Abstract
There are many examples of remote technologies that are clinically effective and provide numerous benefits to adults with hearing loss. Despite this, the uptake of remote technologies for hearing healthcare has been both low and slow until the onset of the COVID-19 pandemic, which has been a key driver for change globally. The time is now right to take advantage of the many benefits that remote technologies offer, through clinical, consumer, or hybrid services and channels. These include greater access and choice, better interactivity and engagement, and tailoring of technologies to individual needs, leading to clients who are better informed, enabled, and empowered to self-manage their hearing loss. This article provides an overview of the clinical research evidence-base across a range of remote technologies along the hearing health journey. This includes qualitative, as well as quantitative, methods to ensure the end-users' voice is at the core of the research, thereby promoting person-centered principles. Most of these remote technologies are available and some are already in use, albeit not widespread. Finally, whenever new technologies or processes are implemented into services, be they clinical, hybrid, or consumer, careful consideration needs to be given to the required behavior change of the key people (e.g., clients and service providers) to facilitate and optimize implementation.
Keywords: remote technologies; connected hearing healthcare; service delivery models; consumer channels; over-the-counter; implementation science, behavior change
In the 2000s, there was a slow introduction of telehealth to provide audiology services including screening, diagnosis, and interventions. 1 The value of such services then, as with now, is that service delivery models that use remote technologies have the potential to maximize limited global healthcare resources by providing greater, and more flexible, opportunities for clients and healthcare professionals, thereby optimizing hearing healthcare. 2 Benefits of telehealth are numerous, most notably greater equity and access to healthcare by overcoming barriers of time, mobility, and geography. The geographical benefit is particularly the case in countries with a large land mass, such as Australia, with scattered populations in rural and remote communities. Other benefits include the potential for more individualized and tailored healthcare, greater interactivity that can lead to improved engagement with services, and greater opportunities for self-monitoring and self-evaluation, leading to increased self-management, which is particularly important for chronic conditions such as hearing loss. 3 More recently, the opportunities to gather huge amounts of “big data” feed into an increasing use of machine learning and artificial intelligence to provide more personalized healthcare. 4
As the field has developed within audiology so has the terminology, whereby telehealth-delivered audiology services can be referred to as connected hearing healthcare (CHH), teleaudiology, and e-audiology, amongst others. 2 5 6 All these terms encompass the broad use of telehealth and ehealth, of which mhealth is a subcategory that delivers healthcare via mobile technologies. In brief, these terms encompass a combination of people, processes, and technology. Here, we use the term “connected hearing healthcare,” which has been defined as “access to hearing healthcare that integrates big data, technology, and machine learning to individualize services for everyone.” 7 We use the term “remote technologies” to refer to component parts of CHH.
The use of remote hearing technologies no longer sits solely within clinical audiology. Since 2016, with a focus on priorities for improving accessibility and affordability for hearing healthcare in adults, 8 there has been an increase in the number and availability of consumer products. These can be delivered through various consumer models, commonly known as over-the-counter (OTC) or direct-to-consumer (DTC). Despite being the subject of many opinion pieces, there is little clarity on how these technologies are evolving or are likely to evolve over the next decade in terms of service (in-person, clinical, user-led) and channel (e.g., clinical, consumer). Thus, it is timely that a framework to conceptualize these has been proposed (see Brice et al 9 ).
There is also a lack of clarity in the terminology for OTC and DTC models, as well as for the hearing devices that are delivered within these models. For example, OTC and DTC are often used interchangeably, and similarly each is often used interchangeably for both devices and services (e.g., an OTC device, an OTC delivery model). This lack of clarity can lead to confusion. To address this, a three-round electronic Delphi review of 21 leading international hearing healthcare experts was conducted in 2019, with the aim of reaching a consensus on the characteristics and definitions of the terms commonly used (see Table 1 ). Although there are characteristics that elicited high agreement or disagreement (≥80%), there remains a lack of consensus in just over half (55%) the characteristic/device combinations. As is also discussed by Penno and Zakis, 10 while there are areas of shared commonalities, the appropriate application of each device type is informed by a clearer understanding of their functionalities and capabilities. Currently, conventional hearing aids are typically differentiated from “alternative hearing or listening devices” in that hearing aids are a registered medical product and the alternatives are not.
Table 1. Consensus relating to device features for hearable, OTC hearing device, PSAP, self-fitting hearing aid, wearable, and hearing aid.
| Hearable | OTC hearing device | PSAP | Self-fitting hearing aid | Wearable | Hearing aid | |
|---|---|---|---|---|---|---|
| Provides amplification |
75%

|


|


|


|
45%

|


|
| Has fixed preprograms |
45%

|
65%

|
75%

|
35%
|
60%

|
55%

|
| Can be programed to a prescriptive target |
40%
|
55%

|
45%

|

|
75%
|


|
| User can adjust frequency/gain using controls on device |
50%

|

|


|


|
50%
|


|
| User can adjust frequency/gain using remote technologies (e.g., smartphone app) |

|
60%

|
65%

|

|
55%

|
75%

|
| Device itself can be customized to physically fit user's ear canal |
|
50%

|
55%
|
70%

|
65%
|


|
| Is only for people with hearing-related difficulties |
|

|
50%
|


|
75%
|


|
| Is considered a medical/healthcare device |
|
45%

|
|

|
75%
|


|
Abbreviations: OTC, over-the-counter; PSAP, personal sound amplification product.
Notes:

 , >90% agreement;
, >90% agreement;
 , 80–90% agreement;
, 80–90% agreement;
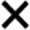
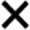 , >90% disagreement;
, >90% disagreement;
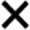 , 80–90% disagreement. For <80% agreement, both percentage and direction (
, 80–90% disagreement. For <80% agreement, both percentage and direction (
 agree;
agree;
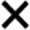 disagree;
disagree;
 neither agree/disagree) are provided.
neither agree/disagree) are provided.
Despite the proliferation of remote technologies over the last decade, uptake within clinical services has remained low, as has the development of clinical audiology services and new delivery models to implement these new technologies effectively. 11 Three key considerations in developing and implementing such services are (1) a robust research evidence-base, (2) input from key stakeholders to ensure new technologies and services are aligned to the needs of end-users (e.g., clients, consumers, hearing care professionals, consumer industry), 12 and (3) access to the necessary connectivity and infrastructure to deliver these services. The global COVID-19 pandemic that took hold early in 2020 was an unexpected and substantial driver for change. This resulted in a need to consider alternative models of care to the conventional high-touch audiology services because of the need of services to use low-touch and no-touch options. 13 14 The increase in the use of remote technologies and CHH services in the first half of 2020, as clinical services strived to continue to provide for their clients, 15 16 17 suggested that the reticence around their uptake was over (or at least substantially reduced), and clinical need would drive a new delivery paradigm (see Fig. 1 ). Remote services have been established for every aspect along the hearing health journey and across a variety of service models. 9 However, while there appears to be acceptance of these services, with a need to make them commercially viable and successful, there is limited evidence on preferences between remote and in-person services when both are offered. One study showed a preference for in-person over remote services, although notably those who tried remote services reported they would be more likely use them in future. 16
Figure 1.
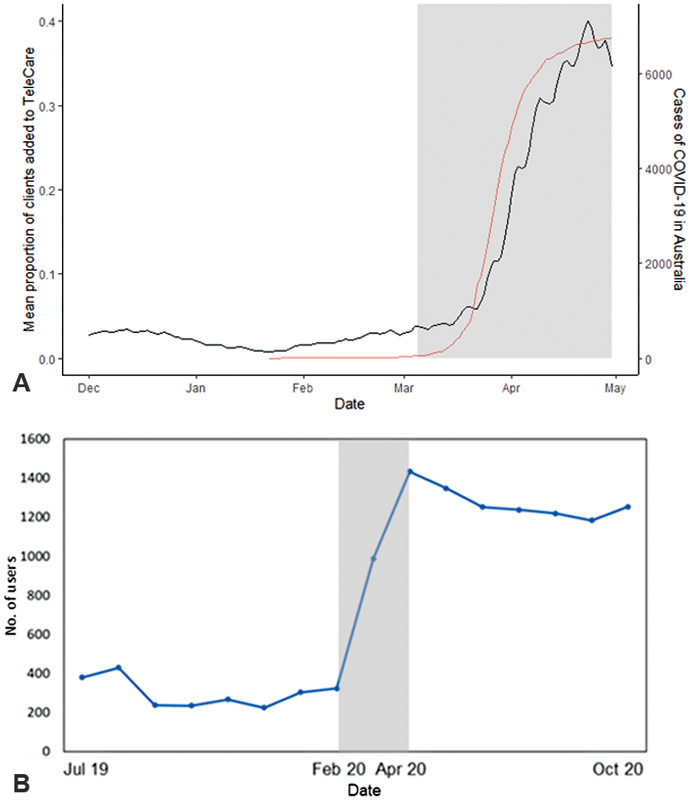
Impact of COVID-19 on the uptake of ( A ) user-controlled hearing aids in Australia. Smooth line = increase in cases of COVID-19. ( B ) m2Hear, a remote educational program for hearing aid users in the United Kingdom. Gray area—start of the pandemic in Australia and the United Kingdom.
As the world has learned to live with the new “COVID-19 normal,” and pandemic-related restrictions have subsided somewhat, there has generally been a subsequent decrease in the use of CHH services. 18 To further support clinicians in adapting to the “new normal” of providing such services, Audiology Australia has developed teleaudiology guidelines to support the safe and effective delivery of hearing services through teleaudiology. 19 The guidelines provide practice operations and clinical guidance on the use of teleaudiology practices, and are accompanied by a series of consumer resources (written and video) and a resource for audiologists that lists organizations and Web sites to assist them with skill development and implementation planning.
Research on hybrid service delivery models, whereby there is a combination of, and interplay between, in-person and remote technologies at various points along the client pathway, suggests that hybrid (or blended) models result in positive experiences. 20 21 22 An example of a hybrid model, described by Ratanjee-Vanmali et al, 20 involved online delivery through Whats-App for hearing screening and motivational engagement, whereas hearing aid trial and fitting were delivered in-person, and aural rehabilitation (AR) was delivered using both delivery methods. Arnold et al 22 delivered a hybrid service model through a manufacturer-developed app (myPhonak) to obtain outcome measures and real ear aided responses. As such, it is likely that we will see more focus on hybrid systems in the future with a greater interplay of in-person and remote technology.
To date, there has been limited research on hybrid systems and much of the CHH research has been conducted for specific interventions at various points along the hearing health journey (i.e., awareness, take action, assessment, intervention, ongoing support). Therefore, the aim of this article is to provide an overview of the research evidence for remote technologies along the hearing health journey, consider the effectiveness of these alongside barriers and facilitators, and discuss what works from a clinical, hybrid, or consumer perspective. This is of particular relevance to audiologists and service providers because most of the technologies discussed are already available, and some are already in use in clinical, hybrid, and consumer services. In short, these technologies have an evidence-base, are a reality, available, and ready to use now.
Awareness
Awareness and understanding of hearing loss among the general public is poor. 23 The insidious nature of hearing loss means that many people are not aware they have hearing difficulties, often blaming others for “mumbling.” However, even if people become aware of having hearing difficulties, they often do not know where to go for advice or where to go to get their hearing tested, and even if they do know what to do, they are not aware of the range of options available to them. 24
In Australia, there have been calls for a national hearing awareness campaign 25 similar to the Australian “slip slop slap” campaign for skin cancer that showed that it is possible to increase the awareness of public health issues through the media and other routes. 26 Identifying target groups is a first step by using the TARPARE framework (T, number of people in the group; AR, proportion at-risk of a health issue; P, persuasibility of the target group; A, the accessibility of the target audience; R, resources required to meet the group's need; and E, equity considerations). A recent study identified six target groups relating to hearing loss, of which the top three were young children and caregivers, people aged 50 to 75 years, and teenagers and young adults. Three themes were identified: (1) accessibility and availability of hearing services, (2) deciding on a preventive or treatment-focused approach, and (3) the difficulty of changing behavior. 27 Accessibility, availability, and prevention are likely to be increased by the provision of online hearing and screening tests. Changing behavior is key to most aspects of hearing healthcare, 28 which we discuss throughout this article.
Hearing screening programs have traditionally been used to raise awareness of hearing loss in the community and workplace. 29 Awareness of hearing loss is the first step to changing the behavior of individuals and taking action for rehabilitation of their hearing loss. 30 Examples of models to reach the population include mobile screening programs for rural and remote regions 31 ; noise awareness programs for children in their schools 32 ; the World Health Organization's app to prevent hearing loss in the 1 billion young people at risk of exposure to entertainment-related noise 33 ; and nationally available screening programs by phone 34 or online. 35 Screening in primary care centers has also been shown to be a cost-effective way to identify hearing loss in older adults. 36
Take Action
A major challenge is that many people do not seek help for hearing problems even when they are aware of hearing loss. 37 CHH may be a route to address this due to increased convenience and access, greater opportunities to provide important information for decision-making, and reduced time and costs to travel for clinic appointments. But what is the willingness to use CHH?
Experience using connected health among the general population has increased due to the COVID-19 pandemic, and clinicians have indicated a high level of willingness to consider CHH for audiology. 17 However, the same levels of use are not seen among adults with hearing loss. Our recent online survey exploring telehealth use, experiences, and preferences for service provision among Australian adults with hearing loss showed only 27% had used telehealth, predominantly over the phone (75%), and only 15% had used CHH for audiology services. Similar results were shown by Saunders and Oliver. 38 The uptake of CHH is substantially lower than that seen in the general Australian population aged over 60 years. 39 However, our survey also found that around 40% had a strong interest in accessing audiology services via CHH in the future, with 75% of these people showing interest in remote hearing aid fittings and adjustments, and 25% for counseling and support. 40 Despite clients' interest in CHH, one of the barriers to using CHH lies with inherent biases of healthcare professionals. As seen in other health disciplines, audiologists can be reluctant to offer remote services to older adults due to a perception of poor digital literacy and low confidence to use remote devices and services (the digital divide), 41 which is discussed later.
Identifying individual needs and understanding motivations for help-seeking and taking action are core to person-centered care in audiology, 42 43 and motivational engagement is a means to understand and improve motivation to use hearing aids. 44 45 46 The Ida Institute Motivation Tools, designed to support, engage, and coach hearing aid users, have been shown to reduce anxiety, improve self-efficacy, and increase engagement with the audiologist early on in the hearing health journey. 45 While this has typically sat within the remit of audiologists in clinic, the online “Why Improve My Hearing?” (WIMH) tool ( https://apps.idainstitute.com/apps/wimh_en ), based on the Ida Institute Motivation Tools, has been developed to encourage adults with hearing loss to reflect on their individual needs and perceived abilities prior to coming into clinic. A randomized controlled trial (RCT) showed improvements in client readiness to use hearing aids (medium to large effect size) in those who used the WIMH tool prior to attending their initial audiology appointment compared with those who received standard care. 47 These results were supported by semistructured interviews of clients and audiologists, which identified that the online WIMH tool (1) enhanced preparation before the audiology appointment and (2) provided a better understanding and acceptance of hearing difficulties, which then led to enriched discussions, 48 which are important to encourage clients to take action to improve their hearing difficulties.
Hearing aids are the most common form of management for hearing loss 49 ; however, there is currently very little coordinated and trusted information available online about the different hearing aid options (e.g., range and function) nor other technological and nontechnological options. 24 Similarly, there is limited information available about hearing healthcare pathways, how to access them, and the available options, which limits people's ability to make informed decisions, and have choice and control over their personal hearing health. With the many changes taking place currently, and on the HHC horizon, the development of well-designed and codeveloped online decision aids to improve knowledge, informed-choice, and decision-making is a key need, identified as a priority in national guidelines. 25 50
Assessment
Hearing screening for adults is often conducted using speech tests in background noise. A commonly used test is the digits-in-noise (DIN), also known as the digit triplet test, 51 that can be delivered without calibrated equipment using consumer products, such as a standard telephone, mobile phone, or an internet browser on a home computer. The value of the DIN test as a hearing screen has increased in recent years in line with new developments. Advice can now be provided automatically without the need for interpretation by an audiologist, and some applications of the DIN test also incorporate sharing of contact information for nearby audiologists. The DIN test has been shown to be largely insensitive to language proficiency, 52 and is available in several languages. 53 This platform is also well-suited to adoption in low-resource settings, 54 55 and has been adopted by the World Health Organization who developed a multi-language version delivered via a smartphone app.
In terms of diagnosis, the three primary elements of an audiology assessment are (1) audiometry, (2) tympanometry, and (3) otoscopy. The ability to conduct each of these elements remotely has been well-reported, but its importance came to the fore during the COVID-19 pandemic as clinics sought to establish how to continue audiology services.
The feasibility of live remote pure-tone audiometry has been demonstrated 56 but is limited by the need to replicate audiometry facilities and equipment in remote sites. Automated audiometry has been shown to be reliable and accurate 57 58 with variations from the gold standard shown to be within acceptable limits. Automation lessens the demand on audiologists, as testing can be facilitated by health workers. 29 Prominent implementations that have been validated include AMTAS, 59 60 KUDUwave, 61 Shoebox, 62 and HearTest. 63 Both AMTAS and KUDUwave include the facility for bone-conduction audiometry. Extended high-frequency assessment using a smartphone has also been demonstrated. 64 Automating the interpretation may also be useful to screen straightforward audiograms from more complex cases, to triage urgent from nonurgent cases, and may also avoid the variations that occur within and between audiologists. 65
Most of these solutions are attractive to CHH implementations because they include ambient noise attenuation and active noise monitoring that enables testing to be paused until the noise abates. 59 61 The KUDUwave uses insert earphones (alongside headphones) that provide ambient sound attenuation equivalent to a soundproof room. Implementation on mobile devices, such as smartphones or tablets, makes applications such as ShoeBox and HearTest attractive as they are portable, less expensive than standard audiometry equipment, not reliant on a power source or connectivity to the internet for cloud storage of results, and report to the facilitator or client. Although modern electronics are less prone to variations over time than they were previously, standards still demand that calibration is to be done regularly. More recently, alongside smartphone apps for hearing assessment, some internet-browser applications have become available, based on tone, speech, or self-report or a combination of these. Evidence to support their use is available for only a few of these, mainly those based on the DIN tests and presentation of words or sentences in quiet or noise. 66 For those responsible for delivering audiology services to clients located remotely, there is now a range of effective tools that are available for screening and assessment, most of them designed to be operated by non-audiologists and even by the client themselves in their own home.
Remote assessment of the middle ear status by tympanometry has been shown to be effective for urban community screening. 67 The incorporation of a tympanometer into the earcups of an automated audiometer, and use of an insert earphone both for pure-tone audiometry testing and for tympanometry, has been demonstrated. 68 However, more evidence is required for tympanometry to be recommended for incorporation into a CHH service, as it relies on a facilitator to perform the tympanometry.
Video otoscopes are effective for both synchronous and asynchronous delivery, 69 and a facilitator can be trained to produce high-quality images or videos by an audiologist or ENT. The automatic assessment or classification of video otoscope images using machine learning (or artificial intelligence) has been reported, 70 71 which enables images showing no abnormalities to be distinguished from those with wax, tympanic membrane perforation, or otitis media. The accuracy of these approaches is over 80%. This opens opportunities for greater involvement of primary care providers to be involved in the assessment of clients in under-resourced communities. For example, automatic classification can initially be used to ensure that good-quality images are captured for later assessment by an audiologist or ENT. 72 It can also assist trained healthcare workers to interpret otoscopic images as part of an assessment of hearing with a view to fitting hearing aids. 73
INTERVENTION
Fifteen years ago, Arthur Boothroyd wrote a seminal paper on adult AR, asking what is it and does it work. 74 He concluded that combining the four pillars of AR—sensory management, instruction, perceptual training, and counseling—to provide a holistic approach was the best way to meet the goals of AR. Today, this is still true; however, the field has moved on enormously since then in terms of technology, practice, and philosophy. We reviewed some of the changes across all four pillars a decade on from Boothroyd's paper, 75 noting several changes, such as emerging technologies to support greater personalization and interactivity with e- and mhealth, the role of cognition in devices and training, the two-way exchange of information to enhance knowledge, the role of underpinning theories to inform research, and a focus on person-centered care and self-management. We extend this to highlight some key recent developments in online interventions for Sensory Management and Auditory Training, and in the next section “Ongoing Support.”
Sensory Management
Hearing aids, which form the basis of “sensory management,” now extend to smartphone-connected hearing aids and alternative devices 41 76 77 (see Table 1 ). Hearing devices can also be self-fit, avoiding the need to involve an audiologist, 78 and devices can be fine-tuned remotely without the need to attend an audiology clinic. 79 All three functionalities described below have been shown to be beneficial, but it is important to recognize they also have disadvantages.
Smartphone-connected hearing aids41 face several barriers and facilitators shown in Table 2 , classified according to the COM-B model. 80 Similar results were reported by Ng et al, 81 in particular the perceived “digital divide” where older adults self-perceived poor digital literacy skills. However, the benefits clearly overrode the disadvantages in both studies. Perception of poor digital literacy was also seen as a barrier from an audiologist's perspective, 81 82 reinforcing audiologist's biases for remote technologies in older adults, as discussed earlier. Given the benefits of smartphone-controlled devices, and the high level of success many older hearing aid users have had with them, it would seem that audiologists would be doing clients a disservice by not offering smartphone-connected hearing aids to those who use smartphones. To address this, we are currently developing a validated one-item mhealth digital literacy question for use in clinic, based on the method of Henshaw et al. 83 This may serve as a valuable clinical tool and useful indicator as to a person's digital literacy to help decide which clients might benefit from remote technologies, and who might not.
Table 2. Barriers and facilitators identified for smartphone-connected hearing aids, based on the COM-B model.
| COM-B domain | Facilitator | Barrier |
|---|---|---|
| Capability | App increased users' knowledge and understanding of how to control the hearing aid. This encouraged self-management of hearing loss. |
Self-perception of poor digital literacy and skills. Increased cognitive burden due to deciding which controls to use. |
| Opportunity | By controlling the sound quality, participants were more likely to participate in conversations. Greater likelihood of adjusting their device in noisy situations, and so very useful. |
Smartphone norms and different listening contexts, where people felt “rude” using their smartphones in company. Rapid change in environmental sounds led to a reduction in user-control, so set to automatic. |
| Motivation | User-control to fine-tune hearing aids enabled participants to meet their individual listening and communication needs (i.e., reduce background noise). Led to greater confidence and participation and was also seen as a benefit to others. Smartphone technology helped reduce hearing aid and self-stigma. Empowerment emerged as a key theme as people could control and use their hearing aids how and when they wanted. |
Perceived generational smartphone behaviors where smartphone use is more common for younger generation. |
Self-fitting of hearing devices can be successfully used in the typical hearing aid population. 78 84 Sabin et al 84 showed that self-selected hearing aid parameters were within 1.8 dB of those set by the audiologists, and speech perception and self-reported benefit showed no significant differences between self-fit and audiologist-fit groups. Those who self-fit their hearing aids reported better sound quality than the audiologist-fit group. This was also reported in a recent systematic review, although outcomes from an audiogram-based audiologist-fit method were better than the self-fit outcomes. 85
Convery et al 78 showed that two-thirds (68%) of participants were successfully able to self-fit their hearing aids. However, only one-third (37%) did so without any input from a clinical assistant who was available to provide advice and support to the participants. Seven factors contributed to successful self-fitting: health locus of control, hearing aid self-efficacy, cognitive function, problem-solving skills, age, hearing aid experience, and mobile device ownership, of which hearing aid ownership and mobile device ownership explained 42% of the variance for self-fitting success. Self-fitting resulted in greater feelings of empowerment, also seen in other remote technology studies. 41 77 86 Recently, empowerment along the hearing journey has been conceptualized, 87 and a 5-item (clinical) and 15-item (research) measure for empowerment has been developed. 88 Given the improvement in willingness to use remote services by clients once they have experienced this, greater empowerment could lead to further shifts in attitudes, which could underlie rehabilitation success and adherence.
Remote fine-tuning involves clients being able to provide feedback about their hearing aids, for example via an app, to which audiologists can respond by remotely adjusting the hearing aid prescription, and has been shown to be a feasible clinical tool in terms of usability and client–provider communication. 79 Three-quarters (73%) were able to successfully use this technology and all reported it was easy to use, were satisfied, and preferred their settings to the initial audiologist settings. There were also no significant differences between speech perception in noise and self-report outcome measures. However, nearly half of the problems could not be addressed by the hearing aid settings (e.g., uncomfortable domes, hearing aids slipping out of ears, and itchy ear canals). This highlights the need to better understand remote technologies and its accessibility within a broad, adaptable model of care. 89
Alternative devices to conventional hearing aids (e.g., PSAPs) have increased in number and type over recent years. 76 One of the key questions is, how effective are these alternative devices in comparison to conventional hearing aids offered by audiologists. A systematic review we published in 2018 90 was not able to answer this question due to a paucity of data on PSAPs. However, a more recent review of five studies showed no significant difference for speech perception, sound quality, and listening effort for PSAPs compared with conventional hearing aids, 91 suggesting that PSAPs have the potential to be as beneficial as conventional hearing aids.
However, it is important to be mindful that cheaper PSAPs (<U.S. $150) can have unacceptable electronic characteristics (e.g., input noise, total harmonic distortion) with too little high-frequency amplification, and too much low-frequency amplification, compared with more expensive PSAPs. 76 Similarly, speech perception appears better with more expensive PSAPs (>U.S. $300), 92 and cosmetic acceptance and willingness to wear also appears greater for more expensive devices. 93 There is also an option for amplification through “hearables” (e.g., Nuheara, Apple AirPods) which are also a potential contender in the PSAP market. Undoubtedly, we will see significant changes in the technologies that come onto the market over the next few years, which is likely to be driven by consumer wants, needs, attitude, and choice.
OTC service models were first investigated in a double-blind RCT by Humes et al 94 who showed that when users self-selected pre-programs for their hearing aids in an OTC delivery model, the hearing aids were efficacious with moderate to large effect sizes, although satisfaction was slightly lower compared with audiologist best practice hearing aids. Similar results have been shown in other studies. 95 A recent Delphi review on remote hearing health technologies in the United Kingdom showed that hearing healthcare professionals were positive about these technologies and introducing them into their adult hearing services. 82 This was particularly the case for adults where communication was their main concern and there were no medical contraindications (e.g., unilateral hearing loss), which can be identified by using the Consumer Ear Disease Risk Assessment (CEDRA) questionnaire. 96 In particular, PSAPs and other technologies were seen as “gateway products” that could be delivered through OTC or DTC channels, whereby early use of cheaper alternatives to hearing aids could lead to earlier uptake of hearing aids. 82
In summary, the research evidence for sensory management suggests that relatively new remote technologies are beneficial to a significant number of adults with hearing loss, with numerous benefits in terms of greater participation, satisfaction, self-efficacy, and empowerment. However, there are barriers to all these technologies. In particular, self-perception of poor digital literacy was very common and can be deep-rooted, similar to that seen in other health disciplines. 97 98 In particular, there has been little research on identifying what people want from devices purchased via an OTC channel and how they can best be supported.
Auditory Training
Online, computerized, or mobile auditory training programs can provide added value to sensory management interventions by improving listening that extends beyond the clinic, either conducted independently or supported by clinical care. Although hearing aids are clinically effective, 49 they do not directly address the role that cognition plays in listening, 99 which can be achieved by auditory training. 100 Auditory training programs delivered remotely are interventions that aim to improve and support listening through active engagement with sounds. 101 Early studies showed that computerized auditory training was effective at improving the trained tasks (e.g., phonemes, words, sentences) but not necessarily untrained tasks. 102 A turning point came when auditory training was also shown to improve cognitive function such as working memory and attention, specifically on executive processes such as memory updating and attention switching. 100 103 This suggested that training cognition directly might be an effective method to provide benefit to adults with hearing loss.
However, a high-quality RCT on cognitive training for working memory showed limited improvement in working memory and self-reported hearing benefit. 104 We proposed that a combined auditory-cognitive approach, where cognition embedded within auditory task, is more likely to improve real-world benefits, 103 105 which was subsequently confirmed by a systematic review. 106 From a clinical perspective, auditory-cognitive training is not widely used. There is evidence that Brain HQ, which combines auditory and cognitive tasks, can provide improvements in temporal and speech processing, and speech perception. 107 108 Another well-known program is LACE (Listening and Communication Enhancement) that includes some aspects of listening and cognition, although it is not clear which aspects of this program are effective, or not. A well-designed RCT concluded that LACE was not an effective intervention for adults with hearing loss 109 ; however, it may be that other, more sensitive outcome measures targeting executive processes may have shown different results. There are several current developments to bring auditory and/or cognitive training into remote clinical care. For example, new training paradigms for involving communication partners, 110 111 and we are currently developing an ecologically valid auditory-cognitive training program to optimize use and adherence in addition to improving listening and cognitive outcomes.
Ongoing Support
Interventions that target ongoing Educational and Rehabilitation Support and Remote Follow-up and Monitoring are discussed in the following section.
Educational and Rehabilitation Support
Hearing devices alone are not sufficient to address the acoustic, communication, and well-being impacts of hearing loss. Hearing loss and its consequences are complex, communication is complex, and hearing devices are complex. Hence, there is a need for additional and ongoing support for both first-time and existing hearing device users. High-quality information and education to enhance knowledge and skill is a key component to self-management of hearing loss, 112 113 health literacy, 114 and empowerment, 87 all of which are essential for successful rehabilitation in adults with hearing loss. Patient's knowledge and skill are also recognized as core to national clinical guidelines. 43 50
Several remote, online rehabilitation programs have been developed and evaluated to support hearing aid users in their hearing-knowledge and rehabilitation needs (e.g., C2Hear/m2Hear 12 15 86 115 116 117 118 ; Internet-based Aural Rehabilitation 119 120 ; Support Program (SUPR) 121 122 ). These programs provide a mix of interactive videos (or reusable learning objects [RLOs]) that cover both practical and psychosocial aspects of hearing loss (e.g., see Fig. 2 ), hearing aids and communication, including acclimatization, benefits and limitations of hearing aids, client testimonials, as well as directed reading and interaction with peers and audiologists. There were clear benefits to clients in using these supplementary programs, resulting in significant improvements (with medium to large effect sizes) in knowledge, practical and hearing aid handling skills, hearing aid use and self-efficacy, social participation, communication, and hearing disability and participation. It is clear that the benefits are much more than simply knowledge gain, and memorably one of the early participants in our C2Hear RCT commented “if it was not for [C2Hear] I would have given up wearing my hearing aids.” For C2Hear/m2Hear, both codeveloped using participatory human-centered design approaches, participants also reported that usability was high and valued by users, which is a likely impact of the co-design approach. m2Hear was viewed more favorably than C2Hear with additional benefits including shorter and more concise RLOs, and being more accessible, convenient, individualized, interactive, and easier to use through the mhealth delivery platform, leading to greater confidence, self-efficacy, participation, and empowerment. 86
Figure 2.

The five overarching themes of m2Hear and exemplar questions, one from each theme, which are addressed by short (e.g., 1 minute) video clips.
There is a clear need and desire from hearing aid users for good-quality information to support them because hearing aids and communication with others are complex, and retention of information given verbally is poor. 30 115 123 124 125 Accessing this remotely, without the need to attend an audiology clinic, was seen as a key advantage of m2Hear, alongside numerous other advantages ( Table 3 ). 86 While most studies have offered educational support at the time of hearing aid fitting, we trialed the early delivery of C2Hear by offering it at the assessment appointment. 117 The RCT showed that there was a highly significant improvement in both self-efficacy and knowledge of hearing aids (with large effect sizes), and a borderline increase in readiness for hearing aids, in those who received C2Hear compared with the standard care group who received a printed booklet on hearing aids and how to use them. This suggests that online educational resources can prime people, even before they obtain their hearing aids, on what hearing aids can do and how hearing aids can benefit them in communicating in everyday life.
Table 3. Benefits of m2Hear, a theoretically-driven, individualized, and interactive educational program for hearing aid users delivered through mobile technologies, based on the COM-B model.
| Capability | Opportunity | Motivation |
|---|---|---|
| Comprehensive, facilitating knowledge | Empowering, better self-management | Greater self-efficacy |
| Concise, easy to retain | Inclusive, shared with others | Better coping |
| Interactive, improved memory | Personalized, tailored to individual needs | Set expectations |
One of the challenges in research when an intervention shows beneficial findings is implementing the intervention into clinical practice. C2Hear was made freely available via YouTube in December 2015 ( https://www.youtube.com/c2hearonline ), and in 2019, we developed a standalone version ( https://c2hearonline.com/ ) to overcome some of the disadvantages of YouTube. Together, there have been more than 1 million views globally to date from across more than 50 countries. The benefits of online educational support were clearly obvious during the start of the COVID-19 pandemic when there was a fourfold increase in the number of views (see Fig. 1 ). Both C2Hear and m2Hear are included in national UK guidelines, 14 43 and C2Hear is the information for patients in the NICE guidelines. 50 C2Hear is on over 50 UK audiology Web sites, and we would encourage people to add the link to either C2Hear Online or m2Hear ( https://www.nottingham.ac.uk/helm/dev-test/m2hear/ ) onto their own audiology service Web site, no permission is required. The benefits of C2/m2Hear also suggest that online educational resources could also be a key element in providing essential support for those accessing hearing devices through OTC or DTC channels. 77
All the education-rehabilitation online programs addressed some aspects of psychosocial consequences of hearing loss, although information retention on this was poorer compared with practical aspects. 124 Furthermore, our understanding of the psychosocial and mental health impacts of hearing loss has grown over recent years, both for adults with hearing loss and their communication partners. 126 127 128 129 130 There are few interventions that focus on psychosocial aspects of hearing loss, one that uses booklet-based cognitive behavioral therapy, 131 and another that delivers internet-based Acceptance Commitment Therapy program by a mental health professional, 132 with indications that online interventions for psychosocial and emotional support would be valuable. 129 133 134 We are currently conducting some research to address this and improvement of user-engagement with remote technologies.
Remote Follow-up and Monitoring
Follow-up and monitoring of adults who receive hearing devices has considerable potential for both hearing aid and cochlear implant (CI) users, and has been identified as a NICE research priority. 50 There are considerable advantages in being able to offer follow-up and monitoring services remotely, either via an audiology clinic or remote support system. Using design thinking principles, the discovery phase of using smart voice technology (e.g., Alexa, GoogleHome) revealed that post-fitting support would increase motivation of hearing aid users, offer encouragement, assist in joint goal-setting, achieve goals, and record experiences of the hearing aid. All of which could also be used to monitor after-care and progress by audiologists. 135
A recent pilot study using a manufacturer's myPhonak app was used to conduct internet-based follow-up appointments by collecting outcome measures (Client-Oriented Scale of Improvement [COSI], Hearing Handicap Inventory for the Elderly [HHIE], QuickSIN, and real-ear aided responses). 22 Most follow-ups were completed, with improvements in the COSI goals, and no significant difference for satisfaction across the in-person and internet-based appointments. As we gain more insights into remote delivery services for hearing aid users from research and clinical practice, these will further guide targeted research and clinical implementation.
There have been similar developments for CI users. Remote Check is an app that offers home-based CI testing incorporating subjective and objective assessments in a comprehensive review of CI function. 136 Assessments include the Speech and Spatial Qualities Questionnaire-Short version (SSQ12), impedance testing, aided hearing test, DIN, and data logging. Users can also upload photos of their implant site for medical review. Studies have shown that use of Remote Check can facilitate high quality, regular review of CI recipient outcomes, with most tests providing equivalent outcomes remotely to those obtained in clinic while also facilitating. 136 137 It is important that remote technologies provide services that are at least as good as, and can be easily integrated into, current clinical services. Maruthurkkara et al 136 reported that 94% of all issues arising postsurgery were identified by Remote Check, and there was 99% agreement in cases where there was need to visit the clinic between the clinic and Remote Check. Sucher et al 137 reported that ease of Remote Check use was high, with 100 and 89% rating somewhat/very easy to use at baseline and 6 months, and 90 and 84% highly likely to recommend Remote Check to others. The DIN and standard clinical speech-in-noise tests were significantly correlated, with no clinically significant differences for impedances and aided thresholds measured clinically and via Remote Check. Views of clients and audiologists on Remote Check were also sought, and key themes are shown in Table 4 .
Table 4. Key themes arising from focus groups of clients and audiologists on the use of Remote Check.
| Clients | Clinician continuity preferred when reviewing results due to the concern that nonfamiliar clinicians may miss subtle issues | Personalized, hybrid models of care are preferred, with Remote Check used in conjunction with in-person appointments. |
| Audiologists | Training and experience with Remote Check, and CHH in general, improves ease of use and increases the likelihood of uptake both among clinicians and CI users | Improved integration between Remote Check and clinical outcome measures will facilitate tracking of client progress |
| Clients and audiologists | Understanding and trust of Remote Check outcomes for both clients and audiologist is essential | Choice of the client is key in the use of any remote technology, including Remote Check |
A similar program for personalized long-term follow-up for the management of CI users is the CHOICE CI home-care program that is manufacturer-agnostic. 138 An RCT showed equivalent, if not better, outcomes for CI users using CHOICE compared with the current in-clinic standard of care for objective and subjective measures of speech recognition. There were also increased levels of empowerment in relation to management of their hearing loss, and most were keen to continue receiving their implant management in this manner.
Studies involving both services clearly reveal a willingness to, and acceptance of, receiving care remotely. Remote CI monitoring enables better management of individuals who are progressing slowly with their implant, as well as removing unnecessary clinic visits if all is going well. Further consideration needs to be given to the necessary integration of outcomes obtained in clinic and remotely to enable direct comparison. As such, we are about to embark on a study to identify a Core Outcome Set to measure outcomes for remote technologies used by CI users.
In general, the financial implications of remote technologies needs to be better understood. While use of remote technologies for ongoing support has the potential to free up clinic capacity, thus allowing clinicians to see more clients, monitoring must still be reviewed and follow-up assessments may still be needed. At present, from an Australian perspective, there is no funding available for asynchronous CHH. Loss of income from in-person review appointments, in conjunction with the possible need to absorb the cost of monitoring and reviewing results, may make the CHH service cost prohibitive. Unless an acceptable funding model is developed for CHH services, there is a possibility that it will not be implemented. More generally, implementation of remote technologies into clinic systems to provide a hybrid model needs to be understood as implementation science has shown that clinicians and clients need to be ready for change, and optimal conditions are required for an intervention to be taken up and maintained. We cannot simply expect to put new systems into clinics and assume they will work, although this is common, based on a desire to “get on with it.”
IMPLEMENTATION AND BEHAVIORAL SCIENCE: FROM RESEARCH TO PRACTICE
Improving healthcare and ensuring that policy on healthcare service delivery is well-grounded requires structured and comprehensive processes for the development, evaluation, and implementation of innovative interventions, such as remote technologies. Healthcare interventions are complex, and it is challenging to implement them effectively within healthcare settings. The UK's Medical Research Council (MRC) developed a framework to guide the development and evaluation of complex health interventions. 139 The four key elements are as follows: Development (identifying the evidence base and theory); Feasibility/piloting (testing procedures, estimating recruitment/retention, calculating sample size); Evaluation (assessing clinical- and cost-effectiveness, understanding the change process); and Implementation (dissemination, monitoring, and long-term follow-up). This framework has guided some of our research, 15 115 140 141 and has been recently updated 142 to align with important aspects that have been introduced into research over the last decade. These include stakeholder engagement, 12 116 143 144 145 identifying key uncertainties, 146 147 iterative refinement of the intervention, 15 116 144 145 148 and economic considerations. 115
While intervention development and evaluation come before implementation, research highlights the benefit of considering implementation needs right from the outset. 149 For example, there is no point developing an effective intervention if the target users are unwilling to use it. This point is crucial, as we see in audiology there are clinically effective remote tools available to help clients to better self-manage their hearing loss, yet their uptake and use often remains limited.
Implementation science holds the key to unlocking what is needed to motivate, equip, and empower clinic staff and service providers so that effective interventions are embedded into routine hearing healthcare so that all clients can benefit. First, it is imperative to clarify the common confusion between what is the clinical intervention and what is the implementation intervention. The clinical intervention is the what (e.g., m2Hear program) and the implementation process is the how (e.g., how the m2Hear program becomes embedded within a clinic). Implementation interventions may include, for example, efforts to change behavior at the client, provider, system, or policy level. Common examples include strategies at the provider level such as education/training, audit-feedback, and performance incentives.
Implementation science is “the scientific study of methods to promote the systematic uptake of research findings and other evidence-based practice into routine practice and, hence, to improve the quality and effectiveness of health services.” 150 There are over 90 implementation theories or conceptual frameworks available to guide research and clinical practice. These can be used to describe and/or guide the process of translating an evidence-based program for delivery into the clinic (process models); understand and explain what influences implementation outcomes (determinant framework); and evaluate aspects of the implementation process (evaluation frameworks). 151 Selection of the appropriate theory, model, or framework will depend on the research question and purpose; the context and implementation setting (e.g., targeting person or organizational-level constructs); and the depth of analysis and operationalization required (e.g., guiding processes, determinants, strategies, evaluation). 152 Reviews and Web sites (e.g., https://dissemination-implementation.org/ ) provide lists of available theories, models, and frameworks 153 and the Theory Comparison and Selection Tool (T-CaST) provides guidance on selection criteria. 152 154 155 All models of care would benefit from a better understanding of implementation requirements as implementation science gains more ground and becomes better understood within audiology. 156 157
One implementation theory that has gained attention in the audiology literature is the Behavior Change Wheel (BCW), an eight-step systematic process guiding intervention development and implementation. The BCW has been widely used to develop interventions that are both acceptable to users and effective in achieving their aims. These include interventions designed to optimize changes in health behaviors, such as audiologists engaging in shared goal planning for adults with hearing loss, 158 159 and engaging hearing aid users with smartphone-connected hearing aids. 41 We recently used the BCW to develop an intervention targeting mental well-being support behaviors in hearing healthcare clinicians, the AIMER (Ask, Inform, Manage, Encourage, Refer). 141 This multifaceted intervention was developed to change hearing healthcare clinicians' behaviors relating to provision of mental well-being support to adults with hearing loss. A follow-up study evaluated the implementation of the AIMER, guided by the RE-AIM framework 160 providing a theoretical structure for the assessment of implementation pathways, and found that after completing the AIMER program, hearing healthcare clinicians were more likely to engage in the target behaviors. All models of care would benefit from a better understanding of implementation requirements as implementation science gains more ground and becomes better understood within audiology. 156 157
At the core of the BCW is the COM-B model of behavior change. 80 The COM-B recognizes that barriers and facilitators of the target Behavior may relate to Capability (e.g., skills, knowledge), Opportunity (e.g., social influences, physical environment), or Motivation (e.g., beliefs, intentions, emotional responses, habitual responses). The COM-B model proposes that if a behavior is not taking place, barriers in one or more of these areas need to be addressed. There is a growing body of work demonstrating the use of the COM-B for identifying barriers and facilitators to implementation of hearing healthcare interventions. 41 77 158 159 161 For example, to address the barriers that users reported for smartphone-connected hearing aids, behavior change techniques (BCTs) were mapped onto the 10 identified themes (e.g., smartphone literacy) to support use of smartphone-connected hearing aids by audiologists. 41 In this case, smartphone literacy could be addressed by BCTs addressing education (for smartphone skills), training (to demonstrate functions), and enablement (provision of behavioral support).
Conclusions
There are many types of remote technologies that have been developed and evaluated along the hearing health journey to provide an evidence-base. Most of these have been developed for clinical systems and some for consumer systems, although the technologies for these are not mutually exclusive. Here, we provide an overview of some of the technologies that have been evaluated through research, of which many are available and some are being used. Yet there is little research that brings these technologies together to form hybrid or fully remote service delivery models. One could envisage a “pick and mix” approach, taking the elements along the hearing journey that best meet individuals' needs, from either a clinical perspective, a consumer perspective, or a mix of the two (see Fig. 3 ). We anticipate that over the next few years, the need to address the huge unmet need within hearing healthcare will continue to drive the development and implementation of remote technologies and new service delivery models and channels. This will almost certainly include new technologies not specifically addressed here (e.g., artificial intelligence 162 and big data 163 ) that are fast moving into our horizon. An evidence-based approach is key for sustainable models, as is the need to implement these models by addressing what works (the facilitators) and what does not (the barriers), which can be at odds with a sense of “just get on with it.” The opportunities to substantially improve hearing healthcare and person-centered outcomes through remote technologies, service models, and channels are great. To quote Matt Mullenweg, founder of WordPress, “technology works best when it brings people together.”
Figure 3.
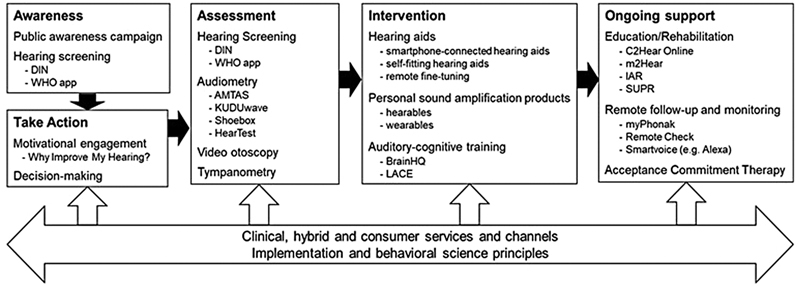
Summary of remote technologies along the hearing health journey, which can be used in clinical, hybrid, and consumer services, underpinned by principles of implementation and behavioral science. Specific examples of technologies are shown.
Acknowledgments
Many thanks to Sophie Brice and an anonymous reviewer for their useful comments and feedback.
Footnotes
Conflict of Interest None declared.
References
- 1.Swanepoel D W, Hall J W. A systematic review of telehealth applications in audiology. Telemedicine journal and e-health. Am Telemed Assoc. 2010;16(02):181–200. doi: 10.1089/tmj.2009.0111. [DOI] [PubMed] [Google Scholar]
- 2.Glista D, Ferguson M, Muñoz K, Davies-Venn E. Connected hearing healthcare: shifting from theory to practice. Int J Audiol. 2021;60 01:S1–S3. doi: 10.1080/14992027.2021.1896794. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson M, Maidment D, Henshaw H, Heffernan E. Thieme Medical Publishers; 2019. Evidence-Based Interventions for Adult Aural Rehabilitation: That Was Then, This Is Now; pp. 68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goudey B, Plant K, Kiral I.A multicenter analysis of factors associated with hearing outcome for 2,735 adults with cochlear implants Trends Hear 20212523312165211037525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eikelboom R, Bennett R, Brennan M. Perth, WA: Ear Science Institute Australia; 2021. Tele-Audiology: An Opportunity for Expansion of Hearing Healthcare Services in Australia. [Google Scholar]
- 6.Montano J, Angley G, Ryan-Bane C. eAudiology: shifting from theory to practice. Hearing Review. 2018;25(09):20–24. [Google Scholar]
- 7.Ferguson M, Glista D, Davies-Venn E. Connected health in audiology: the future of hearing healthcare. ENT Audiol News. 2019;28(05):47. [Google Scholar]
- 8.NASEM Hearing Healthcare for AdultsPriorities for Improving Access and Affordability. NASEM;2016 [PubMed]
- 9.Brice S, Saunders E, Edwards B. Scoping review for a global hearing care framework: matching theory with practice. Semin Hear. 2023;44(03):213–231. doi: 10.1055/s-0043-1769610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penno K, Zakis J. Exploring hearing care technology from clinic to capability. Semin Hear. 2023;44(03):287–301. doi: 10.1055/s-0043-1769741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz K, Nagaraj N K, Nichols N. Applied tele-audiology research in clinical practice during the past decade: a scoping review. Int J Audiol. 2021;60 01:S4–S12. doi: 10.1080/14992027.2020.1817994. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson M, Leighton P, Brandreth M, Wharrad H. Development of a multimedia educational programme for first-time hearing aid users: a participatory design. Int J Audiol. 2018;57(08):600–609. doi: 10.1080/14992027.2018.1457803. [DOI] [PubMed] [Google Scholar]
- 13.Swanepoel D W, Hall J W. Making audiology work during COVID-19 and beyond. Hear J. 2020;73(06):20–22. [Google Scholar]
- 14.AIHHP, BAA, BSA, BSHAA Audiology and Otology Guidance during COVID-19United Kingdom; 2022
- 15.Ferguson M A, Maidment D W, Gomez R, Coulson N, Wharrad H. The feasibility of an m-health educational programme (m2Hear) to improve outcomes in first-time hearing aid users. Int J Audiol. 2021;60 01:S30–S41. doi: 10.1080/14992027.2020.1825839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen D, Ferguson M, Pang J.Clinical outcomes of Hearing Australia in-person and remote services 2020Accessed May 10, 2023 at:https://www.nal.gov.au/wp-content/uploads/sites/2/2020/11/Clinical-outcomes-of-Hearing-Australia-inperson-and-remote-services.pdf
- 17.Eikelboom R H, Bennett R J, Manchaiah V. International survey of audiologists during the COVID-19 pandemic: use of and attitudes to telehealth. Int J Audiol. 2022;61(04):283–292. doi: 10.1080/14992027.2021.1957160. [DOI] [PubMed] [Google Scholar]
- 18.Abrams H B, Callahan C M.Health behavior and motivational engagement models can explain and modify tele-audiology uptake Am J Audiol 202231(3S):1043–1051. [DOI] [PubMed] [Google Scholar]
- 19.Audiology Australia Teleaudiology Guidelines. Accessed May 10, 2023 at:https://teleaudiologyguidelines.org.au/teleaudiology-guidelines/
- 20.Ratanjee-Vanmali H, Swanepoel W, Laplante-Lévesque A. Patient uptake, experience, and satisfaction using web-based and face-to-face hearing health services: process evaluation study. J Med Internet Res. 2020;22(03):e15875. doi: 10.2196/15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao K FM, Swanepoel D W, Brennan-Jones C G, Jayakody D MP, Moriera T C, Coetzee L, Eikelboom R H.Teleaudiology hearing aid fitting follow-up consultations for adults: a single blinded randomised control trial and cohort study Int J Audiol 202160(S10):S49–S60. [DOI] [PubMed] [Google Scholar]
- 22.Arnold M L, Schwartz B, Neil H, Chisolm T H, Sanchez V A.Feasibility and assessment of a hybrid audiology service delivery model for older adult hearing aid users: a pilot study Am J Audiol 202231(3S):892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I. Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models. Health Technol Assess. 2007;11(42):1–294. doi: 10.3310/hta11420. [DOI] [PubMed] [Google Scholar]
- 24.Woods M, Burgess Z. Report of the Independent Review of the Hearing Services Program. 2021.
- 25.HHSC Roadmap for Hearing HealthHHSC;2019
- 26.Montague M, Borland R, Sinclair C. Slip! Slop! Slap! and SunSmart, 1980-2000: skin cancer control and 20 years of population-based campaigning. Health Educ Behav. 2001;28(03):290–305. doi: 10.1177/109019810102800304. [DOI] [PubMed] [Google Scholar]
- 27.Alperstein S, Beach E F. Prioritizing the target audience for a hearing awareness campaign in Australia using the TARPARE model. Health Promot Int. 2022:daac041. doi: 10.1093/heapro/daac041. [DOI] [PubMed] [Google Scholar]
- 28.Coulson N S, Ferguson M A, Henshaw H, Heffernan E. Applying theories of health behaviour and change to hearing health research: time for a new approach. Int J Audiol. 2016;55 03:S99–S104. doi: 10.3109/14992027.2016.1161851. [DOI] [PubMed] [Google Scholar]
- 29.Dawood N, Mahomed Asmail F, Louw C, Swanepoel W. mHealth hearing screening for children by non-specialist health workers in communities. Int J Audiol. 2021;60 01:S23–S29. doi: 10.1080/14992027.2020.1829719. [DOI] [PubMed] [Google Scholar]
- 30.Laplante-Lévesque A, Hickson L, Worrall L. Stages of change in adults with acquired hearing impairment seeking help for the first time: application of the transtheoretical model in audiologic rehabilitation. Ear Hear. 2013;34(04):447–457. doi: 10.1097/AUD.0b013e3182772c49. [DOI] [PubMed] [Google Scholar]
- 31.Brennan-Jones C G, Taljaard D S, Brennan-Jones S E, Bennett R J, Swanepoel W, Eikelboom R H. Self-reported hearing loss and manual audiometry: a rural versus urban comparison. Aust J Rural Health. 2016;24(02):130–135. doi: 10.1111/ajr.12227. [DOI] [PubMed] [Google Scholar]
- 32.Taljaard D S, Leishman N F, Eikelboom R H. Personal listening devices and the prevention of noise induced hearing loss in children: the Cheers for Ears Pilot Program. Noise Health. 2013;15(65):261–268. doi: 10.4103/1463-1741.113523. [DOI] [PubMed] [Google Scholar]
- 33.WHO Check your hearingAccessed May 10, 2023 at:https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/hearwho
- 34.Folmer R L, Vachhani J, McMillan G P, Watson C, Kidd G R, Feeney M P. Validation of a computer-administered version of the digits-in-noise test for hearing screening in the United States. J Am Acad Audiol. 2017;28(02):161–169. doi: 10.3766/jaaa.16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RNID Take our free hearing checkAccessed May 10, 2023 at:https://rnid.org.uk/information-and-support/take-online-hearing-check/
- 36.Dubno J R, Majumder P, Bettger J P. A pragmatic clinical trial of hearing screening in primary care clinics: cost-effectiveness of hearing screening. Cost Eff Resour Alloc. 2022;20(01):26. doi: 10.1186/s12962-022-00360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson A N, Matthews L J, Cassarly C, Dubno J R. Time from hearing aid candidacy to hearing aid adoption: a longitudinal cohort study. Ear Hear. 2019;40(03):468–476. doi: 10.1097/AUD.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders G H, Oliver F. Impact of hearing loss on communication during remote health care encounters. Telemed J E Health. 2022;28(09):1350–1358. doi: 10.1089/tmj.2021.0490. [DOI] [PubMed] [Google Scholar]
- 39.Isautier J M, Copp T, Ayre J. People's experiences and satisfaction with telehealth during the COVID-19 pandemic in Australia: cross-sectional survey study. J Med Internet Res. 2020;22(12):e24531. doi: 10.2196/24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galvin K, Sucher C M, Bennett R J, Ebrahimi-Madiseh A, Crosland P, Eikelboom R H. Willingness to consider and to pay for a variety of telehealth services amongst adult hearing clinic clients. Int J Audiol. 2023;62(03):286–294. doi: 10.1080/14992027.2022.2039965. [DOI] [PubMed] [Google Scholar]
- 41.Gomez R, Habib A, Maidment D W, Ferguson M A. Smartphone-connected hearing aids enable and empower self-management of hearing loss: a qualitative interview study underpinned by the behavior change wheel. Ear Hear. 2022;43(03):921–932. doi: 10.1097/AUD.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 42.Ridgway J, Hickson L, Lind C. Autonomous motivation is associated with hearing aid adoption. Int J Audiol. 2015;54(07):476–484. doi: 10.3109/14992027.2015.1007213. [DOI] [PubMed] [Google Scholar]
- 43.BSA Common Principles of Rehabilitation for Adults in Audiology ServicesBritish Society of Audiology. Accessed May 10, 2023 at:http://www.thebsa.org.uk/wp-content/uploads/2016/10/Practice-Guidance-Common-Principles-of-Rehabilitation-for-Adults-in-Audiology-Services-2016.pdf
- 44.Aazh H. Feasibility of conducting a randomized controlled trial to evaluate the effect of motivational interviewing on hearing-aid use. Int J Audiol. 2016;55(03):149–156. doi: 10.3109/14992027.2015.1074733. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson M, Maidment D, Russell N, Gregory M, Nicholson R. Motivational engagement in first-time hearing aid users: a feasibility study. Int J Audiol. 2016;55 03:S23–S33. doi: 10.3109/14992027.2015.1133935. [DOI] [PubMed] [Google Scholar]
- 46.Ekberg K, Barr C. Identifying clients' readiness for hearing rehabilitation within initial audiology appointments: a pilot intervention study. Int J Audiol. 2020;59(08):606–614. doi: 10.1080/14992027.2020.1737885. [DOI] [PubMed] [Google Scholar]
- 47.Maidment D, Heffernan E, Ferguson M. A randomised controlled clinical trial to assess the benefits of a telecare tool delivered prior to the initial hearing assessment. Int J Audiol. 2023;62(05):400–409. doi: 10.1080/14992027.2022.2059713. [DOI] [PubMed] [Google Scholar]
- 48.Heffernan E, Maidment D W, Ferguson M A. A qualitative study showing that a telecare tool can have benefits before and during the initial hearing assessment appointment. Int J Audiol. 2023;62(04):295–303. doi: 10.1080/14992027.2022.2041740. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson M A, Kitterick P T, Chong L Y, Edmondson-Jones M, Barker F, Hoare D J. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev. 2017;9(09):CD012023. doi: 10.1002/14651858.CD012023.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NICE Hearing loss in adults: assessment and managementNational Institute for Health and Care Excellence. Accessed May 10, 2023 at:https://www.nice.org.uk/guidance/ng98 [PubMed]
- 51.Smits C, Kapteyn T S, Houtgast T. Development and validation of an automatic speech-in-noise screening test by telephone. Int J Audiol. 2004;43(01):15–28. doi: 10.1080/14992020400050004. [DOI] [PubMed] [Google Scholar]
- 52.Potgieter J M, Swanepoel W, Myburgh H C, Hopper T C, Smits C. Development and validation of a smartphone-based digits-in-noise hearing test in South African English. Int J Audiol. 2015;55(07):405–411. doi: 10.3109/14992027.2016.1172269. [DOI] [PubMed] [Google Scholar]
- 53.Van den Borre E, Denys S, van Wieringen A, Wouters J. The digit triplet test: a scoping review. Int J Audiol. 2021;60(12):946–963. doi: 10.1080/14992027.2021.1902579. [DOI] [PubMed] [Google Scholar]
- 54.Swanepoel D, Clark J L. Hearing healthcare in remote or resource-constrained environments. J Laryngol Otol. 2019;133(01):11–17. doi: 10.1017/S0022215118001159. [DOI] [PubMed] [Google Scholar]
- 55.Eksteen S, Launer S, Kuper H, Eikelboom R H, Bastawrous A, Swanepoel W. Hearing and vision screening for preschool children using mobile technology, South Africa. Bull World Health Organ. 2019;97(10):672–680. doi: 10.2471/BLT.18.227876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lancaster P, Krumm M, Ribera J, Klich R. Remote hearing screenings via telehealth in a rural elementary school. Am J Audiol. 2008;17(02):114–122. doi: 10.1044/1059-0889(2008/07-0008). [DOI] [PubMed] [Google Scholar]
- 57.Mahomed F, Swanepoel W, Eikelboom R H, Soer M. Validity of automated threshold audiometry: a systematic review and meta-analysis. Ear Hear. 2013;34(06):745–752. doi: 10.1097/01.aud.0000436255.53747.a4. [DOI] [PubMed] [Google Scholar]
- 58.Wasmann J W, Pragt L, Eikelboom R, Swanepoel W. Digital approaches to automated and machine learning assessments of hearing: scoping review. J Med Internet Res. 2022;24(02):e32581. doi: 10.2196/32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolis R H, Glasberg B R, Creeke S, Moore B C. AMTAS: automated method for testing auditory sensitivity: validation studies. Int J Audiol. 2010;49(03):185–194. doi: 10.3109/14992020903092608. [DOI] [PubMed] [Google Scholar]
- 60.Eikelboom R H, Swanepoel DW, Motakef S, Upson G S. Clinical validation of the AMTAS automated audiometer. Int J Audiol. 2013;52(05):342–349. doi: 10.3109/14992027.2013.769065. [DOI] [PubMed] [Google Scholar]
- 61.Swanepoel DW, Matthysen C, Eikelboom R H, Clark J L, Hall J W., III Pure-tone audiometry outside a sound booth using earphone attenuation, integrated noise monitoring, and automation. Int J Audiol. 2015;54(11):777–785. doi: 10.3109/14992027.2015.1072647. [DOI] [PubMed] [Google Scholar]
- 62.Thompson G P, Sladen D P, Borst B JH, Still O L. Accuracy of a tablet audiometer for measuring behavioral hearing thresholds in a clinical population. Otolaryngol Head Neck Surg. 2015;153(05):838–842. doi: 10.1177/0194599815593737. [DOI] [PubMed] [Google Scholar]
- 63.van Tonder J, Swanepoel DW, Mahomed-Asmail F, Myburgh H, Eikelboom R H. Automated smartphone threshold audiometry: validity and time efficiency. J Am Acad Audiol. 2017;28(03):200–208. doi: 10.3766/jaaa.16002. [DOI] [PubMed] [Google Scholar]
- 64.Bornman M, Swanepoel DW, De Jager L B, Eikelboom R H. Extended high-frequency smartphone audiometry: validity and reliability. J Am Acad Audiol. 2019;30(03):217–226. doi: 10.3766/jaaa.17111. [DOI] [PubMed] [Google Scholar]
- 65.Brennan-Jones C G, Eikelboom R H, Bennett R J, Tao K F, Swanepoel DW. Asynchronous interpretation of manual and automated audiometry: agreement and reliability. J Telemed Telecare. 2018;24(01):37–43. doi: 10.1177/1357633X16669899. [DOI] [PubMed] [Google Scholar]
- 66.Almufarrij I, Dillon H, Dawes P. Web- and app-based tools for remote hearing assessment: a scoping review. Int J Audiol. 2022:1–14. doi: 10.1080/14992027.2022.2075798. [DOI] [PubMed] [Google Scholar]
- 67.Ciccia A H, Whitford B, Krumm M, McNeal K. Improving the access of young urban children to speech, language and hearing screening via telehealth. J Telemed Telecare. 2011;17(05):240–244. doi: 10.1258/jtt.2011.100810. [DOI] [PubMed] [Google Scholar]
- 68.Ramatsoma H, Koekemoer D. Validation of a bilateral simultaneous computer-based tympanometer. Am J Audiol. 2020;29(03):491–503. doi: 10.1044/2020_AJA-20-00013. [DOI] [PubMed] [Google Scholar]
- 69.Alenezi E M, Jajko K, Reid A. The reliability of video otoscopy recordings and still images in the asynchronous diagnosis of middle-ear disease. Int J Audiol. 2022;61(11):917–923. doi: 10.1080/14992027.2021.1983217. [DOI] [PubMed] [Google Scholar]
- 70.Myburgh H C, Jose S, Swanepoel D W, Laurent C. Towards low cost automated smartphone-and cloud-based otitis media diagnosis. Biomed Signal Process Control. 2018;39:34–52. [Google Scholar]
- 71.Sandström J, Myburgh H, Laurent C, Swanepoel W, Lundberg T. A machine learning approach to screen for otitis media using digital otoscope images labelled by an expert panel. Diagnostics (Basel) 2022;12(06):1318. doi: 10.3390/diagnostics12061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alenezi E M, Jajko K, Reid A. Clinician-rated quality of video otoscopy recordings and still images for the asynchronous assessment of middle-ear disease. J Telemed Telecare. 2021:X20987783. doi: 10.1177/1357633X20987783. [DOI] [PubMed] [Google Scholar]
- 73.Frisby C, Eikelboom R H, Mahomed-Asmail F. Community-based adult hearing care provided by community healthcare workers using mHealth technologies. Glob Health Action. 2022;15(01):2.095784E6. doi: 10.1080/16549716.2022.2095784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boothroyd A. Adult aural rehabilitation: what is it and does it work? Trends Amplif. 2007;11(02):63–71. doi: 10.1177/1084713807301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferguson M, Maidment D, Henshaw H, Heffernan E. Evidence-based interventions for adult aural rehabilitation: that was then, this is now. Semin Hear. 2019;40(01):68–84. doi: 10.1055/s-0038-1676784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maidment D W, Amlani A M. Thieme Medical Publishers, Inc.; 2020. Argumentum ad Ignorantiam: Smartphone-Connected Listening Devices; pp. 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maidment D W, Ali Y HK, Ferguson M A. Applying the COM-B model to assess the usability of smartphone-connected listening devices in adults with hearing loss. J Am Acad Audiol. 2019;30(05):417–430. doi: 10.3766/jaaa.18061. [DOI] [PubMed] [Google Scholar]
- 78.Convery E, Keidser G, Hickson L, Meyer C. Factors associated with successful setup of a self-fitting hearing aid and the need for personalized support. Ear Hear. 2019;40(04):794–804. doi: 10.1097/AUD.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 79.Convery E, Keidser G, McLelland M, Groth J. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed J E Health. 2020;26(06):798–804. doi: 10.1089/tmj.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michie S, van Stralen M M, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(01):42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng S L, Phelan S, Leonard M, Galster J. A qualitative case study of smartphone-connected hearing aids: influences on patients, clinicians, and patient–clinician interactions. J Am Acad Audiol. 2017;28(06):506–521. doi: 10.3766/jaaa.15153. [DOI] [PubMed] [Google Scholar]
- 82.Olson A, Maidment D W, Ferguson M A. Consensus on connected hearing health technologies and service delivery models in the UK: a Delphi review. Int J Audiol. 2022;61(04):344–351. doi: 10.1080/14992027.2021.1936223. [DOI] [PubMed] [Google Scholar]
- 83.Henshaw H, Clark D P, Kang S, Ferguson M A. Computer skills and internet use in adults aged 50-74 years: influence of hearing difficulties. J Med Internet Res. 2012;14(04):e113. doi: 10.2196/jmir.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sabin A T, Van Tasell D J, Rabinowitz B, Dhar S.Validation of a self-fitting method for over-the-counter hearing aids Trends Hear 2020242331216519900589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almufarrij I, Dillon H, Munro K J. Do we need audiogram-based prescriptions? A systematic review. Int J Audiol. 2022:1–12. doi: 10.1080/14992027.2022.2064925. [DOI] [PubMed] [Google Scholar]
- 86.Maidment D W, Heyes R, Gomez R, Coulson N S, Wharrad H, Ferguson M A. Evaluating a theoretically informed and co-created mHealth educational intervention for first-time hearing aid users: a qualitative interview study. JMIR Mhealth Uhealth. 2020;8(08):e17193. doi: 10.2196/17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gotowiec S, Larsson J, Incerti P. Understanding patient empowerment along the hearing health journey. Int J Audiol. 2022;61(02):148–158. doi: 10.1080/14992027.2021.1915509. [DOI] [PubMed] [Google Scholar]
- 88.Ferguson M, Gotowiec S, Larsson J, Incerti P, Bennett R, Andrich D.Development of an outcome measure for empowerment for hearing lossPresented at: Hearing Across the Lifespan; June2022Cernobbio, Italy [Google Scholar]
- 89.Glista D, Schnittker J A, Brice S. The modern hearing care landscape: toward the provision of personalized, dynamic, and adaptive care. Semin Hear. 2023;44(03):261–273. doi: 10.1055/s-0043-1769621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maidment D W, Barker A B, Xia J, Ferguson M A. A systematic review and meta-analysis assessing the effectiveness of alternative listening devices to conventional hearing aids in adults with hearing loss. Int J Audiol. 2018;57(10):721–729. doi: 10.1080/14992027.2018.1493546. [DOI] [PubMed] [Google Scholar]
- 91.Chen C H, Huang C Y, Cheng H L. Comparison of personal sound amplification products and conventional hearing aids for patients with hearing loss: a systematic review with meta-analysis. EClinicalMedicine. 2022;46:101378. doi: 10.1016/j.eclinm.2022.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reed N S, Betz J, Kendig N, Korczak M, Lin F R. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA. 2017;318(01):89–90. doi: 10.1001/jama.2017.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Almufarrij I, Munro K J, Dawes P, Stone M A, Dillon H.Direct-to-consumer hearing devices: capabilities, costs, and cosmetics Trends Hear 2019232331216519858301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Humes L E, Rogers S E, Quigley T M, Main A K, Kinney D L, Herring C. The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: a randomized double-blind placebo-controlled clinical trial. Am J Audiol. 2017;26(01):53–79. doi: 10.1044/2017_AJA-16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urbanski D, Hernandez H, Oleson J, Wu Y H. Toward a new evidence-based fitting paradigm for over-the-counter hearing aids. Am J Audiol. 2021;30(01):43–66. doi: 10.1044/2020_AJA-20-00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klyn N AM, Kleindienst Robler S, Bogle J. CEDRA – a tool to help consumers assess risk for ear disease. Ear Hear. 2019;40(06):1261–1266. doi: 10.1097/AUD.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuerbis A, Mulliken A, Muench F, Moore A A, Gardner D. Older adults and mobile technology: factors that enhance and inhibit utilization in the context of behavioral health. Ment Heal Addict Res. 2017;2:1–11. [Google Scholar]
- 98.Wu Y H, Damnée S, Kerhervé H, Ware C, Rigaud A S. Bridging the digital divide in older adults: a study from an initiative to inform older adults about new technologies. Clin Interv Aging. 2015;10:193–200. doi: 10.2147/CIA.S72399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loughrey D G, Kelly M E, Kelley G A, Brennan S, Lawlor B A. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(02):115–126. doi: 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferguson M A, Henshaw H, Clark D P, Moore D R. Benefits of phoneme discrimination training in a randomized controlled trial of 50- to 74-year-olds with mild hearing loss. Ear Hear. 2014;35(04):e110–e121. doi: 10.1097/AUD.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schow R, Nerbonne M. 5th ed. Pearson Education; 2006. Introduction to Audiologic Rehabilitation. [Google Scholar]
- 102.Henshaw H, Ferguson M A. Efficacy of individual computer-based auditory training for people with hearing loss: a systematic review of the evidence. PLoS One. 2013;8(05):e62836. doi: 10.1371/journal.pone.0062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferguson M A, Henshaw H. Auditory training can improve working memory, attention and communication in adverse conditions for adults with hearing loss: perspective. Front Psychol. 2015;6:1–7. doi: 10.3389/fpsyg.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henshaw H, Heinrich A, Tittle A, Ferguson M. Cogmed training does not generalize to real-world benefits for adult hearing aid users: results of a blinded, active-controlled randomized trial. Ear Hear. 2022;43(03):741–763. doi: 10.1097/AUD.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferguson M, Henshaw H. How does auditory training work? Joined up thinking and listening. Semin Hear. 2015;36(04):237–249. doi: 10.1055/s-0035-1564456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawrence B J, Jayakody D MP, Henshaw H.Auditory and cognitive training for cognition in adults with hearing loss: a systematic review and meta-analysis Trends Hear 2018222331216518792096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson S, White-Schwoch T, Choi H J, Kraus N. Training changes processing of speech cues in older adults with hearing loss. Front Syst Neurosci. 2013;7:97. doi: 10.3389/fnsys.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proc Natl Acad Sci U S A. 2013;110(11):4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saunders G H, Smith S L, Chisolm T H, Frederick M T, McArdle R A, Wilson R H. A randomized control trial: Supplementing hearing aid use with listening and communication enhancement (LACE) auditory training. Ear Hear. 2016;37(04):381–396. doi: 10.1097/AUD.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 110.Tye-Murray N, Spehar B, Sommers M, Barcroft J. Auditory training with frequent communication partners. J Speech Lang Hear Res. 2016;59(04):871–875. doi: 10.1044/2016_JSLHR-H-15-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lowe S C, Henshaw H, Wild J, Ferguson M A. Evaluation of home-delivered live-voice auditory training for adult hearing aid users involving their communication partners: a randomised controlled trial. Int J Audiol. 2023;62(01):89–99. doi: 10.1080/14992027.2021.2005834. [DOI] [PubMed] [Google Scholar]
- 112.Convery E, Hickson L, Meyer C, Keidser G. Predictors of hearing loss self-management in older adults. Disabil Rehabil. 2019;41(17):2026–2035. doi: 10.1080/09638288.2018.1457091. [DOI] [PubMed] [Google Scholar]
- 113.Barker F, Mackenzie E, Elliott L, Jones S, de Lusignan S. Interventions to improve hearing aid use in adult auditory rehabilitation. Cochrane Database Syst Rev. 2016;2016(08):CD010342. doi: 10.1002/14651858.CD010342.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bahramian M, Najimi A, Omid A. Association between health literacy with knowledge, attitude, and performance of health-care providers in applying health literacy education strategies for health education delivery. J Educ Health Promot. 2020;9:10. doi: 10.4103/jehp.jehp_199_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferguson M, Brandreth M, Brassington W, Leighton P, Wharrad H. A randomized controlled trial to evaluate the benefits of a multimedia educational programme for first-time hearing aid users. Ear Hear. 2016;37(02):123–136. doi: 10.1097/AUD.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maidment D W, Coulson N S, Wharrad H, Taylor M, Ferguson M A. The development of an mHealth educational intervention for first-time hearing aid users: combining theoretical and ecologically valid approaches. Int J Audiol. 2020;59(07):492–500. doi: 10.1080/14992027.2020.1755063. [DOI] [PubMed] [Google Scholar]
- 117.Gomez R, Ferguson M. Improving knowledge and self-efficacy for hearing aid self-management: the early delivery of a multimedia-based education program in first-time adult hearing aid users. Int J Audiol. 2020;59(04):272–281. doi: 10.1080/14992027.2019.1677953. [DOI] [PubMed] [Google Scholar]
- 118.Malmberg M, Sundewall Thorén E, Öberg M, Lunner T, Andersson G, Kähäri K. Experiences of an Internet-based aural rehabilitation (IAR) program for hearing aid users: a qualitative study. Int J Audiol. 2018;57(08):570–576. doi: 10.1080/14992027.2018.1453171. [DOI] [PubMed] [Google Scholar]
- 119.Thorén E S, Öberg M, Wänström G, Andersson G, Lunner T. A randomized controlled trial evaluating the effects of online rehabilitative intervention for adult hearing-aid users. Int J Audiol. 2014;53(07):452–461. doi: 10.3109/14992027.2014.892643. [DOI] [PubMed] [Google Scholar]
- 120.Malmberg M, Anióse K, Skans J, Öberg M. A randomised, controlled trial of clinically implementing online hearing support. Int J Audiol. 2023;62(05):472–480. doi: 10.1080/14992027.2022.2059712. [DOI] [PubMed] [Google Scholar]
- 121.Meijerink J F, Pronk M, Lissenberg-Witte B I, Jansen V, Kramer S E. Effectiveness of a web-based SUpport PRogram (SUPR) for hearing aid users aged 50+: two-arm, cluster randomized controlled trial. J Med Internet Res. 2020;22(09):e17927. doi: 10.2196/17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meijerink J FJ, Pronk M, Kramer S E.Experiences with and lessons learned from developing, implementing, and evaluating a support program for older hearing aid users and their communication partners in the hearing aid dispensing setting Am J Audiol 202029(3S):638–647. [DOI] [PubMed] [Google Scholar]
- 123.Kelly T B, Tolson D, Day T, McColgan G, Kroll T, Maclaren W. Older people's views on what they need to successfully adjust to life with a hearing aid. Health Soc Care Community. 2013;21(03):293–302. doi: 10.1111/hsc.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferguson M, Brandreth M, Brassington W, Wharrad H. Information retention and overload in first-time hearing aid users: an interactive multimedia educational solution. Am J Audiol. 2015;24(03):329–332. doi: 10.1044/2015_AJA-14-0088. [DOI] [PubMed] [Google Scholar]
- 125.Bennett R J, Eikelboom R H, Sucher C M, Ferguson M, Saunders G H. Barriers and facilitators to delivery of group audiological rehabilitation programs: a survey based on the COM-B model. Int J Audiol. 2022;61(02):130–139. doi: 10.1080/14992027.2021.1928304. [DOI] [PubMed] [Google Scholar]
- 126.Heffernan E, Coulson N, Henshaw H, Barry J, Ferguson M.Understanding the psychosocial experiences of adults with mild-moderate hearing loss: a qualitative study applying Leventhal's self-regulatory model Int J Audiol 201655(Suppl 3, Suppl 3):S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barker A B, Leighton P, Ferguson M A. Coping together with hearing loss: a qualitative meta-synthesis of the psychosocial experiences of people with hearing loss and their communication partners. Int J Audiol. 2017;56(05):297–305. doi: 10.1080/14992027.2017.1286695. [DOI] [PubMed] [Google Scholar]
- 128.Bennett R J, Saulsman L, Eikelboom R H, Olaithe M. Coping with the social challenges and emotional distress associated with hearing loss: a qualitative investigation using Leventhal's self-regulation theory. Int J Audiol. 2022;61(05):353–364. doi: 10.1080/14992027.2021.1933620. [DOI] [PubMed] [Google Scholar]
- 129.Heffernan E, Withanachchi C M, Ferguson M A. ‘The worse my hearing got, the less sociable I got’: a qualitative study of patient and professional views of the management of social isolation and hearing loss. Age Ageing. 2022;51(02):afac019. [Google Scholar]
- 130.Bott A, Saunders G. A scoping review of studies investigating hearing loss, social isolation and/or loneliness in adults. Int J Audiol. 2021;60 02:30–46. doi: 10.1080/14992027.2021.1915506. [DOI] [PubMed] [Google Scholar]
- 131.Garnefski N, Kraaij V. Effects of a Cognitive Behavioral Self-help program on emotional problems for people with acquired hearing loss: a randomized controlled trial. J Deaf Stud Deaf Educ. 2012;17(01):75–84. doi: 10.1093/deafed/enr020. [DOI] [PubMed] [Google Scholar]
- 132.Molander P, Hesser H, Weineland S. Internet-based acceptance and commitment therapy for psychological distress experienced by people with hearing problems: a pilot randomized controlled trial. Cogn Behav Ther. 2018;47(02):169–184. doi: 10.1080/16506073.2017.1365929. [DOI] [PubMed] [Google Scholar]
- 133.Bennett R J, Donaldson S, Kelsall-Foreman I. Addressing emotional and psychological problems associated with hearing loss: perspective of consumer and community representatives. Am J Audiol. 2021;30(04):1130–1138. doi: 10.1044/2021_AJA-21-00093. [DOI] [PubMed] [Google Scholar]
- 134.Bennett R J, Kelsall-Foreman I, Donaldson S, Olaithe M, Saulsman L, Badcock J C. Exploring current practice, knowledge, and training needs for managing psychosocial concerns in the audiology setting: perspectives of audiologists, audiology reception staff, and managers. Am J Audiol. 2021;30(03):557–589. doi: 10.1044/2021_AJA-20-00189. [DOI] [PubMed] [Google Scholar]
- 135.Young T, Pang J, Ferguson M.Hearing from you: design thinking in audiological research Am J Audiol 202231(3S):1003–1012. [DOI] [PubMed] [Google Scholar]
- 136.Maruthurkkara S, Allen A, Cullington H, Muff J, Arora K, Johnson S. Remote check test battery for cochlear implant recipients: proof of concept study. Int J Audiol. 61(06):443–452. doi: 10.1080/14992027.2021.1922767. [DOI] [PubMed] [Google Scholar]
- 137.Sucher C, Bennett R, Coetzee L, Liew A, Ferguson M.Assessments in the cloud: integrating digital technologies into the cochlear implant clinic using implementation sciencePresented at: Hearing Across the Lifespan; June2022Cernobbio, Italy [Google Scholar]
- 138.Cullington H, Kitterick P, Weal M, Margol-Gromada M. Feasibility of personalised remote long-term follow-up of people with cochlear implants: a randomised controlled trial. BMJ Open. 2018;8(04):e019640. doi: 10.1136/bmjopen-2017-019640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Medical Research Council . Medical Research Council; 2006. Developing and Evaluating Complex Interventions: New Guidance. [Google Scholar]
- 140.Maidment D W, Ferguson M.An application of the Medical Research Council's guidelines for evaluating complex interventions: a usability study assessing smartphone-connected listening devices in adults with hearing loss Am J Audiol 201827(3S):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bennett R J, Bucks R S, Saulsman L, Pachana N A, Eikelboom R H, Meyer C J. Use of the Behaviour Change Wheel to design an intervention to improve the provision of mental wellbeing support within the audiology setting. Imp Sci Comm. 2023;4(01):1–22. doi: 10.1186/s43058-023-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skivington K, Matthews L, Simpson S A. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. doi: 10.1136/bmj.n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heffernan E, Coulson N S, Ferguson M A. Development of the Social Participation Restrictions Questionnaire (SPaRQ) through consultation with adults with hearing loss, researchers, and clinicians: a content evaluation study. Int J Audiol. 2018;57(10):791–799. doi: 10.1080/14992027.2018.1483585. [DOI] [PubMed] [Google Scholar]
- 144.Nielsen A C, Rotger-Griful S, Kanstrup A M, Laplante-Lévesque A.User-innovated eHealth solutions for service delivery to older persons with hearing impairment Am J Audiol 201827(3S):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burden R S, Galloway L N, Rothpletz A M, Glasheen K A, Preminger J E.The development of an internet-based decision coaching guide to encourage audiology care: the results of a participatory design approach Am J Audiol 202029(3S):546–563. [DOI] [PubMed] [Google Scholar]
- 146.Henshaw H, Sharkey L, Crowe D, Ferguson M.Research priorities for mild-to-moderate hearing loss in adults Lancet 2015386(10009):2140–2141. [DOI] [PubMed] [Google Scholar]
- 147.Fackrell K, Stratmann L, Kennedy V. Identifying and prioritising unanswered research questions for people with hyperacusis: James Lind alliance hyperacusis priority setting partnership. BMJ Open. 2019;9(11):e032178. doi: 10.1136/bmjopen-2019-032178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ferguson M, Maidment D, Henshaw H, Gomez R. Knowledge is power: improving outcomes for patients, partners, and professionals in the digital age. Perspect ASHA Spec Interest Groups. 2019;4(01):140–148. [Google Scholar]
- 149.Bauer M S, Kirchner J. Implementation science: What is it and why should I care? Psychiatry Res. 2020;283:112376. doi: 10.1016/j.psychres.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 150.Eccles M, Mittman B. Editorial: welcome to implementation science. Implementation Science. 2006;1(01):1–3. [Google Scholar]
- 151.Gitlin L N, Baier R R, Jutkowitz E.Dissemination and implementation of evidence-based dementia care using embedded pragmatic trials J Am Geriatr Soc 202068(Suppl 2, Suppl 2):S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moullin J C, Sabater-Hernández D, Fernandez-Llimos F, Benrimoj S I. A systematic review of implementation frameworks of innovations in healthcare and resulting generic implementation framework. Health Res Policy Syst. 2015;13(01):16. doi: 10.1186/s12961-015-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nilsen P. Springer; 2020. pp. 53–79. [Google Scholar]
- 154.Birken S A, Rohweder C L, Powell B J. T-CaST: an implementation theory comparison and selection tool. Implement Sci. 2018;13(01):143. doi: 10.1186/s13012-018-0836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moullin J C, Dickson K S, Stadnick N A. Ten recommendations for using implementation frameworks in research and practice. Implement Sci Commun. 2020;1(01):42. doi: 10.1186/s43058-020-00023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Studts C R.Implementation science: increasing the public health impact of audiology research Am J Audiol 202231(3S):849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marrone N L, Nieman C L, Coco L. Community-based participatory research and human-centered design principles to advance hearing health equity. Ear Hear. 2022;43 01:33S–44S. doi: 10.1097/AUD.0000000000001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barker F, Lusignan S, Deborah C. Improving collaborative behaviour planning in adult auditory rehabilitation: development of the I-PLAN intervention using the behaviour change wheel. Ann Behav Med. 2018;52(06):489–500. doi: 10.1007/s12160-016-9843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barker F, Atkins L, de Lusignan S.Applying the COM-B behaviour model and behaviour change wheel to develop an intervention to improve hearing-aid use in adult auditory rehabilitation Int J Audiol 201655(3, Suppl 3):S90–S98. [DOI] [PubMed] [Google Scholar]
- 160.Glasgow R E, Harden S M, Gaglio B. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nickbakht M, Meyer C J, Saulsman L. Barriers and facilitators to asking adults with hearing loss about their emotional and psychological well-being: a COM-B analysis. Int J Audiol. 2022:1–9. doi: 10.1080/14992027.2022.2056090. [DOI] [PubMed] [Google Scholar]
- 162.Lesica N A, Mehta N, Manjaly J G, Deng L, Wilson B S, Zeng F G. Harnessing the power of artificial intelligence to transform hearing healthcare and research. Nat Mach Intell. 2021;3(10):840–849. [Google Scholar]
- 163.Saunders G H, Christensen J H, Gutenberg J. Application of big data to support evidence-based public health policy decision-making for hearing. Ear Hear. 2020;41(05):1057–1063. doi: 10.1097/AUD.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]


