Summary
Respiratory syncytial virus (RSV) infection in infants and toddlers is a major public health problem. Here, we provide a protocol for neonatal RSV infection in mice and immune analysis of infected lungs and bronchoalveolar lavage (BAL) fluid. We describe steps for anesthesia and intranasal inoculation, weight monitoring, and whole lung collection. We then detail BAL fluid immune and whole lung analyses. This protocol can be used for neonatal pulmonary infection with other viruses or bacteria.
Subject areas: Flow Cytometry/Mass Cytometry, Immunology, Microbiology, Model Organisms
Graphical abstract

Highlights
-
•
Steps for infecting neonatal mice with respiratory syncytial virus
-
•
BAL fluid isolation and in-depth flow cytometry staining procedures
-
•
Guide to analyzing flow cytometry data from neonatal BAL samples
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Respiratory syncytial virus (RSV) infection in infants and toddlers is a major public health problem. Here, we provide a protocol for neonatal RSV infection in mice and immune analysis of infected lungs and bronchoalveolar lavage (BAL) fluid. We describe steps for anesthesia and intranasal inoculation, weight monitoring, and whole lung collection. We then detail BAL fluid immune and whole lung analyses. This protocol can be used for neonatal pulmonary infection with other viruses or bacteria.
Before you begin
Institutional permissions for animal experiment
All experiments were conducted in accordance with procedures approved by the Institutional Animal Care and Use Committee (IACUC) of Kansas State University, Manhattan, Kansas, USA.
Use of infectious agents was approved by the Institutional Board of Committee (IBC), Kansas State University.
The protocol below describes the steps involved in intranasal inoculation of neonates for respiratory syncytial virus infection and isolation of bronchoalveolar lavage fluid (BALF). The protocol also describes the isolation and analyses of infected neonatal lungs.
Mice
The lung immune cells are affected by respiratory infections,1,2 microbiome,3 as well as various other factors like sex, age, and strain.4 C57BL/6J mice were purchased originally from Jackson laboratories. In-house breeding was performed in one of our animal facilities to expand the colony. Around, 8-week-old male and females were paired for two weeks to get the female pregnant. Male was removed and the female was transferred to the BSL-2 facility and waited until the 7th day (P7) of litters.
Prepare buffers
Timing: 60 min
-
1.
Prepare the necessary buffers before starting the experiment. Recipes and storage conditions for the buffers can be found in the materials and equipment section.
Antibody panel preparation
Timing: 30 min
-
2.
Prepare flow cytometry antibody panels for marker proteins (Table 1).
CRITICAL: Antibodies need to be prepared immediately before the staining step as a master mixture.
Note: Detailed staining panel information and dilution factors can be found in the material and equipment section. Flow cytometry staining buffer will be used for marker proteins followed by fixative buffer to store the cells until data is acquired in the flow cytometer. All antibodies were titrated in a separate experiment to determine the optimal concentration.
Table 1.
Marker antibodies staining panel
| Fluorophore | Marker | Clone | Dilution factor |
|---|---|---|---|
| APC-Cy7 | CD45 | 30-F11 | 1:200 |
| PE | F4/80 | BM8 | 1:133 |
| APC | B220 | RA3-B62 | 1:100 |
| BV 650 | CD11b | H1/70 | 1:200 |
| Pacific blue | CD4 | RM4-5 | 1:200 |
| PE-Cy7 | CD8 | 53–6.7 | 1:200 |
| Per CP- Cy5.5 | Siglec-F | E50-2440 | 1:100 |
| PE-Cy5 | CD11c | N418 | 1:133 |
| Alexa fluor 488 | Ly6G | 1A8 | 1:100 |
| BV 785 | Ly6C | HK1.4 | 1:100 |
Anesthesia setup
Timing: 0.25 h
-
3.Setup anesthesia machine on the side of the biosafety cabinet.
-
a.Keep the isolation chamber inside the cabinet when using pathogens.
-
b.Follow the procedure as described in the step-by-step method details.
-
a.
Preparation setup for BALF isolation
Timing: 0.3 h
-
4.Setup workbench, BALF buffer, 22G catheter, and collection tubes before you euthanize neonates on the final day of experiment.
-
a.Follow the procedure as described in the step-by-step method details.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD45 (Clone ID: 30-F11) APC-Cy7 (1:200) | BioLegend | 103116; AB_312981 |
| Anti-mouse F4/80 (Clone ID: BM8) PE (1:133) | BioLegend | 123110; AB_893486 |
| Anti-mouse B220 (Clone ID: RA3-B62) APC (1:100) | BioLegend | 103212; AB_312997 |
| Anti-mouse CD11b (Clone ID: H1/70) BV 650 (1:200) | BioLegend | 101259; AB_2566568 |
| Anti-mouse CD4 (Clone ID: RM4-5) Pacific Blue (1:200) | BioLegend | 100531; AB_493374 |
| Anti-mouse CD8a (Clone ID: 53-6.7) PE-Cy7 (1:200) | BioLegend | 100722; AB_312761 |
| Anti-mouse Siglec-F (Clone ID: E50-2440) PerCP-Cy5.5 (1:100) | BD Pharma | 565526; AB_2739281 |
| Anti-mouse CD11c (Clone ID: N418) PE-Cy5 (1:133) | BioLegend | 117316; AB_493566 |
| Anti-mouse Ly6G (Clone ID: 1A8) Alexa Fluor 488 (1:100) | BioLegend | 127626; AB_2561340 |
| Anti-mouse Ly6C (Clone ID:HK1.4) BV 785 (1:100) | BioLegend | 128041; AB_2565852 |
| Virus strain | ||
| RSV-A2 (Lot# 70040896) | ATCC | VR1540 |
| Chemicals, peptides, and recombinant proteins | ||
| Isoflurane | Akron Inc. | NDC 59399-106-01 |
| Phosphate buffered saline (PBS) | Hyclone | SH30256.02 |
| EDTA 0.5 M solution | RPI Research | E14000-250.0 |
| Fetal bovine serum (FBS) | Hyclone | SH30396.03IH25-40 |
| Bovine serum albumin (BSA) 30% solution | Sigma-Aldrich | A3299 |
| Dextrose | Fisher Chemicals | D16-500 |
| Heparin sodium salt powder | Sigma-Aldrich | H3393-25KU |
| 4% Paraformaldehyde | N/A | N/A |
| 10% Formalin | Sigma-Aldrich | HT501128 |
| Ultra-pure water | N/A | N/A |
| Critical commercial assays | ||
| LIVE/DEAD Fixable Aqua Dead cell stain kit (1:200) | Thermo Fisher Scientific | L34966 |
| Experimental models: Cell lines | ||
| Hep2 cells | ATCC | CCL-23 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice, age:(P7) 7 days old neonates, sex: male/female | Jackson Laboratories | 000664 |
| Software and algorithms | ||
| FlowJo | BD Biosciences | N/A |
| Prism 9 | GraphPad | N/A |
| Other | ||
| Small animal heating pad | Amazon | B00JHK375E |
| 3× LED Magnification lamp | Amazon | B08J4CWXYP |
| Mini coolers | Heathrow Scientific | 120073 |
| Oxygen cylinder | N/A | N/A |
| Manual pipette | Alphapette | A-10 |
| Weighing balance | N/A | N/A |
| Tissue Lyser II | Qiagen | N/A |
Materials and equipment
Heparin sodium salt stock
| Reagent | Final concentration | Amount |
|---|---|---|
| Heparin Sodium salt powder | 10000 U/mL | N/A |
| ddH2O | N/A | 2.5 mL |
| Total | N/A | 2.5 mL |
Note: Reconstitute the entire vial of heparin sodium salt in 2.5 mL of aqueous solution. Mix the solution completely. The stock can be stored at 4°C for 6 months.
Fixable live/dead viability staining solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Live/dead powder vial (A) | N/A | 1 vial |
| DMSO (B) | N/A | 50 μL |
| Total | N/A | 50 μL |
Note: Fixable live dead staining solution can be prepared early and stored in the −20°C. Thaw and use the required amount on the day of experimentation.
CRITICAL: One component of live/dead contains DMSO, product is hazardous and may be absorbed in the body through the skin.
2.5 mM EDTA stock
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.5 M EDTA | 2.5 mM | 0.5 mL |
| ddH2O | N/A | 99.5 mL |
| Total | N/A | 100 mL |
Note: 2.5 mM EDTA stock can be stored in the 4°C for 6 months.
BALF buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Heparin | 20 U/mL | 200 μL |
| Dextrose | 1 mg/mL | 100 mg |
| PBS (1×) | N/A | 99.8 mL |
| Total | N/A | 100 mL |
Note: BALF buffer can be prepared in advance (not older than 1 month) and stored in 4°C. Keep it in ice until you start the lavage process on the experiment day.
Wash buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 2.5 mM EDTA | 0.2 mM | 8 mL |
| FBS | N/A | 2 mL |
| PBS (1×) | N/A | 90 mL |
| Total | N/A | 100 mL |
Note: Prepare a fresh wash buffer and store it in the 4°C for 1 week.
FACS staining buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA | 2% | 330 μL |
| FBS | 2% | 100 μL |
| PBS (1×) | N/A | 4570 μL |
| Total | N/A | 5 mL |
Note: Prepare fresh FACS staining buffer and keep it in ice until use.
Antibody staining solution (Enough for 10 samples)
| Reagent | Final concentration | Amount |
|---|---|---|
| CD45 (APC-Cy7) | 1 μg/mL | 5 μL (1:200) |
| F4/80 (PE) | 1.5 μg/mL | 7.5 μL (1:133) |
| B220 (APC) | 2 μg/mL | 10 μL (1:100) |
| CD11b (BV 650) | 1 μg/mL | 5 μL (1:200) |
| CD4 (Pacific blue) | 2.5 μg/mL | 5 μL (1:200) |
| CD8 (PE-Cy7) | 1 μg/mL | 5 μL (1:200) |
| Siglec-F (Percp- Cy5.5) | 2 μg/mL | 10 μL (1:100) |
| CD11c (PE-Cy5) | 1.5 μg/mL | 7.5 μL (1:133) |
| Ly6G (Alexa fluor 488) | 5 μg/mL | 10 μL (1:100) |
| Ly6C (BV 785) | 2 μg/mL | 10 μL (1:100) |
| Live/Dead (AmCyan) | N/A | 5 μL (1:200) |
| FACS staining buffer | N/A | 920 μL |
| Total | N/A | 1 mL |
Note: Prepare fresh antibody staining solution right before the staining step and keep it in ice wrapped in aluminum foil until use.
Fixative buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 2.5 mM EDTA | 0.5 mM | 1 mL |
| 4% PFA | 2% | 2.5 mL |
| PBS (1×) | N/A | 1.5 mL |
| Total | N/A | 5 mL |
Note: Prepare a fresh fixative buffer and keep it in ice until use.
CRITICAL: Paraformaldehyde is a highly toxic chemical. Do not inhale or contact bare skin. Discard leftovers in the designated hazardous waste container and request EHS pickup when appropriate.
Step-by-step method details
Setting up the work bench
Timing: 0.5 h
These steps will help prepare the work bench for intranasal infection (Figure 1).
CRITICAL: Follow the BSL-2 safety procedure when you enter the room. All the locations mentioned below will make your work easy, but it is not mandatory. You might have to work the other way around if you are left-handed.
-
1.
Setup the isoflurane machine outside of the hood. Preferably on the left side.
-
2.
Place the anesthesia chamber inside the hood (on the left).
-
3.
Place the small animal heating pad on the middle (front) of the hood and connect it to the electric port.
-
4.
Setup the nose cone in the middle (back) of the heating pad.
-
5.
Place the infectious agent (RSV-A2) on the back right of the hood inside the mini cooler.
-
6.
Setup the pipettor, tips, waste container and animal markers on the right of the bench.
-
7.
Place the lamp with the magnification glass (3× zoom preferred) near the heating pad and nose cone.
-
8.
Place the mouse cage on the left back behind the anesthesia chamber.
-
9.
Mark neonates according to your treatment group and take weight before you start the next step.
CRITICAL: RSV aliquot should be kept in a cold container like dry ice or mini cooler all the time. Only thaw 1 vial every time before you inoculate.
Figure 1.
Anesthesia setup
(A) Set up the anesthesia machine on the left adjacent to the air flow cabinet if you are right-handed.
(B) Set up a 3× magnification LED lamp inside the right side of the hood with appropriate pipette controller.
(C) Set up the small animal heating pad right in front of the lamp and nose cone in the back. Additionally, you can make paper towel V- shape huts if the air flow cabinet is too cold and use it to protect neonates from direct air exposure to cold air until they wake up.
(D) Visual of complete anesthesia setup.
Anesthesia and intranasal inoculation with RSV
Timing: 2.5 h
These steps will guide you through neonatal anesthesia procedure and handling of neonates during the pre-intranasal period. The required time is for 8 neonatal intranasal inoculations.
-
10.Turn on the anesthesia machine (details on the note below) and set it to 2.5% isoflurane in fast mode.
-
a.Use a small anesthesia chamber as much as possible for faster effect.
-
a.
-
11.Place a single mouse pup inside the chamber and wait for 4–5 min.
-
a.Watch for breathing, as the pups are deeply anesthetized, they will breathe slower clearly visible in chest movement.
-
b.Load your sample to be inoculated on the pipette right before you take out the pup from the chamber.
-
c.Remove the pup from the chamber and hold them right below the back of neck (dorsal) gently, keep them straight facing upward.
-
a.
-
12.
Wait 4–5 s and inoculate 5 μL of inoculum on the first nostril looking through a 3× magnification lensed lamp, release steadily until you see complete inoculation.
Note: If you want to be faster for the overall experimental group this is the right time to place another neonate in the anesthesia machine before you hold another pipette with your right hand.
-
13.
Wait for 4–5 s let pup stretch to take the inoculum into the lungs.
-
14.
Keep the pup in an upright position and place them in the nose cone before it wakes up but with little hold time after the inoculation.
-
15.
For the second nostril inoculation, insert the sample dropwise every 10–15 s and keep exposing pup to nose cone for anesthesia frequently until you complete the step.
-
16.Once you are finished, hold the pup for about 30 s upright in the nose cone.
-
a.Hold another 30 s without the nose cone upright.
-
b.Let it breathe and watch for leg movement.
-
a.
Note: This final step ensures all the inoculum is gone through the nostrils.
-
17.Place the pup in the heating pad facing the ventral side upward until they wake up.
-
a.Cover with a boat shaped paper towel or with similar objects to avoid direct air exposure above the heating pad.
-
b.Make sure the towel is not in direct contact with the pup.
-
a.
CRITICAL: Heating pads are recommended. Isoflurane tends to cause hypothermia.5 Since these are small neonates, heating pads will help neonates survive better and recover faster. Also, these neonates really like to stay in the heating pad longer when recovering from anesthesia.
Note: Regarding anesthesia details, VetEquip RC2 Machine and Tec 3 Isoflurane Vaporizer were used to place the mice under general anesthesia. Mice were induced in a small chamber at 2.5% isoflurane set at oxygen flow of 1 mL/circuit flow and transferred into a nose cone mask set at 2.5% isoflurane with 1 mL/circuit flow of oxygen.
Alternatives: Other anesthesia machines can be used as a replacement, but it might need adjustment of the flow rate and the chamber size for faster anesthesia effects.
Daily weight monitoring
Timing: 0.5 h
These steps will guide you through neonatal weight monitoring and calculations. Approximate time required is calculated for 8 neonatal weight monitoring.
-
18.
Set up the weight balance inside the hood.
-
19.
Weigh each neonate daily.
-
20.Calculate the percent weight change/gain.
-
a.Set the weight of infection day (Day 0) to 100% for each neonate.
-
b.Convert everyday weight change to percent change by using the following formula e.g., Day 10.
-
a.
-
21.
Plot the graph accordingly (Figure 2).
Note: Weight of neonates does not decrease with the infection because they are in the developmentally active phase. However, we can observe a difference in the weight gain between infected and non-infected groups.
Figure 2.
Weight change results
Weight change difference between infected (Red) and uninfected (Blue) neonates in percent original weight scale on the Y-axis and number of days post infection in x- axis are shown in Mean ± SEM. Two-way ANOVA with Sidak’s post-test was used for statistical analysis to compare differences in overall weight change versus time. Significant differences between mean comparison of two groups were observed starting from 1 day post infection until day 14 post infection and are shown in asterisks. Asterisks shown in lower row in figure is for odd days and in upper row is for even days. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01.
Whole lung collection
Timing: 2 h
These steps will guide you through neonatal euthanasia procedures for whole lung isolation used for H&E staining and plaque assay. The timing mentioned above is the approximation of required time for 8 neonate whole lung collections.
-
22.
Set up the carbon dioxide chamber and place neonate inside it to euthanize.
-
23.Turn on the gas and manually watch for neonatal breathing inside the chamber.
-
a.Wait until the neonate completely stops breathing.
-
b.Neonatal euthanasia usually takes longer than adults, approximately 5–8 min.
-
c.Take neonate out and lay it over the dissection board.
-
d.Open the skin in the abdomen and continue towards the neck.
-
e.Open the peritoneal cavity and make the incision in the diaphragm and cut the chest rib.
-
f.Slowly remove the lower ribs and diaphragm from both sides.
-
g.Open the rib all the way to the neck.
-
h.Remove thyroid gland and locate the trachea.
-
i.Slowly remove attached tissues in the neck around the trachea.
-
j.Move gently towards the lung lobes without damaging lobes until you detach the lung from the diaphragm.
-
k.Place the collected lung in the ice or directly to 10% buffered formalin.
-
a.
-
24.
Next, follow the procedure for plaque assay by preparing lung homogenate by preparing tissue lysate.
-
25.
Whole lung can also be processed for H&E sectioning by collecting lung in 10% buffered formalin.
Alternatives: Whole lung tissue can be directly lysed using 1 mL of TRIzol reagent for RNA isolation and stored at −80°C for viral load determination or by using a lysis buffer in the manufacturer provided RNA isolation kits.
Note: For RSV plaque assay, we have followed the previously published protocol (Bouillier et al., 2019).6
Bronchoalveolar lavage fluid (BALF) collection
Timing: 2.5 h
These steps will guide you through BALF collection after neonatal euthanasia procedure (Figure 3). The timing above is approximately the required time for 8 neonatal BALF collections.
-
26.Turn on the gas and wait until the neonate stops breathing.
-
a.Take it out and lay it over the dissection board.
-
b.Open the skin in the abdomen and continue towards the neck.
-
c.Open the peritoneal cavity, make the incision in the diaphragm, and cut the chest rib.
-
d.Slowly remove the lower ribs and diaphragm on both sides.
-
e.Remove thyroid gland and locate the trachea.
-
f.Insert the catheter in the trachea (22G is recommended) and lavage the lung with 200 μL BAL buffer three to four times and then transfer the fluid to the collection tube.
-
g.Repeat step 26.f. once again.
-
h.Place the collected fluid in the microcentrifuge tube on ice until further processing.
-
a.
Figure 3.
Dissection setup and BALF isolation
(A) Set up the work bench for dissection inside the hood with required tools.
(B) Expose the abdomen and chest all the way to trachea.
(C) Cut peritoneum, diaphragm, and chest rib cavity all the way to the neck without disturbing lungs and trachea.
(D) Expose lungs and trachea clearly.
(E) Insert catheter to the trachea.
(F) Lavage lung with BALF buffer using 1 mL syringe multiple times, keep your eyes on lung inflation (as seen in picture).
(G) Collect lavage in a 1.5 mL microcentrifuge tube and place on ice.
(H) Centrifuge the lavage and follow the BALF analysis procedure.
Flow cytometry analysis procedure of BALF immune cells
Timing: 3 h
These steps will guide you through BALF processing for single cell flow cytometry analysis to determine the changes in immune cell population. Timing for 8 sample processing in a single experiment may vary depending on the automation.
Note: Follow the required media/buffer preparations prior to starting the procedure that are mentioned in the materials and equipment section.
-
27.
Transfer the supernatant from the previous step to a 1.5 mL microcentrifuge tube.
-
28.
Centrifuge the BALF at 1500 rpm (× 400 g) for 5 min at 4°C and discard the supernatant.
Alternatives: Supernatant can be used for BALF cytokine analysis if desired.
-
29.
Add 300 μL (for each sample) of 1× RBC lysis buffer to the pellet and mix gently to remove the RBC from the pellet.
-
30.
Incubate for 10 min in ice and add 1 mL of wash buffer to the tube.
-
31.
Centrifuge the mixture at 1500 rpm (× 400 g) for 5 min at 4°C and discard the supernatant.
-
32.
Resuspend the pellet in a 1 mL wash buffer and wash the cells one more time.
-
33.
Discard the supernatant and resuspend the pellet in 200 μL wash buffer.
-
34.
Count the cells using trypan blue and seed up to one million cells per well in 96 well V-bottom plates.
-
35.
Centrifuge the 96 well plate at 1500 rpm (× 400 g) for 5 min at 4°C and discard the supernatant.
-
36.
Resuspend the cell pellet in a premade antibody cocktail prepared using FACS staining buffer (100 μL per well) as prepared in the materials and equipment section and incubate on ice for 25 min.
Alternatives: Cell pellets can be directly resuspended in FACS staining buffer (100 μL per well) and stained with desired panel of antibody.
-
37.
Cover the plate and keep it in the dark to protect it from direct light.
Alternatives: Use aluminum foil to cover the plate.
-
38.
Add 100 μL FACS staining buffer to each well, centrifuge at 1500 rpm (× 400 g) for 5 min at 4°C and discard the supernatant.
-
39.
Fix the cells in 200 μL of fixative buffer and transfer the cells to 5 mL FACS tubes or transfer it to 96 U-well plates in an automated plate reader enabled flow cytometer.
-
40.
Gently keep FACS tube/96 U-well plate on ice until you are ready to acquire data in the flow cytometer, acquiring data on the same day of the experiment is recommended.
-
41.
Make separate templates for uninfected and infected cells (Figures 4A and 4B) and use proper gating for your panel when you acquire the data for efficient data acquisition.
-
42.
Vortex each sample before you take the reading in the flow cytometer, all fluorochrome in the antibody table above can be detected by Violet (405 nm), Blue (488 nm), Yellow green (561 nm), Red (640 nm) lasers.
-
43.
Once finished recording, export the FCS file to the desired drive or location.
-
44.
Analyze data using software that can open the FCS file.
-
45.
Use statistical analysis software together with the cell count data to create a graph for percent cells and absolute numbers.
Note: RBC lysis time might need to be adjusted depending upon the experiment. Expect to optimize volume and duration of incubation. It is normal if you do not see any pellets in some samples. Make sure you aspirate out liquid completely from the microcentrifuge tube after RBC lysis. This gating strategy (Figure 4) will help identify leukocytes such as neutrophils, alveolar and interstitial macrophage, eosinophils, monocytes, B cells, CD4+ and CD8+ T cells.
Pause point: Sometimes mice might wake up early if they are not deeply anesthetized, you can hold intranasal and put them back in the nose cone after the first inoculation. Don’t place them back in the chamber. They might bubble out the inoculum.
Alternatives: Fc blockers can be used instead of BSA.U-well 96 plates can be used as an alternative to FACS tubes in an automated system. Different fluorochrome on the marker antibodies can be used if signals need to be optimized for specific cell type detection. Flow cytometers from different companies should also work with these fluorochromes since we have successfully used it in multiple systems. FCS files can be analyzed using other open or paid software. Statistical software that can do unpaired t-test and Two-way ANOVA analyses can be used.
Figure 4.
Gating strategy for BALF immune analysis and expected results
The gating strategy for monocytes, neutrophils, alveolar macrophages, interstitial macrophages, B cells, CD4+ T cells and CD8+ T cell populations. First gated on lymphocytes with SSC and FSC, and single cell gate by SSC-H/SSC-W and FSC-W/FSC-H. Then, live cells were selected by excluding Am Cyan positive cells. Next, CD45 positive cells for pan-leukocytes were gated, then CD11b and Siglec-F were used to differentiate Siglec-F positive cells which were again gated for CD11c and F4/80, giving us the double positive population as alveolar macrophage. On the next side, Siglec-F intermediate were gated for Ly6C and CD11c where negative cells were further gated for side scatter. High side scatter cells were selected as eosinophils. On the other side, CD11b positive cells were gated for F4/80 where positive cells were identified as interstitial macrophage and negative gate was used in Ly6G gating. Furthermore, the positive population was identified as neutrophil, and the negative population was gated using a Ly6C marker giving us Ly6Chi and Ly6C lo monocytes. On the third side, CD11b negative population was gated for B220/SSC-H, positive population was labeled as B cells, negative population was gated for CD4/CD8 gate to identify CD8 positive cells (Q1) and CD4 positive cells (Q3). (A) Uninfected, (B) RSV infected.
Expected outcomes
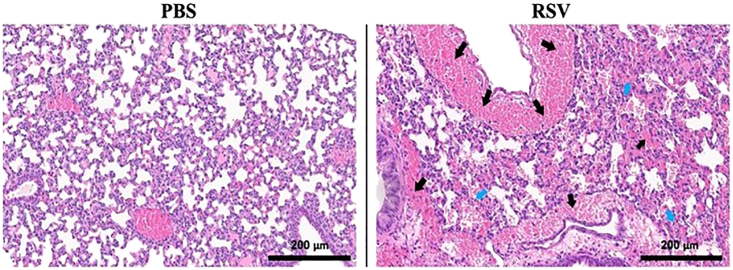
This protocol describes the neonatal infection of RSV in C57BL/6j mice for analyses of infection outcomes (e.g., weight loss), recruited cells in airspaces, and lung damage of infected lungs. We expect to get progressive decline in weight gain by P7 neonates after RSV infection, reaching up to > 100% reduction at Day 14 post-infection (Figure 2). We also expect that the decreased weight gain with RSV infection is consistent with increased lung damage and airway leakage as judged by histopathological analyses of infected lungs and leukocyte recruitment at airspaces. As anticipated, we observed increased accumulation of fluid and RBCs (bright pink cells indicated by the black arrow, the alveolar damage is indicated by blue arrow) in the airways and lung parenchyma after RSV infection as measured by H&E staining of infected lungs at 7 days post-infection (Figure 5). The immune analyses of BAL fluid showed an increased number of total cells, CD45+ cells, CD11b− cells and CD11b+ Cells among the RSV-infected mice (Figure 6). Depending on the nature of study, this protocol enables the study of a range of infection parameters (e.g., weight loss, immune recruitment, lung damage) to evaluate the efficacy of drug-like compounds or vaccine candidates. Given that the current infection protocol is sublethal (no mortality within 14 days of infection), it would be useful for a range of measurements of immune cell status at different time points of infection. For example, this can be used to determine the RSV specific antibody titer, CD4+ T and CD8+ T cell responses.
Figure 5.
H&E staining sections and expected results
The H&E sections were imaged through a Leica Biosystems scanning scope. These images are 20× representative images of PBS and RSV-A2 infected lung samples. Clear pathology like RBC accumulation (bright pink cells as indicated by the black arrow) and disruption of alveolar spaces (indicated by blue arrow) can be seen in the RSV-A2 infected sample. Scale bar, 200 μm.
Figure 6.
BALF cell analysis results
(A) Comparative graph of total BAL cells between uninfected and infected samples. Statistical analyses by unpaired t-test. Error bar is shown in Mean ± SEM.
(B) Comparative graph of total CD11b negative cells between uninfected and infected samples. Statistical analyses by unpaired t-test. Error bar is shown in Mean ± SEM.
(C) Comparative graph of total CD11b positive cells between uninfected and infected samples. Statistical analyses by unpaired t-test. Error bar is shown in Mean ± SEM.
(D) Comparative graph of total leukocytes between uninfected and infected samples. Statistical analyses by unpaired t-test. Error bar is shown in Mean ± SEM.
(E) Representative image of uninfected vs. RSV-A2 infected CD45 gating.
We observed the clear presence of live RSV in the infected lung tissues as measured by plaque forming units, validating the active viral infection in the tissues after RSV inoculation (Figure 7). The viral titers might be changed depending on the time course of infection, especially it will decline at later time points (after day 7 post-infection). Since the developed method is based on C57BL/6J mice, the expected outcomes might be different in other backgrounds of mice.
Figure 7.
Viral load analysis and results
The clear difference in the comparative graph between viral load of uninfected and infected groups is significant and as expected. No visible plaque in the uninfected group and the number of plaques counted in RSV-A2 infected group confirmed the successful inoculation of RSV through intranasal route. Statistical analyses by unpaired t-test. Error bar is shown in Mean ± SEM.
Limitations
Since this is the neonate experiment, there is less likely to have an equal number of sample sizes between the groups for most of the experiment. The sample size in each experiment depends upon the number of pups born in the same litter. Because of microbiota and other factors, it is not recommended to pool the same age pups from different litters to set up the experiment. Neonatal tissues are very soft, delicate and may require extra care while handling. Another limitation is the neonatal survivability during the intranasal inoculation. Some of the neonates might stop breathing in the middle of the experiment and die, needing more time to get the best optimization of the inoculation procedure. The antibody panel used in this protocol is not sufficient to analyze all lymphocyte populations in the lung airspace.7 Overall, this experiment may be influenced by all the factors mentioned above. We suggest working accordingly to troubleshoot the problems.
Troubleshooting
Problem 1
No infection seen after the survival study (step 5).
Potential solution
Check the viability of the stock virus. Make sure aliquots are constantly maintained through the cold chain. Load the virus in the pipette just before the inoculation to avoid longer wait time at room temperature.
Problem 2
Neonate does not get anesthetize (step 10).
Potential solution
Check if the isoflurane is flowing at the appropriate flow rate. Wait 2–3 min longer and see if the issue is resolved. Alternatively, reduce the size of the anesthesia chamber for faster results. If your experiment deals with simultaneous exposure of isoflurane to multiple pups, concentration of isoflurane might need adjustment.
Problem 3
Neonate died in the anesthesia chamber (step 10).
Potential solution
Potential longer exposure to isoflurane than required or too high concentration of isoflurane. Reduce the isoflurane concentration or the duration of exposure to avoid the situation.
Problem 4
Neonate stop breathing during the intranasal inoculation (steps 12–15).
Potential solution
Wait for a few seconds and look for an increase in breathing rate after the neonate is removed from the anesthesia chamber before you start the inoculation procedure. Stop inoculation and wait to see if the neonates start breathing.
Problem 5
Neonate does not get euthanize (step 22).
Potential solution
Expose neonates longer to carbon dioxide gas. Neonates require less air to survive so it will take longer for them to get euthanized compared to adults.
Problem 6
Lung is leaking the fluid during the lavage of BALF (step 26).
Potential solution
Check if you are using the right size of catheter. Make sure you are dissecting on the flat surface and your catheter is a little above the surface and not too deep in the lung. If the issue is due to the expansion of lung during lavage, adjust BALF buffer volume.
Problem 7
Presence of RBC in the sample after the RBC lysis (step 29).
Potential solution
Optimal volume of RBC lysis buffer is recommended for desired results. Check if the RBC lysis buffer is working well. Increase lysis time if the process is slower than expected.
Problem 8
Antibody staining is ineffective (step 43).
Potential solution
Follow the antibody panel design guidelines for better results. Antibody titration might be needed to determine optimal working concentration. A proper gating strategy is also considerable for better results. If the problem persists, check the quality of your antibody and its storage conditions.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Pankaj Baral (baral@ksu.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Kansas State University Veterinary Diagnostic Lab (Histopathology), flow cytometry core, and Comparative Medicine Group (CMG) for their support. We thank all lab members of Baral Lab for their support in this work. This work was supported by (1) Kansas Idea Network for Biomedical Research Excellence K-INBRE (P20 GM103418 to P.B.) and (2) Chemical Biology of Infectious Disease CoBRE (P20GM113117 to P.B.) grants. The graphical abstract was created using BioRender software.
Author contributions
Conceptualization, P.B.; methodology, P.B.; investigation, S.A.; data curation, S.A., P.B.; writing – original draft, S.A.; writing – review and editing, S.A. and P.B.; funding acquisition, P.B.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Sandeep Adhikari, Email: sadhikari@ksu.edu.
Pankaj Baral, Email: baral@ksu.edu.
Data and code availability
All data generated during this study are available upon request.
References
- 1.Bruder D., Srikiatkhachorn A., Enelow R.I. Cellular Immunity and Lung Injury in Respiratory Virus Infection. Viral Immunol. 2006;19:147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 2.Clementi N., Ghosh S., De Santis M., Castelli M., Criscuolo E., Zanoni I., Clementi M., Mancini N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021;34:e00103–e00120. doi: 10.1128/CMR.00103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elderman M., Hugenholtz F., Belzer C., Boekschoten M., van Beek A., de Haan B., Savelkoul H., de Vos P., Faas M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018;9:26. doi: 10.1186/s13293-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rufiange M., Leung V.S.Y., Simpson K., Pang D.S.J. Pre-warming before general anesthesia with isoflurane delays the onset of hypothermia in rats. PLoS One. 2020;15:e0219722. doi: 10.1371/journal.pone.0219722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillier C., Rincheval V., Sitterlin D., Blouquit-Laye S., Desquesnes A., Eléouët J.F., Gault E., Rameix-Welti M.A. Generation, Amplification, and Titration of Recombinant Respiratory Syncytial Viruses. J. Vis. Exp. 2019 doi: 10.3791/59218. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y.-R.A., O’Koren E.G., Hotten D.F., Kan M.J., Kopin D., Nelson E.R., Que L., Gunn M.D. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS One. 2016;11:e0150606. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are available upon request.