Abstract
The development of artificial intelligence (AI) and deep learning provided precise image recognition and classification in the medical field. Ophthalmology is an exceptional department to translate AI applications since noninvasive imaging is routinely used for the diagnosis and monitoring. In recent years, AI-based image interpretation of optical coherence tomography and fundus photograph in retinal diseases has been extended to diabetic retinopathy, age-related macular degeneration, and retinopathy of prematurity. The rapid development of portable ocular monitoring devices coupled with AI-informed interpretations allows possible home monitoring or remote monitoring of retinal diseases and patients to gain autonomy and responsibility for their conditions. This review discusses the current research and application of AI, telemedicine, and home monitoring devices on retinal disease. Furthermore, we propose a future model of how AI and digital technology could be implemented in retinal diseases.
Keywords: Deep learning, digital technology, machine learning, retinal diseases, telemedicine
Introduction
Diagnosing ophthalmic diseases relies on clinical assessment and image interpretation, such as optical coherence tomography (OCT), fundus photographs, and the visual field. The traditional reading of retinal images requires the trained reader to view and report on individual images, which significantly burdens ophthalmologists and highlights the need for artificial intelligence (AI)-assisted clinical interpretation. Among ophthalmic diseases with AI translation, retina disease received the greatest attention and diseases such as diabetic retinopathy (DR), age-related macular degeneration (AMD), and retinopathy of prematurity (ROP) have seen promising advances. Since 2017, the number of publications on AI applications in retinal diseases has skyrocketed,[1] with research on AI in retinal diseases mainly focusing on computer engineering and medical imaging.[1] Many studies have developed DL algorithms that could achieve automated screening, prognostic and treatment prediction of retinal disease in the hope of achieving early detection, further management, and better clinical outcomes.
There are multiple dilemmas in the current health care of retinal diseases. Reports show a shortage of ophthalmologists as we face an aging population and an increasing number of chronic and age-related eye conditions.[2] In Taiwan, the ophthalmology outpatient clinic is overwhelmed with patients, either at the local clinic or in the tertiary center. Due to the chronic character of retina diseases, most patients require multiple follow-up visits for monitoring and management, which is time and resource intensive. Furthermore, the rural areas of Taiwan lack specialists and AI offers the solution to eliminate the geographical barrier to health-care access in screening retinal disease and treating the emergent condition.
DL, coupled with telemedicine and remote home monitoring, might be a long-term solution to screen and monitor the patients with retinal diseases effectively and efficiently. In this review, we discuss the current research and application of AI, telemedicine, and home monitoring devices on retinal disease. Furthermore, we propose a future model of how AI and digital technology could be implemented in retinal diseases [Table 1].
Table 1.
The summary of the current application of artificial intelligence and telemedicine in retinal field
| Types | Applicated diseases/Features | ||
|---|---|---|---|
| AI | Disease screening and monitoring | DR and DME[32-34] | |
| AMD[38-41] | |||
| ROP[62-65] | |||
| Prognostic prediction | AMD[42-45] | ||
| Anti-VEGF treatment prediction | AMD[46-49] | ||
| Telemedicine | Home monitoring applications | Paxos Checkup | Offering users multiple standard vision-assessment tests, such as Snellen visual acuity, dynamic Amsler grid, and color discrimination |
| myVisiontrack | Assessing users’ shape discrimination hyperacuity by presenting the user with three visual stimuli (two circles and one radially distorted circular shape) at the same time and asking the user to identify the distorted shape | ||
| Alleye | Assessing users’ hyperacuity by the user aligning the randomly misaligned dot between two fixed dots to form one straight axis | ||
| Home monitoring device | ForeseeHome | Adopting PHP to quantify vision change by altering the size of the artificial distortions | |
| Tele-laser | Navilas Laser System | A navigated laser photocoagulator that could be preplanned to hit microaneurysm in DME patients | |
| Robotic surgical systems | Preceyes Surgical System | A high-precision device for vitreoretinal surgical procedures | |
| IRISS | Being used to successfully perform anterior lens capsulorhexis, viscoelastic injection, hydrodissection, lens aspiration, retinal vein cannulation, and vitrectomy on ex vivo pig eyes | ||
| Nil | |||
| Telesurgery | Nil | ||
DME = Diabetic macular edema, OCT = Optical coherence tomography, PHP = Preferential hyperacuity perimetry, AI = Artificial intelligence, DR = Diabetic retinopathy, AMD = Age-related macular degeneration, ROP = Retinopathy of prematurity, IRISS = Intraocular robotic interventional surgical system, RAM!S = Robot-assisted microsurgery
Artificial Intelligence, Machine Learning, and Deep Learning
Artificial intelligence
AI is a domain of computer science that simulates human intelligence processes with the goal of problem-solving. The phrase “AI” was coined by John McCarthy as “the science and engineering of making intelligent machines” in 1956.[3]
Machine learning
Machine learning (ML), a branch of AI, is how computer software learns from the data without humans inputting explicit instructions. Arthur Samuel introduced it using the game of checkers as an example in 1959.[4] ML differs from a primary programming language in which we get answers by designing rules. In other words, ML could modify itself when exposed to more data and makes prediction eventually.
Deep learning
Deep learning (DL), a branch of ML, consists of multiple layers of neural networks attempting to mimic the human brain.[5] Neural networks are the sets of algorithms that could view as artificial neuron that processes data by data inputs, weights, summation and bias, activation, and output.[6] DL allows data to go through multiple layers of pattern recognition for accurate judgment.[5,7] In DL, convolutional neural networks (CNN), a type of artificial neural network, is primarily used for image recognition and classification[7] and plays a vital role in medical application.
Digital Technology: Telemedicine and Home Monitoring Devices
Telemedicine
Telemedicine refers to providing health care and transmitting health-care information across distances. Two aspects could classify telemedicine episodes: (1) The interaction between the patient and the expert and (2) the information being transmitted.[8] The type of interaction could be classified as either store-and-forward or real time.[8] Store-and-forward means that patients’ health information was shared in some format and interpreted by an expert later. Real-time interaction means no delay in time for the expert to evaluate the patient. The type of information transmitted includes data and text, audio, images, and video.[8]
Telesurgery
Telesurgery, a subset of telemedicine, connects patients and surgeons who are geographically distant by utilizing wireless networking and robotic technology. ZEUS robotic system (Computer Motion, Galeta, CA, USA) was the first developed functional telesurgery system[9] and was used for the first telesurgery operated by Jacques Marescaux’s surgical team in New York in 2001 performing a remote robot-assisted laparoscopic cholecystectomy on a 68-year-old woman in Strasbourg, France.[10] After undergoing the successful 54-min telesurgery, the patient had an uneventful recovery postoperatively. Moreover, a routine telesurgery service in Canada was established in 2003, and 21 surgeries have taken place in 2 years, including 13 fundoplications, 3 sigmoid resections, 2 right hemicolectomies, 1 anterior resections, and 2 inguinal hernia repairs.[11] For now, there is no telesurgery in the field of ophthalmology; however, based on the recent advance of 5G technology and robotic surgical systems development, telesurgery in the retina field might become a reality in the near future. The 5G technology provides high-speed networks connecting the remote robot and the control console.[12] To date, several ophthalmological robotic surgical systems have been developed, including the Preceyes Surgical System (Preceyes B.V., Netherlands) and the intraocular robotic interventional surgical system (IRISS) (from the University of California, Los Angeles).[13] The Preceyes Surgical System, a high-precision device for vitreoretinal surgical procedures, was used to conduct two clinical trials in the removal of retinal membranes and sub-retinal injection in patients.[14] The IRISS was validated on ex vivo pig eyes and the surgeon could successfully perform anterior lens capsulorhexis, viscoelastic injection, hydrodissection, lens aspiration, retinal vein cannulation, and vitrectomy.[15,16]
In the field of ophthalmology, although telesurgery is still in the infantile stage, there is another teleoperation procedure in great progress. The Navilas® Laser System (OD-OS, Teltow, German), a navigated laser photocoagulator, enables retina specialists to preplan the treatment at a specific location and time. Navilas® combines laser photocoagulation with fluorescein angiographic imaging and can project annotated images with the preplanned treatment spots on the live fundus view in the real time.[17] The laser beam then targets the proposed treatment spots to complete the treatment.[17] It achieved a higher accuracy of hitting rate of microaneurysm in diabetic macular edema (DME) in photocoagulation treatments (92%) than standard manual-technique laser treatment (72%).[18]
Home monitoring device
Self-measurement at home is important in-between consultations as disease fluctuation and progress may be recorded (i.e., blood pressure measurement for hypertension and glucose measurement for diabetes). The Amsler grid is the most commonly used home monitoring tool in ophthalmology. It can detect metamorphopsia, symptoms indicating eyes with mechanically distorted retinas found typical in wet AMD and DME.[19-21] There are three mobile-device telemonitoring applications currently available to patients, Paxos Checkup™ (DigiSight Technologies, Inc., San Francisco, CA, USA), myVisiontrack® (Vital Art and Science, LLC., Richardson, TX, USA) and Alleye (Oculocare Ltd, Switzerland). Paxos Checkup™ offers multiple standard vision-assessment tests, such as Snellen visual acuity (VA), dynamic Amsler grid, and color discrimination, and enables the transmission and storage of patient data to a cloud-based system for the physician to review. The myVisionTrack® application, which assesses shape discrimination hyperacuity,[22] was designed for monitoring patients with diagnosed maculopathy diseases with AMD and DR.[23] Similarly, the Alleye test allows early identification of progression in exudative neovascular AMD and DME by assessing hyperacuity.[24]
ForeseeHome® (Notal Vision Ltd., Manassas, VA, U.S.A.) [Figure 1], the first home telemonitoring device in ophthalmology, can have the earlier detection of AMD-related Choroidal neovascularization and enhance neovascular-AMD detection rate in medical care.[25,26] ForeseeHome is a portable device that adopts Preferential Hyperacuity Perimetry (PHP), a psychophysical test that can detect the changes in the macula. ForeseeHome® connects to an Independent Diagnostic Testing Facility (IDTF) via a wireless network, allowing the pre-assigned staff to promptly schedule a clinical evaluation upon identifying a significant change in the patient’s vision.[27,28]
Figure 1.
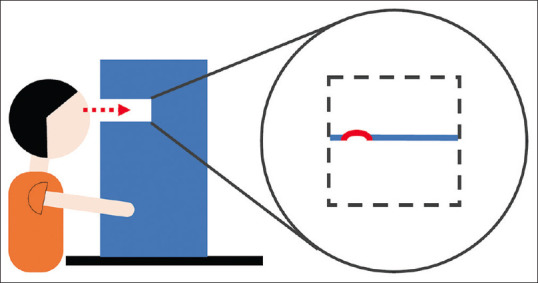
The schematic diagram of a home monitoring device, ForeseeHome. While the patient looks into the LCD monitor of ForeseeHome® (Notal Vision Ltd., Manassas, VA, U.S.A.), Preferential Hyperacuity Perimetry technology developed by Notal Vision was used to detect minute vision changes. In the central visual field of 14 degrees where the patient’s macula is located, the screen presents an “artificial distortion,” a straight line with a wave or bump. However, if a disease-related “wave” in the line is also present, the patient might only perceive the larger distortion. The patient’s vision change can thus be quantified by altering the size of the artificial distortions. (The concept was adopted from website of Notal Vision.)
Deep Learning in Retinal Diseases
Diabetic retinopathy
Diabetes mellitus (DM) prevalence worldwide in 20–79 years old in 2021 was estimated to be 10.5% (536.6 million people) and is anticipated to rise to 12.2% (783.2 million) by the year 2045.[29] DR is a common complication of DM and a leading cause of preventable blindness, with a global prevalence of 28.54 million in 2020 for vision-threatening DR.[30] Annual screening with dilated funduscopy is recommended by the WHO for patients with DM to prevent vision loss.[31] Due to the significant number of DR patients, there is high demand for DR screening, timely referral, and treatment. However, the fundoscopy screening and traditional reading of these images are facing the issues of the availability of human assessors and long-term financial sustainability. Therefore, the development of DL and digital technology is in need to facilitate the process.
DL can be used for screening and even grading DR. The application of DL for DR screening has significantly been studied and has shown good performance with high sensitivity and specificity. In 2016, Google Inc. sponsored Gulshan et al. to train a deep CNN for detecting referable.[32] The algorithm was validated by two large data sets, EyePACS-1 and Messidor-2, with an area under the receiver operating (AUROC) curve of 0.991 and 0.990, respectively. In 2018, the DL algorithm, called IDx-DR, developed by Abràmoff et al., obtained the first approval from the US Food and Drug Administration for the automated detection of DR and diabetic macula edema, achieving 87.2% sensitivity and 90.7% specificity.[33] In addition to the screening of DR, AI can be trained to grade DR, having the potential to offer the prognosis and treatment recommendations. Takahashi et al. developed a GoogLeNet DL neural network to provide a novel AI disease-staging system for grading DR.[34]
Age-related macular degeneration
AMD is a significant cause of vision loss in elderly persons globally. As the aged population grows, the estimated prevalence of early and late AMD in 2050 will be 39.05 million and 6.41 million, respectively.[35] The majority of AMD patients (84%) are unaware of their condition due to its asymptomatic feature in the early stage.[36] The American Academy of Ophthalmology recommends routine screening with at least 2-year intervals for patients aged 65 years or older. The current standard management of wet AMD is the intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents such as bevacizumab or ranibizumab, either monthly or with a more individualized treatment strategy with close follow-up.[37] The number of AMD patient visits is increasing due to the growth of the aged population, the chronic and relapsing nature of the disease, and the frequent follow-up for disease monitoring, evaluation, and treatment. Thus, there is a suppressing need for a robust automatic mechanism to be developed. Fortunately, AMD treatment is primarily determined by the VA and OCT findings, so DL and telemedicine could play a role.
DL could involve initial screening, subsequent monitoring, and treatment prediction of AMD. For AMD screening, Lee et al. developed a DL algorithm that differentiates AMD from normal macula using OCT images,[38] and Treder et al. developed one that determines exudative AMD from the normal macula.[39] DL also showed a promising future in monitoring progression and making the prognostic prediction of AMD. Burlina et al. developed deep CNNs using color fundus images to perform automated AMD grading, comparable to human experts’ performance.[40,41] Bogunovic et al. developed a ML model trained on images of 61 eyes with early/intermediate AMD acquired at 3-month intervals. It can predict drusen regression over the next 2 years with an AUROC curve of 0.75.[42] ML was used to predict VA in a patient with neovascular AMD after treatment.[43-45] Furthermore, some studies develop ML methods to predict the need for anti-VEGF treatment for AMD, which accuracy is similar to a specialist.[46-49] The most relevant feature of OCT scans for treatment prediction is the presence of retinal fluid.[47]
Retinopathy of prematurity
ROP, a leading cause of childhood blindness[50,51] with an incidence of 60%–72.7%,[52-54] is a vasoproliferative disease affecting premature infant which can progress to tractional retinal detachment, resulting in visual loss. However, visual loss is primarily preventable with the early detection of severe ROP and timely treatment.[55,56] “Plus” disease, a critical ICROP parameter to initiate ROP treatment,[57,58] is associated with increased venous dilation and arterial tortuosity of the posterior pole of the retina.[59] “Preplus” disease represents a lesser severity of tortuosity and dilation, forming with plus disease a continuous spectrum of retinal vascular changes. Screening and diagnosis of ROP are made either directly through fundoscopic examination or captured images with portable cameras examined afterward by an expert.
There are two main obstacles in ROP screening: (1) Experienced ROP specialists are scarce; and (2) significant inter-expert variability and inconsistency in ROP diagnosis, leading to differences in management.[60,61] Thus, the application of AI might answer the shortage of trained examiners and minimize the ROP diagnosis variability, effectively implementing ROP screening. Some DL algorithms were developed and showed promise in the accuracy of detecting “plus” or “preplus” disease.[62-64] In 2018, Brown et al. trained a deep CNN on a set of 5,511 retinal photographs to detect “plus” and “preplus” disease, with 93% sensitivity and 94% specificity for “plus” disease and 100% sensitivity and 94% specificity for “pre plus” disease. This fully automated algorithm even outperformed human experts in evaluating the same data set.[65]
Limitation of Artificial Intelligence and Telemedicine
Limitation of artificial intelligence
The major problem of the DL model is the black-box phenomenon. Large-scale adoption of AI in health care is still with great concern that AI and DL use a “black-boxes” approach. DL algorithms do not classify or diagnose disease by the criteria but by the underlying features.[66,67] Due to the “black-box” nature of the neural network, it is challenging for physicians to know what features it utilizes, understands how it reaches a particular decision, and thus identify the inherent error.
Limitation of telemedicine
There are four main barriers to telemedicine implementation that we need to overcome. The first is the infrastructure costs, including ophthalmic imaging equipment or hardware, the high-speed computing facility, and storage. It can be prohibitive as retinal cameras can cost more than 10,000 USD.[68] Second, the digital divide, the availability of internet access and mobile phone or digital device and the capability of using it affect the adoption of telemedicine geographically and socially. People with low incomes, less education or low literacy levels, older adults, people in isolated or rural areas, and people with more chronic conditions are more likely to be left behind by the digital divide.[69,70] Among all groups, the digital divide is particularly obvious among the elderly: only 58% of the elderly use the Internet in the US.[71] Third, whether telemedicine enhances or decreases patient compliance and adherence to medical care is still an issue to be understood. Tele-ophthalmology in the outpatient setting might overburden primary care doctors to perform additional work to ensure patients’ compliance with the telemedicine recommendation.[68] Finally, the remote nature of virtual clinics undermines the quality of the patient–physician interaction. In addition, the absence of a physical examination might lead to physicians overlooking the whole picture of a patient’s illness. Fifty-nine percent of ophthalmologists reported having “low confidence” in making decisions based on the images alone.[72] Telemedicine might force physicians to make decisions with limited information.
Limitation of telesurgery
The major problem with telesurgery is latency time and lack of haptic feedback. Latency time is the delay in sending and receiving the auditory, visual, and even tactile feedback between the two distant locations.[73] Great latency time leads to a lengthy operation, surgical inaccuracy, reductions in surgical performance, and eventually, risk to the safety of the patients.[74,75] According to Xu et al., an ideal latency time for telesurgery is <200 ms, and the surgical performance exponentially deteriorates as latency time increases.[74] The first telesurgery performed by Professor Marescaux in 2001 used a high-speed fiber optic cable with a dedicated asynchronous transfer mode connection with 10-Mbps bandwidth, achieving an average latency of 155 ms.[10,73] However, there are 40 technicians required to maintain the speed.[73] The routine telesurgery service in Canada used a commercially available Internet Protocol-Virtual Private Network with 15-Mbps bandwidth to connect the robotic console in Hamilton with three arms of the Zeus-TS surgical system 400 km away in North Bay.[11] It achieves an overall latency of 135–140 ms, which is noticeable but not difficult for surgeons to adapt.[11]
Haptic feedback technology could compensate for telesurgery’s drawback that surgeons could not feel the tissue in real instruments. Haptic feedback technology conveys tactile information to the users by applying advanced vibration patterns and waveforms. In telesurgery, it allows the operator to not only rely on visual force feedback but feel the texture of the tissue and the tension between the sutures to avoid damage to the tissues, enhance the operator’s confidence, and thus shorten the surgical time.[76,77] The first telesurgical system which integrates haptic feedback technology was introduced in 2015, called Telelap Alf-x (SOFAR S. p. A., ALF-X Surgical Robotics Department, Trezzano Rosa, Milan, Italy).[76-78]
The Future Health-care Model of Retinal Diseases
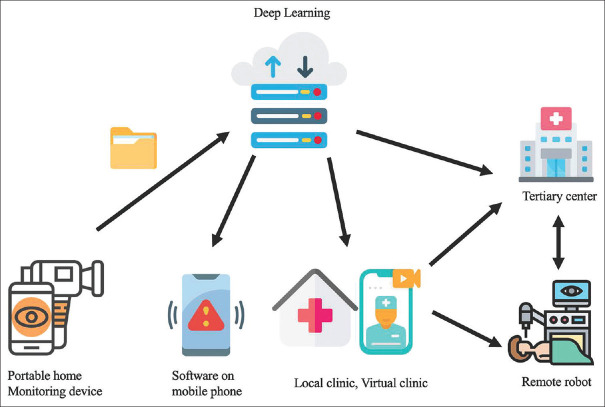
Here, we propose a future healthcare model [Figure 2] for retinal diseases that integrates AI, telemedicine, and remote patient monitoring.
Figure 2.
The future health care model of retinal diseases. The model integrates the application of AI, telemedicine, and remote patient monitoring. The model will rely on (1) home-based self-monitoring with portable imaging devices which provide OCT images, fundus photographs, or so forth, (2) a high-speed computing facility that can run the DL algorithms to read the images and produce the primary report, alert each party and schedule an appointment upon detecting changes, and finally store the information, (3) an internet system that connects to the electronic medical record and transmit information, (4) a referral system between local clinics, virtual clinic, and tertiary centers, and (5) a telesurgery system. Images obtained from the portable home device will be sent to the high-speed computing facility to run the DL algorithms; images and produced reports will be integrated into electronic medical records. Upon detecting significant changes in retinal images, the DL algorithms will alert the patient and their doctor via software on a mobile phone or text message, and schedule an appointment at the local clinic, tertiary center, or even virtual clinic, which can also directly prescribe medicine. Patients needing further management can be referred to a tertiary center either “physically” or “electronically” from the local or virtual clinic. Instead of patients physically visiting tertiary centers to receive treatment, they can receive telesurgery at the local hospital with a remote robot manipulated by another robotic console operated by the retinal specialist at the tertiary center–icons made by Freepik, Pause08, Smashicons, Kerismaker, Juicy_fish, and Eucalyp from www.flaticon.com. OCT: Optical coherence tomography, DL: Deep learning
The model combines portable home monitoring devices and software on mobile phones for remote patient monitoring. The portable home monitoring devices will have the imaging ability to capture OCT images, fundus photography, and so forth. The images will be sent to and stored at a high-speed computing facility. The high-speed computing facility will run the DL algorithm once it receives the images, report the image back to the patient through the software on the mobile phone, and integrate the images into their electronic medical record. The system allows (1) diagnostic screening for patients with associated underlying diseases such as diabetes and (2) follow-up monitoring for patients with already-known retinal diseases. When the DL algorithm detects significant changes in the images, it will alert the patients and their doctors through software on a mobile phone or text messages and arrange an appointment subsequently. Suppose the patient is newly diagnosed by the screening of DL and is primarily seen by family doctors, endocrinologists, or local physicians. In that case, they can be referred to the tertiary center’s retinal specialist from the local clinic. If the patient with already-known retinal diseases is identified with disease progression by the DL algorithm, they can thus schedule a follow-up visit with their retinal specialist.
To avoid unnecessary physical visits and reduced health resources consumption, patients can first “visit” a virtual clinic, a real-time video-based consulting with a retinal specialist. The retinal specialist can review the images from electronic medical records, evaluate clinical symptoms, and treat patients with telesurgery if needed. Telesurgery often involves two parts of the robot: the robot at the remote site and the surgeon’s robotic console at the tertiary center. Telesurgery enables patients in the rural areas to receive surgery at the local hospital with the remote robot, which the retinal specialist in the tertiary center manipulates. Telesurgery of the retina will include routine management such as navigated laser with photocoagulation and intravitreal injection, and more importantly, emergent eye surgery for eyeball rupture, retinal detachment, and so on.
This health-care model involves mixed categories of telemedicine. First, the system stores the images and generates text reports, sending them to doctors for later review. It is the type of store-and-forward model that transmits images and text information. Second, the virtual clinic enables real-time interaction and transmits information from the video. This model connects the doctor and patient consistently and reliably despite time and place and allows both sides to benefit from AI and digital technology.
The model enables clinicians to evaluate and treat their patients remotely. It improves healthcare access in the remote areas that have limited health-care resources and a shortage of certain specialists. Virtual clinic and the image transmitted system allows the retinal specialist to evaluate patients with their retinal images without the patient traveling a far distance. Telesurgery provides timely management in the rural areas of emergent conditions such as eyeball rupture and retinal detachment. Furthermore, during the COVID-19 pandemic, telemedicine and teleconferencing have been adopted into health care to avoid exposure risks and cross-infection between individuals.[79-82] This model strengthens and sustains the idea, allowing the patient to reach medical care more easily by the image-transmission and referral system.
Under regular home monitoring with the DL algorithm, the model can detect the retina change and disease progression before the symptoms appear, prompt treatment to avoid vision loss, and improve long-term visual outcomes. Moreover, by patients gaining self-responsibility and autonomy over their disease status, self-monitoring contributes to higher adherence and compliance to treatment and better disease outcomes.[83,84] It is also a triaging system that could identify patients with acute conditions and prioritize medical care. On the other hand, if the patient is in stable condition, they do not have to pay a visit. However, meanwhile, doctors can still keep track of patients’ retinal images through the electronic medical record.
As the population ages, the number of patients with chronic diseases such as AMD and DR continue to climb, leading to a heavy public health burden. AI and digital technology can facilitate capturing, storing, and interpreting the retina images. It will decrease the high demand for trained technicians and specialists for the traditional reading of fundus/OCT images. Moreover, with the availability to move the frequent monitoring from clinic to home and to reach retinal specialists from virtual clinics, the model aims to decrease follow-up visits to the tertiary center, reduce waiting times for physical appointments, decrease health-care costs, and improve quality of care.
In short, the model implicates four linked trends of the future health-care system. The first is migrating disease monitoring and screening from hospitals and clinics to homes. The second is the application of AI to automate disease diagnosis and predict prognosis and treatment, reducing the requirement for trained human resources and assisting clinical decision-making. The third is to increase access to health care, removing the geographic barrier. The fourth is the expansion of telemedicine from addressing acute conditions to chronic conditions and from medical prescription to surgical intervention. In the foreseeing future, the application of telemedicine with AI technology in retinal diseases is an unstoppable trend and will become a possible reality.
Conclusions
AI has been widely studied in the field of the retina. Either to act as a disease-screening platform or a tool to assist in clinical practices, DL will play an increasingly important role in retinal practice. Thanks to the advance of digital technology, telemedicine was already implemented in our current clinical activities and a home monitoring device was gradually developed for retinal diseases. DL and telemedicine together have the potential to augment the benefit and drastically change the future landscape of retinal practice.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. De-Kuang Hwang, an editorial board member at Taiwan Journal of Ophthalmology, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
References
- 1.Zhao J, Lu Y, Qian Y, Luo Y, Yang W. Emerging trends and research foci in artificial intelligence for retinal diseases: Bibliometric and visualization study. J Med Internet Res. 2022;24:e37532. doi: 10.2196/37532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee PP, Hoskins HD, Jr, Parke DW., 3rd Access to care: Eye care provider workforce considerations in 2020. Arch Ophthalmol. 2007;125:406–10. doi: 10.1001/archopht.125.3.406. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy J, Minsky ML, Rochester N, Shannon CE. A proposal for the dartmouth summer research project on artificial intelligence, August 31, 1955. AI Mag. 2006;27:12. [Google Scholar]
- 4.Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3:210–29. [Google Scholar]
- 5.Krogh A. What are artificial neural networks? Nat Biotechnol. 2008;26:195–7. doi: 10.1038/nbt1386. [DOI] [PubMed] [Google Scholar]
- 6.Krenker A, Bešter J, Kos A. Introduction to the artificial neural networks. Artificial neural networks. Methodol Adv Biomed Appl Tech. 2011 [Google Scholar]
- 7.Min S, Lee B, Yoon S. Deep learning in bioinformatics. Brief Bioinform. 2017;18:851–69. doi: 10.1093/bib/bbw068. [DOI] [PubMed] [Google Scholar]
- 8.Craig J, Patterson V. Introduction to the practice of telemedicine. J Telemed Telecare. 2005;11:3–9. doi: 10.1177/1357633X0501100102. [DOI] [PubMed] [Google Scholar]
- 9.Pugin F, Bucher P, Morel P. History of robotic surgery: From AESOP® and ZEUS® to da Vinci® . J Visc Surg. 2011;148:e3–8. doi: 10.1016/j.jviscsurg.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Marescaux J, Leroy J, Rubino F, Smith M, Vix M, Simone M, et al. Transcontinental robot-assisted remote telesurgery: Feasibility and potential applications. Ann Surg. 2002;235:487–92. doi: 10.1097/00000658-200204000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anvari M, McKinley C, Stein H. Establishment of the world's first telerobotic remote surgical service: For provision of advanced laparoscopic surgery in a rural community. Ann Surg. 2005;241:460–4. doi: 10.1097/01.sla.0000154456.69815.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro EM, Ramos Álvarez AN, Soler Anguiano FI. A new telesurgery generation supported by 5G technology: Benefits and future trends. Procedia Comput Sci. 2022;200:31–8. [Google Scholar]
- 13.Gerber MJ, Pettenkofer M, Hubschman JP. Advanced robotic surgical systems in ophthalmology. Eye (Lond) 2020;34:1554–62. doi: 10.1038/s41433-020-0837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards TL, Xue K, Meenink HC, Beelen MJ, Naus GJ, Simunovic MP, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649–56. doi: 10.1038/s41551-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: Development of the IRISS and feasibility studies in an animal model. Eye (Lond) 2013;27:972–8. doi: 10.1038/eye.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JT, Gerber MJ, Prince SW, Chen CW, Schwartz SD, Hubschman JP, et al. Intraocular Robotic Interventional Surgical System (IRISS): Mechanical design, evaluation, and master-slave manipulation. Int J Med Robot. 2018;14 doi: 10.1002/rcs.1842. [DOI] [PubMed] [Google Scholar]
- 17.Ober MD, Kernt M, Cortes MA, Kozak I. Time required for navigated macular laser photocoagulation treatment with the Navilas. Graefes Arch Clin Exp Ophthalmol. 2013;251:1049–53. doi: 10.1007/s00417-012-2119-0. [DOI] [PubMed] [Google Scholar]
- 18.Kozak I, Oster SF, Cortes MA, Dowell D, Hartmann K, Kim JS, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118:1119–24. doi: 10.1016/j.ophtha.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Associations between metamorphopsia and foveal microstructure in patients with epiretinal membrane. Invest Ophthalmol Vis Sci. 2012;53:6770–5. doi: 10.1167/iovs.12-9683. [DOI] [PubMed] [Google Scholar]
- 20.Xu K, Gupta V, Bae S, Sharma S. Metamorphopsia and vision-related quality of life among patients with age-related macular degeneration. Can J Ophthalmol. 2018;53:168–72. doi: 10.1016/j.jcjo.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Kalinowska A, Nowomiejska K, Brzozowska A, Maciejewski R, Rejdak R. Metamorphopsia score and central visual field outcomes in diabetic cystoid macular edema. Biomed Res Int. 2018;2018:4954532. doi: 10.1155/2018/4954532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser PK, Wang YZ, He YG, Weisberger A, Wolf S, Smith CH. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: Results of a pilot study. Retina. 2013;33:1863–70. doi: 10.1097/IAE.0b013e3182899258. [DOI] [PubMed] [Google Scholar]
- 23.Chhetri AP, Wen F, Wang Y, Zhang K. Shape Discrimination Test on Handheld Devices for Patient Self-Test. Proceedings of the 1st ACM International Health Informatics Symposium. 2010:502–6. [Google Scholar]
- 24.Faes L, Islam M, Bachmann LM, Lienhard KR, Schmid MK, Sim DA. False alarms and the positive predictive value of smartphone-based hyperacuity home monitoring for the progression of macular disease: A prospective cohort study. Eye (Lond) 2021;35:3035–40. doi: 10.1038/s41433-020-01356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew EY, Clemons TE, Harrington M, Bressler SB, Elman MJ, Kim JE, et al. Effectiveness of different monitoring modalities in the detection of neovascular age-related macular degeneration: The home study, report number 3. Retina. 2016;36:1542–7. doi: 10.1097/IAE.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AREDS2-HOME Study Research Group. Chew EY, Clemons TE, Bressler SB, Elman MJ, Danis RP, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization Home Monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–44. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holekamp NM. Moving from clinic to home: What the future holds for ophthalmic telemedicine. Am J Ophthalmol. 2018;187:xxviii–xxxv. doi: 10.1016/j.ajo.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 28.ForeseeHome. Virginia, USA: Notal Vision; 2023. [Last accessed on 2023 May 08]. Available from:https://notalvision.com/technology/foreseehome . [Google Scholar]
- 29.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91. doi: 10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Thylefors B. A global initiative for the elimination of avoidable blindness. Community Eye Health. 1998;11:1–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 33.Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi H, Tampo H, Arai Y, Inoue Y, Kawashima H. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS One. 2017;12:e0179790. doi: 10.1371/journal.pone.0179790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhong Y, Zhang L, Wu Q, Tham Y, Rim TH, et al. Global incidence, progression, and risk factors of age-related macular degeneration and projection of disease statistics in 30 years: A modeling study. Gerontology. 2022;68:721–35. doi: 10.1159/000518822. [DOI] [PubMed] [Google Scholar]
- 36.Gibson DM. Diabetic retinopathy and age-related macular degeneration in the U. S. Am J Prev Med. 2012;43:48–54. doi: 10.1016/j.amepre.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Kovach JL, Schwartz SG, Flynn HW, Jr, Scott IU. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol. 2012;2012:786870. doi: 10.1155/2012/786870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CS, Baughman DM, Lee AY. Deep learning is effective for classifying normal versus age-related macular degeneration OCT Images. Ophthalmology Retina. 2017;1:322–327. doi: 10.1016/j.oret.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treder M, Lauermann JL, Eter N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefes Arch Clin Exp Ophthalmol. 2018;256:259–65. doi: 10.1007/s00417-017-3850-3. [DOI] [PubMed] [Google Scholar]
- 40.Burlina P, Pacheco KD, Joshi N, Freund DE, Bressler NM. Comparing humans and deep learning performance for grading AMD: A study in using universal deep features and transfer learning for automated AMD analysis. Comput Biol Med. 2017;82:80–6. doi: 10.1016/j.compbiomed.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burlina PM, Joshi N, Pekala M, Pacheco KD, Freund DE, Bressler NM. Automated grading of age-related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. 2017;135:1170–6. doi: 10.1001/jamaophthalmol.2017.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogunovic H, Montuoro A, Baratsits M, Karantonis MG, Waldstein SM, Schlanitz F, et al. Machine learning of the progression of intermediate age-related macular degeneration based on oct imaging. Invest Ophthalmol Vis Sci. 2017;58:O141–50. doi: 10.1167/iovs.17-21789. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Erfurth U, Bogunovic H, Sadeghipour A, Schlegl T, Langs G, Gerendas BS, et al. Machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol Retina. 2018;2:24–30. doi: 10.1016/j.oret.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Rohm M, Tresp V, Müller M, Kern C, Manakov I, Weiss M, et al. Predicting visual acuity by using machine learning in patients treated for neovascular age-related macular degeneration. Ophthalmology. 2018;125:1028–36. doi: 10.1016/j.ophtha.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 45.Aslam TM, Zaki HR, Mahmood S, Ali ZC, Ahmad NA, Thorell MR, et al. Use of a neural net to model the impact of optical coherence tomography abnormalities on vision in age-related macular degeneration. Am J Ophthalmol. 2018;185:94–100. doi: 10.1016/j.ajo.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarthy U, Goldenberg D, Young G, Havilio M, Rafaeli O, Benyamini G, et al. Automated identification of lesion activity in neovascular age-related macular degeneration. Ophthalmology. 2016;123:1731–6. doi: 10.1016/j.ophtha.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Bogunovic H, Waldstein SM, Schlegl T, Langs G, Sadeghipour A, Liu X, et al. Prediction of anti-VEGF treatment requirements in neovascular AMD using a machine learning approach. Invest Ophthalmol Vis Sci. 2017;58:3240–8. doi: 10.1167/iovs.16-21053. [DOI] [PubMed] [Google Scholar]
- 48.Prahs P, Märker D, Mayer C, Helbig H. Deep learning to support therapy decisions for intravitreal injections. Ophthalmologe. 2018;115:722–7. doi: 10.1007/s00347-018-0708-y. [DOI] [PubMed] [Google Scholar]
- 49.Schlegl T, Waldstein SM, Bogunovic H, Endstraßer F, Sadeghipour A, Philip AM, et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology. 2018;125:549–58. doi: 10.1016/j.ophtha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020–The right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. The incidence and course of retinopathy of prematurity: Findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 53.Austeng D, Källen KB, Ewald UW, Jakobsson PG, Holmström GE. Incidence of retinopathy of prematurity in infants born before 27 week's gestation in Sweden. Arch Ophthalmol. 2009;127:1315–9. doi: 10.1001/archophthalmol.2009.244. [DOI] [PubMed] [Google Scholar]
- 54.Zin A, Gole GA. Retinopathy of prematurity-incidence today. Clin Perinatol. 2013;40:185–200. doi: 10.1016/j.clp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110–8. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- 56.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 57.Good WV, Hardy RJ. The multicenter study of Early Treatment for Retinopathy Of Prematurity (ETROP) Ophthalmology. 2001;108:1013–4. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 58.Solarte CE, Awad AH, Wilson CM, Ells A. Plus disease: Why is it important in retinopathy of prematurity? Middle East Afr J Ophthalmol. 2010;17:148–55. doi: 10.4103/0974-9233.63080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, 3rd edition. Ophthalmology. 2021;128:e51–68. doi: 10.1016/j.ophtha.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds JD, Dobson V, Quinn GE, Fielder AR, Palmer EA, Saunders RA, et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–6. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]
- 61.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125:875–80. doi: 10.1001/archopht.125.7.875. [DOI] [PubMed] [Google Scholar]
- 62.Gelman R, Jiang L, Du YE, Martinez-Perez ME, Flynn JT, Chiang MF. Plus disease in retinopathy of prematurity: Pilot study of computer-based and expert diagnosis. J AAPOS. 2007;11:532–40. doi: 10.1016/j.jaapos.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajwa A, Aman R, Reddy AK. A comprehensive review of diagnostic imaging technologies to evaluate the retina and the optic disk. Int Ophthalmol. 2015;35:733–55. doi: 10.1007/s10792-015-0087-1. [DOI] [PubMed] [Google Scholar]
- 64.Ataer-Cansizoglu E, Bolon-Canedo V, Campbell JP, Bozkurt A, Erdogmus D, Kalpathy-Cramer J, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity: Performance of the “i-ROP”system and image features associated with expert diagnosis. Transl Vis Sci Technol. 2015;4:5. doi: 10.1167/tvst.4.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RV, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–10. doi: 10.1001/jamaophthalmol.2018.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong TY, Bressler NM. Artificial intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA. 2016;316:2366–7. doi: 10.1001/jama.2016.17563. [DOI] [PubMed] [Google Scholar]
- 67.Froomkin AM, Kerr I, Pineau J. When AIs outperform doctors: Confronting the challenges of a tort-induced over-reliance on machine learning. Ariz Law Rev. 2019;61:33. [Google Scholar]
- 68.Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The current state of teleophthalmology in the United States. Ophthalmology. 2017;124:1729–34. doi: 10.1016/j.ophtha.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cullen R. Addressing the digital divide. Online Inf Rev. 2001;25:311–20. [Google Scholar]
- 70.Susannah Fox KP. Chronic Disease and the Internet. Washington, DC: Pew Research Center; 2020. [Last accessed on 2023 May 08]. Available from:https://www.pewresearch.org/internet/2010/03/24/chronic-disease-and-the-internet/ [Google Scholar]
- 71.Andrew Perrin MD. Americans Internet Access: 2000-2015. Washington, DC: Pew Research Center; 2015. [Last accessed on 2023 May 07]. Available from:https://www.pewresearch.org/internet/2015/06/26/americans-internet-access-2000-2015/ [Google Scholar]
- 72.Shaw J. Teleophthalmology: Ready for prime time. Eye Net Mag. 2016 [Google Scholar]
- 73.Raison N, Khan MS, Challacombe B. Telemedicine in surgery: What are the opportunities and hurdles to realising the potential? Curr Urol Rep. 2015;16:43. doi: 10.1007/s11934-015-0522-x. [DOI] [PubMed] [Google Scholar]
- 74.Fabrizio MD, Lee BR, Chan DY, Stoianovici D, Jarrett TW, Yang C, et al. Effect of time delay on surgical performance during telesurgical manipulation. J Endourol. 2000;14:133–8. doi: 10.1089/end.2000.14.133. [DOI] [PubMed] [Google Scholar]
- 75.Xu S, Perez M, Yang K, Perrenot C, Felblinger J, Hubert J. Determination of the latency effects on surgical performance and the acceptable latency levels in telesurgery using the dV-Trainer(®) simulator. Surg Endosc. 2014;28:2569–76. doi: 10.1007/s00464-014-3504-z. [DOI] [PubMed] [Google Scholar]
- 76.Stark M, Benhidjeb T, Gidaro S, Morales ER. The future of telesurgery: A universal system with haptic sensation. J Turk Ger Gynecol Assoc. 2012;13:74–6. doi: 10.5152/jtgga.2012.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stark M, Morales ER, Gidaro S. Telesurgery is promising but still need proof through prospective comparative studies. J Gynecol Oncol. 2012;23:134–5. doi: 10.3802/jgo.2012.23.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stark M, Pomati S, D’mbrosio A, Giraudi F, Gidaro S. A new telesurgical platform preliminary clinical results. Minim Invasive Ther Allied Technol. 2015;24:31–6. doi: 10.3109/13645706.2014.1003945. [DOI] [PubMed] [Google Scholar]
- 79.Bourdon H, Jaillant R, Ballino A, El Kaim P, Debillon L, Bodin S, et al. Teleconsultation in primary ophthalmic emergencies during the COVID-19 lockdown in Paris: Experience with 500 patients in March and April 2020. J Fr Ophtalmol. 2020;43:577–85. doi: 10.1016/j.jfo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hollander JE, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679–81. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 81.Wickham L, Hay G, Hamilton R, Wooding J, Tossounis H, da Cruz L, et al. The impact of COVID policies on acute ophthalmology services-experiences from moorfields eye hospital nhs foundation trust. Eye (Lond) 2020;34:1189–92. doi: 10.1038/s41433-020-0957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ting DS, Carin L, Dzau V, Wong TY. Digital technology and COVID-19. Nat Med. 2020;26:459–61. doi: 10.1038/s41591-020-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaptein AA, Fischer MJ, Scharloo M. Self-management in patients with COPD: Theoretical context, content, outcomes, and integration into clinical care. Int J Chron Obstruct Pulmon Dis. 2014;9:907–17. doi: 10.2147/COPD.S49622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The Effect of Self-Monitoring of Blood Pressure on Medication Adherence and Lifestyle Factors: A Systematic Review and Meta-Analysis. American Journal of Hypertension. 2015;28:1209–1221. doi: 10.1093/ajh/hpv008. [DOI] [PubMed] [Google Scholar]