Abstract
The retinal pigment epithelium-photoreceptor interaction is one of the most studied cell-to-cell interfaces in nature. While photoreceptors use photosensitive pigments to convert light into electrical signals, the RPE supports photoreceptors in their function by phagocytizing shed photoreceptor tips, regulating the blood-retina barrier, and modulating inflammatory responses, as well as regenerating the 11-cis-retinal chromophore via the classical visual cycle. These processes involve multiple protein complexes, tightly regulated ligand-receptors interactions, and a plethora of lipids and protein-lipids interactions. The role of lipids in maintaining a healthy interplay between the RPE and photoreceptors has not been fully delineated. In recent years, novel technologies have resulted in major advancements in understanding several facets of this interplay, including the involvement of lipids in phagocytosis and phagolysosome function, nutrient recycling, and the metabolic dependence between the two cell types. In this review, we aim to integrate the complex role of lipids in photoreceptor and RPE function, emphasizing the dynamic exchange between the cells as well as discuss how these processes are affected in aging and retinal diseases.
Keywords: phospholipids, membranes, docosahexaenoic acid (DHA), polyunsaturated fatty acid (PUFA), retina, rod outer segment, aging
Introduction
Photoreceptor and retinal pigment epithelium (RPE) cells are crucially dependent on tightly controlled lipid homeostasis to maintain cellular function. Their interdependence relies on the constant incorporation and processing of each other’s membranes. Each day, roughly a tenth of the rod outer segment (OS) is shed and phagocytized by the RPE, which is able to repurpose the membranes. This effort is expended to keep the membrane composition, as well as the protein inhabitants, physiologically optimized for the demanding process of vision. The rod OS has a unique organization of membranes that have been studied extensively for decades. Stacked disks pile on top of each other within the rod OS plasma membrane, creating a multi-layered photon-trap of rhodopsin-laden membranes. The effect is to create a highly sensitive system that can accommodate the activity of a para-crystalline membrane protein population (millimolar in concentration) that is both ordered and fluid. The oft-noted balance of order and fluidity maintained by photoreceptors is largely due to the composition of the membranes themselves. Each element plays a role in maintaining this delicate balance, and many will be discussed in this review, emphasizing the biogenesis, transportation, distribution, and metabolism of several vital components.
As noted by Boesze-Battaglia and Schimmel in their seminal review (1997) of membrane composition and distribution, “Membrane protein activity is influenced by the surrounding lipid matrix and specifically by the association between lipids and proteins at the lipid-protein interface.” (Boesze-Battaglia and Schimmel, 1997). Since the publication of that review, more data has confirmed the ability of annular lipids to directly affect the function or stability of membrane proteins and complexes (Bechara et al., 2015; Schmidt et al., 2015). There has also been important work done identifying several “modes” of lipid binding by the Robinson group (Bechara and Robinson, 2015; Bolla et al., 2020). What this progress has yielded, though, is greater nuance of understanding regarding classical theories with respect to the purpose of the lipid components in membranes. It is well established that membrane proteins have varying degrees of contact with the surrounding membrane components. Lipids directly in contact with the transmembrane domains of proteins are said to be ‘restricted’ or non-annular if they do not also exchange with nearby lipids. Such interactions have been shown many times biochemically, and even structurally when lipids have been identified co-crystallizing in binding pockets of some transporters (Gonen et al., 2005; Gulati et al., 2017). Annular lipids form a shell around the protein, and, as discussed above, can have a substantial impact on membrane proteins without binding tightly to the protein itself.
Whether restricted, annular, or bulk, the lipid bilayers of most membranes consist of a few major classes, namely, phospholipids, sterols, and free fatty acids (FFAs). Phospholipids are usually discussed in the context of their charge/polarity; while phosphatidylcholine (PC), phosphatidylserine (PS), and phosphatidylinositol (PI) are all charged, phosphatidylethanolamine (PE) is not. This split makes PC/PS/PI suitable constituents for surfaces requiring good hydration and solubility, while PE-rich membranes can promote membrane fusion events and interfacial interactions between hydrophobic regions. These components are usually segregated to inner and outer leaflets of the plasma membrane, with PC on the outer layer and PE/PI on the inner. The segregation is maintained via an ATP-transporter, and the increased presence of PS on the outer leaflet can signal apoptosis (Devaux, 1991; Mariño and Kroemer, 2013). Work done in the 1990s by Boesze-Battaglia and Albert (thanks to the development of a disk-plasma membrane separation protocol by Molday and Molday five years prior) gave the first indication of what the relative distribution of the four major phospholipids was in rod OS (Boesze-Battaglia and Albert, 1992; Molday and Molday, 1987). Interestingly, the rod OS disks showed a nearly 4-fold higher relative percent of PE than the photoreceptor plasma membrane (42% vs. 11%, roughly). The precise lipid sorting between the two membranes is particularly striking given that nascent disks themselves are made from the closure of plasma membrane invaginations at the base of the OS. (Spencer et al., 2020).
The sterol component of lipid membranes is mostly cholesterol, which helps create space in the membrane. Cholesterol often acts as a foil for the rest of the membrane. In warmer temperatures, the phospholipid components are much more dynamic than the bulky and locked cholesterol, and the fatty acid (FA) tails tend to allow for more movement when warmed. Cholesterol, then, acts as a buffer by retaining some of the rigidity and structure of the membrane. Conversely, when membranes are cooled, the phospholipids are less dynamic and may harm membrane protein function through stiffening of the membrane if not for cholesterol, which is able to maintain space between the contracting portions of the cooled membrane. Cholesterol’s ability to mediate the extremes makes it a vital component of most membranes, including those of the photoreceptors and RPE (Albert and Boesze-Battaglia, 2005).
The effect of the FA chain length and saturation has been studied and reviewed several times, and it is a critical component of the unique environment used by photoreceptors. Docosahexaenoic acid (DHA, 22:6) is vitally important to many systems of the body, including the retina. DHA is incorporated into multiple lipid species and, given the high level of unsaturation, is able to create a very fluid environment in rod OS disks. Here again, Boesze-Battaglia and Albert were able to give the first indication that the disks were able to sort for a completely different profile of lipids, this time by FA chain length (Boesze-Battaglia and Albert, 1989). Of the components they could detect, DHA represented 35% of the isolated disk membranes, while the plasma membrane had about 5%. This was additional evidence that the hyper-specific and dynamic membrane environment of rhodopsin-laden membranes requires, or perhaps selects for, unique components able to facilitate phototransduction.
The structure and biophysical properties of membranes throughout the body are dictated by the composition of lipid building blocks - including both their chemical structures and stoichiometries. Even a single membrane can feature a dramatically different lipidome across its inner and outer leaflet (Lorent et al., 2020). Membrane properties depend on the lipid headgroup charge and size, as well as their acyl chains. These individual attributes affect aggregate membrane attributes such as viscosity, which is known to vary across cellular compartments (Saffman and Delbrück, 1975). Membranes rich in saturated lipids and cholesterol pack tightly and are rigid and ordered, whereas high unsaturation levels yield relatively fluid and more disordered membranes. Polyunsaturated FAs (PUFAs) reduce membrane viscosity but are also highly susceptible to peroxidation by reactive oxygen species (ROS). Some PUFAs, most notably arachidonic acid (AA), can also be used for the synthesis of different signaling lipids, including eicosanoids, docosanoids, and elovanoids. These soluble compounds bind to cellular receptors, initiating important physiological processes ranging from inflammation to fertility (Bazan, 2018; Mouchlis and Dennis, 2019).
Lipids are also a source of energy and secondary signaling, both of which have been thoroughly reviewed elsewhere (Houten et al., 2016; Houtkooper and Vaz, 2008; Ploumi et al., 2017; Schönfeld and Wojtczak, 2016; van der Veen et al., 2017). To enable their transport to the inner mitochondrial membrane carnitine binds FAs, creating acyl-carnitine, where the FA is then subjected to a cycle of beta-oxidation to produce acetyl CoA and NADH/FADH2. Those products are used in the generation of ATP via the electron transport chain. As mentioned, lipids also act as messengers in several essential signaling pathways. Lysophospholipid signaling has been shown to directly affect GPCR signaling in the Gi and Gq pathways (Jelsema, 1987). Phosphatidylinositol 4,5-diphosphate (PIP2) can be cleaved by phospholipase C (PLC) to form two second messengers, diacylglycerol (DAG) and the soluble metabolite 1,4,5-inositol triphosphate (IP3) (Berridge, 2009; Raucher et al., 2000). Signaling through IP3 is essential for the release of intracellular calcium stores, among other duties (Berridge and Irvine, 1984). Of particular note to vision research is the light-dependent production of IP3 in photoreceptors (Das et al., 1987; Fein et al., 1984; Ghalayini and Anderson, 1984; Hayashi and Amakawa, 1985; Millar et al., 1988). PLC activity has been shown to be the major cause of phototransduction in Drosophila melanogaster, but it is uncertain if IP3 or DAG is responsible for transmitting the signal with both having been hypothesized and contradicted in the literature (Ghalayini and Anderson, 1984; Wensel, 2020). The purpose of IP3 production in the photoreceptor of mammals is not yet settled (Yoshioka et al., 1983).
The metabolism, transport, and function of all these membrane components will be discussed in more detail below (Figure 1). To put it briefly, all these processes accomplish a balance in support of phototransduction. The presence of each component relies upon diet, certainly, but also several classes of enzymes, primarily desaturases, elongases, dehydrogenases, and oxygenases. As will be discussed, the composition of photoreceptors and RPE membranes cannot be established without methods of transport through the body via proteins such as high-density and low-density lipoproteins (HDL and LDL, respectively). Transport then becomes a matter of joining the lipids to the target membranes, which is accomplished by several lipoprotein receptors. Other proteins like adiponectin receptor 1 (ADIPOR1) also play an important role in the photoreceptor and RPE lipid homeostasis. Being a regulator for DHA uptake and retention in photoreceptors and RPE, ADIPOR1 is indispensable for the healthy retina function. The rest of this review will address the uptake, synthesis, transport, and function of lipids in the retina and RPE, with a final discussion of the visual pathologies observed in cases of lipid dysregulation. While retinoids are certainly an important class of lipids in this context, we will not focus on them in this review and instead refer the reader to previous reviews (Kiser et al., 2014; Kiser and Palczewski, 2016; Palczewski and Kiser, 2020; Travis et al., 2007).
Figure 1. Lipid environment at the RPE/photoreceptor interface.
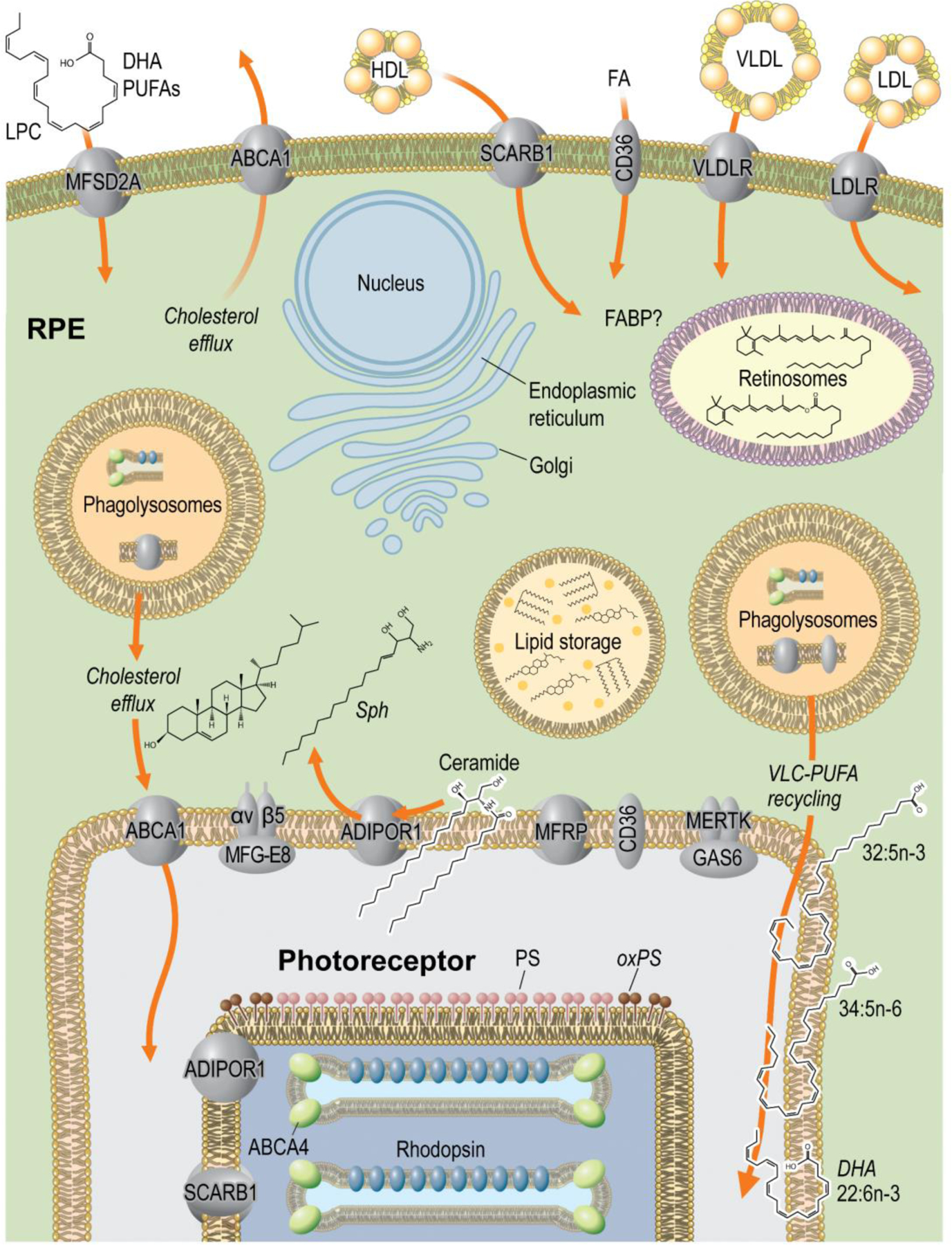
The dynamic interplay between photoreceptors and the RPE enables the efficient and necessary recycling and biogenesis of lipid components. Transporters on the basal side of RPE cells bring in several lipid components from circulation while also allowing for cholesterol efflux. The RPE also accepts lipids from photoreceptors from shedding disk tips, which become phagolysosomes in the RPE. The RPE uses both sources of lipid precursors to produced vital components of photoreceptor membranes, which are then transported back to photoreceptors for their incorporation and support of phototransduction.
I -. Role of lipids in the photoreceptors and RPE
I.1. Lipids as structural components
I.1.1. Rod outer segment membranes
The rod OS consists predominantly of glycerophospholipids (>90% by weight), cholesterol, and glycolipids. The phospholipids are dominated by PC (∼30–40 mol%), PE (∼30–40 mol%), PS (∼10–12 mol%), and PI (∼1–2 mol%). In addition, non-sialylated sphingolipids and gangliosides comprise about 1 mol% each, and cholesterol accounts for ∼10 mol% of the total OS lipids (Boesze-Battaglia et al., 1989; Fliesler and Schroepfer, 1982). The retina as a whole contains 6% to 7% FAs that are N-linked to sphingosine, or roughly 11–13 mole% in comparison to phospholipids (Brush et al., 2010). Interestingly, OS membranes consist of an unusually high level of LC-PUFAs, especially DHA, which can represent up to 50% of the total acyl groups (Anderson and Maude, 1970; Aveldano and Bazan, 1983; Goldberg et al., 2016).
The rod OS plasma and disk membranes each have diverse functions that differ from one another, a fact reflected in their distinct lipid compositions. The disk membrane is the site of light absorption and phototransduction, whereas the plasma membrane primarily maintains appropriate ion permeability and hyperpolarization under dark and light conditions, respectively (Albert et al., 2016). As a cell limiting barrier, rod OS plasma membranes possess significantly higher rigidity/lower permeability than the disk membranes due to higher levels of saturated FA species, cholesterol, and PC. OS membranes also contain approximately twice as much PS as disks or typical mammalian plasma membranes (Boesze-Battaglia and Albert, 1992; Van Meer et al., 2008). The constitution of the rod OS plasma membrane gives it a high surface charge. It was postulated that increased PS exposure preceding rod shedding promotes rod OS tip phagocytosis by RPE (Ruggiero et al., 2012). PS also regulates Müller glia responses after retina injury by initiating phagocytosis, proliferation, and gliotic responses (Nomura-Komoike et al., 2020).
I.1.2. Disk OS membranes
Similar to other eukaryotic cell membranes, rod OS disks possess interleaflet phospholipid asymmetry, displaying elevated concentrations of PS on their outer/cytosolic leaflets (Hessel et al., 2000; Wu and Hubbell, 1993). One particular study reported that at least 73% of PS and PE are located in the cytosolic leaflet of disk membranes (Miljanich et al., 1981). Rhodopsin can preferentially bind to specific lipid species, thereby influencing membrane asymmetry (Palczewski, 2012). For instance, rhodopsin activation can change PS distribution between the intradiscal and cytosolic membrane leaflets (Hessel et al., 2001). Rhodopsin is localized in the center of rod OS disks and is surrounded by a protein-free lipid ring, which separates rhodopsin from disk rim composed of ATP-binding cassette protein, family A, 4 (ABCA4), rod outer membrane protein 1(ROM-1), and peripherin 2 (PRPH2) proteins (Buzhynskyy et al., 2011).
I.1.3. Compartmentalization of lipids and FAs in photoreceptor disks
A recent report from our groups has further distinguished the major membrane domains of the rod OS disks (Sander et al., 2021). Prior work by Falk and Fatt several decades ago on the ultra-structure of rod OS membranes showed that the outer rim region of rod OS disks resist disruption after OsO4 fixation (Falk and Fatt, 1969). Their work indicated that membranes in the rim region are distinct from the disk center, but concrete evidence in support of that idea was still lacking. We chose to study this difference more closely because the differences in membrane components between the rim and lamellar domains of rod OS disks could provide critical information regarding protein-lipid interactions supporting phototransduction. One question of particular interest was whether two forms of Stargardt disease (Table 1), namely STGD1 and STGD3, shared a common molecular etiology. STGD1 is caused by mutations in ABCA4, known as a lipid and retinoid flippase, and mutations in ABCA4 can lead to the accumulation of toxic levels of free retinal and bis-retinoids, later causing lipofuscin deposits to form and the RPE to deteriorate. However, STGD3 is caused by mutations in the elongation of very long FAs 4 (ELOVL4), an enzyme that elongates long-chain PUFAs (LC-PUFAs) into very long-chain PUFAs (VLC-PUFAs). The question naturally arises as to whether ABCA4 may have necessary interactions with VLC-PUFAs when functioning properly. If so, the two disease types could be tied together by the common element of ABCA4 dysregulation. VLC-PUFAs are relatively rare FAs, so their presence in rod OS disks has been studied for many decades. One such study found that VLC-PUFAs copurified with rhodopsin when both were extracted in hexanes, suggesting that the VLC-PUFAs may have a role in the center of rod OS disks as well (Aveldano, 1988). In turn, work by Hopiavuori et al. hypothesized that the saturated version of VLC-FAs (VLC-SFAs) may stabilize curved regions of membranes by reaching across the bilayer and forming enhanced van der Waals interactions that could then modulate the synaptic release of vesicles (Hopiavuori et al., 2019). Because the rod OS disk rim is a bulbous, curved structure, we initially hypothesized that a similar mechanism may be at work in stabilizing the disk rim. Until the advent of styrene maleic acid (SMA), however, there was little hope of resolving if VLC-PUFAs resided in a particular domain of rod OS disks.
Table 1.
Eye disorders associated with lipid metabolism.
| Disease | Genomic mutation | Lipids families affected | Phenotype | References | |
|---|---|---|---|---|---|
| Age-related macular degeneration (AMD) | genetic associations: APOE2, CETP, LIPC, CFH, ABCR, IGF1 | Lipid accumulation, lipid oxidation, drusen | Reduced light sensitivity or contrast sensitivity is the primary visual complaint, while in advanced disease, visual acuity loss is the main complaint. | (Ratnapriya and Chew, 2013) (Fletcher et al., 2014) (Rivera et al., 2000) (Jun et al., 2019) (Arroba et al., 2018) (Skowronska-Krawczyk and Chao, 2019) |
|
| Bestrophinopathy | BEST1 | increase in unesterified cholesterol | Abnormal accumulation of autofluorescent material within RPE cells and bilateral macular or multifocal lesions. | (Guziewicz et al., 2017) | |
| Bietti Crystalline dystrophy | CYP4V2 | High levels of triglycerides and cholesterol storage, decreased metabolism of FA precursors into n-3 PUFAs | Multiple glistening intraretinal crystals scattered over the fundus, a characteristic degeneration of the retina, and sclerosis of the choroidal vessels, ultimately resulting in progressive night blindness and constriction of the visual field. | (Li et al., 2004) (Lee et al., 2001) |
|
| Farber’s disease | ASAH1 | Increased level of ceramide was found in retinas | Retinal impairment and vision loss | (Sugita et al., 1972) (Koch et al., 1996) (Yu et al., 2019) |
|
| Gaucher disease | L444P | Accumulation of glucosylsphingosine | Tapeto-retinal degeneration, pigmented retinal lesions, intra-retinal white dots and inner retinal Gaucher cells. | (Winter et al., 2019) | |
| Macular telangiectasia 2 |
SPTLC1
SPTLC2 PHGDH |
Elevated deoxysphingolipid levels in circulation and RPE | Gradual loss of central vision. | (Gantner et al., 2019) (Fallon et al., 2018) (Eade et al., 2021) |
|
| Niemann-Pick disease (NPD) |
ASM (SMPD1)-Types A, B NPC1, NPC2-Type C |
Accumulation of sphingomyelin (ceramide-phosphocholine), phospholipid, bis(monoacylglycero)phosphate (BMP), lysosphingomyelin (sphingosine phosphocholine) cholesterol, glucocerebroside, lactosylceramide, and gangliosides | Types A-cherry red macula Types B-retinal stigmata Type C- retinal axonal degeneration |
(Schuchman and Desnick, 2017) (McGovern et al., 2004) (Vanier, 2015) (Havla et al., 2020) |
|
| Retinitis pigmentosa | changes in any one of more than 70 genes | Decreased retinal dolichol, hyperlipidemia or hypolipidemia, reductions in DHA, and differences in AA levels in the plasma | Night blindness and progressive vision loss. Progressive loss of rod photoreceptor cells, followed by loss of cone photoreceptor cells. | (McColl and Converse, 1995) (Ramachandra Rao et al., 2020b) |
|
| Bassen-Kornzweig syndrome (abetalipoproteinemia: MTTP | Low cholesterol and triglyceride levels; absence of low-density lipoprotein, a deficiency in fat-soluble vitamins and essential FAs | Retinal degeneration, hearing impairment, renal cysts and hepatomegaly. Untreated individuals may develop atypical retina pigmentation that may present with progressive loss of night vision and/or color vision in adulthood. Neuromuscular findings in untreated individuals, including progressive loss of deep tendon reflexes, vibratory sense, and proprioception; muscle weakness; dysarthria; and ataxia typically manifest in the first or second decades of life. | (McColl and Converse, 1995) (Kayden, 1972) |
||
| Batten’s disease: CLN3 | Neuronal ceroid lipofuscinoses | Affects the nervous system with increasing seizures, movement disorders, altered thought processes, and cognitive decline. Childhood neuronal ceroid lipofuscinoses include vision loss but adult-onset forms of the disease typically do not. | (Lerner et al., 1995) | ||
| Refsum’s disease: PHYH or PEX7 |
Deficiency in phytanic acid α-oxidation. As a result, toxic levels of phytanic acid build up in the brain, blood, and other tissues. | Night blindness, with eventual weakness in arms and legs or unsteadiness (cerebellar ataxia). Other common symptoms include a loss of sense of smell (anosmia), rough, scaly skin (ichthyosis) and after many years, deafness. | (van den Brink et al., 2003) (Jansen et al., 1997) |
||
| RP59: Dhdds |
Decreased retina dolichol levels | Night and peripheral vision loss, constriction of visual fields, and retinal degeneration | (Zelinger et al., 2011) (Ramachandra Rao et al., 2020b) |
||
| RP26: CERKL |
Decreased spgingolipids | photoreceptors neurodegeneration and progressive vision loss | (Tuson et al., 2004) (Mirra et al., 2021) |
||
| Peroxisomal diseases (Zellweger syndrome) : |
PEX1
PEX5 PEX13 PEX26 |
The predominant VLC-PUFAs present in Zellweger brains are penta- and hexaenoic acids, whereas a normal brain contains C32 to C38 tetra- and pentaenoic acids. Zellweger brains also contained trace amounts of C40 n-6 VLC-PUFAs, which are absent in normal brains. | Psychomotor delay, dysmorphia, neonatal seizures, retinopathy, cataracts and hearing loss. | (FitzPatrick, 1996) (Ebberink et al., 2011) (Poulos et al., 1988) |
|
| PEX1 | Elevated C26:0 lyso-PC, 18: 2 n6 and 20: 3 n6, and very low levels of 22: 6 n3, 22: 5 n3, 22: 5 n6 in retina. Elevated C26:0 and higher ratio of C24:0/C22:0 and C26:0/C22:0 in plasma | Progressive retinopathy leading to blindness. | (Argyriou et al., 2019) (Martinez, 1992) |
||
| Usher syndrome: Usher 1B MYO7A Usher 1C USH1C Usher 1D CDH23 Usher 1F PCDH15 Usher 1G USH1G |
Lower blood levels of long-chain polyunsaturated FAs (PUFAs). | Partial or total hearing loss and vision loss that worsens over time. Vision loss occurs as the light-sensing cells of the retina gradually deteriorate (RP). | (Geng et al., 2009) (Geng et al., 2009) (Maude et al., 1998) (Géléoc and El-Amraoui, 2020) |
||
| Sandhoff disease | HEXB | Deficient degradation of glycolipids, accumulation of GM2 ganglioside | The infantile forms are characterized by progressive muscular weakness, mental retardation, blindness, and death in early childhood | (Mahuran, 1999) | |
| Smith-Lemli-Opitz syndrome (SLOS) | DHCR7 | Depletion of cholesterol, abnormal accumulation of 7-dehydrocholesterol Deceased DHA in rod OS |
Progressive retinal degeneration. | (Boesze-Battaglia et al., 2008) (Tulenko et al., 2006) |
|
| Stargardt-like macular dystrophy (STGD1) | ABCA4 | Accumulation of bis(monoacylgylercoro)phosphate lipids, bisretinoid and lipofuscin | Juvenile macular degeneration - reduced light sensitivity or contrast sensitivity and gradual loss of central vision. | (Anderson et al., 2017) (Zhao et al., 2021) (Cideciyan et al., 2004) |
|
| Stargardt-like macular dystrophy (STGD3) | ELOVL4 | Reduced levels of C28–C38 VLC-PUFAs | Juvenile macular degeneration - reduced light sensitivity or contrast sensitivity and gradual loss of central vision. | (Bernstein et al., 2001) (McMahon et al., 2007) (Zhang et al., 2001) (Agbaga et al., 2008) |
|
SMA has rapidly become a popular agent for nanodisc creation from native membranes. When SMA is mixed with membranes, it forms SMA lipid particles (SMALPs) roughly 10–12 nm in diameter. Prior to our study, this method of proteoliposome nanodisc extraction had yet to be proven as a way to copurify native lipids with target membrane proteins of interest. To this end, there were many prior studies trying to address the lipid exchange dynamics of polymer-bound lipid nanodiscs (Cuevas Arenas et al., 2017; Danielczak and Keller, 2018; Schmidt and Sturgis, 2018). Those earlier studies of SMALPs and diisobutylene maleic acid lipid particles (DIBMALPs) showed that phospholipids can exchange more rapidly at ambient temperatures (i.e., 20–30 °C) as compared to those prepared in large unilamellar vesicles (LUVs) or membrane scaffold protein (MSP) nanodiscs. The findings suggested that native membrane proteins, once extracted by SMA, might reside in a lipid environment that reflects the average lipid environment of the extracted tissue, not the domain from which it came. However, more recent data provided evidence that SMALPs of various bacterial proteins formed and isolated under lower temperature conditions (≤ 4 °C) have distinct lipid profiles (Teo et al., 2019), indicating the native local membrane environment composition is retained in samples prepared in this manner. With the addition of our manuscript, there have been no reports of lipid swapping in SMALP-protein nanodiscs. We further validated that the SMALP method could isolate distinct domains from within continuous membranes extracted by leveraging the known localizations of 3 proteins from the rod OS disk. We not only checked for differences between rhodopsin (representing the center of the disks) and ABCA4 (which is found solely on the rim), but added the PRPH2/ROM1 complex, which helps maintain the disk rim curvature and is segregated to the rim region with ABCA4. We hypothesized that native distinctions between the center and rim samples should be more common than those between the rim samples. Our results confirmed this hypothesis, with over 20 statistically significant instances of the rim sample lipid species differing from the rhodopsin samples.
We isolated the central lamellar and rim regions of the disks by immunopurifying integral membrane proteins in SMALPs, and the copurifying lipids from each sample were extracted and analyzed for differences. We found systematic differences between the two rim samples (ABCA4 and PRPH2/ROM1) and the center samples (rhodopsin), the most striking being the proclivity of the rim to have short, saturated FAs (Figure 2). The center contained the vast majority of the LC- and VLC-PUFAs, including DHA. This finding helped clarify the outstanding question as to where VLC-PUFAs were having their effect in rod OS disks, near rhodopsin or perhaps primarily interacting with ABCA4 on the rim. We showed that VLC-PUFAs are nearly undetectable in ABCA4 and PRPH2/ROM1 native nanodiscs, lending credence that VLF-PUFAs exert the majority of their influence in the center of rod OS disks, likely interacting with rhodopsin.
Figure 2. Rod OS disks have regionally distinct microenvironments.
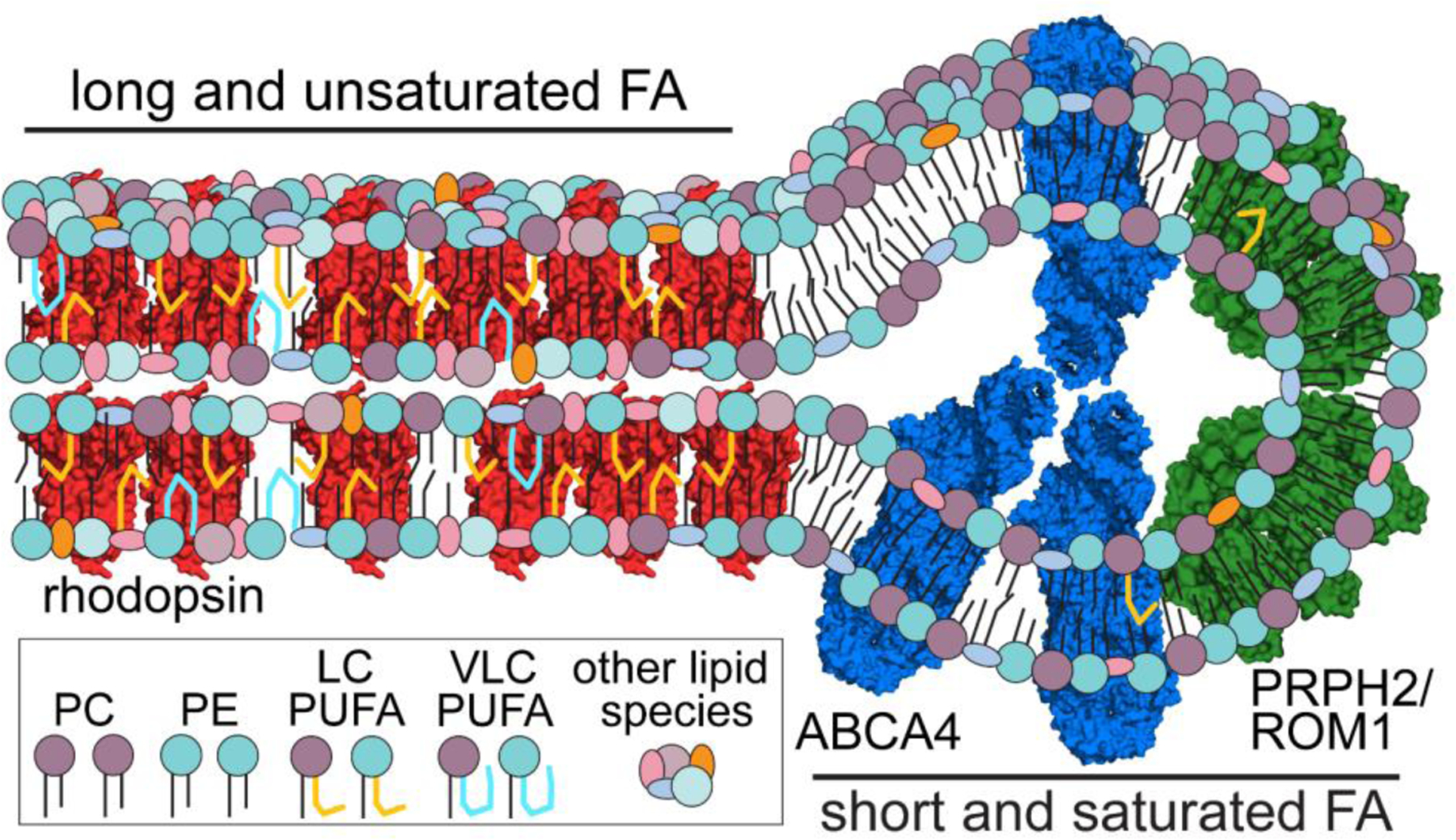
The central region of rod OS disks, rich in rhodopsin (red, PDB: 1F88), has an abundance of long and unsaturated FAs. Rim regions of rod OS disks containing ABCA4 (blue, PDB: 7LKP) and PRPH2/ROM1 (tetramer of human tetraspanin CD81 used as a model in green, PDB: 5TCX) have relatively high amounts of short and saturated FAs. There are many other distinctions in lipid species between the two regions, including relative amounts of PC and PE. ©2021 Sander et al. Adapted from an article originally published in the Journal of Cell Biology. https://doi.org/10.1083/jcb.202101063
The described lipid compartmentalization in the disk suggests the existence of a sorting mechanism that guarantees correct lipid distribution. This likely happens during the disk formation, as specific proteins assume their particular localization very early during the process (H. Y. Chen et al., 2021). One of the possibilities is that vesicles harboring disk proteins during disk formation/regeneration are already enriched in the population of lipids characteristic for each protein. This would suggest that lipid composition of the center and rim is established directly by protein composition. This hypothesis is partially supported by studies highlighting the role of PRPH2 in the initial disc formation and enclosure (Lewis et al., 2021; Salinas et al., 2017). Recent studies reinforced the role of proteins themselves being responsible for creating and maintaining the rim curvature (Pöge et al., 2021) further emphasizing the protein-dependent lipid composition of membrane microenvironments. Interestingly, it may suggest that disk shape and size might be regulated by the protein availability, i.e. high expression of rhodopsin is correlated with low expression of PRPH2 in order to allow growing of the flat center of the disc. How this process is regulated has yet to be explained. The most probable explanation is that proteins occupying the subcompartments of the disk are expressed in their correct proportions, possibly by a regulated feedback mechanism allowing proper daily photoreceptor regeneration. In addition, release of protein/lipid vesicles from the Golgi is likely dependent on FA availability, allowing another level of regulation of the disc formation.
I.1.4. Rod OS cholesterol gradient
The lipid composition of the disk changes during disk transport from the rod OS basal to apical side, enabling functional regulation without affecting the protein composition. For instance, rhodopsin activation is affected by the lipid composition. In addition to the aforementioned lipid asymmetry across disk membrane leaflets, there are reports on changes in cholesterol and sphingomyelin concentrations from the basal to apical rod OS disks.
Cholesterol has a dual role in maintaining the cell membranes’ function by changing the lipid bilayer’s fundamental properties through interacting with phospholipids, as well as directly interacting with specific membrane proteins (Yeagle, 1991). Cholesterol increases the bilayer thickness and reduces the membrane permeability to small uncharged polar molecules and to ions (Jedlovszky and Mezei, 2003). It is suggested that the effective sealing of the plasma membrane with cholesterol against sodium ion leakage is an important factor in maintaining membrane hyperpolarization (Albert and Boesze-Battaglia, 2005). In the basal disks, there are cholesterol-rich, particle-free patches that appear to exclude rhodopsin. These patches had reduced prevalence in distal disks, reflecting a basal-to-distal cholesterol gradient (Andrews and Cohen, 1983, 1979). They contained concentrated cholesterol and sphingomyelin, which were shown to spontaneously segregate into ordered membrane phases called lipid rafts (Simons and Ikonen, 1997). The rod OS plasma membrane has similar cholesterol content to other cell membranes and the newly synthesized disks, which is ~30 mol%. However, a significant reduction in the cholesterol content is observed as the disks age, decreasing from ~30 mol% (basal) to ~5 mol% (distal) (Boesze-Battaglia et al., 1990, 1989).
Cholesterol has a higher affinity to saturated fatty acyl chains than unsaturated ones. The phospholipid species favored by cholesterol are primarily sphingomyelin, then PS, PC, and lastly PE (Yeagle and Young, 1986); and it is suggested that heterogeneous distribution of cholesterol in disk membranes, but also other membranes, is determined by phospholipid fatty acyl composition (Albert and Boesze-Battaglia, 2005). As the disks age during apical displacement, the phospholipid acyl chain unsaturation increases, creating unfavorable conditions for the cholesterol, which leads to decrease in its concentration in the disk membranes. The most notable alteration occurs within the PC fatty acyl chains, where the abundance of saturated palmitic (16:0) FA drops markedly, while the highly unsaturated DHA content doubles as disks age. On the other hand, PE, PS, and PI do not show significant changes in acyl chain composition, and the headgroups structure of the major phospholipid species stays largely unchanged during disk maturation (Albert et al., 2016). Cholesterol directly interacts with rhodopsin. The postulated function of the high cholesterol content in the newly synthesized disks is to stabilize rhodopsin by decreasing the partial free volume in the hydrocarbon core of the bilayer, which inhibits rhodopsin activation. As disks are apically displaced, there is a decrease in cholesterol but an increase in unsaturated FA concentrations favored by rhodopsin and resulting in its higher activity (Albert et al., 2016; Polozova and Litman, 2000).
I.1.5. DHA in photoreceptors
DHA is the most abundant polyunsaturated FA in the mammalian body. In the human retina, DHA constitutes over 15% of total FAs and is the most abundant FA in rod OS (50–70% of FAs) (Bretillon et al., 2008; Fliesler and Anderson, 1983; Makrides et al., 1994). Seminal studies using dietary manipulation discovered the key role of DHA in visual function and established direct dependence of the amount of DHA precursors on DHA levels in the membranes and impact on electroretinogram responses (Benolken et al., 1973; Landis et al., 1973) setting the stage for studies trying to understand the mechanistic and biological role of DHA in the eye. The importance of DHA in vision has been further confirmed in many organisms, notably in non-human primates (Lin et al., 1994; Reisbick et al., 1997) and in preterm and term infants (Birch et al., 1992; Uauy et al., 1992). It has been also found and confirmed by several laboratories that in situations of low DHA availability, this FA is recycled between the RPE and photoreceptors ((Stinson et al., 1991) and see chapter II), again putting DHA in the center of interest.
On the metabolic level, 24:6n-3, a molecule that is converted to DHA, is the shortest FA that undergoes beta oxidation in peroxisomes. At the same time, due to the enzyme specificity, DHA cannot be submitted to further beta-oxidation in peroxisomes and is quickly transported out and involved in further steps of lipids synthesis in the cell. Interestingly, DHA have never been found to be bound to carnitine for mitochondrial beta-oxidation which suggests another level of protection of this FA on the cellular level. Finally, the ratio of DHA to VLC-PUFA has to be tightly regulated, since both pathways use the same ELOVL2 product – 24:6n-3. It would be interesting to decipher this efficient mechanism of 24:6n-3 transport to peroxisomes in order to produce DHA and how modulate the pathway in order to produce more VLC-PUFAs in the cell.
On the biophysical, DHA is a particular molecule. With twelve, out of twenty-two, carbons involved in six double-bonds is the most unsaturated FA in human body. The double bond between two carbons, although by itself providing very rigid structure, allows the adjacent single bond bound carbon to change position with lower energy than if bound to the carbon involved with single bond bound carbon. Therefore, the flexible DHA chain easily changes the conformation, to efficiently support dynamic changes in structure of immersed receptors, channels and other membrane proteins. Of note, with highly abundant DHA levels, retina might be the only tissue in the body that contains double-PFA lipids in their membranes, quality that most probably make the rods disk membranes the most fluid membranes in the body. Our recent finding of the rhodopsin being associated with PUFAs in the disks is well aligned with series of studies showing the increased rhodopsin activity in artificial liposomes enriched with DHA containing phospholipids (Bush et al., 1991; Mitchell et al., 2001). It has been also shown that with concentration of PUFAs increasing towards apical part of rod OS (Albert et al., 1998), rhodopsin activity increases (Williams and Penn, 1985). Finally, several studies using animal models, have shown importance of DHA transporters (such as MFSD2A (Lobanova et al., 2019)) and enzymes involved in PUFA-containing-lipids biogenesis (for example ELOVL2, AGPAT3 (Chen et al., 2020; Shindou et al., 2017) position depends on age, disease state and diet, it is expected that rhodopsin activity in photoreceptor membranes changes as well. This intriguing possibility needs yet to be addressed experimentally.
I.1.6. Lipid membranes microenvironments.
Small cholesterol- and sphingolipid-enriched membrane microdomains are organized into platforms and are resistant to extraction with nonionic detergents (detergent-resistant membrane fraction; DRM). They are involved in compartmentalization and regulation of vital cellular processes (Pike, 2006; Van Meer et al., 2008). Certain lipids interact more favorably with each other than with other lipids because of various chemical and geometric features. The most relevant interactions exist between saturated lipids, sphingolipids, and sterols, which preferentially interact with each other rather than highly unsaturated lipids (Almeida, 2009; Levental et al., 2020). These preferential interactions between lipids generate domains that can drive the sorting of membrane proteins based on their transmembrane domains or lipid moiety modifications (Lorent and Levental, 2015). Lipid domains support membrane-based signaling and other processes by assembling all of the necessary protein and lipid components (Snead and Gladfelter, 2019).
Rhodopsin shows high affinity for specific lipid microenvironments upon light-dependent activation and binding to the G protein transducin. This quality of rhodopsin is dependent on its dimerization, as well as the presence of attached palmitoyl moieties, while monomeric or depalmitoylated rhodopsin favored different domains (Seno and Hayashi, 2017). This and other findings indicate that palmitoylation of many GPCRs plays a role in their compartmentalization (Kaneshige et al., 2020; Kobe et al., 2008; Zheng et al., 2012). Similarly, arrestin and a truncated form of transducin were mainly present in the detergent-soluble membrane fraction in the dark but, upon light exposure, were recruited to detergent-resistant lipid microenvironments (Perdomo and Bubis, 2020). The above reports show the importance of lipid membrane fluidity in regulating signalling pathways and underlies the potential role of liquid-liquid phase separation as a molecular mechanism(Su et al., 2021). It is yet to be discovered whether this biophysical process is involved in regulation of rhodopsin localization and activity.
I.2. Lipids as signaling molecules
Extracellular signals can drive hydrolysis of membrane lipids into lipid second messengers that intiate various downstream effects. Glycerolipid-derived signaling molecules include lysophospholipids, PI phosphates (PIPs), DAG, phosphatidic acid (PA), and FFAs (like AA). AA is the precursor of the signaling eicosanoids and endocannabinoids, and DHA is a substrate for the generation of docosanoids. PIP2 can be further hydrolyzed to generate water-soluble IP3 and DAG or phosphorylated to produce another essential second messenger, phosphatidylinositol (3,4,5)-trisphosphate (PIP3). The sphingolipid-derived signaling molecules include ceramide, sphingosine, and their phosphorylated forms - S1P and ceramide-1-phosphate (C1P).
I.2.1. Sphingolipids
Sphingolipids are a large family of lipids that are critical building blocks of cell membranes, playing important roles in regulating its barrier function, permeability, and fluidity. They also regulate various biological processes such as cell growth, proliferation, death, and survival (Hannun and Obeid, 2008; Simón et al., 2019; Wang and Bieberich, 2018). Sphingolipids constitute roughly 11–13 mol% of the total lipids in the bovine retina, making them the third most abundant type of lipid in the retina after phospholipids and sterols (Brush et al., 2010; Fliesler and Anderson, 1983). Sphingomyelin is the most abundant type of sphingolipid, amounting to about 2.5 mol%, whereas ceramide and glucosylceramide constitute less than 1 mol% of the total lipids.
Ceramide signaling in the retina
Ceramides are considered the base units for sphingolipids and the central hub in the sphingolipid biosynthesis pathways (Harrison et al., 2018; Kanehisa and Goto, 2000). They are comprised of a FA of variable chain length bound to an amino group of sphingosine or other sphingoid bases (Gault et al., 2010). C16-C24 ceramides, which are most common, have very low water solubility; they are one of the least polar and most hydrophobic lipids in biological membranes (Castro et al., 2014). In addition to their structural role, ceramides are potent secondary signaling molecules that activate a diverse set of kinases and phosphatases. Increasing evidence suggests that ceramides can also control photoreceptor cell death decisions by activating the mitochondrial apoptotic pathway, subsequently activating the caspase cascade. This can increase calcium concentration in mitochondria and the cytosol that results in calpain-mediated apoptosis. Ceramides were also shown to trigger photoreceptor death in a caspase-independent manner through the generation of ROS, increase in mitochondrial permeability, activation of Poly(ADP-Ribose) Polymerase 1 (PARP-1) and calpain, accumulation of poly(ADP-ribose) polymers, and nuclear translocation of AIF, all of which are characteristic of the Parthanatos death pathway. Moreover, the inhibition of PARP-1 and calpain activity protected photoreceptors from ceramide-mediated death in a study done after PARP-1 enzyme activity was established as an important contributor in both rd1 and rd2 retinal degeneration mouse models (Prado Spalm et al., 2019; Sahaboglu et al., 2017, 2016). PARP-1 is also reciprocally regulated by calcium, which is one of the mediators of cell death (Bürkle and Virág, 2013; Virág et al., 1998).
Ceramide phosphorylation by ceramide kinase (CERK) leads to the generation of C1P, a sphingolipid with pleiotropic bioactivity (Bornancin, 2011). C1P is synthesized in the trans Golgi and then transported to the plasma membrane to be released for auto- or paracrine signaling (Simanshu et al., 2013). C1P stimulates proliferation, differentiation, and survival of photoreceptor progenitors in vivo, enhancing opsin and PRPH2 expression and promoting the development of OS (Miranda et al., 2011).
Increasing evidence suggests that ceramides are engaged in the onset of retina degeneration in mammals. While studying a rabbit model of retinal detachment, one study found that retinal apoptosis during induced retinal detachment is associated with in vivo production of ceramides (Ranty et al., 2009). Accumulation of ceramides due to deficient acid ceramidase ASAH1 in Farber’s disease leads to retinal dysplasia, inflammation, and rod photoreceptor dysfunction resulting in severe visual impairment (Yu et al., 2019). Many studies indicate that ceramide accumulation leads to retina degeneration, but overexpressing ceramidases or inhibiting ceramide generation pathways can rescue photoreceptor cells and prevent vision loss (Acharya et al., 2008, 2003; Fabiani et al., 2017; German et al., 2006; Piano et al., 2013; Sanvicens and Cotter, 2006; Stiles et al., 2016).
Ceramide imbalance is detrimental for the RPE
One of the critical functions of the RPE is trafficking and sorting cargo originating from the extracellular environment and plasma membrane using endosomes. Endosomes are derived from the RPE plasma membrane; therefore, the plasma membrane composition determines the number and size of nascent endosomes. Excessive accumulation of ceramides in the apical RPE membrane was recognized to play a role in macula diseases, such as age-related macular degeneration (AMD) and STGD (German et al., 2006; Tan et al., 2020; Victoria Simon et al., 2021; Zhu et al., 2010). Kaur and colleagues showed that increased ceramide levels promoted inward budding and fusion of early endosomes in the RPE (Kaur et al., 2018), but lowering ceramide levels with desipramine corrected these endosomal defects in a Stargardt’s disease mouse model (Kaur et al., 2018).
In diabetic retinopathy, acid sphingomyelinase (ASMase, encoded by SMPD1) stimulation by high glucose and subsequent increase in ceramide levels have been shown to cause pathological alterations in RPE cells (Wang et al., 2016). Cholesterol-mediated activation of ASMase also disrupted autophagosome traffic and autophagic flux in the RPE due to ceramide-promoted tubulin acetylation and stiffening of microtubules (Toops et al., 2015). Furthermore, excessive neutral sphingomyelinase activity decreased RPE cells proliferation and increased apoptosis of ARPE-19 cells (Zhu et al., 2010). Another study shows that the addition of membrane-permeable ceramide C2 induced apoptosis-like cell death in the cultured human and rat RPE cells by increasing ROS production, mitochondrial membrane permeabilization, and caspase-3 activation (Kannan et al., 2004; Tomita et al., 2000).
Sphingosine and sphingosine-1-phosphate
Sphingosine is another secondary messenger, potently generated by apoptotic stimuli, that can induce photoreceptor cell death through mitochondrial membrane permeabilization and ROS formation (Rotstein et al., 2010). Reducing sphingosine generation or stimulating its phosphorylation to S1P prevents photoreceptor demise, emphasizing the role of sphingolipids in photoreceptor survival (Simón et al., 2019).
Sphingosine is phosphorylated to S1P through the action of two sphingosine kinases - SphK1 and SphK2. The local concentration of S1P in tissues depends on the balance between its synthesis and degradation. The main enzymes that degrade S1P are S1P lyases and S1P phosphatases (SPPs). SPPs have two isoforms, SPP1 and SPP2, which catalyze the dephosphorylation of S1P, generating sphingosine (Sph) that is then converted to ceramide by ceramide synthases (CERS1–6) (Levy and Futerman, 2010; Pyne et al., 2009) S1P lyase (SGPL1) irreversibly degrades S1P to hexadecenal and ethanolamine-1-phosphate, removing S1P from the process of sphingolipid metabolism (Bandhuvula and Saba, 2007).
S1P emerges as a mediator of photoreceptor development and survival, preventing photoreceptors from oxidative stress (Fabiani et al., 2017; Fang et al., 2018; Miranda et al., 2009). It was shown that cytokines, DHA, glial cell-derived neurotrophic factor, and growth factors such as transforming growth factor b and nerve growth factor stimulate the expression of SphK1, resulting in an increase of S1P that acts as a secondary messenger critical for photoreceptor neurogenesis and survival (Abrahan et al., 2010; Porter et al., 2018). In addition to its pro-survival effect, S1P also potently stimulates angiogenesis and inflammation. Overexpression of SphK2 increases S1P levels, resulting in accelerated retinal angiogenesis and increased neovascularization. Downregulation of S1P in SphK2 KO conditions reverses this effect (Eresch et al., 2018). S1P also promotes the recruitment of immune cells and retention of lymphocytes in inflamed tissues (Aoki et al., 2016). Thus, S1P action through S1P receptors is a driving force in the onset and progression of inflammation and angiogenesis in retinal inflammatory diseases (Simón et al., 2019). Following retinal injury, S1P promotes gliosis that alters the retinal structure, thereby enhancing visual dysfunction instead of preventing it (Lukowski et al., 2013; Swaney et al., 2008).
Ceramides and S1P appear to have opposite cellular roles; while ceramides induce cell cycle arrest or death, S1P promotes cell proliferation and survival (Cuvillier et al., 1996; Gomez-Munoz et al., 1995). The metabolic interconnection of ceramides, C1P, Sph, and S1P, demonstrates how altering the balance of these mediators can affect cell fate. Enzymes interconverting sphingosine and ceramides are located close to each other in the plasma membrane (Gault et al., 2010). The proximity of these enzymes in the plasma membrane suggests that tight regulation of the formation and breakdown of these sphingolipids is critical. The balance between them often referred to as the “sphingolipid rheostat,” can profoundly affect cell death or survival and differentiation (Hait et al., 2006; Lewis et al., 2018; Newton et al., 2015). Understanding how these pathways can be manipulated could lead to novel therapies for treating and preventing different retinal diseases, independent of the underlying etiology.
I.2.2. Signaling of glycerophospholipids
Glycerophospholipids are composed of a glycerol backbone, phosphate group, and two (or less frequently one) esterified FA group (Figure 3A). They account for about 90% (wt/wt) of the total lipids found in rod OS membranes (Fliesler and Anderson, 1983). Besides being a building block of cell membranes, they often act as cellular signal transducers to trigger intracellular signaling events (Van Meer et al., 2008).
Figure 3. Major glycerophospholipids – synthesis and potential signaling applications.
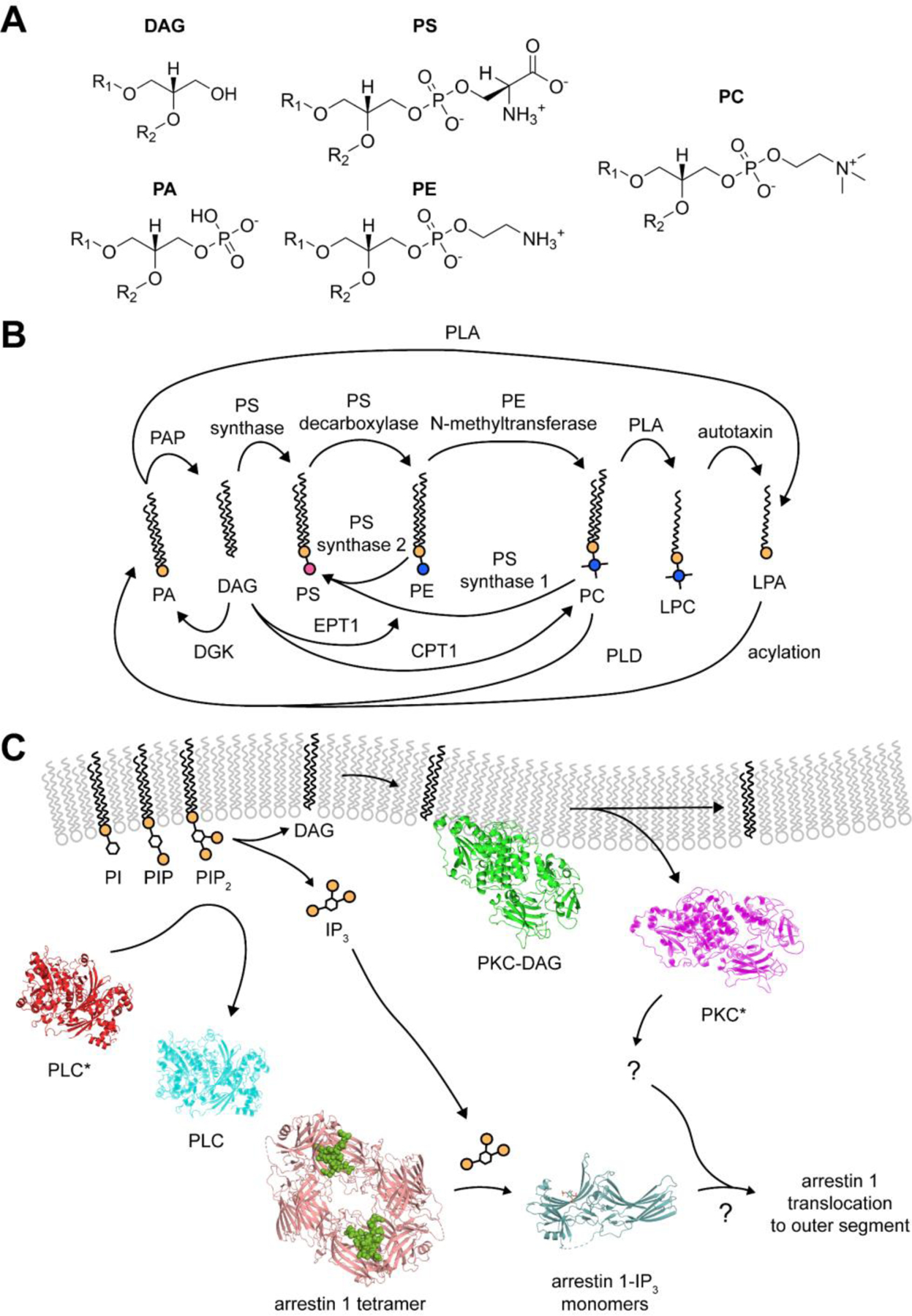
(A) Chemical structure of the five major glycerolipids. (B) Major glycerophospholipid pathways. Many enzymes are involved in the interconversion of each lipid species, including (C) Proposed involvement of IP3 and DAG in arrestin 1 translocation. Structures from a recent study of arrestin 1 in complex with IPs showed their ability to displace the arrestin 1 C-terminus, which is essential for inner-segment localization. The combination of a new basal structure, IP-complex structures, and data from Orisme et al. suggest a possible molecular mechanism for arrestin 1 translocation in cases of PLC activity. PLC structures were made from PDB structure 2ZKM (Hicks et al., 2008). PKC structures made from model predicted using AlphaFold, Uniprot identifier P17252 (Jumper et al., 2021). Basal and IP3-bound arrestin-1 structures taken from PDB structures 7JSM and 7JXA (Sander et al., accepted for puvlication).
Phosphatidylserine (PS) involvement in the phagocytosis signaling
When displayed on the surface of apoptotic cells, PS serves as a classic ‘eat-me’ signal recognized by surface receptors of many phagocytes (Segawa and Nagata, 2015). PS is concentrated in the cytoplasmic leaflet of OS membranes due to the action of PS flippases. One of the flippases keeping the PS distribution asymmetric in the OS membrane is ATPase phospholipid transporting 8A2 (ATP8A2), a member of the P4-ATPase subfamily. ATP8A2 acts on PS and, to a lesser extent, PE without affecting other membrane lipids (Coleman et al., 2009). In the mammalian retina, renewal of the rod OS involves circadian shedding of distal rod OS tips, followed by their subsequent phagocytosis by the RPE. Rod OS tips have PS exposed to the extracellular space, primarily at the onset of light. (Ruggiero et al., 2012). Exposed PS is then bound by the glycoprotein milk fat globule factor-E8 (MFGE8), which is a ligand for αvβ5 integrin, located in the apical RPE (Akakura et al., 2004; Finnemann et al., 1997). OS phagocytosis by the RPE also requires binding of tetraspanin CD81 to integrin αvβ5 (Chang and Finnemann, 2007).
Out of phospholipids, PS have the highest ratio of di-PUFA species (Hopiavuori et al., 2017) which presumably, makes the PS molecule more susceptible to the oxidative stress, therefore prone to the conformational changes. These changes may impact the membrane organization and induce local disturbances. The exact mechanism regulating PS exposure to induce phagocytosis is not well understood. Studies deciphering the molecular mechanism of membrane repair show exposure of PS as one of the first signals of membrane break (Horn and Jaiswal, 2018). Several questions arise: 1. If the exposure of PS is induced similarly in photoreceptors, is it possible that photoreceptor membrane breaks preceed PS exposure also in phagocytosis? 2. Is the PS exposure dependent on oxidation of PS-bound PUFA species? 3. Does PS oxidation induce membrane breaks? These questions await experimentally driven answers.
Phosphatidylinositol phosphates (PIPs)
Phosphatidylinositols are relatively abundant phospholipids in the retinal and RPE membranes (~4–7 mol% of total phospholipids), except for the rod OS where their content is estimated to be ~2 mol% (Anderson, 1970; Anderson et al., 1970). Phosphorylated forms of phosphatidylinositols called PIPs are found at much lower levels. PIPs are a potent class of signaling molecules playing important roles in mediating intracellular membrane trafficking of membrane proteins and lipids in response to environmental changes and cell demands (Wensel, 2020). Phosphatidylinositol-3-phosphate (PI(3)P) was found to regulate the trafficking of proteins to early and recycling endosomes (Carpentier et al., 2013) while also participating in the formation and maturation of autophagosomes (Dall’Armi et al., 2013). Phosphatidylinositol-4-phosphate (PI(4)P) was demonstrated as an essential factor in vesicle formation, lipid metabolism, and membrane trafficking within the trans-Golgi network (Lenoir and Overduin, 2013). PIP2 is a key player in clathrin-dependent endocytosis (McLaughlin et al., 2002), as well as the regulation of ion channels, transporters, and enzymes in membranes (Falkenburger et al., 2010). PIP2 is also a substrate and a regulator of PLC, which hydrolyzes PIP2 to IP3 and DAG – two critical second messengers described in this review (Fukami et al., 2010; Kadamur and Ross, 2013).
It was recently established that PIP2 and PI(4)P were the two major phosphorylated species of PI among retinal phosphoinositides, and 18:0/20:4 and 16:0/20:4 were the most common fatty-acyl chains (Finkelstein et al., 2020). Furthermore, Wensel’s group showed that rods contain 10-fold higher levels of PI(4)P and PIP2 than PI(3)P (Wensel, 2020). It was also demonstrated that PIP2 was enriched in photoreceptor inner segment (IS) and synapses but was scarcely present in OS. PI(4)P was found in the OS, IS, and outer nuclear layer, as well as in the synaptic region to a smaller extent (Finkelstein et al., 2020). However, a study from Rajala lab showed that five PIPs species were found in the murine and bovine rod OS fractions, namely PI(3)P, PI(4)P, phosphatidylinositol 3,4-diphosphate, PIP2, and PIP3, but the PIP2 was the most abundant and PIP3 had the lowest levels (Rajala et al., 2020b). Unlike the retina, they noticed that RPE was the most enriched in PIP3 compared to the other four species suggesting its high importance for RPE cells.
PIP2 acts together with myosin, actin, rac1, and rab8 to regulate the fusion of rhodopsin transport carriers in retinal photoreceptors (Deretic et al., 2004). Impaired PIP metabolism causes dysfunction of the retina, as shown in animal models and human patients (Brooks et al., 2018; Hagstrom et al., 1998). Moreover, several reports suggested that the enzymes involved in PIPs metabolism in the retina are regulated by light (Giusto et al., 2000; Rajala, 2020; Schmidt, 1983a, 1983b). For instance, rod PI(3)P levels increased over 30-fold after the light activation of PI-3 kinase (He et al., 2016). Ablation of the PI-3 kinase Vps34 responsible for PI(3)P production resulted in an impaired fusion of endosomes and autophagosomes with lysosomes, accumulation of abnormal membrane structures, and ultimately photoreceptor degeneration (He et al., 2016), as well as loss of visual function and cone death (Rajala et al., 2020a, 2015). The PI-3 kinase-generated PIPs activate prosurvival Akt kinase, which regulates retinal mitochondrial integrity, triggering anti-apoptotic effects in photoreceptor cells (Li et al., 2007; Rajala et al., 2013), as well as protecting RPE from oxidative stress (Defoe and Grindstaff, 2004; Rajala, 2020). PIPs are also indispensable in the RPE phagocytosis and autophagy processes; however, the molecular details are not yet fully understood (Intartaglia et al., 2021; Ravussin et al., 2021).
Inositol 1,4,5-trisphosphate (IP3)
The retina’s PIP2/IP3/DAG metabolism, especially in the photoreceptors and RPE, is an under-studied component of this well-studied system, as discussed at length in recent studies review by Wensel (Wensel, 2020). The production of inositol polyphosphates during visual processes was first studied using biochemistry and electrophysiology in Limulus polyphemus (Brown et al., 1984). While it is now known that invertebrates such as Limulus and Drosophila use IP3 signaling during phototransduction, comparatively recent work has led to speculation that IP3 is involved in the termination of vertebrate phototransduction. Orisme et al. showed that visual arrestin (known at varying times as S-antigen or rod arrestin, and systematically as arrestin 1) translocates from the IS to OS without exposure to light in the presence of PLC and protein kinase C (PKC) agonists (Orisme et al., 2010). Furthermore, PLC and PKC antagonists reduced arrestin 1 translocation in the light, suggesting that those associated pathways were not merely sufficient but necessary for translocation.
Much earlier work on PIP2, the phospholipid precursor of soluble IP3, reported a light-dependent decrease of PIP2 relative to PI that was attributable to PLC-mediated cleavage, suggesting the release of IP3 upon exposure to light (Ghalayini and Anderson, 1984). Subsequent work found that there was indeed a decrease in PIP2, but they did not detect increases in IP3, which, as highlighted by Wensel, suggested that a phosphatase was acting on PIP2, not PLC (Millar et al., 1988; Wensel, 2020). The aforementioned findings of Orisme et al. came as a surprise, then, as it suggested that PLC activity was responsible for prompting arrestin 1 translocation from the IS to OS. Because the major consequence of PLC activation is IP3 production, there is renewed interest in IP3 as a light-dependent secondary messenger in photoreceptors.
Recent work by one of our groups resolved the molecular details of IP binding to arrestin 1, including IP3 (Sander et al., accepted for publication). Combined with the most complete basal structure to date, we were able to identify candidate residues involved in N-domain binding of the regulatory C-tail of arrestin 1 and which of those residues interact with IP3. R171 and K298 bound both the C-tail and IP3, and, when bound to IP3, the C-tail was removed from the majority of the N-domain, leaving arrestin 1 in a primed conformation. Given the proposed tetrameric structure of arrestin 1, our basal structure suggests that the C-tails are close together in the NN contact sites within the tetramer (Figure 3C). Disturbance of those sites, possibly through IP3 binding, could disrupt the tetramer sufficiently to allow arrestin to translocate from the inner to OS under a restriction-diffusion model as proposed previously. The difference here is that the restriction can potentially be the result of homotetramerization, wherein the protein population shifts in an average size and becomes sterically excluded from diffusion in the rod OS (Najafi et al., 2012; Malhotra et al., 2021).
These results have exciting implications for the PLC pathway in photoreceptor adaptation. First, the underlying mechanism that begins the light-dependent activation of PLC has not yet been explained. The experiments from Orisme, however, give strong evidence that arrestin 1 translocation is tied to PIP2 cleavage by PLC and/or DAG signaling coupled to PKC. If true, components of photoreceptor membrane lipids may play the role of secondary messengers for the termination of phototransduction through the translocation of arrestin 1 to the OS. Light-mediated reduction in PIP2 levels has yet to be fully incorporated into the function of other light-dependent pathways in photoreceptors. Revisiting these older experiments with modern technology may help settle the debate over the levels of PIP2 and IP3 after exposure of photoreceptors to light. Furthermore, the possible connection between rhodopsin signaling and PIP2 cleavage should be studied.
In contrast to photoreceptors, PIP2-derived IP3 has already been confirmed as an important signaling molecule for the essential functions of the RPE. Rodriguez de Turco et al. made an initial observation that light-stimulated RPE cells, at the time of phagocytosis, have increased IP3 production (Rodriguez de Turco et al., 1992). Studies comparing rats without retinal degeneration to Royal College of Surgeons (RCS) rats, a model of retinitis pigmentosa (RP) that lacks phagocytosis, revealed that IP3 signaling increases during RPE phagocytosis (Heth and Marescalchi, 1994). Subsequent work in RCS rats reported that exposure of cultured RCS RPE cells to carbachol (a muscarinic receptor agonist that stimulates IP3 production) restored phagocytic behavior to RCS RPE cells (Heth et al., 1995). These findings were contradicted by Hall et al. one year later, where they reported that, while IP3 increases are a likely result when exposed to carbachol, they could not confirm an increase in phagocytosis when it was incubated with RCS RPE cells (Hall et al., 1996).
The RPE also requires IP3 for several other pathways, including growth factor and neuropeptide signaling (Kuriyama et al., 1991, 1992). P2Y1-mediated inflammation and increased expression of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) in cases of high NaCl levels surrounding the RPE was shown to be produced, in part, by IP3 signaling (Prager et al., 2016). Ion channels also contribute to epithelial transport of Cl- and secretion contain elements of IP3 signaling as well (Strauss et al., 1996; Barro-Soria et al., 2012; Vainio et al., 2015; York et al., 2017; Hollborn et al., 2017), a phenomenon that has been reviewed previously (Constable, 2014).
Diacylglycerol (DAG)
As mentioned above, PIP2 cleavage by PLC generates IP3 and DAG, which is a neutral glyceride containing two FA chains esterified to the glycerol molecule. DAG can also be generated through several other catabolic and anabolic reactions (Figure 3B) (Eichmann and Lass, 2015). DAG is found in cellular membranes as a building block for glycerophospholipids. It can also act as a second messenger at the plasma membrane while also regulating many cellular processes. These processes include insulin signaling, ion channel regulation, and neurotransmitter release (Eichmann and Lass, 2015; Ma et al., 2013; Schuhmacher et al., 2020).
DAG can bind and activate two out of three classes of PKC isozymes, conventional and novel PKC isoforms (Newton, 2018, 1993). DAG-sensitive PKC isozymes are activated at the membrane site, and their action needs to be tightly controlled to warrant healty cell functions (Newton et al., 2016). DAG-activated PKC is present in rod OS (Kelleher and Johnson, 1985; Williams et al., 1997; Wolbring and Cook, 1991), where it phosphorylates rod OS proteins in a light-dependent manner. Proteins from the phototransduction pathway that were shown to be phosphorylated by PKC isozymes are transducin, cGMP-phosphodiesterase (PDE6), rod cGMP-gated channel, arrestin and rhodopsin (Newton and Williams, 1993, 1991; Williams et al., 1997). Giusto group demonstrated that DAG and PA levels depend on the dark vs. light conditions in rod OS due to the light-driven regulation of the activity of enzymes producing these molecules (Pasquaré et al., 2008). Under illumination, there is an increase in DAG kinase activity (Huang et al., 2000) that generates higher PA levels, whereas in darkness DAG concentration increases due to elevated activity of phospholipase D (PLD)/ lipid phosphate phosphatases (LPP), that are otherwise inhibited by light (Salvador and Giusto, 2006). It is also suggested that DAG/PA ratio in rod OS is dependent on the LPPs activity, which is modulated differently based on the concentration of LPA, S1P, or C1P (Pasquaré et al., 2008).
The DAG kinase isoform epsilon (DGKE) is abundantly expressed in the human retina, especially in rods and cones (Figure 4), and a possible connection has been suggested with inherited RP; however, a pilot study did not reveal DGKE gene mutations (Tang et al., 1999). The increased de novo synthesis and abnormal DAG accumulation are driven by high glucose-induced PKC activation. This, in turn, causes microvascular pathologies in the retina of diabetic patients. Increased levels of DAG affect vascular blood flow and cause extracellular matrix buildup, basement membrane thickening, increased permeability, and neovascularization (Mérida et al., 2008; Way et al., 2001).
Figure 4. RPE, rods, and cones express select enzymes involved in lipid metabolism.
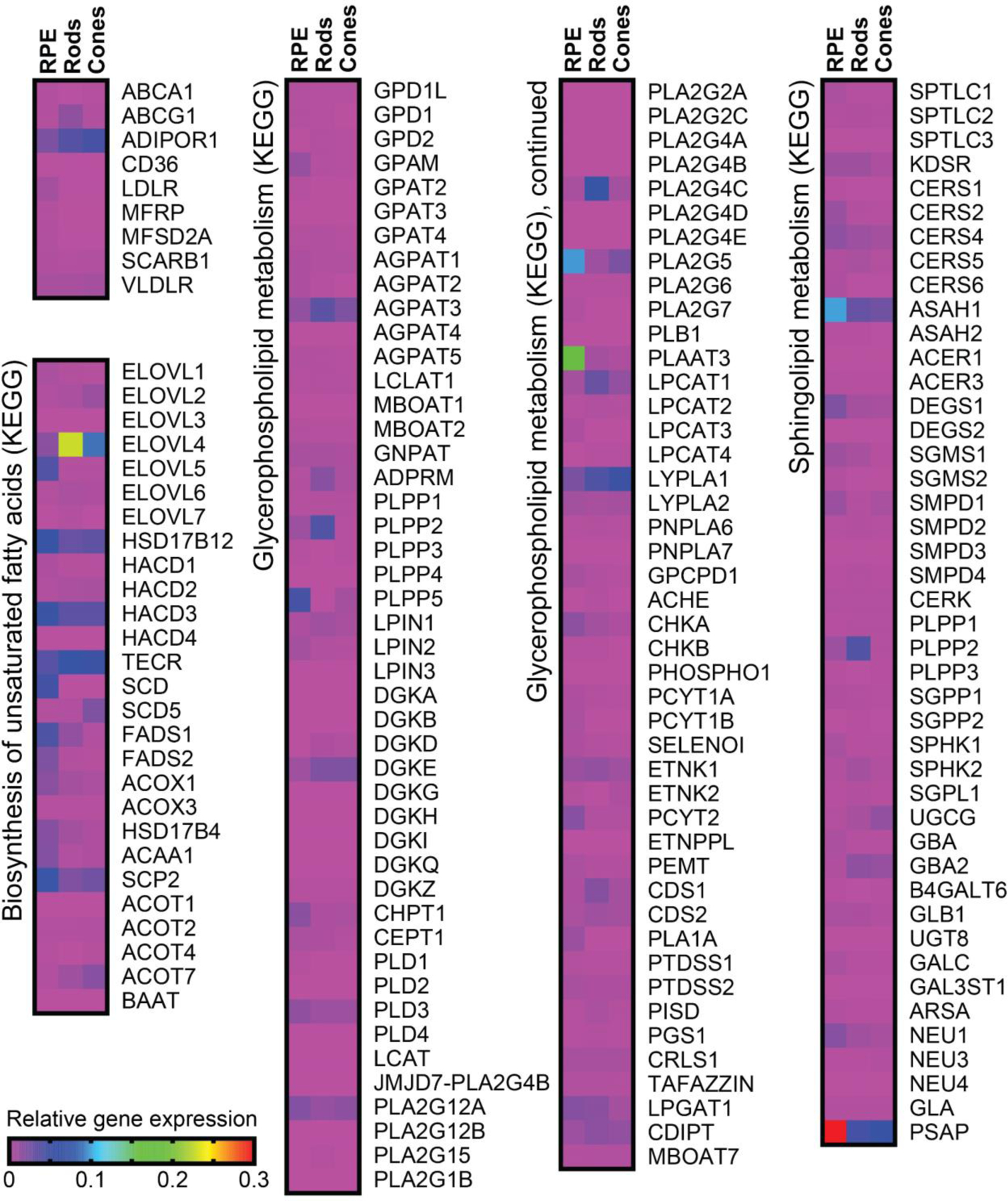
(A) Heatmaps representing gene expression profiles of genes and pathways involved in lipid uptake, biosynthesis, and metabolism. Data were sourced from the human eye single-cell RNA-seq gene expression database (Lu et al., 2020) and normalized to each cell GAPDH expression level.
Phosphatidic acid (PA)
Except for de novo synthesis, PA can be generated by acylation of lysophosphatidic acid (LPA), hydrolysis of PC by PLD, or produced from DAG through phosphorylation by DAG kinases (Figure 3B). Both PA and DAG are maintained in the cell at relatively low levels, and there is a dynamic interconversion between the two molecules (Moine and Vitale, 2019). It is proposed that the de novo pathway generates a pool of a ‘structural’ PA used as a precursor for other glycerophospholipids while the other metabolic pathways produce PA for signaling events (Kim and Wang, 2020). PA can increase its net negative charge from −1 to −2 depending on the pH, which allows for attracting and tethering proteins to membranes via electrostatic interaction. Moreover, PA can induce conformational changes within proteins upon binding, promote protein oligomerization, or hinder ligand binding, thus regulating enzyme catalytic activity (Kim and Wang, 2020; Kooijman et al., 2007; Zhukovsky et al., 2019). The average membrane displacement caused by the structure of PA enables it to induce negative (concave) curvature in the lipid bilayer, which aids membrane fusion and fission and the formation of secretory vesicles (Kooijman et al., 2003). PA is also one of the positive regulators of mTOR signaling (Ávila-Flores et al., 2005). Acting together with insulin, PA activates mTOR survival pathways and protects hyperglycemic neuronal cells from apoptosis in diabetic retinas (Fox et al., 2012).
Lysophosphatidic acid (LPA)
Lysophosphatidic acid (LPA) is not a single entity but a family of naturally occurring glycerophospholipids composed of a glycerol backbone with an esterified single acyl chain and a phosphate group. LPA species can be derived from PA hydrolysis by phospholipase A1, but their primary source is the cleavage of LPC by autotaxin enzyme (encoded by ENPP2 gene) (Yanagida and Valentine, 2020). Autotaxin is a secreted enzyme with lysophospholipase D activity that converts lysophospholipids to LPA and which was found to be highly expressed in the retina (Uhlen et al., 2015). LPA exerts its functions by binding with its GPCR receptors (LPA1–6), and depending on the type of LPA receptor and the type of its coupled G-proteins, it can activate different signaling pathways (Chun et al., 2010). LPA regulates various biological functions such as cell proliferation, migration, inflammation, angiogenesis, metastasis, apoptosis, and others (Chun et al., 2010; Yanagida and Valentine, 2020).
LPA induced a rise in Ca2+ concentration in the neural retina of chick embryos during neurogenesis (Zhou et al., 1999), and promoted growth cone collapse throughout retina development in mice and chick (Birgbauer and Chun, 2010; Fincher et al., 2014). Moreover, LPA was suggested to alter different types of ion channel activity in human and bovine Müller cells (Kusaka et al., 1998). Another finding demonstrated that mutations in the PNPLA6 gene encoding lysophospholipase, which deacetylates PC to LPC to glycerophosphocholine, led to retinal LPA accumulation and photoreceptor degeneration (Kmoch et al., 2015).
It was determined that treatment of the RPE with either PA or LPA stimulated cell proliferation, but the effect was much weaker when LPC was used (Thoreson et al., 1997). Using human pluripotent stem cell (hPSC)-derived retinal cells, it was shown that LPA regulates tight junctions in RPE in a receptor-dependent manner and increases the transepithelial electrical resistance of the RPE. Additionally, the high content of LPA decreased the efficiency of phagocytosis of photoreceptor OS by the RPE. LPA treatment of the stem cell-derived photoreceptors caused morphological changes and reorganization of the actin-myosin cytoskeleton (Lidgerwood et al., 2018).
Eicosanoids, docosanoids, and elovanoids.
Eicosanoids, docosanoids, and elovanoids are generated as breakdown products of membrane lipids. They act as secondary lipid messengers, modulating local inflammatory responses and homeostasis, and are often called bioactive lipids (BL).
Eicosanoids
Inflammatory events trigger the release of AA or other 20-carbon PUFAs (from either the n-3 or n-6 group) derived from membrane phospholipids by phospholipases A2, C, and D. These are further metabolized through cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 monooxygenase (CYP) pathways. From there, the resulting products can generate different types of eicosanoids (Bazan, 2018; Dennis and Norris, 2015; Wang et al., 2021).
Eicosanoids produced by COX include predominantly prostaglandins and thromboxanes. Prostaglandins regulate blood flow in the choroid and inner retina blood vessels. COX enzyme expression and activity is regulated by inflammatory signals, including ROS (Karaa et al., 2006; Li et al., 2011; Martín et al., 2012). In particular, COX2 expression is precisely regulated through the non-coding RNA mechanism and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) dependent initiation of transcription (Krawczyk and Emerson, 2014) and sustained inhibition of NFκB signaling pathway has been shown to protect neuroretina in Akita mice (Homme et al., 2021). It was suggested that prostaglandins could affect choroidal and retinal blood flow regulation in newborn animals, resulting in surplus oxygen delivery and retinal microvascular damage in retinopathy of prematurity (Abran et al., 1995). Moreover, prostaglandin E2 (PGE2) effects on the blood vessels include increased oxidative stress, vasodilation, higher vascular permeability, and elevated production of proinflammatory cytokines (Huang et al., 2016).
The major LOX-derived eicosanoids are hydroxyeicosatetraenoic acids (HETEs), and leukotrienes (LTs). HETEs are involved in the degranulation of neutrophils, skin inflammation, and regulation of blood vessel dilation (Takayama et al., 1987; Tang et al., 1995; M. H. Wang et al., 2020). LTs promote leukocyte chemotaxis and degranulation while also enhancing oxidative stress, vascular permeability, and the production of proinflammatory cytokines (M. H. Wang et al., 2020). Interactions between retinal pericytes and polymorphonuclear leukocytes may lead to the production of sulfidopeptide LTs, which can alter microvascular permeability (Mcmurdo et al., 1998). LTA4 has been considered a contributing factor in chronic inflammation due to diabetic retinopathy (Talahalli et al., 2010).
The CYP pathway comprises many enzymes that contain heme iron, and many of these enzymes are expressed in the liver, eye, and other tissues, where they inactivate and eliminate toxins and metabolites. Eicosanoids produced via the CYP pathway include epoxyeicosatrienoic acids (EETs) and 20-HETE that are recognized as anti-inflammatory, while downstream diHETEs are pro-inflammatory. Various EET species are produced in the retina (Hu et al., 2017). EETs have been implicated in angiogenesis by retinal endothelial cells, especially under hypoxic conditions (Michaelis et al., 2008). Various eicosanoids endogenously produced in damaged tissue initiated the inflammatory response by increasing vascular permeability and stimulating leukocyte chemotaxis in diabetic retinopathy. PGE2, thromboxane B2 (TxB2), LTs, and 12-HETE were among the proinflammatory and angiogenic eicosanoids. In addition, EETs and LOX-derived lipoxins were also found in samples derived from several diabetic vitrei (Schwartzman et al., 2010). Precise regulation of oxygenases and CYP proteins, induced by external factors, is at the base of the composition of eicosanoids in the cell.
Docosanoids
Docosanoids are another group of bioactive lipid mediators derived from DHA or docosapentaenoic acid. Docosanoids have neuroprotective and pro-homeostatic properties. They aim to resolve inflammation by ceasing neutrophil recruitment, promoting tissue debris clearance by macrophages, counter regulating proinflammatory mediators, and stimulating tissue repair (Bazan, 2018; Serhan and Chiang, 2013; Serhan and Savill, 2005). Within this class of proresolving lipids, there are three families of distinct structures, namely protectins, resolvins, and maresins.
Under oxidative stress, DHA is released from membrane phospholipids by PLA2 and converted by 15-LOX-1 to dihydroxylated docosatriene, named neuroprotectin D1 (NPD1). NPD1 promotes photoreceptor and RPE survival by protecting them from oxidative stress, light damage, and inflammatory events (Bazan et al., 2011; Marcheselli et al., 2010; Mukherjee et al., 2004). Similar in structure, maresin 1 was demonstrated to have a beneficial effect on conjunctival goblet cells by maintaining optimal tear film mucin levels in the healthy eye. It also attenuated mucin overproduction, as occurs in ocular allergy, through compensating calcium levels (Olsen et al., 2021).
Resolvins, protectins, and lipoxins, which are derived from various PUFAs, suppress the production of interleukin 6 (IL-6), tumor necrosis factor α (TNFα), and vascular endothelial growth factor (VEGF), and have anti-angiogenic effects. Thus, they were proposed as potential candidates for preventing and treating diabetic macular edema and retinopathy (Das, 2013). Resolvin D1 showed protective effects on primary retinal cells exposed to high glucose by reducing ROS levels, promoting mitochondrial DNA repair by 8-oxoguanine glycosylase (OGG1), and reducing apoptosis (Trotta et al., 2020). Resolvin D1 also showed a beneficial effect in rats with streptozotocin-induced diabetic retinopathy by inhibiting the activation of NLRP3 inflammasome and associated cytokine production (Yin et al., 2017).
Elovanoids
Elovanoids are another class of interesting lipid mediators. They are derived from VLC-PUFAs and show mainly protective functions. Elovanoids have been explicitly covered in the context of their general neuroprotective effects in the retina in several previous reviews (Bazan, 2021; Yeboah et al., 2021). Briefly, the VLC-PUFAs that result from elongation by the ELOVL enzymes can subsequently be incorporated into phospholipids. In a similar way to docosanoid creation beginning with the release from the sn-2 position of phospholipids by PLA2, VLC-PUFAs can be cleaved from phospholipids by PLA1 from the sn-1 position of a phospholipid, and elovanoids are then generated from the dihydroxylated derivatives of 32:6n-3 and 34:6n-3 (Jun et al., 2017). Elovanoids were found to protect RPE cells from uncompensated oxidative stress Bcl-2 and Bcl-xL upregulation and Bax, Bid, and Bim downregulation (Jun et al., 2017). A report in 2019 showed that elovanoids protect photoreceptors by counteracting oligomeric β-amyloid-induced gene expression in a model of simultaneous injection of elovanoids and oligomerized amyloid-β peptide, with subsequent topical administration of elovanoids, in 6-mo-old C57BL/6J WT mice (Do et al., 2019). Interestingly, although VLC-PUFAs are synthesized in situ by ELOVL4, they are also present in actively phagocytic RPE cells, where they can be further converted to elovanoids. Interestingly, because of their particular enzymatic requirements, elovanoids might be molecules present only in limited places in the organism (for example – central nervous system), making them potentially interesting molecule in searching specific pharmacological approaches. There is much work to be done to uncover how elovanoids exert their protective effects, however. Potential binding partners, receptors, and mechanisms involved in blunting the effects of oxidative stress will be important avenues of further research.
Many studies highlight the crucial role of FAs derivatives in cell and tissue biology. These molecules can act as ligands, signaling molecules, and structural components of membranes. The presence of each bioactive lipid is precisely regulated by a cohort of enzymes regulated by intrinsic and extrinsic signals, such as stress, nutrition changes, and others. In addition, the combination of these molecules can trigger different responses to the same stimulus. Complex interdependencies and “feed-forward” reactions, as well as autoregulatory loops, provide many opportunities for modulating the synthesis of eicosanoids, docosanoids and elovanoids. Further interdisciplinary studies involving chemistry, cell biology, and lipid experts are needed to understand the role of these interesting lipids in maintaining the photoreceptor/RPE homeostasis. Such studies can then attempt to manipulate the network of enzymes and products to influence cell health.
I.3. Lipids as an energy source
The retina is one of the highest energy-consuming tissues in the body, requiring abundant energy substrates to support metabolic functions, including light sensing via phototransduction and maintenance of electrical gradients, production of the molecules and structures that allow vision, and managing the oxidative stress arising from these processes (Joyal et al., 2016). As a part of the central nervous system (CNS), it was assumed that the metabolism and energy requirements of the retina rely on glucose, similar to the brain (Mergenthaler et al., 2013). This was supported by studies where the lack of glucose and its transporter in the RPE was associated with photoreceptor degeneration (reviewed in (Hurley, 2021)). Besides glucose, Smith, and colleagues (Joyal et al., 2016) showed that lipids, especially FAs, can serve as another rich source of ATP through oxidative FA oxidation (FAO). The reader can find more details in a recently published, extensive review (Fu et al., 2021). In brief, during this process, FAs are oxidized in the mitochondria to acetyl-CoA and enter the Krebs cycle to produce energy. Lipid turnover plays an essential role in neuronal survival. Peroxisome proliferator-activated receptor-alpha (PPARα), a known lipid metabolism regulator, may be responsible for neovascularization in the retina (Pearsall et al., 2017). The Pparα−/− mouse model showed developing retinal degeneration and indicated that PPARα activity is required to internalize FAs into the mitochondria for oxidation and ATP production. Therefore, PPARα has been proposed to be a potential therapeutic target for treating neurovascular defects in retinal metabolic disorders (Fu et al., 2019; Pearsall et al., 2017). The mechanism underlying how cells balance between glucose and lipid energy sources is still not well understood. The involvement of pathways, such as mTOR, in the nutrient-sensing mechanism (Kim and Guan, 2019) is a great starting point for potential future studies.
Chapter II. Lipid homeostasis in the photoreceptors and RPE
As mentioned earlier, lipids constitute a significant portion of photoreceptors and are especially enriched in the OS structures. Filled with the stacks of densely packed disk membranes, OS exists in an equilibrium between the proximal biosynthesis and elongation of lipid species balanced by distal OS shedding and phagocytosis by the adjacent RPE cells. The continuous renewal of the OS leads to a full renewal of the structure approximately every ten days in the vertebrate eye, drives increased demand for lipid anabolism in the photoreceptor IS, comparable to the levels observed in proliferating cells (Young, 1971, 1967; Young and Bok, 1969). To satisfy this demand, in addition to the systemic delivery of lipids and their precursors, lipid components of the shed OS undergo recovery and are delivered back to OS biogenesis sites, as will be discussed in the following section.
II.1. Lipid exchange with the blood
Transport of hydrophobic lipid compounds through the aqueous blood plasma is enabled by a variety of specialized protein carriers forming lipoproteins, a detailed description of which falls out of the scope of this review but has been extensively reviewed elsewhere (Getz, 2018). In general, lipoproteins in the bloodstream form particles classified based on their size or density: from the smallest albumin complexes with the FFAs or their lysophospholipid derivatives to a wide range of differently sized spheres encapsulating the lipidic cargo in a hydrophilic shell. The latter include (in the order of size) HDL, LDL, intermediate-, and very-low-density lipoproteins (IDL and VLDL respectively) as well as the largest and lowest density chylomicrons (CM), which can exceed 1 µm in diameter.
Photoreceptor OS and IS form the avascular part of the retina is surrounded by two diffusion barriers. Junctional complexes between the Müller cell apical processes and the photoreceptor IS membranes form the outer limiting membrane, while on the other side the tightly joined RPE cell monolayer serves as the outer blood-retina barrier (BRB). The RPE, together with the opposed Bruch’s membrane, separate the OS from a dense network of choroid vasculature, which mediates the majority of photoreceptor metabolite exchange with the systemic circulation. Large, retina-oriented fenestrations in the choriocapillaris facilitate the flow of plasma components into the extracapillary space, as well as further infiltration of the acellular Bruch’s membrane layer. Studies of the permeability of the Bruch’s membrane indicate that while the diffusion of macromolecules might be hindered to some degree by the inner collagenous portion of the membrane, even relatively large particles are capable of crossing it under native conditions (Cankova et al., 2011; Gordiyenko et al., 2004; Sørensen et al., 2019; Starita et al., 1997). Consequently, the RPE monolayer serves a prominent role in regulating the outer retina metabolite influx and efflux, including that of lipidic compounds.
II.1.1. Lipid influx to the RPE
The uptake of lipidic compounds to the RPE is facilitated by a range of specific receptors present in its cellular membranes (Figures 1 and 4). RPE cells express several lipoprotein cognate receptors, including the LDL receptor (LDLR) (Gordiyenko et al., 2004; Hayes et al., 1989; Tserentsoodol et al., 2006b) and VLDL receptor (VLDLR) (Hu et al., 2008), as well as broad-specificity scavenger receptor class B, member 1 (SCARB1, with alternative splicing variants I and II) (Duncan et al., 2009, 2002; Tserentsoodol et al., 2006a), and the cluster of differentiation 36 (CD36) (Kociok and Joussen, 2007; Ryeom et al., 1996). In addition, several other proteins present in RPE membranes were identified as important regulators of FA content and composition in the photoreceptor cells: major facilitator superfamily domain-containing protein 2a (MFSD2A) (Wong and Silver, 2020), ADIPOR1 (Rice et al., 2015), and membrane frizzled-related protein (MFRP) (Kautzmann et al., 2020). Since the tight junction barrier prevents membrane protein diffusion between the apical and basolateral aspects of the cell, both surfaces differ in their receptor composition and lipid uptake characteristics.
The choroid-oriented basolateral membrane is enriched in LDLR, and accordingly, it was observed that LDL constitutes a more efficient source for cholesterol uptake over the more abundant HDL pool (Tserentsoodol et al., 2006b). Loss of LDLR in mice results in lipid accumulation in the Bruch’s membrane and gradual photoreceptor degeneration (Schmidt-Erfurth et al., 2008). This and several other lines of evidence suggest that LDL particles constitute a significant source of lipids taken up from the systemic circulation by RPE cells (Gordiyenko et al., 2004; Hayes et al., 1989; Rodríguez and Larrayoz, 2010; Tserentsoodol et al., 2006a). Loss of VLDLR, abundant in both photoreceptors and the RPE (Figure 4), results in reduced FA levels in the retina, suggesting another important route for the lipid influx (Hu et al., 2008; Joyal et al., 2016). In addition, it was proposed that the influx of albumin-bound unesterified FAs can be to some extent driven by their spontaneous crossing of the cellular membrane followed by binding to the cellular FA transport proteins (FATP) and FA binding proteins (FABP), and subsequent quenching to a water-soluble acyl-CoA form (Hamilton and Brunaldi, 2007).
The systematic distribution of other lipid receptors is described in a single study from the Rodriguez group (Tserentsoodol et al., 2006a). Quantitative RT-PCR and immunohistochemistry analysis have shown that SCARB1 splice variant II and CD36 receptors are expressed on both the apical and basal side of RPE as well as on outer and inner segment of photoreceptors. In contrast, SCARB1 splice variant I is not expressed in the RPE and is only present in photoreceptor membranes (Figure 4). Other studies have shown that VLDLR is expressed highly in RPE cells (Hu et al., 2008); however, its exact distribution between apical and basal membranes was not investigated. Finally, the distribution of ApoE receptor 2 (APOER2), also known as LRP8, is confined to the limiting membrane area (Trotter et al., 2011), suggesting a role in communication with Müller Glia. These sparse but important data show the distribution differences between the membranes further underlying the high specification of RPE in lipid transport.
Interestingly, a dedicated uptake pathway involving the MFSD2A receptor seems to support the selective enrichment of DHA in the photoreceptor cells. MFSD2A, present in the RPE basolateral membrane, mediates the FA uptake primarily in the form of LPC, simultaneously contributing to the transcytosis suppression and the BRB permeability properties (Andreone et al., 2017; Nguyen et al., 2014; Wong et al., 2016). While DHA supplementation in the form of LPC effectively increases its retinal content (Sugasini et al., 2020), loss of Mfsd2a leads to the significant DHA depletion from the mouse retina. This depletion translates to a surprisingly modest retinal degeneration phenotype (Lobanova et al., 2019; Wong et al., 2016). Given the importance of DHA for photoreceptor health, it comes with no surprise that multiple delivery pathways coordinate its uptake to the neural retina. It is, however, intriguing that the retina depleted of nearly half of its original DHA content can retain relatively normal structural and functional characteristics, raising a question about the relation between the level of photoreceptor pathology and the retinal DHA content.
II.1.2. Lipid efflux from the RPE
The apical membrane of RPE mediates further lipid flux towards the neural retina. Thanks to its phagocytic capability, the same membrane mediates the daily clearance of photoreceptor OS tips concomitant with a significant lipid material ingestion by the RPE. Both processes, with opposite directionality, depend on a dedicated set of membrane receptors and transporters. While several proteins involved in the phagocytosis orchestration have been described in detail (see section II.3.4), the mechanisms by which the RPE apical membrane regulates lipid efflux towards the photoreceptors remain poorly understood.
The apical membrane contains ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1), which utilize ATP to flip various classes of lipids (cholesterol and phospholipids in particular) to the lipophilic acceptors located in the extracellular matrix (Figure 1) (Ananth et al., 2014; Tserentsoodol et al., 2006a). RPE-specific depletion of both results in lipid accumulation in the form of lipid droplets in the RPE cells of young mice, coinciding with inflammatory response and retinal degeneration (Storti et al., 2019). Yet, the neural retinas of those animals do not present any major lipid deficiency, suggesting that certain redundancy and compensatory mechanisms exist at this level to ensure an adequate supply of lipids and essential FAs to the retina. Conversely, loss of either AdipoR1 or Mfrp, two other transmembrane proteins abundant in the RPE apical membrane (Figure 1), leads to significant changes in the mouse retinal lipid profile (Kautzmann et al., 2020). These changes are reminiscent of the Mfsd2a KO phenotype (Lobanova et al., 2019), and characterized by significant depletion of multiple phospholipid species, most prominently containing DHA or VLC-PUFAs. Unlike Mfsd2a KO, however, AdipoR1 or Mfrp deficient animals exhibit a gradual photoreceptor loss evident even at an early age (Rice et al., 2015; Won et al., 2008). While ADIPOR1 possesses an intrinsic ceramidase activity leading to the release of sphingosine and FFA (Holland et al., 2011), the role of MFRP in lipid metabolism or transport remains unresolved. Interestingly, ADIPOR1 deficiency has been recently proposed to affect the expression of Elovl2 (Osada et al., 2021), a critical elongase contributing to the DHA synthesis (see Chapter II.2.1). Therefore, Osada and colleagues implicate that the primary cause of photoreceptor degeneration in AdipoR1 KO mice might be related to DHA biosynthesis rather than its transport to the retina. Comparison of Mfsd2a, AdipoR1, and Mfrp KO phenotypes also suggests that a deeper look into the lipid compositional differences at single lipid species levels is needed to fully understand the role of DHA and VLC-PUFAs for retinal structural and functional homeostasis.
II.2. Lipid biosynthesis and metabolism in situ
In many ways, the RPE functions as a life support system for the outer retina, complementing the elevated metabolic requirements of both rods and cones. For example, a direct comparison between the retinal and RPE metabolite content showed elevated β-oxidation of FAs and sphingolipid biosynthesis intermediates in the RPE (Kanow et al., 2017; Sinha et al., 2020). Moreover, the expression of several essential enzymes involved in lipid biosynthesis and metabolism was confirmed in both RPE and photoreceptor cells (Figure 4), indicating the important role of lipid anabolism in situ, as will be further discussed in this chapter.
II.2.1. Free FAs
Photoreceptors exhibit a unique FA composition, being particularly enriched with DHA and other LC- and VLC-PUFAs. Early studies suggested that biosynthesis of DHA occurs predominantly in the liver, but it is also present in the retina and other parts of the CNS (Bazan et al., 2011; Scott and Bazan, 1989). In recent years more light has been shed on the presence, and consequently the role of PUFA biosynthesis enzymes in the retina (Figure 4). As mentioned earlier, all LC- and VLC-PUFAs can be biosynthesized in human body from their more abundant dietary precursors, like the α-Linolenic acid in case of the n-3 series, and part of the elongation reaction catalyzed by a family of ELOVL elongases constitutes the rate-limiting step in this process. ELOVL enzymes involved in PUFA elongation exhibit substrate selectivity: ELOVL5 elongates PUFAs 18–22 carbons in length, ELOVL2, 20–22 carbons, and ELOVL4 acts on VLC-PUFAs of 24 carbons or more (Deák et al., 2019). ELOVL5 exhibits high expression in the liver and comparably low expression in the retina, where is it seemingly confined to the Müller cells. ELOVL2, as a critical enzyme for DHA synthesis, is highly expressed in the liver. In addition, it is expressed in the central nervous system, including retinal cones, and at low level in other cells (see chapter III). ELOVL4 is highly and specifically expressed in photoreceptors, mainly in the IS, and, in contrast to ELOVL2, is absent from the liver (Agbaga et al., 2008; Tikhonenko et al., 2010; Vasireddy et al., 2007). Thus, in great opposition to the DHA supply, biosynthesis of VLC-PUFAs by ELOVL4 occurs predominantly in the photoreceptor cells. ELOVL2 products of 24 carbons in length can serve a dual role as both DHA and VLC-PUFA precursors and, as such, are critical for the homeostasis of photoreceptors. In addition, it was shown that the RPE contains measurable amounts of the 22 carbon intermediate (22:5 n-3) that can be further converted into the DHA (Wang and Anderson, 1993) therefore confirming the presence of ELOVL2 in RPE. Accordingly, mutations in ELOVL4 and ELOVL2, the two most abundant elongases in the retina (Figure 4), affect the health of the photoreceptors. Loss of ELOVL4 significantly decreases the overall VLC-PUFA photoreceptor content, which likely contributes to the Stargardt-like macular dystrophy (STGD3) disease pathogenesis (see Table 1), as will be discussed later in this review (Bennett and Anderson, 2016). However, to this day, no genomic variants or mutations correlate with ocular phenotypes identified in ELOVL2 (see more in chapter III).
To better understand the role of ELOVL2 in the eye, our group generated Elovl2C234W mutant mice lacking enzymatic ability to elongate DHA into its 24 carbon derivative (24:5 n-3), and showed that they exhibit accelerated vision decline as early as 6 months of age (Chen et al., 2020). In addition, we observed the development of sub-RPE deposits containing oxidized lipids and ApoE and altered composition of PUFAs in the retina, all of which are disease features reminiscent of the dry AMD. Intriguingly, our data also pointed to the potential role of ELOVL2 in the senescence induction. Furthermore, the analysis of Elovl2C234W transcriptomes from 4 months old animals (a stage before visual changes can be detected), showed downregulation of genes involved in oxidative phosphorylation (Figure 5A,B) and deregulation of genes engaged in oxidative-stress-induced senescence (Figure 5C). The analysis pointed out a potential connection between PUFAs and inflammation as retinas isolated from mutant mice had higher levels of inflammatory genes activated. This data agrees with studies performed on Elovl2 KO animals (Talamonti et al., 2017), where authors noticed that macrophages isolated from the KO mice have a proinflammatory phenotype compared to healthy macrophages. Continuous inflammation can be the reason for several other phenotypes, including the presence of complement proteins in the sub-RPE deposits in mutant mice. Finally, we have recently obtained electron microscopy images of Bruch’s membrane (BM) in 18 month-old wt and Elovl2C234W females. The data (Figure 5D) show several differences when compared to the WT tissues. First, the BM is distorted and thickened in the mutant animals. The collagen stripes are disturbed, and the elastin layer seems irregular. These phenotypes are accompanied by an accumulation of basal lamina deposits (BLamD) forming a layer on the BM and invading basal infoldings of RPE (Figure 5D, BLamD – red stars). Since the accumulation of BLamD can signify aging and stress (Ramrattan et al., 1994) we conclude that our Elovl2C234W animals express signs of accelerated aging in the eye and as such the model may be considered to study an age component in age-related eye diseases including AMD.
Figure 5. Metabolic and structural changes in ELOVL2 deficient animals.
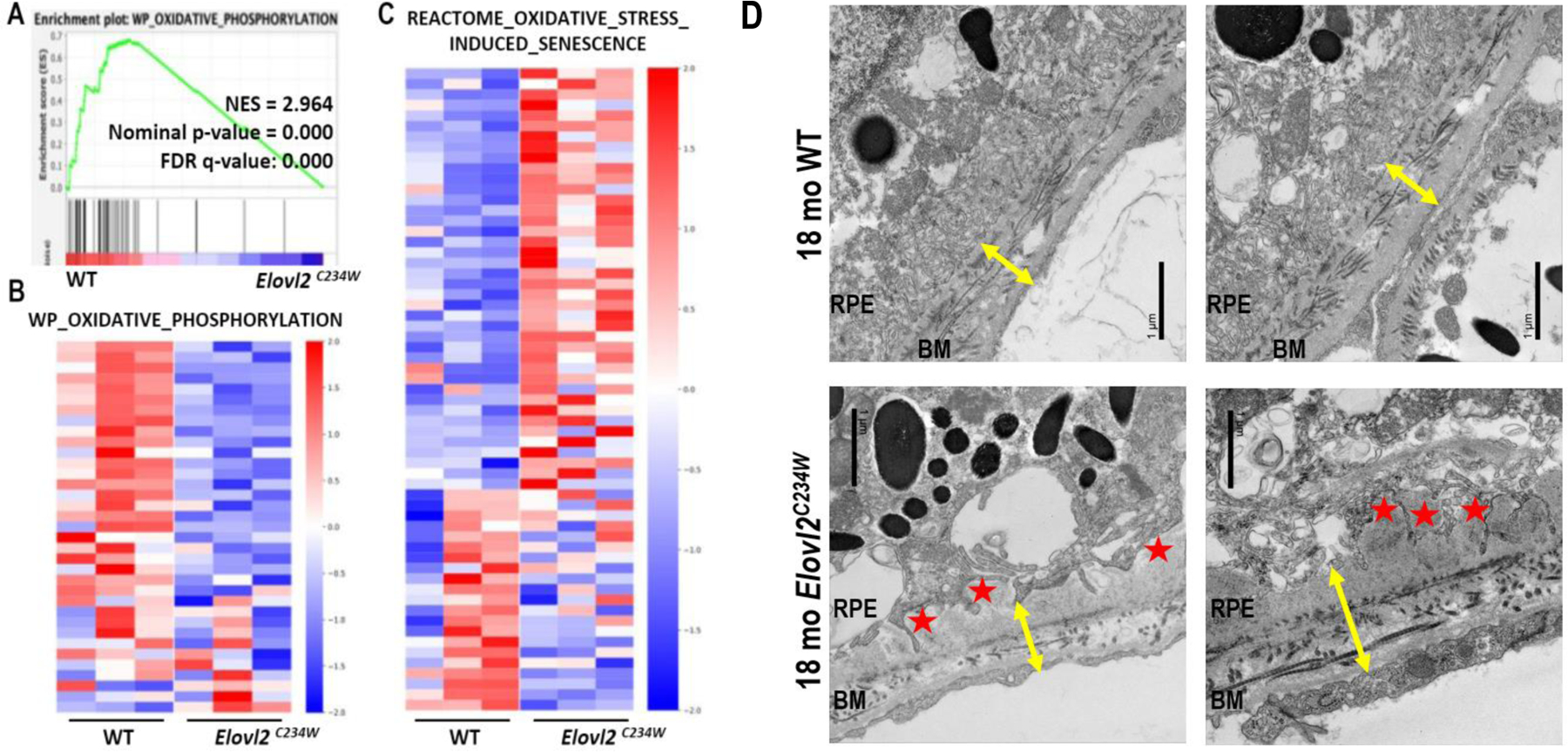
Gene set enrichment analysis (GSEA) was performed on RNA-Seq data from the retina of Elovl2C234W and WT mice. A. “Oxidative Phosphorylation” pathway is significantly downregulated, as shown on the enrichment plot. B. Gene sets “Oxidative Phosphorylation” and C. “Oxidative Stress Induced Senescence’’ with genes ranked by WT/Elovl2C234W in mean log2Fold Change of expression level. Color bars indicate row Z-score. In the analysis, we used 4-month-old mice (N=4 for each genotype) and Molecular Signatures Database (MSigDB). D. EM analysis of Bruch’s Membrane (BM). Comparison of 18-month-old WT and Elovl2C234W RPE/BM surfaces show thickening and disorganization of BM (yellow arrows), including accumulation of basal lamina deposits (BLamD - red stars) and distorted RPE infoldings
II.2.2. Phospholipids
Phospholipids constitute a major fraction of the total retinal lipid content. Photoreceptors express components of both major pathways contributing to the diverse phospholipid composition: the de novo biosynthesis (Kennedy) pathway and the remodeling pathway (known as Land’s cycle) (Figure 4). Biosynthesis first leads to the generation of a common precursor of all phospholipids, PA, through a multistep process involving glycerol-3-phosphate acyltransferase (GPAT) and 1-acylglycerol-3-phosphate acyltransferase (AGPAT, also known as lysophosphatidic acid acyltransferase: LPAAT) enzymes. AGPAT3, the most abundant AGPAT in both photoreceptors and RPE (Figure 4), is responsible for incorporating the majority of DHA into OS phospholipids. Mice devoid of Agpat3 show a specific and almost complete lack of DHA and VLC-PUFA-containing phospholipids in the retina, leading to an abnormal morphology and organization of the OS disks (Shindou et al., 2017). Interestingly, the retinal degeneration and visual function decline associated with DHA and VLC-PUFA deficiency progress faster in those mice, compared to the previously mentioned Mfsd2a KO phenotype (Lobanova et al., 2019), suggesting a pivotal role of AGPAT3 in the retinal phospholipid supply. Such a conclusion, however, awaits further verification with the retina-specific genetic approaches as well as lipid supplementation studies. (Hishikawa et al., 2017; Nagata et al., 2021).
The FA composition of phospholipids can be further modified by the sequential action of PLA2 and lysophospholipid acyltransferase (LPLAT) families of enzymes, involved in the deacylation-reacylation cycles known as the Land’s cycles (Figure 3B). LPCAT1, an LPC-specific LPLAT, was shown to facilitate the conversion of palmitoyl-LPC to dipalmitoyl-PC (DPPC) mainly distributed in the IS structures of the photoreceptor cells (Anderson et al., 2014). KO of Lpcat1 leads to retinal degeneration (known as rd11 mouse model), confirming again the importance of in situ lipid biosynthesis in preserving photoreceptor integrity (Akagi et al., 2016; Friedman et al., 2010). Interestingly, an LRAT-related phospholipase A and acyltransferase 3 (PLAAT3, also known as HRASLS3 or AdPLA) (Golczak et al., 2015), occurs as the most abundant enzyme from the phospholipid metabolism pathway in the RPE layer (Figure 4). PLAAT3 shows both phospholipase A1 and A2 activity leading to the generation of LPC and LPE (Chatterjee et al., 2021), which suggests its important role in the lipolysis process, associated with both uptake and recycling of lipids, as will be described further in this review.
II.2.3. Sphingolipids
The first steps of sphingolipids de novo biosynthesis occur in the endoplasmic reticulum (ER) and involve condensation of serine and palmitoyl-CoA into a common precursor, dihydroceramide, through a sequential action of serine palmitoyltransferase (SPT; encoded by SPTLC1–3), 3-ketodihydrosphingosine reductase (KDSR), and ceramide synthases (CERS1–6) (Gault et al., 2010), all more abundant in RPE than rods or cones (Figure 4). Recently, TLCD3B, a novel ceramide synthase, has been discovered and associated with human recessive retinal dystrophy (Bertrand et al., 2021). SPT constitutes the rate-limiting enzyme in the process, while mutations in SPT lead to the accumulation of toxic atypical deoxysphingolipids (Table 1). Serine deficiency, deoxysphingolipid accumulation, and SPT mutations were associated with macular telangiectasia, a rare macular disease that leads to loss of central vision (Chapter III), underscoring the importance of sphingolipid metabolism for photoreceptor health. Ceramides are further synthesized in the ER, but to begin sphingomyelin production, they must first be transported to the trans-Golgi region by the ceramide transferase (CERT1) or vesicular transport pathways (Hanada et al., 2009). In the trans-Golgi, ceramide can also be phosphorylated to C1P by CERK, an essential enzyme for photoreceptor health.
Apart from the de novo synthesis, ceramide generation can occur through the sphingomyelin hydrolysis or through the salvage pathway in which complex sphingolipids are converted back to sphingosine and ceramides. Catabolism of sphingomyelin and other complex sphingolipids involves the action of sphingomyelin phosphodiesterases (also known as sphingomyelinases; SMases). These enzymes differ in subcellular localization, optimal pH range, and cation dependence (Marchesini and Hannun, 2004). In contrast to the de novo pathway, the sphingomyelinase pathway can rapidly generate ceramides through a single type of enzyme upon activation by various stimuli, a fact which is essential for precise signal transduction (Chapter I). This prompt response is possible due to the abundance of sphingomyelin in the membrane (Canals et al., 2018). Mutations in SMPD1, the most abundant sphingomyelin phosphodiesterase (SMPD) in the photoreceptors and RPE (Figure 4), lead to human lysosomal storage disorder manifested by retinal stigmata, among many other symptoms, suggesting a direct link between photoreceptor health and the retinal sphingolipid hydrolysis capacity (Gault et al., 2010).
The salvage pathway of ceramide generation comes from the breakdown of complex sphingolipids, enzymatically degraded through non-SMase hydrolases such as cerebrosidases, galactosidases, and ceramidases in acidic compartments (late endosomes/lysosomes). The resulting ceramide cannot be released from this compartment and must first be hydrolyzed by N-acylsphingosine amidohydrolase 1 (ASAH1), forming a free FA and sphingosine. The latter is then re-utilized in the ER to regenerate ceramides via CERS (Kitatani et al., 2008). The mouse model with deficient Asah1 shows retinal dysplasia and optic nerve pathology accompanied by abnormal accumulation of ceramides and other sphingolipids in the retinal tissues, which is characteristic of Farber’s disease (Table 1) (Yu et al., 2019). Exogenous ceramide can also be salvaged and converted to endogenous ceramide by the reverse activity of ceramidases (Novgorodov et al., 2011). C1P and S1P are generated through their phosphorylation by CERK or one of the two sphingosine kinases (SPHK), respectively (Hait et al., 2006; Wijesinghe et al., 2005). It was shown that adiponectin receptors (ADIPOR1 and ADIPOR2) possess high structural homology with intracellular alkaline ceramidase ACER3 (Vasiliauskaité-Brooks et al., 2018), and that adiponectin promotes ceramide degradation through its receptors (Holland et al., 2011). This observation was also confirmed by the Granier group, which demonstrated that both ADIPORs have ceramidase activity, which can be further stimulated through the binding of adiponectin (Vasiliauskaité-Brooks et al., 2017). Interestingly, ADIPOR1 mutations in humans are associated with syndromic and non-syndromic RP, and were also identified as a genetic risk factor for AMD in the Finish population (Kaarniranta et al., 2012; Xu et al., 2016; Zhang et al., 2016). Except for retina diseases mentioned above, impaired metabolism of sphingolipids, resulting in their dyshomeostasis, have been observed in AMD patients with choroidal neovascularisation (CNV) and geographic atrophy, in patients with diabetic retinopathy, retinal ischemia and other pathologies (Fan et al., 2016; Shiwani et al., 2021; Wilmott et al., 2019).
II.3. Lipid cycling
II.3.1. Transport through the interphotoreceptor matrix (IPM)
Lipid transport from the RPE to photoreceptor cells is by far the least understood part of the entire lipid cycle. Interphotoreceptor retinoid-binding protein (IRBP), a prevalent protein in the IPM initially recognized as a major retinoid carrier (Zeng et al., 2020), has been shown to efficiently bind various FFAs, including oleic acid and DHA (Chen et al., 1993; Ghosh et al., 2015; Semenova and Converse, 2003). In native conditions, IRBP associates with several FA chains and possesses two retinoid-binding sites, of which one has been shown to release 11-cis-retinal specifically upon DHA binding (Chen et al., 1996). IRBP undergoes rapid turnover in the IPM involving endocytosis into the RPE and photoreceptor IS, suggesting one intake route for the bound cargo (Cunningham and Gonzalez-Fernandez, 2003). The presence of albumin, the common FFA carrier of the blood, has also been postulated in the IPM (Adler and Edwards, 2000; Duncan et al., 2006). Unlike the RPE, both rods and cones lack MFSD2A while at the same time expressing ADIPOR1 at the highest levels in the retina (Figure 1). ADIPOR1 promotes DHA uptake and biosynthesis of VLC-PUFAs in the IS, mostly localizing to the OS structures of photoreceptor cells (Rice et al., 2015; Sluch et al., 2018). Proteins associated with lipoprotein particle formation and transport have also been identified in the subretinal space. Apolipoprotein A1 (APOA1), the major HDL constituent, is present in the apical portion of the RPE cells, rod IS, and the IPM (Tserentsoodol et al., 2006a). Its major transporter, ABCA1, is found in both the RPE apical membrane and photoreceptor IS membranes, suggesting an active HDL-mediated transport through the IPM (Ananth et al., 2014; Tserentsoodol et al., 2006a).
II.3.2. Lipid trafficking in photoreceptor cells
Upon delivery to photoreceptors, FFAs preferentially accumulate in the IS, and their uptake is coupled with subsequent esterification into phospholipids (Rodriguez De Turco et al., 2009). Biosynthesis of phospholipids occurs at a high rate in the ER and Golgi system of the IS, and photoreceptor-expressed FABPs (FABP7 (Su et al., 2016), FABP12 (Liu et al., 2008) likely assist in channeling FFAs towards these sites. OS disk formation, which consumes a significant portion of the newly synthesized phospholipids, takes place in the base of the OS structure, separated from the IS by a narrow connecting cilium (CC). Vesicles transport lipids, as well as newly synthesized OS membrane proteins, across the IS to the periciliary ridge complex at the base of the CC structure (Besharse and Pfenninger, 1980; Papermaster et al., 1985). Both conventional and unconventional (trans-Golgi omitting) secretory pathways seem to be utilized in this process (Imanishi, 2019). Since OS proteins may directly interact with lipids, (a prominent example being rhodopsin’s interaction with cholesterol and DHA (Albert et al., 1996; Grossfield et al., 2006; Soubias and Gawrisch, 2005)), it is plausible to speculate that just as vesicles provide means of transmembrane protein transport to OS, the proteins themselves may provide means of certain lipid trafficking. However, it was shown that some aspects of protein and lipid transport act independently of each other, as was the case for cholesterol precursors (Fliesler and Keller, 1997). The CC creates a barrier against membrane protein and lipid diffusion between the IS and OS compartments (Nachury et al., 2010). Most rhodopsin molecules cross the CC via the ciliary plasma membrane, suggesting the same fate for at least a portion of the accompanying vesicular phospholipids (Burgoyne et al., 2015; Wolfrum and Schmitt, 2000). Nonetheless, vesicular structures have been observed within the CC lumen, implicating the existence of alternative protein and lipid transport modalities through the CC (Chuang et al., 2015; Gilliam et al., 2012). As revealed recently, nascent OS disks are formed through evaginations of the plasma membrane at the neck of OS structure, and this region is devoid of any vesicles (Spencer et al., 2020). Overall, it strongly suggests that most of the OS-destined lipid (and protein) transport occurs via the ciliary plasma membrane, continuous with the OS plasma membrane. Since the OS disk membrane differs in terms of both lipid and protein composition from the plasma membrane surrounding the structure (see Chapter I) it is evident that another sorting mechanism for both classes of molecules must be in place at the site of new disk formation (Nemet et al., 2014). Molecular details of this process, however, remain unknown.
II.3.3. Outer segment lipid dynamics
It takes approximately 10 days for the newly synthesized OS disk in the mammalian eye to reach the apical side of the OS structure, where it ultimately becomes detached and subjected to the RPE-mediated phagocytosis process (Young, 1967; Young and Bok, 1969). As a disk gradually moves along the OS longitudinal axis, its membrane proteins remain stably associated with that particular disk, without any measurable turnover (Hall et al., 1969). In stark contrast, using biochemical and autoradiography assays, disk lipids were shown to freely diffuse to other disks and the plasma membrane of the OS structure (Bibb and Young, 1974a, 1974b). Different rates of OS incorporation and turnover were observed for the individual phospholipid classes, as well as fatty acyl chains (Gordon and Bazan, 1990; Louie et al., 1988). Yet, the disk’s membrane lipid composition undergoes significant changes as it moves from the basal to the apical side of the OS structure, most notably a decrease in cholesterol and increase in the unsaturated fatty acyl chain content (Chapter I). The mechanisms underlying this process are not known and may involve properties of certain proteins present in the OS to bind and translocate lipids between membranes, as well as lipid-lipid interactions. Significant differences in phospholipid headgroup composition between the OS disk and plasma membranes were suggested to favor gradual cholesterol transfer to the latter (Albert et al., 2016). In addition, cholesterol inherently segregates away from the unsaturated lipid acyl chains, DHA in particular, supporting the formation of their respective opposing gradients in the OS structure (Wassall and Stillwell, 2009). As discussed earlier in this review, the role of such gradients is not fully understood. However, since the lipid composition of a particular disk is directly correlated with its age (as opposed to the relatively stable protein composition), it could potentially serve a role in initiating the old disk clearance at the tip of the OS structure. Multiple synergizing factors make the OS membranes especially prone to lipid oxidation (Organisciak and Vaughan, 2010), as also discussed in the context of aging further in this review (Chapter III). Those factors include the exceptionally high PUFA content, light exposure, high metabolic rate of the photoreceptor cells, high overall oxygen tension in the outer retina, and possible lipofuscin accumulation in the RPE with age. In fact, similar reasons help to explain why OS structures are subject to such an intensive renewal process. While lipid oxidation products accumulate over time, it is yet to be determined what their distribution is across the OS structure and whether the already described lipid gradients affect their generation process.
II.3.4. Phagocytosis
Every day the distal portion of the photoreceptor OS, equal to around 7–10% of the entire structure, is removed by the RPE cells through an evolutionarily conserved, multi-step process known as phagocytosis. The process has been the subject of several excellent recent reviews (Kwon and Freeman, 2020; Lakkaraju et al., 2020). PS externalized to the outer leaflet of the OS tip plasma membrane constitutes the earliest known hallmark of the phagocytosis initiation (Ruggiero et al., 2012). Asymmetric PS distribution to the inner leaflet in virtually all healthy cell membranes is tightly maintained by cellular flippases and only lost upon the apoptosis induction. Consequently, localized conditions resembling apoptosis initiation are suspected to occur diurnally at the OS tip, though the molecular details of this process remain unknown. It is further speculated that some form of diffusion barrier needs to be present to prevent the expansion of the externalized PS signal beyond the photoreceptor tip. Once externalized, PS is bound by a number of proteins secreted to the IPM, including growth arrest-specific protein 6 (GAS6), vitamin K-dependent protein S (PROS), and MFGE8. These in turn function as ligands for the two major receptors present in the RPE apical membrane known for their involvement in the phagocytosis initiation: Mer tyrosine kinase (MERTK) and αvβ5 integrin. While roles of MERTK-GAS6/PROS and αvβ5 integrin-MFG-E8 complexes with PS are well established in the context of RPE phagocytosis (Kwon and Freeman, 2020; Lakkaraju et al., 2020), additional pathways involving protein-lipid interaction likely play a supporting role in the orchestration of the process. Lipid scavenger receptor CD36, involved in the oxidized LDL cleanup from the subretinal space (Picard et al., 2010), was shown to specifically bind several oxidized PC and PS species present in the OS, and this binding was suggested to direct the engulfment of the photoreceptor tips by the RPE apical processes (Greenberg et al., 2006; Sun et al., 2006). Although intensely studied, the full mechanism of phagocytosis, the regulation of its onset and decline, as well as precise timing of ingestion vs. maturation of phagosomes have to be reevaluated using contemporary technologies.
II.3.5. Lipid recycling and repurposing in RPE
OS phagosomes formed at the apical membrane of the RPE undergo maturation as they gradually shift towards the basal side of the cell. The process involves fusion with endosomes and lysosomes aimed primarily at fast degradation of the large quantities of the OS material ingested every day. Each RPE cell contacts many photoreceptors, up to 30 in humans and 200 in mice (Gao and Hollyfield, 1992; Volland et al., 2015). Ultimate resolution of the phagolysosomes must occur at the end of each daily cycle to prevent the accumulation of cell debris while simultaneously allowing the recycling and repurposing of many nutrients. The RPE expresses several lysosomal lipid hydrolases at significantly higher levels than rods or cones, reflecting their important role in RPE biology. This includes pH-dependent phospholipases A1 (PLA1) and PLA2, which aid in the release of FFAs from phospho- and glycolipids (Swartz and Mitchell, 1973). The lysosomal acid lipase type A (LIPA) de-esterifies the cholesterol molecules. Breakdown of the ingested lipids constitutes an important step in phagosome maturation, and its inhibition results in both lipid and rhodopsin accumulation in the RPE. This observation, originating from the Pnpla2 conditional knock-out in the RPE (Bullock et al., 2021), suggests that lipase-mediated OS lipid breakdown is required for efficient hydrolysis of the accompanying OS proteins. The abundance of the released FAs comparable to the levels metabolized daily by hepatocytes creates a challenge, but also an opportunity for the postmitotic RPE layer. Similarly, like the liver, RPE expresses enzymes involved in energy production through the saturated and unsaturated FAO and ketogenesis pathways (Reyes-Reveles et al., 2017). At the same time, the presence of the high levels of major glucose transporter GLUT1 in both basal and apical membranes of the RPE combined with the known suppressive role of lactate in the RPE was suggested to support the notion of direct transfer of glucose from the choroid vasculature to photoreceptors instead of its catabolism by RPE cells (Kanow et al., 2017; Swarup et al., 2019). While the extent of glucose utilization by the RPE requires further investigation, the RPE is believed to use FAO to meet a significant portion of its energy requirements (Fu et al., 2021). However, the retina is known to preserve DHA content even upon prolonged n-3 FA deprivation, which early on suggested that DHA and likely other lipids are in part directly recycled back to the photoreceptor cells for non-energy purposes (Bazan et al., 1992; Chen and Anderson, 1993; Tinoco et al., 1977). Further studies utilizing biochemical and autoradiography assays showed that DHA ingested by the RPE upon phagocytosis is transiently converted into TAGs and quickly recycled back to the photoreceptor cells (Gordon et al., 1992; Rodriguez De Turco et al., 1999; Stinson et al., 1991). The process is selective towards the DHA over other FA species, however, it is unclear how this selectivity is obtained and which exactly proteins are involved in its orchestration.
Chapter III. Lipids in retinal aging and disease
As mentioned above (Chapter I), lipid composition affects membrane structure and properties, such as viscosity. Since viscosity is an essential factor in determining the rate of membrane-related reactions including cell signaling, cell adhesion, and enzyme binding/activity, it plays a crucial role in aging processes. Both headgroup and tail chemistry can significantly modulate lipid diffusion (Seu et al., 2006). Therefore, it is not surprising that the the role of lipids, lipid composition and lipid metabolism in aging is under active investigation in several laboratories.
III.1. Lipids in aging
Aging is a complex, multifactorial process characterized by a progressive deterioration of tissue structure and function. Aging changes are driven by intrinsic (genetic) and extrinsic (environmental) factors. Because aging is the single greatest risk factor for the development of age-related eye diseases such as AMD and glaucoma, understanding the molecular mechanisms of aging presents an opportunity to find new therapeutic approaches in biomedical science. Changes in visual capability in later adulthood impacts patients’ ability to perform common everyday visual tasks, thereby influencing a patient’s quality of life and well-being. Several studies have focused on analyzing age-related changes in the lipid composition of human postmortem retinas and in mice.
Lipids play an important role in the mechanism of aging in the central nervous system (Layé et al., 2018; Singh et al., 2019; Wang and Michaelis, 2010). Multiple groups have measured changes in various lipid levels to assess their association with healthy aging, longevity, and disease. Recent development of high-tech methods for detection and quantification of lipids has accelerated the development of the field. However, much is still to be done to parse lipids’ causative vs. correlative roles in the aging process.
The change in membrane lipid composition with age has a number of characteristics. First, there is usually an increase of “rigid” lipids (cholesterol, sphingolipids, and degree of saturation in the FAs acyl chain). Second, several changes in lipid composition observed in one tissue can be translated to other tissues. For example, increases in cholesterol in plasma and decreases of PUFAs in plasma have been correlated to changes in cholesterol levels in the brain and eye. Lastly, it seems that changes in membrane fluidity materialize quite late compared to other cellular functions, which suggests that membrane function is tightly maintained and presents a metabolic challenge to the cell during the aging process (Skowronska-Krawczyk and Budin, 2020).
Photoreceptors
The photoreceptor OS is built from stacks of membranous discs with specific lipid composition (discussed in Chapter I), which fluctuates with age as reported by multiple laboratories. For example, changes in FA composition in the eye were quantified by several groups through the years (Bretillon et al., 2008; Liu et al., 2010; Prokopiou et al., 2019). Very little to no age-related change was observed in FAs such as 16:0, 18:0, 18:1, 18:2, 18:3, 20:4, 22:4, 22:5 and 22:6. The level of DHA is particularly well-maintained in the aging retina (Liu et al., 2010). Intriguingly, levels of VLC-PUFAs (>26C) were consistently lower in aged donors and severely reduced in AMD donor eyes. These changes were consistently accompanied by an altered ratio of n-3/n-6 PUFAs (Liu et al., 2010), with higher n-6 FAs correlating with decreased visual function.
Other changes have also been observed in aged retinas. For example, an age-dependent increase in membrane micro viscosity in rod OS has been reported, suggesting a decrease in membrane fluidity during aging (Ohia et al., 1994). This brings an important question, whether specific membrane composition changes affect the primary function of photoreceptors – the visual cycle. With our recent studies pointing to the specific lipidomic environments for different OS proteins (Sander et al., 2021), this question becomes even tractable experimentally.
Aging of the human retina is accompanied by elevated ROS levels and reduced antioxidant capacity (Ohia et al., 1994) adding to the already high oxygen tension in the outer retina, caused by low oxygen extraction for the venous blood leaving the eye. ROS are mainly produced in mitochondria during normal oxygen metabolism and signaling molecules through the redox pathway (Veal et al., 2007). However, excess ROS causes oxidative damage to lipids, proteins, and DNA, resulting in cell dysfunction and apoptosis. Highly abundant PUFAs in the retina are the primary targets of free radical-induced lipid peroxidation, since the susceptibility of unsaturated FAs to oxidation increases with the number of double bonds. Lipid peroxidation may affect the retina in several ways. Anderson’s group observed the loss of PUFAs and the accumulation of lipid hydroperoxides in rod OS isolated from albino rats exposed to constant illumination (Wiegand et al., 1983). Therefore, they proposed that lipid peroxidation was an important factor in light-induced retinal degeneration (Anderson et al., 1985, 1984; Wiegand et al., 1983). Palczewski group (Maeda et al., 2006) showed that retinol dehydrogenase 12 (RDH12) might play a role in the detoxification of lipid peroxidation products (Belyaeva et al., 2005; Lee et al., 2008). They concluded that RDH12 could protect photoreceptors from light-induced degeneration in mice because the Rdh12−/− mice showed increased sensitivity to light-induced photoreceptor apoptosis. Another group (Nag et al., 2019) detected changes in aged vessels, including degeneration of endothelial cells and pericytes, accumulations of lipofuscin granules, loss of filaments in pericytes, and thickening of the capillary endothelial and pericyte basal lamina. Immunohistochemistry for a biomarker of oxidative stress (4-hydroxy 2-nonenal [4-HNE]) was significantly localized in aged capillaries and arteries, suggesting that in the aged retina, elevated lipid peroxidation may damage vessels, followed by limitation of the energy supply to the neurons and eventually leading to neuronal loss in the inner retina. Oxidative damage of PUFAs generates different products, including malondialdehyde (MDA) and 4-HNE are observed in aged retinas but at increased levels in AMD (Castorina et al., 1992; Dhingra et al., 2018). Peroxidation of DHA-containing lipids generates carboxyethylpyrrole (CEP) easily detectable in AMD retina and in AMD patients plasma (Crabb et al., 2002; Gu et al., 2003). Noteworthy, immunization with a hapten generated by oxidative damage to the DHA present in the drusen is sufficient to produce AMD-like lesions in mouse retina, providing the most robust evidence for the role of these molecules in AMD genesis (Hollyfield et al., 2008). Other metabolites of LC-PUFA peroxidation present in the retina, such as isoprostanes and neuroprostanes, derived from AA and DHA, could contribute to retinal microvascular degeneration by directly inducing the death of retinovascular endothelial cells (Hardy et al., 2005). Finally, lipid peroxidation can also affect phagocytosis. Moderate oxidation induced-phototoxicity in ARPE-19 cells decreases their phagocytic activity (Pawlak et al., 2019). Similarly, in older retinas, increased lipid peroxidation is accompanied by a moderate decrease in the phagocytic activity of RPE cells (Inana et al., 2018).
Not surprisingly, the delivery of antioxidants is regarded as a promising approach to protecting the retina from light damage, aging, and AMD. Early studies of the Tso group have shown that several types of antioxidative molecules can accelerate the retina after photic injury recovery (Lam et al., 1990; Ll et al., 1993), while others have shown an increased lipid peroxidation upon lack of antioxidants, such as vitamin C or E and their protective role in response to retinal light damage (Li et al., 1985; Noell et al., 1987; Wiegand et al., 1986). These and other results precipitated the interest in using antioxidants to protect against age-related eye diseases such as AMD and were at the basis of formulation of the supplement used in Age-Related Eye Disease clinical Study (AREDS). AREDS was a randomized controlled trial set to evaluate the effects of high-dose zinc and antioxidants on the progression of AMD and vision loss. The study found that the proposed combination reduced the risk by 25% in those at high risk of developing advanced AMD and reduced the risk of moderate vision loss by 19%. No benefit was observed in patients with no AMD or early AMD after supplementation (Chew, 2013; Evans, 2008). Further studies have shown that supplementation with natural antioxidants, such as lutein and zeaxanthin (both used in AREDS supplement), can improve macular pigment and visual function in patients with early AMD due to their ability to scavenge free radicals, absorb damaging blue light, and neutralize photosensitizers and ROS (Ma et al., 2012; Widomska, 2014).
Current studies are focused on finding new and more specific antioxidants. Recently, mitochondria-targeted antioxidant SkQ1 has proved effective in protecting rabbit retinas from light-induced oxidative stress (Baksheeva et al., 2019). Other efforts are focused on using deuterated DHA, where deuterium protects unsaturated bonds from oxidation, and only 20–25% deuteration prevents lipid peroxidation (Shchepinov, 2020). Several recent studies seem to confirm this hypothesis, for example, in RPE cell culture settings (Rosell et al., 2019) but it has not yet in animal models of retinal diseases. If successful, supplementation with deuterated DHA could provide an easy way to protect the retina from oxidative stress, and could be conveniently added to any supplement containing PUFAs and other antioxidants.
RPE aging
Age-related changes in the retina are concurrent with the aging of RPE. Aging RPE cells have been shown to change morphology accompanied by early documented cell loss in the fovea at the rate of approximately 0.3% per year(Panda-Jonas et al., 1996) and may increase the metabolic demand on the remaining cells. In addition, RPE endures significant aging-related changes such as the increase in senescence (as detected by senescence-associated b-galactosidase staining), telomere loss, DNA damage, and protein and lipids peroxidation (Cao et al., 2013; Matsunaga et al., 1999). These phagocytic cells have very long and thin microvilli on their apical surface intertwined with the tips of the rod and cone photoreceptor OS (Bosch et al., 1993). With aging, microvilli length is shorter (Bonilha et al., 2006), and the interaction with the photoreceptor OS is less interwoven (Katz and Robison, 1984; Lai and Rana, 1986). This observation is consistent with the hypothesis of change of lipids composition of RPE membrane in aging, but there is no data yet to suggest the potential age-related mechanism that could play a role in this process.
RPE cells are also under constant oxidative stress due to their unusually high levels of oxygen and exposure to light(Algvere et al., 2006; Bazan, 2008; Cai et al., 2000). In addition, the daily recycling of membranes that contain oxidized lipids and proteins as well as high exposure to toxic retinoids provides constant metabolic pressure and involves continuous activity of machinery controlling the stress levels (recently reviewed in (George et al., 2021)). The role of oxidative stress has been further shown in multiple studies with cultured primary or immortalized RPE cells and in many statistical studies correlating the cigarette smoking, i.e., oxidative environment, with RPE dysfunction (e.g. (Bertram et al., 2009; Espinosa-Heidmann et al., 2006) and more recent (Cano et al., 2021; L. Wang et al., 2020). Interestingly, smoke exposure in mice and cultured RPE cells has also been shown to induce lipid accumulation, as shown by the Roher group using several lipid staining procedures (Kunchithapautham et al., 2014). Most importantly, the use of antioxidants protect against the RPE cell death (e.g. (Cai et al., 2000; Kamoshita et al., 2016; Yu et al., 2012), however, whether these treatments are lowering the levels of oxidized lipids in the human retina still needs to be evaluated.
One of the most common age-related phenotypes of RPE is the accumulation of lipofuscin, also called “aging pigment”, within lysosomes and melanolysosomes in the basal side of the cell, accumulating in aged individuals (Feeney-Burns et al., 1984; Feeney, 1978). Specific distribution and number of specific granules have been recently observed by Bermond et al., suggesting different mechanisms of build-up between the fovea region and perifovea (Bermond et al., 2020). Although the exact composition of lipofuscin is not yet resolved, it is well documented that lipids, including peroxidized lipids, lipid-binding proteins, and toxic retinoids, are contained in these structures; however, the ratio of different components may differ depending on the region of the eye (Panda-Jonas et al., 1996). This accumulation of oxidative species in lipofuscine provides continuous low level of oxidative stress in the cell. Lysosomes, organelles extremely active in RPE, when filled with lipofuscin, are less effective in processing photoreceptor OS (Feeney-Burns et al., 1987; Shamsi and Boulton, 2001), delaying the hydrolysis of phospholipids (Finnemann et al., 2002), therefore disturbing the balanced lipid-based interaction between photoreceptors and RPE.
Lipofuscin is also abundant in extracellular deposits below RPE and in Bruch’s membrane, which accumulation with age may be involved in drusen formation (Marshall et al., 1998). Additionally, age-dependent accumulation of lipids deposits has been observed underneath the RPE. These sub-RPE deposits also called basal lamina deposits (BLamD) (Curcio, 2018), increase with the progression of normal aging and are associated with an increased risk of developing AMD. Drusen are focal deposits located below RPE basal lamina. Accumulation of drusen deposits is aggravated in AMD patients where the phenotype is accompanied by thickening of the Bruch’s membrane (Curcio et al., 2011; Li et al., 2005), activation of inflammatory events, and damage of the tissue. Dedicated studies were performed to describe and define the different types of deposits and correlate them with aging and progression of AMD (reviewed in (Curcio, 2018)). The presence and identity of lipids in drusen have been studied for many years. EM studies have detected different types of droplets and lipoproteins aggregates under the RPE. Although it is understood that part of the material in drusen consists of phagocytosed photoreceptor OS, the presence of lipid-like droplets in the exact location has been at the origin of a diet-based hypothesis of drusen (reviewed in (Bergen et al., 2019) and (Curcio, 2018)). While the accumulation of lipid droplet-like deposits in LDL-R KO mice seem to support the diet-origin of deposits in BM (Schmidt-Erfurth et al., 2008), detection of products of DHA oxidation, such as carboxyethylpyrroles (CEPs) and 4-Hydroxy-7-oxo-5-heptenoic acid (HOHA) lactone (Linetsky et al., 2020; Salomon et al., 2011) is congruent with the idea of the phagocytic debris origin of drusen (Bergen et al., 2019). Of note, the above-cited studies with antibody-based detection of CEP were only possible thanks to the development of an antibody specific for the molecule. The availability of such antibodies is scarce but highly desired in the field focused on understanding lipids’ role on the cellular and sub-cellular levels. Finally, the composition of lipoproteins in the deposits also supports the phagocytic origin of lipids. Therefore, it is highly probable that both mechanisms add to the loss of functionality of BM and RPE in age. For example, due to the presence of RPE-derived deposits blocking the delivery of lipids from the circulation, local lipids and lipid-transporter proteins start to accumulate and form lipid-like droplets, adding to the local traffic. With time drusen undergo calcification and show accumulation of hydroxyapatite (Tan et al., 2018) which suggests the contribution of the vitamins and minerals exchange in the process of drusen aging.
One of the major consequences of accumulating deposits in the BM is a blockage of molecules exchange between circulation (choroidal vessels) and the retina. Multiple macromolecules are trafficking from circulation, and this process directly depends on the rate of diffusion of BM called hydraulic conductivity (HC). Studies measuring this process started already in the late 1970s, have established that HC of this compartment declines exponentially with age (Hussain et al., 2010; Starita et al., 1995; STARITA et al., 1996). Very early, it has been suggested that the age-related changes in BM could be responsible for this decrease (Starita et al., 1997). Aquaporin 1 expression has also been shown in the regions containing drusen, most probably to help the fluid transport. Unfortunately, studies involving measurements of HC ceased with time, leaving many questions unanswered. For example, it would be exciting to understand what components of deposits have the highest contribution to the age-related HC decrease. Additionally, population studies could address questions whether this biophysical property of BM is dependent on diet. Finally, it would be interesting to figure out whether any treatments could help increase HC in aging. As an example, Marshall’s group showed that treatment with steroidal glycoside could slightly improve the diffusion rate in BM in vitro (Lee et al., 2015). Notably, lipid deposits in AMD precede disease symptoms; understanding these features is one of the current goals in eye research to better detect the disease and provide early treatment to patients.
Molecular mechanism
Very little is known about the molecular mechanism of age-related changes in lipid synthesis or metabolism regulation. Two recent studies shed light on potential mechanisms involved in lipid-dependent aging phenotype. It is well established that DNA methylation patterns progressively change during aging (Noroozi et al., 2021; Raj and Horvath, 2020). Within the top 10 markers of aging, four are localized in the regulatory element of ELOVL2 gene (Hannum et al., 2013). Consequently, methylation of the ELOVL2 regulatory region has been shown in many studies to correlate with the biological age of individuals (Hao et al., 2021; Sukawutthiya et al., 2021). The correlation has also been confirmed for rodents (Wang et al., 2017). DNA methylation impacts the properties of DNA and therefore affects the chromatin structure and function, implying the changes in gene expression (Campello et al., 2021; Telese et al., 2013). In WT mice, we demonstrated that the expression of Elovl2 gradually declines in the aging retina, concomitant with reduced visual function (Chen et al., 2020). Taken together and with the data generated using the mouse lacking the ELOVL2 enzymatic activity (see chapter II) our work provides a potential direct genetic link between aging and age-related vision loss.
In another study, Swaroop and colleagues investigated epigenetic changes in aging photoreceptors (Corso-Díaz et al., 2020). The group also focused their efforts on the age-related pattern of DNA methylation, specifically in rods. After correlating differentially methylated regions with gene expression in aging rods, they noted a pattern of changes in genes associated with metabolism and mitochondrial oxidative respiration. Their results suggest that the changes in mitochondrial respiration are related to increased reliance on lipid beta-oxidation with age, therefore showing age-related changes in lipid homeostasis. Interestingly, mitochondrial membrane composition may affect mitochondrial function (Budin et al., 2018). Further studies should follow to understand the molecular basis of these changes, including the signals that promote this change in mitochondria metabolism. Of note, the aforementioned Corso-Diaz et al. studies did not detect changes in Elovl2 expression most probably because of its low level of expression in rods (Figure 4) (Weir et al., 2021; Yan et al., 2020).
Overwhelming evidence of the role of lipids in retina aging and several variants or mutations in proteins involved in lipid metabolism (Table 1) brought the attention of researchers to the lipid transporters and lipid-binding proteins. Apolipoproteins, which are major components of lipids transport particles, are at the center of studies related to aging and age-related eye diseases. ApoE is a cholesterol particle transporting protein, expressed predominantly in RPE. Human ApoE protein exists in three variants, namely ApoE2, ApoE3, and ApoE4. Single amino-acid differences between these forms change their affinity to the LDL receptor as well as the ability to form HDL particles. In particular, ApoE2 protein has a low affinity to the LDLR receptors, therefore, it is not efficient in delivering cargo (mostly HDL) through this pathway. This isoform, however, still maintains the ability to bind to other lipid receptors. Population studies show that ApoE2 form correlates with longevity as higher frequencies of APOE2 in elderly individuals and centenarians were detected compared to younger populations, whereas the frequency of APOE4 is lower in the oldest individuals (Sebastiani et al., 2019). This particular correlation may be one of the key factors causing higher correlation of this variant with the risk of AMD (see III.2). Studies dividing the AMD patients into age groups to correlate ApoE2 variant frequency in each group are yet to be performed.
III.1.2. Bioactive lipids in aging
PUFAs and their metabolites, such as eicosanoids, docosanoids and elovanoids (as mentioned in chapter II) are the most common BLs. They are not only components of cellular membranes but also act as signaling molecules (Elmasry et al., 2019) . BLs are involved in several cellular functions and biological processes that are altered in aging. There is usually a decrease in the formation of PUFAs in age-related disorders (Liu et al., 2010), accompanied by an increase in the production of pro-inflammatory PGE2, thromboxanes, and LTs, and a decrease in anti-inflammatory compounds including lipoxins, resolvins, protectins, and maresins (Beauchamp et al., 2001; Behl et al., 2016; Das, 2020; Schoenberger et al., 2012). The imbalance in the concentrations of pro- and anti-inflammatory BLs may result in enhanced local inflammation in aging and increase the risk of aging-associated diseases (Das, 1995; Gundala et al., 2017; Urlić et al., 2020). For example, to understand the role of inflammation in aging, Steinle et al. compared the levels of inflammatory markers, including inducible nitric oxide synthase (iNOS), prostaglandin E2 (PGE2), and TNFα, in the retina and choroid in the young and old rat model. The results showed that PGE2 (pro-inflammatory metabolites of AA) significantly increased in the aging retinas and choroids (Steinle et al., 2009). In other studies, qPCR analysis of lipoxin A4 (LXA4, anti-inflammatory metabolite of AA) biosynthetic enzyme and LXA4 receptor showed that LXA4 biosynthesis and signaling are significantly decreased in the end-stage of Retinal degeneration (RD) in RD1 mice model. Further study showed that LXA4 treated retina maintained the visual function of the mice through reducing photoreceptor apoptosis and inhibiting microglial overactivation (Lu et al., 2019). The above results indicate that metabolites of PUFAs are likely involved in the aging process in the retina and choroid. It has been suggested that with advancing age, pro-inflammatory cytokines, such as TNFα and IL-6, block the activities of desaturases and affect the formation of PUFAs and their metabolites, leading to an imbalance of pro- and anti-inflammatory BLs (Mayer et al., 2002). Das summarized the potential relationship among AA and its metabolites with age (Das, 2020), indicating there is decreased concentration of AA and LXA4 accompanied by decreased activity of desaturases, and increase of the pro-inflammatory PGE2, leukotriene B4 (LTB4), TNFα, and IL-6 in aging. In contrast, increased levels of DHA in the retina favor the production of docosanoids, neuroprotective molecules that leads to induction of reparative phenotype in macroglia, therefore helping the rescue of photoreceptors (Ebert et al., 2009). The failure to maintain the homeostasis of these molecules contributes to an increase of inflammatory events in aging and expansion of age-related diseases. This also suggests, that potential manipulations of the levels of PUFAs in the retina can still became a therapeuting options in some diseases.
III.1.4. Measuring lipids in the plasma – lipids supplementation
There is significant interest in studying the lipid composition of plasma isolated from patients as a marker of retinal health. There are several advantages to this approach: (i). Measuring lipid content can be done multiple times on the same model animal or patient; (ii) Access to the material is straightforward; (iii) The abundance of material helps in evaluating levels of several lipids; (iv) Longitudinal studies are more feasible; (v) The feasibility of measuring the impact of a given intervention on the lipid levels in the retina.
Multiple epidemiological studies have suggested that diets rich in n-3 LC-PUFAs are associated with lower rates of AMD. Low dietary intake of n-3 LC-PUFAs has conversely been associated with a higher risk of developing AMD (for example (Chong et al., 2008; Bénédicte M J Merle et al., 2015; van Leeuwen et al., 2018)). In addition, two extensive studies demonstrated that high plasma levels of n-3 LC-PUFAs were correlated with decreased risk of AMD (Merle et al., 2014, 2013). Interestingly, in the Age-Related Eye Disease Study (AREDS), a large prospective study investigating factors of progression to advanced AMD, subjects with the highest self-reported intake of foods rich in n-3 LC-PUFAS were 30% less likely to develop central GA and 50% less likely to develop AMD than subjects with the lowest self-reported intake (AREDS Research Group, 2009). Later, the impact of more defined n-3 LC-PUFA supplementation was investigated by two large prospective studies. The AREDS2 study and the nutritional AMD treatment study (NAT-2) examined the effect of n-3 PUFA supplementation to prevent progression to advanced AMD or wet AMD (Benedicte M J Merle et al., 2015; Souied et al., 2016). Surprisingly, neither study showed a significant difference between oral supplementation of PUFAs and the placebo in progression to wet AMD (Chew et al., 2012; Souied et al., 2013), suggesting that other FAs or other molecules present in foods rich in n-3 LC-PUFAs may have a preventive role in AMD progression. Similarly, Similarly in the openlabel study with (n-3) PUFA supplementation of 11 STDG3 patients, no significant beneficial effect has been observed; however, this study was limited by poor compliance with supplementation (Choi et al., 2018). Of note, recent studies showing the positive effect of oral supplementation of VLC-PUFA in animal models of STDG3 bring new hope in this line of pharmacological approaches(Gorusupudi et al., 2021). Future molecular studies focused on the specific roles of enzymes involved in lipid metabolism should help understand these clinical findings’ results.
Among all the lipids, cholesterol is gaining more attention since studies showed that failure to maintain cholesterol homeostasis is associated with many retina diseases, such as Bietti Crystalline Dystrophy, Bassen-Kornzweig syndrome (abetalipoproteinemia), Smith-Lemli-Opitz syndrome, and AMD (Table 1). Fluorescently-labeled lipoprotein particles injection indicated that the circulating cholesterol could cross the RPE and reach the retina with LDL as the major carrier (Tserentsoodol et al., 2006b). With aging, the clean-up of deposited lipids becomes less efficient, and the accumulation of cholesterol in Bruch’s membrane is reported to be the primary reason for AMD (Curcio et al., 2001). A population-based study containing 963 elderly individuals showed that higher plasma high-density lipoprotein cholesterol was significantly associated with an increased risk of AMD (Cougnard-Grégoire et al., 2014). Accumulated oxidized cholesterol, such as 7-ketocholesterol, may also probably relate to the pathogenesis of AMD (Rodríguez and Larrayoz, 2010).
Lipids in body fluids are also assessed as a potential biomarker for aging and disease processes happening in the retina. Several correlative studies support this. For example, a plasma-based metabolomics study showed that AMD patients had altered plasma metabolomic profiles compared to the control group. Among them, the most significant metabolites map to the glycerophospholipid pathway, indicating the potential role of lipids as novel targets for early diagnosis of AMD (Laíns et al., 2018). A comparison of circulating n-3 FAs between AMD patients and controls showed lower serum eicosapentaenoic acid (EPA) and red blood cell membrane EPA/DHA levels in AMD patients, suggesting that circulating EPA and DHA may be related to the status of AMD. The differences are not sufficient as evidence that EPA and DHA represent markers for diagnosis of AMD) (Gorusupudi et al., 2016; Merle et al., 2014). However, strong evidences indicate that dietary intake of n-3 long-chain polyunsaturated fatty acid may decrease the risk of AMD and influence the development of advanced AMD (Cho et al., 2001; SanGiovanni et al., 2008).
The concentration of lipids composition in body fluids closely correlates with aging and disease processes happening in the retina. For example, a plasma-based metabolomics study showed that AMD patients had altered plasma metabolomic profiles compared to the control group. Among them, the most significant metabolites map to the glycerophospholipid pathway (Laíns et al., 2018). A comparison of circulating n-3 FAs between AMD patients and controls showed lower serum eicosapentaenoic acid (EPA) and red blood cell membrane EPA/DHA levels in AMD patients, suggesting that circulating EPA and DHA may be used as an indication for further diagnosis (Gorusupudi et al., 2016; Merle et al., 2014). Recent studies, aimed at describing the blood biomarkers correlating with AMD, pointed out the positive correlation of n-3 cholesteryl esters in the plasma compared to n-3 PUFAs levels in the retina. In contrast, n-6 cholesteryl esters levels seem to correlate with lower levels of n-3 in the retina, therefore a potential higher risk of disease (Acar et al., 2021). More extensive follow-up studies are needed to establish precise biomarkers that can be used to predict the disease.
Besides lipids, lipoproteins can also act as biomarkers for the aging retina and AMD. There is an increase in the serum concentration of total cholesterol and LDL accompanied by a decrease of HDL in AMD patients, suggesting they may be useful as markers of AMD (Reynolds et al., 2010). Fauser et al.(Fauser et al., 2011) reported elevated apolipoprotein B levels in AMD patients’ serum. Curcio et al. (Curcio et al., 2010) addressed the role of apolipoprotein-B lipoproteins in the aging retina, especially its role in the formation of drusen associated with AMD. Some LC-PUFA peroxidation metabolites such as isoprostanes (IsoPs), which are derived from AA, are found to be applicable as diagnostic markers of oxidative stress in the mammalian eye due to their stability in healthy conditions and noticeably increased production upon exposure of the retina to oxidative stress (Dentchev et al., 2007; Njie-Mbye et al., 2013). This also suggests that since oxidative stress has been implicated as an integral part of aging processes, lipid peroxidation metabolites could also be used as potential indicators of aging in future studies.
III.2. Lipids in eye diseases
Several inherited diseases related to lipid metabolism and transport have been described. STGD is an autosomal recessive retinal disorder characterized by juvenile-onset macular degeneration, reduced light sensitivity or contrast sensitivity, and gradual loss of central vision (Allikmets et al., 1997b). Mutations in the ABCA4 (STGD1), ELOVL4 (STGD2, STGD3) (Small, 2001), PROM1 (STGD4) (Yang et al., 2008), BEST1 (Zolnikova et al., 2017), and PRPH2 (STGD1) (Boon et al., 2007) genes have been detected in patients with autosomal recessive or dominant STGD. Among them, mutations in ELOVL4 and ABCA4 cause related lipid disorders in RPE and photoreceptors. Mutations in ABCA4 result in the accumulation of A2E, a component of lipofuscin known to be toxic to RPE and photoreceptors. The autosomal dominant form of STGD3 is caused by mutations in ELOVL4 (Molday and Zhang, 2010). Three independent mutations of ELOVL4 are associated with juvenile macular degeneration causing STGD3 in humans (Bernstein et al., 2001; McMahon et al., 2007; Zhang et al., 2001). Due to the autosomal dominant nature of STGD3 mutations and the fact that VLC-PUFAs are associated with rhodopsin (Figure 2) (Sander et al., 2021), the lack of VLC-PUFAs in mutant animals may impair rhodopsin function, resulting in reduced visual function and photoreceptor degeneration. Because of the autosomal dominant nature of STGD3 mutations and the fact that VLC-PUFAs are associated with rhodopsin (Figure 2) (Sander et al., 2021), the lack of VLC-PUFAs in mutant animals may impair rhodopsin function, resulting in reduced visual function and photoreceptor degeneration. Interestingly, the dominant-negative mutant of ELOVL4 protein itself is not anchored to the ER but fills the OS of photoreceptors, impacting phagocytosis and recycling (Esteve-Rudd et al., 2018; Mandal et al., 2014). Further studies, however, are still needed to dissect the molecular steps affected and allow for looking for novel, effective strategies in treating patients. Mutation of BEST1 was also identified in bestrophinopathy, which is one of the most common forms of macular degenerations. This disease was identified by abnormal accumulation of autofluorescent material within RPE cells was identified and leading to bilateral macular or multifocal lesions (Table 1) (Guziewicz et al., 2017).
Similarly, different species of lipids, including VLC-PUFAs, have been shown to play a role in AMD, which has been recently reviewed in depth (Skowronska-Krawczyk and Chao, 2019; van Leeuwen et al., 2018). Recently we have looked at the potential involvement of ELOVL2 in AMD disease. Our RNAscope analysis has shown that ELOVL2 is abundantly expressed in cones, including the perifoveal subtype (Figure 7A). Therefore, we have looked at whether the DNA methylation of cg16867657, where methylation levels faithfully correlate with the person’s age, changes in the samples isolated from AMD donors but could not detect any change (Figure 7B). In contrast, chromatin accessibility on ELOVL2 promoter significantly decreases in macula and periphery (Figure 7C) of in retinas from AMD donors compared to age-matched healthy tissue. This change is specific to the promoter region of ELOVL2 as the intron region accessibility does not change in both groups. This intriguing loss of accessibility in AMD patients may suggest the secondary, disease but not age-related impact on the chromatin in ELOVL2 locus and further downregulation of the gene in the disease. For example, given the multifactorial character of AMD, it is not excluded that particular phenotype of the disease, such as oxidative stress, elicits an additional mechanism repressing ELOVL2 promoter. Further studies need to establish what transcriptional and metabolic pathways are involved in age-related chromatin remodeling on ELOVL2 locus.
Figure 7. ELOVL2 and AMD.
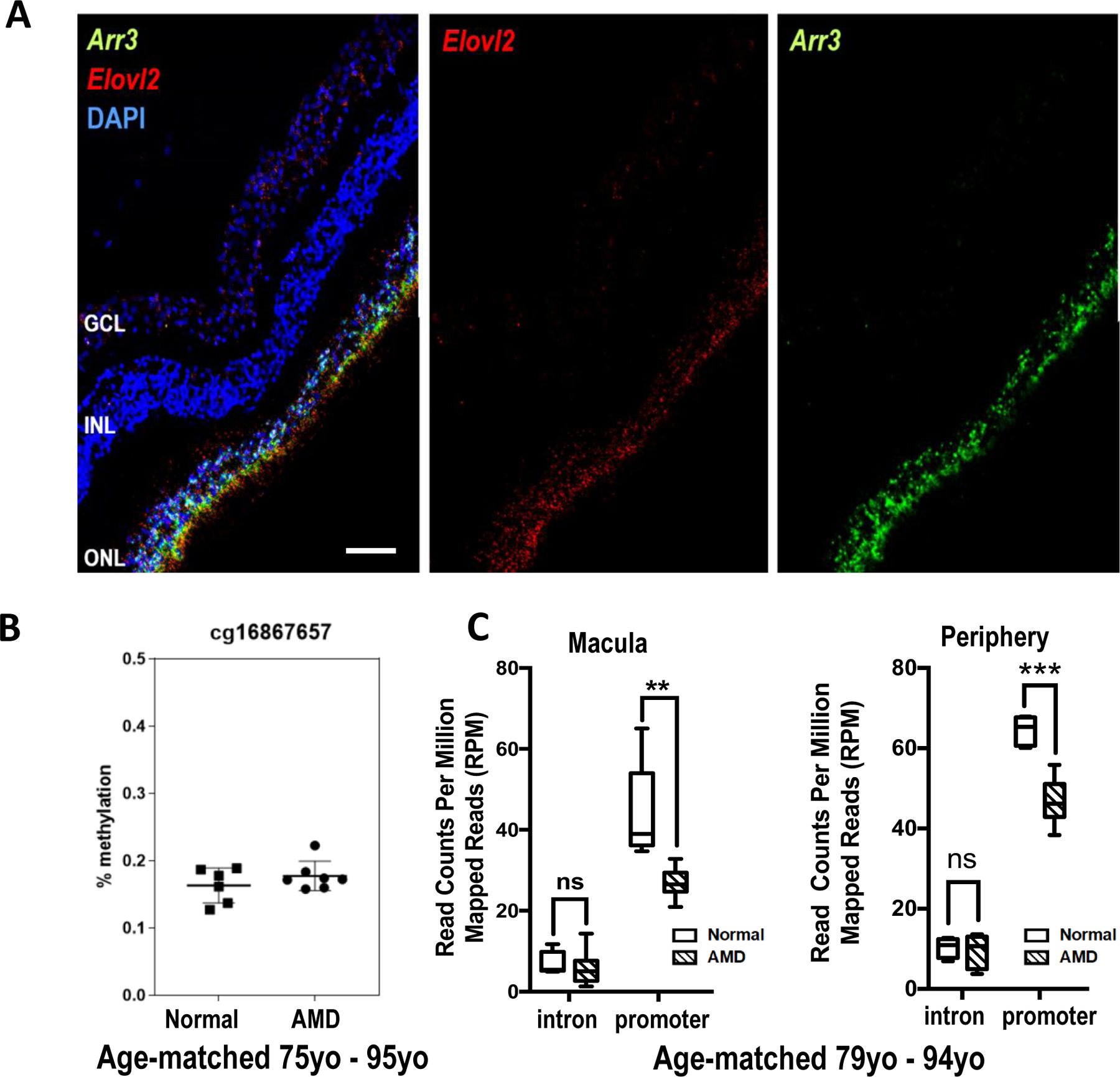
(A) RNAscope detection of Elovl2 (red) and Arr3 (green) mRNA shows high expression of Elovl2 in perifoveal cones and low expression in ganglion cells. (B) DNA methylation level of the cg16867657 in ELOVL2 promoter is not increased in samples isolated from AMD donors (GSE102952 (Oliver et al., 2015)); (C) Chromatin accessibility data measured by ATAC-seq (GSE99287 (Wang et al., 2018)) shows significantly decreased signal of ELOVL2 promoter in samples isolated from AMD donors when compared to age-matched healthy donors both in the macula and in the periphery. No change is observed in the intron region.
To date, despite numerous genome-wide studies, no detected ELOVL2 mutations or variants are correlating with the risk of AMD. There are several possible explanations: 1. ELOVL2 is an essential gene for population survival. It has been shown that complete deletion of the gene in a mouse model causes infertility in C57BL/6 mice that can be potentially overcome by keeping the genotype in the mixed background (Pauter et al., 2014). Therefore, variants that potentially can be correlated with the disease are rare and yet to be discovered. 2. ELOVL2 is regulated on the transcriptional level, and the downregulation of its expression is not specific for AMD. Potential variants affecting the activity or expression of ELOVL2 may correlate rather with the onset of the disease and not the disease itself. This type of analysis has yet to be performed. 3. Other ways to deregulate ELOVL2 transcription are employed and correlate with the disease. As shown in (Figure 7), the promoter accessibility significantly decreases in retinas isolated from AMD donors. What is the potential mechanism of this phenotype remains to be elucidated.
Accumulations of lipids, lipoproteins, and oxidized lipids within Bruch’s membrane are considered as pathogenesis of AMD. Though the pathogenesis of AMD is complex and still unknown, many genetic variants in genes that regulate lipid metabolism and lipid transfer between lipoproteins have been proved to be associated with the risk of advanced AMD, such as APOE2 (Baird et al., 2006), CETP, LIPC(Neale et al., 2010), CFH (Zareparsi et al., 2005), ABCR (Allikmets et al., 1997a), IGF1(Castellino et al., 2018), ADIPOR1 (Castellino et al., 2018), and other. The molecular connection of AMD with lipid metabolism opened new opportunities to search for pharmaceutical and nutritional interventions for affected patients.
One of the most interesting cases is the association of ApoE variants and AMD (Baird et al., 2004). As mentioned above, human ApoE is encoded by three alleles – E2, E3, and E4. The association of APOE4 isoform with Alzheimer’s disease has been very well established and further correlated with the molecular mechanism of accumulation of beta-amyloid plaques (recently reviewed in (Yassine and Finch, 2020). Surprisingly, it is the APOE2 isoform that is associated with a higher risk of developing and progression of AMD (Baird et al., 2006; Levy et al., 2015b, 2015a). Several studies using the humanized mice expressing specifically E2, E3, or E4 isoforms have been performed to understand the mechanism of the disease in the presence of a high-fat diet (Ding et al., 2011; Malek et al., 2005). In particular, studies have shown that APOE isoforms are differentially associated with AMD-like phenotypes in mice than in humans. Opposite to what was observed in humans, mice carrying APOE4 allele had the highest levels of subRPE diffused and drusenoid deposits, and AMD-like RPE/retinal phenotypes. Several explanations are possible. In addition to the high-fat diet as an environmental factor, rarely considered in studies related to APOE alleles, specific differences in mice and humans physiology can help to explain the differences and help understand the disease. One of the main factors is the low levels of VLDL particles in the mice plasma, due to the extremely low levels of APOB100 expression, apolipoprotein indispensable for VLDL formation. High-fat diet and lack of ability to process the lipids via VLDL might have a specific impact on transmembrane lipid transport and lipid metabolism. Nevertheless, when knowing caveats, the humanized APOE mouse model can be used to test different therapies such as anti-amyloid therapy to rescue vision (Ding et al., 2011).
In parallel, studies focused on understanding the ApoE-dependent AMD phenotype in human iPS-RPE cell culture, and human tissues elegantly suggested that due to the conformational change upon oxidative stress, ApoE2 isoform poorly performs its primary function in the retina – cholesterol efflux from the RPE (La Cunza et al., 2021). This study connects mitochondrial stress to the APOE function, therefore bringing together two critical processes known to be at the basis of AMD development. This work also seems to support the RPE-origin of drusen (Bergen et al., 2019); however, as mentioned above, ApoE2 protein has very low or no affinity to the LDLR (Weisgraber et al., 1982), the most abundant receptor of the lipid particles on the basal side of the RPE (Tserentsoodol et al., 2006b, 2006a). It is, therefore, possible that uptake of HDL particles containing ApoE2 is slower, therefore ADDINg to the accumulation of the subRPE protein-lipid deposits.
One of the most known genetic variants associated with AMD is the tyrosine (Y) to histidine (H) substitution at amino acid 402 (Y402H) in CFH. It was believed that CFH variant induced AMD by activation of complement in the eye. Nevertheless, the limited success of the clinical trials of complement inhibitors in the treatment of AMD patients challenged this theory. To investigate the relationship between Cfh mutation and complement dysregulation and lipid homeostasis in BM, an aged, Cfh haploinsufficiency (Cfh+/−), supplied with high fat, cholesterol-enriched diet mice model was developed (Toomey et al., 2015). Studies showed that this mice model presents many phenotypes resembling the human AMD phenotypes, including increased basal lamina deposits, accompanied by accumulation of lipoproteins and lipids in BM, RPE damage, and vision loss. This model revealed that rather than complement activation, Cfh variant caused the AMD-like symptoms through lack of activity and disturbing the complement pathway causing accumulation of lipoproteins and lipids and induction of sub-RPE deposit formation in aged mice (Landowski et al., 2019; Toomey et al., 2015). The use of high-fat, cholesterol-enriched diet (HFC) confirms the previous studies emphasizing the role of lipid balance in preserving vision. Fluorescence-based measurement and Western blot showed that the CHF polymorphism affects the total level of plasma VLDLs and LDLs containing ApoB100 and ApoE, as well as ApoB48 and ApoA1 retention in aged murine BM in the eyecup (rather than complement activation) in response to the HFC diet. To date, this is the best animal model of AMD, and great hope in the field seeking for models to understand human disease.
RP is a group of inherited retinopathies characterized by tunnel vision (loss of peripheral vision) and night blindness due to the loss of rods from the retina. Over 70 genes have been associated with the development of RP so far (McColl and Converse, 1995). The panoply of genetic causes of RP has been reviewed elsewhere (Daiger et al., 2015). Interestingly, several mutations are located in genes encoding proteins involved in lipid metabolism and homeostasis. For example, variants in MTTP (encoding microsomal triglyceride transfer protein) were characterized in Bassen-Kornzweig syndrome, associated with low LDL-cholesterol, triglyceride and apolipoprotein, and a deficiency in fat-soluble vitamins and essential FAs. Batten disease caused by mutation of CLN3 gene is characterized by accumulation of ceroid and lipofuscin in the neuronal system, including the retina. Variations in PHYH (encoding Phytanoyl‐CoA Hydroxylase) and PEX7 (encoding the Peroxisome targeting signal 2 (PTS2) receptor) were identified in Refsum’s disease, causing deficiency in phytanic acid α-oxidation. Other RPs, including Zellweger syndrome and Usher syndrome, are also described as associated with lipid imbalance, such as abnormal saturated and unsaturated FA levels. Table 1 highlights the list of eye diseases associated with mutations in genes involved in lipid metabolism.
Macular telangiectasia is a rare degenerative retinal disease affecting the macula, causing loss of central vision. There are three types of Macular Telangiectasia: type 1, 2, and 3. Among them, macular telangiectasia type 2 (MacTel) is the most common of the three types, and it occurs in one out of 1,000 people over 40 years old. Phenotypic characterization of MacTel showed lack of foveolar reflex, reduced retinal transparency (graying), crystalline deposits and ectatic capillaries (Charbel Issa et al., 2013). Many familial cases of MacTel indicated genetic factors in the pathogenesis of this disease. Genetic and metabolism works showed a link between MacTel and serine metabolism (Scerri et al., 2017). However, the related genes were not described until very recently. Variant SPTLC1 or SPTLC2 (encoding a subunit of serine palmitoyltransferase) was identified through exome sequencing analysis of genomic DNA from MacTel patients and their family members (Gantner et al., 2019). The two variants led to elevated levels of circulating deoxysphingolipids, which negatively correlated with serine levels. A mouse model confirmed that the decreased level of serine is sufficient to cause increased retinal deoxysphingolipid levels and retina degeneration. Genome-wide association studies (GWAS) identified a new rare PHGDH (phosphoglycerate dehydrogenase) variant in MacTel patients (Fallon et al., 2018). PHGDH encodes a rate-limiting enzyme that performs the first step of the phosphorylated pathway of serine biosynthesis, converting 3-phosphoglycerate into phosphohydroxy pyruvate. The PHGDH variant also causes decreased serum levels of serine and accumulation of neurotoxic deoxysphingolipids (deoxySLs) in retinal pigmented epithelial cells, which is correlated with the extent of disease in MacTel (Eade et al., 2021; Gantner et al., 2019). These two findings help to understand the basis of the disease phenotype of MacTel and indicate the potential treatment targets.
Of the lipid storage disorders, Gaucher disease, Farber’s disease and Sandhoff’s disease are lysosomal storage disorders accompanied by the accumulation of harmful lipids and ocular phenotypes. For example, Gaucher disease is an inherited glycolipid storage disorder that affects many of the organs including retina. It is associated with mutation in GBA that encodes acid β-glucosidase (lysosome glucocerebrosidase) (GRABOWSKI, 1997). L444P is the most common mutation on GBA in Gaucher disease, it causes accumulation of glucosylsphingosine leading to tapeto-retinal degeneration, pigmented retinal lesions, intra-retinal white dots and inner retinal Gaucher cells (Table 1) (Winter et al., 2019). Optical coherence tomography (OCT) was utilized in a pilot study showed that retinal thinning can be detected in patients and carriers(McNeill et al., 2013). Farber’s disease and Sandhoff’s disease are rare lysosomal storage disorders. Farber’s disease is caused by a mutation in ASAH1 gene that encodes acid ceramidase enzyme (Koch et al., 1996). Deficiency of acid ceramidase causes ceramide accumulation in various tissues, including the retina (Sugita et al., 1972). Mouse model of ASAH1 human mutation shows progressive retinal pathology, including progressive inflammation and retinal dysplasia accompanied by abnormal accumulation of ceramides and other sphingolipids followed by severe visual impairment (Yu et al., 2019). Sandhoff’s disease, which is another disease related to pathologic sphingolipid build-up, is caused by variants in the hexosaminidase-B (HEXB) gene. The mutations led to the deficient hexosaminidase activity, resulting in the accumulation of GM2 ganglioside in the lysosomes of neuronal cells, progressive neural degradation, and vision deterioration (Mahuran, 1999; Sandhoff and Harzer, 2013). These two examples emphasize the importance of animal models when studying human diseases, especially for rare syndromes where gathering a substantial amount of data from patients is very limited.
Retinal diseases may also induce changes in membrane fluidity and further progression of retinal degeneration. For example, Smith-Lemli-Opitz syndrome (SLOS), caused by mutations in the DHCR7 gene, is accompanied by abnormal accumulation of 7-dehydrocholesterol and decreased cholesterol levels, affecting most organ systems, including eye and brain, extremities, skin, lung, heart, and other (Porter, 2008). Fliesler and colleagues (Fliesler and Xu, 2018) generated a rat model by treating rats with AY9944, a selective inhibitor of the enzyme affected in SLOS. Steady-state anisotropy measurements of the model showed that the fluidity of rod OS membranes is significantly reduced, along with a marked decrease in membrane lipid unsaturation due to losses in DHA. Compromised rhodopsin regeneration and retinal degeneration have also been observed in this model (Table 1) (Boesze-Battaglia et al., 2008; Tulenko et al., 2006).
Also, other types of retinal diseases are connected to the lipids serving as co-factors in specific reactions. For example, mutation of the dehydrodolichyl diphosphate synthase (Dhdds) gene causes RP (RP59), which contains symptoms including night and peripheral vision loss, constriction of visual fields, and retinal degeneration (Zelinger et al., 2011). Animal experiments suggested that the knockdown of Dhdds in rod cells showed retinal dysfunction initiated by decreased retina dolichol levels, lipid molecule involved in N-glycosylation of proteins including rhodopsin. However, neither the human point mutation nor removal of Dhdds from rods affect the N-glycosylation of retinal proteins, which was considered as a key factor of the pathogenesis of RP59. (Ramachandra Rao et al., 2020a, 2020b). Further studies need to decipher whether the results are due to the differences between the mice and human or there are other factors contributing in the development of the disease in human.
Mutations of genes involved in lipid metabolism have been detected in other retinal phenotypes. For example, ACBD5 (encoding Acyl-CoA binding domain-containing 5) mutation, a peroxisomal tail-anchored membrane protein, was observed in patients with a syndromic form of retinal dystrophy (Abu-Safieh et al., 2013). Genome editing experiment on patient-derived fibroblasts and HeLa cells indicated that ACBD5 deficiency causes defect in peroxisomal β-oxidation of very-long-chain FAs (VLCFAs) and accumulation of cellular phospholipids containing VLCFAs. It is proposed that ACBD5 plays a vital role in transporting VLCFA-CoAs from the cytosol into peroxisomes for subsequent β-oxidation (Yagita et al., 2017). Given the complexity of lipid metabolism and the vast number of enzymes involved therein, it is highly probable that a deficit in these pathways causes many eye pathologies.
Besides the imbalance of lipids, lipid peroxidation has been reported to contribute to degenerative ocular diseases like AMD, glaucoma, and diabetic retinopathy. For example, the age-dependent susceptibility of the macula to lipid peroxidation and its products is related to the attenuation of antioxidant defense systems with aging. Long-term oxidation of PUFAs results in impairment in the function and structure of cell membranes, leading finally to degeneration of photoreceptors (De La Paz and Anderson, 1992). With age, oxidated PUFAs are not efficiently digested in the lysosomes of aged RPE cells and become deposited in the form of lipofuscin (see above). Several clinical findings showed that excessive accumulation of lipofuscin granules initiates RPE cells damage (Kopitz et al., 2004; Malek et al., 2005; Sparrow et al., 2000). For instance, Batten’s disease and Stargardt disease have been attributed to excessive accumulation of lipofuscin-like materials in the RPE, causing loss of visual function. Besides the imbalance of lipids, lipid peroxidation has also been reported to contribute to degenerative ocular diseases like AMD, glaucoma, and diabetic retinopathy. For example, the age-dependent susceptibility of the macula to lipid peroxidation and its products is related to the attenuation of antioxidant defense systems with aging. Long-term oxidation of PUFAs results in impairment in the function and structure of cell membranes, leading finally to degeneration of photoreceptors (De La Paz and Anderson, 1992). With age, oxidized PUFAs are not efficiently digested in the lysosomes of aged RPE cells and become deposited in the form of lipofuscin (see above). Several clinical findings showed that excessive accumulation of lipofuscin granules initiates RPE cells damage (Kopitz et al., 2004; Malek et al., 2005; Sparrow et al., 2000). For example, Batten’s disease and Stargardt disease have been attributed to excessive accumulation of lipofuscin-like materials in the RPE, causing loss of visual function.
Lipid deposits are one of the most distinctive and earliest features of AMD. Studies indicate that unesterified and esterified cholesterol and triacylglycerols (Curcio et al., 2005), PC (Wang et al., 2010), and apolipoproteins (Anderson et al., 2001; Curcio et al., 2010) are abundant components in deposits and drusen of AMD eyes. Among them, esterified cholesterol and PC accounted for at least 40% of the drusen volume (Wang et al., 2010). As mentioned above, these lipids can be derived from the OS, the nutrient supply system, or produced directly by RPE cells (Pikuleva and Curcio, 2014). Ultrastructural techniques indicated that esterified cholesterol is only located in Bruch’s membrane, while unesterified cholesterol and phospholipids are located in nearby cellular membranes (Rudolf and Curcio, 2009). A high concentration of oxidized lipids in the drusen triggered several ideas regarding the involvement of inflammation in disease progression. Curcio et al. has proposed that the deposits may interact with ROS, form pro-inflammatory oxidized lipids, activate the complement system, and trigger angiogenesis (Curcio et al., 2011). Current studies focus on correlating subretinal lipid deposits with a patient’s stage of AMD aim to discover potential pathways involved in deposit formation (L. Chen et al., 2021; Sura et al., 2020) to suggest the potential ways to interrupt this process in the aged retina.
Final comments
As is the case with all biomedical studies, a major challenge in the ophthalmology field is translating data from animal models to human subjects. Studies on mice and rats were instrumental in acquiring our current knowledge level, and studies on cone-rich retinas (e.g., large animals, diurnal rodents) have allowed more accurate modeling of several human conditions. Research on primates, although extremely valuable, is limited due to the high cost. Studies on human organoids can answer questions relevant for human retina development but cannot yet be used in studying aging and age-related eye diseases. In short, there are still no attractive, comprehensive model with developed macula that would facilitate studies of macular function and degeneration. While there is an urgent need to develop appropriate animal models for these studies, current models, despite their shortcomings, can be extremely useful provided that steps are taken to limit the risk of overinterpreting data. With the development of the new technologies, new animal models and outstanding progress in systems biology approaches the field is poised to new discoveries in sufficient mechanistic detail to help initiate novel approaches to prevent and even reverse the age and genetic predisposition to lipid-related eye diseases.
Figure 6. Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) signals consistent with localization to photoreceptor and RPE compartments.
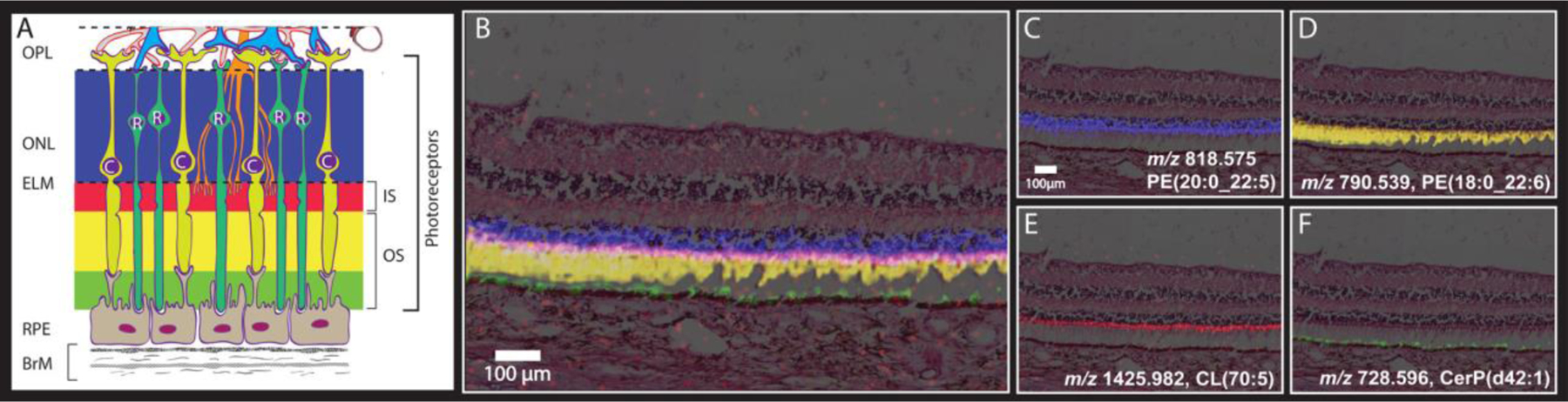
(A) Schematic diagram of outer retina and Bruch’s membrane. Blue, pink, yellow, and green bands indicate layers formed by highly compartmentalized and vertically aligned photoreceptors and RPE cells in panels B and C. Layers: OPL, outer plexiform layer; ONL, outer nuclear layer; ELM, external limiting membrane; RPE, retinal pigment epithelium; BrM, Bruch’s membrane; R, Rod; C, cone photoreceptors. (B–F) Overlaid MALDI-MSI images and H&E stained cross-section of the peripheral retina display signals from multiple lipid classes that localize to specific subcellular compartments of the photoreceptor cells and RPE. (B) Overlay showing four separate signals defined in panels C–F, localized to (C) ONL, (D) photoreceptor inner and outer segments, (E) mitochondria-rich photoreceptor inner segments, and (F) RPE apical processes. ©2021 Anderson et al. Reprinted from an article originally published in J. Am. Soc. Mass Spectrom. https://doi.org/10.1021/jasms.0c00119.
Acknowledgments
The authors wish to thank Drs. Philip Kiser and Krzysztof Palczewski (University of California, Irvine) for their helpful comments, Kelly O’Neil (Penumbra Design, Inc.) for her help in figure preparation, and anonymous reviewers for their insightful comments that helped to improve the manuscript.Research in the D.S.K. laboratory is funded by NIH R01EY027011, Thome Foundation and BrightFocus Foundation.
Abbreviations
- 4-HNE
4-hydroxy 2-nonenal
- AA
arachidonic acid
- ABCA1
ATP-binding cassette transporters, family A, 1
- ABCA4
ATP-binding cassette protein, family A, 4
- ABCG1
ATP-binding cassette transporters, family G, 1
- ACER3
Alkaline ceramidase 3
- ADIPOR1
adiponectin receptor 1
- AGPAT
1-acylglycerol-3-phosphate acyltransferase
- AMD
age-related macular degeneration
- APOA1
apolipoprotein A1
- ApoE
apolipoprotein E
- APOER2
ApoE receptor 2
- AREDS
Age-Related Eye Disease Study
- ASAH1
N-acylsphingosine amidohydrolase 1
- ASMase
acid sphingomyelinase
- ATP8A2
ATPase phospholipid transporting 8A2
- BL
bioactive lipid
- BLamD
basal lamina deposits
- BM
Bruch’s membrane
- BRB
blood-retina barrier
- C1P
ceramide-1-phosphate
- CC
connecting cilium
- CEP
carboxyethylpyrrole
- CERK
ceramide kinase
- CERS
ceramide synthases
- CERT
ceramide transferase
- CFH
complement factor H
- CM
chylomicron
- CNS
central nervous system
- COX
cyclooxygenase
- CPT1
choline phsphotransferase 1
- CYP
cytochrome P450 monooxygenase
- DAG
diacylglycerol
- deoxySL
deoxysphingolipid
- DGK
DAG kinase
- DGKE
DAG kinase isoform epsilon
- DHA
docosahexaenoic acid
- DIBMALP
diisobutylene maleic acid lipid particles
- DPPC
dipalmitoyl-phosphatidylcholine
- DRM
detergent-resistant membrane fraction
- EET
epoxyeicosatrienoic acid
- ELOVL4
elongation of very long FAs 4
- EPA
eicosapentaenoic acid
- EPT1
ethanolamine phosphotransferase 1
- ER
endoplasmic reticulum
- FABP
fatty acid binding protein
- FAO
fatty acid oxidation
- FATP
fatty acid transfer protein
- FFA
free fatty acid
- GAS6
Growth arrest-specific protein 6
- GLUT1
glucose transporter type 1
- GPAT
glycerol-3-phosphate acyltransferase
- HC
hydraulic conductivity
- HDL
high-density lipoprotein
- HETE
hydroxyeicosatetraenoic acid
- hPSC
human pluripotent stem cell
- IDL
intermediate-density lipoprotein
- IL-6
interleukin 6
- IP3
(1,4,5)-inositol triphosphate
- IPM
interphotoreceptor matrix
- IRBP
interphotoreceptor retinoid-binding protein
- IS
inner segment
- IsoP
isoprostane
- KDSR
3-ketodihydrosphingosine reductase
- KO
knock-out
- LC-PUFA
long chain polyunsaturated fatty acid
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LIPA
lysosomal acid lipase type A
- LOX
lipoxygenase
- LPA
lysophosphatidic acid
- LPA1–6
LPA-binding GPCR 1–6
- LPAAT
lysophosphatidic acid acyltransferase
- LPC
lysophosphatidylcholine
- LPLAT
lysophospholipid acyltransferase
- LPP
lipid phosphate phosphatases
- LRAT
lecithin retinol acyltransferase
- LT
leukotriene
- LTA4
leukotriene A4
- LUV
large unilamellar vesicles
- LXA4
Lipoxin A4
- MacTel
macular telangiectasia 2
- MDA
malondialdehyde
- MERTK
MER tyrosine kinase
- MFGE8
milk fat globule factor-E8
- MFRP
membrane frizzled-related protein
- MFSD2A
major facilitator superfamily domain-containing protein 2a
- MSP
membrane scaffold protein
- mTOR
mechanistic target of rapamycin
- n-3
omega-3
- NAT-2
nutritional AMD treatment study
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NOD-like receptor family pyrin domain-containing 3
- NPD1
neuroprotectin D1
- OGG1
8-oxoguanine glycosylase
- OS
outer segment
- PA
phosphatidic acid
- PAP
phosphatidate phosphatase
- PARP-1
Poly(ADP-Ribose) Polymerase 1
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PGE2
prostaglandin E2
- PI(3)P
phosphatidylinositol-3-phosphate
- PI(4)P
phosphatidylinositol-4-phosphate
- PI
phosphatidylinositol
- PIP
PI phosphate
- PIP2
phosphatidylinositol 4,5-diphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PKC
protein kinase C
- PLAAT3
phospholipase A and acyltransferase 3
- PLC
phospholipase C
- PPARα
Peroxisome proliferator activated receptor-α
- PRPH2
peripherin 2
- PROS
vitamin K-dependent protein S
- PS
phosphatidylserine
- PTS2
peroxisome targeting signal 2
- PUFA
polyunsaturated fatty acid
- RCS
Royal College of Surgeons
- RDH12
retinol dehydrogenase 12
- ROM-1
rod outer membrane protein 1
- ROS
reactive oxygen species
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- S1P
sphingosine-1-phosphate
- SCARB1
scavenger receptor class B, member 1
- SLOS
Smith-Lemli-Opitz syndrome
- SMA
styrene maleic acid
- SMALP
styrene maleic acid lipid particle
- SMase
sphingomyelinase
- SMPD
sphingomyelin phosphodiesterase
- Sph
sphingosine
- SPHK
sphingosine kinase
- SPP
S1P phosphatase
- SPT
serine palmitoyltransferase
- STGD
Stargardt-like macular dystrophy
- TNFα
tumor necrosis factor α
- TRP
transient receptor potential
- TxB2
thromboxane B2
- VEGF
vascular endothelial growth factor
- VLC-PUFA
very long chain polyunsaturated fatty acid
- VLC-SFA
very long chain saturated fatty acid
- VLDL
very low density-lipoprotein
- VLDLR
very low-density lipoprotein receptor
- WT
wild-type
Footnotes
Disclosures
D.S.K. is a scientific advisor for Visgenx, Inc.
LITERATURE
- Abrahan CE, Miranda GE, Agnolazza DL, Politi LE, Rotstein NP, 2010. Synthesis of Sphingosine Is Essential for Oxidative Stress-Induced Apoptosis of Photoreceptors. Invest. Ophthalmol. Vis. Sci 51, 1171–1180. 10.1167/iovs.09-3909 [DOI] [PubMed] [Google Scholar]
- Abran D, Li DY, Varma DR, Chemtob S, 1995. Characterization and ontogeny of PGE2 and PGF2α receptors on the retinal vasculature of the pig. Prostaglandins 50, 253–267. 10.1016/0090-6980(95)00132-8 [DOI] [PubMed] [Google Scholar]
- Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, Al-Zahrani J, Al-Abdi L, Hashem M, Al-Tarimi S, 2013. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res 23, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acar N, Merle BMJ, Ajana S, He Z, Grégoire S, Hejblum BP, Martine L, Buaud B, Bron AM, Creuzot-Garcher CP, Korobelnik J-F, Berdeaux O, Jacqmin-Gadda H, Bretillon L, Delcourt C, Group, for the B. of L.S.A. metabolism in R. ageing (BLISAR) S., 2021. Predicting the retinal content in omega-3 fatty acids for age-related macular-degeneration. Clin. Transl. Med 11, e404. 10.1002/ctm2.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya JK, Dasgupta U, Rawat SS, Yuan C, Sanxaridis PD, Yonamine I, Karim P, Nagashima K, Brodsky MH, Tsunoda S, Acharya U, 2008. Cell-Nonautonomous Function of Ceramidase in Photoreceptor Homeostasis. Neuron 10.1016/j.neuron.2007.10.041 [DOI] [PMC free article] [PubMed]
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK, 2003. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science (80-. ) 10.1126/science.1080549 [DOI] [PubMed]
- Adler AJ, Edwards RB, 2000. Human interphotoreceptor matrix contains serum albumin and retinol-binding protein. Exp. Eye Res 70, 227–234. 10.1006/exer.1999.0780 [DOI] [PubMed] [Google Scholar]
- Agbaga M-P, Brush RS, Mandal MNA, Henry K, Elliott MH, Anderson RE, 2008. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci 105, 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi S, Kono N, Ariyama H, Shindou H, Shimizu T, Arai H, 2016. Lysophosphatidylcholine acyltransferase 1 protects against cytotoxicity induced by polyunsaturated fatty acids. FASEB J 30, 2027–2039. 10.1096/fj.201500149 [DOI] [PubMed] [Google Scholar]
- Akakura S, Singh S, Spataro M, Akakura R, Kim J Il, Albert ML, Birge RB, 2004. The opsonin MFG-E8 is a ligand for the αvβ5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. Cell Res 292, 403–416. 10.1016/j.yexcr.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Albert A, Alexander D, Boesze-Battaglia K, 2016. Cholesterol in the rod outer segment: A complex role in a “simple” system. Chem. Phys. Lipids 199, 94–105. 10.1016/j.chemphyslip.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Albert AD, Boesze-Battaglia K, 2005. The role of cholesterol in rod outer segment membranes. Prog. Lipid Res 10.1016/j.plipres.2005.02.001 [DOI] [PMC free article] [PubMed]
- Albert AD, Young JE, Paw Z, 1998. Phospholipid fatty acyl spatial distribution in bovine rod outer segment disk membranes. Biochim. Biophys. Acta - Biomembr 10.1016/S0005-2736(97)00200-9 [DOI] [PubMed]
- Albert AD, Young JE, Yeagle PL, 1996. Rhodopsin-cholesterol interactions in bovine rod outer segment disk membranes. Biochim. Biophys. Acta - Biomembr 1285, 47–55. 10.1016/S0005-2736(96)00145-9 [DOI] [PubMed] [Google Scholar]
- Algvere PV, Marshall J, Seregard S, 2006. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand 84, 4–15. 10.1111/j.1600-0420.2005.00627.x [DOI] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, 1997a. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science (80-. ) 277, 1805–1807. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR, 1997b. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Starqardt macular dystrophy. Nat. Genet 15, 236–246. 10.1038/ng0397-236 [DOI] [PubMed] [Google Scholar]
- Almeida PFF, 2009. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta - Biomembr 10.1016/j.bbamem.2008.08.007 [DOI] [PubMed]
- Ananth S, Gnana-Prakasam JP, Bhutia YD, Veeranan-Karmegam R, Martin PM, Smith SB, Ganapathy V, 2014. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim. Biophys. Acta - Mol. Basis Dis 1842, 603–612. 10.1016/j.bbadis.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV, 2001. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am. J. Ophthalmol 131, 767–781. [DOI] [PubMed] [Google Scholar]
- Anderson DMG, Ablonczy Z, Koutalos Y, Hanneken AM, Spraggins JM, Calcutt MW, Crouch RK, Caprioli RM, Schey KL, 2017. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci. Rep 7, 17352. 10.1038/s41598-017-17402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DMG, Ablonczy Z, Koutalos Y, Spraggins J, Crouch RK, Caprioli RM, Schey KL, 2014. High Resolution MALDI Imaging Mass Spectrometry of Retinal Tissue Lipids. J. Am. Soc. Mass Spectrom 25, 1394–1403. 10.1007/s13361-014-0883-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RE, 1970. Lipids of ocular tissues. IV. A comparison of the phospholipids from the retina of six mammalian species. Exp. Eye Res 10.1016/S0014-4835(70)80046-X [DOI] [PubMed]
- Anderson RE, Feldman LS, Feldman GL, 1970. Lipids of ocular tissues. II. The phospholipids of mature bovine and rabbit whole retina. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 202, 367–373. 10.1016/0005-2760(70)90200-6 [DOI] [PubMed] [Google Scholar]
- Anderson RE, Maude MB, 1970. Phospholipids of Bovine Rod Outer Segments. Biochemistry 10.1021/bi00820a019 [DOI] [PubMed]
- Anderson RE, Maude MB, Nielsen JC, 1985. Effect of lipid peroxidation on rhodopsin regeneration. Curr. Eye Res 4, 65–71. 10.3109/02713688508999969 [DOI] [PubMed] [Google Scholar]
- Anderson RE, Rapp LM, Wiegand RD, 1984. Lipid peroxidation and retinal degeneration. Curr. Eye Res 3, 223–227. [DOI] [PubMed] [Google Scholar]
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C, 2017. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94, 581–594.e5. 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews LD, Cohen AI, 1983. Freeze-fracture studies of photoreceptor membranes: New observations bearing upon the distribution of cholesterol. J. Cell Biol 10.1083/jcb.97.3.749 [DOI] [PMC free article] [PubMed]
- Andrews LD, Cohen AI, 1979. Freeze-fracture evidence for the presence of cholesterol in particle-free patches of basal disks and the plasma membrane of retinal rod outer segments of mice and frogs. J. Cell Biol 10.1083/jcb.81.1.215 [DOI] [PMC free article] [PubMed]
- Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K, 2016. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm 10.1155/2016/8606878 [DOI] [PMC free article] [PubMed]
- AREDS Research Group, 2009. ω–3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr 90, 1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou C, Polosa A, Cecyre B, Hsieh M, Di Pietro E, Cui W, Bouchard J-F, Lachapelle P, Braverman N, 2019. A longitudinal study of retinopathy in the PEX1-Gly844Asp mouse model for mild Zellweger Spectrum Disorder. Exp. Eye Res 186, 107713. 10.1016/j.exer.2019.107713 [DOI] [PubMed] [Google Scholar]
- Arroba AI, Campos-Caro A, Aguilar-Diosdado M, Valverde ÁM, 2018. IGF-1, Inflammation and Retinal Degeneration: A Close Network. Front. Aging Neurosci 10, 203. 10.3389/fnagi.2018.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveldano MI, 1988. Phospholipid Species Containing Long and Very Long Polyenoic Fatty Acids Remain with Rhodopsin after Hexane Extraction of Photoreceptor Membranes. Biochemistry 27, 1229–1239. 10.1021/bi00404a024 [DOI] [PubMed] [Google Scholar]
- Aveldano MI, Bazan NG, 1983. Molecular species of phosphatidylcholine, -ethanolamine, -serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J. Lipid Res [PubMed]
- Ávila-Flores A, Santos T, Rincón E, Mérida I, 2005. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J. Biol. Chem 280, 10091–10099. 10.1074/jbc.M412296200 [DOI] [PubMed] [Google Scholar]
- Baird PN, Guida E, Chu DT, Vu HTV, Guymer RH, 2004. The ε2 and ε4 Alleles of the Apolipoprotein Gene Are Associated with Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci 45, 1311–1315. 10.1167/iovs.03-1121 [DOI] [PubMed] [Google Scholar]
- Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, McCarty CA, Guymer RH, 2006. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD). Hum. Mutat 27, 337–342. 10.1002/humu.20288 [DOI] [PubMed] [Google Scholar]
- Baksheeva VE, Tiulina VV, Tikhomirova NK, Gancharova OS, Komarov SV, Philippov PP, Zamyatnin AA, Senin II, Zernii EY, 2019. Suppression of light-induced oxidative stress in the retina by mitochondria-targeted antioxidant. Antioxidants 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandhuvula P, Saba JD, 2007. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol. Med 10.1016/j.molmed.2007.03.005 [DOI] [PubMed]
- Barro-Soria R, Stindl J, Müller C, Foeckler R, Todorov V, Castrop H, Strauß O, 2012. Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS One 7, e49624. 10.1371/journal.pone.0049624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, 2021. Overview of how n32 and n34 elovanoids sustain sight by protecting retinal pigment epithelial cells and photoreceptors. J. Lipid Res 10.1194/JLR.TR120001137 [DOI] [PMC free article] [PubMed]
- Bazan NG, 2018. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Aspects Med 10.1016/j.mam.2018.09.003 [DOI] [PMC free article] [PubMed]
- Bazan NG, 2008. Neurotrophins Induce Neuroprotective Signaling in the Retinal Pigment Epithelial Cell by Activating the Synthesis nl of the Anti-inflammatory and Anti-apoptotic Neuroprotectin D1 BT - Recent Advances in Retinal Degeneration, in: Anderson RE, LaVail MM, Hollyfield JG (Eds.), . Springer New York, New York, NY, pp. 39–44. 10.1007/978-0-387-74904-4_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Gordon WC, Rodriguez de Turco EB, 1992. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv. Exp. Med. Biol 318, 295–306. 10.1007/978-1-4615-3426-6_26 [DOI] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC, 2011. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr 31, 321–351. 10.1146/annurev.nutr.012809.104635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Martinez-Bermudez AK, Gobeil F Jr, Marrache AM, Hou X, Speranza G, Abran D, Quiniou C, Lachapelle P, Roberts J, 2001. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. J. Appl. Physiol 90, 2279–2288. [DOI] [PubMed] [Google Scholar]
- Bechara C, Nöll A, Morgner N, Degiacomi MT, Tampé R, Robinson CV, 2015. A subset of annular lipids is linked to the flippase activity of an ABC transporter. Nat. Chem 7, 255–262. 10.1038/nchem.2172 [DOI] [PubMed] [Google Scholar]
- Bechara C, Robinson CV, 2015. Different modes of lipid binding to membrane proteins probed by mass spectrometry. J. Am. Chem. Soc 137, 5240–5247. 10.1021/jacs.5b00420 [DOI] [PubMed] [Google Scholar]
- Behl T, Kaur I, Kotwani A, 2016. Role of leukotrienes in diabetic retinopathy. Prostaglandins Other Lipid Mediat 122, 1–9. [DOI] [PubMed] [Google Scholar]
- Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY, 2005. Biochemical properties of purified human retinol dehydrogenase 12 (RDH 12): Catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the ox. Biochemistry 44, 7035–7047. 10.1021/bi050226k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett LD, Anderson RE, 2016. Current progress in deciphering importance of VLC-PUFA in the retina, in: Advances in Experimental Medicine and Biology Springer; New York LLC, pp. 145–151. 10.1007/978-3-319-17121-0_20 [DOI] [PubMed] [Google Scholar]
- Benolken RM, Anderson RE, Wheeler TG, 1973. Membrane fatty acids associated with the electrical response in visual excitation. Science (80-. ) 182, 1253–1254. [DOI] [PubMed] [Google Scholar]
- Bergen AA, Arya S, Koster C, Pilgrim MG, Wiatrek-Moumoulidis D, van der Spek PJ, Hauck SM, Boon CJF, Emri E, Stewart AJ, Lengyel I, 2019. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res 70, 55–84. 10.1016/j.preteyeres.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Bermond K, Wobbe C, Tarau I-S, Heintzmann R, Hillenkamp J, Curcio CA, Sloan KR, Ach T, 2020. Autofluorescent Granules of the Human Retinal Pigment Epithelium: Phenotypes, Intracellular Distribution, and Age-Related Topography. Invest. Ophthalmol. Vis. Sci 61, 35. 10.1167/iovs.61.5.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Tammur J, Singh N, Hutchinson A, Dixon M, Pappas CM, Zabriskie NA, Zhang K, Petrukhin K, Leppert M, Allikmets R, 2001. Diverse Macular Dystrophy Phenotype Caused by a Novel Complex Mutation in the ELOVL4 Gene. Invest. Ophthalmol. Vis. Sci 42, 3331–3336. [PubMed] [Google Scholar]
- Berridge MJ, 2009. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta - Mol. Cell Res 10.1016/j.bbamcr.2008.10.005 [DOI] [PubMed]
- Berridge MJ, Irvine RF, 1984. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321. 10.1038/312315a0 [DOI] [PubMed] [Google Scholar]
- Bertram KM, Baglole CJ, Phipps RP, Libby RT, 2009. Molecular regulation of cigarette smoke induced-oxidative stress in human retinal pigment epithelial cells: implications for age-related macular degeneration. Am. J. Physiol. Physiol 297, C1200–C1210. 10.1152/ajpcell.00126.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand RE, Wang J, Xiong KH, Thangavel C, Qian X, Ba-Abbad R, Liang Q, Simões RT, Sampaio SAM, Carss KJ, Lucy Raymond F, Robson AG, Webster AR, Arno G, Porto FBO, Chen R, 2021. Ceramide synthase TLCD3B is a novel gene associated with human recessive retinal dystrophy. Genet. Med 23, 488–497. 10.1038/s41436-020-01003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Pfenninger KH, 1980. Membrane assembly in retinal photoreceptors: I. Freeze-fracture analysis of cytoplasmic vesicles in relationship to disc assembly. J. Cell Biol 87, 451–463. 10.1083/jcb.87.2.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C, Young RW, 1974a. Renewal of glycerol in the visual cells and pigment epithelium of the frog retina. J. Cell Biol 62, 378–389. 10.1083/jcb.62.2.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C, Young RW, 1974b. Renewal of fatty acids in the membranes of visual cell outer segments. J. Cell Biol 61, 327–348. 10.1083/jcb.61.2.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch DG, Birch EE, Hoffman DR, Uauy RD, 1992. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Invest. Ophthalmol. Vis. Sci 33, 2365–2376. [PubMed] [Google Scholar]
- Birgbauer E, Chun J, 2010. Lysophospholipid receptors LPA1–3 are not required for the inhibitory effects of LPA on mouse retinal growth cones. Eye Brain 2, 1. 10.2147/eb.s7666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD, 1992. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp. Eye Res 10.1016/0014-4835(92)90040-Y [DOI] [PubMed]
- Boesze-Battaglia K, Albert AD, 1989. Fatty acid composition of bovine rod outer segment plasma membrane. Exp. Eye Res 49, 699–701. 10.1016/S0014-4835(89)80064-8 [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Damek-Poprawa M, Mitchell DC, Greeley L, Brush RS, Anderson RE, Richards MJ, Fliesler SJ, 2008. Alteration of retinal rod outer segment membrane fluidity in a rat model of Smith-Lemli-Opitz syndrome. J. Lipid Res 49, 1488–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD, 1990. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J. Biol. Chem [PMC free article] [PubMed]
- Boesze-Battaglia K, Hennessey T, Albert AD, 1989. Cholesterol heterogeneity in bovine red outer segment disk membranes. J. Biol. Chem [PMC free article] [PubMed]
- Boesze-Battaglia K, Schimmel R, 1997. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol 200, 2927–2936. [DOI] [PubMed] [Google Scholar]
- Bolla JR, Corey RA, Sahin C, Gault J, Hummer A, Hopper JTS, Lane DP, Drew D, Allison TM, Stansfeld PJ, Robinson CV, Landreh M, 2020. A Mass‐Spectrometry‐Based Approach to Distinguish Annular and Specific Lipid Binding to Membrane Proteins. Angew. Chemie 132, 3551–3556. 10.1002/ange.201914411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Rayborn ME, Bhattacharya SK, Gu X, Crabb JS, Crabb JW, Hollyfield JG, 2006. The retinal pigment epithelium apical microvilli and retinal function. Retin. Degener. Dis 519–524. [DOI] [PMC free article] [PubMed]
- Boon CJF, van Schooneveld MJ, den Hollander AI, van Lith-Verhoeven JJC, Zonneveld-Vrieling MN, Theelen T, Cremers FPM, Hoyng CB, Klevering BJ, 2007. Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. Br. J. Ophthalmol 10.1136/bjo.2007.115659 [DOI] [PMC free article] [PubMed]
- Bornancin F, 2011. Ceramide kinase: The first decade. Cell. Signal 10.1016/j.cellsig.2010.11.012 [DOI] [PubMed]
- Bosch E, Horwitz J, Bok D, 1993. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J. Histochem. Cytochem 41, 253–263. 10.1177/41.2.8419462 [DOI] [PubMed] [Google Scholar]
- Bretillon L, Thuret G, Grégoire S, Acar N, Joffre C, Bron AM, Gain P, Creuzot-Garcher CP, 2008. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res 87, 521–528. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Zein WM, Thompson AH, Mokhtarzadeh M, Doherty DA, Parisi M, Glass IA, Malicdan MC, Vilboux T, Vemulapalli M, Mullikin JC, Gahl WA, Gunay-Aygun M, 2018. Joubert Syndrome: Ophthalmological Findings in Correlation with Genotype and Hepatorenal Disease in 99 Patients Prospectively Evaluated at a Single Center. Ophthalmology 125, 1937–1952. 10.1016/j.ophtha.2018.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Rubin LJ, Ghalayini AJ, Tarver AP, Irvine RF, Berridge MJ, Anderson RE, 1984. myo-inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature 311, 160–163. 10.1038/311160a0 [DOI] [PubMed] [Google Scholar]
- Brush RS, Tran JTA, Henry KR, McClellan ME, Elliott MH, Mandal MNA, 2010. Retinal sphingolipids and their very-long-chain fatty acid-containing species. Investig. Ophthalmol. Vis. Sci 10.1167/iovs.09-5134 [DOI] [PMC free article] [PubMed]
- Budin I, Rond T. de, Chen Y, Chan LJG, Petzold CJ, Keasling JD, 2018. Viscous control of cellular respiration by membrane lipid composition. Science (80-. ) 362, 1186–1189. 10.1126/science.aat7925 [DOI] [PubMed] [Google Scholar]
- Bullock J, Polato F, Abu-Asab M, Bernardo-Colón A, Aflaki E, Agbaga MP, Patricia Becerra S, 2021. Degradation of Photoreceptor Outer Segments by the Retinal Pigment Epithelium Requires Pigment Epithelium-Derived Factor Receptor (PEDF-R). Investig. Ophthalmol. Vis. Sci 62. 10.1167/IOVS.62.2.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, Meschede IP, Burden JJ, Bailly M, Seabra MC, Futter CE, 2015. Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc. Natl. Acad. Sci. U. S. A 112, 15922–15927. 10.1073/pnas.1509285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle A, Virág L, 2013. Poly(ADP-ribose): PARadigms and PARadoxes. Mol. Aspects Med 34, 1046–1065. 10.1016/J.MAM.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Bush RA, Remé CE, Malnoë A, 1991. Light damage in the rat retina: The effect of dietary deprivation of n-3 fatty acids on acute structural alterations. Exp. Eye Res 10.1016/0014-4835(91)90109-R [DOI] [PubMed]
- Buzhynskyy N, Salesse C, Scheuring S, 2011. Rhodopsin is spatially heterogeneously distributed in rod outer segment disk membranes. J. Mol. Recognit 10.1002/jmr.1086 [DOI] [PubMed]
- Cai J, Nelson KC, Wu M, Sternberg P, Jones DP, 2000. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res 19, 205–221. 10.1016/S1350-9462(99)00009-9 [DOI] [PubMed] [Google Scholar]
- Campello L, Singh N, Advani J, Mondal AK, Corso-Diaz X, Swaroop A, 2021. Aging of the Retina: Molecular and Metabolic Turbulences and Potential Interventions. Annu. Rev. Vis. Sci 7. 10.1146/annurev-vision-100419-114940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals D, Salamone S, Hannun YA, 2018. Visualizing bioactive ceramides. Chem. Phys. Lipids 10.1016/j.chemphyslip.2018.09.013 [DOI] [PMC free article] [PubMed]
- Cankova Z, Huang JD, Kruth HS, Johnson M, 2011. Passage of low-density lipoproteins through Bruch’s membrane and choroid. Exp. Eye Res 93, 947–955. 10.1016/j.exer.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Datta S, Wang L, Liu T, Flores-Bellver M, Sachdeva M, Sinha D, Handa JT, 2021. Nrf2 deficiency decreases NADPH from impaired IDH shuttle and pentose phosphate pathway in retinal pigmented epithelial cells to magnify oxidative stress-induced mitochondrial dysfunction. Aging Cell 20, e13444. 10.1111/acel.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Walker GB, Wang X, Cui JZ, Matsubara JA, 2013. Altered cytokine profiles of human retinal pigment epithelium: oxidant injury and replicative senescence. Mol. Vis 19, 718–728. [PMC free article] [PubMed] [Google Scholar]
- Carpentier S, N’Kuli F, Grieco G, Van Der Smissen P, Janssens V, Emonard H, Bilanges B, Vanhaesebroeck B, Gaide Chevronnay HP, Pierreux CE, Tyteca D, Courtoy PJ, 2013. Class III phosphoinositide 3-kinase/VPS34 and dynamin are critical for apical endocytic recycling. Traffic 10.1111/tra.12079 [DOI] [PubMed]
- Castellino N, Longo A, Avitabile T, Russo A, Fallico M, Bonfiglio V, Toro MD, Rejdak R, Nowomiejska K, Murabito P, 2018. Circulating insulin-like growth factor-1: a new clue in the pathogenesis of age-related macular degeneration. Aging (Albany NY) 10, 4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina C, Campisi A, Di Giacomo C, Sorrenti V, Russo A, Vanella A, 1992. Lipid peroxidation and antioxidant enzymatic systems in rat retina as a function of age. Neurochem. Res 17, 599–604. 10.1007/BF00968789 [DOI] [PubMed] [Google Scholar]
- Castro BM, Prieto M, Silva LC, 2014. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res 54, 53–67. 10.1016/j.plipres.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Chang Y, Finnemann SC, 2007. Tetraspanin CD81 is required for the αvβ5-integrin-dependent particle-binding step of RPE phagocytosis. J. Cell Sci 10.1242/jcs.006361 [DOI] [PMC free article] [PubMed]
- Charbel Issa P, Gillies MC, Chew EY, Bird AC, Heeren TFC, Peto T, Holz FG, Scholl HPN, 2013. Macular telangiectasia type 2. Prog. Retin. Eye Res 34, 49–77. 10.1016/j.preteyeres.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SD, Zhou J, Dasgupta R, Cramer-Blok A, Timmer M, Van Der Stelt M, Ubbink M, 2021. Protein Dynamics Influence the Enzymatic Activity of Phospholipase A/Acyltransferases 3 and 4. Biochemistry 60, 1178–1190. 10.1021/acs.biochem.0c00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Chao DL, Rocha L, Kolar M, Nguyen Huu VA, Krawczyk M, Dasyani M, Wang T, Jafari M, Jabari M, 2020. The lipid elongation enzyme ELOVL2 is a molecular regulator of aging in the retina. Aging Cell 19, e13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Anderson RE, 1993. Differential incorporation of docosahexaenoic and arachidonic acids in frog retinal pigment epithelium. J. Lipid Res 34, 1943–1955. [PubMed] [Google Scholar]
- Chen HY, Kelley RA, Li T, Swaroop A, 2021. Primary cilia biogenesis and associated retinal ciliopathies. Semin. Cell Dev. Biol 110, 70–88. 10.1016/j.semcdb.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Messinger JD, Ferrara D, Freund KB, Curcio CA, 2021. Stages of Drusen-Associated Atrophy in Age-Related Macular Degeneration Visible via Histologically Validated Fundus Autofluorescence. Ophthalmol. Retin 10.1016/j.oret.2020.11.006 [DOI] [PMC free article] [PubMed]
- Chen Y, Houghton LA, Brenna JT, Noy N, 1996. Docosahexaenoic acid modulates the interactions of the interphotoreceptor retinoid-binding protein with 11-cis-retinal. J. Biol. Chem 271, 20507–20515. 10.1074/jbc.271.34.20507 [DOI] [PubMed] [Google Scholar]
- Chen Y, Saari JC, Noy N, 1993. Interactions of All-i/Ym-retinol and Long-Chain Fatty Acids with Interphotoreceptor Retinoid-Binding Protein1, Biochemistry. Adler & Martin [DOI] [PubMed]
- Chew EY, 2013. Nutrition Effects on Ocular Diseases in the Aging Eye. Invest. Ophthalmol. Vis. Sci 54, ORSF42–ORSF47. 10.1167/iovs13-12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL, Group AR, 2012. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 119, 2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE, 2001. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr 73, 209–218. 10.1093/ajcn/73.2.209 [DOI] [PubMed] [Google Scholar]
- Choi R, Gorusupudi A, Bernstein PS, 2018. Long-term follow-up of autosomal dominant Stargardt macular dystrophy (STGD3) subjects enrolled in a fish oil supplement interventional trial. Ophthalmic Genet 39, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong EWT, Kreis AJ, Wong TY, Simpson JA, Guymer RH, 2008. Dietary ω−3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch. Ophthalmol 126, 826–833. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Hsu YC, Sung CH, 2015. Ultrastructural visualization of trans-ciliary rhodopsin cargoes in mammalian rods. Cilia 4, 4. 10.1186/s13630-015-0013-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH, 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev 10.1124/pr.110.003111 [DOI] [PMC free article] [PubMed]
- Cideciyan AV, Aleman TS, Swider M, Schwartz SB, Steinberg JD, Brucker AJ, Maguire AM, Bennett J, Stone EM, Jacobson SG, 2004. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum. Mol. Genet 13, 525–534. 10.1093/hmg/ddh048 [DOI] [PubMed] [Google Scholar]
- Coleman JA, Kwok MCM, Molday RS, 2009. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine Flippase in photoreceptor disc membranes. J. Biol. Chem 10.1074/jbc.M109.047415 [DOI] [PMC free article] [PubMed]
- Constable PA, 2014. A perspective on the mechanism of the light-rise of the electrooculogram. Invest. Ophthalmol. Vis. Sci 55, 2669–2673. 10.1167/iovs.14-13979 [DOI] [PubMed] [Google Scholar]
- Corso-Díaz X, Gentry J, Rebernick R, Jaeger C, Brooks MJ, van Asten F, Kooragayala K, Gieser L, Nellissery J, Covian R, Cogliati T, Mondal AK, Jiang K, Swaroop A, 2020. Genome-wide Profiling Identifies DNA Methylation Signatures of Aging in Rod Photoreceptors Associated with Alterations in Energy Metabolism. Cell Rep 31. 10.1016/j.celrep.2020.107525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnard-Grégoire A, Delyfer M-N, Korobelnik J-F, Rougier M-B, Le Goff M, Dartigues J-F, Barberger-Gateau P, Delcourt C, 2014. Elevated high-density lipoprotein cholesterol and age-related macular degeneration: the Alienor study. PLoS One 9, e90973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG, 2002. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci 99, 14682 LP – 14687. 10.1073/pnas.222551899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas Arenas R, Danielczak B, Martel A, Porcar L, Breyton C, Ebel C, Keller S, 2017. Fast Collisional Lipid Transfer Among Polymer-Bounded Nanodiscs. Sci. Rep 7, 45875. 10.1038/srep45875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Gonzalez-Fernandez F, 2003. Internalization of Interphotoreceptor Retinoid-Binding Protein by the Xenopus Retinal Pigment Epithelium. J. Comp. Neurol 466, 331–342. 10.1002/cne.10861 [DOI] [PubMed] [Google Scholar]
- Curcio CA, 2018. Soft Drusen in Age-Related Macular Degeneration: Biology and Targeting Via the Oil Spill Strategies. Invest. Ophthalmol. Vis. Sci 59, AMD160–AMD181. 10.1167/iovs.18-24882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang J-D, Rudolf M, 2010. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J. Lipid Res 51, 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang J-D, 2011. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol 95, 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS, 2001. Accumulation of cholesterol with age in human Bruch’s membrane. Invest. Ophthalmol. Vis. Sci 42, 265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Millican CL, Medeiros NE, 2005. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp. Eye Res 80, 761–775. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Cosot OA, Gutkind JS, Spiegel S, 1996. Suppression of ceramide-mediated programmed cell death by sphingosine- 1-phosphate. Nature 10.1038/381800a0 [DOI] [PubMed]
- Daiger SP, Bowne SJ, Sullivan LS, 2015. Genes and mutations causing autosomal dominant retinitis pigmentosa. Cold Spring Harb. Perspect. Med 5, a017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Armi C, Devereaux KA, Di Paolo G, 2013. The role of lipids in the control of autophagy. Curr. Biol 10.1016/j.cub.2012.10.041 [DOI] [PMC free article] [PubMed]
- Danielczak B, Keller S, 2018. Collisional lipid exchange among DIBMA-encapsulated nanodiscs (DIBMALPs). Eur. Polym. J 109, 206–213. 10.1016/j.eurpolymj.2018.09.043 [DOI] [Google Scholar]
- Das ND, Yoshioka T, Samuelson D, Cohen RJ, Shichi H, 1987. Immunochemical Evidence for the Light-Regulated Modulation of Phosphatidylinositol-4, 5-Bisphosphate in Rat Photoreceptor Cells. Cell Struct. Funct 12, 471–481. 10.1247/csf.12.471 [DOI] [PubMed] [Google Scholar]
- Das UN, 2020. Bioactive lipids in age-related disorders. Rev. New Drug Targets Age-Related Disord 33–83. [DOI] [PubMed]
- Das UN, 2013. Lipoxins, resolvins, and protectins in the prevention and treatment of diabetic macular edema and retinopathy. Nutrition 10.1016/j.nut.2012.02.003 [DOI] [PubMed]
- Das UN, 1995. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins, Leukot. Essent. Fat. Acids 52, 387–391. 10.1016/0952-3278(95)90066-7 [DOI] [PubMed] [Google Scholar]
- De La Paz M, Anderson RE, 1992. Region and age-dependent variation in susceptibility of the human retina to lipid peroxidation. Invest. Ophthalmol. Vis. Sci 33, 3497–3499. [PubMed] [Google Scholar]
- Deák F, Anderson RE, Fessler JL, Sherry DM, 2019. Novel Cellular Functions of Very Long Chain-Fatty Acids: Insight From ELOVL4 Mutations. Front. Cell. Neurosci 10.3389/fncel.2019.00428 [DOI] [PMC free article] [PubMed]
- Defoe DM, Grindstaff RD, 2004. Epidermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Exp. Eye Res 79, 51–59. 10.1016/j.exer.2004.02.017 [DOI] [PubMed] [Google Scholar]
- Dennis EA, Norris PC, 2015. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol 15, 511–523. 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentchev T, Yao Y, Praticó D, Dunaief JL, 2007. Isoprostane F2α-VI, a new marker of oxidative stress, increases following light damage to the mouse retina. Mol. Vis 13, 190. [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Traverso V, Parkins N, Jackson F, Rodriguez De Turco EB, Ransom N, 2004. Phosphoinositides, Ezrin/Moesin, and rac1 Regulate Fusion of Rhodopsin Transport Carriers in Retinal Photoreceptors. Mol. Biol. Cell 10.1091/mbc.E03-04-0203 [DOI] [PMC free article] [PubMed]
- Devaux PF, 1991. Static and Dynamic Lipid Asymmetry in Cell Membranes. Biochemistry 30, 1163–1173. 10.1021/bi00219a001 [DOI] [PubMed] [Google Scholar]
- Dhingra A, Bell BA, Peachey NS, Daniele LL, Reyes-Reveles J, Sharp RC, Jun B, Bazan NG, Sparrow JR, Kim HJ, Philp NJ, Boesze-Battaglia K, 2018. Microtubule-Associated Protein 1 Light Chain 3B, (LC3B) Is Necessary to Maintain Lipid-Mediated Homeostasis in the Retinal Pigment Epithelium. Front. Cell. Neurosci [DOI] [PMC free article] [PubMed]
- Ding J-D, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Rickman CB, 2011. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci 108, E279 LP–E287. 10.1073/pnas.1100901108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KV, Kautzmann MAI, Jun B, Gordon WC, Nshimiyimana R, Yang R, Petasis NA, Bazan NG, 2019. Elovanoids counteract oligomeric β-amyloid-induced gene expression and protect photoreceptors. Proc. Natl. Acad. Sci. U. S. A 116, 24317–24325. 10.1073/pnas.1912959116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KG, Bailey KR, Kane JP, Schwartz DM, 2002. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun 292, 1017–1022. 10.1006/bbrc.2002.6756 [DOI] [PubMed] [Google Scholar]
- Duncan KG, Hosseini K, Bailey KR, Yang H, Lowe RJ, Matthes MT, Kane JP, LaVail MM, Schwartz DM, Duncan JL, 2009. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br. J. Ophthalmol 93, 1116–1120. 10.1136/bjo.2008.144006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan T, Fariss RN, Wiggert B, 2006. Confocal immunolocalization of bovine serum albumin, serum retinol-binding protein, and interphotoreceptor retinoid-binding protein in bovine retina. Mol. Vis 12, 1632–9. [PubMed] [Google Scholar]
- Eade K, Gantner ML, Hostyk JA, Nagasaki T, Giles S, Fallon R, Harkins-Perry S, Baldini M, Lim EW, Scheppke L, Dorrell MI, Cai C, Baugh EH, Wolock CJ, Wallace M, Berlow RB, Goldstein DB, Metallo CM, Friedlander M, Allikmets R, 2021. Serine biosynthesis defect due to haploinsufficiency of PHGDH causes retinal disease. Nat. Metab 3, 366–377. 10.1038/s42255-021-00361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebberink MS, Mooijer PAW, Gootjes J, Koster J, Wanders RJA, Waterham HR, 2011. Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder. Hum. Mutat 32, 59–69. 10.1002/humu.21388 [DOI] [PubMed] [Google Scholar]
- Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BHF, Langmann T, 2009. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J. Neurochem 110, 1863–1875. 10.1111/j.1471-4159.2009.06286.x [DOI] [PubMed] [Google Scholar]
- Eichmann TO, Lass A, 2015. DAG tales: The multiple faces of diacylglycerol - Stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci 10.1007/s00018-015-1982-3 [DOI] [PMC free article] [PubMed]
- Elmasry K, Ibrahim AS, Abdulmoneim S, Al‐Shabrawey M, 2019. Bioactive lipids and pathological retinal angiogenesis. Br. J. Pharmacol 176, 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eresch J, Stumpf M, Koch A, Vutukuri R, Ferreirós N, Schreiber Y, Schröder K, Devraj K, Popp R, Huwiler A, Hattenbach L, Pfeilschifter J, Pfeilschifter W, 2018. Sphingosine Kinase 2 Modulates Retinal Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci 59, 653–661. 10.1167/iovs.17-22544 [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW, 2006. Cigarette Smoke–Related Oxidants and the Development of Sub-RPE Deposits in an Experimental Animal Model of Dry AMD. Invest. Ophthalmol. Vis. Sci 47, 729–737. 10.1167/iovs.05-0719 [DOI] [PubMed] [Google Scholar]
- Esteve-Rudd J, Hazim RA, Diemer T, Paniagua AE, Volland S, Umapathy A, Williams DS, 2018. Defective phagosome motility and degradation in cell nonautonomous RPE pathogenesis of a dominant macular degeneration. Proc. Natl. Acad. Sci 115, 5468–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, 2008. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye 22, 751–760. 10.1038/eye.2008.100 [DOI] [PubMed] [Google Scholar]
- Fabiani C, Zulueta A, Bonezzi F, Casas J, Ghidoni R, Signorelli P, Caretti A, 2017. 2-Acetyl-5-tetrahydroxybutyl imidazole (THI) protects 661W cells against oxidative stress. Naunyn. Schmiedebergs. Arch. Pharmacol 10.1007/s00210-017-1374-3 [DOI] [PubMed]
- Falk G, Fatt P, 1969. Distinctive properties of the lamellar and disk-edge structures of the rod outer segment. J. Ultrastruct. Res 28, 41–60. 10.1016/s0022-5320(69)90005-7 [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B, 2010. Phosphoinositides: Lipid regulators of membrane proteins, in: Journal of Physiology 10.1113/jphysiol.2010.192153 [DOI] [PMC free article] [PubMed]
- Fallon R, Zernant J, Nagasaki T, Gantner M, harkins-perry sarah, Eade K, Allikmets R, Friedlander M, 2018. Rare variants in the phosphoglycerate dehydrogenase (PHGDH) gene in MacTel patients lead to decreased enzymatic activity. Invest. Ophthalmol. Vis. Sci 59, 5409. [Google Scholar]
- Fan J, Wu BX, Crosson CE, 2016. Suppression of acid sphingomyelinase protects the retina from ischemic injury. Investig. Ophthalmol. Vis. Sci 57, 4476–4484. 10.1167/iovs.16-19717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Bian G, Ren P, Xiang J, Song J, Yu C, Zhang Q, Liu L, Chen K, Liu F, Zhang K, Wu C, Sun R, Hu D, Ju G, Wang J, 2018. S1p transporter spns2 regulates proper postnatal retinal morphogenesis. FASEB J 10.1096/fj.201701116R [DOI] [PubMed]
- Fauser S, Smailhodzic D, Caramoy A, van de Ven JPH, Kirchhof B, Hoyng CB, Klevering BJ, Liakopoulos S, den Hollander AI, 2011. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 52, 5525–5528. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Gao CL, Tidwell M, 1987. Lysosomal enzyme cytochemistry of human RPE, Bruch’s membrane and drusen. Invest. Ophthalmol. Vis. Sci 28, 1138–1147. [PubMed] [Google Scholar]
- Feeney-Burns L, Hilderbrand ES, Eldridge S, 1984. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest. Ophthalmol. Vis. Sci 25, 195–200. [PubMed] [Google Scholar]
- Feeney L, 1978. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest. Ophthalmol. Vis. Sci 17, 583–600. [PubMed] [Google Scholar]
- Fein A, Payne R, Wesley Corson D, Berridge MJ, Irvine RF, 1984. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature 311, 157–160. 10.1038/311157a0 [DOI] [PubMed] [Google Scholar]
- Fincher J, Whiteneck C, Birgbauer E, 2014. G-Protein-Coupled Receptor Cell Signaling Pathways Mediating Embryonic Chick Retinal Growth Cone Collapse Induced by Lysophosphatidic Acid and Sphingosine-1-Phosphate. Dev. Neurosci 36, 443–453. 10.1159/000364858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein S, Gospe SM, Schuhmann K, Shevchenko A, Arshavsky VY, Lobanova ES, 2020. Phosphoinositide Profile of the Mouse Retina. Cells 9, 1417. 10.3390/cells9061417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E, 1997. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. U. S. A 94, 12932–12937. 10.1073/pnas.94.24.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E, 2002. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci 99, 3842 LP – 3847. 10.1073/pnas.052025899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzPatrick DR, 1996. Zellweger syndrome and associated phenotypes. J. Med. Genet 33, 863 LP – 868. 10.1136/jmg.33.10.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Jobling AI, Greferath U, Mills SA, Waugh M, Ho T, de Iongh RU, Phipps JA, Vessey KA, 2014. Studying Age-Related Macular Degeneration Using Animal Models. Optom. Vis. Sci 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler AJ, Anderson RE, 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res 22, 79–131. 10.1016/0163-7827(83)90004-8 [DOI] [PubMed] [Google Scholar]
- Fliesler S, Xu L, 2018. Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention. Molecules 23, 2720. 10.3390/molecules23102720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Keller RK, 1997. Isoprenoid metabolism in the vertebrate retina. Int. J. Biochem. Cell Biol 29, 877–94. 10.1016/s1357-2725(97)00018-6 [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Schroepfer GJ, 1982. Sterol composition of bovine retinal rod outer segment membranes and whole retinas. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 10.1016/0005-2760(82)90020-0 [DOI] [PubMed]
- Fox TE, Young MM, Pedersen MM, Han X, Gardner TW, Kester M, 2012. Diabetes diminishes phosphatidic acid in the retina: A putative mediator for reduced mTOR signaling and increased neuronal cell death. Investig. Ophthalmol. Vis. Sci 53, 7257–7267. 10.1167/iovs.11-7626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JS, Chang B, Krauth DS, Lopez I, Waseem NH, Hurd RE, Feathers KL, Branham KE, Shaw M, Thomas GE, Brooks MJ, Liu C, Bakeri HA, Campos MM, Maubaret C, Webster AR, Rodriguez IR, Thompson DA, Bhattacharya SS, Koenekoop RK, Heckenlively JR, Swaroop A, 2010. Loss of lysophosphatidylcholine acyltransferase 1 leads to photoreceptor degeneration in rd11 mice. Proc. Natl. Acad. Sci. U. S. A 107, 15523–15528. 10.1073/pnas.1002897107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Chen CT, Cagnone G, Heckel E, Sun Y, Cakir B, Tomita Y, Huang S, Li Q, Britton W, Cho SS, Kern TS, Hellström A, Joyal J, Smith LE, 2019. Dyslipidemia in retinal metabolic disorders. EMBO Mol. Med 11, e10473. 10.15252/emmm.201910473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Kern TS, Hellström A, Smith LEH, 2021. Fatty acid oxidation and photoreceptor metabolic needs. J. Lipid Res 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, Nakamura Y, 2010. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res 10.1016/j.plipres.2010.06.001 [DOI] [PubMed]
- Gantner ML, Eade K, Wallace M, Handzlik MK, Fallon R, Trombley J, Bonelli R, Giles S, Harkins-Perry S, Heeren TFC, Sauer L, Ideguchi Y, Baldini M, Scheppke L, Dorrell MI, Kitano M, Hart BJ, Cai C, Nagasaki T, Badur MG, Okada M, Woods SM, Egan C, Gillies M, Guymer R, Eichler F, Bahlo M, Fruttiger M, Allikmets R, Bernstein PS, Metallo CM, Friedlander M, 2019. Serine and Lipid Metabolism in Macular Disease and Peripheral Neuropathy. N. Engl. J. Med 381, 1422–1433. 10.1056/NEJMoa1815111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG, 1992. Aging of the human retina: Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci 33, 1–17. [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA, 2010. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol 10.1007/978-1-4419-6741-1_1 [DOI] [PMC free article] [PubMed]
- Géléoc GGS, El-Amraoui A, 2020. Disease mechanisms and gene therapy for Usher syndrome. Hear. Res 394, 107932. 10.1016/j.heares.2020.107932 [DOI] [PubMed] [Google Scholar]
- Geng R, Geller SF, Hayashi T, Ray CA, Reh TA, Bermingham-McDonogh O, Jones SM, Wright CG, Melki S, Imanishi Y, Palczewski K, Alagramam KN, Flannery JG, 2009. Usher syndrome IIIA gene clarin-1 is essential for hair cell function and associated neural activation†. Hum. Mol. Genet 18, 2748–2760. 10.1093/hmg/ddp210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SM, Lu F, Rao M, Leach LL, Gross JM, 2021. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res 100969. 10.1016/j.preteyeres.2021.100969 [DOI] [PMC free article] [PubMed]
- German OL, Miranda GE, Abrahan CE, Rotstein NP, 2006. Ceramide is a mediator of apoptosis in retina photoreceptors. Investig. Ophthalmol. Vis. Sci 10.1167/iovs.05-1310 [DOI] [PubMed]
- Getz GS, 2018. Lipid transfer proteins: Introduction to the thematic review series. J. Lipid Res 10.1194/jlr.R084020 [DOI] [PMC free article] [PubMed]
- Ghalayini A, Anderson RE, 1984. Phosphatidylinositol 4,5-bisphosphate: Light-mediated breakdown in the vertebrate retina. Biochem. Biophys. Res. Commun 124, 503–506. 10.1016/0006-291X(84)91582-1 [DOI] [PubMed] [Google Scholar]
- Ghosh D, Haswell KM, Sprada M, Gonzalez-Fernandez F, 2015. Structure of zebrafish IRBP reveals fatty acid binding. Exp. Eye Res 140, 149–158. 10.1016/j.exer.2015.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG, 2012. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151, 1029–1041. 10.1016/j.cell.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusto NM, Pasquaré SJ, Salvador GA, Castagnet PI, Roque ME, Ilincheta de Boschero MG, 2000. Lipid metabolism in vertebrate retinal rod outer segments. Prog. Lipid Res 39, 315–391. 10.1016/S0163-7827(00)00009-6 [DOI] [PubMed] [Google Scholar]
- Golczak M, Sears AE, Kiser PD, Palczewski K, 2015. LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3. Nat. Chem. Biol 11, 26–32. 10.1038/nchembio.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AFX, Moritz OL, Williams DS, 2016. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res 10.1016/j.preteyeres.2016.05.003 [DOI] [PMC free article] [PubMed]
- Gomez-Munoz A, Waggoner DW, O’Brien L, Brindley DN, 1995. Interaction of ceramides, sphingosine, and sphingosine 1-phosphate in regulating DNA synthesis and phospholipase D activity. J. Biol. Chem 10.1074/jbc.270.44.26318 [DOI] [PubMed]
- Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T, 2005. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638. 10.1038/nature04321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordiyenko N, Campos M, Lee JW, Fariss RN, Sztein J, Rodriguez IR, 2004. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Investig. Ophthalmol. Vis. Sci 45, 2822–2829. 10.1167/iovs.04-0074 [DOI] [PubMed] [Google Scholar]
- Gordon W, Bazan N, 1990. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. J. Neurosci 10, 2190–2202. 10.1523/JNEUROSCI.10-07-02190.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WC, de Turco EBR, Bazan NG, 1992. Retinal pigment epithelial cells play a central role in the conservation of docosahexaenoic acid by photoreceptor cells after shedding and phagocytosis. Curr. Eye Res 11, 73–83. 10.3109/02713689209069169 [DOI] [PubMed] [Google Scholar]
- Gorusupudi A, Liu A, Hageman GS, Bernstein PS, 2016. Associations of human retinal very long-chain polyunsaturated fatty acids with dietary lipid biomarkers. J. Lipid Res 57, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorusupudi A, Rallabandi R, Li B, Arunkumar R, Blount JD, Rognon GT, Chang F-Y, Wade A, Lucas S, Conboy JC, 2021. Retinal bioavailability and functional effects of a synthetic very-long-chain polyunsaturated fatty acid in mice. Proc. Natl. Acad. Sci 118. [DOI] [PMC free article] [PubMed]
- GRABOWSKI GA, 1997. Gaucher Disease: Gene Frequencies and Genotype/Phenotype Correlations. Genet. Test 1, 5–12. 10.1089/gte.1997.1.5 [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL, 2006. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med 203, 2613–2625. 10.1084/jem.20060370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossfield A, Feller SE, Pitman MC, 2006. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. U. S. A 103, 4888–93. 10.1073/pnas.0508352103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG, 2003. Carboxyethylpyrrole Protein Adducts and Autoantibodies, Biomarkers for Age-related Macular Degeneration*. J. Biol. Chem 278, 42027–42035. 10.1074/jbc.M305460200 [DOI] [PubMed] [Google Scholar]
- Gulati S, Jastrzebska B, Banerjee S, Placeres ÁL, Miszta P, Gao S, Gunderson K, Tochtrop GP, Filipek S, Katayama K, Kiser PD, Mogi M, Stewart PL, Palczewski K, 2017. Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism. Proc. Natl. Acad. Sci. U. S. A 114, E2608–E2615. 10.1073/pnas.1617446114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundala NKV, Naidu VGM, Das UN, 2017. Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. BioFactors 43, 251–271. 10.1002/biof.1336 [DOI] [PubMed] [Google Scholar]
- Guziewicz KE, Sinha D, Gómez NM, Zorych K, Dutrow EV, Dhingra A, Mullins RF, Stone EM, Gamm DM, Boesze-Battaglia K, Aguirre GD, 2017. Bestrophinopathy: An RPE-photoreceptor interface disease. Prog. Retin. Eye Res 58, 70–88. 10.1016/j.preteyeres.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom SA, North MA, Nishina PM, Berson EL, Dryja TP, 1998. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with Retinitis pigmentosa. Nat. Genet 18, 174–176. 10.1038/ng0298-174 [DOI] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S, 2006. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta - Biomembr 10.1016/j.bbamem.2006.08.007 [DOI] [PubMed]
- Hall MO, Bok D, Bacharach ADE, 1969. Biosynthesis and assembly of the rod outer segment membrane system. Formation and fate of visual pigment in the frog retina. J. Mol. Biol 45, 397–406. 10.1016/0022-2836(69)90114-4 [DOI] [PubMed] [Google Scholar]
- Hall MO, Burgess BL, Abrams TA, Martinez MO, 1996. Carbachol does not correct the defect in the phagocytosis of outer segments by Royal College of Surgeons rat retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci 37, 1473–1477. [PubMed] [Google Scholar]
- Hamilton JA, Brunaldi K, 2007. A model for fatty acid transport into the brain. J. Mol. Neurosci 10.1007/s12031-007-0050-3 [DOI] [PubMed]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda SV, Klotzle B, Bibikova M, Fan J-BB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K, 2013. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 49, 359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2008. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol 10.1038/nrm2329 [DOI] [PubMed]
- Hao T, Guo J, Liu J, Wang J, Liu Z, Cheng X, Li J, Ren J, Li Z, Yan J, Zhang G, 2021. Predicting human age by detecting DNA methylation status in hair. Electrophoresis 42, 1255–1261. 10.1002/elps.202000349 [DOI] [PubMed] [Google Scholar]
- Hardy P, Beauchamp M, Sennlaub F, Gobeil FJ, Mwaikambo B, Lachapelle P, Chemtob S, 2005. Inflammatory lipid mediators in ischemic retinopathy. Pharmacol. Reports 57, 169. [PubMed] [Google Scholar]
- Harrison PJ, Dunn T, Campopiano DJ, 2018. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep 10.1039/c8np00019k [DOI] [PMC free article] [PubMed]
- Havla J, Moser M, Sztatecsny C, Lotz-Havla AS, Maier EM, Hizli B, Schinner R, Kümpfel T, Strupp M, Bremova-Ertl T, Schneider SA, 2020. Retinal axonal degeneration in Niemann–Pick type C disease. J. Neurol 267, 2070–2082. 10.1007/s00415-020-09796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Amakawa T, 1985. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem. Biophys. Res. Commun 128, 954–959. 10.1016/0006-291X(85)90139-1 [DOI] [PubMed] [Google Scholar]
- Hayes KC, Lindsey S, Stephan ZF, Brecker D, 1989. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest. Ophthalmol. Vis. Sci 30, 225–32. [PubMed] [Google Scholar]
- He F, Agosto MA, Anastassov IA, Tse DY, Wu SM, Wensel TG, 2016. Phosphatidylinositol-3-phosphate is light-regulated and essential for survival in retinal rods. Sci. Rep 6, 1–12. 10.1038/srep26978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessel E, Herrmann A, Müller P, Schnetkamp PPM, Hofmann KP, 2000. The transbilayer distribution of phospholipids in disc membranes is a dynamic equilibrium: Evidence for rapid flip and flop movement. Eur. J. Biochem 10.1046/j.1432-1327.2000.01147.x [DOI] [PubMed]
- Hessel E, Müller P, Herrmann A, Hofmann KP, 2001. Light-induced Reorganization of Phospholipids in Rod Disc Membranes. J. Biol. Chem 10.1074/jbc.M009061200 [DOI] [PubMed]
- Heth CA, Marescalchi PA, 1994. Inositol triphosphate generation in cultured rat retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci 35, 409–416. [PubMed] [Google Scholar]
- Heth CA, Marescalchi PA, Ye L, 1995. IP3 generation increases rod outer segment phagocytosis by cultured Royal College of Surgeons retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci 36, 984–989. [PubMed] [Google Scholar]
- Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J, 2008. General and Versatile Autoinhibition of PLC Isozymes. Mol. Cell 31, 383–394. 10.1016/j.molcel.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa D, Valentine WJ, Iizuka‐Hishikawa Y, Shindou H, Shimizu T, 2017. Metabolism and functions of docosahexaenoic acid‐containing membrane glycerophospholipids. FEBS Lett 591, 2730–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo M-S, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE, 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17, 55–63. 10.1038/nm.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollborn M, Fischer S, Kuhrt H, Wiedemann P, Bringmann A, Kohen L, 2017. Osmotic regulation of NFAT5 expression in RPE cells: The involvement of purinergic receptor signaling. Mol. Vis 15. [PMC free article] [PubMed]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL, 2008. Oxidative damage–induced inflammation initiates age-related macular degeneration. Nat. Med 14, 194–198. 10.1038/nm1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homme RP, Sandhu HS, George AK, Tyagi SC, Singh M, 2021. Sustained Inhibition of NF-κB Activity Mitigates Retinal Vasculopathy in Diabetes. Am. J. Pathol 191, 947–964. 10.1016/j.ajpath.2021.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopiavuori BR, Agbaga M-P, Brush RS, Sullivan MT, Sonntag WE, Anderson RE, 2017. Regional changes in CNS and retinal glycerophospholipid profiles with age: a molecular blueprint. J. Lipid Res 58, 668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopiavuori BR, Anderson RE, Agbaga M-P, 2019. ELOVL4: Very long-chain fatty acids serve an eclectic role in mammalian health and function. Prog. Retin. Eye Res 69, 137–158. 10.1016/j.preteyeres.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Jaiswal JK, 2018. Cellular mechanisms and signals that coordinate plasma membrane repair. Cell. Mol. Life Sci 75, 3751–3770. 10.1007/s00018-018-2888-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Violante S, Ventura FV, Wanders RJA, 2016. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol 10.1146/annurev-physiol-021115-105045 [DOI] [PubMed]
- Houtkooper RH, Vaz FM, 2008. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci 10.1007/s00018-008-8030-5 [DOI] [PMC free article] [PubMed]
- Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, De Mos J, Awwad K, Frömel T, Jungmann A, Devraj K, Cheng Z, Wang L, Fauser S, Eberhart CG, Sodhi A, Hammock BD, Liebner S, Müller OJ, Glaubitz C, Hammes HP, Popp R, Fleming I, 2017. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 552, 248–252. 10.1038/nature25013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, Qiao X, 2008. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s retinal angiomatous proliferation. Investig. Ophthalmol. Vis. Sci 49, 407–415. 10.1167/iovs.07-0870 [DOI] [PubMed] [Google Scholar]
- Huang H, Al-Shabrawey M, Wang MH, 2016. Cyclooxygenase- and cytochrome P450-derived eicosanoids in stroke. Prostaglandins Other Lipid Mediat 10.1016/j.prostaglandins.2015.12.007 [DOI] [PMC free article] [PubMed]
- Huang Z, Ghalayini AJ, Guo XX, Alvarez KM, Anderson RE, 2000. Light-mediated activation of diacylglycerol kinase in rat and bovine rod outer segments. J. Neurochem 75, 355–362. 10.1046/j.1471-4159.2000.0750355.x [DOI] [PubMed] [Google Scholar]
- Hurley JB, 2021. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci 7, 665–692. 10.1146/annurev-vision-100419-115156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AA, Starita C, Hodgetts A, Marshall J, 2010. Macromolecular diffusion characteristics of ageing human Bruch’s membrane: Implications for age-related macular degeneration (AMD). Exp. Eye Res 90, 703–710. 10.1016/j.exer.2010.02.013 [DOI] [PubMed] [Google Scholar]
- Imanishi Y, 2019. Protein Sorting in Healthy and Diseased Photoreceptors. Annu. Rev. Vis. Sci 5, 73–98. 10.1146/annurev-vision-091718-014843 [DOI] [PubMed] [Google Scholar]
- Inana G, Murat C, An W, Yao X, Harris IR, Cao J, 2018. RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells. J. Transl. Med 16, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intartaglia D, Giamundo G, Conte I, 2021. Autophagy in the retinal pigment epithelium: a new vision and future challenges. FEBS J. febs 16018. 10.1111/febs.16018 [DOI] [PMC free article] [PubMed]
- Jansen GA, Oftnan R, Ferdinandusse S, Ijlst L, Muijsers AO, Skjeldal OH, Stokke O, Jakobs C, Besley GTN, Wraith JE, Wanders RJA, 1997. Refsum disease is caused by mutations in the phytanoyl–CoA hydroxylase gene. Nat. Genet 17, 190–193. 10.1038/ng1097-190 [DOI] [PubMed] [Google Scholar]
- Jedlovszky P, Mezei M, 2003. Effect of Cholesterol on the Properties of Phospholipid Membranes. 1. Structural Features. J. Phys. Chem. B 107, 5311–5321. 10.1021/jp0219505 [DOI] [PubMed] [Google Scholar]
- Jelsema CL, 1987. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J. Biol. Chem 262, 163–168. 10.1016/s0021-9258(19)75904-3 [DOI] [PubMed] [Google Scholar]
- Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G, Juan AM, Hurst CG, Hatton CJ, Cui Z, Pierce KA, Bherer P, Aguilar E, Powner MB, Vevis K, Boisvert M, Fu Z, Levy E, Fruttiger M, Packard A, Rezende FA, Maranda B, Sapieha P, Chen J, Friedlander M, Clish CB, Smith LEH, 2016. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med 22, 439–445. 10.1038/nm.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D, 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun B, Mukherjee PK, Asatryan A, Kautzmann MA, Heap J, Gordon WC, Bhattacharjee S, Yang R, Petasis NA, Bazan NG, 2017. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Sci. Rep 7, 1–14. 10.1038/s41598-017-05433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Datta S, Wang L, Pegany R, Cano M, Handa JT, 2019. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp. Eye Res 181, 346–355. 10.1016/j.exer.2018.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Paananen J, Nevalainen T, Sorri I, Seitsonen S, Immonen I, Salminen A, Pulkkinen L, Uusitupa M, 2012. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett 10.1016/j.neulet.2012.02.050 [DOI] [PubMed]
- Kadamur G, Ross EM, 2013. Mammalian phospholipase C. Annu. Rev. Physiol 10.1146/annurev-physiol-030212-183750 [DOI] [PubMed]
- Kamoshita M, Toda E, Osada H, Narimatsu T, Kobayashi S, Tsubota K, Ozawa Y, 2016. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep 6, 30226. 10.1038/srep30226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed]
- Kaneshige Y, Hayashi F, Morigaki K, Tanimoto Y, Yamashita H, Fujii M, Awazu A, 2020. Affinity of rhodopsin to raft enables the aligned oligomer formation from dimers: Coarse-grained molecular dynamics simulation of disk membranes. PLoS One 10.1371/journal.pone.0226123 [DOI] [PMC free article] [PubMed]
- Kannan R, Jin M, Gamulescu MA, Hinton DR, 2004. Ceramide-induced apoptosis: Role of catalase and hepatocyte growth factor. Free Radic. Biol. Med 10.1016/j.freeradbiomed.2004.04.011 [DOI] [PubMed]
- Kanow MA, Giarmarco MM, Jankowski CSR, Tsantilas K, Engel AL, Du J, Linton JD, Farnsworth CC, Sloat SR, Rountree A, Sweet IR, Lindsay KJ, Parker ED, Brockerhoff SE, Sadilek M, Chao JR, Hurley JB, 2017. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6. 10.7554/eLife.28899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaa A, Kamoun WS, Xu H, Zhang J, Clemens MG, 2006. Differential effects of oxidative stress on hepatic endothelial and kupffer cell eicosanoid release in response to endothelin-1. Microcirculation 13, 457–466. 10.1080/10739680600776278 [DOI] [PubMed] [Google Scholar]
- Katz ML, Robison WG, 1984. Age-related changes in the retinal pigment epithelium of pigmented rats. Exp. Eye Res 38, 137–151. 10.1016/0014-4835(84)90098-8 [DOI] [PubMed] [Google Scholar]
- Kaur G, Tan LX, Rathnasamy G, Cunza N. La, Germer CJ, Toops KA, Fernandes M, Blenkinsop TA, Lakkaraju A, 2018. Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc. Natl. Acad. Sci. U. S. A 115, 9014–9019. 10.1073/pnas.1805039115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzmann MI, Gordon WC, Jun B, Do KV, Matherne BJ, Fang Z, Bazan NG, 2020. Membrane‐type frizzled‐related protein regulates lipidome and transcription for photoreceptor function. FASEB J 34, 912–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayden HJ, 1972. Abetalipoproteinemia. Annu. Rev. Med 23, 285–296. 10.1146/annurev.me.23.020172.001441 [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Johnson GL, 1985. Purification of protein kinase C from bovine rod outer segments. J. Cyclic Nucleotide Protein Phosphor. Res 10, 579–591. [PubMed] [Google Scholar]
- Kim J, Guan K-L, 2019. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol 21, 63–71. 10.1038/s41556-018-0205-1 [DOI] [PubMed] [Google Scholar]
- Kim SC, Wang X, 2020. Phosphatidic acid: An emerging versatile class of cellular mediators. Essays Biochem 10.1042/EBC20190089 [DOI] [PubMed]
- Kiser PD, Golczak M, Palczewski K, 2014. Chemistry of the retinoid (visual) cycle. Chem. Rev 10.1021/cr400107q [DOI] [PMC free article] [PubMed]
- Kiser PD, Palczewski K, 2016. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci 10.1146/annurev-vision-111815-114407 [DOI] [PMC free article] [PubMed]
- Kitatani K, Idkowiak-Baldys J, Hannun YA, 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal 10.1016/j.cellsig.2007.12.006 [DOI] [PMC free article] [PubMed]
- Kmoch S, Majewski J, Ramamurthy V, Cao S, Fahiminiya S, Ren H, MacDonald I, Lopez I, Sun V, Keser V, Khan A, StráneckyStránecky V, Hartmannová H, Přistoupilová A, Hodaňová K, Piherová L, Kuchař L, Baxová A, Chen R, Barsottini O, Pyle A, Griffin H, Splitt M, Sallum J, Tolmie J, Sampson J, Chinnery P, Canada R, Banin E, Sharon D, Dutta S, Grebler R, Helfrich-Foerster C, Pedroso J, Kretzschmar D, Cayouette M, Koenekoop R, 2015. ARTICLE Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness. Nat. Commun 10.1038/ncomms6614 [DOI] [PMC free article] [PubMed]
- Kobe F, Renner U, Woehler A, Wlodarczyk J, Papusheva E, Bao G, Zeug A, Richter DW, Neher E, Ponimaskin E, 2008. Stimulation- and palmitoylation-dependent changes in oligomeric conformation of serotonin 5-HT1A receptorsi. Biochim. Biophys. Acta - Mol. Cell Res 10.1016/j.bbamcr.2008.02.021 [DOI] [PubMed]
- Koch J, Gärtner S, Li C-M, Quintern LE, Bernardo K, Levran O, Schnabel D, Desnick RJ, Schuchman EH, Sandhoff K, 1996. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase: identification of the first molecular lesion causing Farber disease. J. Biol. Chem 271, 33110–33115. [DOI] [PubMed] [Google Scholar]
- Kociok N, Joussen AM, 2007. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefe’s Arch. Clin. Exp. Ophthalmol 245, 101–113. 10.1007/s00417-006-0266-x [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Chupin V, de Kruijff B, Burger KNJ, 2003. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4, 162–174. 10.1034/j.1600-0854.2003.00086.x [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DTS, Burger KNJ, De Kruijff B, 2007. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem 282, 11356–11364. 10.1074/jbc.M609737200 [DOI] [PubMed] [Google Scholar]
- Kopitz J, Holz FG, Kaemmerer E, Schutt F, 2004. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie 86, 825–831. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Emerson BM, 2014. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-κB complexes. Elife 3. 10.7554/eLife.01776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunchithapautham K, Atkinson C, Rohrer B, 2014. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J. Biol. Chem 289, 14534–14546. 10.1074/jbc.M114.564674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S, Ohuchi T, Yoshimura N, Honda Y, 1991. Growth factor-induced cytosolic calcium ion transients in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci 32, 2882–2890. [PubMed] [Google Scholar]
- Kuriyama S, Yoshimura N, Ohuchi T, Tanihara H, Ito S, Honda Y, 1992. Neuropeptide-induced cytosolic Ca2+ transients and phosphatidylinositol turnover in cultured human retinal pigment epithelial cells. Brain Res 579, 227–233. 10.1016/0006-8993(92)90055-e [DOI] [PubMed] [Google Scholar]
- Kusaka S, Kapousta-Bruneau N, Green DG, Puro DG, 1998. Serum-induced changes in the physiology of mammalian retinal glial cells: Role of lysophosphatidic acid. J. Physiol 506, 445–458. 10.1111/j.1469-7793.1998.445bw.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon W, Freeman SA, 2020. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol 10.3389/fimmu.2020.604205 [DOI] [PMC free article] [PubMed]
- La Cunza N, Tan LX, Thamban T, Germer CJ, Rathnasamy G, Toops KA, Lakkaraju A, 2021. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 6. 10.1172/jci.insight.142254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YL, Rana MW, 1986. A Study of Photoreceptor–Retinal Pigment Epithelium Complex: Age-Related Changes in Monkeys. Proc. Soc. Exp. Biol. Med 181, 371–381. 10.3181/00379727-181-42267 [DOI] [PubMed] [Google Scholar]
- Laíns I, Kelly RS, Miller JB, Silva R, Vavvas DG, Kim IK, Murta JN, Lasky-Su J, Miller JW, Husain D, 2018. Human plasma metabolomics study across all stages of age-related macular degeneration identifies potential lipid biomarkers. Ophthalmology 125, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Umapathy A, Tan LX, Daniele L, Philp NJ, Boesze-Battaglia K, Williams DS, 2020. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res 10.1016/j.preteyeres.2020.100846 [DOI] [PMC free article] [PubMed]
- Lam S, Tso MOM, Gurne DH, 1990. Amelioration of Retinal Photic Injury in Albino Rats by Dimethylthiourea. Arch. Ophthalmol 108, 1751–1757. 10.1001/archopht.1990.01070140105039 [DOI] [PubMed] [Google Scholar]
- Landis DJ, Dudley PA, Anderson RE, 1973. Alteration of disc formation in photoreceptors of rat retina. Science (80-. ) 182, 1144–1146. [DOI] [PubMed] [Google Scholar]
- Landowski M, Kelly U, Klingeborn M, Groelle M, Ding J-D, Grigsby D, Rickman CB, 2019. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. Proc. Natl. Acad. Sci 116, 3703–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layé S, Nadjar A, Joffre C, Bazinet RP, 2018. Anti-inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharmacol. Rev 70, 12–38. [DOI] [PubMed] [Google Scholar]
- Lee J, Jiao X, Hejtmancik JF, Kaiser-Kupfer M, Gahl WA, Markello TC, Guo J, Chader GJ, 2001. The Metabolism of Fatty Acids in Human Bietti Crystalline Dystrophy. Invest. Ophthalmol. Vis. Sci 42, 1707–1714. [PubMed] [Google Scholar]
- Lee SA, Belyaeva OV, Kedishvili NY, 2008. Effect of lipid peroxidation products on the activity of human retinol dehydrogenase 12 (RDH12) and retinoid metabolism. Biochim. Biophys. Acta - Mol. Basis Dis 1782, 421–425. 10.1016/j.bbadis.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hussain AA, Seok J-H, Kim S-H, Marshall J, 2015. Modulating the Transport Characteristics of Bruch’s Membrane With Steroidal Glycosides and its Relevance to Age-Related Macular Degeneration (AMD). Invest. Ophthalmol. Vis. Sci 56, 8403–8418. 10.1167/iovs.15-16936 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Overduin M, 2013. PtdIns(4)P signalling and recognition systems. Adv. Exp. Med. Biol 10.1007/978-94-007-6331-9_5 [DOI] [PubMed]
- Lerner TJ, Boustany R-MN, Anderson JW, D’Arigo KL, Schlumpf K, Buckler AJ, Gusella JF, Haines JL, 1995. Isolation of a novel gene underlying batten disease, CLN3. Cell 82, 949–957. 10.1016/0092-8674(95)90274-0 [DOI] [PubMed] [Google Scholar]
- Levental I, Levental KR, Heberle FA, 2020. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol 10.1016/j.tcb.2020.01.009 [DOI] [PMC free article] [PubMed]
- Levy M, Futerman AH, 2010. Mammalian ceramide synthases. IUBMB Life 10.1002/iub.319 [DOI] [PMC free article] [PubMed]
- Levy O, Calippe B, Lavalette S, Hu SJ, Raoul W, Dominguez E, Housset M, Paques M, Sahel J-A, Bemelmans A-P, Combadiere C, Guillonneau X, Sennlaub F, 2015a. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol. Med 7, 211–226. 10.15252/emmm.201404524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O, Lavalette S, Hu SJ, Housset M, Raoul W, Eandi C, Sahel J-A, Sullivan PM, Guillonneau X, Sennlaub F, 2015b. APOE Isoforms Control Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration. J. Neurosci 35, 13568 LP – 13576. 10.1523/JNEUROSCI.2468-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AC, Wallington-Beddoe CT, Powell JA, Pitson SM, 2018. Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies. Cell Death Discov 10.1038/s41420-018-0075-0 [DOI] [PMC free article] [PubMed]
- Lewis TR, Makia MS, Castillo CM, Al-Ubaidi MR, Naash MI, Arshavsky VY, 2021. Photoreceptor Disc Enclosure Is Tightly Controlled by Peripherin-2 Oligomerization. J. Neurosci 41, 3588 LP – 3596. 10.1523/JNEUROSCI.0041-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Jiao X, Munier FL, Schorderet DF, Yao W, Iwata F, Hayakawa M, Kanai A, Shy Chen M, Alan Lewis R, Heckenlively J, Weleber RG, Traboulsi EI, Zhang Q, Xiao X, Kaiser-Kupfer M, Sergeev YV, Hejtmancik JF, 2004. Bietti Crystalline Corneoretinal Dystrophy Is Caused by Mutations in the Novel Gene CYP4V2. Am. J. Hum. Genet 74, 817–826. 10.1086/383228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-M, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, 2005. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest. Ophthalmol. Vis. Sci 46, 2576–2586. [DOI] [PubMed] [Google Scholar]
- Li G, Anderson RE, Tomita H, Adler R, Liu X, Zack DJ, Rajala RVS, 2007. Nonredundant Role of Akt2 for Neuroprotection of Rod Photoreceptor Cells from Light-Induced Cell Death. J. Neurosci 27, 203–211. 10.1523/JNEUROSCI.0445-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Han JY, Jiang W, Wang J, 2011. Selenium inhibits high glucose-induced cyclooxygenase-2 and P-selectin expression in vascular endothelial cells. Mol. Biol. Rep 38, 2301–2306. 10.1007/s11033-010-0362-1 [DOI] [PubMed] [Google Scholar]
- Li ZY, Tso MO, Wang HM, Organisciak DT, 1985. Amelioration of photic injury in rat retina by ascorbic acid: a histopathologic study. Invest. Ophthalmol. Vis. Sci 26, 1589–1598. [PubMed] [Google Scholar]
- Lidgerwood GE, Morris AJ, Conquest A, Daniszewski M, Rooney LA, Lim SY, Hernández D, Liang HH, Allen P, Connell PP, Guymer RH, Hewitt AW, Pébay A, 2018. Role of lysophosphatidic acid in the retinal pigment epithelium and photoreceptors. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1863, 750–761. 10.1016/j.bbalip.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Lin DS, Anderson GJ, Connor WE, Neuringer M, 1994. Effect of dietary N-3 fatty acids upon the phospholipid molecular species of the monkey retina. Invest. Ophthalmol. Vis. Sci 35, 794–803. [PubMed] [Google Scholar]
- Linetsky M, Guo J, Udeigwe E, Ma D, Chamberlain AS, Yu AO, Solovyova K, Edgar E, Salomon RG, 2020. 4-Hydroxy-7-oxo-5-heptenoic acid (HOHA) lactone induces apoptosis in retinal pigment epithelial cells. Free Radic. Biol. Med 152, 280–294. 10.1016/j.freeradbiomed.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Chang J, Lin Y, Shen Z, Bernstein PS, 2010. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res 10.1194/jlr.M007518 [DOI] [PMC free article] [PubMed]
- Liu RZ, Li X, Godbout R, 2008. A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: transcription in rat retina and testis. Genomics 92, 436–445. 10.1016/j.ygeno.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Ll J, Edward DP, Lam TT, Tso MOM, 1993. Amelioration of Retinal Photic Injury by a Combination of Flunarizine and Dimethylthiourea. Exp. Eye Res 56, 71–78. 10.1006/exer.1993.1010 [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Schuhmann K, Finkelstein S, Lewis TR, Cady MA, Hao Y, Keuthan C, Ash JD, Burns ME, Shevchenko A, Arshavsky VY, 2019. Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction. J. Neurosci 39, 9689–9701. 10.1523/JNEUROSCI.1142-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent JH, Levental I, 2015. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem. Phys. Lipids 10.1016/j.chemphyslip.2015.07.022 [DOI] [PubMed]
- Lorent JH, Levental KR, Ganesan L, Rivera-Longsworth G, Sezgin E, Doktorova M, Lyman E, Levental I, 2020. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol 10.1038/s41589-020-0529-6 [DOI] [PMC free article] [PubMed]
- Louie K, Wiegand RD, Anderson RE, 1988. Docosahexaenoate-Containing Molecular Species of Glycerophospholipids from Frog Retinal Rod Outer Segments Show Different Rates of Biosynthesis and Turnover. Biochemistry 27, 9014–9020. 10.1021/bi00425a020 [DOI] [PubMed] [Google Scholar]
- Lu Y, Shiau F, Yi W, Lu S, Wu Q, Pearson JD, Kallman A, Zhong S, Hoang T, Zuo Z, Zhao F, Zhang M, Tsai N, Zhuo Y, He S, Zhang J, Stein-O’Brien GL, Sherman TD, Duan X, Fertig EJ, Goff LA, Zack DJ, Handa JT, Xue T, Bremner R, Blackshaw S, Wang X, Clark BS, 2020. Single-Cell Analysis of Human Retina Identifies Evolutionarily Conserved and Species-Specific Mechanisms Controlling Development. Dev. Cell 53, 473–491.e9. 10.1016/j.devcel.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Zhang H, Zhang X, Gao Y, Yin ZQ, 2019. Lipoxin A4 delays the progression of retinal degeneration via the inhibition of microglial overactivation. Biochem. Biophys. Res. Commun 516, 900–906. [DOI] [PubMed] [Google Scholar]
- Lukowski ZL, Min J, Beattie AR, Meyers CA, Levine MA, Stoller G, Schultz GS, Samuelson DA, Sherwood MB, 2013. Prevention of ocular scarring after glaucoma filtering surgery using the monoclonal antibody LT1009 (Sonepcizumab) in a rabbit model. J. Glaucoma 10.1097/IJG.0b013e31822e8c83 [DOI] [PMC free article] [PubMed]
- Ma C, Su L, Seven AB, Xu Y, Rizo J, 2013. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science (80-. ) 10.1126/science.1230473 [DOI] [PMC free article] [PubMed]
- Ma L, Yan S-F, Huang Y-M, Lu X-R, Qian F, Pang H-L, Xu X-R, Zou Z-Y, Dong P-C, Xiao X, 2012. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 119, 2290–2297. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, 2006. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem 281, 37697–37704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuran DJ, 1999. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta - Mol. Basis Dis 1455, 105–138. 10.1016/S0925-4439(99)00074-5 [DOI] [PubMed] [Google Scholar]
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA, 1994. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr 10.1093/ajcn/60.2.189 [DOI] [PubMed]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Rickman CB, 2005. Apolipoprotein E allele-dependent pathogenesis: A model for age-related retinal degeneration. Proc. Natl. Acad. Sci. U. S. A 102, 11900–11905. 10.1073/pnas.0503015102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra H, Barnes CL, Calvert PD, 2021. Functional compartmentalization of photoreceptor neurons. Pflugers Arch. Eur. J. Physiol 473, 1493–1516. 10.1007/s00424-021-02558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal NA, Tran J-TA, Zheng L, Wilkerson JL, Brush RS, McRae J, Agbaga M-P, Zhang K, Petrukhin K, Ayyagari R, 2014. In vivo effect of mutant ELOVL4 on the expression and function of wild-type ELOVL4. Invest. Ophthalmol. Vis. Sci 55, 2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG, 2010. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fat. Acids 82, 27–34. 10.1016/j.plefa.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Hannun YA, 2004. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol 10.1139/o03-091 [DOI] [PubMed]
- Mariño G, Kroemer G, 2013. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res 10.1038/cr.2013.115 [DOI] [PMC free article] [PubMed]
- Martín A, Pérez-Girón JV, Hernanz R, Palacios R, Briones AM, Fortuño A, Zalba G, Salaices M, Alonso MJ, 2012. Peroxisome proliferator-activated receptor-γ activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress. J. Hypertens 30, 315–326. 10.1097/HJH.0b013e32834f043b [DOI] [PubMed] [Google Scholar]
- Martinez M, 1992. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res 583, 171–182. 10.1016/S0006-8993(10)80021-6 [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Handa JT, Aotaki-Keen A, Sherwood SW, West MD, Hjelmeland LM, 1999. Beta-galactosidase histochemistry and telomere loss in senescent retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci 40, 197–202. [PubMed] [Google Scholar]
- Maude MB, Anderson EO, Anderson RE, 1998. Polyunsaturated fatty acids are lower in blood lipids of Usher’s type I but not Usher’s type II. Invest. Ophthalmol. Vis. Sci 39, 2164–2166. [PubMed] [Google Scholar]
- Mayer K, Schmidt R, Muhly-Reinholz M, Bögeholz T, Gokorsch S, Grimminger F, Seeger W, 2002. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFα impact of ω−3 versus ω−6 fatty acids. J. Lipid Res 43, 944–951. 10.1016/s0022-2275(20)30469-7 [DOI] [PubMed] [Google Scholar]
- McColl AJ, Converse CA, 1995. Lipid studies in retinitis pigmentosa. Prog. Lipid Res 34, 1–16. [DOI] [PubMed] [Google Scholar]
- McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, Schuchman EH, Wasserstein MP, 2004. Lipid abnormalities in children with types A and B Niemann Pick disease. J. Pediatr 145, 77–81. 10.1016/j.jpeds.2004.02.048 [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D, 2002. PIP2 and proteins: Interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct 10.1146/annurev.biophys.31.082901.134259 [DOI] [PubMed] [Google Scholar]
- McMahon A, Butovich IA, Mata NL, Klein M, Ritter R 3rd, Richardson J, Birch DG, Edwards AO, Kedzierski W, 2007. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol. Vis 13, 258–272. [PMC free article] [PubMed] [Google Scholar]
- Mcmurdo L, Stephenson AH, Baldassare JJ, Sprague RS, Lonigro AJ, 1998. Biosynthesis of Sulfidopeptide Leukotrienes Via the Transfer of Leukotriene A4 from Polymorphonuclear Cells to Bovine Retinal Pericytes. J. Pharmacol. Exp. Ther 285, 1255–12599. [PubMed] [Google Scholar]
- McNeill A, Roberti G, Lascaratos G, Hughes D, Mehta A, Garway-Heath DF, Schapira AHV, 2013. Retinal thinning in Gaucher disease patients and carriers: Results of a pilot study. Mol. Genet. Metab 109, 221–223. 10.1016/j.ymgme.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A, 2013. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci 10.1016/j.tins.2013.07.001 [DOI] [PMC free article] [PubMed]
- Mérida I, Ávila-Flores A, Merino E, 2008. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J 10.1042/BJ20071040 [DOI] [PubMed]
- Merle BMJ, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH, 2014. Circulating Omega-3 Fatty Acids and Neovascular Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci 55, 2010–2019. 10.1167/iovs.14-13916 [DOI] [PubMed] [Google Scholar]
- Merle BMJ, Delyfer M-N, Korobelnik J-F, Rougier M-B, Malet F, Féart C, Le Goff M, Peuchant E, Letenneur L, Dartigues J-F, Colin J, Barberger-Gateau P, Delcourt C, 2013. High Concentrations of Plasma n3 Fatty Acids Are Associated with Decreased Risk for Late Age-Related Macular Degeneration. J. Nutr 143, 505–511. 10.3945/jn.112.171033 [DOI] [PubMed] [Google Scholar]
- Merle Benedicte M J, Richard F, Benlian P, Puche N, Delcourt C, Souied EH, 2015. CFH Y402H and ARMS2 A69S polymorphisms and oral supplementation with docosahexaenoic acid in neovascular age-related macular degeneration patients: the NAT2 study. PLoS One 10, e0130816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle Bénédicte M J, Silver RE, Rosner B, Seddon JM, 2015. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am. J. Clin. Nutr 102, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR, Fleming I, 2008. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Investig. Ophthalmol. Vis. Sci 49, 1242–1247. 10.1167/iovs.07-1087 [DOI] [PubMed] [Google Scholar]
- Miljanich GP, Nemes PP, White DL, Dratz EA, 1981. The asymmetric transmembrane distribution of phosphatidylethanolamine, phosphatidylserine, and fatty acids of the bovine retinal rod outer segment disk membrane. J. Membr. Biol 10.1007/BF01992562 [DOI] [PubMed]
- Millar FA, Fisher SC, Muir CA, Edwards E, Hawthorne JN, 1988. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. BBA - Mol. Cell Res 970, 205–211. 10.1016/0167-4889(88)90180-2 [DOI] [PubMed] [Google Scholar]
- Miranda GE, Abrahan CE, Agnolazza DL, Politi LE, Rotstein NP, 2011. Ceramide-1-Phosphate, a New Mediator of Development and Survival in Retina Photoreceptors. Invest. Ophthalmol. Vis. Sci 52, 6580–6588. 10.1167/iovs.10-7065 [DOI] [PubMed] [Google Scholar]
- Miranda GE, Abrahan CE, Politi LE, Rotstein NP, 2009. Sphingosine-1-phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Invest. Ophthalmol. Vis. Sci 50, 4416–4428. 10.1167/iovs.09-3388 [DOI] [PubMed] [Google Scholar]
- Mirra S, García-Arroyo R, Domènech B, Gavaldà-Navarro A, Herrera-Úbeda C, Oliva C, Garcia-Fernàndez J, Artuch R, Villarroya F, Marfany G, 2021. CERKL, a retinal dystrophy gene, regulates mitochondrial function and dynamics in the mammalian retina. Neurobiol. Dis 156, 105405. 10.1016/j.nbd.2021.105405 [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ, 2001. Optimization of receptor-G protein coupling by Bilayer lipid composition I: Kinetics of rhodopsin-transducin binding. J. Biol. Chem 10.1074/jbc.M105772200 [DOI] [PubMed]
- Moine H, Vitale N, 2019. Of local translation control and lipid signaling in neurons. Adv. Biol. Regul 10.1016/j.jbior.2018.09.005 [DOI] [PubMed]
- Molday RS, Molday LL, 1987. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J. Cell Biol 105, 2589–2601. 10.1083/jcb.105.6.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Zhang K, 2010. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog. Lipid Res 49, 476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Dennis EA, 2019. Phospholipase A 2 catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 10.1016/j.bbalip.2018.08.010 [DOI] [PMC free article] [PubMed]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG, 2004. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. U. S. A 101, 8491–8496. 10.1073/pnas.0402531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H, 2010. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed]
- Nag TC, Maurya M, Roy TS, 2019. Age-related changes of the human retinal vessels: Possible involvement of lipid peroxidation. Ann. Anatomy-Anatomischer Anzeiger 226, 35–47. [DOI] [PubMed] [Google Scholar]
- Nagata K, Hishikawa D, Sagara H, Saito M, Watanabe S, Shimizu T, Shindou H, 2021. Lysophosphatidylcholine acyltransferase 1 controls the mitochondrial reactive oxygen species generation and survival of the retinal photoreceptor cells. bioRxiv [DOI] [PMC free article] [PubMed]
- Najafi M, Maza NA, Calvert PD, 2012. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc. Natl. Acad. Sci 109, 203–208. 10.1073/pnas.1115109109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM, 2010. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (<em>LIPC</em>). Proc. Natl. Acad. Sci 107, 7395 LP – 7400. 10.1073/pnas.0912019107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet I, Tian G, Imanishi Y, 2014. Organization of cGMP sensing structures on the rod photoreceptor outer segment plasma membrane. Channels 8, 528–535. 10.4161/19336950.2014.973776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, 2018. Protein kinase C: perfectly balanced. Crit. Rev. Biochem. Mol. Biol 10.1080/10409238.2018.1442408 [DOI] [PMC free article] [PubMed]
- Newton AC, 1993. Interaction of proteins with lipid headgroups: lessons from protein kinase C. Annu. Rev. Biophys. Biomol. Struct 22, 1–25. 10.1146/annurev.bb.22.060193.000245 [DOI] [PubMed] [Google Scholar]
- Newton AC, Bootman MD, Scott JD, 2016. Second Messengers. Cold Spring Harb. Perspect. Biol 8, a005926. 10.1101/CSHPERSPECT.A005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, Williams DS, 1993. Rhodopsin is the major in situ substrate of protein kinase C in rod outer segments of photoreceptors. J. Biol. Chem 268, 18181–18186. 10.1016/s0021-9258(17)46827-x [DOI] [PubMed] [Google Scholar]
- Newton AC, Williams DS, 1991. Involvement of protein kinase C in the phosphorylation of rhodopsin. J. Biol. Chem 266, 17725–17728. 10.1016/s0021-9258(18)55183-8 [DOI] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, Spiegel S, 2015. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res 10.1016/j.yexcr.2015.02.025 [DOI] [PMC free article] [PubMed]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL, 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506. 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- Njie-Mbye YF, Chitnis MK, Opere CA, Ohia SE, 2013. Lipid peroxidation: pathophysiological and pharmacological implications in the eye. Front. Physiol 4, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK, Organisciak DT, Ando H, Braniecki MA, Durlin C, 1987. Ascorbate and dietary protective mechanisms in retinal light damage of rats: electrophysiological, histological and DNA measurements. Prog. Clin. Biol. Res 247, 469–483. [PubMed] [Google Scholar]
- Nomura-Komoike K, Saitoh F, Fujieda H, 2020. Phosphatidylserine recognition and Rac1 activation are required for Müller glia proliferation, gliosis and phagocytosis after retinal injury. Sci. Rep 10.1038/s41598-020-58424-6 [DOI] [PMC free article] [PubMed]
- Noroozi R, Ghafouri-Fard S, Pisarek A, Rudnicka J, Spólnicka M, Branicki W, Taheri M, Pośpiech E, 2021. DNA methylation-based age clocks: From age prediction to age reversion. Ageing Res. Rev 10.1016/j.arr.2021.101314 [DOI] [PubMed]
- Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM, 2011. Novel pathway of ceramide production in mitochondria: Thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J. Biol. Chem 10.1074/jbc.M110.214866 [DOI] [PMC free article] [PubMed]
- Ohia SE, Bagchi M, Stohs SJ, 1994. Age-related oxidative damage in Long-Evans rat retina. Res. Commun. Mol. Pathol. Pharmacol 85, 21–31. [PubMed] [Google Scholar]
- Oliver VF, Jaffe AE, Song J, Wang G, Zhang P, Branham KE, Swaroop A, Eberhart CG, Zack DJ, Qian J, Merbs SL, 2015. Differential DNA methylation identified in the blood and retina of AMD patients. Epigenetics 10, 698–707. 10.1080/15592294.2015.1060388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MV, Lyngstadaas AV, Bair JA, Hodges RR, Utheim TP, Serhan CN, Dartt DA, 2021. Maresin 1, a specialized proresolving mediator, stimulates intracellular [Ca2+] and secretion in conjunctival goblet cells. J. Cell. Physiol 236, 340–353. 10.1002/jcp.29846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK, 2010. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res 10.1016/j.preteyeres.2009.11.004 [DOI] [PMC free article] [PubMed]
- Orisme W, Li J, Goldmann T, Bolch S, Wolfrum U, Smith WC, 2010. Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell. Signal 22, 447–456. 10.1016/j.cellsig.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada H, Toda E, Homma K, Guzman NA, Nagai N, Ogawa M, Negishi K, Arita M, Tsubota K, Ozawa Y, 2021. ADIPOR1 deficiency-induced suppression of retinal ELOVL2 and docosahexaenoic acid levels during photoreceptor degeneration and visual loss. Cell Death Dis 12, 458. 10.1038/s41419-021-03741-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, 2012. Chemistry and biology of vision. J. Biol. Chem 10.1074/jbc.R111.301150 [DOI] [PMC free article] [PubMed]
- Palczewski K, Kiser PD, 2020. Shedding new light on the generation of the visual chromophore. Proc. Natl. Acad. Sci. U. S. A 10.1073/PNAS.2008211117 [DOI] [PMC free article] [PubMed]
- Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M, 1996. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol 121, 181–189. 10.1016/S0002-9394(14)70583-5 [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC, 1985. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest. Ophthalmol. Vis. Sci 26, 1386–404. [PubMed] [Google Scholar]
- Pasquaré SJ, Salvador GA, Giusto NM, 2008. Involvement of lysophosphatidic acid, sphingosine 1-phosphate and ceramide 1-phosphate in the metabolization of phosphatidic acid by lipid phosphate phosphatases in bovine rod outer segments. Neurochem. Res 33, 1205–1215. 10.1007/s11064-007-9569-5 [DOI] [PubMed] [Google Scholar]
- Pauter AM, Olsson P, Asadi A, Herslöf B, Csikasz RI, Zadravec D, Jacobsson A, 2014. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice[S]. J. Lipid Res 55, 718–728. 10.1194/jlr.M046151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak AM, Olchawa M, Koscielniak A, Zadlo A, Broniec A, Oles T, Sarna TJ, 2019. Oxidized Lipids Decrease Phagocytic Activity of ARPE‐19 Cells In Vitro. Eur. J. Lipid Sci. Technol 121, 1800476. [Google Scholar]
- Pearsall EA, Cheng R, Zhou K, Takahashi Y, Matlock HG, Vadvalkar SS, Shin Y, Fredrick TW, Gantner ML, Meng S, Fu Z, Gong Y, Kinter M, Humphries KM, Szweda LI, Smith LEH, Ma J. xing, 2017. PPARα is essential for retinal lipid metabolism and neuronal survival. BMC Biol 15, 1–14. 10.1186/s12915-017-0451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo D, Bubis J, 2020. Light or tyrosine phosphorylation recruits retinal rod outer segment proteins to lipid rafts. Biochimie 10.1016/j.biochi.2020.07.016 [DOI] [PubMed]
- Piano I, Novelli E, Gasco P, Ghidoni R, Strettoi E, Gargini C, 2013. Cone survival and preservation of visual acuity in an animal model of retinal degeneration. Eur. J. Neurosci 10.1111/ejn.12196 [DOI] [PubMed]
- Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, Ong H, Sennlaub F, Chemtob S, 2010. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging (Albany. NY) 2, 981–989. 10.18632/AGING.100218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ, 2006. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res 10.1194/jlr.E600002-JLR200 [DOI] [PubMed]
- Pikuleva IA, Curcio CA, 2014. Cholesterol in the retina: The best is yet to come. Prog. Retin. Eye Res 41, 64–89. 10.1016/j.preteyeres.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploumi C, Daskalaki I, Tavernarakis N, 2017. Mitochondrial biogenesis and clearance: a balancing act. FEBS J 284, 183–195. 10.1111/febs.13820 [DOI] [PubMed] [Google Scholar]
- Pöge M, Mahamid J, Imanishi SS, Plitzko JM, Palczewski K, Baumeister W, 2021. Determinants shaping the nanoscale architecture of the mouse rod outer segment. bioRxiv 2021.08.18.456753 10.1101/2021.08.18.456753 [DOI] [PMC free article] [PubMed]
- Polozova A, Litman BJ, 2000. Cholesterol Dependent Recruitment of di22:6-PC by a G Protein-Coupled Receptor into Lateral Domains. Biophys. J 79, 2632–2643. 10.1016/S0006-3495(00)76502-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, 2008. Smith–Lemli–Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet 16, 535–541. [DOI] [PubMed] [Google Scholar]
- Porter H, Qi H, Prabhu N, Grambergs R, McRae J, Hopiavuori B, Mandal N, 2018. Characterizing sphingosine kinases and sphingosine 1-phosphate receptors in the mammalian eye and retina. Int. J. Mol. Sci 10.3390/ijms19123885 [DOI] [PMC free article] [PubMed]
- Poulos A, Sharp P, Johnson D, Easton C, 1988. The occurrence of polyenoic very long chain fatty acids with greater than 32 carbon atoms in molecular species of phosphatidylcholine in normal and peroxisome-deficient (Zellweger’s syndrome) brain. Biochem. J 253, 645–650. 10.1042/bj2530645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado Spalm FH, Vera MS, Dibo MJ, Victoria Simón M, Politi LE, Rotstein NP, 2019. Ceramide Induces the Death of Retina Photoreceptors Through Activation of Parthanatos. Mol. Neurobiol 10.1007/s12035-018-1402-4 [DOI] [PubMed]
- Prager P, Hollborn M, Steffen A, Wiedemann P, Kohen L, Bringmann A, 2016. P2Y1 Receptor Signaling Contributes to High Salt-Induced Priming of the NLRP3 Inflammasome in Retinal Pigment Epithelial Cells. PLoS One 11, e0165653. 10.1371/journal.pone.0165653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopiou E, Kolovos P, Georgiou C, Kalogerou M, Potamiti L, Sokratous K, Kyriacou K, Georgiou T, 2019. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ open Ophthalmol 4, e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Lee SC, Long J, Pyne NJ, 2009. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell. Signal 10.1016/j.cellsig.2008.08.008 [DOI] [PubMed]
- Raj K, Horvath S, 2020. Current perspectives on the cellular and molecular features of epigenetic ageing. Exp. Biol. Med 245, 1532–1542. 10.1177/1535370220918329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Gupta VK, Anderson RE, Rajala RVS, 2013. Light activation of the insulin receptor regulates mitochondrial hexokinase. A possible mechanism of retinal neuroprotection. Mitochondrion 13, 566–576. 10.1016/j.mito.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, He F, Anderson RE, Wensel TG, Rajala RVS, 2020a. Loss of class iii phosphoinositide 3-kinase vps34 results in cone degeneration. Biology (Basel) 9, 1–14. 10.3390/biology9110384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, McCauley A, Brush RS, Nguyen K, Rajala RVS, 2020b. Phosphoinositide Lipids in Ocular Tissues. Biology (Basel) 9, 125. 10.3390/biology9060125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RVS, 2020. Signaling roles of phosphoinositides in the retina. J. Lipid Res 62, 100041. 10.1194/jlr.TR120000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RVS, Ranjo-Bishop M, Wang Y, Rajala A, Anderson RE, 2015. The p110α isoform of phosphoinositide 3-kinase is essential for cone photoreceptor survival. Biochimie 112, 35–40. 10.1016/j.biochi.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra Rao S, Fliesler SJ, Kotla P, Nguyen MN, Pittler SJ, 2020a. Lack of Overt Retinal Degeneration in a K42E Dhdds Knock-In Mouse Model of RP59. Cells 10.3390/cells9040896 [DOI] [PMC free article] [PubMed]
- Ramachandra Rao S, Skelton LA, Wu F, Onysk A, Spolnik G, Danikiewicz W, Butler MC, Stacks DA, Surmacz L, Mu X, Swiezewska E, Pittler SJ, Fliesler SJ, 2020b. Retinal Degeneration Caused by Rod-Specific Dhdds Ablation Occurs without Concomitant Inhibition of Protein N-Glycosylation. iScience 23, 101198. 10.1016/j.isci.2020.101198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, De Bruijn WC, Mulder PG, De Jong PT, 1994. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest. Ophthalmol. Vis. Sci 35, 2857–2864. [PubMed] [Google Scholar]
- Ranty M-L, Carpentier S, Cournot M, Rico-Lattes I, Malecaze F, Levade T, Delisle M-B, Quintyn J-C, 2009. Ceramide production associated with retinal apoptosis after retinal detachment. Graefe’s Arch. Clin. Exp. Ophthalmol 247, 215–224. 10.1007/s00417-008-0957-6 [DOI] [PubMed] [Google Scholar]
- Ratnapriya R, Chew EY, 2013. Age-related macular degeneration—clinical review and genetics update. Clin. Genet 84, 160–166. 10.1111/cge.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T, 2000. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100, 221–228. 10.1016/S0092-8674(00)81560-3 [DOI] [PubMed] [Google Scholar]
- Ravussin A, Brech A, Tooze SA, Stenmark H, 2021. The phosphatidylinositol 3-phosphate-binding protein SNX4 controls ATG9A recycling and autophagy. J. Cell Sci 134. 10.1242/JCS.250670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ, 1997. Visual attention in infant monkeys: Effects of dietary fatty acids and age. Dev. Psychol 10.1037/0012-1649.33.3.387 [DOI] [PubMed]
- Reyes-Reveles J, Dhingra A, Alexander D, Bragin A, Philp NJ, Boesze-Battaglia K, 2017. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem 292, 8038–8047. 10.1074/jbc.M116.770784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Rosner B, Seddon JM, 2010. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology 117, 1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA, Bazan NG, 2015. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun 6. 10.1038/ncomms7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, White K, Stöhr H, Steiner K, Hemmrich N, Grimm T, Jurklies B, Lorenz B, Scholl HPN, Apfelstedt-Sylla E, Weber BHF, 2000. A Comprehensive Survey of Sequence Variation in the ABCA4 (ABCR) Gene in Stargardt Disease and Age-Related Macular Degeneration. Am. J. Hum. Genet 67, 800–813. 10.1086/303090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Gordon WC, Bazan NG, 1992. Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Exp. Eye Res 55, 719–725. 10.1016/0014-4835(92)90176-s [DOI] [PubMed] [Google Scholar]
- Rodriguez De Turco EB, Gordon WC, Bazan NG, 2009. Docosahexaenoic acid is taken up by the inner segment of frog photoreceptors leading to an active synthesis of docosahexaenoyl inositol lipids: similarities in metabolism in vivo and in vitro Docosahexaenoic acid is taken up by the inner segment of frog p 10.3109/02713689409042394 [DOI] [PubMed]
- Rodriguez De Turco EB, Parkins N, Ershov AV, Bazan NG, 1999. Selective retinal pigment epithelial cell lipid metabolism and remodeling conserves photoreceptor docosahexaenoic acid following phagocytosis. J. Neurosci. Res 57, 479–486. [DOI] [PubMed] [Google Scholar]
- Rodríguez IR, Larrayoz IM, 2010. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. Lipid Res 51, 2847–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell M, Giera M, Brabet P, Shchepinov MS, Guichardant M, Durand T, Vercauteren J, Galano J-M, Crauste C, 2019. Bis-allylic Deuterated DHA Alleviates Oxidative Stress in Retinal Epithelial Cells. Antioxidants 10.3390/antiox8100447 [DOI] [PMC free article] [PubMed]
- Rotstein NP, Miranda GE, Abrahan CE, German OL, 2010. Regulating survival and development in the retina: Key roles for simple sphingolipids. J. Lipid Res 10.1194/jlr.R003442 [DOI] [PMC free article] [PubMed]
- Rudolf M, Curcio CA, 2009. Esterified cholesterol is highly localized to Bruch’s membrane, as revealed by lipid histochemistry in wholemounts of human choroid. J. Histochem. Cytochem 57, 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC, 2012. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc. Natl. Acad. Sci 109, 8145–8148. 10.1073/pnas.1121101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Sparrow JR, Silverstein RL, 1996. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci 109, 387–395. [DOI] [PubMed] [Google Scholar]
- Saffman PG, Delbrück M, 1975. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. U. S. A 72, 3111–3113. 10.1073/pnas.72.8.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahaboglu A, Barth M, Secer E, Del Amo EM, Urtti A, Arsenijevic Y, Zrenner E, Paquet-Durand F, 2016. Olaparib significantly delays photoreceptor loss in a model for hereditary retinal degeneration. Sci. Rep 10.1038/srep39537 [DOI] [PMC free article] [PubMed]
- Sahaboglu A, Sharif A, Feng L, Secer E, Zrenner E, Paquet-Durand F, 2017. Temporal progression of PARP activity in the Prph2 mutant rd2 mouse: Neuroprotective effects of the PARP inhibitor PJ34. PLoS One 10.1371/journal.pone.0181374 [DOI] [PMC free article] [PubMed]
- Salinas RY, Pearring JN, Ding J-D, Spencer WJ, Hao Y, Arshavsky VY, 2017. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J. Cell Biol 216, 1489–1499. 10.1083/jcb.201608081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RG, Hong L, Hollyfield JG, 2011. Discovery of carboxyethylpyrroles (CEPs): critical insights into AMD, autism, cancer, and wound healing from basic research on the chemistry of oxidized phospholipids. Chem. Res. Toxicol 24, 1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador GA, Giusto NM, 2006. Phospholipase D from photoreceptor rod outer segments is a downstream effector of RhoA: Evidence of a light-dependent mechanism. Exp. Eye Res 83, 202–211. [DOI] [PubMed] [Google Scholar]
- Sander CL, Sears AE, Pinto AFM, Choi EH, Kahremany S, Gao F, Salom D, Jin H, Pardon E, Suh S, 2021. Nano-scale resolution of native retinal rod disk membranes reveals differences in lipid composition. J. Cell Biol 220, e202101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K, Harzer K, 2013. Gangliosides and Gangliosidoses: Principles of Molecular and Metabolic Pathogenesis. J. Neurosci 33, 10195 LP – 10208. 10.1523/JNEUROSCI.0822-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Agrón E, Clemons TE, Ferris III FL, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, Sperduto RD, Group A-REDSR, 2008. The Relationship of Dietary ω−3 Long-Chain Polyunsaturated Fatty Acid Intake With Incident Age-Related Macular Degeneration: AREDS Report No. 23. Arch. Ophthalmol 126, 1274–1279. 10.1001/archopht.126.9.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvicens N, Cotter TG, 2006. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J. Neurochem 10.1111/j.1471-4159.2006.03977.x [DOI] [PubMed]
- Scerri TS, Quaglieri A, Cai C, Zernant J, Matsunami N, Baird L, Scheppke L, Bonelli R, Yannuzzi LA, Friedlander M, Egan CA, Fruttiger M, Leppert M, Allikmets R, Bahlo M, Consortium MP, 2017. Genome-wide analyses identify common variants associated with macular telangiectasia type 2. Nat. Genet 49, 559–567. 10.1038/ng.3799 [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Rudolf M, Funk M, Hofmann-Rummelt C, Franz-Haas NS, Aherrahrou Z, Schlötzer-Schrehardt U, 2008. Ultrastructural changes in a murine model of graded bruch membrane lipoidal degeneration and corresponding VEGF164 detection. Investig. Ophthalmol. Vis. Sci 49, 390–398. 10.1167/IOVS.07-0227 [DOI] [PubMed] [Google Scholar]
- Schmidt SY, 1983a. Light- and cytidine-dependent phosphatidylinositol synthesis in photoreceptor cells of the rat. J. Cell Biol 97, 832–837. 10.1083/jcb.97.3.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SY, 1983b. Phosphatidylinositol synthesis and phosphorylation are enhanced by light in rat retinas. J. Biol. Chem 258, 6863–6868. 10.1016/s0021-9258(18)32303-2 [DOI] [PubMed] [Google Scholar]
- Schmidt T, Suk JE, Ye F, Situ AJ, Mazumder P, Ginsberg MH, Ulmer TS, 2015. Annular anionic lipids stabilize the integrin αiIbβ3 Transmembrane Complex. J. Biol. Chem 290, 8283–8293. 10.1074/jbc.M114.623504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt V, Sturgis JN, 2018. Modifying styrene-maleic acid co-polymer for studying lipid nanodiscs. Biochim. Biophys. Acta - Biomembr 1860, 777–783. 10.1016/j.bbamem.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E, 2012. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest. Ophthalmol. Vis. Sci 53, 5906–5911. [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Wojtczak L, 2016. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res 10.1194/jlr.R067629 [DOI] [PMC free article] [PubMed]
- Schuchman EH, Desnick RJ, 2017. Types A and B Niemann-Pick disease. Mol. Genet. Metab 120, 27–33. 10.1016/j.ymgme.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher M, Grasskamp AT, Barahtjan P, Wagner N, Lombardot B, Schuhmacher JS, Sala P, Lohmann A, Henry I, Shevchenko A, Coskun Ü, Walter AM, Nadler A, 2020. Live-cell lipid biochemistry reveals a role of diacylglycerol side-chain composition for cellular lipid dynamics and protein affinities. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1912684117 [DOI] [PMC free article] [PubMed]
- Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, Pertile MG, Leonardi A, Sathe S, Beaton A, Trieu L, Sack R, 2010. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes 59, 1780–1788. 10.2337/db10-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BL, Bazan NG, 1989. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. U. S. A 86, 2903–2907. 10.1073/pnas.86.8.2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Gurinovich A, Nygaard M, Sasaki T, Sweigart B, Bae H, Andersen SL, Villa F, Atzmon G, Christensen K, 2019. APOE alleles and extreme human longevity. Journals Gerontol. Ser. A 74, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, Nagata S, 2015. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell Biol 10.1016/j.tcb.2015.08.003 [DOI] [PubMed]
- Semenova EM, Converse CA, 2003. Comparison between oleic acid and docosahexaenoic acid binding to interphotoreceptor retinoid-binding protein. Vision Res 43, 3063–3067. 10.1016/j.visres.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Seno K, Hayashi F, 2017. Palmitoylation is a prerequisite for dimerization-dependent raftophilicity of rhodopsin. J. Biol. Chem 10.1074/jbc.M117.804880 [DOI] [PMC free article] [PubMed]
- Serhan CN, Chiang N, 2013. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol 10.1016/j.coph.2013.05.012 [DOI] [PMC free article] [PubMed]
- Serhan CN, Savill J, 2005. Resolution of inflammation: The beginning programs the end. Nat. Immunol 10.1038/ni1276 [DOI] [PubMed]
- Seu KJ, Cambrea LR, Everly RM, Hovis JS, 2006. Influence of lipid chemistry on membrane fluidity: tail and headgroup interactions. Biophys. J 91, 3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi FA, Boulton M, 2001. Inhibition of RPE Lysosomal and Antioxidant Activity by the Age Pigment Lipofuscin. Invest. Ophthalmol. Vis. Sci 42, 3041–3046. [PubMed] [Google Scholar]
- Shchepinov MS, 2020. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends Pharmacol. Sci 41, 236–248. 10.1016/j.tips.2020.01.010 [DOI] [PubMed] [Google Scholar]
- Shindou H, Koso H, Sasaki J, Nakanishi H, Sagara H, Nakagawa KM, Takahashi Y, Hishikawa D, Iizuka-Hishikawa Y, Tokumasu F, Noguchi H, Watanabe S, Sasaki T, Shimizu T, 2017. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. J. Biol. Chem 10.1074/jbc.M117.790568 [DOI] [PMC free article] [PubMed]
- Shiwani HA, Elfaki MY, Memon D, Ali S, Aziz A, Egom EE, 2021. UpdaShiwani, H.A., Elfaki, M.Y., Memon, D., Ali, S., Aziz, A., Egom, E.E., 2021. Updates on sphingolipids: Spotlight on retinopathy. Biomed. Pharmacother 143, 112197. https://doi.org/10.1016/j.biopha.2021.112197tes on sphingolipids: Spotlight on retinopa. Biomed. Pharmacother. 143, 112197. 10.1016/j.biopha.2021.112197 [DOI] [PubMed] [Google Scholar]
- Simanshu DK, Kamlekar RK, Wijesinghe DS, Zou X, Zhai X, Mishra SK, Molotkovsky JG, Malinina L, Hinchcliffe EH, Chalfant CE, Brown RE, Patel DJ, 2013. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 10.1038/nature12332 [DOI] [PMC free article] [PubMed]
- Simón MV, Prado Spalm FH, Vera MS, Rotstein NP, 2019. Sphingolipids as emerging mediators in retina degeneration. Front. Cell. Neurosci 10.3389/fncel.2019.00246 [DOI] [PMC free article] [PubMed]
- Simons K, Ikonen E, 1997. Functional rafts in cell membranes. Nature 10.1038/42408 [DOI] [PubMed]
- Singh A, Kukreti R, Saso L, Kukreti S, 2019. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Naash MI, Al-Ubaidi MR, 2020. The Symbiotic Relationship between the Neural Retina and Retinal Pigment Epithelium Is Supported by Utilizing Differential Metabolic Pathways. iScience 23, 101004. 10.1016/j.isci.2020.101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Budin I, 2020. Aging membranes: Unexplored functions for lipids in the lifespan of the central nervous system. Exp. Gerontol 131, 110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Chao DL, 2019. Long-Chain Polyunsaturated Fatty Acids and Age-Related Macular Degeneration, in: Bowes Rickman C, Grimm C, Anderson RE, Ash JD, LaVail MM, Hollyfield JG (Eds.), Retinal Degenerative Diseases Springer International Publishing, Cham, pp. 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch VM, Banks A, Li H, Crowley MA, Davis V, Xiang C, Yang J, Demirs JT, Vrouvlianis J, Leehy B, Hanks S, Hyman AM, Aranda J, Chang B, Bigelow CE, Rice DS, 2018. ADIPOR1 is essential for vision and its RPE expression is lost in the Mfrprd6mouse. Sci. Rep 8, 14339. 10.1038/s41598-018-32579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KW, 2001. Once Again High Tech Meets Low Tech on Chromosome 6. Arch. Ophthalmol 119, 573–575. 10.1001/archopht.119.4.573 [DOI] [PubMed] [Google Scholar]
- Snead WT, Gladfelter AS, 2019. The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation. Mol. Cell 10.1016/j.molcel.2019.09.016 [DOI] [PMC free article] [PubMed]
- Sørensen NB, Christiansen AT, Kjær TW, Klemp K, la Cour M, Heegaard S, Kiilgaard JF, 2019. Bruch’s membrane allows unhindered passage of up to 2 μm latex beads in an in vivo porcine model. Exp. Eye Res 180, 1–7. 10.1016/j.exer.2018.11.019 [DOI] [PubMed] [Google Scholar]
- Soubias O, Gawrisch K, 2005. Probing specific lipid-protein interaction by saturation transfer difference NMR spectroscopy. J. Am. Chem. Soc 127, 13110–13111. 10.1021/ja0538942 [DOI] [PubMed] [Google Scholar]
- Souied EH, Aslam T, Garcia-Layana A, Holz FG, Leys A, Silva R, Delcourt C, 2016. Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res 55, 62–69. [DOI] [PubMed] [Google Scholar]
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P, Group, N.A.M.D.T. 2 S., 2013. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 120, 1619–1631. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA, 2000. The Lipofuscin Fluorophore A2E Mediates Blue Light–Induced Damage to Retinal Pigmented Epithelial Cells. Invest. Ophthalmol. Vis. Sci 41, 1981–1989. [PubMed] [Google Scholar]
- Spencer WJ, Lewis TR, Pearring JN, Arshavsky VY, 2020. Photoreceptor Discs: Built Like Ectosomes. Trends Cell Biol 10.1016/j.tcb.2020.08.005 [DOI] [PMC free article] [PubMed]
- Starita C, Hussain AA, Marshall J, 1995. Decreasing hydraulic conductivity of Bruch’s membrane: Relevance to photoreceptor survival and lipofuscinoses. Am. J. Med. Genet 57, 235–237. 10.1002/ajmg.1320570224 [DOI] [PubMed] [Google Scholar]
- STARITA C, HUSSAIN ALIA, PAGLIARINI S, MARSHALL J, 1996. Hydrodynamics of Ageing Bruch’s Membrane: Implications for Macular Disease. Exp. Eye Res 62, 565–572. 10.1006/exer.1996.0066 [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AUA, Patmore A, Marshall J, 1997. Localization of the site of major resistance to fluid transport in Bruch’s membrane. Investig. Ophthalmol. Vis. Sci [PubMed]
- Steinle JJ, Sharma S, Smith CP, McFayden-Ketchum LS, 2009. Normal aging involves modulation of specific inflammatory markers in the rat retina and choroid. Journals Gerontol. Ser. A Biomed. Sci. Med. Sci 64, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles M, Qi H, Sun E, Tan J, Porter H, Allegood J, Chalfant CE, Yasumura D, Matthes MT, Lavail MM, Mandal NA, 2016. Sphingolipid profile alters in retinal dystrophic P23H-1 rats and systemic FTY720 can delay retinal degeneration. J. Lipid Res 10.1194/jlr.M063719 [DOI] [PMC free article] [PubMed]
- Stinson A, Wiegand R, Anderson R, 1991. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J. Lipid Res 32, 2009–2017. 10.1016/S0022-2275(20)41904-2 [DOI] [PubMed] [Google Scholar]
- Storti F, Klee K, Todorova V, Steiner R, Othman A, Van Der Velde-Visser S, Samardzija M, Meneau I, Barben M, Karademir D, Pauzuolyte V, Boye SL, Blaser F, Ullmer C, Dunaief JL, Hornemann T, Rohrer L, Den Hollander A, Von Eckardstein A, Fingerle J, Maugeais C, Grimm C, 2019. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. Elife 8. 10.7554/eLife.45100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, Wiederholt M, Wienrich M, 1996. Activation of Cl- currents in cultured rat retinal pigment epithelial cells by intracellular applications of inositol-1,4,5-triphosphate: differences between rats with retinal dystrophy (RCS) and normal rats. J. Membr. Biol 151, 189–200. 10.1007/s002329900069 [DOI] [PubMed] [Google Scholar]
- Su Q, Mehta S, Zhang J, 2021. Liquid-liquid phase separation: Orchestrating cell signaling through time and space. Mol. Cell 10.1016/j.molcel.2021.09.010 [DOI] [PMC free article] [PubMed]
- Su X, Tan QSW, Parikh BH, Tan A, Mehta MN, Wey YS, Tun SBB, Li LJ, Han XY, Wong TY, Hunziker W, Luu CD, Owada Y, Barathi VA, Zhang SS, Chaurasia SS, 2016. Characterization of fatty acid binding protein 7 (FABP7) in the murine retina. Investig. Ophthalmol. Vis. Sci 57, 3397–3408. 10.1167/iovs.15-18542 [DOI] [PubMed] [Google Scholar]
- Sugasini D, Yalagala PCR, Subbaiah PV, 2020. Efficient enrichment of retinal DHA with dietary lysophosphatidylcholine-DHA: Potential application for retinopathies. Nutrients 10.3390/nu12103114 [DOI] [PMC free article] [PubMed]
- Sugita M, Dulaney JT, Moser HW, 1972. Ceramidase deficiency in Farber’s disease (lipogranulomatosis). Science (80-. ) 178, 1100–1102. [DOI] [PubMed] [Google Scholar]
- Sukawutthiya P, Sathirapatya T, Vongpaisarnsin K, 2021. A minimal number CpGs of ELOVL2 gene for a chronological age estimation using pyrosequencing. Forensic Sci. Int 318, 110631–110631. 10.1016/j.forsciint.2020.110631 [DOI] [PubMed] [Google Scholar]
- Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, Silverstein RL, Hazen SL, 2006. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem 281, 4222–4230. 10.1074/jbc.M509769200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sura AA, Chen L, Messinger JD, Swain TA, McGwin G, Bailey Freund K, Curcio CA, 2020. Measuring the contributions of basal laminar deposit and bruch’s membrane in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci 61. 10.1167/IOVS.61.13.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Moreno KM, Gentile AM, Sabbadini RA, Stoller GL, 2008. Sphingosine-1-phosphate (S1P) is a novel fibrotic mediator in the eye. Exp. Eye Res 10.1016/j.exer.2008.07.005 [DOI] [PubMed]
- Swartz JG, Mitchell JE, 1973. Phospholipase Activity of Retina and Pigment Epithelium. Biochemistry 12, 5273–5278. 10.1021/bi00750a008 [DOI] [PubMed] [Google Scholar]
- Swarup A, Samuels IS, Bell BA, Han JYS, Du J, Massenzio E, Abel ED, Boesze-Battaglia K, Peachey NS, Philp NJ, 2019. Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: Impact on photoreceptors and müller glial cells. Am. J. Physiol. - Cell Physiol 316, C121–C133. 10.1152/ajpcell.00410.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, Gimbrone MA, Schafer AI, 1987. Vascular lipoxygenase activity: synthesis of 15-hydroxyeicosatetraenoic acid from arachidonic acid by blood vessels and cultured vascular endothelial cells. Thromb. Res 45, 803–816. 10.1016/0049-3848(87)90090-9 [DOI] [PubMed] [Google Scholar]
- Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA, 2010. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 51, 1699–1708. 10.1167/iovs.09-3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamonti E, Pauter AM, Asadi A, Fischer AW, Chiurchiù V, Jacobsson A, 2017. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell. Mol. Life Sci 74, 2815–2826. 10.1007/s00018-017-2498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ACS, Pilgrim MG, Fearn S, Bertazzo S, Tsolaki E, Morrell AP, Li M, Messinger JD, Dolz-Marco R, Lei J, 2018. Calcified nodules in retinal drusen are associated with disease progression in age-related macular degeneration. Sci. Transl. Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LX, Germer CJ, La Cunza N, Lakkaraju A, 2020. Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration. Redox Biol 10.1016/j.redox.2020.101781 [DOI] [PMC free article] [PubMed]
- Tang DG, Renaud C, Stojakovic S, Diglio CA, Porter A, Honn KV, 1995. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: Its potential role in angiogenesis. Biochem. Biophys. Res. Commun 211, 462–468. 10.1006/bbrc.1995.1836 [DOI] [PubMed] [Google Scholar]
- Tang W, Bardien S, Bhattacharya SS, Prescott SM, 1999. Characterization of the human diacylglycerol kinase ε gene and its assessment as a candidate for inherited retinitis pigmentosa. Gene 239, 185–192. 10.1016/S0378-1119(99)00345-5 [DOI] [PubMed] [Google Scholar]
- Telese F, Gamliel A, Skowronska-Krawczyk D, Garcia-Bassets I, Rosenfeld MG, 2013. Seq-ing Insights into the Epigenetics of Neuronal Gene Regulation. Neuron 10.1016/j.neuron.2013.01.034 [DOI] [PMC free article] [PubMed]
- Teo ACK, Lee SC, Pollock NL, Stroud Z, Hall S, Thakker A, Pitt AR, Dafforn TR, Spickett CM, Roper DI, 2019. Analysis of SMALP co-extracted phospholipids shows distinct membrane environments for three classes of bacterial membrane protein. Sci. Rep 9, 1–10. 10.1038/s41598-018-37962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Khandalavala BN, Manahan RG, Polyak IA, Liu JL, Chacko DM, 1997. Lysophosphatidic acid stimulates proliferation of human retinal pigment epithelial cells. Curr. Eye Res 16, 698–702. 10.1076/ceyr.16.7.698.5056 [DOI] [PubMed] [Google Scholar]
- Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, Mcsorley KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV, 2010. Remodeling of Retinal Fatty Acids in an Animal Model of Diabetes A Decrease in Long-Chain Polyunsaturated Fatty Acids Is Associated With a Decrease in Fatty Acid Elongases Elovl2 and Elovl4. Diabetes 59, 219–227. 10.2337/db09-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco J, Miljanich P, Medwadowski B, 1977. Depletion of docosahexaenoic acid in retinal lipids of rats fed a linolenic acid-deficient, linoleic acid-containing diet. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 486, 575–578. 10.1016/0005-2760(77)90111-4 [DOI] [PubMed] [Google Scholar]
- Tomita H, Abe T, Tamai M, 2000. Ceramide-induced cell death in cultured rat retinal pigment epithelial cells. Tohoku J. Exp. Med 10.1620/tjem.190.223 [DOI] [PubMed]
- Toomey CB, Kelly U, Saban DR, Rickman CB, 2015. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc. Natl. Acad. Sci 112, E3040–E3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A, 2015. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol. Biol. Cell 10.1091/mbc.E14-05-1028 [DOI] [PMC free article] [PubMed]
- Travis GH, Golczak M, Moise AR, Palczewski K, 2007. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol 10.1146/annurev.pharmtox.47.120505.105225 [DOI] [PMC free article] [PubMed]
- Trotta MC, Pieretti G, Petrillo F, Alessio N, Hermenean A, Maisto R, D’Amico M, 2020. Resolvin D1 reduces mitochondrial damage to photoreceptors of primary retinal cells exposed to high glucose. J. Cell. Physiol 235, 4256–4267. 10.1002/jcp.29303 [DOI] [PubMed] [Google Scholar]
- Trotter JH, Klein M, Jinwal UK, Abisambra JF, Dickey CA, Tharkur J, Masiulis I, Ding J, Locke KG, Rickman CB, Birch DG, Weeber EJ, Herz J, 2011. ApoER2 Function in the Establishment and Maintenance of Retinal Synaptic Connectivity. J. Neurosci 31, 14413–14423. 10.1523/JNEUROSCI.3135-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR, 2006a. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis 12, 1319–1333. [PubMed] [Google Scholar]
- Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR, 2006b. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis 12, 18. [PubMed] [Google Scholar]
- Tulenko TN, Boeze-Battaglia K, Mason RP, Tint GS, Steiner RD, Connor WE, Labelle EF, 2006. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res 47, 134–143. [DOI] [PubMed] [Google Scholar]
- Tuson M, Marfany G, Gonzàlez-Duarte R, 2004. Mutation of CERKL, a Novel Human Ceramide Kinase Gene, Causes Autosomal Recessive Retinitis Pigmentosa (RP26). Am. J. Hum. Genet 74, 128–138. 10.1086/381055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R, Birch E, Birch D, Peirano P, 1992. Visual and brain function measurements in studies of n-3 fatty acid requirements of infants. J. Pediatr 120, S168–S180. 10.1016/S0022-3476(05)81252-1 [DOI] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA-K, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, 2015. Tissue-based map of the human proteome. Science (80-. ) 347, 1260419–1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Urlić M, Urlić I, Urlić H, Mašek T, Benzon B, Uljević MV, Vukojević K, Filipović N, 2020. Effects of different n6/n3 pufas dietary ratio on cardiac diabetic neuropathy. Nutrients 12, 1–20. 10.3390/nu12092761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio I, Abu Khamidakh A, Paci M, Skottman H, Juuti-Uusitalo K, Hyttinen J, Nymark S, 2015. Computational Model of Ca2+ Wave Propagation in Human Retinal Pigment Epithelial ARPE-19 Cells. PLoS One 10, e0128434. 10.1371/journal.pone.0128434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink DM, Brites P, Haasjes J, Wierzbicki AS, Mitchell J, Lambert-Hamill M, de Belleroche J, Jansen GA, Waterham HR, Ronald Wanders JA, 2003. Identification of PEX7 as the Second Gene Involved in Refsum Disease. Am. J. Hum. Genet 72, 471–477. 10.1086/346093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL, 2017. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta - Biomembr 10.1016/j.bbamem.2017.04.006 [DOI] [PubMed]
- van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, Dammeier S, Meester-Smoor M, Pool FM, de Jong EK, Delcourt C, Rodrigez-Bocanegra E, Biarnés M, Luthert PJ, Ueffing M, Klaver CCW, Nogoceke E, den Hollander AI, Lengyel I, 2018. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res 10.1016/j.preteyeres.2018.04.006 [DOI] [PubMed]
- Van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol 10.1038/nrm2330 [DOI] [PMC free article] [PubMed]
- Vanier MT, 2015. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis 38, 187–199. 10.1007/s10545-014-9794-4 [DOI] [PubMed] [Google Scholar]
- Vasiliauskaité-Brooks I, Healey RD, Rochaix P, Saint-Paul J, Sounier R, Grison C, Waltrich-Augusto T, Fortier M, Hoh F, Saied EM, Arenz C, Basu S, Leyrat C, Granier S, 2018. Structure of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. Nat. Commun 9, 1–13. 10.1038/s41467-018-07864-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliauskaité-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, De Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S, 2017. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 10.1038/nature21714 [DOI] [PMC free article] [PubMed]
- Vasireddy V, Uchida Y, Salem N, Kim SY, Mandal MNA, Reddy GB, Bodepudi R, Alderson NL, Brown JC, Hama H, Dlugosz A, Elias PM, Holleran WM, Ayyagari R, 2007. Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet 16, 471–482. 10.1093/hmg/ddl480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal EA, Day AM, Morgan BA, 2007. Hydrogen peroxide sensing and signaling. Mol. Cell 26, 1–14. [DOI] [PubMed] [Google Scholar]
- Victoria Simon M, Basu SK, Qaladize B, Grambergs R, Rotstein NP, Mandal N, 2021. Sphingolipids as critical players in retinal physiology and pathology. J. Lipid Res 62, 100037–100038. 10.1194/JLR.TR120000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virág L, Salzman AL, Szabó C, 1998. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J. Immunol 161, 3753–9. [PubMed] [Google Scholar]
- Volland S, Esteve-Rudd J, Hoo J, Yee C, Williams DS, 2015. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS One 10, e0125631. 10.1371/journal.pone.0125631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, Hu J, Fleming I, Wang DW, 2021. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther 6, 1–30. 10.1038/s41392-020-00443-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Bieberich E, 2018. Sphingolipids in neurodegeneration (with focus on ceramide and S1P). Adv. Biol. Regul 10.1016/j.jbior.2018.09.013 [DOI] [PMC free article] [PubMed]
- Wang J, Zibetti C, Shang P, Sripathi SR, Zhang P, Cano M, Hoang T, Xia S, Ji H, Merbs SL, Zack DJ, Handa JT, Sinha D, Blackshaw S, Qian J, 2018. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat. Commun 9, 1364. 10.1038/s41467-018-03856-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA, 2010. Abundant lipid and protein components of drusen. PLoS One 5, e10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kaya KD, Kim S, Brooks MJ, Wang J, Xin Y, Qian J, Swaroop A, Handa JT, 2020. Retinal pigment epithelium transcriptome analysis in chronic smoking reveals a suppressed innate immune response and activation of differentiation pathways. Free Radic. Biol. Med 156, 176–189. 10.1016/j.freeradbiomed.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Hsiao G, Al-Shabrawey M, 2020. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 9. 10.3390/antiox9060520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Anderson RE, 1993. Synthesis of Docosahexaenoic Acid by Retina and Retinal Pigment Epithelium. Biochemistry 32, 13703–13709. 10.1021/bi00212a040 [DOI] [PubMed] [Google Scholar]
- Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, Chen YE, Yin KJ, Powell FL, Martin PM, Grant MB, Busik JV, 2016. Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy. EBioMedicine 10.1016/j.ebiom.2016.08.013 [DOI] [PMC free article] [PubMed]
- Wang T, Tsui B, Kreisberg JF, Robertson NA, Gross AM, Yu MK, Carter H, Brown-Borg HM, Adams PD, Ideker T, 2017. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol 18, 1–11. 10.1186/s13059-017-1186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK, 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W, 2009. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochim. Biophys. Acta - Biomembr 10.1016/j.bbamem.2008.10.011 [DOI] [PubMed]
- Way KJ, Katai N, King GL, 2001. Protein kinase C and the development of diabetic vascular complications. Diabet. Med 18, 945–959. 10.1046/j.0742-3071.2001.00638.x [DOI] [PubMed] [Google Scholar]
- Weir K, Leavey P, Santiago C, Blackshaw S, 2021. Multiplexed analysis of retinal gene expression and chromatin accessibility using scrna-seq and scatac-seq. J. Vis. Exp 2021. 10.3791/62239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH, Innerarity TL, Mahley RW, 1982. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J. Biol. Chem 257, 2518–2521. 10.1016/S0021-9258(18)34954-8 [DOI] [PubMed] [Google Scholar]
- Wensel TG, 2020. Phosphoinositides in Retinal Function and Disease. Cells 10.3390/cells9040866 [DOI] [PMC free article] [PubMed]
- Widomska J, 2014. Why has Nature Chosen Lutein and Zeaxanthin to Protect the Retina? J. Clin. Exp. Ophthalmol 05, 326. 10.4172/2155-9570.1000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand RD, Giusto NM, Rapp LM, Anderson RE, 1983. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest. Ophthalmol. Vis. Sci 24, 1433–1435. [PubMed] [Google Scholar]
- Wiegand RD, Joel CD, Rapp LM, Nielsen JC, Maude MB, Anderson RE, 1986. Polyunsaturated fatty acids and vitamin E in rat rod outer segments during light damage. Invest. Ophthalmol. Vis. Sci 27, 727–733. [PubMed] [Google Scholar]
- Wijesinghe DS, Massiello A, Subramanian P, Szulc Z, Bielawska A, Chalfant CE, 2005. Substrate specificity of human ceramide kinase. J. Lipid Res 10.1194/jlr.M500313-JLR200 [DOI] [PubMed]
- Williams DS, Liu X, Schlamp CL, Ondek B, Jaken S, Newton AC, 1997. Characterization of protein kinase C in photoreceptor outer segments. J. Neurochem 69, 1693–1702. 10.1046/j.1471-4159.1997.69041693.x [DOI] [PubMed] [Google Scholar]
- Williams TP, Penn JS, 1985. Intracellular topography of rhodopsin regeneration in vertebrate rods. J. Gen. Physiol 86, 413–422. 10.1085/jgp.86.3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N, 2019. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J. Diabetes Complications 33, 195–201. 10.1016/j.jdiacomp.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter AW, Salimi A, Ospina LH, Roos JCP, 2019. Ophthalmic manifestations of Gaucher disease: the most common lysosomal storage disorder. Br. J. Ophthalmol 103, 315 LP – 326. 10.1136/bjophthalmol-2018-312846 [DOI] [PubMed] [Google Scholar]
- Wolbring G, Cook NJ, 1991. Rapid purification and characterization of protein kinase C from bovine retinal rod outer segments. Eur. J. Biochem 201, 601–606. 10.1111/j.1432-1033.1991.tb16320.x [DOI] [PubMed] [Google Scholar]
- Wolfrum U, Schmitt A, 2000. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil. Cytoskeleton 46, 95–107. [DOI] [PubMed] [Google Scholar]
- Won J, Smith RS, Peachey NS, Wu J, Hicks WL, Naggert JK, Nishina PM, 2008. Membrane frizzled-related protein is necessary for the normal development and maintenance of photoreceptor outer segments. Vis. Neurosci 25, 563–574. 10.1017/S0952523808080723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DLA, Ghosh S, Nguyen LN, Barathi VA, Yeo SW, Luu CD, Wenk MR, Silver DL, 2016. Mfsd2a is a transporter for the essential ω−3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J. Biol. Chem 291, 10501–10514. 10.1074/jbc.M116.721340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BH, Silver DL, 2020. Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye. Adv. Exp. Med. Biol 1276, 223–234. 10.1007/978-981-15-6082-8_14 [DOI] [PubMed] [Google Scholar]
- Wu G, Hubbell WL, 1993. Phospholipid Asymmetry and Transmembrane Diffusion in Photoreceptor Disc Membranes. Biochemistry 10.1021/bi00054a020 [DOI] [PubMed]
- Xu M, Eblimit A, Wang J, Li J, Wang F, Zhao L, Wang X, Xiao N, Li Y, Wong L-JC, Lewis RA, Chen R, 2016. ADIPOR1 Is Mutated in Syndromic Retinitis Pigmentosa. Hum. Mutat 37, 246–249. 10.1002/humu.22940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Shinohara K, Abe Y, Nakagawa K, Al-Owain M, Alkuraya FS, Fujiki Y, 2017. Deficiency of a Retinal Dystrophy Protein, Acyl-CoA Binding Domain-containing 5 (ACBD5), Impairs Peroxisomal β-Oxidation of Very-long-chain Fatty Acids*. J. Biol. Chem 292, 691–705. 10.1074/jbc.M116.760090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Peng YR, van Zyl T, Regev A, Shekhar K, Juric D, Sanes JR, 2020. Cell Atlas of The Human Fovea and Peripheral Retina. Sci. Rep 10. 10.1038/s41598-020-66092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Valentine WJ, 2020. Druggable Lysophospholipid Signaling Pathways, in: Advances in Experimental Medicine and Biology Springer, pp. 137–176. 10.1007/978-3-030-50621-6_7 [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen Yali, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen H, Zhao C, Chen Yuhong, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S, Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT, Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K, 2008. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Invest 118, 2908–2916. 10.1172/JCI35891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine HN, Finch CE, 2020. APOE Alleles and Diet in Brain Aging and Alzheimer’s Disease . Front. Aging Neurosci . [DOI] [PMC free article] [PubMed]
- Yeagle PL, 1991. Modulation of membrane function by cholesterol. Biochimie 73, 1303–1310. 10.1016/0300-9084(91)90093-G [DOI] [PubMed] [Google Scholar]
- Yeagle PL, Young JE, 1986. Factors contributing to the distribution of cholesterol among phospholipid vesicles. J. Biol. Chem 261, 8175–8181. 10.1016/s0021-9258(19)83893-0 [DOI] [PubMed] [Google Scholar]
- Yeboah GK, Lobanova ES, Brush RS, Agbaga MP, 2021. Very long chain fatty acid-containing lipids: A decade of novel insights from the study of ELOVL4. J. Lipid Res 62, 100030. 10.1016/J.JLR.2021.100030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Chen F, Wang W, Wang H, Zhang X, 2017. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis 14, 242–250. [PMC free article] [PubMed] [Google Scholar]
- York N, Halbach P, Chiu MA, Bird IM, Pillers D-AM, Pattnaik BR, 2017. Oxytocin (OXT)-stimulated inhibition of Kir7.1 activity is through PIP2-dependent Ca2+ response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cell. Signal 37, 93–102. 10.1016/j.cellsig.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Inoue H, Hotta Y, 1983. Defective phospholipid metabolism in the retinular cell membrane of norpA (no receptor potential) visual transduction mutants of Drosophila. Biochem. Biophys. Res. Commun 111, 567–573. 10.1016/0006-291X(83)90344-3 [DOI] [PubMed] [Google Scholar]
- Young RW, 1971. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol 49, 303–18. 10.1083/jcb.49.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, 1967. The renewal of photoreceptor cell outer segments. J. Cell Biol 33, 61–72. 10.1083/jcb.33.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D, 1969. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol 42, 392–403. 10.1083/jcb.42.2.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-C, Nandrot EF, Dun Y, Finnemann SC, 2012. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking αvβ5 integrin. Free Radic. Biol. Med 52, 660–670. 10.1016/j.freeradbiomed.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FPS, Sajdak BS, Sikora J, Salmon AE, Nagree MS, Gurka J, Kassem IS, Lipinski DM, Carroll J, Medin JA, 2019. Acid Ceramidase Deficiency in Mice Leads to Severe Ocular Pathology and Visual Impairment. Am. J. Pathol 10.1016/j.ajpath.2018.10.018 [DOI] [PMC free article] [PubMed]
- Zareparsi S, Branham KEH, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A, 2005. Strong Association of the Y402H Variant in Complement Factor H at 1q32 with Susceptibility to Age-Related Macular Degeneration. Am. J. Hum. Genet 77, 149–153. 10.1086/431426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinger L, Banin E, Obolensky A, Mizrahi-Meissonnier L, Beryozkin A, Bandah-Rozenfeld D, Frenkel S, Ben-Yosef T, Merin S, Schwartz SB, Cideciyan AV, Jacobson SG, Sharon D, 2011. A Missense Mutation in DHDDS, Encoding Dehydrodolichyl Diphosphate Synthase, Is Associated with Autosomal-Recessive Retinitis Pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet 88, 207–215. 10.1016/j.ajhg.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Zhang T, Madigan MC, Fernando N, Aggio-Bruce R, Zhou F, Pierce M, Chen Y, Huang L, Natoli R, Gillies MC, Zhu L, 2020. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell. Neurosci 10.3389/fncel.2020.577935 [DOI] [PMC free article] [PubMed]
- Zhang J, Wang C, Shen Y, Chen N, Wang L, Liang L, Guo T, Yin X, Ma Z, Zhang B, Yang L, 2016. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. Hum. Genet 135, 1375–1387. 10.1007/s00439-016-1730-2 [DOI] [PubMed] [Google Scholar]
- Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, Kakuk LE, Lagali PS, Wong PW, MacDonald IM, Sieving PA, Figueroa DJ, Austin CP, Gould RJ, Ayyagari R, Petrukhin K, 2001. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet 27, 89–93. 10.1038/83817 [DOI] [PubMed] [Google Scholar]
- Zhao J, Kim HJ, Ueda K, Zhang K, Montenegro D, Dunaief JL, Sparrow, 2021. A vicious cycle of bisretinoid formation and oxidation relevant to recessive Stargardt disease. J. Biol. Chem 296, 100259. 10.1016/j.jbc.2021.100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Pearsall EA, Hurst DP, Zhang Y, Chu J, Zhou Y, Reggio PH, Loh HH, Law PY, 2012. Palmitoylation and membrane cholesterol stabilize μ-opioid receptor homodimerization and G protein coupling. BMC Cell Biol 10.1186/1471-2121-13-6 [DOI] [PMC free article] [PubMed]
- Zhou WL, Sugioka M, Yamashita M, 1999. Lysophosphatidic acid-induced Ca2+ mobilization in the neural retina of chick embryo. J. Neurobiol 41, 495–504. [DOI] [PubMed] [Google Scholar]
- Zhu DH, Sreekumar PG, Hinton DR, Kannan R, 2010. Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vision Res 10.1016/j.visres.2009.09.002 [DOI] [PMC free article] [PubMed]
- Zhukovsky MA, Filograna A, Luini A, Corda D, Valente C, 2019. Phosphatidic acid in membrane rearrangements. FEBS Lett 10.1002/1873-3468.13563 [DOI] [PubMed]
- Zolnikova IV, Strelnikov VV, Skvortsova NA, Tanas AS, Barh D, Rogatina EV, Egorova IV, Levina DV, Demenkova ON, Prikaziuk EG, Ivanova ME, 2017. Stargardt disease-associated mutation spectrum of a Russian Federation cohort. Eur. J. Med. Genet 60, 140–147. 10.1016/j.ejmg.2016.12.002 [DOI] [PubMed] [Google Scholar]


