Abstract
Ziziphus mauritiana is used as food and medicine. It is very nutritive and contains high amounts of calcium, phosphorus, iron, vitamin C, and beta-carotene. To choose an appropriate technique for fruit preservation, this study evaluated the effect of temperature and time on the nutritional and anti-nutritional qualities of Ziziphus mauritiana fruit harvested from Kobo Woreda in North-Eastern Ethiopia. This study assessed the impact of preservation parameters on the nutritional and anti-nutritional properties of Ziziphus mauritiana fruit, such as storage temperature and preservation day (time). Ziziphus mauritiana fruit was also analyzed to evaluate storage temperature and preservation day on its nutritional and antinutritional composition and found moisture content (5.25–10.99%), ash content (4.74–10.70%), crude fiber (3.81–17.88), fat (0.49–2.16), nitrogen content (1.01–1.8%), protein content (6.33–11.27), phytate content (67.27–659.33 mg/100 g m), and tannin content (3842.46–16577.80 mg/100 g m). The findings revealed that both individual and interaction effects were highly significant differences (p-value, 0.0001) in each nutritional and antinutritional content of the fruit. The results of this study showed that it was possible to store Ziziphus mauritiana fruit using plastic bags (High-Density Polyethylene Bags), and further deep-freezing the fruit had the best effect on preserving it in its fresh state (without damage) for up to 45 days.
Keywords: Antinutritional, Nutritional, Preservation, Ziziphus mauritiana
1. Introduction
Globally, approximately one billion people derive livelihoods and food from forests [1], and over 300 million rely heavily on non-timber forest products (NTFPs) for their sustenance and livelihood. According to Refs. [2,3], NTFPs are widely used for nutritional and medical purposes in tropical and low-income countries. According to Ref. [4], one in six people worldwide relies on wild foods such as bushmeat, insects, honey, fungi, wild vegetables, and wild edible fruits (WEFs) for nourishment. The main NTFPs, either raw or processed, used to supplement daily calorie needs are WEFs [5,6].
Furthermore, there is strong evidence that wild fruits (food) play a significant role in the global food market and prevent malnutrition, which is a serious health concern in poor countries such as Ethiopia [7,8]. Because WEFs are accessible during the dry season, when many domestic fruits and vegetables are in short supply and expensive, they are essential for guaranteeing the security of food and livelihoods for countless families and communities worldwide [9,10]. Additionally, WEFs are important for human nutrition, particularly as a source of carbohydrates, proteins, vitamins, minerals, and dietary fiber, and they have a vast array of therapeutic benefits [11,12]. For instance, edible wild plants have been reported to have higher fat, protein, mineral, and vitamin content than cultivated species [13].
Africa has abundant novel plant species that are known to be rich in health-promoting chemicals and compounds, many of which have yet to be explored or utilized. Sub-Saharan Africa is home to hundreds of different native fruit tree species one of the key WEFs, Ziziphus mauritiana is a multipurpose species with economic potential in the Sahel region and potential as an agroforestry candidate species [14]. There is a variety of Ziziphus species and cultivars. One of the most important species, Ziziphus mauritiana, is very nutritious and contains high levels of iron and vitamin C, beta (β)-carotene, calcium, and phosphorus [15]. The Jujube (Zizphus mauritiana Lam.) species is native to northern China, but today its geographic range occupied arid and semi-arid and was introduced in West Asia, Africa, and the West in the Mediterranean region, where it would eventually become naturalized [16,17].
Ziziphus mauritiana, also known as Jujube in English and Kurkura in Amharic, is a spiny tree or shrub that grows between 8 and 15 m (m) and is a member of the Rhamnaceae family with a wide geographical spread. It grows in most semi-arid areas of Africa, where rainfall ranges from 200 to 1200 mm per year. This species is also common in the arid regions of South and Southeast Asia. Ziziphus mauritiana is tolerant of drought, water-logging, salinity, and a wide range of soil pH. It is common in Gonder, Gojam, Welo, Shewa, Arsi, Illubabor, GamoGofa, Bale, Sidamo, and Harerge, and widely dispersed in dry regions of tropical and South Africa, Madagascar, and Arabia [18]. Ziziphus mauritiana is a multi-purpose tree. It has a long history of use as a vital food and in traditional medicine. Various parts of the Ziziphus mauritiana plant, including roots, stems, leaves, flowers, and fruits, are used as pharmacological agents [19].
Fresh fruits and vegetables are perishable commodities because of their inherent tendency to spoilage for physiological reasons. Postharvest losses can occur at any stage of the marketing process between harvest and consumption. Post-harvest losses of fresh products range from 25 to 50% of the entire production in developing countries, depending on the commodity where there is a severe lack of infrastructure and marketing facilities. Because proper fruit handling increases the economic importance of WEFs and improves the production of value-added products from these widely available WEFs, it should be prioritized. Another objective for the development of valuable fruit trees is to determine their nutrient availability and nutritional quality of fruit trees [20].
Therefore, there is a need to assess post-harvest handling, study fruit preservation techniques, and process the fruit and associated products of the Ziziphus mauritiana fruit tree to recommend for utilization. Therefore, the objective of this study was to determine the best storage conditions for Ziziphus mauritiana, including the preservation time, temperature, and packaging material. Furthermore, this study provides information that can be used by the local community, development partners, researchers, and individual farmers to design appropriate programs for improving income and food security in rural communities using the Ziziphus mauritiana tree, especially in Ethiopia.
2. Materials and methods
2.1. Study area description
Physiologically mature wild edible Ziziphus mauritiana fruits were harvested from Kobo in the Amhara National Regional State in Ethiopia (12°09′N, 39°38′E with an elevation of 1468 m above sea level).
2.2. Sample collection and preparation
A sample of Ziziphus mauritiana fruit was collected from Kobo in the Amhara National Regional State of Ethiopia in November 2021. During the harvesting period, mature (fully ripe) and undamaged (with a good appearance) fresh fruit, as shown in Fig. 1A, were collected, placed in a cooled icebox, and immediately transported to Addis Ababa, Forest Products Innovation Center's laboratory for nutritional and anti-nutritional analysis. Sorting was performed by hand, and any moldy/rotting fruit, insect damage, mechanical damage, or foreign matter was removed. The fruits were washed with water, cleaned with a dry cloth, spread over a thickness of 4–6 cm on an aluminum tray, and allowed to dry for 2 h at room temperature under shade. Furthermore, the Ziziphus mauritiana fruit was packed using a high-density polyethylene bag (HDPB) and preserved in a deep freezer. On 15, 30, and 45 days, the fruit samples were removed from the refrigerator and dried in an oven for 48 h at 60 °C. The dried fruit samples were manually crushed and ground using a mortar and pestle to a sieve size of approximately 2 mm. The powdered fruit was then placed in aluminum foil and refrigerated at 4 °C until nutritional and anti-nutritional analyses were performed.
Fig. 1.
Picture of Ziziphus mauritiana fruit at the time of harvesting.
2.3. Nutritional and antinutrition composition analysis
2.3.1. Determination of moisture content
The moisture content of the samples was determined using the oven-drying method. Accordingly, three replicates of Ziziphus mauritiana fruit pulp samples were accurately weighed (2 g) from each preservation technique and dried in an oven overnight at 105 °C for 12 h. The moisture content of the fruit pulp is expressed as follows eqn: (1) [21]:
| (1) |
Where: W1 is the weight (g) of the sample before drying, and W2 is the weight (g) of the sample after drying.
2.3.2. Determination of ash
Three replications of ground Ziziphus mauritiana fruit pulp samples (moisture-free) that accurately weighed 2 g were heated on a burner in the air to remove smoke. Each fruit pulp sample was then burned in a furnace at 550 °C for 4 h [22]. The ash content was expressed as indicated below, eqn. (2):
| (2) |
Where: W1 is the weight (g) of the moisture-free sample before burned, and W2 is the weight (g) of the sample after burned.
2.3.3. Determination of total nitrogen and crude protein
The Kjeldahl method was used for nitrogen determination taking 0.5 g of ground Ziziphus mauritiana fruit pulp sample was added to the digestion flask in three replicates for each preservation technique. The nitrogen content was then calculated [23]. As suggested by Ref. [24], a nitrogen conversion factor of 6.25 [24],was used to compute the protein content.
2.3.4. Determination of crude fiber
The crude fiber content was determined by acid-base digestion or the Coarse Fiber method using 2 g of Ziziphus mauritiana fruit pulp sample (moisture-free). The crude fiber content was calculated as the difference between the weight of the residues and ash, and which then converted to a fraction and represented as a percentage loss in weight on ignition [22].
2.3.5. Determination of crude fat
Two grams of moisture-free Ziziphus mauritiana fruit pulp sample in triplicate were weighed into a porous thimble, and its mouth was covered with cotton. The thimble was then placed in a Soxhlet apparatus. A dry pre-weighed flask was connected beneath the apparatus to which the required volume of solvent (hexane or petroleum ether) was added, which was then connected to the condenser. An adequate amount of hexane (boiling point of 40–60 °C) was added to the flask. To extract the crude fat, the flask was heated in a heating mantle for 8 h. After removing the thimble, the solvent was placed inside the apparatus. Excess solvent was evaporated from the flask in a hot water bath, and the flask was dried in an oven at 40–60 °C. The samples were then cooled in a desiccator and weighed [25].
2.3.6. Phytate determination
The method described by Ref. [26] was used to determine phytate content. With three replications, an accurate 0.5 g of ground Ziziphus mauritiana fruit pulp sample was weighed into a 100 ml conical flask. A conical flask was filled with 10 ml of 0.2 normal hydrochloric acids (HCl). Subsequently, the sample was extracted using a mechanical shaker and soaked for 1 h at ambient temperature before being filtered. Then, 3 ml of the filtered solution was mixed with 2 ml of the Wade reagent solution. After homogenization, the mixture was centrifuged at 3000 rpm for 10 min. A UV-VIS spectrophotometer was used to measure the absorbance of the clear mixed solution (CECL 1021 Model, England) at 500 nm.
2.3.7. Tannin determination
The dried fruit pulp of Ziziphus mauritiana (1 g) was extracted with 10 ml of 1% HCl in methanol using a mechanical shaker for 24 h at room temperature, followed by a 5-min centrifugation. After 1 ml of clear supernatant solution and 5 ml of Vanillin HCl reagent were mixed, the reaction was allowed to proceed for 20 min. The absorbance of the clear supernatant solution at 500 nm was measured using a UV-VIS spectrophotometer (CECL 1021, England). Finally, the amount of tannin was determined using the slope of the SPSS plot, the intercept of the standard curve, and Eq. (3) [26].
| (3) |
Where: As is Sample absorbance; Ab is Blank absorbance; d is Density of solution (0.791 g/ml); and w is Weight of sample in gram.
2.3.8. Data analysis
Design expert software version 13 was used to examine the effect of storage temperature and preservation time on the nutritional and antinutritional composition of Ziziphus mauritiana fruit.
3. Results and discussion
3.1. Variation in nutritional and antinutritional composition of Ziziphus mauritiana
Analysis of variance (ANOVA) was used to test the significance of the developed models. The summary analysis of variance (ANOVA) in Table 1 shows that temperature and preservation time affect the nutritional and anti-nutritional composition of Ziziphus mauritiana fruit. In this study, for the response nutritional and antinutritional composition, the Model F-value of 11 implies that the model is significant. Values of p-value less than 0.05 showed model terms were significant. In this study, it was found that the individual factors and their interaction effects were significant model terms. It shows that table temperature, preservation time, and their interaction affect the nutritional and antinutritional composition of Ziziphus mauritiana.
Table 1.
Summary of variance analysis for Ziziphus mauritiana fruit nutritional and antinutritional composition.
| MC |
Ash |
CF |
F |
N |
P |
TAN |
PHY |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value | Sum of Squares | Mean Square | F-value | p-value |
| M | 11 | 151.60 | 13.78 | 166.72 | < 0.0001 | 117.54 | 10.69 | 1149.31 | < 0.0001 | 645.05 | 58.64 | 348.24 | < 0.0001 | 8.81 | 0.8005 | 1455.47 | < 0.0001 | 2.35 | 0.2135 | 19.36 | < 0.0001 | 92.06 | 8.37 | 19.34 | < 0.0001 | 5.201 E+08 | 4.728 E+07 | 1029.78 | < 0.0001 | 8.192 E+05 | 74474.88 | 5022.79 | < 0.0001 |
| A | 3 | 27.13 | 9.04 | 109.39 | < 0.0001 | 93.49 | 31.16 | 3351.89 | < 0.0001 | 145.94 | 48.65 | 288.88 | < 0.0001 | 7.09 | 2.36 | 4298.40 | < 0.0001 | 1.34 | 0.4455 | 40.40 | < 0.0001 | 52.37 | 17.46 | 40.35 | < 0.0001 | 1.378 E+08 | 4.593 E+07 | 1000.35 | < 0.0001 | 3.470 E+05 | 1.157 E+05 | 7801.08 | < 0.0001 |
| B | 2 | 6.26 | 3.13 | 37.86 | < 0.0001 | 7.06 | 3.53 | 379.47 | < 0.0001 | 182.15 | 91.08 | 540.86 | < 0.0001 | 0.5106 | 0.2553 | 464.16 | < 0.0001 | 0.2193 | 0.1097 | 9.94 | < 0.0007 | 8.59 | 4.30 | 9.93 | < 0.0007 | 1.088 E+08 | 5.440 E+07 | 1184.76 | < 0.0001 | 2.204 E+05 | 1.102 E+05 | 7430.68 | < 0.0001 |
| AB | 6 | 118.21 | 19.70 | 238.34 | < 0.0001 | 16.99 | 2.83 | 304.63 | < 0.0001 | 316.96 | 52.83 | 313.71 | < 0.0001 | 1.20 | 0.2004 | 364.43 | < 0.0001 | 0.7928 | 0.1321 | 11.98 | < 0.0001 | 31.09 | 5.18 | 11.97 | < 0.0001 | 2.735 E+08 | 4.559 E+07 | 992.84 | < 0.0001 | 2.519 E+05 | 41976.52 | 2831.01 | < 0.0001 |
| PE | 24 | 1.98 | 0.0827 | 0.2231 | 0.0093 | 4.04 | 0.1684 | 0.0132 | 0.0005 | 0.2647 | 0.0110 | 10.39 | 0.4327 | 1.102 E+06 | 45917.01 | 355.86 | 14.83 | ||||||||||||||||
| CT | 35 | 153.59 | 117.76 | 649.09 | 8.82 | 2.61 | 102.44 | 5.212 E+08 | 8.196 E+05 | ||||||||||||||||||||||||
* = Significant at p < 0.05, ns = Non-significant at p > 0.05.
MC=Moisture content, CF=Crude fiber, F=Fat, N= Crude nitrogen, P= Crude protein; PHY= Phytate content, TAN= Tannin, M = Model. A = Temperature, B=Preservation time, AB= Interaction of Temperature and Preservation time, PE=Pure Error, and CT=Cor Total.
3.2. Effect of independent factors on the nutritional and anti-nutritional composition of Ziziphus mauritiana
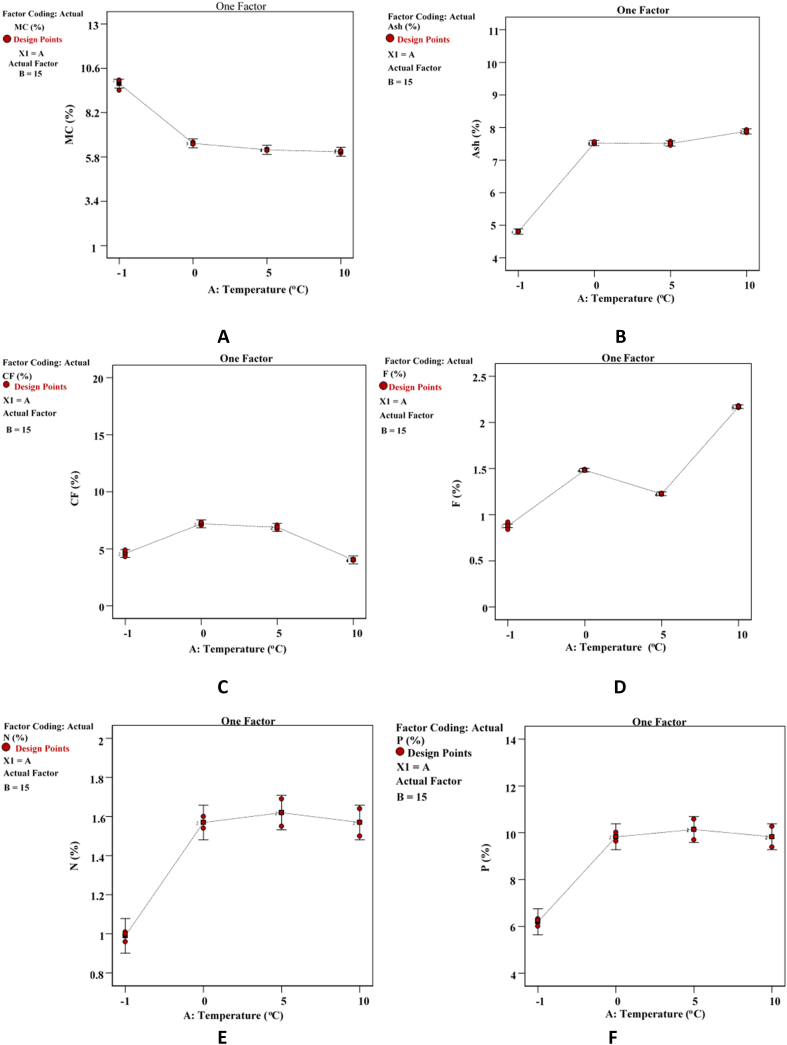
Fig. 2a, Fig. 3b show the effects of temperature and preservation time on moisture content, respectively. As shown in Fig. 2A, the moisture content of the fruit ranged from 5.8 to 10.6%, depending on temperature. It decreased as the temperature increased; however, the graph shows that above 5 °C, the moisture content become constant. Lower moisture contents of 6.08% and higher 9.77% were observed at 10 °C and below −1 °C (deep freezer), respectively. On the other hand, the effect of preservation time on moisture content showed a decreasing pattern until 30 days and then started to increase until 45 days of preservation time, as shown in Fig. 3A. With 10.46 and 5.16% moisture contents after 45- and 30-days of preservation time respectively. This fluctuating change can be attributed to the storage duration, owing to the ingress of moisture through the packaging material and environmental conditions [27].
Fig. 2a.
Effect of Temperature on A) Moisture content, B) Ash content, C) Crude fiber content, D) Fat Content, E) Nitrogen content, F) Protein content.
Fig. 3b.
Effect of Preservation time on C) Crude fiber content, D) Fat Content, E) Nitrogen content, F) Protein content, G) Phytate, and H) Tannin.
Fig. 3a.
Effect of Preservation time on A) Moisture content, B) Ash content.
The ash content of Ziziphus mauritiana fluctuated from 15 to 45 days of preservation. It slightly increased from 4.81 to 4.98% until 30 days of preservation time and started to decrease to 4.60% when it reached 45 days of preservation time, as illustrated in Fig. 3B. As shown in Fig. 2B, the ash content sharply increases from 4.81 to 7.52% as the temperature increased from below 0 °C–5 °C, and it increased at a constant rate after 5 °C until it reached 7.88% at 10 °C. This may be due to the increase in moisture content with an increase in storage period [27].
Fig. 3C illustrates that the crude fiber content of Ziziphus mauritiana increases from 4.60 to 7.61% until day 30 of preservation and thereafter decreased to 3.86% after 45 days. Similar to the preservation time, temperature also showed a similar effect on the crude fiber (Fig. 2C). The crude fiber content of Ziziphus mauritiana increased from 4.6 to 7.20% as the temperature increased and reached 5 °C. After that, the crude fiber content started to decrease from 7.20 to 4.03% as the temperature increased from 5 to 10 °C. The reduction in crude fiber may be a result of the conversion of cellulose to carbohydrates and due to the fluctuation of moisture [28].
As shown in Fig. 2a, Fig. 3bD, the fat content of Ziziphus mauritiana was affected by the preservation time and temperature, respectively. The fat content of Ziziphus mauritiana increased from 0.88 to 2.17% as the temperature increased from deep-freezing to 10 °C. This result may be due to the increase in moisture content with increasing storage temperature. Whereas the Ziziphus mauritiana fat content in this study increases from 0.88 to 0.97% as the preservation time was prolonged from 15 to 45 days.
Fig. 2a, Fig. 3bE illustrate how preservation time and temperature affected nitrogen, respectively. On the other hand, Fig. 2a, Fig. 3bF show how temperature and preservation time affected the crude protein content of Ziziphus mauritiana in this study. Nitrogen content increased with the shelf life of Ziziphus mauritiana fruit increasing from 1.00 to 1.22% when the preservation time was prolonged from 15 to 30 days and slightly decreases to 1.21% when the preservation time increased from 30 to 45 days. The same trend was observed for preservation temperature.
The nitrogen content increases from 0.99 to 1.67% when the temperature increases from deep freezing (which is less than −1 °C) to 5 °C and drops to 1.57% when the temperature rises to 10 °C, which is the preservation temperature.
The same result was also observed for the protein content of Ziziphus mauritiana, which is shown in Fig. 2a, Fig. 3bF for both preservation temperature and time, respectively. The protein content of this fruit increases from 6.19 to 10.14% when the temperature increased and from 6.19 to 7.63% when the preservation time was prolonged.
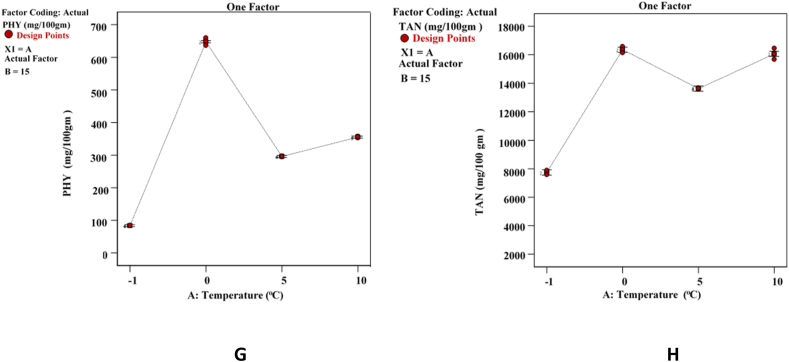
The effect of preservation parameters on the antinutritional composition of Ziziphus mauritiana, mainly the phytate and tannin content, was analyzed. Fig. 2G shows that the phytate content increases until the temperature reaches 0 °C from 84.00 to 647.67 mg/100 g m and starts to decrease to 294.77 mg/100 g m when the temperature increases to 5 °C. However, in the case of preservation time, the amount of phytate in Ziziphus mauritiana decreases from 84.00 to 67.27 mg/100 g m when the preservation time was prolonged from 15 to 30 days, and started to increase to 95.80 mg/100 g m when the time was prolonged from 30 to 45 days (Fig. 3G). In contrast, the tannin content of Ziziphus mauritiana increases from 7738.26 to 16357.90 mg/100 g m when the until the temperature reached 0 °C and started to decrease, as shown in Fig. 2H. It is also increased as the preservation time is prolonged from 15 to 30 days and starts to decrease when the preservation time is prolonged to 45 days with a lower of 7270.20 mg/100 g m and a higher 11371.80 mg/100 g m tannin content (Fig. 3H). The decrease in phytate content may be due to the gradual action of the phytase enzyme, which hydrolyzes a portion of the phosphate ester groups [29].
Fig. 2b.
Effect of temperature on G) phytate, and H) tannin.
3.3. Interaction effect of storage temperature and preservation time on the nutritional and anti-nutritional composition of Ziziphus mauritiana
The interaction effect of preservation time and temperature on the moisture content is shown in Fig. 4A. A maximum moisture content of 10.99% was observed for Ziziphus mauritiana preserved in a deep refrigerator (less than −1 °C) after 45 days of preservation. In this study, a low moisture content (5.25%) was observed at a temperature of −1 after 30 days of preservation.
Fig. 4a.
Interaction effect of Temperature and preservation time on A) Moisture content, B) Ash content, C) Crude fiber content, D) Fat Content.
The interaction effect of preservation time and temperature on the composition of Ziziphus mauritiana is shown in Fig. 4B. After 30 days of storage at 10 °C, a maximum ash content of 10.70% in Ziziphus mauritiana. The lowest ash content (4.74%) was observed after 45 days of preservation at a lower temperature (deep freezing).
As shown in Fig. 4C, preserving Ziziphus mauritiana at 5 °C for more than 45 days resulted in a higher crude fiber content of 17.88%. However, prolonged storage of Ziziphus mauritiana for 45 days at a low temperature (less than −1 °C) decreased the fruit's crude fiber content to 3.81%.
The study showed that the maximum fat content of 2.16% of Ziziphus mauritiana was observed at 10 °C after 15 days of preservation. Preserving Ziziphus mauritiana at 5 °C temperature and after 30 days of preservation time on the other hand shows a lower amount (0.49%) of fat content as shown in Fig. 4D.
As shown in Fig. 4E, the maximum nitrogen content (1.8%) of Ziziphus mauritiana was observed at 0 °C after 45 days of preservation, followed by 5 °C after 45 days. A lower nitrogen content (1.01%) was observed in the deep refrigerator (less than −1 °C) after 15 days of preservation.
The same trend as the nitrogen content of Ziziphus mauritiana was also observed in Fig. 4F for the crude protein content of Ziziphus mauritiana. The lower content (6.33%) of crude protein was observed with a preserving Ziziphus mauritiana for 15 days at deep freezing whereas preserving Ziziphus mauritiana for 45 days at 0 °C shows higher protein content (11.27%).
Fig. 4b.
Interaction effect of Temperature and preservation time on E) Nitrogen content, F) Protein content, G) Phytate, and H) Tannin.
A high amount (659.33 mg/100 g m) of phytate content of Ziziphus mauritiana was observed after 15 days of preservation at 0 °C (Fig. 4G). Preserving Ziziphus mauritiana in a deep refrigerator (which is less than −1 °C) for 30 days of preservation time on the other hand decreases the phytate content of Ziziphus mauritiana to 67.27 mg/100 g m.
The interaction effect of temperature and preservation time on the tannin content is shown in Fig. 4H. The maximum tannin content (16577.80 mg/100 g m) was observed at 0 °C after 15 days of preservation. A lower tannin content (3842.46 mg/100 g m) of Ziziphus mauritiana was observed after 30 days of preservation at 10 °C.
The findings cannot be directly compared to those of other similar studies conducted in the country because there is no literature specifically relevant to this topic. However, it makes sense to compare the findings to existing studies conducted on Ziziphus ssp. Fruit. A considerable number of papers have been published on Ziziphus ssp. Fruit, however, much of the literature has focused on the diversification, variation, growth, nutritional and anti-nutritional value, processing, and application of the fruit [[30], [31], [32], [33], [34], [35], [36], [37]]. Most studies have reported Ziziphus mauritiana as an underutilized, less known, and perishable fruit [38]. To increase the commercial value and shelf life of Ziziphus mauritiana fruit, fruit drying using different techniques has been suggested [39]. In their study, they investigated the effect of different drying methods on the physicochemical properties and sensory analysis of various Indian jujube (Zizyphus mauritiana) and found higher vitamin C, phenol content, antioxidant activity using oven and sun drying; but lower antioxidant activity, and higher antioxidant activity for oven and sun drying respectively [39].
A study on the effect of freeze drying, conventional oven drying, and sun drying on total phenolic content, proanthocyanidins, vitamin C content, cyclic adenosine monophosphate, and antioxidant activities of different cultivars of Ziziphus jujuba Mill was also conducted by Sapkota et al. [40]. The results indicate that most of the proanthocyanidins and vitamin C content of Ziziphus jujuba Mill. Lost in sun and oven drying than in freeze drying; however, antioxidant activities showed a higher retention rate in the oven drying than in sun drying. The results also show that oven drying is temperature dependent and that increasing the temperature affects the total phenolic content, proanthocyanidins, vitamin C content, cyclic adenosine monophosphate, and antioxidant activities [40]. Tembo et al. [40] reported the effect of storage on the Zizyphus mauritiana fruit. In their first study, they evaluated the effect of storage temperature and duration (3–12 weeks) on the weight loss, color, vitamin C, reducing sugars, and titratable acids of Zizyphus mauritiana; the results showed that fruit preserved at low temperature (5 °C) lost only 48% of their weight compared to others stored at ambient (22 °C) and intermediate (15 °C) temperatures. The above trend also works for color change in fruits stored at lower temperatures to maintain their color than in fruits stored at ambient and intermediate temperatures. In contrast, vitamin C, reducing sugar, and titratable acid are significantly affected by the temperature and duration of storage of Zizyphus mauritiana fruit [41]. Their second report determined the effect of pre-drying treatment, drying method, and duration on the quality and vitamin C content of Zizyphus mauritiana and found that both quality and vitamin C were affected by the drying method and duration [42].
In another study, improved crop storage and polypropylene bags as controls were used, and the findings showed that the improved crop storage bags showed comparable pest infections and damages as the initial stage after four-month. There was no significant difference in sugar content after four months of treatment using both improved crop storage and polypropylene bags, but a change in organoleptic properties was observed in Zizyphus mauritiana preserved using polypropylene bags [43]. Cold atmospheric plasma was used to treat Zizyphus jujuba Mill. In order to preserve postharvest quality and lengthen storage time [44]. This treatment delayed ripening and preserved weight loss, moisture content, and bacterial growth. However, the report shows that cold atmospheric plasma treatment is dependent on exposure time.
In recent years, studies on the coating of Ziziphus ssp. With edible films have been reported from edible materials and their mixtures, such as gums, proteins, and lipids, to extend the post-harvest shelf life [45]. A study by Ref. [46] using chitosan (0, 1, and 2%) as a coating film followed by packing in polyethylene bags for four weeks showed that Ziziphus fruit treated with 2% chitosan had the highest concentration of vitamin C and low-weight loss. A similar study [47] on the effect of chitosan coating (0, 0.5, and 1%), storage temperature (5 and 25 °C), and 28 days on the physicochemical properties of Zizyphus mauritiana revealed that chitosan coating at low temperature had a positive effect on the physicochemical quality and prolonged shelf life. Overall, result differences could be attributed to differences in experimental protocol and conditions or setup, drying method, and duration, environmental conditions where the species are grown, and type of subspecies. However, all the above study shows that postharvest handling affects the nutritional and antinutritional content of Zizyphus ssp.
4. Conclusion and recommendation
The results of the investigation demonstrated that the preservation method used in this study had a substantial impact on the nutritional composition of Ziziphus mauritiana fruits, as well as their antinutritional content (preservation time and temperature). The nutritional composition of Ziziphus mauritiana fruit that was preserved with different preservations techniques was not comparable (not the same) to the nutritional composition of the fresh fruit. The study shows that there is a significant effect of the individual and interaction effect of preservation parameters on the nutritional value. When compared to fresh fruit, Ziziphus mauritiana fruit's anti-nutritional composition, namely, its Tannin and Phytate values, were typically the least for deep-freeze preservation techniques. Moreover, when the temperature at which the fruit was stored increased, the amount of tannin and phytate in Ziziphus mauritiana fruit increased continually. Increased phytate and tannin contents will affect (decrease) the disease-resistance capacity of the human body and growth. Storage time and temperature are factors that influence the nutritional and anti-nutritional content of the fruit, reducing or increasing their value as a function of time and temperature. Therefore, it is clear that storage of deep-frozen fruit is essential. Fruits should be packed in plastic bags (HDPB), and the fruit can be preserved for up to 45 days.
This study shows that Ziziphus mauritiana can be preserved for a long time without significant decay, damage, or loss of its nutritional and anti-nutritional composition. This makes it a valuable wild fruit in rural livelihoods to supplement food, ensure food security, and generate household income. However, the study does not cover the social, economic, and livelihood impacts of the Ziziphus mauritiana fruit.
Author contribution statement
Mahelete Tsegaye, Tewabech Alemu, Abraham Dilnesa, Amsalu Tolessa, Tegene Tantu, Yihunie Bekalu, Fikiremariam Haile: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Shackleton C., Delang C.O., Shackleton S., Shanley P. 2011. Non-timber Forest Products: Concept and Definitions; pp. 3–21. [Google Scholar]
- 2.Sakai S., et al. Social and ecological factors associated with the use of non-timber forest products by people in rural Borneo. Biol. Conserv. 2016;204:340–349. doi: 10.1016/j.biocon.2016.10.022. [DOI] [Google Scholar]
- 3.Toda M., Masuda M., Rengifo E.L. Medicinal plant use influenced by health care service in mestizo and indigenous villages in the Peruvian amazon. J. Sustain. Dev. 2017;10(3):19. doi: 10.5539/jsd.v10n3p19. [DOI] [Google Scholar]
- 4.Sardeshpande M., Shackleton C. Wild edible fruits: a systematic review of an under-researched multifunctional NTFP (Non-Timber Forest Product) Forests. 2019;10(6):1–24. doi: 10.3390/f10060467. [DOI] [Google Scholar]
- 5.Agrawal A., Cashore B., Hardin R., Shepherd G., Benson C., Miller D. United Nations Forum For. Tenth Sess.; 2013. Economic Contributions of Forests; pp. 1–132. [Google Scholar]
- 6.Duguma H.T. Wild edible plant nutritional contribution and consumer perception in Ethiopia. Int. J. Food Sci. 2020;2020 doi: 10.1155/2020/2958623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yumkham S.D., Chakpram L., Salam S., Bhattacharya M.K., Singh P.K. Edible ferns and fern–allies of North East India: a study on potential wild vegetables. Genet. Resour. Crop Evol. 2017;64(3):467–477. doi: 10.1007/s10722-016-0372-5. [DOI] [Google Scholar]
- 8.Mavengahama S., McLachlan M., de Clercq W. The role of wild vegetable species in household food security in maize based subsistence cropping systems. Food Secur. 2013;5(2):227–233. doi: 10.1007/s12571-013-0243-2. [DOI] [Google Scholar]
- 9.Lulekal E., Asfaw Z., Kelbessa E., Van Damme P. Wild edible plants in Ethiopia: a review on their potential to combat food insecurity. Afr. Focus. 2011;24(2):71–121. doi: 10.21825/af.v24i2.4998. [DOI] [Google Scholar]
- 10.Neudeck L., Avelino L., Bareetseng P., Ngwenya B.N., Teketay D., Motsholapheko M.R. The contribution of edible wild plants to food security, dietary diversity and income of households in Shorobe Village, Northern Botswana. Ethnobot. Res. Appl. 2012;10:449–462. [Google Scholar]
- 11.Ghedira K., Chemli R., Caron C., Nuzilard J.M., Zeches M., Le Men-Olivier L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry. 1995;38(3):767–772. doi: 10.1016/0031-9422(94)00669-K. [DOI] [Google Scholar]
- 12.Ohiokpehai Omo. Promoting the nutritional goodness of traditional food products. Pakistan J. Nutr. 2003;2(4):267–270. doi: 10.3923/pjn.2003.267.270. [DOI] [Google Scholar]
- 13.Kibar B., Temel S. Evaluation of mineral composition of some wild edible plants growing in the eastern anatolia region grasslands of Turkey and consumed as vegetable. J. Food Process. Preserv. 2016;40(1):56–66. doi: 10.1111/jfpp.12583. [DOI] [Google Scholar]
- 14.Abasse T., et al. Morphological and phenological characterization for 5 improved varieties of zizyphusmauritiana in Niger. Int. J. Dev. Res. 2017;7(10):16224–16230. http://www.journalijdr.com [Google Scholar]
- 15.Julia F. 1987. Morton, Fruits Of Warm Climates. [Google Scholar]
- 16.Koné B., Kalinganire A., Doumbia M. vol. 10. 2009. (La culture du jujubier : un manuel pour l’hor ticulteur sahélien). [Google Scholar]
- 17.Kalinganire A., Weber J.C., Uwamariya A., Kone B. Indigenous Fruit Trees in the Tropics: Domestication. Utillization and Commercialization; 2007. Improving rural livelihoods through domestication of indigenous fruit trees in the parklands of the sahel; pp. 186–203. [Google Scholar]
- 18.Orwa . 2009. “Ziziphus Mauritiana,” Edible Med. Non-medicinal Plants; pp. 605–613. [DOI] [Google Scholar]
- 19.Rashwan A.K., Karim N., Shishir M.R.I., Bao T., Lu Y., Chen W. Jujube fruit: a potential nutritious fruit for the development of functional food products. J. Funct.Foods. 2020;75 doi: 10.1016/j.jff.2020.104205. Elsevier Ltd, Dec. 01. [DOI] [Google Scholar]
- 20.McMullin S., et al. Developing fruit tree portfolios that link agriculture more effectively with nutrition and health: a new approach for providing year-round micronutrients to smallholder farmers. Food Secur. 2019;11(6):1355–1372. doi: 10.1007/s12571-019-00970-7. [DOI] [Google Scholar]
- 21.AOAC Official Method 2007.04: fat, moisture, and protein in meat and meat products. Off. Methods Anal. AOAC Int. 2007;90(4):1073–1083. [PubMed] [Google Scholar]
- 22.AOAC 1990 “No more riffling with a,” 75 Years report. Anal. Sci. 1990;73(1):1–192. [Google Scholar]
- 23.Ibrahim M.A., Koorbanally N.A., Islam M.S. In vitro anti-oxidative activities of the various parts of Parkia biglobosa and GC-MS analysis of extracts with high activity. Afr. J. Tradit., Complementary Altern. Med. 2013;10(5):283–291. doi: 10.4314/ajtcam.v10i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenfield H., Southgate D.A.T. Food composition data and food composition data bases. Food Compos. Data. 2003:3–14. doi: 10.1007/978-1-4615-3544-7_2. [DOI] [Google Scholar]
- 25.Zhu W., et al. United States department of agriculture food safety and inspection service , office of public health science United States department of agriculture food safety and inspection service , office of public health science. J. Chromatogr. A. 2009;1216(1–2):1–15. http://link.springer.com/10.1134/S1061934813030027%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3771199&tool=pmcentrez&rendertype=abstract%5Cn http://xlink.rsc.org/?DOI=C4AY02499K%5Cn [Google Scholar]
- 26.Latta M., Eskin M. A simple and rapid colorimetric method for phytate determination. J. Agric. Food Chem. 1980;28(6):1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- 27.Gupta N., Rn S. Preparation and quality evaluation of dehydrated carrot and onion slices. J. Food Process. Technol. 2017;8(9) doi: 10.4172/2157-7110.1000692. [DOI] [Google Scholar]
- 28.Adetuyi F.O., Osagie A.U., Adekunle A.T. Effect of postharvest storage techniques on the nutritional properties of Benin indigenous okra abelmoschus esculentus (L) moench. Pakistan J. Nutr. 2008;7(5):652–657. doi: 10.3923/pjn.2008.652.657. [DOI] [Google Scholar]
- 29.Melese Mulu Abay . 2021. Effects of Storage Temperature and Relative Humidity on Physico Chemical Qualities of Selected Common Bean (Phaseolus vulgaris L.) Varieties. [Google Scholar]
- 30.Vardhan P.H. vol. 11. 2022. (Effect of Different Drying Techniques on Nutritional Composition of Arid Zone Fruits). no. 7, pp. 2435–2447. [Google Scholar]
- 31.Sapkota G., et al. Dynamics of nutrients in jujube (Ziziphus jujuba Mill.) at different maturity stages, cultivars, and locations in the southwest United States. Hortscience. 2023;58(2):155–163. doi: 10.21273/HORTSCI16880-22. [DOI] [Google Scholar]
- 32.Yao S., Heyduck R., Guldan S., Sapkota G. Early performance of jujube drying and multipurpose cultivars in the Southwestern United States. Hortscience. 2020;55(11):1804–1810. doi: 10.21273/HORTSCI15344-20. [DOI] [Google Scholar]
- 33.Pal R., Abrol G., Singh A.K., Punetha S., Sharma P., Pandey A.K. Nutritional and medicinal value of underutilized fruits. Acta Sci. Agric. 2019;3(1):16–22. [Google Scholar]
- 34.Uddin Mb, Hussain I. Development of diversified technology for jujube (Ziziphus jujuba L) processing and preservation. World J. Dairy Food Sci. 2012;7(1):74–78. doi: 10.5829/idosi.wjdfs.2012.7.1.62115. [DOI] [Google Scholar]
- 35.Abdel-Sattar M., Almutairi K.F., Al-Saif A.M., Ahmed K.A. Fruit properties during the harvest period of eleven Indian jujube (Ziziphus mauritiana Lamk.) cultivars. Saudi J. Biol. Sci. 2021;28(6):3424–3432. doi: 10.1016/j.sjbs.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moradinezhad F., Setayesh F., Mahmoodi S., Khayyat M. Physicochemical properties and nutritional value of jujube (Ziziphus jujuba Mill.) fruit at different maturity and ripening stages. Int. J. Hortic. Sci. Technol. 2016;3(1):43–50. [Google Scholar]
- 37.Adilah H.N., Saleh M.I., Az-Zahra N.D.A., Cho E., Sinaga E. Total phenolic and total flavonoid content, antioxidant activity, and nutritional profile of Ziziphus mauritiana fruit juice. Int. J. Biol. Phys. Chem. Stud. 2023;5(1):1–8. doi: 10.32996/ijbpcs.2023.5.1.1. [DOI] [Google Scholar]
- 38.Okigbo R.N., Ugwu C.S. Neglected crops of Africa. Int. J. Agric. Technol. 2021;17(6):2197–2210. [Google Scholar]
- 39.Anjum M.A., Haram A., Ahmad R., Bashir M.A. Physico-chemical attributes of fresh and dried indian jujube (Zizyphus mauritiana) fruits. Pakistan J. Agric. Sci. 2020;57(1):165–176. doi: 10.21162/PAKJAS/20.7845. [DOI] [Google Scholar]
- 40.Sapkota G., Delgado E., Vanleeuwen D., Holguin F.O., Flores N., Yao S. Preservation of phenols, antioxidant activity, and cyclic adenosine monophosphate in jujube (Ziziphus jujuba Mill.) fruits with different drying methods. Plants. 1804;12:2023. doi: 10.3390/plants12091804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tembo L., Chiteka Z.A., Kadzere I., Akinnifesi F.K., Tagwira F. Storage temperature affects fruit quality attributes of Ber (Ziziphus mauritiana Lamk.) in Zimbabwe. Afr. J. Biotechnol. 2008;7(8):3092–3099. http://www.academicjournals.org/AJB [Google Scholar]
- 42.Tembo L., Chiteka Z.A., Kadzere I., Akinnifesi F.K., Tagwira F. Blanching and drying period affect moisture loss and vitamin C content in Ziziphus mauritiana (Lamk.) Afr. J. Biotechnol. 2008;7(17):3100–3106. doi: 10.5897/AJB08.429. [DOI] [Google Scholar]
- 43.Amadou I., Baoua I.B., Amadou L., Baributsa D. Hermetic bags effectively preserve dried Ziziphus mauritiana Lam. Fruits in Niger. J. Agric. Sci. Apr. 2022;14(5):15. doi: 10.5539/jas.v14n5p15. [DOI] [Google Scholar]
- 44.Jin T., Dai C., Xu Y., Chen Y., Xu Q., Wu Z. Applying cold atmospheric plasma to preserve the postharvest qualities of winter jujube (Ziziphus jujuba Mill. Cv. Dongzao) during cold storage. Front. Nutr. 2022;9(Jul) doi: 10.3389/fnut.2022.934841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar Rout C., Singh S., Singh S. Efficacy of edible coatings on jujube (ziziphus mauritiana lamk.) fruits : a review. PLANT Arch. 2020;21(supplement 1) doi: 10.51470/plantarchives.2021.v21.s1.434. [DOI] [Google Scholar]
- 46.D. A. Taain, E. A. Al-Sareh, A. Dhia, and A. Taain, “THE EFFECT OF TREATMENT WITH CHITOSAN ON THE STORAGE ABILITY OF JUJUBE FRUITS (Ziziphus spp) CV.CHIBCHAB,” Orig. Res. Artic. Plant Cell Biotechnol. Mol. Biol., vol. 22, no. 14, pp. 1–6, [Online]. Available: https://www.researchgate.net/publication/349692766.
- 47.Hesami A., Kavoosi S., Khademi R., Sarikhani S. Effect of chitosan coating and storage temperature on shelf-life and fruit quality of Ziziphus mauritiana. Int. J. Fruit Sci. 2021;21(1):509–518. doi: 10.1080/15538362.2021.1906825. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.