Abstract
Milk yield and composition are critical determining factors for the early growth and development of neonates. The objective of this experiment was to comprehensively evaluate the effects of dietary sodium acetate (SA) supplementation on the milk yield and composition of sows and the growth performance of their offspring. A total of 80 sows (Landrace × Yorkshire, 3 to 6 parity) were randomly assigned to 2 groups (with or without 0.1% SA) from d 85 of gestation to d 21 of lactation. The result shows that maternal 0.1% SA supplementation significantly increased sows milk yield, milk fat, immunoglobulin A (IgA) and IgG content in milk (P < 0.05), with the up-regulation of short-chain fatty acids receptors (GPR41 and GPR43) expression and the activation of mammalian target of rapamycin complex C1 (mTORC1) signaling pathway. Consistently, in our in vitro experiment, SA also activated mTORC1 signaling in porcine mammary epithelial cells (P < 0.05). Furthermore, the improvement of milk quality and quantity caused by maternal SA supplementation led to the increase in body weight (BW) and average daily weight gain (ADG) of weaning piglets, with the improvement of gut health and colonization of the beneficial bacteria (P < 0.05). In conclusion, maternal supplementation of 0.1% SA improved the lactation performance (milk yield and milk fat) of sows, possibly with the activation of GPR41/GPR43-mTORC1 signaling. Furthermore, enhanced milk quality improved growth performance, gut health and the colonization of beneficial microbial flora of their piglets.
Keywords: Sow, Sodium acetate, Lactation performance, Milk fat, Gut health
1. Introduction
Over the past decade, advances in breeding technology have resulted in a dramatic increase in sows’ litter size, which led to a great challenge for milk yield. The lactation performance of sows is critical for early growth and development of their piglets (Harrell et al., 1993). Insufficient milk intake leads to the reduction of weaning body weight (BW) and the decrease in weaning survival rate of piglets during lactation (Farmer, 2018).
Maternal nutrient intake directly affects milk yield and milk composition in lactating sows. Insufficient maternal energy intake could induce AMP-activated protein kinase (AMPK) activation, which further inhibits mammary gland milk synthesis by targeting prolactin receptor (PrlR) and proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) (Wu et al., 2022). Dietary supplementation of functional amino acids (such as branched-chain amino acids, methionine, arginine, and lysine) has been widely demonstrated to regulate milk synthesis (Wu et al., 2020). However, it is still unknown whether other nutrients participate in the regulation of milk synthesis.
Rumen microbiota-derived short-chain fatty acids (SCFA) (especially acetate and butyrate) have been recognized as substrates for milk fat synthesis for a long time. In monogastric animals, serum SCFA is much lower, which is mainly derived from the fermentation of dietary fiber in the hindgut. Intriguingly, feeding high-fiber diets during gestation also increases serum acetic acid level with the upregulation of colostrum and milk fat content in sows (Jensen et al., 2012). In addition, studies have shown that acetic acid is more effective in promoting milk fat synthesis in bovine mammary epithelial cells than other SCFA (Sheng et al., 2016). However, the specific mechanism remains unclear. One possible mechanism is that SCFA induce the mobilization of fatty acids in mammary adipose tissue, and promote fatty acid transport from adipose tissue to mammary acinar (Feyera et al., 2019; Jensen et al., 2012). Sterol regulatory element binding proteins (SREBP1) is a critical regulator to promote milk fat synthesis (Li et al., 2014). Mechanistically, SCFA might modulate the expression of SREBP1 through the activation of mTORC1 pathway in the mammary gland (Düvel et al., 2010; Li et al., 2014; Park et al., 2015; Wang et al., 2014).
The main purpose of this experiment was to study the effect and underlying mechanism of maternal sodium acetate (SA) supplementation on milk yield and composition of sows. Furthermore, growth performance, gut health and intestinal colonization of microbial flora of their piglets were also determined.
2. Materials and methods
2.1. Animal ethics statement
All of the procedures performed in animal feeding and sample harvesting during this study were approved by the South China Agricultural University Animal Care and Use Committee (Guangzhou, China).
2.2. In vivoanimal experiment
2.2.1. Animals and experimental design
As shown in Fig. S1, a total of 80 Landrace × Yorkshire, 3 to 6 parity) sows were divided into 2 treatments (control treatment and 0.1% SA, 40 replicates per treatment) by parity, backfat thickness and historical reproductive performance. The feeding experiment started from d 85 of gestation to d 21 of lactation, which was conducted on a modern commercial swine farm in Qingyuan City, Guangdong Province, China.
2.2.2. Diets and management
According to NRC (2012), diets were formulated to meet or exceed the nutritional needs of sows during gestation and lactation. The ingredient and chemical composition are shown in Table S1. During gestation, all sows were housed in individual gestation stalls (2.1 m × 0.6 m), and fed twice a day (08:00 and 14:00). Then they were transferred to the farrowing crates at d 110 of gestation. According to the backfat thickness, sows were fed 3 to 3.5 kg/d diets from the d 85 to 114 of gestation. After farrowing, the feed supply for the sows was increased by 1 kg/d until d 3 and then ad libitum feeding. The litter size was standardized to approximately 12 ± 1 piglets by cross-fostering within 24 h of farrowing.
2.2.3. Sow and litter performance
At farrowing, the number of litter size, litter weight, live births, stillbirths, mummy and weak (BW < 0.8 kg) piglets were recorded. Litter weight were recorded again at 24 h after farrowing of sows and cross-fostering of piglets. While, sows' litter size and litter weight were recorded on d 7, 14 and 21 of lactation. Piglets' BW, survival rate, average daily gain (ADG) and sows' average daily feed intake (ADFI) during lactation were recorded and calculated, n = 40. On d 85 of gestation, day of farrowing and d 21 of lactation, the backfat thickness of the sow was measured at P2 point (left side of the 10th rib and 6 cm away from the spine) according to the previous method (Mateo et al., 2007).
2.2.4. Sow lactation performance
Colostrum yield and colostrum intake were calculated using the method provided by Theil et al. (2014):
where WG = weight gain of individual piglets (g) from the first piglet 24 h after birth, BWB = the birth weight of piglets (kg), D = colostrum intake time per piglet (min).
The total milk yield of each sow is calculated based on the ADG and litter size of the piglets using the following formula (Miao et al., 2019):
2.2.5. Milk sampling and analysis
Samples were collected from 6 representative sows per group, according to the average BW and parity of sows in each treatment group. Colostrum was collected within 12 h after farrowing the first piglet without oxytocin injection, and milk was collected on d 21 of lactation after the intramuscular injection of 20 IU oxytocin per sow. Milk samples were rapidly placed in liquid nitrogen, then transferred to –80 °C refrigerator preservation.
The composition of milk (lipids, crude protein, lactose, solids-not-fat) were determined by automatic milk composition analyzer (Milko-Scan 134 A/B, Foss Company, Denmark). And the concentrations of immunoglobulin A (IgA), IgG and IgM in milk were determined using ELISA kit (Wuhan Huamei), according to a method described in literature (Zanello et al., 2013).
2.2.6. Plasma sampling and analysis
On the day of farrowing and the d 21 of lactation, 20 mL blood was collected from ear veins of sows and anterior vena cava of piglets (select 1 piglet, near the average BW in each treatment group, from each of the 6 representative sows) using vacuum blood collection vessel containing EDTA, n = 6. After centrifugation at 3000 × g at 25 °C for 15 min, the plasma was transferred to a 1.5-mL cryovial, then quickly placed in liquid nitrogen and transferred to –80 °C refrigerator for storage. The concentrations of prolactin and immunoglobulin in plasma of sows and piglets were determined using Wuhan Huamei ELISA kit.
2.2.7. Tissue sample and intestinal morphology
After blood collection on d 21 of lactation, these piglets were sacrificed by electrocution for subsequent experimental sample collection. Duodenum, jejunum and ileum were collected according to the method provided by Ren et al. (2020). Formalin-fixed, paraffin-embedded intestinal samples were stained with hematoxylin and eosin, then made into 4 to 6 μm thick sections. Villous height and crypt depth were measured for 6 well-oriented crypt-villus units per sample, and the ratio of villous height to crypt depth (VCR) was calculated.
2.2.8. Sows milk-derived cells isolation
Sows milk-derived cells were isolated from milk using the method provided by Twigger et al. (2022). Briefly, milk samples were diluted in an equal volume of sterile phosphate-buffered saline (PBS) and centrifuged at 870 × g at 20 °C for 20 min to isolate milk cells. Then the supernatant was removed and the pellet was resuspended in 5 to 10 mL of pre-chilled PBS. Finally, the sample was transferred to a new 10 mL tube and centrifuged at 490 × g at 4 °C for 5 min to collect the milk-derived cells after removing the supernatant.
2.3. In vitrocell culture experiment
The porcine mammary epithelial cells (PMEC) used in this study were isolated and characterized in our laboratory from the mammary glands of sows (G 90). PMEC used in this study were cultured in the complete medium consisted of DMEM/F12 (Thermo), containing 10% fetal bovine serum (FBS), 5 μg/mL insulin-like growth factor-1 (IGF-1), 10 ng/mL epidermal growth factor (EGF), 5 μg/mL insulin-Transferrin-Selenium (ITS) and 10 μg/mL penicillin-streptomycin (PS) in a cell incubator at 37 °C and 5% CO2 concentration. When the cells reached to around 50% confluence, they were seeded in culture plates and incubated with in DMEM/F12 containing 10% FBS, 1 μg/mL hydrocortisone, 10 μg/mL PS, 0.5 μg/mL insulin and 2 μg/mL prolactin (Ma et al., 2018). The cell differentiation process was carried out for about 2 d until the cells reach 80% confluency. Then cells were used for the following experiments. PMEC were cultured with different concentrations (0, 0.25, 0.5, 0.75, 1.0 and 1.5 mM) of SA, n = 3. After 24 h incubation, cells were harvested using EZ-press RNA Purification Kit (EZBioscience, China) and RIPA lysis buffer for real-time PCR and western-blotting respectively.
2.4. RNA isolation and real-time PCR
Total RNA was isolated from cell samples using EZ-press RNA Purification Kit (EZBioscience, China) according to the manufacturer's instructions. The mRNA concentration was measured by NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Each component's 260:280 ratio is between 1.6 and 1.8. The cDNA was prepared using an RNA reverse transcription kit (EZBioscience, China) according to instructions. Real-time PCR uses ABI StepOnePlus real-time PCR Systems. The reaction system was 10 μL Real-Time PCR Master Mix, 2 μL cDNA, 0.8 μL of each PCR primer and 7.2 μL DEPC water. The following thermal profile was used for RT-PCR, 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 59 °C for 15 s, and extension at 72 °C for 40 s. The primers used in the experiment were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). Primer sequences are shown in Table S2.
2.5. Western-blotting
Milk-derived cells and PMEC were homogenized in RIPA lysis buffer containing 1% PMSF and 1% phosphatase inhibitor. Subsequently, 5 × protein loading buffer was added, fully shaken and mixed. Finally, the protein samples were boiled and denatured, and stored at –20 °C. The proteins were separated by electrophoresis on a 10% polyacrylamide gel and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween (TBST), followed by overnight probing with primary antibodies. After washing, membranes were incubated with secondary antibodies. The chemiluminescent signal was detected by using ECL reagents (P1020), and bands were quantified by ImageJ Software (ImageJ 1.52a). The antibodies used in this experiment were as follows. Primary antibodies: fatty acid synthase (FASN) (1:2000, ab99359), acetyl-CoA carboxylα (ACACA) (1:1000, ab72046), fatty acid binding protein 3 (FABP3), (1:1000, ab231568), diacylglycerol O-Acyltransferase 1 (DGAT1) (1:500, ab100982), sterol-regulatory element binding protein1 (SREBP1) (1:1000, ab28481), 4E-binding protein 1 (4EBP1) (1:1000, ab2606), ribosomal protein S6 kinase 1 (S6K1) (1:1000, ab9366), GPR41 (1:1000, ab236654) and GPR43 (1:1000, ab131003) all purchased from Abcam, USA; Mammalian target of rapamycin (mTOR) (1:1000, #2983), P-mTOR (1:1000, #5356), P-4EBP1 (1:1000, 9451S) and P-S6K1 (1:1000, #9234) all purchased from Cell Signaling Technology; beta-actin (1:2000, bs-0061R) purchased from Beijing Bo Osen Biotechnology Co., Ltd.; Secondary antibodies: goat anti-rabbit IgG (1:5000, 511203) and goat Anti-mouse IgG (1:5000, 511103) purchased from ZenBio.
2.6. Oil red O staining of lipid droplets and triglyceride content
In this experiment, the oil red O staining method was used to detect the effect of different concentrations of SA on the synthesis of lipid droplets in cells. Specifically, cells were seeded into 24-well plates with a pipette and cultured as described above. The collected cells were rinsed 3 times with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature, then oil red O solution was carefully added to the cells (150 μL per well). Samples were stained for 3 h under room temperature. Afterward, the staining solution was discarded and cells were washed once with 65% isopropanol and 5 times with PBS. Finally, 250 μL of distilled water was added, and pictures of samples were observed and taken under a 400× inverted microscope. Triglyceride Assay Kit (Jiancheng, Nanjing, China) was used to detect the content of triglycerides (TAG) in PMEC.
2.7. 16S rDNA sequencing
Microbiome analysis using 16S rDNA high-throughput sequencing technology after collecting ileal digesta from piglets on d 21 of lactation (performed by Gene Denovo [Guangzhou, China]). After genomic DNA was extracted from the sample, the V3 + V4 region of 16S rDNA was amplified with barcode-specific primers. The primer sequence is 341F: CCTACGGGNGGCWGCAG and 806R: GGACTACHVGGGTATCTAAT. Connect the purified amplification products (i.e., amplicons) to sequencing adapters to construct a sequencing library, which was sequenced on a NovaSeq 60000 (PE250, Illumina Inc., CA, United States). Finally, the 16S rDNA sequencing data were analyzed using the Omicsmart (Gene Denovo, Guangzhou, China) online tool.
2.8. Statistical analysis
Data from this study were analyzed based on a randomized complete block design using the MIXED procedure of SAS (Cary, NC). Treatment (control group and SA group) was the fixed effect and the parity was the block. The sows and their litters were used as the experimental units for the analysis of data of reproductive performance. The pre-weaning survival was analyzed using the Chi-square test. Other in vivo experimental data were analyzed by independent samples t-test. Data from in vitro experiments were processed using one-way ANOVA analysis. Composition and diversity of microbial communities expressed as standardized operational taxonomic unit (OTU) reads were analyzed using R software (version 3.2.2; R Software Inc., Auckland, New Zealand). Analysis of relative abundance of phyla and order at different levels using the Kruskal–Wallis Test. All analysis results were expressed in the form of mean ± SEM, and P < 0.05 was used as the criterion of the significance of difference.
3. Results
3.1. Sow reproductive performance
As shown in Table 1, ADFI and backfat thickness of sows were not affected by maternal diets during the experiment (P > 0.05). Besides, dietary SA supplementation during gestation had no effect on total litter size, number of mummies, birth weight, litter weight, and the estrus interval after weaning (P > 0.05). Maternal SA numerically reduced the number of weak offspring by 51% compared to the control group.
Table 1.
Effect of dietary sodium acetate (SA) supplementation on reproductive performance of sows.
| Item | Treatment group1 |
SEM | P-value | |
|---|---|---|---|---|
| CON | SA | |||
| Parity | 3.43 | 3.40 | 0.16 | 0.912 |
| Total litter size | 13.88 | 13.65 | 0.61 | 0.797 |
| Weak offspring | 1.03 | 0.53 | 0.19 | 0.065 |
| Stillbirths | 0.80 | 0.55 | 0.14 | 0.222 |
| Mummy | 0.20 | 0.25 | 0.08 | 0.674 |
| Live births | 13.03 | 13.40 | 0.53 | 0.375 |
| Litter weight, kg | 18.82 | 19.55 | 0.77 | 0.506 |
| Individual birth weight, kg | 1.47 | 1.47 | 0.04 | 0.993 |
| Weaning to estrus interval, d | 4.08 | 4.23 | 0.11 | 0.325 |
| ADFI, kg | ||||
| Week 1 of lactation | 3.48 | 3.51 | 0.12 | 0.876 |
| Week 2 of lactation | 4.72 | 4.72 | 0.17 | 0.989 |
| Week 3 of lactation | 5.60 | 5.58 | 0.13 | 0.889 |
| Lactation | 4.60 | 4.60 | 0.10 | 0.991 |
| Sow backfat thickness, mm | ||||
| Day 85 of gestation | 18.60 | 18.98 | 0.60 | 0.663 |
| Day 114 of gestation | 17.73 | 18.18 | 0.66 | 0.629 |
| Day 21 of lactation | 16.33 | 17.00 | 0.62 | 0.445 |
| Late pregnancy loss, mm | 0.88 | 0.80 | 0.26 | 0.839 |
| Lactation loss, mm | 1.40 | 1.18 | 0.31 | 0.605 |
ADFI = average daily feed intake; SEM = standard error of the mean.
CON, sows were fed a base diet; SA, sows were fed a basal diet supplemented with 0.1% sodium acetate (n = 40).
3.2. Sodium acetate supplementation regulated yield and composition of colostrum and milk
As shown in Table 2, SA supplementation significantly increased colostrum (P = 0.028) and total milk yield (P = 0.044) of sows during lactation. Furthermore, we measured the colostrum and d 21 milk composition. The results showed that SA supplementation significantly increased the contents of colostrum fat (P = 0.039), colostrum crude protein (CP) (P < 0.001), colostrum lactose (P = 0.012) and colostrum solids-not-fat (SNF) (P < 0.001) and milk fat content on day 21 of lactation (P = 0.015).
Table 2.
Effect of dietary sodium acetate (SA) supplementation on yield and composition of colostrum and milk in sows.
| Item | Treatment group1 |
SEM | P-value | |
|---|---|---|---|---|
| CON | SA | |||
| Average piglet colostrum intake on d 1, g | 444.00b | 497.77a | 12.34 | 0.025 |
| Colostrum yield for 24 h, kg/d | 5.46b | 6.68a | 0.28 | 0.028 |
| Total milk yield, kg | 200.72b | 216.52a | 3.94 | 0.044 |
| Colostrum composition, % | ||||
| Fat | 4.04b | 4.77a | 0.24 | 0.039 |
| CP | 6.98b | 7.86a | 0.15 | <0.001 |
| Lactose | 3.34b | 3.66a | 0.07 | 0.012 |
| SNF | 18.56b | 20.94a | 0.39 | <0.001 |
| Milk on d 21 composition, % | ||||
| Fat | 6.96b | 7.46a | 0.14 | 0.015 |
| CP | 4.60 | 4.58 | 0.02 | 0.539 |
| Lactose | 6.51 | 6.48 | 0.04 | 0.665 |
| SNF | 11.98 | 11.93 | 0.07 | 0.604 |
CP = crude protein; SNF = solids-not-fat; SEM = standard error of the mean.
a,b Different letters indicate significant differences between the 2 data.
CON, sows were fed a base diet; SA, sows were fed a basal diet supplemented with 0.1% sodium acetate (n = 6).
3.3. Sodium acetate supplementation regulated Ig composition of plasma, colostrum and milk
As shown in Table 3, SA supplementation significantly increased the plasma IgA and IgG content of sows on the day of farrowing and the d 21 after farrowing (P < 0.05), but had no effect on IgM. Consistently, SA supplementation significantly increased IgA and IgG levels in colostrum and d 21 of milk (P < 0.05; Table 4). It is worth noting that IgG in the plasma of the offspring piglets was numerically increased both at born (P = 0.086) and weaning (P = 0.054) (Table S3).
Table 3.
Effect of dietary sodium acetate (SA) supplementation on plasma immunoglobulin and prolactin levels of sows.
| Item | Treatment group1 |
SEM | P-value | |
|---|---|---|---|---|
| CON | SA | |||
| Farrowing | ||||
| IgA, mg/mL | 0.41b | 1.09a | 0.08 | <0.001 |
| IgG, mg/mL | 1.48b | 2.01a | 0.15 | 0.032 |
| IgM, mg/mL | 0.14 | 0.15 | 0.02 | 0.692 |
| Prolactin, ng/mg | 4.99b | 5.87a | 0.22 | 0.021 |
| Day 21 after farrowing | ||||
| IgA, mg/mL | 0.50b | 1.31a | 0.07 | <0.001 |
| IgG, mg/mL | 0.77b | 1.41a | 0.13 | 0.006 |
| IgM, mg/mL | 0.41 | 0.44 | 0.04 | 0.536 |
| Prolactin, ng/mg | 4.78b | 6.43a | 0.35 | 0.008 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; SEM = standard error of the mean.
a,b Different letters indicate significant differences between the 2 data.
CON, sows were fed a base diet; SA, sows were fed a basal diet supplemented with 0.1% sodium acetate (n = 6).
Table 4.
Effect of dietary sodium acetate (SA) supplementation on immunoglobulin levels in colostrum and milk of sows.
| Item | Treatment group1 |
SEM | P-value | |
|---|---|---|---|---|
| CON | SA | |||
| Colostrum | ||||
| IgA, mg/mL | 3.61b | 5.25a | 0.46 | 0.032 |
| IgG, mg/mL | 20.50b | 27.10a | 1.60 | 0.034 |
| IgM, mg/mL | 1.27 | 1.37 | 0.16 | 0.718 |
| Day 21 of milk | ||||
| IgA, mg/mL | 0.32b | 0.42a | 0.03 | 0.038 |
| IgG, mg/mL | 1.05b | 1.70a | 0.16 | 0.020 |
| IgM, mg/mL | 0.12 | 0.13 | 0.01 | 0.582 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; SEM = standard error of the mean.
a,b Different letters indicate significant differences between the 2 data.
CON, sows were fed a base diet; SA, sows were fed a basal diet supplemented with 0.1% sodium acetate (n = 6).
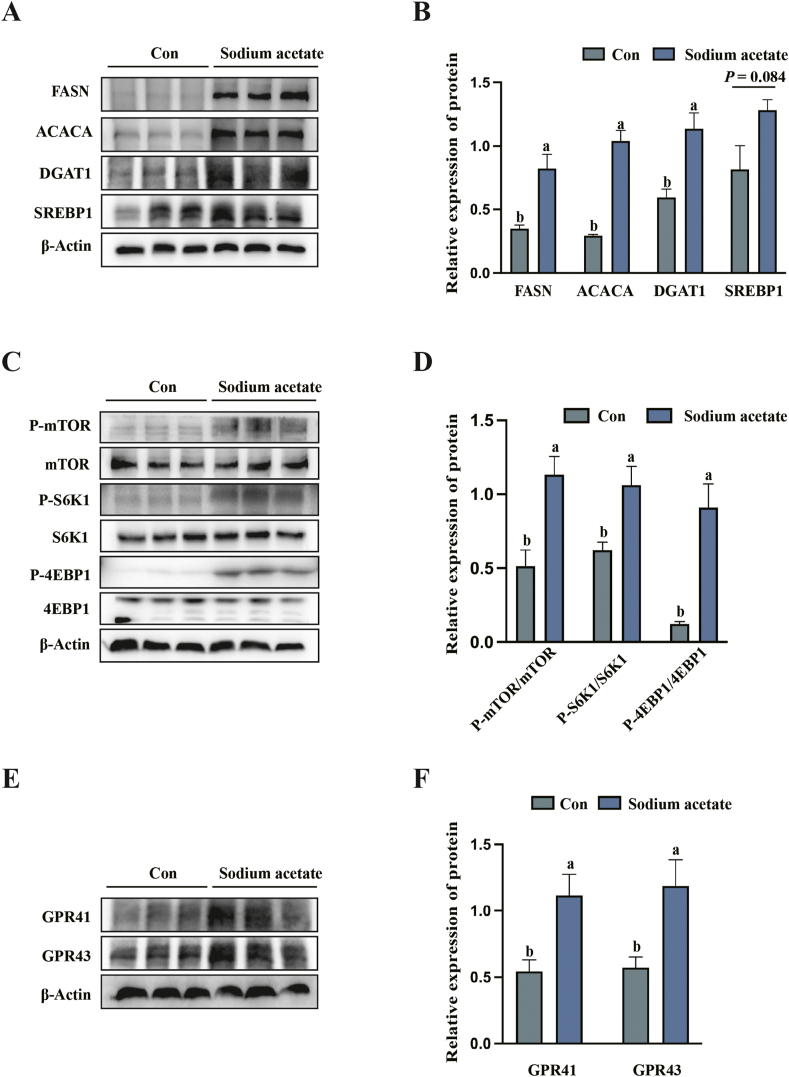
3.4. Sodium acetate regulated milk fat synthesis and activated mTORC1 pathway in milk-derived cells isolated from sow milk
As shown in Fig. 1A and B, SA supplementation significantly increased (P < 0.05) the expression of milk fat synthesis-related proteins (FASN, ACACA and DGAT1). Compared with the control group, the addition of SA significantly activates (P < 0.05) mTORC1 signaling pathway (as indicated by the increased level of P-mTOR/mTOR, P-S6K1/S6K1 and P-4EBP1/4EBP1) (Fig. 1C and D). Furthermore, SA increased the expression of SCFA receptors (GPR41 and GPR43) in milk-derived cells (Fig. 1E and F), which indicates SA might regulate through GPR41/GPR43-mTORC1.
Fig. 1.
Sodium acetate (SA) addition promotes the expression of milk fat synthesis-related proteins in milk-derived cells, accompanied by activation of GPR41/43-mTORC1 signaling pathway. (A and B) Relative protein expression abundance of milk fat synthesis-related genes in milk-derived cells (FASN = fatty acid synthase; ACACA = acetyl-CoA carboxylα; DGAT1 = diacylglycerol O-Acyltransferase 1; SREBP1 = sterol-regulatory element binding protein1). (C and D) Phosphorylation of mTORC1 pathway-related target proteins in milk derived cells (mTORC1 = mammalian target of rapamycin complex C1; S6K1 = ribosomal protein S6 kinase 1; 4EBP1 = 4E-binding protein 1). (E and F) Relative protein expression abundance of GPR41 and GPR43 receptors in milk-derived cells. All data with error bars are averages ± SEM (n = 3). In histograms, no letter or the same letter above the bar indicates no significant difference (P > 0.05), and different letters indicate significant difference (P < 0.05).
3.5. Maternal sodium acetate supplementation modulates offspring growth performance, gut health and intestinal microbiota
As indicated by Table 5, the addition of SA had no effect on litter size and survival rate at weaning (P > 0.05). However, there was a significant increase on piglets' BW at the second week of born (P = 0.013), weaning BW (P = 0.007) and ADG during lactation (P = 0.025).
Table 5.
Effect of dietary sodium acetate (SA) supplementation on lactation performance of sows and growth performance of piglets.
| Item | Treatment group1 |
SEM | P-value | |
|---|---|---|---|---|
| CON | SA | |||
| Litter size | ||||
| After cross-foster | ||||
| Day 7 | 11.95 | 11.70 | 0.15 | 0.249 |
| Day 14 | 11.84 | 11.52 | 0.14 | 0.126 |
| Day 21 | 11.66 | 11.29 | 0.16 | 0.070 |
| Pre-weaning survival, % | 98.7 | 97.7 | 0.004 | 0.235 |
| Litter weight | ||||
| After cross-foster | ||||
| Day 7 | 34.28 | 34.08 | 0.96 | 0.885 |
| Day 14 | 49.52 | 51.66 | 1.25 | 0.231 |
| Day 21 | 70.70 | 73.36 | 1.61 | 0.248 |
| Piglet BW 2, kg | ||||
| Day 7 | 2.87 | 2.91 | 0.07 | 0.713 |
| Day 14 | 4.16b | 4.56a | 0.11 | 0.013 |
| Day 21 | 6.04b | 6.62a | 0.15 | 0.007 |
| Piglet ADG 2, g/d | ||||
| Week 1 | 163.99 | 170.67 | 5.71 | 0.411 |
| Week 2 | 195.94b | 224.65a | 7.02 | 0.028 |
| Week 3 | 268.49b | 294.31a | 11.25 | 0.036 |
| Piglet ADG during lactation | 209.47b | 229.87a | 6.29 | 0.025 |
BW = body weight; ADG = average daily weight gain; SEM = standard error of the mean.
a,b Different letters indicate significant differences between the 2 data.
CON, sows were fed a base diet; SA, sows were fed a basal diet supplemented with 0.1% sodium acetate (n = 40).
The weight per litter was divided by the number of pigs to calculate piglet BW and then piglet ADG, n = 40.
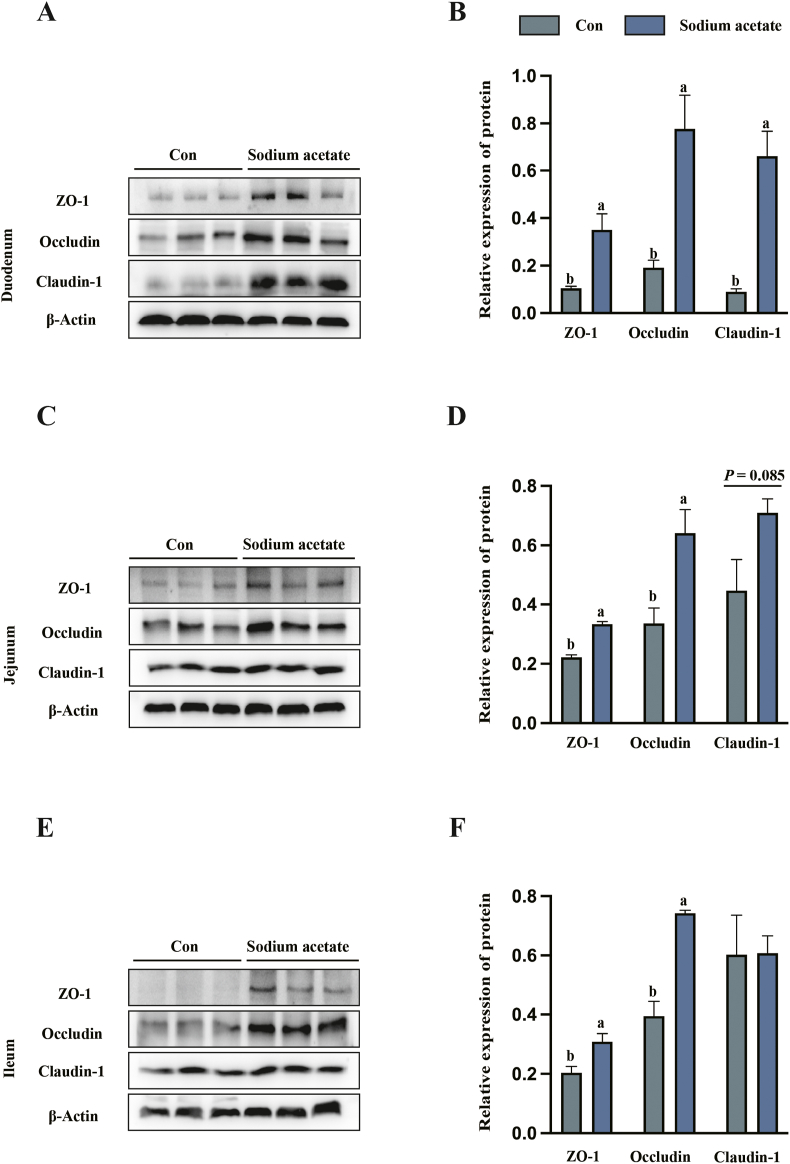
In duodenum (Fig. 2A and B), the VCR value was significantly increased in SA group compared with the control group (P < 0.05). In jejunum (Fig. 2C and D), VCR was significantly increased in SA group compared with the control group (P < 0.05). Furthermore, in the duodenum, SA addition significantly upregulated the protein expression of tight junctions such as ZO-1, occludin and cladin-1 (Fig. 3A and B). In the jejunum (Fig. 3C and D) and ileum (Fig. 3E and F), the addition of SA significantly increased the tight junction protein expression of ZO-1 and occludin (P < 0.05).
Fig. 2.
Effects of maternal sodium acetate (SA) addition supplementation on intestinal morphology of piglets. (A, C and E) H&E staining of duodenum, jejunum and ileum. (B, D and F) Villous height, crypt depth, and ratio of villous height to crypt depth of the intestine. All data with error bars are averages ± SEM (n = 6). In histograms, no letter or the same letter above the bar indicates no significant difference (P > 0.05), and different letters indicate significant difference (P < 0.05).
Fig. 3.
Maternal sodium acetate (SA) addition supplementation promotes the expression of tight junctions in piglet intestines. (A and B) The relative expression of tight junction proteins in duodenum. (C and D) The relative expressions of tight junction proteins in jejunum. (E and F) The relative expression of tight junction proteins in ileum. All data with error bars are averages ± SEM (n = 3). In histograms, no letter or the same letter above the bar indicates no significant difference (P > 0.05), and different letters indicate significant difference (P < 0.05).
A total of 1,273,031 valid sequences were generated from 10 ileal content samples (collected from piglets at d 21 of lactation) (2 treatments, n = 5), with an average of 127,303 sequences per sample and obtained 9,925 OTU (97% similarity) after removing noise sequences. The Venn diagram was used to analyze and compare the common and unique species of each group, so as to get a preliminary understanding of the species composition characteristics between the groups. As shown in Fig. 4A, there were 252 and 243 elements unique to the control and SA groups, respectively. The alpha diversity of the ileal microflora indicated by Shannon (Fig. 4B). Compared with the control group, there was no significant difference in alpha diversity in the SA group. As shown by the beta diversity results (Fig. 4C), there was a trend to separate between control group and SA group by PCoA analysis based on Bray–Curtis distance, which shows the similarity between samples to find the main sample difference distance in complex samples. At the phylum level (Fig. 4D), the 4 most common bacteria in the ileal contents of piglets are: Firmicutes (45.37% to 50.58%), Bacteroidetes (40.10% to 32.72%), Euryarchaeota (4.20% to 5.88%) and Proteobacteria (3.71% to 4.77%). At the order level (Fig. 4E), the 5 most common bacteria in the ileal contents of piglets are: Bacteroidales (39.76% to 32.64%), Clostridiales (33.61% to 36.95%), Selenomonadales (7.33% to 6.34%), Methanobacteriales (4.17% to 5.86%) and Lactobacillales (1.59% to 5.29%). Within the changes in Firmicutes, the most influential at the order level were Clostridiales and Lactobacillales. The proportion of genus level is shown in Fig. 4F, among them we screened 2 bacteria with the most obvious differences (P < 0.05) are Anaerovibrio and Subdoligranulum (Fig. 4G).
Fig. 4.
Maternal sodium acetate (SA) addition supplementation affects intestinal microbes of piglets, n = 5. (A) Venn graph of gut microbes in the 2 groups. (B) Alpha diversity index: Shannon. (C) Principal coordinate analysis based on Bray–Curtis distance. (D) Relative abundance at the phylum level. (E) Relative abundance at the order level. (F) Relative abundance at the genus level. (G) Differential bacteria in genus.
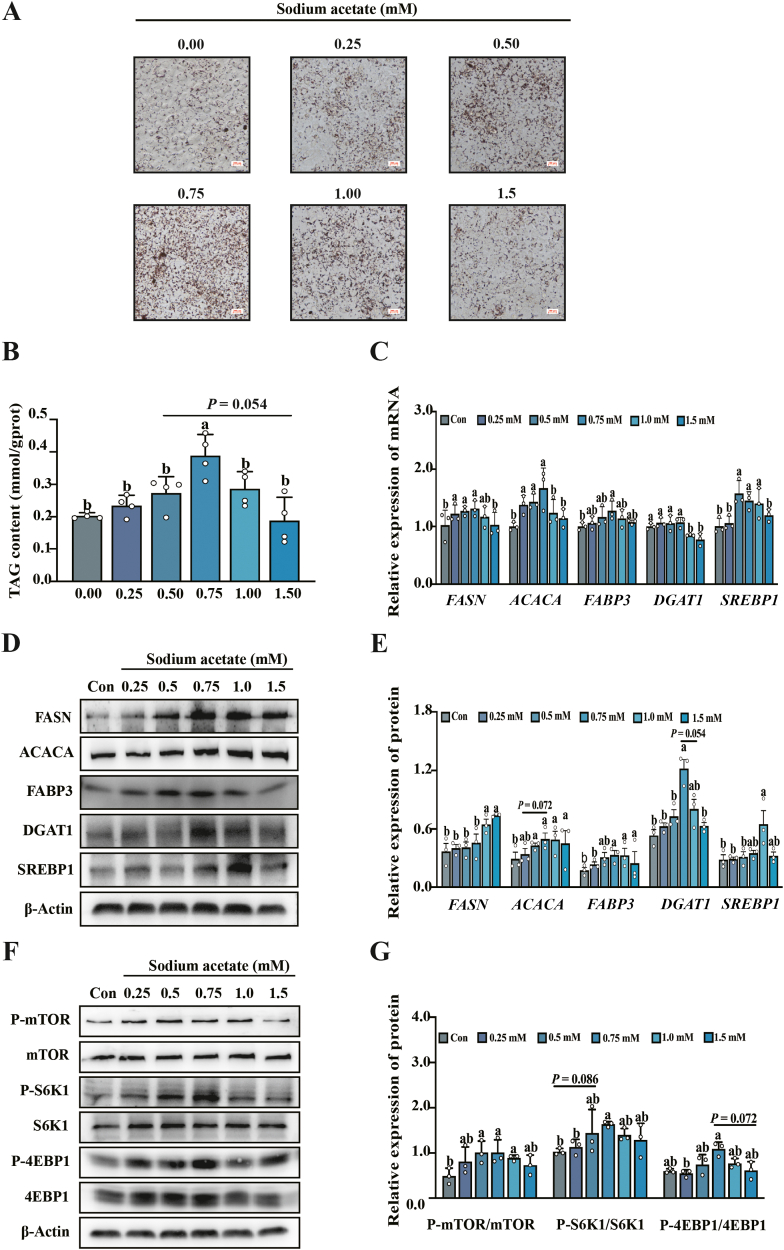
3.6. Sodium acetate regulated milk fat synthesis and activated mTORC1 signaling pathway in PMEC in vitro
To further verify the effect of SA on milk fat synthesis, PMEC were used as an in vitro model. PMEC were treated with different concentrations of SA (0, 0.25, 0.5, 0.75, 1.0 and 1.5 mM) for 24 h. The results indicated 0.75 mM SA efficiently promoted the synthesis of lipid droplets (P < 0.05; Fig. 5A) and TAG content (P < 0.05; Fig. 5B). Furthermore, 0.75 mM SA significantly upregulated the mRNA expression abundance of FASN, ACACA, FABP3 and SREBP1 genes in PMEC (P < 0.05; Fig. 5C). Consistently, as shown in Fig. 5D and E, 0.75 mM SA also upregulated the protein expression of ACACA, FABP3 and DGAT1 (P < 0.05). Phosphorylation level of mTOR, S6K1 and 4EBP1 was also upregulated under the treatment of 0.75 mM SA (P < 0.05; Fig. 5F and G), which indicates the activation of mTORC1 signaling pathway.
Fig. 5.
Sodium acetate (SA) addition promoted milk fat synthesis in porcine mammary epithelial cells (PMEC) and was accompanied by activation of mTORC1 signaling pathway. Treatment of PMEC with different concentrations (0, 0.25, 0.5, 0.75, 1.0 and 1.5 mM) of SA. (A) Oil red O staining images of PMEC. Scale bar is 100 μm. (B) The content of TAG in PMEC after treatment with different concentrations of SA (TAG = triacylglycerol). (C) mRNA abundance of milk fat synthesis-related genes in PMEC (FASN = fatty acid synthase; ACACA = acetyl-CoA carboxylα; FABP3 = fatty acid binding protein 3; DGAT1 = diacylglycerol O-Acyltransferase 1; SREBP1 = sterol-regulatory element binding protein1). (D and E) Relative protein expression abundance associated with milk fat synthesis in PMEC (FASN = fatty acid synthase; ACACA = acetyl-CoA carboxylα; FABP3 = fatty acid binding protein 3; DGAT1 = diacylglycerol O-acyltransferase 1; SREBP1 = sterol-regulatory element binding protein1). (F and G) Phosphorylation levels of mTORC1 signaling pathway-related target proteins in PMEC (mTORC1 = mammalian target of rapamycin complex C1; S6K1 = ribosomal protein S6 kinase 1; 4EBP1 = 4E-binding protein 1). All data with error bars are averages ± SEM (n = 3). In histograms, no letter or the same letter above the bar indicates no significant difference (P > 0.05), and different letters indicate significant difference (P < 0.05).
4. Discussion
Maternal milk yield and composition directly affect early growth and development of piglets. Previously, addition of SA has been reported to significantly increase milk yield in ruminants, such as in dairy cows (Aii et al., 1990). In this study, we first reported that maternal SA supplementation in monogastric animals (sows) also promotes maternal milk yield and milk fat.
Milk fat is an important nutrient component in milk, which provides a large amount of energy for early mammalian growth and development (Zhang et al., 2018). Sodium acetate is the main product of gastrointestinal bacterial fermentation of dietary fiber (Dalile et al., 2019). As a critical substrate for milk fat synthesis, SA efficiently promotes the synthesis of TAG in bovine mammary epithelial cells (Sheng et al., 2016) with upregulation of the expression abundance of de novo fatty acid synthesis genes (ACACA, FASN) and transcriptional regulation genes involved in lipid synthesis (SREBP1) (Zhao et al., 2021). Consistently, dietary supplementation of SA also increases plasma SA content and significantly increases milk fat content mainly by increasing the production of de novo fatty acids in the mammary gland in ruminants (Matamoros et al., 2021). Similarly, in our experiments, maternal SA supplementation significantly increased the milk fat content and the expression abundance of milk fat synthesis-related proteins (ACACA, FASN, DGAT) in milk-derived cells of sows. Although SA increased the milk fat synthesis both in ruminants and monogastric animals, the underlying mechanism might not be the same. It should be noted that the concentration of SCFA in the blood of sows is much lower than that in dairy cows (Urrutia and Harvatine, 2017). Therefore, in sows, the proportion of SCFA act as substrates that are directly involved in fat synthesis is relatively small. In monogastric animals, SA can be recognized by mammary epithelial cell-specific receptors as a signaling molecule, thereby regulating process of milk synthesis.
G protein coupled receptors are the largest family of cell membrane receptors, which involved in the regulation of multiple cellular and physiological functions (Wettschureck and Offermanns, 2005). G protein coupled receptors directly binds to G proteins in the membrane, which is composed of 3 subunits, including Gα (bound to GTP or GDP), Gβ and Gγ (Reimann et al., 2012; Tomita et al., 2014). Gα subunit is consisted of Gαi, Gαs, Gαq and Gα12/13. GPR41 and GPR43 have been identified as SCFA receptors in recent years. GPR43 is coupled to Gαi and Gαq, while GPR41 only coupled to Gαi (Flodgren et al., 2007; Tian et al., 2022b). It is worth noting that, the activity of mTORC1 is inhibited after the specific activation of Gαs by AKAP13, which implies that activation of Gαi can inversely increase the activity of mTORC1 (Zhang et al., 2021). The mTORC1 signaling pathway is not only involved in the regulation of protein translation, but also an important regulator of lipid anabolism (Soliman, 2013). Furthermore, mTORC1 signal pathway has been reported to regulate mammary gland development, protein translation and milk fat synthesis of dairy cows (Düvel et al., 2010; Wang et al., 2014). Importantly, in bovine mammary epithelial cells, sodium butyrate induced the increase of milk fat synthesis and activation of mTORC1 signaling was abolished when GPR41 was knocked out (Cheng et al., 2020). In this study, addition of SA significantly increased the expression of GPR41 and GPR43 proteins, accompanied by the activation of the mTORC1 signaling pathway. This evidence suggests that SA might promote milk fat synthesis in sows by activating the GPR41/43-mTORC1 pathway, which still requires further investigation.
Sows lactation performance is closely related to piglets growth performance (Hojgaard et al., 2020). In this study, maternal SA supplementation-induced increases of ADG and weaning BW in piglets during lactation are partially attributed to the increase in milk synthesis. Previously, sows milk production and milk IgA content have been reported to promote weaning BW, ADG and intestinal health of piglets (Rezaei et al., 2022; Shang et al., 2019). In addition, sufficient milk fat intake could prevent neonates from gastrointestinal disease (Brink and Lönnerdal, 2020; Koopman et al., 1984). Intestinal morphology directly reflects the development and health of gut (Yi et al., 2021). The intestinal barrier of piglets is mainly composed of mechanical barrier, immune barrier and biological barrier (Tian et al., 2022a). As the first physical barrier, tight junctions are the most important connections between cells, which are primarily composed of claudin 1, occludin, and ZO-1 (Suzuki, 2013). Tight junctions only allow soluble and small molecular substances to pass through, hindering the passage of macromolecular substances and microorganisms, thus ensuring homeostasis (Lee, 2015). Consistent with the growth performance of piglets, maternal SA supplementation increased the VCR values and promoted the expression abundance of tight junction proteins in small intestine of piglets.
Intestinal microorganisms play a crucial role to maintain the health and growth of piglets. Milk has been identified as a crucial factor contributing to the establishment of intestinal microbial community (Gresse et al., 2017). Previously, it has been demonstrated that milk fat content can increase the proportion of Firmicutes in the ileal microbes of piglets, which is consistent with the finding in this study (Thum et al., 2020). In our experiments, the increased abundance of Firmicutes in the piglet gut was mainly due to the increase in Clostridiales and Lactobacillales in order level. Clostridiales are negatively relative to inflammatory bowel disease (Baumgart et al., 2007), while Lactobacillales are widely considered as beneficial bacteria that contribute to the maintenance of normal intestinal function and piglet BW increase (Li et al., 2018). At the genus level, Anaerovibrio was significantly decreased while Subdoligranulum was significantly increased in the SA group. Anaerovibrio was associated with intestinal epithelial damage, tissue inflammation, and leaky gut (Rocafort et al., 2019). However, Subdoligranulum abundance was positively correlated with microbial abundance and negatively correlated with interleukin-6 level (Van Hul et al., 2020).
5. Conclusion
In conclusion, maternal supplementation of 0.1% SA from late gestation to lactation (D85 to L21) significantly increased sows' milk production, milk fat and immunoglobulins, which might attribute to the activation of GPR41/43-mTORC1 signaling pathway. An improvement in milk quality further promoted growth performance, gut health and the colonization of beneficial microbial flora of their piglets.
Author contributions
Yingao Qi, Shihai Zhang and Wutai Guan: designed the study. Yingao Qi, Tenghui Zheng, Yongxing Zhong and Siwang Yang: conducted research. Yingao Qi and Qianzi Zhang: analyzed the data. Yingao Qi and Baofeng Li: wrote the manuscript. Xiangfang Zeng and Fang Chen: critically reviewed the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This study was financially supported by the National Key R&D Program of China (2021YFD1300700), Guangdong Basic and Applied Basic Research Foundation (2021A1515010440 and 2023A1515012098), Science and Technology Program of Guangzhou (202102020056). We thank the members of our laboratory for their assistance during the animal experiment and sample collection.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.04.003.
Appendix. Supplementary data
The following is the Supplementary data to this article:
References
- Aii T., Kurihara M., Kume S., Tomita M., Hayasawa H. The effect of feeding calcium soap of fatty acids and sodium acetate on the physiological responses of dairy cows. Jpn J Zootech Sci. 1990;61:959–962. [Google Scholar]
- Baumgart M., Dogan B., Rishniw M., Weitzman G., Bosworth B., Yantiss R., Orsi R.H., Wiedmann M., Mcdonough P., Kim S.G., Berg D., Schukken Y., Scherl E., Simpson K.W. Culture independent analysis of ileal mucosa reveals a selective increase in invasive escherichia coli of novel phylogeny relative to depletion of clostridiales in crohn's disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- Brink L.R., Lönnerdal B. Milk fat globule membrane: the role of its various components in infant health and development. J Nutr Biochem. 2020;85 doi: 10.1016/j.jnutbio.2020.108465. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhang Y., Ge Y., Li W., Cao Y., Qu Y., Liu S., Guo Y., Fu S., Liu J. Sodium butyrate promotes milk fat synthesis in bovine mammary epithelial cells via gpr41 and its downstream signalling pathways. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118375. [DOI] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M.G., Mackeigan J.P., Finan P.M., Clish C.B., Murphy L.O., Manning B.D. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer C. Nutritional impact on mammary development in pigs: a review. J Anim Sci. 2018;96:3748–3756. doi: 10.1093/jas/sky243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera T., Zhou P., Nuntapaitoon M., Sørensen K.U., Krogh U., Bruun T.S., Purup S., Jørgensen H., Poulsen H.D., Theil P.K. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period1. J Anim Sci. 2019;97:231–245. doi: 10.1093/jas/sky395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgren E., Olde B., Meidute-Abaraviciene S., Winzell M.S., Ahrén B., Salehi A. Gpr40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem Biophys Res Commun. 2007;354:240–245. doi: 10.1016/j.bbrc.2006.12.193. [DOI] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van De Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Harrell R.J., Thomas M.J., Boyd R.D. Limitations of sow milk yield on baby pig growth. Proceedings. 1993:156–164. [Google Scholar]
- Hojgaard C.K., Bruun T.S., Theil P.K. Impact of milk and nutrient intake of piglets and sow milk composition on piglet growth and body composition at weaning. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa060. skaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.B., Pedersen L.J., Theil P.K., Yde C.C., Bach Knudsen K.E. Feeding motivation and plasma metabolites in pregnant sows fed diets rich in dietary fiber either once or twice daily. J Anim Sci. 2012;90:1910–1919. doi: 10.2527/jas.2010-3289. [DOI] [PubMed] [Google Scholar]
- Koopman J.S., Turkisk V.J., Monto A.S., Thompson F.E., Isaacson R.E. Milk fat and gastrointestinal illness. Am J Publ Health. 1984;74:1371–1373. doi: 10.2105/ajph.74.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Int Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhao F., Wei C., Liang M., Zhang N., Wang C., Li Q.-Z., Gao X.-J. Function of srebp1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int J Mol Sci. 2014;15:16998–17013. doi: 10.3390/ijms150916998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu X., Ma X., Geng S., Jiang X., Huang Q., Hu C., Han X. Intestinal microbiome-metabolome responses to essential oils in piglets. Front Microbiol. 2018;9:1988. doi: 10.3389/fmicb.2018.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Hu S., Bannai M., Wu G. L-arginine regulates protein turnover in porcine mammary epithelial cells to enhance milk protein synthesis. Amino Acids. 2018;50:621–628. doi: 10.1007/s00726-018-2541-7. [DOI] [PubMed] [Google Scholar]
- Matamoros C., Cai J., Patterson A.D., Harvatine K.J. Comparison of the effects of short-term feeding of sodium acetate and sodium bicarbonate on milk fat production. J Dairy Sci. 2021;104:7572–7582. doi: 10.3168/jds.2020-19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo R.D., Wu G., Bazer F.W., Park J.C., Shinzato I., Kim S.W. Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007;137:652–656. doi: 10.1093/jn/137.3.652. [DOI] [PubMed] [Google Scholar]
- Miao J., Adewole D., Liu S., Xi P., Yang C., Yin Y. Tryptophan supplementation increases reproduction performance, milk yield, and milk composition in lactating sows and growth performance of their piglets. J Agric Food Chem. 2019;67:5096–5104. doi: 10.1021/acs.jafc.9b00446. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Nutrient requirements of swine. 11th ed. The National Academy Press; Washington, DC, USA: 2012. [Google Scholar]
- Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. Short-chain fatty acids induce both effector and regulatory t cells by suppression of histone deacetylases and regulation of the mtor–s6k pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F., Tolhurst G., Gribble Fiona M. G-protein-coupled receptors in intestinal chemosensation. Cell Metabol. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Ren C., Wang Y., Lin X., Song H., Zhou Q., Xu W., Shi K., Chen J., Song J., Chen F., Zhang S., Guan W. A combination of formic acid and monolaurin attenuates enterotoxigenic escherichia coli induced intestinal inflammation in piglets by inhibiting the nf-κb/mapk pathways with modulation of gut microbiota. J Agric Food Chem. 2020;68:4155–4165. doi: 10.1021/acs.jafc.0c01414. [DOI] [PubMed] [Google Scholar]
- Rezaei R., Gabriel A.S., Wu G. Dietary supplementation with branched-chain amino acids enhances milk production by lactating sows and the growth of suckling piglets. J Anim Sci Biotechnol. 2022;13:65. doi: 10.1186/s40104-022-00718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocafort M., Noguera-Julian M., Rivera J., Pastor L., Guillén Y., Langhorst J., Parera M., Mandomando I., Carrillo J., Urrea V., Rodríguez C., Casadellà M., Calle M.L., Clotet B., Blanco J., Naniche D., Paredes R. Evolution of the gut microbiome following acute hiv-1 infection. Microbiome. 2019;7:73. doi: 10.1186/s40168-019-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q., Liu H., Liu S., He T., Piao X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets1. J Anim Sci. 2019;97:4922–4933. doi: 10.1093/jas/skz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Yan S.M., Qi L.Z., Zhao Y.L., Jin L., Guo X.Y. Effect of the ratios of acetate and β-hydroxybutyrate on the expression of milk fat- and protein-related genes in bovine mammary epithelial cells. Czech J Anim Sci. 2016;60:531–541. [Google Scholar]
- Soliman G.A. The role of mechanistic target of rapamycin (mtor) complexes signaling in the immune responses. Nutrients. 2013;5:2231–2257. doi: 10.3390/nu5062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil P.K., Flummer C., Hurley W.L., Kristensen N.B., Labouriau R.L., Sørensen M.T. Mechanistic model to predict colostrum intake based on deuterium oxide dilution technique data and impact of gestation and prefarrowing diets on piglet intake and sow yield of colostrum. J Anim Sci. 2014;92:5507–5519. doi: 10.2527/jas.2014-7841. [DOI] [PubMed] [Google Scholar]
- Thum C., Young W., Montoya C.A., Roy N.C., Mcnabb W.C. In vitro fermentation of digested milk fat globule membrane from ruminant milk modulates piglet ileal and caecal microbiota. Front Nutr. 2020;7:91. doi: 10.3389/fnut.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Li L., Tian Z., Zhao H., Chen F., Guan W., Zhang S. Glyceryl butyrate attenuates enterotoxigenic escherichia coli-induced intestinal inflammation in piglets by inhibiting the nf-κb/mapk pathways and modulating the gut microbiota. Food Funct. 2022;13:6282–6292. doi: 10.1039/d2fo01056a. [DOI] [PubMed] [Google Scholar]
- Tian M., Wu Z., Heng J., Chen F., Guan W., Zhang S. Novel advances in understanding fatty acid-binding g protein-coupled receptors and their roles in controlling energy balance. Nutr Rev. 2022;80:187–199. doi: 10.1093/nutrit/nuab021. [DOI] [PubMed] [Google Scholar]
- Tomita T., Hosoda K., Fujikura J., Inagaki N., Nakao K. The g-protein-coupled long-chain fatty acid receptor gpr40 and glucose metabolism. Front Endocrinol. 2014;5:152. doi: 10.3389/fendo.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigger A.-J., Engelbrecht L.K., Bach K., Schultz-Pernice I., Pensa S., Stenning J., Petricca S., Scheel C.H., Khaled W.T. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. Nat Commun. 2022;13:562. doi: 10.1038/s41467-021-27895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia N.L., Harvatine K.J. Acetate dose-dependently stimulates milk fat synthesis in lactating dairy cows. J Nutr. 2017;147:763–769. doi: 10.3945/jn.116.245001. [DOI] [PubMed] [Google Scholar]
- Van Hul M., Le Roy T., Prifti E., Dao M.C., Paquot A., Zucker J.-D., Delzenne N.M., Muccioli G.G., Clément K., Cani P.D. From correlation to causality: the case of subdoligranulum. Gut Microb. 2020;12 doi: 10.1080/19490976.2020.1849998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Lin Y., Bian Y., Liu L., Shao L., Lin L., Qu B., Zhao F., Gao X., Li Q. Leucyl-trna synthetase regulates lactation and cell proliferation via mtor signaling in dairy cow mammary epithelial cells. Int J Mol Sci. 2014;15:5952–5969. doi: 10.3390/ijms15045952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N., Offermanns S. Mammalian g proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wu Z., Heng J., Tian M., Song H., Chen F., Guan W., Zhang S. Amino acid transportation, sensing and signal transduction in the mammary gland: Key molecular signalling pathways in the regulation of milk synthesis. Nutr Res Rev. 2020;33:287–297. doi: 10.1017/S0954422420000074. [DOI] [PubMed] [Google Scholar]
- Wu Z., Li Q., Yang S., Zheng T., Shao J., Guan W., Chen F., Zhang S. Energy deprivation-induced ampk activation inhibits milk synthesis by targeting prlr and pgc-1α. Cell Commun Signal. 2022;20:25. doi: 10.1186/s12964-022-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q., Liu J., Zhang Y., Qiao H., Chen F., Zhang S., Guan W. Anethole attenuates enterotoxigenic escherichia coli-induced intestinal barrier disruption and intestinal inflammation via modification of tlr signaling and intestinal microbiota. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanello G., Meurens F., Serreau D., Chevaleyre C., Melo S., Berri M., D’inca R., Auclair E., Salmon H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet Immunol Immunopathol. 2013;152:20–27. doi: 10.1016/j.vetimm.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen F., Zhang Y., Lv Y., Heng J., Min T., Li L., Guan W. Recent progress of porcine milk components and mammary gland function. J Anim Sci Biotechnol. 2018;9:77. doi: 10.1186/s40104-018-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang H., Melick C., Jeong M.-H., Curukovic A., Tiwary S., Lama-Sherpa T., Meng D., Servage K., James N., Jewell J. Akap13 couples gpcr signaling to mtorc1 inhibition. PLoS Genet. 2021;17:10. doi: 10.1371/journal.pgen.1009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Guo X., Yan S., Shi B., Sheng R. Acetate regulates milk fat synthesis through the mammalian target of rapamycin/eukaryotic initiation factor 4e signaling pathway in bovine mammary epithelial cells. J Dairy Sci. 2021;104:337–345. doi: 10.3168/jds.2020-18246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.