Abstract
Background and aim
Although studies have associated elevated prenatal obesity with increased risk of various diseases in offspring, little is known regarding the immune system. The aim of this study was to evaluate the relationship between prenatal obesity and levels of cytokines in umbilical cord blood and development of allergic disease during the first 10 years of life in an offspring.
Methods
A cohort of term infants born at the ShaoXing Women and Children Hospitals in China in 2011 was enrolled in this study. Flow cytometry was performed to measure levels of various cord blood cytokines, namely IL1β, IL2, IL10, IL6, IL8, IL17, IL12, TNF-α and IFN-γ. Next, logistic regression was used to explore the association of prenatal BMI with the development of allergic disease. The relationship between levels of each cord blood cytokine with prenatal BMI, and allergic disease development was tested using linear and logistic regression analyses, respectively.
Results
After 10 years of follow-up, higher prenatal BMI was significantly associated with development of allergic disease in children (HR = 2.45, 95% CI:1.08–5.57, P = 0.033). We also adjusted for maternal age, education and infant gender, and found that prenatal BMI was significantly associated with higher levels of IL12 (P = 0.023) and IL1β (P = 0.049) in cord blood. Moreover, we adjusted for maternal age, education, allergic dermatitis, gestation age and infant gender, and found that increase in each unit (1.26 pg/ml) in IL17 was associated with a 55.5% higher risk of allergic disease in 10-year-old children (HR = 1.55, 95%Cl: 0.99–2.45, P = 0.056). Meanwhile, after adjusting for maternal age, education level, gestation age, prenatal BMI, gestational weight gain, infant gender and birthweight, we found that for every unit increase in IL10, IL6 and IL1β, the risk of overweight/obesity in children after 10-year follow-up increased by 18.7% (HR = 1.19, 95%Cl: 1.01–1.40, P = 0.042), 13.9% (HR = 1.14, 95%Cl: 1.02–1.27, P = 0.021) and 41.3% (HR = 1.41, 95%Cl: 1.02–1.95, P = 0.036), respectively.
Conclusions
Prenatal obesity was positively correlated with allergic diseases in offspring. Cord blood cytokine may play mediating roles in the associations of prenatal obesity with offspring allergic diseases.
Keywords: Allergic diseases, Cord blood, Cytokine levels, Prenatal obesity
1. Introduction
Obesity is a current global health problem that is becoming more common among pregnant women and children [1,2]. Recent studies have associated obesity with asthma and allergies [3,4], while other investigations have demonstrated that prenatal obesity can cause obesity and changes of immunophenotype in offspring [[5], [6], [7]]. These alterations are considered part of the fetal programming (or fetal imprinting) phenomenon. Fetal programming is a series of adaptive mechanisms incited by stimuli acting during critical periods of growth and development, that affect local fetal cellular environments through changes in gene expression, with profound and permanent consequences on tissues structure and function. The ensuing changes can be transmitted from offspring to the next generation. Studies have shown that obesity can affect several factors, including growth factors, cytokines and hormones.
Allergic diseases are characterized by skewed balance of the Th1/Th2 ratio, away from Th1 and towards allergy-promoting Th2 cells. Numerous studies, targeting cord blood mononuclear cells (CBMCs) obtained at birth, have demonstrated that newborns who subsequently develop allergy and atopy exhibit obviously low levels of Th1-associated cytokines, such as IFN-γ and IL-12 [[8], [9], [10]]. Several studies have quantified levels of cord blood immunoglobulin E (IgE) and phytohemagglutinin (PHA) stimulated cytokine-response profiles and demonstrated their potential as early predictive markers for allergic diseases [11]. To date, however, no consensus has been reached on the subject, and information regarding the apparent relationship between prenatal BMI umbilical cord blood of cytokine and development of allergic diseases in children remains dearth. In this study, we hypothesized that prenatal obesity not only alters cytokine levels in cord blood, but this alteration also contributes to development of allergic diseases or obesity in children aged 0–10 years.
2. Methods
2.1. Study participants and selection criteria
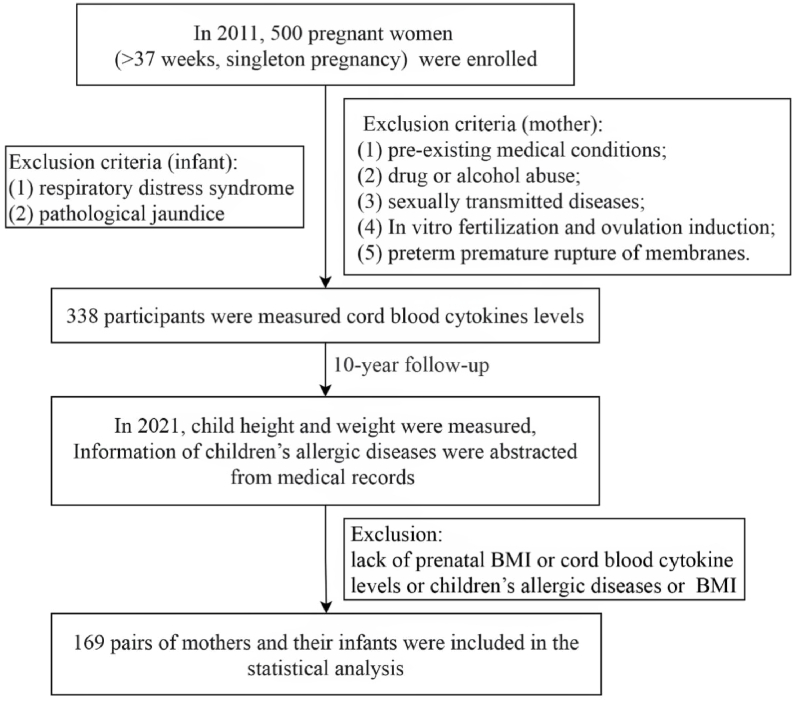
We recruited mothers and infants in this prospective newborn study. A total of 500 pregnant women (>37 weeks, singleton pregnancy) who visited the ShaoXing Women and Children Hospitals in 2011 were enrolled. The study objectives were explained to the subjects, who later voluntarily signed a written informed consent prior to enrolment. The study was approved by the Ethics Committee of Shaoxing Maternity and Child Health Care Hospital (Approval No. 2018035). Pregnant women were excluded from the study if they met the following criteria: had pre-existing medical conditions such as diabetes mellitus, seizures, and serious psychiatric disorders; drug or alcohol abuse; sexually transmitted diseases; in vitro fertilization and ovulation induction; and preterm premature rupture of membranes. Neonates with respiratory distress syndrome and pathological jaundice after birth were also excluded. Analysis was limited to 169 of 338 enrolled participants in whom cytokines were measured and pregnancy BMI and childhood anthropometric measurements were available. Selection criteria of the enrolled subjects are summarized using a flow chart in Fig. 1.
Fig. 1.
Flow diagram showing selection of study subjects.
2.2. Data collection
Women were interviewed at enrollment, and information on their education as well as current and previous pregnancies collected. We also recorded maternal height and weight at enrollment, with the latter assessed based on maternal recall. Maternal pregnancy BMI (kg/m2) was calculated. Data involving the parity, mode of delivery, pregnancy complications, intrapartum complications, allergic dermatitis, asthma, and smoking were collected. A sterile needle puncture was used to obtain cord blood samples immediately after cord clamping, which typically occurred within 60 s of delivery. Serum was separated from the umbilical cord blood within 6–24 h, by centrifuging it for 10 min at 300 g. The serum was immediately stored at −80 °C until further analysis. Levels of IL-2, IL4, IL-6, IL-8, I-10, IL-1β, IL-12, IL-17, IFN-γ, and TNF-α in cord blood serum were determined via flow cytometry microsphere chip capture technology level. The human T helper cell 1/2 cytokine kit II (BDTM CBA Human Th1/Th2 Cytokine Kit II, product of BD Biosciences) and FACSCalibur flow cytometer (American BD company) were used according to the manufacturer's instructions with minor modifications. Briefly, 96-well filter-bottom plates were first wetted with buffer, and beads conjugated with capture antibodies targeting each cytokine added. Serum (in duplicate wells) was then added to the wells, followed by serial dilutions of cytokine standards. Plates were incubated at room temperature on a shaker for 2 h, then for another 18 h at 4 °C. Next, the plates were washed on a vacuum manifold, then incubated with a biotin-labeled detector antibody cocktail for 2 h at room temperature on a shaker. The plates were washed again and incubated with streptavidin-PE for 40 min. A final set of washes was performed and the beads resuspended in reading buffer. Samples were acquired on the Luminex MAP200 instrument, with collection criteria set for 100 beads per analyte (2000 beads total). Data were analyzed using MasterPlex software (Hitachi Software Engineering America Ltd., MiraiBio Group). Each infant's height and weight were also measured at every visit. We also extracted each child's status on allergic diseases from medical records and information collected during study visits. Allergic diseases included food allergies, atopic dermatitis, allergies rhinitis and allergic asthma. Offspring's data were compiled annually for 10 years.
2.3. Statistical analysis
We used the median prenatal BMI (26.36 kg/m2) to stratify the mothers into two groups. Continuous variables were presented as means ± standard deviation (SD), whereas categorical data were presented as frequencies and proportions. Levels of inflammatory factors were log-transformed prior to subsequent regression analysis. Comparisons between L-BMI and H-BMI groups were performed using t-test or non-parametric test for continuous variables and with χ2 or Fisher's exact tests for categorical variables.
The relationship between prenatal obesity, and cord blood cytokine levels with children's allergic diseases was analyzed using a 3-step statistical analysis. Firstly, we employed logistic regression to determine the association between prenatal BMI and children's allergic disease, then applied linear regression models to determine the relationship between maternal prenatal BMI and levels of each cytokine in umbilical cord blood (log-transformed). Next, we employed logistic regression models to evaluate the relationship between levels of each cytokine in umbilical cord blood (10-fold log-transformed) with child anaphylactic disease, and overweight/obesity. All analyses were performed using IBM SPSS software version 23, and packages implemented in R version 4.0.3. All tests were two-tailed, and data followed by P < 0.05 considered statistically significant.
3. Results
3.1. Participant characteristics
A total of 169 subject pairs (a mothers and her infant) completed the 10-year follow-up and were included in the final analysis. The mean maternal and gestational ages of the study group at delivery were 27 years and 39 weeks, respectively, with an average weight gain of 15.3 kg during pregnancy. The mean ages of the children were 10.3 years. A summary of demographic and clinical characteristics of mothers and infants is shown in Table 1. The mean prenatal BMI for the L-BMI and H-BMI groups were 24.43 and 28.81 kg/m2, respectively. There were significant differences in gestational weight gain between the L-BMI and H-BMI groups (14.37 kg vs. 16.33 kg, P < 0.05). Notably, patients in the H-BMI group had significantly higher numbers of cesarean section deliveries than those in the L-BMI group (P < 0.05). Moreover, subjects in the H-BMI group exhibited higher SBP (P = 0.073) and more infections during pregnancy (P = 0.099) than their L-BMI counterparts, although the differences were not statistically significant. Similarly, we found no statistically significant differences between the two groups with regard to prenatal characteristics, including parity, gestational age, pregnancy complications, intrapartum complications, allergic dermatitis, asthma, smoking, and maternal education (P > 0.05). Analysis of infants revealed that half of them were males, with those in the H-BMI group exhibiting significantly higher birthweights than their L-BMI counterparts (P < 0.05).
Table 1.
Demographic and clinical characteristics of mothers and infants.
| L-BMI (n = 84) | H-BMI (n = 85) | P-value | |
|---|---|---|---|
| Mothers | |||
| Prenatal BMI (kg/m2) | 24.43 ± 1.51 | 28.81 ± 2.99 | <0.001 |
| Gestational weight gain (kg) | 14.37 ± 3.10 | 16.33 ± 3.36 | <0.001 |
| Gestational age (weeks) | 39.20 ± 1.12 | 39.26 ± 1.87 | 0.812 |
| Maternal age (years) | 26.96 ± 3.47 | 27.56 ± 3.59 | 0.271 |
| 18–24 | 34 (40.5) | 28 (32.9) | 0.591 |
| 25–34 | 48 (57.1) | 55 (64.7) | |
| 35+ | 2 (2.4) | 2 (2.4) | |
| Parity | |||
| 1 | 66 (78.6) | 67 (78.8) | 0.833 |
| 2 | 16 (19.0) | 17 (20.0) | |
| 3 | 2 (2.4) | 1 (1.2) | |
| Mode of delivery | |||
| Vaginal | 73 (86.9) | 59 (69.4) | 0.006 |
| Cesarean section | 11 (13.1) | 26 (30.6) | |
| SBP (mmHg) | 123.32 ± 10.11 | 126.05 ± 9.54 | 0.073 |
| DBP (mmHg) | 75.49 ± 6.82 | 76.28 ± 7.59 | 0.475 |
| Infection | 4 (4.8) | 10 (11.8) | 0.099 |
| Pregnancy complications | 27 (32.1) | 31 (36.5) | 0.554 |
| Intrapartum complications | 24 (28.6) | 22 (25.9) | 0.695 |
| Allergic dermatitis | 14 (16.7) | 17 (20.0) | 0.576 |
| Asthma | 0 (0.0) | 2 (2.4) | 0.497 |
| Smoking | 38 (45.2) | 37 (43.5) | 0.823 |
| Education (graduate and above) | 48 (57.1) | 49 (57.6) | 0.947 |
| Infants | |||
| Child's sex | |||
| Male | 41 (48.8) | 43 (50.6) | 0.817 |
| Female | 43 (51.2) | 42 (49.4) | |
| Child's age | 10.31 ± 0.27 | 10.30 ± 0.28 | 0.813 |
| Birthweight, kg | 3.25 ± 0.34 | 3.42 ± 0.40 | 0.004 |
| High birthweight (≥4000 g) | 2 (2.4) | 5 (5.9) | 0.450 |
L-BMI, prenatal BMI<26.36 kg/m2; H-BMI, prenatal BMI≥26.36 kg/m2.
3.2. Relationship between prenatal BMI and development of allergic diseases in children
Profiles of the relationship between prenatal BMI (exposure) and childhood allergic diseases (outcome) are presented in Table 2. In summary, high prenatal BMI was associated with children's allergic disease (HR = 2.45, 95% CI:1.08–5.57, P = 0.033) after 10 years of follow-up.
Table 2.
Association between prenatal BMI (exposure) and childhood anaphylactic disease.
| Childhood anaphylactic diseaseb |
||||
|---|---|---|---|---|
| β | SE | OR (95%CI) | P-value | |
| L-BMI (n = 84)a | 1.00 | |||
| H-BMI (n = 85) | 0.895 | 0.420 | 2.45 (1.08, 5.57) | 0.033 |
Mothers were divided into two groups based on the median prenatal BMI (26.36 kg/m2).
Adjusted for maternal age, parity, mode of delivery, gestational age, infant sex and birthweight.
3.3. Prenatal BMI and levels of cytokines in cord blood
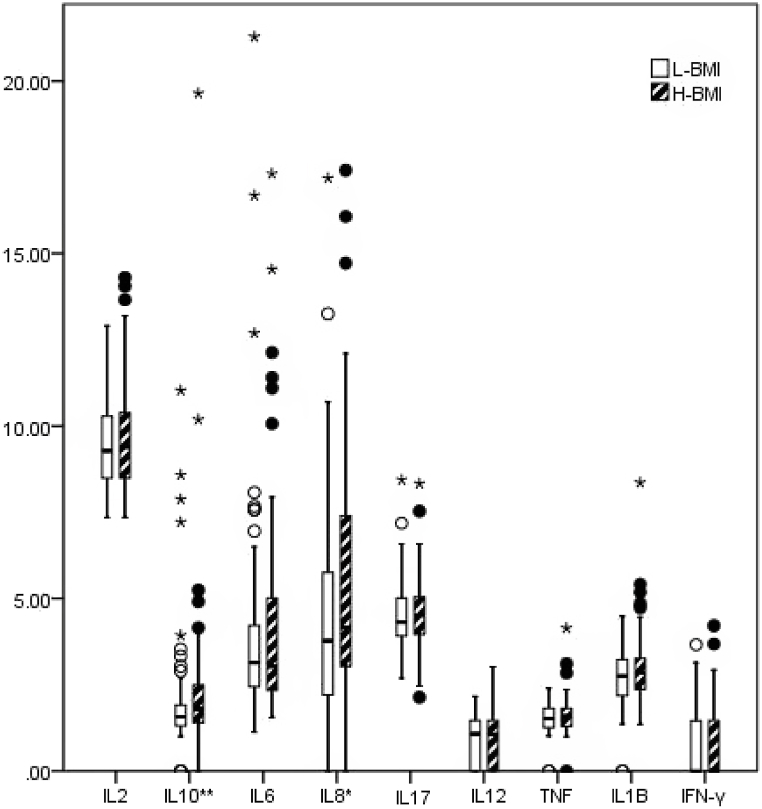
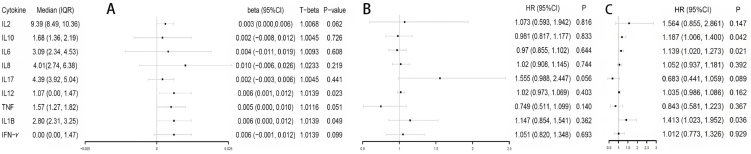
The distribution profile of cytokines in cord blood for patients in the L-BMI and H-BMI groups are presented in Fig. 2. Nonparametric tests showed that subjects in the H-BMI group had higher IL-8 (P = 0.083) and IL-10 (P = 0.029) than their counterparts in the L-BMI group. Levels of cytokines in cord blood caused by prenatal BMI, based on Linear regression, are presented in Fig. 3A. After adjusting for maternal age, education and infant gender, prenatal BMI was significantly associated with higher concentrations of IL12 (β = 0.006, P = 0.023) and IL1β (β = 0.006, P = 0.049) in cord blood. In addition, prenatal BMI was marginally associated with high levels of IL2 (β = 0.003, P = 0.062), TNF (β = 0.005, P = 0.051), and IFN-γ (β = 0.006, P = 0.099). Epidemiologically, these results indicated that for every 1 kg/m2 increase in prenatal BMI, IL12, IL1β, IL2, TNF, and IFN-γ increased by 0.0139, 0.0139, 0.0068, 0.0116, and 0.0139 pg/ml, respectively.
Fig. 2.
Profiles of distribution of nine inflammatory factors in umbilical cord blood of subject in L-BMI (n = 84) and H-BMI (n = 85) groups. *, P < 0.1; **, P < 0.05.
Fig. 3.
(A) Association of prenatal BMI (exposure) with each umbilical cord blood inflammatory factor (outcome); (B) Association of each inflammatory factor in umbilical cord blood (exposure) with childhood anaphylactic disease (outcome); (C) Association of levels of each inflammatory factor in umbilical cord blood (exposure) with childhood overweight/obesity (outcome).
Model A: Adjusted for maternal age, infant sex and education. Model B: Adjusted for Model A + gestation age and maternal allergic dermatitis. Model C: Adjusted for Model A + prenatal BMI, gestational weight gain, gestation age and infant birthweight. Cytokine data were log-transformed in linear regression of model A, and 10-fold log-transformed in logistic regression models B and C. N = 169. T-beta, back-transformed after regression. HR, hazard ratio.
3.4. Relationship between cytokine levels in cord blood and development of allergic diseases in children
The association of cytokine levels in cord blood with childhood allergic diseases and overweight/obesity are presented in Fig. 3B and C, respectively. In summary, after adjusting for maternal age, education, allergic dermatitis, gestation age, and infant gender, each unit increase (1.259 pg/ml) in IL17 was associated with a 55.5% increased risk of allergic diseases in 10-year-old children (HR = 1.55, 95%Cl: 0.99–2.45, P = 0.056). Meanwhile, after adjusting for maternal age, education level, gestation age, prenatal BMI, gestational weight gain, infant gender and birthweight, for every unit increase in IL10, IL6 and IL1β, the risk of overweight/obesity in children after 10-year follow-up increased by 18.7% (HR = 1.19, 95%Cl: 1.01–1.40, P = 0.042), 13.9% (HR = 1.14, 95%Cl: 1.02–1.27, P = 0.021) and 41.3% (HR = 1.41, 95%Cl: 1.02–1.95, P = 0.036), respectively.
4. Discussion
This is the first study to demonstrate the cytokines profile in cord blood of obese pregnant women, and explore their impact on the development of allergic diseases and obesity in their offspring. Based on our results, we conclude with the following three points: (1) prenatal obesity is positively related with levels of IL12 and IL1β in cord blood; (2) high prenatal BMI may affect the occurrence of allergic diseases in offspring; and (3) cytokines in umbilical cord blood can mediate the effect of prenatal obesity on the development of childhood allergic diseases.
Studies have shown that overweight before or excessive weight gain after pregnancy can affect the growth and development of children, possibly through the inflammatory pathway [5,[12], [13], [14]]. Our results showed that prenatal BMI was positively correlated with the development of allergic diseases in offspring, consistent with previous studies which showed that high maternal BMI was linked to postnatal wheeze and eczema [15,16]. In the present study, we provide the first report of the impact of BMI levels in mothers before delivery on the immune system and their possible inflammatory factor pathways. Analysis of cytokine levels in cord blood in 169 pairs of mothers demonstrated that prenatal BMI was positively correlated with levels of IL12 (P < 0.05) and IL1β (P < 0.05) in cord blood. These results were consistent with findings from previous reports, and support the hypothesis that maternal obesity is associated with low-grade chronic systemic inflammation due to higher levels of pro-inflammatory cytokines, such as IL-1β and induce placental inflammation [[17], [18], [19]]. Studies have also shown that obesity induces IL12 production [20]. However, the specific magnitude of the increase in inflammatory factors may vary slightly among different studies [5,7,21], which may be due to several factors such as race or other unknown variables. Nevertheless, it is generally acknowledged that obesity leads to elevated cytokine levels. Levels of maternal cytokines were more skewed to the Th2 response in cases where their offspring had allergic disease. Previous studies have shown that IL17 not only plays an important role in Th2 differentiation [22], but is also involved in pathogenesis of allergic skin diseases, extrinsic atopic dermatitis, and asthma [[23], [24], [25]]. IL17A might can stimulate the Th2 cytokine [26]. Our results showed that each unit increase in IL17 was marginally increased risk of allergic disease in 10-year-old children. Our results were consistent with findings from previous studies, where IL17 produced by Th2 cells was not only highly expressed in sensitized mice, but was also associated with food allergy [27,28]. However, only a handful of studies have described the relationship between IL17 levels and development of allergic diseases in children. To our knowledge, this is the first report to demonstrate the association of maternal cytokine profiles with the development of allergic disease in 10-year-old children.
Our results further showed that every unit increase in IL10, IL6 and IL1β, mediated an 18.7%, 13.9% and 41.3% increase in the risk of overweight/obesity in children after 10-year follow-up. A previous study showed that IL-6 promoted amino acid transport in the placental system and upregulated fatty acid uptake in human trophoblast cells, thereby enhancing nutrient transport and fetal growth [5,29,30]. Moreover, Lisa et al. [31] demonstrated that Blimp-1-regulated IL-10 secretion by Tregs white adipose tissue homeostasis.
5. Limitations
This study has several limitations that should be noted. Firstly, we found no evidence of mediating effect of cytokine, which cannot constitute the pathway. Secondly, sample size enrolled in the present study was relatively small, larger studies are needed to confirm this evidence of mediating effect of cytokines. Thirdly, our follow-up duration was only 10 years. Therefore, the long-term effects of high prenatal BMI on children's adulthood need to be further investigated.
6. Conclusion
Prenatal BMI was positively correlated with levels of IL12 and IL1β in cord blood. After 10 years of follow-up, levels of IL17 in cord blood were associated with occurrence of allergic diseases. Although we were unable to directly link cytokine levels to maternal obesity and development of allergic diseases in offspring, our results provide new insights into the relationship between cord blood cytokines and maternal obesity and offspring allergic diseases. Further research is needed to validate these findings.
Author contribution statement
Jian-Wei Zhang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jie-Qiong Guana: Analyzed and interpreted the data; Wrote the paper.
Yong-Xing Zhong: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests.
Contributor Information
Jian-Wei Zhang, Email: sxfyzjw@163.com.
Yong-Xing Zhong, Email: yyfmsheji@163.com.
References
- 1.Poston L., et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 2.Moreno L.A. Obesity: early severe obesity in children. Nat. Rev. Endocrinol. 2018;14(4):194–196. doi: 10.1038/nrendo.2018.15. [DOI] [PubMed] [Google Scholar]
- 3.Sansone F., et al. Asthma and obesity in children. Biomedicines. 2020;8(7) doi: 10.3390/biomedicines8070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visness C.M., et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005-2006. J. Allergy Clin. Immunol. 2009;123(5):1163–1169. doi: 10.1016/j.jaci.2008.12.1126. 1169 e1-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell K.R., Powell T.L. Effects of maternal obesity on placental function and fetal development. Reproduction. 2017;153(3):R97–R108. doi: 10.1530/REP-16-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pruszynska-Oszmalek E., et al. Obesity is associated with increased level of kisspeptin in mothers' blood and umbilical cord blood - a pilot study. Eur. Rev. Med. Pharmacol. Sci. 2021;25(19):5993–6002. doi: 10.26355/eurrev_202110_26877. [DOI] [PubMed] [Google Scholar]
- 7.Maguire R.L., et al. Associations between maternal obesity, gestational cytokine levels and child obesity in the NEST cohort. Pediatr Obes. 2021;16(7) doi: 10.1111/ijpo.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood R.A., et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) J. Allergy Clin. Immunol. 2011;127(4):913. doi: 10.1016/j.jaci.2010.12.1122. 919 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo N., et al. Reduced interferon gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders--6-year follow-up study. Clin. Exp. Allergy. 1998;28(11):1340–1344. doi: 10.1046/j.1365-2222.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 10.Paalanne N., et al. Cord blood cytokine profile is associated with the risk of asthma at the age of 8 years. Acta Paediatr. 2020;109(6):1271–1272. doi: 10.1111/apa.15141. [DOI] [PubMed] [Google Scholar]
- 11.Neaville W.A., et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J. Allergy Clin. Immunol. 2003;112(4):740–746. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 12.Marginean C.O., et al. The adipokines and inflammatory status in the era of pediatric obesity. Cytokine. 2020;126 doi: 10.1016/j.cyto.2019.154925. [DOI] [PubMed] [Google Scholar]
- 13.He H., et al. Body mass index was linked with multi-cardiometabolic abnormalities in Chinese children and adolescents: a community-based survey. BMC Pediatr. 2022;22(1):33. doi: 10.1186/s12887-021-03092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez C.E., et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes. Rev. 2018;19(4):464–484. doi: 10.1111/obr.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudarzi H., et al. Contrasting associations of maternal smoking and pre-pregnancy BMI with wheeze and eczema in children. Sci. Total Environ. 2018;639:1601–1609. doi: 10.1016/j.scitotenv.2018.05.152. [DOI] [PubMed] [Google Scholar]
- 16.Dumas O., et al. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy. 2016;71(9):1295–1304. doi: 10.1111/all.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sano M., et al. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in mice. J. Reprod. Dev. 2020;66(3):241–248. doi: 10.1262/jrd.2020-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tersigni C., et al. Impact of maternal obesity on the risk of preterm delivery: insights into pathogenic mechanisms. J. Matern. Fetal Neonatal Med. 2020:1–6. doi: 10.1080/14767058.2020.1817370. [DOI] [PubMed] [Google Scholar]
- 19.Xue Y., et al. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J. Nutr. Biochem. 2014;25(7):758–764. doi: 10.1016/j.jnutbio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louer C.R., et al. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 2012;64(10):3220–3230. doi: 10.1002/art.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaramillo-Ospina Á., et al. Maternal obesity is associated with higher cord blood adipokines in offspring most notably in females. J. Pediatr. Gastroenterol. Nutr. 2021;73(2):264–270. doi: 10.1097/MPG.0000000000003172. [DOI] [PubMed] [Google Scholar]
- 22.Kamijo S., et al. Innate IL-17a enhances IL-33-independent skin eosinophilia and IgE response on subcutaneous papain sensitization. J. Invest. Dermatol. 2021;141(1):105–113 e14. doi: 10.1016/j.jid.2020.05.088. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann M.A., Kiecker F., Zuberbier T. A systematic review of the role of interleukin-17 and the interleukin-20 family in inflammatory allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2016;16(5):451–457. doi: 10.1097/ACI.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 24.Molet S., et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 25.Li C.W., et al. In vivo and in vitro studies of Th17 response to specific immunotherapy in house dust mite-induced allergic rhinitis patients. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima S., et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Invest. Dermatol. 2014;134(8):2122–2130. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., et al. Role of immune tolerance in BALB/c mice with anaphylactic shock after Echinococcus granulosus infection. Immunol. Res. 2016;64(1):233–241. doi: 10.1007/s12026-015-8741-2. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., et al. Activation-induced cytidine deaminase plays crucial role in ovalbumin-induced food allergy and promoted by IL-21. Mol. Immunol. 2019;114:369–377. doi: 10.1016/j.molimm.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Jones H.N., Jansson T., Powell T.L. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol. Cell Physiol. 2009;297(5):C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 30.Lager S., et al. Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta. 2011;32(2):121–127. doi: 10.1016/j.placenta.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Beppu L.Y., et al. Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight. 2021;6(3) doi: 10.1172/jci.insight.140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.