584.34 (df = 11, p < 0.001), indicating that PCA would be suitable for reducing the dimensionality of the measured dataset. The results of the rotated component matrix are depicted in Table 7 and Fig. 4. Four major components were obtained from the analysis with 93.39% cumulative variance. The first principal component (F1) had an eigenvalue of 8.42, which explained 54.22% of the total variance, while the second principal component (F2) explained 19.34% (eigenvalue: 2.67) of the total variation present in the measured dataset. Similarly, the third (F3) and fourth (F4) components have eigenvalues 1.87 and 1.67 and explained 10.93% and 8.90%; respectively.
Table 7.
Rotated component matrix of the hydrochemical variables in the thermal springs of the Gandaki Province, Nepal.
| Parameters | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| pH | −0.30 | −0.08 | −0.95 | −0.01 |
| EC | 0.89 | 0.37 | 0.21 | −0.02 |
| TDS | 0.93 | 0.32 | 0.15 | 0.02 |
| Ca2+ | 0.88 | 0.07 | 0.40 | −0.15 |
| Mg2+ | 0.92 | 0.25 | 0.18 | 0.03 |
| K+ | 0.93 | 0.31 | 0.09 | −0.08 |
| Na+ | 0.73 | 0.62 | 0.11 | 0.01 |
| Cl− | 0.97 | 0.06 | 0.00 | 0.10 |
| NO3− | 0.15 | 0.27 | 0.10 | 0.88 |
| SO42- | 0.22 | 0.85 | 0.41 | 0.09 |
| HCO3− | 0.12 | 0.94 | −0.15 | 0.03 |
| HN | 0.92 | 0.14 | 0.33 | −0.09 |
| Fe | 0.96 | −0.04 | 0.08 | 0.12 |
| As | −0.21 | −0.46 | −0.17 | 0.63 |
| % of Variance | 54.22 | 19.34 | 10.93 | 8.90 |
| Cumulative % | 54.22 | 73.56 | 84.49 | 93.39 |
Fig. 4.
Loading plot based on hydrochemical variables observed during the present study.
The first two PCs explained 73.56% of the total variation in the data set and F1 has significant loadings (>0.75) on EC, TDS, HN, Ca2+, Mg2+, K+, Cl−, and Fe. The F1 has moderate positive loading (0.50–0.75) on Na+. High loadings on F1 along with EC and TDS were due to the carbonate-dominated underlying lithology which may have contributed to the increased TDS in the thermal springs [35]. Moreover, the factor loadings of F1 specified that it mainly comprised carbonate weathering rocks. Interestingly the HCO3− has not been clustered in this group signifying the role of high temperature during the process of carbonation reactions [20].
The F2 is strongly associated with SO42− and HCO3−.The main sources of SO42− and HCO3− were due to various carbonate and silicate weathering processes and their grouping in the same factor (F2) is due to the moderate pH and high temperature [35]. Similar observations were reported in the literature study [23]. At the same time, F2 is also moderately associated with Na+ indicating the role of silicate weathering in the region. F3 is strongly negatively associated with only one variable i.e., pH indicating that the moderate values of pH has no significant role in altering the overall hydrochemistry of the springs.
Finally, F4 is strongly associated with NO3− indicating that its source could be different from the other measured ions. At the same time, F4 is also moderately positively associated with As signifying their similar sources. The major source As in the springs could be the increased interaction of water with the arsenic-rich rock at elevated temperature [23].
4.5. Cluster analysis
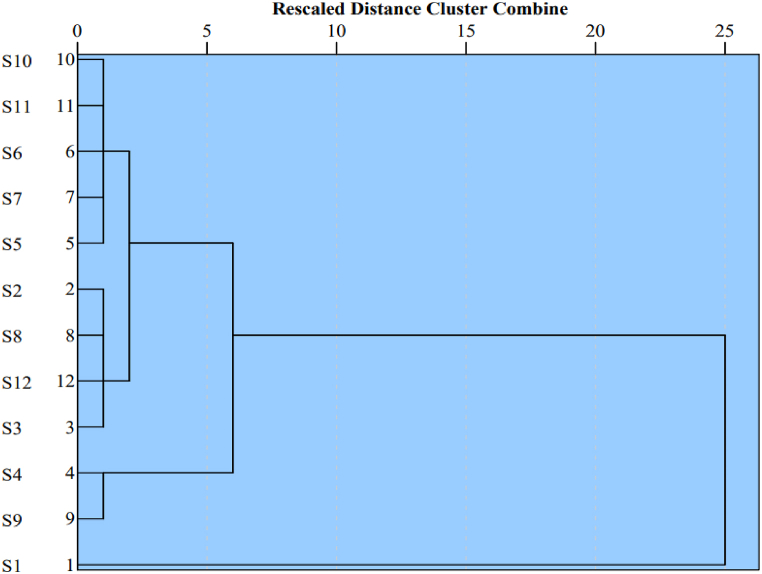
Cluster analysis is one the commonly used statistical methods to group the sampling sites according to their similarity or dissimilarity in contamination source [36,45]. In this study, hierarchical clustering was done to evaluate the water quality of different sampling points. The resulted dendrogram plot is provided in Fig. 5. The study revealed three significant clusters from the low distance criteria i.e., 0–5. In cluster 1, the majority of the sample sites (9/12) were found (S2, S3, S5, S6, S7, S8, S10, S11, and S12). Clustering of the majority of samples indicates that hydrochemical characteristics of the same reach changed slightly but mostly possess the same features. Interestingly, the TDS values of all the sampling points clustered in the different groups were significantly different. The mean TDS of cluster 1 was found to be 647 mg/L. Similarly, in cluster 2, S4 and S9 sampling sites were clustered. The mean TDS of this cluster was found to be 3008 mg/L. A similar environment may be the reason for clustering the S4 and S9 samples together. For instance, the major ionic compositions of S4 and S9 is more or less similar but the turbidity content is markedly low in S9 which could be due to low content of Fe resulting in lower possibility of colloidal formation. The only sampling point S1 was clustered in cluster 3. The mean TDS of cluster 3 was found to be 6637 mg/L. This site is characterized by garnet and kyanite deposits which may have formed by multiple metamorphisms and dynamic crystallization of minerals. Additionally, turbidity, EC, TDS, TH and Fe contents in this site is markedly high as compared to the other sites. The elevated level of Ca2+, Mg2+ and Fe could directly contribute to high turbidity due to colloidal formation. Furthermore, the presence of high content of dissolved ions could be responsible for the dramatically elevated concentration of electrical conductivity, leading to higher TDS in S1.
Fig. 5.
Clustering of sampling points of thermal springs on the basis of hydrochemical variables.
Among, the three clusters, the lowest TDS was reported in cluster 1. In this cluster, the mean TDS was found to be <1000 mg/L, which is acceptable for drinking purposes as per the WHO guideline (Table S2). The main reason for low TDS in this cluster could be low ionic concentrations in the springs. Additionally, the anthropic pressure was also low in the regions. The moderate TDS among the three clusters was obtained in cluster 2. The TDS of this cluster was significantly lower than cluster 3 and but higher than cluster 1. The sampling points of this cluster were in carbonate and silicate regions, so the carbonate and silicates minerals could also play a major role in the elevated concentrations of TDS in the springs.
The markedly high mean value of TDS in cluster 3 could be due to the carbonate-dominated under-lying lithology in the area. The reported TDS in one of the river basin close to cluster 3 sites was found to be 255 mg/L [46], but the mean values of geothermal spring were around 26 times higher in this study. Another river system, which is also located in current study areas, a mean TDS value of 120 mg/L was found [35]. The distinctly different TDS concentrations in the geothermal springs as compared to the river water is mainly due to the elevated temperature, enhanced geogenic interactions, and low dilution.
4.6. Classification of hydrochemical facies
The results of assessment of hydrochemical facies of samples are shown as a Piper diagram (Fig. 6). In the diagram, the milli-equivalent percentage (meq %) of major cations and anions is plotted and further projected into the central diamond field to evaluate the types of water. It is found that the hydrogeochemical results are confined only to three types i.e., most of the samples (58.33%) lies in the type 2 zone (Table 8 and Fig. 6) in the diamond diagram, indicating the dominance of Na–Cl type water. The result also suggested that the majority of the water samples are enriched with Cl− and SO42−. Four out of 12 samples (S5, S7, S10 and S11) were found in zone 1 indicating Ca–HCO3 type water. Only one water sample (S1) was found in zone 5 with high concentrations of calcium and chloride ions. This suggested that the sampling site has Ca–Cl type water. Most of the hydrochemical facies in the Himalayan region are characterized by Ca–HCO3 type [35]. However, the overall characteristics of this study are not consistent with them owing to the influence of elevated temperature of the thermal springs. Thus, the results plotted in the central diamond field (Fig. 6) showed the overall characteristics of spring water chemistry: the dominance of the alkalines (Na+ and K+) over the alkaline earth elements (Ca2+ and Mg2+) and the strong acids (Cl− and SO42−) over the weak acids (HCO3−). Also, spatial heterogeneity is visible in the springs especially the S1 is distinct from the other sites.
Fig. 6.
Piper diagram for spring water samples.
Table 8.
Results of Piper diagram for spring water samples.
| Type | Chemicals facies | Sampling points (S) | % Contribution |
|---|---|---|---|
| 1 | Ca–HCO3 | 5, 7, 10 and 11 | 33.33 |
| 2 | Na–Cl | 2, 3, 4, 6, 8, 9 and 12 | 58.33 |
| 3 | Mixed Ca–Na–HCO3 | ||
| 4 | Mixed Ca–Mg–Cl | ||
| 5 | Ca–Cl | 1 | 8.33 |
| 6 | Na–HCO3 |
The enrichment of Na–Cl in thermal springs is a complex process that depends on a variety of geological and hydrological factors. In general, thermal springs are formed when groundwater is heated by the earth's geothermal heat and rises to the surface. As the water travels through the earth's crust, it can pick up minerals and salts from the rocks it passes through, which can contribute to the water's chemical composition. Importantly, Na–Cl are two of the most common elements found in thermal springs, and their presence can be linked to the dissolution of rocks containing sodium chlorides, such as halite or rock salt. The heated water dissolves these minerals and carries them to the surface. This could be the main reason for the enrichment of the Na–Cl water type in this study.
Analysis of hydrochemical facies of hot springs form other part of the globe is also reported in few studies. Munoz et al. [23] studied the thermal springs on the Bolivian Altiplano (n = 16) are found principal water types Na–Cl (37.5%) and Na–Cl–HCO3 (37.5%). Guo et al. [24] analyzed the thermal ground water from two distinct reservoirs form Yangbajing geothermal field, Tibet, China and found that the water is Na–Cl type.
5. Conclusions
In this study, water quality and hydrogeochemistry of twelve thermal springs from Gandaki Province, Nepal was systematically assessed. The concentration of major cations Na+, Ca2+, K+, and Mg2+ ranged from 7.90 to 370 mg/L, 8.00–364 mg/L, 2.80–137 mg/L, and 1.90–121 mg/L; respectively. The dominance order of major cations, and anions was found to be: Na+ > Ca2+ > K+ > Mg2+; and Cl− > HCO3− > SO42− > NO3−, respectively. It is found that most physicochemical characteristics are significantly influenced by elevated temperature in the springs along with lithological, climatic, and multifarious interactions with anthropogenic activities. This is evidenced by the markedly high ionic concentrations in the thermal springs as compared to the river water from the same region Principal component analysis suggested that four major components are required to explain the hydrogeochemistry. For example, the hydrogeochemistry of sampling sites S1, S4 and S9 is found to be controlled by Ca2+, Cl−, HCO3−, Na+, K+ and Fe. Cluster analysis suggested that the sampling sites can be grouped into three distinct clusters. Interestingly, the classification of hydrochemical facies suggested that majority of hot water springs (58.33%, 7 out of 12) have Na–Cl type water. Out of the 9 heavy metals and trace elements analyzed, Fe (total), Zn2+, Ni2+, Cr3+, Cd+2, Hg (total), and Pb2+ were found below WHO safe limit. Only one sample (S7) showed a significantly elevated level of As (∼140 ppb) but in the rest of the samples As was found at or near WHO safe limit. The water quality index (WQI) of the thermal springs ranged from 40.9 to 573. The WQI for the majority of springs (9/12) was >50; suggesting that the spring water samples are not good for drinking purpose.
The hot water spring samples analyzed here have moderate temperature, close to neutral pH, and low to moderate turbidity, low concentration of potentially toxic trace elements (except S7), and optimal concentration of other ions (except S1); suggesting potentially low dermal adsorption or inhalation hazards. These indicators suggested that 10/12 hot water springs explored here could qualify for hot water baths and balneotherapy. The hot water springs are human-reachable and lie close to: 1) world-famous Annapurna trekking route, 2) Annapurna, Manaslu and Dhaulagiri; three major Himalayas of Nepal with elevation >8000 m, 3) Muktinath; a world-famous religious site. In these respects, both national and international tourists can entertain hot water spas while engaging in other activities.
The water quality parameters of the thermal springs can have seasonal variation. This was not explored in this study. It would be interesting to explore the microbial communities in the thermal springs and correlate their existence with the temperature, biological and chemical oxygen demands. Additionally, the geothermal energy content and possible ways to harness the energy could be systematically explored in future. It is also important to assess the social, aesthetic, and economical values of the hot water springs located in the different geographical region of the Himalayas. Therefore, additional in-depth study is recommended to guarantee the wellbeing and upgrading the water quality of the thermal springs in the Himalayas. Furthermore, proper policies and management plans are required for the sustainable management and use of thermal springs.
Author contribution statement
Conceived and designed the experiments (Bhanu Neupane); performed the experiments (Baburam Chalise, Prem Paudyal, and Buddha Bahadur Kunwar); analyzed and interpreted the data (Bhanu Neupane, Ramesh Raj Pant, Baburam Chalise, Prem Paudyal, Buddha Bahadur Kunwar, Kiran Bishwakarma, and Bina Thapa); contributed reagents, materials, analysis tools or data (Bhanu Neupane, Ramesh Raj Pant); wrote the paper (Baburam Chalise, Bhanu Neupane, Ramesh Raj Pant, Bina Thapa, and Kiran Bishwakarma).
Funding statement
This work was partly supported by National Youth Council, Nepal (to BC) and University Grants Commission, Nepal (to BBN, # FRG-76/77-S&T-3).
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
The quality control data (Table S1), individual data sets (as measured) for all the physical parameters, major anions and cations, and trace elements (Tables S2 and S3) are provided as a supporting file.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e17353.
Contributor Information
Ramesh Raj Pant, Email: rpant@cdes.edu.np.
Bhanu Bhakta Neupane, Email: bbneupane@cdctu.edu.np.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bhateria R., Jain D. Water quality assessment of lake water: a review. Sustain. Water Resour. Manag. 2016;2:161–173. [Google Scholar]
- 2.Yan T., Shen S.-L., Zhou A. Indices and models of surface water quality assessment: review and perspectives. Environ. Pollut. 2022 doi: 10.1016/j.envpol.2022.119611. [DOI] [PubMed] [Google Scholar]
- 3.Giri S. Water quality prospective in twenty first century: status of water quality in major river basins, contemporary strategies and impediments: a review. Environ. Pollut. 2021;271 doi: 10.1016/j.envpol.2020.116332. [DOI] [PubMed] [Google Scholar]
- 4.Haque M., Jewel M., Sayed A., Sultana M. Assessment of physicochemical and bacteriological parameters in surface water of Padma River, Bangladesh. Appl. Water Sci. 2019;9:1–8. [Google Scholar]
- 5.Eliku T., Leta S. Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Appl. Water Sci. 2018;8:1–13. [Google Scholar]
- 6.Peterson E.W., Nicodemus P., Spooner E., Heath A. The effectiveness of an artificial floating wetland to remove nutrients in an urban stream: a pilot-study in the Chicago River, Chicago, IL USA. Hydrology. 2021;8:115. [Google Scholar]
- 7.Zeng J., Han G., Wu Q., Tang Y. Geochemical characteristics of dissolved heavy metals in Zhujiang River, Southwest China: spatial-temporal distribution, source, export flux estimation, and a water quality assessment. PeerJ. 2019;7 doi: 10.7717/peerj.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvat Z., Horvat M., Pastor K., Bursić V., Puvača N. Multivariate analysis of water quality measurements on the danube river. Water. 2021;13:3634. [Google Scholar]
- 9.Cattin R., Martelet G., Henry P., Avouac J.P., Diament M., Shakya T.R. Gravity anomalies, crustal structure and thermo-mechanical support of the Himalaya of Central Nepal. Geophys. J. Int. 2001;147:381–392. [Google Scholar]
- 10.Rai S.M., Bhattarai T.N., Khatiwada D. Hot water springs (thermal springs) in Nepal: a review on their location, origin, and importance. J. Dev. Innov. 2020;4:24–42. [Google Scholar]
- 11.Verma J., Sourirajan A., Dev K. Bacterial diversity in 110 thermal hot springs of Indian Himalayan Region (IHR) 3 Biotech. 2022;12:1–21. doi: 10.1007/s13205-022-03270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhimwal M., Kaur S., Srivastava R.K., Hagare D., Shiva Prasad H.J. Water quality of springs and lakes in the Kumaon lesser Himalayan region of Uttarakhand, India. J. Water Health. 2022;20:737–754. doi: 10.2166/wh.2022.028. [DOI] [PubMed] [Google Scholar]
- 13.Hamzah Z., Rani N.L.A., Saat A., Wood A.K. Determination of hot springs physico-chemical water quality potentially use for balneotherapy. Malays. J. Anal. Sci. 2013;17:436–444. [Google Scholar]
- 14.Kim D.-G., Heo S.-K., Kim E.-G., Heo Y.-J., Kong I.-P., Han S.-H., Cho Y.-H., Kong K.-H., Jeong S.-H., Cha Y.-Y. A literature review and study on effect of balneotherapy. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2007;20:132–141. [Google Scholar]
- 15.Pelić M., Puvača N., Kartalović B., Živkov Baloš M., Novakov N., Ljubojević Pelić D. Antibiotics and sulfonamides in water, sediment and fish in an integrated production system. J. Agron. Technol. Eng. Manag. 2023;6:851–856. [Google Scholar]
- 16.Amarouche-Yala S., Benouadah A., el Ouahab Bentabet A., Moulla A.S., Ouarezki S.A., Azbouche A. Physicochemical, bacteriological, and radiochemical characterization of some Algerian thermal spring waters. Water Qual. Expo. Health. 2015;7:233–249. [Google Scholar]
- 17.Dupuy P., Casse M., Andre F., Dhivert-Donnadieu H., Pinton J., Hernandez-Pion C. Low-salt water reduces intestinal permeability in atopic patients. Dermatology. 1999;198:153–155. doi: 10.1159/000018092. [DOI] [PubMed] [Google Scholar]
- 18.Nasermoaddeli A., Kagamimori S. Balneotherapy in medicine: a review. Environ. Health Prev. Med. 2005;10:171–179. doi: 10.1007/BF02897707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najar I.N., Sharma P., Das S., Sherpa M.T., Kumar S., Thakur N. Bacterial diversity, physicochemical and geothermometry of South Asian hot springs. Curr. Res. Microb. Sci. 2022 doi: 10.1016/j.crmicr.2022.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherpa M.T., Das S., Thakur N. Physicochemical analysis of hot water springs of Sikkim-Polok tatopani, Borong tatopani and Reshi tatopani. Recent Res. Sci. Technol. 2013;5 [Google Scholar]
- 21.Baioumy H., Nawawi M., Wagner K., Arifin M.H. Geochemistry and geothermometry of non-volcanic hot springs in West Malaysia. J. Volcanol. Geoth. Res. 2015;290:12–22. [Google Scholar]
- 22.Farhat N., Hussain S., Faisal F., Batool I., Noreen M. Physico-chemical characteristics and therapeutic potential of Chutrun thermal springs in Shigar Valley, Gilgit-Baltistan (Pakistan) Appl. Water Sci. 2021;11:1–8. [Google Scholar]
- 23.Munoz M.O., Bhattacharya P., Sracek O., Ramos O.R., Aguirre J.Q., Bundschuh J., Maity J.P. Arsenic and other trace elements in thermal springs and in cold waters from drinking water wells on the Bolivian Altiplano. J. South Am. Earth Sci. 2015;60:10–20. [Google Scholar]
- 24.Guo Q., Wang Y., Liu W. Major hydrogeochemical processes in the two reservoirs of the Yangbajing geothermal field, Tibet, China. J. Volcanol. Geoth. Res. 2007;166:255–268. [Google Scholar]
- 25.Ghobadi A., Cheraghi M., Sobhanardakani S., Lorestani B., Merrikhpour H. Hydrogeochemical characteristics, temporal, and spatial variations for evaluation of groundwater quality of Hamedan–Bahar Plain as a major agricultural region, West of Iran. Environ. Earth Sci. 2020;79:428. [Google Scholar]
- 26.Ranjit M. Status of geothermal energy in Nepal. Res. Cent. Appl. Sci. Technol. Kirtipur Kathmandu Nepal. 1995 [Google Scholar]
- 27.Rai S.M., Bhattarai T.N., Khatiwada D. Hot water springs (thermal springs) in Nepal: a review on their location, origin, and importance. J. Dev. Innov. 2020;4:24–42. [Google Scholar]
- 28.Perrier F., Chitrakar G.R., Froidefond T., Tiwari D., Gautam U., Kafle B., Trique M. Estimating streaming potentials associated with geothermal circulation at the Main Central Thrust: an example from Tatopani-Kodari hot spring in central Nepal. J. Nepal Geol. Soc. 2002;26:17–27. [Google Scholar]
- 29.Tamang S., Thapa S., Paudyal K.R., Girault F., Perrier F. Geology and mineral resources of Khudi-Bahundanda area of west-central Nepal along Marshyangdi Valley. J. Nepal Geol. Soc. 2019;58:97–103. [Google Scholar]
- 30.Pant R.R., Qaiser F.U.R., Wang G., Adhikari S., Bishwakarma K., Baral U., Rimal B., Bhatta Y.R., Rijal K. Hydrochemical appraisal and solute acquisitions in seti River Basin, central Himalaya, Nepal. Environ. Monit. Assess. 2021;193:1–21. doi: 10.1007/s10661-021-09437-9. [DOI] [PubMed] [Google Scholar]
- 31.Sahu P., Sikdar P.K. Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ. Geol. 2008;55:823–835. [Google Scholar]
- 32.Ramesh K., Elango L. Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ. Monit. Assess. 2012;184:3887–3899. doi: 10.1007/s10661-011-2231-3. [DOI] [PubMed] [Google Scholar]
- 33.Singh K.P., Malik A., Mohan D., Sinha S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res. 2004;38:3980–3992. doi: 10.1016/j.watres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S., Bajracharya R.M., Sitaula B.K., Merz J. Water quality in the central Himalaya. Curr. Sci. 2005:774–786. [Google Scholar]
- 35.Pant R.R., Zhang F., Rehman F.U., Wang G., Ye M., Zeng C., Tang H. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, central Himalaya Nepal. Sci. Total Environ. 2018;622:770–782. doi: 10.1016/j.scitotenv.2017.12.063. [DOI] [PubMed] [Google Scholar]
- 36.Pant R.R., Zhang F., Rehman F.U., Koirala M., Rijal K., Maskey R. Spatiotemporal characterization of dissolved trace elements in the Gandaki River, central Himalaya Nepal. J. Hazard Mater. 2020;389 doi: 10.1016/j.jhazmat.2019.121913. [DOI] [PubMed] [Google Scholar]
- 37.Rao N.S., Das R., Gugulothu S. Understanding the factors contributing to groundwater salinity in the coastal region of Andhra Pradesh, India. J. Contam. Hydrol. 2022;250 doi: 10.1016/j.jconhyd.2022.104053. [DOI] [PubMed] [Google Scholar]
- 38.Rai S.K. Water Qual. Third Pole. Elsevier; 2020. Geochemical constituents in hot spring waters in the Third Pole; pp. 211–235. [Google Scholar]
- 39.Sharma C.M., Kang S., Tripathee L., Paudyal R., Sillanpää M. Major ions and irrigation water quality assessment of the Nepalese Himalayan rivers. Environ. Dev. Sustain. 2021;23:2668–2680. [Google Scholar]
- 40.Malkemus D., Perkins R.B., Palmer C.D. Geochemistry and geothermometry of breitenbush hot springs, Oregon, USA. Geothermics. 2021;95 [Google Scholar]
- 41.Husain M.S., Umar R., Ahmad S. A comparative study of springs and groundwater chemistry of Beas and Parbati valley, Kullu District, Himachal Pradesh, India. HydroResearch. 2020;3:32–47. [Google Scholar]
- 42.Olivier J., Venter J.S., Jonker C.Z. Thermal and chemical characteristics of hot water springs in the northern part of the Limpopo Province, South Africa. WaterSA. 2011;37:427–436. [Google Scholar]
- 43.Bengraine K., Marhaba T.F. Using principal component analysis to monitor spatial and temporal changes in water quality. J. Hazard Mater. 2003;100:179–195. doi: 10.1016/s0304-3894(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 44.Rao N.S., Sunitha B., Das R., Kumar B.A. Monitoring the causes of pollution using groundwater quality and chemistry before and after the monsoon. Phys. Chem. Earth, Parts A/B/C. 2022 [Google Scholar]
- 45.Ravindra B., Subba Rao N., Dhanamjaya Rao E.N. Groundwater quality monitoring for assessment of pollution levels and potability using WPI and WQI methods from a part of Guntur district, Andhra Pradesh, India. Environ. Dev. Sustain. 2022:1–31. [Google Scholar]
- 46.Singh R., Kayastha S.P., Pandey V.P. Water quality of marshyangdi river, Nepal: an assessment using water quaity index (WQI) J. Instr. Sci. Technol. 2021;26:13–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.