Abstract
Biofilms formed by antibiotic-resistant bacteria in wound beds present unique challenges in terms of treating wound infections. In this work, the in vivo activity of a novel electrochemical bandage (e-bandage) composed of carbon fabric and controlled by a wearable potentiostat, designed to continuously deliver low amounts of hydrogen peroxide (H2O2) was evaluated against methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Pseudomonas aeruginosa (MDR-PA) and mixed-species (MRSA and MDR-PA) wound infections. Wounds created on Swiss Webster mice were infected with the above-named bacteria and biofilms allowed to establish on wound beds for 3 days. e-Bandages, which electrochemically reduce dissolved oxygen to H2O2 when polarized at −0.6 VAg/AgCl, were placed atop the infected wound bed and polarized continuously for 48 hours. Polarized e-bandage treatment resulted in significant reductions (p <0.001) of both mono-species and mixed-species wound infections. After e-bandage treatment, electron microscopy showed degradation of bacterial cells, and histopathology showed no obvious alteration to the inflammatory host response. Blood biochemistries showed no abnormalities. Taken all together, results of this work suggest that the described H2O2-producing e-bandage can effectively reduce in vivo MRSA, MDR-PA and mixed-species wound biofilms, and should be further developed as a potential antibiotic-free strategy for treatment of wound infections.
Keywords: electrochemical bandage, hydrogen peroxide, Staphylococcus aureus, Pseudomonas aeruginosa, biofilm, wound infection, mouse
Graphical Abstract
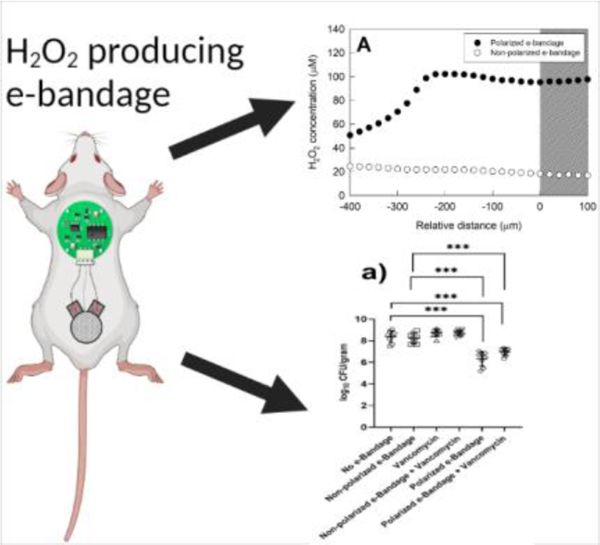
Wound biofilms are difficult to treat using conventional antibiotics. In this work, the authors developed and evaluated the in vivo anti-biofilm activity of H2O2 producing electrochemical bandage (e-bandage) against wound biofilms formed by methicillin-resistant S. aureus, multi-drug resistant P. aeruginosa and mixed-species. 48 hr treatment with e-bandage resulted in significant reduction of biofilm CFU counts.
Introduction
Antibiotic-resistant bacteria are a challenging global issue. According to the Centers for Disease Control and Prevention, ~2.8 million antibiotic-resistant infections are reported in the United States each year, with more than 35,000 patients dying due to these infections annually (1). Given the dearth of new antibiotics and waning interest in the research and development of new antibiotic classes, various strategies are required to address antibiotic resistance (2). Numbers of infections associated with antibiotic-resistant bacteria have risen during the COVID-19 pandemic (3).
Chronic wound infections are commonly encountered in hospitals and wound clinics and are complex to manage. In the United States alone, ~ 6.5 million patients are affected by chronic wounds annually, with treatment costing more than $25 billion per year (4). Wound healing typically proceeds through several stages: homeostasis, inflammation, granulation, and tissue remodeling (5). The presence of microbes in wounds exacerbates the overall wound healing process (6, 7). Often, wound infections harbor microbes in biofilm form, further complicating the healing process as well as reducing the efficacy of various antimicrobial strategies (8–10). Microorganisms found in biofilms produce extracellular polymeric substances (EPS), made up of glycoproteins, glycolipids, carbohydrate molecules, and/or extracellular DNA (11, 12). The inner layers of biofilm contain dormant microbial cells which are recalcitrant to treatment with many antibiotics and other drugs. Furthermore, the overall mechanical and chemical structure of EPS and biofilm protect microbial cells associated with them through various strategies, which may also increase selection of antibiotic-resistance bacteria (13–16). Thus, new topical treatment approaches which do not rely on antibiotics are needed to treat chronic wound infections.
Biocides, topical antimicrobials, and antiseptics are used for treatment of wound infections. Some commonly used compounds for topical wound application and debridement include hypochlorous acid (HOCl), medicinal honey, povidone iodine, hydrogen peroxide (H2O2), phenols, and chlorhexidine gluconate (17–19). Also, wound dressings based on hydrogels, silver, and copper are reported in treating wound infections (20–22). Among the above-mentioned chemicals and dressings, there is particular interest in H2O2, a natural biocide found in wound beds produced in low concentrations as part of the cellular inflammatory response (23, 24). Several studies have reported both bactericidal and wound healing properties of H2O2 (14, 15). It has been shown that H2O2 produced by host immune cells can improve the migration of keratinocytes and fibroblasts cells to wound sites along with improving differentiation of keratinocytes, thereby promoting wound healing (25–28). However, a drawback of H2O2-based wound treatments to date has been that H2O2 concentrations decline rapidly over time, limiting activity. Administration of high H2O2 concentrations might overcome this limitation but these are toxic to mammalian cells. Therefore, to be effectively used to treat wounds, H2O2 ideally needs to be continuously supplied/applied to wound beds in low concentrations not toxic to mammalian cells.
In our previous work, we designed and developed an electrochemical bandage (e-bandage) composed of carbon and cotton fabric embedded with hydrogel to deliver controlled amounts of H2O2 using a small, lightweight battery-operated potentiostat (29). This e-bandage was designed so that the e-bandage can be applied on the skin like traditional adhesive bandages used clinically to cover wounds. The H2O2-producing e-bandage was evaluated for its in vitro anti-biofilm activity against several bacterial and fungal mono- and dual-species biofilms (30, 31), including those formed by methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Acinetobacter baumannii, multidrug-resistant Pseudomonas aeruginosa (MDR-PA), and Escherichia coli. In this work, we report for the first time the in vivo efficacy of H2O2-producing e-bandages in a murine model of skin wound infection. Wounds were infected with mono- MRSA and MDR-PA alone or together. The mixed-species combination was selected based on the frequency with which these bacterial species are associated with polymicrobial wound infections (32).
Methods and Materials
Electrochemical bandage:
The e-bandage and wearable potentiostat were previously described in detail (29). Briefly, the e-bandage is comprised of three electrodes embedded in a circular bandage-like structure: conductive carbon fabric makes up the working and counter electrode (Panex 30 PW-06, Zoltek Companies Inc., St. Louis, MO), and a silver/silver chloride (Ag/AgCl) wire which acts as quasi reference electrode (QRE). A wearable potentiostat controls the working electrode of e-bandage system at −0.6 V Ag/AgCl which results in generation of H2O2 on the working electrode via reduction of dissolved O2. Further details can be found in our previous work (29).
Murine wound infection model:
All animal experiments were approved by the Mayo Clinic Institutional Animal Care and Use Committee under protocol approval number, A00003272-18. Full thickness 5-mm skin wounds were created on Swiss Webster mice (Charles River, Wilmington, MA). Groups of eight mice were assigned to no e-bandage, non-polarized e-bandage, polarized e-bandage, non-polarized e-bandage with antibiotic, or polarized e-bandage with antibiotics. Uninfected mice with e-bandages served as a negative control group. Mice were anesthetized by intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Injection of buprenorphine SR-Lab was given subcutaneously as an analgesic. A previously-described skin wound infection model was used (33). Briefly, the dorsum of the mice was shaved and disinfected with povidone-iodine and alcohol swabs thrice. A sterile 5-mm diameter circular, full thickness skin wound was created on each mouse using a skin puncture biopsy tool (Acuderm Inc., Fort Lauderdale, FL). Excised skin was homogenized and quantitatively cultured to check for sterility. For the mono-species infection model, freshly grown MRSA IDRL-6169 or MDR-PA IDRL-11442 were suspended in 0.9% sterile saline and adjusted to ~108 CFU/ml. 10 μl of this suspension was inoculated onto the wound bed. Control mice were inoculated with 10 μl of 0.9% sterile saline. For mixed-species infections, 5 μl of MRSA adjusted to 0.5 McFarland in 0.9% saline and 5 μl of MDR-PA adjusted to 106 CFU/ml in 0.9% saline were mixed and inoculated onto the wound bed. Bacterial suspensions were allowed to soak onto the wound bed for 5 minutes after which wound beds were covered with semi-occlusive transparent Tegaderm® (3M, St. Paul, MN) using liquid adhesive Mastisol® (Eloquest Health care, Ferndale, MI). Photographs of wounds were taken, and wound diameters recorded daily. Wound infections were allowed to establish over 3 days. On day 3, mice were anesthetized with isoflurane and the Tegaderm® carefully removed without disturbing the wound bed. A wearable potentiostat was sutured (Ethicon LLC Cat. #1668G, San Lorenzo, Puerto Rico) onto the mice as shown in figure 1.
Figure 1.
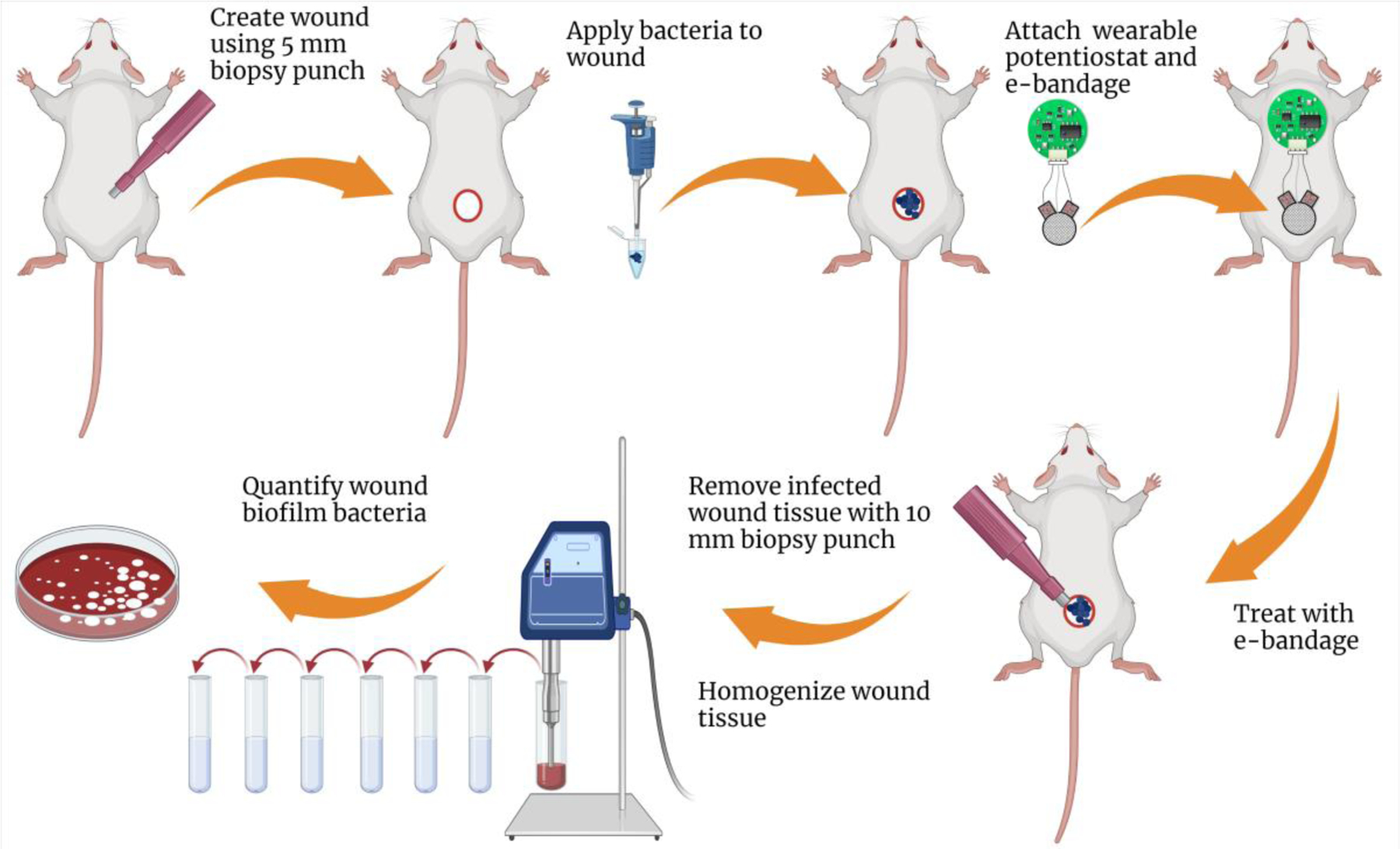
Graphical representation of the overall experimental design for evaluating the efficacy of H2O2-producing e-bandages in mice. Created using Biorender.com.
e-Bandage preparation for in vivo treatment:
e-Bandages were steam sterilized by autoclaving at 121°C for 20 minutes. Sterile e-bandages were pre-hydrated for 15 minutes in 1X phosphate buffer saline (1X PBS). Prior to starting e-bandage treatment, 100 μl of sterile hydrogel was added to the e-bandage fabric layers as previously described (29). Additionally, 100 μl of hydrogel was added on top of the wound bed. Next, e-bandages were placed on top of the wound beds and connected to the potentiostats. 100 μl of hydrogel was added on top of the e-bandages, and the wound bed, along with the e-bandage, covered with Tegaderm®.
e-Bandage treatment:
Wearable potentiostats were connected to the e-bandage electrodes and a 3V battery inserted into each potentiostat to start treatment (polarized e-bandage treatment). Wound biofilms were treated for 48 hours. Controls included wounds exposed to non-polarized e-bandages (e-bandages connected to a wearable potentiostat without a battery) and wound biofilms without e-bandages/wearable potentiostats. The potential of the working electrode relative to the QRE was measured for each bandage at the start and end of each experiment. Additionally, after 24 hours, an additional 100 μl of hydrogel was added on top of the e-bandages, and an additional layer of Tegaderm® applied. The working electrode potential was again measured for each bandage, and a new battery inserted into each wearable potentiostat after 24 hours of polarization.
Treatment with vancomycin and amikacin alone and in combination with e-bandages:
The pharmacokinetic profile of vancomycin and amikacin in Swiss Webster mice was determined to determine the treatment dose. Vancomycin (150 mg/kg) was administered intraperitoneally every 12 hours for 4 doses and blood collected at 0.5, 1, 2, 4, 8, and 12 hours after the final dose. Amikacin (15 mg/kg) was administered subcutaneously every 6 hours for 4 doses, and blood was collected at 0.25, 0.5, 1, 2, 4, and 6 hours after the final dose. Three animals were studied for each time point. Blood was collected via cardiac punctre after euthanasia and serum was separated using centrifugation. Bioassays were performed using drug diffusion on agar plates seeded with Bacillus subtilis subsp. spizizenii (ATCC® 6633™) with zones of inhibition compared to a standard curve to determine vancomycin and amikacin serum concentrations (34). Area under the curve calculations were done in GraphPad Prism (software version 8.0, GraphPad Software). Wound infections were treated with vancomycin alone (for MRSA infected mice) or amikacin alone (for MDR-PA infected mice) (total of 4 doses given every 12 hours) or in combination (vancomycin and amikacin for mixed-species infections) with polarized or non-polarized e-bandages.
H2O2 measurements in wounds infected with MRSA:
H2O2 concentrations were measured using amperometric microelectrodes fabricated as previously described (35). Briefly, each microelectrode was constructed using a 50 µm platinum wire sealed in a glass capillary. The sensing tip was a platinized platinum bulb covered with a cellulose acetate membrane. A commercial leakless Ag/AgCl reference electrode was used during measurements (ET072-1, eDAQ, Colorado Springs, CO). Before and after measurements, microelectrodes were calibrated using known H2O2 concentrations in PBS.
Mice were euthanized with CO2 before microelectrode measurements. Wound beds were prepared prior to microelectrode measurement by creating an opening in the solid scab. This prevented breakage of microelectrode tip and allowed its visualization. Microelectrode position was controlled using a stepper motor guided by custom LabVIEW software (Physik Instrumente©, PI M-230.10 S, part no. M23010SX). The vertical range of the stepper motor was 8 mm, with a resolution of 40 nm. A stereomicroscope (Zeiss Stemi 2000) was used to monitor the position of the microelectrode tip relative to the surface of the wound bed. First, the microelectrode was positioned at ~50 µm above the wound bed using a manual micromanipulator platform, and then the microelectrode was lowered in steps of 5 µm using the stepper motor until it reached to the wound surface. After that, the microelectrode was moved 4 mm above the wound bed using the stepper motor, 100 µl hydrogel added, and the reference electrode placed within the hydrogel to maintain electrolytic connection with the microelectrode. Finally, the microelectrode was lowered to 100 µm above the wound bed and polarized at 0.8 VAg/AgCl using a Gamry Interface 1000E potentiostat (Gamry Instruments, Warminster, PA). The microelectrode was polarized for 5 min to minimize interference from the transient current response. The polarized microelectrode was then gradually moved using the stepper motor with a step size of 20 µm to obtain the H2O2 concentration-depth profiles. Five second measurements were recorded at each depth.
Wound biofilm quantification:
After treatment, both Tegaderm™ and e-bandages were removed. Wound tissue was removed using a 10 mm biopsy punch tool (Acuderm Inc., Fort Lauderdale, FL). The tissue was placed in a pre-weighed sterile tube and weighed. 1 ml of sterile 0.9% saline and the tissue homogenized using a tissue-homogenizer (Omni International, Kennesaw, GA). This suspension was vortexed for 30 seconds, sonicated in a water bath for 5 minutes, and vortexed again for 30 seconds. 100 µl was serially diluted (10-fold dilutions) in 0.9% saline and colony forming units (CFUs) determined by spread-plating 100 µl of each dilution tube onto sterile sheep blood agar plates for enumerating mono-species bacteria. For the mixed species infections, eosin methylene blue and colistin nalidixic acid plates were used to enumerate MRSA and MDR-PA, respectively. Plates were incubated at 37°C for 24 hours and results reported as log10 CFU/g.
Quantitative wound area measurements and clinical scoring of wounds:
Immediately after creating wounds, quantitative wound area measurements of the wound bed were taken by utilizing a Silhouette wound imaging assessment system (Aranz Medical Ltd., Christchurch, New Zealand). Wound images were taken before e-bandage application and at the end of treatment. Reductions in wound areas in comparison to controls were considered to be indicative of wound healing. Infected mice were clinically scored for purulence on a daily basis using a previously-described scoring system (33) which uses the following scaling model: 0 - no exudate in the wound bed; 1 - slight turbid exudate at the wound site; 2 - mild amount of white exudate at the wound site; 3 - moderate amount of white exudate at the wound site; 4 - moderate amount of yellowish exudate at the wound site; 5 - large amount of turbid yellow exudate extending beyond the wound bed.
Scanning electron microscopy:
After e-bandage treatment, wound tissues for both treatment and control groups (3 animals from each group) were removed using a 10 mm biopsy punch tool (Acuderm Inc., Fort Lauderdale, FL), and placed in sterile tubes containing a fixative solution comprised of 4% formaldehyde with 1% glutaraldehyde in phosphate buffer. Samples were rinsed in phosphate buffer and dehydrated in a series of ethanol washes (10%, 30%, 50%, 70%, 90%, 95%, and 100% - 2×). Samples underwent critical point drying in a vacuum sputter coater (Bio-Rad E5100) and were sputter-coated with gold/palladium (60/40%). Imaging was performed using a Hitachi S4700 cold-field emission scanning electron microscope (Hitachi High Technologies America, Inc., Schaumburg, IL). Magnification ranged between 4000X and 10,000X.
Wound histopathology:
Tissue samples exposed to e-bandage treatment or control groups (3 animals from each group) were removed using a 10 mm biopsy punch tool (Acuderm Inc., Fort Lauderdale, FL) and fixed in 10% formalin for histopathological analysis. Samples were stained with hematoxylin and eosin and blindly analyzed by a board-certified clinical infectious diseases pathologist.
Toxicity and inflammatory panel screening:
After sacrificing the animals, blood was collected via cardiac puncture, separated by centrifugation and serum samples analyzed on a Piccolo® Xpress™ Chemistry Analyzer to assess levels of glucose, amylase, blood urea nitrogen, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase, lactate dehydrogenase, C-reactive protein, blood urea nitrogen, total bilirubin, creatinine, uric acid, albumin, total protein, calcium, chloride, magnesium, potassium, sodium, and total CO2 (performed by Mayo Clinic Central Clinical Laboratories). Serum was also analyzed on a MesoScale Discovery SQ 120 to assess levels of interferon-γ, interleukin-10, interleukin-12p70, interleukin-2, interleukin-4, interleukin-5, interleukin-6, tumor necrosis factor-α, and keratinocyte chemoattractant/human growth-regulated oncogene.
Statistical analysis:
Comparisons across all experimental groups were first performed using the Kruskal Wallis test. Further comparisons between groups were performed in a pairwise manner using the Wilcoxon rank sum test. Non-parametric tests were used due to small sample sizes and inability to support the assumption of normal distribution of the data. Analysis was performed for each bacterial isolate and treatment time. All tests were two sided; p-values less than 0.05 were considered statistically significant. Analysis was performed using SAS software (version 9.4; SAS Institute). Graphs were generated in GraphPad Prism (software version 8.0, GraphPad Software).
Results
H2O2 concentrations were increased in wound beds of MRSA infected mice treated with polarized e-bandages:
As shown in figure 2a, after 24 hours of polarized e-bandage treatment, H2O2 levels were ~113 µM whereas the animals treated with non-polarized e-bandage only showed ~20 µM H2O2, with similar concentrations in the no e-bandage control group. Surprisingly, after 48 hours of polarized e-bandage treatment, there was a reduction in H2O2 levels in the wound bed, although levels remained higher than in the non-polarized group.
Figure 2.

H2O2 concentration profiles in MRSA infected mice treated with polarized or non-polarized e-bandages for a) 1 day or b) 2 days. The approximate wound surface is reported as zero relative distance; H2O2 measurements within the wound tissue are reported as negative relative distances.
Polarized e-bandage treatment reduced bacterial counts in all infected wounds:
Wounds exposed to polarized H2O2-producing e-bandages had a significant reduction (p<0.001) in log10 CFU/g (figure 3). The mean quantity of MRSA in wounds after 48-hour exposure to polarized H2O2-producing e-bandage was 6.32 ±0.67 log10 CFU/g compared to 8.35 ±0.55 log10 CFU/g for the no e-bandage control group (figure 3a). The mean quantity of MRSA in wounds after 48-hour exposure to non-polarized H2O2-producing e-bandages was 8.24 ±0.49 log10 CFU/g, which when compared to the no e-bandage control group was not statistically significantly different. Moreover, there were significant differences in the group treated with vancomycin alone (mean counts of 8.73 ±0.34 log10 CFU/g) compared to the no e-bandage control group. When MRSA infected wounds were treated with a polarized e-bandage and vancomycin, there was no additional decrease in overall CFU counts (mean 6.97 ±0.34 log10 CFU/g) compared to polarized e-bandages alone.
Figure 3.
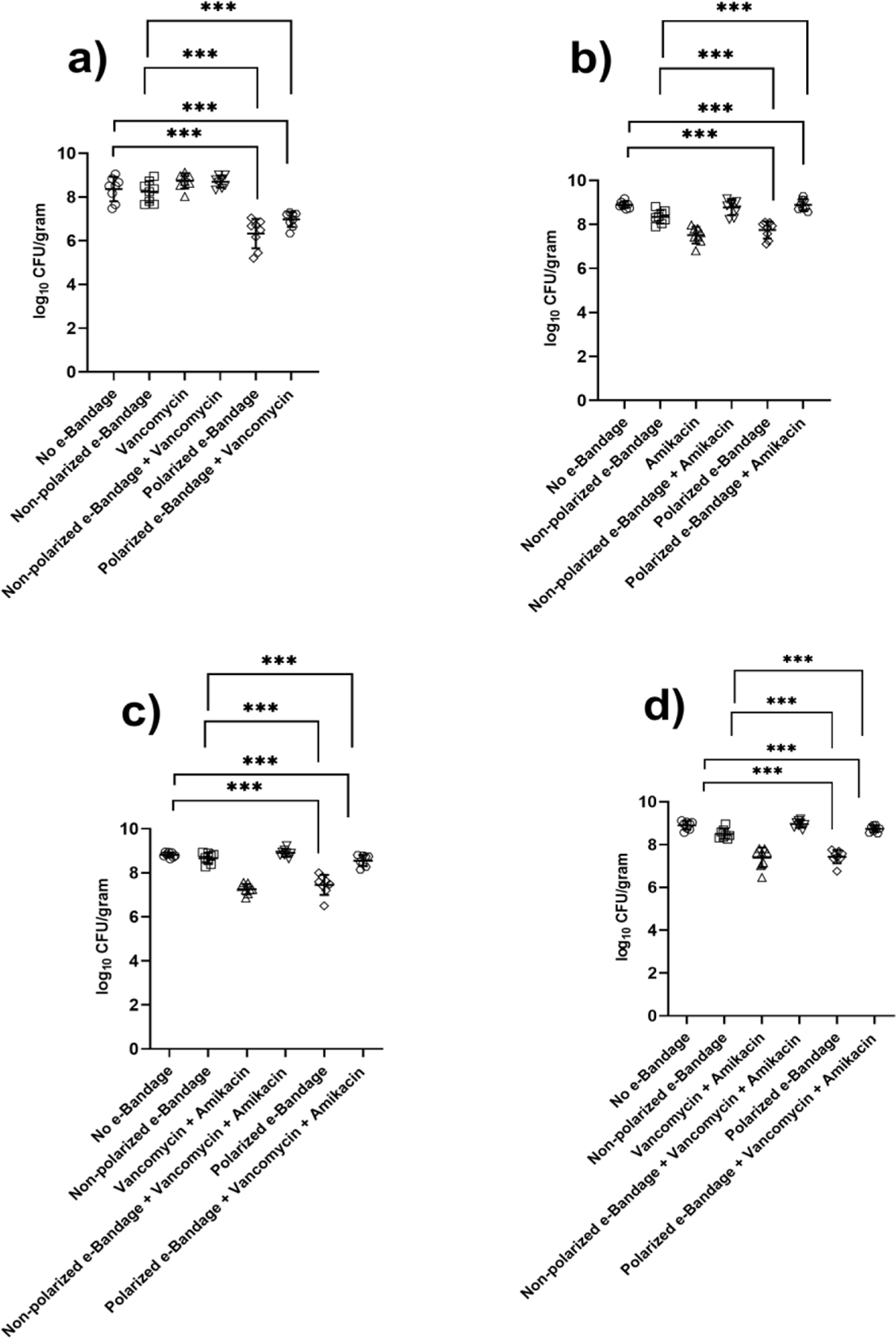
Results of quantitative culture of a) Methicillin-resistant Staphylococcus aureus (MRSA) in MRSA infection; b) Multidrug-resistant Pseudomonas aeruginosa (MDR-PA) in MDR-PA infection; c) MRSA in mixed species infection; and d) MDR-PA in mixed species infection after 48 hours of treatment. N = 8 for each treatment group. Statistical analysis performed by Wilcoxon rank sum test. ***P ≤ 0.001
The mean count of MDR-PA after exposure to polarized H2O2-producing e-bandages was 7.51 ±0.37 log10 CFU/g compared to 8.89 ±0.14 log10 CFU/g for the no e-bandage control group (figure 3b). The mean CFU count of the non-polarized experimental MDR-PA group was 8.76 ±0.34 log10 CFU/g. The group treated with amikacin showed a ~0.55 log reduction in CFU counts compared to the control no e-bandage group, which was significant (mean CFU counts for amikacin group - 8.34 ±0.30 log10 CFU/g; p<0.01). When MDR-PA-infected wounds were treated with the combination of polarized e-bandage and amikacin, there was a significant decrease in overall CFU counts compared to the control no e-bandage group (mean CFU counts - 7.74 ±0.38 log10 CFU/g; p<0.01). However, no significant decreases in CFU counts were observed when comparing results of polarized e-bandage treatment with those of polarized e-bandage in combination with amikacin.
In the mixed-species wound infection model, CFU counts of both MRSA and MDR-PA were significantly reduced after exposure to polarized H2O2-producing e-bandages (p<0.05) (figure 3c and 3d). Mean MRSA CFU counts in the polarized treatment group were 7.26 ±0.25 log10 CFU/g, while those of MDR-PA were 7.51 ±0.31 log10 CFU/g. Mean CFU counts of MRSA and MDR-PA in the no e-bandage control group were significantly higher than in the polarized treatment groups (8.81 ±0.10 log10 CFU/g and 8.90 ±0.19 log10 CFU/g, respectively; p<0.01). The reduction in wound counts was significant in the groups treated with polarized e-bandage alone or with vancomycin and amikacin when compared to the no e-bandage control group (p<0.05).
Based on the above reduction in CFU counts, polarized e-bandage treatment effectively reduces both mono- and mixed-species wound infections caused by MRSA and MDR-PA, and combining antibiotics with an e-bandage does not necessarily augment CFU reduction.
The vancomycin area under the curve (AUC) from 0 to 12 hours, with dosing every 12 hours was 455.4 μg•h/mL. With a MIC of 1 μg/mL, the AUC/MIC ratio is 455.4, within range of the recommended AUC/MIC ratio of 400–600 for achieving clinical efficacy in humans (36, 37). The therapeutic peak concentration of amikacin was 33.6 μg/ml (0.25 hour), also within the reported range achieved with standard human dosing (34).
Treatment of infected wounds with polarized e-bandages increased wound closure and reduced purulence:
Figure 4 shows differences in overall wound area measurements observed in different treatment groups. As shown in figure 4, the most visible change in overall wound area measurement was in treatment groups involving polarized e-bandages. Average wound area reductions (in mm2) of the polarized treatment group for MRSA, MDR-PA and mixed-species infections were all statistically significant (p-value <0.05), whereas reductions for the control groups were not. Similarly, the average wound area reduction of the group treated with polarized e-bandages in combination with vancomycin and/or amikacin was also found to be statistically significant (p-value <0.05). The non-polarized e-bandage groups with and without concomitant antibiotics showed a marginal decrease (not statistically significant) in average wound area when compared with pre-treatment measurements.
Figure 4.

Wound area measurements of a) Methicillin-resistant Staphylococcus aureus; b) Multidrug-resistant Pseudomonas aeruginosa; c) Mixed species infections after 48 hours of treatment. N = 8 for each treatment group. Statistical analysis performed by Wilcoxon rank sum test. *P ≤ 0.05
Interestingly, treatment with vancomycin or amikacin alone showed no marked decrease in average wound area measurements. Moreover, as shown in figure 5, there was a marked decrease in average wound purulence scores when mice were treated with polarized e-bandages with or without antibiotics. The average purulence scores of the e-bandage polarized treatment groups were significantly lower (p<0.01) compared to the pre-treatment group. In all polarized treatment groups, mice infected with MRSA, MDR-PA, or both, showed significantly lower purulence scores (p<0.01) regardless of the presence or absence of antibiotics. Infected mice treated with non-polarized e-bandages with and without antibiotics did not show any significant change in purulence scores.
Figure 5.
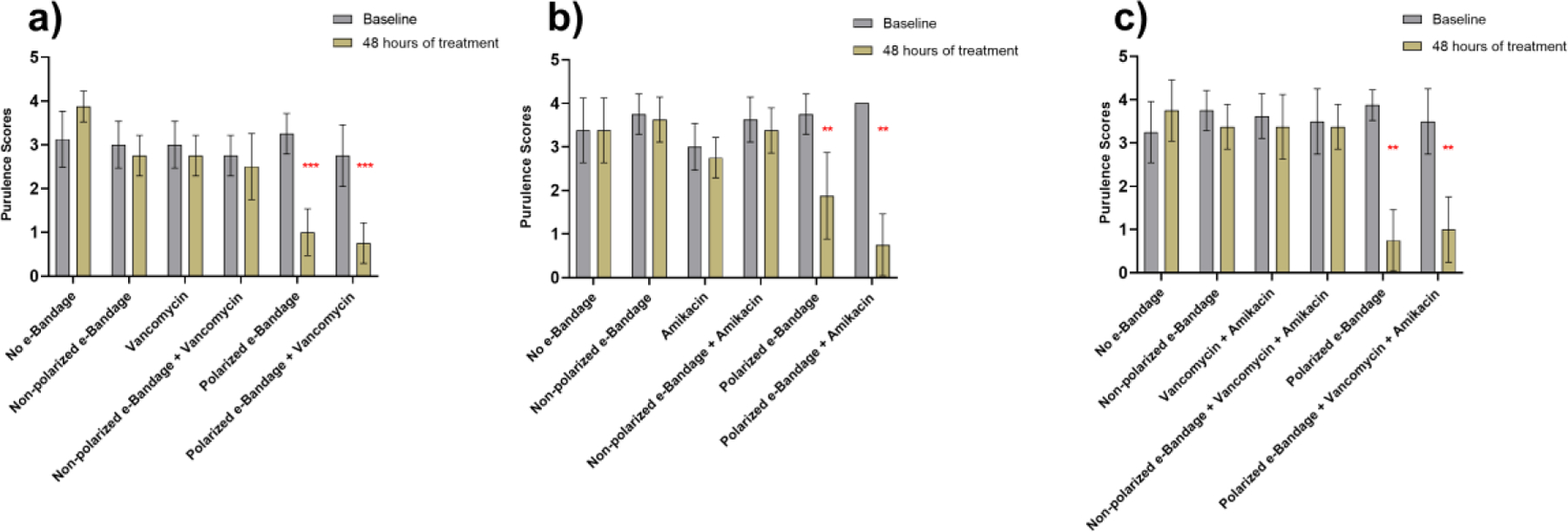
Purulence scores associated with a) Methicillin-resistant Staphylococcus aureus; b) Multidrug-resistant Pseudomonas aeruginosa; c) Mixed species infections after 48 hours of treatment. N = 8 for each treatment group. Statistical analysis performed by Wilcoxon rank sum test. ** - P ≤ 0.01, *** - P ≤0.001.
Collectively, results of quantitative wound area measurements and purulence scores suggest that treating wound infections with H2O2-producing polarized e-bandages improves wound-healing in infected animals.
Scanning electron microscopy revealed wound biofilms treated with polarized e-bandages to be degraded, and resident bacterial cells to be damaged:
Scanning electron microscopy was performed on wound tissue after exposure to e-bandage treatment. As seen in figure 6, an abundance of bacteria was observed throughout the wound bed. Dense MRSA and MDR-PA biofilms comprised a large portion of the wound tissue. Biofilm matrix components were visualized as were host-tissue cells. Biofilms treated with polarized e-bandages showed a different morphology when compared to the no e-bandage control group, with biofilm-matrix components being reduced or of irregular shapes and bacterial cells being irregular in shape and size, and with damaged outer cell surfaces, suggesting H2O2-induced bacterial cell lysis.
Figure 6.
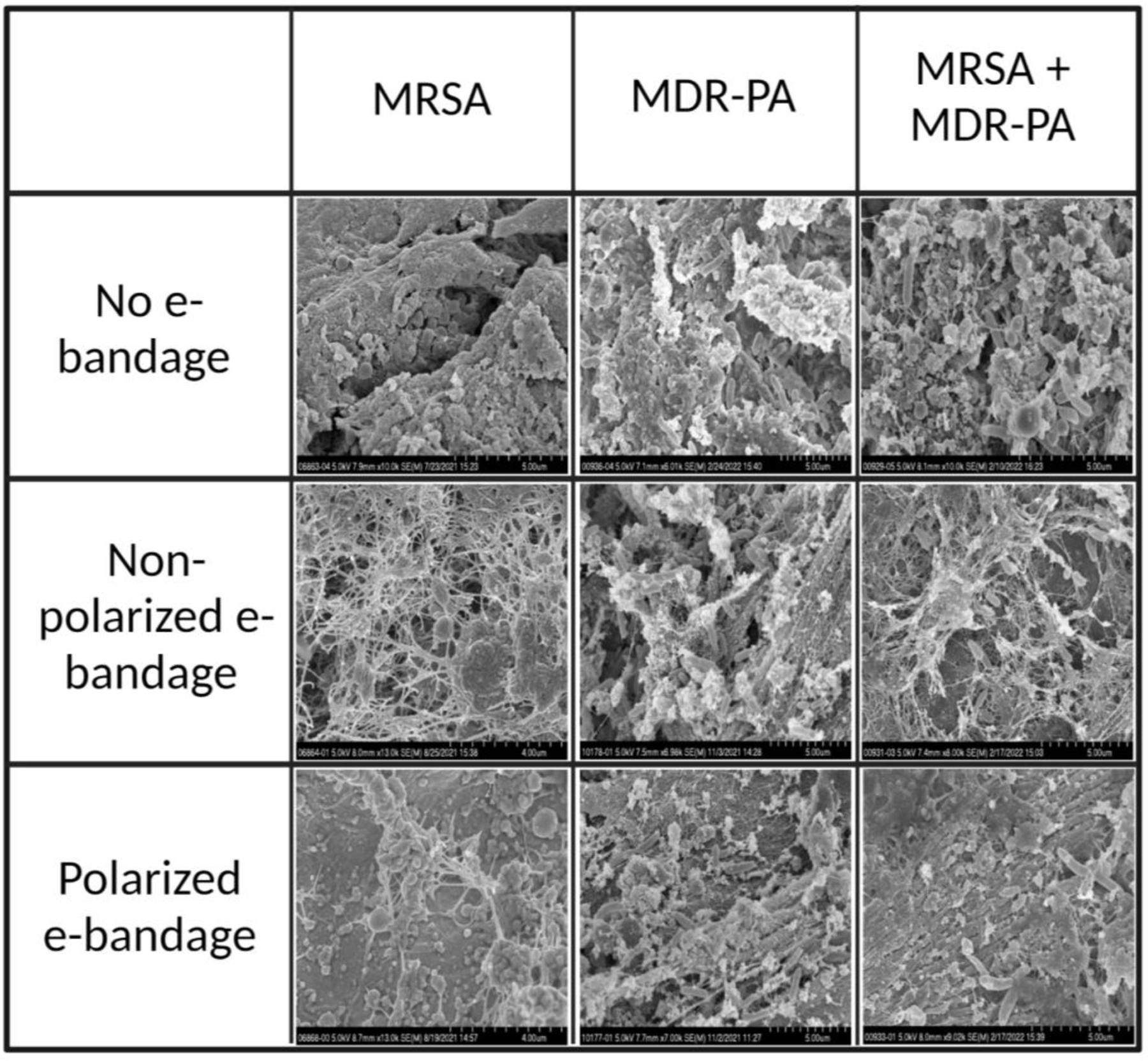
Scanning electron microscopy images of infected wound beds without and with non-polarized and polarized e-bandages after 48 hours of treatment. MRSA, methicillin-resistant Staphylococcus aureus; MDR-PA, multidrug-resistant Pseudomonas aeruginosa. Scale bar – 5µm.
Appropriate tissue inflammatory response to bacterial pathogens was observed:
Figure 7 shows the histopathology of infected wound tissues stained with hematoxylin and eosin. Moderate to acute inflammation affecting the epidermal and dermal layers was observed in all tissue samples with either mono- or mixed-species infections. Tissue samples were ulcerated with inflammatory regions characterized by an influx of neutrophils, macrophages, and occasional lymphocytes. Tissue samples from all experimental groups, including no e-bandage control group and non-polarized and polarized treatment groups, showed similar degrees of inflammation. However, there was a slight increase in neutrophils and macrophages in tissue samples infected with MRSA alone or with MDR-PA. These results suggest that the presence of an e-bandage does not cause adversely alter the expected antibacterial inflammatory response present in the underlying tissue.
Figure 7.
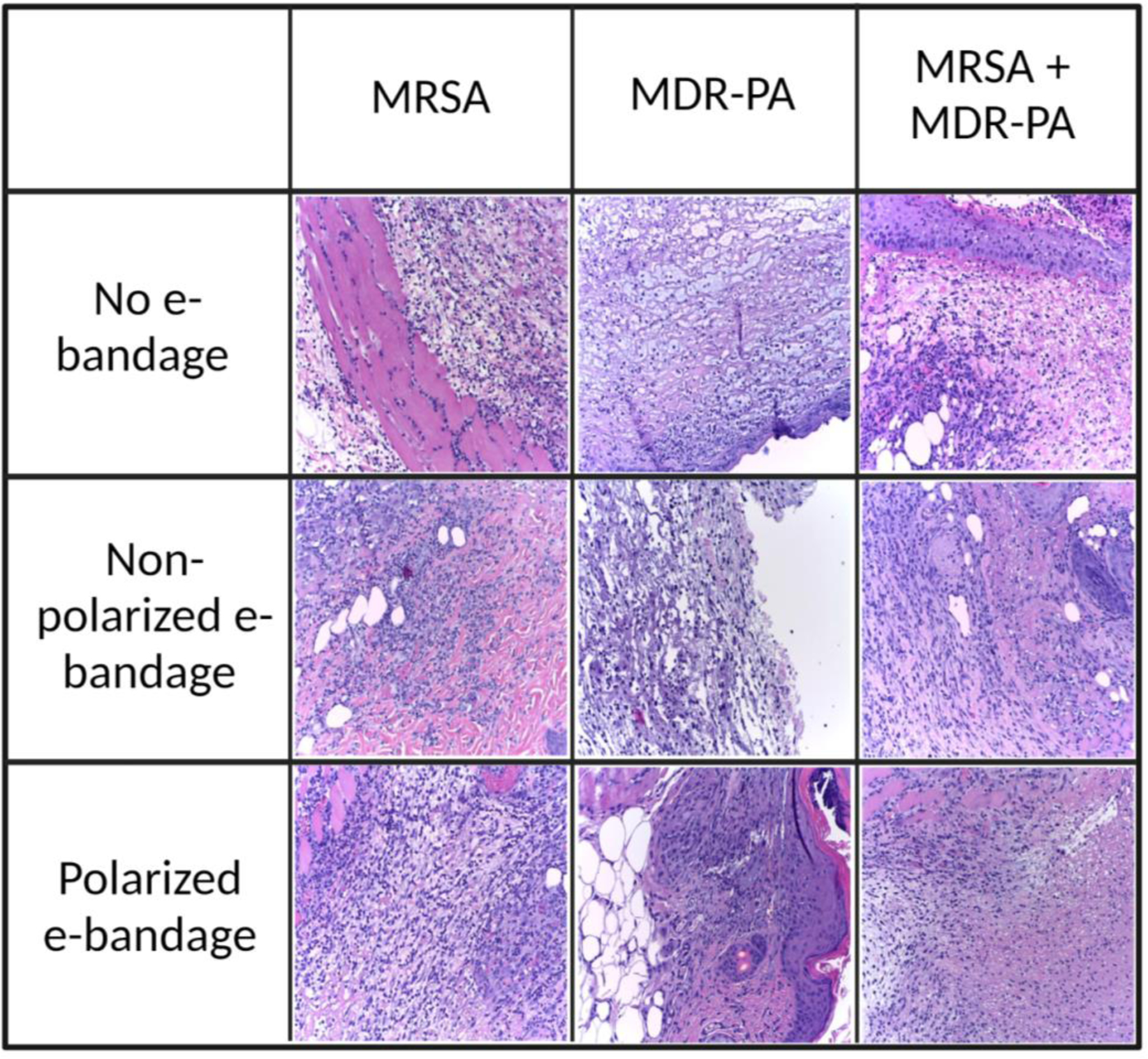
Histopathological images of infected wound beds without and with non-polarized and polarized e-bandages after 48 hours of treatment. Images were taken at 200× total magnification. MRSA, methicillin-resistant Staphylococcus aureus; MDR-PA, multidrug-resistant Pseudomonas aeruginosa.
Toxicity and inflammatory panel screens:
There were no significant changes in serum blood chemistry or enzyme levels between animals from different experimental treatment groups in mixed-species infection (table S1). Also, levels of proinflammatory cytokines in mice serum were not significantly different between various experimental groups (table S2 and figure S1), although slightly elevated levels of interleukin-5, interleukin-6, keratinocyte chemoattractant/human growth-regulated oncogene, and tumor necrosis factor-α were observed in animals exposed to either non-polarized or polarized e-bandages.
Discussion
S. aureus and/or P. aeruginosa are commonly present in wounds and can be associated with persistent infections; together, these two species are responsible for a majority of skin and soft tissue infections (38). MRSA wound colonization is estimated to occur in 3% of wounds in the US (39, 40). Infections caused by MDR-PA have been increasing in the US over the last two decades (41, 42). Although treatment of wound infections caused by mono-species bacterial infection has been pursued (43), more than one bacterial species is typically found in wound beds. Therefore, in this study, the activity of H2O2 producing e-bandages against wound infections caused by single as well as dual species was assessed. Concurrent wound infection by MRSA and P. aeruginosa has been reported to hinder wound healing (44–46). Even with regular debridement, complete removal of biofilms from wound beds can be difficult. Hence, additional measures, such as cleansing with antiseptic solutions like H2O2 are often deployed. Low concentrations of H2O2 can accelerate wound-healing mechanism beyond acting as an antibacterial agent (47). However, a drawback of using H2O2 in this way is that it is rapidly decomposed due to inherent instability, requiring frequent reapplication. Thus, novel strategies for continuous delivery of H2O2 to infected wound beds represent an innovative wound infection treatment option. Our group has developed a state-of-the-art electrochemical anti-bacterial/anti-biofilm bandage (e-bandage) that can continuously generate low amounts of H2O2 at nontoxic concentrations (29). The e-bandage is powered by inexpensive small (less than a size of penny) Li-ion batteries, via a custom-built wearable potentiostat. In previous work, we showed that this e-bandage could sterilize in vitro bacterial biofilms within 48 hours of treatment (30). Here, we assessed this H2O2 producing e-bandage in a mouse wound infection model.
Traditional antibiotics are often rendered poorly active in wound biofilm environments due to low pH, low water availability, production of enzymes that degrade antibiotics, decreased bacterial metabolism, etc. (48, 49). The polarized e-bandage under control of wearable potentiostat described in this work reduced MRSA wound biofilms by ~2-logs after 48 hours in vivo. In comparison, the same e-bandage eliminated >8-logs of the same MRSA strain in vitro (30). Such a difference between in vitro and in vivo efficacy is not unexpected. For example, Dong et. al 2019 assessed the anti-bacterial and anti-biofilm activity of staphyloxanthin photolysis in the presence of H2O2 and low-level 460 nm blue laser light (50). Synergistic addition of H2O2 and staphyloxanthin photolysis in the presence of blue light eliminated in vitro MRSA biofilms within 40 minutes. When evaluated against MRSA in a murine wound infection model, ~1-log and ~2-log reduction in colony counts was observed when treated with H2O2 alone and in combination with 460 nm blue laser light, respectively. Bacteria present in biofilms produce various enzymes and chemicals to protect themselves against external stress-inducing factors, including temperature, pH, chemicals, and antibiotics (11, 14). Bacterial-produced peroxidases, catalases, and glutathione reductase can degrade H2O2 (51–53), and possibly reduce overall effectiveness of the e-bandage system. Eukaryotic cells can also mount responses against H2O2 produced by e-bandages, and this could initiate a cascade of events that could result in the production of enzymes which reduce the antibiofilm activity of the system. In addition to this, during in vitro experiments, e-bandages cover biofilms perfectly and have a direct contact with their entire surface. In contrast, in the animal model, e-bandages imperfectly cover wound surfaces because of physical movements of the mice.
Combination treatment of some biofilm infections (i.e., with multiple antimicrobial agents) may be more effective than single agent treatments, especially with regard to biofilm infections. For example, Fasiku et al. evaluated the efficacy of a hydrogel loaded with H2O2 against MRSA in a mouse wound infection model (54). A ~0.5-log reduction in MRSA colony counts was found with H2O2-loaded hydrogels alone; however, when the hydrogel was combined with antimicrobial peptide and chitosan, the anti-biofilm activity was improved with a ~2-log reduction in MRSA colony counts achieved. In the current study, treatment of MRSA wound biofilms with vancomycin in combination with polarized e-bandages revealed a marginal improvement in reduction of viable colony counts.
In comparison to MRSA-infected wounds, wounds infected with MDR-PA only showed ~1.3-logs reduction in biofilm CFU counts when treated with polarized e-bandages; combination treatment with amikacin and e-bandages did not improve bacterial killing. P. aeruginosa can mount a strong SOS response in the presence of sub-lethal H2O2 concentrations (55, 56). P. aeruginosa has several catalase producing genes (e.g., katA, katB) which can confer protection against H2O2. These factors might explain the lower CFU reductions observed with e-bandage treatment of MDR-PA compared to MRSA infection.
The presence of bacterial in biofilms in wound beds decreases the efficacy of many treatment regimens employed in clinical settings. The presence of more than one bacterial species impedes wound healing compared to wound infections caused by a single species. In one study, mice wounds infected with a combination of S. aureus, P. aeruginosa, Enterococcus faecalis, and Finegoldia magna showed lower wound closure rates compared to those infected with P. aeruginosa alone (57). Moreover, when such wounds were treated with either bleach or gentamicin, the polymicrobial infection group had higher numbers of viable bacteria compared to mono-species P. aeruginosa infections. In another study, the presence of biofilms formed by MRSA and P. aeruginosa in murine wound beds resulted in delayed epithelialization, as evidenced by staining of epidermal marker K14 (46). In the present study, mixed-species wound infections treated with polarized e-bandages with and without antibiotics had CFU reductions of each bacterial species similar to that of mono-species infection, suggesting that the presence of mixed-species biofilms in wounds did not offer additional protection against e-bandage treatment compared to the individual bacterial species .
The inherent wound healing properties of H2O2 in both in vitro and in vivo settings have been studied (28, 58–60). For example, Loo et al. performed experiments on skin wounds generated on C57Bl/6 mice and observed that low concentrations of topically applied H2O2 improved wound closure rates and enhanced angiogenesis (61). 10 mM H2O2 enhanced wound healing, whereas 166 mM delayed wound closure. In the current study, enhanced wound area reduction was observed with polarized e-bandage treatment compared to nonpolarized e-bandage or no e-bandage controls. The presence of H2O2-producing e-bandages on wounds resulted in similar wound reduction/closure rates in mice infected with MRSA or MDR-PA alone or together. This suggests that application of e-bandages to infected wounds may be beneficial whether infections are mono-species or mixed-species in nature. Although the results presented here are promising, continued optimization of the e-bandage system is needed. One possible way to further increase the in vivo anti-biofilm activity would be to increase the treatment time. Additionally, future studies are warranted to assess e-bandage effects in conjunction with antimicrobial agents beyond those studied here, including those which can be applied topically.
In conclusion, H2O2-producing e-bandages reduced MRSA and MDR-PA wound infections in mice both in mono- and mixed-species infections, improved wound closure rates, and were associated with decreased purulence in wound beds without apparent toxicity as assessed by scanning electron microscopy, histopathology, and blood chemistry analyses. Based on these promising results, H2O2-producing e-bandages offer a potential new treatment strategy for wound infections.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under the grant number R01 AI091594. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Aaron Barnes (University of Minnesota) for review of scanning electron microscopy images. The authors also acknowledge the technical assistance provided by Mayo Clinic Microscopy and Cell Analysis Core and Scott Gamb. R.P. reports grants from ContraFect, TenNor Therapeutics Limited, Hylomorph, BioFire and Shionogi. R.P. is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, Torus, Mammoth Biosciences and Qvella; monies are paid to Mayo Clinic. R.P. is also a consultant to Netflix. In addition, R.P. has patents on Bordetella pertussis/parapertussis PCR, a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and an antibiofilm substance. R.P. receives an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. H.B. holds a patent (US20180207301A1), “Electrochemical reduction or prevention of infections,” which refers to the electrochemical scaffold upon which the current design of e-bandage is based.
References
- 1.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States [Google Scholar]
- 2.Butler MS, Gigante V, Sati H, Paulin S, Al-Sulaiman L, Rex JH, Fernandes P, Arias CA, Paul M, Thwaites GE. 2022. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: despite progress, more action is needed. Antimicrobial agents and chemotherapy 66:e01991–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2022. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. [Google Scholar]
- 4.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. 2009. Human skin wounds: A major and snowballing threat to public health and the economy. Wound repair and regeneration 17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. 2008. Growth factors and cytokines in wound healing. Wound repair and regeneration 16:585–601. [DOI] [PubMed] [Google Scholar]
- 6.James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wound Repair and regeneration 16:37–44. [DOI] [PubMed] [Google Scholar]
- 7.Edwards R, Harding KG. 2004. Bacteria and wound healing. Current opinion in infectious diseases 17:91–96. [DOI] [PubMed] [Google Scholar]
- 8.Metcalf DG, Bowler PG. 2013. Biofilm delays wound healing: a review of the evidence. Burns & Trauma 1:2321–3868.113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson CH. 2011. Biofilms: do they affect wound healing? International wound journal 8:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhoads DD, Wolcott RD, Percival SL. 2008. Biofilms in wounds: management strategies. Journal of wound care 17:502–508. [DOI] [PubMed] [Google Scholar]
- 11.Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS matrix: the “house of biofilm cells”. Journal of bacteriology 189:7945–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flemming H-C, Wingender J 2010. The biofilm matrix. Nature reviews microbiology 8:623–633. [DOI] [PubMed] [Google Scholar]
- 13.Gil C, Solano C, Burgui S, Latasa C, García B, Toledo-Arana A, Lasa I, Valle J. 2014. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infection and immunity 82:1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison DG. 2003. The biofilm matrix. Biofouling 19:139–150. [DOI] [PubMed] [Google Scholar]
- 15.Mah T-F. 2012. Biofilm-specific antibiotic resistance. Future microbiology 7:1061–1072. [DOI] [PubMed] [Google Scholar]
- 16.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. International journal of antimicrobial agents 35:322–332. [DOI] [PubMed] [Google Scholar]
- 17.Lipsky BA, Hoey C. 2009. Topical antimicrobial therapy for treating chronic wounds. Clinical infectious diseases 49:1541–1549. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Turnbull L, Burke CM, Liu M, Carter DA, Schlothauer RC, Whitchurch CB, Harry EJ. 2014. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2:e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campeau ME, Patel R. 2014. Antibiofilm activity of Manuka honey in combination with antibiotics. International journal of bacteriology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip M, Lui SL, Poon VK, Lung I, Burd A. 2006. Antimicrobial activities of silver dressings: An in vitro comparison. Journal of medical microbiology 55:59–63. [DOI] [PubMed] [Google Scholar]
- 21.Tavakoli S, Klar AS. 2020. Advanced hydrogels as wound dressings. Biomolecules 10:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borkow G, Gabbay J, Dardik R, Eidelman AI, Lavie Y, Grunfeld Y, Ikher S, Huszar M, Zatcoff RC, Marikovsky M. 2010. Molecular mechanisms of enhanced wound healing by copper oxide‐impregnated dressings. Wound repair and regeneration 18:266–275. [DOI] [PubMed] [Google Scholar]
- 23.Hampton MB, Kettle AJ, Winterbourn CC. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood, The journal of the american society of hematology 92:3007–3017. [PubMed] [Google Scholar]
- 24.Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C. 2007. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. Journal of burns and wounds 6. [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q, Qiu WY, Huo YN, Yao YF, Lou MF. 2011. Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing. Investigative ophthalmoly & visual science 52:1723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy EC, Friedman AJ. 2019. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. Journal of the american academy of dermatology 81:1379–1386. [DOI] [PubMed] [Google Scholar]
- 27.van der Vliet A, Janssen-Heininger YM. 2014. Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? Journal of cellular biochemistry 115:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo AEK, Halliwell B. 2012. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochemical and biophysical research communications 423:253–258. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed A, Anoy MMI, Tibbits G, Raval YS, Flurin L, Greenwood-Quaintance KE, Patel R, Beyenal H. 2021. Hydrogen peroxide-producing electrochemical bandage controlled by a wearable potentiostat for treatment of wound infections. Biotechnology and Bioengineering 118:2815–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raval YS, Mohamed A, Flurin L, Mandrekar JN, Greenwood Quaintance KE, Beyenal H, Patel R. 2021. Hydrogen-peroxide generating electrochemical bandage is active in vitro against mono- and dual-species biofilms. Biofilm 3:100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raval YS, Mohamed A, Mandrekar JN, Fisher C, Greenwood-Quaintance KE, Beyenal H, Patel R. 2022. In vitro antibiofilm activity of hydrogen peroxide-generating electrochemical bandage against yeast biofilms. Antimicrobial agents and chemotherapy 66:e0179221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flurin L, Raval YS, Mohamed A, Greenwood-Quaintance KE, Cano EJ, Beyenal H, Patel R. 2021. An integrated HOCl-producing e-scaffold is active against monomicrobial and polymicrobial biofilms. Antimicrobial agents and chemotherapy 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim CK, Karau MJ, Greenwood-Quaintance KE, Tilahun AY, Krogman A, David CS, Pritt BS, Patel R, Rajagopalan G. 2015. Superantigen-producing Staphylococcus aureus elicits systemic immune activation in a murine wound colonization model. Toxins 7:5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anhalt J, Washington II J. 1985. Bactericidal tests. Laboratory procedures in clinical microbiology Springer-Verlag, New York:731–745. [Google Scholar]
- 35.Lewandowski Z, Beyenal H. 2013. Fundamentals of biofilm research CRC press. [Google Scholar]
- 36.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clinical infectious diseases 49:325–327. [DOI] [PubMed] [Google Scholar]
- 37.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. American journal of health-system pharmacy 66:82–98. [DOI] [PubMed] [Google Scholar]
- 38.Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, De Franciscis S. 2015. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert review of anti-infective therapy 13:605–613. [DOI] [PubMed] [Google Scholar]
- 39.Tong SY, McDonald MI, Holt DC, Currie BJ. 2008. Global implications of the emergence of community-associated methicillin-resistant Staphylococcus aureus in indigenous populations. Clinical infectious diseases 46:1871–1878. [DOI] [PubMed] [Google Scholar]
- 40.Kalra L, Camacho F, Whitener CJ, Du P, Miller M, Zalonis C, Julian KG. 2013. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. American journal of infection control 41:1253–1257. [DOI] [PubMed] [Google Scholar]
- 41.Tam VH, Chang K-T, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, Weston JS, Caeiro J-P, Garey KW. 2010. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy 54:1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsch EB, Tam VH. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert review of pharmacoeconomics & outcomes research 10:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seth AK, Geringer MR, Hong SJ, Leung KP, Mustoe TA, Galiano RD. 2012. In vivo modeling of biofilm-infected wounds: A review. Journal of surgical research 178:330–338. [DOI] [PubMed] [Google Scholar]
- 44.Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. 2012. Increasing the presence of biofilm and healing delay in a porcine model of MRSA‐infected wounds. Wound repair and regeneration 20:537–543. [DOI] [PubMed] [Google Scholar]
- 45.Scales BS, Huffnagle GB. 2013. The microbiome in wound repair and tissue fibrosis. The journal of pathology 229:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, Valdes J, Stojadinovic O, Plano LR, Tomic-Canic M, Davis SC. 2013. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PloS one 8:e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schreml S, Landthaler M, Schäferling M, Babilas P. 2011. A new star on the H2O2rizon of wound healing? Experimental dermatology 20:229–231. [DOI] [PubMed] [Google Scholar]
- 48.Davies D 2003. Understanding biofilm resistance to antibacterial agents. Nature reviews drug discovery 2:114–122. [DOI] [PubMed] [Google Scholar]
- 49.Del Pozo J, Patel R. 2007. The challenge of treating biofilm‐associated bacterial infections. Clinical pharmacology & therapeutics 82:204–209. [DOI] [PubMed] [Google Scholar]
- 50.Dong PT, Mohammad H, Hui J, Leanse LG, Li J, Liang L, Dai T, Seleem MN, Cheng JX. 2019. Photolysis of staphyloxanthin in methicillin‐resistant Staphylococcus aureus potentiates killing by reactive oxygen species. Advanced science 6:1900030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fomenko DE, Koc A, Agisheva N, Jacobsen M, Kaya A, Malinouski M, Rutherford JC, Siu K-L, Jin D-Y, Winge DR. 2011. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proceedings of the national academy of sciences 108:2729–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho Y-S, Xiong Y, Ma W, Spector A, Ho DS. 2004. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. Journal of biological chemistry 279:32804–32812. [DOI] [PubMed] [Google Scholar]
- 53.de Haan JB, Bladier C, Griffiths P, Kelner M, O’Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS. 1998. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. Journal of biological chemistry 273:22528–22536. [DOI] [PubMed] [Google Scholar]
- 54.Fasiku VO, Omolo CA, Devnarain N, Ibrahim UH, Rambharose S, Faya M, Mocktar C, Singh SD, Govender T. 2021. Chitosan-based hydrogel for the dual delivery of antimicrobial agents against bacterial methicillin-resistant Staphylococcus aureus biofilm-infected wounds. ACS omega 6:21994–22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elkins JG, Hassett DJ, Stewart PS, Schweizer HP, McDermott TR. 1999. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Applied and environmental microbiology 65:4594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong P-T, Jusuf S, Hui J, Zhan Y, Zhu Y, Liu GY, Cheng J-X. 2022. Photoinactivation of catalase sensitizes a wide range of bacteria to ROS-producing agents and immune cells. JCI insight 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP. 2011. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PloS one 6:e27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan Q, Qiu W-Y, Huo Y-N, Yao Y-F, Lou MF. 2011. Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing. Investigative ophthalmology & visual science 52:1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy S, Khanna S, Sen CK. 2008. Redox regulation of the VEGF signaling path and tissue vascularization: Hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free radical biology and medicine 44:180–192. [DOI] [PubMed] [Google Scholar]
- 60.Kanta J 2011. The role of hydrogen peroxide and other reactive oxygen species in wound healing. Acta Medica (Hradec Kralove) 54:97–101. [DOI] [PubMed] [Google Scholar]
- 61.Loo AEK, Wong YT, Ho R, Wasser M, Du T, Ng WT, Halliwell B. 2012. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PloS one 7:e49215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


