Abstract
Parthenolide (PTL) is a new compound extracted from traditional Chinese medicine. In recent years, it has been proven to play an undeniable role in tumors, autoimmune diseases, and inflammatory diseases. Similarly, an increasing number of experiments have also confirmed the biological mechanism of PTL in these diseases. In order to better understand the development trend and potential hot spots of PTL in cancer and other diseases, we conducted a detailed bibliometric analysis. The purpose of presenting this bibliometric analysis was to highlight and inform researchers of the important research directions, co-occurrence relationships and research status in this field. Publications related to PTL research from 2002 to 2022 were extracted on the web of science core collection (WoSCC) platform. CiteSpace, VOSviewers and R package “bibliometrix” were applied to build relevant network diagrams. The bibliometric analysis was presented in terms of performance analysis (including publication statistics, top publishing countries, top publishing institutions, publishing journals and co-cited journals, authors and co-cited authors, co-cited references statistics, citation bursts statistics, keyword statistics and trend topic statistics) and science mapping (including citations by country, citations by institution, citations by journal, citations by author, co-citation analysis, and keyword co-occurrence). The detailed discussion of the results explained the focus and latest trends from the bibliometric analysis. Finally, the current status and shortcomings of the research field on PTLwere clearly pointed out for reference by scholars.
Keywords: Parthenolide, Bibliometrics, Apoptosis, Antiinflammatory, Anticancer
1. Introduction
Parthenolide (PTL) is a pure natural sesquiterpene lactone derived from Compositae (Daisy) as a secondary metabolite [1]. It was first extracted from the feverfew plant (Tanacetum parthenium). Feverfew, as a herbal medicine, has a wide history of application before modern medicine was fully developed [2]. As traditional oral Chinese medicine, feverfew can relieve fever, headache, rheumatoid arthritis, dry eyes, and other diseases [3]. However, this process needs to be guided by the theories of traditional medicine and requires the mixed application of other traditional herbs [4]. Obviously, this mixed agent hinders the exploration of the mechanism of action. Therefore, extracting compounds with pharmacological effects and single components from feverfew has become an effective means of modernizing traditional medicine. Among the numerous compounds extracted from the feverfew plant, PTL is one of the most bioactive. It includes a ten-membered α-methylene-γ-lactone hoop and epoxy compounds, which can interact with the nucleophilic loci of biomolecules [5]. In particular, unsaturated double bonds conjugated with carbonyl groups have been shown to interact with free proteins, leading to changes in intracellular environmental homeostasis and induction of cell death [6]. Moreover, it has been observed that these interactivities of PTL correlate with its ability to induce oxidative stress; they reveal a variety of anticancer and antiinflammatory properties [7].
Recently, many studies have confirmed that PTL can inhibit inflammation by blocking IkB kinase and then inhibiting the activity of NF-κB [8,9]. Subsequently, PTL has been observed to be a good blocker of inflammasome to reduce the release of proinflammatory factors. This process is independent of the NF-κB pathway and uncovers a new path for the antiinflammatory mechanism of PTL [10]. Interestingly, the anticancer mechanism of PTL has a similar aspect. PTL is believed to play an anticancer role by affecting the NF-κB pathway, thereby decreasing the expression of the hypoxia-inducible factor 1a (a tumor-related antiapoptosis gene) [11]. With further research, an increasing number of studies have revealed its antitumor role, such as interfering with microtubule formation, inducing reactive oxygen species (ROS), and reducing STAT protein expression [12,13]. In summary, the current role of PTL is no longer limited to the aforementioned effects, and its mechanisms are not limited to the NF-κB pathway. Although the mechanism of the multitarget and multipathway pharmacological action of PTL is unclear, PTL and its derivatives are potential first-line therapeutic drugs.
As a qualitative and quantitative evaluation method, bibliometric analysis is broadly applied to measure the influence of academic journals and track the development of a certain discipline in a specific period [14]. The main role of bibliometrics research is to systematically and objectively evaluate the status of specific research fields. This can encourage researchers, policy makers, and funding institutions to identify areas of strengths and weaknesses, and make reasonable decisions on the allocation of resources and support [15]. Meanwhile, it mainly also summarizes and points out the future research directions in a field according to the contribution of individuals/countries/institutions, the influence of publications, the frequency of keywords, and other indicators. This can help researchers identify new opportunities and challenges while keeping their research up-to-date with the times. PTL research has rapidly developed in the past 20 years. Although a large number of scientific research achievements were generated during this period, they belong to different diseases and mechanisms. Therefore, the lack of systematic review of research achievements in this field will make it difficult for researchers to fully understand the current research status and accurately grasp future trends. Over time, this is detrimental to the development of PTL research. As a result, bibliometric analysis is an effective way to deal with this problem. However, up to now, there have been no relevant reports on PTL research in the field of library information science. Here, we conducted a bibliometric analysis of PTL studies for nearly 20 years worldwide to clarify the academic panorama and potential progress trends.
2. Methods
2.1. Data source and retrieval strategy
Keywords and search criteria are first confirmed to define the scope of the study. The search entries were “Parthenolide” and “Dimethylamino Parthenolide.” The retrieval period was 2002–2022. Only English-language literature was considered. The literature types were confined to articles and reviews in journals.
2.2. Database search
All the raw data were extracted from the Web of Science Core Collection (WoSCC) on November 24, 2022. Subsequently, the literature retrieved within a week was downloaded. Ultimately, 1148 articles were included in the analysis (Fig. 1).
Fig. 1.
Flow chart of document retrieval.
2.3. Data analysis
VOSviewer (version 1.6.18) is a bibliometric analysis platform that can retrieve important messages from multiple journals [16]. Here, this software was mainly utilized to analyze the relevant information on countries, institutions, journals, keywords, and authors. In the graph generated by VOSviewer, a node represents a class of elements, such as authors and journals. The color and area of the node represent the classification and quantity of the element, respectively. The thickness of the lines between the nodes represents the intensity of cocitation and collaboration between elements.
CiteSpace (version 6.1.R3) is a platform invented by Professor Chen C for bibliometric research [17]. Here, it was used to draw the dual-map overlay of journals and display references with citation bursts.
The R package, “bibliometrix” (version 3.2.1), was employed to build the relationship between published articles among countries and research trends in this field. In addition, Excel 2019 was applied to map the number of documents issued in the past 20 years. For the generated network graph, in addition to changing the threshold for clearer visualization, default software parameters were also used.
3. Results
3.1. Trends in the issuance of global publications
Based on the search terms and exclusion criteria, 1148 articles were included in the analysis. As shown in Fig. 2, the annual number of documents issued in this field exhibited an intense growth trend. From 2002 to 2011, the number of documents issued increased yearly and had maintained a growth rate of over 10% in most years. From 2012 to 2022, the annual number of documents issued significantly fluctuated but was never below 50. In particular, in 2013 and 2020, the number of publications in this field exceeded 80 per year and the growth rate in both years was greater than 30%. In addition, the median number of publications before 2011 was 51, while in the following 11 years this number was 65.
Fig. 2.
Annual output of PTL research.
3.2. Analysis of countries and institutions
The research literature in this field was distributed in 60 countries. The top 10 countries were located in Europe, Asia, North America, and South America(Table 1). Among these countries, the United States (n = 343) had the largest number of documents, followed by China (n = 250), Germany (n = 69), and Italy (n = 66). Thereafter, we screened and mapped these countries, according to the number of papers not less than 2, and built a collaboration network, according to the number and relationship of papers in each country (Fig. 3). A clear cooperative relationship was observed between different countries. In this intricate map of cooperation, the United States had extensive cooperative relations with countries on all continents in this field, especially with China.
Table 1.
Top 10 countries and institutions for PTL research.
| Rank | Country | Count | Institution | Count |
|---|---|---|---|---|
| 1 | USA (North America) | 343 (29.8%) | Nankai University (China) | 31 (2.7%) |
| 2 | China (Asia) | 250 (21.7%) | University of Rochester (USA) | 28 (2.4%) |
| 3 | Germany (Europe) | 69 (6.0%) | Medical University of Lodz (Poland) | 28 (2.4%) |
| 4 | Italy (Europe) | 66 (5.7%) | University of Kentucky (USA) | 23 (2.0%) |
| 5 | Japan (Asia) | 58 (5.0%) | Indiana University (USA) | 22 (1.9%) |
| 6 | South Korea (Asia) | 58 (5.0%) | University of Arkansas For Medical Science (USA) | 20 (1.7%) |
| 7 | Spain (Europe) | 44 (3.8%) | University of Palermo (Italy) | 20 (1.7%) |
| 8 | Poland (Europe) | 44 (3.8%) | Universidad Autónoma de Madrid (Spain) | 16 (1.3%) |
| 9 | Brazil (South America) | 40 (3.4%) | Chinese Academy of Sciences (China) | 16 (1.3%) |
| 10 | France (Europe) | 35 (3.0%) | Temple University (USA) | 15 (1.3%) |
Fig. 3.
Geographical distribution (A) and visualization of countries (B) of PTL research.
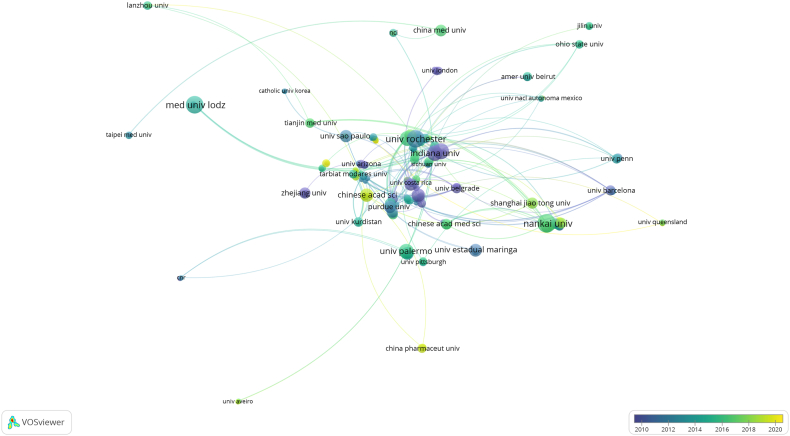
The top 10 institutions were located in five countries, half of which were in the United States. The top three institutions publishing papers in this field were Nankai University (n = 31), the University of Rochester (n = 28), and the Medical University of Lodz (n = 28). Subsequently, we screened 162 institutions for visualization according to the minimal number of publications (3) and built a collaborative network (Fig. 4). Fig. 4 shows very close cooperation among the University of Rochester, the University of Kentucky, Indiana University, and other research institutions in Western Europe and North America. Interestingly, Nankai University, ranking first in the number of published papers, only cooperated closely with domestic research institutions. The same rules applied to the Medical University of Lodz in Poland.
Fig. 4.
Visualization of institutions on PTL research.
3.3. Analysis of journals and cocited journals
Documents on PTL have been issued in 531 journals over the past two decades. Molecules published the most relevant documents (n = 26), followed by Bioorganic & Medicinal Chemistry Letters (n = 16) and Journal of Biological Chemistry (n = 15) (Table 2). Among the top 10 journals, Cancer Research had the highest impact factor (IF) (13.31), followed by Cancer Letters (IF = 9.75) and Journal of Medical Chemistry (IF = 8.03). Subsequently, we visualized 208 articles according to the minimal number of articles (2) and constructed a network diagram (Fig. 5A). Fig. 5A shows that Bioorganic & Medical Chemistry Letters exhibited a significant reference relationship with Molecules, Bioorganic & Medical Chemistry, and Journal of Medicinal Chemistry.
Table 2.
Top 10 journals and cocited journals for PTL research.
| Rank | Journal | Count | IF | Q | Cocited Journal | Cocitation | IF | Q |
|---|---|---|---|---|---|---|---|---|
| 1 | Molecules | 26 | 4.92 | Q2 | Journal of Biological Chemistry | 2009 | 5.48 | Q2 |
| 2 | Bioorganic & Medicinal Chemistry Letters | 16 | 2.94 | Q3 | Cancer Research | 1124 | 13.31 | Q1 |
| 3 | Journal of Biological Chemistry | 15 | 5.48 | Q2 | Blood | 1025 | 25.66 | Q1 |
| 4 | European Journal of Medicinal Chemistry | 14 | 7.08 | Q1 | Oncogene | 861 | 8.75 | Q1 |
| 5 | Molecular Cancer Therapeutics | 12 | 6.01 | Q1 | Proceedings of The National Academy of Sciences of The United States of America | 833 | 12.77 | Q1 |
| 6 | International Journal of Oncology | 12 | 5.88 | Q2 | Nature | 739 | 69.50 | Q1 |
| 7 | Bioorganic & Medicinal Chemistry | 11 | 3.46 | Q3 | Science | 554 | 63.83 | Q1 |
| 8 | Journal of Medicinal Chemistry | 11 | 8.03 | Q1 | Cell | 550 | 66.85 | Q1 |
| 9 | Cancer Letters | 11 | 9.75 | Q1 | Journal of Medicinal Chemistry | 516 | 8.03 | Q1 |
| 10 | Cancer Research | 11 | 13.31 | Q1 | Clinical Cancer Research | 504 | 13.80 | Q1 |
Fig. 5.
Visualization of journals (A) and cocited journals (B) on PTL research.
The top 10 cocited journals (Table 2) had been cited over 500 times. The number of citations in the Journal of Biological Chemistry ranked first (cocitation = 2009), followed by those of Cancer Research (cocitation = 1124) and Blood (cocitation = 1025). Additionally, according to the IF rankings, Nature (IF = 69.5), Cell (IF = 66.85), and Science (IF = 63.83) ranked in the top three. Based on the minimal number of citations (25), 386 journals were screened and visualized to build a citation network diagram (Fig. 5B). As shown in Fig. 5B, the Journal of Biological Chemistry exhibited an active cocitation correlation with Nature, Journal of Clinical Investigation, and Journal of Immunology.
A dual-map overlay was employed to analyze and display the distribution of papers, citation trajectory, and center of gravity drift. As shown in Fig. 6, the orange pathway, as the major citation pathway, elucidated the citation relationship between journals.
Fig. 6.
Dual-map overlay of journals on PTL research.
4. Analysis of authors and cocited authors
A total of 5976 authors conducted PTL studies. Each of the top 10 authors issued at least 10 relevant papers (Table 3). We constructed a collaborative network according to the minimum number of publications issued by the scholars (no less than 4) (Fig. 7A). Peter Crooks, Yue Chen, and Craig Jordan had the most prominent positions in the network because they had published the most articles in this field. In addition, Fig. 7A illustrates the collaboration among numerous authors. For instance, Monica Guzman exhibited strong cooperation with Duane Hassane, and Michele Yip-Schneider exhibited intense cooperation with Max Schmidt.
Table 3.
Top 10 authors and cocited authors for PTL research.
| Rank | Author | Count | Cocited Author | Citation |
|---|---|---|---|---|
| 1 | Peter Crooks | 36 | Guzman Ml | 423 |
| 2 | Craig Jordan | 24 | Hehner Sp | 334 |
| 3 | Yue Chen | 23 | Ghantous A | 245 |
| 4 | Quan Zhang | 20 | Kwok Bhb | 205 |
| 5 | Anna Janecka | 15 | Wen J | 159 |
| 6 | Antonella D’anneo | 12 | Garcia-Pineres Aj | 157 |
| 7 | Christopher Sweeney | 12 | Yip-Schneider Mt | 138 |
| 8 | Marianna Lauricella | 11 | Nakshatri H | 137 |
| 9 | Yahui Ding | 11 | Shanmugam R | 121 |
| 10 | Daniela Carlisi | 10 | Zhang S | 117 |
Fig. 7.
Visualization of authors (A) and cocited authors (B) on PTL research.
Out of 30330 cocited authors, four authors were cocited over 200 times (Table 3). The top three cocited authors were Monica Guzman (n = 423), Steffen Hehner (n = 334), and Akram Ghantous (n = 245). Authors who had minimal cocitations equivalent to 15 were visualized to draw a cocitation diagram (Fig. 7B). Similarly, there were many strong collaborative relationships among multiple cocited authors, such as Steffen Hehner and Benjamin Kwok, Monica Guzman, and Craig Jordan.
4.1. Analysis of cocited references
There were 40176 cocited PTL references. Each of the top 10 cited references (Table 4) was cited over 100 times, and the top three were cited over 200 times. We extracted references with the minimum number of cocitations (13) to construct a cocitation network graph (Fig. 8). According to Fig. 8, “Wen J, 2002, J Biol Chem” exhibited intense cocited correlations with “Kwok Bhb, 2001, Chem Biol” and “Hehner Sp, 1999, J Immunol.”
Table 4.
Top 10 cocited references for PTL research.
| Rank | Cocited Reference | Citation |
|---|---|---|
| 1 | Kwok Bhb, 2001, Chem Biol, V8, P759 | 205 |
| 2 | Guzman Ml, 2007, Blood, V110, P4427 | 201 |
| 3 | Hehner Sp, 1999, J Immunol, V163, P5617 | 200 |
| 4 | Wen J, 2002, J Biol Chem, V277, P38954 | 157 |
| 5 | Ghantous A, 2013, Drug Discov Today, V18, P894 | 135 |
| 6 | Guzman Ml, 2005, Blood, V105, P4163 | 132 |
| 7 | Hehner Sp, 1998, J Biol Chem, V273, P1288 | 132 |
| 8 | Bork Pm, 1997, Febs Lett, V402, P85 | 115 |
| 9 | Zhang S, 2004, Cancer Lett, V208, P143 | 105 |
| 10 | Garcia-Pineres Aj, 2001, J Biol Chem, V276, P39713 | 104 |
Fig. 8.
Visualization of cocited references on PTL research.
4.2. Analysis of citation bursts
Citation bursts reflect references that are continually cited by authors on a certain topic within a specific period. According to the current study, 15 references with intense citation bursts were analyzed by CiteSpace (Fig. 9). As shown in Fig. 9, the red bar reflects the duration and intensity of the citation burst [18]. The citation bursts of references started in 2002 at the earliest and in 2020 at the latest. The article titled “Parthenolide: from plant shoots to cancer roots” written by Akram Ghantous had the strongest citation burst (strength = 30.82), and its citation bursts were from 2015 to 2018. In short, the burst intensities of 15 references ranged from 13.38 to 30.82, while the sustained intensity ranged from 3 to 5 years. Table 5 lists the main contents of the 15 references in detail.
Fig. 9.
Top 15 references with strong citation bursts.
Table 5.
The main research contents of the 15 references with strong citation bursts.
| Rank | Strength | Main research contents |
|---|---|---|
| 1 | 23.26 | Parthenolide can inhibit NF-kappa B by targeting the I kappaB kinase complex |
| 2 | 15.12 | Parthenolide from the medicinal herb, Feverfew, directly binds to and inhibits I kappaB kinase |
| 3 | 13.38 | Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by parthenolide |
| 4 | 23.26 | Parthenolide mediates apoptosis through oxidative stress |
| 5 | 16.03 | Parthenolide reverses the resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligands through the sustained activation of c-Jun N-terminal kinase |
| 6 | 22.36 | Parthenolide can induce the apoptosis of human acute myelogenous leukemia stem and progenitor cells |
| 7 | 15.29 | Parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer |
| 8 | 15.78 | Parthenolide analog can selectively eradicate acute myelogenous leukemia stem and progenitor cells |
| 9 | 16.45 | Parthenolide plays an antileukemia role as an inhibitor of NF kappaB |
| 10 | 15.32 | Chemical and biological characteristics of parthenolide and its role in antiinflammation and antitumor |
| 11 | 17.22 | The anticancer and antiinflammatory mechanism of parthenolide |
| 12 | 13.93 | Multitarget anticancer mechanism of parthenolide |
| 13 | 30.82 | The discovery process of parthenolide and its anticancer mechanism |
| 14 | 13.39 | The development of parthenolide as an anticancer drug |
| 15 | 14.64 | Advances in the chemistry and bioactivity of parthenolide |
Analysis of Keywords and Trend Topics; Through the cooccurrence research of keywords, we can promptly understand the cutting-edge trends in this field. The top 20 keywords in PTL studies are summarized in Table 6. Among these keywords, “apoptosis” and “NF Kappa B” were mentioned over 90 times, which portrayed the frontier direction in this field from another aspect. We visualized keywords with the minimum number of occurrences (2) and applied occurrence analysis to draw a network graph (Fig. 10A). As shown in Fig. 10A, the area of the nodes and the thickness of the lines represent the frequency of keywords and the intensity between words, respectively. Subsequently, we drew a trend topic map using the R package, “bibliometrix” (Fig. 10B). Prior to 2009, the keywords mainly included potent inhibitors, nitric-oxide synthase, transcription factor, and tumor necrosis factor. However, after 2009, the keywords mainly included oxidative stress, apoptosis, multidrug resistance, cell cycle arrest, and immunotherapy. Therefore, we can understand the current research focus from the conversion of keywords in different periods.
Table 6.
Top 20 keywords for PTL research.
| Rank | Keyword | Count | Rank | Keyword | Count |
|---|---|---|---|---|---|
| 1 | parthenolide | 320 | 11 | reactive oxygen species | 20 |
| 2 | apoptosis | 129 | 12 | breast cancer | 20 |
| 3 | NF-Kappa B | 95 | 13 | Autophagy | 20 |
| 4 | sesquiterpene lactones | 64 | 14 | Cancer | 20 |
| 5 | tanacetum parthenium | 39 | 15 | Composite | 16 |
| 6 | inflammation | 37 | 16 | Leukemia | 16 |
| 7 | feverfew | 34 | 17 | oxidative stress | 14 |
| 8 | asteraceae | 33 | 18 | Lipopolysaccharide | 13 |
| 9 | cytotoxicity | 28 | 19 | pancreatic cancer | 11 |
| 10 | natural products | 21 | 20 | allergic contact dermatitis | 10 |
Fig. 10.
Keyword cluster analysis (A) and trend topic analysis (B).
5. Discussion
Some conventional reviews have already discussed the role of PTL. Liu et al. (2022) elaborated on the various antitumor activities of PTL, and pointed out that it stimulated the apoptosis pathway of pathological cells without damaging normal cells [19]. Joanna et al. (2020) also summarized the antioxidant properties of PTL and its anti-inflammatory effect in respiratory diseases [20]. Maria et al. (2012) analyzed the multi-target nature of PTL in treating diseases and elucidated its molecular biological mechanisms in various cells [21]. These studies will help us to better understand the role of PTL, while the bibliometrics will give us a new understanding of PTL from the perspective of information management.
5.1. Basic information
In 2002, the annual publication volume reached 20, indicating that scholars had conducted preliminary studies on PTL. In the following 10 years, the annual publishing volume in this field maintained a steady increase. This showed that PTL had attracted the attention of researchers, and the exploration of the drug had deepened. However, since 2012, the annual publishing volume has been in a dynamic fluctuation. The reason may be a shortage of research funding or a bottleneck in research. Although the publishing volume has not increased yearly, it has remained at a high level. This indicated that certain objective factors may have hindered the research in this field, but scholars still had considerable interest in PTL exploration.
The PTL papers in the United States and China accounted for half of the total, which showed that the research in this field had evident regional characteristics. The general rule that the United States and China are the main exporting countries of papers can also be found in other bibliometric reviews [22,23]. This may be due to the fact that the United States is a global technology giant with more outstanding research institutions than other countries. In addition to its increasing scientific research strength, China is also the main birthplace of traditional medicine, thus possessing unique advantages. Germany and Italy, on the other hand, may have increased their research on PTL due to the impact of the traditional medicine boom in recent years. We observed that the United States was the center, which had extremely positive cooperative relations with China, Australia, Italy, Spain, and Germany. This citation relationship may be due to the fact that research in the United States is often regarded as the cornerstone of this field, while other countries are willing to cite relevant achievements. China, which ranks second in the number of articles, exhibited less active cooperation with other countries, which may have had something to do with language and article quality. Obviously, it is not surprising that research institutions from the United States and China appear among the top ranked research institutions. However, the Lodz Medical University from Poland ranks third, indicating that although some countries and institutions do not have a broad research platform, their enthusiasm for traditional medicine has also driven them to conduct extensive research. In addition, universities in Western Europe and North America had many effective cooperative relationships. This may mainly be due to the consistency of language and the similarity of academic thinking. However, although Nankai University in Asia and the Medical University of Lodz in Central Europe were at the forefront of publishing articles, they lacked positive contact with western universities. Regional and cultural differences should not be an obstacle to academic exchanges. In the long run, this phenomenon will delay the study of PTL. Therefore, research institutions in different countries should adopt active, broad exchanges and cooperate to promote PTL research.
The largest number of PTL research papers was published in Molecules, showing that the journal paid significant attention to this field, and scholars highly recognized this journal. Another reason might be that as an open-access journal, it had a fast review speed and a high acceptance rate. The Q1 journals accounted for half of the top 10 journals. Interestingly, the top three publishing journals also have strong citation relationships, indicating that high publication volume can help provide references for other journals. For the cocited journals, most of them were Q1 journals with high impact. Journal of Biological Chemistry not only leads in the number of cocitations, but also has a strong cocitation relationship with top journals such as Nature, indicating its extensive research in the field of PTL. These authoritative journals provide a platform for academic exchange on PTL research. In addition, PTL research was mainly published in the journals related to basic medicine, such as immunology and biology. However, a few clinical research papers have been published, indicating that the PTL research is still in the initial stage with a lot of room for improvement. Meanwhile, these studies mainly cover the impact of PTL on specific target genes, which is consistent with the drift trajectory of the overlay map. This also indicates that research on PTL has gradually shifted to the level of exploring mechanisms through gene regulation. Moreover, the left side of the overlay represents the citing literature, which reflects the cutting-edge field of PTL research. The right side represents the cited literature, which reflects the foundation of PTL research. The results obtained in Fig. 6 are also basically consistent with those in Fig. 5.
From the perspective of the current study, Peter Crooks, Yue Chen, Craig Jordan, and Quan Zhang published the most articles in this field (at least 20 articles per person). Professor Peter Crooks published 36 articles. His main research direction is the inhibitory effect and mechanism of dimethylamino (DMAPTL) as a water-soluble analog of PTL on various tumor cells in vitro [[24], [25], [26], [27]]. Craig Jordan published 24 articles, mainly focusing on the research of PTL on acute myeloid leukemia through the PI3K/mTOR pathway, NF-κB pathway, and other mechanisms [[28], [29], [30], [31]]. In addition, Professor Peter Crooks participated in the writing of several articles. Yue Chen and Quan Zhang jointly published many articles, mainly on the molecular structure, biological activity, and chemical synthesis of PTL [[32], [33], [34], [35]]. Therefore, we observed that many high-yield authors in this field had cooperative relationships. This may be due to the high yield of the paper, which to some extent represents the author's authority in the field, and other authors are also willing to cite relevant achievements. In addition, the research in this field focused on basic scientific research and lacked first-line clinical experiments.
For cocited authors, Professor Monica Guzman had the highest number of citations (423). As early as 2005, Professor Monica Guzman, as the first author, published a review on PTL, detailing its characteristics [36]. In the same year, Monica Guzman published a study on the apoptosis of human myeloid leukemia cells induced using PTL, explaining that its mechanism was closely related to the inhibition of the nuclear factor, sensitization of p53, and upregulation of ROS [30]. In addition, this study laid a foundation for the mechanism of PTL inhibiting blood tumors. In 2007 and 2016, Professor Monica Guzman conducted research on the inhibition of acute myeloid leukemia in vivo using oral analogs of PTL and PTL-loaded nanoparticles, indicating that he had gradually moved from basic scientific research to clinical research [27,37]. Therefore, Professor Monica Guzman performed excellent preliminary work on the anticancer mechanism of PTL and its transformation from basic research to clinical research. In addition, there is a strong collaborative relationship between the top cocited authors, indicating that their research content intersects and supplements each other.
5.2. Knowledge domain
Cocited references are often cited together by many journals; therefore, cocited references can be regarded as the cornerstone of a discipline. In this study, we screened 10 cocited references to explore the current research status of PTL. Benjamin Kwok published the most cocited articles in 2001 [8]. He was the first to discover that PTL could inhibit I kappaB kinase to achieve an antiinflammatory effect. Monica Guzman published two papers in the top 10. The content of the articles has been described in the previous paragraph. Steffen Hehner also published two articles in the top 10 list. He first discovered that tumor necrosis factor-alpha (TNF-α) could cause the activation of transcription factor NF-kappaB [38]. In the following year, he confirmed that PTL could target TNF-α to inhibit NF-kappaB and ultimately play an antiinflammatory role [39]. These two papers had a connection. Jing Wen's paper ranked fourth in the number of cocitations [40]. He confirmed in vitro that the oxidative stress mediated by PTL promoted cell apoptosis. Akram Ghantous reviewed the discovery process and research status of PTL and pointed out the direction for future research [41]. In eighth place was the paper written by Peter Bork in 1997. It was verified by experiments that PTL could be used as an inhibitor of transcription factor NF-kappaB, but its antiinflammatory and antitumor mechanisms were not further explored [5]. Siyuan Zhang published the ninth cocited article, which discussed the key role of intracellular thiols and calcium balance on the apoptosis of colorectal cancer cells induced by PTL [42]. The experimental result enriched the research on the anticancer mechanism of PTL. The last cocited article was written by Alfonso Garcia-Pineres, which revealed the important role of Cysteine 38 in the inhibition of the NF-kappaB pathway by PTL [43]. In addition, just like cocited authors, there is a clear collaborative relationship between references with the highest number of cocitations, indicating that important references in this field will attract scholars' attention and complement each other academically. Summarily, the top 10 cocited articles mainly focus on the biological activity, pharmacological effect, and mechanism of PTL, which constitute the current research basis of PTL.
5.3. Focus and frontiers
References with citation bursts reflect the latest trends in specific research directions because scholars frequently cite these references for a specific period [44]. As shown in Table 5, we concluded that the biological activity of PTL and its mechanism of antiinflammation and anticancer are the frontier research directions in this field. In particular, scholars conducted the most extensive and in-depth studies on the inhibition of the NF-kappaB pathway by PTL. Moreover, from 2002 to 2020, the hot topics have continued to demonstrate that there are many unknowns in these fields that we can continue to explore.
Apart from references, keywords help analyze the progress in the field of PTL. After excluding drug names such as PTL, sesquiterpene lactones, and feverfew, the following keywords were mainly used (Table 6): apoptosis, inflammation, cancer, composite, and cytotoxicity. This indicates that research on PTL mainly focuses on the mechanisms and safety of anticancer and antiinflammatory effects. Based on the analysis shown in Fig. 10, we concluded that the current research on PTL primarily focused on the following points.
5.4. Delivery and synthesis of parthenolide
PTL has shown great prospects in the field of antitumors, but it has not been widely used in clinical practice. This is mainly caused by its hydrophobicity, which leads to its rapid elimination in the blood and its poor pharmacological properties [29]. Nanotechnology is regarded as a feasible method to enhance the water solubility and bioavailability of PTL. Baranello et al. (2015) developed PTL-loaded micelles, which released cytotoxicity for a longer time than free PTL [29]. Moreover, the cytotoxicity of the PTL-loaded micelles did not reduce because of the reduction of thiol on the cell surface; therefore, they exhibited good stability and bioavailability. In the same year, Karmakar et al. (2015) overcame the hydrophobicity of PTL through the use of carbon-functionalized nanographene (fGn). The PTL-fGn complex was confirmed to significantly promote the production of ROS and mitochondrial membrane rupture in human pancreatic cancer cell lines [45]. Darwish et al. (2019) expanded the application of nanotechnology by combining poly lactide coglycolide (PLGA) nanoparticles with antibody CD44 and encapsulated PTL to induce good targeting and selectivity in leukemia cell lines [46]. Similarly, PTL could be combined with Fe3O4 nanoparticles to exhibit a magnetic targeting effect [47]. In addition to micelles and nanoparticles, nanocrystals were also used as a new carrier-free strategy to improve the stability and release time of PTL [48]. The precipitation–high-pressure homogenization method was employed to prepare PTL nanocrystals, combined with sorafenib in vitro tests to play an extremely good role in inhibiting malignant biological behaviors in HepG2 cells [49].
Apart from changing the way drugs are delivered, the chemical modification of drugs can be employed to improve potency and reduce by-effects [50]. Quy et al. (2020) discussed the therapeutic effect of PTL derivatives with different structures containing aniline on chronic leukemia cells. Although the experimental results were satisfactory, the differences in efficacy caused by different subtypes were not further investigated [51]. Similarly, for leukemic cells, many studies have used PTL in combination with certain chemical components, such as dithiocarbamate ester, C1–C10 olefin, and functionalized the aliphatic positions (C9 and C14) of PTL. The specific subtypes of these complexes obtained by chemical synthesis and modification exhibited strong anticancer activity and weak toxicity to normal cells [[52], [53], [54]]. Apart from leukemia, compounds based on PTL have shown potential anticancer activity for various cancers [55]. PTL-5-fluorouracil (5-FU) conjugates and a PTL–cycloabine complex exhibited significant cytotoxicity for various carcinoma cell lines [56,57]. Other studies also confirmed that the heterozygotes of PTL with thiazolidinedione and micheliolide could be used as NF-κB inhibitors; thus, they can play a role in inhibiting tumor proliferation [58,59]. At the gene level, it was observed that a specific gene could code PTL 3β-hydroxylase, which uncovered a new way to improve the water solubility of PTL [60]. Majdi et al. (2015) confirmed that exogenous methyl jasmonate and salicylic acid could upregulate the expression of genes related to germane A synthase, thereby promoting the accumulation of PTL [61]. Presently, a few in vitro experiments have been conducted on the water-soluble analog of PTL, DMAPTL, which confirmed that its oral bioavailability and drug stability in blood were better than those of free PTL [24,25]. The production of DMAPTL showed that PTL research had gradually shifted from basic scientific research to clinical practice. However, to be widely used in clinical practice, its mechanism should be clarified, and more in-depth randomized controlled trials should be conducted.
The ultimate goal of scholars is to improve the effectiveness of PTL, whether it is to change the transportation route of PTL or chemically synthesize a new molecular structure. Although many achievements have been made, there is still a long way to go before PTL can be used in clinical applications.
5.5. Antiinflammatory effect of parthenolide
Feverfew was initially used to treat inflammatory diseases, and PTL is the main active ingredient of feverfew. Therefore, scholars conducted in-depth studies on the antiinflammatory effect of PTL [9]. In a study on vascular atherosclerosis, in vivo and in vitro experiments confirmed that PTL could reduce the expression of MCP-1 and attenuate the activity of NF-κB, thereby delaying damage and fighting against vascular injury [62]. Khare et al. (2017) confirmed that in the model of type-2 diabetes mice, the application of PTL could improve their cognitive deficits, significantly reduce the expression of TNF-a and IL-6, and alleviate the imbalance of glutamate [63]. Zhang et al. (2014) used PTL in human periodontal ligament-derived cells. It was observed that the activation of NF-κB and extracellular signal-regulated kinase (ERK) was inhibited, and the corresponding gene expression of osteoclasts was reduced. These phenomena evidenced the PTL treatment of periodontitis [64]. Another study revealed that PTL inhibited the activities of NF-κB and IκB kinase in pulmonary cystic fibrosis cells, indicating that PTL may be a promising drug for the treatment of excessive inflammation [65]. Summarily, many papers have reported that PTL plays an antiinflammatory role through the NF-κB pathway. However, this is not the only way [[66], [67], [68], [69]].
Li et al. (2015) observed that PTL attenuated liposaccharide (LPS)-induced inflammatory cytokines in THP-1 cells through the toll-like receptor 4 signaling pathway [70]. In macrophages, PTL directly inhibited the protease activity of caspase-1 and the activity of NLRP3 inflammatory bodies, independent of their inhibition of NF-κB activity [10]. Liu et al. (2020a) believed that PTL could improve the severity of colitis in rats with inflammatory bowel disease by regulating the Treg/Th17 balance. Therefore, PTL can be regarded as a microbial regulator rather than an antiinflammatory drug [71]. A study revealed that PTL could protect the liver in mice with acute hepatitis, and its antiinflammatory mechanism involved the inhibition and enhancement of the STAT3/p38 and p53 signals, respectively [72]. However, not every study yielded gratifying positive results. Zhang et al. (2017a) confirmed that PTL played a dual role in experimental autoimmune neuritis (EAN). Although PTL could reduce the production of TNF-α and IFN-γ in the early stage of the disease, it hindered the recovery of nerves in the late stage of the disease. Therefore, it was unsuitable for the treatment of EAN [73].
In conclusion, the antiinflammatory mechanism of PTL involves multiple pathways, and understanding the interaction of these pathways is a major direction in future studies on PTL.
5.6. Antitumor effect of parthenolide
With further research, the antitumor effect of PTL has attracted increasing attention [74]. In particular, the molecular biological mechanism of the PTL antitumor effect is the current research focus [75]. For oral cancer, Yu et al. (2015) observed that PTL induced the apoptosis of cancer cells by increasing the expression of Bim and death receptor 5 proteins [76]. Another in vivo experiment showed that PTL induced the apoptosis of oral cancer cells by significantly increasing the proapoptotic gene, bcl-2 associated X-protein (BAX), and significantly decreasing the antiapoptotic gene, bcl-2 [77]. Similarly, PTL activated the proapoptotic Bcl-2 family in colorectal cancer cell lines and alleviated the cachexia symptoms of this cancer model [78,79]. In other digestive tract tumors such as hepatocellular carcinoma, cholangiocarcinoma, and pancreatic cancer, PTL could also significantly increase the expression of proapoptosis related proteins, thereby exhibiting a strong antitumor effect [[80], [81], [82]].
Cheng and Xie discovered that PTL could cause apoptosis and cell cycle arrest in bladder cancer cells by regulating bcl-2 family proteins [83]. In addition, it was discovered that PTL could cause ROS-mediated autophagic cell death in human osteosarcoma cells [84]. Two studies on breast cancer confirmed that PTL could cause the apoptosis of cancer cells by producing ROS and starting the apoptosis pathway (Lu et al., 2014; D’Anneo et al., 2013). Two other papers on cervical cancer and renal cell carcinoma showed that PTL could cause apoptosis by inhibiting the PI3K/Akt signaling pathway [[85], [86], [87], [88]]. Zhao et al. (2014) confirmed that PTL induced the apoptosis of human lung cancer cells through the TNFRSF10B and PMAIP1 pathways [89]. In addition, the NF-κB pathway plays a significant role in the antitumor mechanism of PTL. Many studies have revealed that PTL leads to tumor cell apoptosis by inhibiting the activation of the NF-κB pathway [[90], [91], [92]]. Interestingly, a study showed that PTL induced the apoptosis of glioblastoma cells using caspase 3/7 without suppressing NF-κB activity [93].
Thus, the antitumor process of PTL involves multiple pathways and targets [94]. In the future, basic experiments are required to verify this complex pharmacological process.
5.7. Parthenolide in multiple disease models
Presently, PTL has been applied in basic medical experiments on many tissues and diseases [95]. In the field of neurology, PTL alleviated neuralgia in mice by promoting M2 microglia/macrophage polarization [96]. Moreover, PTL exhibited a neuroprotective effect through the activation of the Akt/GSK-3b pathway in PC12 cells [97]. Liu et al. (2020b) observed that PTL increased the level of Bcl-2 and reduced the expression of BAX in Parkinson's disease rats [98]. Many studies have also confirmed that PTL can repair damaged nerves after nerve injury [99,100].
Two studies on fatty-liver disease confirmed that PTL could protect the liver by reducing the levels of proinflammatory factors and oxidative stress markers [101,102]. Similarly, Jiang et al. (2016) believed that PTL could reduce the infiltration of proinflammatory factors to achieve a protective effect against acute lung injury [103]. It has been reported that PTL slowed down left ventricular hypertrophy and pulmonary fibrosis by inhibiting the STAT3 and NF-κB/Snail signal paths, respectively [104,105]. In particular, the NF-κB pathway plays an important role in the PTL treatment of certain disease models, such as renal injury, sepsis, and endometriosis [[106], [107], [108]]. Interestingly, Zhang et al. (2020) reported that PTL, as an NF-κB inhibitor, alleviated peritoneal fibrosis by inhibiting the TGF-β/Smad pathway [109]. However, exciting experimental results were not obtained in a few studies. Arıkan-Ayyıldız et al. (2017) believed that although PTL could alleviate certain pathological changes in asthmatic rats, its efficacy was not as good as that of dexamethasone, and its combination with dexamethasone could not enhance the efficacy [110].
In conclusion, currently, PTL research is limited to disease models, and more in-depth clinical trials should be conducted to determine its effectiveness.
5.8. Advantages and limitations
This study is relevant in the following ways. First, to the best of our knowledge, this is the first bibliometric study on PTL; it provides a detailed reference for scholars in this field. Second, we applied three bibliometric tools in this study and analyzed the current situation in this field from different perspectives. Finally, this study captured the forward position more comprehensively than general reviews.
This study also encountered certain limitations. First, the data in this study were only obtained from the WoSCC database, which led to the omission of certain papers. Second, we only selected English studies, implying that non-English studies may have been underestimated. Finally, as this is a developing field, we may have underestimated the contribution of recently published studies because their citation frequency is extremely low, although a few papers are published in high-quality journals.
6. Conclusions
This manuscript made a detailed bibliometric review of the research on PTL in the past 20 years. The performance analysis and science mapping were conducted on 1148 articles extracted from WoSCC. The performance analysis showed that there had been a rapid growth in publications in this field since 2002. Although there had been some fluctuations in the middle, the number of publications had remained stable. This indicates that although it may be affected by a lack of funding, the researchers' enthusiasm for exploring PTL has not decreased. In terms of geographical distribution, the United States and China have become the two countries with the highest numbers of publications. This trend may be related to the country's emphasis on scientific research and the amount of funding invested. Correspondingly, research institutions affiliated with both countries were also at the top of the rankings. It was worth mentioning that the Lodz Medical University in Poland had also published many articles in this field, which may indicate that traditional medical research is increasingly widely recognized. In terms of journals and cocited journals, Molecules and Journal of Biological Chemistry ranked first, respectively. As for the author and cocited author, Peter Crooks and Guzman Ml were also in the first place, respectively. Interestingly, the top ranked countries, institutions, journals, and authors had strong collaborative relationships in science mapping, indicating that extensive and in-depth research is easily recognized as benchmark research and used as a reference. In addition, cocited references, citation bursts, keyword co-occurrence, and trend topics all demonstrate the current research status and future development direction of PTL in recent years.
PTL has broad research prospects, particularly in the field of antiinflammatory and antitumor effects. The continuous publication of articles on PTL indicates that its role is valued by an increasing number of scholars. From the analysis results, the cooperation and exchange between institutions and countries need to be strengthened, particularly among regions and continents. On the one hand, further study of the molecular mechanism of the antiinflammatory and antitumor pharmacological effects of PTL is required. Attention should be paid to the characteristics of multiple targets and pathways in the treatment of diseases with PTL. On the other hand, PTL research should focus on basic experiments and the clinical application of PTL.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Funding
This study was supported by the 345 Talent Project of Shengjing Hospital.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to all the scholars who have conducted studies on PTL.
Contributor Information
Yibing Wang, Email: ybwang@cmu.edu.cn.
Jiahe Wang, Email: wangjh1@sj-hospital.org.
References
- 1.Freund R.R.A., et al. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020;37(4):541–565. doi: 10.1039/c9np00049f. [DOI] [PubMed] [Google Scholar]
- 2.Pareek A., et al. Feverfew (Tanacetum parthenium L.): a systematic review. Phcog. Rev. 2011;5(9):103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sztiller-Sikorska M., Czyz M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals. 2020;13(8) doi: 10.3390/ph13080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur K., et al. The efficacy of herbal supplements and nutraceuticals for prevention of migraine: can they help? Cureus. 2021;13(5) doi: 10.7759/cureus.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bork P.M., et al. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997;402(1):85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- 6.Carlisi D., et al. Parthenolide and its soluble analogues: multitasking compounds with antitumor properties. Biomedicines. 2022;10(2) doi: 10.3390/biomedicines10020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathema V.B., et al. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35(2):560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 8.Kwok B.H., et al. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001;8(8):759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang M., Li Q. Parthenolide could become a promising and stable drug with anti-inflammatory effects. Nat. Prod. Res. 2015;29(12):1092–1101. doi: 10.1080/14786419.2014.981541. [DOI] [PubMed] [Google Scholar]
- 10.Juliana C., et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285(13):9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacchini L., et al. Hepatocyte growth factor-activated NF-kappaB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis. 2004;25(11):2089–2100. doi: 10.1093/carcin/bgh227. [DOI] [PubMed] [Google Scholar]
- 12.Tapia P.C. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: "Mitohormesis" for health and vitality. Med. Hypotheses. 2006;66(4):832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Carlisi D., et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J. Cell. Physiol. 2011;226(6):1632–1641. doi: 10.1002/jcp.22494. [DOI] [PubMed] [Google Scholar]
- 14.Akmal M., et al. Glioblastome multiforme: a bibliometric analysis. World Neurosurg. 2020;136:270–282. doi: 10.1016/j.wneu.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z., Lin H., Li Y. Exploiting the performance of dictionary-based bio-entity name recognition in biomedical literature. Comput. Biol. Chem. 2008;32(4):287–291. doi: 10.1016/j.compbiolchem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Yeung A.W.K., Mozos I. The innovative and sustainable use of dental panoramic radiographs for the detection of osteoporosis. Int. J. Environ. Res. Publ. Health. 2020;17(7) doi: 10.3390/ijerph17072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Synnestvedt M.B., Chen C., Holmes J.H. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu. Symp. Proc. 2005;2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X., et al. Emerging trends and research foci in gastrointestinal microbiome. J. Transl. Med. 2019;17(1):67. doi: 10.1186/s12967-019-1810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Wang X. Recent advances on the structural modification of parthenolide and its derivatives as anticancer agents. Chin. J. Nat. Med. 2022;20(11):814–829. doi: 10.1016/S1875-5364(22)60238-3. [DOI] [PubMed] [Google Scholar]
- 20.Wieczfinska J., et al. The anti-inflammatory potential of selected plant-derived compounds in respiratory diseases. Curr. Pharmaceut. Des. 2020;26(24):2876–2884. doi: 10.2174/1381612826666200406093257. [DOI] [PubMed] [Google Scholar]
- 21.Kreuger M.R., et al. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anti Cancer Drugs. 2012;23(9):883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- 22.Goerlandt F., Li J., Reniers G. The landscape of risk communication research: a scientometric analysis. Int. J. Environ. Res. Publ. Health. 2020;17(9) doi: 10.3390/ijerph17093255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng C., et al. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J. Contr. Release. 2022;345:625–645. doi: 10.1016/j.jconrel.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Lamture G., Crooks P.A., Borrelli M.J. Actinomycin-D and dimethylamino-parthenolide synergism in treating human pancreatic cancer cells. Drug Dev. Res. 2018;79(6):287–294. doi: 10.1002/ddr.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanmugam R., et al. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkappaB and generating reactive oxygen species. Prostate. 2010;70(10):1074–1086. doi: 10.1002/pros.21141. [DOI] [PubMed] [Google Scholar]
- 26.Yip-Schneider M.T., et al. Effect of celecoxib and the novel anti-cancer agent, dimethylamino-parthenolide, in a developmental model of pancreatic cancer. Pancreas. 2008;37(3):e45–e53. doi: 10.1097/MPA.0b013e318172b4dd. [DOI] [PubMed] [Google Scholar]
- 27.Guzman M.L., et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110(13):4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Y., et al. The NF (Nuclear factor)-κB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br. J. Haematol. 2010;151(1):70–83. doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baranello M.P., et al. Micelle delivery of parthenolide to acute myeloid leukemia cells. Cell. Mol. Bioeng. 2015;8(3):455–470. doi: 10.1007/s12195-015-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman M.L., et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105(11):4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei S., et al. Rational design of a parthenolide-based drug regimen that selectively eradicates acute myelogenous leukemia stem cells. J. Biol. Chem. 2016;291(48) doi: 10.1074/jbc.A116.750653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai J.D., et al. Biomimetic semisynthesis of arglabin from parthenolide. J. Org. Chem. 2012;77(16):7103–7107. doi: 10.1021/jo300888s. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z.J., et al. Syntheses and biological evaluation of costunolide, parthenolide, and their fluorinated analogues. J. Med. Chem. 2015;58(17):7007–7020. doi: 10.1021/acs.jmedchem.5b00915. [DOI] [PubMed] [Google Scholar]
- 34.Long J., et al. Protection-group-free semisyntheses of parthenolide and its cyclopropyl analogue. J. Org. Chem. 2013;78(20):10512–10518. doi: 10.1021/jo401606q. [DOI] [PubMed] [Google Scholar]
- 35.Ge W., et al. Design and synthesis of parthenolide-SAHA hybrids for intervention of drug-resistant acute myeloid leukemia. Bioorg. Chem. 2019;87:699–713. doi: 10.1016/j.bioorg.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Guzman M.L., Jordan C.T. Feverfew: weeding out the root of leukaemia. Expet Opin. Biol. Ther. 2005;5(9):1147–1152. doi: 10.1517/14712598.5.9.1147. [DOI] [PubMed] [Google Scholar]
- 37.Zong H., et al. In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche. Leukemia. 2016;30(7):1582–1586. doi: 10.1038/leu.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hehner S.P., et al. Tumor necrosis factor-alpha-induced cell killing and activation of transcription factor NF-kappaB are uncoupled in L929 cells. J. Biol. Chem. 1998;273(29):18117–18121. doi: 10.1074/jbc.273.29.18117. [DOI] [PubMed] [Google Scholar]
- 39.Hehner S.P., et al. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J. Immunol. 1999;163(10):5617–5623. [PubMed] [Google Scholar]
- 40.Wen J., et al. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002;277(41):38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- 41.Ghantous A., et al. Parthenolide: from plant shoots to cancer roots. Drug Discov. Today. 2013;18(17–18):894–905. doi: 10.1016/j.drudis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S., Ong C.N., Shen H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004;208(2):143–153. doi: 10.1016/j.canlet.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 43.García-Piñeres A.J., et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001;276(43):39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 44.Miao Y., Zhang Y., Yin L. Trends in hepatocellular carcinoma research from 2008 to 2017: a bibliometric analysis. PeerJ. 2018;6:e5477. doi: 10.7717/peerj.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karmakar A., et al. Nanodelivery of parthenolide using functionalized nanographene enhances its anticancer activity. RSC Adv. 2015;5(4):2411–2420. doi: 10.1039/c4ra10871j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darwish N.H.E., et al. Novel targeted nano-parthenolide molecule against NF-kB in acute myeloid leukemia. Molecules. 2019;24(11) doi: 10.3390/molecules24112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao W., et al. Nanomagnetic liposome-encapsulated parthenolide and indocyanine green for targeting and chemo-photothermal antitumor therapy. Nanomedicine (Lond) 2020;15(9):871–890. doi: 10.2217/nnm-2019-0038. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H., et al. Preparation and antitumor study of camptothecin nanocrystals. Int. J. Pharm. 2011;415(1–2):293–300. doi: 10.1016/j.ijpharm.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 49.Liang P., et al. Preparation and characterization of parthenolide nanocrystals for enhancing therapeutic effects of sorafenib against advanced hepatocellular carcinoma. Int. J. Pharm. 2020;583 doi: 10.1016/j.ijpharm.2020.119375. [DOI] [PubMed] [Google Scholar]
- 50.Obata T., et al. Structure-activity relationship of indomethacin derivatives as Ido1 inhibitors. Anticancer Res. 2021;41(5):2287–2296. doi: 10.21873/anticanres.15004. [DOI] [PubMed] [Google Scholar]
- 51.Quy A.S., et al. Aniline-containing derivatives of parthenolide: synthesis and anti-chronic lymphocytic leukaemia activity. Tetrahedron. 2020;76(48) doi: 10.1016/j.tet.2020.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Y., et al. Synthesis and biological evaluation of dithiocarbamate esters of parthenolide as potential anti-acute myelogenous leukaemia agents. J. Enzym. Inhib. Med. Chem. 2018;33(1):1376–1391. doi: 10.1080/14756366.2018.1490734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kempema A.M., et al. Synthesis and antileukemic activities of C1-C10-modified parthenolide analogues. Bioorg. Med. Chem. 2015;23(15):4737–4745. doi: 10.1016/j.bmc.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyagi V., et al. Chemoenzymatic synthesis and antileukemic activity of novel C9- and C14-functionalized parthenolide analogs. Bioorg. Med. Chem. 2016;24(17):3876–3886. doi: 10.1016/j.bmc.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ou Y., et al. Synthesis and biological evaluation of parthenolide derivatives with reduced toxicity as potential inhibitors of the NLRP3 inflammasome. Bioorg. Med. Chem. Lett. 2020;30(17) doi: 10.1016/j.bmcl.2020.127399. [DOI] [PubMed] [Google Scholar]
- 56.Taleghani A., Nasseri M.A., Iranshahi M. Synthesis of dual-action parthenolide prodrugs as potent anticancer agents. Bioorg. Chem. 2017;71:128–134. doi: 10.1016/j.bioorg.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Ding Y., et al. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur. J. Med. Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111706. [DOI] [PubMed] [Google Scholar]
- 58.Qiu J., et al. Design, synthesis, and cytotoxic activities of novel hybrids of parthenolide and thiazolidinedione via click chemistry. J. Asian Nat. Prod. Res. 2020;22(5):425–433. doi: 10.1080/10286020.2019.1597055. [DOI] [PubMed] [Google Scholar]
- 59.Zeng B., et al. Design, synthesis and in vivo anticancer activity of novel parthenolide and micheliolide derivatives as NF-κB and STAT3 inhibitors. Bioorg. Chem. 2021;111 doi: 10.1016/j.bioorg.2021.104973. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q., et al. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab. Eng. 2014;23:145–153. doi: 10.1016/j.ymben.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Majdi M., Abdollahi M.R., Maroufi A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant Cell Rep. 2015;34(11):1909–1918. doi: 10.1007/s00299-015-1837-2. [DOI] [PubMed] [Google Scholar]
- 62.López-Franco O., et al. Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26(8):1864–1870. doi: 10.1161/01.ATV.0000229659.94020.53. [DOI] [PubMed] [Google Scholar]
- 63.Khare P., Datusalia A.K., Sharma S.S. Parthenolide, an NF-κB inhibitor ameliorates diabetes-induced behavioural deficit, neurotransmitter imbalance and neuroinflammation in type 2 diabetes rat model. NeuroMolecular Med. 2017;19(1):101–112. doi: 10.1007/s12017-016-8434-6. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X., et al. Anti-inflammatory and antiosteoclastogenic activities of parthenolide on human periodontal ligament cells in vitro. Evid. Based Complement Alternat. Med. 2014;2014 doi: 10.1155/2014/546097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saadane A., et al. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am. J. Respir. Cell Mol. Biol. 2007;36(6):728–736. doi: 10.1165/rcmb.2006-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalia M., et al. Exploring the impact of parthenolide as anti-quorum sensing and anti-biofilm agent against Pseudomonas aeruginosa. Life Sci. 2018;199:96–103. doi: 10.1016/j.lfs.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Kiuchi H., et al. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. J. Urol. 2009;181(5):2339–2348. doi: 10.1016/j.juro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 68.Nam Y.J., et al. Sesquiterpene lactone parthenolide attenuates production of inflammatory mediators by suppressing the Toll-like receptor-4-mediated activation of the Akt, mTOR, and NF-κB pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388(9):921–930. doi: 10.1007/s00210-015-1132-3. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Z.J., et al. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice. Int. Immunopharm. 2012;12(1):169–174. doi: 10.1016/j.intimp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Li S., et al. Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim. Biophys. Sin. 2015;47(5):368–375. doi: 10.1093/abbs/gmv019. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y.J., et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10(12):5225–5241. doi: 10.7150/thno.43716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D., et al. Parthenolide ameliorates Concanavalin A-induced acute hepatitis in mice and modulates the macrophages to an anti-inflammatory state. Int. Immunopharm. 2016;38:132–138. doi: 10.1016/j.intimp.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M., et al. Parthenolide inhibits the initiation of experimental autoimmune neuritis. J. Neuroimmunol. 2017;305:154–161. doi: 10.1016/j.jneuroim.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Pajak B., Gajkowska B., Orzechowski A. Molecular basis of parthenolide-dependent proapoptotic activity in cancer cells. Folia Histochem. Cytobiol. 2008;46(2):129–135. doi: 10.2478/v10042-008-0019-2. [DOI] [PubMed] [Google Scholar]
- 75.Dong Y., Qian X., Li J. Sesquiterpene lactones and cancer: new insight into antitumor and anti-inflammatory effects of parthenolide-derived dimethylaminomicheliolide and micheliolide. Comput. Math. Methods Med. 2022;2022 doi: 10.1155/2022/3744837. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Yu H.J., et al. Induction of apoptosis by parthenolide in human oral cancer cell lines and tumor xenografts. Oral Oncol. 2015;51(6):602–609. doi: 10.1016/j.oraloncology.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Baskaran N., et al. Parthenolide attenuates 7,12-dimethylbenz[a]anthracene induced hamster buccal pouch carcinogenesis. Mol. Cell. Biochem. 2018;440(1–2):11–22. doi: 10.1007/s11010-017-3151-5. [DOI] [PubMed] [Google Scholar]
- 78.Zhang S., Ong C.N., Shen H.M. Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett. 2004;211(2):175–188. doi: 10.1016/j.canlet.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 79.Yang Q., et al. Parthenolide from Parthenium integrifolium reduces tumor burden and alleviate cachexia symptoms in the murine CT-26 model of colorectal carcinoma. Phytomedicine. 2013;20(11):992–998. doi: 10.1016/j.phymed.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 80.Kim J.H., et al. Susceptibility of cholangiocarcinoma cells to parthenolide-induced apoptosis. Cancer Res. 2005;65(14):6312–6320. doi: 10.1158/0008-5472.CAN-04-4193. [DOI] [PubMed] [Google Scholar]
- 81.Sun J., et al. Parthenolide-induced apoptosis, autophagy and suppression of proliferation in HepG2 cells. Asian Pac. J. Cancer Prev. APJCP. 2014;15(12):4897–4902. doi: 10.7314/apjcp.2014.15.12.4897. [DOI] [PubMed] [Google Scholar]
- 82.Liu W., et al. Parthenolide suppresses pancreatic cell growth by autophagy-mediated apoptosis. OncoTargets Ther. 2017;10:453–461. doi: 10.2147/OTT.S117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng G., Xie L. Parthenolide induces apoptosis and cell cycle arrest of human 5637 bladder cancer cells in vitro. Molecules. 2011;16(8):6758–6768. doi: 10.3390/molecules16086758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang C., et al. Parthenolide induces reactive oxygen species-mediated autophagic cell death in human osteosarcoma cells. Cell. Physiol. Biochem. 2016;40(1–2):146–154. doi: 10.1159/000452532. [DOI] [PubMed] [Google Scholar]
- 85.Lu C., et al. Inhibition of AMPK/autophagy potentiates parthenolide-induced apoptosis in human breast cancer cells. J. Cell. Biochem. 2014;115(8):1458–1466. doi: 10.1002/jcb.24808. [DOI] [PubMed] [Google Scholar]
- 86.D'Anneo A., et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013;4(10):e891. doi: 10.1038/cddis.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeyamohan S., et al. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol. Lett. 2016;38(8):1251–1260. doi: 10.1007/s10529-016-2102-7. [DOI] [PubMed] [Google Scholar]
- 88.Liu D., et al. Parthenolide inhibits the tumor characteristics of renal cell carcinoma. Int. J. Oncol. 2021;58(1):100–110. doi: 10.3892/ijo.2020.5148. [DOI] [PubMed] [Google Scholar]
- 89.Zhao X., Liu X., Su L. Parthenolide induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung cancer cells. J. Exp. Clin. Cancer Res. 2014;33(1):3. doi: 10.1186/1756-9966-33-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao K., et al. Parthenolide inhibits cancer stem-like side population of nasopharyngeal carcinoma cells via suppression of the NF-κB/COX-2 pathway. Theranostics. 2015;5(3):302–321. doi: 10.7150/thno.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuch D., et al. Targeting radioresistant osteosarcoma cells with parthenolide. J. Cell. Biochem. 2012;113(4):1282–1291. doi: 10.1002/jcb.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karam L., et al. Anticancer activities of parthenolide in primary effusion lymphoma preclinical models. Mol. Carcinog. 2021;60(8):567–581. doi: 10.1002/mc.23324. [DOI] [PubMed] [Google Scholar]
- 93.Anderson K.N., Bejcek B.E. Parthenolide induces apoptosis in glioblastomas without affecting NF-kappaB. J. Pharmacol. Sci. 2008;106(2):318–320. doi: 10.1254/jphs.sc0060164. [DOI] [PubMed] [Google Scholar]
- 94.Won Y.K., et al. Chemopreventive activity of parthenolide against UVB-induced skin cancer and its mechanisms. Carcinogenesis. 2004;25(8):1449–1458. doi: 10.1093/carcin/bgh151. [DOI] [PubMed] [Google Scholar]
- 95.Benassi-Zanqueta É., et al. Parthenolide influences herpes simplex virus 1 replication in vitro. Intervirology. 2018;61(1):14–22. doi: 10.1159/000490055. [DOI] [PubMed] [Google Scholar]
- 96.Popiolek-Barczyk K., et al. Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast. 2015;2015 doi: 10.1155/2015/676473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J.F., et al. Parthenolide attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β pathway in PC12 cells. Biomed. Pharmacother. 2017;89:1159–1165. doi: 10.1016/j.biopha.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 98.Liu Q., et al. The parthenolide derivative ACT001 synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson's disease in mice. Behav. Brain Res. 2020;379 doi: 10.1016/j.bbr.2019.112337. [DOI] [PubMed] [Google Scholar]
- 99.Jinling D., et al. Parthenolide promotes expansion of Nestin+ progenitor cells via Shh modulation and contributes to post-injury cerebellar replenishment. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Materazzi S., et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain. 2013;154(12):2750–2758. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W., He Y., Liu Q. Parthenolide plays a protective role in the liver of mice with metabolic dysfunction-associated fatty liver disease through the activation of the HIPPO pathway. Mol. Med. Rep. 2021;24(1) doi: 10.3892/mmr.2021.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bahabadi M., et al. Hepatoprotective effect of parthenolide in rat model of nonalcoholic fatty liver disease. Immunopharmacol. Immunotoxicol. 2017;39(4):233–242. doi: 10.1080/08923973.2017.1327965. [DOI] [PubMed] [Google Scholar]
- 103.Jang Y.J., et al. Protective effect of sesquiterpene lactone parthenolide on LPS-induced acute lung injury. Arch Pharm. Res. (Seoul) 2016;39(12):1716–1725. doi: 10.1007/s12272-016-0716-x. [DOI] [PubMed] [Google Scholar]
- 104.Skoumal R., et al. Parthenolide inhibits STAT3 signaling and attenuates angiotensin II-induced left ventricular hypertrophy via modulation of fibroblast activity. J. Mol. Cell. Cardiol. 2011;50(4):634–641. doi: 10.1016/j.yjmcc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Li X.H., et al. Parthenolide attenuated bleomycin-induced pulmonary fibrosis via the NF-κB/Snail signaling pathway. Respir. Res. 2018;19(1):111. doi: 10.1186/s12931-018-0806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheehan M., et al. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit. Care Med. 2003;31(9):2263–2270. doi: 10.1097/01.CCM.0000085186.14867.F7. [DOI] [PubMed] [Google Scholar]
- 107.Francescato H.D., et al. Parthenolide reduces cisplatin-induced renal damage. Toxicology. 2007;230(1):64–75. doi: 10.1016/j.tox.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 108.Takai E., et al. Parthenolide reduces cell proliferation and prostaglandin E2 [corrected] in human endometriotic stromal cells and inhibits development of endometriosis in the murine model. Fertil. Steril. 2013;100(4):1170–1178. doi: 10.1016/j.fertnstert.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y., et al. Parthenolide, an NF-κB inhibitor, alleviates peritoneal fibrosis by suppressing the TGF-β/Smad pathway. Int. Immunopharm. 2020;78 doi: 10.1016/j.intimp.2019.106064. [DOI] [PubMed] [Google Scholar]
- 110.Arıkan-Ayyıldız Z., et al. Efficacy of parthenolide on lung histopathology in a murine model of asthma. Allergol. Immunopathol. 2017;45(1):63–68. doi: 10.1016/j.aller.2016.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.