Abstract
Purpose
To examine the association between choroidal thickness and myopic maculopathy in a general Japanese population.
Design
Population-based cross-sectional study.
Participants
A total of 2841 residents of a Japanese community aged ≥ 40 years, who consented to participate and had available data of choroidal thickness and fundus photographs, were enrolled in this study.
Methods
The choroidal thickness was measured by swept-source OCT. Participants were divided into quartiles of choroidal thickness. Myopic maculopathy was defined according to the classification system of the Meta-analysis of Pathologic Myopia Study Group. Main outcome measures were odds ratios (ORs) of choroidal thickness for prevalent myopic maculopathy. The ORs and 95% confidence intervals (CIs) were estimated using a logistic regression model.
Main Outcome Measures
Prevalent myopic maculopathy.
Results
Eighty-one participants had myopic maculopathy (45 diffuse chorioretinal atrophy, 31 patchy chorioretinal atrophy, and 5 macular atrophy). Individuals in the lowest quartile of choroidal thickness had a significantly greater OR for the presence of myopic maculopathy than those in the highest quartile of choroidal thickness (OR: 4.78 [95% CI: 1.78–16.72]) after adjusting for confounders, including axial length. The sensitivity analysis among the 1176 myopic individuals with axial length of ≥ 24.0 mm also showed that thinner choroidal thickness was significantly associated with prevalent myopic maculopathy.
Conclusions
The present study demonstrated the significant inverse association between choroidal thickness and the likelihood of myopic maculopathy, suggesting that the measurement of choroidal thickness in addition to axial length would be useful for assessing the risk of myopic maculopathy and elucidating its pathogenesis.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: Choroidal thickness, Myopia, Myopic maculopathy, Population-based study, SS-OCT
Myopic maculopathy is one of the most important complications of myopia for middle-aged and older people, often causing significant visual impairment.1, 2, 3, 4 The prevalence of myopic maculopathy has been estimated to increase globally owing to the rising prevalence of myopia among the world population.5,6 Therefore, the prevention and early treatment of myopic maculopathy are important issues to reduce the burden of this condition worldwide. However, the pathogenesis of myopic maculopathy has not been fully elucidated, and a curative treatment has not been established.
The choroidal layer is a tissue filled with blood vessels and delivers nutrients to photoreceptor cells in the outer retina. Recently, the thickness of the choroidal layer has been measured noninvasively, quantitatively, and reproducibly by using swept-source OCT (SS-OCT).7,8 Some neuropathological studies using a chick model of high myopia and myopic maculopathy have revealed the reduced choroidal blood flow in these conditions.9,10 Several hospital-based epidemiological studies have reported that the thinning of these choroidal layers is associated with a higher risk of the presence and the progress of myopic maculopathy.11, 12, 13, 14 These findings suggest that the choroidal thinning plays an important role in the pathogenesis of myopic maculopathy. On the other hand, these hospital-based studies were conducted on a relatively limited sample size of patients with high myopia or those with more advanced myopic maculopathy, since patients in the early stage of myopic maculopathy are less likely to visit the ophthalmic hospital because they have fewer ocular symptoms such as visual impairment. In addition, there has been only 1 population-based study addressing this issue, and that study enrolled only participants with high myopia with an axial length of ≥ 26 mm.15 Therefore, we thought that it would be of great value to examine this issue using the survey data from a community-based epidemiological study that included individuals in different stages of myopic maculopathy ranging from early to advanced, with consideration for the mutual influence of axial length, including axial length < 26 mm, on the myopic maculopathy.
The Hisayama Study is an ongoing, population-based epidemiological study of noncommunicable diseases, including cardiovascular disease, dementia, and eye disease, in a Japanese community.16 The present study sought to investigate the association between choroidal thickness and myopic maculopathy by using SS-OCT data from approximately 3000 people living in a community in Japan.
Materials and Methods
Study Population
The Hisayama Study was established in 1961 in the town of Hisayama, a suburban community adjacent to Fukuoka City in a metropolitan area of Kyushu Island in southern Japan.16 As a part of the study, an epidemiologic study of eye disease among the residents ≥ 40 years has been underway since 1998.17 In 2017, a total of 3246 Hisayama residents aged ≥ 40 years received the eye examinations (participation rate: 65.7% of all residents of the relevant age group). After excluding 7 individuals who did not provide their consent for study participation, 386 individuals without available data of choroidal thickness and with ungradable eye-photographs, and 12 individuals with poor-quality SS-OCT scans, 2841 individuals (1255 men and 1586 women) were enrolled in the present study.
Measurements of Choroidal Thickness Levels and Other Ophthalmic Examination
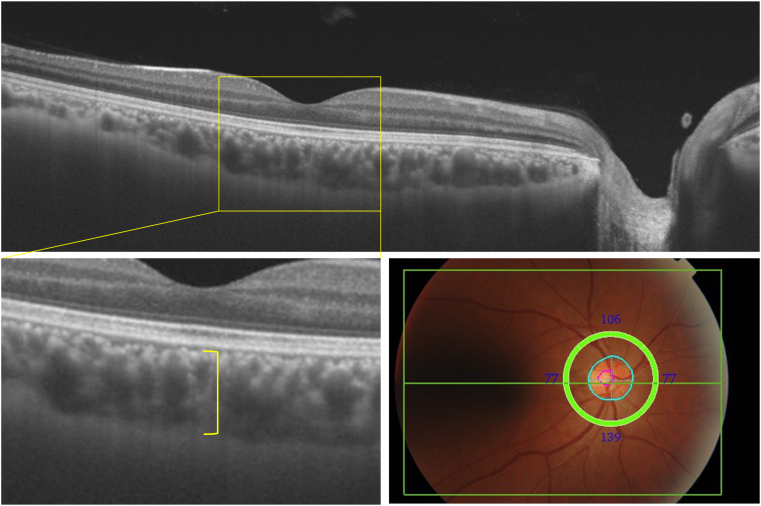
In 2017, imaging was performed using an SS-OCT instrument, for example, DRI-OCT Triton (Topcon Corporation). The principles of SS-OCT have been well described.18,19 Wide scanning over a 12 × 9 mm area was performed at a scan density of 512 A-scans (horizontal) × 128. The thickness of the choroid was evaluated at the subfoveal region, which was measured vertically from the outer border of a hyperreflective line corresponding to the retinal pigment epithelium to the inner border of the sclera (Figure 1). Image quality values < 30 (as recommended by the manufacturer) were excluded from the analyses. All scans were reviewed, and 2 ophthalmologists (E.U. and K.F.), who were masked to the excluded poor-quality scans, manually corrected the centering of all images if necessary. Choroidal thickness levels were divided into 4 quartile categories (Q1: < 138.5 μm; Q2: 138.5–197.8 μm; Q3: 197.9–267.4 μm; and Q4: ≥ 267.5 μm). At the other ophthalmic examination, measurements of the axial lengths and color fundus photography of both eyes were performed for each participant. Axial length measurements were performed with noncontact partial coherence laser interferometry (OA-2000; Tomey GmbH). Objective refraction was measured using an AR-660 automatic refractometer (Nidek) without cycloplegia. A spherical equivalent refraction was used to calculate refractive error and was defined as a sphere plus half of the cylindrical refraction. Each participant underwent ophthalmic examination after pupil dilatation with 1.0% tropicamide and 10% phenylephrine. Nonstereoscopic fundus photographs (45°) were taken using a Topcon digital fundus camera (DRI-OCT Triton; Topcon Corporation). We photographed 1 field, centered at a point midway between the temporal edge of the optic disc and the fovea in both eyes.
Figure 1.
Measurements of choroidal layer thickness on swept-source OCT, 2017. The thickness of the choroid was evaluated at the subfoveal region (yellow lines), which was measured vertically from the outer border of a hyperreflective line corresponding to the retinal pigment epithelium to the inner border of the sclera. Wide scanning over a 12 × 9 mm area (green rectangle) and a circle with a diameter of 3.4 mm centered on the optic disc (green circle, blue circle, pink circle) was performed at a scan density of 512 A-scans (horizontal) × 128.
Definition of Myopic Maculopathy
The presence of myopic maculopathy was determined by using the color fundus photograph. Myopic maculopathy was defined by myopic macular changes equal to or more serious than diffuse chorioretinal atrophy and was graded into 3 categories: diffuse chorioretinal atrophy (category 2), patchy chorioretinal atrophy (category 3), and macular atrophy (category 4), according to the classification system from the Meta-analysis of Pathologic Myopia Study Group (Supplemental figure 2).20 Three additional features supplementing these categories were defined as plus lesions (including lacquer cracks, myopic choroidal neovascularization, and Fuchs spot). When 1 eye of a participant was categorized differently than the other, the more severe category was assigned. All photographs were evaluated independently by 2 experienced ophthalmologists (E.U. and S.H.). When their judgments disagreed, the photographs were reexamined by 3 retinal specialists (E.U., S.H., and M.Y.) and the final judgment was determined after discussion. Clinical data of participants were blinded in the process of the photograph evaluation.
Data Collection
Body height was measured in light clothing without shoes. Blood pressure was measured 3 times after the individual had rested for ≥ 5 minutes in the sitting position. The average of the 3 measurements was used for the analysis. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of antihypertensive medication. Blood samples were collected from an antecubital vein after an overnight fast of ≥ 12 hours. After taking the fasting blood specimen, a 75-g oral glucose tolerance test was performed with a 75-g glucose equivalent carbohydrate load (Trelan G; Shimizu Pharmaceutical Inc). Diabetes mellitus was defined as follows: fasting glucose level ≥ 7.0 mmol/L, casual or 2-hour postload glucose levels ≥ 11.1 mmol/L, and/or use of antidiabetic medications. Serum total cholesterol was determined enzymatically using an autoanalyzer (TBA-80S; Toshiba Inc). Information on smoking habits and alcohol intake was obtained using a standard questionnaire by trained interviewers at the initial examination (J.H. and T.N.). Smoking habits and alcohol intake were classified as either current habitual use or not.
Statistical Methods
The SAS software package version 9.4 (SAS Institute) was used to carry out all statistical analyses. The age- and sex-adjusted frequencies or mean values of risk factors across the quantiles of choroidal thickness were computed by using a logistic or linear regression analysis, respectively. The odds ratios (ORs) and their 95% confidence intervals (CIs) of the quantiles of choroidal thickness levels for the presence of myopic maculopathy were estimated by using a logistic regression model. In the multivariable-adjusted analysis, the following covariates were included in the relevant model: age, sex, height, axial length, hypertension, diabetes mellitus, serum total cholesterol, smoking habits, and drinking habits. Essentially, we used data gathered from the right eye for the analysis of choroidal thickness and axial length but data from the left eye when only the left eye showed myopic maculopathy. To estimate the shape of the associations between choroidal thickness and the risk of the presence of myopic maculopathy, a logistic regression analysis with restricted cubic splines was also performed, where 3 knots were placed at the 10th, 50th, and 90th percentiles of the choroidal thickness (96.5, 197.8, and 330.7 μm) and the 50th percentile was set as the reference value.21 A 2-tailed value of P < 0.05 was considered statistically significant in all analyses.
Ethical Considerations
This study was approved by the Kyushu University Institutional Review Board for Clinical Research (approval number: 2021-457), and was carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Results
The mean (standard deviation) of choroidal thickness was 205.8 (88.2) μm. Among the study participants, 81 participants (2.9%) had myopic maculopathy. With regard to the category of myopic maculopathy, 45 participants (1.6%) presented with diffuse chorioretinal atrophy (category 2), 31 participants (1.1%) with patchy chorioretinal atrophy (category 3), and 5 participants (0.2%) with macular atrophy (category 4).
The age- and sex-adjusted baseline characteristics of the study population according to each quantile of thickness are shown in Table 1. The mean age, axial length and height, and the ratio of women increased significantly with lower levels of choroidal thickness (P for trend < 0.05 for all).
Table 1.
Age- and Sex-Adjusted Baseline Characteristics of Participants According to the Quartiles of Choroidal Thickness, 2017
| Variable | Choroidal Thickness Levels (μm) |
P for Trend | |||
|---|---|---|---|---|---|
| < 138.5 |
138.5–197.8 |
197.9–267.4 |
≥ 267.5 |
||
| (n = 710) | (n = 710) | (n = 711) | (n = 710) | ||
| Age, mean (SE), yrs | 69 (0.4) | 64 (0.4) | 62 (0.4) | 58 (0.4) | < 0.001 |
| Women, % | 58.9 | 57.5 | 54.7 | 52.0 | 0.01 |
| Axial length, mean (SE), mm | 24.6 (0.04) | 24.0 (0.04) | 23.8 (0.04) | 23.3 (0.04) | 0.01 |
| Height, mean (SE), cm | 159.1 (9.1) | 159.2 (9.3) | 158.8 (9.5) | 158.5 (9.2) | < 0.001 |
| Hypertension, % | 47.9 | 49.5 | 46.9 | 45.8 | 0.35 |
| Diabetes mellitus, % | 17.2 | 17.7 | 16.5 | 16.6 | 0.63 |
| Serum total cholesterol, mean (SE), mmol/L | 5.37 (0.03) | 5.38 (0.03) | 5.41 (0.03) | 5.38 (0.03) | 0.69 |
| Smoking habits, % | 47.3 | 44.2 | 44.5 | 49.6 | 0.52 |
| Drinking habits, % | 71.9 | 70.9 | 67.7 | 68.6 | 0.11 |
SE = standard error.
All values are given as the mean or as a percentage adjusted for age and sex.
The mean age was sex-adjusted. Percentage of women was age-adjusted.
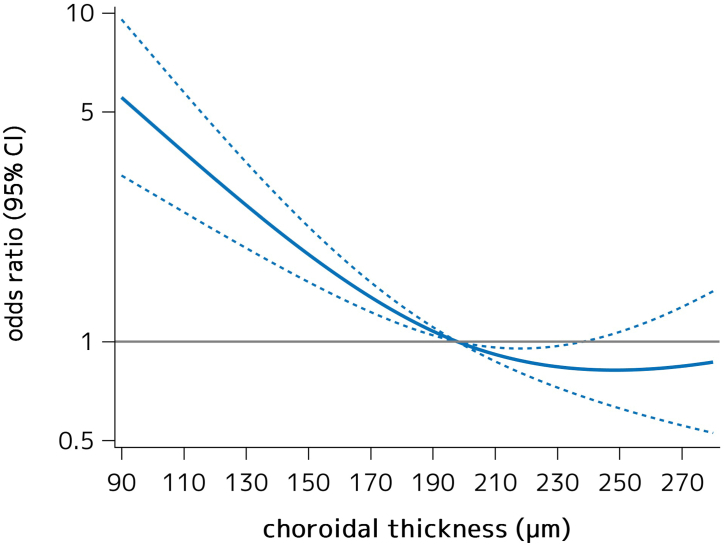
Table 2 shows the age-, sex-, and axial-length-adjusted and multivariable-adjusted ORs for the presence of myopic maculopathy according to the quantiles of choroidal thickness. In the age-, sex-, and axial length-adjusted model, the ORs of prevalent myopic maculopathy significantly increased in individuals in the lowest quartile of choroidal thickness, as compared with those in the highest quartile (OR = 4.83, 95% CI = 1.80–16.90, P = 0.005). These associations were unchanged after adjustment for age, sex, axial length, height, hypertension, diabetes mellitus, serum total cholesterol, smoking habits, and drinking habits (OR = 4.94, 95% CI = 1.80–17.63, P = 0.004). Furthermore, we assessed the shape of the associations between choroidal thickness and the presence of myopic maculopathy by using a logistic regression analysis with restricted cubic spline analyses, and found that the multivariable-adjusted OR of the presence of myopic maculopathy tended to gradully increase with lower choroidal thickness from about ≤ 190 μm (Figure 3).
Table 2.
Adjusted Odds Ratios and Their 95% CIs for the Presence of Myopic Maculopathy According to the Quartiles of Choroidal Thickness, 2017
| Choroidal Thickness Levels (μm) | No. of Events/Individuals | Age-, Sex-, and Axial Length-Adjusted |
Multivariable-Adjusted∗ |
||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | ||
| Q1 (< 138.5) | 62/710 | 4.83 (1.80–16.90) | 0.005 | 4.94 (1.80–17.63) | 0.004 |
| Q2 (138.5–197.8) | 10/710 | 1.11 (0.34–4.27) | 0.87 | 1.13 (0.34–4.44) | 0.84 |
| Q3 (197.9–267.4) | 5/711 | 0.66 (0.16–2.90) | 0.57 | 0.60 (0.13–2.73) | 0.50 |
| Q4 (≥ 267.5) | 4/710 | 1.00 (reference) | 1.00 (reference) | ||
| P for trend | < 0.001 | < 0.001 | |||
CI = confidence interval.
Adjusted for age, sex, axial length, height, hypertension, diabetes mellitus, serum total cholesterol, smoking habits, and drinking habits.
Figure 3.
A logistic regression analysis with restricted cubic splines for the association between choroidal thickness and the multivariable-adjusted odds ratio of the presence of myopic maculopathy, 2017. Solid lines represent the odds ratio and dashed lines represent the 95% confidence interval (CI). Knots were placed at the 10th, 50th, and 90th percentiles of the choroidal thickness (96.5, 197.8, and 330.7 μm). A reference point was set at 197.8 μm of the choroidal thickness. The risk estimates were adjusted for age, sex, height, axial length, hypertension, diabetes mellitus, serum total cholesterol, smoking habits, and drinking habits.
We also examined the association between choroidal thickness and each category of myopic maculopathy (Table 3). Among the 81 individuals with myopic maculopathy, the numbers of individuals with diffuse chorioretinal atrophy (category 2), patchy chorioretinal atrophy (category 3), and macular atrophy (category 4) were 45, 31, and 5. Individuals in the first quartile were significantly more likely to have diffuse chorioretinal atrophy (category 2) and patchy chorioretinal atrophy (category 3) than those in the second to fourth quartiles after adjusting for potential confounders, including axial length (OR = 7.99, 95% CI = 3.69–17.26, P < 0.001 for diffuse chorioretinal atrophy; OR = 6.12, 95% CI = 2.28–16.38, P < 0.001 for patchy chorioretinal atrophy). The association between choroidal thickness and macular atrophy (category 4) could not be assessed due to the small number of participants with macular atrophy (category 4) in the present study.
Table 3.
Adjusted Odds Ratios and Their 95% CIs for the Presence of Each Category for Myopic Maculopathy in Choroidal Thickness Level, 2017
| No. of Events/Individuals |
Age-, Sex-, and Axial Length-Adjusted |
Multivariable-Adjusted∗ |
||||
|---|---|---|---|---|---|---|
| Q1 (CT < 138.5 μm) | Q2–Q4 (CT ≥ 138.5 μm) | Odds Ratio (95% CI) for Q1 (vs. Q2–Q4) | P | Odds Ratio (95% CI) for Q1 (vs. Q2–Q4) | P | |
| Myopic maculopathy | 62/710 | 19/2131 | 5.19 (2.89–9.32) | < 0.001 | 5.39 (2.96–9.81) | < 0.001 |
| Diffuse chorioretinal atrophy (category 2) | 35/693† | 10/2122† | 7.64 (3.57–16.35) | < 0.001 | 7.99 (3.69–17.26) | < 0.001 |
| Patchy chorioretinal atrophy (category 3) | 25/708‡ | 6/2128‡ | 5.50 (2.09–14.47) | < 0.001 | 6.12 (2.28–16.38) | < 0.001 |
| Macular atrophy (category 4) | 2/710 | 3/2131 | NA | NA | ||
CI = confidence interval; CT = choroidal thickness; NA = not assessed.
Adjusted for age, sex, axial length, height, hypertension, diabetes mellitus, serum total cholesterol, smoking habits, and drinking habits.
Individuals with patchy chorioretinal atrophy (category 3) or macular atrophy (category 4) were excluded from the analysis.
Individuals with macular atrophy (category 4) were excluded from the analysis.
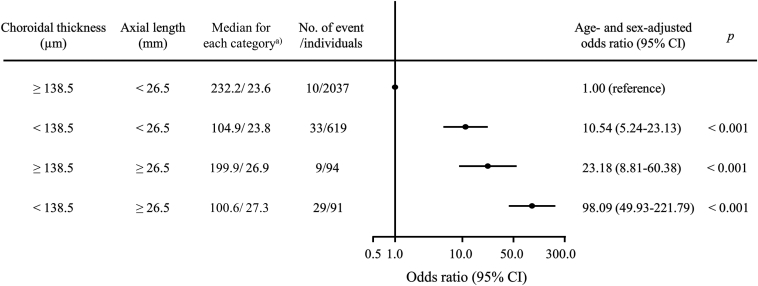
In addition, we estimated the combined influence of choroidal thickness levels and axial length levels on myopic maculopathy (Figure 4). We divided the individuals into 4 groups according to the status of longer axial length (axial length ≥ 26.5 mm) and the choroidal thickness values (the first quantile [< 138.5 μm] versus the others [≥ 138.5 μm]). When individuals with a choroidal thickness level of ≥ 138.5 μm and axial length of < 26.5 mm were used as a reference group, those with choroidal thickness of ≥ 138.5 μm and axial length level of ≥ 26.5 mm had significantly higher OR of myopic maculopathy. Moreover, the ORs for prevalent myopic maculopathy significantly increased in individuals with choroidal thickness of < 138.5 μm, even when they had axial length of < 26.5 mm (OR = 10.54, 95% CI = 5.24–23.13, P < 0.001), and the ORs for prevalent myopic maculopathy appeared to further increase in those with both choroidal thickness of < 138.5 μm and axial length of ≥ 26.5 mm (OR = 98.09, 95% CI = 49.93–221.79, P < 0.001).
Figure 4.
Age- and sex-adjusted odds ratios and 95% confidence intervals (CIs) (dots and lines) for the presence of myopic maculopathy according to choroidal thickness levels and axial length levels, 2017. a) Values are shown as a median value of choroidal thickness (μm)/axial length (mm) for each category.
As a sensitivity analysis, we investigated the association between choroidal thickness and the presence of myopic maculopathy after excluding individuals with glaucoma and retinal disease and found that similarly significant associations were also observed between choroidal thickness and myopic maculopathy (Supplemental table 4). In addition, we performed similar analyses among the 1176 myopic individuals with axial length of ≥ 24.0 mm, because individuals with an axial length of < 24.0 mm did not have myopic maculopathy (Supplemental Tables 5 and 6, Supplemental figure 5). As a consequence, myopic individuals in the lowest quartile of choroidal thickness were at significantly higher risk for the presence of myopic maculopathy than those in the highest quartile (Supplemental table 5), and there was a significant increase in the age- and sex-adjusted OR for the presence of myopic maculopathy in individuals with a choroidal thickness of < 138.5 μm as compared to those with a choroidal thickness of ≥ 138.5 μm and axial length of < 24.0 to 26.4 mm, regardless of axial length of 24.0 to 26.4 mm or ≥ 26.5 mm (Supplemental table 6). Similar significant associations were also observed among the 1089 individuals with a spherical equivalent refraction < −0.5 diopters (Supplemental table 7). In the restricted cubic spline analyses, the multivariable-adjusted OR of the presence of myopic maculopathy tended to increase gradually with lower choroidal thickness from about ≤ 190 μm among myopic individuals (Supplemental figures 5 and 6).
Discussion
The present study clearly demonstrated that lower choroidal thickness estimated by using SS-OCT was significantly associated with greater likelihood of myopic maculopathy after adjusting for potential confounding factors, including axial length, in a general Japanese population. Regarding the categories of myopic maculopathy, the risk of diffuse and patchy atrophy significantly increased with the thinning of choroidal thickness levels. Most notably, the present study found that individuals with lower choroidal thickness were at higher likelihood of myopic maculopathy even if they had relatively mild-to-moderate myopia with axial length of 24.0 to 26.5 mm; their risk of prevalent myopic maculopathy was further increased if they had both lower choroidal thickness and longer axial length of ≥ 26.5 mm. These findings suggested that the measurement of choroidal thickness provides additional information beyond that provided by axial length for assessing myopic maculopathy and elucidating its pathological processes.
Our results showed that lower choroidal thickness was significantly associated with the presence of myopic maculopathy. In several hospital-based cross-sectional studies, individuals with myopic maculopathy were found to have lower choroidal thickness than those without myopic maculopathy.11, 12, 13 A hospital-based longitudinal study also reported that lower choroidal thickness was significantly associated with the progression of myopic maculopathy over 2 years among patients with high myopia.14 In addition, 1 population-based study, the Shanghai Eye study, revealed that the OR for the presence of myopic maculopathy significantly increased with lower choroidal thickness in individuals with high myopia with axial length of ≥ 26.0 mm.15 Our findings were consistent with the results of these previous studies. On the other hand, the Shanghai Eye study enrolled only 496 participants with high myopia with an axial length of ≥ 26 mm. The present study enrolled 2841 residents of a Japanese community in different stages of myopic maculopathy ranging from early to advanced, including axial length < 26 mm (1176 of whom had myopia) and showed that the multivariable-adjusted risk of having myopic maculopathy was significantly higher with lower choroidal thickness in individuals with axial length of < 26 mm and increased steeply with the combination of thinner choroidal thickness and axial length of ≥ 26 mm. Therefore, it is possible to examine the association between choroidal thickness and myopic maculopathy with consideration for the mutual influence of axial length on the myopic maculopathy. The present study also revealed that lower choroidal thickness was significantly associated with diffuse chorioretinal atrophy and patchy chorioretinal atrophy, which are observed during the early stage on the progression patterns of myopic maculopathy. These findings suggest that choroidal thinning may play a key role in the early stage of myopic maculopathy.
The exact mechanisms underlying the association of choroidal thickness with the likelihood of the presence of myopic maculopathy are unclear, but changes in the choroidal vascular structure may be involved in the link. Animal experiments using a chick model with myopic maculopathy have reported the obliteration of precapillary arterioles or postcapillary venules and fibrotic changes in the choroid.9,10 Several clinical studies using indocyanine green angiograms revealed a decrease in the number of choroidal arteries and veins in the chorioretinal atrophic lesions in individuals with myopic maculopathy.22,23 Other clinical studies using OCT angiography have also demonstrated the association between choroidal thickness and flow reduction in the choriocapillaris within the group of myopic eyes.24,25 In addition, Pang C.E. et al have reported that there is a group of myopia patients with severe choroidal thinning in which myopic maculopathy never develops, and they retain flow of the inner choroidal circulation, which provides oxygen and support to the outer nuclear layer and photoreceptor layer in the retina.26 Therefore, myopic chorioretinal atrophy may be caused by ischemia in the posterior fundus. Intriguingly, the present study found no significant associations between choroidal thickness and systematic vascular risk factors known to cause ischemia in the retina, such as diabetes and hypertension. Taken together, these findings support the idea that the choroidal localized vasculature, rather than systemic vascular change, may cause choroidal thinning and may be the main player in the pathogenesis of myopic maculopathy.
Importantly, the present study revealed that individuals with lower choroidal thickness were at greater risk of the presence of myopic maculopathy, even if they had relatively mild-to-moderate myopia with an axial length of 24.0 to 26.5 mm, and that the risk of myopic maculopathy was further increased in those with choroidal thinning combined with a longer axial length, which is widely known to be a major risk factor for myopic maculopathy.27, 28, 29, 30, 31 Several pathological studies have revealed that mechanical stretching stress due to the elongation of axial length can lead to rupture of the capillary network and attenuation of fibroblasts in the choroidal layer.32,33 Therefore, our findings suggest that choroidal thinning and longer axial length may contribute to the etiology of myopic maculopathy through the choroidal vascular changes and ocular stretching stress.
The strengths of our study are the population-based design, the large sample size with advanced SS-OCT measurement of choroidal thickness, the accurate determination of myopic maculopathy by expert ophthalmologists, and the detailed evaluation of confounding factors. However, there are potential limitations that should be noted. First, the current study was a cross-sectional study, and thus an interpretation of the causal relationship between choroidal thickness and the presence of myopic maculopathy could not be made. Second, the prevalence and classification of myopic chorioretinal atrophy were determined using only fundus photography, not fluorescein and indocyanine green angiography, and this could have led to an underestimation of ocular lesions related to myopic maculopathy, such as the plus lesions. However, since the diagnosis of myopic maculopathy depended predominantly on the ophthalmoscopic appearance of the fundus on the fundus photographs, we believe that this limitation may not have exerted a meaningful influence on our findings. Third, we could not assess the association of choroidal thickness with the presence of macular atrophy (category 4) and the plus lesions because of the small number of individuals with these lesions. Macular atrophy (category 4) and the plus lesions have been acknowledged to be strongly associated with visual impairment and central vision loss.34 Some clinical studies have reported that choroidal thinning was linked with the presence of these lesions.35,36 Further large-scale studies will be needed to elucidate this issue. Finally, the generalizability of our findings may be limited, because the present study was conducted in only 1 community of Japan.
In conclusion, the present data revealed that lower choroidal thickness on SS-OCT was significantly associated with a higher likelihood of myopic maculopathy in a general Japanese population. Moreover, it is noteworthy that the risk of the presence of myopic maculopathy was high even in individuals with relatively mild-to-moderate myopia with an axial length of 24.0 to 26.5 mm, and the combination of choroidal thinning and longer axial length further increased the risk of myopic maculopathy. Choroidal thinning in combination with longer axial length has been proposed to play an important role in the pathogenesis of myopic maculopathy. Further prospective studies are required to validate the findings of this study, particularly the potential use of choroidal thickness to predict the development of myopic maculopathy.
Manuscript no. XOPS-D-23-00018.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors made the following disclosures:
This study was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (JSPS KAKENHI Grant Number JP21H03200, JP19K07890, JP20K10503, JP20K11020, JP21K07522, JP21K11725, JP21K10448, JP22K07421, JP22K09767, and JP22K17396); by the Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (JPMH20FA1002); and by the Japan Agency for Medical Research and Development (JP22dk0207053). The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: This study was approved by the Kyushu University Institutional Review Board for Clinical Research (approval number: 2021-457), and was carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants. No animal subjects were used in this study.
Author Contributions:
Conception and design: Ueda, Yasuda, Ninomiya, Sonoda; Data collection: Ueda, Yasuda, Fujiwara, Hashimoto, Honda, Nakamura, Hata, Ninomiya, Sonoda
Analysis and interpretation: Ueda, Yasuda, Ninomiya, Sonoda
Obtained funding: N/A; Overall responsibility: Ueda, Yasuda, Fujiwara, Hashimoto, Honda, Nakamura, Hata, Ninomiya, Sonoda
Supplementary data
References
- 1.Iwase A., Araie M., Tomidokoro A., et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113:1354–1362. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Xu L., Wang Y., Li Y., et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing eye study. Ophthalmology. 2006;113:1134–1145. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Buch H., Vinding T., La Cour M., et al. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City eye study. Ophthalmology. 2004;111:53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Cotter S.A., Varma R., Ying-Lai M., et al. Causes of low vision and blindness in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2006;113:1574–1582. doi: 10.1016/j.ophtha.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Ueda E., Yasuda M., Fujiwara K., et al. Trends in the prevalence of myopia and myopic maculopathy in a Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 2019;60:2781–2786. doi: 10.1167/iovs.19-26580. [DOI] [PubMed] [Google Scholar]
- 6.Holden B.A., Fricke T.R., Wilson D.A., et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Obadă O., Pantalon A.D., Rusu-Zota G., et al. Posterior choroidal boundary morphology and segmentation errors influence on choroidal thickness assessment in diabetic patients - a swept-source OCT study. Rom J Ophthalmol. 2021;65:222–229. doi: 10.22336/rjo.2021.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansouri K., Medeiros F.A., Tatham A.J., et al. Evaluation of retinal and choroidal thickness by swept-source optical coherence tomography: repeatability and assessment of artifacts. Am J Ophthalmol. 2014;157:1022–1032. doi: 10.1016/j.ajo.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata A., Negi A. Lacquer crack lesions in experimental chick myopia. Graefes Arch Clin Exp Ophthalmol. 1998;236:138–145. doi: 10.1007/s004170050054. [DOI] [PubMed] [Google Scholar]
- 10.Shih Y.F., Fitzgerald M.E., Reiner A. Choroidal blood flow is reduced in chicks with ocular enlargement induced by corneal incisions. Curr Eye Res. 1993;12:229–237. doi: 10.3109/02713689308999468. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X., Ding X., Lyu C., et al. Morphological characteristics and visual acuity of highly myopic eyes with different severities of myopic maculopathy. Retina. 2020;40:461–467. doi: 10.1097/IAE.0000000000002418. [DOI] [PubMed] [Google Scholar]
- 12.Wang N.K., Lai C.C., Chu H.Y., et al. Classification of early dry-type myopic maculopathy with macular choroidal thickness. Am J Ophthalmol. 2012;153:669–677. doi: 10.1016/j.ajo.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y., Du R., Nagaoka N., et al. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology. 2019;126:1018–1032. doi: 10.1016/j.ophtha.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Wang W., Liu R., et al. Choroidal thickness predicts progression of myopic maculopathy in high myopes: a 2-year longitudinal study. Br J Ophthalmol. 2021;105:1744–1750. doi: 10.1136/bjophthalmol-2020-316866. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q., He J., Hu G., et al. Morphological characteristics and risk factors of myopic maculopathy in an older high myopia population-based on the new classification system (ATN) Am J Ophthalmol. 2019;208:356–366. doi: 10.1016/j.ajo.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya T. Japanese legacy cohort studies: the Hisayama study. J Epidemiol. 2018;28:444–451. doi: 10.2188/jea.JE20180150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda M., Kiyohara Y., Hata Y., et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology. 2009;116:2135–2140. doi: 10.1016/j.ophtha.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Huang D., Swanson E.A., Lin C.P., et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choma M., Sarunic M., Yang C., et al. Sensitivity advantage of swept source and fourier domain optical coherence tomography. Opt Express. 2003;11:2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 20.Ohno-Matsui K., Kawasaki R., Jonas J.B., et al. META-analysis for pathologic myopia (META-PM) study group. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–883. doi: 10.1016/j.ajo.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Durrleman S., Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama M., Ohno-Matsui K., Futagami S., et al. Morphology and long-term changes of choroidal vascular structure in highly myopic eyes with and without posterior staphyloma. Ophthalmology. 2007;114:1755–1762. doi: 10.1016/j.ophtha.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Quaranta M., Arnold J., Coscas G., et al. Indocyanine green angiographic features of pathologic myopia. Am J Ophthalmol. 1996;122:663–671. doi: 10.1016/s0002-9394(14)70484-2. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sheikh M., Phasukkijwatana N., Dolz-Marco R., et al. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Invest Ophthalmol Vis Sci. 2017;58:2063–2069. doi: 10.1167/iovs.16-21289. [DOI] [PubMed] [Google Scholar]
- 25.Su L., Ji Y.S., Tong N., et al. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258:1173–1180. doi: 10.1007/s00417-020-04639-2. [DOI] [PubMed] [Google Scholar]
- 26.Pang C.E., Sarraf D., Freund K.B. Extreme choroidal thinning in high myopia. Retina. 2015;35:407–415. doi: 10.1097/IAE.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 27.Ueda E., Yasuda M., Fujiwara K., et al. Five-year incidence of myopic maculopathy in a general Japanese population: the Hisayama study. JAMA Ophthalmol. 2020;138:887–893. doi: 10.1001/jamaophthalmol.2020.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asakuma T., Yasuda M., Ninomiya T., et al. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama study. Ophthalmology. 2012;119:1760–1765. doi: 10.1016/j.ophtha.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto S., Yasuda M., Fujiwara K., et al. Association between axial length and myopic maculopathy: the Hisayama study. Ophthalmol Retina. 2019;3:867–873. doi: 10.1016/j.oret.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Chang L., Pan C.W., Ohno-Matsui K., et al. Myopia-related fundus changes in Singapore adults with high myopia. Am J Ophthalmol. 2013;155:991–999. doi: 10.1016/j.ajo.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka N., Ohno-Matsui K., Saka N., et al. Clinical characteristics of patients with congenital high myopia. Jpn J Ophthalmol. 2011;55:7–10. doi: 10.1007/s10384-010-0896-8. [DOI] [PubMed] [Google Scholar]
- 32.Hirata A., Negi A. Morphological changes of choriocapillaris in experimentally induced chick myopia. Graefes Arch Clin Exp Ophthalmol. 1998;236:132–137. doi: 10.1007/s004170050053. [DOI] [PubMed] [Google Scholar]
- 33.Shih Y.F., Fitzgerald M.E., Norton T.T., et al. Reduction in choroidal blood flow occurs in chicks wearing goggles that induce eye growth toward myopia. Curr Eye Res. 1993;12:219–227. doi: 10.3109/02713689308999467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang Y., Yokoi T., Nagaoka N., et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;12:863–877. doi: 10.1016/j.ophtha.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang N.K., Lai C.C., Chou C.L., et al. Choroidal thickness and biometric markers for the screening of lacquer cracks in patients with high myopia. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikuno Y., Jo Y., Hamasaki T., et al. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci. 2010;51:3721–3725. doi: 10.1167/iovs.09-3493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.