Abstract
INTRODUCTION:
In the published studies of early liver transplantation (LT) for alcohol-associated hepatitis (AH), patients with a prior liver decompensation are excluded. The appropriateness of this criteria is unknown.
METHODS:
Among 6 American Consortium of Early Liver Transplantation for Alcohol-Associated Hepatitis sites, we included consecutive early LT for clinically diagnosed AH between 2007 and 2020. Patients were stratified as first vs prior history of liver decompensation, with the latter defined as a diagnosis of ascites, hepatic encephalopathy, variceal bleeding, or jaundice, and evidence of alcohol use after this event. Adjusted Cox regression assessed the association of first (vs prior) decompensation with post-LT mortality and harmful (i.e., any binge and/or frequent) alcohol use.
RESULTS:
A total of 241 LT recipients (210 first vs 31 prior decompensation) were included: median age 43 vs 38 years (P = 0.23), Model for End-Stage Liver Disease Sodium score of 39 vs 39 (P = 0.98), and follow-up after LT 2.3 vs 1.7 years (P = 0.08). Unadjusted 1- and 3-year survival among first vs prior decompensation was 93% (95% confidence interval [CI] 89%–96%) vs 86% (95% CI 66%–94%) and 85% (95% CI 79%–90%) vs 78% (95% CI 57%–89%). Prior (vs first) decompensation was associated with higher adjusted post-LT mortality (adjusted hazard ratio 2.72, 95% CI 1.61–4.59) and harmful alcohol use (adjusted hazard ratio 1.77, 95% CI 1.07–2.94).
DISCUSSION:
Prior liver decompensation was associated with higher risk of post-LT mortality and harmful alcohol use. These results are a preliminary safety signal and validate first decompensation as a criterion for consideration in early LT for AH patients. However, the high 3-year survival suggests a survival benefit for early LT and the need for larger studies to refine this criterion. These results suggest that prior liver decompensation is a risk factor, but not an absolute contraindication to early LT.
INTRODUCTION
Alcohol-associated liver disease (ALD) accounts for approximately half of all liver disease mortality in the United States (1,2). Alcohol-associated hepatitis (AH) is an acute manifestation of ALD, with the most severe classification carrying up to 70% mortality at 6 months (3–5). Although corticosteroids have been shown to improve short-term survival, no medications have been shown to improve long-term survival (6–10). For patients deemed nonresponders to corticosteroid treatment, liver transplantation (LT) provides definitive therapy. Historically, mandated periods of sobriety were a key component in patient selection in transplant for ALD. The high rate of mortality in AH has prompted challenges to these conventions. An early study demonstrated that a carefully selected population of patients transplanted for AH without a mandated period of sobriety had a 6-month survival of 77% vs 22% in those not transplanted (11). The American Consortium of Early Liver Transplantation for Alcohol-Associated Hepatitis (ACCELERATE-AH) is a multicenter observational study group evaluating LT and alcohol-associated outcomes in early LT for severe AH (12). ACCELERATE-AH has found that any alcohol use was found in 25% of patients at 1 year, with 11% of patients returning to sustained drinking (12,13). Further modeling has supported the idea that early LT provides survival benefits when compared with mandating a period of sobriety (14).
The promising outcomes of this shift in patient selection have been reflected in major organization guidelines, with the American Association for the Study of Liver Disease and the European Association for the Study of Liver Diseases recommending early transplant evaluation for selected patients with AH (15). Given the shortage of available organs for transplant, further work has aimed to identify criteria that can be applied to guide candidate selection of patients with acceptable rates of post-LT survival and alcohol use. Central to inclusion criteria in previous studies has been the requirement that the episode of severe AH be the first decompensating event in a patient without previous knowledge of liver disease (11,12). The utility of this empiric criterion for first decompensating event is still unclear and should be investigated. Cessation of alcohol use in patients with AH has been shown to have a strong positive impact on long-term mortality. Incidence of alcohol use after hospitalization for severe AH exceeds 25% at 1 year and exceeds 50% with long-term follow-up (3,4). Patients who return to alcohol use after one episode of AH have a high risk of recurrent AH and a higher overall mortality rate (3,4). In the population of LT recipients for AH, attempts have been made to predict risk of alcohol use, including derivation of the Sustained Alcohol Use After Liver Transplantation score (16). It is largely unknown, however, how outcomes compare among early LT recipients with severe AH with prior vs first decompensation with respect to mortality and alcohol use. The need to address this knowledge gap is heightened by the increase in alcohol consumption during the coronavirus disease 2019 pandemic and reported 325% increase in transplant listings for AH in this period compared with prepandemic trends (17–20).
To inform this knowledge gap, we aimed to investigate outcomes among early LT recipients with severe AH with prior vs first decompensation. We have previously demonstrated that early LT can be lifesaving in carefully selected patients. This study expands the selected patient population by providing updated follow-up and additional patients from the original ACCELERATE-AH cohorts, in addition to expanding inclusion criteria to patients who underwent early LT for severe AH with subsequent alcohol use, despite previous decompensating events and knowledge of liver disease.
METHODS
Study population
The methods for derivation of the ACCELERATE-AH cohort have been previously described (12). Briefly, adult (age > 18 years) patients were included if they received LT for clinically diagnosed severe AH with no prior liver decompensation (identified as “first decompensation” group within this article). Exclusion criteria were other proven etiologies of liver disease (such as viral hepatitis), human immunodeficiency virus infection, or other medical contraindications to LT. This current study includes additional patients and updated follow-up from previous ACCELERATE-AH publications, which expands the total number of patients analyzed (12,13). We included patients who received early LT for AH with prior liver decompensation during this same period, defined as the “prior decompensation” cohort. The prior decompensation cohort includes patients with a prior diagnosis of ascites, hepatic encephalopathy, variceal bleeding, or jaundice, and evidence of any alcohol use between this liver decompensating event and the index AH episode leading to early LT. All patients carried a diagnosis of ALD, but the prior decompensation was not necessarily because of AH. Formal selection criteria and protocols did not differ between the “prior” and “first” decompensation cohorts among ACCELERATE-AH sites included in this study. The focus of this current study is to compare outcomes from the prior decompensation group to the first decompensation group. Given the retrospective and deidentified nature of data collection, exemption from individualized informed consent was approved, and this study was conducted in accordance with the 1975 Declaration of Helsinki. No organs for transplant were obtained from executed prisoners or other institutionalized persons.
To mitigate confounding by center-effect, only centers who performed early LT for patients with prior decompensation were included in this study. Thus, data from 6 ACCELERATE-AH sites were analyzed retrospectively for consecutive patients with a prior liver decompensating event who underwent LT for severe AH from 2007 to 2020 without a mandated period of sobriety. The interval of alcohol abstinence before LT was defined as the time between last drink and transplantation date. Severity of the AH episode was based on Maddrey discriminant function, with severe AH defined as a score of 32 or higher. All patients included in this study had severe disease by this criterion.
Investigators from each center retrospectively collected data about baseline characteristics, psychosocial profiles (e.g., substance abuse history, family history of alcohol use disorder, history of alcohol-related legal issues, and history of rehabilitation attempts), quantification of pre-LT alcohol use, and post-LT outcomes (i.e., graft failure, survival, and post-LT alcohol use). There was no mandated or prescribed abstinence period for any patient included in this study. Laboratory values were recorded at initial hospitalization for severe AH, transplant listing, and transplantation date. Variables were abstracted from outside hospital records if patient initially presented to an outside hospital before transfer to the liver transplantation center. All variables that could not be gathered were coded as missing. Psychosocial profiles and quantification of pre-LT alcohol use were drawn from LT evaluation records. Records of explant histology were evaluated for steatohepatitis (e.g., ballooning hepatocytes, Mallory bodies, and neutrophil predominance) and fibrosis. To address potential misclassification bias related to lack of liver biopsy, we categorized patients whether they met or did not meet inclusion criteria for AH as defined by the National Institute on Alcohol Abuse and Alcoholism Alcoholic Hepatitis Consortia using explant pathology to assess for histologic findings of steatohepatitis (13). All patients were evaluated by a transplantation social worker with detailed substance abuse evaluation. One drink is defined as a US standard drink (14 g of alcohol in one unit).
Assessment of alcohol use after transplant
Methods for assessment of alcohol use after transplant have been reported previously (12,13). In brief, all centers performed routine interview at every post-LT visit to elicit alcohol use, and almost all patients had biomarker screening for alcohol metabolites (either urine ethylglucuronide or blood phosphatidylethanol). These combined methods were well used with only 5% missing longitudinal alcohol use data when captured in 3-month intervals for the first year after LT and in 6-month intervals thereafter (13). Quantity of alcohol use after LT was captured with the average daily alcohol consumption based on patient report. Harmful alcohol use was defined as presence of binge drinking, which was defined as $5 drinks in men, $4 drinks in women in one setting, and/or frequent drinking, which was defined as alcohol use $ 4 days per week.
Statistical analysis
Demographic, clinical characteristics, and pre-LT disease management were described using frequency (percentage) and median (interquartile range [IQR]). Post-LT survival rates and post-LT alcohol use were estimated with time-to-event analysis using the Kaplan-Meier method, with follow-up time starting at LT date and ending at date of death or date of first alcohol use, respectively. Patients without the event of interest were censored at last follow-up, whichever occurred first. Schoenfeld residuals test verified that the assumption of proportionality was met for all variables in the multivariable Cox regression models. To assess for potential bias because of differential follow-up times, post-LT survival and alcohol use were compared across groups at 1, 2, and 3 years after LT, in addition to overall post-LT survival and alcohol use. To evaluate factors associated with post-LT mortality and post-LT alcohol use, Cox proportional hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs), with robust SEs to account for center clustering. Factors with univariate P < 0.1 were included in initial multivariable models, with final models selected using backward elimination (P for removal >0.05). All variables had 5% or less missing data.
RESULTS
Study cohort
A total of 241 (210 with first decompensation, and 31 with prior decompensation) LT recipients between 2007 and 2020 from 6 LT centers were included in this study. Demographics, clinical characteristics, and psychosocial profiles were similar across study groups, except patients with first (vs prior) were less likely to have had multiple rehabilitation attempts (6 vs 27%, P < 0.001) and had more drinks per day before abstinence (12 vs 9, P = 0.03). Patients meeting National Institute on Alcohol Abuse and Alcoholism inclusion criteria for AH clinical trials were similar among first (83%) vs prior (87%) decompensation groups (P = 0.60) (21). Among LT recipients with explant histology available (N = 236), the proportion of LT recipients with features of steatohepatitis were similar among first (149 of 206 [72%]) vs prior (24 of 30 [80%]) decompensation groups (P = 0.38). All explants had signs of advanced fibrosis. Median Sustained Alcohol Use After Liver Transplantation score for first (4; IQR 0, 4) vs prior (4; IQR 1, 4) decompensation groups was similar. Loss to follow-up because of nonadherence for first (5 of 210 [2%]) vs prior (1 of 31 [3%]) decompensation groups was similar (P = 0.78). Median follow-up time after LT was 2.3 years (IQR 1.1, 4.0) in the first decompensation group and 1.7 years (IQR 0.6, 2.9) in the prior decompensation group (P = 0.08). Patient characteristics among first vs prior decompensation groups are summarized in Table 1.
Table 1.
Baseline characteristics at LT among first vs prior decompensation groups
| Factor | First decompensation n = 210 (100%) | Prior decompensation N = 31 (100%) | P value |
|---|---|---|---|
| Agea | 43 (35, 52) | 38 (36, 47) | 0.23 |
| Male | 143 (68) | 16 (52) | 0.07 |
| Race | |||
| White | 177 (85) | 27 (87) | 0.97 |
| African American | 13 (6) | 2 (6) | |
| Latinx | 8 (4) | 1 (3) | |
| Asian | 8 (4) | 1 (3) | |
| Other | 3 (1) | 0 (0) | |
| Insurance | |||
| Private | 138 (66) | 22 (71) | 0.73 |
| Medicare | 14 (7) | 1 (3) | |
| Medicaid | 56 (27) | 8 (26) | |
| Body mass indexa | 29 (25–34) | 31 (25–38) | 0.16 |
| Sodiuma | 136 (133, 140) | 138 (134, 141) | 0.42 |
| INRa | 2.2 (1.9, 2.8) | 2.4 (2.1, 2.9) | 0.35 |
| Bilirubina | 26 (16, 35) | 26 (19, 34) | 0.78 |
| Creatininea | 2.3 (1.4, 3.9) | 1.7 (1.5, 2.8) | 0.28 |
| Renalreplacement therapy | 121 (58) | 20 (67) | 0.35 |
| MELD-Na Scorea | 39 (35, 40) | 39 (36, 40) | 0.98 |
| On ventilator | 33 (17) | 9 (30) | 0.08 |
| Overt hepatic encephalopathy | 100 (50) | 13 (43) | 0.46 |
| Days of abstinencea | 45 (25, 73) | 52 (25, 109) | 0.09 |
| Married or significant other | 134 (64) | 23 (74) | 0.51 |
| History of psychiatric disease | 99 (47) | 17 (57) | 0.33 |
| Current smoker | 42 (21) | 9 (31) | 0.23 |
| Current marijuana | 18 (9) | 4 (13) | 0.47 |
| History of illicit drug use | 28 (14) | 5 (17) | 0.65 |
| History of failed rehab attempts | |||
| No priors | 146 (71) | 16 (53) | <0.001 |
| 1 prior | 47 (23) | 6 (20) | |
| Multiple | 12 (6) | 8 (27) | |
| Family history of alcohol use disorder | |||
| None | 113 (56) | 13 (46) | 0.51 |
| Extended family | 24 (12) | 3 (11) | |
| Immediate family | 64 (32) | 12 (43) | |
| Employment immediately before presentation | 114 (54) | 13 (42) | 0.20 |
| Legal history (e.g., DUI and custody loss because of alcohol) | |||
| None | 167 (80) | 22 (73) | 0.21 |
| 1 prior | 26 (12) | 3 (10) | |
| Multiple | 15 (7) | 5 (17) | |
| Alcoholunits perdaya | 12 (7, 18) | 9 (6, 14) | 0.03 |
| Years of heavy drinking | 12 (6, 20) | 11 (7, 20) | 0.79 |
| Meets NIAAA criteria for AH | 174 (83) | 26 (87) | 0.60 |
| Follow-up time in yearsa | 2.3 (1.1, 4.0) | 1.7 (0.6, 2.9) | 0.08 |
AH, alcohol-associated hepatitis; INR, international normalized ratio; IQR, interquartile range; MELD-Na, Model for End-Stage Liver Disease-Sodium score; NIAAA, National Institute on Alcohol Abuse and Alcoholism.
Median (IQR).
Prior decompensation characteristics
Among the 31 LT recipients with prior decompensation, the index decompensating event occurred a median of 283 days before LT (IQR 182, 679 days). These prior episodes of decompensation, for which 28 (90%) patients were hospitalized, took the form of combined AH and decompensated alcohol-associated cirrhosis in 13 patients (42%), whereas the remainder was equally divided between AH alone or decompensated cirrhosis alone, each in 9 patients (29%). The median maximal Model for End-Stage Liver Disease Sodium score during the prior decompensation was 22 (IQR 18–25). Jaundice (77%) and ascites (42%) were the most prevalent manifestations of liver decompensation. Extrahepatic complications because of alcohol relapse after prior decompensation occurred in a substantial minority of patients (alcohol withdrawal: 32%, alcohol-related pancreatitis: 19%, and alcohol-related seizures: 16%). Contemporaneous narratives from the selection process indicated that the factors mitigating in favor of listing for LT despite prior decompensation included young age of LT candidate in 73% of candidates, exceptional social support in 47%, and passionate advocacy by members of the transplant team in 40%. Characteristics of the prior decompensation episodes are summarized in Table 2.
Table 2.
Characteristics of prior decompensation among prior decompensation group (N = 31)
| Characteristic | N = 31 (100%) |
|---|---|
| Prior decompensation diagnosis | |
| Decompensated alcohol-associated cirrhosis | 9 (29) |
| AH | 9 (29) |
| AH and decompensated alcohol-associated cirrhosis | 22 (42) |
| Days from prior decompensation to LT, median (IQR) | 283 (182–679) |
| Hospitalized for prior decompensation? | 28 (90) |
| MELD-Na score at prior decompensation, median (IQR) | 22 (18, 25) |
| Ascites at prior decompensation | 13 (42) |
| Overt HE at prior decompensation | 3 (10) |
| Varicealbleeding at prior decompensation | 5 (16) |
| Jaundice present at prior decompensation | 24 (77) |
| Admissions for EtOH withdrawalafter prior decompensation | 9 (32) |
| Admissions for alcohol-related seizures after prior decompensation | 5 (16) |
| Admissions for alcohol-related pancreatitis after prior decompensation | 6 (19) |
AH, alcohol-associated hepatitis; EtOH, alcohol; HE, hepatic encephalopathy; IQR, interquartile range, MELD-Na, Model for End-Stage Liver Disease-Sodium score.
Post-transplant survival
There were 41 deaths in total; causes of death by first vs prior decompensation groups are detailed in Table 3. Unadjusted probability of 1-year survival was 93% (95% CI 89%–96%) vs 86% (66%–94%) and for 2-year survival was 88% (95% CI 82%–92%) vs 78%(95%CI57%–89%)among first vs prior decompensation groups (Figure 1). In an adjusted multivariable model (Table 4), prior decompensation (adjusted hazard ratio [aHR] 2.72, 95% CI 1.61–4.59), nonwhite race/ethnicity (aHR 2.51, 95% CI 1.40–4.50), prescribed medications for comorbid psychiatric disease (aHR 2.73, 95% CI 1.18–6.32), and harmful alcohol use after LT (aHR 3.02, 95% CI 1.21–7.51) were independently associated with overall post-LT mortality. In a series of sensitivity analyses, adding history of failed rehabilitation attempts into the model did not significantly change the association of prior decompensation with post-LT mortality (aHR 2.62, 95% CI 1.74–3.94), whereas dropping harmful alcohol use after LT did not change the association of the prior decompensation with post-LT mortality (aHR 2.80, 95% CI 1.55–5.04). Finally, adjusting for any alcohol use after LT did not change the association of prior decompensation with post-LT mortality (aHR 2.74, 95% CI 1.67–4.49). In multivariable analysis, there was no significant interaction between prior decompensation and harmful (P = 0.72) or any (P = 0.88) alcohol use after LT to predict post-LT death.
Table 3.
Causes of death in first and prior decompensation groups
| Cause of death, n (%) | First decompensation (31 deaths) | Prior decompensation (7 deaths) |
|---|---|---|
| Sepsis | 9 (29) | 3 (43) |
| Recurrent ALD | 6 (19) | 1 (14) |
| Found dead at home, unclearcause | 2 (6) | 1 (14) |
| Myocardial infarction | 2 (6) | 1 (14) |
| Intracranialhemorrhage | 0 (0) | 1 (14) |
| Opioid overdose | 2 (6) | 0 (0) |
| Intraoperative | 2 (6) | 0 (0) |
| Unknown | 2 (6) | 0 (0) |
| Chronic rejection after developing delusionaldisorder | 1 (3) | 0 (0) |
| Pulmonary embolism | 1 (3) | 0 (0) |
| Esophagealcancer | 1 (3) | 0 (0) |
| Hepatic artery thrombosis | 1 (3) | 0 (0) |
| Status epilepticus | 1 (3) | 0 (0) |
| Pancreatic cancer | 1 (3) | 0 (0) |
ALD, alcohol-associated liver disease.
Figure 1.
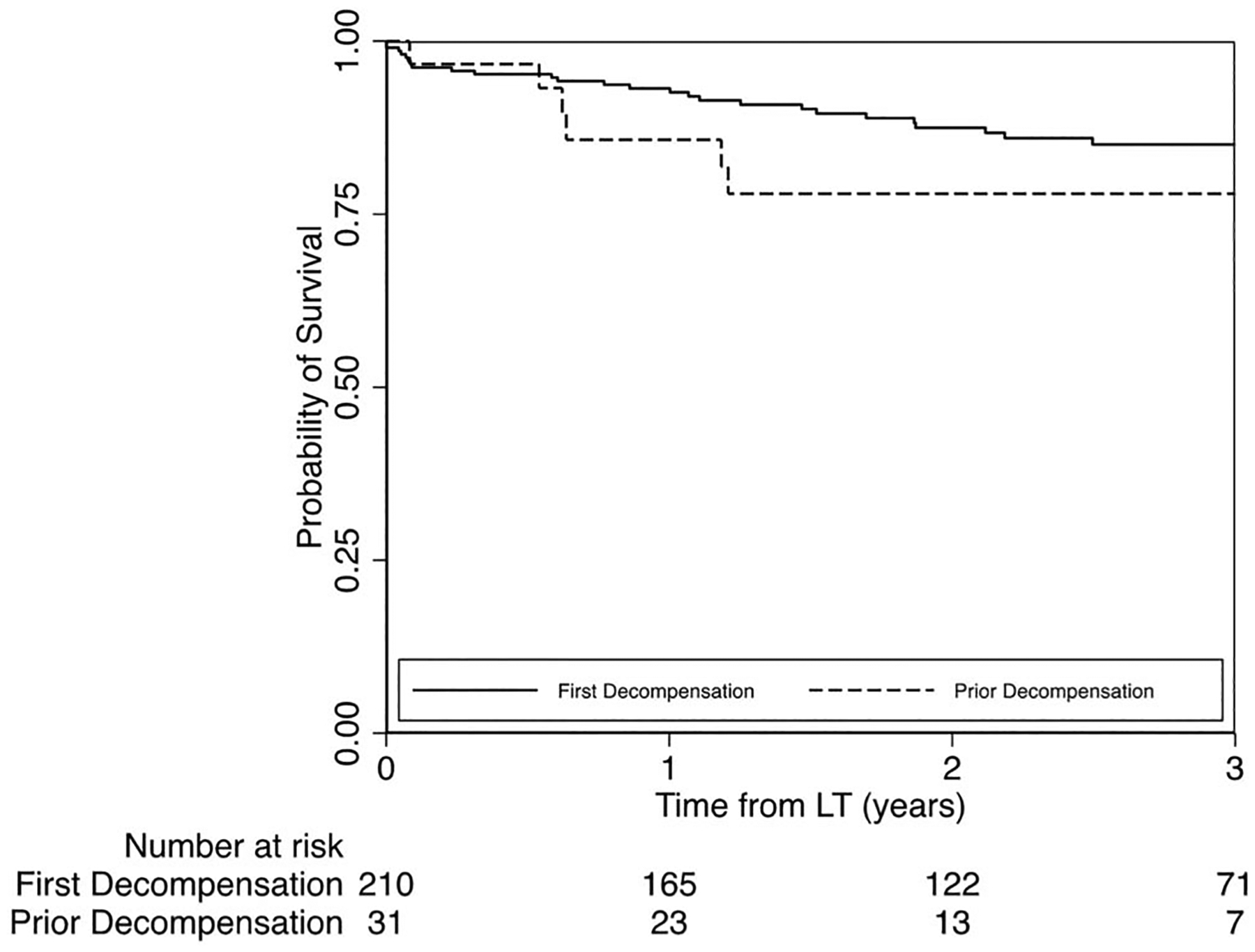
Probability of survival after LT among first vs prior liver decompensation groups.
Table 4.
Univariable and multivariable analysis for mortality following LT hospital discharge
| Factor | Univariable HR (95% CI) | P value | Multivariable aHR (95% CI) | P value |
|---|---|---|---|---|
| Prior decompensation | 2.48 (1.28–4.80) | 0.007 | 2.71 (1.61–4.59) | <0.001 |
| Age, per year | 1.02 (0.98–1.06) | 0.37 | — | — |
| Male | 0.81 (0.65–1.00) | 0.05 | — | — |
| Non-White race/ethnicity | 2.47 (1.34–4.53) | 0.004 | 2.51 (1.40–4.50) | 0.002 |
| Insurance | ||||
| Medicare (vs private) | 1.28 (0.41–4.06) | 0.67 | — | — |
| Medicaid (vs private) | 1.14 (0.75–1.74) | 0.54 | — | — |
| Body mass index | 0.98 (0.93–1.03) | 0.44 | — | — |
| Renalreplacement therapy | 1.32 (0.76–2.29) | 0.32 | — | — |
| MELD-Na | 0.93 (0.89–0.98) | 0.004 | — | — |
| On ventilator | 1.36 (0.32–5.71) | 0.68 | — | — |
| Overt hepatic encephalopathy | 0.82 (0.43–1.60) | 0.57 | — | — |
| Days of abstinence | 1.00 (1.00–1.01) | 0.12 | — | — |
| Married or significant other | 0.52 (0.15–1.78) | 0.30 | — | — |
| History of psychiatric disease | 1.85 (0.92–3.71) | 0.08 | — | — |
| On medications for psychiatric disease | 2.74 (1.59–4.71) | <0.001 | 2.73 (1.18–6.33)a | 0.02 |
| Current smoker | 1.40 (0.97–2.01) | 0.08 | — | — |
| Current marijuana | 0.86 (0.65–1.15) | 0.32 | — | — |
| History of illicit drug use | 1.10 (0.46–2.60) | 0.84 | — | — |
| History of failed rehab attempts | ||||
| 1 only (vs none) | 2.24 (0.85–5.89) | 0.10 | — | — |
| ≥ 2 (vs none) | 2.71 (1.34–5.50) | 0.006 | — | — |
| Family history of alcoholuse disorder | ||||
| Immediate family (vs none) | 1.20 (0.29–5.06) | 0.80 | — | — |
| Extended family only | 0.64 (0.19–2.15) | 0.47 | — | — |
| Employment immediately before presentation | 0.59 (0.28–1.23) | 0.16 | — | — |
| Legal history (e.g., DUI and custody loss because of alcohol) | ||||
| 1 only (vs none) | 0.92 (0.18–4.59) | 0.91 | — | — |
| ≥2 (vs none) | 1.14 (0.35–3.68) | 0.83 | — | — |
| >10 alcoholunits per day | 1.38 (0.55–3.45) | 0.49 | — | — |
| >15 years of heavy drinking | 0.73 (0.34–1.56) | 0.42 | — | — |
| Harmfulalcoholuse after LT | 3.31 (1.49–7.35) | 0.003 | 3.02 (1.21–7.51) | 0.02 |
DUI, driving under the influence; HR, hazard ratio; LT, liver transplantation; MELD-Na, Model for End-Stage Liver Disease-Sodium score.
On medications for psychiatric disease and history of psychiatric disease were co-linear, thus only medications for psychiatric disease was included in the multivariable model given its higher association.
In unadjusted logistic regression, odds of episodes of rejection (OR 1.60, 95% CI 0.72–3.56), vascular (OR 0.34, 95% CI 0.06–1.80), and biliary (OR 0.69, 95% CI 0.28–1.66) complications within 1 year after LT were similar in prior vs first decompensation groups.
Post-transplant alcohol use
Among LT recipients surviving initial hospitalization, unadjusted probability of harmful alcohol use 1 year after LT was 13% (95% CI 9%–19%) vs 22% (95% CI 10%–42%) and at 2 years after LT was 23% (95% CI 17%–30%) and 33% (95% CI 17%–56%), each for first vs prior decompensation, respectively (Figure 2). In multivariable analysis, prior decompensation (aHR 1.77, 95% CI 1.07–2.94), history of past failed rehab attempts (aHR 1.77, 95% CI 1.07–2.92, for 1 past attempt; aHR 3.54, 95% CI 2.52–4.95, for multiple past attempts), and >10 drinks per day (aHR 2.18, 95% CI 1.44–3.29) were associated with harmful alcohol use after LT. Univariable and multivariable models are summarized in Table 5. In multivariable analysis, prior decompensation (aHR 1.72, 95% CI 1.11–2.68), history of past failed rehab attempts (aHR 1.56, 95% CI 1.41–1.73, for 1 past attempt; aHR 2.99, 95% CI 2.41–3.72, for multiple past attempts), and >10 drinks per day (aHR 1.74, 95% CI 1.14–2.65) were associated with any alcohol use after LT. Univariable and multivariable models for any alcohol use after LT are summarized in Table 6.
Figure 2.
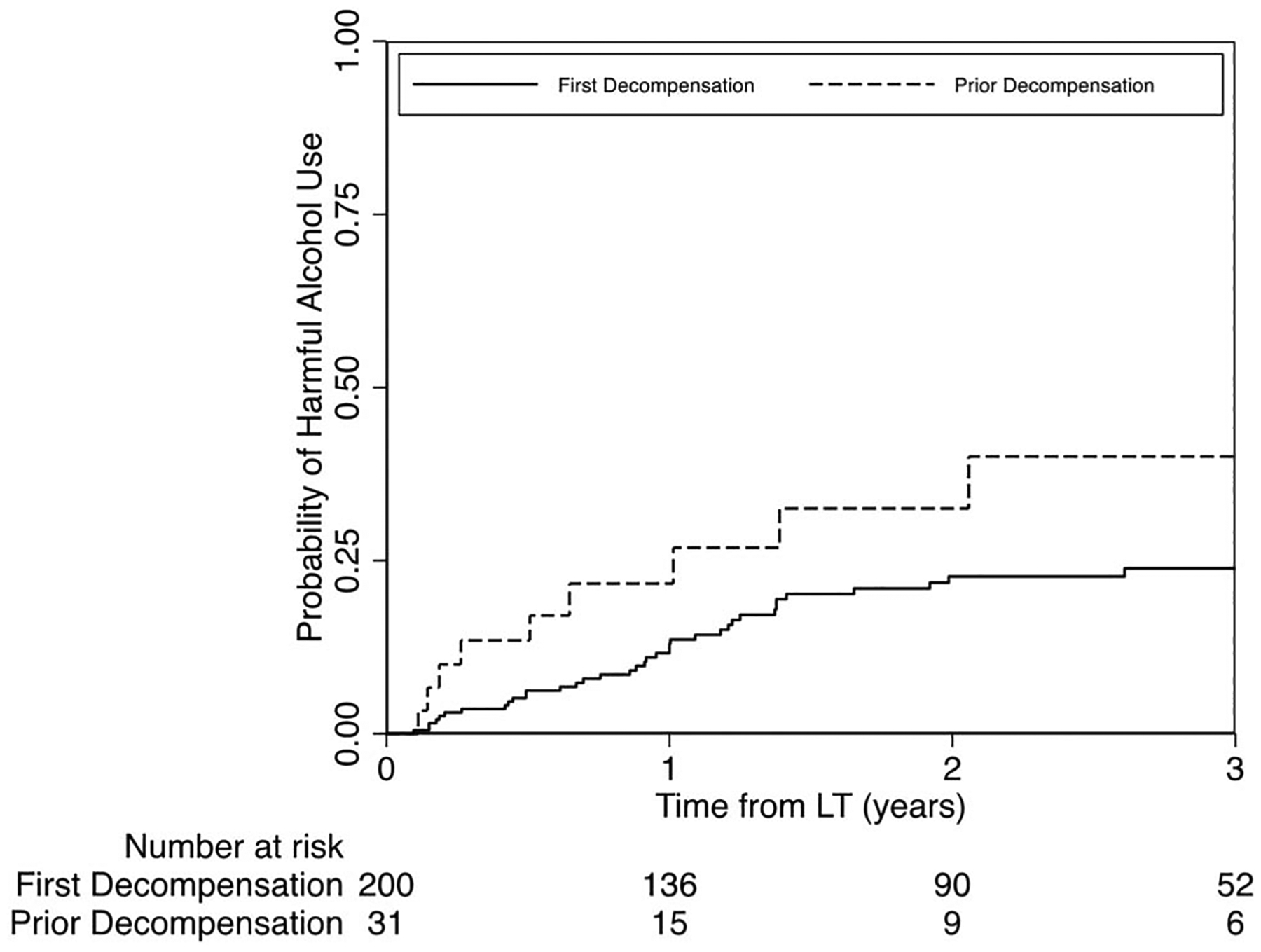
Probability of harmful alcohol use after LTamong first vs prior liver decompensation groups. LT, liver transplantation.
Table 5.
Univariable and multivariable analysis for harmful alcohol use following LT hospital discharge
| Factor | Univariable HR (95% CI) | P value | Multivariable aHR (95% CI) | P value |
|---|---|---|---|---|
| Prior decompensation | 1.84 (1.01–3.33) | 0.05 | 1.77 (1.07–2.94) | 0.03 |
| Age, per year | 0.99 (0.96–1.01) | 0.30 | — | — |
| Male | 0.89 (0.59–1.36) | 0.59 | — | — |
| Non-White race/ethnicity | 1.57 (0.59–4.17) | 0.37 | — | — |
| Private insurance | 0.80 (0.49–1.31) | 0.38 | ||
| Body mass index | 1.01 (0.94–1.08) | 0.75 | — | — |
| Renalreplacement therapy | 0.92 (0.63–1.34) | 0.66 | — | — |
| MELD-Na | 0.94 (0.87–1.01) | 0.11 | — | — |
| On ventilator | 1.85 (1.30–2.62) | 0.001 | — | — |
| Overt hepatic encephalopathy | 1.32 (0.77–2.26) | 0.32 | — | — |
| Days of abstinence | 1.00 (0.99–1.01) | 0.78 | — | — |
| Married or significant other | 1.25 (0.71–2.21) | 0.04 | — | — |
| History of psychiatric disease | 1.68 (0.91–3.11) | 0.10 | — | — |
| On medications for psychiatric disease | 1.50 (1.01–2.23) | 0.05 | — | — |
| Current smoker | 0.71 (0.26–1.88) | 0.48 | — | — |
| Current marijuana | 1.15 (0.54–2.44) | 0.71 | — | — |
| History of illicit drug use | 0.58 (0.24–1.42) | 0.23 | — | — |
| History of failed rehab attempts | ||||
| 1 only (vs none) | 2.06 (1.36–3.13) | 0.001 | 1.77 (1.07–2.92) | 0.02 |
| ≥2 (vs none) | 3.82 (2.27–6.42) | <0.001 | 3.54 (2.52–4.95) | <0.001 |
| Family history of alcoholuse disorder | ||||
| Immediate family (vs none) | 0.87 (0.48–1.58) | 0.64 | — | — |
| Extended family only | 1.18 (0.75–1.84) | 0.48 | — | — |
| Employment immediately before presentation | 0.86 (0.51–1.44) | 0.56 | — | — |
| Any legal history (e.g., DUI and custody loss because of alcohol) | 1.90 (1.14–3.17) | 0.01 | — | — |
| >10 alcoholunits per day | 2.41 (1.52–3.82) | <0.001 | 2.18 (1.44–3.29) | <0.001 |
| >15 years of heavy drinking | 1.08 (0.54–2.19) | 0.82 | — | — |
DUI, driving under the influence; HR, hazard ratio; LT, liver transplantation; MELD-Na, Model for End-Stage Liver Disease-Sodium score..
Table 6.
Univariable and multivariable analysis for any alcohol use following LT hospital discharge
| Factor | Univariable HR (95% CI) | P value | Multivariable aHR (95% CI) | P value |
|---|---|---|---|---|
| Prior decompensation | 1.72 (1.01–3.33) | 0.01 | 1.72 (1.11–2.68) | 0.02 |
| Age, per year | 0.99 (0.97–1.01) | 0.22 | — | — |
| Male | 0.75 (0.50–1.10) | 0.14 | — | — |
| Non-White race/ethnicity | 1.40 (0.54–3.59) | 0.49 | — | — |
| Private insurance | 0.85 (0.56–1.28) | 0.43 | ||
| Body mass index | 0.99 (0.94–1.04) | 0.64 | — | — |
| Renalreplacement therapy | 0.92 (0.64–1.32) | 0.65 | — | — |
| MELD-Na | 0.97 (0.89–1.06) | 0.55 | — | — |
| On ventilator | 1.49 (1.13–1.96) | 0.004 | — | — |
| Overt hepatic encephalopathy | 1.15 (0.76–1.72) | 0.51 | — | — |
| Days of abstinence before LT | 1.00 (0.99–1.01) | 0.92 | — | — |
| Married or significant other | 1.38 (1.02–1.87) | 0.04 | — | — |
| History of psychiatric disease | 1.46 (0.90–2.38) | 0.12 | — | — |
| On medications for psychiatric disease | 1.49 (1.10–2.03) | 0.01 | — | — |
| Current smoker | 0.69 (0.31–1.54) | 0.37 | — | — |
| Current marijuana | 0.91 (0.37–2.26) | 0.85 | — | — |
| History of illicit drug use | 0.84 (0.38–1.84) | 0.66 | — | — |
| History of failed rehab attempts | ||||
| 1 only (vs none) | 1.68 (1.51–1.86) | <0.001 | 1.56 (1.41–1.73) | <0.001 |
| ≥2 (vs none) | 3.02 (2.14–4.27) | <0.001 | 2.99 (2.41–3.72) | <0.001 |
| Family history of alcoholuse disorder | ||||
| Immediate family (vs None) | 1.10 (0.58–2.06) | 0.77 | — | — |
| Extended family only | 1.06 (0.76–1.48) | 0.73 | — | — |
| Employment immediately before presentation | 0.95 (0.60–1.49) | 0.81 | — | — |
| Any legal history (e.g., DUI and custody loss because of alcohol) | 1.54 (0.92–2.56) | 0.01 | — | — |
| >10 alcoholunits per day | 1.90 (1.25–2.89) | 0.003 | 1.74 (1.14–2.65) | 0.01 |
| >15 years of heavy drinking | 1.02 (0.68–1.53) | 0.92 | — | — |
DUI, driving under the influence; HR, hazard ratio; LT, liver transplantation; MELD-Na, Model for End-Stage Liver Disease-Sodium score.
DISCUSSION
Although early LT for AH is known to be lifesaving among carefully selected patients with no prior liver decompensation and has been adopted by major organization consensus guidelines, this current study provides further insights into the expansion of early LT for severe AH to those with prior liver decompensation (12,18). Despite relatively similar baseline demographic and clinical characteristics, this study found that patients in the prior decompensation cohort had a higher risk of mortality and harmful alcohol use after post-LT hospital discharge. These findings persisted even after adjusting for psychosocial factors that might otherwise suggest higher risk patients (previous alcohol rehab attempts, >10 drinks per day). Furthermore, the association of prior decompensation with post-LT mortality was robust in sensitivity analysis that included history of failed rehabilitation attempts in the model or dropped harmful or any alcohol use after LT from the model. This suggests that history of prior decompensation may be an independent risk factor beyond these other psychosocial risk factors or that there are other risk factors yet to be uncovered.
Despite higher risk of post-LT mortality and harmful alcohol use, among LT recipients with prior decompensation, their 1- and 3-year survival was greater than 85% and 75%, respectively, which is higher than those who undergo LT for HCC with expanded criteria (22). With MELD score of 39 at LT and history of multiple decompensations, these were patients at exceptionally high risk (>80%) for short-term mortality without early LT. Although longer follow-up is desirable as graft failure related to alcohol is most apparent after 5 years after LT, these results suggest that prior decompensation alone should not be considered an absolute contraindication to early LT (12,13).
The mortality risk persisted despite no difference in 1-year vascular, biliary, or rejection complications, or differences in loss to follow-up, and adjustment for center clustering. We did not examine post-LT nonhepatic alcohol-related complications (e.g., pancreatitis and motor vehicle accidents), and we were not powered to assess multiple patterns of alcohol use after LT. This suggests that the higher risk of mortality may not necessarily be related to technical or procedural factors, ascertainment bias, or center-level practices. In addition, although rates of harmful alcohol use were higher among LT recipients with prior decompensation, prior decompensation remained independently associated with higher risk of post-LT mortality even after adjusting for post-LT harmful alcohol use. Further research regarding variables that were not measured in this study, including prospective monitoring of exposure to alcohol and tobacco, adherence to immunosuppression, cardiovascular and oncologic risk factors and events after LT, and sustained engagement with treatment for AUD after LT, will be critical to advancing insight regarding differences in outcomes among LT recipients with prior decompensation.
Our results raise important questions regarding mechanisms to explain the differential outcomes observed among LT recipients with prior decompensation. Return to alcohol use after the initial decompensating event may lead to negative pathophysiologic effects that predispose this patient population to higher risk of mortality, which has been observed in the non-LT AH population independent of demographic and clinical characteristics (3,4). It is also possible that the higher risk of return to alcohol use in the prior decompensation cohort reflects a different pathobiology of addiction, which is more resistant to treatment efforts for alcohol use disorder. The 2 independent predictors of post-LT hospital discharge mortality were nonwhite race and baseline prescription of medication for comorbid psychiatric disease. Racial differences in post-LT mortality exist broadly across the United States, especially among ALD and independent of socioeconomic measures, and have worsened in recent years (22,23). Baseline prescription for comorbid psychiatric disease was also independently associated with mortality, which may suggest that these patients carry a higher burden of addiction or other comorbid psychiatric illness influencing survival outcomes. Future larger prospective studies should focus on these knowledge gaps to better elucidate mechanisms and to inform selection processes and post-LT management for patients with history of prior decompensation.
Our data draw attention to the selection process for LT in patients with liver failure and comorbid AUD. Because abandoning an arbitrary required interval of abstinence, programs (including those in ACCELERATE-AH) have adopted a psychosocial assessment to determine the likelihood of returning to alcohol use and appropriateness for transplant. In a recent analysis from one of the contributing programs (University of Wisconsin), retrospective analysis of the selection process showed that a greater proportion of patients with ALD were declined placement on the transplant waiting list compared with patients without ALD, despite higher MELD scores in the patients with ALD (23). Psychosocial assessment was the main reason for declining to list these patients. Furthermore, the outcome for patients with ALD with high MELD scores who are declined placement on the list was severe, with the majority dying within 90 days, and very few returning to a compensated state of liver disease (24). These data put into context our observation that young age and a passionate advocate on the LT team were identified as the most common reasons to proceed with early LT, despite prior decompensation. These findings likely reflect the moral dilemma faced by the LT community—young age has been consistently found to be a significant risk factor for alcohol relapse, but younger patients also have the most life-years to gain from a successful lifesaving liver transplantation (25). Our study should encourage development of objective tools to more accurately weigh the risks and benefits of early LT, regarding both survival benefit and risk of harmful alcohol use after LT. Such future research may help LT providers faced with these challenging decisions and limit the potential for implicit subjective biases to influence decision-making during the selection process. Also, studies and the practice of post-LT AUD treatment may be tailored to this highest risk population with aggressive and early psychosocial interventions that may potentially attenuate this risk.
This study has limitations. The data are retrospective, and differential reporting of alcohol use across study groups is possible. However, all patients were closely monitored with clinical interview, and there was high use of frequent biomarker-based screening of alcohol metabolites across centers according to early LT policies. The use of standardized questionnaires (e.g., AUDIT-C) and protocolized biomarkers in the future may help to further standardize this process. Second, although patients with prior decompensation had clear decompensating liver events, we could not reliably establish the extent of counseling they had regarding their liver disease and importance of abstinence. It is thus assumed that these decompensating events were definitively presented to patients as evidence of liver disease induced by alcohol use, but this cannot be proven. Third, the prior decompensation group provides modest sample size with intermediate-term follow-up. These patients are very infrequent in cohorts of transplanted patients because they are typically excluded secondary to stringent selection criteria but represent a common clinical scenario. ACCELERATE-AH is the largest and only multicenter US consortium to study this research question, and our preliminary data are important to encourage further attention and research and to inform ongoing debate regarding potential expansion of selection criteria for early LT. In particular, although our study found no differences between groups in the relatively rare events of post-LT rejection, vascular complications, or biliary complications, these outcomes would benefit from further study in larger cohorts.
Fourth, practices among centers regarding pre-LT selection and post-LT management of alcohol use disorder may vary. Our analyses adjusted for center clustering, and we specifically restricted this study to ACCELERATE-AH sites performing early LT among patients with prior decompensation. Examining differences in post-LT treatment for alcohol use disorder were outside the scope of this study and would benefit from prospective studies. Standardized and rigorous approaches to post-LT monitoring of alcohol use and treatment for alcohol use disorder are desirable for future clinical practice. Finally, this study reflects very carefully selected subpopulations of AH; specific to this study, prior decompensation was defined as evidence of overt liver decompensation (jaundice, ascites, encephalopathy, and variceal bleeding), and patients in a more “gray zone” (e.g., patients diagnosed with compensated cirrhosis, with subsequent alcohol relapse) would need further research.
Overall, our preliminary results suggest that patients who undergo early LT despite prior liver decompensation seem to have higher risk of mortality and harmful alcohol use after LT. These findings validate the value of the “first decompensation” criteria in published experiences regarding early LT for AH. However, relatively high intermediate-term survival despite these higher risks suggests that absolute exclusion of these patients may not be appropriate and warrants further research. Further larger and prospective studies with longer-term follow-up will be needed to assess ways to optimally select patients in this cohort who may benefit most from early LT and ways to manage patients at highest risk of worse outcomes after LT.
Study Highlights.
WHAT IS KNOWN
Alcohol-associated hepatitis (AH) is now the fastest growing indication for liver transplant in the United States and Europe.
Early liver transplantation for AH can be lifesaving.
Patients presenting with AH prior liver decompensation have been excluded from studies, and the use of early liver transplantation in this unique subpopulation is largely unknown.
WHAT IS NEW HERE
In this expanded cohort, early liver transplantation for AH continues to provide high intermediate-term post-transplant survival.
Prior (vs first) liver decompensation is associated with higher mortality and harmful alcohol use after early liver transplantation.
Despite higher mortality, patients with prior decompensation still have high survival rates at 3 years after transplant.
Financial support:
Research reported in this publication was supported by the National Institute On Alcohol Abuse And Alcoholism of the National Institutes of Health under Award Number K23AA029752 (BPL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C605.
CONFLICTS OF INTEREST
Potential competing interest: None to report.
REFERENCES
- 1.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150(8):1786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2018;16(8):1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altamirano J, López-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology 2017;66(6):1842–53. [DOI] [PubMed] [Google Scholar]
- 4.Louvet A, Labreuche J, Artru F, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology 2017;66(5):1464–73. [DOI] [PubMed] [Google Scholar]
- 5.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45(6):1348–54. [DOI] [PubMed] [Google Scholar]
- 6.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372(17):1619–28. [DOI] [PubMed] [Google Scholar]
- 7.Mathurin P Corticosteroids for alcoholic hepatitis—what’s next?. J Hepatol 2005;43(3):526–33. [DOI] [PubMed] [Google Scholar]
- 8.Soultati AS, Dourakis SP, Alexopoulou A, et al. Predicting utility of a model for end stage liver disease in alcoholic liver disease. World J Gastroenterol 2006;12(25):4020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41(2):353–8. [DOI] [PubMed] [Google Scholar]
- 10.Forrest EH, Evans CDJ, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the glasgow alcoholic hepatitis score. Gut 2005;54(8):1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365(19):1790–800. [DOI] [PubMed] [Google Scholar]
- 12.Lee BP, Mehta N, Platt L, et al. Outcomes of early liver transplantation for patientswithseverealcoholichepatitis. Gastroenterology 2018;155(2):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BP, Im GY, Rice JP, et al. Patterns of alcohol use after early liver transplantation for alcoholic hepatitis. Clin Gastroenterol Hepatol 2022; 20(2):409–18. e5. doi: [DOI] [PubMed] [Google Scholar]
- 14.Lee BP, Samur S, Dalgic OO, et al. Model to calculate harms and benefits of early vs delayed liver transplantation for patients with alcohol-associated hepatitis. Gastroenterology 2019;157(2):472–80.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Disease. Hepatology 2020;71(1):306–33. [DOI] [PubMed] [Google Scholar]
- 16.Lee BP, Vittinghoff E, Hsu C, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: The sustained alcohol use Post–Liver transplant score. Hepatology 2019; 69(4):1477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittermann T, Mahmud N, Abt P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Netw Open 2021;4(7):e2118713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson MS, Valbuena VSM, Brown CS, et al. Association of COVID-19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Netw Open 2021; 4(10):e2131132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholankeril G, Goli K, Rana A, et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology 2021;74(6):3316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open 2020;3(9):e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: Recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 2016;150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piñero F, Anders M, Boin IF, et al. Liver transplantation for hepatocellular carcinoma: Impact of expansion criteria in a multicenter cohort study from a high waitlist mortality region. Transpl Int 2021; 34(1):97–109. [DOI] [PubMed] [Google Scholar]
- 23.Daniel KE, Matthews LA, Deiss Yehiely N, et al. Psychosocial assessment rather than severity of liver failure dominates selection for liver transplantation in patients with alcohol-associated liver disease. Liver Transplant 2022; 28(6):936–44. [DOI] [PubMed] [Google Scholar]
- 24.Musto J, Stanfield D, Ley D, et al. Recovery and outcomes of patients denied early liver transplantation for severe alcohol-related hepatitis. Hepatology 2022;75(1):104–14. [DOI] [PubMed] [Google Scholar]
- 25.Im GY, Cameron AM, Lucey MR. Liver transplantation for alcoholic hepatitis. J Hepatol 2019;70(2):328–34. [DOI] [PubMed] [Google Scholar]


