Abstract
Background:
In recent years, various studies have been conducted to investigate the role of the influenza vaccine in reducing the risk of hospitalization and mortality; however, the results of these studies are clearly contradictory. Accordingly, we aimed to investigate the effect of monovalent flu vaccines on the risk of hospitalization and all-cause mortality.
Methods:
This study was a systematic review and meta-analysis of Randomized Clinical Trial (RCT) studies published in databases (Web of Science (ISI), Scopus, PubMed, Cochrane, Science Direct, Google Scholar) from 1980 to Dec 2022. All analyzes were performed by Stata15 statistical software and the significance level in this study was considered 0.05.
Results:
In the initial search, 375 articles were retrieved which, considering the study criteria, finally 8 RCT were included in the meta-analysis of the effects of monovalent Flu vaccine on the risk of hospitalization, and 10 RCT on the risk of all-cause mortality. Based on the results of meta-analysis, the overall Odds Ratio (OR) of hospitalization is equal to 0.71 (95% CI: 0.56–0.90; P <0.001) and the overall OR of all-cause mortality is equal to 0.82 (95% CI: 0.68–0.98; P=0.033). There was no publication bias in the study of the effect of monovalent flu vaccine on the risk of hospitalization and all-cause mortality
Conclusion:
Getting the flu vaccine can reduce the risk of hospitalization by 29% and the risk of overall death by 18%. Therefore, it may be promising to receive this vaccine as a preventive intervention for deaths and hospitalizations.
Keywords: Influenza vaccine, Hospitalization, All-cause mortality, Meta-analysis
Introduction
Influenza is an acute viral disease of the respiratory system that has a global spread (1). The importance of influenza is in the speed of spreading of its epidemics, the extent and number of patients and the severity of its complications, especially viral and bacterial pneumonia (2). Since this virus has the ability to create genetic changes, there are always many concerns about the occurrence of pandemics caused by it in the world (3). Influenza pandemics have always been a threat to public health around the world (4).
Influenza viruses can cause mild to severe illness and even death, especially in high-risk individuals such as the elderly, people at risk for cardiovascular disease, stroke, kidney disease, and immune system deficiency diseases (5). Therefore, due to the weak control of this disease by health systems and the resulting severe economic and health damage, it was finally decided to targeted vaccination against influenza (6). According to studies, vaccination is one of the most effective ways to prevent the disease and reduce its medical costs (5).
In recent years, with the improvement of nutrition and health in different communities, we are witnessing an increase in the average age of the population and, in other words, an aging population. In the near future, the elderly will make up the bulk of the world's population. One of the important challenges related to the phenomenon of population aging is the issue of providing health services for this segment of the population (7–11). The elderly are a major consumer of health services due to their reduced physical function and mental vulnerability, and their increase in population is accompanied by an increase in demand for health services (12–14). The treatment costs of the age group over 65 years are more than 5 times the total treatment costs of the age group less than 65 yr (15). In recent years, various studies have been conducted around the world to investigate the role of the influenza vaccine in reducing the risk of hospitalization and mortality due to various diseases in the elderly. A high proportion of these studies have shown that influenza vaccination reduces the risk of hospitalization or mortality in people receiving the vaccine (16–18). However, a number of other studies have shown that receiving the flu vaccine increases the risk of hospitalization (19–21) or death (19, 22) in vaccine recipients. Nevertheless, some studies have shown that the risk of hospitalization or death does not differ between those who receive the vaccine and those who do not (23, 24).
Therefore, the results of studies conducted in this field are clearly contradictory, and based on these studies; no general conclusion can be reached about the effect of influenza vaccine on the risk of hospitalization and all-cause mortality in the people receiving the vaccine. Since one of the best ways to achieve a clear answer to a scientific question in the field of health is to use systematic review and meta-analysis studies using the results of clinical trial investigations, the present study, using the results of researches conducted in this field by systematic review and meta-analysis, investigated the effect of flu vaccine on the risk of hospitalization and all-cause mortality according to the results of randomized clinical trials.
Methods
Type of study and population studied
This systematic review and meta-analysis used data from clinical trial studies to investigate the effect of flu vaccine on the risk of hospitalization and all-cause mortality from 1980 to Dec 2022.
Search strategies
A comprehensive search of the texts published in the databases of Web of Science (ISI), Scopus, PubMed, Cochrane, Science Direct and Google Scholar was performed in the period from 1980 to Dec 2022, in this study, the keywords Influenza Vaccine, Hospitalization and mortality and their synonyms based on PubMed MeSH were used to perform the search.
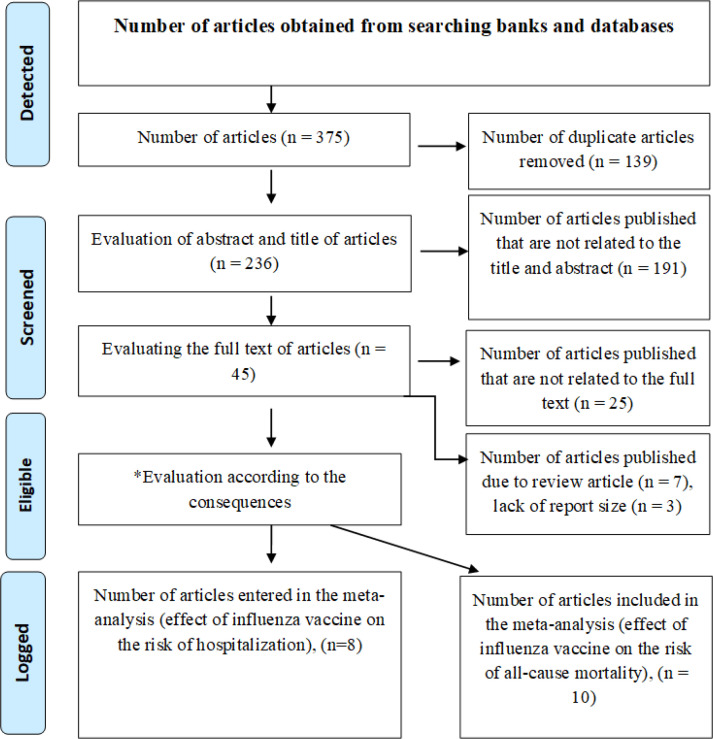
Moreover, to ensure the availability of all published studies in this regard; the list of references of articles retrieved in the electronic search was reviewed to access related studies. In addition, in order to access articles whose full text could not be received through databases, we contacted the relevant authors by e-mail to receive the full text of the articles. After collecting the documents and articles, their characteristics and abstracts were entered into the Endnote software and duplicate items were removed using this software as well as re-reading the titles. In the next step, by reviewing the titles, articles unrelated to the purpose of the research were excluded, and then among the remaining studies, by referring to the abstract and also the full text of the article, it was relevant to the purpose of the study. Figure 1 shows the process of identifying and selecting studies as well as how to examine them in order to enter a systematic review and meta-analysis schematically.
Fig. 1:
Diagram of selected clinical trial studies for meta-analysis
Criteria for inclusion and exclusion of clinical trial studies
Only articles from clinical trials that examined the effect of flu vaccine on the risk of hospitalization and all-cause mortality were evaluated. Moreover, the relative risk, risk ratio, or odds ratio of the effect of exposure on the outcomes under consideration should be measured in the article, taking into account the 95% confidence interval (CI), or it should be calculable based on the information in the article. Articles that did not provide sufficient data to calculate the effect size or standard deviation (SD) for the relevant estimates were excluded from the study.
Information extracted from the clinical trial studies
From the final articles included in this study, information such as study title, type of study, name of the first author of the article, year of publication, country of study, sample size, number of exposed and non-exposed groups, duration of follow-up of patients, the status of receiving or not receiving influenza vaccine in participants, hospitalization rate, the incidence of total mortality, RR and OR with 95% CI related to hospitalization and overall mortality, the percentage of women in the study population, the average age of the participants in the study, the prevalence of diabetes, the prevalence of blood pressure and the proportion of smokers; and variables that were adjusted in the multivariate models, were extracted and collected. In many studies, the vaccine effectiveness (VE) was calculated and presented based on the relevant formula VE = (OR −1) × 100 (25). In these cases, the OR and the relevant 95% CI were calculated based on the presented VE values. In studies where the effect size reports were calculated and presented separately for time or seasonal periods, using the meta-analysis method, an overall effect size was calculated from the presented values and considered in the analysis. Moreover, in studies where the effect size was not reported but information about the number of participants and injected vaccines were available, the effect size and relevant 95% CI were calculated using a 2×2 table.
Evaluating the quality of the Clinical trial studies
To evaluate the quality of studies, the Jadad scale checklist was used due to its quantitative scoring capability (26, 27). This checklist is used to evaluate the quality of randomized clinical trial (RCT) studies. The maximum score that can be given to an article using this checklist is 5 and the minimum is 0. Based on the Jadad scale, scores range from 0 to 2, 3 to 4, and more than 4 were defined for low, moderate, and good-quality articles, respectively (26, 27)
Statistical Analysis
Due to the low incidence of outcomes (hospitalization and mortality), the rate ratio and the risk ratio in various studies were considered as equal of odds ratio (OR). The presence of heterogeneity in the studies included in the meta-analysis was assessed using I2 or Q-Test (Chi-square). I2 test was used to report a quantitative amount of heterogeneity. In addition, Forest Plot was used to investigate the heterogeneity graphically. Using the Chi-square test, the differences in the results of the studies entered in the met-analysis were investigated and according to results of this test determined the type of model (fixed or random). Egger’s test and Begg's test were used to evaluate publication bias. All analysis were performed by Stata statistical software (version 15.0, Stata Corp, College Station, TX), and the significance level in all tests were considered 0.05.
Results
Clinical trial studies included in the systematic review and meta-analysis
According to Fig. 1; 375 articles were collected by electronic search in databases with keywords created in Mesh with Title/Abstract. By examining the effect size reported in studies related to the purpose of the study, 8 studies for hospitalization (28–35) and 10 studies for general mortality (28–37) were included in the meta-analysis, eight articles reported the effect size associated with hospitalization and mortality (28–35).
Effect of influenza vaccine on the risk of hospitalization
Overall, eight clinical trial studies were conducted to investigate the effect of influenza vaccine on the risk of hospitalization (28–35). These studies were performed on 14,396 people between 1994 and 2022. The follow-up time of participants in different studies varied from 5 to 24 months. Moreover, 6 study are classified in terms of quality assessment in the group of good-quality studies (Table 1). The adjusted variables in the study of the effect of influenza vaccine on the risk of hospitalization in different studies can be seen in Table 2.
Table 1:
Characteristics of clinical trial studies included in the meta-analysis to investigate the effect of influenza vaccine on the risk of hospitalization
| First author (Reference number) | Year | Country | Sample size | Mean age | Women, % | Hypertension, % | Diabetes Mellitus, % | Smoker, % | OR (95% CI) | Duration of follow-up | Jadad Scale (Score) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Govaert (29) | 1994 | Netherlands | 1838 | 65 | - | - | - | - | 0.42 (0.24–0.74) | 5 months | 5 |
| Flucad(31) | 2008 | Finland | 658 | 60 | 26 | 67 | 20 | 18 | 0.55 (0.22 – 1.37) | 10 months | 5 |
| IVCAD (33) | 2009 | Iran | 266 | 54.7 | 33 | 83 | - | - | 1.94 (0.36 – 10.42) | 6 months | 4 |
| De Villiers (32) | 2009 | South Korea | 3242 | 69.5 | 33 | 57 | 26 | 17 | 0.91 (0.36 – 2.27) | 8 months | 5 |
| Phrommintikul (34) | 2011 | Thailand | 439 | 66 | 43 | 62 | 31 | 12 | 0.70 (0.57 – 0.82) | 24 months | 4 |
| FLUVACS (30) | 2004 | Argentina | 292 | 64.5 | 28 | 52 | 18 | 44 | 0.44 (0.28 – 0.71) | 24 months | 5 |
| Fröbert (35) | 2021 | Sweden, Denmark, Norway, Latvia, the United Kingdom, Czech Republic, Bangladesh, and Australia | 2532 | 60 | 18 | 49 | 21 | 35 | 1.38 (0.78–2.49) | 12 months | 5 |
| Loeb (28) | 2022 | India-China-Africa | 5129 | 57 | 51 | - | 23 | - | 0.83 (0.72–0.97) | 12 months | 5 |
Table 2:
Adjusted variables in the studies that investigate the effect of influenza vaccine on the risk of hospitalization
| First author (Reference number) | Year | Adjusted variables |
|---|---|---|
| Govaert (29) | 1994 | Age, sex, previous influenza vaccination |
| Flucad (31) | 2008 | Age, gender, weight, height, residence, employment status, hypertension, diabetes, smoking, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, creatinine, clinical history, actual treatment, inflammatory markers and infectious burden |
| IVCAD (33) | 2009 | Age, sex, previous influenza vaccination, hypertension, diabetes, smoking |
| De Villiers (32) | 2009 | Fever ≥37 degrees, cough, sore throat, age, sex, runny nose, stuffy nose, headache |
| Phrommintikul (34) | 2011 | Age, sex, serum creatinine, treatment with angiotensin converting enzyme inhibitors and coronary arteries |
| FLUVACS (30) | 2004 | Age, sex, anterior infarction, stemi, hypertension, diabetes |
| Fröbert (35) | 2021 | - |
| Loeb (28) | 2022 | - |
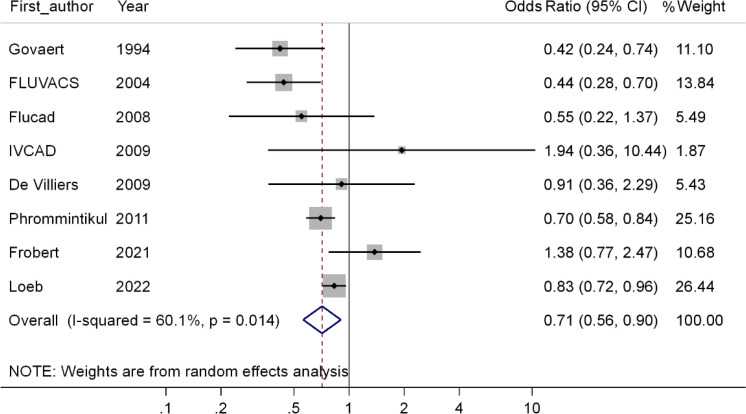
Based on meta-analysis of the results of RCT studies included in the meta-analysis (28–35), the OR of hospitalization is equal to 0.71 (CI 95 %; 0.56–0.90, P<0.001), in other words, this meta-analysis show that compared to the people did not receive the flu vaccine, the odds of hospitalization in persons receiving the flu vaccine is reduced by 29%, which is statistically significant (Fig. 2).
Fig. 2:
Forest plot of the effect of receiving the flu vaccine on the risk of hospitalization
Assessment of publication bias in the valuation of the effect of receiving the flu vaccine on the risk of hospitalization according to the results of clinical trial studies
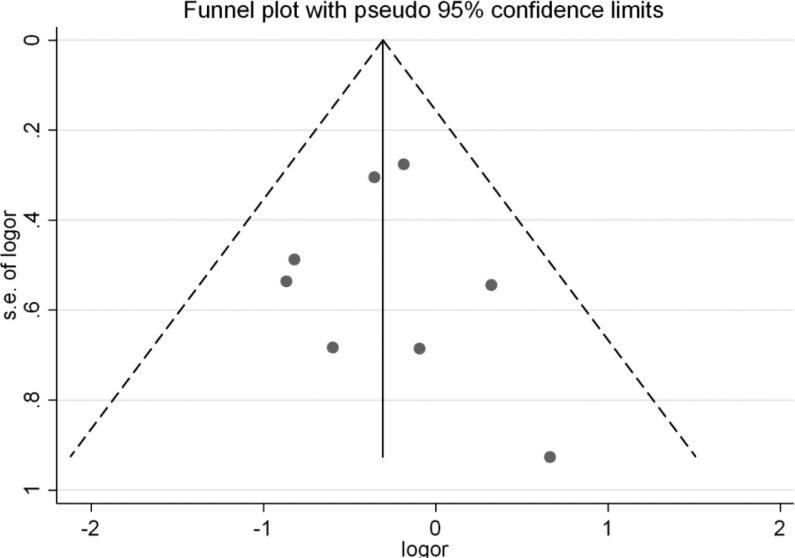
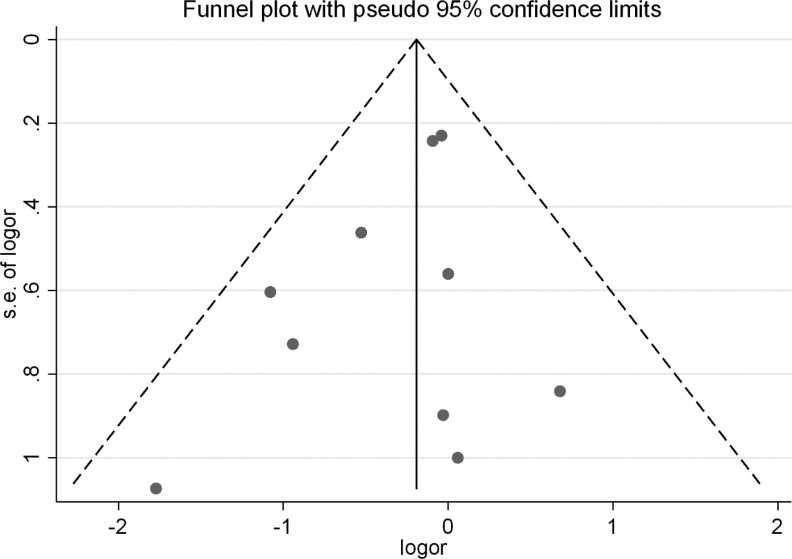
In the study of the effect of receiving the flu vaccine on the risk of hospitalization, using Begg's test (P=0.536) and Egger’s test (P=0.790), no Publication bias was observed. The funnel plot of the effect of receiving the flu vaccine on the risk of hospitalization is presented in Fig. 3.
Fig. 3:
Funnel plot for evaluation of Publication bias in investigating the effect of receiving the flu vaccine on the risk of hospitalization according to the results of clinical trial studies
Effect of influenza vaccine on the risk of all-cause mortality
In general, ten clinical trial studies (28–37) have been conducted to investigate the effect of influenza vaccine on the risk of all-cause mortality. These studies were performed on 21,155 people between 1994 and 2022. The follow-up time of participants in different studies varied from 5 to 24 months. Moreover, 8 study are classified in terms of quality evaluation in the group of good-quality studies (Table 3). The adjusted variables in the study of the effect of influenza vaccine on the risk of all-cause mortality can be seen in Table 4.
Table 3:
Characteristics of the clinical trial studies included in the meta-analysis to investigate the effect of influenza vaccine on the risk of all-cause mortality
| First author (Reference number) | Year | Country | Sample size | Mean age | Women, % | Hypertension, % | Diabetes Mellitus, % | Smoker, % | OR (95% CI) | Duration of follow-up | Jadad Scale (Score) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Govaert (29) | 1994 | Netherlands | 1838 | 65 | - | - | - | - | 1.97(0.49–7.84) | 5 months | 5 |
| FLUVACS (30) | 2004 | Argentina | 292 | 64.5 | 28 | 52 | 18 | 44 | 0.34 (0.17 – 0.71) | 24 months | 5 |
| FLUCAD (31) | 2008 | Finland | 658 | 60 | 26 | 67 | 20 | 18 | 1.06 (0.15 – 7.56) | 10 months | 5 |
| IVCAD (33) | 2009 | Iran | 266 | 54.7 | 33 | 83 | - | - | 0.97 (0.20 – 4.72) | 6 months | 4 |
| De Villiers (32) | 2009 | South Korea | 3242 | 69.5 | 33 | 57 | 26 | 17 | 1 (0.54 – 1.85) | 8 months | 5 |
| Phromminti kul(34) | 2011 | Thailand | 439 | 66 | 43 | 62 | 31 | 12 | 0.39 (0.14 – 1.12) | 24 months | 4 |
| Verhees (37) | 2019 | Netherlands | 2198 | 67 | 53 | 45 | 3 | 23 | 0.96 (0.87 – 1.07) | 24 months | 5 |
| Fröbert (35) | 2021 | Sweden, Denmark, Norway, Latvia, the United Kingdom, Czech Republic, Bangladesh, and Australia | 2532 | 60 | 18 | 49 | 21 | 35 | 0.59(0.39–0.90) | 12 months | 5 |
| Langley (36) | 2011 | North america | 4561 | 50 | 55 | - | - | - | 0.17(0.02–1.83) | 12 months | 5 |
| Loeb (28) | 2022 | India-China-Africa | 5129 | 57 | 51 | - | 23 | - | 0.91(0.81–1.02) | 12 months | 5 |
Table 4:
Adjusted variables in the studies that investigate the effect of influenza vaccine on the risk of all-cause mortality
| First author (Reference number) | Year | Adjusted variables |
|---|---|---|
| Govaert (29) | 1994 | Age, gender, previous influenza vaccination, health status |
| FLUVACS (30) | 2004 | Age, sex, anterior infarction, stemi, hypertension, diabetes |
| FLUCAD (31) | 2008 | Age, gender, weight, height, residence, employment status, hypertension, diabetes, smoking, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, creatinine, clinical history, actual treatment, inflammatory markers and infectious burden |
| IVCAD (33) | 2009 | Age, sex, previous influenza vaccination, hypertension, diabetes, smoking |
| De villiers (32) | 2009 | Fever ≥37 degrees, cough, sore throat, age, sex, runny nose, stuffy nose, headache |
| Phrommintikul(34) | 2011 | Age, sex, serum creatinine, treatment with angiotensin-converting enzyme inhibitors and coronary arteries |
| Verhees (37) | 2019 | Gender, age, smoking status, lung disease, heart disease, diabetes mellitus, and previous vaccinations |
| Fröbert (35) | 2021 | Age, gender, st-segment–elevation myocardial infarction, non–st-segment–elevation myocardial infarction, stable coronary artery disease, body mass index, diabetes, smoking, hyperlipidemia, hypertension, previous myocardial infarction, previous percutaneous coronary intervention, previous coronary artery bypass graft, killip class, number of diseased vessels |
| Langley (36) | 2011 | - |
| Loeb (28) | 2022 | - |
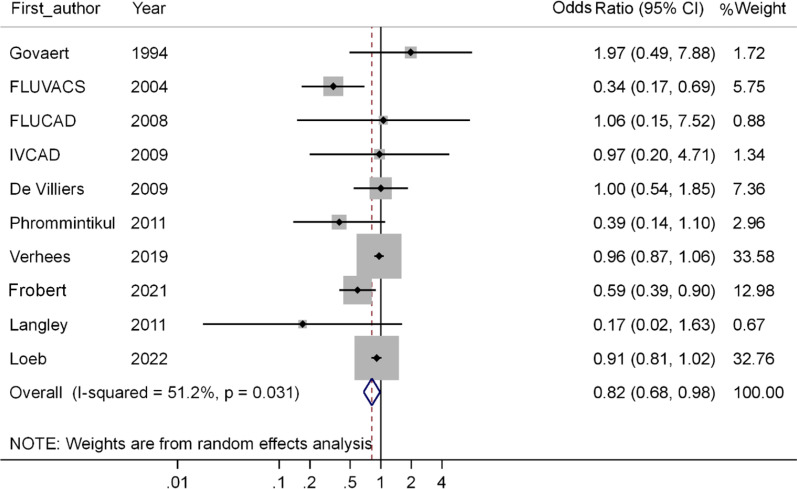
Based on the results of RCT studies included in the meta-analysis (28–37), the OR of all-cause mortality is equal to 0.82 (95% CI; 0.68 – 0.98, P=0.033), in other words, this meta-analysis show that compared to the people who did not receive the flu vaccine, the odds of death in the persons receiving the influenza vaccine decreased by 18%, which is statistically significant (Fig. 4).
Fig. 4:
Forest plot of the effect of influenza vaccine on the risk of all-cause mortality
Assessment of publication bias in the valuation of the effect of influenza vaccine on the risk of all-cause mortality according to the results of clinical trial studies
In the study of the effect of influenza vaccine on the risk of all-cause mortality, using Begg's test (P=0.858) and Egger’s test (P=0.145), no Publication bias was observed (Fig. 5).
Fig. 5:
Funnel plot for evaluation of Publication bias in investigating the effect of influenza vaccine on the risk of all-cause mortality according to the results of clinical trial studies
Discussion
The purpose of this systematic review and meta-analysis was to investigate the effect of influenza vaccine on the risk of hospitalization and all-cause mortality. Based on the meta-analysis of the results of RCT studies included in the meta-analysis (28–37), compared to the individuals who did not receive the flu vaccine, the odds of hospitalization and mortality in people receiving the flu vaccine was decreased by 29% and 18%, respectively. Similarly, a systematic review and meta-analysis by Kyu Rae Lee and et al., entitled “Effect of influenza vaccination on risk of stroke: a systematic review and meta-analysis”, showed that receiving the flu vaccine had protective effects against stroke (6).
In the same way, the protective effects of influenza vaccine against the chance of hospitalization or mortality have also been confirmed in other studies, in a cohort study that conducted in patients with ischemic heart disease in Taiwan, influenza vaccine has protective effects against hospitalization and mortality, so that influenza vaccination leads to a reduction in the OR of hospitalization (0.84 (95% CI; 0.76–0.93)) and mortality (0.42 (95% CI ; 0.35–0.49)) due to cardiovascular diseases (38).
Moreover, in another cohort study was conducted on 4,454 vaccinated diabetic patients and 4,571 unvaccinated diabetic patients. The risk of pneumonia, respiratory failure, the need for intensive care, hospitalization, and overall mortality was assessed during the first year after vaccination. Fewer Pneumonia and respiratory failure are seen in the vaccinated group. Moreover, in the vaccinated group, the rate of hospitalization is 11% lower than the non-vaccinated group (incidence rate of 29.6 vs. 33.1 per 100 people per year) with an adjusted Hazard Ratio (HR) 0.88 (95% CI; 0.81–0.96). In addition, the HR of hospitalization in the intensive care unit in the vaccinated group is equal to 0.30 (95% CI; 0.19–0.47), and the HR of overall mortality is equal to 0.44 (95% CI; 0.36–0.54). Furthermore, in comparison with non-vaccinated group, receiving the flu vaccine led to a reduction of $ 1283 hospitalization costs per person in vaccinated group (39).
In addition, receiving the flu vaccine in the elderly reduced the need for hospitalization as well as the length of hospitalization (40). In most observational studies with a sample size of more than 10,000 people, the protective role of the influenza vaccine in preventing the hospitalization of the elderly has been confirmed (41, 42).
However, some studies have not shown a protective effect of the flu vaccine against the risk of hospitalization or overall mortality. For example, in a study conducted on 1,000 people in Japan, in comparison with non-vaccinated individuals, the HR of hospitalization in vaccinated people is equal to 1.25 (95% CI; 0.29–5.37) (43), which of course this risk increases is not statistically significant. Another study as a prospective cohort study in Hong Kong on 27,469 people found that compared to non-vaccinated people, the HR of hospitalization in the vaccinated individuals was 0.85 (95% CI; 0.61–1.17) (44). However, the reason for the lack of a significant relationship between exposure and outcome in some observational studies (cohort, cross-sectional, case-control, etc.) is probably a random error due to sampling or lack of control of potential confounder variables or the presence of residual confounder. However, in RCT studies, due to the randomization phase, the confounding variables are evenly distributed among the groups under study, if the randomization is performed well and the sample size is appropriate.
Usually, the elderly have a higher prevalence of underlying or chronic diseases such as cardiovascular diseases (35, 37), high blood pressure (35), diabetes (30–32) and blood lipid disorders (31, 32), on the other hand, people who have such chronic diseases are more likely to suffer from more severe forms of the influenza and its complications; that ultimately leads to an increase in the possibility of hospitalization or death in the elderly (29, 31, 33, 35, 37). However, the results of this study show that receiving the influenza vaccine can effectively reduce the chance of hospitalization, which indicates the effectiveness of the vaccine in preventing the disease or preventing severe forms of the disease. This success eventually leads to improve the health of the elderly and reduce treatment costs. Although the primary goal of this study was not to investigate the effect of the influenza vaccine on the risk of hospitalization and mortality exclusively in the elderly, the average age of the participants in all the studies included in the analysis was equal to or greater than 50 years. Therefore, the results of this study can be extended to elderly people as well.
The results of studies on the relationship between influenza vaccination and hospitalization and mortality in the elderly are inconsistent, especially in studies with observational design. Therefore, this study can be very helpful in providing an appropriate answer in this regard based on the result of RCT studies as golden-standard studies in medical science and extracted clear results for evidence-based medicine and scientific medical advice, therefore the results of this study can be very useful in the decision of the elderly to receive the flu vaccine as well as providing preventive advice with health experts based on scientific evidence.
Nevertheless, this study has its limitations. One of the main limitations of this study is the small number of RCT studies that have examined the effect of influenza vaccine on the risk of hospitalization (8 studies) and all-cause mortality (10 studies). Due to the small number of studies conducted in this field, current studies cannot provide a good pattern of the effect of exposure and the outcomes in different parts of the world.
Conclusion
Getting the flu vaccine can reduce the risk of hospitalization by 29% and the risk of all-cause mortality by 18%. Therefore, receiving this vaccine as a preventive intervention may be promising. However, more clinical studies are needed to evaluate these relationships and their potential contribution to improving health outcomes.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This is to acknowledge that the project leading to the publication of this paper is fully funded by the research deputy of Shahrekord University of Medical Sciences, Iran with grant number: 5574 and ethical code: IR.SKUMS.REC.1399.176. However, we have conducted a new and updated systematic search and meta-analysis in Dec 2022 based on the topic of the above thesis.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Data availability
The data used in this systematic review and meta-analysis can be retrieved in the tables provided in the text of the article. In addition, the data used for meta-analysis in the present study is freely available in the text of the articles used.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Clark NM, Lynch JP., 3rd (2011). Influenza: epidemiology, clinical features, therapy, and prevention. Semin Respir Crit Care Med, 32:373–392. [DOI] [PubMed] [Google Scholar]
- 2.Lagacé-Wiens PR, Rubinstein E, Gumel A. (2010). Influenza epidemiology--past, present, and future. Crit Care Med, 38(4 Suppl):e1–9. [DOI] [PubMed] [Google Scholar]
- 3.Eden JS, Tanaka MM, Boni MF, et al. (2013). Recombination within the pandemic norovirus GII.4 lineage. J Virol, 87:6270–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilbourne ED. (2006). Influenza pandemics of the 20th century. Emerg Infect Dis, 12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pakzad R, Mohammadian-Hafshejani A, Khosravi B, et al. (2016). The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann Transl Med, 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KR, Bae JH, Hwang IC, et al. (2017). Effect of influenza vaccination on risk of stroke: a systematic review and meta-analysis. Neuroepidemiology, 48:103–110. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadian M, Allah Bakeshei K, Mohammadian-Hafshejani A. (2020). International epidemiology of liver cancer: geographical distribution, secular trends and predicting the future. J Prev Med Hyg, 61:E259–E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakzad R, Khani Y, Pakzad I, et al. (2016). Spatial Analysis of Stomach Cancer Incidence in Iran. Asian Pac J Cancer Prev, 17(S3):27–32. [DOI] [PubMed] [Google Scholar]
- 9.Pakzad R, Moudi A, Pournamdar Z, et al. (2016). Spatial Analysis of Colorectal Cancer in Iran. Asian Pac J Cancer Prev, 17(S3):53–58. [DOI] [PubMed] [Google Scholar]
- 10.Rafiemanesh H, Maleki F, Mohammadian-Hafshejani A, et al. (2016). The Trend in Histological Changes and the Incidence of Esophagus Cancer in Iran (2003–2008). Int J Prev Med, 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salehiniya H, Ghobadi Dashdebi S, Rafiemanesh H, et al. (2016). Time Trend Analysis of Cancer Incidence in Caspian Sea, 2004 – 2009: A Population-based Cancer Registries Study (northern Iran). Caspian J Intern Med, 7:25–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Hertzman L, Hayes M. (1985). Will the elderly really bankrupt us with increased health care costs? Can J Public Health, 76:373–377. [PubMed] [Google Scholar]
- 13.Mohammadian M, Soroush A, Mohammadian-Hafshejani A, et al. (2016). Incidence and Mortality of Liver Cancer and Their Relationship with Development in Asia. Asian Pac J Cancer Prev, 17:2041–2047. [DOI] [PubMed] [Google Scholar]
- 14.Rafiemanesh H, Mehtarpoor M, Mohammadian-Hafshejani A, et al. (2015). Cancer epidemiology and trends in Sistan and Baluchestan province, Iran. Med J Islam Repub Iran, 29:254. [PMC free article] [PubMed] [Google Scholar]
- 15.Russell M, Ardalan A. (2007). Aging and future health care costs: a warning for the country's health system. Iran J Ageing, 4:300–305. [Google Scholar]
- 16.Chen CM, Chen HJ, Chen Ws, et al. (2018). Clinical effectiveness of influenza vaccination in patients with rheumatoid arthritis. Int J Rheum Dis, 21:1246–1253. [DOI] [PubMed] [Google Scholar]
- 17.Mulpuru S, Li L, Ye L, et al. (2019). Effectiveness of influenza vaccination on hospitalizations and risk factors for severe outcomes in hospitalized patients with COPD. Chest, 155:69–78. [DOI] [PubMed] [Google Scholar]
- 18.Wang I-K, Lin C-L, Lin P-C, et al. (2016). Seasonal influenza vaccination is associated with reduced morbidity and mortality in peritoneal dialysis patients. Nephrol Dial Transplant, 31:269–274. [DOI] [PubMed] [Google Scholar]
- 19.Anderson ML, Dobkin C, Gorry D. (2020). The effect of influenza vaccination for the elderly on hospitalization and mortality: an observational study with a regression discontinuity design. Ann Intern Med, 172:445–452. [DOI] [PubMed] [Google Scholar]
- 20.Gilca R, Skowronski DM, Douville-Fradet M, et al. (2015). Mid-season estimates of influenza vaccine effectiveness against influenza A (H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS One, 10:e0132195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaselitz TB, Martin ET, Power LE, et al. (2019). Impact of vaccination on morbidity and mortality in adults hospitalized with influenza A, 2014–2015. Infect Dis Clin Pract, 27:328–333. [Google Scholar]
- 22.Yokomichi H, Kurihara S, Yokoyama T, et al. (2014). The pandemic influenza A (H1N1) 2009 vaccine does not increase the mortality rate of idiopathic interstitial pneumonia: a matched case-control study. PLoS One, 9:e88927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song JY, Noh JY, Lee JS, et al. (2018). Effectiveness of influenza and pneumococcal polysaccharide vaccines against influenza-related outcomes including pneumonia and acute exacerbation of cardiopulmonary diseases: Analysis by dominant viral subtype and vaccine matching. PLoS One, 13:e0207918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Zhang Y, Wang Q, et al. (2019). The effectiveness of influenza vaccination in preventing hospitalizations in elderly in Beijing, 2016–18. Vaccine, 37:1853–1858. [DOI] [PubMed] [Google Scholar]
- 25.Pebody R, Zhao H, Whitaker H, et al. (2020). Effectiveness of influenza vaccine in children in preventing influenza associated hospitalisation, 2018/19, England. Vaccine, 38:158–164. [DOI] [PubMed] [Google Scholar]
- 26.Jadad AR, Moore RA, Carroll D, et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials, 17:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG, Schulz KF, Moher D, et al. (2001). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med, 134:663–694. [DOI] [PubMed] [Google Scholar]
- 28.Loeb M. Influenza vaccine to prevent adverse vascular events - IVVE. Presented at the American College of Cardiology Annual Scientific Session (ACC 2022), Washington, DC, April 3, 2022. [Google Scholar]
- 29.Govaert TM, Thijs C, Masurel N, et al. (1994). The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA, 272:1661–1665. [PubMed] [Google Scholar]
- 30.Gurfinkel EP, Leon de la Fuente R, Mendiz O, et al. (2004). Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J, 25:25–31. [DOI] [PubMed] [Google Scholar]
- 31.Ciszewski A, Bilinska ZT, Brydak LB, et al. (2008). Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J, 29:1350–1358. [DOI] [PubMed] [Google Scholar]
- 32.De Villiers PJ, Steele AD, Hiemstra LA, et al. (2009). Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine, 28:228–234. [DOI] [PubMed] [Google Scholar]
- 33.Keshtkar-Jahromi M, Vakili H, Rahnavardi M. (2009). The efficacy of influenza vaccination in reducing cardiovascular events in patients with coronary artery diseases: IVCAD study. Clin Microbiol Infect, 15:S395–396. [Google Scholar]
- 34.Phrommintikul A, Kuanprasert S, Wongcharoen W, et al. (2011). Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J, 32:1730–1735. [DOI] [PubMed] [Google Scholar]
- 35.Fröbert O, Götberg M, Erlinge D, et al. (2021). Influenza Vaccination After Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Circulation, 144:1476–1484. [DOI] [PubMed] [Google Scholar]
- 36.Langley JM, Risi G, Caldwell M, et al. (2011). Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis, 203:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhees RAF, Thijs C, Ambergen T, et al. (2019). Influenza vaccination in the elderly: 25 years follow-up of a randomized controlled trial. No impact on long-term mortality. PLoS One, 14:e0216983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu I-F, Huang C-C, Chan W-L, et al. (2012). Effects of annual influenza vaccination on mortality and hospitalization in elderly patients with ischemic heart disease: a nationwide population-based study. Prev Med, 54:431–433. [DOI] [PubMed] [Google Scholar]
- 39.Wang I-K, Lin C-L, Chang Y-C, et al. (2013). Effectiveness of influenza vaccination in elderly diabetic patients: a retrospective cohort study. Vaccine, 31:718–724. [DOI] [PubMed] [Google Scholar]
- 40.Puig-Barberà J, Díez-Domingo J, Varea ÁB, et al. (2007). Effectiveness of MF59™-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine, 25:7313–7321. [DOI] [PubMed] [Google Scholar]
- 41.Örtqvist Å, Granath F, Askling J, et al. (2007). Influenza vaccination and mortality: prospective cohort study of the elderly in a large geographical area. Eur Respir J, 30:414–422. [DOI] [PubMed] [Google Scholar]
- 42.Nichol KL, Nordin J, Mullooly J, et al. (2003). Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med, 348:1322–1332. [DOI] [PubMed] [Google Scholar]
- 43.Hara M, Sakamoto T, Tanaka K. (2006). Effectiveness of influenza vaccination in preventing influenza-like illness among community-dwelling elderly: population-based cohort study in Japan. Vaccine, 24:5546–5551. [DOI] [PubMed] [Google Scholar]
- 44.Hung IF, Leung AY, Chu DW, et al. (2010). Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis, 51:1007–1016. [DOI] [PubMed] [Google Scholar]