Abstract
Pesticides like Mancozeb are being increasingly indispensable in the control of crop pests. Unfortunately, they have been implicated in genotoxicity due to their ubiquity, toxicological properties, persistence and presence in the food chain. This study sought to evaluate the efficacy of powdered avocado seed on reproductive parameters in the management of oxidative stress in female rabbits caused by the herbicide Mancozeb. Twenty-eight female rabbits aged 7–8 months and weighing between 2780.4 g and 3143.7 g were randomly divided into four groups of seven rabbits each. Each group received for 90 consecutive days distilled water or Mancozeb associated or not with avocado seed powder orally as follows: T1: 10 ml distilled water; T2, T3 and T4: 100 mg/kg bw Mancozeb. This was followed by oral administration of 250, 500, and 0 mg/kg of avocado seed powder for T2, T3, and T4, respectively. Water and feed were distributed ad libitum. Collected data concerned growth, carcass and reproductive performances, hematological and biochemistry characteristics. Results demonstrated that pregnant and lactating female rabbits administered Mancozeb exhibited a significant decrease (P < 0.05) in food intake, body weight, and body weight gain. Female rabbits exposed to Mancozeb had a decrease in litter size and weight from birth to weaning, as well as in weaning body weight and weight increase, fertility and prolificacy rate, milk yield, and daily milk efficiency. However, administration of avocado seed powder reversed (P < 0.05) the trends in these parameters in a dose-dependent manner. The increase in relative weight of the kidney and liver, concentrations of urea, creatinine, alanine and aspartate aminotransferases, mean cell volume, white blood cells, and lymphocytes were all associated with increased Mancozeb rates (P < 0.05). On the contrary, administration of the Mancozeb caused decrease in hemoglobin (Hb), Red blood Cell (RBC) and protein content. Administration of avocado seed powder significantly (P < 0.05) ameliorated the Mancozeb effects on these parameters. Applying 500 mg/kg b.w Avocado seed powder may be suggested as an alternative therapy for reproductive defects induced by Mancozeb in female rabbits.
Keywords: Female rabbit, Oxidative stress, Persea americana seed, Pesticide, Phytotherapy, Reprotoxicity
1. Introduction
Pesticides use for control pests in crop production has increased in sub-Saharan Africa to levels implicated in genotoxicity. This is due to their ubiquity, toxicological properties, persistence and presence in the food chain [1]. Human and livestock exposed to pesticides contaminated food or environment face several reproductive disorders including spermatogenesis impairment, decrease of viability and motility of male spermatozoa [2]; and disruption of female oestrus cycle [3].
Mancozeb is among the most popular pesticides used in sub-Saharan Africa in vegetable production. It is a fungicide belonging to the carbamate class, a polymeric complex of manganese and zinc compounds of ethylene-bis-dithiocarbamate [4]. It inhibits enzyme activity in fungi by forming a complex with metal-containing enzymes, including those involved in the production of adenosine triphosphate (ATP) [4]. However, it has been linked to cutaneous contact toxicity, resulting in contact dermatitis and dermal sensitization [5]. It has also been reported that Mancozeb alters reproductive and endocrine structures, resulting in decreased fertility [6]. Mice administered 500 mg/kg bw of Mancozeb exhibited a decrease in the total number of collected oocytes, oocyte maturation, fertilization rate, implantation rate, fecundity rate, and embryo development [7]. Female Wistar rats exposed to 500, 600, 700, and 800 mg/kg bw of Mancozeb exhibited the same number of oestrus cycles, proestrus duration, number of healthy follicles, and increase in the number of atretic follicles, followed by a decrease in ovary total lipids, glycogen, and phospholipids [8].
Farmers in sub-Saharan Africa favor rabbits due to their early maturity, rapid growth rate, high genetic selection potential, high feed conversion efficiency, economical use of space [9]; and simplicity of feeding using primarily crop residues and food crop weeds [10]. Unfortunately, Mancozeb is generally used in vegetable gardens, which constitute the main source of rabbit feeds especially in dry season. Dietary exposure to Mancozeb through the consumption of contaminated produce or potable water is the most common route of exposure [11]. Thus, rabbits are highly exposed to Mancozeb contamination risks and the associate reproductive defects due to this pesticide [10].
The use of plants is more promising than synthetic antioxidants that have been shown to be carcinogenic (butylate hydroxyanisole, tert-butylate hydroxyquinone, and butylate hydroxytoluene) [12] because of their accessibility and low toxicity [13]. Their phytochemical properties, such as antioxidant properties, minerals, and vitamins [14], work synergistically to protect against the damaging effects of free radicals [15]. The presence of bioactive compounds such as iridoid, phenylpropanoid glycosides, phenols, flavonoids, alkaloids, and labdane diterpenoids [16] is responsible for these protective properties. The interior of the avocado (Persea americana Mill.), one of the most widely cultivated fruits, is a well-known source of carotenoids, minerals, phenolics, vitamins, fatty acids, saponin, tannin, flavonoid, glycoside cyanogenic, alkaloid, phenol, and steroids [17,18]. Its seed has been reported to extend the oestrus phase, increase serum Luteinizing Hormone (LH) and estradiol as well as uterus catalase and superoxide dismutase in female cavies [19]. In addition, significant effects were observed on urea, creatinine, protein, fat, cholesterol contents when female quail ratio was supplemented at 9% of avocado seed powder [20]. Given its composition, avocado seed could therefore be used to alleviate oxidative damages induced by Mancozeb. However, the pharmacological efficacy of powdered avocado seed extract on reproductive parameters of female rabbits exposed to mancozeb is not well documented in the scientific literature. The objective of this study was to assess the efficacy of powdered avocado seed on reproductive parameters in female rabbits suffering from oxidative stress caused by Mancozeb (herbicide). More specifically, the aim of the study was to assess the efficacy of powdered avocado seed on reproductive performance, hematological, and biochemical parameters in the management of oxidative stress induced by Mancozeb in female rabbits.
2. Materials and methods
2.1. Study site
This study was conducted in Bukavu, Democratic Republic of Congo (DRC), at the experimental facility of the Department of Animal Production at the Université Evangélique en Afrique (UEA). It is located at latitude 02°32′24.0″S, longitude 028°51′25.0″E, and 1714 m above sea level. It has a mountainous tropical climate and moderate temperature. Under a bimodal regime, the average annual precipitation ranges between 1300 mm and 1800 mm [21]. All laboratory work was conducted in the Animal Physiology Laboratory of the Faculty of Agriculture and Environmental Sciences at the UEA.
2.2. Ethical approval
The experimental protocol (reference number CEUEA/CIRE/2022/123) was authorized by the Université Evangelique en Afrique's Ethical Committee. The experiments were conducted in accordance with the internationally acknowledged standard ethical guidelines for the protection of animals used for scientific purposes, as outlined in the guidelines of the European Community; EEC Directive 2010/63/EU, effective January 1, 2013.
2.3. Plant material
Avocado seeds were gathered from local vendors in Bukavu, Democratic Republic of the Congo. As described by Talabi [22], they were cleaned and pulverized into fine particles before being boiled in a constant pressure pot (100 °C) for 15 min, after which the crushed seeds were dried in the shade. This treatment intended to reduce the concentration of glycoside cyanogenic and cyanhydric acid in avocado seed. The crushed and desiccated seeds were then ground into a powder. The resulting homogenous powder was then combined with potable water for animal gavage.
2.4. Chemical (Mancozeb)
The herbicide used was Mancozeb (commercial grade of 75% wettable powder) and manufactured by Rohm and Haas Company, US company. Throughout the experiment, Mancozeb was maintained in its original container and stored in a cool, dry, well-ventilated area at room temperature.
2.5. Animals and husbandry
A local breeder was contacted and ordered twenty-eight female New Zealand White rabbits (7–8 months old and weighing an average of 2780.4–3143.7 g). During the experimental period, they were confined in individual specialized wire cages (0.6 × 0.5 × 0.4 m) at room temperature (23–27 °C) and relative humidity of 73 ± 6% in the animal house. Before introducing the females, each cage was cleansed and disinfected with bio-safe disinfectant. After two weeks of acclimation, the animals were ear-marked for identification and arbitrarily divided into four groups of seven animals of comparable weight. The National Research Council [23] outlined the nutritional needs of rabbits, so a control diet consisting of corn and soy meal was prepared. This fundamental diet's composition was determined by the Association of Official and Analytical Chemists (AOAC) [24] method. All ingredients were commercially available in Bukavu, Democratic Republic of the Congo. Water and feed were provided ad libitum, while the diet was served according the animal body weight. Table 1 presents the diet composition and the nutritional profile.
Table 1.
Composition (%) of the control diet fed to rabbit bucks in South Kivu, the Democratic Republic of Congo.
| Ingredients (%) | Proportion (%) |
|---|---|
| Maize grain | 28 |
| Rice polish | 28 |
| Soybean meal | 10 |
| Palm kernel cake | 24 |
| Fish meal | 6 |
| Bone meal | 2 |
| Salt | 1 |
| Vitamin mineral premix | 1 |
| Calculated composition | |
| Crude protein (g) | 16.39 |
| Metabolizable energy (kcal/kg) | 2746.5 |
| Crude fiber (%) | 9.54 |
| Ether extract | 6.58 |
2.6. Experimental design
After an acclimatization period of two weeks, female rabbits were randomly placed into four groups of seven animals each and orally administered with Mancozeb and avocado seed powder as follows: T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb. The selected dose of Mancozeb in the present study was reported to be in the range of developmental toxicity studies in laboratory animals [11], while the different doses of avocado seed powder were selected according to its pharmacological effects at such doses [19,25]. Mancozeb and avocado seed powder were diluted in 10 ml of water and administrated orally by gavaging to the female rabbits daily for 90 consecutive days as follows: five weeks from the beginning of the experiment to the mating day, five weeks for the gestational period and five weeks from farrowing to the weaning period. Feed intake (F.I.) and weight gain (W.G.) were monitored weekly during the experimentation period. Using a Terraillon scale (capacity 5 kg and 1 g accuracy), F.I. was calculated as the difference between the provided food and the leftovers and the W.G. as the difference between the final body weight and the initial body weight.
2.7. Reproductive parameters
Five weeks after exposing animals to Mancozeb and administering avocado seed powder orally, female rabbits were transferred into the cages of male rabbits proved to be fertile. Eight males of the same genetic strain and weighing approximately 600 ± 50 g were used for this purpose. They were obtained from the experimental farm of Université Evangélique en Afrique and acclimatized for 15 days in individual enclosures. Ten days after mating, pregnancy was assessed by abdominal palpation by hands feeling around until the discovery of small lumps that can pass through fingers as they gingerly probe and explore [26]. The infertile females returned promptly to the same buck for another service.
The gestation length was determined by calculating the number of days between the date of observation of copulation and the date of birth [27]. The litter size was determined by tallying the number of young rabbits produced by each female at farrowing and weekly thereafter [28]. Fertility rate was calculated by dividing the number of expectant females by the number of females coupled with a male, while prolificacy rate was calculated by dividing the number of children born by the number of females who have given birth. The stillbirth rate was calculated by dividing the number of stillborn young rabbits by the litter size at delivery, while the weight evolution of the young rabbits was determined by weighing them at birth and weekly until five weeks post-partum (weaning) [27,28]. For each female, milk yield was calculated by comparing the weight of the young before and after nursing [29]. Milk intake per kit was calculated by dividing the milk yield by the number of kits in the litter for each female rabbit, and the efficacy of converting milk into body-weight gain per kit was calculated by multiplying the kit weight gain by the milk intake per kit [26]. The litter weight was determined by weighing the entire litter, the kit body weight was estimated by weighing each kitten individually, and the kit body weight gain was calculated as the difference between the actual kitten weight and the kitten weight from the previous week [29]. These parameters were estimated at weekly intervals from birth until weaning (35 days).
2.8. Blood and organ collections
At the end of the 15-week study, all animals were fasted for 24 h prior to being euthanized with ether vapor. Following this, a sterile instrument was used to directly collect 10 ml of blood for hematological and biochemical analysis by cardiac puncture. Each animal was then dissected, and its kidneys, liver, uterus, and ovaries were collected, freed of adipose tissue, rinsed with saline solution, and blot-dried to determine their weight. The relative weights of the organs were calculated and expressed as a percentage of the carcass weight.
2.9. Blood parameters
Blood was collected in EDTA-coated test tubes for hematological evaluation of haemoglobin (Hb), white blood cells (WBCs), red blood cells (RBCs), lymphocytes (LYM), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and packed cell volume (PCV) utilizing an automated hematimeter MINDARY BC 3000. Analyzers determine the quantity and distribution of white blood cells, basophils, red blood cells, and platelets using the coulter principle. The concentration of hemoglobin is determined by colorimetry, and the statistical count of white blood cells is obtained by semiconductor laser flow cytometry. Using this information, the analyzer computes the results for the remaining parameters [30].
Blood for biochemical analysis was collected in anticoagulant-free containers and kept at room temperature. After 24 h, the serum was collected and stored at −20 °C for analysis of albumin, total cholesterol, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), creatinine, urea, glucose, and protein content using CHRONOLAB® commercial kits (Ref: 101–0576).
2.10. Data analysis
The collected data were analyzed using R software (version 4.0.5). All results were expressed as mean ± SD (standard deviation) and treatment effects on experimental parameters among experimental groups and controls were assessed using one-way analysis of variance. The means of the observed parameters were compared as a function of treatment. Prior to the analysis of variance, the Shapiro-Wilk test and the Bartlett test were respectively used to test the normality and the homoscedasticity of the data. If there were significant differences between the means at α = 0.05 significance level, the Tukey HSD test was applied to identify homogeneous groups means. The Effect Rate (ER) was calculated at two levels: i) ER1 being the level of performance impairment for the positive treatment having received exclusively the mancozeb dose compared to the negative treatment having received only distilled water while ii) ER2 was calculated as the rate of aleviation of induced maconzeb impairment by avocado powder and calculated from the treatment having received the highest dose of combined mancozeb and avocado powder. The applied formulas are as follow: ER1= (T4-T1)*100/T1 and ER2= (T3-T4)*100/T4.
3. Results
3.1. Growth performances
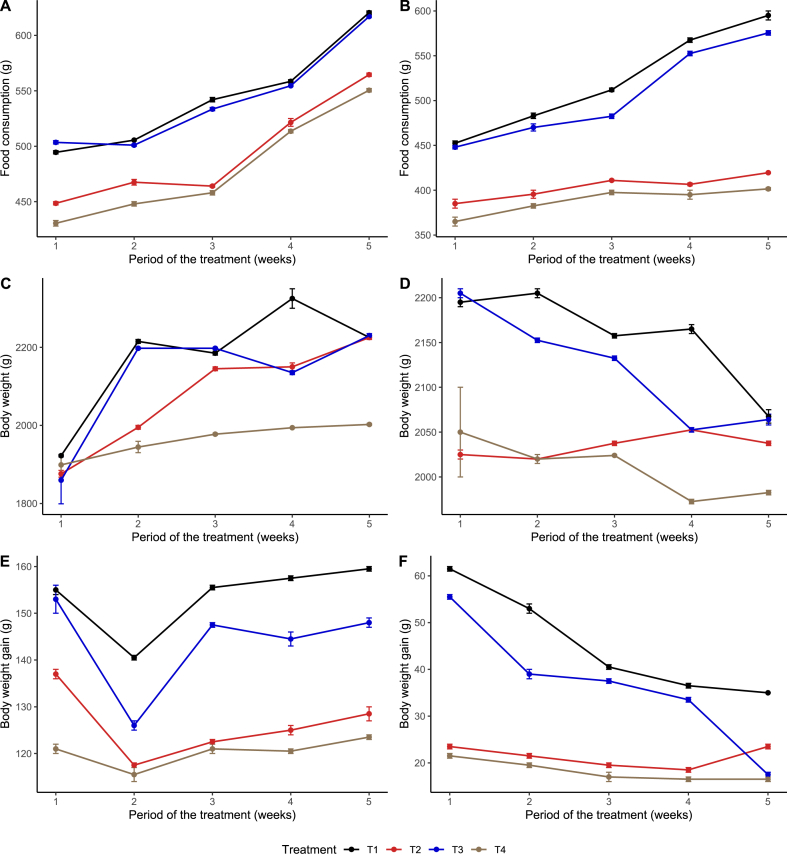
It emerges in Fig. 1 that compared to the control group, the food intake of pregnant (A) and lactating (B) female rabbits receiving Mancozeb decreased by 16% and 28%, respectively. However, this parameter increased dose-dependently in animals treated with avocado seed powder. Following administration of Mancozeb, the bodyweight of pregnant (C) and lactating (D) female rabbits decreased by 9 and 22%, respectively. In contrast, the administration of avocado seed powder reversed the trend. There was a dose-dependent decrease of body weight gain following exposure to Mancozeb in pregnant (E) and lactating (F) female rabbits. However, an increase of this parameter was also observed following the administration of avocado seed powder to these animals.
Fig. 1.
Variation of the food consumption, body weight and body weight gain following different doses of the avocado seed powder in pregnant and lactating female rabbits submitted to Mancozeb. A and B: Food consumption in pregnant and lactating female rabbits respectively; C and D: Body weight in pregnant and lactating female rabbits respectively; E and F: Body weight gain in pregnant and lactating female rabbits respectively. T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb; n = 7.
3.2. Litter size at different ages
In comparison to the control group, the litter size of rabbits exposed to Mancozeb from birth (−27.9%) to weaning (35 days) (−686%) decreased significantly (P < 0.05). However, in animals treated with Mancozeb and avocado seed powder, this parameter increased dose-dependently (12.3% and 128.6% at birth and weaning, respectively), but did not reach the values of the control group (Table 2).
Table 2.
Litter size at different ages of litter from birth to weaning following administration of different doses of the avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 (n = 7) | T2 (n = 7) | T3 (n = 7) | T4 (n = 7) | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Birth | 7.9 ± 1.2a | 6.2 ± 1.1b | 6.4 ± 1.6ab | 5.7 ± 1.3b | −27.9 | 12.3 |
| 7 days | 7.1 ± 0.3a | 4.6 ± 0.3b | 6.4 ± 1.6a | 4.2 ± 0.2b | −40.8 | 52.3 |
| 14 days | 6.7 ± 0.3a | 4.5 ± 0.3c | 5.7 ± 0.5b | 3.4 ± 0.3d | −49.2 | 67.6 |
| 21 days | 6.7 ± 0.3a | 3.6 ± 0.3c | 4.8 ± 0.4b | 2.7 ± 0.2d | −59.7 | 77.8 |
| 28 days | 6.7 ± 0.3a | 3.6 ± 0.3c | 4.8 ± 0.3b | 2.1 ± 0.2d | −68.6 | 128.6 |
| 35 days | 6.7 ± 0.3a | 3.6 ± 0.3c | 4.8 ± 0.3b | 2.1 ± 0.2d | −68.6 | 128.6 |
The superscripts a, b, c, d on the same line: means the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate; n = 7.
3.3. Litter weight, kit body weight and kit body weight gain
The litter weight of animals administered Mancozeb decreased significantly (P < 0.05) from birth (−30.7%) to weaning (−80.7%) when compared to the control group. However, when co-treated with avocado seed powder, this parameter increased dose-dependently (11.4% at birth and 194.6% at weaning) but did not reach the levels of the control group (T1). The body weight of weaned kits and the daily weight gain of weaned kits decreased markedly (P < 0.05) in the Mancozeb-treated groups compared to the control group (−16.7% and −33.3%, respectively). Nonetheless, these parameters were increased in animals receiving avocado seed powder (15.6% and 37.1% for body weight and weight gain, respectively) as compared to those only subjected to Mancozeb (Table 3).
Table 3.
Litter weight, kit body weight and kit body weight gain from birth to weaning following administration of different doses of the avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 | T2 | T3 | T4 | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Litter weight (kg) | ||||||
| Birth | 329.6 ± 15.4a | 250.8 ± 14.3b | 254.6 ± 15.8b | 228.6 ± 15.5b | −30.7 | 11.4 |
| 7 days | 549.6 ± 26.8a | 269.8 ± 30.3c | 452.6 ± 13.9b | 233.6 ± 13.3d | −57.5 | 93.9 |
| 14 days | 1088.3 ± 22.8a | 811.4 ± 15.9c | 881.9 ± 29.7b | 335.7 ± 25.4d | −69.2 | 162.9 |
| 21 days | 1479.8 ± 33.7a | 626.1 ± 15.1c | 861.8 ± 25.8b | 435.6 ± 15.9d | −70.6 | 97.9 |
| 28 days | 2453.6 ± 33.9a | 935.2 ± 21.4c | 1332.8 ± 23.9b | 496.2 ± 23.6d | −79.8 | 168.5 |
| 35 days | 3072.6 ± 57.3a | 1226.3 ± 32.2c | 1762.3 ± 30.1b | 598.5 ± 24.4d | −80.5 | 194.6 |
| Kid body weight (g) | ||||||
| At birth | 40.7 ± 1.6a | 38.6 ± 1.4a | 39.4 ± 1.8a | 39.1 ± 1.5a | −3.9 | 0.7 |
| 35 days | 453.5 ± 10.2a | 408.3 ± 11.1b | 436.3 ± 5.7b | 377.3 ± 12.5c | −16.7 | 15.6 |
| Kid weight gain (g/day) | 14.5 ± 0.6a | 11.7 ± 0.5b | 13.3 ± 0.6ab | 9.7 ± 0.5c | −33.1 | 37.1 |
The superscripts a, b, c, d on the same line: means the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate; n = 7.
3.4. Fertility and milk production
Female rabbits submitted to Mancozeb showed a decrease in fertility rate (−28.8%), prolificity rate (−32.2%), milk yield (−44.3%) and milk efficiency (−68.7%) compared to those of the control group (Table 4). Moreover, these parameters increased in animals receiving avocado seed powder (P < 0.05) compared to those solely exposed to Mancozeb (20.4, 44.6, 50.1 and 120% for fertility rate, prolificity rate, milk yield and milk efficiency respectively). An opposite trend was observed in milk intake which was higher compared to control group (101.2%) and those treated with the avocado seed powder (36.5%).
Table 4.
Fertility and milk production rates following administration of avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 | T2 | T3 | T4 | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Fertility rate (%) | 100 ± 0.00ᵃ | 57.4 ± 6.9 ͨ | 85.7 ± 14.2ᵇ | 71.2 ± 26.9ᵇ | −28.8 | 20.4 |
| Prolificity rate (%) | 91.1 ± 4.6ᵃ | 66.8 ± 14.3ᵇ | 89.4 ± 6.8ᵃ | 61.8 ± 26.8ᵇ | −32.2 | 44.6 |
| Milk yield (g per litter per day) | 74.6 ± 3.7a | 56.1 ± 5.4c | 62.3 ± 6.5b | 41.5 ± 7.2d | −44.3 | 50.1 |
| Milk intake (g per litter per day) | 9.8 ± 1.1c | 14.2 ± 1.5b | 12.5 ± 0.8b | 19.7 ± 2.2a | 101.2 | 36.5 |
| Milk efficiency per day | 1.5 ± 0.3a | 0.8 ± 0.2b | 1.1 ± 0.3ab | 0.5 ± 0.3c | −66.7 | 120 |
The superscripts a, b, c, d on the same line: mean the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate; n = 7.
3.5. Relative internal organs weights
A significant increase (P < 0.05) in the relative weight of kidney (41.3%) and liver (35.7%) was observed in female rabbits exposed to Mancozeb compared to the control group (Table 5). However, these parameters decreased by 24.6 and 22.8% respectively for kidney and liver following the treatment with avocado seed powder compared to those solely submitted to Mancozeb.
Table 5.
Relative weight of uterus, ovary, kidney and liver following the administration of different doses of the avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 | T2 | T3 | T4 | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Uterus | 0.45 ± 0.14a | 0.43 ± 0.11a | 0.43 ± 0.15a | 0.42 ± 0.12a | −6.7 | 2.4 |
| Ovary | 0.31 ± 0.09a | 0.29 ± 0.02a | 0.29 ± 0.06a | 0.28 ± 0.09a | −9.6 | 3.6 |
| Kidney | 0.46 ± 0.02ᵃ | 0.53 ± 0.04ᵇ | 0.49 ± 0.06ᵃᵇ | 0.65 ± 0.06c | 41.3 | −24.6 |
| Liver | 3.95 ± 0.32ᵃ | 5.12 ± 0.55ᵇ | 4.14 ± 0.24ᵃ | 5.36 ± 0.33ᵇ | 35.7 | −22.8 |
The superscripts a, b, c on the same line: mean the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate; n = 7.
3.6. Biochemical parameters
The serum concentration of urea, creatinine, ALAT, ASAT increased significantly (P < 0.05) by 62.5%, 36.5%, 41.2% and 88.2% respectively in Mancozeb-treated groups relatively to controls (Table 6). However, these parameters decreased in a dose-dependent manner by 25.8%, 23.7%, 15.5% and 22.7% respectively for urea, creatinine, ALAT, ASAT in animals receiving avocado seed powder by compared to those solely treated with Mancozeb. A contrasting trend was observed for total protein in response to Mancozeb treatment (decrease of 21.4%) versus that of avocado seed powder (increase of 23.1%). There was no significant difference in cholesterol and serum total albumin in treated groups compared to controls (Table 6).
Table 6.
Biochemical parameters following administration of different doses of the avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 | T2 | T3 | T4 | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Albumin (g/dl) | 3.28 ± 0.48a | 3.42 ± 0.48a | 3.57 ± 0.77a | 3.85 ± 0.89a | 17.4 | −7.3 |
| Urea (mg/dl) | 86.12 ± 3.22d | 116.3 ± 5.52b | 103.81 ± 4.29c | 139.93 ± 8.33a | 62.5 | −25.8 |
| Creatinine (mg/dl) | 1.45 ± 0.19a | 1.95 ± 0.22b | 1.51 ± 0.13a | 1,98 ± 0.26ᵇ | 36.5 | −23.7 |
| ALAT (U/L) | 41.29 ± 3.15d | 54.19 ± 2.11b | 48.97 ± 1.26c | 58.32 ± 2.49a | 41.2 | −15.5 |
| ASAT (U/L) | 16.99 ± 2.31c | 30.15 ± 2.88a | 24.77 ± 2.91b | 32.04 ± 3.67a | 88.2 | −22.7 |
| Total protein (g/L) | 61.78 ± 3.22a | 51.17 ± 2.98b | 59.73 ± 3.44a | 48.55 ± 3.86b | −21.4 | 23.1 |
| Cholesterol (mg/dl) | 120.43 ± 34.32a | 167.57 ± 27.51a | 158.29 ± 36.32a | 94.86 ± 38.65a | −21.2 | 68.1 |
The superscripts a, b, c, d on the same line: mean the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate. ALAT: Alanine aminotransferase; ASAT: Aspartate aminotransferase; n = 7.
3.7. Hematological parameters
Mancozeb administration significantly decreased (P < 0.05) the Hb (−42.4%) and RBC (−24.1%) while increasing total WBC (76.1%), LYM (39.7%) and MCV (10.7%) of the animals of animals submitted to Mancozeb compared to those of the control group (Table 7). However, in groups co-treated with avocado seed powder, Hb and RBC concentrations increased by 59.8 and 28.5% respectively. In contrast, the concentration in WBC, LYM and MCV decreased by 26.6, 10.5 and 8.1% respectively compared to the group solely submitted to Mancozeb.
Table 7.
Hematological parameters following administration of different doses of the avocado seed powder in female rabbits submitted to Mancozeb.
| Parameters | T1 | T2 | T3 | T4 | ER1 (%) | ER2 (%) |
|---|---|---|---|---|---|---|
| Hb (g/dl) | 11.18 ± 1.21a | 7.27 ± 1.91b | 10.29 ± 1.33a | 6.44 ± 1.35b | −42.4 | 59.8 |
| PCV (%) | 32.25 ± 2.16a | 33.19 ± 2.99a | 32.44 ± 3.81a | 32.18 ± 1.97a | −0.2 | 8.1 |
| RBC (x1012/l) | 5.40 ± 0.25ᵃ | 4.23 ± 0.44ᵇ | 5.27 ± 0.53ᵃ | 4.10 ± 0.46ᵇ | −24.1 | 28.5 |
| MCV (fl) | 68.00 ± 2.28ᵇ | 71.85 ± 1.59ᵃᵇ | 69.21 ± 4.29ᵇ | 75.27 ± 2.28ᵃ | 10.7 | −8.1 |
| MCH (pg) | 17.14 ± 2.12a | 17.74 ± 0.94a | 16.85 ± 1.59a | 16.72 ± 1.36a | −2.5 | 0.7 |
| MCHC (g/dl) | 33.31 ± 2.06a | 32.14 ± 5.26a | 33.22 ± 4.62a | 30.17 ± 2.74a | −9.4 | 10.1 |
| WBC (x109/l) | 8.12 ± 0,86 ͨ | 13.84 ± 0.77ᵃ | 10.50 ± 0.82ᵇ | 14.30 ± 0.37ᵃ | 76.1 | −26.6 |
| PLT ( × 103/μl) | 156.22 ± 8.49a | 155.12 ± 11.39a | 159.01 ± 6.37a | 161.67 ± 7.26a | 3.5 | −1.2 |
| LYM (%) | 34.06 ± 1.45c | 46.04 ± 1.35a | 42.57 ± 1.74ᵇ | 47.58 ± 5,81ᵃ | 39.7 | −10.5 |
The superscripts a, b, c on the same line: mean the mean values are significantly different at P < 0.05; T1: 10 ml of distilled water; T2: 100 mg/kg bw of Mancozeb and 250 mg/kg bw of Avocado seed powder; T3: 100 mg/kg bw of Mancozeb and 500 mg/kg bw of Avocado seed powder; T4: 100 mg/kg bw of Mancozeb, ER: Effect Rate. Hb: Hemoglobin; PCV: Packed cell volume; RBC: Red blood Cell; MCV: Mean cell volume; MCHC: Mean corpuscular hemoglobin concentration; MCH: Mean corpuscular hemoglobin; WBC: White blood cells; PLT: Platelet count; LYM: Lymphocytes; n = 7.
3.8. Discussion
Primarily through contact, mancozeb exerts effects on multiple organ systems. It has teratogenic and reproductive effects and alters reproductive and endocrine structures, causing infertility. Oral administration of mancozeb induces hyperplasia of the thyroid, most likely due to its ability to inhibit thyroxine synthesis [31]. It is also believed to induce neurotoxicity and has chelating properties, enabling it to potentially interfere with a variety of enzyme systems containing metals, including zinc, copper, and iron (e.g. dopamine -hydroxylase). Mancozeb increases oxidative stress, and the increased oxidative stress results in cell mortality through multiple pathways, including the mitochondrial apoptosis pathway, proteotoxicity, and mitochondrial dysfunction [32].
This is the first study to evaluate the ameliorative effect of avocado seed powder on the management of the deleterious effects of Mancozeb (herbicide) on reproductive performance in rabbits. Compared to the control group, expectant and lactating female rabbits receiving Mancozeb (100 mg/kg bw) exhibited a reduction in food consumption, body weight, and body weight gain. This result is consistent with previous findings [5] in female rats receiving 0, 50, 100, or 150 mg mancozeb/kg b.w from gestation day 7 to postnatal day; in another study [33], rats exposed to 500 mg/kg b.w of Mancozeb for 42 days exhibited a decrease in body weight gain and food consumption. According to Chahoud et al. [34], weight loss is an important indicator of toxicity; consequently, it can be inferred that Mancozeb induced systemic toxicity, which may be related to its ability to stimulate reactive oxygen species (ROS) production. In fact, Mancozeb is reported to induce oxidative stress by generating both reactive oxygen and nitrogen species (ROS and RNS). ROS and RNS can activate at least five independent signaling pathways, including apoptosis induced by mitochondria. The decrease in these parameters is attributable to the injury caused by Mancozeb to the appetite centre of the hypothalamus, which consequently decreases feed intake [35]. Hazard et al. [36] and Gore [37] reported that numerous environmental toxicants altered reproductive functions concurrently with effects on the central nervous system and behavior, the so-called neuroendocrine disruptors operating through the hypothalamus-pituitary-gonadal axis. In contrast, this parameter increased dose-dependently in animals administered powdered avocado seed. These results are consistent with those reported by Tendonkeng [38], who found that the addition of phytobiotics to an animal's diet increased its feed intake. The presence of flavonoids in avocado seed [25] may account for the observed increase in these parameters following administration of powdered avocado seed in this study. In fact, flavonoids have been linked to the protection of tissue from the deleterious effects of external agents and, by extension, the structures of the central nervous system that control appetite [39]. Stimulation of this region increases food intake, whereas bilateral injury causes a total cessation of food intake [40].
This study demonstrated a significant reduction in litter size and weight from birth to weaning, kitten body weight at weaning, and daily kid weight gain in animals administered Manconzeb. These results corroborate the findings of Kodithuwakku [41] in mice receiving 30 mg/kg bw of mancozeb. The decrease in litter weight may be attributable to a reduction in food intake by lactating female rabbits, which affects milk production and results in inadequate or insufficient nutrition for the young. The decrease in litter weight has direct effects on other relevant parameters, such as the body weight of weaned kits and the daily weight gain of youngsters. However, the increase in these parameters after administration of avocado seed powder could be attributed to the phenolic compounds and vitamin C it contains [19]. In fact, avocado seed contains 150.6–265.75 mg ascorbic acid equivalents (AAE)/100 g antioxidant, 1.92 mg quercetin 100 g flavonoid, and >70% phenolic [42,43] compound that is 44.89 mg/kg, which is greater than the leaves or flesh [43]. These compounds can protect the cell membranes of the digestive system from oxidative stress by scavenging O2 radicals, thereby restoring feed utilization [44] and enhancing reproductive performance [45]. Phytoestrogens (flavonoids, triterpenes, and sterols) found in certain plants, such as avocado seed, may be responsible for the increased litter size, fertility rate, and prolificacy rate in animals receiving avocado seed powder compared to those receiving only Mancozeb [46]. These compounds have similar molecular structures to estrogens, allowing them to bind to estrogen receptors in specific tissues and function as estrogen agonists or antagonists, depending on their concentrations and the tissues to which they bound [19]. In the present investigation, they would have increased estrogen production (including estradiol) in response to a rise in reproductive hormones (FSH and LH) in the blood. Indeed, excessive FSH promotes the recruitment and growth of primordial follicles up until the preovulatory stage [47]. Subsequently, the elevated levels of LH and estrogens triggered the rupture of a large number of mature follicles (increased ovulation rate), thereby enhancing the likelihood of an increased number of fertilized follicles [48].
There was a decrease in both weaning body weight and daily weight gain in animals administered Mancozeb compared to the control group (Table 3). The correlations between these parameters and milk production, milk ingestion, and milk efficiency were strong. In fact, the low milk production in Mancozeb-treated groups was likely a result of the decrease in feed consumption observed in this study, which resulted in a low energy intake that was insufficient to cover both animal body metabolism and daily milk production requirements [35]. Therefore, the decrease in kid body weight and daily kid weight gain was a result of the observed low milk production, as children's metabolic needs become increasingly dependent on milk quantity, and this circumstance results in the daily milk efficiency of converting milk into body-weight gain. The enhancement of these parameters may be attributable to the antioxidant activity of bioactive compounds present in avocado seed powder that inhibit lipid peroxidation in the cell membrane [49]. This is also essential for the development of the immune system in juvenile animals [50] and for pre-weaning children, who have been reported to be more sensitive to oxidative stress than adults. In addition, the elevated daily milk intake in animals receiving Mancozeb alone compared to those receiving avocado seed powder and the control group is primarily attributable to the high infant mortality rate observed in this group. At weaning, only 36.8% of children in the group receiving only Mancozeb were still alive, compared to 84.8% in the control group. Therefore, the small amount of milk generated by these females was sufficient to meet the nutritional needs of their young, in contrast to the animals in the control group, which produced a large amount of milk, but not enough to meet the needs of their young.
In this study, the relative weight of the liver and kidney in animals administered Mancozeb was significantly greater than that of the control group. Similar results were observed in guinea pigs administered glyphosate-based herbicide WILLOSATE (186, 280, and 560 mg/kg body weight) for 60 days [2]. The increase in weight of these organs may be attributable to their intensive detoxification of Mancozeb. However, the decrease in these parameters following administration of avocado seed powder may be attributable to the antioxidant activity of flavonoids found in these seeds, as flavonoids have been reported to inhibit the metabolism of xenobiotics by stimulating detoxification systems [51]. The decreased concentrations of urea, creatinine, ALAT, and ASAT were directly caused by the decreased weight of these organs, which was induced by the administration of avocado seed powder. In fact, urea, creatinine, ALAT, and ASAT are commonly utilized biochemical indicators in the investigation of renal and liver degeneration [52]. Compared to animals receiving only Mancozeb, avocado seed powder decreased these markers in a dose-dependent manner in the current study. These results corroborate the findings of Nienga [53] in female guinea pigs exposed to 12 mg/kg b.w. of lead acetate and treated with 50, 100, and 200 mg/kg b.w. of hydroethanolic extract of Spirulina platensis. The decrease in these parameters may be attributable to bioactive compounds, such as flavonoids, alkaloids, and gallic acid, which are antioxidant-rich [49]. These compounds may reduce hepatic and renal tissue damage, thereby preserving their integrity [54]. A decrease in protein content was also observed in animals administered Mancozeb, whereas avocado seed powder administration increased this parameter dose-dependently. Proteins are a class of molecules that perform a variety of functions within an organism, such as hormone transporters, cellular receptors, enzymes, and antibodies [55]. Increase in blood protein levels due to the administration of avocado seed powder may be attributable to its anti-oxidative and hepato-protective activities that scavenge reactive oxygen species, as avocado seed contains antioxidants and scavengers of oxidative stress [56].
This study revealed a decrease in Hb and RBC levels, followed by an increase in MCV, WBC, and LYM, in female rabbits administered Mancozeb. The reduction in RBC can be attributed to the presence of few antioxidant defenses on the cell membranes of RBCs, which favored the formation of methemoglobin, lipoperoxidation, and lysis of RBCs [57] or to the cytotoxicity caused by Mancozeb, which resulted in DNA damage due to elevated levels of ROS [58]. The deterioration of RBCs may result in hypoxia, as RBCs play a significant role in the transport of blood gases, conveying approximately 98% of oxygen throughout the body system [59], and as Hb is a protein molecule in RBCs, it may have contributed to the decrease in Hb reported in this study. It is also plausible that Mancozeb's inhibition of erythropoiesis and heme synthesis, as well as the destruction of erythrocytes in hemopoietic tissue [60] or chromosomal aberrations in bone marrow cells [61], contributed to the decrease in Hb. This study indicates that avocado seed powder supports or does not interfere with normal hemopoiesis [62] and/or may be associated with the high iron content of avocado seed [49].
Both humoral and cellular immunity are mediated by lymphocytes and WBC. The increase in their content reported in the present study may be attributable to the stress caused by pathogens during Mancozeb exposure, which may increase blood viscosity and generate allergic effects that induce an increase in WBC [63]. The increase in MCV results from the decrease in sodium concentration in the blood plasma of rabbits administered Mancozeb [64].
As WBC counts possess phagocytic function and serve as biomarkers of immune functions [62], the increase in WBC in female rabbits following administration of avocado seed powder may be the result of immunostimulatory effects of avocado seed. Among the biological activities associated with phytogenic feed additives [65] is immunostimulatory activity in animals. The high concentration of Vitamin C in avocado seed, as previously reported [19], endows it with a potent antioxidant capacity that mitigates the negative responses of rabbits to stressors. This could also contribute to the healthy upbringing of rabbits by reducing LYM concentrations to the normal level in order to combat infection.
4. Conclusion
Mancozeb (herbicide) induces oxidative stress, which negatively impacts the reproductive function of female rabbits. However, treatment with powdered Persea americana (avocado) seed combats the action of free radicals and improves reproductive characteristics. Therefore, avocado seed could be used as an alternative treatment to mitigate the effects of reproductive stress caused by Mancozeb (herbicide) in female rabbits. A dose of 500 mg/kg bw is suggested for its administration. The authors acknowledge that this is a preliminary study on the evaluation of avocado seed powder's potential in the management of oxidative stress, and they recommend that more animals be used in future studies, as well as other physiological parameters, in order to arrive at more reliable conclusions.
Author contribution
ARBB and MVB conceived and designed the experiments, MVB, BBF, KHS, SDW performed the experiments, MY and KVW analyzed and interpreted the data, ARBB, MY and KVW contributed reagents, materials, analysis tools or data, ARBB, BBF, KHS, SDW, MY and MVB wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgement
The authors acknowledge the multiple support of the Université Evangélique en Afrique to this work mainly through the granting of the experimental framework and the laboratories facilities for the different analyses.
References
- 1.Benbrook C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016;28(3):15. doi: 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutwedu V.B., Nyongesa A.W., Azine P.C., Chiregereza D.K., Ngoumtsop V.H., Mugumaarhahama Y., Ayagirwe R.B.B. Growth performance and reproductive function impairment of glyphosate‐based herbicide in male Guinea pig (Cavia porcellus) Veterinary Medicine and Science. 2021;7(3):1047–1055. doi: 10.1002/vms3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hamdani N.M.H., Yajurvedi H.N. Effect of cypermethrin on the ovarian activity and its impact on fertility and pubertal onset of offspring. Beni-Suef Univ. Journal of Basic and Applied Sciences. 2017;6:374–382. [Google Scholar]
- 4.Yahia D., El-Amir Y.O., Rushdi M. Mancozeb fungicide-induced genotoxic effects, metabolic alterations, and histological changes in the colon and liver of Sprague Dawley rats. Toxicol. Ind. Health. 2019;35(4):265–276. doi: 10.1177/0748233719834150. [DOI] [PubMed] [Google Scholar]
- 5.Axelstad M., Boberg J., Nellemann C., Kiersgaard M., Jacobsen P.R., Christiansen S., Hougaard K.S., Hass U. Exposure to the widely used fungicide mancozeb causes thyroid hormone disruption in rat dams but No behavioral effects in the offspring. Toxicol. Sci. 2011;120(2):439–446. doi: 10.1093/toxsci/kfr006. [DOI] [PubMed] [Google Scholar]
- 6.Hashem M.A., Mohamed W.A.M., Attia E.S.M. Assessment of protective potential of Nigella sativa oil against carbendazim and/or mancozeb-induced hematotoxicity, hepatotoxicity, and genotoxicity. Environ. Sci. Pollut. Control Ser. 2018;25(2):1270–1282. doi: 10.1007/s11356-017-0542-9. [DOI] [PubMed] [Google Scholar]
- 7.Esmaiel S., Tahereh H., Noreddin N.M.S., Massood E. Mancozeb exposure during development and lactation periods results in decreased oocyte maturation, fertilization rates, and implantation in the first-generation mice pups: protective effect of vitamins E and C. Toxicol. Ind. Health. 2019;35(11–12):714–725. doi: 10.1177/0748233719890965. [DOI] [PubMed] [Google Scholar]
- 8.Baligar P.N., Kaliwal B.B. Induction of gonadal toxicity to female rats after chronic exposure to mancozeb. Ind. Health. 2001;39:235–243. doi: 10.2486/indhealth.39.235. [DOI] [PubMed] [Google Scholar]
- 9.Hassan H.E., Elamin K.M., Yousif I.A., Musa A.M., Elkhairey M.A. Evaluation of body weight and some morphometric traits at various ages in local rabbits of Sudan. J. Anim. Sci. Adv. 2012;2:407–415. [Google Scholar]
- 10.Mutwedu V.B., Ayagirwe R.B.B., Metre K.T., Mugumaarhahama Y., Sadiki J.M., Bisimwa E.B. Systèmes de production cunicole en milieu paysan au Sud-Kivu, Est de la RD Congo. Livest. Res. Rural Dev. 2015;27(10):14. http://www.lrrd.org/lrrd27/10/mutw27206.html [Google Scholar]
- 11.Runkle J., Flocks J., Economos J., Dunlop A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017;99:29–42. doi: 10.1016/j.envint.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Molla M.R., Rahman M.M., Akter F., Mostofa M. Effects of Nishyinda, black pepper and cinnamon extract as growth promoter in broilers. Bangladesh Vet. 2012;29(2):69–77. [Google Scholar]
- 13.Ikpeme E.V., Ekaluo U.B., Udensi O.U., Ekerette E.E. Screening fresh and dried fruits of avocado pear (Persea americana) for antioxidant activities: an alternative for synthetic antioxidant. Journal of Life Sciences Research and Discovery. 2014;1:19–25. [Google Scholar]
- 14.Machebe N.S., Iweh P., Onyimonyi A.E., Ekere O.S., Abonyi F. Zinc oxide as an effective mineral for induced moulting: effects on post moult performance of laying hens in the humid tropics. J. Vet. Sci. Technol. 2013;11 doi: 10.4172/2157-7579.S11-003. [DOI] [Google Scholar]
- 15.Mutwedu Valence B., Nyongesa Albert W., Kitaa Jafred M., Ayagirwe Rodrigue B.B., Baharanyi Chasinga, Mbaria James M. Effects of Moringa oleifera aqueous seed extracts on reproductive traits of heat-stressed New Zealand white female rabbits. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.883976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indumathi P., Vijayalakshmi K.M. Quantification of phytochemicals and antioxidant potential of Persea americana and Actinidia deliciosa. Int. J. Biol. Pharmaceut. Res. 2015;6(1):6–11. [Google Scholar]
- 17.Tabeshpour J., Razavi B.M., Hosseinzadeh H. Effect of avocado (Persea americana) on metabolic syndrome: a comprehensive systematic. Phytother Res. 2017;31(6):819–837. doi: 10.1002/ptr.5805. [DOI] [PubMed] [Google Scholar]
- 18.Anggraeny D., Inneke F.M.R., Gregoria S.S.D., Pipih S. Antioxidant's activity of avocado (Persea americana Mill.) seeds extract coating by nanochitosan. Jurnal Ilmu dan Teknologi Pangan. 2017;5(2):6–11. [Google Scholar]
- 19.Baulland D., Narcisse V.B., Hervé T., Adamou M., Momo C., Nadège D.M., Adam M., Ferdinand N. Influence of ethanolic extract of avocado (Persea americana Mill.) seed flour on the estrous cycle, the serum concentrations of reproductive hormones, and the activities of oxidative stress markers in female cavies (Cavia porcellus L.) Journal of advanced veterinary and animal research. 2021;8(3):501–510. doi: 10.5455/javar.2021.h540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tugiyanti E., Iriyanti N., Apriyanto Y.S. The effect of avocado seed powder (Persea americana Mill.) on the liver and kidney functions and meat quality of culled female quail (Coturnix coturnix japonica) Vet. World. 2019;12(10):1608–1615. doi: 10.14202/vetworld.2019.1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansoms A., Marivoet W. 2009. Profil socio-économique du Sud-Kivu et futures pistes de recherche. L'Afrique des grands lacs: annuaire 2009/2010; pp. 259–271. [Google Scholar]
- 22.Talabi J., Olukemi A.O., Ajayi O.O., Adegoke G.O. Nutritional and antinutritional compositions of processed avocado (Persea americana Mill) seeds. Asian J. Plant Sci. Res. 2016;6(2):6–12. [Google Scholar]
- 23.NRC . The National Academy Press; Washington, DC: 1977. Nutrient Requirements of Rabbits. [Google Scholar]
- 24.Association of Official and Analytical Chemist (A.O.A.C . fifteenth ed. Washington; DC, USA: 1990. Official Methods of Analysis. [Google Scholar]
- 25.Tatsinkou A.S., Miegoue E., Tendonkeng F., Mube H., Noumbissi M.N.B., Mouchili M., Fossi J., Pamo E.T. Effect of aqueous and hydroethanolic extracts of avocado seeds (Persea americana) on nutrient digestibility in Guinea pigs (Cavia Porcellus) Animal Husbandry, Dairy and Veterinary Science. 2020;4:1–5. [Google Scholar]
- 26.Marai I.F.M., Habeeb A.A.M., Gad A.E. Reproductive traits of female rabbits as affected by heat stress and lighting regime under subtropical conditions of Egypt. Anim. Sci. 2004;78:119–127. [Google Scholar]
- 27.Attia Y.A., Bovera F., El-Tahawy W.S., El-Hanoun A.M., Al-Harthi M.A., Habiba H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015;23:273–282. doi: 10.4995/wrs.2015.3644. [DOI] [Google Scholar]
- 28.Celia C., Cullere M., Gerencsér Z., Matics Z., Zotte A.D., Giaccone V., Szendrö Z. Effect of Digestarom® dietary supplementation on the reproductive performances of rabbit does: preliminary results. Ital. J. Anim. Sci. 2015;14:4138. [Google Scholar]
- 29.Marai I.F.M., Ayyat M.S., Abd El-Monem U.M. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001;33:1–12. doi: 10.1023/a:1012772311177. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Zhao S., Su Z., Liu X. Analytical comparison between two hematological analyzer systems: mindray BC-5180 vs Sysmex XN-1000. J. Clin. Lab. Anal. 2019 Oct;33(8) doi: 10.1002/jcla.22955. Epub 2019 Jun 20. PMID: 31218736; PMCID: PMC6805265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sule R.O., Condon L., Gomes A.V. Pohanish, in Sittig's Handbook of Pesticides and Agricultural Chemicals. second ed. 2022. A common feature of pesticides: oxidative stress-the role of oxidative stress in pesticide-induced toxicity. Oxidative medicine and cellular longevity 5563759; p. 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmoslemany A.M., El-Magd M.A., Ghamry H.I., Alshahrani M.Y., Zidan N.S., Zedan A.M.G. Avocado seeds relieve oxidative stress-dependent nephrotoxicity but enhance immunosuppression induced by cyclosporine in rats. Antioxidants. 2021;10(8):1194. doi: 10.3390/antiox10081194. Jul 27. PMID: 34439442; PMCID: PMC8388998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayed M.A. The protective effect of Zn-metallothionein (Zn-Mt) on the toxicity of diazinon, mancozeb. Journal of the Egyptian Society of Toxicology. 2007;36:35–41. [Google Scholar]
- 34.Chahoud I., Ligensa A., Dietzel L., Faqi A.S. Correlation between maternal toxicity and embryo/fetal effects. Reprod. Toxicol. 1999;13(5):375–381. doi: 10.1016/s0890-6238(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 35.Das R., Sailo L., Verma N., Bharti P., Saikia J., Imtiwati Kumar R. Impact of heat stress on health and performance of dairy animals: a review. Vet. World. 2016;9(3):260–268. doi: 10.14202/vetworld.2016.260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazard J., Perlemuter L., Abramovici Y., Muriel B. Masson; Paris: 2001. Endocrinologie; p. 484. 2000. [Google Scholar]
- 37.Gore A.C. Environmental toxicant effects on neuroendocrine function. Endocrine. 2000;14:235. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- 38.Tendonkeng F., Mekuiko W.H., Ngoula F., Miegoué E. Effet de l’huile essentielle de Zingiber officinale sur la digestibilité in vivo du foin de Pennisetum clandestinum chez le mouton djallonke. Journal of Animal and Plant Science. 2018;1:6074–6085. [Google Scholar]
- 39.Weston L.A., Mathesius U. Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013;39(2):283–297. doi: 10.1007/s10886-013-0248-5. [DOI] [PubMed] [Google Scholar]
- 40.Ganong W.F. Masson; Paris: 2001. Physiologie Médicale; pp. 408–411. [Google Scholar]
- 41.Kodithuwakku S.P., Akthar I., Ratnayake C., Lee K.F., Wijayagunawardane M.P.B. The effects of commonly used fungicide “Mancozeb” on embryo implantation and fertility: an in vivo mice study. Conference paper Ipurse. 2015:33–37. [Google Scholar]
- 42.Anggraeny D., Inneke F.M.R., Gregoria S.S.D., Pipih S. Antioxidant's activity of avocado (Persea americana Mill.) seeds extract coating by nanochitosan. Jurnal Teknologi dan Industri Pangan. 2017;5(2):6–11. [Google Scholar]
- 43.Antasionasti I., Riyanto S., Rohman A. Antioxidant activities and phenolics contents of avocado (Persea americana Mill.) peel in vitro. Res. J. Med. Plant. 2017;11(2):55–56. [Google Scholar]
- 44.Abou-Zeid A.E., Isshak A., Badawy N., Abou-Ouf N. The potential effect of vitamin C supplementation in quail. Egyptian Journal of Poultry Science. 2000;20:817–838. [Google Scholar]
- 45.Abdel-Moneim A.A., Osama M.A., Hanaa I.F., Eman E.M. The preventive effects of avocado fruit and seed extracts on cardio-nephrotoxicity induced by diethylnitrosamine/2-acetylaminoflurine in Wistar rats. Basic Sci. Med. 2017;6(1):4–13. doi: 10.5923/j.medicine.20170601.02. [DOI] [Google Scholar]
- 46.Ramawat K.G., Dass S., Mathur M. In: Ramawat K., editor. Springer; Berlin, Heidelberg: 2009. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. (Herbal Drugs: Ethnomedicine to Modern Medicine). 8–32. [DOI] [Google Scholar]
- 47.Gayrard V. vol. 198. Ecole Normale Vétérinaire de Toulouse; France: 2007. Physiologie de la reproduction des mammifères. Polycopié. Unité de physiologie-physiopathologie. [Google Scholar]
- 48.Retana-Márquez S., Hernández H., Flores J.A., Muñoz-Gutiérrez M., Duarte G., Vielma J., Fitz-Rodríguez G., Fernández I., Keller M., Delgadillo J. Effects of phytoestrogens on mammalian reproductive physiology. Tropical and Subtropical Agroecosystems. 2012;15(1):129–145. [Google Scholar]
- 49.Melgar B., Dias M.I., Ciric A., Sokovic M., Garcia-Castello E.M., Rodriguez-Lopez A.D., Barros L., Ferreira I.C.R.F. Bioactive characterisation of Persea americana Mill. By-producs: a rich source of inherent antioxidants. Ind. Crop. Prod. 2018;111:212–218. doi: 10.1016/j.indcrop.2017.10.024. [DOI] [Google Scholar]
- 50.Debier C., Pottier J., Goffe C., Larondelle Y. Present knowledge and unexpected behaviours of vitamins A and E in colostrum and milk. Seventh international workshop in the biology of lactation in farm animals. Livest. Prod. Sci. 2005;98:135–147. [Google Scholar]
- 51.Makkar H., Francis G., Becker K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal. 2007;1:1371–1391. doi: 10.1017/S1751731107000298. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Wahab M., Aly S. Antioxidant property of Nigella sativa (black curcumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J. Appl. Toxicol. 2005;25(3):218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 53.Nienga D.S., Ngoula F., Tsague M.T.P., Kenfack N.O., Nguemo M.N.L., Vemo B., Tchoumboue J. Oxidative stress and reproductive damage induced by lead acetate in female Guinea pig (Cavia porcellus): curative effects of hydroethanolic extract of Spirulina platensis. Am. J. Anim. Vet. Sci. 2019;14(1):69–77. doi: 10.3844/ajavsp.2019.69.77. [DOI] [Google Scholar]
- 54.Albrakati A. Protective effect of moringa oleifera leaves against tramadol induced nephrotoxicity in mice. International Journal of Toxicological and Pharmacological Research. 2017;9(2):156–162. doi: 10.25258/ijtpr.v9i02.9053. [DOI] [Google Scholar]
- 55.Tissier M. Contribution à l’étude du stress oxydant chez le chien de cross canin. Thèse de Doctorat en Médecine Vétérinaire, Université Claude-Bernard-Lyon, Villeurbanne, France. 2011;1:173. –9. [Google Scholar]
- 56.Mahadeva R.U.S., Mainul H., Atif A.B. Insulin stimulative and anti-oxidative effects of Persea americana fruit extract on streptozotocin induced hyperglycemic rats. Journal of Medical and Biological Sciences. 2011;4(1):1–10. [Google Scholar]
- 57.Jasper R., Locatelli G.O., Pilati C., Locatelli C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate- Roundup. Interdiscipl. Toxicol. 2012;5(3):133–140. doi: 10.2478/v10102-012-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortiz-Ordoñez E., Uría-Galicia E., Ruiz-Picos R.A., Duran A.G.S., Trejo Y.H. Effect of Yerbimat herbicide on lipid peroxidation, catalase activity, and histological damage in gills and liver of the freshwater fish Goodea atripinnis. Arch. Environ. Contam. Toxicol. 2011;1(3):443–452. doi: 10.1007/s00244-011-9648-0. [DOI] [PubMed] [Google Scholar]
- 59.Jensen F.B. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009;212:3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 60.Fetoui H., Garoui E.M., Makni-Ayadi F., Zeghal N. Oxidative stress induced by lambda-cyhalothrin (L.T.C.) in rat erythrocytes and brain: attenuation by vitamin C. Environ. Toxicol. Pharmacol. 2008;26:225–231. doi: 10.1016/j.etap.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Prasad S., Srivastava S., Singh M., Shukla Y. Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J. Toxicol. 2009:1–6. doi: 10.1155/2009/308985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oloruntola O.D., Ayodele S.O., Adeyeye S.A., Agbede J.O. Performance, haemato-biochemical indices and antioxidant status of growing rabbits fed on diets supplemented with Mucuna pruriens leaf meal. World Rabbit Sci. 2018;26(4):277–285. doi: 10.4995/wrs.2018.10182. [DOI] [Google Scholar]
- 63.Okab A.B., El-Banna S.G., Koriem A.A. Influence of environmental temperatures on some physiological and biochemical parameters of New-Zealand Rabbit Male. Slovak Journal of Animal Science. 2008;41(1):12–19. [Google Scholar]
- 64.Badawi Y.K., El-Aasar T.A. Effects of breed and air conditioning on some productive and reproductive performance during hot summer season in rabbits. Journal of Animal and Poultry Production. 2018;9(3):163–174. [Google Scholar]
- 65.Valenzuela-Grijalva N.V., Pinelli-Saavedra A., Muhlia Almazan A., Domínguez-Díaz D., González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017;58:1–8. doi: 10.1186/s40781-017-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.