Abstract
Background:
Autism Spectrum Disorders (ASDs) are common behavioral syndromes but limited critical evidence in Vietnam. This study aimed to identify ante-, peri- and neonatal factors for ASDs amongst children in Vietnam.
Methods:
This population-based study applied the cross-sectional design with a multistage sampling in 21 urban and rural districts in seven cities/provinces in Vietnam during 2017–2018. Overall, 42,551 children age 18 to 30 months were enrolled in the study. Two phases of assessment using Modified Checklist for Autism in Toddlers (M-CHAT) for screening and diagnostic assessment using Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for confirmation. We employed univariate and binary logistic regression to identify.
Results:
Our study showed a fast-growing trend of ASDs amongst children age 18 and 30 months (75.8 per 10,000 individuals). Nine ante-, peri-, and neonatal factors were associated with ASDs: five factors of antenatal period (history of miscarriage/abortion or stillbirth, children conceived by assisted reproduction technologies, having cold, flu or acquiring virus during pregnancy, having gestational diabetes, toxemia, high blood pressure or pre-eclampsia during pregnancy, and having stress or mental disorders during pregnancy); one factors of perinatal period (mode of delivery); and three factors of neonatal period (jaundice, respiratory distress, and newborn seizures)
Conclusion:
This first large-scale survey in Vietnam confirms some prenatal, perinatal, and postnatal factors with ASDs amongst children age 18 and 30 months. Future interventions should focus on these factors to early diagnosis and intervention to improve functional outcomes for risky children.
Keywords: Autism spectrum disorder (ASD), Population-based survey, Vietnam
Introduction
Autism spectrum disorders (ASD) refers to a range of chronic neurodevelopmental disorders characterized by impaired social behavior, communication and language and stereotyped, repetitive patterns of behavior characterized (1). Symptoms often appear early by the age of 16 months and manifest in severe lifelong impairments (2). Affected children require long term and constant care from their family members and professionals. Raising a child with an ASD could affect the entire family and considerably modifies the lifestyle of each member of the family.
ASDs is currently recognized as one of the most common public health problem amongst children with various degrees of severity (1). The global prevalence is children worldwide is 1 in 160 children has an ASD (3). Since then, a substantial increase of ASD prevalence estimates over many studies worldwide (4). In Vietnam, number of cases in clinical settings is double during the period 2000–2013 (5). Some previous studies estimated the ASDs among children under five years old ranged from 0.46%–0.52% (5, 6). ASD has become one of the biggest challenges amongst children in many countries including Vietnam, as it has severe consequences on both health and wealthy of the children and their families (3). Besides, shortage of resources for healthcare and evidences about the epidemiology, etiology, and natural course of ASD put the situation worse in the country.
The associated factors for ASD amongst children are incomplete. ASD have no single factor reported to be a positive predictor. Genetic, maternal and environment are proposed to account for its development (7). A study reports that up to two-thirds of ASD are likely to be resulted from antenatal (ante-), perinatal (peri-), and neonatal environmental factors (8, 9). For example, advanced parental age, maternal diabetes and hypertension, threatened abortion are associated with increased risk of ASD (9–12).
Worldwide studies investigated the relationship between antenatal, perinatal, and neonatal, and ASDs (13). In Vietnam, a recent publication has shown different socio-demographic factors of infants and their parents could increase the risk of ASD amongst children age 18 to 30 months (14). There are, however, no studies, to our best knowledge, exploring the relationship between antenatal, perinatal, and neonatal associate factors and ASD in a large-scale study in Vietnam. No risk factors were consistently reported in Vietnam. A population-based survey with data on pregnancy and delivery outcomes could help to further explore this association.
As a result, this national representative study was conducted to identify the relationship between ante-, peri- and neonatal factors and ASD among children age 18 and 30 months.
Materials and Methods
Design, study setting, and sampling
The population-based study applied a cross-sectional design with a multistage sampling in 21 urban and rural districts in seven cities/provinces in Vietnam during 2017–2018. First, we chose randomly 6 provinces (Thai Binh, Hoa Binh, Quang Nam, Dong Nai, Dong Thap, and Dak Lak) representing the six socio-economic regions in Vietnam. Hanoi, the capital of Vietnam, was purposively chosen as it is one of the most populous urban provinces in Vietnam. Later, one urban district and two rural districts (considering the proportion of urban (1/3) and rural (2/3) populations in Vietnam) were randomly chosen from each study province. Finally, about 2,000 children age 18 to 30 months in a district were chosen randomly from sampling frame provided by the District Health Center.
Sample size
We calculated the sample size using the following formula (15):
We used 95% confidence interval (CI) (z(1- α/2) = 1.96) with a relative precision of 3.5% (ɛ). From a previous population-based study in 2012 in Hanoi, the prevalence of ASD amongst children was 0.46% (p) (16). After adding a 15% of refusal rate, 5,918 children age 18 and 30 months were randomly chosen from each province. Counting for all 7 provinces, this study was conducted in 42,551 children age 18 to 30 months.
Measurements of ASD
Phase I: Screening
For screening the ASD amongst children age 18 to 30 months, our study applied the Modified Checklist for Autism in Toddlers (M-CHAT), a widely-applied screening tools in both primary and tertiary hospitals with a high positive predictive value (17, 18). Literature has also reported a high reliability and validity of M-CHAT in Vietnam (6, 16).
Phase II: Comprehensive diagnostic assessment
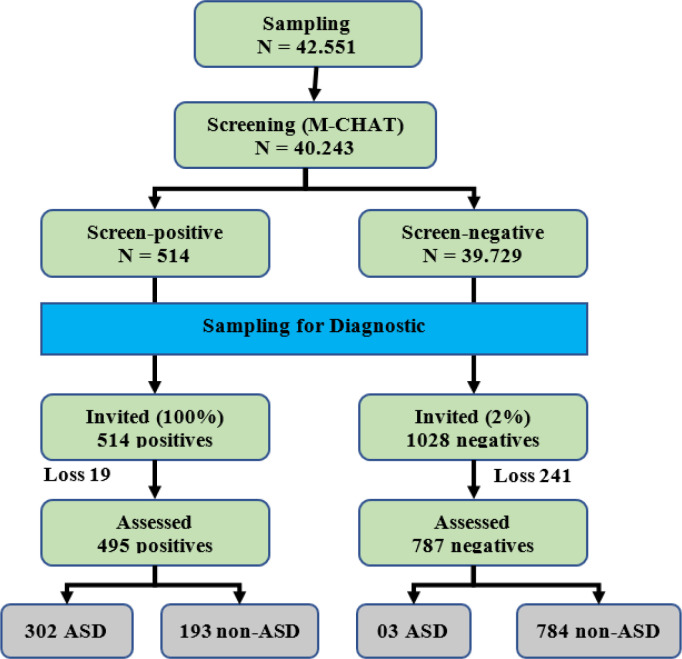
In phase II, we performed comprehensive diagnostic assessment (Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)) to confirm ASD among the children with positive-screen results of M-CHAT. A multidisciplinary team including eight leading professionals in ASD diagnosis and intervention performed this task (19). DSM-IV has also been applied widely in Vietnam and other countries to clinically diagnosis ASD (20). The average length of time for a child was 35 min. A further result of the screening and diagnostic process was presented in Fig. 1.
Fig. 1:
Screening and Diagnostic Process
Study variables
The dependent variable was ASD, as initially identified by screening and subsequently diagnosed by pediatric neurologists, among the study participants. Independent variables included:
Antenatal period: History of miscarriage, abortion or stillbirth (Yes/No); Children conceived by assisted reproduction technologies (Yes/No); Having cold, flu or acquiring virus during pregnancy (Yes/No); Having gestational diabetes, toxemia, high blood pressure or preeclampsia during pregnancy (Yes/No); Having stress or mental disorders during pregnancy (Yes/No); Contacting with chemical toxic or second-hand smoke (Yes/No).
Perinatal period: Mode of delivery (Assisted delivery/Normal delivery); Time of active labor (Prolonged (>24 h)/Normal (≤24 h)), Pre-term childbirth (Yes/No); Low-birth weight (Yes/No); and Perinatal asphyxia (Yes/No).
Neonatal period: Neonatal jaundice (Yes/No); Neonatal respiratory distress syndrome (Yes/No); and Neonatal seizures (Yes/No).
Data collection
In each city/province, 46 local health workers were recruited and trained. 12 supervisors were assigned to supervise and monitor the survey data collectors throughout the process of data collection and to confirm positive screened cases at the household level. A team of pediatric neurologists, public health specialists and child development therapists provided one day of training on data collection to all the field data collection staff. The data collectors conducted face-to-face interviews with the mother/father or caregivers of the child using a structured questionnaire with three vital components including the children and their parents’ socio-demographic information, and 23 items of M-CHAT. As noted above, M-CHAT-23 was used as the primary screening tool for ASD and M-CHAT-positive cases were diagnosed by pediatric neurologists from National Pediatrics Hospital using DSM-IV criteria.
Statistical method
The data analyses were carried out using Stata version 14 (Stata Corporation, College Station, TX). Descriptive and analytical statistics were performed. Prevalence estimates were derived and stratified by ante-, peri- and neonatal variables. The independent and outcome variable were regarded as dichotomous variables. The association tests were carried out using: (1) Univariate logistic regression to explore the empirical relationship between independent variables and the outcome variable (ASDs among children age 18 and 30 months); and (2) Binary logistic regression models to control for confounding and interaction effect. We followed the Hosmer and Lemeshow approach to purposeful select and decide the variables for the binary modeling process (21). The magnitude of the association between the outcome and the independent variables were estimated by the Odds Ratio (OR), with respective 95% confidence intervals (95% CI). P-value<0.05 was considered significant.
Ethics approval
Ethical permission for this study was obtained in Decision 319/2016/YTCC-HD3 of the Hanoi University of Public Health Ethics Committee dated 16th November 2016. As participants of the study were children aged between 18–30 months, written informed consent was obtained from each of the parents or caregivers to provide information about their children.
Results
In each province, about 5,500 to 5,900 children resulting in a total of 40,243 children were enrolled. For screening phase using M-CHAT, 495 children had positive results. Out of these children, 302 children (0.76%) were confirmed as ASD-positive using DSM-IV criteria.
An analysis of 14 previously identified associate factors for ASD was performed using univariate and multivariate logistic regression to identify the associations with a diagnosis of ASD (followed the guideline of Sperandei S (22)). In Table 1, 14 factors had significant associations with children with confirmed ASD in our univariate analysis. These factors with statistical significance with ASD status was then further analyzed using binary logistic regression in Table 2. The multivariate analysis showed that nine factors were associated with ASD amongst children age 18 to 30 months.
Table 1:
Ante-, peri-, and neonatal characteristics & their association with Autism Spectrum Disorders (ASD) in Children age 18 to 30 months
| Variables | N (%) | ASD prevalence** | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Frequency (N) | Percentage (%) | Frequency (N) | Percentage (%) | OR | 95%CI | ||
| Lower | Upper | ||||||
| Total number of participants | 40,243 | 100% | 302 | 0.76 | |||
| Antenatal period* | |||||||
| History of miscarriage, abortion or stillbirth | 2,216 | 5.5 | 118 | 3.20 | 6.48c | 5.13 | 8.18 |
| Children conceived by assisted reproduction technologies | 208 | 0.5 | 12 | 5.77 | 8.28c | 4.57 | 14.99 |
| Having cold, flu or acquiring virus during pregnancy | 1,851 | 4.6 | 45 | 2.50 | 3.68c | 2.67 | 5.07 |
| Having gestational diabetes, toxemia, high blood pressure or preeclampsia during pregnancy | 517 | 1.3 | 39 | 6.9 | 13.9c | 9.38 | 20.85 |
| Having stress or mental disorders during pregnancy | 307 | 0.8 | 31 | 10.10 | 16.22c | 10.98 | 23.95 |
| Contacting with chemical toxic or second-hand smoke | 2,976 | 6.1 | 47 | 8.3 | 4.87c | 2.48 | 9.56 |
| Perinatal period | |||||||
| Mode of delivery | |||||||
| Assisted delivery (C-section, forcept) | 12,760 | 31.7 | 143 | 1.12 | 1.91c | 1.52 | 2.40 |
| Normal delivery | 27,483 | 68.3 | 162 | 0.59 | 1 | - | - |
| Time of active labour | |||||||
| Prolonged (>24 h) | 21,345 | 53.0 | 19 | 2.44 | 3.04c | 1.90 | 4.86 |
| Normal (≤24 h) | 18,898 | 47.0 | 286 | 0.82 | 1 | - | - |
| Pre-term childbirth* | 2,086 | 5.2 | 50 | 2.40 | 3.63c | 2.67 | 4.93 |
| Birth weight | |||||||
| Low-birth weight (<2500 gram) | 1,307 | 3.3 | 24 | 1.84 | 2.56c | 1.68 | 3.90 |
| Normal (≥2500 gram) | 38,773 | 96.3 | 281 | 0.72 | 1 | - | - |
| Perinatal asphyxia* | 348 | 0.9 | 14 | 4.02 | 5.65c | 3.27 | 9.76 |
| Neonatal period | |||||||
| Neonatal jaundice* | 619 | 1.5 | 29 | 4.68 | 7.01c | 4.74 | 10.36 |
| Neonatal respiratory distress syndrome* | 167 | 0.4 | 27 | 16.17 | 27.60c | 17.98 | 42.36 |
| Neonatal seizures* | 550 | 1.4 | 51 | 9.60 | 16.43c | 12.00 | 22.50 |
Data show for Yes, in which
represents statistically significant at P-value <0.05;
represents statistically significant at P-value <0.01;
represents statistically significant at P-value < 0.001
Table 2:
Multivariate logistic regression analyses of ante-, peri-, and Neonatal variables for association with Autism Spectrum Disorders (ASD) in Children age 18 to 30 months
| Variables | β (SE) | OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|---|
| Antenatal period | |||
| History of miscarriage, abortion or stillbirth (Yes/No) | 1.5 (0.13) | 6.5 (5.1–8.2)c | 4.5 (3.4–5.8)c |
| Children conceived by assisted reproduction technologies (Yes/No) | 1.18 (0.39) | 8.3 (4.6–15.0)c | 3.3 (1.5–7.0)b |
| Having cold, flu or acquiring virus during pregnancy (Yes/No) | 0.59 (0.2) | 3.7 (2.7–5.1)c | 1.8 (1.2–2.7)b |
| Having gestational diabetes, toxemia, high blood pressure or preeclampsia during pregnancy (Yes/No) | 2.04 (0.4) | 10.4 (5.4–20.0)c | 7.7 (3.5–16.9)c |
| Having stress or mental disorders during pregnancy | 1.42 (0.29) | 16.2 (11.0–24.0)c | 4.1 (2.4–7.3)c |
| Contacting with chemical toxic or second-hand smoke | 0.2 (0.2) | 2.5 (1.8–3.4)b | 1.2 (0.8–1.8) |
| Perinatal period | |||
| Mode of delivery (Assisted delivery/Normal) | 0.5 (0.13) | 1.9 (1.5–2.4)c | 1.6 (1.3–2.1)c |
| Time of active labour (Abnormal/Normal) | 0.53 (0.28) | 3.0 (1.9–4.9)c | 1.7 (0.9–2.9) |
| Full-term childbirth (Preterm/Full-term) | 0.26 (0.17) | 3.6 (2.7–4.9)c | 1.3 (0.9–1.8) |
| Birthweight (Low-birth weight/Normal) | −0.1 (0.29) | 2.6 (1.7–3.9)c | 0.9 (0.5–1.6) |
| Perinatal asphyxia (Yes/No) | 0.71 (0.39) | 5.6 (3.3–9.8)c | 2 (0.9 – 4.3) |
| Neonatal period | |||
| Neonatal jaundice (Yes/No) | 0.56 (0.27) | 7.0 (4.7 – 10.4)c | 1.7 (1.1–2.9)a |
| Neonatal respiratory distress syndrome (Yes/No) | 1.82 (0.33) | 27.6 (18–42.4)c | 6.2 (3.3–11.7)c |
| Neonatal seizures (Yes/No) | 2.11 (0.21) | 16.4 (12.0–22.5)c | 8.2 (5.5–12.3)c |
result from multivariate logistic regression, in which
represents statistically significant at P<0.05;
represents statistically significant at P<0.01;
represents statistically significant at P<0.001
During antenatal period, the following five factors were consistently associated with ASD: 1) history of miscarriage, abortion or stillbirth (OR: 4.5, 95%CI: 3.4–5.8), 2) children conceived by assisted reproduction technologies (OR: 3.3, 95%CI: 1.5–7.0), 3) having cold, flu or acquiring virus during pregnancy (OR: 1.8, 95%CI: 1.2–2.7), 4) having gestational diabetes, toxemia, high blood pressure or preeclampsia during pregnancy (OR: 7.7, 95%CI: 3.5–16.9), and 5) having stress or mental disorders during pregnancy (OR: 4.1, 95%CI: 2.4–7.3).
In term of perinatal period, in adjusted analysis, only one variable was associated with ASD (mode of delivery, OR: 1.6, 95%CI: 1.3–2.1). For neonatal period, three neonatal factors associated with ASD were neonatal jaundice (OR: 1.7, 95%CI: 1.1–2.9), neonatal respiratory distress syndrome (OR: 6.2, 95%CI: 3.3–11.7), and newborn seizures (OR: 8.2, 95%CI: 5.5–12.3).
Discussion
To our knowledge, this is the first survey with a nationally representative sample showing the relationship between antenatal, perinatal, and neonatal associate factors and ASD amongst children age 18 to 30 months in Vietnam. ASD-positive survey was confirmed by a two-phase procedure: identify high-risk children in the general population using M-CHAT and confirm ASD status using comprehensive diagnostic assessment (DSM-IV). Our study revealed that ASD prevalence in children age 18 and 30 months is 75.8 per 10,000 individuals. This suggested a fast-growing trend of ASD in Vietnam and it should be recognized as a national public health issue. We also found the nine prenatal, neonatal, and postnatal associate factors which were more likely to occur in children diagnosed with ASD than in healthy children. All of our found associate factors are consistent with the potential list of ante-, peri-, and postnatal factors in a recent meta-analysis in 2017 (8).
In our study, children born from mother with a history of miscarriage, abortion or stillbirth raised risk by 4.5 times for ASD. The finding is in line with previous studies highlighting that a history of spontaneous or provoked abortions is associated with heightened risk of ASD and mental retardation, learning disorder (23, 24). The underlying mechanisms of higher risk of abortion or stillbirth to ASD remain to be elucidated. An explanation could be that a history of miscarriage or abortions is associated with a lifestyle of the mother that places all her babies at greater risk (23).
Our study indicated that the use of assisted reproductive technology (ART) associated with higher risk of ASD amongst children. This association has been explored and highlighted in several studies (25, 26). In a recent review, use of ART is associated with higher percentage of ASD with relative risk (RR) as 1.35 (95% CI: 1.09–1.68) (25). ART may be an independent risk factor for ASD. Further, ART had a higher risk of ASD in European and Asian populations than that of remaining populations in the world. A possible mechanism linking ART and ASD is genetic changes due to procedure of ART such as repeated hormone exposure, semen preparation, delayed insemination, etc.
Our study found five prenatal conditions associated with high risk of ASD including having cold or acquiring virus, gestational hypertension, gestational diabetes, and having mental disorders during pregnancy. Hypertensive disorders of pregnancy (HDP), a disorder including gestational or chronic hypertension, ante-eclampsia and eclampsia (27), is associated with many mental disorders, including neurologic, psychiatric disorders, and ASD (28). Gestational diabetes mellitus is associated with an increased risk of ASD in offspring with RR as 1.48 (95% CI: 1.26–1.75) (29). To explain the underlying mechanism, several biological mechanisms are proposed in order to understand how gestational diabetes may cause brain malformation and aberrant neurodevelopment (30). Consistent with our results, women with mental disorders such as depression during pregnancy were more likely to have a child with ASD compared to healthy women (31). There are also differences in risk by the severity of mental disorders. The risk of ASD in children increases with increasing severity (31).
Our study found only one perinatal risk factor for ASD in children, that is, mode of delivery. This confirms the previous findings that children born by C-section are approximately 20% of increased risk of ASD compared with vaginal delivery (32, 33). However, the mechanisms behind these associations appear to be vague. Babies born by C-section have different exposures that can subtly alter neonatal physiology (34). This association may be due to familial confounding by genetic and/or environmental factors (33).
In term of neonatal associate factors, we found three factors, including neonatal jaundice, respiratory distress, and newborn seizures associated with the higher risk of ASD in children. There is ample evidence in the previous reports about all these associate factors. Further, studies suggest that exposure to multiple neonatal complications may rocket ASD risk (35). Neonatal jaundice may be linked to ASD and affected the prevalence of ASD. The severity of jaundice may determine the risk of ASD in neonates (36). The relationship between ASD and seizures can be explained by structural changes in the brain function (37).
Strengths of the present study is the large sample size which is adequate to perform a multivariate analysis to explore the antenatal, perinatal and neonatal risk factors for ASD. Secondly, this study provided that clinical status of pregnant women is associated with ASD status of children age 18 to 30 months. They were gestational diseases including diabetes, hypertension and mental disorder in children. Thirdly, all data collectors were trained and closely supervised by researchers to establish high reliability and ensure the quality of the interviews. Fourthly, the multidisciplinary team included eight professionals with longtime experiences in ASD diagnosis and intervention in Vietnam.
In terms of limitation of the study, although ten prenatal, perinatal, and neonatal factors were identified as a risk factors for ASD, it was not known that whether these factors are causal in the development of autism. Although one of the largest studies to date, it is nevertheless limited by a small ASD prevalence. Moreover, health staff was trained to become the data collectors of the study. This could increase the reliability of the structured M-CHAT interview but could be difficult to replicate the methods for future large-scale study. Further, since this was a large-scale survey (with a nationally representative sample), it took time for cleaning, analyzing, and reporting the data. Due to the limitation of resources, the sample size was not selected independently in each province using probability proportional to size (PPS) sampling.
Conclusion
This is the first survey with a nationally representative sample showing the relationship between antenatal, perinatal, and neonatal associate factors and ASD amongst children age 18 to 30 months in Vietnam. We found 9 prenatal, neonatal, and postnatal associate factors which were more likely to occur in children diagnosed with ASD than in healthy children. The results suggested interventions to improve the screening and prognosis for children with abnormalities during ante-, peri- and neonatal period. It was, however, still unclear that whether these factors are causal or play a secondary role in the development of ASD. Further retrospective cohort studies are needed to verify our findings, and investigate the effects of multiple factors on ASD.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We would like to thank data collectors and the participants in this study, as well as Department of Health in Hanoi, Thai Binh, Hoa Binh, Quang Nam, Dong Thap, Dong Nai, Dak Lak for supporting us to implement this study. We also send thank to Ministry of Science and Technology, Vietnam for providing both technical and financial support to the study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.World Health Organization (2021) Autism spectrum disorders. (https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders).
- 2.Ozonoff S, Heung K, Byrd R, et al. (2008). The onset of autism: patterns of symptom emergence in the first years of life. Autism Res, 1:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsabbagh M, Divan G, Koh YJ, et al. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Res, 5:160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiarotti F, Venerosi A. (2020). Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci, 10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen NTH. (2014). Early intervention for autism spectrum disorder and inclusive education for children with Autism in Vietnam for the period 2011–2020. ed. Hanoi National University of Education, Hanoi. [Google Scholar]
- 6.Kien PT, Dung LTK, Dung DV, Yen PT. (2014) Prevalence and Treatment Results of Children with Autism Spectrum Disorder in Thai Nguyen Province, Vietnam. (ed)^(eds), [Google Scholar]
- 7.Bertrand J, Mars A, Boyle C, et al. (2001). Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics, 108:1155–61. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Geng H, Liu W, Zhang G. (2017). Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore), 96:e6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadjkacem I, Ayadi H, Turki M, et al. (2016). Prenatal, perinatal and postnatal factors associated with autism spectrum disorder. J Pediatr (Rio J), 92:595–601. [DOI] [PubMed] [Google Scholar]
- 10.Roberts AL, Lyall K, Hart JE, et al. (2013). Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses' Health Study II participants. Environ Health Perspect, 121:978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. (2009). Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics, 123:1293–300. [DOI] [PubMed] [Google Scholar]
- 12.Williams K, Helmer M, Duncan GW, Peat JK, Mellis CM. (2008). Perinatal and maternal risk factors for autism spectrum disorders in New South Wales, Australia. Child Care Health Dev, 34:249–56. [DOI] [PubMed] [Google Scholar]
- 13.Kolevzon A, Gross R, Reichenberg A. (2007). Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med, 161:326–33. [DOI] [PubMed] [Google Scholar]
- 14.Hoang VM, Le TV, Chu TTQ, et al. (2019). Prevalence of autism spectrum disorders and their relation to selected socio-demographic factors among children aged 18–30 months in northern Vietnam, 2017. Int J Ment Health Syst, 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binu VS, Mayya SS, Dhar M. (2014). Some basic aspects of statistical methods and sample size determination in health science research. Ayu, 35:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giang NTH. (2012) Study of early detection of autism with M-CHAT23, epidemiological-clinical characteristics and early intervention with rehabilitation for autistic children. Thesis for Doctor of Medicine. Hanoi Medical University. [Google Scholar]
- 17.Kleinman JM, Robins DL, Ventola PE, et al. (2008). The modified checklist for autism in toddlers: a follow-up study investigating the early detection of autism spectrum disorders. J Autism Dev Disord, 38:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canal-Bedia R, Garcia-Primo P, Martin-Cilleros MV, et al. (2011). Modified checklist for autism in toddlers: cross-cultural adaptation and validation in Spain. J Autism Dev Disord, 41:1342–51. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CP, Myers SM, American Academy of Pediatrics Council on Children With D (2007). Identification and evaluation of children with autism spectrum disorders. Pediatrics, 120:1183–215. [DOI] [PubMed] [Google Scholar]
- 20.Hossain MD, Ahmed HU, Jalal Uddin MM, et al. (2017). Autism Spectrum disorders (ASD) in South Asia: a systematic review. BMC Psychiatry, 17:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bursac Z, Gauss CH, Williams DK, Hosmer DW. (2008). Purposeful selection of variables in logistic regression. Source Code Biol Med, 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperandei S. (2014). Understanding logistic regression analysis. Biochem Med (Zagreb), 24:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton WW, Mortensen PB, Thomsen PH, Frydenberg M. (2001). Obstetric complications and risk for severe psychopathology in childhood. J Autism Dev Disord, 31:279–85. [DOI] [PubMed] [Google Scholar]
- 24.Glasson EJ, Bower C, Petterson B, et al. (2004). Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry, 61:618–27. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Gao J, He X, Cai Y, Wang L, Fan X. (2017). Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep, 7:46207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hvidtjorn D, Schieve L, Schendel D, et al. (2009). Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolesc Med, 163:72–83. [DOI] [PubMed] [Google Scholar]
- 27.Brown MA, Lindheimer MD, de Swiet M, et al. (2001). The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy, 20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 28.Xu RT, Chang QX, Wang QQ, et al. (2017). Association between hypertensive disorders of pregnancy and risk of autism in offspring: a systematic review and meta-analysis of observational studies. Oncotarget, 9:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan H, Zhang C, Li H, Luan S, Liu C. (2018). Association of maternal diabetes with autism spectrum disorders in offspring: A systemic review and meta-analysis. Medicine (Baltimore), 97:e9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braunschweig D, Ashwood P, Krakowiak P, et al. (2008). Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology, 29:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagberg KW, Robijn AL, Jick S. (2018). Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol, 10:1599–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Sidorchuk A, Sevilla-Cermeno L, et al. (2019). Association of Cesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring: A Systematic Review and Meta-analysis. JAMA Netw Open, 2:e1910236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran EA, Dalman C, Kearney PM, et al. (2015). Association Between Obstetric Mode of Delivery and Autism Spectrum Disorder: A Population-Based Sibling Design Study. JAMA Psychiatry, 72:935–42. [DOI] [PubMed] [Google Scholar]
- 34.Sandall J, Tribe RM, Avery L, et al. (2018). Short-term and long-term effects of caesarean section on the health of women and children. Lancet, 392:1349–1357. [DOI] [PubMed] [Google Scholar]
- 35.Gardener H, Spiegelman D, Buka SL. (2011). Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics, 128:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin SB, Smith T, Wang H. (2011). Is neonatal jaundice associated with Autism Spectrum Disorders: a systematic review. J Autism Dev Disord, 41:1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartley-McAndrew M, Weinstock A. (2010). Autism Spectrum Disorder: Correlation between aberrant behaviors, EEG abnormalities and seizures. Neurol Int, 2:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]