Abstract
Repairing significant bone defects remains a critical challenge, raising the clinical demand to design novel bone biomaterials that incorporate osteogenic and angiogenic properties to support the regeneration of vascularized bone. Bioactive glass scaffolds can stimulate angiogenesis and osteogenesis. In addition, natural or synthetic polymers exhibit structural similarity with extracellular matrix (ECM) components and have superior biocompatibility and biodegradability. Thus, there is a need to prepare composite scaffolds of hydrogels for vascularized bone, which incorporate to improve the mechanical properties and bioactivity of natural polymers. In addition, those composites' 3-dimensional (3D) form offer regenerative benefits such as direct doping of the scaffold with ions. This review presents a comprehensive discussion of composite scaffolds incorporated with BaG, focusing on their effects on osteo-inductivity and angiogenic properties. Moreover, the adaptation of the ion-doped hydrogel composite scaffold into a 3D scaffold for the generation of vascularized bone tissue is exposed. Finally, we highlight the challenges and future of manufacturing such biomaterials.
Keywords: Bioactive glasses, Ion-doped hydrogel scaffold, 3D scaffold, Vascularized bone
1. Introduction
Treatment of significant bone defects resulting from trauma, infections, tumors, congenital malformations, or skeletal diseases represents a significant challenge for clinicians worldwide [1]. All bone grafts, either as autografts or allografts, are associated with common limitations, which mostly include limited availability of donors, morbidity at the donor site, disease transmission, and a high incidence of non-union healing [2,3].
The bone matrix is comprised of organic (mainly collagen) and inorganic (mainly hydroxyapatite) components. Different natural and synthetic materials have been developed to prepare an ideal scaffold for bone repair; however, these materials have their shortcoming in inducing bio-mineralization, inadequate mechanical properties, and flexibility [2,4]. Moreover, various natural and synthetic materials, such as metals [5], ceramics, and hydrogel, have been applied in bone tissue engineering [6]. However, the lack of rapid vascularization after implantation remains a critical hurdle in bone tissue engineering. Vascularization is needed to supply the implanted construct and osteoblasts with oxygen and nutrients and remove waste products [7]. In the case of significant bone defects, designing bone biomimetic materials, which incorporates both inorganic and organic components to simulate the typical structure and mechanical support offered by natural bone matrix, remains a critical challenge for complete repair [8].
Recent developments in bone tissue engineering have attempted to tackle this problem by designing materials that benefit bone components and structure [7]. In this context, efforts have been focused on incorporating mineral components such as bioactive glasses [9] into organic scaffolds [10,11]. In the 1970s, Hench and coworkers fabricated the first bioactive glasses known as 45S5 Bioglass® (BG). The latter was composed of four components 45 SiO2–24.5 CaO–24.5 Na2O–6 P2O5 (weight %) [10]. Bioactive glasses that are being utilized for bone tissue engineering belong to a group of surface reactive amorphous materials identified with good biocompatibility, biodegradation properties, and the property of stimulating the osteogenic differentiation of stem cells [11]. Modification of BaG could change the biological and chemical structure. In bone regeneration, BaGs can bind firmly to bone tissue, consequently inducing angiogenesis and osteogenesis. However, due to the fast dissolution behavior and fragility of BaG, they are applied for coatings of implants and composite polymers [12,13]. Relatedly, BG-based scaffolds were shown to have angiogenic properties that promote neo-vascularization and vascular ingrowth within the bone tissue [[13], [14], [15]], fulfilling the osteogenic and angiogenic properties required for bone repair [14].
Indeed, the result of adding inorganic ions dissolved from S53P4 bioactive glass into cell. In addition, Silicate or phosphate-based BGs, are inorganic bioactive biomaterials that have been used as scaffolds for bone tissue engineering [15]. These glasses have a high level of bioactivity, and biological fixation enables bonding with soft and hard tissues. BGs are composed of elements such as silica (Si), calcium (Ca), phosphorus (P), and sodium (Na) that are naturally present in the body. The surface of a BaGs implant is mineralized to a carbonated hydroxyapatite layer when subjected to body fluids, and is thus capable of inducing differentiation of osteoblasts and deposition of new bone [16]. Different metal ions such as strontium (Sr) [17], zinc (Zn) [18], fluorine (F) [19], magnesium [20,21], tantalum (Ta) [22] silver (Ag), and boron (B) [20] have been doped within BaG to induce specific biological responses by changing intracellular ionic concentrations [9]. Recently, BaG scaffold doped with potentially therapeutic elements such strontium and silicon was shown to enhance osteogenesis and induce angiogenesis [23].
Adding inorganic ions dissolved from S53P4 bioactive glass into cell culture media promoted both endothelial and osteogenic processes, supporting the simultaneous formation of vascular-like structures and mineralization. Furthermore, it has been indicated that the angiogenic effects of BaG, such an increased secretion of vascular endothelial growth factor (VEGF) induced endothelial cells proliferation and tubules formation [24]. Moreover, a model of 3D co-culture in the same medium indicated the formation of functional vessel-like structures characterized by the presence of an internal lumen [[25], [26], [27]], showing that BaGs can induce vascularization and promote osteogenesis in co-culture systems [26]. Besides its osteogenic and angiogenic abilities in co-culture systems, combination of BaG with bioactive nanoparticles (NPs), or its incorporation with synthetic polymers was demonstrated to enhance the mechanical and biological properties of BaG composite scaffolds [2,28]. For example, when BaG and NPs are combined for bone repair, they can induce osteogenesis by releasing active Si and Ca ions [29]. It was found that including ions such as Si, Ca, and Cu into BaG has improved osteogenic and angiogenic properties of BaG [30]. This review aims to highlight the recent progress in research on the use of BaGs to stimulate vascularization and osteogenic properties of bone-engineered constructs, particularly the application of BaGs in 3D co-culture hydrogel-based scaffolds for the induction of angiogenesis and osteogenesis.
2. Angiogenesis and osteogenic properties of bioactive glass
Mesoporous BaG (MBG) has attracted enormous attention for bone regeneration. In comparison to traditional Bioglass, synthesized MBG incorporated with CaO–P2O5–SiO2 system, has been suggested to exhibit superior osteogenic bioactivity, degradation and enhanced drug loading [31]. However, the angiogenesis properties of MBGs seem to be non prominent, and therefore require combinition of additional angiogenesis enhancers. It can be seen from various studies that incorporation of ions, including Co and Rb greatly promote angiogenesis and osteogenesis capacities [32,33]. He et al. investigated the effect of Rb doped MBG on proliferation, angiogenesis, and osteogenesis human bone marrow mesenchymal stem cells (hBMSCs). Superior ostogenicpropoerties of this MBG were characterized by analyzing genes of bone formation (alkaline phosphatase (ALP), type 1 (COL-1), angiogenesis (VEGF hypoxia-inducible factor (HIF-1α), and Wnt/β-catenin related-signaling pathway [32]. This study indicated that incorporating Rb could increase the expression of ALP and HIF-1α, the secretion of VEGF, and COL-1 in hBMSCs through the Wnt/β-catenin signal pathway. In this context, Rb-MBG was probably restrain GSK3β activity, thus activating Wnt pathway [32]. Furthermore, the application of ions such as Cu2+has been proposed as a potential strategy to enhance neovascularization, especially stimulating the proliferation of endothelial cells, and VEGF expression by stabilizing HIF-1a expression. Hypoxic condition is critical in cell recruitment, cell differentiation, and blood vessel formation, thus linking osteogenesis to angiogenesis.
Moreover, Hypoxia can be artificially mimicked by applying Cu2+, which leads to stabilized HIF-1a expression [34]. In a study conducted by Wu et al. the addition of Cu2+ into MBG scaffolds significantly promoted hypoxia-like tissue conditions and consequently led to enhanced cell recruitment , cell differentiation, and blood vessel formation, thus coupling of angiogenesis and osteogenesis. This study indicates that Cu-MBG scaffolds could induce HIF1 alfa [35] and VEGF expression hBMSCs [33]. Moreover, boron-containing MBG (B-MBG) is an excellent scaffold with significantly enhanced osteogenic properties. Boron can facilitate the release of osteoinductive growth factors and cytokines. Chen et al. indicated that a composite scaffold composed of novel p (N-isopropylacrylamide-co-butyl methylacrylate) (PIB) nanogels combined with p (N-isopropylacrylamide-co-butyl methylacrylate) (PIB) nanogels, was able to treat defects of irregular shapes as an injectable, thermoresponsive hydrogel that undergoes rapid thermal gelation once body temperature is reached [36].
3. Hydrogel -based scaffold incorporated with BaG
Since bone consists of inorganic and organic components, e.g., calcium phosphate and collagen, bioactive inorganic materials and naturally-derived polymers are considered a suitable choice as the scaffolding matrices for bone tissue engineering [36]. Natural polymers have attracted much attention in bone tissue engineering due to their specific properties, such as biocompatibility, biodegradability, and non-toxicity [2]. However, they exhibit low mechanical characteristics and limited bio-mineralization, which are critically needed for bone repair [4]. In this regard, fabrication of multifunctional composite scaffolds composed of hydrogel combined with inorganic BaG and encapsulated with cells and growth factors could enhance bone regeneration by promoting osteogenesis and angiogenesis. In addition, BG dispersed in natural polymer scaffold were shown to improve mechanical properties, and osteogenic induction, and stimulate the production of growth factors, proliferation, and angiogenesis in bone tissue repair [37]., Previous studies have shown the multifunctional properties of scaffolds consisting of BG nanoparticles and alginate cross-linked with various cations in bone tissue engineering [38,39]. One study has shown that BG incorporated in an oxidized alginate-gelatin hydrogel (ADA-GEL) had a great impact on the covalent crosslinking between ADA and GEL that was displayed by the higher degree of crosslinking and shorter gelation time of the fabricated hydrogels and further increasing mechanical properties of the resulting scaffold [40].
In addition to the above mentioned benefits of hydrogel/BG composites, an injectable hydrogel has gel properties at physiological temperatures without requiring any chemical and environmental treatment. This allows the gel to fully repaired critical-size bone defects in vivo [41,42]. Among injectable hydrogels, chitosan (CH)/glycerophosphate [43] with thermal sensitivity has been used in combination with other materials such as collagen, gelatin, silk fibroin, and BaG to obtain better performance of critical-size injured bone tissues [29,[44], [45], [46]]. For example, Wu et al. fabricated an injectable nanocomposite hydrogel of (CH/silk fibroin (SF)/GP (CH/SF/GP gel) incorporated with Cu-BG. This nanocomposite hydrogel exhibited control release of Si, Ca, and Cu ions when serving as a cell-free injectable scaffold at a critical-size calvarias bone defect in a rat model, supporting the neovascularization and full repair of the defective area.
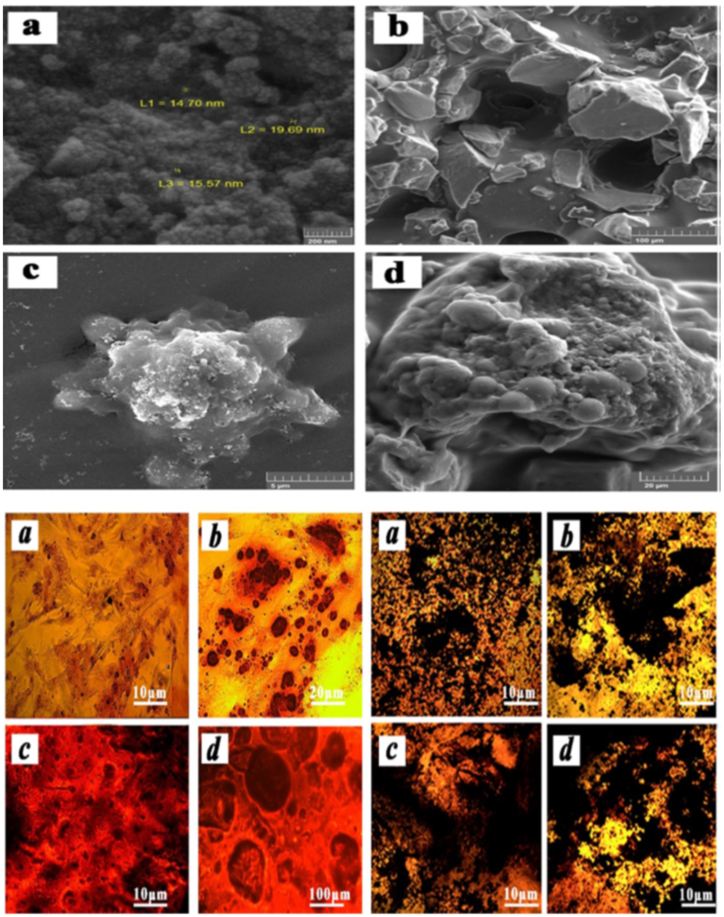
Moreover, in vitro experiments of cell seeded-nanocomposite hydrogel scaffold showed apatite formation and upregulation of expression of angiogenic and osteogenic genes. In addition, Cu-BG/CH/SF/GP gel exhibited complete restoration of the bone defect with the formation of vascularized bone tissue and mineralized collagen deposition during 8 weeks [14]. A bioactive glass nanowhisker (BGnW) composed of 58% SiO2, 33% CaO, and 9% P2O5 (based on mol%) displayed excellent binding to hard and soft tissues. Thus it could be combined with a hydrogel-based scaffold to increase osteogenic differentiation. Azizipour et al. incorporated BGnW into 3D porous hydrogel nanocomposite scaffold, which consisted of gelatin-glutaraldehyde-collagen (Gel-Glu-Co). The result of the study showed the hydrophilic properties of Gel-Glu-Col/BGnW hydrogel scaffold, which increase the viability and proliferation of hMSCs seeded on the scaffold. In addition, MSCs cultured on the scaffold showed osteoblastic differentiation that upregulated the expression of COL-1, Runx-2, and ALP and the protein expression of osteocalcin and osteopontin (Fig. 1 a-d) [47].
Fig. 1.
Top panel show Scanning electron microscopy images. a) BGnW, b) Gel-Glu-Col/BGnW, c) Gel-Glu-Col (pure), d) Gel-Glu-Col/BGnW. Bottom panel show Alizarin red staining to confirm the osteogenic differentiation of hMSCs. Cells were cultured in presence of osteogenic induction media for 14 days: TCPS (a), BGnW100 μg/ml (b), Gel-Glu-Col (c), Gel-Glu-Col/BGnW1% (d). b) von Kossa staining to confirm the osteogenic differentiation of human MSCs during 14 days culturing in presence of osteogenic induction media: TCPS (a), BGnW100 μg/ml (b), Gel-Glu-Col (c), Gel-Glu-Col/BGnW1% (d) [47]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1. Proteins
3.1.1. Gelatin-BGs
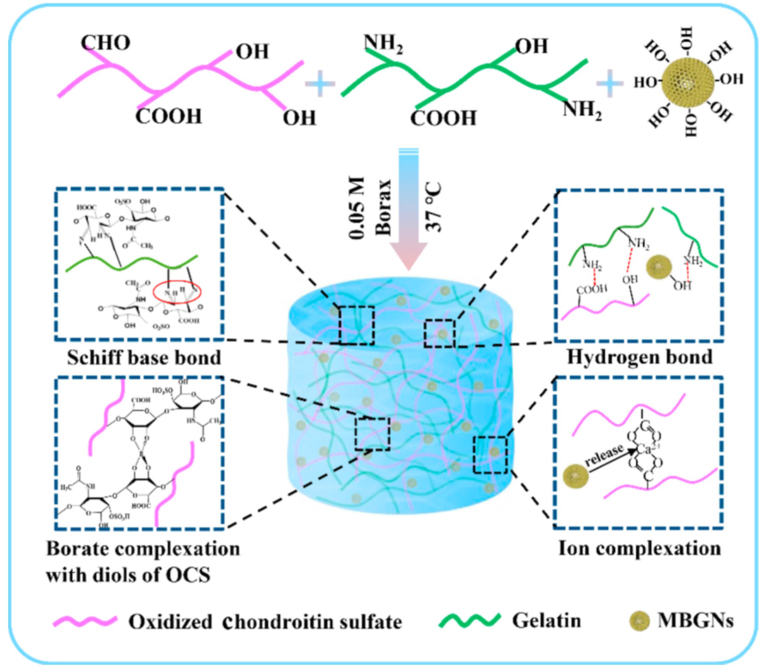
Gelatin, a partial collagen derivative, has been used as a nanocomposite scaffold in combination with BaGs. Embedding BG within gelatin increased its bioactivity and mechanical properties, facilitating their application for bone tissue engineering [48,49]. Moreover, gelatin promotes cell attachment, improving tissue regeneration in vivo. Zare jalize et al. fabricated Sr-delivering BGs in a SiO2–CaO–SrO–P2O5 structure, in combination with gelatin as a composite scaffold. This composite scaffold exhibited enhanced compressive strength and elastic modulus. In addition, this strontium-enriched BG improved neovascularization and enhanced osteoblast cell viability and differentiation [9]. Zhou et al. synthesized self-cross-linking hybrid gelatin/oxidized chondroitin sulfate (OCS) hydrogels that incorporated MBG nanoparticles (MBGNs) (Fig. 2). The addition of MBGNs enhanced crosslinking and the gelation process.
Fig. 2.
Schematic depicting the mechanisms involved in the gelation of hybrid Gel-OCS/MBGN hydrogels [50].
Moreover, the storage modulus and compressive strength increased after including MBGNs. Further analyzing the biological activity of Gel-OCS/MBGN hydrogels revealed osteogenic differentiation of bone marrow mesenchymal stem cell (BMSCs) in vitro as well as effective bone regeneration in vivo compared with Gel-CS hydrogels without MBGNs (Fig. 3 a-d) [50]. Apart from that, polymer coating may reduce the bioactivity and osteogenesis activity of the scaffold. To overcome this issue, researcher incorporated BaGs in gelatin-coated scaffolds. In this context, Zheng e. al; prepared a gelatin-coated scaffold with the addition of Cu-containing BaG nanoparticles (Cu-BGN: 95SiO2-2.5CaO-2.5CuO, in mol %), as bioactive fillers. The Cu-BGN/gelatin nanocomposite-coated BGs revealed high bioactivity, appropriate mechanical properties, and osteogenic potential. In addition, the results showed that the cells could attach to the BG scaffolds and the incorporation of Cu-BGN in the coating did not significantly affect cell attachment (Fig. 3a–c) [51].
Fig. 3.
Osteoblast-related gene and protein expressions of MSCs cultured on the hydrogel surfaces on day 14. (a) RT-PCR analysis of osteogenic-related markers such as osteopontin (OPN), Osteocalcin (OCN), RunX2, and COL-1. (b) immunofluorescent images of osteogenic-associated proteins RunX2 and OPN in different groups. RunX2 and OPN are marked by red fluorescence, and the cell nuclei were stained blue by the Hoechst. (c) Quantitative analysis of RunX-2 and OPN fluorescence intensity. (d) Protein expression of RunX2 in BMSCs and GAPDH was used as a reference. [50]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Polysaccharides
3.2.1. Alginate-BGs
Alginate is a natural polysaccharide polymer widely used in foods, industry, and tissue engineering materials. It can be cross-linked in the presence of certain divalent cations, e.g., Ca2+, Sr2+, and Ba2+, leading to the formation of a hydrogel [52]. Alginate with Ca2+ crosslinks has been used as a polymer matrix to compensate for the lack of Ca ions in Zn- and Mg-doped bioactive glasses. Although alginate is used in bone tissue engineering, it has the disadvantages of inadequate mechanical properties and the lack of bio-mineralization and flexibility, which are required for bone regeneration [3]. In this regard, Zamani et al. fabricated an alginate scaffold incorporated with BGs composed of Zn and Mg ions. This scaffold exhibited antibacterial effects, enhanced the mechanical properties and the bioactivity of the Alginate/BG composite. In addition, the antibacterial activity was related to the released Zn and Mg ions, restricting the growth of both S. aureus and E. coli [2].
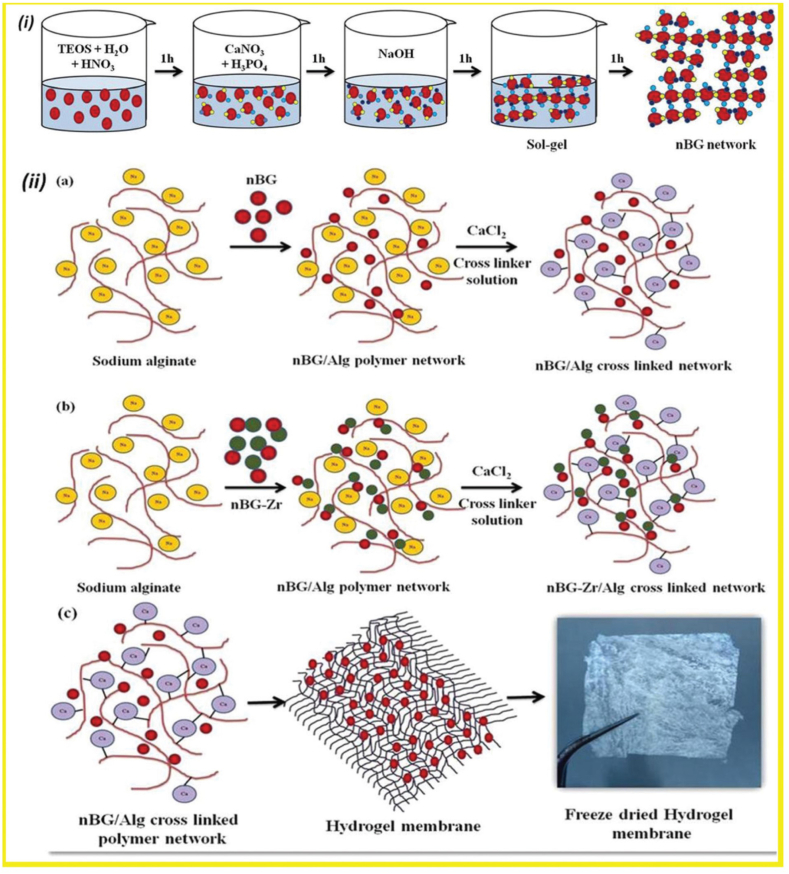
Alginate lacks a cell-binding ligand, attributing it with inferior cell adhesion properties. Hence, combination of alginate and a hydrogel improved vascular cell adhesion and proliferation in vitro [53]. In addition, alginate has an intrinsic non-degradable nature in mammalian tissues, because of the lack of alginase enzyme excreted from the body. The lack of mammalian enzymes that degrade alginate has its shortcoming. In this context, Alginate/gelatin scaffolds combined with BG showed immediate release at ∼1 h by the process in which oxidation of alginate generates reactive aldehyde groups in the backbone of alginate, allowing in vivo degradation of the hydrogel by making covalent bonds with ε-amino groups of lysine or hydroxylysine of gelatin [54,55]. A study conducted by Rottensteiner-Brandl et al. showed that the combination of gelatin and oxidized alginate (alginate dialdehyde, ADA) with BG had beneficial effects on cell survival and angiogenesis of bone marrow-derived MSCs encapsulated within the composite hydrogel. Further, in vivo implantation of the composite hydrogel revealed the recruitment of endothelial cells and a consequent increase in angiogenesis [54]. Bioglass/Alg composites are generated using sol-gel and freeze-drying, 3D printing [56], and surfactant foaming [57]. The addition of Zirconia (Zr2+) as one of the strongest nanoparticles in hard and soft tissue enhanced biocompatibility. Ramya et al. fabricated a freeze-dried nanosheet of Bioglass and alginate (45S5 Bioglass®)/Alg composite doped with Zr. The inclusion of Zr in the hybrid hydrogel promoted the growth rate of spheroid when scaffold was co-cultured with HDF and KB-3-1 cell lines by increasing surface roughness and changing porosity. This study concluded that Alg-BG/Alg-nBG-Zr could be used as hemostats, soft and hard tissue grafts, and composite scaffold for organotyping [58].
3.2.2. Dextran-BG
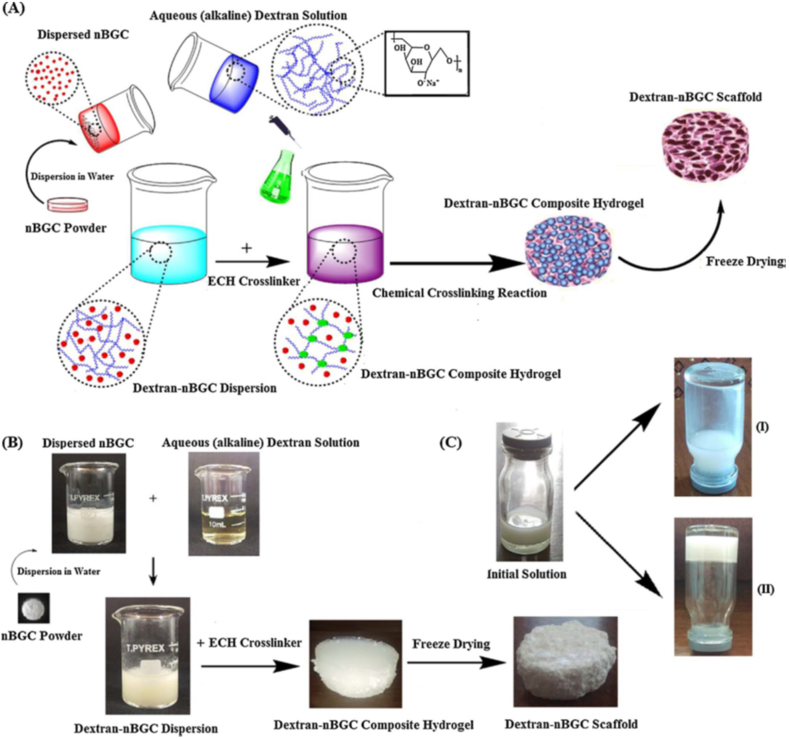
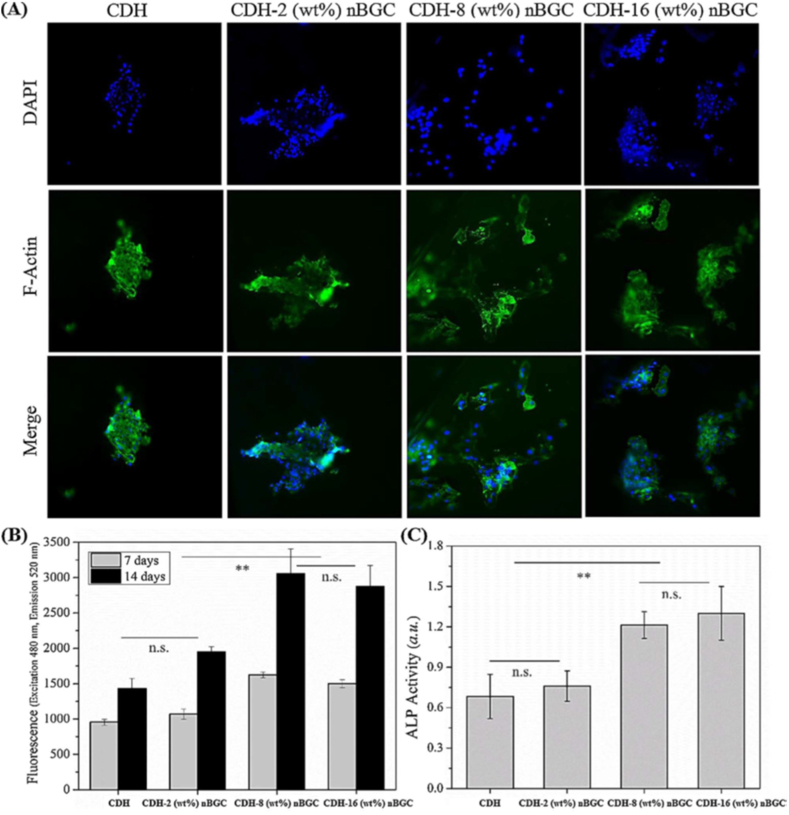
Dextran, a hydrophilic carbohydrate biopolymer showed good degradation properties in certain physical environments without any effect on the cell viability. Cells proliferated in clumps on dextran rather than spreading, thus inorganic materials such as hydroxyapatite has been incorporated with dextran to increase the bioactivity and mechanical properties [59]. Bioactive glass ceramics (BGC) containing SiO2–CaO–P2O5 networks have drawn much attention among inorganic materials due in part to their biocompatibility, bioactivity and osteoconductive properties, which bonds to both hard and soft tissues through the formation of surface hydroxy carbonate apatite layer. Nikpour et al. synthesized Cross-linked dextran hydrogels (CDH)- bioactive glass ceramic nanoparticles (nBGC) for bone tissue engineering (Fig. 4 a-c). The concentration of nBGC nanoparticles affects their distribution in the composite scaffold in which nBGC at low contents (2 wt%) is dispersed homogenously within the Dex matrix. However, nBGC nanoparticles at higher concentrations revealed agglomeration and increased water uptake. The composite scaffold supported the growth and improved ALP [60] activity of human osteoblasts (HOBs) at a concentration up to 16 (wt%) over two weeks (Fig. 5 a-c) [61].
Fig. 4.
Schematic illustration of synthesized composite scaffolds (A), fabrication process of CDH-nBGC composite scaffolds (B) CDH-nBGC composite hydrogels before (I) and after [62] gelation [61].
Fig. 5.
(A) Immunofluorescence images of seeded HOB cells on nanocomposite scaffolds. (B) the PrestoBlue viability of HOB cells on composite scaffold over two weeks and (C) Alkaline phosphatase (ALP) of HOB cells at day 14 of culture [60].
3.3. Biodegradable polymers
3.3.1. Poly D, l-lactide (PDLLA) doped-BGs
Biodegradable thermoplastic polymers, especially poly D, l-lactide (PDLLA) has been used for bone regeneration. Bejarano et al. fabricated a biodegradable PDLLA scaffold incorporated with sol-gel BG of chemical composition 60 SiO2; 25 CaO; 11 Na2O; and 4 P2O5 (mol %) doped with 1 CuO or ZnO (1 mol %). This study indicated that PDLLA scaffolds with Cu-doped BG increased angiogenic potential confirmed by VEGF secretion, while scaffolds with Zn-doped BG showed the higher potential of osteogenic properties confirmed by enhancing ALP [60] expression. In addition, scaffolds prepared with co-dopping of both ions revealed enhanced osteogenic and angiogenic properties, and antibacterial activity against methicillin-resistant S. aureus bacteria [63]. In another study, Meretoja et al. fabricated e-caprolactone/D, l-lactide-based scaffolds in combination with BG filler (70/30 caprolactone/lactide ratio and related composites with <45 μm BaG filler size). When implanted in rats, the scaffold enhanced osteogenic response and in-growth vascularization within the macroporous scaffold [64].
3.3.2. β-tricalcium phosphate (β-TCP)-doped BGs
Synthetic β-tricalcium phosphate (β-TCP; commercially available as Vitoss), which have been used as the most common bone substitutes, are limited by their inadequate stimulation of angiogenesis and osteogenic differentiation and insufficient filling of the bone defect due to imbalance between resorption and osseous regeneration [65,66]. Studies have shown that the fabrication of composite scaffold of β-TCPs and BGs nanoparticles was able to overcome these obstacles and improve the properties of β-TCP scaffolds; for example, by enhancing osseointegration, osteogenic differentiation in vitro, bone formation within implants in vivo. These composite scaffolds were also able to enhance the mechanical characteristics of nanoparticles of β-TCPs-incorporated BG. In this context, the BG nanoparticles in the Vitoss-based scaffold promoted osteogenic differentiation of MSCs [67]. Westhauser et al. described a stimulatory effect of 45S5-BG particles in Vitoss BA on vascularization. Furthermore, Tartrate-Resistant Acid Phosphatase-Positive (TRAP+) cells in Vitoss BA scaffolds were responsible for the maturation of the osteoid [68].
4. Bioactive glass inorganic ions in Co-culture system
Three-dimensional (3D) models are superior to 2D models as they have been demonstrated to be vital to simulate the native tissue environment [69]. 3D cell cultures incorporate the additional spatial dimension to simulate the tissue microenvironment, enhancing cell-cell andcell–matrix interactions [70]. BGs are stable in harsh condition involved in scaffold preparation, do not show any toxicity effects upon application compared to the application of solid growth factors, and the application of inorganic ions is more cost-effective than the addition of limited number of growth factors [71]. Alginate scaffolds have been commonly used as a 3D matrix in biomedicine, drug discovery, and tissue engineering studies [72]. Recently, Bargavi et al. have used the sol-gel process to fabricate a nano-membrane of BG (45S5 Bioglass®)/Alginate composite, and zirconium (Zr) ions were introduced into BG in the composite scaffold. This 3D scaffold exhibited enhanced bioactivity and cell adhesion efficiency (Fig. 6). In vitro 3D co-culture of HDF (human dermal fibroblast cell lines) and KB-3-1 cell line (human epithelial cells) cultures revealed that 3D nBG/Alg and nBG-Zr/Alg hydrogel membrane served a suitable matrix for cells resulting growth spheroids over the composite hydrogel membrane (Fig. 7). Toldrà et al. exploited the osteogenic and angiogenic properties of S53P4 BG in a co-culture system in which dental pulp pluripotent-like stem cells were cultured in S53P4 BG-conditioned media containing different concentrations of inorganic ions dissolved from S53P4 BG. Vascular-like structures and osteogenesis were induced under the stimulatory effects of BG ions from the S53P4 BG-conditioned media [26]. In another study, Rath et al. fabricated copper ions-doped 45S5 BG scaffolds co-cultured with the MSCs and human dermal microvascular endothelial cells (HDMECs). In this system, MSCs secreted VEGF into the culture media in the presence of 1% Cu2+, which in turn induced HDMECs to display endothelial phenotype; thus, the Cu2+- doped BGs acted indirectly as angiogenic growth factor delivery system, suggesting a potential stimulatory effect of Cu2+ on MSCs in the MSC- HDMECs co-culture system (Fig. 8). Deb et al. fabricated Bioglass derived porous scaffolds made of Bioglass 45S5 with polyvinyl alcohol (PVA) as the porogen. The scaffolds were derived from 4:1 and 3:1 glass-polymer compositions, which referred as BG1 and BG2, respectively. In this study, the proliferation of human umbilical vein endothelial cells (HUVECS) and human osteoblasts (HOBS) were investigated in both co-culture and mono-culture conditions and compared with a commercial hyalouronic acid (HA) (SynHApor HA) scaffolds and demonstrated that a porous scaffold prepared from 45S5 Bioglass supported the proliferation responses of HUVECS cultured with human osteoblasts in both co-culture and mono-culture system [73].
Fig. 6.
Bioactive glass nanoparticles synthesized by a sol-gel process. (a–c) the network formation of nano-bioactive glass (nBG)- alginate(Alg) and nBG-zirconium (Zr/Alg) composite hydrogel membranes [58].
Fig. 7.
A schematic illustrating 3D spheroid formation of a co-culture of HDF cells (human dermal fibroblast cells lines) and KB-3-1 cells (human epithelial cell lines) [58].
Fig. 8.
Light microscopic images showing tube formation of human dermal microvascular endothelial cells (HDMECs) cultured in the presence of both MSCs and Cu2+-doped Bioglass (arrows). (A) Group I, (B) group II, (C) group III, (D) group IV, and (E) group V [74].
5. Conclusion and future challenge
The research reported in this review highlights the incorporation of bioactive glass particles into natural and biodegradable polymers-based composite scaffold and their effects on osteogenesis and angiogenesis processes in bone tissue engineering. Natural polymers including collagen, gelatin, and alginate, are biocompatible and non-cytotoxic and have been applied for medical devices for soft and hard tissues. However, such natural polymers suffer from low bioactivity and poor mechanical properties that limit their applications. To tackle these drawbacks, natural polymers can be combined with bioactive glass (BG) nanoparticles and microparticles to produce composites scaffolds. More importantly, incorporating BGs improves the mechanical properties of the resulting scaffold and its bioactivity and osteogenic differentiation properties compared to pure scaffolds without BG. Additionally, 3D co-culture of hydrogel-based BGs provides the additional spatial dimensions required to mimic a tissue microenvironment and enhance hierarchical cell-cell and cell–matrix interactions. Such a 3D structure serves as an ideal substrate for cell adhesion and proliferation. In the 3D structure, a synergistic effect of ions (released from BaG) promoted vascularization, which could alleviate the problem of high cost and potential disadvantages of using growth factor dependent models. Hence, the accurate methods of 3D co-culture system using natural-based biomaterials and BaG scaffold to investigate the formation of vascular-like structures is of paramount importance. Further research is required to determine the most advantageous combination of natural polymers and BG to obtain good mechanical properties with specific biological performance. Moreover, the response of specific natural polymers should be investigated to avoid undesired adverse effects after implantation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The corresponding author acknowledges the Faculty of Chemical and Process Engineering Technology at the University of Malaysia Pahang (UMP) for supporting him with a Postdoctoral Fellowship under the grant number ID (UIC190806).
Table of Abbreviations
- ALP
Alkaline phosphatase
- BG
Bioactive glass
- BGC
Bioactive glass ceramics
- BMSC
Bone marrow mesenchymal stem cell
- CDH
Cross-linked dextran hydrogels
- DPPSC
Dental pulp pluripotent-like stem cells
- HCA
Hydroxy carbonate apatite
- HDMEC
Human dermal microvascular endothelial cells
- HUVECS
Human umbilical vein endothelial cells
- ICBME
Iranian conference on biomedical engineering
- MBG
Mesoporous bioactive glass
- MBGN
Mesoporous bioactive glass nanoparticles
- OCS
Oxidized chondroitin sulfate
- VEGF
Vascular endothelial growth factor
References
- 1.Moses J.C., Nandi S.K., Mandal B.B. Multifunctional cell instructive silk‐bioactive glass composite reinforced scaffolds toward osteoinductive, proangiogenic, and resorbable bone grafts. Adv. Healt. Mater. 2018;7(10) doi: 10.1002/adhm.201701418. [DOI] [PubMed] [Google Scholar]
- 2.Zamani D., Moztarzadeh F., Bizari D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019;137:1256–1267. doi: 10.1016/j.ijbiomac.2019.06.182. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh Z., Najeeb S., Khurshid Z., Verma V., Rashid H., Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials. 2015;8(9):5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajiali F., Tajbakhsh S., Shojaei A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym. Rev. 2018;58(1):164–207. [Google Scholar]
- 5.Chiu T.-W., Chang C.-H., Yang L.-W., Wang Y.-P. Preparation of transparent Cu2Y2O5 thin films by RF magnetron sputtering. Appl. Surf. Sci. 2015;354:110–114. [Google Scholar]
- 6.Deux J.-F., Prigent-Richard S., d'Angelo G., Feldman L.J., Puvion E., Logeart-Avramoglou D., Pellé A., Boudghène F.P., Michel J.-B., Letourneur D. A chemically modified dextran inhibits smooth muscle cell growth in vitro and intimal in stent hyperplasia in vivo. J. Vasc. Surg. 2002;35(5):973–981. doi: 10.1067/mva.2002.123093. [DOI] [PubMed] [Google Scholar]
- 7.Quinlan E., Partap S., Azevedo M.M., Jell G., Stevens M.M., O'Brien F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials. 2015;52:358–366. doi: 10.1016/j.biomaterials.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Jonker A.M., Löwik D.W., Van Hest J.C. Peptide-and protein-based hydrogels. Chem. Mater. 2012;24(5):759–773. [Google Scholar]
- 9.Jalise S.Z., Baheiraei N., Bagheri F. The effects of strontium incorporation on a novel gelatin/bioactive glass bone graft: in vitro and in vivo characterization. Ceram. Int. 2018;44(12):14217–14227. [Google Scholar]
- 10.Hench L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006;17(11):967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 11.Baino F., Hamzehlou S., Kargozar S. Bioactive glasses: where are we and where are we going? J. Funct. Biomater. 2018;9(1):25. doi: 10.3390/jfb9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamani D., Razmjooee K., Moztarzadeh F., Bizari D. IEEE; 2017. Synthesis and Characterization of Alginate Scaffolds Containing Bioactive Glass for Bone Tissue Engineering Applications. 2017 24th National and 2nd International Iranian Conference on Biomedical Engineering (ICBME) pp. 330–333. [Google Scholar]
- 13.Badr-Mohammadi M.-R., Hesaraki S., Zamanian A. Mechanical properties and in vitro cellular behavior of zinc-containing nano-bioactive glass doped biphasic calcium phosphate bone substitutes. J. Mater. Sci. Mater. Med. 2014;25(1):185–197. doi: 10.1007/s10856-013-5062-7. [DOI] [PubMed] [Google Scholar]
- 14.Wu J., Zheng K., Huang X., Liu J., Liu H., Boccaccini A.R., Wan Y., Guo X., Shao Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019;91:60–71. doi: 10.1016/j.actbio.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Bellucci D., Anesi A., Salvatori R., Chiarini L., Cannillo V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C. 2017;79:286–295. doi: 10.1016/j.msec.2017.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan V., Lakshmi T. Bioglass: a novel biocompatible innovation. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2013;4(2):78. doi: 10.4103/2231-4040.111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentleman E., Fredholm Y., Jell G., O'Donnell M., Lotfibakhshaiesch N., Hill R., Stevens M., King’s Research Portal Biomaterials. 2010;31(14):3949–3956. doi: 10.1016/j.biomaterials.2010.01.121. [DOI] [PubMed] [Google Scholar]
- 18.Huang M., Hill R.G., Rawlinson S.C. Zinc bioglasses regulate mineralization in human dental pulp stem cells. Dent. Mater. 2017;33(5):543–552. doi: 10.1016/j.dental.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Pessan J.P., Al-Ibrahim N.S., Buzalaf M.A.R., Toumba K.J. Slow-release fluoride devices: a literature review. J. Appl. Oral Sci. 2008;16:238–244. doi: 10.1590/S1678-77572008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agathopoulos S., Tulyaganov D., Ventura J., Kannan S., Karakassides M., Ferreira J. Formation of hydroxyapatite onto glasses of the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. Biomaterials. 2006;27(9):1832–1840. doi: 10.1016/j.biomaterials.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich E., Oudadesse H., Lucas‐Girot A., Mami M. In vitro bioactivity of melt‐derived glass 46S6 doped with magnesium. J. Biomed. Mater. Res. Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009;88(4):1087–1096. doi: 10.1002/jbm.a.31901. [DOI] [PubMed] [Google Scholar]
- 22.Alhalawani A.M., Towler M.R. A novel tantalum-containing bioglass. Part I. Structure and solubility. Mater. Sci. Eng. C. 2017;72:202–211. doi: 10.1016/j.msec.2016.11.066. [DOI] [PubMed] [Google Scholar]
- 23.Mao L., Xia L., Chang J., Liu J., Jiang L., Wu C., Fang B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017;61:217–232. doi: 10.1016/j.actbio.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Gorustovich A., Roether J., Boccaccini A. vitro; 2010. Effect of Bioactive Glasses on Angiogenesis: A Review of. [DOI] [PubMed] [Google Scholar]
- 25.Coelho M., Cabral A.T., Fernandes M. Human bone cell cultures in biocompatibility testing. Part I: osteoblastic differentiation of serially passaged human bone marrow cells cultured in α-MEM and in DMEM. Biomaterials. 2000;21(11):1087–1094. doi: 10.1016/s0142-9612(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 26.Núñez-Toldrà R., Montori S., Bosch B., Hupa L., Atari M., Miettinen S. S53P4 Bioactive glass inorganic ions for vascularized bone tissue engineering by dental pulp pluripotent-like stem cell cocultures. Tissue Eng. 2019;25(17–18):1213–1224. doi: 10.1089/ten.TEA.2018.0256. [DOI] [PubMed] [Google Scholar]
- 27.Shahabipour F., Oskuee R.K., Dehghani H., Shokrgozar M.A., Aninwene G.E., Bonakdar S. Cell–cell interaction in a coculture system consisting of CRISPR/Cas9 mediated GFP knock‐in HUVECs and MG‐63 cells in alginate‐GelMA based nanocomposites hydrogel as a 3D scaffold. J. Biomed. Mater. Res. 2020;108(8):1596–1606. doi: 10.1002/jbm.a.36928. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z., Meng Z., Wu Q., Zeng D., Guo Z., Yao J., Bian Y., Gu Y., Cheng S., Peng L. Biomimetic and osteogenic 3D silk fibroin composite scaffolds with nano MgO and mineralized hydroxyapatite for bone regeneration. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420967791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreira C.D., Carvalho S.M., Mansur H.S., Pereira M.M. Thermogelling chitosan–collagen–bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater. Sci. Eng. C. 2016;58:1207–1216. doi: 10.1016/j.msec.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 30.Zheng K., Dai X., Lu M., Hüser N., Taccardi N., Boccaccini A.R. Synthesis of copper-containing bioactive glass nanoparticles using a modified Stöber method for biomedical applications. Colloids Surf. B Biointerfaces. 2017;150:159–167. doi: 10.1016/j.colsurfb.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Gao W., Zhang Y., Zhang Q., Zhang L. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016;44:2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X., Liu Y., Tan Y., Grover L.M., Song J., Duan S., Zhao D., Tan X. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng. C. 2019;105 doi: 10.1016/j.msec.2019.110155. [DOI] [PubMed] [Google Scholar]
- 33.Wu C., Zhou Y., Xu M., Han P., Chen L., Chang J., Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34(2):422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 34.Finney L., Vogt S., Fukai T., Glesne D. Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009;36(1):88–94. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueiredo J.C., Hirsch F.R., Kushi L.H., Nembhard W.N., Crawford J.M., Mantis N., Finster L., Merin N.M., Merchant A., Reckamp K.L., Melmed G.Y., Braun J., McGovern D., Parekh S., Corley D.A., Zohoori N., Amick B.C., Du R., Gregersen P.K., Diamond B., Taioli E., Sariol C., Espino A., Weiskopf D., Gifoni A., Brien J., Hanege W., Lipsitch M., Zidar D.A., Scheck Mcalearney A., Wajnberg A., Labaer J., Yvonne Lewis E., Binder R.A., Moormann A.M., Forconi C., Forrester S., Batista J., Schieffelin J., Kim D., Biancon G., Vanoudenhove J., Halene S., Fan R., Barouch D.H., Alter G., Pinninti S., Boppana S.B., Pati S.K., Latting M., Karaba A.H., Roback J., Sekaly R., Neish A., Brincks A.M., Granger D.A., Karger A.B., Thyagarajan B., Thomas S.N., Klein S.L., Cox A.L., Lucas T., Furr-Holden D., Key K., Jones N., Wrammerr J., Suthar M., Yu Wong S., Bowman N.M., Simon V., Richardson L.D., McBride R., Krammer F., Rana M., Kennedy J., Boehme K., Forrest C., Granger S.W., Heaney C.D., Knight Lapinski M., Wallet S., Baric R.S., Schifanella L., Lopez M., Fernandez S., Kenah E., Panchal A.R., Britt W.J., Sanz I., Dhodapkar M., Ahmed R., Bartelt L.A., Markmann A.J., Lin J.T., Hagan R.S., Wolfgang M.C., Skarbinski J. Mission, organization, and future direction of the serological sciences network for COVID-19 (SeroNet) epidemiologic cohort studies. Open Forum Infect. Dis. 2022;9(6) doi: 10.1093/ofid/ofac171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Zhao Y., Geng S., Miron R.J., Zhang Q., Wu C., Zhang Y. In vivo experimental study on bone regeneration in critical bone defects using PIB nanogels/boron-containing mesoporous bioactive glass composite scaffold. Int. J. Nanomed. 2015;10:839. doi: 10.2147/IJN.S69001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vichery C., Nedelec J.-M. Bioactive glass nanoparticles: from synthesis to materials design for biomedical applications. Materials. 2016;9(4):288. doi: 10.3390/ma9040288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattalini J.P., Hoppe A., Pishbin F., Roether J., Boccaccini A.R., Lucangioli S., Mouriño V. Novel nanocomposite biomaterials with controlled copper/calcium release capability for bone tissue engineering multifunctional scaffolds. J. R. Soc. Interface. 2015;12(110) doi: 10.1098/rsif.2015.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erol M., Mouriňo V., Newby P., Chatzistavrou X., Roether J., Hupa L., Boccaccini A.R. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2012;8(2):792–801. doi: 10.1016/j.actbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Sarker B., Li W., Zheng K., Detsch R., Boccaccini A.R. Designing porous bone tissue engineering scaffolds with enhanced mechanical properties from composite hydrogels composed of modified alginate, gelatin, and bioactive glass. ACS Biomater. Sci. Eng. 2016;2(12):2240–2254. doi: 10.1021/acsbiomaterials.6b00470. [DOI] [PubMed] [Google Scholar]
- 41.Reakasame S., Boccaccini A.R. Oxidized alginate-based hydrogels for tissue engineering applications: a review. Biomacromolecules. 2018;19(1):3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., Li S., Deng Y., He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5(1):1–20. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L., Li S., Xu M., Yu P., Zheng S., Duan Z., Liu J., Chen Y., Li J. 2020. Risk Factors Related to Hepatic Injury in Patients with Corona Virus Disease 2019. MedRxiv. [Google Scholar]
- 44.Zhou H.Y., Jiang L.J., Cao P.P., Li J.B., Chen X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Poly. 2015;117:524–536. doi: 10.1016/j.carbpol.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 45.Wu J., Liu J., Shi Y., Wan Y. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels. J. Mech. Behav. Biomed. Mater. 2016;64:161–172. doi: 10.1016/j.jmbbm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Kondiah P.J., Choonara Y.E., Kondiah P.P., Marimuthu T., Kumar P., Du Toit L.C., Pillay V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules. 2016;21(11):1580. doi: 10.3390/molecules21111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azizipour E., Aghamollaei H., Halabian R., Poormoghadam D., Saffari M., Entezari M., Salimi A. A novel hydrogel scaffold contained bioactive glass nanowhisker (BGnW) for osteogenic differentiation of human mesenchymal stem cells (hMSCs) in vitro. Int. J. Biol. Macromol. 2021;174:562–572. doi: 10.1016/j.ijbiomac.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Peter M., Binulal N., Nair S., Selvamurugan N., Tamura H., Jayakumar R. Novel biodegradable chitosan–gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem. Eng. J. 2010;158(2):353–361. [Google Scholar]
- 49.Nadeem D., Kiamehr M., Yang X., Su B. Fabrication and in vitro evaluation of a sponge-like bioactive-glass/gelatin composite scaffold for bone tissue engineering. Mater. Sci. Eng. C. 2013;33(5):2669–2678. doi: 10.1016/j.msec.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L., Fan L., Zhang F.-M., Jiang Y., Cai M., Dai C., Luo Y.-A., Tu L.-J., Zhou Z.-N., Li X.-J. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021;6(3):890–904. doi: 10.1016/j.bioactmat.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng K., Wu J., Li W., Dippold D., Wan Y., Boccaccini A.R. Incorporation of Cu-containing bioactive glass nanoparticles in gelatin-coated scaffolds enhances bioactivity and osteogenic activity. ACS Biomater. Sci. Eng. 2018;4(5):1546–1557. doi: 10.1021/acsbiomaterials.8b00051. [DOI] [PubMed] [Google Scholar]
- 52.Rottensteiner U., Sarker B., Heusinger D., Dafinova D., Rath S.N., Beier J.P., Kneser U., Horch R.E., Detsch R., Boccaccini A.R. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials. 2014;7(3):1957–1974. doi: 10.3390/ma7031957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh R., Sarker B., Silva R., Detsch R., Dietel B., Alexiou C., Boccaccini A.R., Cicha I. Evaluation of hydrogel matrices for vessel bioplotting: vascular cell growth and viability. J. Biomed. Mater. Res. 2016;104(3):577–585. doi: 10.1002/jbm.a.35590. [DOI] [PubMed] [Google Scholar]
- 54.Sarker B., Papageorgiou D.G., Silva R., Zehnder T., Gul-E-Noor F., Bertmer M., Kaschta J., Chrissafis K., Detsch R., Boccaccini A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B. 2014;2(11):1470–1482. doi: 10.1039/c3tb21509a. [DOI] [PubMed] [Google Scholar]
- 55.Jeong S.I., Krebs M.D., Bonino C.A., Samorezov J.E., Khan S.A., Alsberg E. Electrospun chitosan–alginate nanofibers with in situ polyelectrolyte complexation for use as tissue engineering scaffolds. Tissue Eng. 2011;17(1–2):59–70. doi: 10.1089/ten.TEA.2010.0086. [DOI] [PubMed] [Google Scholar]
- 56.Luo Y., Wu C., Lode A., Gelinsky M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication. 2012;5(1) doi: 10.1088/1758-5082/5/1/015005. [DOI] [PubMed] [Google Scholar]
- 57.Mishra R., Basu B., Kumar A. Physical and cytocompatibility properties of bioactive glass–polyvinyl alcohol–sodium alginate biocomposite foams prepared via sol–gel processing for trabecular bone regeneration. J. Mater. Sci. Mater. Med. 2009;20(12):2493–2500. doi: 10.1007/s10856-009-3814-1. [DOI] [PubMed] [Google Scholar]
- 58.Bargavi P., Ramya R., Chitra S., Vijayakumari S., Chandran R.R., Durgalakshmi D., Rajashree P., Balakumar S. Bioactive, degradable and multi-functional three-dimensional membranous scaffolds of bioglass and alginate composites for tissue regenerative applications. Biomater. Sci. 2020;8(14):4003–4025. doi: 10.1039/d0bm00714e. [DOI] [PubMed] [Google Scholar]
- 59.Varoni E., Canciani E., Palazzo B., Betti V., Dellavia C., Rimondini L. Nanostructured hydroxyapatite-dextran composite scaffolds for tissue engineering. Acad. Dent. Mater. Ann. Meet. 2010;26:e83–e84. [Google Scholar]
- 60.Stevenson M., Halpin K., Heuer C. Emerging and endemic zoonotic diseases: surveillance and diagnostics. OIE Rev. Sci. Tech. 2021;40(1):119–129. doi: 10.20506/rst.40.1.3212. [DOI] [PubMed] [Google Scholar]
- 61.Nikpour P., Salimi-Kenari H., Fahimipour F., Rabiee S.M., Imani M., Dashtimoghadam E., Tayebi L. Dextran hydrogels incorporated with bioactive glass-ceramic: nanocomposite scaffolds for bone tissue engineering. Carbohydr. Poly. 2018;190:281–294. doi: 10.1016/j.carbpol.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 62.Mäkinen O.E., Wanhalinna V., Zannini E., Arendt E.K. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016;56(3):339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- 63.Bejarano J., Detsch R., Boccaccini A.R., Palza H. PDLLA scaffolds with Cu‐and Zn‐doped bioactive glasses having multifunctional properties for bone regeneration. J. Biomed. Mater. Res. 2017;105(3):746–756. doi: 10.1002/jbm.a.35952. [DOI] [PubMed] [Google Scholar]
- 64.Meretoja V.V., Tirri T., Malin M., Seppälä J.V., Närhi T.O. Ectopic bone formation in and soft‐tissue response to P (CL/DLLA)/bioactive glass composite scaffolds. Clin. Oral Implants Res. 2014;25(2):159–164. doi: 10.1111/clr.12051. [DOI] [PubMed] [Google Scholar]
- 65.Karadjian M., Essers C., Tsitlakidis S., Reible B., Moghaddam A., Boccaccini A.R., Westhauser F. Biological properties of calcium phosphate bioactive glass composite bone substitutes: current experimental evidence. Int. J. Mol. Sci. 2019;20(2):305. doi: 10.3390/ijms20020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellucci D., Sola A., Cannillo V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: state of the art and current applications. J. Biomed. Mater. Res. 2016;104(4):1030–1056. doi: 10.1002/jbm.a.35619. [DOI] [PubMed] [Google Scholar]
- 67.Westhauser F., Karadjian M., Essers C., Senger A.-S., Hagmann S., Schmidmaier G., Moghaddam A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented β-TCP composite scaffold: an in-vitro comparison of Vitoss and Vitoss BA. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westhauser F., Essers C., Karadjian M., Reible B., Schmidmaier G., Hagmann S., Moghaddam A. Supplementation with 45S5 bioactive glass reduces in vivo resorption of the β-tricalcium-phosphate-based bone substitute material Vitoss. Int. J. Mol. Sci. 2019;20(17):4253. doi: 10.3390/ijms20174253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pampaloni F., Reynaud E.G., Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007;8(10):839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 70.Simian M., Bissell M.J. Organoids: a historical perspective of thinking in three dimensions. JCB (J. Cell Biol.) 2017;216(1):31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakubietz R.G., Jakubietz D.F., Horch R.E., Gruenert J.G., Meffert R.H., Jakubietz M.G. The microvascular peroneal artery perforator flap as a" lifeboat" for pedicled flaps. Plas. Reconstr. Surg. Glob. Open. 2019;7(9) doi: 10.1097/GOX.0000000000002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao J., Wang B., Huang Y., Qu Y., Peng J., Qian Z. Injectable alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega. 2017;2(2):443–454. doi: 10.1021/acsomega.6b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deb S., Mandegaran R., Di Silvio L. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J. Mater. Sci. Mater. Med. 2010;21(3):893–905. doi: 10.1007/s10856-009-3936-5. [DOI] [PubMed] [Google Scholar]
- 74.Rath S.N., Brandl A., Hiller D., Hoppe A., Gbureck U., Horch R.E., Boccaccini A.R., Kneser U. Bioactive copper-doped glass scaffolds can stimulate endothelial cells in co-culture in combination with mesenchymal stem cells. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113319. [DOI] [PMC free article] [PubMed] [Google Scholar]