Abstract
Heart failure affects over 2.6 million women and 3.4 million men in the United States with known sex differences in epidemiology, management, response to treatment and outcomes across a wide spectrum of cardiomyopathies that include peripartum cardiomyopathy, hypertrophic cardiomyopathy, stress cardiomyopathy, cardiac amyloidosis and sarcoidosis. Some of these sex-specific considerations are driven by the cellular effects of sex hormones on the renin-angiotensin-aldosterone system, endothelial response to injury, vascular aging, and left ventricular remodeling. Other sex differences are perpetuated by implicit bias leading to undertreatment and underrepresentation in clinical trials. The goal of this narrative review is to comprehensively examine the existing literature over the last decade regarding sex differences in various heart failure syndromes from pathophysiological insights to clinical practice.
Subject Terms: Cardiomyopathy, Heart Failure, Women, Sex, Gender
Introduction
Heart failure (HF) affects over 2.6 million women and 3.4 million men in the United States (U.S.).1 There are major sex differences in the prevalence of HF, response to treatment, and mortality across the spectrum of left ventricular ejection fraction (LVEF). Additionally, peripartum cardiomyopathy is a sex specific disease with unique considerations. Other conditions such as stress cardiomyopathy, hypertrophic cardiomyopathy, sarcoidosis, and amyloidosis have important but less well-recognized sex differences in prevalence, management, and outcomes. Some of these sex-specific considerations are driven by the cellular effects of sex hormones on the renin-angiotensin-aldosterone system, endothelial response to injury, vascular aging, and left ventricular remodeling. Others are a result of implicit bias leading to underdiagnosis, undertreatment, and underrepresentation in clinical trials. The objective of this narrative review is to discuss the sex differences with respect to pathophysiology, epidemiology, treatment and prognosis as well as limitations in the field including under-representation of women in HF studies, under-utilization of treatment, and selection bias for rare diseases.
Heart Failure with Reduced Ejection Fraction
Epidemiology
Heart failure with reduced ejection fraction (HFrEF) is usually defined as HF with LVEF <40%2 although studies have included patients with LVEF <45%3 and LVEF <50%.4 The lifetime risk for HFrEF is lower in women compared to men (5.8% versus 10.6%) based on two large prospective cohort studies (Cardiovascular Health Study and the Multiethnic Study of Atherosclerosis [MESA]).3 HF incidence has declined over the years with a greater reduction in women than men with HFrEF according to a population study in Olmsted County, Minnesota.4 There are also sex differences in underlying disease with women more likely to have non-ischemic cardiomyopathy while men are more likely to have an ischemic cardiomyopathy.5,6 Among hospitalized HFrEF patients, women compared to men are older, more likely to have hypertension and valvular disease, less likely to have coronary artery disease, peripheral vascular disease and tobacco usage, and have higher natriuretic peptides (Figure 1).7 Morbidity and mortality associated with HFrEF is significant with important sex differences. Based on two large HFrEF trials (PARADIGM-HF and ATMOSPHERE), women compared to men have worse quality of life despite lower mortality after adjusting for possible confounders (adjusted HR: 0.68, 95% CI 0.62–0.74; p < 0.001) and lower risk of HF hospitalization (HR 0.80, 95% CI 0.72–0.89; p < 0.001).8 Older studies have also found similar sex differences in prognosis.5,6
Figure 1.
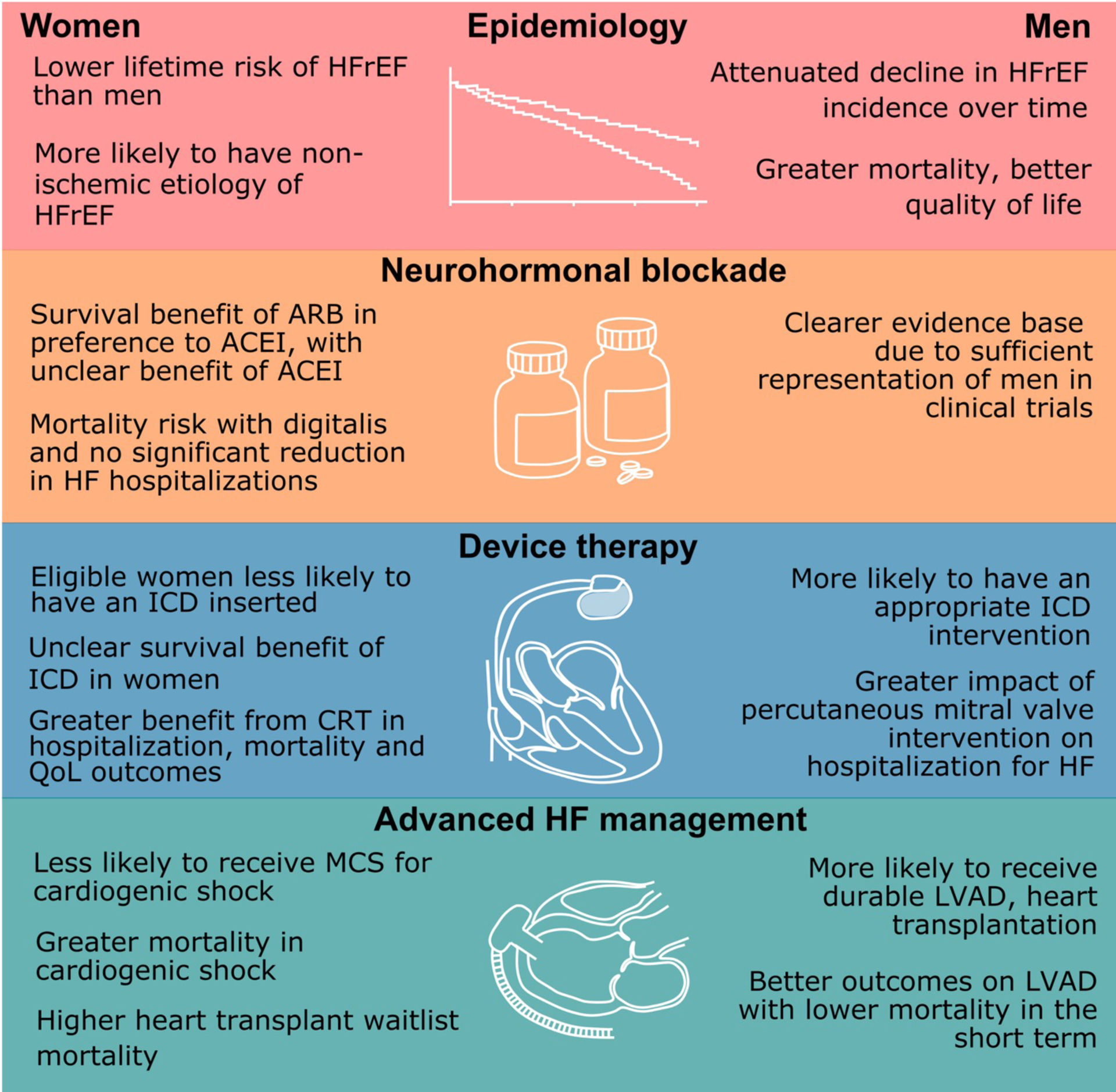
Sex Differences in the Pathophysiology and Treatment of Heart Failure with Reduced Ejection Fraction
Notable sex differences exist with respect to the epidemiology of heart failure with reduced ejection fraction. Additionally, trials have shown differential effect of neurohormonal blockade and device therapy in men and women.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CRT = cardiac resynchronization therapy; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LVAD = left ventricular assist device
Medical Therapy
Medical therapy for HFrEF has been shown to reverse remodeling of the LV, improve quality of life, reduce HF hospitalizations, and reduce mortality.9–11 Our understanding of sex differences in response to therapy is limited by retrospective and post-hoc subgroup analyses of landmark HF clinical trials that often had under-representation of women.6,7 Despite these limitations, review of these studies suggest that not all HF medications benefit men and women equally (Table 1).11–17 For instance, two large angiotensin converting enzyme inhibitors (ACEI) meta-analyses showed no definitive benefit of an ACEI in women12,13 and a large observational HFrEF study in Canada with 10,223 women and 9,475 men was notable for women having better survival on angiotensin receptor blockers (ARB) than ACEI (adjusted HR 0.69, 95% CI 0.59–0.80; p <0.0001) with no difference in survival for men (adjusted HR 1.10, 95% CI 0.95–1.30, p=0.21).18 This analysis and the two large ACEI meta-analyses raise the importance of choosing optimal controls when performing clinical trials. For example, in the PARADIGM-HF trial which compared Angiotensin Receptor-Neprilysin Inhibitor (ARNI) to ACEI (enalapril) in symptomatic HFrEF patients, the combined endpoint (mortality and HF hospitalization) among the women taking ARNI was driven by a reduction in HF hospitalizations. Due to the choice of control (ACEI instead of valsartan), it remained unclear if the benefit of ARNI in women was due to the sacubitril-valsartan combination therapy versus valsartan alone since valsartan also was shown to reduce HF hospitalizations in women in Val-HeFT (Table 1).
Table 1.
Sex Differences in Risk of Mortality and/or Hospitalization in Heart Failure with Reduced Ejection Fraction
| Study | Endpoint | Risk (95% CI) | |
|---|---|---|---|
| Women | Men | ||
| ACEI | |||
| ACEI meta-analysis12 | Mortality | OR 0.79 (0.59–1.06) | OR 0.76 (0.65–0.88) |
| ACEI meta-analysis12 | Mortality and HF Hospitalizations | OR 0.78 (0.59–1.04) | OR 0.63 (0.55–0.73) |
| ACEI meta-analysis13 | Mortality | RR 0.92 (0.81–1.04) | RR 0.82 (0.74–0.90) |
| ARB | |||
| Val-HeFT† | Mortality | HR 0.93 (0.68–1.27) | HR 1.04 (0.90–1.19) |
| Val-HeFT† | HF Hospitalizations | HR 0.74 (0.55–0.98) | HR 0.73 (0.62–0.86) |
| CHARM- Trials† | CV Mortality or HF hospitalization | HR 0.81 (0.67–0.98) | HR 0.82 (0.73–0.91) |
| ELITE II158 | Mortality | HR 1.14 (0.8–1.8) | HR 1.12 (0.9–1.4) |
| ARNI | |||
| PARADIGM-HF10 | CV Mortality or HF Hospitalization | Not reported but p-value for interaction = 0.63 | Not reported but p-value for interaction = 0.63 |
| Aldosterone blockers | |||
| Meta-analysis159 | CV Mortality or HF hospitalization | aHR0.73 (0.62–0.86) | aHR0.69 (0.62–0.77) |
| Nitrate + Hydralazine | |||
| A-HEFT22 | Mortality | HR 0.33 (0.16–0.71) | HR 0.79 (0.46–1.35) |
| A-HEFT22 | Mortality or HF hospitalization | HR 0.58(0.39– 0.86) | HR 0.67 (0.49–0.92) |
| B-blocker therapy | |||
| US Carvedilol HF160 | Mortality | HR 0.23 (0.07–0.69) | HR 0.41 (0.22–0.80) |
| COPERNICUS161 | Mortality | RR 0.63 (0.39–1.04) | RR 0.68 (0.54–0.86) |
| CIBIS II162 | Mortality | RH 0.53 (0.42–0.67) | RH 0.37 (0.19–0.69) |
| MERIT-HF163 | Mortality | RR 0.93 (0.58–1.49) | RR 0.63 (0.50–0.78) |
| Ivabradine | |||
| Ivabradine23 | CV Mortality or HF hospitalization | HR 0.74 (0.60–0.91) | HR 0.84 (0.76–0.94) |
| Digitalis | |||
| Digitalis19 | Mortality | aHR1.23 (1.02–1.47) | aHR0.93 (0.85–1.02) |
| Digitalis19 | HF Hospitalizations | HR 0.87 (0.72–1.04) | HR 0.66 (0.60– 0.73) |
| SGLT2 inhibitors | |||
| DAPA-HF11 | HF Hospitalization, IV therapy or CV Mortality | HR 0.79 (0.59–1.06) | HR 0.73 (0.63–0.85) |
| EMPEROR17 | CV Mortality or HF hospitalization | HR 0.59 (0.44–0.80) | HR 0.80 (0.68–0.93) |
Unpublished data provided by the principal investigators
Sex hormones may be responsible for some of these observed differences in HF medical therapies.24 Plasma renin levels are lower in women compared to men (Figure 2). ACE activity, vascular response to angiotensin II, and aldosterone secretion are all decreased by estrogen and increased by androgens. Additionally, expression of the Angiotensin II type 2 (AT2) receptor, which is on the X chromosome, increases much more in women than men in response to injury.24 Therefore, in pathophysiological conditions such as heart failure, estrogen stimulates increased AT2 receptor expression and activation. As a result, the inhibitory effect of ARB is more pronounced in women than men. This may explain the greater efficacy of ARBs, and subsequently ARNIs, relative to ACE inhibitors in women.
Figure 2.
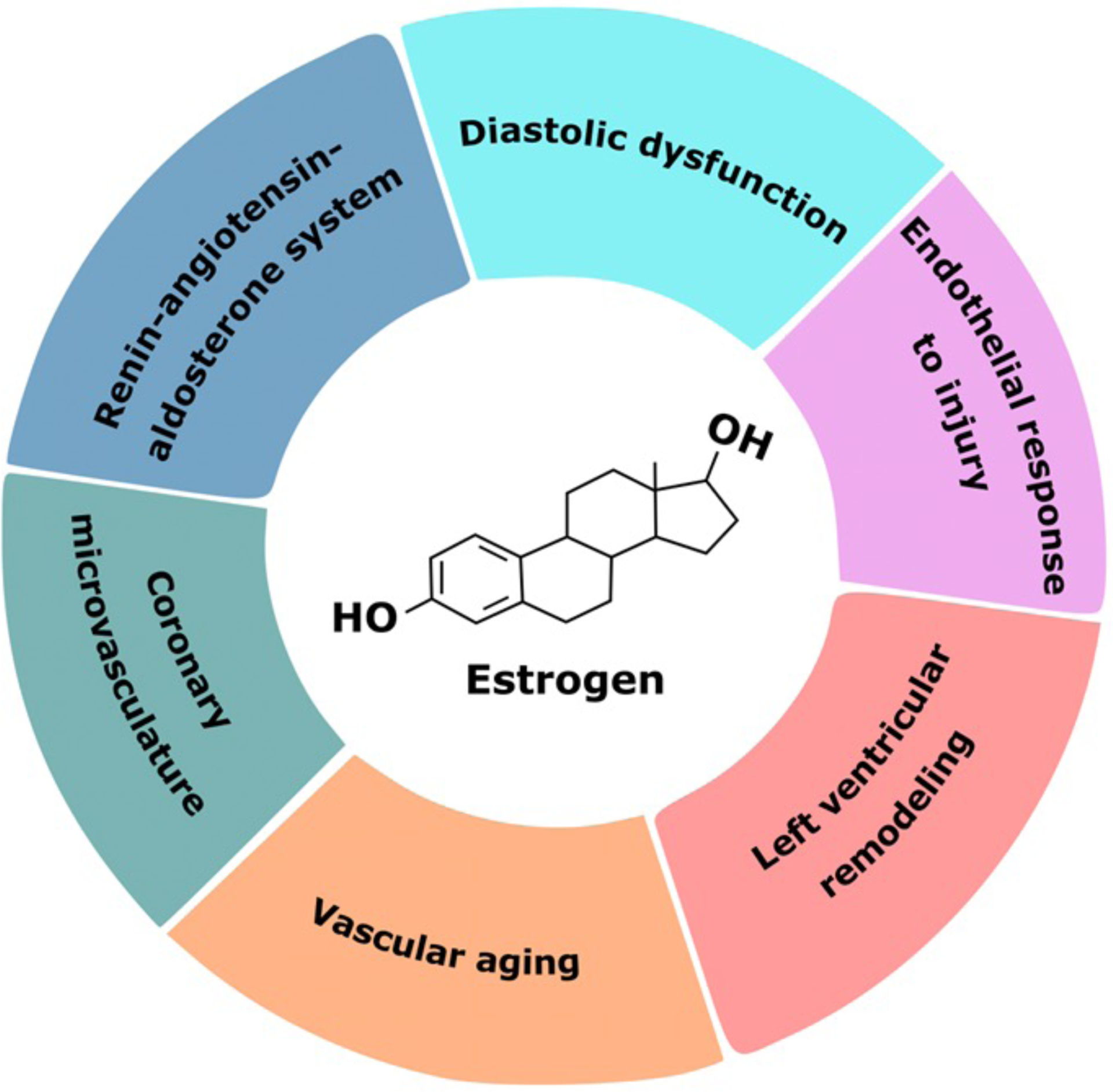
Postulated Estrogen-Mediated Mechanisms in Heart Failure
Estrogen modulates a variety of pathophysiologic processes including endothelial response to injury, the renin-angiotensin-aldosterone system, and left ventricular remodeling. Additionally, estrogen has roles in maintaining diastolic function and in the coronary microvasculature. These may explain sex differences in heart failure pathophysiology at a mechanistic level.
Knowledge of sex differences in HF therapy is also necessary to prevent harm. Among the 1,519 women participants in the Digitalis Investigation Group (DIG) trial there was a higher risk of mortality with digitalis and no significant reduction in HF hospitalizations.19 An in-depth analysis attributed the higher mortality in women to higher serum digoxin levels despite similar doses. Digoxin was deemed safe when serum concentrations ranged from 0.5 to 0.9 ng/ml emphasizing the importance of recognizing the considerable sex differences in pharmacokinetics.19,20
Under-representation of women in clinical trial has been another concern5 with some trials such as Vasodilator-Heart Failure Trial I (V-HeFT 1) not even including women when comparing prazosin, isosorbide and hydralazine to placebo in heart failure patients.21 In fact, isosorbide and hydralazine were studied only in women participating in the African American Heart Failure Trial (A-HEFT) but have not been studied among women with HFrEF of other races. In A-HEFT which included 420 women with moderately severe HF symptoms (New York Heart Association [NYHA] class III-IV) already on guideline medical therapy, the combination of isosorbide and hydralazine therapy reduced mortality and HF hospitalization.22 There was improvement in quality of life with treatment but it did not reach statistical significance in both sexes which may be due to relatively small number of patients in both subgroups.
Finally, newer therapies like ivabradine and sodium-glucose cotransporter-2 inhibitors (SGLT2i) have limited sex specific data that are important to review. Ivabradine is recommended for symptomatic HFrEF patients in normal sinus rhythm with resting heart rate ≥ 70 beats per minute despite maximal β-blocker therapy. Based on the Systolic Heart Failure Treatment with the Ir Inhibitor Ivabradine Trial (SHIFT) that included 1,535 women, ivabradine reduced combined endpoint of cardiovascular death and HF hospitalization in women and had similar benefit in men.23 SGLT2i are indicated in symptomatic HFrEF patients with or without diabetes mellitus type II. In the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced), empagliflozin reduced a combined endpoint of cardiovascular death or HF hospitalization among the 893 women participating.17 Dapagliflozin is another SGLT2i with limited sex-specific data, that showed among the 1,109 women with HFrEF a trend towards reduced combined endpoint of HF hospitalizations, intravenous outpatient therapy for HF or cardiovascular death in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial.11 These studies show some benefit despite under-representation of women in HFrEF trials.
Sex Hormones and Heart Failure
Sex hormones have many effects on the vasculature and renin-angiotensin-aldosterone system as discussed earlier (Figure 2). Estrogen in the form of hormone replacement therapy (HRT) has also been shown to effect postmenopausal women with symptomatic HF.25 In a secondary analysis of Beta-Blocker Evaluation of Survival Trial (BEST), which included patients with NYHA Class III or IV HF with an LVEF of 35% or less, there was a significant survival benefit for HRT users among the 237 nonischemic postmenopausal women even after adjustment for potential confounders (HR = 0.35, 95% CI 0.14–0.87).25 Posited mechanisms included reduced activation of neurohormonal systems and suppression of sympathetic activity.25
Implantable Cardioverter Defibrillators (ICD)
There are sex differences in the utilization, outcomes, and adverse events with ICDs, which are recommended to prevent sudden death for all HF patients NYHA class II-III with LVEF ≤ 35% (Class I indication) or those with NYHA class III-IV eligible for biventricular pacemaker (CRT).2 Eligible women are less likely than men to have an ICD based on many studies,26–31 with one large analysis showing no sex differences in ICD utilization among those who received counseling.27 The benefit of an ICD to prevent sudden death in women remains controversial because the landmark primary ICD trials included few women. In one meta-analysis of five primary prevention ICD trials (DEFINITE, SCD-HeFT, DINAMIT, MUSTT, and MADIT-II) that included 934 women there was no survival benefit for women with an ICD (HR 1.01, 95% CI 0.76–1.33).32 In another meta-analysis that included 1,145 women, there was also no survival benefit among the women with an ICD (HR 0.78, 95% CI 0.57–1.05, p= 0.1). Women were also less likely to receive appropriate ICD therapies compared to men (HR 0.63, 95% CI 0.49–0.82, p=0.001).33 In the EU-CERT-ICD project that included 957 women from 11 European countries, women with an ICD for primary prevention had fewer appropriate ICD shocks when compared to men (8% women vs 14% men) yet better survival even after adjusting for confounders such as age, biventricular pacemaker, and ischemic cardiomyopathy (adjusted HR 0.65, 95% CI 0.53–0.79; p<0.0001).34 The sex differences in the benefit of ICDs for primary prevention may be attributed to sex differences in mode of death with one study showing a 32% lower risk of sudden death in women than men and no sex difference in pump failure.35
The risk of complications with an ICD remains low but two large registries have reported a higher likelihood of women having adverse events. In the National Cardiovascular Data Registry that included Medicare patients with an ICD for primary prevention, women were more likely than men to have 30- and 90-day adverse events such as bleeding and mechanical complications (aOR:1.39, 95% CI 1.26–1.53; p <0.001) as well as hospital readmissions within 6 months (aOR: 1.32, 95% CI 1.23–1.42, p <0.001).36 In the Ontario ICD Database with 1,106 women implanted with an ICD for primary and secondary prevention, women compared to men had a higher likelihood of major complications within 45 days including the risk of lead dislodgement (odds ratio, 1.78 [CI, 1.24 to 2.58]; p = 0.002).37 The cause of sex differences in adverse events may be due to differences in vascular access with smaller vessels and body size in women compared to men.
Cardiac Resynchronization Therapy (CRT)
Similar underutilization in women compared to men has been seen with CRT despite greater benefit. CRT is recommended for symptomatic HF patients (NYHA II-IV) with LVEF ≤35%, sinus rhythm, and LBBB with QRS ≥150 ms (Class I indication) and may be helpful in those with LBBB with QRS 120–149ms, non-LBBB with QRS ≥ 150 ms, or those with significant pacemaker dependency regardless of underlying rhythm (Class IIa).2 Eligible women with HFrEF are less likely than men to receive CRT and this sex disparity in utilization has increased over time in the U.S.38–41 However, women are more likely than men to benefit from CRT with improved quality of life, ventricular remodeling, HF hospitalizations, and mortality. In the Management of Atrial Fibrillation Suppression in AF-HF Comorbidity Therapy (MASCOT) study that included 82 women with LVEF ≤ 35% and QRS ≥ 130 ms, women compared to men with CRT had more improvement in quality of life, greater reduction in left ventricular end-diastolic dimension (−8.27 +/− −11.14% vs −1.14 +/− 22.05%, p=0.02), and fewer HF hospitalizations (p = 0.045).42 In another study including 105 women, women compared to men had greater reduction in LV end-systolic dimension (0.85 +/−1.2 cm vs 0.34 +/− 0.91 cm, p<0.01) and greater increase in LVEF (12+/− 13% vs 0.89+/−9%).43 A meta-analysis of three CRT studies (REVERSE, MADIT-CRT, RAFT) also demonstrated sex differences in the benefits of CRT with a narrower QRS. In this meta-analysis with HFrEF, LBBB, and QRS 130–149 ms, only women had reduced HF and mortality (HR 0.24, 95% CI 0.11–0.53, P < 0.001) and reduced mortality alone with CRT (HR 0.24, 95% CI, 0.06–0.89, P = 0.03).44
Baroreflex Activation Therapy
Baroreflex activation therapy (BAT) with an electrode attached to the bifurcation of the carotid artery reduces sympathetic activity and increases parasympathetic activity resulting in reduction in blood pressure and improved venous and arterial compliance.45,46 This therapy has been deemed safe and effective in HFrEF patients based on a multicenter study that showed improvements in quality of life and reduced HF hospitalizations with BAT, especially for patients without CRT therapy.47 In the BeAT-HF (Baroreflex Activation Therapy for Heart Failure) that included 53 women randomized to BAT vs guideline directed medical therapy (GDMT) followed for 6 months, BAT was deemed safe and effective with women benefiting based on improvement in quality of life, improvement in 6 minute walk test (44 +/− 45 m BAT vs −32 +/− 118 m GDMT, p<0.01), improvement in NYHA functional class (70% BAT vs 27% GDMT, p <0.01) and NT-proBNP levels (−43% BAT vs 7% GDMT; p<0.01).46 Sex differences in mortality or HF hospitalization have not been fully evaluated and will need a larger female cohort.
Transcatheter Mitral Valve Repair
There are similarly limited sex specific data on response to transcatheter edge-to-edge mitral valve repair (TMVr) for patients with functional mitral regurgitation. Among 614 patients in the COAPT trial, 221 (36.0%) were women. Women were more likely to have NYHA Class III or IV symptoms than men with lower baseline KCCQ scores and 6-min walk distances despite being younger with fewer comorbidities.48 Although treatment with TMVr reduced HF hospitalization compared to treatment with GDMT alone, the effect was less in women than in men (P interaction = 0.002).48 Even after adjusting for clinically relevant covariates, a significant interaction persisted between sex and treatment modality for outcome of HF hospitalization at 2 years (adjusted P interaction = 0.009).48 In contrast to these findings a similar analysis from the real-world European Registry of Transcatheter Repair for Secondary Mitral Regurgitation found equal effectiveness in both sexes with similar all-cause mortality and durability of MR reduction.49
Advanced Heart Failure
Advanced HF refers to patients with severe HF despite optimal medical guideline therapy and compliance with diet and fluid restrictions. Therapeutic options are limited to mechanical circulatory support (MCS) and heart transplantation with known sex differences in utilization and outcomes. A recent hospital study to assess disparities in temporary mechanical support for cardiogenic shock due to heart failure using the National Inpatient Sample (N=57,742, 40% women) was notable for women less likely to receive these devices than men (aOR 0.72, p<0.0167). Women with cardiogenic shock due to HF also had a higher mortality than men (aOR 1.17, p< 0.05) even after adjusting for race, insurance, income, age, comorbidities, hospital characteristics and type of temporary MCS.50 Among patients waiting for heart transplantation in the U.S., fewer women than men were supported with durable continuous flow left ventricular assist devices (LVAD) and were less likely to undergo transplantation. Waitlist mortality was also higher among women compared to men with LVAD even after adjusting for device type (HR 1.51; p<0.001).51 Sex differences in adverse events with durable LVADs have recently been demonstrated with a contemporary international database that included 2,066 women with continuous flow-LVADs. In this analysis, women had a higher risk of mortality during the first 4 months after LVAD implantation compared to men (aHR 1.74; p <0.0001) with the cause of death mainly due to neurologic complications (aHR 2.62, 95% CI 1.80–3.81;p= 0.006).52
Heart Failure with Preserved Ejection Fraction
Epidemiology
There are more women than men with HF and preserved ejection fraction (HFpEF). In the EPidemiologia da Insuficiência Cardiaca e Aprendizagem —Epidemiology of Heart Failure and Learning (EPICA) study in Portugal,53 the prevalence of HFpEF was higher in women (2.42%; 95% CI 1.86–2.98%) compared to men (0.88%; 95% CI, 0.57–1.20%) at any given age. As for incidence of HFpEF, the age- and sex-adjusted incidence of HF in Olmsted County, Minnesota from 2000 to 2010 declined from 3.2 to 2.2 cases per 1000 person years, corresponding to a 37.5% decline over the decade-long study period. The reduction was more pronounced in women (43%) than in men (29%), and smaller for HFpEF (LVEF EF ≥50%; 28% reduction in incidence) than for HFrEF (45% reduction in incidence).4 Overall, HFpEF constituted an increasing proportion of both prevalent and incident HF cases over time.4,54–56
Risk Factors and Comorbidities
While population-based longitudinal studies have established the well-known clinical risk factors for incident HF, few have taken into account different HF types and sex differences in comorbidities. In the Framingham Heart Study (FHS),57 risk factors specific to HFpEF included higher body mass index, smoking, and a history of atrial fibrillation. Older age was also associated with a higher risk of HFpEF than HFrEF.57 In a larger analysis pooling individual level data from FHS, Prevention of Renal and Vascular End-stage Disease (PREVEND) and Cardiovascular Health Study (CHS),58 independent predictors of incident HFpEF included older age, higher systolic blood pressure, increased body mass index, antihypertensive treatment, and previous myocardial infarction. Older age was more strongly associated with HFpEF than HFrEF. Female sex was not a predictor of HFpEF suggesting that the predominance of women is strongly related to aging.
One important risk factor with sex differential associations with HFpEF is obesity. In a sex-pooled analysis of FHS, CHS, PREVEND and MESA,59 each one standard deviation increase in body mass index (BMI) was associated with 34% increase in incident HFpEF (vs 18% for HFrEF). Sex stratified analyses revealed that the differential association between BMI and HFpEF versus HFrEF was more apparent in women than men. Similarly, waist circumference was associated with HFpEF but not HFrEF in women and with both HF subtypes in men. This is consistent with findings from the Women’s Health Initiative showing that the population attributable risk of obesity was larger for HFpEF compared to HFrEF in this population of exclusively women.60 An inflammatory-metabolic hypothesis has been proposed, wherein an expanded epicardial adipose tissue mass, microvascular endothelial dysfunction, and (possibly) altered activity of adipocyte-associated inflammatory mediators, may predispose women to greater risk of HFpEF in the presence of systemic inflammatory conditions such as obesity, diabetes and the metabolic syndrome.61
Atrial fibrillation (AF) is another comorbidity that often exists in conjunction with heart failure and is associated with higher morbidity and mortality in women compared with men. Women with AF and HF have a significantly higher risk of mortality compared to men with both conditions. Additionally, women with new-onset AF without HF had a 9-fold risk of developing HF.62 Women also remain less likely to receive anticoagulation with DOACs for AF than men at all levels of CHADS-VASc score.63 Additionally, women are less likely to be treated with rhythm control and ablation which may explain the higher symptom burden reported in women. Sex specific data regarding left atrial appendage closure is limited with the Watchman trial only included 30% women. Nonetheless, population analyses have showed that women compared to men had higher rates of in-hospital adverse outcomes, including bleeding, vascular, cardiac complications, post-procedure stroke and acute kidney injury after left atrial appendage closure.64
Differences in cardiac aging and changes in LV remodeling
Sex differences in cardiac aging underscore the relative protection against the development of HFrEF in women as compared with men. Coupled with the lower incidence of obstructive coronary artery disease, women exhibit lower rates of myocardial apoptosis and necrosis in response to myocardial infarction (MI), and lower rates of adverse cardiac remodeling in response to MI and aging.65,66
Conversely, cardiac aging in women serves as fertile ground for the development of HFpEF. Women have higher systolic and diastolic elastance which increases to a greater extent with aging than men. This is alongside greater arterial elastance, higher pulse pressure, smaller arterial caliber, and earlier wave reflection than men.67 Furthermore, women exhibit greater concentric remodeling and heightened load-induced impairment of left ventricular relaxation.68,69 Dysfunction of the coronary microvasculature also plays a central role in HFpEF pathophysiology,70 and is seen to a greater extent in cardiac aging in women than men.71
Intrinsic differences in remodeling and reactivity of the pulmonary vasculature may also affect HFpEF development and severity. Women have higher rates of pulmonary arterial hypertension,72 and women are overrepresented amongst HFpEF patients with combined precapillary-postcapillary pulmonary hypertension,73 suggestive of underling sex differences in the pulmonary circulation.
The role of sex hormones in cardiac ageing may also contribute to the development of HFpEF; whilst estrogen has an overwhelmingly protective effect on the cardiovascular system, the sudden loss of these effects with menopause could potentially contribute to HFpEF development.74 Loss of ovarian estrogens has been linked to activation of the renin-angiotensin-aldosterone system.75 Estrogen both induces the production of nitric oxide via non-nuclear estrogen receptor alpha signaling, and prolongs the half-life of cyclic guanosine monophosphate. Both pathways are central to HFpEF pathophysiology with effects on coronary vasodilation, and inhibition of inflammation and cardiomyocyte proliferation.76 Accordingly, mouse models of pressure overload have demonstrated that estrogen protects against the development of cardiac hypertrophy, and response to phosphodiesterase 5 (PDE5) inhibition is dependent on estrogen receptor signaling, supporting a role for estrogen in the development of HFpEF in postmenopausal women and dictating response to PDE5 inhibition. Loss of estrogen with menopause may also contribute to the development of coronary microvascular dysfunction due its pro-angiogenic properties, with implications for HFpEF development given the strong association between HFpEF and microvascular dysfunction.77 Additionally, women have a higher age-adjusted incidence of LV diastolic dysfunction than men. Parameters of diastolic dysfunction in postmenopausal women are attenuated by the use of hormone replacement therapy, highlighting the important role of estrogen in maintaining normal diastolic function.74
Sex Differences in Aortic Stenosis
Similar sex differences in LV remodeling are seen in the context of aortic stenosis. Studies using cardiac magnetic resonance show greater concentric remodeling in men resulting in greater LV volumes and LV mass. Comparatively, women have less concentric remodeling with lower LV volumes. As a result, women likely develop higher wall stress and filling pressures which leads to more advanced symptoms.78 Even when adjusted for body area, women have less aortic valve calcification with no difference in aortic valve areas. Perhaps due to lower levels of myocardial fibrosis, studies have also shown greater LV mass regression after TAVR in women compared to men.78
Sex Differences in Exercise Hemodynamics
Several studies have investigated sex differences in the hemodynamic response of HFpEF patients to exercise. Two have identified attenuated cardiac output reserve with exercise, along with greater rises in pulmonary capillary wedge pressure indexed to cardiac output and workload in women compared to men.79,80 Poorer pulmonary and systemic arterial compliance were also seen79 which may reflect greater ventricular-vascular stiffening in women.67 Both poorer diastolic reserve and adverse pulmonary vascular remodeling have been associated with HFpEF symptom severity81,82 and may contribute to the poorer quality of life observed amongst women compared to men.83
Conversely, males with HFpEF have higher mortality84 which may parallel right ventricular (RV) dysfunction.85 An invasive cardiopulmonary exercise testing study of patients with HFpEF identified adverse RV-pulmonary arterial (RV-PA) coupling in men as compared to women at peak exercise.86 This RV-PA uncoupling was attributable to greater increases in RV afterload in conjunction with poorer contractile reserve and was associated with poorer peak exercise aerobic capacity. This mirrors findings of poorer resting RV systolic function in males,87 which appears to be independent of the degree of RV afterload.88 These studies highlight differing hemodynamic phenotypes between the sexes and may point to differences in underlying mechanisms of HFpEF (Figure 3).
Figure 3.
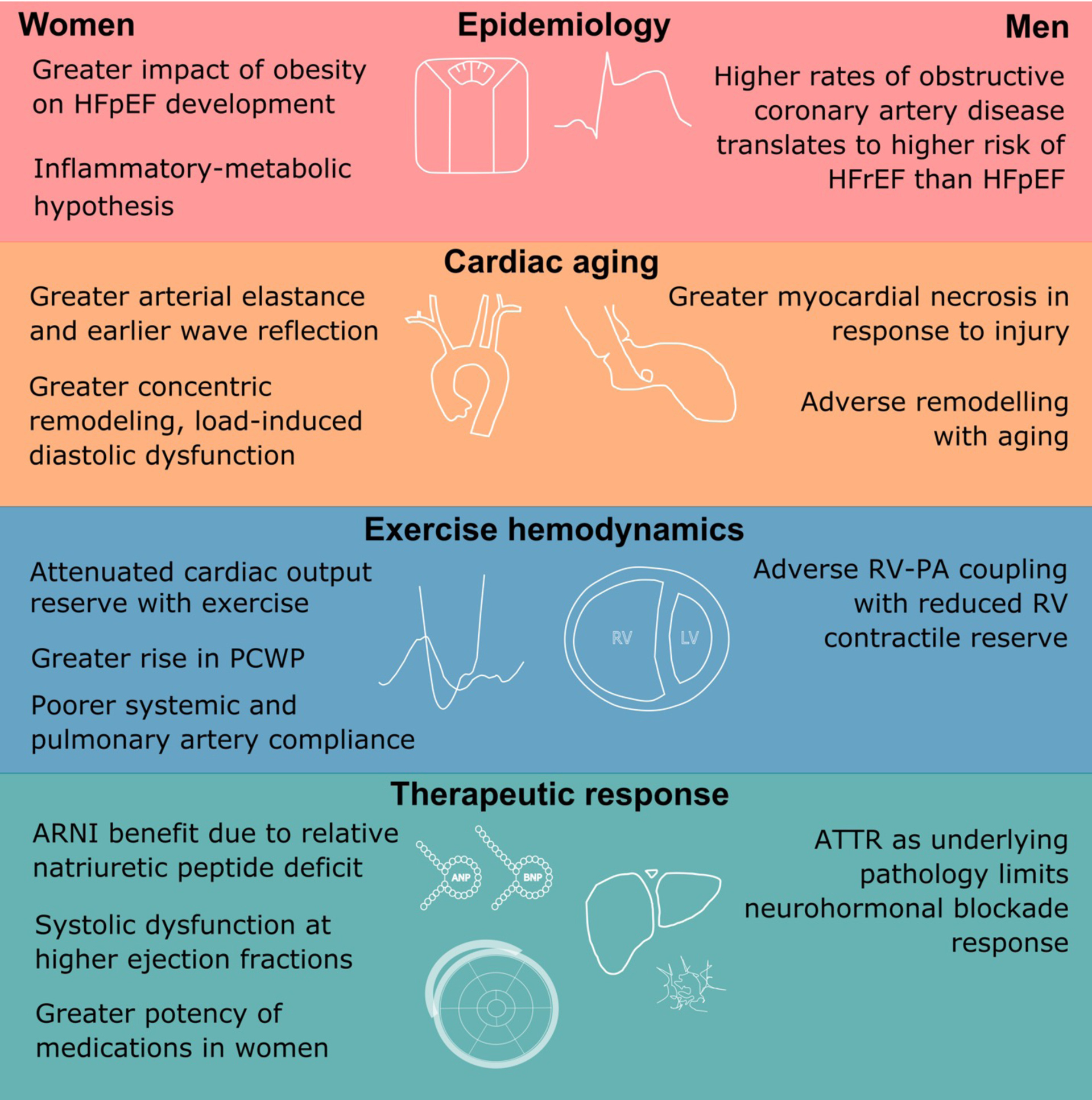
Sex Differences in the Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction
Sex differences also exist in the epidemiology of heart failure with preserved ejection fraction. Differences in vascular aging, concomitant comorbidities, and therapeutic response have all been demonstrated in the literature.
ARNI =angiotensin receptor neprilysin inhibitor; HFpEF = heart failure with preserved ejection fraction; RV = right ventricle; PCWP = pulmonary capillary wedge pressure
Sex Interactions in Medical Therapy
While trials of pharmacologic treatment for HFpEF have been largely neutral, there are notable sex differences.89 An exploratory, post hoc, non-pre-specified analysis of the Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function (TOPCAT) trial evaluated sex differences in outcomes and responses to spironolactone in patients with HFpEF.90 Of the 1,757 participants, 882 (49.9%) were women and were older with fewer comorbidities compared to the male participants. There were no sex differences in the primary outcome of a composite of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization. However, there was a significant reduction in all-cause mortality associated with spironolactone in women (HR: 0.66, 95% CI: 0.48–0.90, p = 0.01) but not in men with a significant sex-treatment interaction (Pinteraction = 0.024).90
As we have seen in trials of neurohormonal blockade in women with HFrEF, there was also a sex treatment interaction observed with angiotensin-neprilysin inhibitors (ARNIs). In PARAGON-HF, ARNIs showed a reduction in the primary outcome in women but not in men, independent of baseline differences.91 The effect was mainly driven by reduction in HF hospitalization rather than mortality and was significant in women with an ejection fraction (EF) of 45–60%. This may indicate that the women who responded had a mildly reduced, rather than preserved, EF given women’s higher EFs than men.92 Alternative explanations include a relative natriuretic peptide deficit in women, translating to a greater benefit from ARNIs91 or more transthyretin amyloidosis among men with HFpEF associated with poorer response to neurohormonal blockade.93
SGLT2 inhibitors are a new therapy for both men and women with HFpEF. The Empaglifozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) evaluated the effects of SGLT2i with empaglifozin on major HF outcomes in HFpEF patients. Among 5,988 patients with HFpEF (LVEF >40%) and New York Heart Association functional class II-IV followed for a median of 26.2 months, empaglifozin 10 mg per day reduced the combined risk of cardiovascular death or hospitalization (HR: 0.79, 95% CI 0.69–0.90, p<0.001). In a pre-specified sub-group analysis, 162 of 1,338 women receiving empagliflozin experienced a composite of cardiovascular death or hospitalization for heart failure compared to 214 of 1,338 women in the placebo group (HR 0.75, 95% CI 0.61–0.92). This landmark trial demonstrates a new therapeutic option for women with HFpEF.94
Remote Hemodynamic Monitoring Systems
Therapy guided by remote monitoring of pulmonary artery (PA) pressures using the CardioMEMS PA pressure sensor has been demonstrated to reduce hospitalization rates in HF patients but many clinical subgroups including women were underrepresented in the CHAMPION trial.95,96 The CardioMEMS Post-Approval Study, a multi-center, prospective, open-label, single-arm trial evaluating the use of CardioMEMS pressure-guided therapy in routine clinical practice subsequently enrolled 452 women of 1,200 adults (38% of total).97 Both men and women demonstrated similar reductions in pulmonary artery pressures, significant decreases in HF hospitalizations over 12 months compared with the year before the study (interaction p = 0.13), and improved quality of life.97 In the more recent GUIDE-HF, there were 37.5% women. The primary outcome was a composite of all-cause mortality, HF hospitalizations, and urgent HF visits. The hazard ratio for women was 0.65 (95% CI 0.47–0.87) compared to 1.05 (95% CI 0.84–1.31) in men with interaction p-value of 0.01.98
Specific Cardiomyopathies
There are many cardiomyopathies worthy of separate discussion because they are unique to women (peripartum cardiomyopathy), reversible (takosubo cardiomyopathy) or cross the spectrum of HFpEF and HFrEF (hypertrophic cardiomyopathy and amyloidosis). Data in some of these cardiomyopathies is ample (peripartum cardiomyopathy) while others is sparse (sarcoidosis). Below is a summary of the literature with a focus on sex differences.
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a pregnancy-associated, idiopathic cardiomyopathy presenting with HF due to left ventricular systolic dysfunction, typically early after delivery but also during pregnancy or up to a few months after the delivery.99,100 PPCM is the leading cause of HF in pregnancy and the post-partum period and an important cause of non-obstetrical maternal mortality.101 The incidence of PPCM varies widely, between approximately 1:100 live births in Nigeria and 1:20,000 in Japan. In the U.S., the incidence is approximately 1:3,000 live births and is increasing due to advancing maternal age, increased rate of multifetal pregnancies, and an increased recognition of the disease.99
There is a strong association between PPCM and older maternal age, pregnancy-associated hypertension, preeclampsia, multifetal pregnancies, African American race, and possibly diabetes mellitus and anemia. Familial clustering has been reported and recent studies have shown that some women with PPCM share a genetic profile with familial non-ischemic dilated cardiomyopathy (NICM).102
The diagnosis of PPCM is often missed or delayed because many of the signs and symptoms of normal pregnancy can mimic those of HF, resulting in preventable complications103 such as severe HF, cardiogenic shock, thromboembolic complications, arrythmias and death.99 The incidence of intra-cardiac thrombi is higher compared to other forms of NICM, emphasizing the importance of cardiac imaging. The rate of recovery of cardiac function is variable. In the U.S., it is higher than 50% and recovery mostly occurs within 2 to 6 months after diagnosis although later recovery is possible.104 Recovery of myocardial function greatly depends on the degree of the initial myocardial insult reflected by low LVEF (<30%), enlarged LV diastolic dimension (>6.0 cm), RV dysfunction, high levels of natriuretic peptides, and troponin elevation.99,104,105 In addition, late diagnosis and African American ethnicity adversely affect recovery. The rate of LV recovery is significantly lower and the timing longer in African American patients compared with non- African American women.106 Reports of rate of recovery in other countries are variable with high recovery rates in Germany Japan, China, Pakistan and low recovery rates in Africa, Turkey, and Haiti.99
Goals of therapy are symptomatic improvement, recovery of cardiac function, prevention of thromboembolic complications and sudden death (Figure 4).99 Management of PPCM is similar to other forms of NICM and includes early use of guideline-directed medical therapy, considering fetal safety during pregnancy and lactation.99 The prolactin inhibitor, bromocriptine, has been shown in animal studies to prevent the development of a pregnancy associated cardiomyopathy. In a small clinical trial in African women bromocriptine improved the rate of recovery and outcome of patients with PPCM and has received a IIb recommendation for use in women with PPCM by the European society of cardiology guidelines.107 No information is available about the safety and efficacy of the drug in North America. A National Institute of Health supported randomized, double-blind study for the evaluation of the effect of bromocriptine in women with PPCM (The REBIRTH study) is about to start soon in the U.S. and Canada. Because of the increased incidence of thromboembolic complications, the use of prophylactic anticoagulation may be useful in women with PPCM receiving bromocriptine.108 Similarly, anticoagulation is recommended during pregnancy and for 6 weeks postpartum for women with severe left ventricular dysfunction (LVEF <35%). Data are lacking regarding stopping HF medications after normalization of LV function in this population. It may be possible to extrapolate from the TRED-HF trial, which included patients with dilated cardiomyopathy who had recovered LVEF from <40% to 50% or greater. Within 6 months of withdrawal of medications, 44% relapsed.109 Therefore, in patients with PPCM, discontinuation of medications should only be done gradually with close monitoring of LV function followed by annual assessment of LV function for early detection of recurrent cardiomyopathy.99
Figure 4.
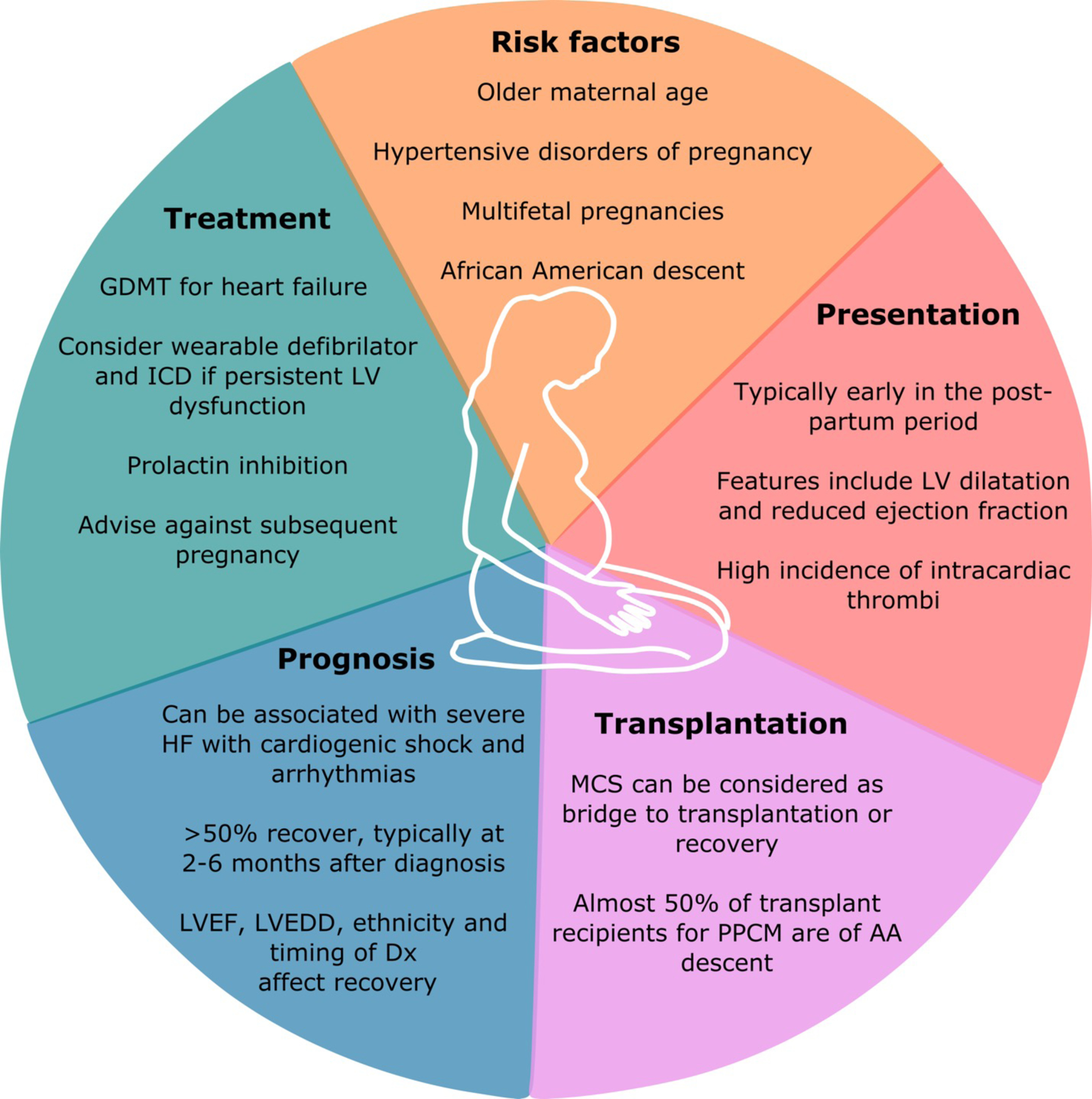
Approach to Peripartum Cardiomyopathy
Peripartum cardiomyopathy is the leading cause of HF in pregnancy. Risk factors, presentation, treatment, prognosis, and the role of transplantation are depicted here. GDMT = guideline-directed medical therapy; LV = left ventricular; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; PPCM = peripartum cardiomyopathy
Mortality is caused by sudden death in 30% of cases and most deaths occur within the first 6 months after diagnosis and in women with LVEF < 30%. Criteria for ICD and CRT-D are similar to other causes of HF, but given the high risk of sudden cardiac death, may warrant usage of wearable external defibrillators in patients with severe LV dysfunction as a bridge to recovery or permanent ICD.110 In women with persistent LV dysfunction, ICD placement is recommended.
MCS devices and heart transplantation have been used successfully in patients with cardiogenic shock not responding to medical therapy including vasoactive medications.111,112 Because the rate of recovery in patients with PPCM is higher than in those with other forms of NICM, temporary devices should be considered as a bridge to recovery before referral for cardiac transplantation. Review of the UNOS registry of patients receiving heart transplantation between 1987 and 2019 identified 666 with PPCM. These patients were younger and 48% were African American. They had fewer days on the wait list and demonstrated a greater incidence of pre-transplant panel reactive antibodies. The overall post-transplant survival was slightly lower in the PPCM group than other etiologies.113
Subsequent pregnancy in women with a history of PPCM can lead to deterioration of LV function and symptoms.114 The risk of relapse is higher in women with persistent LV dysfunction compared to those with recovered function and can be associated with significant decline in LVEF, clinical deterioration and death. Approximately 20% of women with recovered systolic function also have a decline in LVEF with the subsequent pregnancy. The change is usually mild but persists in about half of the patients. Isolated cases of severe deterioration of LV function and life-threatening arrythmias have been described; fortunately there have been no reported deaths.114
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic cardiomyopathy with a prevalence that is estimated between 1:500 and 1:200.115 Various cohort studies have suggested a slight male predominance (55–65%) both globally and in the U.S.116–118
One cohort study investigating sex differences among 969 patients with HCM in Italy and the U.S. found that women compared to men were older at diagnosis and initial evaluation.116 In this cohort, men were more likely to be diagnosed in the setting of routine medical examinations while women were more likely to be diagnosed after clinical manifestations.116 Women also were more likely than men to have exertional dyspnea, chest pain and syncope with higher outflow gradients and more likely to functionally decline or experience death from HF or stroke.116 There were no sex differences in the rate of ICD implantation, myectomy or alcohol septal ablation. There were similar findings in a Mayo Clinic study including 3,673 patients with HCM (45% women). In this study women compared to men underwent more frequent alcohol septal ablation with similar frequency of myectomy yet had significantly higher mortality at 5- and 10-years even when adjusted for age, NYHA Class III-IV symptoms, and comorbidities (aHR 1.13[1.03–1.22], p = 0.01).118 Given the sex differences in mortality in both cohorts despite similar therapy, there remain concerns that sex differences in outcome are due to sex differences in disease phenotype or progression at time of diagnosis and therapy (Figure 5).
Figure 5.
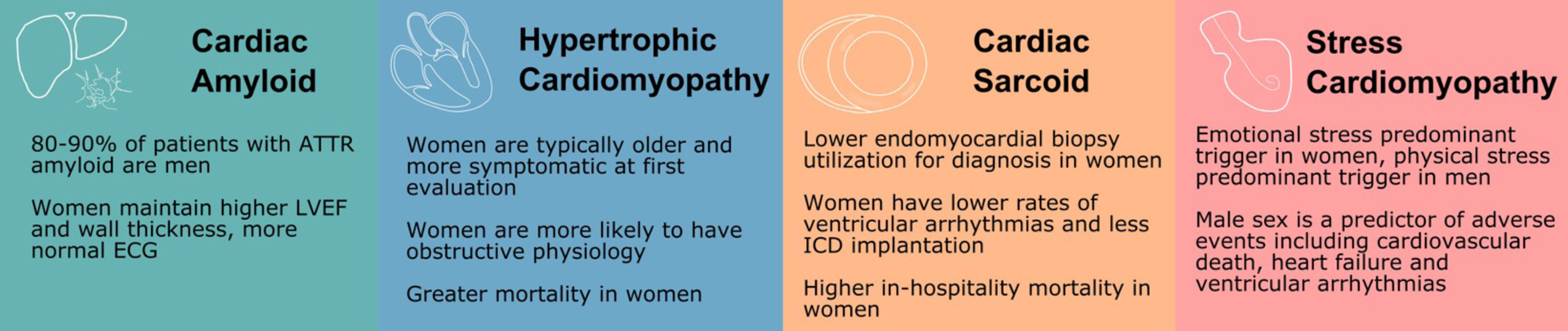
Sex Differences in Other Cardiomyopathies
Although overall less data are available in women, infiltrative and restrictive cardiomyopathies as well as stress cardiomyopathy have unique sex differences. In cardiac amyloidosis, it is unclear to what extent the male predominance of the disease is due to ascertainment bias and the potential role for sex-specific diagnostic criteria. Similarly, women with HCM are typically more symptomatic at first evaluation which may be due to the use of similar wall thickness cutoffs for both sexes.
The diagnostic criterion of LV wall thickness of at least 15 mm for HCM does not account for body surface area which may be why disease severity is underestimated in women.115,119 Among patients referred for myectomy with MYH7 or MYBPC3 mutations, women were, on average, 7 years older than men with more advanced diastolic dysfunction.119 On a cellular level, women also had a greater amount of fibrosis compared with male patients.
Takotsubo Cardiomyopathy
Stress cardiomyopathy, also referred to as Takotsubo cardiomyopathy, refers to a transient and usually reversible form of cardiomyopathy typically instigated by a physical or emotional stress. Studies suggest a strong female predominance (female to male ratio of 9:1).120 Diagnostic criteria include the Mayo Clinic Criteria as well as the International Expert Consensus Document on Takotsubo Syndrome, both of which include female sex as a risk factor.121,122 Additionally, the majority of cases occur in postmenopausal women with mean age of 67 years old. Hypotheses include estrogen deficiency and a heightened autonomic nervous system leading to increased sympathetic stimulation.123 In one case-control study of women with self-reported reproductive histories, a history of irregular menses, number of pregnancies, and use of post-menopausal hormone replacement therapy were associated with the development of Takotsubo cardiomyopathy.124
Other studies have noted sex differences in risk factors and outcome. In Japanese studies, emotional stress was a more common trigger for women while prior physical stress was more common in men. Men were younger than women and were more likely to have severe pump failure or require mechanical circulatory support. In multivariable analysis, male sex was an independent predictor of adverse cardiac events including cardiovascular death, severe pump failure and ventricular arrhythmias (adjusted OR: 4.32 (1.41–13.6), p = 0.011).125 Similar sex differences in outcomes have also been shown in other cohorts.126
Cardiac Amyloidosis
Light-chain amyloidosis (AL) and Transthyretin amyloid cardiomyopathy (ATTR-CM) represent the two most common types of cardiac amyloidosis, an infiltrative myocardial disease caused by the deposition of misfolded amyloid fibrils within the heart muscle.127–129 Light-chain amyloidosis arises from secretion and overproduction of immunoglobin light chains. Cardiac involvement has been observed in 50–75% of AL amyloid cases.130,131 There is a slight male predominance of AL cardiac amyloidosis, and the disease generally presents from the fifth to seventh decade.130 There is sparse data regarding the treatment responsiveness and prognosis of AL amyloid in women versus men. There is also limited data on the sex differences in cardiac transplantation for cardiac amyloidosis, however, single-center studies suggest similar post-transplantation outcomes in ATTR, AL, and non-amyloid cardiomyopathy.132
ATTR-CM has shifted from a rare disease entity to an increasingly recognized cardiomyopathy with sex differences in the presentation, natural history, and, more recently, enrollment in clinical studies.129,132–134 Sex differences in ATTR-CM are most marked within wild-type ATTR-CM (ATTRwt) with 80–90% of patients men.135–137 In an autopsy study of patients with an ante-mortem diagnosis of HFpEF without known amyloidosis, 17% had ATTRwt deposition with 5% having at least moderate deposition to suggest causative etiology.135,138 Males had nearly a 3 times higher risk of having amyloid deposition post-mortem. However, in a prospective screening study of patients over 60 years of age admitted with HFpEF and left ventricular wall thickness (LVWT) >12 mm, there was an equal proportion of women and men identified as having ATTR-CM by scintigraphy, suggesting ascertainment bias in longitudinal cohorts reporting sex differences.139 Furthermore, in a prospective endomyocardial biopsy study of 108 patients with HFpEF (61% women) 40% of the ATTR-CM patients were women.136
There is some evidence for increased male prevalence among familial ATTR-CM (ATTRv) amyloidosis with selected mutations associated with ATTR-CM including Leu111Met, Ileu68Leu, Thr60Ala, and Val122Ile where only 30% of cases were observed in women.140 A recent analysis from the Transthyretin Amyloidosis Outcomes Survey (THAOS) registry posited that given the higher prevalence of ATTRv in men compared to women with cardiac phenotypes and transthyretin mutations, female sex could be protective against the degree of myocardial involvement in ATTRv amyloidosis. Notably, women presented with higher LVEF, lower interventricular septal thickness, posterior wall thickness, and were less likely to have an abnormal electrocardiogram. They note that the proportion of men with ATTRv increased progressively with severity of disease based on quartiles utilizing natriuretic peptide levels (NT-proBNP), LVEF, mean left-ventricular wall thickness divided by height, and LV mass index divided by height.141 The THAOS study authors acknowledged that using LV wall thickness > 12 mm to define ATTRv cardiomyopathy may partially account for the lower proportion of women (27.8%) in the THAOS registry, given women may require a longer deposition period to reach this pre-specified wall thickness measurement. LV wall thickness is typically not indexed to height for registry and trial purposes and therefore does not account for baseline sex differences in this parameter. However, the investigators reasonably contend this measurement bias does not fully explain increasing prevalence of males in higher risk cardiac phenotypes.
Other genetic studies have raised similar concerns regarding sex differences. A study of patients with ATTR V30M found that women with type A fibrils had significantly lower median septal and posterior wall thicknesses and lower median LV mass compared to men, suggesting lower rates of cardiac infiltration.142 In another study involving Val122Ile patients , there were no sex differences in HF severity markers including NYHA class, cardiac troponin, and mortality despite women being older than men (76 vs. 69 years of age)143 , also suggesting slower disease progression in women compared to men. However, there may have been referral bias given that the proportion of women diagnosed with Val122Ile cardiac amyloid increased from 22% early in the longitudinal study (2007–2012) to 50% from 2013–2018.143
While sex-specific screening guidelines may have biologic justification and have already been proposed by amyloidosis experts,128 ascertainment biases and ATTR-CM definitions that do not account for known sex differences may alter our perception regarding ATTR-CM. Based on the existing data, it remains unknown whether ATTR-CM occurs predominantly in men or whether we have failed to ascertain the true prevalence of the disease.
Cardiac Sarcoidosis (CS)
Cardiac sarcoid has had an evolving definition that typically involves either histologic diagnosis on endomyocardial biopsy or a combination of clinical cardiac manifestations in the presence of biopsy-confirmed extra-cardiac sarcoid.144,145 Sarcoidosis tends to be more common in African-Americans, particularly women, and tends to effect residents of northern latitudes more commonly.144,146,147 The prevalence of CS has increased partly because of new imaging modalities for diagnosis and better disease understanding and recognition of CS over time.144,148,149 Prospective screening of patients with unexplained conduction abnormalities with fluorodeoxyglucose-positron emission tomography (FDG-PET) found that 34% of patients with atrioventricular block (AVB) had CS. Though this study had relatively small number of CS patients (n=11) there was no significant difference in the sex of those with CS vs. idiopathic AVB.150
In the longitudinal Myocardial Inflammatory Diseases in Finland (MIDFIN) study CS prevalence has risen more than 20-fold from 1988–2012.149 Of 110 patients with CS in this cohort, 71 had isolated CS, while 39 patients had CS with extra-cardiac disease. Those with isolated CS were more often women and had a higher frequency of LV dysfunction and septal wall motion abnormalities. Despite sex differences in the constellation of organ involvement in patients with CS, sex was not an independent predictor of outcomes in the Finnish cohort.149
The incidence of supraventricular tachycardia was comparable between men and women in a cohort in patients with CS and known cardiac involvement151, however U.S. national survey data showed that women, who constituted the significant majority of patients with documented sarcoidosis (n=200,241, 67% women), had lower rates of ICD, CRT-D and endomyocardial biopsy compared to males.152 Interestingly, women had higher rates of atrial arrhythmia, lower rates of ventricular arrhythmia, and had significantly higher in-hospital mortality compared to men (64% vs. 36%, p<.0001).152
Patients with CS have worse prognosis (i.e. sudden death and heart failure death) compared to sarcoid patients without cardiac involvement. In patients with clinical CS, LV dysfunction is the strongest predictor of survival.153 Although sex differences in outcome for CS remain limited, the underutilization of devices (ICD, CRT-D) in CS women compared to men may contribute to poor cardiac outcomes.152 Cardiac transplantation for CS in the U.S. increased from 0.1% in the mid 1990’s to 0.5% in the mid 2000’s.154 There is limited data on comparative outcomes between women and men post-transplantation with most transplant centers using more immunotherapy for CS patients compared to other transplant patients to prevent reoccurrence of disease. Potential toxicities during pregnancy and lactation should be considered, especially with steroid sparing therapeutic regimens.155,156
Conclusions
Women and men have different phenotypes of HF with unique risk factors, epidemiology, prognosis, and response to interventions. Women continue to be undertreated with guideline-directed medical therapy and device therapy. Exploratory analyses suggest that sex specific criteria may be helpful given the benefit of biventricular pacing at lower QRS in women compared to men. Additionally, existing data in the areas of hypertrophic cardiomyopathy and cardiac amyloidosis may suggest benefit of sex differential diagnostic criteria, to avoid first identifying women at a later and more advanced stage of disease. Despite the data that currently exist, there remain gaps in knowledge regarding factors driving the underuse of medical and device therapies in clinical practice and how we can mitigate these disparities. Additionally, we need increased representation of women in heart failure clinical trials. Potential strategies to achieve this goal include minimizing the burden of study visits via telehealth given sex differences in responses to in-person clinical trials and increasing female representation in clinical trials by including more women leaders.157 Furthermore, national and international guidelines should account for differential responses to treatment and outcomes by sex.
Table 2.
Sex Differences in Risk of Mortality and/or Hospitalization in Heart Failure with Preserved Ejection Fraction
| Study | Endpoint | Risk (95% CI) | |
|---|---|---|---|
| Women | Men | ||
| ARNI | |||
| PARAGON-HF91 | CV Mortality or HF Hospitalization | RR 0.73 (0.59–0.90) | RR 1.03 (0.85–1.25) |
| Aldosterone blockers | |||
| TOPCAT90 | CV Mortality or HF hospitalization | aHR 0.81 (0.63–1.05) | aHR 0.85(0.67–1.08) |
| SGLT2 inhibitors | |||
| EMPEROR-Preserved94 | CV Mortality or HF hospitalization | HR 0.75 (0.61–0.92) | HR 0.81 (0.69–0.96) |
Disclosures:
DeFilippis: No disclosures
Beale: No disclosures
Martyn: TM receives grant/research support from Akcea Therapeutics.
Agarwal: AA plans to submit a patent for heart failure polypills.
Elkayam: UE has served on a steering committee for the NIH, DSMB for V-Wave and a speakers bureau of Zoll Medical, Merck and Astra Zeneca
Lam: C.S.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Actelion, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Roche Diagnostics, Sanofi and Us2.ai; and serves as co-founder & non-executive director of Us2.ai.
Hsich: Dr. Hsich is supported by the National Heart, Lung and Blood Institute of the National Institute of Health under Award Number R01HL141892.
Abbreviations
- ACEI
angiotensin converting enzyme inhibitors
- AL
light chain amyloidosis
- ARB
angiotensin receptor blockers
- ARNI
angiotensin receptor neprilysin inhibitor
- ATTR-CM
Transthyretin amyloid cardiomyopathy
- ATTRv
familial ATTR-CM
- BAT
baroreflex activation therapy
- CRT
Cardiac Resynchronization Therapy
- CS
cardiac sarcoidosis
- LVEF
left ventricular ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ICD
implantable cardioverter defibrillator
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- NYHA
New York Heart Association
- PA
pulmonary artery
- PPCM
peripartum cardiomyopathy
- RV
right ventricle
- SGLT2i
Sodium glucose cotransporter 2 inhibitors
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW, On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation [Internet]. 2021. [cited 2021 Dec 13];143. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 3.Pandey A, Omar W, Ayers C, LaMonte M, Klein L, Allen NB, Kuller LH, Greenland P, Eaton CB, Gottdiener JS, Lloyd-Jones DM, Berry JD. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation. 2018;137:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsich EM, Piña IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–498. [DOI] [PubMed] [Google Scholar]
- 6.Mentzer G, Hsich EM. Heart Failure with Reduced Ejection Fraction in Women: Epidemiology, Outcomes, and Treatment. Heart Fail Clin. 2019;15:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsich EM, Grau-Sepulveda MV, Hernandez AF, Eapen ZJ, Xian Y, Schwamm LH, Bhatt DL, Fonarow GC. Relationship between sex, ejection fraction, and B-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: findings from the Get With The Guideline-Heart Failure Registry. Am Heart J. 2013;166:1063–1071.e3. [DOI] [PubMed] [Google Scholar]
- 8.Dewan P, Rørth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, Mogensen UM, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Differential Impact of Heart Failure With Reduced Ejection Fraction on Men and Women. J Am Coll Cardiol. 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M, DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 12.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 13.Shekelle PG, Rich MW, Morton SC, Atkinson CSW, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 17.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 18.Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure--a population study. Eur J Heart Fail. 2007;9:602–609. [DOI] [PubMed] [Google Scholar]
- 19.Adams KF, Patterson JH, Gattis WA, O’Connor CM, Lee CR, Schwartz TA, Gheorghiade M. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. [DOI] [PubMed] [Google Scholar]
- 20.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. [DOI] [PubMed] [Google Scholar]
- 21.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of Vasodilator Therapy on Mortality in Chronic Congestive Heart Failure. N Engl J Med. 1986;314:1547–1552. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AL, Lindenfeld J, Ziesche S, Walsh MN, Mitchell JE, Adams K, Tam SW, Ofili E, Sabolinski ML, Worcel M, Cohn JN, A-HeFT Investigators. Outcomes by gender in the African-American Heart Failure Trial. J Am Coll Cardiol. 2006;48:2263–2267. [DOI] [PubMed] [Google Scholar]
- 23.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 24.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24:687–698. [DOI] [PubMed] [Google Scholar]
- 25.Lindenfeld J, Ghali JK, Krause-Steinrauf HJ, Khan S, Adams K, Goldman S, Peberdy MA, Yancy C, Thaneemit-Chen S, Larsen RL, Young J, Lowes B, Rosenberg YD. Hormone replacement therapy is associated with improved survival in women with advanced heart failure. Journal of the American College of Cardiology. 2003;42:1238–1245. [DOI] [PubMed] [Google Scholar]
- 26.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–1524. [DOI] [PubMed] [Google Scholar]
- 27.Hess PL, Hernandez AF, Bhatt DL, Hellkamp AS, Yancy CW, Schwamm LH, Peterson ED, Schulte PJ, Fonarow GC, Al-Khatib SM. Sex and Race/Ethnicity Differences in Implantable Cardioverter-Defibrillator Counseling and Use Among Patients Hospitalized With Heart Failure: Findings from the Get With The Guidelines-Heart Failure Program. Circulation. 2016;134:517–526. [DOI] [PubMed] [Google Scholar]
- 28.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED, Get With the Guidelines Steering Committee and Hospitals. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaPointe NMA, Al-Khatib SM, Piccini JP, Atwater BD, Honeycutt E, Thomas K, Shah BR, Zimmer LO, Sanders G, Peterson ED. Extent of and reasons for nonuse of implantable cardioverter defibrillator devices in clinical practice among eligible patients with left ventricular systolic dysfunction. Circ Cardiovasc Qual Outcomes. 2011;4:146–151. [DOI] [PubMed] [Google Scholar]
- 30.MacFadden DR, Tu JV, Chong A, Austin PC, Lee DS. Evaluating sex differences in population-based utilization of implantable cardioverter-defibrillators: role of cardiac conditions and noncardiac comorbidities. Heart Rhythm. 2009;6:1289–1296. [DOI] [PubMed] [Google Scholar]
- 31.Patel NJ, Edla S, Deshmukh A, Nalluri N, Patel N, Agnihotri K, Patel A, Savani C, Patel N, Bhimani R, Thakkar B, Arora S, Asti D, Badheka AO, Parikh V, Mitrani RD, Noseworthy P, Paydak H, Viles-Gonzalez J, Friedman PA, Kowalski M. Gender, Racial, and Health Insurance Differences in the Trend of Implantable Cardioverter-Defibrillator (ICD) Utilization: A United States Experience Over the Last Decade. Clin Cardiol. 2016;39:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S, Machado C. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–1506. [DOI] [PubMed] [Google Scholar]
- 33.Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, Santarelli P, Di Biase L, Natale A. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7:876–882. [DOI] [PubMed] [Google Scholar]
- 34.Sticherling C, Arendacka B, Svendsen JH, Wijers S, Friede T, Stockinger J, Dommasch M, Merkely B, Willems R, Lubinski A, Scharfe M, Braunschweig F, Svetlosak M, Zürn CS, Huikuri H, Flevari P, Lund-Andersen C, Schaer BA, Tuinenburg AE, Bergau L, Schmidt G, Szeplaki G, Vandenberk B, Kowalczyk E, Eick C, Juntilla J, Conen D, Zabel M, EU-CERT-ICD Investigators. Sex differences in outcomes of primary prevention implantable cardioverter-defibrillator therapy: combined registry data from eleven European countries. Europace. 2018;20:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni AP, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–2407. [DOI] [PubMed] [Google Scholar]
- 36.Russo AM, Daugherty SL, Masoudi FA, Wang Y, Curtis J, Lampert R. Gender and outcomes after primary prevention implantable cardioverter-defibrillator implantation: Findings from the National Cardiovascular Data Registry (NCDR). Am Heart J. 2015;170:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee NA, Borgquist R, Chang Y, Lewey J, Jackson VA, Singh JP, Metlay JP, Lindvall C. Increasing sex differences in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator. Eur Heart J. 2017;38:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randolph TC, Hellkamp AS, Zeitler EP, Fonarow GC, Hernandez AF, Thomas KL, Peterson ED, Yancy CW, Al-Khatib SM. Utilization of cardiac resynchronization therapy in eligible patients hospitalized for heart failure and its association with patient outcomes. Am Heart J. 2017;189:48–58. [DOI] [PubMed] [Google Scholar]
- 40.Lund LH, Braunschweig F, Benson L, Ståhlberg M, Dahlström U, Linde C. Association between demographic, organizational, clinical, and socio-economic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail. 2017;19:1270–1279. [DOI] [PubMed] [Google Scholar]
- 41.Sridhar ARM, Yarlagadda V, Parasa S, Reddy YM, Patel D, Lakkireddy D, Wilkoff BL, Dawn B. Cardiac Resynchronization Therapy: US Trends and Disparities in Utilization and Outcomes. Circ Arrhythm Electrophysiol. 2016;9:e003108. [DOI] [PubMed] [Google Scholar]
- 42.Schuchert A, Muto C, Maounis T, Frank R, Ella RO, Polauck A, Padeletti L, MASCOT Study Group. Gender-related safety and efficacy of cardiac resynchronization therapy. Clin Cardiol. 2013;36:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma N, Manne M, Nguyen D, He J, Niebauer M, Tchou P. Probability and magnitude of response to cardiac resynchronization therapy according to QRS duration and gender in nonischemic cardiomyopathy and LBBB. Heart Rhythm. 2014;11:1139–1147. [DOI] [PubMed] [Google Scholar]
- 44.Zusterzeel R, Selzman KA, Sanders WE, Caños DA, O’Callaghan KM, Carpenter JL, Piña IL, Strauss DG. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–1348. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe UC, Brandt M-C, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG, Haller H. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–276. [DOI] [PubMed] [Google Scholar]
- 46.Lindenfeld J, Gupta R, Grazette L, Ruddy JM, Tsao L, Galle E, Rogers T, Sears S, Zannad F. Response by Sex in Patient-Centered Outcomes With Baroreflex Activation Therapy in Systolic Heart Failure. JACC Heart Fail. 2021;9:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zile MR, Abraham WT, Weaver FA, Butter C, Ducharme A, Halbach M, Klug D, Lovett EG, Müller-Ehmsen J, Schafer JE, Senni M, Swarup V, Wachter R, Little WC. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail. 2015;17:1066–1074. [DOI] [PubMed] [Google Scholar]
- 48.Kosmidou I, Lindenfeld J, Abraham WT, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Gaba P, Rogers JH, Shahim B, Redfors B, Zhang Z, Mack MJ, Stone GW. Sex-Specific Outcomes of Transcatheter Mitral-Valve Repair and Medical Therapy for Mitral Regurgitation in Heart Failure. JACC: Heart Failure. 2021;9:674–683. [DOI] [PubMed] [Google Scholar]
- 49.Park S-D, Orban M, Karam N, Lubos E, Kalbacher D, Braun D, Stolz L, Neuss M, Butter C, Praz F, Kassar M, Petrescu A, Pfister R, Iliadis C, Unterhuber M, Lurz P, Thiele H, Baldus S, von Bardeleben S, Blankenberg S, Massberg S, Windecker S, Hausleiter J, EuroSMR Investigators. Sex-Related Clinical Characteristics and Outcomes of Patients Undergoing Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc Interv. 2021;14:819–827. [DOI] [PubMed] [Google Scholar]
- 50.Thangam M, Luke AA, Johnson DY, Amin AP, Lasala J, Huang K, Joynt Maddox KE. Sociodemographic differences in utilization and outcomes for temporary cardiovascular mechanical support in the setting of cardiogenic shock. Am Heart J. 2021;236:87–96. [DOI] [PubMed] [Google Scholar]
- 51.DeFilippis EM, Truby LK, Garan AR, Givens RC, Takeda K, Takayama H, Naka Y, Haythe JH, Farr MA, Topkara VK. Sex-Related Differences in Use and Outcomes of Left Ventricular Assist Devices as Bridge to Transplantation. JACC Heart Fail. 2019;7:250–257. [DOI] [PubMed] [Google Scholar]
- 52.Nayak A, Hu Y, Ko Y-A, Mehta A, Liu C, Pennington J, Xie R, Cowger J, Kirklin JK, Kormos RL, Simon MA, Morris AA. Gender Differences in Mortality After Left Ventricular Assist Device Implant: A Causal Mediation Analysis Approach. ASAIO J. 2021;67:614–621. [DOI] [PubMed] [Google Scholar]
- 53.Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A, EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4:531–539. [DOI] [PubMed] [Google Scholar]
- 54.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction: Prevalence, Therapies, and Outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 56.Oktay AA, Rich JD, Shah SJ. The Emerging Epidemic of Heart Failure with Preserved Ejection Fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of New-Onset Heart Failure: Differences in Preserved Versus Reduced Ejection Fraction. Circ: Heart Failure. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ: Heart Failure [Internet]. 2016. [cited 2021 Aug 16];9. Available from: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.115.003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC: Heart Failure. 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu W-C, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ: Heart Failure [Internet]. 2016. [cited 2021 Aug 16];9. Available from: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.115.002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madan N, Itchhaporia D, Albert CM, Aggarwal NT, Volgman AS. Atrial Fibrillation and Heart Failure in Women. Heart Fail Clin. 2019;15:55–64. [DOI] [PubMed] [Google Scholar]
- 63.Thompson LE, Maddox TM, Lei L, Grunwald GK, Bradley SM, Peterson PN, Masoudi FA, Turchin A, Song Y, Doros G, Davis MB, Daugherty SL. Sex Differences in the Use of Oral Anticoagulants for Atrial Fibrillation: A Report From the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry. J Am Heart Assoc. 2017;6:e005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanjoy SS, Choi Y-H, Sparrow RT, Baron SJ, Abbott JD, Azzalini L, Holmes DR, Alraies MC, Tzemos N, Ayan D, Mamas MA, Bagur R. Sex Differences in Outcomes Following Left Atrial Appendage Closure. Mayo Clin Proc. 2021;96:1845–1860. [DOI] [PubMed] [Google Scholar]
- 65.Biondi-Zoccai GGL. Reduced post-infarction myocardial apoptosis in women: a clue to their different clinical course? Heart. 2005;91:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunlay SM, Roger VL. Gender Differences in the Pathophysiology, Clinical Presentation, and Outcomes of Ischemic Heart Failure. Curr Heart Fail Rep. 2012;9:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and Gender-Related Ventricular-Vascular Stiffening: A Community-Based Study. Circulation. 2005;112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 68.Gori M, Lam CSP, Gupta DK, Santos ABS, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJV, Solomon SD, PARAMOUNT Investigators. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542. [DOI] [PubMed] [Google Scholar]
- 69.Shim CY, Park S, Choi D, Yang W-I, Cho I-J, Choi E-Y, Chung N, Ha J-W. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–1233. [DOI] [PubMed] [Google Scholar]
- 70.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart. 2016;102:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 73.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction - PubMed. Circ Heart Failure. 2011;4:257–65. [DOI] [PubMed] [Google Scholar]
- 74.Sabbatini AR, Kararigas G. Menopause-Related Estrogen Decrease and the Pathogenesis of HFpEF: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:1074–1082. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barton M, Meyer MR. Heart Failure With Preserved Ejection Fraction in Women: New Clues to Causes and Treatment. JACC Basic Transl Sci. 2020;5:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sickinghe AA, Korporaal SJA, den Ruijter HM, Kessler EL. Estrogen Contributions to Microvascular Dysfunction Evolving to Heart Failure With Preserved Ejection Fraction. Front Endocrinol. 2019;10:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saeed S, Dweck MR, Chambers J. Sex differences in aortic stenosis: from pathophysiology to treatment. Expert Review of Cardiovascular Therapy. 2020;18:65–76. [DOI] [PubMed] [Google Scholar]
- 79.Beale AL, Nanayakkara S, Segan L, Mariani JA, Maeder MT, van Empel V, Vizi D, Evans S, Lam CSP, Kaye DM. Sex Differences in Heart Failure With Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail. 2019;7:239–249. [DOI] [PubMed] [Google Scholar]
- 80.Lau ES, Cunningham T, Hardin KM, Liu E, Malhotra R, Nayor M, Lewis GD, Ho JE. Sex Differences in Cardiometabolic Traits and Determinants of Exercise Capacity in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2020;5:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D. Functional Status, Pulmonary Artery Pressure, and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:189–199. [DOI] [PubMed] [Google Scholar]
- 82.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, Lefkowitz MP, Shi VC, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV, Solomon SD, PARAGON-HF Investigators. Health-Related Quality of Life in Heart Failure With Preserved Ejection Fraction: The PARAGON-HF Trial. JACC Heart Fail. 2019;7:862–874. [DOI] [PubMed] [Google Scholar]
- 84.Lam CSP, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile MR, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2012;5:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh I, Oliveira RKF, Heerdt PM, Pari R, Systrom DM, Waxman AB. Sex-Related Differences in Dynamic Right Ventricular-Pulmonary Vascular Coupling in Heart Failure With Preserved Ejection Fraction. Chest. 2021;159:2402–2416. [DOI] [PubMed] [Google Scholar]
- 87.Duca F, Zotter-Tufaro C, Kammerlander AA, Aschauer S, Binder C, Mascherbauer J, Bonderman D. Gender-related differences in heart failure with preserved ejection fraction. Sci Rep. 2018;8:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel RB, Li E, Benefield BC, Swat SA, Polsinelli VB, Carr JC, Shah SJ, Markl M, Collins JD, Freed BH. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020;7:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer P, Beale AL. Sex differences in heart failure with preserved ejection therapy: potential mechanisms and clinical implications. EMJ Cardiology. 2020;82–91. [Google Scholar]
- 90.Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex Differences in Outcomes and Responses to Spironolactone in Heart Failure With Preserved Ejection Fraction: A Secondary Analysis of TOPCAT Trial. JACC Heart Fail. 2019;7:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS, Solomon SD. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation. 2020;141:338–351. [DOI] [PubMed] [Google Scholar]
- 92.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH. Women Have Higher Left Ventricular Ejection Fractions Than Men Independent of Differences in Left Ventricular Volume: The Dallas Heart Study. Circulation. 2006;113:1597–1604. [DOI] [PubMed] [Google Scholar]
- 93.Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin Cardiac Amyloidosis in Older Americans. J Card Fail. 2016;22:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021; [Google Scholar]
- 95.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 96.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. [DOI] [PubMed] [Google Scholar]
- 97.DeFilippis EM, Henderson J, Axsom KM, Costanzo MR, Adamson PB, Miller AB, Brett M-E, Givertz MM. Remote Hemodynamic Monitoring Equally Reduces Heart Failure Hospitalizations in Women and Men in Clinical Practice: A Sex-Specific Analysis of the CardioMEMS Post-Approval Study. Circ Heart Fail. 2021;14:e007892. [DOI] [PubMed] [Google Scholar]
- 98.Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB, Costanzo MR. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. The Lancet. 2021;398:991–1001. [DOI] [PubMed] [Google Scholar]
- 99.Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:207–221. [DOI] [PubMed] [Google Scholar]
- 100.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, Shotan A. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. [DOI] [PubMed] [Google Scholar]
- 101.Hameed AB, Lawton ES, McCain CL, Morton CH, Mitchell C, Main EK, Foster E. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. 2015;213:379.e1–10. [DOI] [PubMed] [Google Scholar]
- 102.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley-Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J, McNamara DM, Seidman CE, Seidman JG, Arany Z. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N Engl J Med. 2016;374:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LSC, Illum S, Hatamizadeh P, Elkayam U. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–650. [DOI] [PubMed] [Google Scholar]
- 104.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J, Wu W-C, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD, IPAC Investigators. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goland S, Bitar F, Modi K, Safirstein J, Ro A, Mirocha J, Khatri N, Elkayam U. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011;17:426–430. [DOI] [PubMed] [Google Scholar]
- 106.Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA, Arany Z. Comparison of Clinical Characteristics and Outcomes of Peripartum Cardiomyopathy Between African American and Non-African American Women. JAMA Cardiol. 2017;2:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA ESC Scientific Document Group, Deaton C, Simpson IA, Aboyans V, Agewall S, Barbato E, Calda P, Coca A, Coman IM, De Backer J, Delgado V, Di Salvo G, Fitzsimmons S, Fitzsimons D, Garbi M, Gevaert S, Hindricks G, Jondeau G, Kluin J, Lionis C, McDonagh TA, Meier P, Moons P, Pantazis A, Piepoli MF, Rocca B, Roffi M, Rosenkranz S, Sarkozy A, Shlyakhto E, Silversides CK, Sliwa K, Sousa-Uva M, Tamargo J, Thorne S, Van de Velde M, Williams B, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Zamorano JL, Hammoudi N, Piruzyan A, Mascherbauer J, Samadov F, Prystrom A, Pasquet A, Caluk J, Gotcheva N, Skoric B, Heracleous H, Vejlstrup N, Maser M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. European Heart Journal. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 108.Ricke-Hoch M, Pfeffer TJ, Hilfiker-Kleiner D. Peripartum cardiomyopathy: basic mechanisms and hope for new therapies. Cardiovasc Res. 2020;116:520–531. [DOI] [PubMed] [Google Scholar]
- 109.Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. The Lancet. 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016;133:1397–1409. [DOI] [PubMed] [Google Scholar]
- 111.Elkayam U, Schäfer A, Chieffo A, Lansky A, Hall S, Arany Z, Grines C. Use of Impella heart pump for management of women with peripartum cardiogenic shock. Clin Cardiol. 2019;42:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naoum EE, Chalupka A, Haft J, MacEachern M, Vandeven CJM, Easter SR, Maile M, Bateman BT, Bauer ME. Extracorporeal Life Support in Pregnancy: A Systematic Review. J Am Heart Assoc. 2020;9:e016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Genyk PA, Liu GS, Nattiv J, Banankhah P, Kingsford P, Li JP, Wolfson AM, Vucicevic D, DePasquale EC, Vaidya AS. Heart Transplantation Outcomes in Adults with Postpartum Cardiomyopathy. The Journal of Heart and Lung Transplantation. 2020;39:S223–S224. [Google Scholar]
- 114.Elkayam U Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014;64:1629–1636. [DOI] [PubMed] [Google Scholar]
- 115.van Driel B, Nijenkamp L, Huurman R, Michels M, van der Velden J. Sex differences in hypertrophic cardiomyopathy: new insights. Curr Opin Cardiol. 2019;34:254–259. [DOI] [PubMed] [Google Scholar]
- 116.Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender-Related Differences in the Clinical Presentation and Outcome of Hypertrophic Cardiomyopathy. Journal of the American College of Cardiology. 2005;46:480–487. [DOI] [PubMed] [Google Scholar]
- 117.Husser D, Ueberham L, Jacob J, Heuer D, Riedel-Heller S, Walker J, Hindricks G, Bollmann A. Prevalence of clinically apparent hypertrophic cardiomyopathy in Germany-An analysis of over 5 million patients. PLoS One. 2018;13:e0196612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, Miller VM, Nishimura RA, Oh JK, Schaff HV, Gersh BJ, Ommen SR. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38:3434–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nijenkamp LLAM, Bollen IAE, van Velzen HG, Regan JA, van Slegtenhorst M, Niessen HWM, Schinkel AFL, Krüger M, Poggesi C, Ho CY, Kuster DWD, Michels M, van der Velden J. Sex Differences at the Time of Myectomy in Hypertrophic Cardiomyopathy. Circ: Heart Failure [Internet]. 2018. [cited 2021 Oct 5];11. Available from: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.117.004133 [DOI] [PubMed] [Google Scholar]
- 120.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 121.Ghadri J-R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y.-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. European Heart Journal. 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. American Heart Journal. 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 123.Agdamag AC, Patel H, Chandra S, Rao A, Suboc TM, Marinescu K, Ledsky C, Volgman AS. Sex Differences in Takotsubo Syndrome: A Narrative Review. Journal of Women’s Health. 2020;29:1122–1130. [DOI] [PubMed] [Google Scholar]
- 124.Salmoirago-Blotcher E, Dunsiger S, Swales HH, Aurigemma GP, Ockene I, Rosman L, Wittstein IS. Reproductive History of Women With Takotsubo Cardiomyopathy. Am J Cardiol. 2016;118:1922–1928. [DOI] [PubMed] [Google Scholar]
- 125.Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, Nagao K, Yamamoto T, Takayama M. Gender Differences in Patients with Takotsubo Cardiomyopathy: Multi-Center Registry from Tokyo CCU Network. PLoS One. 2015;10:e0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schneider B, Athanasiadis A, Stöllberger C, Pistner W, Schwab J, Gottwald U, Schoeller R, Gerecke B, Hoffmann E, Wegner C, Sechtem U. Gender differences in the manifestation of tako-tsubo cardiomyopathy. International Journal of Cardiology. 2013;166:584–588. [DOI] [PubMed] [Google Scholar]
- 127.Donnelly JP, Hanna M. Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med. 2017;84:12–26. [DOI] [PubMed] [Google Scholar]
- 128.Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, Gospodinova M, Obici L, Rapezzi C, Garcia-Pavia P. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Heart Fail. 2019;7:709–716. [DOI] [PubMed] [Google Scholar]
- 129.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Falk RH, Alexander KM, Liao R, Dorbala S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol. 2016;68:1323–1341. [DOI] [PubMed] [Google Scholar]
- 131.Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, Hoffman J, Jaber W, Soman P, Witteles RM, Grogan M. Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:2851–2862. [DOI] [PubMed] [Google Scholar]
- 132.Barrett CD, Alexander KM, Zhao H, Haddad F, Cheng P, Liao R, Wheeler MT, Liedtke M, Schrier S, Arai S, Weisshaar D, Witteles RM. Outcomes in Patients With Cardiac Amyloidosis Undergoing Heart Transplantation. JACC Heart Fail. 2020;8:461–468. [DOI] [PubMed] [Google Scholar]
- 133.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR-ACT Study Investigators. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018; [DOI] [PubMed] [Google Scholar]
- 134.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Planté-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C, THAOS Investigators. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Planté-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceição I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA, Coelho T. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 136.Bruno M, Castaño A, Burton A, Grodin JL. Transthyretin amyloid cardiomyopathy in women: frequency, characteristics, and diagnostic challenges. Heart Fail Rev. 2021;26:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.González-López E, Gagliardi C, Dominguez F, Quarta CC, de Haro-Del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo-Marcos M, Lorenzini M, Lara-Pezzi E, Foffi S, Alonso-Pulpon L, Rapezzi C, Garcia-Pavia P. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38:1895–1904. [DOI] [PubMed] [Google Scholar]
- 138.Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG, Caringal-Galima J, Manwani R, Sharpley FA, Wechalekar AD, Lachmann HJ, Mahmood S, Sachchithanantham S, Drage EPS, Jenner HD, McDonald R, Bertolli O, Calleja A, Hawkins PN, Gillmore JD. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation. 2019;140:16–26. [DOI] [PubMed] [Google Scholar]
- 139.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020;8:712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, Wixner J, Maurer MS, Garcia-Pavia P, Tournev I, Planté-Bordeneuve V, Chapman D, Amass L, THAOS Investigators. Sex-Related Risk of Cardiac Involvement in Hereditary Transthyretin Amyloidosis: Insights From THAOS. JACC Heart Fail. 2021;S2213-1779(21)00229–8. [DOI] [PubMed] [Google Scholar]
- 142.Arvidsson S, Pilebro B, Westermark P, Lindqvist P, Suhr OB. Amyloid Cardiomyopathy in Hereditary Transthyretin V30M Amyloidosis - Impact of Sex and Amyloid Fibril Composition. PLoS One. 2015;10:e0143456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Batra J, Rosenblum H, Defilippis EM, Griffin JM, Saith SE, Gamino D, Teruya S, Santos JDL, Helmke S, Burkhoff D, Maurer MS. Sex Differences in the Phenotype of Transthyretin Cardiac Amyloidosis Due to Val122Ile Mutation: Insights from Noninvasive Pressure-Volume Analysis. J Card Fail. 2021;27:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, Baughman R, Fayad ZA, Judson MA. Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1878–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 146.Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018;9:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–127. [PubMed] [Google Scholar]
- 148.Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021;107:1591–1599. [DOI] [PubMed] [Google Scholar]
- 149.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietilä-Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 150.Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, Davis D, Ohira H, Gollob MH, Leung E, Healey JS, Birnie DH. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 151.Viles-Gonzalez JF, Pastori L, Fischer A, Wisnivesky JP, Goldman MG, Mehta D. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013;143:1085–1090. [DOI] [PubMed] [Google Scholar]
- 152.Durugu S, Gonuguntla K, Patil S, Rojulpote C, Vyata V, Karambelkar P, Narayanareddy P, Vuthaluru K, Bhattaru A. Gender Differences in Rates of Arrhythmias, Cardiac Implantable Electronic Devices, and Diagnostic Modalities Among Sarcoidosis Patients. Cureus. 2020;12:e7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. [DOI] [PubMed] [Google Scholar]
- 154.Birnie DH, Nery PB, Ha AC, Beanlands RSB. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 155.Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, da Silva J, Nelson-Piercy C, Cetin I, Costedoat-Chalumeau N, Dolhain R, Förger F, Khamashta M, Ruiz-Irastorza G, Zink A, Vencovsky J, Cutolo M, Caeyers N, Zumbühl C, Østensen M. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 156.Birru Talabi M, Clowse MEB. Antirheumatic medications in pregnancy and breastfeeding. Curr Opin Rheumatol. 2020;32:238–246. [DOI] [PubMed] [Google Scholar]
- 157.Whitelaw S, Sullivan K, Eliya Y, Alruwayeh M, Thabane L, Yancy CW, Mehran R, Mamas MA, Van Spall HGC. Trial characteristics associated with under‐enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur J Heart Fail. 2021;23:15–24. [DOI] [PubMed] [Google Scholar]
- 158.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. [DOI] [PubMed] [Google Scholar]
- 159.Rossello X, Ferreira JP, Pocock SJ, McMurray JJV, Solomon SD, Lam CSP, Girerd N, Pitt B, Rossignol P, Zannad F. Sex differences in mineralocorticoid receptor antagonist trials: a pooled analysis of three large clinical trials. Eur J Heart Fail. 2020;22:834–844. [DOI] [PubMed] [Google Scholar]
- 160.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 161.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 162.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II). Circulation. 2001;103:375–380. [DOI] [PubMed] [Google Scholar]
- 163.Ghali JK, Piña IL, Gottlieb SS, Deedwania PC, Wikstrand JC, MERIT-HF Study Group. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in Metoprolol Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF). Circulation. 2002;105:1585–1591. [DOI] [PubMed] [Google Scholar]


