Introduction
The resectability of pancreatic ductal adenocarcinoma (PDAC) is mostly defined by anatomical factors, but not all small PDACs are early tumours1. After surgical resection, early tumour recurrence develops in approximately one-third of patients2. However, some patients with borderline resectable or locally advanced neoplasms may enjoy long-term survival after effective neoadjuvant treatments and radical tumour resection3–5. Hence, a more recent definition of resectability is based on response to chemotherapy and tumour biology6,7.
Increasing experience in pancreatectomy with vein resection has gradually evolved into resection of peripancreatic arteries in selected situations8–10. In a previous study9, the authors reported the oncological outcomes of pancreatectomy with arterial resection (PAR). The aim of this short report was to demonstrate the feasibility of pancreatectomy with superior mesenteric artery (SMA) resection (P-SMA).
Methods
The main objective of this study was to report the 90-day outcomes of P-SMA performed between 1 January 1993 and 28 February 2021. Comparison with matched cohorts of patients undergoing pancreatectomy with resection of either superior mesenteric/portal vein (SM/PV) or coeliac trunk/hepatic artery (CT/HA) is also provided. Outcomes were recorded according to agreed definitions11–15. A detailed description of the methods and results is provided in the supplementary material (Tables S1–S4 and Figs S1–S11). Figs. 1 and 2 provide examples of P-SMA with resection and reconstruction of multiple vascular segments.
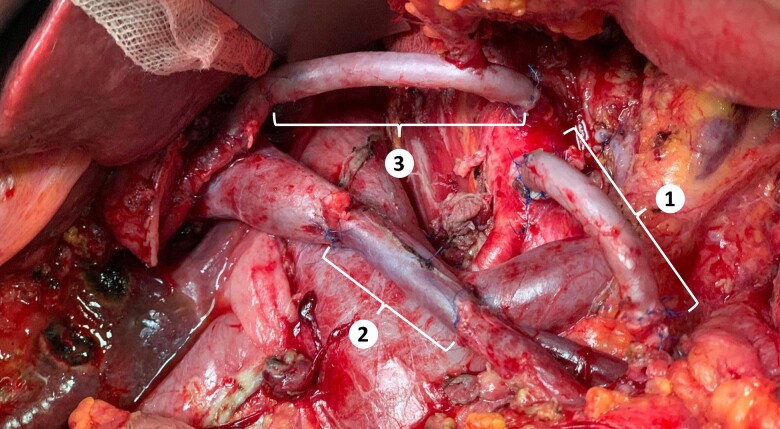
Figure 1.
The SMA (n. 1) and the CT/HA (n. 3) are reconstructed using two jump grafts of the greater saphenous vein. The superior mesenteric/portal vein (n = 2) was reconstructed using an interpositional conduit made of the left internal jugular vein. SMA, superior mesenteric artery CT/HA, celiac trunk/hepatic artery.
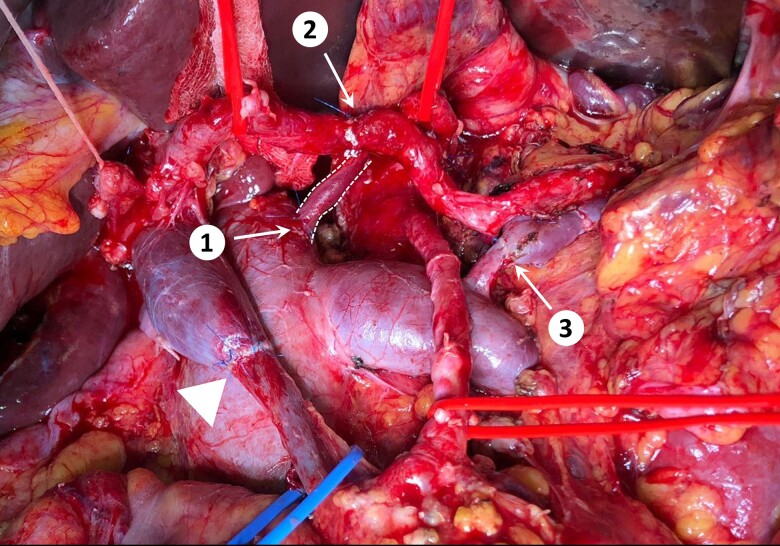
Figure 2.
In this patient the left gastric vein (shown between dotted lines) was drained into the inferior vena to relieve gastric congestion (end-to-side anastomosis; n.1), despite the splenic vein had already been anastomosed end-to-end to an enlarged left adrenal vein (n.3). The patient also underwent superior mesenteric/portal vein resection (end-to-end anastomosis; triangle) and celiac trunk resection. The spleen and liver were revascularized using the splenic artery. The proximal stump of the splenic artery was anastomosed end-to-end to the hepatic artery (n = 2), and the stump of the celiac trunk was anastomosed to the posterior aspect of the splenic artery (celiac trunk anastomosis not visible in this picture owing to splenic artery overlap).
Results
During the study interval, pancreatectomy with resection of peripancreatic vessels was performed in 587 of 2595 patients (22.7 per cent), including 327 SM/PV resections (55.7 per cent), 86 CT/HA resections (15 per cent), and 69 P-SMAs (12 per cent). Concurrent SM/PV resection was required in the majority of PARs (80.6 per cent).
In the P-SMA group, primary chemotherapy (57 patients, 97 per cent) or chemoradiation (2, 3 per cent) was administered to 59 of the 63 patients (94 per cent) operated after 2002.
Technical refinements and 90-day outcomes of pancreatectomy with superior mesenteric artery resection
Total gastrectomy was performed in 16 patients (23 per cent). All gastrectomies were undertaken in patients who had surgery before 2014. After then, gastrectomy was avoided owing to revascularization of the left gastric artery (2 patients), drainage of the left gastric vein in either the inferior vena cava (2) or reconstructed SM/PV (3), reimplantation of the right gastroepiploic vein (1), and spleen preservation (10) (Table S1).
Other time-related technical refinements (before versus after 2014) included more direct vein reconstructions (59 versus 85 per cent; P = 0.028) and fewer direct SMA reconstructions (74 versus 31 per cent; P < 0.001), mostly because of the more frequent implementation of clockwise rotated splenic artery (6 versus 54 per cent; P < 0.001). No significant variation was observed in the CT/HA reconstruction technique (end-to-end anastomosis: 36 versus 18 per cent; interposition grafts: 45 versus 55 per cent; rotated splenic artery: 18 versus 27 per cent).
Postoperative complications developed in 52 patients (75 per cent), with a median comprehensive complication index (CCI) score of 24.2 (i.q.r. 4.4–33.5). Severe postoperative complications occurred in 19 patients (28 per cent). Eight patients died during the index hospital stay (12 per cent), and 2 after readmission. The overall 90-day postoperative mortality rate was 15 per cent (failure-to-rescue rate 53 per cent). The primary causes of postoperative death were intestinal ischemia (2), sepsis (2), liver failure (1), respiratory failure (1), bowel perforation (1), digestive haemorrhage (1), grade C postoperative pancreatic fistula (POPF) with SM/PV thrombosis (1), and SMA pseudoaneurysm (1). Mortality at 90 days declined after the first 21 P-SMAs, but the difference was not statistically significant (6 of 21 versus 4 of 48; P = 0.057). In an analysis restricted to deaths that occurred during the index hospital stay, the mortality rate clearly decreased after 36 procedures (8 of 36 versus 2 of 33). Including the 2 patients who died after readmission to hospital, the postoperative rate among the last 33 consecutive P-SMAs was 6.0 per cent.
Repeat surgery was required in six patients (9 per cent) because of bowel perforation (3), SMA pseudoaneurysm (1), intestinal ischaemia (1), and gastrointestinal bleeding (1). Endoscopic and interventional radiology procedures were required in two (3 per cent) and seven (10 per cent) patients respectively.
Severe postoperative complications were recorded in 69 of 327 patients (21.1 per cent) after SM/PV resection and in 29 of 86 (33 per cent) after CT/HA resection The equivalent figures for rates of failure to rescue and 90-day postoperative mortality were 33.3 and 7.0 per cent, and 40.0 and 9.3 per cent, respectively. Neither P-SMA nor CT/HA resection increased the unadjusted and adjusted ORs for severe complications compared with SM/PV resection. Postoperative mortality was equivalent for P-SMA and CT/HA resections in all analyses. However, the postoperative mortality rate for P-SMA approached statistical significance compared with that for SM/PV resection (Tables 1 and S2).
Table 1.
Postoperative outcomes after pancreatectomy with resection of superior mesenteric/portal vein, coeliac trunk/hepatic artery, or superior mesenteric artery
| SM/PV resection (n = 327) |
CT/HA resection (n = 86) |
P-SMA (n = 69) |
P† | |
|---|---|---|---|---|
| Type of pancreatic resection | - | |||
| Pancreatoduodenectomy | 224 (68.5) | 13 (15) | 7 (10) | |
| Distal pancreatectomy | 21 (6.4) | 24 (28) | 0 (0) | |
| Total pancreatectomy | 82 (25.0) | 49 (57) | 62 (90) | |
| Postoperative complications (during index hospital stay) | 218 (66.7) | 63 (73) | 52 (75) | 0.238 |
| Grade I | 20 (6.1) | 7 (8) | 6 (9) | 0.648 |
| Grade II | 129 (39.5) | 36 (42) | 29 (42) | 0.873 |
| Grade IIIa | 21 (6.4) | 5 (6) | 3 (4) | 0.802 |
| Grade IIIb | 15 (4.6) | 4 (5) | 4 (6) | 0.911 |
| Grade IVa | 8 (2.5) | 3 (3) | 1 (1) | 0.718 |
| Grade IVb | 2 (0.6) | 0 (0) | 1 (1) | 0.522 |
| Grade V | 23 (7.0) | 8 (9) | 8 (12) | 0.407 |
| ≥ grade III | 69 (21.1) | 20 (23) | 19 (27) | 0.774 |
| CCI score, median (i.q.r.) | 22.6 (0–33.5) | 22.6 (0–35) | 24.2 (4.4–33.5) | 0.487 |
| POPF* | 39 (15.9) | 7 (19) | 1 (14) | 0.890 |
| Biochemical leak | 11 (4.5) | 3 (8) | 0 (0) | 0.528 |
| Grade B | 24 (9.8) | 3 (8) | 0 (0) | 0.655 |
| Grade C | 4 (1.6) | 1 (3) | 1 (14) | 0.066 |
| Delayed gastric emptying | 120 (36.7) | 26 (30) | 22 (32) | 0.462 |
| Grade A | 54 (16.5) | 8 (9) | 4 (6) | 0.031 |
| Grade B | 43 (13.2) | 14 (16) | 11 (16) | 0.668 |
| Grade C | 23 (7) | 4 (5) | 7 (10) | 0.409 |
| PPH | 50 (15.3) | 19 (22) | 21 (30) | 0.009 |
| Grade A | 0 (0) | 0 (0) | 0 (0) | – |
| Grade B | 35 (10.7) | 15 (17) | 17 (25) | 0.006 |
| Grade C | 15 (4.6) | 4 (5) | 4 (6) | 0.911 |
| Perioperative blood transfusion | 111 (33.9) | 28 (33) | 27 (39) | 0.853 |
| No. of blood transfusions, median (i.q.r.) | 2 (2–4) | 2 (2–4) | 3 (2–5) | 0.358 |
| Bile leak | 2 (0.6) | 0 (0) | 0 (0) | 0.621 |
| Chyle leak | 2 (0.6) | 0 (0) | 1 (1) | 0.522 |
| Enteric leak | 5 (1.5) | 4 (5) | 2 (3) | 0.211 |
| Surgical-site infection | 33 (10.1) | 11 (13) | 8 (12) | 0.968 |
| Vascular thrombosis | 3 (0.9) | 4 (5) | 5 (7) | 0.003 |
| Vein thrombosis | 2 (0.6) | 4 (5) | 3 (4) | 0.013 |
| Artery thrombosis | 1 (0.3) | 0 (0) | 2 (3) | 0.033 |
| Interventional radiology procedures | 27 (8.3) | 5 (6) | 7 (10) | 0.606 |
| Reoperation | 24 (7.3) | 8 (9) | 6 (9) | 0.822 |
| Duration of hospital stay (days), median (i.q.r.) | 19 (14–26) | 19 (14.8–26) | 22 (14.5–30) | 0.135 |
| 90-day readmission | 10 (3.1) | 4 (5) | 10 (14) | 0.004 |
| 90-day mortality | 23 (7) | 8 (9) | 10 (14) | 0.125 |
| 90-day failure to rescue | 23 (33.3) | 8 (40) | 10 (53) | 0.301 |
Values are n (%) unless otherwise indicated. *Postoperative pancreatic fistula (POPF) data refer to pancreatoduodenectomy and distal pancreatectomy. SM/PV, superior mesenteric/portal vein; CT/HA, coeliac trunk/hepatic artery; P-SMA, pancreatectomy with superior mesenteric artery resection. CCI, comprehensive complication index; PPH, postpancreatectomy haemorrhage. †?.
The number of resected vessels did not influence either overall postoperative complications or severe postoperative complications. Similarly, there was no relationship between these outcome metrics and the number of reconstructed SMA branches or type of vascular reconstruction. However, higher CCI scores were recorded in patients who did not undergo direct vein reconstruction (Table S3). Data on pathological outcomes are reported in Table S4.
Ischaemic complications
Nine patients (13 per cent) were on chronic antiaggregant (5, 7 per cent) or anticoagulant (4, 6 per cent) therapy before surgery. All the patients received postoperative antithrombotic prophylaxis.
Ischaemic complications occurred in five patients (7 per cent). One additional patient developed intestinal ischaemia after a failed attempt at endovascular stenting of an SMA pseudoaneurysm, and one patient developed occlusive SM/PV thrombosis as a consequence of grade C POPF. Overall, seven patients had ischaemic complications (10 per cent).
Treatments included repeat surgery (2), heparin infusion (3), heparin infusion followed by reoperation (1), and angioplasty followed by reoperation (1). Three patients were rescued, and four died.
Discussion
P-SMA was feasible in selected patients, and associated with postoperative outcomes similar to those recorded after pancreatectomy with either SM/PV or CT/HA resection. The 90-day mortality rate remained quite high, but 2 deaths among the last 33 P-SMAs demonstrated that the risk of death can be reduced. A trend toward lower 90-day mortality was observed after 21 procedures with a clear reduction after 36 procedures. Ability to preserve the stomach also improved after 34 P-SMAs, suggesting that more than 30 procedures are required to surpass the learning curve of P-SMA. In a study16 of 195 PARs (30 P-SMAs), the mortality rate dropped below 10 per cent after 15 procedures. In another study17 of 111 PARs (15 P-SMAs), the mortality rate was 9 per cent after 24 procedures.
In P-SMA, postoperative complications, severe postoperative complications, and CCI scores were not influenced by the associated CT/HA resection, number of reconstructed SMA branches, technique of SMA reconstruction, or resection of a patch of the inferior vena cava. Data from the literature17,18 have confirmed that P-SMA resection does not significantly increase operative risk compared with other complex pancreatic resections.
Five patients developed primary ischaemic complications. Reported rates of ischaemic complications after PAR range between 4 and 16 per cent16,18,19. A study19 showed that the incidence of ischaemic complications was similar irrespective of anticoagulation type (aspirin alone, aspirin plus unfractionated heparin, and aspirin plus low molecular weight heparin). Guidelines20 for prevention of venous thromboembolism in pancreatic surgery state that the data for anticoagulation after vein reconstruction are inconclusive. No information on anticoagulation after PAR is provided. Well designed studies are clearly needed.
This study has several limitations. First, the oncological results of P-SMA have not been presented. However, the authors’ previous study9 showed that long-term survival is possible in selected patients. Second, because of the retrospective study design, it was not possible to obtain information regarding quality of life, intestinal function, and nutritional status. Third, the results have shown that postoperative outcomes of P-SMA are affected by a learning curve. Therefore, the generalizability of these results remains to be established.
Supplementary Material
Contributor Information
Ugo Boggi, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Niccolò Napoli, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Emanuele F Kauffmann, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Sara Iacopi, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Michael Ginesini, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Cesare Gianfaldoni, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Daniela Campani, Division of Pathology, University of Pisa, Pisa, Italy.
Gabriella Amorese, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy.
Fabio Vistoli, Division of General and Transplant Surgery, University of Pisa, Pisa, Italy.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data are stored in an institutional database and are available upon reasonable request to the corresponding author.
References
- 1. Egawa S, Takeda K, Fukuyama S, Motoi F, Sunamura M, Matsuno S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004;28:235–240 [DOI] [PubMed] [Google Scholar]
- 2. Nishio K, Kimura K, Amano R, Yamazoe S, Ohrira G, Nakata Bet al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nigri G, Petrucciani N, Belloni E, Lucarini A, Aurello P, D’Angelo Fet al. Distal pancreatectomy with celiac axis resection: systematic review and meta-analysis. Cancers (Basel) 2021;13:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshiya S, Fukuzawa K, Inokuchi S, Kosai-Fujimoto Y, Sanefuji K, Iwaki Ket al. Efficacy of neoadjuvant chemotherapy in distal pancreatectomy with en bloc celiac axis resection (DP-CAR) for locally advanced pancreatic cancer. J Gastrointest Surg 2020;24:1605–1611 [DOI] [PubMed] [Google Scholar]
- 5. Klompmaker S, van Hilst J, Gerritsen SL, Adham M, Teresa Albiol Quer M, Bassi Cet al. Outcomes after distal pancreatectomy with celiac axis resection for pancreatic cancer: a pan-European retrospective cohort study. Ann Surg Oncol 2018;25:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oba A, Croce C, Hosokawa P, Meguid C, Torphy RJ, Al-Musawi MHet al. Prognosis based definition of resectability in pancreatic cancer. A road map to new guidelines. Ann Surg 2022;275:175–181 [DOI] [PubMed] [Google Scholar]
- 7. Boggi U. Resection for pancreatic cancer with arterial involvement: a paradigm shift away from unresectable to ‘how to do it’. Surgery 2021;169:1036. [DOI] [PubMed] [Google Scholar]
- 8. Boggi U, Del Chiaro M, Croce C, Vistoli F, Signori S, Moretto Cet al. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery 2009;146:869–881 [DOI] [PubMed] [Google Scholar]
- 9. Napoli N, Kauffmann E, Cacace C, Menonna F, Caramella D, Cappelli Cet al. Factors predicting survival in patients with locally advanced pancreatic cancer undergoing pancreatectomy with arterial resection. Updates Surg 2021;73:233–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boggi U, Truty M, Zyromski NJ. 2021 SSAT debate: selective approach to resection of the superior mesenteric artery in pancreatic cancer vs superior mesenteric artery encasement is not an absolute contraindication for surgery in pancreatic cancer. J Gastrointest Surg 2022;26:523–531 [DOI] [PubMed] [Google Scholar]
- 11. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham Met al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591 [DOI] [PubMed] [Google Scholar]
- 12. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JRet al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768 [DOI] [PubMed] [Google Scholar]
- 13. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJet al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25 [DOI] [PubMed] [Google Scholar]
- 14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7 [DOI] [PubMed] [Google Scholar]
- 16. Loos M, Kester T, Klaiber U, Mihaljevic AL, Mehrabi A, Müller-Stich BMet al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann Surg 2022;275:759–768 [DOI] [PubMed] [Google Scholar]
- 17. Tee MC, Krajewski AC, Groeschl RT, Farnell MB, Nagorney DM, Kendrick MLet al. Indications and perioperative outcomes for pancreatectomy with arterial resection. J Am Coll Surg 2018;227:255–269 [DOI] [PubMed] [Google Scholar]
- 18. Bachellier P, Addeo P, Faitot F, Nappo G, Dufour P. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it be done safely and with which outcomes? A single institution’s experience with 118 patients. Ann Surg 2020;271:932–940 [DOI] [PubMed] [Google Scholar]
- 19. Alva-Ruiz R, Abdelrahman AM, Starlinger PP, Yonkus JA, Moravec DN, Busch JJet al. Patency rates of hepatic arterial resection and revascularization in locally advanced pancreatic cancer. HPB (Oxford) 2022;S1365-182X(22)01505-2. doi: 10.1016/j.hpb.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 20. Clancy TE, Baker EH, Maegawa FA, Raoof M, Winslow E, House MG. AHPBA guidelines for managing VTE prophylaxis and anticoagulation for pancreatic surgery. HPB (Ofxord) 2022;24:575–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are stored in an institutional database and are available upon reasonable request to the corresponding author.