Abstract
Background:
Exploring and analyzing the cost of medicines is an important tool for their management and planning. This study aims to analyze the utilization and costs of parenteral anti-diabetic medications during the past decade and predict the future trend of these medications from 2021 to 2031 in people that are covered by Iran Health Insurance Organization (IHIO).
Methods:
This study was based on secondary analysis of data routinely reported to IHIO from 2011 to 2019. For each drug, the Defined Daily Dose (DDDs) and DDDs per 1000 inhabitants per day were calculated for the last 9 years according to the WHO protocol. Then a regression analysis was used to predict the utilization trend of each drug for the following 10 years.
Results:
The overall utilization of injectable antidiabetic drugs has constantly increased during the last nine years. This increasing trend is estimated to continue during the next decade.
Conclusion:
In Iran, the increase in the diabetic population and better access in the future will be the main reasons for the increase in the utilization of various insulins. The increasing trend of utilizing injectable anti-diabetic drugs in Iran might be partly due to new patients and partly because of improvement in patient access to new treatments. This also suggests that, compared to the average in the commonwealth countries, Iranian diabetic patients has faced lack of drug utilization in the past decade that is gradually reducing.
Keywords: Trend analysis, Drug utilizations, Defined daily dose, Diabetes mellitus
Introduction
Diabetes is a relatively common disease (1); and a top priority for achieving Universal Health Coverage (UHC) especially in the low and middle income countries (1, 2). In Iran in 2016 the prevalence of diabetes was reported 8.87% for men, 11.03% for women, and 9.95% in total (3). This disease influences low and middle income countries in particular, as 77% of all diabetic patients worldwide live in those countries (4). Among the six WHO regions, the Eastern Mediterranean Region (EMRO) had the highest diabetes rate of 13.8% in 2016. The prevalence of diabetes is estimated to enhance by an average of 9.9% by 2045 (95% CI: 7.5 to 12.5%).
In the United States, it was estimated that, out of the 7$ spent on health, one dollar is spent on direct or indirect care associated with diabetes (5). In Iran currently over 90% of the population are insured by a basic insurance organization; about half of the population (about 40 million) are insured by the Iran Health Insurance Organization (IHIO) (6). Diabetes is one of the most burdensome diseases in Iran but the majority of anti-diabetic medications are available in Iran; although a significant proportion of them are imported. The IHIO covers a wide range of anti-diabetic drugs including the relatively more expensive injectable pen arbitrators. The cost of anti-diabetic drugs of the IHIO in 2017 were 2,253,660 million Rials, that was about 10% of the total drug costs (7). In the same year, in IHIO, the top four more expensive anti-diabetic drugs, including three injectable insulin drugs (glargine, aspart Mix, aspart fast), and an oral drug (metformin), accounted for about 83% of the total cost of anti-diabetic drugs (7, 8).
Diabetes is a very costly disease, especially insulins or parenteral anti-diabetic. Major plan and decisions for cost containment and rational drug use of the parenteral antidiabetic drugs depend on trend analysis of utilization and cost of them. Despite the importance of utilization and cost trend of the parenteral antidiabetic drugs in Iran, there remains a paucity of evidence on future parenteral utilization antidiabetic drugs in the nation scale and for the IHIO. Assessing the trend of anti-diabetic drugs utilization can assist policy makers in the process of resource allocation and planning for the future which is the subject of this study.
The main aim of this study was to investigate utilization analysis of parenteral anti-diabetic drugs in coverage of the IHIO in the past decade and to predict the future trend of the utilization between 2021 and 2031 on a national scale.
Methods
Ethical code was IR.TUMS.SPH.REC.1399.257. This study was based on secondary analysis of data that are routinely collected for all the medications prescribed and used by the people covered through the IHIO. The central database of the IHIO was used to collect data for injectable anti-diabetic drugs used by the insurance from 2011 to 2019 and were covered by the IHIO. The data was collected and analyzed according to the recommended WHO Drug Utilization Study method (9, 10). The cost of prescribed injectable drugs that were received by the patient and were covered by the IHIO were calculated. By designing the data collection form, the necessary data for processing and analysis were collected and in the next step these data were sanitized. Because the data were related to the individual use of the insured and included in the details, Defined Daily Dose (DDD) calculations were summarized first in terms of urban and provincial geography and later in national and national regions. For each studied drug the “DDD” and “DDDs per 1000 inhabitants per day”(DID) were calculated according to the WHO Anatomical Therapeutic Chemical(ATC)/DDD methodology or Drug Utilization Study method as follows. a) The standard ATC codes and the names of the anti-diabetes drugs were identified as declared by the WHO. b) The ATC codes were used to collect data from the IHIO database and import them into a pre-structured excel datasheet.
Drug names that were inconsistent or outdated were removed from the list using a systematic pre-defined process. c) The DDD level of each selected drug was calculated for the years 2008 to 2019. d) The DID of each drug was also measured at both provincial and national level. The following formula was used to calculate the DID (9, 10):
e) In the last step, the trend of DDD and DID was compared for each drug and each year from 2011 to 2019. Then a regression model was used for each drug or drug group to predict the future trends for the years 2021 to 2031. The goodness of fit, statistical method, and the following assumptions were used to run this model.
The best regression model was identified based on the maximum amount of R2.
In selecting the best model, equations that intersected the axis of lengths (time) over the next decade were avoided as they equated the value to zero and negative.
The annual population growth rate was considered in each of the funds and the total population covered by the IHIO. This was particularly essential for calculating DIDs.
Structurally important conditions, processes, and results including diagnosis, control, and treatment management as well as other factors (prevention, treatment, and rehabilitation) of diabetes have been supposed to be constant in both decades.
Sanction conditions and lack of national funding for valuable injectable anti-diabetic drugs (both vials and pens) were not considered as they were the subject of other studies.
f) After the best regression model was identified and selected, the prediction of each utilization indicator for each injectable antidiabetic drug was predicted for years 2012 to 2019. The trend analyses were carried out using Excel 2016, STATISTICA V10, and Joinpoint Trend Analysis Version 4.7.0.0 software.
Results
Results for years 2011 to 2019
By 2020, a total of 105 anti-diabetic drugs with ATC code were defined by the WHO (11). 76 Seventy six of these drugs had a DDD, of which 69 drugs were oral use, 35 were injection, and 1 was inhaled drug. From this list of 105 anti-diabetic drugs, a 22.9% of the injectable drugs and 8.7% of the oral drugs were covered by the IHIO.
From 2011, the list of anti-diabetic drugs that have been covered by the IHIO has gradually expanded. The total population covered by the IHIO has also increased significantly. Therefore the utilization of injectable analog anti-diabetic drugs has increased significantly in the past decade. This increasing trend is expected to continue over the next 10 years. The greatest increase was seen in pen insulin and to some extent in insulin vials. Long-acting and fast-acting insulins would be associated with the greatest increased utilization in the next decade. The highest utilization in the trend of the last and the next ten years belongs to aspart and the lowest utilization belongs to beef insulin vial.
Table 1 displays number of DDDs per 1000 inhabitants per day for diabetes injectable drugs. In this indicator, the population factor was taken into account and the amount of utilization in a unit of one thousand people was assessed. The amount of this indicator is similar to the DDD indicator, where vial insulins were decreasing and pen insulins were increasing in the last decade. There are however some exceptions to this marker similar to the previous marker that was described.
Table 1:
Total number of DDDs per 1000 inhabitants per day for diabetes injectable drugs (insulins)
| DIDs | ATC Code | TU | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Short-Acting Insulin (Vials) | 2.954 | 3.304 | 2.466 | 2.491 | 2.991 | 2.867 | 2.209 | 2.424 | 2.366 | ||
| Insulin (beef) | A10AB02 | 1000 | 0.125 | 0.132 | 0.005 | 0.008 | 0.012 | 0.007 | 0.001 | 0.000 | 0.000 |
| Insulin (human) | A10AB01 | 1000 | 0.736 | 0.864 | 0.731 | 0.802 | 1.009 | 0.876 | 0.794 | 0.957 | 0.930 |
| Combination Insulin | A10AB30 | 1000 | 2.093 | 2.308 | 1.730 | 1.681 | 1.969 | 1.984 | 1.414 | 1.466 | 1.437 |
| Rapid-Acting Insulin (Vials) | 0.0000 | 0.0000 | 0.0000 | 0.00010 | 0.00002 | 0.00003 | 0.00038 | 0.00015 | 0.00024 | ||
| Insulin glulisine (Vial) | A10AB06 | 300 | 0.0000 | 0.0000 | 0.0000 | 0.00010 | 0.00020 | 0.00030 | 0.00038 | 0.00015 | 0.00024 |
| Rapid-Acting Insulin (Pens) | 0.000 | 0.059 | 0.351 | 0.377 | 1.140 | 1.691 | 2.024 | 2.154 | 2.417 | ||
| Insulin aspart | A10AD05 | 300 | 0.000 | 0.059 | 0.351 | 0.377 | 1.132 | 1.669 | 1.957 | 2.085 | 2.352 |
| Insulin glulisine (Pen) | A10AB06 | 300 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.021 | 0.067 | 0.069 | 0.065 |
| Long-Acting Insulin (Vials/Pens) | 0.000 | 0.043 | 0.246 | 0.314 | 0.777 | 1.157 | 1.379 | 1.611 | 1.721 | ||
| Insulin detemir (Pen) | A10AE05 | 300 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.049 | 0.092 | 0.064 |
| Insulin glargine (Vials/Pens) | A10AE04 | 300 | 0.000 | 0.043 | 0.264 | 0.314 | 0.777 | 1.152 | 1.330 | 1.519 | 1.657 |
| Total Insulins | 2.954 | 3.407 | 3.063 | 3.181 | 4.908 | 5.715 | 5.612 | 6.189 | 6.505 |
Predicting the results for years 2021 to 2031
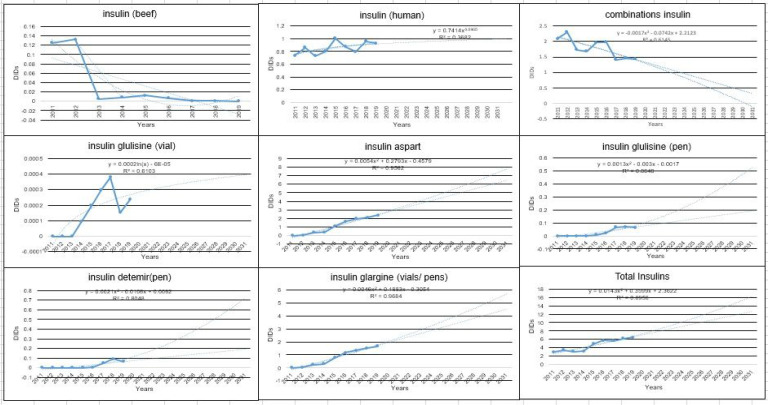
According to the Fig. 1, the utilization indicator for all selected injectable antidiabetic drugs were associated with an increasing trend in the next 10 years.
Fig. 1:
Total amount of the prediction of the DDDs in anti-diabetes parenteral drugs (insulins)
DDDs in1000 inhabitants per day (DID) in All Type of Insulins
Table 2 represents the regression equations of the best model for “DDD” in each of the injectable drugs and classes of anti-diabetes (insulin). Table 3 displays the regression equations of the best model for the “DIDs “in each of the injectable drugs and classes of anti-diabetes (insulin).
Table 2:
Regression equations of the best model in the utilization indicator of “DDDs” in each of the parenteral anti-diabetes drugs and classifications (insulins)
| DDDs for Total Insulins - Regression Analysis | |||
|---|---|---|---|
| Drug Name | Regression Type | Regression Equation | R2 |
| Insulin (beef) | Polynomial | y = 52091x2 - 709873x + 2E+06 | R2 = 0.7928 |
| Insulin aspart | Polynomial | y = 194885x2 + 3E+06x − 6E+06 | R2 = 0.9688 |
| Insulin glargine | Polynomial | y = 149075x2 + 2E+06x − 4E+06 | R2 = 0.9786 |
| Insulin (human) | Polynomial | y = −81.161x2 + 1403.6x − 2241.7 | R2 = 0.7005 |
| Combinations Insulin | Linear | y = −551827x + 3E+07 | R2 = 0.2367 |
| Insulin detemir | Polynomial | y = 33237x2 − 170685x + 155513 | R2 = 0.817 |
| Insulin glulisine (Pen) | Polynomial | y = 22100x2 − 63183x + 850.45 | R2 = 0.8801 |
| Insulin glulisine (Vial) | Linear | y = 634458x + 9E+06 | R2 = 0.6795 |
| Total Insulins | Power | y = 3E+07x0.5057 | R2 = 0.8087 |
| DDDs for Total Insulin Classifications - Regression Analysis | |||
|---|---|---|---|
| Drug Name | Regression Type | Regression Equation | R2 |
| Short-Acting Insulin (Vials) | Polynomial | y = −23613x2 + 129796x + 4E+07 | R2 = 0.0071 |
| Rapid-Acting Insulin (Vials) | Polynomial | y = −81.161x2 + 1403.6x - 2241.7 | R2 = 0.7005 |
| Rapid-Acting Insulin (Pens) | Polynomial | y = 216985x2 + 3E+06x − 6E+06 | R2 = 0.969 |
| Long-Acting Insulin (Vials/Pens) | Polynomial | y = 182313x2 + 2E+06x − 4E+06 | R2 = 0.9796 |
| Total Insulins | Polynomial | y = 375603x2 + 5E+06x + 3E+07 | R2 = 0.9446 |
Table 3:
Regression equations of the best model for “DDDs per 1000 inhabitants per day “in each of the parenteral anti-diabetes drugs and classifications (insulins)
| DIDs for Total Insulins - Regression Analysis | |||
|---|---|---|---|
| Drug Name | Regression Type | Regression Equation | R2 |
| Insulin (beef) | Polynomial | y = 0.0042x2 − 0.0573x + 0.1851 | R2 = 0.7949 |
| Insulin aspart | Polynomial | y = −0.0056x2 + 0.389x − 0.6532 | R2 = 0.9936 |
| Insulin glargine | Polynomial | y = −0.0039x2 + 0.2731x − 0.457 | R2 = 0.9952 |
| Insulin (human) | Polynomial | y = 2E-05x2 + 0.0335x + 0.622 | R2 = 0.6686 |
| Combinations Insulin | Exponential | y = 2.282e−0.052x | R2 = 0.6291 |
| Insulin detemir | Polynomial | y = −0.0001x2 + 0.0101x − 0.0093 | R2 = 0.7548 |
| Insulin glulisine (Pen) | Polynomial | y = −0.0001x2 + 0.0121x − 0.0303 | R2 = 0.9754 |
| Insulin glulisine (Vial) | Polynomial | y = 1E-05x2 − 0.0004x + 0.004 | R2 = 0.0866 |
| Total Insulins | Polynomial | y = −0.0073x2 + 0.5802x + 2.0044 | R2 = 0.988 |
| DIDs for Total Insulin Classifications - Regression Analysis | |||
|---|---|---|---|
| Drug Name | Regression Type | Regression Equation | R2 |
| Short-Acting Insulin (Vials) | Polynomial | y = 0.0009x2 − 0.0942x + 3.1181 | R2 = 0.4127 |
| Rapid-Acting Insulin (Vials) | Polynomial | y = −6E-06x2 + 0.0001x − 0.0002 | R2 = 0.6849 |
| Rapid-Acting Insulin (Pens) | Polynomial | y = −0.0057x2 + 0.401x − 0.6835 | R2 = 0.9936 |
| Long-Acting Insulin (Vials/Pens) | Polynomial | y = 0.0067x2 + 0.1777x − 0.2962 | R2 = 0.9701 |
| Total Insulins | Polynomial | y = −0.0073x2 + 0.5802x + 2.0044 | R2 = 0.988 |
Based on Fig. 2, insulin aspart had the highest utilization or DDD in the last decade and is expected to have the highest utilization in the next decade as well. The lowest levels of the DDD were for beef insulin in the last decade and possibly in the next decade. Vial insulins, except human analog insulin, which is classified as short-acting, had a decreasing trend in the past decade. Vial mixed insulin had also a declining trend, and this trend is expected for the next decade.
Fig. 2:
Total amount of DDDs for injectable diabetes drugs (insulins) and prediction of this indicator for the next decade
Fig. 2 also shows that the total amount of DDDs for each of the injectable diabetes drugs (insulins) and their prediction for the next decade are increasing for all drugs; apart from the beef and mixed insulins that are expected to have a decreasing trend. In addition, the largest rise in the next decade for the “DDD” belongs to injectable drugs (aspart and glargine, both of which are of the pen-type).
Fig. 3 shows that the total amount of the DIDs for each of the injectable diabetes drugs (insulins) and their prediction for the next decade are increasing for all drugs, except for the beef and mixed insulins that are expected to have a decreasing trend. In addition, the main increase in the next decade for the “DIDs” belongs to injectable drugs (aspart and glargine, both of them are the pen-type).
Fig. 3:
Total amount of DIDs in injectable diabetes drugs (insulins) and prediction of this indicator in the next decade
Fig. 4 shows that the total amount of DIDs in each of the classes of short-acting (vial), fast-acting (vial), fast-acting (pen), and long-acting (vial and pen) in injectable diabetes drugs (insulins) and prediction of this indicator in the next decade are increasing for all drugs, except for vial short-acting insulins that are expected to have a decreasing trend. The main increase in the next decade for the “DIDs” belongs to the fast-acting pen drugs and long-acting pen and vial drugs.
Fig. 4:
Total amount of DIDs of injectable diabetes drugs (insulins)
This study shows that the utilization trend of the vial insulin is decreasing, and the utilization trend of the pen insulin is increasing with a significant slope for the next 10 years.
Discussion
This study explored the utilization trend of injectable anti-diabetic drugs covered by the IHIO in the past decade and predicted the trend for the next ten years using standard regression models.
This study showed that the use of all injectable antidiabetic drugs is increasing, except for vial insulins, which include beef, mixed, and glulisine insulins which had a decreasing trend. The highest increase were for pen insulins including aspart, glulisine, detemir, and glargine. On the other hand, vial insulins including beef and mixed insulins had a decreasing trend in the past and are expected to have the same decreasing trend in the future. However, pen insulins, especially aspart, glulisine, detemir, and glargine are expected to have an increasing trend in the coming years. These findings are in line with similar studies in other countries that reported an increasing trend for pen insulins both when it was used as monotherapy (12) and when it was combined with an oral drug (13–15). In the Commonwealth countries, and regional countries such as the Arab countries of the Persian Gulf, the utilization of injectable drugs, including pen insulins in the form of monotherapy or combination with oral drugs, have been very high with an increasing trend (7, 12, 13, 16–21). Previous studies reported similar results for Iran (6, 7, 20, 22, 23); and worldwide (13, 24). These investigations have highlighted the affordability of oral antidiabetic drugs against pen insulins and concluded that their utilization will be higher (2, 4, 20).
In Iran, because of the economic conditions and income of people covered by different Health Insurance Organizations, the availability and ability to pay for oral anti-diabetic drugs covered by the organizations is perhaps higher than injectable drugs (6, 20) and this might enhance the utilization of oral anti-diabetic drugs, or a combination pattern of two oral drugs, or a combination pattern of an oral and injectable drug, or a monotherapy pattern with an oral drug, as has been reported by studies from Southeast Asian countries(21, 25). Thus, paying attention to the selection of efficient oral drugs to be covered by IHIO is very important.
Comparing the findings of this research with other similar international studies, the intensity and annual growth rate of injectable drug use in Iran are less than the international experience (11). Possible reasons for this might be the difficult access (presence of sanctions and the foreignness of pen insulins) and the fact that these drugs are more expensive in Iran, compared to North and Central American countries.
Based on the results of this research, aspart pen insulin (fast-acting) and then glargine pen insulin (long-acting) had the highest growth rate in the past decade and they will perhaps have the same trend in the next decade, but aspart pen insulin alone will have the highest increase and use. Maybe one of the reasons for the increased use of this drug is the increase in gestational diabetes because of the increase in age and weight of pregnant mothers over the next decade. Thus, it is very important to pay attention to the lifestyle and quality of life of mothers to avoid and decrease the use of this drug along with paying attention to the production, quality, and cost of this insulin.
On the other hand, the rate of use of pen insulin drugs with long-term effects in the last and next decades, as in commonwealth countries, has an increasing trend (11). Possible reasons for this increase are easier use and fewer injections, superior technology, advertisement of pharmaceutical companies for diabetics, especially doctors, facilitating the prescription of such drugs by holding training courses on how to prescribe these medications by drug companies, as well as successful experience of prescribing and familiarity of physicians (with these drugs).
In Iran and the pharmaceutical market, anti-diabetic drugs that exist but are not covered by health insurance include three oral drugs and eight injectable or mixed drugs. The absence of all injectable antidiabetic drugs in the insurance commissioner has probably put the most pressure on the items that have been covered by the health insurance coverage. The compliance rate of the list covered by WHO (11) and IHIO in both oral and injectable parts is less than a quarter. Thus, studies are required to assess the effectiveness of the anti-diabetes drug list covered by this insurance organization.
The major limitation of this study mainly was in the data collection stage and included the existence of unrelated and discontinuous data and inconsistencies of old and new insulin codes, in other words, dirty and unbalanced data, lack of access to other insurers data on a national scale, and in the statistical analysis, the limitation of this study was the existence of static preset assumptions and lack of attention to dynamic variables for modeling each insulin.
Conclusion
In this study, the utilization model of each injectable insulin was determined and the rate of increase in the utilization of each in the next ten years was determined. Accordingly, managing the process of detection, diagnosis and treatment of diabetic patients can be the first and most basic step in containment and rationalizing the utilization of injectable insulin. Next steps will be adopting a policy of rationalizing utilization of anti-diabetic drugs by reducing the prevalence of diabetes and altering the lifestyle and quality of life of the insurers by using health incentives, and second stage will be to develop clinical guidelines for high-utilization or expensive anti-diabetic drugs could be provided by IHIO, and strategic purchases should be made based on guidelines and clinical protocol. In the third stage, considering the increase of the new generation of anti-diabetic drugs that are inexpensive or more effective and can be produced in this country, these drugs should be selected through selection in health technology evaluation studies and added to the benefit package of the IHIO or removed from the package in necessary cases. In the last stage, because of the increasing and upward trend of drug use, more attention should be paid by IHIO to the accessibility, justice, availability, and affordability of anti-diabetic drugs in Iran, especially for fast and long-acting pen insulins, that had and will have an increasing trend in the last and next ten years, and they are mainly produced outside of Iran and are expensive.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was a part of PhD thesis supported by School of Public Health, Tehran University of Medical Sciences. Ethical code was IR.TUMS.SPH.REC.1399.257. The authors strongly appreciate all contributors to this study especially from Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, National Center for Health Insurance Research, and the executives of IHIO.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.WHO (2020). Diagnosis and Management of Type 2 Diabetes. https://www.who.int/publications-detail-redirect/who-ucn-ncd-20.1
- 2.Mapa-Tassou C, Katte JC, Mba Maadjhou C, Mbanya JC. (2019). Economic Impact of Diabetes in Africa. Curr Diab Rep, 19:5. [DOI] [PubMed] [Google Scholar]
- 3.Education MoHaM (2016) The Project of the Burden of Diseases Center for the Investigation of Non-Communicable Diseases, STEPS. [Google Scholar]
- 4.Organisation IDF (2019). International Diabetes Federation Organisation Partnership Annual Report 2019. https://www.idf.org/component/attachments/attachments.html?id=2569&task=download
- 5.(2020). National Diabetes Statistics Report 2020 Estimates of Diabetes and Its Burden in the United States.
- 6.-IHIO IHIO (2017). Proceedings of the National Conference on Health Insurance; Public coverage and financial resource management. ed.
- 7.Shajari Pour Mousavi Masoud, Zahra S. (2019). FACT SHEET: The Cost of Expensive Diabetes Drugs in the Health Insurance Organization, 2017. In: -NCHIR NCfHIR, Iran Health Insurance Organisation -IHIO, Iran Health Insurance Organisation -IHIO [Google Scholar]
- 8.Ebrahimi H, Pishgar F, Yoosefi M, et al. (2019). Insulin pen use and diabetes treatment goals: A study from Iran STEPS 2016 survey. PLoS One, 14:e0221462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elseviers M, Almarsdi AB, Andersen M, Benko R, Bennie M, Eriksson I, Godman B, Krska J, Poluzzi E, Taxis K. (2016). Drug utilization research: methods and applications. ed. John Wiley & Sons. [Google Scholar]
- 10.Organization WH (2003). Introduction to drug utilization research. ed. World Health Organization. [Google Scholar]
- 11.ORGANISATION WH (2020) ATC/DDD Index 2020. https://www.whocc.no/atc_ddd_index/
- 12.Cheen HH, Lim SH, Huang MC, Bee YM, Wee HL. (2014). Adherence to premixed insulin in a prefilled pen compared with a vial/syringe in people with diabetes in Singapore. Clin Ther, 36:1043–53. [DOI] [PubMed] [Google Scholar]
- 13.Parekh W, Hoskins N, Baker-Knight J, et al. (2017). The Economic Burden of Insulin-Related Hypoglycemia in Spain. Diabetes Ther, 8:899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soppi A, Heino P, Kurko T, et al. (2018). Growth of diabetes drug expenditure decomposed-A nationwide analysis. Health Policy, 122:1326–1332. [DOI] [PubMed] [Google Scholar]
- 15.Organization WH (2018). Guidelines on second-and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. [PubMed]
- 16.Danaei G, Farzadfar F, Kelishadi R, et al. (2019). Iran in transition. Lancet, 393:1984–2005. [DOI] [PubMed] [Google Scholar]
- 17.Salman RA, AlSayyad AS, Ludwig C. (2019). Type 2 diabetes and healthcare resource utilisation in the Kingdom of Bahrain. BMC Health Serv Res, 19:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang C, Mortensen MB, Lauridsen KG, Bruun JM. (2020). Trends in antidiabetic drug utilization and expenditure in Denmark: A 22-year nationwide study. Diabetes Obes Metab, 22:167–172. [DOI] [PubMed] [Google Scholar]
- 19.Sarayani A, Rashidian A, Gholami K. (2014). Low utilisation of diabetes medicines in Iran, despite their affordability (2000–2012): a time-series and benchmarking study. BMJ Open, 4:e005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu C-H, Hsu C-C, Lin S-Y, et al. (2019). Trends in antidiabetic medical treatment from 2005 to 2014 in Taiwan. J Formos Med Assoc, 118 Suppl 2:S74–S82. [DOI] [PubMed] [Google Scholar]
- 21.Ou HT, Chang KC, Liu YM, Wu JS. (2017). Recent trends in the use of antidiabetic medications from 2008 to 2013: A nationwide population-based study from Taiwan. J Diabetes, 9:256–266. [DOI] [PubMed] [Google Scholar]
- 22.Javanbakht M, Baradaran HR, Mashayekhi A, et al. (2011). Cost-of-illness analysis of type 2 diabetes mellitus in Iran. PLoS One, 6:e26864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. (2014). Use of antidiabetic drugs in the U.S., 2003–2012. Diabetes Care, 37:1367–74. [DOI] [PubMed] [Google Scholar]
- 24.organisation wh (2016). GLOBAL REPORT ON DIABETES. https://www.who.int/publications-detail-redirect/9789241565257
- 25.OECD.Stat (2020) Pharmaceutical Market: Pharmaceutical consumption.