Abstract
The trophic strategies of cold-water planktonic foraminifera are not well understood due to the challenge of culturing them in polar conditions. Here, we identify previously unknown ectoplasmic and cytoplasmic projections in three species of planktonic foraminifera thriving in polar and subpolar marine environments: Globigerina bulloides, Neogloboquadrina incompta and Neogloboquadrina pachyderma. These structures were observed during routine monitoring of cultured specimens sampled from the Norwegian coast, Greenland Sea and Baffin Bay. Two types of projections were discovered, including permanent and non-permanent structures such as ectoplasmic roots, twigs and twig-like projections, similar to those observed in benthic taxa Cibicides and Cibicidoides. Additionally, a previously undescribed filopodia-like projection was observed in N. pachyderma. We discuss the function, the ecological significance and the potential impact on pelagic processes of the presence of these structures in foraminifera species that occupy diverse niches in the water column. Our findings suggest that these structures may play an important role in the trophic strategies of cold-water planktonic foraminifera, and further research and observations are necessary to fully comprehend their significance in the carbon cycle.
Keywords: planktonic foraminifera, ectoplasmic roots, trophic strategy, laboratory observations
INTRODUCTION
Eukaryotic microbes exhibit a remarkable array of structural adaptations that enable them to carry out a diverse range of complex behaviors including defense and attack mechanisms, infection, modes of locomotion, feeding, reproduction, buoyancy control and more (Keeling and del Campo, 2017). By leveraging their structural diversity, these microorganisms can execute remarkable repertoire strategies essential for their survival and success in various ecological niches.
Foraminifera (Rhizaria) are microbial eukaryotes thriving in aquatic environments and soil that use cytoplasmic projections named reticulopodia (or rhizopodia) as the primary means of locomotion (Travis et al., 2002; Holzmann et al., 2021). These structures, once extended in the surrounding environment, can anastomose, creating complex networks of pseudopodia characterized by a constant bidirectional cytoplasmic flow (Hedley et al., 1967; Bowser and Travis, 2002). The movement of these web-like formations is powered by cytoplasmic microtubules that also enable intracellular transport, generating the tracks that regulate the bidirectional transit of the organelles (Travis et al., 2002).
In the pelagic environment, the abrupt shed of reticulopodia allows planktonic species to reduce the fluid drag and move downward in the water column (Furbish and Arnold, 1997). However, the exact mechanism of how planktonic foraminifera achieve and regulate their buoyancy throughout their life cycle is poorly understood.
Next to reticulopodia, other cytoplasmic protrusions have been described in foraminifera, including frothy pseudopodia involved in the calcification process and reported in both benthic and planktonic species (Schiebel and Hemleben, 2017; Tyszka et al., 2019). In addition, structures named filopodia and axopodia have been identified in planktonic foraminifera (Adshead, 1966; Schiebel and Hemleben, 2017), but notably, the adoption of these terms varied across investigations and has been recently revisited (Zlatogursky, 2021).
The transport of food particles is one of the major functions of reticulopodia; however, for benthic taxa that live in the sediment, they also represent a vital substrate adhesion apparatus (Goldstein, 1999). A recent study has shown that, in some deep-sea benthic species, reticulopodia can build permanent formations, called ectoplasmic structures, that serve as an anchor in highly dynamic ecosystems and that, when conditions change, the foraminifera leaves behind (Wollenburg et al., 2021). Importantly, since they have only been observed in benthic species of the family Cibicididae, interpretations of ectoplasmic structures’ ecological and evolutionary significance have been limited to their specific habitat (i.e. the deep-sea marine sediment) (Wollenburg et al., 2021).
To date, no evidence of the occurrence of such structures has been reported in any planktonic species.
Here, we describe for the first time overlooked ectoplasmic structures and cytoplasmic projections in the planktonic foraminifera species Globigerina bulloides, Neogloboquadrina incompta and Neogloboquadrina pachyderma sampled off the coast of Norway, in the Greenland Sea and in the Baffin Bay. We serendipitously observed these structures in cultured specimens during routine monitoring of their rhizopodial activity in the autumn of 2018 (Greco et al., 2020) and in the summer of 2021 and 2022 (Ezat et al., 2022a, 2022b). Life-history observations on these species are particularly scarce given the high complexity of keeping cold-adapted foraminifera alive in culture (Manno et al., 2012). Additionally, cultivating Non-Spinose foraminifera of the Neogloboquadrina genus is challenging (Von Langen et al., 2005), primarily due to their small size. These species behave differently from Spinose foraminifera, such as G. bulloides, as they tend to stick to the walls of the flask rather than floating in the culture media (e.g. Davis et al., 2017; Fehrenbacher et al., 2018). The wide distribution of these species in the water column (Rebotim et al., 2017; Greco et al., 2019) suggests a need to reconsider the function of foraminiferal ectoplasmic structures in a pelagic context. The pelagic ecosystem comprises a multitude of micro-habitats (Boero et al., 2019), such as sea ice and marine snow, where planktonic foraminifera are known to thrive (Dieckmann et al., 1991; Fehrenbacher et al., 2018; Greco et al., 2021). By describing ectoplasmic structures and novel projections, we can enhance our understanding of planktonic foraminiferal trophic strategies and explore how this behavior could affect foraminifera-related carbon fluxes, as well as buoyancy control. Thus, this paper aims to document the existence of different cytoplasmic projections and ectoplasmic structures in planktonic species, discuss their ecological significance and explore their potential impact on pelagic processes, including intra and interspecific interactions and carbon export.
METHODS
Sampling and experimental settings
Sample collection and experimental setup are described in detail by Greco et al., 2020 and Westgård et al. (in prep). Briefly, specimens of N. incompta, N. pachyderma and G. bulloides were collected during three cruises on the RV Helmer Hanssen in autumn 2018 and summer 2021 and 2022, respectively, conducted off Tromsø (Greco et al., 2020, Ezat et al., 2022b), in the Greenland Sea (Ezat et al., 2022a) and at the end of summer 2022, in the Baffin Bay onboard the RV Maria S. Merian (Table I). Specimens were sampled from the production zone, between 0 and 50 or 100 m, using a WP2 plankton net (64-μm mesh size, HydroBios) towed vertically or a multi net equipped with a 100-μm mesh size (Multi Plankton Sampler, HydroBios type Midi). The retrieved specimens were picked on board and incubated in jars (70 or 150 mL) containing seawater collected at the site of collection and filtered through a 0.22-μm nitrate cellulose filter. Temperature and salinity were kept such that they would mimic the environmental conditions. The collected foraminifera were then transferred to different temperature and salinity treatments for specific scientific projects (Greco et al., 2020; Meilland et al., 2022; Westegård et al. in prep) and kept in a cold room directly onboard and/or in one of the facilities of UiT—The Arctic University of Norway in Tromsø or in incubators where experiments and microscope observations were performed. Cytoplasm-bearing specimens were cultured individually in 75 mL Falcon flasks or in 10 mL wells (culture Figures) and under constant temperature (2, 4.5, 6, 7, 9 or 9.5°C), salinity (31, 32.1, 34.8, 35, 36.7) and pH (7.8, 8, and 8.1) conditions. All specimens were exposed to their in situ light cycles (e.g. Manno et al., 2012; Greco et al., 2020). They were fed daily with autoclaved marine microalgae Nannochloropsis food mix (30–50 μL Nannochloropsis concentrate: 200 mL filtered seawater), which had undergone autoclave steps to prevent bacteria and algal proliferation, or with living diatoms (Pseudonitzschia turgidula). This approach aimed to simulate a diet that closely mimicked their natural environment. The water of all N. pachyderma and G. bulloides specimens sampled and cultured in 2022 was partly or fully replaced once a week to ensure stable carbonate chemistry.
Table I.
Sampling area of origin and culturing conditions of the foraminifera specimens included in the study
| Species | ID | Year | Origin | t (°C) | pH | Salinity | Day | Structure | Figure |
|---|---|---|---|---|---|---|---|---|---|
| N. incompta | B-h2 | 2018 | Norwegian Sea | 6 | – | 31 | 13–18 | Root | Fig. 1 |
| N. pachyderma | 89 | 2021 | Greenland Sea | 5 | 8 | 34.8 | 18 | Root | Fig. 2e |
| N. pachyderma | 295 | 2021 | Greenland Sea | 5 | 8 | 34.8 | 11 | Root | Fig. 2f |
| N. pachyderma | 254 | 2021 | Greenland Sea | 5 | 8 | 34.8 | 4 | Filopodia-like | Fig. 2d |
| N. pachyderma | 4 | 2021 | Greenland Sea | 6 | 7.8 | 35 | 40 | Twig-like | Fig. 2b |
| N. pachyderma | 15 | 2021 | Greenland Sea | 2 | 8.1 | 35 | 21 | Twig-like | Fig. 2a |
| N. incompta | B-h1 | 2018 | Norwegian Sea | 6 | - | 31 | 12 | Root fragment | Fig. 2c |
| N. pachyderma | 9 | 2021 | Greenland Sea | 6 | 8.1 | 36.7 | 26 | Filopodia-like | Fig. 3 |
| N. pachyderma | 11 | 2021 | Greenland Sea | 6 | 8.1 | 32.1 | 16 | Twig-like | Supplementary Fig. 1c–d |
| N. pachyderma | 257 | 2022 | Baffin Bay | 5 | 8 | 34.8 | 11 | Twig-like | Fig. 4 |
| G. bulloides | Gc100_18 | 2022 | Norwegian Sea | 10 | 8.1 | 35 | 18 | Twigs | Fig. 5 |
| N. pachyderma | C250 | 2021 | Greenland Sea | 6 | 8.1 | 35 | 17 | Root and filopodia-like | Supplementary Fig. 1b |
| N. pachyderma | 12 | 2021 | Greenland Sea | 9 | 8.1 | 35 | 4 | Twig-like | Supplementary Fig. 1a |
All specimens were checked at least bi-weekly under the inverted microscope (AxioVert 0.1, Zeiss), and information relative to their cytoplasm color, rhizopodial extent and activities were recorded.
All measurements were performed in ImageJ v1.8.0 (Schneider et al., 2012).
Since no standardized methodology exists for characterizing the diversity, composition and potential role of reticulopodia in planktonic foraminifera, we decided to use the terminology from research on benthic species and other microbial groups to describe our observations.
RESULTS
Ectoplasmic roots in N. incompta
Photographic evidence of ectoplasmic roots (as defined in Wollenburg et al., 2021) was obtained for two specimens of N. incompta cultured at a salinity of 31. For one specimen (Fig. 1), it was possible to capture the root formation process, while for the other, the root structure could only be observed as a torn fragment (Fig. 2c). The torn root fragments and root formation were observed after 11 days in the culture. The roots, easily distinguishable from inadvertently introduced artificial fibers by their organic and motile base (as shown in Fig. 1c and d), exhibited a significant thickness and demonstrated a remarkable extension. They ranged from three times (515 μm) to more than five times (885.7 μm) the size of the specimen (as depicted in Fig. 1a and e), securely anchoring it to the walls of the flask. Interestingly, the specimen in Fig. 1 built the new root in the same position as the previously torn one (Fig. 1b). Specimen’s rhizopodia were extended before, during and after root formation. The root projection of the specimen in Fig. 1a appears to be composed of several fiber-like fragments of ectoplasm that have intertwined to form a robust structure. After a few days, this stable root structure detached from the specimen and could be observed floating next to the foraminifera in the culture flask (Fig. 1b).
Fig. 1.
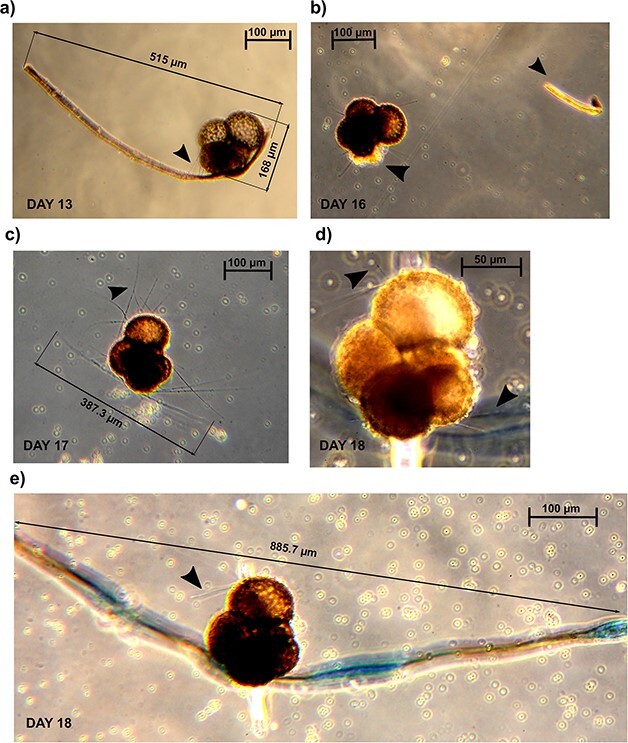
Serial observations of a cultured specimen of Neogloboquadrina incompta collected in the Norwegian Sea presenting and forming ectoplasmic roots. Panels (a) to (e) depict different stages of ectoplasmic root formation observed over the course of 6 days in culture. Black arrows in panels (a), (c), (d) and (e) indicate the rhizopodial activity. The black arrows in panel (b) indicate the root fragment and the location of the fracture on the test. The contrast in the pictures has been artificially enhanced to visualize the reticulopodia and the ectoplasmic roots. Scale bars: 100 μm.
Fig. 2.
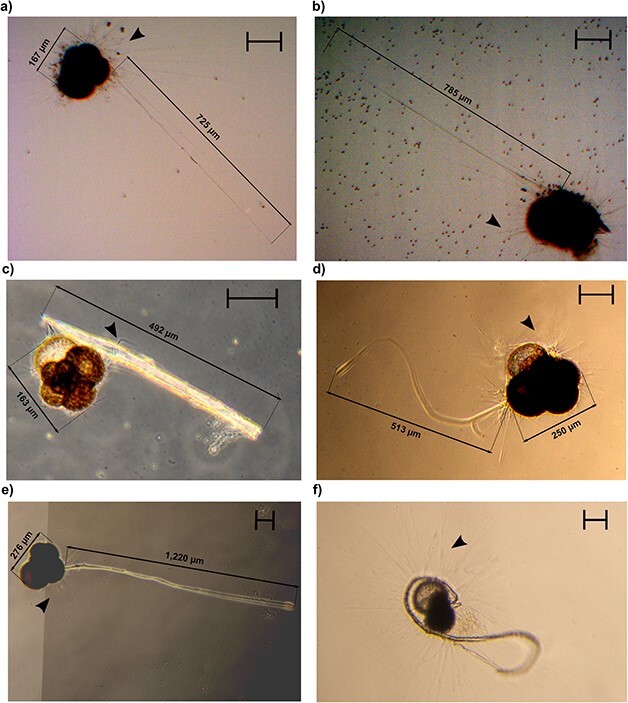
Permanent and Non-Permanent structures observed in specimens of the Genus Neogloboquadrina. Panels (a) and (b) depict two Neogloboquadrina pachyderma specimens displaying extended twig-like structures. The Neogloboquadrina incompta specimen in panel (c) is attached to an ectoplasmic root fragment. Panel (d) illustrates a N. pachyderma specimen extending a filopodia-like projection. Panels (e) and (f) show two N. pachyderma specimens exhibiting fully extended ectoplasmic roots. The maximum diameter of the specimens in panels (b) and (f) measured 210 and 251.5 μm, respectively. Black arrows indicate the rhizopodial activity. The contrast in the pictures has been artificially enhanced to visualize the reticulopodia and the other structures. Scale bars: 100 μm.
Non-permanent structures in N. pachyderma
Several specimens of N. pachyderma displayed ectoplasmic structures that differed from roots in both structure and durability. We could divide them into two categories using the term “twig-like structures”, referring to the long and thick arrangement of rhizopods (Fig. 2a and b, Supplementary Fig. 1), and the term filopodia-like structures, referring to retractile cytoplasm protrusion (Figs 2d– 4, Supplementary Fig. 1b, video on figshare: 10.6084/m9.figshare.22665031). Both types of structures were retractable as opposed to the ectoplasmic roots that were not reabsorbed once extended. Twigs-like structures could reach up to four times the size of the shell, with their distal extremity anchored to the flask wall. At their proximal end, we could observe modified rhizopods converging and eventually generating the twigs (Supplementary Fig. 1a–d). They were systematically associated with food particles at their surface.
Fig. 4.

Time-lapse microscopy images of a specimen of Neogloboquadrina pachyderma using a filopodia-like structure to collect and gather food particles close to its position. The orange arrow indicates the direction of the movement. The black arrow indicates the rhizopodial activity. The contrast in the images has been artificially enhanced to visualize the filopodia-like projection. Scale bars: 100 μm.
The filopodia-like projections observed in Fig. 3 appeared to be extremely dynamic and thicker than the twigs. Furthermore, no modified rhizopods could be observed at their proximal extremity, suggesting that these structures are not associated with a specialized attachment mechanism. The specimen in Fig. 3 entirely retracted the structure in less than three minutes, accompanied by an intense lateral motion (see video on figshare: 10.6084/m9.figshare.22665031). These structures were freely moving, not attached to the jar wall at any time and were also observed in association with food particles on their surface and at their distal extremity, which appeared to be composed of at least three anastomizing filopodia (Fig. 3c). This trifurcation might have a prehensile-like function allowing the foraminifera to collect food and transport it closer to its position, as shown in the N. pachyderma specimen in Fig. 4.
Fig. 3.

Time-lapse microscopy images of a specimen of Neogloboquadrina pachyderma projecting a filopodia-like structure. Panels (a) to (d) depict snapshots of a 3-min process (see video on figshare 10.6084/m9.figshare.22665031) that occurred 26 days after the foraminifera was introduced to its culture. The inset in panel (c) shows a close-up of the distal end of the projection. The orange arrow indicates the direction of the movement. The black arrows indicate the rhizopodial activity The contrast in the images has been artificially enhanced to visualize the filopodia-like projection. Scale bars: 100 μm.
Ectoplasmic roots in N. pachyderma
Roots protrusions were also observed in three cultured specimens of N. pachyderma (Fig. 2d and f, Supplementary Fig. 1b). Even if no time series of the observation was recorded, the photographed structures clearly resemble the ones observed for N. incompta, differing in both extension and thickness from other rhizopodia. In fact, the projections measured more than two times the shell of the specimens and were found to be sticky (Fig. 2f), promoting the aggregation of particles around the foraminifera. These ectoplasmic roots were observed to be attached to the walls of the flask, providing anchorage for the specimens. In all instances, the observation of roots occurred after more than 4 days, before the water in the flask was changed. For one specimen (Supplementary Fig. 1b), the extension of the root structure changed its overall buoyancy leading to its floatation. As for N. incompta, the rhizopodial activity was evident when these specimens extended the structures.
Twigs in G. bulloides
Fully extended twigs consisting of anastomizing rhizopodia and algal particles were observed surrounding a specimen of G. bulloides after 18 days in culture (Fig. 5). These twigs stretched well beyond the spines of the specimen, likely using them as a base, and formed loop structures. Interestingly, although in the benthic genus Cibicidoides, these twigs were extended when the foraminifera was attached to the flask walls, G. bulloides presented these protrusions while suspended in the flask medium.
Fig. 5.
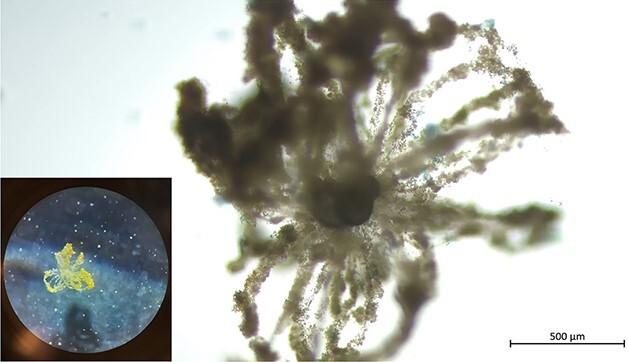
Twigs on a specimen of Globigerina bulloides. The inset shows a photograph of the microscope ocular, indicating that the twigs were extended, while the foraminifera was suspended in the culture flask. Scale bar: 500 μm.
DISCUSSION
Potential origins of ectoplasmic projections
The presence of previously undocumented structures in planktonic foraminifera raises the question of whether they are genuine biological structures or merely artifacts of culture flasks. However, the fact that these structures were observed across multiple years and expeditions, and in specimens of different species and populations, provides strong evidence for their authenticity. This is further supported by the presence of similar structures in benthic lineages of the order Rotaliida (Wollenburg et al., 2021). Recent molecular investigations have suggested that the current diversity of planktonic foraminifera is the result of multiple invasions of the water column by benthic taxa from the order Rotaliida (Morard et al., 2022). In addition, a previous experiment documented benthic-like behaviors, such as reorientation, crawling and burrowing, in the planktonic foraminifera species Globigerinella siphonifera (Spinose) and Globorotalia menardii (Non-Spinose) cultivated in the presence of sediment (Hilbrecht and Thierstein, 1996). Thus, a shared toolkit of projections between benthic and planktonic species can be expected. These structures could represent evolutionary relics deriving from planktonic foraminifera’s benthic ancestors, which could have potentially assumed novel functions in the pelagic environment. The functionality of roots and twigs observed in the benthic genus Cibicidoides has been previously explored in the ecological context of the investigated species, particularly with regard to their deep-sea habitat and epibenthic lifestyle. Specifically, these projections are thought to be used by the deep-sea sediment-dwelling taxa to anchor themselves in the presence of strong currents (Wollenburg et al., 2021). In the following sections, we will discuss the potential functions of these structures in the pelagic species N. pachyderma, N. incompta and G. bulloides, and their ecological implications.
Potential functions of the observed structures
Our observations indicate that species of planktonic foraminifera belonging to the genera Neogloboquadrina and Globigerina can build ectoplasmic systems, as recently reported in the benthic taxa Cibicidoides wuellerstorfi, Cibicides lobatulus and Cibicidoides pachyderma (Wollenburg et al., 2021).
The presence of ectoplasmic roots was confirmed in two specimens of N. incompta and three specimens of N. pachyderma. As reported in benthic species, these structures are permanent once extended and are the longest and the thickest we observed. Furthermore, the sequence of photographs in Fig. 1 shows how roots can be rebuilt within 24 hours if torn, consistent with what was previously observed for benthic lineages (Wollenburg et al., 2021). Although we cannot resolve the molecular composition of the ectoplasmic roots from our observations, we note that they appeared in the absence or ahead of flask water exchange, leading us to believe that the amount of algal particles present affects the ability of the foraminifera to build these protrusions. From our observations, it is clear that the ectoplasmic roots help the specimen anchor to the culture flasks, providing support to its rhizopodial network that can be extended canonically or arise from the structures themselves (Fig. 2c).
The twig-like structures detected in three specimens of N. pachyderma are non-permanent projections that are considerably long (>700 μm). Although they are not identical to the twigs described by Wollenburg et al. (2021), as these specimens could retract them, we believe they serve a similar function in stabilizing the specimen while the rhizopodial network is extended and assisting in the food gathering of the reticulopodia, as shown in Fig. 2a and b, where specimens display a fully extended network of reticulopodia. Conversely, the twigs observed in G. bulloides closely resemble, in both extent and shape, the benthic twigs observed in C. pachyderma, also because of the fact that they are directed in the water column once formed (Wollenburg et al., 2021). Indeed, the projections are visible even at minimal magnification (Fig. 5) and are extended while the specimen is floating in the culture medium, not attached to any of the flask walls. This might suggest that by increasing the drag, twigs, similarly to calcareous spines (on which they are based), could help the foraminifera maintain its position in the water column.
To our knowledge, filopodia-like structures have previously not been photo-documented for any species within the phylum and we observed them in three specimens of N. pachyderma. These protrusions seem rather flexible and appear to swing during retraction causing the specimen to spin about its axis with some displacement (see video on figshare 10.6084/m9.figshare.22665031). The distal extremity magnified in Fig. 3c indicates that these projections actually consist of multiple structures merged in a slender protrusion. However, contrary to reticulopodia, filopodia-like projections do not ramify, hinting at a different cytological composition. Among the structures presented, these are the ones more directly involved in the motility of the foraminifera (see video on figshare 10.6084/m9.figshare.22665031). In absence of analogous observations within the phylum, we looked for resembling structures in other microbial eukaryotes to make inferences on the potential functions of filopodia-like projections.
Similar cytoplasmic structures have been reported in diatoms where they have been described as “cytoplasmic strands”, functioning as spatial determinants for cells and male gametes (Pollock and Pickett-Heaps, 2005; Davidovich et al., 2012). Thus, it is possible that filopodia-like projections function as sensory structures that help the foraminifera search and attach to a target surface far from its position. Another possibility is that filopodia-like projections are the foraminiferal analogous to the axoflagellum described in radiolarians (Ichinohe et al., 2018, 2019). The axoflagellum is long, a thick pseudopodium that radiolaria use not only for capturing food particles but also for reorienting the cell in presence of a current (Ichinohe et al., 2018, 2019). In this scenario, filopodia-like protrusions may enable foraminifera to adjust their buoyancy by projecting them to realign themselves in response to changes in water flow.
Ecological significance of our observations and outlook for future research
Most planktonic foraminifera species are omnivorous, and use their spines and/or extended reticulopodia to ensnare food particles or capture their prey (Schiebel and Hemleben, 2017). Thus, as passive drifters, the efficiency of planktonic foraminifera feeding strategy highly relies on random encounters with their food source. However, in culture, specimens of the Non-Spinose species Globorotalia truncatulinoides have been observed using their rhizopodial network to move from a position where they had consumed food to another one rich in diatoms (Nitzschia spp.) (Schiebel and Hemleben, 2017). Such behavior might indicate that planktonic foraminifera have receptor structures allowing them to detect a food source (chemosensing) and the capacity to displace toward it. This observation is consistent with filopodia-like structures functioning as sensory projections that help planktonic foraminifera reorient toward the preferred food source or collect and gather food particles (see Fig. 4).
The presence of ectoplasmic twigs as the ones we observe in G. bulloides could also impact the planktonic foraminifera feeding strategy. As shown in Fig. 5, twigs significantly increase the overall size of the foraminifera, multiplying, in this way, the chances of potential prey encounters. Like foraminiferal spines, pseudopodia and ectoplasmic structures in planktonic foraminifera, such as those found in G. bulloides, may provide a competitive advantage in low-density prey environments (Grigoratou et al., 2021).
In addition, the projection of root protrusions could increase the likelihood of mate encounters in planktonic foraminifera, despite their low abundance and patchy distribution in the global oceans (Keeling and del Campo, 2017). These organisms primarily reproduce sexually by releasing hundreds of thousands of gametes. A recent investigation into their reproduction dynamics, based on mathematical modeling, has shown that the spatial concentration of gamete release is critical for the successful production of zygotes, overcoming the limitations that result from their sparse distribution (Weinkauf et al., 2022). Wollenburg et al. (2021) demonstrated that the production of roots from multiple benthic individuals can result in the combination of these structures into a single braid-like ectoplasmic root, connecting the specimens for months and they sometimes used it to reposition themselves. As we cultured specimens individually, we can only speculate that the connection of two or multiple foraminifera specimens in braid-like roots can occur in planktonic species as well, potentially representing a significant advantage for successful reproduction events in the water column by granting spatial concentration of gamete release. Furthermore, the previously inferred potential propensity of planktonic foraminifera to descend and congregate in the chlorophyll-dense deep layer during gamete production and release may facilitate this process (Bijma and Hemleben, 1994; Schiebel et al., 1997).
Planktonic foraminifera are among the most represented groups associated with sinking particles, according to a recent metagenomic investigation based on sediment trap samples (Boeuf et al., 2019). We have exclusively observed ectoplasmic roots, which play a crucial role in anchoring foraminifera firmly to their substrate (Figs 1 and 2), in Non-Spinose species that are known to feed on detritus material found in marine aggregates (Greco et al., 2021). Based on our observations, we hypothesize that the development and utilization of roots represent a primary feeding strategy for these organisms, enabling them to extract vital nutrients from the surrounding marine snow and maintain a stable position in the turbulent ocean environment. Furthermore, the high concentrations of particles in the flask coincided with the projection of roots by the specimens, providing further support for our hypothesis. Interestingly, the high concentration of nutrients and the projection of these structures in both G. bulloides (twigs) and N. pachyderma (ectoplasmic roots) could cause a change in the buoyancy of the foraminifera.
Moreover, the ectoplasmic projections observed in planktonic foraminifera may have significant implications for the marine carbon cycle. These structures, extruded by the organism and potentially left behind as it drifts through the water column, can become incorporated into marine aggregates and enhance the vertical flux of organic matter to the deep ocean. This is especially significant as foraminifera are already recognized as major contributors to the carbon pump, particularly in the inorganic carbon pathway (Neukermans et al., 2023).
It is important to note that the observations presented in this study represent the initial step toward the understanding of the functions of the described structures of planktonic foraminifera. To gain a better understanding of these structures, future investigations could employ techniques such as live actin staining (Tyszka et al., 2019) to elucidate the cellular structure and composition of these extensions. Additionally, the function of the described structures could be further studied by implementing different experimental designs to culture foraminifera under various conditions (e.g. different particulate concentrations) and with different substrates to uncover the factors that regulate the different types of projections. The contribution of filopodia-like projections and twigs to the buoyancy of foraminifera could be recorded and tested by placing single specimens in glass cells, similar to the experimental setup in Ichinohe et al. (2019). Ideally, these experiments could be combined with genomics and transcriptomics approaches to understand the genetic and molecular basis of the formation and function of the different structures.
CONCLUSIONS
In this study, we report the presence of previously unnamed types of endoplasmic and ectoplasmic projections in three species of cold-water planktonic foraminifera: N. pachyderma, N. incompta and G. bulloides. Our experiments revealed two types of projections: permanent and non-permanent. Some of these structures, such as ectoplasmic roots, twigs and twig-like projections, closely resemble to those recently described in benthic taxa Cibicides and Cibicidoides, suggesting that they may play a role in anchoring planktonic foraminifera to a substrate or enhancing the chances of prey and mate encounter. Additionally, we discovered a previously undescribed, non-permanent structure that we named the filopodia-like projection in the species N. pachyderma. We hypothesize that this species uses this projection to locate food particles or to reorient itself in the presence of a current. The existence of roots, twigs and filopodia-like projections broadens the trophic toolkit of planktonic foraminifera. Our findings highlight the importance of life-history observations of this group and the need for further studies on these structures that could inform trait-based models (e.g. Grigoratou et al., 2019), improving our understanding of the role of planktonic foraminifera in the carbon cycle.
Supplementary Material
Contributor Information
Mattia Greco, Institute of Marine Sciences (ICM), CSIC, Passeig MARítim de la Barceloneta, 37-49, Barcelona 08003, Spain.
Adele Westgård, Cage – Centre for Arctic Gas Hydrate, Environment and Climate, Department of Geosciences, UIT, The Arctic University of Norway, Dramsveien 201, Tromso 9010, Norway.
Freya E Sykes, Cage – Centre for Arctic Gas Hydrate, Environment and Climate, Department of Geosciences, UIT, The Arctic University of Norway, Dramsveien 201, Tromso 9010, Norway.
Mohamed M Ezat, Cage – Centre for Arctic Gas Hydrate, Environment and Climate, Department of Geosciences, UIT, The Arctic University of Norway, Dramsveien 201, Tromso 9010, Norway.
Julie Meilland, Marum – Center for Marine Environmental Sciences, University of Bremen, Leoberner Str. 8, Bremen 28359, Germany.
ACKNOWLEDGEMENTS
We are grateful to the captains and crews of the teaching cruise for courses GEO-3111 and GEO-3122, which was financially supported by the Department of Geosciences, UiT—The Arctic University of Norway, Tromsø, Norway. We thank the captain, the crew and the scientific parties of the R/V Helmer Hanssen for their support during the CAGE 21-2 and CAGE-ARCLIM cruises. We also thank the captain and crew of the expedition MSM111 on the R/V Maria S. Merian to the Baffin Bay. We would like to extend our appreciation to Professor Michal Kucera for his insightful contributions to our discussions on ectoplasmic structures.
FUNDING
A.W, F.S and M.M.E are supported by the Tromsø Research Foundation [Grant Number A31720 awarded to M.M.E.]. J.M. was funded through the Cluster of Excellence “The Ocean Floor – Earth’s Uncharted Interface”. MG was supported by a Juan de la Cierva-formación 2021 fellowship (FJC2021–047494-I/MCIN/AEI/10.13039/501100011033) from the European Union “NextGenerationEU”/PRTR.
DATA AVAILABILITY
Video in supplement to this article is available on FigShare (10.6084/m9.figshare.22665031).
REFERENCES
- Adshead, P. C. (1966) Taxonomic significance of pseudopodial development in living planktonic foraminiferida. AAPG Bull., 50, 642–643. [Google Scholar]
- Bijma, J. and Hemleben, C. (1994) Population dynamics of the planktic foraminifer Globigerinoides sacculifer (Brady) from the central Red Sea. Deep Sea Res. Part Oceanogr. Res. Pap., 41, 485–510. 10.1016/0967-0637(94)90092-2. [DOI] [Google Scholar]
- Boero, F., De Leo, F., Fraschetti, S. and Ingrosso, G. (2019) The cells of ecosystem functioning: towards a holistic vision of marine space. In Sheppard, C. (ed.), Advances in Marine Biology. Elsevier Ltd, pp. 129–153, 10.1016/bs.amb.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Boeuf, D., Edwards, B. R., Eppley, J. M., Hu, S. K., Poff, K. E., Romano, A. E., Caron, D. A., Karl, D. M. et al. (2019) Biological composition and microbial dynamics of sinking particulate organic matter at abyssal depths in the oligotrophic open ocean. Proc. Natl. Acad. Sci. U. S. A., 116, 11824–11832. 10.1073/pnas.1903080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser, S. S. and Travis, J. L. (2002) Reticulopodia: structural and behavioral basis for the suprageneric placement of granuloreticulosan protists. J. Foraminifer. Res., 32, 440–447. 10.2113/0320440. [DOI] [Google Scholar]
- Davidovich, N. A., Kaczmarska, I., Karpov, S. A., Davidovich, O. I., MacGillivary, M. L. and Mather, L. (2012) Mechanism of male gamete motility in Araphid pennate diatoms from the genus Tabularia (Bacillariophyta). Protist, 163, 480–494. 10.1016/j.protis.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Davis, C. V., Fehrenbacher, J. S., Hill, T. M., Russell, A. D. and Spero, H. J. (2017) Relationships between temperature, pH, and crusting on mg/ca ratios in laboratory-grown Neogloboquadrina foraminifera. Paleoceanography, 32, 1137–1152. 10.1002/2017PA003111. [DOI] [Google Scholar]
- Dieckmann, G. S., Spindler, M., Lange, M. A., Ackley, S. F. and Eicken, H. (1991) Antarctic Sea ice: a habitat for the foraminifer Neogloboquadrina pachyderma. J. Foraminifer. Res., 21, 182–189. 10.2113/gsjfr.21.2.182. [DOI] [Google Scholar]
- Ezat, M. M., Meilland, J., Westgård, A., Chalk, T., Rasmussen, T. L., El Bani Altuna, N. and Lockwood-Ireland, C. (2022a) CAGE21-2 cruise report: planktic foraminifera sampling for culturing experiments, central Greenland Sea 75°N. CAGE, 9. 10.7557/cage.6714. [DOI] [Google Scholar]
- Ezat, M. M., Meilland, J., Westgård, A., Chalk, T., Sykes, F., El Bani Altuna, N., Nadar, P., Tell, F. et al. (2022b) CAGE-ARCLIM cruise: culturing (sub)Arctic planktic foraminifera Neogloboquadrina pachyderma and Globigerina bulloides: implications for ocean acidification and paleoceanography reconstructions. CAGE, 10. 10.7557/cage.6768. [DOI] [Google Scholar]
- Fehrenbacher, J. S., Russell, A. D., Davis, C. V., Spero, H. J., Chu, E. and Hönisch, B. (2018) Ba/ca ratios in the non-spinose planktic foraminifer Neogloboquadrina dutertrei: evidence for an organic aggregate microhabitat. Geochim. Cosmochim. Acta, 236, 361–372. 10.1016/j.gca.2018.03.008. [DOI] [Google Scholar]
- Furbish, D. J. and Arnold, A. J. (1997) Hydrodynamic strategies in the morphological evolution of spinose planktonic foraminifera. GSA Bull., 109, 1055–1072. . [DOI] [Google Scholar]
- Goldstein, S. T. (1999) Foraminifera: A biological overview. In: Modern Foraminifera. Springer, Dordrecht. 10.1007/0-306-48104-9_3. [DOI] [Google Scholar]
- Greco, M., Jonkers, L., Kretschmer, K., Bijma, J. and Kucera, M. (2019) Depth habitat of the planktonic foraminifera Neogloboquadrina pachyderma in the northern high latitudes explained by sea-ice and chlorophyll concentrations. Biogeosciences, 16, 3425–3437. 10.5194/bg-16-3425-2019. [DOI] [Google Scholar]
- Greco, M., Meilland, J., Zamelczyk, K., Rasmussen, T. L. and Kucera, M. (2020) The effect of an experimental decrease in salinity on the viability of the subarctic planktonic foraminifera Neogloboquadrina incompta. Polar Res., 39, 1–8. [Google Scholar]
- Greco, M., Morard, R. and Kucera, M. (2021) Single-cell metabarcoding reveals biotic interactions of the Arctic calcifier Neogloboquadrina pachyderma with the eukaryotic pelagic community. J. Plankton Res., 43, 113–125. 10.1093/plankt/fbab015. [DOI] [Google Scholar]
- Grigoratou, M., Monteiro, F. M., Ridgwell, A. and Schmidt, D. N. (2021) Investigating the benefits and costs of spines and diet on planktonic foraminifera distribution with a trait-based ecosystem model. Mar. Micropaleontol., 166, 102004. 10.1016/j.marmicro.2021.102004. [DOI] [Google Scholar]
- Grigoratou, M., Monteiro, F. M., Schmidt, D. N., Wilson, J. D., Ward, B. A. and Ridgwell, A. (2019) A trait-based modelling approach to planktonic foraminifera ecology. Biogeosciences, 16, 1469–1492. 10.5194/bg-16-1469-2019. [DOI] [Google Scholar]
- Hedley, R. H., Parry, D. M. and Wakefield, J. S. T. J. (1967) Fine structure of Shepheardella taeniformis (foraminifera: protozoa). J. R. Microsc. Soc., 87, 445–456. 10.1111/j.1365-2818.1967.tb04522.x. [DOI] [Google Scholar]
- Hilbrecht, H. and Thierstein, H. R. (1996) Benthic behavior of planktic foraminifera. Geology, 24, 200–202. . [DOI] [Google Scholar]
- Holzmann, M., Gooday, A. J., Siemensma, F. and Pawlowski, J. (2021) Review: freshwater and soil foraminifera—a story of long-forgotten relatives. J. Foraminifer. Res., 51, 318–331. 10.2113/gsjfr.51.4.318. [DOI] [Google Scholar]
- Ichinohe, R., Shiino, Y. and Kurihara, T. (2018) The passive spatial behaviour and feeding model of living nassellarian radiolarians: morpho-functional insights into radiolarian adaptation. Mar. Micropaleontol., 140, 95–103. 10.1016/j.marmicro.2018.02.002. [DOI] [Google Scholar]
- Ichinohe, R., Shiino, Y., Kurihara, T. and Kishimoto, N. (2019) Active floating with buoyancy of pseudopodia versus passive floating by hydrodynamic drag force: a case study of the flat-shaped spumellarian radiolarian dictyocoryne. Paleontol. Res., 23, 236–244. 10.2517/2018PR023. [DOI] [Google Scholar]
- Keeling, P. J. and del Campo, J. (2017) Marine protists are not just big bacteria. Curr. Biol., 27, R541–R549. 10.1016/j.cub.2017.03.075. [DOI] [PubMed] [Google Scholar]
- Manno, C., Morata, N. and Bellerby, R. (2012) Effect of ocean acidification and temperature increase on the planktonic foraminifer Neogloboquadrina pachyderma (sinistral). Polar Biol., 35, 1311–1319. 10.1007/s00300-012-1174-7. [DOI] [Google Scholar]
- Meilland, J., Ezat, M. M. Westgård, A., Manno, C., Morard, R., Siccha, M. and Kucera, M. (2022) Rare but persistent asexual reproduction explains the success of planktonic foraminifera in polar oceans. J. Plankton Res., 45, 15–32. [Google Scholar]
- Morard, R., Hassenrück, C., Greco, M., Fernandez-Guerra, A., Rigaud, S., Douady, C. J. and Kucera, M. (2022) Renewal of planktonic foraminifera diversity after the cretaceous Paleogene mass extinction by benthic colonizers. Nat. Commun., 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukermans, G., Bach, L. T., Butterley, A., Sun, Q., Claustre, H. and Fournier, G. R. (2023) Quantitative and mechanistic understanding of the open ocean carbonate pump - perspectives for remote sensing and autonomous in situ observation. Earth Sci. Rev., 239, 104359. 10.1016/j.earscirev.2023.104359. [DOI] [Google Scholar]
- Pollock, F. M. and Pickett-Heaps, J. D. (2005) Spatial determinants in morphogenesis: recovery from plasmolysis in the diatom Ditylum. Cell Motil. Cytoskeleton, 60, 71–82. 10.1002/cm.20044. [DOI] [PubMed] [Google Scholar]
- Rebotim, A., Voelker, A. H. L., Jonkers, L., Waniek, J. J., Meggers, H., Schiebel, R., Fraile, I., Schulz, M. et al. (2017) Factors controlling the depth habitat of planktonic foraminifera in the subtropical eastern North Atlantic. Biogeosciences, 14, 827–859. 10.5194/bg-14-827-2017. [DOI] [Google Scholar]
- Schiebel, R., Bijma, J. and Hemleben, C. (1997) Population dynamics of the planktic foraminifer Globigerina bulloides from the eastern North Atlantic. Deep Sea Res. Part Oceanogr. Res. Pap., 44, 1701–1713. 10.1016/S0967-0637(97)00036-8. [DOI] [Google Scholar]
- Schiebel, R. and Hemleben, C. (2017) Planktic Foraminifers in the Modern Ocean. Springer-Verlag, Berlin. 10.1007/978-3-662-50297-6. [DOI] [Google Scholar]
- Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012) NIH image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis, J. L., Welnhofer, E. A. and Orokos, D. D. (2002) Autonomous reorganization of foraminiferan reticulopodia. J. Foraminifer. Res., 32, 425–433. 10.2113/0320425. [DOI] [Google Scholar]
- Tyszka, J., Bickmeyer, U., Raitzsch, M., Bijma, J., Kaczmarek, K., Mewes, A., Topa, P. and Janse, M. (2019) Form and function of F-actin during biomineralization revealed from live experiments on foraminifera. Proc. Natl. Acad. Sci. U S A, 116, 4111–4116. 10.1073/pnas.1810394116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Langen, P. J., Pak, D. K., Spero, H. J. and Lea, D. W. (2005) Effects of temperature on mg/ca in neogloboquadrinid shells determined by live culturing. Geochem. Geophys. Geosyst., 6. 10.1029/2005GC000989. [DOI] [Google Scholar]
- Weinkauf, M. F. G., Siccha, M. and Weiner, A. K. M. (2022) Reproduction dynamics of planktonic microbial eukaryotes in the open ocean. J. R. Soc. Interface, 19, 20210860. 10.1098/rsif.2021.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenburg, J. E., Bijma, J., Cremer, C., Bickmeyer, U. and Zittier, Z. M. C. (2021) Permanent ectoplasmic structures in deep-sea Cibicides and Cibicidoides taxa—long-term observations at in situ pressure. Biogeosciences, 18, 3903–3915. 10.5194/bg-18-3903-2021. [DOI] [Google Scholar]
- Zlatogursky, V. V. (2021) Microtubular cytoskeleton-based cell outgrowths: from pseudopodia to axons and dendrites. Protistology, 15, 195–205. 10.21685/1680-0826-2021-15-4-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Video in supplement to this article is available on FigShare (10.6084/m9.figshare.22665031).


