Abstract
Nicotinamide riboside is a precursor to the important cofactor nicotinamide adenine dinucleotide and has elicited metabolic benefits in multiple preclinical studies. In 2016, the first clinical trial of nicotinamide riboside was conducted to test the safety and efficacy of human supplementation. Many trials have since been conducted aiming to delineate benefits to metabolic health and severe diseases in humans. This review endeavors to summarize and critically assess the 25 currently published research articles on human nicotinamide riboside supplementation to identify any poorly founded claims and assist the field in elucidating the actual future potential for nicotinamide riboside. Collectively, oral nicotinamide riboside supplementation has displayed few clinically relevant effects, and there is an unfortunate tendency in the literature to exaggerate the importance and robustness of reported effects. Even so, nicotinamide riboside may play a role in the reduction of inflammatory states and has shown some potential in the treatment of diverse severe diseases.
The putative clinical potential of oral nicotinamide riboside supplementation in humans remains to be understood.
INTRODUCTION
The purpose of this review is to highlight the currently available literature on nicotinamide riboside (NR) supplementation in humans. Specifically, the intent is to critically assess this body of literature to identify any baseless or poorly founded claims and assist the field in delineating the future potential of NR supplementation. We believe that we have assessed all currently available literature on the topic (Table 1), with the exception of a series of studies where NR made up a small part (~5% w/w) of a set of so-called “combined metabolic activators” or “metabolic cofactors” (1–3). These studies were excluded because of the complexity of this treatment, making it difficult to attribute any effects to the individual compounds. In the interest of transparency, it should be noted that the authors of this review have been involved with several of the included studies (4–8).
Table 1. Overview of the reports from clinical NR trials included in this review.
| First author | Year published | Group treated | Treatment group size | Placebo group size | Daily NR dose (mg) | Duration | Reference in review |
|---|---|---|---|---|---|---|---|
| Trammell | 2016 | Healthy | 12 | 0 | 100, 300, 1000 | 24 hours | (24) |
| Airhart | 2017 | Healthy | 8 | 0 | 2000 | 8 days | (25) |
| Dellinger | 2017 | Elderly | 40 | 40 | 250, 500 | 60 days | (40) |
| Dollerup | 2018 | Obese, insulin resistant | 20 | 20 | 2000 | 12 weeks | (4) |
| Martens | 2018 | Elderly | 24 | 24 | 1000 | 6 weeks | (37) |
| Conze | 2019 | Healthy | 32–34 | 34 | 100, 300, 1000 | 8 weeks | (32) |
| Dollerup | 2019 | Obese, insulin resistant | 20 | 20 | 2000 | 12 weeks | (5) |
| Elhassan | 2019 | Elderly | 12 | 12 | 1000 | 21 days | (33) |
| de la Rubia | 2019 | Amyotrophic lateral sclerosis | 10–14 | 10–13 | 1000* | 4 months | (43) |
| Zhou | 2020 | Heart failure | 4 | 0 | 2000 | 9 days | (26) |
| Dollerup | 2020 | Obese, insulin resistant | 20 | 20 | 2000 | 12 weeks | (6) |
| Remie | 2020 | Obese | 13 | 13 | 1000 | 6 weeks | (34) |
| Dolopikou | 2020 | Young/elderly | 12 | 12 | 500 | 2 hours | (36) |
| Simic | 2020 | Acute kidney injury | 3–5 | 0–2 | 250, 500, 1000, 2000 | 2 days | (42) |
| Veenhuis | 2021 | Ataxia telangiectasia | 24 | 0 | 25/kg (max 900) | 4 months | (27) |
| Stocks | 2021 | Healthy | 8 | 8 | 1000 | 7 days | (35) |
| Nascimento | 2021 | Obese | 8 | 8 | 1000 | 6 weeks | (39) |
| Wang | 2022 | Heart failure | 20 | 10 | 2000 | 12 weeks | (29) |
| Brakedal | 2022 | Parkinson’s disease | 15 | 15 | 1000 | 30 days | (30) |
| Wu | 2022 | Healthy | 12 | 9 | 1000 | 7 days | (31) |
| Vreones | 2022 | Elderly | 24 | 24 | 1000 | 6 weeks | (38) |
| Dellinger | 2022 | Nonalcholic fatty liver disease | 24–36 | 27–31 | 250, 500 | 6 months | (41) |
| Jensen | 2022 | Elderly | 16 | 15 | 1000 | 44 days | (8) |
| Lapatto | 2023 | Obese | 3–16 | 0 | 1000 | 5 months | (28) |
| Peluso | 2023 | Obese, insulin resistant | 20 | 20 | 2000 | 12 weeks | (7) |
*Not formally disclosed.
The pleiotropic roles of NAD+
NR is primarily of interest due to its relation to nicotinamide adenine dinucleotide (NAD+). In biochemistry textbooks, NAD+ is lauded as an important cofactor in all cell types due to its ability to supply the mitochondrial electron transport chain by transferring electrons from glycolysis and the tricarboxylic acid cycle in its reduced form, NADH. This fuels the process of oxidative phosphorylation in the mitochondria, whereby adenosine diphosphate (ADP) is converted to adenosine triphosphate (ATP). Another facet of NAD+ is its role as a cosubstrate in multiple enzymatic processes. In particular, two protein families, namely, the sirtuins and the ADP-ribosyltransferases, use NAD+ as a cosubstrate, whereby NAD+ is consumed in a process that utilizes the ADP-ribosyl (ADPR) moiety and releases nicotinamide (NAM). In addition to these two protein families, SARM1, CD38, and CD157/BST1 can consume NAD+, but in these cases, both NAM and either ADPR or cyclic ADPR are released (9, 10).
NAD+ metabolism
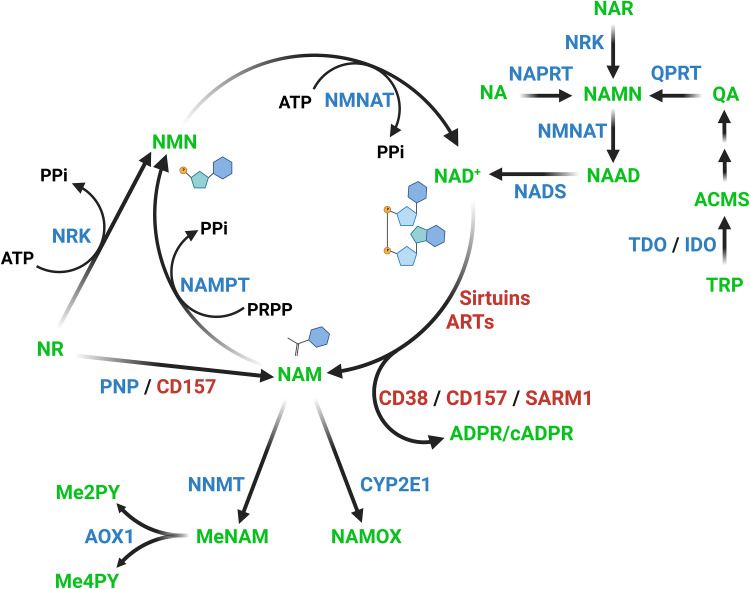
With such a multitude of NAD+ consumers, naturally there are also pathways in place to replenish the cellular NAD+ level (Fig. 1). The three canonical pathways responsible are the Preiss-Handler pathway, the de novo or kynurenine pathway, and the salvage pathway (11). The Preiss-Handler pathway converts nicotinic acid (NA) through nicotinic acid mononucleotide (NAMN) and nicotinic acid adenine dinucleotide (NAAD) to NAD+. The de novo pathway is a multistep process starting from tryptophan and converging with the Preiss-Handler pathway at the level of NAMN. Nicotinic acid riboside (NAR), the deamidated version of NR, also converges at the level of NAMN (12). The last pathway is the salvage pathway, in which NAM is converted to nicotinamide mononucleotide (NMN), and from there to NAD+. Instead of being salvaged, NAM can also be targeted for excretion by conversion to NAM N-oxide (NAMOX) or methyl-NAM (MeNAM) (13), the latter of which can be oxidized to N-methyl-2-pyridone-5-carboxamide (Me2PY) and N-methyl-4-pyridone-5-carboxamide (Me4PY) (14). The relevant enzymes for these conversions are indicated in Fig. 1. Not all enzymes are present or active in all tissues. As an example, the contribution of tryptophan and NA and, thus, of the de novo and Preiss-Handler pathways to NAD biosynthesis is primarily important in liver and kidney and has low to no contribution in muscle (15, 16). Similarly, the only enzyme that has been demonstrated to convert NAM to NAMOX, CYP2E1 (13), appears to be exclusively expressed in the liver (17).
Fig. 1. Pathways of mammalian NAD+ biosynthesis and metabolism.
Metabolites are shown in green, and enzymes are shown in blue. Major NAD+-consuming enzymes are in red, and CD157 degrades both NAD+ and NR. Abbreviations: TRP, tryptophan; ACMS, 2-amino-3-carboxymuconate semialdehyde; QA, quinolinic acid; NAMN, nicotinic acid mononucleotide; NAR, nicotinic acid riboside; NA, nicotinic acid; NAAD, nicotinic acid adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMOX, nicotinamide N-oxide; MeNAM, methyl-nicotinamide; Me2PY: N-methyl-2-pyridone-5-carboxamide; Me4PY, N-methyl-4-pyridone-5-carboxamide; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; TDO, tryptophan-2,3-dioxygenase; IDO, indoleamine-2,3-dioxygenase; QRPT, quinolinic acid phosphoribosyl-transferase; NRK, nicotinamide riboside kinase (1 and 2); NAPRT, nicotinic acid phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyltransferase (1, 2, and 3); NADS, nicotinamide adenine dinucleotide synthase; CYP2E1, cytochrome P450 2E1; NNMT, nicotinamide N-methyltransferase; AOX1, aldehyde oxidase 1; PNP, purine nucleoside phosphorylase; NAMPT, nicotinamide phosphoribosyltransferase; PRPP, phosphoribosyl pyrophosphate; PPi, inorganic pyrophosphate; ATP, adenosine triphosphate.
Absorption and incorporation of NR
At the cellular level, NR uptake is mediated by the equilibrative nucleoside transporter protein family (18). Once inside the cell, NR can be phosphorylated by the NR kinases (NRK1 and NRK2) to become NMN (19) or be deribosylated by the purine nucleoside phosphorylase to become NAM (20). In either case, the conversion in principle allows entry into the salvage pathway and thus production of NAD+. However, the incorporation of NAM into the NAD+ pool is highly tissue dependent (15), and few tissues are routinely exposed to large amounts of NR since the majority of orally administered NR is converted to NAM by CD157 in the small intestine and then to NA in an interaction with the gut microbiota (21–23). The hepatic uptake of NAD+ precursors, at least in the case of large oral doses, is primarily in the form of NA as oral gavage with both NAM and NR was much less potent in increasing hepatic NAD+ levels in NA phosphoribosyltransferase knockout mice (21). These findings are supported by studies showing that oral administration of labeled NAM and NR led to large quantities of labeled NA and other deamidated metabolites in serum and whole blood from the portal vein and in the liver (22, 23). The studies in question further demonstrated the importance of the microbiota since the rise in labeled NAD and other NAD-related metabolites in the liver was severely blunted or absent in germ-free and antibiotics-treated mice. As a result, the primary circulating NAD+ precursors resulting from oral NR administration are NA and NAM (22), and what little orally administered NR enters the blood in its native form is rapidly converted to NAM in whole blood (half-life of ~3 min), so the concentration of NR in the blood is very low (15).
HUMAN TRIALS
So far, NR has only been given to humans as an oral supplement, and this will not be reiterated for each trial in the following sections.
Trials without placebo groups
Before addressing the results of the trials performed without placebo groups, a few general comments on this type of trial is in order. Excluding placebo controls from human trials can be acceptable, but it changes how the data should be interpreted. If a single group is tested, it means that comparisons have to be performed between measurements taken at baseline and measurements taken during or after treatment. This affects the interpretation of data because any observed change during such a trial is confounded by, for example, the passage of time and the very act of partaking in the study. The latter could, for example, cause stress to the participants or inspire a change in diet or physical activity, subconsciously or otherwise. As a result, while these baseline-controlled trials can serve as pilot studies, idea-generating ventures, and limited support for findings in properly controlled trials, their results should be considered preliminary. If additional nonplacebo groups are added to the study, these may serve to elucidate whether the different groups react differently during an intervention; however, without placebo controls, the findings will still be confounded as described above. In other words, an observed difference after a specific treatment is always of potential interest, but without a matched placebo group, the difference cannot be attributed to the treatment per se. On this note, there are specific examples where the likelihood that an observed difference is a direct result of treatment is very high. Changes to the NAD metabolome after supplementation with NAD precursors is one such example, but it is worth bearing in mind that even this could, in principle, be confounded.
Trammel et al. (24) performed the first study of human NR supplementation on just a single individual. More specifically, a 52-year-old, healthy, male volunteer weighing 65 kg received 1000 mg of NR every morning for a week. Blood and urine samples were collected at multiple time points throughout this period and demonstrated a relatively stable increase in NAD+ and NAAD levels of peripheral blood mononuclear cells (PBMCs). More temporary increases were observed for NMN and NAD phosphate (NADP+) as well as for the excretion-bound MeNAM, Me2PY, and Me4PY, while NAM and ADPR levels seemed to fluctuate throughout the period. In urine, NAM, MeNAM, Me2PY, and Me4PY were increased, highlighting an increased excretion of NAD-related compounds after NR supplementation. The authors followed up with a small human trial using 100 to 1000 mg of NR. The results from the 12 participants showed that in PBMCs, MeNAM and Me2PY were consistently increased by NR during the first 24 hours after an oral NR dose, whereas NAD+ and NAAD were increased only at 24 and 8 hours, respectively, and NAM and NMN showed no change. A more robust response may have been observed had all participants received the highest dose of NR, but nonetheless, the trial suggested that orally ingested NR can increase the presence of NAD metabolites in PBMCs.
Airhart et al. (25) later assessed the general safety and efficacy of NR in eight human volunteers. It was reported that the steady-state levels of NR and NAD+ in whole blood were increased by oral NR after 8 days of escalating NR doses (250 to 2000 mg/day) followed by a single administration of 1000 mg on the ninth day. The way the steady state was calculated in this study was simply the average level of each compound during the first 12 hours after the last dose of NR. During this period, NAD+ did appear to reach a steady state in the blood, but this was not the case for NR, which seemed to peak and then trough within this interval. While still a useful measure of the average systemic exposure to NR during the 12 hours between twice-daily doses, the NR levels in blood cannot be said to have been in a steady state during this period. Nevertheless, the authors note that the level of NR in the blood was highly variable among participants and speculate that either a type of active transport of NR or its degradation in the gut could result in distinct populations of responders and nonresponders with regard to oral NR supplementation. Considering the immense importance of the microbiota for the breakdown and effective utilization of oral NR, as detailed previously (21–23), variations in the microbiota could very well explain the observed differences. A variety of additional measurements was performed on these eight participants, and decreases were observed in blood potassium, hematocrit, hemoglobin, and platelet count after the intervention, although the changes were slight and, according to the authors, not clinically relevant (25). Meanwhile, body weight, blood pressure, blood glucose, and various markers of liver and kidney damage were unaltered during the intervention.
Another pilot study by Zhou et al. (26), including many of the same researchers as above, gave four patients with heart failure escalating doses of NR (500 to 2000 mg daily) for 5 to 9 days. Whole blood NAD+ levels and basal and maximal respiration of PBMCs were increased after the intervention. In addition, mRNA expression levels of two markers of inflammation, interleukin-6 (IL-6) and IL-18, were decreased in PBMCs from these patients, while NLRP3 and IL-1B did not reach statistical significance. One potential issue with the interpretation of these data is that it appears as if the gene expression values for each individual was independently normalized to reach a posttreatment result of 1, rather than using a common normalization factor to reach a mean of 1. Since the paired t test, as used here, bases its calculation on the absolute differences between data point pairs, such a normalization strategy will result in incorrect P values. Even so, gene expression changed in the same direction for all subjects, and considering the small sample size, this is probably just as meaningful an observation.
In a study including more participants, Veenhuis et al. (27) investigated NR for the treatment of ataxia telangiectasia in 24 children (above 2 years of age) and adults. For this trial, participants were treated with NR for 4 months at a daily NR dose of 25 mg/kg up to a maximum of 900 mg. This is an atypical approach to dosing as it means that the participants with a body weight lower than 36 kg, assumedly a subgroup entirely made up of children, received a larger relative dose than those with a higher body weight. The authors used multiple scoring systems to assess the development of ataxia telangiectasia through evaluation of motor skills and speech. Of these, the ICARS and SARA systems suggested improvement during the intervention, which disappeared during the washout period, whereas 9-HPT, RDA/P-RDA, and ICS scores displayed no change. Unfortunately, as commendably brought up in the report, the longitudinal development of ataxia telangiectasia has not been studied in detail. In conjunction with the absence of a placebo group, this raises the question of whether the change can truly be attributed to NR or could result from uncontrolled parameters, such as the placebo effect or seasonal dependence of disease severity. There was no change with NR treatment in biomarkers of ataxia telangiectasia, quality of life assessment, HbA1c, or various markers of renal and hepatic function. However, NAM, MeNAM, Me2PY, and Me4PY were increased in the plasma after NR treatment.
A more recent study of NR was performed by Lapatto et al. (28) and composed of two study arms, of which one was placebo controlled. Unfortunately, the placebo-controlled arm of the study cannot be meaningfully interpreted in its current form. Despite their sample size of 4, the authors applied the Wilcoxon signed-rank test, which has a minimum sample size requirement of 6. This means that reaching statistical significance was a mathematical impossibility, for which reason the results from this arm of the study will not be discussed. The other arm of the study was focused on body mass index (BMI)–discordant monozygotic twins without placebo controls. Data from this part of the study were also analyzed mostly by the Wilcoxon signed-rank test, and a comprehensive table was provided to support the notion that NR acts irrespective of BMI. However, the sample sizes range from 3 to 16 without specification for each individual parameter. Thus, some of the reported parameters fall below the required sample size for the test, and it is unclear how many parameters are affected. Nevertheless, on the basis of their results, the authors conclude that “[…] NR acts as a potent modifier of NAD+ metabolism, muscle mitochondrial biogenesis and stem cell function, gut microbiota, and DNA methylation in humans irrespective of BMI.” This is an overinterpretation as the absence of placebo controls precludes this type of conclusion. A concrete example of the problem is that the authors attribute correlations between changes in 5'-C-phosphate-G-3' (CpG) methylation and expression of associated genes to the treatment with NR. The reason this is problematic is illustrated by one of the highlighted genes in the paper, PGC-1α, for which it is shown that hypomethylation correlates with a change in expression. However, the figure detailing PGC-1α expression shows that it does not change according to any discernible pattern during the intervention with NR. Thus, the observed correlation was caused by unmodeled, individual factors. In addition, the statement that NR is a potent modifier of the gut microbiota was made despite the absence of changes in α diversity, β diversity, or bacterial abundance at either the phylum or family level. Instead, it is based on a change in the genus Faecalibacterium, which did not reach statistical significance after false discovery rate correction. The authors proceeded to investigate the only known species of the genus, F. prausnitzii, but rather than providing a P value, they use Cohen’s d due to “great interindividual variation.” This is problematic because Cohen’s d does not assess the strength of the evidence for a difference between two means like a statistical test but only describes the difference between two means measured using standard deviations as the unit. Moreover, interindividual variation should not be a concern for the paired statistical testing used in the paper. If instead the authors meant that it was due to “great interindividual variation in response,” it effectively means that F. prausnitzii did not change abundance according to any pattern during the intervention. Lastly, the reported results of the metabolomics analysis were based on uncorrected P values despite the availability of properly false discovery rate–corrected values in the provided table. The issues outlined here were exacerbated by an unfortunate tendency in the report to attribute beneficial changes to the effect of NR, whereas detrimental changes, such as the increase in insulin resistance and bodyweight, were glossed over or attributed to the effect of time.
Collectively, most of the reports on human NR trials without a placebo group are cognizant and honest about the limitations imposed by this inferior design, and, together, they indicate that NR is safe to consume, that multiple NAD-related metabolites are increased in blood after supplementation with NR, and that NR potentially reduces inflammation and improves ataxia telangiectasia.
Parallel-design trials with placebo control
In a follow-up trial to the heart failure pilot study (26), Wang et al. (29) split patients with clinically stable heart failure into unequal groups receiving escalating doses of NR (500 to 2000 mg/day, n = 20) or placebo (n = 10) for 12 weeks. A range of prespecified variables of interest, including potassium, glucose, homeostatic model assessment for insulin resistance (HOMA-IR), blood pressure, body weight, hematocrit, hemoglobin, and platelet count, as well as renal and hepatic markers were not affected by NR in comparison to the placebo group. Whole blood NAD+ levels were stably increased after 12 weeks of NR treatment, albeit with highly individual magnitude, and appeared to have reached this new steady state already in week 4—1 week after switching to the largest NR dose. Whole blood NR did not reach a new steady state during this period but did increase acutely, as had been previously observed and misrepresented (25). The ratio between NAD post- and preintervention correlated well with the log-transformed post/pre ratio of both basal and maximal respiration of PBMCs isolated from NR-receiving patients (29). Similarly, the log-transformed post/pre ratio of NLRP3 mRNA correlated with the post/pre ratio of NAD+, although other inflammatory markers did not reach statistical significance in this regard. Direct comparisons of basal and maximal respiration as well as of inflammatory marker levels between placebo and NR groups were not reported. The lack of such comparisons is highly unfortunate since it follows that the effect of NR on respiration and inflammation was not properly assessed.
In addition to heart failure, NR has also been tested in the setting of Parkinson’s disease by Brakedal et al. (30). In this study, 30 newly diagnosed (mainly male) patients were split into two groups receiving either NR (1000 mg/day) or placebo for 30 days. Treatment with NR increased NAD levels in the brain, as assessed by magnetic resonance spectroscopy. In cerebrospinal fluid, Me2PY levels were increased by NR treatment, but other NAD-related metabolites were below the limit of detection. In muscle, NR treatment increased the levels of NAAD, NAMOX, Me2PY, and Me4PY. MeNAM was also increased in muscle compared to baseline, but this was not unique to the NR-treated patients. The presence of NAMOX in skeletal muscle is unexpected considering that no NAMOX-producing enzymes have been identified in this tissue (13, 17). In PBMCs, NAAD and MeNAM were increased by NR. In both muscle and PBMCs, a host of targets were assessed, including NAD+, NADP+, NA, NR, NAM, NMN, NAMN, and ADPR, but none of these changed with NR treatment. Nonetheless, the authors reported an NR-associated change in cerebral metabolism, and when only the subset for whom cerebral NAD was increased by NR treatment was analyzed, treatment could be associated with clinical improvement of Parkinson’s disease. This type of post hoc exploratory subsetting is arguably not appropriate, but the finding is interesting and supports the previously published notion of responders and nonresponders when it comes to oral administration of NR (25, 29). In patients with Parkinson’s disease, a reduction in cerebrospinal fluid inflammatory markers was observed with NR treatment (30). Serum inflammatory markers were also reduced but not uniquely in NR-treated patients. Nevertheless, on the basis of RNA sequencing data, NR affected the expression of 58 genes in muscle and 13 genes in PBMCs. The differentially expressed genes in muscle were generally associated with proteasomal function and RNA transport, whereas those in PBMCs were associated with mitochondrial, ribosomal, lysosomal, and proteasomal pathways. Intriguingly, type I interferon signaling was also represented in the supplemental lists for both muscle and PBMCs. This supports a separate report by Wu et al. (31) where RNA sequencing was performed on isolated monocytes from people supplemented for 7 days with NR (1000 mg/day; n = 12) or placebo (n = 9). Specifically, these results suggested that NR supplementation reduced type 1 interferon signaling and autophagy. Samples of whole blood from these volunteers confirmed that NR increased NAD+, NAAD, ADPR, and Me4PY.
The largest trial of NR in humans to date was conducted by Conze et al. (32) and tested three different doses of NR (100, 300, and 1000 mg/day) against placebo in 140 (n = 32 to 34 for the analysis) healthy, middle-aged volunteers for 8 weeks. This study was primarily concerned with the safety of NR administration to humans. The results showed a dose-dependent increase of NAD+ in whole blood as well as increases of NAM and MeNAM in plasma and of MeNAM and Me2PY in urine. The authors observed some changes in hematology during the study, but these differences were generally only from baseline rather than between NR and placebo groups. Similarly, there were no detectable differences between groups in markers of hepatic and renal function, potassium levels, triglycerides, or cholesterol levels, and the waste product from methylation of NAM, homocysteine, was unchanged in plasma. Blood pressure and heart rate likewise remained unaltered.
Another relatively well-powered trial by Dollerup et al. (4–7) resulted in several published reports generally concerned with metabolism. For this trial, 40 middle-aged, obese, insulin-resistant but otherwise healthy men received either placebo or NR (2000 mg/day) for 12 weeks. In the first report, NR treatment was shown to not affect body composition, and a hyperinsulinemic euglycemic clamp did not detect differences in insulin sensitivity (4). Moreover, fasting glucose, HbA1c, cholesterol, and alanine aminotransferase (ALT) in plasma remained unaffected. In contrast, plasma triglycerides and the levels of NR, NAM, NAMOX, MeNAM, Me2PY, Me4PY, and NAR in urine were increased by NR. While NAR had the largest fold increase in urine, MeNAM, Me2PY, and Me4PY constituted the bulk of the excreted NAD-related metabolites. In the follow-up report, with a relatively large topical overlap, it was reported that NR did not affect glucose tolerance or the levels of insulin, glucagon, C-peptide, glucagon-like peptide 1 (GLP-1), or gastric inhibitory polypeptide (GIP) during a glucose tolerance test (GTT) (5). Likewise, eight separate indices of β cell function showed no effect of NR, and plasma levels of 15 bile acids and the adipokine, adipsin, remained unaltered. The third report had a more muscle-centric focus and reported that NAD+, NADH, NADP+, and NADPH were unchanged in skeletal muscle following NR supplementation (6). Nicotinamide phosphoribosyltransferase (NAMPT) protein was slightly reduced in the NR group, but NRK2, SIRT3, and the total amount of acetylated proteins remained unchanged. mRNA levels of NMRK1, NMRK2, and PGC1α were similarly unaffected by NR treatment, and while insulin increased the phosphorylation level of glycogen synthase and mammalian target of rapamycin (mTOR), NR treatment did not augment this or the levels of GLUT4 and HK2. In addition, NR did not affect mitochondrial respiration, abundance, fractional area, or network organization, and lipid deposition in muscle remained unaffected. Lastly, the most recent report based on this study was performed by Peluso et al. (7) and investigated how orally supplemented NR affects the gut microbiota by analyzing stool samples from before and after treatment. The results showed that NR did not induce differential abundance at any of the assessed taxonomic levels. Together, the differences associated with NR treatment in this study were that it increased plasma triglycerides and reduced muscle NAMPT slightly, and that NAD metabolites were effectively excreted via the urine.
Crossover-design trials with placebo control
The findings described above regarding mitochondrial function and bioavailability of NR to muscle were generally supported by a previous study by Elhassan et al. (33). In this study, 12 elderly and healthy men were supplemented with 1000 mg of NR daily for a period of 21 days. NAD metabolites were measured in whole blood, muscle, and urine 14 hours after the last dose was ingested. MeNAM, Me2PY, Me4PY, and NAAD were increased by NR in both muscle and blood, but despite 14 and 15 measured metabolites, respectively, no further changes were observed in muscle, although both NAD+ and NMN increased in the blood. In contrast, the excretion of NAD+, NMN, NAM, NR, NAR, and NAMOX as well as MeNAM, Me2PY, and Me4PY through urine was increased by NR supplementation. The authors found no differences in muscle mitochondrial function, substrate utilization, or blood flow as a result of the supplementation. Likewise, grip strength, glucose tolerance as well as nonesterified fatty acid levels, and respiratory exchange ratio (RER) during the GTT were unaltered by NR. In the biochemical analysis, various markers of hepatic, renal, and thyroid functions were all unchanged. This was also true for glucose, insulin, and the resulting HOMA-IR as well as cholesterol levels, potassium, and platelet counts to name just a few from a comprehensive list of unchanged blood variables. The authors also reported that 1088 genes (of which ~885 were protein coding) had changed expression in muscle as a result of NR supplementation. It can be expected, however, that most of the detected differences in gene expression were type I errors due to multiple testing without proper correction of P values. Nonetheless, the levels of the inflammatory markers, IL-6, IL-5, IL-2, and tumor necrosis factor–α, in serum were decreased by NR, in general agreement with several other studies (26, 29–31). Unfortunately, it appears that the results after both phases were compared to the phase 1 baseline. On that topic, the authors note that the effect of NR had a tendency to carry over beyond the washout period, which affected the measurements after placebo in the cases where NR was given first. The truth of this statement can of course not be verified since the phase 2 baseline (after washout) was not included in the analysis.
Remie et al. (34) used a similar setup, albeit with 6 weeks of 1000 mg of NR, on 13 overweight/obese, middle-aged people. The results showed an NR-induced increase in MeNAM and NAAD in muscle, whereas NAD+, NADH, NADP, NADPH, NAM, and NMN were unaffected. NR administration did not result in any changes to muscle mitochondrial respiration, hepatic lipid deposition, or various measures of cardiac function including blood pressure and heart rate. Similarly unaffected were a range of inflammatory markers as well as cholesterol, triglycerides, and glucose in the blood. There was no detectable effect of NR on whole-body or tissue-specific insulin sensitivity as measured by a hyperinsulinemic euglycemic clamp, and substrate utilization was likewise unchanged. They did find a decrease in fat mass and a corresponding increase in fat-free mass after the NR phase. The latter of these was associated with an increased sleeping metabolic rate. However, especially considering the small effect sizes, it would have been prudent to analyze body composition at day 0 in addition to the measurement at day 40. This would have effectively ruled out the possibility that the data were confounded by changes taking place during the washout period. Nonetheless, the authors used magnetic resonance spectroscopy to demonstrate that NR decreased muscle acetylcarnitine in the rested, 3-hour fasted state during the early evening; however, in muscle samples taken 5 days later, after an overnight fast, NR was shown to increase acetylcarnitine. It bears mentioning that all of the significant differences reported in this study had small effect sizes and that the strength of the evidence was not particularly compelling with P values ranging from 0.02 to 0.05 despite the increased statistical power afforded by the paired analysis. Thus, whether resulting from the two different types of assessment, the differences in fasting period, or simply day-to-day fluctuations, it seems clear that especially the results on acetylcarnitine should be viewed with some amount of skepticism. The rest of the acylcarnitine panel, including free carnitine, showed no effects of NR.
Maintaining the somewhat muscle-centric focus, Stocks et al. (35) investigated the effects of 7 days of 1000 mg of NR supplementation on acute exercise in eight recreationally active men. In this setting, NR slightly increased the levels of NAMN and NAR and markedly increased the levels of Me2PY and Me4PY in muscle, whereas NR, NAD+, NADP+, NAM, NMN, MeNAM, and ADPR remained unchanged. No other NR-associated changes were reported. Basic metabolic rate and substrate utilization and plasma levels of nonesterified fatty acids, glycerol, glucose, and lactate were unchanged during rest and exercise. As were mitochondrial function and protein levels in the rested state. Likewise, acetylation levels of sirtuin targets and phosphorylation levels of multiple exercise-related proteins during rest and exercise were unaffected by NR treatment. mRNA levels of genes associated with NAD metabolism were generally unaffected by NR, although there was a tendency for NR to reduce an exercise-induced increase in NNMT expression. This study had a relatively small sample size considering the commendable but intricate study design, but this was mitigated somewhat by the crossover nature of the study, and their findings are in general alignment with previous studies (6, 33, 34).
In contrast, Dolopikou et al. (36) demonstrated some potential with acute delivery of NR. In this study, 12 young and 12 old participants received 500 mg of NR 2 hours before a performance test. Blood samples were collected immediately before supplementation and again immediately before performance testing. NADH and NADPH were convincingly increased in blood cells from both the young and old group, since in both cases, the reponse to NR were different from placebo and the levels increased from baseline. Moreover, VO2max lactate and isokinetic lactate were increased from baseline in both young and old individuals, but this was the case in response to both placebo and NR, albeit the change was larger in the NR-treated groups in the case of isokinetic lactate. The remainder of the tested parameter changes were generally not as convincing, but there were some potential effects. F2 isoprostanes were reduced compared to baseline but not compared to placebo treatment, and glutathione (reduced form) tended to increase both compared to baseline and placebo control. Superoxide dismutase level was affected differently in old compared to young individuals, but this was seemingly driven by a difference between the response to placebo and NR in young participants and was potentially a result of large variations in the baseline level. Glutathione peroxidase was reduced by NR in the young participants only. Lactate dehydrogenase levels responded differently to placebo and NR treatment in the old participants, but this difference was driven by a change with placebo. In general, many P values were close to the conventional cutoff of 0.05, and the authors decided to use eight separate t tests for each parameter without correcting P values, so it is uncertain how many of these were false positives. Given the experimental setup, analysis of variance (ANOVA) accounting for both experimental factors and the repeated measurements simultaneously would have increased the interpretability. For the functional readouts, the authors reported that isometric peak torque was increased, and the fatigue index was reduced specifically for the old participants, while concentric peak torque and VO2max were unaffected. For the latter, it should be mentioned that the reported variation (SEM > 200) seems to be incorrect considering that VO2max cannot go below 0 and that the highest levels ever recorded remaint shy of 100 ml kg−1 min−1. Nevertheless, the interpretation suggests a performance-enhancing effect of NR in old individuals, but the foundation for interpretation could have been improved considerably by using ANOVAs.
In a study by Martens et al. (37), 24 middle-aged and elderly volunteers were supplemented daily with 1000 mg of NR for 6 weeks. NAD-related metabolites were measured in PBMCs and showed that NAD+ and NAAD were increased by NR, whereas NADP, NAM, and NMN were not. Intriguingly, NR supplementation reduced blood pressure and arterial stiffness, which visually was greatest in participants with higher-than-normal levels of these variables. These findings have not been supported by other reports (25, 29, 32, 34), but none of these focused specifically on the treatment of elevated blood pressure or atherosclerosis. There were no observable changes in overall motor function, exercise performance, or measures of glucose regulation, substrate utilization, markers of hepatic and renal function, or cholesterol levels (37). Likewise, potassium, platelet count, hemoglobin, and hematocrit were unchanged by NR treatment. Newer experiments by Vreones et al. (38) on samples from the same original study focused on extracellular vesicles isolated from plasma and enriched for neuronal origin. Only 10 of the participants had quantifiable levels of NAD+ in these vesicles, but for this subgroup, NAD+ levels were increased after NR treatment. NADH was measured in 22 participants and remained unchanged by the treatment. Changes in NAD+ levels were found to positively correlate with abundance changes in pAKT, tGSK3β, pGSK3β, tp70S6K, pp70S6K, pERK1/2, and pJNK. Negative correlations were found between NADH changes and the same targets except for tGSK3β. Curiously, only the 10 individuals for which NAD+ was quantifiable were used for the investigation of NADH correlations. Investigating the phosphorylation status and total levels of IRS-1, AKT, GSK3β, and p70S6 revealed no effect of NR, either in the full group of participants or in the “responder” subgroup (the nine participants for whom NAD+ levels were increased by NR). The phosphorylation status of ERK1/2, JNK, and p38 also remained unchanged by NR when investigating the whole group, but pERK1/2 and pJNK were reduced specifically in the responder subgroup. Lastly, there were no correlations between changes in NAD+ or NADH and the markers of Alzheimer’s disease: Aβ42, total Tau, and p-Tau-181, and their total levels were also unaffected by NR when the whole group was assessed. Aβ42 was, however, reduced with NR among responders. Overall, this experiment highlights an interesting method for future studies, but the authors are right to report their other findings with caution since their observed differences with NR are dependent on stratification of the test group.
Nascimento et al. (39) assessed effects of NR at a dose of 1000 mg/day for its ability to enhance brown adipose tissue activity after 6 weeks of supplementation in eight overweight/obese volunteers. This was done by measuring the uptake of radiolabeled 2-deoxyglucose in brown adipose tissue as well as energy expenditure and nonshivering thermogenesis in response to cold exposure. NR did not affect these readouts.
Clinical trials of NR in combination with PT
In addition to the human trials of NR alone, there are also five reports using a combination between NR and pterostilbene (PT) (8, 40–43). These studies all used parallel designs with placebo control but did not include test groups where NR and PT were assessed individually. As a result, aside from perhaps the metabolites directly associated with either compound, observed effects cannot be attributed to NR or PT specifically.
The first study of combined NR and PT (NRPT) in humans was conducted by Dellinger et al. (40) and focused on the safety of the supplementation and the supposed ability to increase NAD+ levels in whole blood of 120 elderly volunteers. NRPT supplement was administered as 250 mg of NR and 50 mg of PT or as double these doses daily for 60 days and was compared against placebo. Both doses of NRPT increased whole blood NAD+ levels in comparison to baseline and placebo, while other metabolites were not tested. In the case of diastolic blood pressure and ALT, the authors reported a decrease from baseline specifically in the low-dose NRPT group. Likewise, in the case of two tests of motor function, the high-dose NRPT group showed an improvement from baseline. Nevertheless, in all these cases, no difference could be observed between the NRPT and placebo groups, so the evidence is not compelling. When total cholesterol was assessed, the delta value was significantly higher in the high-dose NRPT group than in the placebo group. The same was true for both NRPT doses in relation to low-density lipoprotein (LDL) levels. This perhaps indicates a detrimental effect of NRPT, albeit the clinical relevance is uncertain since none of the groups changed from baseline in these parameters. Blood triglyceride levels were lowered in the placebo group, and the delta value was significantly different from that of the low-dose NRPT group. This perhaps indicates that the reduction was a natural progression that was prevented by NRPT, although this was not adressed in the report. The authors then stratified the individuals by BMI post hoc and showed that the change in LDL was different from baseline in the case of overweight receivers of either placebo or low-dose NRPT and in the case of normal weight and overweight but not obese, receivers of high-dose NRPT. The clinical significance of these findings is uncertain, but it is interesting that the observed tendencies of LDL, total cholesterol, and blood pressure were in agreement with the only investigation of solo treatment with PT (44). Together, these findings should prompt a thorough investigation of the potentially detrimental effect of PT on human cholesterol levels, as others have also pointed out (45). Other variables, including systolic blood pressure, hemoglobin, platelet count, potassium, and fasting glucose, were unaltered by NRPT supplementation (40).
A substantially longer study using the same general setup was conducted by Dellinger et al. (41) to test the effect of 6 months of NRPT treatment on 111 adults with nonalcoholic fatty liver disease. The primary end point of hepatic fat fraction (HFF) was improved from baseline in placebo and low-dose NRPT groups, but no differences were found in final values or the change from baseline between any of the groups. Fatty liver index, HOMA-IR, and high-sensitivity C-reactive protein (hsCRP) were significantly different from baseline, specifically in the low-dose NRPT group, but as with HFF, neither the final values nor the changes from baseline differed between groups, calling into question the clinical relevance. The authors proceeded to stratify the population based on a baseline HFF of 27%. Subgroup analysis demonstrated that in the population with lower than 27% HFF at baseline, only the group receiving low-dose NRPT reduced HFF and fatty liver index, and this was significantly different from both baseline and the placebo group. In the population with higher than 27% HFF at baseline, the placebo group demonstrated reduced HFF compared with baseline, but no differences from the other groups in either this change or the final values were reported. The rationale provided for the choice to stratify at an HFF level of 27% was to specifically remove certain individuals in the placebo group from the statistical analysis because of an observation that more people with high HFF had been randomly assigned to this group. While the authors are clear that this is a post hoc exploratory analysis, it still raises the question of how the results would look if another cutoff point had been arbitrarily chosen. Moreover, this stratification created relatively large differences in sample size between compared subgroups. These reservations challenge the validity of the analysis. Nevertheless, levels of ALT and γ-glutamyltransferase were seemingly reduced with NRPT without arbitrary stratification although only to a small extent. In addition, one specific ceramide (14:0) was significantly reduced from baseline in the low-dose NRPT group, and this change was significantly different from the change in the placebo group. However, visually, the level of this ceramide was increased in the low-dose NRPT group at baseline, and a statistical comparison at baseline was not reported, so this result may be confounded. Triglycerides and total ceramides were unchanged by NRPT, and unlike the previous study (40), total cholesterol and total LDL were not affected either (41). No measurements of NAD-related metabolites were performed.
Simic et al. (42) assessed the short-term safety of NRPT specifically for patients with acute kidney injury. For this study, 24 patients were divided into eight “groups” that received either placebo or NRPT for 2 days at a daily dose of 250 mg of NR and 50 mg of PT or two, three, or four times this amount. This meant only one placebo-receiving participant and five NRPT-receiving participants for each dose. Moreover, there were dropouts such that only two placebo recipients were included in the final analysis. These two were therefore combined to constitute a single group. Nevertheless, when all NRPT groups were combined, they demonstrated a significant relative increase from baseline in whole blood NAD+ levels after 48 hours, whereas there was a relative decrease for the placebo participants. However, the average baseline NAD+ level of the NRPT groups was ~50% lower from the outset than that of the placebo participants. No clinical markers were significantly affected by NRPT treatment, but this could not be expected with such a low-powered study design. Still, it is interesting that after the 2 days, unlike NRPT receivers, the two placebo-receiving patients had marked nominal reduction in average estimated glomerular filtration rate and increased blood area nitrogen in comparison to baseline. Unfortunately, a much larger study would be necessary to draw any conclusions.
NRPT has also been used by de la Rubia et al. (43) to treat patients with amyotrophic lateral sclerosis. In this study, daily supplementation of 1200 mg of EH301 (NRPT) was tested against placebo in 32 adult patients (43). The specific ratio between NR and PT was seemingly not disclosed, but considering the involvement of personnel from Elysium Health, the standard 5:1 ratio was likely used, resulting in 1000 mg of NR and 200 mg of PT per day. NRPT treatment was associated with a general improvement in amyotrophic lateral sclerosis, assessed using a scoring index, and with protection of pulmonary function and muscle strength. The treatment was further associated with a reduction in fat mass and an increase in muscle mass. NAD and PT-related metabolites as well as biochemical markers were not reported for this trial.
The most recently published study was conducted by Jensen et al. (8) and tested NRPT (1000 mg of NR and 200 mg of PT per day) in a setting of experimentally induced muscle injury in 32 elderly volunteers. For this study, NRPT supplementation was initiated 14 days before the induction of muscle injury, and participants had their last muscle biopsy 30 days after injury. Reported results demonstrated an NRPT-induced increase of NAD+, NAAD, Me2PY/Me4PY, and PT sulfate, a metabolite of PT, in whole blood both before and after injury. Multiple other metabolites were measured but not reported since the measurements in blood were used only to confirm participant compliance and uptake. Measurements of NAD+, NADH, NADP+, and NADPH in muscle revealed no change with NRPT treatment, but no additional NAD-related metabolites were assessed in this tissue. Muscle PT sulfate was significantly increased in the NRPT group as a whole but was below the limit of detection in some individuals. Blood markers, including ALT, platelets, and hemoglobin were unaffected by NRPT treatment. Likewise, NRPT treatment did not improve muscle function, satellite cell response, or the histological appearance of the muscles after injury. There was a difference between NRPT and placebo groups in the muscle fiber area distribution, but this was present before supplementation.
SUMMARY OF CLINICAL FINDINGS
The general finding in human NR trials, especially those concerned with metabolism, is that NR supplementation has very few clinically relevant effects. That said, it bears mentioning that only ~7 years have passed since the first report of an exploratory human NR trial was published, and since then, many of the conducted studies have been focused on safety, used small sample sizes, had generally healthy, albeit in some cases elderly or obese, participants, or all of the above. The single somewhat reproducible beneficial effect of NR in these trials has been a reduction of inflammatory markers in whole blood or immune cells (26, 29–31, 33). In addition, oral NR supplementation has been shown multiple times to increase NAD+ and a range of its related metabolites in whole blood and, occasionally, PBMCs. Data on the NAD-boosting effect of NR in other tissues than blood is unfortunately limited to muscle and brain at this time, and while the report regarding the brain is highly encouraging for further study (30), there is no indication that oral NR increases muscle NAD+ levels.
Unfortunately, some of the human NR trial reports are rather uncritical of their own results with a propensity to overinterpret results. As an example of this, increased levels of NAAD, MeNAM, Me2PY, and Me4PY are in some reports taken as indicators that NAD+ metabolism is increased. However, these NAD-related metabolites do not necessarily indicate flux through NAD+. As mentioned previously, the primary circulating NAD+ precursors after oral NR administration are NA and NAM (22). NAM can, in principle, be salvaged and incorporated in the NAD+ pool, but it can also be directly methylated and excreted. The latter appears to occur to a rather large extent, as the increase in urinary MeNAM, Me2PY, and Me4PY is well established (4, 32, 33) and occurs shortly after NR supplementation (24). This is particularly important in relation to muscle, because while NAM is efficiently absorbed into the muscle, it is not readily converted to NAD+ (15, 46, 47). As a result, observed increases in MeNAM, Me2PY, and Me4PY in muscle are more likely indications of an overload of NAM that needs to be excreted rather than of increased flux through NAD+. Meanwhile, NA can be incorporated into the NAD+ pool via the Preiss-Handler pathway in various tissues, but utilization of NA in muscle is low or nonexistent (15, 16). Even if the observed accumulation of NAAD in skeletal muscle is a true reflection of myocyte NAAD and not just an artifact of tissue heterogeneity, its ability to supply the NAD+ pool is highly uncertain as the activity of NADS, the enzyme responsible for transforming NAAD to NAD+, appears to be lacking in this tissue (16). It is notable that despite this, oral NA treatment of patients with mitochondrial myopathy and NAD+ deficiency has been demonstrated to rescue muscle NAD+ levels and improve muscle strength, mitochondrial function, and exercise performance (48). Similarly, multiple preclinical investigations of NR have led to improved mitochondrial function in muscle (46, 49, 50), but as detailed previously, human studies of NR supplementation have failed to replicate this finding (6, 33–35). The use of relatively healthy individuals for these NR studies could potentially explain the discrepancy, so future human trials may benefit from identifying a population with NAD+ deficiency.
Thus, despite some reservations regarding the current literature, it is clear that oral treatment with NR in humans has not been fully explored. NR has shown some promise in the reduction of inflammatory markers in blood (26, 29–31, 33), as well as in the treatment of diverse severe diseases (26, 27, 29, 30) and, potentially, hypertension (37). These findings all deserve rigorous follow-up studies. Likewise, NRPT may be beneficial for patients with acute kidney injury (42) or amytrophic lateral sclerosis (43), although further studies are needed to ascertain this, as well as to delineate the individual effects of these two compounds.
Acknowledgments
Funding: This work was supported by a PhD scholarship to M.V.D. from the Danish Diabetes Academy, which was funded by the Novo Nordisk Foundation (NNF17SA0031406). M.V.D. and J.T.T. are supported by Novo Nordisk Foundation Center for Basic Metabolic Research (CBMR). CBMR is an independent Research Center at the University of Copenhagen, which is partially funded by an unrestricted donation from the Novo Nordisk Foundation (NNF18CC0034900).
Author contributions: M.V.D. wrote the first draft, which was then discussed and edited by both M.V.D. and J.T.T. to produce the final version.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.B. Yulug, O. Altay, X. Li, L. Hanoglu, S. Cankaya, S. Lam, H. A. Velioglu, H. Yang, E. Coskun, E. Idil, R. Nogaylar, A. Ozsimsek, C. Bayram, I. Bolat, S. Oner, O. O. Tozlu, M. E. Arslan, A. Hacimuftuoglu, S. Yildirim, M. Arif, S. Shoaie, C. Zhang, J. Nielsen, H. Turkez, J. Boren, M. Uhlen, A. Mardinoglu, Combined metabolic activators improve cognitive functions in Alzheimer's disease patients: A randomised, double-blinded, placebo-controlled phase-II trial. Transl. Neurodegener. 12, 4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O. Altay, M. Arif, X. Li, H. Yang, M. Aydin, G. Alkurt, W. Kim, D. Akyol, C. Zhang, G. Dinler-Doganay, H. Turkez, S. Shoaie, J. Nielsen, J. Boren, O. Olmuscelik, L. Doganay, M. Uhlen, A. Mardinoglu, Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19. Adv. Sci. 8, 2101222 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C. Zhang, E. Bjornson, M. Arif, A. Tebani, A. Lovric, R. Benfeitas, M. Ozcan, K. Juszczak, W. Kim, J. T. Kim, G. Bidkhori, M. Stahlman, P. O. Bergh, M. Adiels, H. Turkez, M. R. Taskinen, J. Bosley, H. U. Marschall, J. Nielsen, M. Uhlen, J. Boren, A. Mardinoglu, The acute effect of metabolic cofactor supplementation: A potential therapeutic strategy against non-alcoholic fatty liver disease. Mol. Syst. Biol. 16, e9495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O. L. Dollerup, B. Christensen, M. Svart, M. S. Schmidt, K. Sulek, S. Ringgaard, H. Stodkilde-Jorgensen, N. Moller, C. Brenner, J. T. Treebak, N. Jessen, A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 108, 343–353 (2018). [DOI] [PubMed] [Google Scholar]
- 5.O. L. Dollerup, S. A. J. Trammell, B. Hartmann, J. J. Holst, B. Christensen, N. Moller, M. P. Gillum, J. T. Treebak, N. Jessen, Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J. Clin. Endocrinol. Metab. 104, 5703–5714 (2019). [DOI] [PubMed] [Google Scholar]
- 6.O. L. Dollerup, S. Chubanava, M. Agerholm, S. D. Sondergard, A. Altintas, A. B. Moller, K. F. Hoyer, S. Ringgaard, H. Stodkilde-Jorgensen, G. G. Lavery, R. Barres, S. Larsen, C. Prats, N. Jessen, J. T. Treebak, Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 598, 731–754 (2020). [DOI] [PubMed] [Google Scholar]
- 7.A. A. Peluso, A. T. Lundgaard, P. Babaei, F. Mousovich-Neto, A. L. Rocha, M. V. Damgaard, E. G. Bak, T. Gnanasekaran, O. L. Dollerup, S. A. J. Trammell, T. S. Nielsen, T. Kern, C. B. Abild, K. Sulek, T. Ma, Z. Gerhart-Hines, M. P. Gillum, M. Arumugam, C. Orskov, D. McCloskey, N. Jessen, M. J. Herrgard, M. A. S. Mori, J. T. Treebak, Oral supplementation of nicotinamide riboside alters intestinal microbial composition in rats and mice, but not humans. NPJ Aging 9, 7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. B. Jensen, O. L. Dollerup, A. B. Moller, T. B. Billeskov, E. Dalbram, S. Chubanava, M. V. Damgaard, R. W. Dellinger, K. Trost, T. Moritz, S. Ringgaard, N. Moller, J. T. Treebak, J. Farup, N. Jessen, A randomized placebo-controlled trial of nicotinamide riboside+pterostilbene supplementation in experimental muscle injury in elderly subjects. JCI Insight 7, e158314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Z. Y. Zhao, X. J. Xie, W. H. Li, J. Liu, Z. Chen, B. Zhang, T. Li, S. L. Li, J. G. Lu, L. Zhang, L.-h. Zhang, Z. Xu, H. C. Lee, Y. J. Zhao, A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15, 452–466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.V. Quarona, G. Zaccarello, A. Chillemi, E. Brunetti, V. K. Singh, E. Ferrero, A. Funaro, A. L. Horenstein, F. Malavasi, CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytometry B Clin. Cytom. 84, 207–217 (2013). [DOI] [PubMed] [Google Scholar]
- 11.A. J. Covarrubias, R. Perrone, A. Grozio, E. Verdin, NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.V. Kulikova, K. Shabalin, K. Nerinovski, C. Dölle, M. Niere, A. Yakimov, P. Redpath, M. Khodorkovskiy, M. E. Migaud, M. Ziegler, A. Nikiforov, Generation, release, and uptake of the NAD precursor nicotinic acid riboside by human cells. J. Biol. Chem. 290, 27124–27137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A. M. Real, S. Hong, P. Pissios, Nicotinamide N-oxidation by CYP2E1 in human liver microsomes. Drug Metab. Dispos. 41, 550–553 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R. L. Felsted, S. Chaykin, N1-methylnicotinamide oxidation in a number of mammals. J. Biol. Chem. 242, 1274–1279 (1967). [PubMed] [Google Scholar]
- 15.L. Liu, X. Su, W. J. Quinn 3rd, S. Hui, K. Krukenberg, D. W. Frederick, P. Redpath, L. Zhan, K. Chellappa, E. White, M. Migaud, T. J. Mitchison, J. A. Baur, J. D. Rabinowitz, Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 27, 1067–1080.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.V. Mori, A. Amici, F. Mazzola, M. Di Stefano, L. Conforti, G. Magni, S. Ruggieri, N. Raffaelli, G. Orsomando, Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLOS ONE 9, e113939 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. Uhlén, L. Fagerberg, B. M. Hallström, C. Lindskog, P. Oksvold, A. Mardinoglu, Å. Sivertsson, C. Kampf, E. Sjöstedt, A. Asplund, I. Olsson, K. Edlund, E. Lundberg, S. Navani, C. A.-K. Szigyarto, J. Odeberg, D. Djureinovic, J. O. Takanen, S. Hober, T. Alm, P.-H. Edqvist, H. Berling, H. Tegel, J. Mulder, J. Rockberg, P. Nilsson, J. M. Schwenk, M. Hamsten, K. von Feilitzen, M. Forsberg, L. Persson, F. Johansson, M. Zwahlen, G. von Heijne, J. Nielsen, F. Pontén, Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 18.A. Kropotov, V. Kulikova, K. Nerinovski, A. Yakimov, M. Svetlova, L. Solovjeva, J. Sudnitsyna, M. E. Migaud, M. Khodorkovskiy, M. Ziegler, A. Nikiforov, Equilibrative nucleoside transporters mediate the import of nicotinamide riboside and nicotinic acid riboside into human cells. Int. J. Mol. Sci. 22, 1391 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.P. Bieganowski, C. Brenner, Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 (2004). [DOI] [PubMed] [Google Scholar]
- 20.P. Belenky, K. C. Christensen, F. Gazzaniga, A. A. Pletnev, C. Brenner, Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J. Biol. Chem. 284, 158–164 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.K. Yaku, S. Palikhe, H. Izumi, T. Yoshida, K. Hikosaka, F. Hayat, M. Karim, T. Iqbal, Y. Nitta, A. Sato, M. E. Migaud, K. Ishihara, H. Mori, T. Nakagawa, BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat. Commun. 12, 6767 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.K. Chellappa, M. R. McReynolds, W. Lu, X. Zeng, M. Makarov, F. Hayat, S. Mukherjee, Y. R. Bhat, S. R. Lingala, R. T. Shima, H. C. Descamps, T. Cox, L. Ji, C. Jankowski, Q. Chu, S. M. Davidson, C. A. Thaiss, M. E. Migaud, J. D. Rabinowitz, J. A. Baur, NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 34, 1947–1959.e5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.I. Shats, J. G. Williams, J. Liu, M. V. Makarov, X. Wu, F. B. Lih, L. J. Deterding, C. Lim, X. Xu, T. A. Randall, E. Lee, W. Li, W. Fan, J. L. Li, M. Sokolsky, A. V. Kabanov, L. Li, M. E. Migaud, J. W. Locasale, X. Li, Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 31, 564–579.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.S. A. J. Trammell, M. S. Schmidt, B. J. Weidemann, P. Redpath, F. Jaksch, R. W. Dellinger, Z. Li, E. D. Abel, M. E. Migaud, C. Brenner, Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 7, 12948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.S. E. Airhart, L. M. Shireman, L. J. Risler, G. D. Anderson, G. A. Nagana Gowda, D. Raftery, R. Tian, D. D. Shen, K. D. O'Brien, An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLOS ONE 12, e0186459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.B. Zhou, D. D. Wang, Y. Qiu, S. Airhart, Y. Liu, A. Stempien-Otero, K. D. O'Brien, R. Tian, Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Invest. 130, 6054–6063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S. J. G. Veenhuis, N. J. H. van Os, A. Janssen, M. van Gerven, K. L. M. Coene, U. F. H. Engelke, R. A. Wevers, G. H. Tinnevelt, R. Ter Heine, B. P. C. van de Warrenburg, C. M. R. Weemaes, N. Roeleveld, M. A. A. P. Willemsen, Nicotinamide riboside improves ataxia scores and immunoglobulin levels in ataxia telangiectasia. Mov. Disord. 36, 2951–2957 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.H. A. K. Lapatto, M. Kuusela, A. Heikkinen, M. Muniandy, B. W. van der Kolk, S. Gopalakrishnan, N. Pollanen, M. Sandvik, M. S. Schmidt, S. Heinonen, S. Saari, J. Kuula, A. Hakkarainen, J. Tampio, T. Saarinen, M. R. Taskinen, N. Lundbom, P. H. Groop, M. Tiirola, P. Katajisto, M. Lehtonen, C. Brenner, J. Kaprio, S. Pekkala, M. Ollikainen, K. H. Pietilainen, E. Pirinen, Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci. Adv. 9, eadd5163 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D. D. Wang, S. E. Airhart, B. Zhou, L. M. Shireman, S. Jiang, C. Melendez Rodriguez, J. N. Kirkpatrick, D. D. Shen, R. Tian, K. D. O’Brien, Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. JACC Basic Transl. Sci. 7, 1183–1196 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.B. Brakedal, C. Dölle, F. Riemer, Y. Ma, G. S. Nido, G. O. Skeie, A. R. Craven, T. Schwarzlmüller, N. Brekke, J. Diab, L. Sverkeli, V. Skjeie, K. Varhaug, O.-B. Tysnes, S. Peng, K. Haugarvoll, M. Ziegler, R. Grüner, D. Eidelberg, C. Tzoulis, The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 34, 396–407.e6 (2022). [DOI] [PubMed] [Google Scholar]
- 31.J. Wu, K. Singh, A. Lin, A. M. Meadows, K. Wu, V. Shing, M. Bley, S. Hassanzadeh, R. D. Huffstutler, M. S. Schmidt, L. P. Blanco, R. Tian, C. Brenner, M. Pirooznia, M. J. Kaplan, M. N. Sack, Boosting NAD+ blunts TLR4-induced type I IFN in control and systemic lupus erythematosus monocytes. J. Clin. Invest. 132, e139828 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D. Conze, C. Brenner, C. L. Kruger, Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 9, 9772 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Y. S. Elhassan, K. Kluckova, R. S. Fletcher, M. S. Schmidt, A. Garten, C. L. Doig, D. M. Cartwright, L. Oakey, C. V. Burley, N. Jenkinson, M. Wilson, S. J. E. Lucas, I. Akerman, A. Seabright, Y. C. Lai, D. A. Tennant, P. Nightingale, G. A. Wallis, K. N. Manolopoulos, C. Brenner, A. Philp, G. G. Lavery, Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 28, 1717–1728.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.C. M. E. Remie, K. H. M. Roumans, M. P. B. Moonen, N. J. Connell, B. Havekes, J. Mevenkamp, L. Lindeboom, V. H. W. de Wit, T. van de Weijer, S. Aarts, E. Lutgens, B. V. Schomakers, H. L. Elfrink, R. Zapata-Pérez, R. H. Houtkooper, J. Auwerx, J. Hoeks, V. B. Schrauwen-Hinderling, E. Phielix, P. Schrauwen, Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 112, 413–426 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.B. Stocks, S. P. Ashcroft, S. Joanisse, L. C. Dansereau, Y. C. Koay, Y. S. Elhassan, G. G. Lavery, L. E. Quek, J. F. O'Sullivan, A. M. Philp, G. A. Wallis, A. Philp, Nicotinamide riboside supplementation does not alter whole-body or skeletal muscle metabolic responses to a single bout of endurance exercise. J. Physiol. 599, 1513–1531 (2021). [DOI] [PubMed] [Google Scholar]
- 36.C. F. Dolopikou, I. A. Kourtzidis, N. V. Margaritelis, I. S. Vrabas, I. Koidou, A. Kyparos, A. A. Theodorou, V. Paschalis, M. G. Nikolaidis, Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: A double-blind cross-over study. Eur. J. Nutr. 59, 505–515 (2020). [DOI] [PubMed] [Google Scholar]
- 37.C. R. Martens, B. A. Denman, M. R. Mazzo, M. L. Armstrong, N. Reisdorph, M. B. McQueen, M. Chonchol, D. R. Seals, Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M. Vreones, M. Mustapic, R. Moaddel, K. A. Pucha, J. Lovett, D. R. Seals, D. Kapogiannis, C. R. Martens, Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell 22, e13754 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.E. B. M. Nascimento, M. P. B. Moonen, C. M. E. Remie, K. Gariani, J. A. Jörgensen, G. Schaart, J. Hoeks, J. Auwerx, W. D. van Marken Lichtenbelt, P. Schrauwen, Nicotinamide riboside enhances in vitro beta-adrenergic brown adipose tissue activity in humans. J. Clin. Endocrinol. Metab. 106, 1437–1447 (2021). [DOI] [PubMed] [Google Scholar]
- 40.R. W. Dellinger, S. R. Santos, M. Morris, M. Evans, D. Alminana, L. Guarente, E. Marcotulli, Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: A randomized, double-blind, placebo-controlled study. NPJ Aging Mech. Dis. 3, 17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R. W. Dellinger, H. E. Holmes, T. Hu-Seliger, R. W. Butt, S. A. Harrison, D. Mozaffarian, O. Chen, L. Guarente, Nicotinamide riboside and pterostilbene reduces markers of hepatic inflammation in NAFLD: A double-blind, placebo-controlled clinical trial. Hepatology, (2022). [DOI] [PubMed] [Google Scholar]
- 42.P. Simic, X. F. Vela Parada, S. M. Parikh, R. Dellinger, L. P. Guarente, E. P. Rhee, Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): A randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol. 21, 342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.J. E. de la Rubia, E. Drehmer, J. L. Platero, M. Benlloch, J. Caplliure-Llopis, C. Villaron-Casales, N. de Bernardo, J. AlarcÓn, C. Fuente, S. Carrera, D. Sancho, P. GarcÍa-Pardo, R. Pascual, M. JuÁrez, M. Cuerda-Ballester, A. Forner, S. Sancho-Castillo, C. Barrios, E. Obrador, P. Marchio, R. Salvador, H. E. Holmes, R. W. Dellinger, L. Guarente, J. M. Estrela, Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 20, 115–122 (2019). [DOI] [PubMed] [Google Scholar]
- 44.D. M. Riche, K. D. Riche, C. T. Blackshear, C. L. McEwen, J. J. Sherman, M. R. Wofford, M. E. Griswold, Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evid. Based Complement. Alternat. Med. 2014, 459165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.C. Brenner, A. C. Boileau, Pterostilbene raises low density lipoprotein cholesterol in people. Clin. Nutr. 38, 480–481 (2019). [DOI] [PubMed] [Google Scholar]
- 46.D. W. Frederick, E. Loro, L. Liu, A. Davila Jr., K. Chellappa, I. M. Silverman, W. J. Quinn 3rd, S. J. Gosai, E. D. Tichy, J. G. Davis, F. Mourkioti, B. D. Gregory, R. W. Dellinger, P. Redpath, M. E. Migaud, E. Nakamaru-Ogiso, J. D. Rabinowitz, T. S. Khurana, J. A. Baur, Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269–282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R. S. Fletcher, J. Ratajczak, C. L. Doig, L. A. Oakey, R. Callingham, G. Da Silva Xavier, A. Garten, Y. S. Elhassan, P. Redpath, M. E. Migaud, A. Philp, C. Brenner, C. Canto, G. G. Lavery, Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 6, 819–832 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.E. Pirinen, M. Auranen, N. A. Khan, V. Brilhante, N. Urho, A. Pessia, A. Hakkarainen, J. Kuula, U. Heinonen, M. S. Schmidt, K. Haimilahti, P. Piirilä, N. Lundbom, M.-R. Taskinen, C. Brenner, V. Velagapudi, K. H. Pietiläinen, A. Suomalainen, Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 31, 1078–1090.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 49.M. Agerholm, M. Dall, B. A. H. Jensen, C. Prats, S. Madsen, A. L. Basse, A. S. Graae, S. Risis, J. Goldenbaum, B. Quistorff, S. Larsen, S. G. Vienberg, J. T. Treebak, Perturbations of NAD+ salvage systems impact mitochondrial function and energy homeostasis in mouse myoblasts and intact skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 314, E377–E395 (2018). [DOI] [PubMed] [Google Scholar]
- 50.K. L. Seldeen, A. Shahini, R. Thiyagarajan, Y. Redae, M. Leiker, N. Rajabian, A. Dynka, S. T. Andreadis, B. R. Troen, Short-term nicotinamide riboside treatment improves muscle quality and function in mice and increases cellular energetics and differentiating capacity of myogenic progenitors. Nutrition 87-88, 111189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]