Abstract
The presence of poor-quality veterinary drugs hampers the effectiveness of animal health care systems. It may produce danger to human safety through animal-derived food products that are consumed by the people. Thus, this study assessed the quality of veterinary albendazole, fenbendazole and ivermectin boluses/tablets marketed in Gondar Zones, North West Ethiopia. A total of 42 samples were collected from all government veterinary clinics and private veterinary pharmacies in Gondar zones by mystery shoppers from October, 2020 to January, 2021. All samples were visually and physically inspected for proper labeling and packaging. Samples were evaluated based on Pharmacopoeias and manufacturers' methods for identification, assay, dosage uniformity, dissolution, disintegration, hardness, and friability tests. All samples passed the visual inspection criteria outlined in the joint WHO/FIP/USP checklist. In general, 80.95% (34/42) of the products examined were substandard failing one or more quality test parameters, including all albendazole (30/30) and fenbendazole (4/4) samples. However, all of the ivermectin samples (8/8) passed the quality test parameters investigated in this work. The study had indicated that low quality veterinary albendazole and fenbendazole products are incredibly common in the study sites. The most crucial quality features investigated as failure were friability and disintegration. Thus, regulations and enforcements that guarantee quality of veterinary anthelminthic medications should be strictly in place in the study area and in the country.
Keywords: Albendazole, Ivermectin fenbendazole, Quality evaluation, Ethiopia
1. Introduction
Livestock production has been projected to support roughly three-quarters of severely impoverished people throughout the world. Therefore, livestock is a highly significant aspect of the economy of many developing nations [1]. Ethiopia is a home for various populations of animals. The country is well suited for livestock production. The nation possesses the greatest livestock population in Africa [2].
The livestock subsector is vital to Ethiopia’s economy. Many livelihoods rely on it. The industry is nevertheless promising for the country’s future economic growth [3]. However, diseases, ineffective nutrition and husbandry systems, and lack of effective veterinary services keep productivity low for this huge livestock population [4].
Globally, parasitic diseases are a major threat to grazing livestock. Clinical and sub-clinical helminthic parasite infections cause low productivity. Poor feed utilization and mortality contribute to low productivity and reduced economic benefits from livestock [5].
Strategic parasite management is required for maintaining proper animal health. Various parasites can be controlled with anti-parasitic medications, which are widely used in animal production. There are many broad-spectrum anthelmintics available to treat helminthiasis. Among these anthelmintic medications, imidothiazoles (levamisole), tetrahydropyrimidines (pyrantel), and avermectins are the most widely utilized ones in most countries [5]. Although veterinary anthelmintic medications provide a remedy to these animal health issues in case they are used appropriately, there is increasing usage of ineffective treatments owing to the existence of poor-quality pharmaceuticals in the supply chain system [6]. Health for animals and the global animal medicines association has predicted the illicit veterinary drugs trade amounts to be around 1 billion USD annually, nearly 3% of the value of the legitimate veterinary market [7]. This presents a major danger to animal health and welfare: not only they are inefficient at treating ailments, but also, they can be hazardous for animals. Furthermore, in respect to human health, the use of such poor-quality veterinary drugs in food-producing animals might affect food safety [8].
The WHO provides recommendations to nations to adopt veterinary medicine quality assurance systems. The International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products initiative helps nations to set global standards for veterinary medicine. Global veterinary product specialists have been assembled by the World Organization for Animal Health (OIE), with each member country represented by a local authority on veterinary health and medicine [8].
There is evidence of counterfeiting on both branded and generic products [9]. Veterinary drugs available on the market may contain the right components, but inadequate quantity of the active component, or no active ingredients at all, or packaging that is deceptive. They may potentially include hazardous or poisonous contaminants [10].
Ivermectin, fenbendazole, and albendazole are the most extensively used anthelmintics in Ethiopia [11]. Ivermectin (Ashiver 5 mg) is indicated for the treatment and control of gastrointestinal roundworms (adult & larval stages), eye worms, and lungworms. It is also effective against ectoparasites such as ticks, mites & suckling lice in sheep, goats, cattle, and pigs. Fenbendazole (fenacure 750 mg) is indicated for the removal & control of gastrointestinal roundworms (adult & 4th stage larvae), lungworms (adult & larval stages), hookworms and tapeworms (heads & segments) in cattle, horses and camel. Albendazole is a synthetic anthelmintic which belongs to the group of benzimidazole-derivatives with activity against a broad range of worms. It is also used at a higher dosage level against adult stages of liver fluke in cattle sheep and goat. Privately imported veterinary anthelmintic medications dominate the Ethiopian market, with little local production. Ethiopian Veterinary Drug and Animal Feed Administration and Control Authority which is recently re-organized as Ethiopian Agricultural Authority (EAA) oversee the quality of veterinary medications imported, produced, and dispensed in Ethiopia [12].
The Authority monitors possible access and entry points for veterinary medicines and animal feeds to the country. However, low-quality medications may be imported and distributed [13]. Professionals and animal owners alike have expressed concerns about the efficacy of currently available medications [14]. Treatment failures are a problem for many stakeholders in animal health [15]. This is exemplified by in vivo studies that document treatment failures in Ethiopia [11]. The reported low effectiveness may be due to parasite medication resistance or poor medicine quality. This demands further research and comparisons.
There has been no comprehensive quality assessment of ivermectin, fenbendazole, and albendazole boluses available in the study area. Therefore, the objective of this study was to assess the quality of albendazole, fenbendazole, and ivermectin products sold in Gondar zones, Northwest Ethiopia.
2. Materials and methods
2.1. Materials
2.1.1. Standard drugs, chemicals and solvents
HPLC grade methanol (B. No. 4549892, Mumbai, India), HPLC grade acetonitrile (B. No. 2189056, Mumbai, India and B. No. D6L012106L, Carlo Erba, France) were used. The other reagents utilized include, hydrochloric acid 37% (B. No V1N797131 N, Carlo Erba, France), sulfuric acid 98% (B. No 7664939, Mumbai India). The chemicals used include ammonium di-Hydrogen Phosphate (B. No 7722-76-1, Mumbai, India), sodium hydroxide (B. No 15530806, Mumbai India), sodium dodecyl sulfate (B. No. 9840973F, BDH chemicals ltd Poole, England), Sodium dihydrogen orthophosphate (Bulux laboratories Ltd. 121005). The primary reference standards used were parbendazole United States Pharmacopoeia Reference Standard (USP RS) (Lot No. R02810, China), albendazole USP RS (Lot No. R110MO, Mexico with potency of 99.6%), Ivermectin British Pharmacopoeia Reference Standard (BP RS) (batch No. 3549, UK with potency of 90.6%), Fenbendazole BP RS (batch No. 3808, UK with potency of 99.6%).
2.1.2. Instrumentation
The following instruments were used for the experiments: Ultrasonic cleaner (S. No 0083311134PO04, Dahan scientific Co., Ltd, Korea), pH meter (S. No 31092, Biby scientific Ltd. Co., UK), shaker (S. No 100077495220240V, Germany), vortex mixer (S. No F202A0173FI, Italy), vacuum pump (S No AP0013735, China), HPLC (S. No. L20705312035IX, Shimadzu Corporation, Japan), Polaris 5 μm C18 column of 25 cm × 2.6 mm (S.No. 524649, Netherlands), analytical C18 column of 25 cm × 2.6 mm, 5 μm (S. No. USUXA20680, USA), UV-VIS detector (S. No. L20145302582AE, Shimadzu Corporation, Japan), UV- VIS spectrophotometer (S No. 50038, UK), UV-1800 Shimadzu UV- spectrophotometer (S. No. A1145602220 CD, Japan), Whatman® qualitative filter paper (Grade 1, diameter 125 mm), Sartorius analytical balance (S. No. 26504358, Germany), Disintegration apparatus (model No. DZF-6050, Zenith Lab, P.R.C), Erweka dissolution tester apparatus (S. No. 135658-2217, Germany), Caleva hardness tester (S. No. 111436, Boston laboratory equipment), Erweka friability tester (S. No. 101821, Germany). The following glasswares:beakers, volumetric flasks, conical flasks, measuring cylinders, pipettes, and funnels. were also used in the experiment.
2.1.3. Sample collection
All sample boluses/tablets were collected by mystery shoppers from all government veterinary clinic and private veterinary pharmacy shops in Gondar zones, North West Ethiopia from October 2020 to January 2021. The study sites include Metema, Genda wuha, Debark, Gondar (including Azezo, Maksegnit suburb localities' around the town), Addis Zemen, Woreta, Debretabor and Gaynt (as shown on the map in Fig. 1). From all sites, a total of 42 samples (brands/batches) of albendazole, fenbendazole and ivermectin boluses were collected using prescription papers. The samples were kept at room temperature until they were tested and none of the products had expired at the time of testing. The countries of origins i.e. manufacturer country in terms of generics (total 11) for boluses are Ethiopia (1/11), India (4/11) and China (6/11). Bolus is a round mass of medicinal material larger than an ordinary pill. Details of information about the samples were described in Table 1, Table 2, Table 3.
Fig. 1.
Map of the study area.
Table 1.
General information on albendazole tablet samples used for the study.
| S. No. | Sample site | Sample code | Brand name | Manufacturing date | Expiry date | Country of manufacturer | Batch No | Manufacturer |
|---|---|---|---|---|---|---|---|---|
| 1 | Metema vet. HC | 881 | Albenda – QK 2500 mg | 10/01/2020 | 09/01/2023 | China | B2050110 | Chengdu Qiankun Vet. Pharmaceuticals co., LTD |
| 2 | Metema vet. HC | 868 | AshialbenR 300 mg | 12/2019 | 11/2023 | India | ALT- 7973 | Ashish life science pvt limited |
| 3 | Metema vet. HC | 865 | Albenda-QK 300 mg | 22/05/2020 | 21/05/2023 | China | B2030522 | Chengdu Qiankun Vet. Pharmaceuticals co., LTD |
| 4 | Genda wuha vet. HC | 882 | Petazole 2500™ mg | 02/2020 | 01/2023 | India | DB022012 | Dips Bioscience private limited |
| 5 | Genda wuha vet. HC | 866 | AshialbenR 300 mg | 03/2020 | 02/2024 | India | ALT- 8156 | Ashish life science pvt limited |
| 6 | Debark vet. HC | 883 | YZ-albendazole 300 mg | 09/2020 | 09/2025 | China | 200920B | Hebei Yuanzheng pharmaceutical Co., Ltd |
| 7 | Debark vet. HC | 869 | AshialbenR 300 mg | 03/2020 | 02/2024 | India | ALT- 8156 | Ashish life science pvt limited |
| 8 | Debark vet. HC | 870 | Albenda-QK 300 mg | 26/05/2020 | 25/05/2023 | China | B2030526 | Chengdu Qiankun Vet. Pharmaceuticals co., LTD |
| 9 | Gondar town Priv | 884 | AshialbenR 2500 mg | 06/2020 | 05/2024 | India | ALT- 8260 | Ashish life science pvt limited |
| 10 | Gondar town priv | 871 | AshialbenR 300 mg | 07/2020 | 06/2024 | India | ALT- 8394 | Ashish life science pvt limited |
| 11 | Gondar town priv | 872 | AlzoleR 300 mg | 07/2020 | 06/2024 | Ethiopia | J6324 | East Africa pharmaceuticals PLC |
| 12 | Gondar town vet. HC | 885 | Albenda – QK 2500 mg | 03/2018 | 03/2021 | China | B8550322 | Chengdu Qiankun Vet. Pharmaceuticals co., Ltd |
| 13 | Gondar town vet. HC | 873 | Albentong 300 mg | 07/2020 | 07/2024 | China | 200725 | Chongqing Fangtong animal Pharmaceutical Co., Ltd |
| 14 | Azezo vet. HC | 886 | Alben –LH 2500 mg | 08/2019 | 09/2022 | China | 1908001 | Hebei Lihua pharmaceutical Co., Ltd |
| 15 | Azezo vet. HC | 867 | Albentong 300 mg | 07/2020 | 07/2024 | China | 200725 | Hebei Lihua pharmaceutical Co., Ltd |
| 16 | Maksegnit vet. HC | 887 | Albenda – QK 2500 mg | 10/01/2020 | 09/01/2023 | China | B2050110 | Chengdu Qiankun Vet. Pharmaceuticals co., Ltd |
| 17 | Maksegnit vet. HC | 874 | Albentong 300 mg | 07/2020 | 07/2024 | China | 200725 | Hebei Lihua pharmaceutical Co., Ltd |
| 18 | Maksegnit vet. HC | 875 | Alben –LH 300 mg | 05/2020 | 05/2023 | China | 2005101 | Hebei Lihua pharmaceutical Co., Ltd |
| 19 | Addis zemen vet. HC | 888 | AshialbenR 2500 mg | 06/2020 | 05/2024 | India | ALT- 8260 | Ashish life science pvt limited |
| 20 | Addis zemen vet. HC | 876 | Alben 300 mg | 09/2020 | 08/2024 | China | 200901 | Baoding sunlight herb medicament Co., Ltd. |
| 21 | Weretta vet. HC | 890 | AshialbenR 2500 mg | 05/2020 | 04/2024 | India | ALT- 8229 | Ashish life science pvt limited |
| 22 | Weretta vet. HC | 877 | AshialbenR 300 mg | 07/2020 | 06/2024 | India | ALT-8389 | Ashish life science pvt limited |
| 23 | Debretabor vet. HC | 891 | YZ-albendazole 2500 mg | 07/2019 | 07/22024 | China | 190730 | Hebei Yuanzheng pharmaceutical Co., Ltd |
| 24 | Debretabor vet. HC | 878 | YZ-albendazole 300 mg | 07/2020 | 07/2025 | China | 200716B | Hebei Yuanzheng pharmaceutical Co., Ltd |
| 25 | Debretabor vet. HC | 879 | Alben 300 mg | 06/2020 | 05/2024 | China | 200601 | Baoding sunlight herb medicament Co., Ltd. |
| 26 | Gaynt vet. HC | 892 | Petazole 2500™ mg | 02/2020 | 01/2023 | India | DB022029 | Dips Bioscience private limited |
| 27 | Gaynt vet. HC | 880 | Alben 300 mg | 06/2020 | 05/2024 | China | 200601 | Baoding sunlight herb medicament Co., Ltd. |
| 28 | Azezo vet. HC | 906 | AlzoleR 300 mg | 06/2020 | 05/2024 | Ethiopia | JF301 | East Africa pharmaceuticals PLC |
| 29 | Gondar town priv | 918 | YZ-albendazole 300 mg | 07/2020 | 07/2025 | China | 200716B | Hebei Yuanzheng pharmaceutical Co., Ltd |
| 30 | Gondar town priv | 919 | AlbenC 2500 mg | 09/05/2020 | 08/05/2023 | China | 200509 | Hebei new century pharmaceutical co., Ltd |
Vet HC=Veterinary Health center (owned and run by Government).
Priv: Private veterinary pharmacy (owned by private individuals).
Table 2.
General information on ivermectin tablets samples used for the study.
| S. No. | Sample site | Sample code | Brand name | Manufacturing date | Expiry date | Country of manufacturer | Batch No | Manufacturer |
|---|---|---|---|---|---|---|---|---|
| 1 | Metema vet. HC | 896 | Ashiver TM 5 mg | 04/2020 | 03/2024 | India | ALT- 8184 | Ashish life science pvt limited |
| 2 | Gondar town vet. HC | 897 | Ashiver TM 5 mg | 08/2020 | 07/2024 | India | ALT- 8437 | Ashish life science pvt limited |
| 3 | Azezo vet. HC | 8981 | Ashiver TM 5 mg | 06/2020 | 05/2024 | India | ALT - 8296 | Ashish life science pvt limited |
| 4 | Weretta vet. HC | 899 | Ashiver TM 5 mg | 08/2020 | 07/2024 | India | ALT- 8437 | Ashish life science pvt limited |
| 5 | Debretabor vet. HC | 8891 | Ashiver TM 5 mg | 06/2020 | 05/2024 | India | ALT- 8297 | Ashish life science pvt limited |
| 6 | Gaynt vet. HC | 8982 | Ashiver TM 5 mg | 06/2020 | 05/2024 | India | ALT - 8296 | Ashish life science pvt limited |
| 7 | Debark vet. HC | 8892 | Ashiver TM 5 mg | 06/2020 | 05/2024 | India | ALT- 8297 | Ashish life science pvt limited |
| 8 | Maksegnit vet. HC | 909 | Ashiver TM 5 mg | 06/2020 | 05/2024 | India | ALT - 8296 | Ashish life science pvt limited |
Vet HC=Veterinary Health center (owned and run by Government).
Priv: Private veterinary pharmacy (owned by private individuals).
Table 3.
General information on fenbendazole tablets samples used for the study.
| S. No | Sample site | Sample code | Brand name | Manufacturing date | Expiry date | Country of manufacturer | Batch No | Manufacturer |
|---|---|---|---|---|---|---|---|---|
| 1 | Debark vet. HC | 893 | Fenacure 750 mg | 02/2020 | 01/2024 | India | ALT- 8067 | Ashish life science pvt limited |
| 2 | Debretabor vet. HC | 894 | Fenacure 750 mg | 02/2020 | 01/2024 | India | ALT-8069 | Ashish life science pvt limited |
| 3 | Gaynt vet. HC | 895 | Fenacure 750 mg | 01/2019 | 12/2022 | India | ALT- 7466 | Ashish life science pvt limited |
| 4 | Weretta vet. HC | 921 | Fenacure 750 mg | 02/2020 | 01/2024 | India | ALT- 8068 | Ashish life science pvt limited |
Vet HC=Veterinary Health center (owned and run by Government).
Priv: Private veterinary pharmacy (owned by private individuals).
2.1.4. Quality control test methods
The quality control laboratory works were conducted at the National Animal Products, Veterinary Drug and Feed Quality Assessment Center (APVD-FQAC), School of Pharmacy Addis Ababa University and Ethiopian Food and Drug Administration laboratories (EFDA). The laboratory tests were undertaken in accordance with the Pharmacopoeial and manufacturer’s methods. The system suitability tests for HPLC methods as well as analytical instrument performance were conducted successfully.
The following quality characteristics were used to assess the products: identification, dissolution, dosage uniformity, assay, disintegration, friability and hardness tests. A product failing any of the above tests was classified as a substandard. A description of quality control test methods employed to assess the study veterinary anthelminthic medications is given in supplementary file. Quality characteristics of albendazole and ivermectin samples were examined based on USP specifications. For dissolution, the percentage of drug released was taken into account. Albendazole boluses should release more than 80% of the indicated quantity within 30 min [16], whereas ivermectin should release 80% within 45 min, according to the USP acceptance specification [17]. Content uniformity or weight variation can be used to show dosage uniformity as per USP requirements. Weight variation is employed when products containing 25 mg or more of active components while content uniformity test is used for products with active ingredients less than 25 mg. However, quality characteristics of fenbendazole products were performed according to the manufacturer’s specifications (Ashish life science private limited company, Dist-Palghar, India) [18]. For hardness test, the tester was set to crush each tablet and the force required to crush each tablet was measured in newton. Boluses are expected to fulfill the requirement for a hardness value of greater than 40 N [19].
2.2. Identification test
2.2.1. Identification of albendazole
An accurately weighed portion of sample powder which is equivalent to about 100 mg of albendazole was transferred to a 50 mL volumetric flask. 5.0 mL of sulfuric acid in methanol and 20 mL of methanol were added and shaken by mechanical shaker for about 15 min. It was diluted with methanol to volume, mixed and filtered. 1.0 mL of the filtrate was transferred to 200 mL volumetric flask and diluted to volume by acidified methanol to obtain solutions containing about 10 μg of albendazole per mL [16]. The absorbance of prepared sample solution was determined using a UV/Visible spectrophotometer. Similarly standard solution was prepared per the above procedure. The light absorption of the solution was in the range of 200–350 nm. Acidified methanol was used as the blank solution.
2.2.2. Identification of ivermectin
For identification of ivermectin, the retention times of the avermectin B1a (H2B1a) and avermectin B1b (H2B1b) peaks in the chromatogram of the assay preparation correspond to those in the chromatogram of the standard preparation, as obtained in the assay test were used [17].
2.2.3. Identification of fenbendazole bolus
Bolus powder which is equivalent to 100 mg of fenbendazole was weighed and transferred into 100 mL volumetric flask. 50 mL of 0.1 M methanolic HCl was added, sonicated for 30 min to dissolve fenbendazole completely. After sonication, the solution was diluted to 100 mL with 0.1 M methanolic HCl. Then the solution was shaken and filtered with Whatman filter paper No. 41. Then 1.0 mL of the filtrate was transferred into 100 mL volumetric flask and diluted with 0.1 M NaOH to get the final concentration of 10 μg/mL. Finally, the absorbance of prepared sample solution was determined using a UV/Visible spectrophotometer. A 0.1 M NaOH was used as the blank solution. According to the manufacturer’s method specification, the light absorption of the solution should be in the range of 280–350 nm and should exhibit maxima at about 309 nm [18].
2.3. Assay
2.3.1. Assay for albendazole boluses
About 20 boluses were weighed and finely powdered. An accurately weighed portion of the powder, equivalent to about 100 mg of albendazole was transferred to a 50 mL volumetric flask. 5 mL of Sulfuric acid in methanol and 20 mL of methanol were added, and shaken by mechanical means for about 15 min. Then the solution was diluted with methanol to volume, mixed, and filtered. The first 15 mL of the filtrate was discarded. 5.0 mL of the clear filtrate and 5.0 mL of internal standard solution were transferred to a second 50 mL volumetric flask and diluted with methanol to volume, and mixed. The mixture solution was filtered and transferred in to HPLC vials [16]. Equal volumes (about 20 μL) of the standard preparation and the assay preparation were separately injected into the chromatograph. The chromatograms were recorded, and the responses for the major peaks were measured.
The quantity, in mg, of C12H15N3O2S was calculated in the portion of tablets taken by the formula:
| (1) |
where, C is the concentration, in mg per ml, of USP albendazole RS in the standard preparation and RU and RS are the peak response ratios of the albendazole peak to the parbendazole peak obtained from the assay preparation and the standard preparation, respectively (Peak heights were used as indicated by USP 38/NF 33).
2.4. Assay of ivermectin
2.4.1. Sample preparation
Twelve boluses were transferred into a 250 mL volumetric flask according to the accompanying table described in USP [17]. Approximately 25 mL of distilled water was added and sonicated for 10 min. Then methanol was added and filled the flask three-quarters full, sonicated for 5 min and the boluses were completely disintegrated and shaken on a mechanical shaker for 10 min and mixed well. The solution was allowed to cool at room temperature. Diluted with methanol to volume, a magnetic stirrer was added, and mixed until no lumps are present in the solution. A portion of the solution was passed through chemically resistant filter paper prior to injection. Equal volumes (about 10 μL) of the standard preparation and the assay preparation were separately injected into the chromatograph. The chromatograms were recorded, and the peak areas for component H2B1a plus component H2B1b were measured. The percentage of component H2B1a (C48H74O14) plus component H2B1b (C47H72O14) were calculated as a percentage of the label claim of ivermectin per tablet taken by the formula:
| (2) |
where
AU is the total peak response of H2B1a plus H2B1bobtained from the assay preparation
WS is the weight of the USP ivermectin RS, in mg, taken to prepare the standard preparation
P is the purity of the USP ivermectin RS (percent [w/w] H2B1a plus percent [w/w] H2B1b) expressed as a decimal
DU is the sample dilution factor
AS is the total peak area of H2B1a plus H2B1bobtained from the standard preparation
DS is the standard dilution factor
N is the number of tablets taken to prepare the assay preparation; and
L is the label claim of ivermectin, in mg per tablet.
2.5. Assay of fenbendazole
2.5.1. Sample preparation
Five boluses were weighed and powdered. An accurately weighed portion of the powder, equivalent to about 100 mg of fenbendazole was transferred to a 100 mL volumetric flask. 50 mL of 0.1 M methanolic HCl was added. The solution was sonicated for 30 min to dissolve fenbendazole completely and diluted to 100 mL with 0.1 M methanolic HCl. The solution was shaken and filtered with Whatman filter paper No. 41.1.0 mL of the filtrate was taken into a 100 mL volumetric flask and diluted to 100 mL with 0.1 M NaOH (10 μg/mL).
The absorbance of standard and sample solution was measured at the wavelength maxima at 309 nm using 0.1 M NaOH as a blank. The content of fenbendazole was calculated by comparison of sample and standard. The content of fenbendazole in mg/bolus was calculated as:
| (3) |
| (4) |
where
Spl Abs is absorbance of sample
Std Abs is absorbance standard
Stdwt is standard weight
Splwt is the weight of sample taken
Av. Wt is average weight of five boluses
2.6. Dissolution profile
2.6.1. Dissolution profiles of albendazole
The dissolutions of all brands of albendazole were evaluated using a dissolution apparatus type II (paddle) following United States Pharmacopoeia protocol [20]. The dissolution medium (900 mL of 0.1 N HCl) was transferred to vessels of dissolution apparatus. The temperature and the spindle rotation speed were set to 37 ± 0.5 °C and 50 rpm for 30 min, respectively. This dissolution medium was used because of the higher solubility of albendazole at acidic pH compared with neutral or basic media [16]. Boluses from each brand were randomly assigned to the six dissolution vessels. Five (5.0) mL samples were withdrawn at predetermined time points (5, 10, 15, 20, 30, 45, 60 min). After each withdrawal, an equal volume of 0.1 N HCl medium that had been maintained at the same temperature was replaced in order to maintain the total volume of the medium constant. The samples (5 mL) were immediately filtered using syringe filter and 3 mL (for label claim 300 mg) of the filtered sample solution was quantitatively taken in to 100 mL volumetric flask. It was diluted to volume with 0.1 N NaOH. The absorbance of each sample was determined at 308 nm and 350 nm using a UV/Visible spectrophotometer. The difference was taken as absorbance value. A 0.1 N sodium hydroxide was used as the blank solution. The quantity in mg of C12H15N3O2S dissolved was calculated by the formula:
| (5) |
where
C is the concentration in mg per ml of USP albendazole RS in the standard solution
AU and AS are the differences in absorbance between 308 nm and 350 nm obtained from the solution under test and the standard solution respectively [16].
2.7. Dissolution of ivermectin
The dissolutions of ivermectin boluses were evaluated using a dissolution apparatus using a dissolution apparatus type II (paddle) following the United States Pharmacopoeia recommendation [17]. The dissolution medium was prepared 0.01 M phosphate buffer, pH 7, with 0.5% of sodium dodecyl sulfate (prepared by dissolving 50 g of sodium dodecyl sulfate in approximately 9 L of water, adding 100 mL of 1 M monobasic sodium phosphate monohydrate, adjusted with sodium hydroxide to a pH of 7, and diluted with water to 10 L). 900 mL of the medium was transferred to vessels of dissolution apparatus and the temperature and the spindle rotation speed was set to 37 ± 0.5 °C and 50 rpm for 45 min, respectively.
Ivermectin boluses from each batch were randomly assigned to the six dissolution vessels and five (5.0) mL samples were withdrawn at predetermined time points (5, 10, 15, 20, 30 45 and 60 min) and replaced with an equal volume of fresh dissolution medium at the same temperature. The samples (5.0 mL) were immediately filtered using syringe filter and the filtrate was used for analysis [17]. Equal volumes (about 100 μL) of the test solution and the standard solution were transferred to the HPLC vials and separately injected into the chromatograph. The chromatograms were recorded, and the responses for the major peaks were measured. The dissolved combined quantities, in percentage, of H2B1a plus H2B1bwas calculated based on the peak responses obtained from the test solution and the standard solution by the formula:
| (6) |
where:
AU is the total peak area of H2B1a plus H2B1bobtained from the test solution
WS is the weight, in mg, of the USP ivermectin RS taken to prepare the standard stock solution P is the purity of the USP ivermectin RS (percent [w/w] H2B1a plus percent [w/w] H2B1b) expressed as a decimal
DU is the test solution dilution factor
AS is the total peak area of H2B1a plus H2B1bobtained from the standard solution
DS is the standard solution dilution factor; and
L is the label claim of ivermectin, in mg per tablet.
Tolerances: - Not less than 80% of the labeled amount of C48H74O14 (H2B1a) plus C47H72O14 (H2B1b) is dissolved in 45 min [17].
2.8. Uniformity of dosage units
The term uniformity of dosage unit is defined as the degree of uniformity in the amount of the drug substance among dosage units. The uniformity of dosage units can be demonstrated by either of two methods, content uniformity or weight variation. When products contain 25 mg or more of an active ingredient comprising 25% or more, by weight, of the dosage unit, USP 38/NF 33 recommends uniformity of dosage units can be demonstrated by weight variation. Whereas, products containing active ingredients less than 25 mg will be demonstrated by content uniformity test [21].
The uniformity of dosage units of albendazole and fenbendazoe were demonstrated by weight variation as their active ingredient content is greater than 25 mg. The uniformity of dosage units of ivermectin tablets was demonstrated by content uniformity test (individual assay of tablets) as its active ingredient content is less than 25 mg [21].
2.9. The uniformity of dosage units of albendazole
For uniformity of dosage units of albendazole, a weight variation test was done using a method specified in USP (2015). According to USP (2015) specification, the requirements for dosage uniformity are met if the acceptance value of the first 10 dosage units is less than or equal to L1%, which is 15.
First, 10 boluses were selected randomly [21]. Each bolus was weighed individually. Then, the average weight of 10 boluses was determined. Then, assay (content) of individual bolus (% xi), was obtained by the formula:
| (7) |
where
%Xi is content percentage of each tablet/bolus
A is total percent assay which is obtained from assay conducted on representative drug samples of each brand as indicated on monograph for albendazole.
Finally, average () for ten boluses was determined by dividing the sum of individual bolus content percentage (%xi) by 10, which is the number of boluses used for weight uniformity test in the study
Acceptance value: Acceptance value was determined by formula:
| (8) |
where
k = acceptability constant which is 2.4, for number of boluses is 10.
S = standard deviation of % xi
= average content percentage of 10 boluses
M = reference value
If 98.5% ≤ ≤ 101.5%, then M = (AV = ks)
If < 98.5%, then M = 98.5% (AV = 98.5 − + ks)
If > 101.5%, then M = 101.5% (AV = − 101.5 + ks)
2.10. The uniformity of dosage units of fenbendazole boluses
Five boluses were weighed individually; their total and average weight was calculated according to manufacturer’s method recommendations. The weight of five boluses should be between 16.250 ± 2%. The average weight of five boluses should be between 3.250 ± 5% [18].
2.11. The uniformity of dosage units of ivermectin
For uniformity of dosage units of ivermectin, content uniformity test was done using a method specified in USP [21]. The requirements for dosage uniformity are met if the acceptance value of the first 10 dosage units is less than or equal to L1%, which is 15.
Ten boluses were selected randomly [21]. Each bolus was assayed for its content percentage individually (% Xi). Then, the average percentage content () of ten boluses was determined by dividing the sum of individual bolus content percentage (% Xi) by 10.
Acceptance value: Acceptance value was determined by using the same formula which is given in Equation (8):
2.12. Disintegration time
Six boluses from each brand of albendazole, six boluses from each batch of ivermectin and fenbendazole were randomly selected and each bolus was placed in one of six separate tubes. The basket rack assembly was set to move up and down. The medium (900 mL of distilled water) temperature was maintained at 37 ± 1 °C. The disintegration time was taken to be the time when no particles remained in the basket. The time in minutes required for each bolus to disintegrate was recorded.
2.13. Friability test
Friability is the tendency for a tablet to chip, crumble or break following compression. Friability is designed to evaluate the ability of the tablet to withstand abrasion in packaging, handling and shipping. It is usually measured by the use of the friability apparatus [22]. Ten boluses from each brands of albendazole, ten boluses from each batch of ivermectin and fenbendazole were randomly selected and weighed initially before undergoing friability test on an analytical balance. The boluses were then placed in the drum of the tester which was adjusted to rotate at 25 rpm for 4 min for a total of 100 revolutions. After the procedure was completed, the boluses were dedusted and reweighed. The percent loss in weight was calculated as friability. According to USP for friability, the weight loss should not be more than 1%. Percentage of weight loss was calculated as follows [23].
| (9) |
2.14. Hardness test
The hardness of boluses was determined using a hardness tester. Ten boluses from each brand of albendazole, ten boluses from each batch of ivermectin and fenbendazole were randomly taken and each bolus was then placed in the hardness tester. The tester was set to crush each tablet and the force required to crush each tablet was measured in newton [24].
3. Results and discussion
3.1. Quality of the examined samples
Out of a total of 42 samples collected, 30 were albendazole, 8 were ivermectin and 4 were fenbendazole samples (as described in Table 1, Table 2, Table 3 respectively). According to the results of the identification tests, all of the samples contained the indicated active ingredient. All products passed for visual and physical inspection for packaging and labelling. All ivermectin samples (8/8) meet all quality criteria assessed in this study per USP specification. The laboratory tests, on the other hand, revealed that 80.95% (34/42) of the samples did not meet the anticipated pharmacopoeial quality criteria including all albendazole (30/30) and fenbendazole (4/4) samples. The findings of the various quality control tests performed on the samples are summarized in Table 4 and given in detail as supplementary file.
Table 4.
Quality control test results of products.
| Product | Samples failing quality criteria test |
|||||||
|---|---|---|---|---|---|---|---|---|
| Identification | Dissolution | Dosage uniformity | Assay | Disintegration | Friability | Hardness | Overall | |
| Albendazole | 0% (0/30) | 100% (30/30) | 23.33% (7/30) | 30% (9/30) | 10% (3/30) | 96.66% (29/30) | 0% (0/30) | 100%(30/30) |
| Ivermectin | 0% (0/8) | 0% (0/8) | 0% (0/8) | 0% (0/8) | 0% (0/8) | 0% (0/8) | 0% (0/8) | 0%(0/8) |
| Fenbendazole | 0% (0/4) | – | 0% (0/4) | 0% (0/4) | 0% (0/4) | 100% (4/4) | 0% (0/4) | 100% (4/4) |
| Overall | 0% (0/42) | 71.43% (30/42) | 16.66% (7/42) | 21.43% (9/42) | 7.14% (3/42) | 78.57% (33/42) | 0%(0/42) | 80.95%(34/42) |
3.2. Identification test
All samples met the specification for identification test.
3.2.1. Assay
According to the findings, around two-thirds (9/30) of albendazole samples did not fulfill the pharmacopoeial acceptability criteria for the test. Albendazole medication products had test results ranging from 73.91 to 104.84%. According to the USP [16] standard, the product assay (i.e. drug content) should fall between 90% and 110% of the label claims [16]. Ivermectin medication products had test results ranging from 92.26 to 96.12%. This result indicates that all batches meet the USP standard limit (90–110%) [17]. Similarly, all batches of fenbendazole samples passed the manufacturer’s specified limit (95–105%), according to the test findings.
3.3. Uniformity of dosage units
Dosage uniformity is assessed in order to guarantee that the same amount of medication is administered in each individual dosage form. In terms of dosage uniformity, all albendazole, ivermectin, and fenbendazole samples met pharmacopoeial acceptance requirements, although only 23.33% (7/30) of albendazole samples did not satisfy these parameters, as seen in Table 4 and the details in supplementary file.
3.4. Disintegration test
All samples passed the disintegration time test, with the exception of 10% (3/30) of albendazole samples, which did not pass the regulatory criteria, as indicated in Table 4 and more details in supplementary files.
3.5. Dissolution test
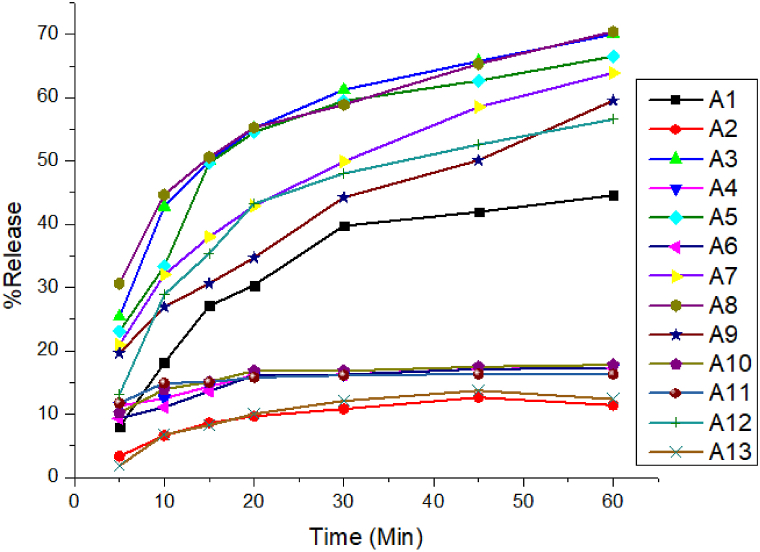
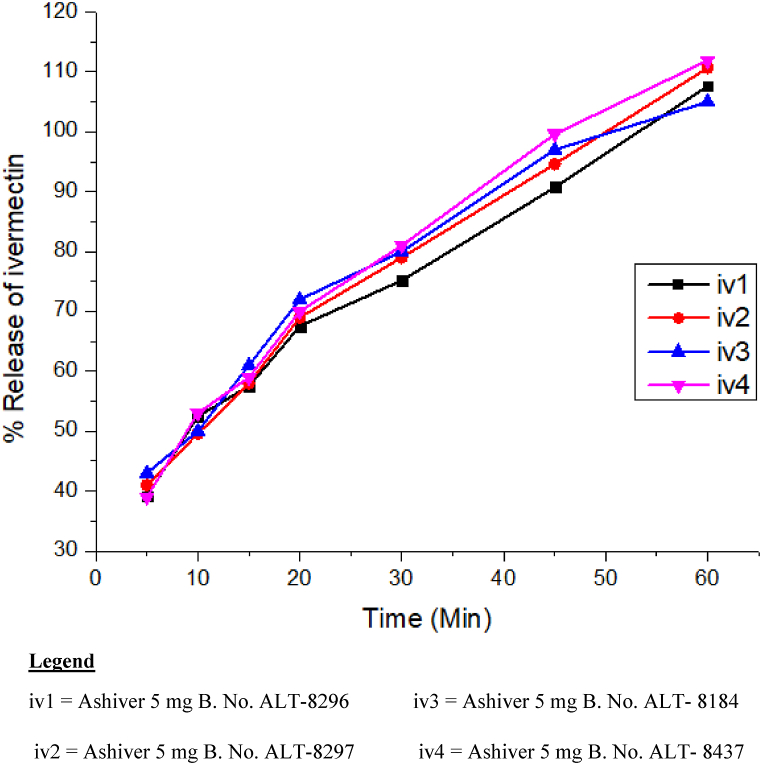
None of the albendazole samples met the regulatory tolerance levels as indicated in Table 4. In 30 min, all albendazole products released less than 80% (the official tolerance limit) of the dosage. Even in 60 min, they did not release the required quantity, as presented in Fig. 2 for albendazole samples. However, all ivermectin samples tested for in-vitro dissolution released more than 80% within 45 min, meeting the stated tolerance limits, as shown in Fig. 3.
Fig. 2.
Dissolution profile of different albendazole products.
Fig. 3.
Dissolution profiles of different batches of ivermectin boluses. Legend: iv1 = Ashiver 5 mg B. No. ALT-8296 iv3 = Ashiver 5 mg B. No. ALT- 8184; iv2 = Ashiver 5 mg B. No. ALT-8297 iv4 = Ashiver 5 mg B. No. ALT- 8437.
3.6. Friability test
All albendazole brands failed the friability test, with the exception of one (1/30). Additionally, none of the fenbendazole samples were found to be compliant. All ivermectin samples, on the other hand, passed the friability test. As demonstrated in Table 4, 78.57% (33/42) of the evaluated samples failed to pass the friability test.
3.7. Hardness test
All samples of albendazole, ivermectin and fenbendazole passed the hardness test. This test measures the ability of tablets/boluses to withstand pressure or stress during handling, packaging, and transportation.
The results of this study revealed a significant prevalence of low-quality veterinary drugs, which included all albendazole and fenbendazole samples analyzed. In all, 80.95% (34/42) of the studied medication samples failed to comply with the regulatory tolerance levels for friability, dissolution, dosage uniformity, assay and disintegration. A similar work done on albendazole tablets in Addis Ababa, Ethiopia revealed that all batches of veterinary albendazole circulating in the city did not fulfill either physical or chemical quality requirements [12]. A nationwide survey in Ethiopia also found that 45.3% of the tested samples did not meet the required pharmacopoeial quality requirements for human anthelminthic medications (mebendazole and albendazole) and antiprotozoal drug (tinidazole) [25]. A study in Burkina Faso, Cote d’Ivoire, Ghana, and Tanzania demonstrated that 61.4% of human anthelminthic drugs failed in at least one area (assay, dissolution, dosage uniformity and disintegration) [26]. Inadequate storage environments, unsatisfactory quality assurance, inadequate conformance with good manufacturing practice requirements, lack of scientific competence in manufacturing, restricted technical capacity and regulatory system may be the causes of poor drug quality in developing countries like Ethiopia [25]. Quality problems in veterinary drugs may have a huge effect. Inability to produce therapeutic effect is one of them. The animals' welfare and production are compromised, and the farmer is forced to repeat treatments at a higher expense. In long-terms, it may enhance resistant parasite population [12].
Visual examination of the studied samples revealed no evidence of counterfeit drugs. Similar findings were obtained for packaging and labeling compliance in several brands of albendazole tablets marketed in Jimma, Ethiopia [27]. Conversely, problems were observed with labelling and physical characteristic of tested samples in other studies [12,26].
All analyzed albendazole, ivermectin and fenbendazole samples contained the intended active ingredient. These findings are consistent with those of previous investigations [12,27]. However, 21.43% (9/42) of the samples (i.e. 30% (9/30) of the albendazole samples) failed to meet the pharmacopoeial assay acceptance requirements. A similar result was reported in Addis Ababa, Ethiopia [12] and even higher percentages of samples failed assay test, with 48% failing in Jimma, Ethiopia [25] and 71% failing in Yemen [28].
To ensure dosage unit homogeneity, each batch should have an API level in uniform way. Variations are allowed within a small range of the label claim [26]. Pharmaceutical dosage unit uniformity is dependent on formulations and production procedures. So, it is unreasonable to anticipate that every unit has exactly the same amount of active ingredient as stated on the label. Because of this, pharmacopeial standards and specifications have been established to set general limitations on allowed changes for active components quantity in single dosage units [29]. The findings of this investigation demonstrated that all ivermectin and fenbendazole samples met the dosage uniformity acceptance criteria, which are consistent with findings from Yemen [28], Cambodia [9] and India [30]. However, in the current investigation, 23.33% (7/30) of albendazole samples did not meet the acceptance requirements. Previous investigations in Ethiopia [12], Nigeria [31], Korea and Cambodia have shown comparable results [32]. The discrepancy might be attributable to manufacturing procedures such as using various quantities of excipients and/or API in varied proportions for different drug formulations.
Disintegration in the gastrointestinal tract is an essential step for drug absorption and bioavailability, and subsequently therapeutic efficacy of medicines [19]. It is known that the type of binder used will affect the disintegration time of a tablet/bolus. In the present study, all analyzed samples for disintegration have complied with the acceptance requirements [26] except 10% (3/30) albendazole samples, which is in agreement with the results of previous studies [9,31]. Dissolution is a solid dosage form quality control feature that predicts absorption and bioavailability. It is consequently critical to analyze in vitro dissolution. It distinguishes acceptable and unsuitable items [31]. Apparently, dissolution also limits parasite uptake into the circulation. Thus, inadequate solubility may impair effectiveness [26]. In the present study, all ivermectin samples passed the requirements but all albendazole samples failed the specification limit. Studies on albendazole dissolution profile revealed similar findings to the previous reports [26,28,31,32]. A modest dose of API is available for absorption into the systemic circulation when a considerable component of a medication fails dissolution. However, the unabsorbed fraction of the drug produces the negative effects [33].
Hardness is used to ascertain the quality of a tablet/bolus; it is an important parameter since tablets must have sufficient ability to survive the handling forces during packaging, breakage under conditions of storage and transportation. In this study, all samples fulfilled the limits of the hardness test. Similar results were reported in studies done in Bangladesh and India [34]. However, 78.57% (33/42) of the samples failed the specification of friability test including all fenbendazole and 96.66% (29/30) of albendazole samples [35]. These findings are in agreement with the findings of studies conducted in Ethiopia [25] and Nigeria [31]. On the other hand, all ivermectin products passed the friability requirements. Failure to meet specification of friability test may be due to low binder concentration, resulting in loose inter particulate bonding or the use of low compression pressure in the tablet machine during tableting step.
The overall failure rate found in this study is 80.95% which is very high compared the recent report from the summary analyses that had performed a trend analysis on all quality control works of veterinary medicines in Ethiopia (from 16 November 2016 and 22 June 2021) for which it had reported 8.2% overall quality failure rate [36]. The discrepancy may be due to the fact that the high numbers of samples in this report are from pre-market regulatory procedures where samples for analysis are supplied sometimes selectively by importers in consultation with manufacturers. In addition, it may be attributed to the wide range of test parameters assessed in this study while limited test parameters were assessed by the regulatory authority. This finding insights the need for rigorous post marketing surveillance that guarantees the veterinary medicines in use in Ethiopia are of appropriate quality.
4. Conclusion
As a conclusion, this investigation demonstrated that all tested albendazole and fenbendazole products were low quality products owing to non-compliant friability test and assay, insufficient drug release of needed dose and lack of dosage consistency. The existence of such veterinary anthelminthic drugs in the market contributes to treatment failure and the development of parasitic resistance. Therefore, remedial actions must be implemented towards proper execution of regulatory and legal frameworks. Quality control of veterinary anthelmintic medicines and enhancing the level and quality of veterinary services and routine monitoring of anthelmintic drug effectiveness are desperately required.
Author contribution statement
Melaku Getahun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ayenew Ashenef: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Belachew Bacha: Performed the experiments.
Belachew Tefera: Contributed reagents, materials, analysis tools or data.
Tadele Eticha: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Veterinary Drug and animal Feed Administration and Control Authority (VDFACA) currently renamed as Ethiopian Agricultural Authority and Ethiopian Food and Drug Administration (EFDA) for permitting us to conduct our work in their laboratories. The work was supported by the postgraduate studies thesis research support grant, Addis Ababa University, Ethiopia and Ministry of Innovation and Technology, Government of Ethiopia National Innovation Award to Ayenew Ashenef. However the funders did not have any role in the design of the experiments and publication of this research output.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Turner R.L. 2005. Livestock, Liberalization and Democracy: Constraints and Opportunities for Rural Livestock Producers in a Reforming Uganda. [Google Scholar]
- 2.Leta S., Mesele F. Spatial analysis of cattle and shoat population in Ethiopia: growth trend, distribution and market access. SpringerPlus. 2014;3(1):1–10. doi: 10.1186/2193-1801-3-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumsa B., Tolera A., Nurfeta A. Comparative efficacy of seven brands of albendazole against naturally acquired gastrointestinal nematodes in sheep in Hawassa, southern Ethiopia. Turk. J. Vet. Anim. Sci. 2010;34(5):417–425. [Google Scholar]
- 4.Getachew T., Muktar Y., Mekonnen N., Tesma F. Prevalence of gastrointestinal nematodes and efficacy of commonly used anthelmintics in different sheep breeds in Areka Agricultural Research Center, Areka, Ethiopia. Livest. Res. Rural Dev. 2016;28:117. [Google Scholar]
- 5.Desta A.H. Veterinary drugs handling, management and supply chain assessment in Afar pastoral region of North East Ethiopia. Am. J. Biosci. Bioeng. 2015;3(6):142–148. [Google Scholar]
- 6.Seear M., Gandhi D., Carr R., Dayal A., Raghavan D., Sharma N. The need for better data about counterfeit drugs in developing countries: a proposed standard research methodology tested in Chennai, India. J. Clin. Pharm. Therapeut. 2011;36(4):488–495. doi: 10.1111/j.1365-2710.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 7.OIE. (Health for animals global animal medicines association) 2017. Illegal Veterinary Medicines Impact and Effective Control. [Google Scholar]
- 8.LEGS. (Livestock Emergency Guidelines and Standards) 2020. The Quality of Veterinary Pharmaceuticals a Discussion Paper. [Google Scholar]
- 9.Khan M.H., Okumura J., Sovannarith T., Nivanna N., Akazawa M., Kimura K. Prevalence of counterfeit anthelminthic medicines: a cross‐sectional survey in Cambodia. Trop. Med. Int. Health. 2010;15(5):639–644. doi: 10.1111/j.1365-3156.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- 10.Roeber F., Jex A.R., Gasser R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance-an Australian perspective. Parasites Vectors. 2013;6(1):1–13. doi: 10.1186/1756-3305-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyoum Z., Demessie Y., Bogale B., Melaku A. Field evaluation of the efficacy of common anthelmintics used in the control of gastrointestinal nematodes of sheep in Dabat district, Northwest Ethiopia. Ir. Vet. J. 2017;70:1–8. doi: 10.1186/s13620-017-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifu A., Kebede E., Bacha B., Melaku A., Setegn T. Quality of albendazole tablets legally circulating in the pharmaceutical market of Addis Ababa, Ethiopia: physicochemical evaluation. BMC Pharmacol. Toxicol. 2019;20(1):1–7. doi: 10.1186/s40360-019-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desie S., Dejene G., Jemere B., Yifat D. Assessment of anthelmentic resistance in gastirointestinal nematodes of small ruminants, Dale district, Southern Ethiopia. J. Vet. Med. Anim. Health. 2013;5(9):257–261. [Google Scholar]
- 14.Wakayo B., Dewo T. Anthelmintic resistance of gastrointestinal parasites in small ruminants: a review of the case of Ethiopia. Vet. Sci. Technol. 2015;10(4) [Google Scholar]
- 15.Babushkina E.A., Belokopytova L.V., Grachev A.M., Meko D.M., Vaganov E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Change. 2017;17:1725–1737. [Google Scholar]
- 16.USP . vol. 27. 2015. USP 38/NF 33. USP Monographs: Albendazole Tablets. The United States Pharmacopoeial Convention; pp. 2066–2505. [Google Scholar]
- 17.USP . 2015. USP 38/NF33. USP Monograph: Ivermectin Tablets. The United states Pharmacopeial Convention; p. 3993. [Google Scholar]
- 18.ALS . MIDC; Tarapur, Boisar Dist-Palghar, India: 2017. ALS/FP/B/10/B. Ashish Life Science Pvt Limited: Fenbendazole Tablets/boluses. . J-137. [Google Scholar]
- 19.Allen . In: LV PN, Ansel HC Pharmaceutical Dosage Forms and Drugs Delivery System. ninth ed. Troy D.B., editor. Walters Kluwer/Lipincott Williams & Wilkins; 2009. p. 233. [Google Scholar]
- 20.USP . vol. 27. 2015. USP 38/NF 33. USP Monographs: Albendazole Tablets. The United States Pharmacopoeial Convention; pp. 2066–2505. [Google Scholar]
- 21.USP . The United States pharmacopeial Convention; 2015. USP 38/NF 33. USP General Chapters: Uniformity of Dosage Units; pp. 675–724. [Google Scholar]
- 22.Shirsand S., Suresh S., Swamy P. Formulation design and optimization of fast dissolving clonazepam tablets. Indian J. Pharmaceut. Sci. 2009;71(5):567. doi: 10.4103/0250-474X.58189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.USB . 2015. USP 38/NF 33. USP General Chapters: Tablet Friability. The United States Pharmacopeia Convention; p. 1432. [Google Scholar]
- 24.Abebe K., Beressa T.B., Yimer B.T. In-vitro evaluations of quality control parameters of paracetamol tablets marketed in Gondar city, Northwest Ethiopia. Drug Healthc. Patient Saf. 2020;12:273–279. doi: 10.2147/DHPS.S282420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suleman S., Zeleke G., Deti H., Mekonnen Z., Duchateau L., Levecke B., et al. Quality of medicines commonly used in the treatment of soil transmitted helminths and giardia in Ethiopia: a nationwide survey. PLoS Neglected Trop. Dis. 2014;8(12):e3345. doi: 10.1371/journal.pntd.0003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitzer M., Klapper S., Mazigo H.D., Holzgrabe U., Mueller A. Quality and composition of albendazole, mebendazole and praziquantel available in Burkina Faso, Côte d’Ivoire, Ghana and Tanzania. PLoS Neglected Trop. Dis. 2021;15(1) doi: 10.1371/journal.pntd.0009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belew S., Suleman S., Wynendaele E., D’Hondt M., Kosgei A., Duchateau L., et al. Quality of anthelminthic medicines available in Jimma Ethiopia. Acta Trop. 2018;177:157–163. doi: 10.1016/j.actatropica.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Othman G.Q. Quality assessment of seven brands of albendazole tablets marketed in Yemen. Yemeni J. Med. Sci. 2017;11(1):46–52. [Google Scholar]
- 29.Martins J.M., Farinha A. Uniformity of dosage units—comparative study of methods and specifications between Eur. Pharm. 3rd and USP 23. J. Pharmaceut. Biomed. Anal. 1998;18(4–5):487–495. doi: 10.1016/s0731-7085(98)00269-6. [DOI] [PubMed] [Google Scholar]
- 30.Matsyagiri L., Kumar B.P., Pranitha D., Kumar P.V. Design and evaluation of albendazole matrix tablets for colon specific drug release. Adv. J. Pharm. Life Sci. Res. 2014;4:17–28. [Google Scholar]
- 31.Gberindyer F.A., Onyeyili P.A., Bosha J.A. Quality control properties of some brands of veterinary albendazole boluses common in Nigeria. J. Pharm. Pharmacol. 2014;2:135–139. [Google Scholar]
- 32.Khan M.H., Hatanaka K., Sovannarith T., Nivanna N., Casas L.C.C., Yoshida N., et al. Effects of packaging and storage conditions on the quality of amoxicillin-clavulanic acid–an analysis of Cambodian samples. BMC Pharm. Toxicol. 2013;14:1–7. doi: 10.1186/2050-6511-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takano R., Furumoto K., Shiraki K., Takata N., Hayashi Y., Aso Y., et al. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs; prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharmaceut. Res. 2008;25:2334–2344. doi: 10.1007/s11095-008-9637-9. [DOI] [PubMed] [Google Scholar]
- 34.Fidelis G.A., Oladipo O.O., Sunday O.C. Indo American Journal of Pharmaceutical Research; 2017. In-vitro Dissolution and Some Physical Properties of Two Generics of Levamisole Bolus Formulations for Large Animals. ISSN NO: 2231-6876. [Google Scholar]
- 35.Nasrin N., Asaduzzaman M., Mowla R., Rizwan F., Alam A. A comparative study of physical parameters of selected ketorolac tromethamine tablets available in the pharma market of Bangladesh. J. Appl. Pharmaceut. Sci. 2011:101–103. [Google Scholar]
- 36.Tefera B., Bacha B., Belew S., Ravinetto R., Andualem T., Abegaz Z., et al. Study on identification, assay and organoleptic quality of veterinary medicines in Ethiopia. J. Pharm. Policy Pract. 2022;15(1):17. doi: 10.1186/s40545-022-00410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.