Abstract
Inflammatory caspases sensing lipopolysaccharide (LPS) to drive gasdermin (GSDM)–mediated pyroptosis is an important immune response mechanism for anti-infection defense in mammals. In this work, we resolved an LPS-induced and GSDM-gated pyroptosis signaling cascade in Cnidarians. Initially, we identified a functional GSDM protein, HyGSDME, in Hydra, executing cytosolic LPS-induced pyroptosis in a caspase-dependent manner. Further, we identified a proinflammatory caspase, HyCaspA, capable of sensing cytosolic LPS by an uncharacterized N-terminal domain relying on its unique hydrophobic property, thereby triggering its oligomerization and self-activation. Subsequently, the LPS-activated HyCaspA cleaved an apoptotic caspase, HyCARD2, to trigger HyGSDME-gated pyroptosis. Last, HyGSDME exhibited an enriched distribution on the ectodermal layer of Hydra polyps, exerting a canonical immune defense function against surface-invading bacteria. Collectively, our work resolved an ancient pyroptosis signaling cascade in Hydra, suggesting that inflammatory caspases sensing cytosolic LPS to initiate GSDM-gated pyroptosis are a conserved immune defense mechanism from Cnidarians to mammals.
Hydra inflammatory caspase sensing LPS activates gasdermin-gated pyroptotic cell death defending against invading bacteria.
INTRODUCTION
Lipopolysaccharide (LPS), a major component of Gram-negative bacteria outer membrane, can directly trigger the proteolysis of human caspase-4/5 (1) and murine caspase-11 (2) to activate the noncanonical inflammasome, thereby inducing pyroptotic cell death, which is characterized by rapid cell swelling, plasma membrane disruption, and massive intracellular contents release (3, 4). A large amount of data has revealed that caspase-11 activation induced by cytoplasmic LPS is critical for endotoxic shock and sepsis in the mice model (5–7). The caspase-11–initiated noncanonical inflammasome can recognize various invading pathogens, including Burkholderia thailandensis (8), Legionella pneumophila (8–10), Salmonella typhimurium SL1344 (11), and Escherichia coli (2), by sensing cytoplasmic LPS (5, 6), which plays an important role in anti-infection immunity. Notably, caspase-11 activation is also required for neutrophil extracellular trap formation and NETosis (12, 13), which is essential for neutrophil-mediated bactericidal effects. Recent studies reported that, in multiple carnivorans (14) and some lower vertebrates, such as zebrafish (15) and turbot (16), LPS sensory caspases were also identified to induce cell pyroptosis by recognizing cytosolic LPS or invading bacteria. These findings demonstrate the importance of LPS sensory caspase–mediated proptosis in vertebrates and raise the question as to the evolutionary origin of this protease for LPS sensing in the animal kingdom.
Inflammatory caspase-4/5/11 consist of an N-terminal (NT) caspase recruitment domain (CARD) and a C-terminal (CT) catalytic domain. Previous studies indicate that the activation of inflammatory caspase-4/5/11 following the LPS stimulation depends on the binding of their NT CARD to the core element of LPS, lipid A (1). The affinity to LPS is a prerequisite for subsequent oligomerization and self-activation of caspase-4/5/11 (1, 17). Although caspase-1 has a similar NT CARD domain and is also capable of mediating the cleavage of gasdermin D (GSDMD) to trigger pyroptosis (18, 19), it is incapable of being directly activated by recognizing LPS (1). Different from mammalian caspase-4/5/11, zebrafish caspy2 activation induced by LPS is indispensable of the binding of caspy2 NT pyrin domain to LPS. Likewise, zebrafish caspy, a caspase-1 homolog, also has an NT pyrin domain, but no interaction with LPS (15). These findings suggest that containing an NT CARD or pyrin domain is not a sufficient condition for caspases to be identified as an LPS receptor. Since no common NT motif of LPS sensory caspase shared by these vertebrates was defined, it is hard for us to accurately predict caspase-like LPS sensor by bioinformatics. Whether the functional caspases for LPS detection exist in primitive organisms remains unknown.
The GSDM proteins are identified as the direct pyroptosis executors following caspase activation in mammals (18–23). To date, six members (GSDMA to GSDME and PJVK) of the GSDM family are found in mammals. Apart from PJVK, these GSDMs share a similar autoinhibited structure consisting of pore-forming NT and inhibitory CT domains (21). GSDMD is first reported to be cleaved by inflammatory caspases sensing bacterial invasion (18–20). Mechanistically, active GSDMD-NTs form pores in the plasma membrane to provide a channel for the release of alarmin signal molecules (24), such as cytokines interleukin-1β (IL-1β) and IL-18, to amplify tissue inflammation by recruiting immune cells (25, 26) and can also directly permeabilize bacterial cell membrane to exert pathogen clearance (21, 22, 27). In-depth phylogenetic analysis reveals the presence of extensive GSDM duplications in particular taxa (28). However, the GSDMD gene is only present in the mammals, while the GSDME gene can be traced back to the phylum Cnidaria. Infection-induced GSDME cleavage has been found in invertebrates, such as oyster (29) and coral (30), which also plays a role in defending against bacterial infection. The activation mechanisms of pyroptosis signaling cascade and what kind of pathogen-associated molecular patterns (PAMPs) is recognized in invertebrates remain unknown.
As a representative species of the phylum Cnidaria, Hydra vulgaris has been used as an important model organism for fundamental studies on evolutionary mechanisms of development, regeneration, and neurology, as well as programmed cell death (31). In this work, we reported an LPS-induced and GSDM-gated pyroptosis signaling cascade in Hydra, providing insights into the evolution of pyroptosis signaling mechanisms in the animal kingdom.
RESULTS
HyGSDME engages LPS-induced and caspase-dependent pyroptosis in Hydra
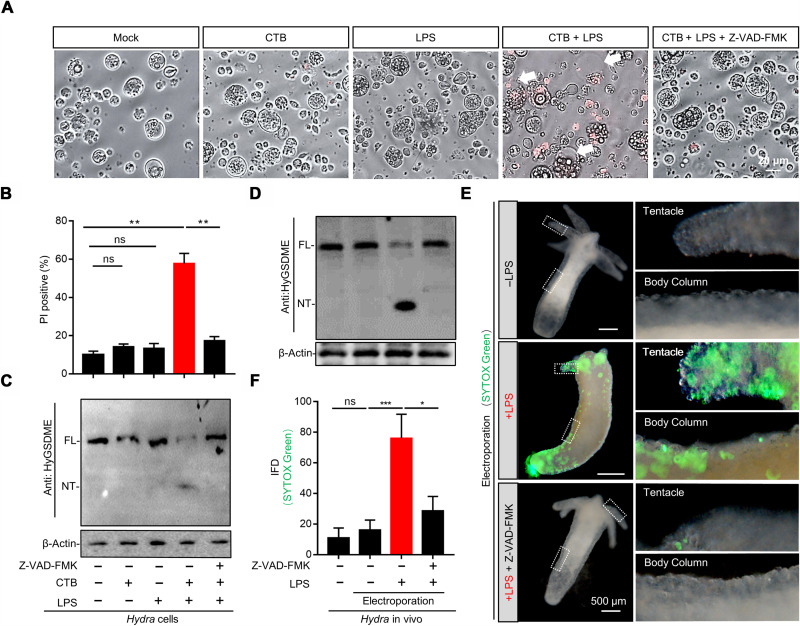
To explore the ancestral pyroptosis signaling in the animal kingdom, we traced one of the earliest GSDME gene to the well-established Cnidarian model, Hydra. Here, we found that Hydra encodes a conserved GSDME protein, HyGSDME (fig. S1A), which shares a highly similar tertiary structure to its mammalian homologs (fig. S1B). The N terminus of HyGSDME could induce pyroptosis in human embryonic kidney (HEK) 293T cells (fig. S2, A and B) and bactericidal activities against E. coli (fig. S2, C and D). To investigate whether HyGSDME mediates pyroptosis in Hydra, the primary cells dissociated from the freshwater Hydra polyp were subjected to various pyroptosis stimuli. Cholera toxin subunit B (CTB)–mediated LPS cytosolic delivery induced typical pyroptosis phenotypes, including membrane blebbing (Fig. 1A), membrane permeability (Fig. 1B), and HyGSDME cleavage (Fig. 1C). Further, cleavage of HyGSDME was obviously detected in Hydra polyps after LPS electroporation in vivo (Fig. 1D). In addition, SYTOX Green signals were clearly observed in the head, body column, and foot during LPS electroporation (Fig. 1, E and F), indicating the presence of pyroptotic cells in the above sites. Notably, these pyroptosis-related phenotypes were impaired by the pan-caspase inhibitor, Z-VAD-FMK (Fig. 1, A to F), suggesting that the caspase activity is necessary for pyroptosis activation. Together, these results determined the existence of an LPS-induced HyGSDME-gated pyroptosis pathway in Hydra.
Fig. 1. LPS triggers HyGSDME-gated pyroptosis signaling in Hydra.
(A to C) Pyroptotic morphology of Hydra cells pretreated with Z-VAD-FMK or not upon CTB plus LPS treatment, compared to CTB, LPS treated alone, or mock (no stimulation). Loss of plasma membrane integrity was detected by propidium iodide (PI) staining (A), and percentage of PI-positive cells (B) was calculated by approximately 250 cells in each sample (n = 3, two-tailed t test). HyGSDME cleavage was probed with anti-HyGSDME antibody via immunoblots (C). Arrows indicate pyroptotic cells. (D) Hydra polyps pretreated with Z-VAD-FMK or not were electroporated with LPS in vivo followed by immunoblots to detect HyGSDME cleavage. (E and F) Pyroptosis detection in vivo by SYTOX Green staining. Hydra polyps electroporated in (D) were stained by SYTOX Green to detect pyroptosis in vivo. The images were taken and measured by ImageJ to quantify the integrated fluorescence density (IFD), 30 polyps for each group (F), and images of representative SYTOX Green–positive Hydra polyps were shown (E). Representative SYTOX Green–positive cells in Hydra tentacle and body column were marked by dotted rectangular box and enlarged on the right (E). Values are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001. ns, not significant.
HyCaspA engages a high affinity to LPS/lipid A undergoing oligomerization and self-activation
In mammals, the intracellular LPS-mediated pyroptosis activation is dependent on inflammatory caspase-4/5/11–GSDMD axis signaling (1, 2, 18, 20); in Hydra, it encodes at least eight potential caspase proteins (32), among which HyCaspA and HyCaspD were clustered into an inflammatory caspase group (fig. S3A), exhibiting caspase-5 substrate cleavage activity (fig. S3B), while HyCaspB, HyCaspC, HyCARD1, and HyCARD2 were in an apoptotic caspase group (fig. S3A), exhibiting caspase-3 substrate cleavage (fig. S3B). Subsequently, these HyCasps were expressed and purified, and their ability of binding to LPS was analyzed. Compared to the other HyCasps, HyCaspA had significantly higher ability of LPS binding (Fig. 2, A and B). Moreover, endogenous HyCaspA also specifically bound to LPS/lipid A but not to Pam3CSK4 or MDP (Fig. 2C). Correspondingly, HyCaspA showed a dose-dependent resonance signal and rapid association with chip-immobilized LPS/lipid A but not MDP in the biolayer interferometry (BLI) assay (Fig. 2, D to F). The detected dissociation constants (Kd) between HyCaspA and LPS/lipid A were 1.5 ± 0.7 × 10−7 M and 6.5 ± 0.3 × 10−8 M. HyCaspA binding to LPS featured a similar slower dissociation rate constant in accord with caspase-4/5/11 (1). These results indicate that HyCaspA engages a high affinity to LPS/lipid A. Notably, coincubation with either LPS or lipid A directly induced its oligomerization and activation of Flag-purified wild-type HyCaspA (fig. S4, A and B). Collectively, HyCaspA is identified to be the Hydra LPS sensing receptor.
Fig. 2. Inflammatory HyCaspA directly and specially binds to LPS/lipid A.
(A and B) Determine the high affinity to LPS of HyCaspA. Pull-down assays using Flag-purified catalytic-cysteine mutated HyCasps bound to precoated LPS in (A) (n = 3, two-tailed t test) or biotin-conjugated LPS in (B). (C) LPS bound to endogenous HyCaspA. Immunoblots were probed with anti-HyCaspA/B for the pulled-down proteins with indicated PAMPs and total lysates. (D to F) Affinity of HyCaspA to PAMPs measured by BLI. The affinity curves of LPS or lipid A, or MDP binding to HyCaspA are expressed in nanometers (response unit) versus time. Recombinant catalytic-cysteine mutated HyCaspA(C211A) was purified from insect cells. The gradient concentrations (12.5, 25, 50, 100, and 200 nM) of HyCaspA (C211A) were incubated with LPS (D), lipid A (E), and controlled by MDP (F) to detect the surface plasmon resonance (SPR) signal. Red lines indicate SPR signals from different concentrations and were fitted by black lines. Values are expressed as mean ± S.E.M. (**P < 0.01 and ***P < 0.001).
Inflammatory HyCaspA recognizes LPS via a unique LBD domain
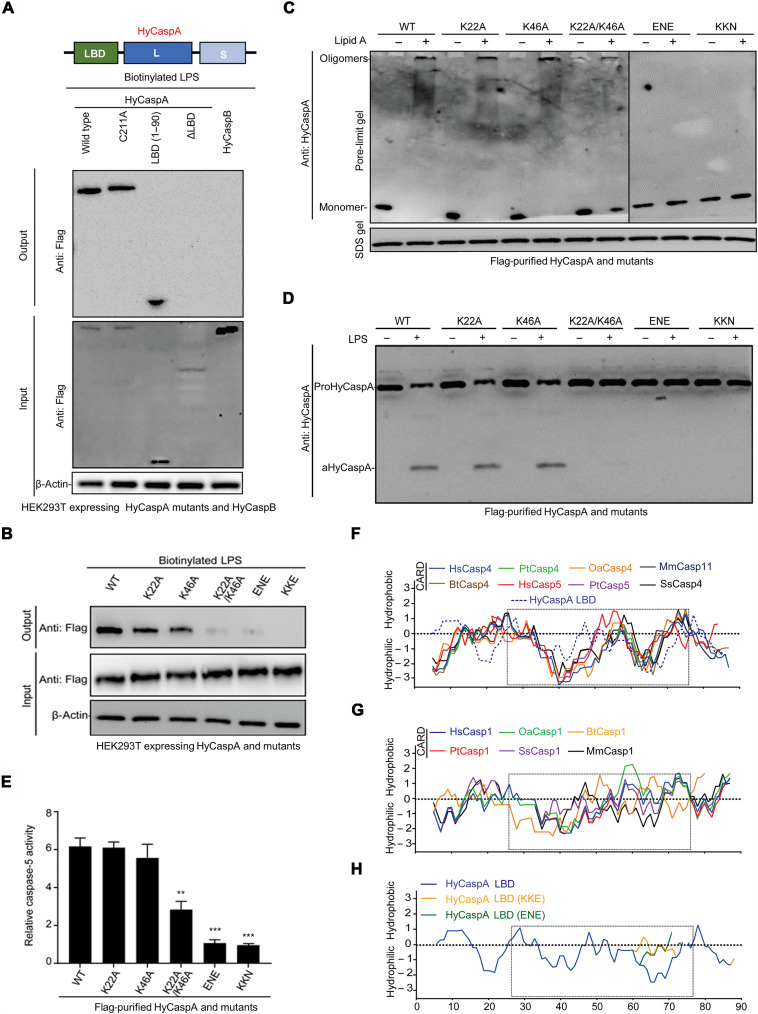
In mammals, the binding of caspase-4/5/11 to LPS/lipid A depends on the NT CARD domains (1). Here, although showing a low similarity to the conserved caspase-4/11 CARD domains in both sequence (fig. S5A) and tertiary structure (fig. S5B), the HyCaspA NT domain (1 to 90 amino acids) was found to directly bind to LPS and thus defined as the LPS-binding domain (LBD) (Fig. 3A). Several positively charged residues of caspase-4/11 CARDs were found to be indispensable for lipid A–induced oligomerization, indicating that the charge interaction plays a role in this process (1). Mutation of the positive-charged amino acid residues (K22A or/and K46A) within LBD only partially impaired its LPS affinity (Fig. 3B) and downstream oligomerization (Fig. 3C) and activation (Fig. 3, D and E), indicating that the LPS-binding mechanism of HyCaspA LBD may not be limited to charge interaction. Further analysis of amino acid hydrophobicity revealed that all the LPS-binding caspases harbor two distinct hydrophobic grooves in their NT domains (Fig. 3, F and G). Destruction of the second hydrophobic groove by mutating the KKE or ENE in HyCaspA LBD (Fig. 3H) significantly abrogated its LPS-binding ability (Fig. 3B), oligomerization (Fig. 3C), and activation (Fig. 3, D and E), suggesting that hydrophobic interaction plays important roles in the LPS-binding activity for HyCaspA LBD. Collectively, these results determined that HyCaspA uses a unique hydrophobic LBD to achieve oligomerization and self-activation in Hydra.
Fig. 3. HyCaspA recognizes LPS via its ancestral LBD.
(A) Streptavidin-based pull-down assay to detect biotin-conjugated LPS binding to Flag-tagged HyCaspA wild type, C211A, ΔLBD, LBD, and HyCaspB in transfected HEK293T cell lysates. Immunoblots were performed using total cell lysates and anti-Flag antibodies to detect HyCasps pulled-down proteins. (B) Streptavidin-based pull-down assay to detect the binding of biotin-conjugated LPS to Flag-tagged HyCaspA mutants in transfected HEK293T cell lysates. (C) Pore-limit native gel analysis of Flag-purified HyCaspA [wild type (WT) or mutants] oligomerization in response to lipid A. (D and E) LPS-induced activation of Flag-purified HyCaspA mutants. Flag-purified wild type, K22A, K46A, K22A/K46A, ENE, and KKN mutated HyCaspA were coincubated with LPS for 60 min at 37°C, followed by immunoblots probed with anti-HyCaspA antibodies to detect the activation (D). Caspase activity (E) was also measured by detecting free pNA from hydrolyzed Ac-WEHD-pNA (n = 3). (F to H) Hydropathy plot of mammalian caspase-1/4/5/11 CARD and HyCaspA LBD mutants generated by a ProtScale tool from Expasy database (https://web.expasy.org/protscale/pscale/Hphob.Doolittle.html) using the method developed by J. Kyte and R. F. Doolittle. The dotted box indicated the distinctive hydrophobic grooves within LBD domains of caspase-1 and caspase-4 homologs and HyCaspA. Values are expressed as means ± SEM (**P < 0.01 and ***P < 0.001; two-tailed t test).
HyCaspA sensing LPS activates HyCARD2 to cleave HyGSDME in Hydra
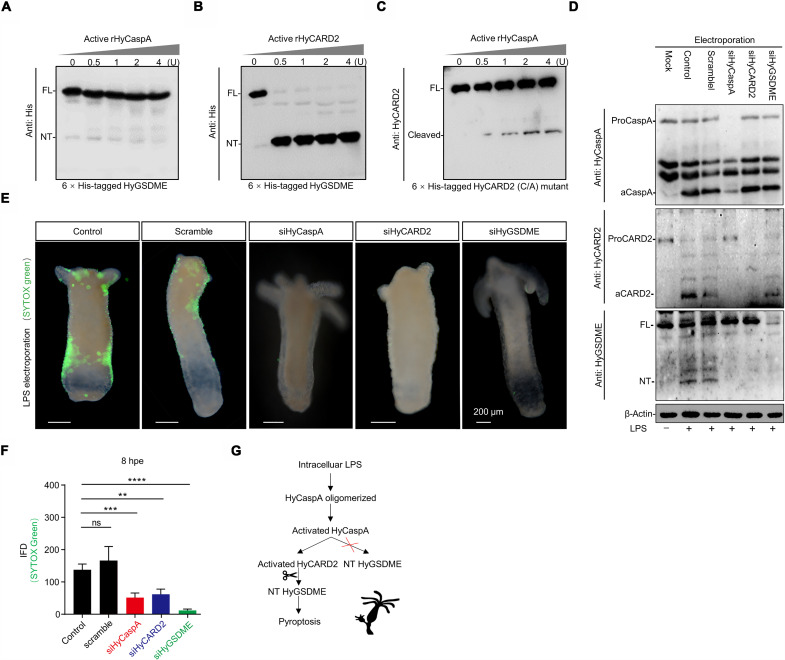
Mammalian inflammatory caspases were activated to directly mediate GSDMD cleavage (18–20). However, incubation of active HyCaspA with HyGSDME did not produce cleaved HyGSDME (Fig. 4A), suggesting the presence of an unknown mediator downstream of HyCaspA to execute the cleavage of HyGSDME. Further, we analyzed HyGSDME cleavage by incubation with the four apoptotic HyCasps of Hydra. Each of them was able to cleave HyGSDME directly, with HyCARD2 showing the highest cleavage activity (Fig. 4B and fig. S5, A to C). Furthermore, upon incubation with active HyCaspA, HyCARD2 was obviously cleaved (Fig. 4C), suggesting that HyCaspA might activate HyCARD2 to perform HyGSDME cleavage. To further examine the activation of HyCaspA-HyCARD2-HyGSDME axis in vivo, LPS was delivered by electroporation into living Hydra polyps. Cleavage of HyCaspA, HyCARD2, and HyGSDME was detected in LPS-treated Hydra lysates (Fig. 4D). Electroporation of HyCaspA small interfering RNA (siRNA) (fig. S6, A and B) hindered the cleavage of HyCARD2 and HyGSDME, while RNA interference of HyCARD2 (fig. S6, A and B) only blocked HyGSDME cleavage but did not affect HyCaspA activation (Fig. 4D). The LPS-induced pyroptosis activation was also verified by SYTOX Green staining of living Hydra polyps (Fig. 4, E and F). Together, these results indicated that LPS-induced pyroptosis is mediated by HyCaspA-HyCARD2-HyGSDME signaling axis in Hydra (Fig. 4G).
Fig. 4. HyCaspA activates apoptotic HyCARD2 to mediate HyGSDME cleavage sensing cytosolic LPS in vivo.
(A and B) Cleavage of HyGSDME by HyCasps. His-tagged HyGSDME was incubated with active recombinant HyCaspA (A) and HyCARD2 (B) followed by immunoblotting. (C) Cleavage of HyCARD2 by active recombinant HyCaspA. His-tagged HyCARD2 (catalytic-cysteine mutant) was incubated with active rHyCaspA followed by immunoblotting. (D to F) Polyps pre-electroporated with indicated siRNAs were subjected to LPS transfection and probed with anti-HyGSDME and HyCaspA/B antibodies (D). Images of representative SYTOX Green–stained polyps are shown in (E), and IFD of indicated images in (F) was measured using ImageJ, 30 polyps for each group (two-tailed t test). (G) Schematic representation of LPS-induced pyroptosis signaling axis in Hydra. Values are expressed as means ± SEM (**P < 0.01, ***P < 0.001, and ****P < 0.0001).
HyCaspA-HyCARD2-HyGSDME–gated pyroptosis protects Hydra from bacterial infection
To explore the physiological function of pyroptosis signaling in Hydra, we analyzed the distribution of HyGSDME transcripts based on the single-cell transcriptome data (33). HyGSDME showed higher expression level in the cell lineage of ectoderm, compared to that of endoderm and interstitial (Fig. 5A). Further, immunofluorescent staining demonstrated that HyGSDME signals were enriched on the ectodermal layer of Hydra polyps (Fig. 5B), which was known as the surface barrier to protect Hydra from external stimuli (34). These data hinted that HyGSDME-gated pyroptosis may play roles in the mucosal immune defense in Hydra.
Fig. 5. HyCaspA-initiated pyroptosis signaling protects Hydra against invading bacterial insults.
(A) Spatial distribution of HyGSDME expression in Hydra according to public single-cell database (https://singlecell.broadinstitute.org/single_cell/study/SCP260/stem-cell-differentiation-trajectories-in-Hydra-resolved-at-single-cell-resolution). (B) HyGSDME expression patterns obtained using immunofluorescent staining. Confocal images of whole Hydra polyp (left) and frozen section (right) following immunofluorescent staining were shown. Phalloidin staining (red) represents actin filaments running along the ectoderm; 4′,6-diamidino-2-phenylindole (DAPI) (blue) represents nuclei. Arrowheads indicate HyGSDME-positive cells in the body column and tentacle. The dotted line was used to distinguish the ectoderm and endoderm. (C) Diagram of RNA interference model following E. piscicida infection. At 96 hours after the siRNA electroporation when the genes were effectively knocked down, Hydra polyps were infected with E. piscicida [2 × 108 colony-forming units (CFUs)/ml]. Hydra polyps were harvested for testing at indicate time points. (D) RT-qPCR analysis of HyGSDME, HyCaspA, and HyCARD2 at 24 hours post-infection (hpi) (n = 3, two-tailed t test). (E) Bacterial colonization of siRNA-electroporated Hydra polyps after infection. E. piscicida–infected polyps were homogenized under aseptic conditions at 24 hpi for CFU counts (n = 6, two-tailed t test). (F) Temporal phenotypic score of Hydra polyps following E. piscicida EIB202 infection. Infected polyps in (C) were scored at indicated time points following the criteria shown in fig. S8C, 60 polyps for each group (two-tailed Mann-Whitney test). P values are shown in fig. S8D. (G) Representative phenotypic scores of infected polyps at 96 hpi as shown in (D), 60 polyps for each group (two-tailed Mann-Whitney test). Values are expressed as means ± SEM (**P < 0.01, ***P < 0.001, and ****P < 0.0001).
To verify this hypothesis, we used an aquatic pathogen (Edwardsiella piscicida), which could induce pyroptosis in mammals (35) and teleosts (15, 16), to establish an immersion infection model in Hydra polyps (Fig. 5C). E. piscicida significantly up-regulated the expression of HyCaspA and HyGSDME in vivo (Fig. 5D). SYTOX Green staining of EIB202-infected Hydra polyps showed obvious pyroptosis activation on the ectodermal layer (fig. S8, A and B). RNA interference of HyCaspA, HyCARD2, or HyGSDME significantly reduced pyroptosis activation (fig. S8, A and B), which notably increased the bacterial burdens (Fig. 5E) and exacerbated the infection-induced injury in Hydra polyps (Fig. 5, F and G, and fig. S8, C and D). Collectively, our data proved that bacterial immersion infection could activate pyroptosis signaling on the ectodermal layer of Hydra polyps and provide important mucosal immune defense against invading bacteria.
DISCUSSION
To detect the conservative LPS molecules derived from Gram-negative bacterial cell wall, the mammalian innate immune systems have evolved a series of pattern recognition receptors (PRRs), such as CD14, LPS-binding protein (LBP), MD-2, and Toll-like receptor 4 (TLR4) (36, 37). Unlike these previously identified membrane-bound or extracellular LPS sensors, caspase-4/5/11 are cytosolic LPS sensory proteases that recognize LPS at the cytoplasmic space of host cells (1). After the definition of mammalian caspase-4/5/11 for LPS sensing, the LPS sensory caspases are also found in other vertebrates, such as carnivorans (14) and teleost (15, 16), to drive pyroptosis. It remains unknown whether the LPS sensory caspase exists in invertebrates. Here, we first reported an inflammatory caspase, HyCaspA, in Hydra, as an ancient PRR for cytosolic LPS recognition. Previous studies indicated that an NT pro-domain (CARD or Pyrin) capable of affinity to LPS is a common precondition for LPS sensing by these sensory proteases (1, 15, 16). Here, the NT LBP domain of HyCaspA was also indispensable for the recognition of LPS. The HyCaspA LBD domain is defined to be neither CARD nor pyrin, representing an uncharacterized LPS-binding motif. Although caspases containing an NT CARD domain (HyCARD1/2) were also present in the Hydra, they did not show a high affinity to LPS compared to HyCaspA. These results collectively demonstrate the diversity and complexity of caspase LBD domains used for LPS detection in animal kingdom.
In consistent with TLR4/MD-2 complex (38), caspase-4/5/11 are capable of direct binding to the lipid A motif of LPS, thereby resulting in their oligomerization and self-activation (1). Whereas the structural basis of TLR4 complex–mediated lipid A recognition has been profoundly investigated and well characterized, the mechanisms underlying LPS/lipid A interaction with caspase-4/11 are largely unknown. Previous studies indicate that charge interaction plays a role in the LPS-caspase binding process (1). However, these identified conservative positively charged lipid A–binding residues in caspase-4 are not conserved in caspase-11, as well as HyCaspA. Apart from charge interaction, lessons from the structure of the TLR4-MD-2–LPS complex suggest that hydrophobic interaction is also essential for LPS sensing (38). Here, we demonstrated that the NT domains of caspase-4/5/11 have a common hydrophobic property compared to that of caspase-1 homologs. HyCaspA LBD also shared a similar hydrophobic property with that of caspase-4/5/11, instead of caspase-1. Residues of hydrophobic groove mutation changed the hydrophobic property of HyCaspA, leading to the failure of its oligomerization and activation. These results indicate that hydrophobic interaction may be a conservative acting force for LPS interaction with LBD.
In mammals, LPS-activated caspase-4/5/11 directly cleaves GSDMD to drive noncanonical inflammasome–induced pyroptosis that plays an important role in anti-infection immune defense (12, 18, 20). Recently, the discovery of GSDM homologs was reported in fish (16, 39–41), oyster (29), coral (30), and even microbes (42). In teleost, zebrafish caspy2, a human caspase-5–like homolog, is directly activated by LPS to induce direct DrGSDMEb cleavage and pyroptosis, which is critical for septic acute kidney injury (15, 43). Here, we also found that Hydra HyGSDME is activated by cytosolic LPS to trigger pyroptosis in vivo. Conversely, we found that LPS-activated HyCaspA did not induce direct cleavage of HyGSDME; instead, it activated apoptotic HyCARD2 to execute subsequent HyGSDME cleavage and thus triggered pyroptosis, which reveals an ancestral caspase-mediated cytosolic LPS sensing and signaling cascade in Cnidarians. Furthermore, we demonstrated that HyGSDME was significantly expressed on the ectodermal layer of Hydra, which is similar to the high expression of human GSDMs in mucosal surfaces (28), such as skin and respiratory and gastrointestinal tracts. Immersion-invading bacteria elevated HyGSDME expression and activated HyCasps to cleave HyGSDME, thus triggering pyroptosis on the ectodermal layer, which notably decreased the bacterial burdens and relieved infection-induced injury. This evidence collectively indicates that LPS–inflammatory caspase–GSDM axis–mediated cell necrosis is an ancient host immune defense mechanism shared between vertebrates and invertebrates.
MATERIALS AND METHODS
Study design
Our study aims to identify the invertebrate LPS sensory caspase and unravel the ancestral pyroptosis signaling pathway in animal kingdom. We traced one of the earliest GSDME gene to the well-established Cnidarian model, Hydra, and demonstrated that cytosolic LPS delivered by CTB or electroporation can result in HyGSDME cleavage, thereby inducing Hydra cell pyroptosis in vitro and in vivo in a caspase-dependent manner. Thus, we subsequently determined the LPS sensory caspase HyCaspA by LPS affinity assays and characterized the unique LBD domain of HyCaspA. To further examine the activation of HyGSDME, rHyGSDME was purified and incubated with active rHyCasps for cleavage analysis to speculate LPS-induced pyroptosis axis in Hydra. The speculated pyroptosis signaling axis was verified by RNA interference in vivo by LPS electroporation. In a Hydra immersion infection model, morphological, bacterial colonization, and transcriptions analyses were performed to identify the canonical role of HyGSDME in anti-infection immune defense.
Hydra culture
Hydra polyps were cultured at 18°C in Hydra medium composed of 0.1 mM KCl, 1 mM NaCl, 1 mM CaCl2, 0.1 mM MgSO4, and 1 mM tris-HCl (pH 7.6). They were fed with fresh Artemia nauplii larvae. Before infection, Hydra polyps were incubated for 4 days in Hydra medium supplemented with ampicillin (50 μg/μl), streptomycin, rifampicin, and neomycin, as previously described (44), to remove potential EIB202 contamination, as verified by plating homogenized polyps on Deoxycholate Hydrogen Sulfide Lactose Agar (DHL)–selective plates.
Bacterial strains and cell lines
E. piscicida EIB202 (CCTCC no. M208068) (45) was grown at 30°C on DHL-selective agar plates containing colistin (12.5 μg/μl). HEK293T cells (CRL-11268, American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium (OPM Biosciences) with 10% (v/v) fetal bovine serum (HyClone) at 37°C in a 5% CO2 incubator.
Sequence alignment and phylogenetic analyses
Sequences of GSDMs and caspases (table S1) were retrieved from the National Center for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov/RefSeq/). GSDM sequences were aligned with the ClustalW program with the neighbor-joining strategy using MEGA 5.0 and visualized by ESPript 3.0 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). For phylogenetic analysis, GSDMs or caspases from various species were aligned using MEGA 5.0 and presented by the Interactive Tree of Life tool (http://itol.embl.de).
Gene cloning
The codon-optimized sequences encoding Hydra proteins—HyCaspA (NM_001287792.1), HyCaspB (AF155128.1), HyCaspC (NM_001309754.1), HyCaspD (NM_001309780.1), HyCARD1 (NM_001287356.1), HyCARD2 (NM_001280856.1), and HyGSDME (XM_012702131.1)—were artificially synthesized by GenScript Biotech (Nanjing, China). These Hydra genes were inserted into a modified pCDH vector with a CT 3× Flag tag for transient expression in HEK293T cells, into a modified pET-28a vector for recombinant expression in E. coli, and into a modified pFastBac vector (Life Technologies) for recombinant expression in insect cells. Truncation mutants were constructed by polymerase chain reaction (PCR) cloning. Point mutations were constructed by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Purification of recombinant proteins
The insect cell–expressed HyCaspA was purified using the Bac-to-Bac Baculovirus Expression System (Life Technologies) according to the manufacturer’s instructions. Briefly, Sf9 insect cells (7 × 105 to 5 × 106 cells/ml) were cultured in Sf-900 II serum-free medium. Bacmid harboring HyCaspA was transfected into Sf9 cells by CellFECTIN II reagent for recombinant baculovirus production. One-liter culture of insect cells (1.5 × 106 cells/ml) was infected with the P3 baculovirus [multiplicity of infection = 1 infective particle (IP) per cell] and grown at 28°C for 72 hours. For protein expression in E. coli, BL21 (DE3) strains harboring indicated plasmids were cultured in antibiotic-supplemented LB medium, followed by 16 to 18 hours of induction at 16°C with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) after OD600 (optical density at 600 nm) reached 0.6 to 0.8. To harvest recombinant proteins, both insect cells and bacteria were collected and lysed in a buffer composed of 300 mM NaCl, 50 mM imidazole, 5 mM 2-mercaptoethanol, and 50 mM tris-HCl (pH 7.6). Next, 1% Triton X-100 (v/v) was added into the lysis buffer for insect cell lysis. His-tagged HyCaspA was purified by affinity chromatography using Ni–nitrilotriacetic acid beads (Qiagen) and subsequently eluted with 250 mM imidazole in 300 mM NaCl and 50 mM tris-HCl (pH 7.6). Eluted HyCaspA proteins were dialyzed against a buffer containing 150 mM NaCl and 50 mM tris-HCl (pH 7.6) to remove imidazole. Glutathione S-transferase (GST)–tagged proteins were purified using a GST-Sefinose kit (Yubo Biotech).
For purifying Flag-tagged proteins expressed in HEK293T cells, transfected cells were harvested and lysed for 30 min in a buffer containing 100 mM KCl, 20 mM tris, 1 mM EDTA, 10% glycerol, 10 mM tetrasodium pyrophosphate, 0.1% NP-40, and protease inhibitor cocktail (Roche Molecular Biochemicals). The cell lysates were incubated with anti-Flag magnetic beads (Thermo Fisher Scientific) for 6 hours at 4°C. After three washes with lysis buffer, Flag-tagged proteins were eluted using Flag peptides (Apexbio) following the manufacturer’s instruction. To prepare active forms of recombinant HyCasps, NT GST-tagged subunits were constructed as follows: HyCaspA, 1 to 232 (large subunit), 215 to 347 (small subunit); HyCaspB, 1 to 225 (large subunit), 205 to 326 (small subunit); HyCaspC, 1 to 225 (large subunit), 205 to 326 (small subunit); HyCaspD, 1 to 209 (large subunit), 146 to 269 (small subunit); HyCARD1, 1 to 314 (large subunit), 254 to 402 (small subunit); HyCARD2, 1 to 372 (large subunit), 350 to 476 (small subunit), and purified using GST-Sefinose. The purified subunits of each caspase were diluted to 100 μg/ml in a buffer composed of 150 mM NaCl, 100 mM Hepes, 0.1% CHAPS, 10 mM dithiothreitol (DTT), 1% Triton X-100, and 10% sucrose (pH 7.5) and then mixed overnight at room temperature, as previously described (46).
Hydra single-cell dissociation
Hydra polyps were dissociated into single cells using a previously described dissociation solution containing pronase E (75 units/ml; MedChemExpress) (33). Briefly, 120 to 140 Hydra polyps were washed twice in sterile-filtered Hydra medium and transferred into a 5-ml tube. The medium was replaced with 2 ml of pronase solution, and the cells were hydrolyzed for 90 min with slight agitation on a shaker at room temperature.
Antibody preparation
The rabbit polyclonal antibodies used in this study for Hydra protein immunoblots were generated by GenScript Biotech based on epitope prediction. The predicted top antigenic determinants were as follows: HyCaspA, 167-HGSESGILGIDSSEC-180; HyCaspB, 98-SNKHEYPRLGTDVD-111; HyGSDME, 175-GSADLNVTTDDSVS-188; HyCARD1, 148-MSSRRGSERDAENL-161; and HyCARD2, 432-ATKKSQTGQLSSHN-446. The other antibodies used were as follows: mouse anti–β-actin monoclonal antibody, mouse anti-Flag monoclonal antibody, mouse anti-His monoclonal antibody, goat anti-mouse immunoglobulin G (IgG)–horseradish peroxidase (HRP) antibody, and goat anti-rabbit IgG-HRP antibody (all purchased from Abcam).
LPS transfection and cell viability measurement
To induce pyroptosis in Hydra cells, dissociated Hydra cells were seeded into 12-well plates (approximately 107 cells per well) and stimulated with CTB (20 μg/μl; List Biological Laboratories) plus ultrapure LPS (1 μg/μl; E. coli O111:B4, InvivoGen). For inhibitor treatment, Hydra cells were pretreated with 20 μM Z-VAD-FMK (Sigma-Aldrich) for 30 min before adding CTB plus LPS. For propidium iodide (PI) staining, PI (5 ng/ml; Thermo Fisher Scientific) was added into medium to detect the loss of cell membrane integrity. The cytotoxicity induced by overexpression of HyGSDME wild type and its truncation mutants in HEK293T cells was determined by a CytoTox 96 assay kit (Promega) to detect the lactate dehydrogenase release. Static bright-field images of pyroptotic cells were captured by Nikon A1R microscope and processed by ImageJ.
For LPS transfection in vivo, Hydra polyps were pulsed as previously described with minor modification (47), using the Bio-Rad Gene Pulser (Gene Pulser XCell with CE Module; Bio-Rad) set at 25 mF and 300 V. Briefly, 20 polyps were chilled to 4°C for 60 min and then placed in a 0.2 cm–gapped plastic cuvette containing 200 μg of LPS diluted in 100 μl of ice-cold Hydra medium. Two pulses were applied, each lasting 20 ms. After electroporation, polyps were immediately transferred into 10 ml of Hydra medium supplemented with 20% (v/v) hyperosmotic dissociation medium (pH 6.9) composed of 3.6 mM KCl, 6 mM CaCl2, 1.2 mM MgSO4, 6 mM sodium pyruvate, 6 mM sodium citrate, 12.5 mM N-tris (hydroxymethyl)methyl-2-aminoethanesulfonic acid, 6 mM glucose, and rifampicin (50 mg/ml). Eight hours after electroporation, Hydra polyps were stained for 5 min in the dark with SYTOX Green (1:2000, Thermo Fisher Scientific) and then washed three times with Hydra medium before immunofluorescence analysis. For inhibitor treatment in vivo, Hydra polyps were pretreated with 100 μM Z-VAD-FMK (Sigma-Aldrich) for 2 hours at 18°C.
siRNA electroporation
For the knockdown of adult Hydra genes in vivo, Hydra polyps were electroporated with siRNAs, as previously described (47, 48). Briefly, three siRNAs for each gene were designed and synthesized with a TT overhang (Genepharma Biotech) and tested for in vivo efficiency by reverse transcription quantitative PCR (RT-qPCR). siRNAs specific for each gene were selected as follows: control siRNA labeled by 6-carboxy-fluorescein (FAM) or not, 5′-AGGUAGUGUAAUCGCCUUG-3′; siHyCaspA, 5′-CUCGGAAGUAGACGUUCAATT-3′; siHyCARD2, 5′-CGGGCCUCUAAGAUCUUUATT-3′; siHyGSDME, 5′-CACCAUGUUUCAGAUUUAATT-3′, and scrambled sequence was designed as control: scrHyCaspA, 5′-CUACGAGUAGAUGACUGCATT-3′; scrHyCARD2, 5′-CGCCGGUCUGAUCUUAAUATT-3′; siHyGSDME, 5′-CCAUGUGAUUCUAUUACAATT-3′. For subsequent electroporation, 20 polyps were chilled on ice and then placed in a 0.4 cm–gapped plastic cuvette containing siRNAs (3 μM) diluted in 200 μl of double-distilled water. One square wave pulse (250 V) was applied for 25 ms (Gene Pulser XCell with CE Module; Bio-Rad). After electroporation, polyps were immediately transferred into 10 ml of Hydra medium supplemented with 20% (v/v) hyperosmotic dissociation medium to recover overnight at 18°C. Subsequently, the medium was changed with custom Hydra medium.
Hydra lysate preparation and immunoblotting
To investigate LPS transfection or E. piscicida infection–induced pyroptosis, immunoblotting was performed referring to a previous study (49). Approximately 100 to 120 polyps were exposed to each condition. Hydra polyps were homogenized on ice through a 2.5-gauge needle (approximately 30 times) and centrifuged for 10 min at 13,000g in Dignam buffer composed of 1.5 mM NaCl, 10 mM KCl, 10 mM Hepes, 0.5 mM phenylmethylsulfonyl fluoride, 0.1% NP-40, and 0.5 mM DTT (pH 8.0). The supernatants were harvested, aliquoted, and stored at −80°C. The concentration of total protein was quantified using a bicinchoninic acid protein assay kit (Abcam). Equal amounts of protein samples were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) using 12% resolving gel, followed by conventional immunoblotting.
LPS pull-down assays
Biotin-conjugated lipid A (10 μg), LPS (50 μg), Pam3CSK4 (50 μg), and MDP (50 μg) from E. coli O111:B4 (InvivoGen) were immobilized onto 8 μl of streptavidin Sepharose beads (Thermo Fisher Scientific). For pull-down assay, the beads were washed three times in lysis buffer to remove unconjugated ligands and incubated with transfected cell lysates at 4°C for 12 hours. For the competition assay, 20 μg of unlabeled LPS, lipid A, Pam3CSK4, or MDP from E. coli O111:B4 (InvivoGen) was incubated with cell lysates for 60 min at room temperature before adding biotinylated LPS-conjugated streptavidin beads. The beads were washed in lysis buffer, and the precipitates were eluted in 1 × SDS loading buffer followed by immunoblotting analysis. To evaluate LPS binding to endogenous HyCaspA, 120 to 150 polyps were pretreated with 100 μM Z-VAD-FMK (Sigma-Aldrich) for 2 hours and lysed in the Hydra lysis buffer. For standard pull-down assay, 50 μg of biotin-conjugated LPS, lipid A, or MDP was immobilized onto streptavidin Sepharose beads followed by incubation with Hydra lysates. To examine LPS binding to purified recombinant HyCasps, 1 μg of Flag-purified HyCasp proteins (catalytic-cysteine mutants) was added to 250 μl of cell lysis buffer to perform pull-down assay.
Enzyme-linked immunosorbent assay–based LPS-binding assays
To investigate the LPS-binding ability of Flag-purified HyCasps expressed in HEK293T cells, a 96-well microplate (Costar) was precoated with LPS (100 μg per well) in 100 μl of carbonate-bicarbonate buffer (pH 9.6) at 4°C for 12 hours. After four washes with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST) to remove uncoated LPS, the nonspecific binding sites were blocked using 100 μl of PBST supplemented with 3% bovine serum albumin (BSA) (PBSTB) at 37°C for 60 min. Next, 1 μg of each Flag-purified protein was added into different wells and incubated at 37°C for 90 min. After four washes with PBST, the wells were incubated with anti-Flag antibody (diluted 1:500) in PBSTB at 37°C for 60 min. Subsequently, the microplate was washed four times with PBST and incubated with goat anti-mouse IgG-HRP antibody (diluted 1:500). After five washes with PBST, the microplate was incubated with trimethylboron substrate solution (Beyotime Biotech) to detect the absorbance at 450 nm.
BLI assays
Binding kinetics of HyCaspA and bacterial ligands were determined by BLI (ForteBio Octet RED96e) as previously described with minor modification (50). Briefly, the biotin-conjugated lipid A (5 μg/μl), LPS (20 μg/μl), and MDP (20 μg/μl) were diluted at indicated concentrations and immobilized to streptavidin biosensors after prewetting with 1 × kinetic buffer (ForteBio). The equilibrated streptavidin biosensors were incubated with the insect cell–derived HyCaspA (C211A) diluted by buffer at indicated concentrations. The association and dissociation process were then performed for 300 or 400 s at 25°C with shaking, respectively. All these data were recorded and analyzed using Octet RED96e data analysis software.
Oligomerization analysis by native gel
Pore-limit native PAGE was performed to analyze HyCaspA oligomerization as described (51). Briefly, 4 to 20% precast polyacrylamide gels (Epizyme Biotech) were run at 90 V for 8 hours in an ice-cold commercial Hepes-tris buffer (Epizyme Biotech), followed by immunoblotting analysis.
Caspase-substrate cleavage assay
Hydrolysis of fluorogenic or chromogenic substrates by recombinant HyCasps was performed in a reaction buffer composed of 150 mM NaCl, 50 mM Hepes, 10 mM DTT, 3 mM EDTA, and 0.005% Tween 20 (46). To measure the activity of LPS-activated HyCaspA or recombinant HyCasps, each ligand was incubated with 0.3 μM HyCaspA (wild type or mutants) or active HyCasps (100 μg/ml) in 100 μl of reaction buffer at 37°C for 60 min. Each fluorogenic or chromogenic substrate (pan-caspase, Z-VAD-AMC; caspase-1, Ac-YVAD-pNA; caspase-2, Ac-VDQQD-pNA; caspase-3/7, Ac-DEVD-pNA; caspase-4, Ac-LEVD-pNA; caspase-5, Ac-WEHD-pNA; caspase-8, Ac-IETD-pNA; caspase-9, Ac-LEHD-pNA) was added at a final concentration of 200 mM and incubated at 37°C for another 3 hours following the manufacturer’s instructions (BioVision). Substrate cleavage was detected by measuring the emission at 450 nm after excitation at 365 nm or the absorbance at 405 nm using a SpectraMax M2 microplate reader.
To examine recombinant HyGSDME cleavage by active HyCasps, HyGSDME (5 μg) was incubated with increasing amounts of active HyCasps in a 25-μl reaction buffer system at 37°C for 2 hours. The cleaved fragments were analyzed by immunoblotting. Similarly, to examine rHyCasp (catalytic-cysteine mutants) cleavage by active rHyCaspA, rHyCasps were incubated with increasing amounts of active rHyCaspA in a 25-μl reaction buffer system at 37°C for 3 hours.
Reverse transcription PCR
Complementary DNA (cDNA) was extracted by a conventional method described before (52). One microliter of cDNA was used as the template to perform standard RT-qPCR analysis in 20 μl of MonAmp SYBR Green qPCR Mix (ANHE GENE Biotech; https://m.instrument.com.cn/netshow/SH104279/q4844810.html) using the QuantStudio 3 Real-Time PCR System (Applied Biosystems). The following primer sets were used for each gene: HyCaspA, AAGCTGATAATGCTCCTGCCT (forward primer) and AATCGCTTAACATGGGAATGGT (reverse primer); HyGSDME, AAGTAAATACTCGCCATCCCTTGT (forward primer) and TGGACACACTGTCATCTGTTGT (reverse primer); HyCARD2, CAGAAGGGGTTCCGAGAGAG (forward primer) and AAACAACACCCGTGGTCAGG (reverse primer); HyActin, TCAGACAAATGAATTGTCCATG (forward primer) and TCCAATGTATCATGTAACGT (reverse primer).
Hydra infection and morphological observations
E. piscicida strain EIB202 was cultured in tryptic soy broth (TSB) medium overnight at 30°C with aeration. Fifty milliliters of EIB202 broth was centrifuged for 3 min at 8000g. The harvested pellets were resuspended in sterile Hydra medium and diluted to an OD600 of 0.2. Ten polyps were placed in each well of 24-well plate and immersion-infected with 1 ml of diluted E. piscicida broth at 20°C for 24 hours. To evaluate the effects of gene knockdown by siRNA electroporation on pyroptosis induced by LPS or EIB202 infection, polyps were pre-electroporated with indicated siRNAs 96 hours before LPS transfection or infection. LPS-transfected or LPS-infected polyps were then scored by following the criteria shown in fig. S8C as previously described (44) or ground with PBS containing 1% Triton X-100 to prepare homogenates that were plated on the DHL-selective agar plate for colony-forming unit counts or stained with SYTOX Green (1:2000, Thermo Fisher Scientific) for 5 min followed by several washes before observation under a Zeiss Stereo Lumar V12 fluorescence microscope.
Immunofluorescence
Hydra polyps were incubated in 2% urethane (MedChemExpress) solution for 3 min and fixed with 4% paraformaldehyde for 60 min, as previously described (33). After three washes for 10 min in PBS, the fixed Hydra polyps were placed in 0.5% Triton X-100 in PBS for 15 min and then incubated in blocking solution (1% BSA, 10% fetal bovine serum, and 0.1% Triton X-100) for 1 hour. Subsequently, the blocked polyps were then incubated overnight at 4°C in anti-HyGSDME antibody (1:250). Following three washes for 10 min in washing buffer (1% BSA and 0.5% Tween), polyps were then incubated in Alexa Fluor 488 goat anti-rabbit IgG (1:1000, Invitrogen) and phalloidin (1:200, Yisen Biotech) for 1 hour at room temperature. After one wash for 10 min with PBS, polyps were incubated with 4′,6-diamidino-2-phenylindole (1:1000, Beyotime Biotech) to stain nuclei for 10 min and then washed with PBS for 10 min. The slides were mounted in a mounting solution (Beyotime Biotech) and observed under a Zeiss Stereo Lumar V12 fluorescence microscope.
Acknowledgments
We thank X. B. Hu, J. M. Xu, S. L. Gu, and Y. Z. Chen for assistance with microscope experiments and W. Wu and J. M. Xu for assistance with Hydra experiments.
Funding: This work was supported by the Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism grant 2021 Sci & Tech 03-28 (Shanghai Municipal Education Commission), the National Natural Science Foundation of China (nos. 32025038 and 32002429), the China Postdoctoral Science Foundation (no. 2020 M671033), and the ECUST-OPM Open Fund (no. 20220701).
Author contributions: Q.L. and S.C. conceived the study; S.C. designed and performed most of the experiments, assisted by H.C. and S.L. Data curation was performed by S.C. H.C. and S.L. contributed reagents and analytic tools. Q.L., Y.Z., D.Y., and S.C. analyzed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S9
Table S1
REFERENCES AND NOTES
- 1.J. Shi, Y. Zhao, Y. Wang, W. Gao, J. Ding, P. Li, L. Hu, F. Shao, Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014). [DOI] [PubMed] [Google Scholar]
- 2.N. Kayagaki, S. Warming, M. Lamkanfi, W. L. Vande, S. Louie, J. Dong, K. Newton, Y. Qu, J. Liu, S. Heldens, J. Zhang, W. P. Lee, M. Roose-Girma, V. M. Dixit, Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). [DOI] [PubMed] [Google Scholar]
- 3.J. Shi, W. Gao, F. Shao, Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 4.P. Broz, V. M. Dixit, Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016). [DOI] [PubMed] [Google Scholar]
- 5.J. A. Hagar, D. A. Powell, Y. Aachoui, R. K. Ernst, E. A. Miao, Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N. Kayagaki, M. T. Wong, I. B. Stowe, S. R. Ramani, L. C. Gonzalez, S. Akashi-Takamura, K. Miyake, J. Zhang, W. P. Lee, A. Muszynski, L. S. Forsberg, R. W. Carlson, V. M. Dixit, Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013). [DOI] [PubMed] [Google Scholar]
- 7.S. Wang, M. Miura, Y. K. Jung, H. Zhu, E. Li, J. Yuan, Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Y. Aachoui, I. A. Leaf, J. A. Hagar, M. F. Fontana, C. G. Campos, D. E. Zak, M. H. Tan, P. A. Cotter, R. E. Vance, A. Aderem, E. A. Miao, Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.C. L. Case, L. J. Kohler, J. B. Lima, T. Strowig, M. R. de Zoete, R. A. Flavell, D. S. Zamboni, C. R. Roy, Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl. Acad. Sci. U.S.A. 110, 1851–1856 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A. Akhter, K. Caution, K. A. Abu, M. Tazi, B. A. Abdulrahman, D. H. Abdelaziz, O. H. Voss, A. I. Doseff, H. Hassan, A. K. Azad, L. S. Schlesinger, M. D. Wewers, M. A. Gavrilin, A. O. Amer, Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.P. Broz, T. Ruby, K. Belhocine, D. M. Bouley, N. Kayagaki, V. M. Dixit, D. M. Monack, Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.K. W. Chen, M. Monteleone, D. Boucher, G. Sollberger, D. Ramnath, N. D. Condon, J. B. von Pein, P. Broz, M. J. Sweet, K. Schroder, Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 3, eaar6676 (2018). [DOI] [PubMed] [Google Scholar]
- 13.G. Sollberger, A. Choidas, G. L. Burn, P. Habenberger, R. Di Lucrezia, S. Kordes, S. Menninger, J. Eickhoff, P. Nussbaumer, B. Klebl, R. Kruger, A. Herzig, A. Zychlinsky, Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 3, eaar6689 (2018). [DOI] [PubMed] [Google Scholar]
- 14.P. Devant, A. Cao, J. C. Kagan, Evolution-inspired redesign of the LPS receptor caspase-4 into an interleukin-1β–converting enzyme. Sci. Immunol. 6, eabh3567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D. Yang, X. Zheng, S. Chen, Z. Wang, W. Xu, J. Tan, T. Hu, M. Hou, W. Wang, Z. Gu, Q. Wang, R. Zhang, Y. Zhang, Q. Liu, Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish. Nat. Commun. 9, 3052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S. Chen, P. Jin, H. Chen, D. Wu, S. Li, Y. Zhang, Q. Liu, D. Yang, Dual function of a turbot inflammatory caspase in mediating both canonical and non-canonical inflammasome activation. Dev. Comp. Immunol. 121, 104078 (2021). [DOI] [PubMed] [Google Scholar]
- 17.J. An, S. H. Kim, D. Hwang, K. E. Lee, M. J. Kim, E. G. Yang, S. Y. Kim, H. S. Chung, Caspase-4 disaggregates lipopolysaccharide micelles via LPS-CARD interaction. Sci. Rep. 9, 826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J. Shi, Y. Zhao, K. Wang, X. Shi, Y. Wang, H. Huang, Y. Zhuang, T. Cai, F. Wang, F. Shao, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 19.W. T. He, H. Wan, L. Hu, P. Chen, X. Wang, Z. Huang, Z. H. Yang, C. Q. Zhong, J. Han, Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.N. Kayagaki, I. B. Stowe, B. L. Lee, K. O'Rourke, K. Anderson, S. Warming, T. Cuellar, B. Haley, M. Roose-Girma, Q. T. Phung, P. S. Liu, J. R. Lill, H. Li, J. Wu, S. Kummerfeld, J. Zhang, W. P. Lee, S. J. Snipas, G. S. Salvesen, L. X. Morris, L. Fitzgerald, Y. Zhang, E. M. Bertram, C. C. Goodnow, V. M. Dixit, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 21.J. Ding, K. Wang, W. Liu, Y. She, Q. Sun, J. Shi, H. Sun, D. C. Wang, F. Shao, Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 22.X. Liu, Z. Zhang, J. Ruan, Y. Pan, V. G. Magupalli, H. Wu, J. Lieberman, Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D. M. Cerqueira, M. Gomes, A. Silva, M. Rungue, N. Assis, E. S. Guimaraes, S. B. Morais, P. Broz, D. S. Zamboni, S. C. Oliveira, Guanylate-binding protein 5 licenses caspase-11 for gasdermin-D mediated host resistance to Brucella abortus infection. PLOS Pathog. 14, e1007519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.X. Chen, W. T. He, L. Hu, J. Li, Y. Fang, X. Wang, X. Xu, Z. Wang, K. Huang, J. Han, Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. R. Kurtz, J. A. Goggins, J. B. Mclachlan, Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 190, 42–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L. A. Knodler, S. M. Crowley, H. P. Sham, H. Yang, M. Wrande, C. Ma, R. K. Ernst, O. Steele-Mortimer, J. Celli, B. A. Vallance, Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.J. Wang, K. Deobald, F. Re, Gasdermin D protects from melioidosis through pyroptosis and direct killing of bacteria. J. Immunol. 202, 3468–3473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.E. De Schutter, R. Roelandt, F. B. Riquet, G. Van Camp, A. Wullaert, P. Vandenabeele, Punching holes in cellular membranes: Biology and evolution of gasdermins. Trends Cell Biol. 31, 500–513 (2021). [DOI] [PubMed] [Google Scholar]
- 29.X. Li, X. Yan, J. Leng, W. Wang, Y. Li, C. Yang, J. Sun, L. Wang, L. Song, CgCaspase-3 activates the translocation of CgGSDME in haemocytes of Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 131, 757–765 (2022). [DOI] [PubMed] [Google Scholar]
- 30.S. Jiang, Z. Zhou, Y. Sun, T. Zhang, L. Sun, Coral gasdermin triggers pyroptosis. Sci. Immunol. 5, eabd2591 (2020). [DOI] [PubMed] [Google Scholar]
- 31.B. Galliot, Hydra, a fruitful model system for 270 years. Int. J. Dev. Biol. 56, 411–423 (2012). [DOI] [PubMed] [Google Scholar]
- 32.M. Lasi, B. Pauly, N. Schmidt, M. Cikala, B. Stiening, T. Kasbauer, G. Zenner, T. Popp, A. Wagner, R. T. Knapp, A. H. Huber, M. Grunert, J. Soding, C. N. David, A. Bottger, The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 20, 812–825 (2010). [DOI] [PubMed] [Google Scholar]
- 33.S. Siebert, J. A. Farrell, J. F. Cazet, Y. Abeykoon, A. S. Primack, C. E. Schnitzler, C. E. Juliano, Stem cell differentiation trajectories in Hydra resolved at single-cell resolution. Science 365, eaav9314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.K. Schroder, T. C. Bosch, The origin of mucosal immunity: Lessons from the holobiont Hydra. MBio 7, e01184-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.H. Chen, D. Yang, F. Han, J. Tan, L. Zhang, J. Xiao, Y. Zhang, Q. Liu, The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-Jnk pathway. Cell Host Microbe 21, 47–58 (2017). [DOI] [PubMed] [Google Scholar]
- 36.J. C. Kagan, Lipopolysaccharide detection across the kingdoms of life. Trends Immunol. 38, 696–704 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A. E. Gauthier, R. D. Rotjan, J. C. Kagan, Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biol. 12, 220146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.B. S. Park, D. H. Song, H. M. Kim, B. S. Choi, H. Lee, J. O. Lee, The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 (2009). [DOI] [PubMed] [Google Scholar]
- 39.S. Jiang, H. Gu, Y. Zhao, L. Sun, Teleost gasdermin E is cleaved by caspase 1, 3, and 7 and induces pyroptosis. J. Immunol. 203, 1369–1382 (2019). [DOI] [PubMed] [Google Scholar]
- 40.J. Y. Li, Y. Y. Wang, T. Shao, D. D. Fan, A. F. Lin, L. X. Xiang, J. Z. Shao, The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis. J. Biol. Chem. 295, 1120–1141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.H. Chen, X. Wu, Z. Gu, S. Chen, X. Zhou, Y. Zhang, Q. Liu, Z. Wang, D. Yang, Zebrafish gasdermin E cleavage-engaged pyroptosis by inflammatory and apoptotic caspases. Dev. Comp. Immunol. 124, 104203 (2021). [DOI] [PubMed] [Google Scholar]
- 42.A. G. Johnson, T. Wein, M. L. Mayer, B. Duncan-Lowey, E. Yirmiya, Y. Oppenheimer-Shaanan, G. Amitai, R. Sorek, P. J. Kranzusch, Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Z. Wang, Z. Gu, Q. Hou, W. Chen, D. Mu, Y. Zhang, Q. Liu, Z. Liu, D. Yang, Zebrafish GSDMEb cleavage-gated pyroptosis drives septic acute kidney injury in vivo. J. Immunol. 204, 1929–1942 (2020). [DOI] [PubMed] [Google Scholar]
- 44.S. Franzenburg, S. Fraune, S. Kunzel, J. F. Baines, T. Domazet-Loso, T. C. Bosch, MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proc. Natl. Acad. Sci. U.S.A. 109, 19374–19379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Q. Wang, M. Yang, J. Xiao, H. Wu, X. Wang, Y. Lv, L. Xu, H. Zheng, S. Wang, G. Zhao, Q. Liu, Y. Zhang, Genome sequence of the versatile fish pathogen Edwardsiella tarda provides insights into its adaptation to broad host ranges and intracellular niches. PLOS ONE 4, e7646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.M. Garcia-Calvo, E. P. Peterson, D. M. Rasper, J. P. Vaillancourt, R. Zamboni, D. W. Nicholson, N. A. Thornberry, Purification and catalytic properties of human caspase family members. Cell Death Differ. 6, 362–369 (1999). [DOI] [PubMed] [Google Scholar]
- 47.T. C. Bosch, R. Augustin, K. Gellner, K. Khalturin, J. U. Lohmann, In vivo electroporation for genetic manipulations of whole Hydra polyps. Differentiation 70, 140–147 (2002). [DOI] [PubMed] [Google Scholar]
- 48.H. Watanabe, H. A. Schmidt, A. Kuhn, S. K. Hoger, Y. Kocagoz, N. Laumann-Lipp, S. Ozbek, T. W. Holstein, Nodal signalling determines biradial asymmetry in Hydra. Nature 515, 112–115 (2014). [DOI] [PubMed] [Google Scholar]
- 49.W. Buzgariu, S. Chera, B. Galliot, Chapter twenty-six methods to investigate autophagy during starvation and regeneration in Hydra. Methods Enzymol. 451, 409–437 (2008). [DOI] [PubMed] [Google Scholar]
- 50.H. Hara, S. S. Seregin, D. Yang, K. Fukase, M. Chamaillard, E. S. Alnemri, N. Inohara, G. Y. Chen, G. Nunez, The NLRP6 inflammasome recognizes lipoteichoic acid and regulates Gram-positive pathogen infection. Cell 175, 1651–1664.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.K. M. Boatright, M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, G. S. Salvesen, A unified model for apical caspase activation. Mol. Cell 11, 529–541 (2003). [DOI] [PubMed] [Google Scholar]
- 52.P. Chomczynski, A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15, 536–537 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S9
Table S1