Abstract
Traumatic brain injury (TBI) is the major and leading cause of mortality and an alarming public health challenge. TBI leads to permanent cognitive, motor, sensory and psychotic disabilities. Patients suffering from the various and long-term repercussions of TBI currently have limited therapy choices. The current research work was designed to evaluate the beneficial and neuroprotective role of Troxerutin (Trox) (a natural flavonoid) in a closed brain injury mouse model. The male BALB/c 8-weeks old mice (n꞊150) were randomly distributed in three experimental groups. Control group of mice (n꞊50), TBI group (n꞊50) and Trox pre-treated mice group (Trox + TBI, n꞊50). The mice in Trox + TBI were pre-treated with Trox (150 mg/kg, 7 days) before TBI. The weight-drop mechanism was used to induce mild-moderate injury in mice in both the groups. Our results showed that the mice pre-treated with troxerutin significantly improved neurological severity score, blood glucose level, food intake and brain edema as compared to the mice in the TBI group. Furthermore, compared to the TBI group, the mice treated with troxerutin improved cognitive behavior as evaluated by Open field test, Shallow Water Maze and Y-Maze, decreased brain-infarct volume and blood-brain barrier (BBB) permeability, significantly decreased Reactive Oxygen Species (ROS), improved neuronal morphology and survival in the brain regions such as cortex and hippocampus. In summary, our data provided evidence that pre-treatment with troxerutin improved neurological functions, decreased the BBB permeability, improved behavior, reduced ROS and increased neuronal survival in the weight-drop close head traumatic injury mouse model.
Keywords: Troxerutin, Neuroprotection, Brain injury, Memory
Highlights
-
•
Weight drop traumatic brain injury induced motor, sensory and behavioral abnormalities.
-
•
Troxerutin pre-treatment improved memory and reduced ROS/BBB leakage in TBI mice model.
-
•
Troxerutin pre-treatment reduced brain edema, improved food intake and neurological severity score.
-
•
Troxerutin pre-treatment increased neuronal survival in the brain against TBI induced neurodegeneration.
1. Introduction
Traumatic brain injury (TBI) is one of the leading causes of disabilities and mortalities around the world, posing a huge public health and economic challenge [1,2]. TBI is a series of complicated spectrum mild-moderate disorders having fewer risks of permanent disability to the most severe form of TBI linked with long-lasting brain damage [3]. Worldwide reported cases of mild brain injuries vary from 100 to 749 cases per 100,000 persons/year and are the real burden on the global economy. Particularly, the TBI cases are more common in developing countries [[4], [5], [6]]. The pathological mechanism of TBI is very complex and initially involves primary injury due to mechanical or external collision that leads to structural damage, brain trauma, tissue deformation and tissue loss [7]. The primary injury is followed by secondary and post-traumatic consequences that involve the activation of cellular pathways leading to cognitive decline, neuronal cell death, inflammation, increased blood-brain barrier permeability, oxidative stress and dementia [[8], [9], [10]]. Besides, severe/moderate TBI results in epileptic seizures, peripheral neuropathy, dementia, aggression, impaired depth perception and post-traumatic hypopituitarism [11].
Due to ethical and other limitations in clinical settings, there is a need for pre-clinical research to understand the complex pathology of TBI and is based mainly on rodent models [12]. Different TBI animal models are used in research such as the weight-drop model, controlled cortical impact (CCI), blast injury and fluid percussion using both mice and rats [13,14]. Various TBI animal models have different co-relationships with human brain injuries. The current work is based on using a controlled closed-head injury in mice using a weight-drop model that developed the neurological severity score (NSS) ranging from mild to moderate, behavioral impairment, histological abnormalities, increased blood-brain barrier (BBB) permeability and oxidative stress. The weight-drop model in mice and rats is clinically acceptable and relevant due to the controlled mechanism to induce traumatic axonal injury and provides a suitable platform for testing novel molecules and drugs against TBI [14]. The previous research demonstrated that the weight drop method of inducing TBI in mice is in-expensive and can generate the desired severity of injury i.e. mild, moderate and severe by changing the height and size of dropping weight [15].
TBI is currently treated with a very limited number of therapeutic options and there is a need of screening small molecules, having the properties and characteristics of potential candidate drug molecules against TBI associated devastating neurological conditions. The best way to search for such potential drug candidates is to look for naturally occurring compounds which have neuroprotective capabilities i.e. flavonoids. Troxerutin is a naturally occurring bioflavonoid isolated and derived from Sophora japonica, some vegetables, coffee and green tea [16,17]. Previous research has shown that troxerutin exhibits a number of pharmacological activities i.e. anti-inflammatory, anticancer, anti-apoptotic and anti-oxidant [[18], [19], [20]]. Recent studies have demonstrated that troxerutin improved synaptic plasticity in Alzheimer's β-amyloid mice model [21] and d-galactose induced kidney damage in mice [22]. The current study was designed and aimed to investigate the neuroprotective effect of troxerutin in the weight-drop TBI mice model and its possible mechanism linked with blood glucose level, brain edema, cognitive impairment, oxidative stress, BBB permeability and brain lesions. Such kind of studies can be of significant help in developing novel treatment approaches against TBI.
2. Materials and methods
2.1. Animals
BALB/c male mice (n = 150) having age of (6–8 weeks) were used in this study. The mice were housed in the animal house at Center for Interdisciplinary Research in Basic Sciences (CIRBS) by providing a constant 25 ± 2 °C temperature and 12 h cycle of dark and light. Mice were given two weeks for acclimatization and provided with access to water and food ad libitum. The international guidelines for the use of laboratory animals by National Institute of Health, USA were followed in true spirit. The experimental procedures and protocols were approved in advance from the Ethical Committee with approval number (IIUI-SA-CIRBS/FBAS-EC 2020/1) of International Islamic University, Islamabad.
2.2. Mice groups and drug treatment
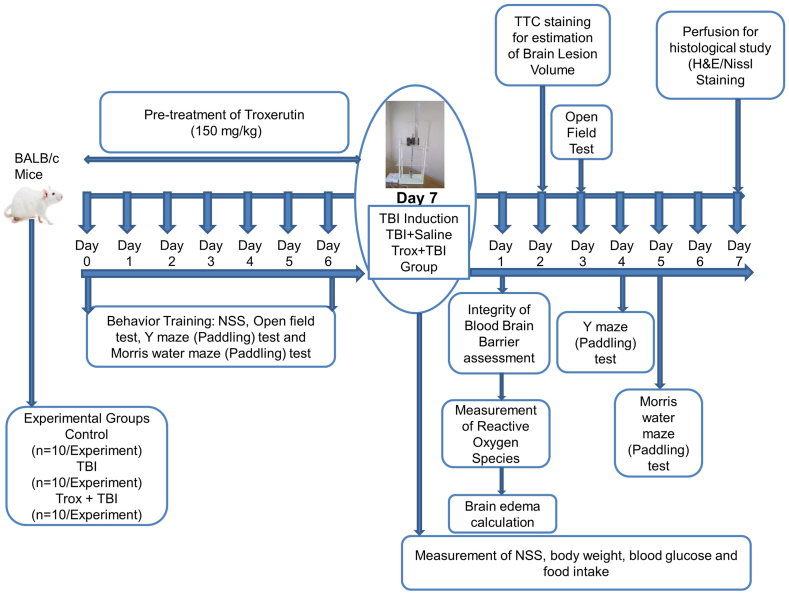
The mice were placed in to three experimental groups and 50 animals in each group with the following symbolic representations group I: control group (saline injection intraperitoneally for 7 days), group II: TBI group (saline injection intraperitoneally for 7 days, followed by TBI induction), group III: Troxerutin (Trox) + TBI (pre-treatment of trox 150 mg/kg dissolved in saline, injected intraperitoneally for 7 days duration, followed by TBI induction). Troxerutin was purchased from (Sigma; Catalog number: Y0000497). The mice treated with troxerutin alone were excluded from the current study on the basis of previous research findings [21]. The detail of study design such as behavior training, drug treatment, staining and the induction of TBI is given in (Fig. 1).
Fig. 1.
Schematic presentation of the experimental design.
2.3. TBI model for brain injury
We adopted the weight drop model used by Khalin [23] with some modifications. The device has two compartments supported by four vertical metal-supported rods. The top and middle of the device is designed using acrylic glass and a rod (metal) with a round tip caged in plastic covering is passing through the center. In our modified weight drop model, the weight for dropping instead of being on the top is modified and shifted to the lower compartment and controlled by magnetic and electric currents. TBI was induced in mice without craniotomy. The mice were anesthetized using the cocktail containing Ketamine/xylazine (Sigma, Aldrich Saint Louis, MO 63103, USA). Single intraperitoneal (i.p) injection of the ketamine/xylazine cocktail at a dose of 60 mg/kg ketamine, 9 mg/kg xylazine was administered. Initially, for the induction of TBI, 400 g weight was used from 2 cm height but due to high mortality, the height was reduced to 1.6 cm and fixed for further experiments. After adjusting the height to 1.6 cm and weight to 400 g, the right hemisphere of the mice's skull was fixed and a 3 mm plastic tip was attached to a metal rod by pushing the button dropped on the skull and TBI was induced. The experiment was performed in an area where fresh air entrance was maximum. After weight drop, the mice were kept under observation for some time to recover from anesthesia and then returned to the designated cages.
2.4. Assessment of neurological severity score (NSS)
The NSS was assessed by using the already established protocol of [24]. The NSS was evaluated at different time intervals ranging from 1 to 168 h (1, 4, 24, 48, 72 and 168 h) according to previous research with slight modifications [25,26]. The NSS measures the motor, balance and other activities such as alertness. Three days of initial training trials were given to all experimental groups. The mice were given different NSS scores grading based on the intensity of trauma and the grading. The NSS was calculated by investigators unaware of the treatment protocols and experimental groups.
2.5. Measurement of body weight, food intake and blood glucose
The body weights of the mice were measured before the start of experiments and then at the time of injury and post-injury till day 7 with these time intervals (0 h, 24 h, 48 h, and 168 h). The food intake was calculated till the end of the experiments using an electronic balance for the purpose. Blood glucose level was measured in all experimental groups. The blood was obtained from the tail vein and the first drop was discarded and blood glucose levels were measured at different time intervals i.e. before the injury and after injury with these time intervals (0 h, after 24 h, 48 h, and 168 h) using glucometer (On Call, Germany).
2.6. Behavioral studies (shallow water maze)
The Shallow Water Maze (SWM) pool design and method of performance were adapted from Deacon [27]. The SWMT time points were adapted from a previous research report with slight changes [28]. We used the water pool having a total of eight exits on the sides of the pool and there is only one true exit available and the rest of seven false exits were closed using designed plugs. The true exit is connected with a plastic pipe to make it easy for the transfer of mice to their home cages. The mice were trained on the SWM pool for 3 days for at least 3 trials in each training session before the start of experimental test trials. The exit time in (seconds) and errors were calculated and recorded on day 5 after TBI.
2.7. Y-maze (Paddling)
The Y-Maze paddling was adapted from the protocol used by Deacon [27]. The Maze was made using transparent acrylic sheets with a dimension of 30 × 8 × 20 cm having three arms. The floor or the base of the maze was laminated with a white sheet to make it easy for mice to escape from the shallow water. The Y-Maze was kept in the behavior room and mice were trained for 3 days with three consecutive trials for 60 s. During the training session, the maze was filled with water (2 cm) deep. The Y-Maze consists of three exits (two false and one true exit). During the training session, initially the animals were placed at the end of one of the selected closed arm that by design is opposite to the middle and frequently in each trial the position of the arm was changed and each trial last 60s. After this, the mice were returned to their cages. The test trials were performed on day 4 of brain injury in different experimental groups in triplicate and each test trial last 60s. The entries in different arms i.e. true exit arm and false exit were recorded. The escape time and error were recorded and calculated by investigators blind of the treatment protocols and animal groups.
2.8. Open field test (OFT)
OFT is the most important task used for anxiety and locomotor behavior in mice. The time point window was adopted from previous studies in a TBI model with slight modifications [28]. The open field square box was designed using transparent acrylic sheets 60 cm × 60 cm in width and 40 cm heightened walls. The central area of the open field was 30 cm with white background and the remaining 15 cm are from each side of the walls of the square was designated as a peripheral area. Before the start of experiments, the mice were provided with a training session for 3 days and each individual mouse was positioned in the center of the box and allowed for 35 min and videos were recorded. For the next mouse, the open field box was cleaned using ethanol (70%) and dried. On day three of the brain injuries, the test was performed in an open field for 30 min for each mouse in the arena. Different parameters such as time spent on a maze, total distance traveled in the open field, total rearing, total movement in the field and total time spent in central area were recorded and then calculated manually according to the previous protocol with some modifications [6].
2.9. Brain lesion volume using 2,3,4-triphenyl tetrazolium chloride staining (TTC)
The mice (n = 10/group) were sacrificed 48 h post TBI i.e. on day 2 post TBI and brains were collected and a coronal section (2 mm) thick was made using the surgical blade position from the central bregma. The post TBI sacrifice time point i.e. 48 h was decides on the basis of previous reports [[29], [30],]. The serial coronal sections were kept in PBS until transferred to the triphenyl tetrazolium chloride (TTC) for staining. The fresh TTC solution (0.25%) was prepared in PBS and the desired sections incubated at (37 °C) in dark for a period of 30 min. The slices, postfixed with 4% paraformaldehyde solution. The slices, placed on a plate having black background were photographed using a digital camera. Furthermore, the photographs were analyzed for brain lesion area using the (SAT, Software) and data was calculated in % according to an already established method [31].
2.10. The brain water content assay
For brain edema/brain water content, the mice (n = 10/group) were sacrificed 24 h post-TBI and brains were taken out and dissected to the desired left hemispheres as well as right according to the previous reported time point [32]. Immediately, wet weight of both hemispheres were measured on an analytical balance. The tissues were further dried in a drying oven at (80 °C) for 72 h (dry weight). The cumulative % brain water content of both hemispheres was calculated by the formula:
Brain water content = [wet weight-dry weight]/wet weight ×100. The data is presented in %.
2.11. Measurement of reactive oxygen species (ROS)
The mice (n = 10/group) were sacrificed 24 h post TBI and brain homogenate was tested for ROS and was measured using 2,7-dichlorofluorescein diacetate (DCF, Sigma Aldrich). The brains were removed in temperature controlled environment and from the anesthetized mice the right hemisphere was extracted/isolated followed by homogenization using the Teflon (grinder). The brain homogenate was centrifuged at 4 °C at 10000 rpm for 10 min to collect the supernatant for measurement of ROS with some modifications in the methods reported by Refs. [33,34]. Using ice-cold Locke's buffer the resultant homogenate diluted 1:20 to get the desired 5 mg tissue/ml concentration. The composition of the Locke's buffer for our experiment was (5.6 mM KCl, 154 mM NaCl, 3.6 mM NaHCO3, 2.0 mM CaCl2, 10 mM d-glucose, and 5 mM HEPES) and finally the pH was adjusted to 7.4. A reaction mixture containing 1 ml Locke's buffer, 0.2 ml of the brain resultant homogenate, 10 ml DCFH-DA (5 mM) and then the reaction mixture incubated at normal room temperature for esterase cleavage of diacetate group. The reaction mixture was additionally incubated for 30 min and the conversion of DCFH-DA to DCF was calculated using a Multimode Microplate reader (Thermo Scientific, Finland) with an excitation/emission at 484 and 530 nm. The blanks without homogenate were subtracted as justification for extra or background fluorescence. The DCF standard curve was used for the quantification of ROS formation.
2.12. Analysis of blood-brain barrier integrity
The permeability and disruption of Blood-Brain Barrier (BBB) was assessed using the Evan's Blue dye (Sigma-Aldrich). Ten mice in each group (n = 10) for BBB integrity/permeability were used. The BBB integrity was assessed by using a time window defined previously in TBI model [35]. The dye (2%, 5 ml/kg) was gently injected in the tail vein, 24 h post-TBI and was allowed 4 h circulation. Mice were then intracardially perfused with a volume of 50 ml of cold heparinized phosphate-buffered saline. The affected side of injury i.e. cerebral cortex dissected, weighed and then homogenized in 1.1 ml of PBS followed by sonication and centrifuged at 14000 rpm for 35 min at 4 °C. The supernatant (0.5 ml) was mixed with an equal volume of trichloroacetic acid (TCA) 50% and kept overnight at 4 °C. Following overnight incubation, the samples were centrifuged at a speed of 14000 rpm for 35 min again; the EB dye concentrations in the brain tissue were measured using the 650 nm wavelengths by (Multimode Microplate reader, Thermo Scientific, Finland). The data and values were quantified by plotting the absorbance of the samples and matched with standard curve of EB in both TCA and PBS. Results are calculated and expressed as given (μg of EB stain/g of brain tissue).
2.13. Histological analysis
The mice were anesthetized and sacrificed on the 7th day post-TBI. Transcardially, mice were perfused with 0.9% saline solution and fixed with 4% paraformaldehyde solution. Carefully, the brains were isolated and kept at 4 °C in 4% paraformaldehyde solution for post-fixation (24–48 h). Later on the brains were transferred to 20% sucrose solution almost (48–72 h). Furthermore, the brains for cutting were embedded in paraffin and solid blocks were shaped. The thin coronal sections of 14 μm were made from cortex and hippocampus regions and were collected on gelatin-coated slides using a manual rotary microtome. The following staining's performed for morphology studies. For Nissl and H&E staining the every fifth coronal section of cortex and hippocampus was stained for the histology and for correct anatomic orientation. The cortex and hippocampus section before staining were confirmed by bright field microcopy according to the stereotaxic atlas.
2.14. Hematoxylin and Eosin (H&E) staining
For H&E staining the tissue sections were deparaffinized using xylenes (twice) for 5 min followed by rehydration using 95%, 70% and 50% ethanol solution 3 min in each. The sections were stained with hematoxylin solution for 8 min. The slides were washed with distilled water and were de-stained using 95% ethanol solution. Further, the tissue sections were counter-stain with eosin for almost 1 min followed by dehydration with 95% ethanol (twice) and with absolute alcohol for 5 min. The samples were cleared with xylene for 5 min and coverslips were gently placed using the few drops of paramount. The slides were photographed with a fluorescence-light microscope (Optika, Italy, B-383FL) at different magnifications. The cellular morphology in terms of shape and size is explained.
2.15. Nissl staining
For the purpose of Nissl staining, the cut 14 μm thin sections were treated with Nissl staining solution. Samples were deparaffinized in xylene with 2 changes for 10 min each following hydration in absolute alcohol for 2 to 10 min, 95% alcohol 3 min and 70% alcohol 3 min. Slides were rinsed in tap water and in the distilled water followed by staining in 0.1% cresyl violet solution for 3 to 10 min. Then rinsed quickly in tap water and then differentiated in ethyl alcohol for 2 to 30 min. All samples were dehydrated with absolute alcohol and then cleared with xylene 2 to 10 min each and then placed a drop of paramount over the tissue on each slide by placing coverslips. The slides were photographed with a fluorescence-light microscope (Optika, Italy, B-383FL) at different magnifications.
2.16. Statistical analysis
The Graph Pad prism 6 software was used for data analysis and Statistics. One-way and two-way ANOVA was performed for data analysis as well Tukey's test. The results are expressed as mean ± standard error of the mean (SEM). The value p < 0.05 was considered significant. * Symbol is used for significant difference Control vs TBI and Trox + TBI. The value of p < 0.0001 is denoted by ****, *** means p < 0.001 and ** means p < 0.01. For comparison of TBI group with Trox + TBI # Symbol is used for significant difference Control vs TBI and Trox + TBI. The value of p < 0.0001 is denoted by ####, ### means p < 0.001 and ## means p < 0.01.
3. Results
3.1. Administration of troxerutin improved motor performance
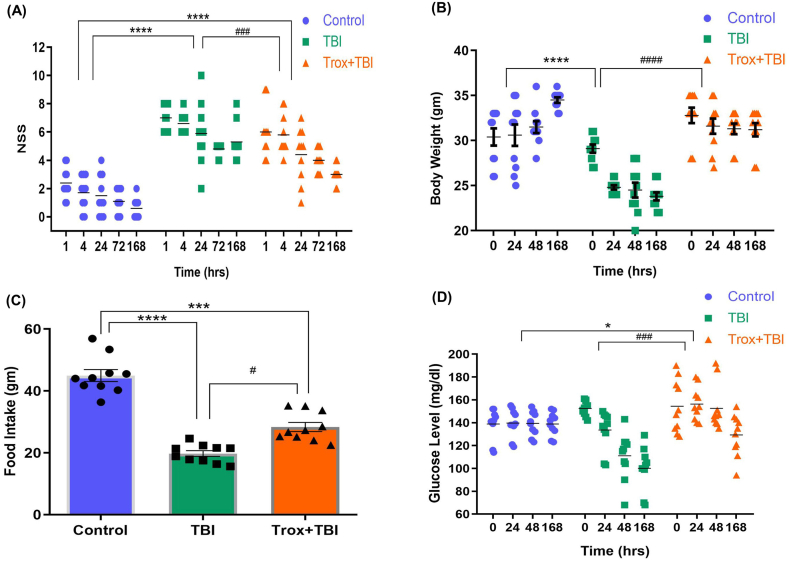
The motor activity of Trox was evaluated on NSS in a mild-moderate model of TBI. The mice pre-treated with Trox significantly improved the motor and behavior performance with time i.e. (1, 4, 24, 72 and 168 h) post-TBI. The statistical data revealed that there were significant changes in the NSS score among these three groups (F = 103.6, df = 2, p < 0.0001). Trox pre-treatment significantly (p < 0.001) decreased the NSS (4.64 ± 0.56) compared with the TBI group (5.92 ± 0.42) (Fig. 2A). Furthermore, the TBI and TBI + Trox groups were compared with the control group and significant changes were observed (Fig. 1A). The body weights were measured before and after TBI i.e. (0, 24, 48 and 168 h) in all experimental groups. The overall significant changes were observed from these statistical interpretation among these three groups in terms of body weight (F = 46.76, df = 2, p < 0.0001). A significant decrease was observed in mean body weight in the mice only with TBI (p < 0.0001) 25.5g ± 1.23 compared to Trox + TBI 31.8g ± 0.89. No significant changes in body weights of the control and Trox + TBI groups were observed. However, a significant difference in mean body weights was observed in the TBI group versus the control (p < 0.0001) (Fig. 2B). The food intake in grams (g) was measured daily for 7 days post-TBI in all experimental groups. The statistical analysis of food intake supported our results that among all these three groups significant changes were observed (F = 36.51, df = 2, p < 0.0001). A significant decrease was observed in food intake in the TBI group 19.67g ± 1.43 in seven days compared with Trox + TBI group 28.33g ± 2.15 (p < 0.05), (Fig. 2C). Furthermore, we measured the blood glucose level in all experimental groups at different time intervals before TBI (0 h) and post-TBI (24, 48 and 168 h). The blood glucose level among these three groups was significantly changed and the data analyzed by two-way ANOVA (F = 8.978, df = 2, p = 0.0005). Overall, there was a decrease in blood glucose level in the TBI group compared with the control and Trox + TBI group. The average blood glucose value for the TBI group and Trox + TBI group was 124.9 mg/dl ± 11.2 and 147.8 mg/dl ± 6.27 respectively, which showed that Trox significantly improved blood glucose level in mice model of TBI with the passage of time. Similarly, no significant difference was observed in control group in comparison with Trox + TBI group (Fig. 2D).
Fig. 2.
Administration of troxerutin improved motor performance after TBI. (A) Troxerutin pre-treatment significantly improved the motor performance compared with TBI group. (B) Representative graph of measurement of body weight prior to injury and post TBI. (C) The average food intake and consumption in 7 days in different groups. (D) Blood glucose level in mice prior to injury and post TBI in different groups at different time intervals. Data is presented as mean ± SEM (n = 10 animals/group); *P < 0.05, ***P < 0.001 ****P < 0.0001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by #P < 0.05, ###P < 0.001, ####P < 0.0001.
3.2. Troxerutin improved behavioral deficit after TBI
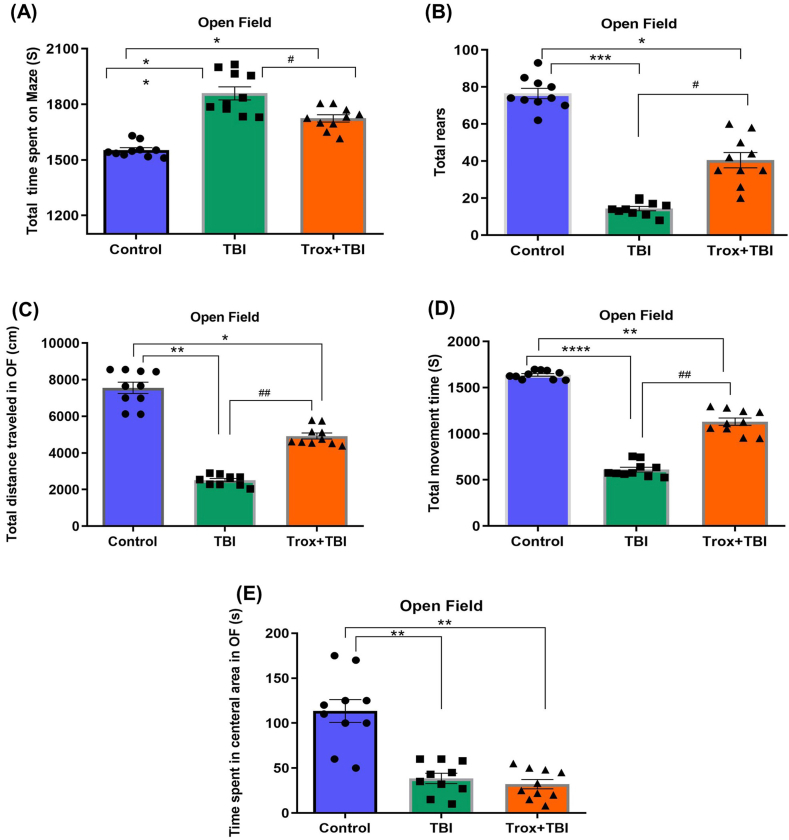
To assess the behavioral deficit induced by TBI, the open-field (OF) test was performed. The anxiety-like behavior was determined by recording the total time spent on the maze (peripheral area), total rears, total distance traveled in OF, total movement time and time spent in the central area of the OF. The mice alertness was measured by using the total time spent on the maze in seconds (S) in each group. The total time actually spent in (peripheral area) by the three groups of mice were statistically analyzed (F = 19.50, df = 2, p = 0.0002). After TBI on day 3, the mice showed less alertness and reduced locomotion as we observed from the total time spent in (peripheral area) of the OF in the TBI group (1860 ± 49.7 S, **p < 0.01) compared with the control group (1553 ± 17.7 S). Similarly, the Trox treatment significantly increased the alertness in terms of movement and less time spent in the peripheral area of the maze Trox + TBI 1724 ± 29.26, *p < 0.05) compared to the TBI group (Fig. 3A). Next, we observed the rearing activity of mice in the OF in each experimental group for 35 min (F = 38.89, df = 2, p = 0.0025). The total number of vertical rearing activities of mice was calculated (control vs TBI and Trox + TBI vs TBI). The number of rearing events were significantly less in TBI group (14 ± 2.2, ***p < 0.001) vs control (77 ± 4.7) and Trox + TBI (40.60 ± 5.0, *p < 0.05). The Trox significantly improved the rearing events in mice with TBI. The mice in the TBI group reared less than mice in the control group and Trox + TBI group (Fig. 3B). A one-way ANOVA test was performed for the distance traveled by the mice in each group in the (OF) maze and the data is expressed mean total distance traveled in cm (F = 46.02, df = 2, p = 0.0008). The mice in the TBI group showed a reduction in total distance traveled in a maze (2513 ± 167.0 cm) compared with a control group (7554 ± 454.4 cm). Furthermore, the mice treated with Trox increased the total traveled distance compared with Trox + TBI group (4924 ± 246.2 cm). The data is presented in (Fig. 3C). The mobility time for each mice group was calculated after 3 days post TBI for 35 min (F = 111.2, df = 2, p < 0.0001). The number and time of movements (mobility) were significantly reduced in the TBI group (612 ± 37.60 S) compared with a control group (1638 ± 21.42 S). Similarly, the troxerutin treatment increased the mobility time (1131 ± 60.89 S) compared with the TBI group (Fig. 3D). The time spent in the central area of the OF was calculated for each group of mice (F = 19.50, df = 2, p = 0.0002). The central area time was reduced (Fig. 3E) in case of TBI group (240 ± 49.70 S) compared to control (547 ± 17.72 S) and Trox + TBI group (376 ± 29.26 S).
Fig. 3.
Effects of troxerutin pre-treatment on exploratory and motor behavior in mice in an open field post-TBI. (A) Represents the total time spent by the animals on maze in seconds (S). (B) Represent total number of rears in the open field (C) Total distance traveled by mice in each group measured in centimeters (cm). (D) The total active time by mice in each group in the open field calculated in seconds (S). (E) Total time spent in central area in seconds (S). The data is statistically presented as mean ± SEM (n = 10 animals/group); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by #P < 0.05, ##P < 0.01.
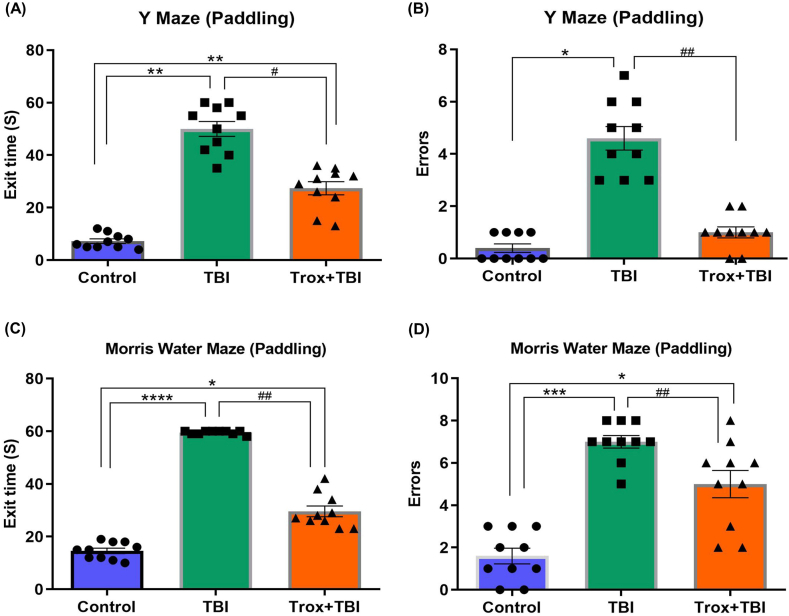
3.3. Troxerutin treatment enhanced learning and spatial memory deficits induced by TBI
The learning and memory behavior in the experimental mice was evaluated using the Y-Maze (Paddling) and Shallow Water Maze test after injury. The Y-maze paradigm was used to assess spatial memory. The mice treated with Trox significantly improved the memory in terms of exit time for 60 s (S) on day 4th post-TBI (F = 32.69, df = 2, p = 0.0009) In Y-maze (Paddling) the average exit time for the TBI group is (50 ± 6.32 S) compared with control group and Trox + TBI group exit time (7.2 ± 1.39 S) and (27.40 ± 3.80 S) respectively. Our results showed that Trox treatment decreases the exit time significantly (Fig. 4A). Furthermore, the number of errors by mice in each group were recorded having statistical results (F = 32.25, df = 2, p = 0.0015) and calculated by observing the entry in the false exit (60 Seconds). The mice in the TBI group having more errors in the given time compared with the control and Trox + TBI group. In the case of TBI group the number of errors were (4.6 ± 0.50) compared with control group (0.40 ± 0.24) and Trox + TBI (1.0 ± 0.31) group (Fig. 4B). Next, we used the shallow water maze test for evaluation of memory deficit on day 5th post-TBI (F = 171.5, df = 2, p < 0.0001). Our result indicated that the mice in the TBI group have a longer exit time (escape latency) to find out the true exit platform in the maze. The exit time recorded for the TBI group was (60.0 ± 0.0 S) compared with the control group (14.60 ± 0.92 S). Furthermore, treatment with Trox shortened the exit time (escape latency) in Trox + TBI group (29.60 ± 3.01 S) compared with the TBI group (Fig. 4C) which showed the improved memory in terms of finding the true exit. The number of errors were counted in each treated group in the shallow water maze test (F = 65.55, df = 2, p = 0.0006). TBI group mice having more number of errors that is moving towards the false exit in the maze in 1 min time period (8.0 ± 0.0 errors) as compared to the control (1.8 ± 0.37 errors). Similarly, the mice treated with Trox significantly decreased the number of errors as shown by the Trox + TBI group (29.60 ± 3.01 errors) compared with the TBI group (Fig. 4D).
Fig. 4.
Effect of troxerutin on memory and learning via using the Y maze and Morris water maze (peddling) tests. The panel (A) represents the total exit time in seconds (S) utilized by mice in Y maze (peddling) and panel (B) represents the total number of errors by mice in each group in Y maze. The panel (C) shows the exit time in seconds in the Morris water maze peddling and (D) shows the total number of errors by mice in each group. The data is presented as mean ± SEM (n = 10 animals/group); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by #P < 0.05, ##P < 0.01.
3.4. Troxerutin treatment reduced the brain injury, brain edema and ROS after TBI
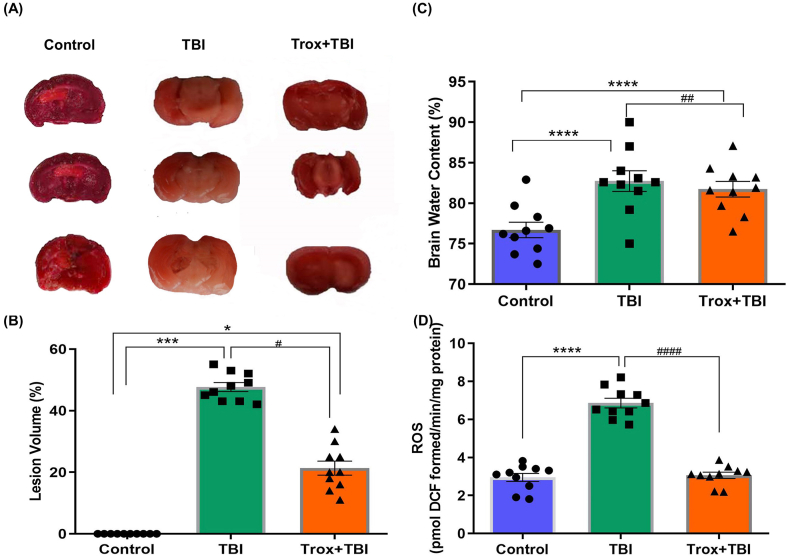
The brain injury after 24 h of TBI was evaluated using the TTC staining in coronal sections of the brain. The TTC stained sections were photographed and the brain lesion volume was calculated to understand the severity of the injury and the statistical data revealed that a significant difference exist among groups (F = 31.35, df = 2, p = 0.0007). The lesion volume was reduced in case of mice treated with troxerutin (21.33 ± 6.38%) compared with TBI group (47.67 ± 3.71%). The mice in the control group showed no lesion when compared with the TBI group (Fig. 5A and B). There was a significant difference (p < 0.001, p < 0.05) observed when the control group compared with TBI and Trox + TBI groups respectively (Fig. 5A and B). Similarly, a significant (p < 0.05) difference was observed when TBI group was compared with the Trox + TBI treated group.
Fig. 5.
Troxerutin decrease lesion volume, brain water content and ROS levels in the brain of mice with mild-moderate TBI. (A) Comparison of TTC stains coronal sections (2 mm thick) in photographs in different experimental groups on day 2 after TBI and (B) The % lesion volume in the brain. (C) Represents brain edema (D) Represents the comparison of ROS level. The data is presented as mean ± SEM (n = 10 animals/group); *P < 0.05, ***P < 0.001, ****P < 0.0001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by #P < 0.05, ##P < 0.01, ####P < 0.0001.
Next, we determined that troxerutin treatment reduced the brain water content which is a sensitive measure for brain edema (F = 504.1, df = 2, p < 0.001). There was a significant increase in brain water content at 24 h post-TBI in TBI group (82.7 ± 0.13%) compared with the control group (76.7 ± 0.15%, p < 0.0001) in the left and right hemisphere of the brain injury. Similarly the troxerutin pre-treatment significantly reduced brain water content in TBI model of mice (Trox + TBI group 81.6 ± 0.14% vs TBI group 82.7 ± 0.13%, p < 0.01). The brain water contents in % are presented in (Fig. 5C). Next, we carried out the ROS assay which is a hallmark of neurodegeneration particularly in the case of traumatic brain injuries and leading cause of neuronal cell death. The effect of troxerutin treatment on TBI-induced oxidative stress was assessed in mice brain particularly the right hemisphere at the site of injury (F = 237.1, df = 2, p < 0.0001). Our results showed that TBI significantly enhanced the ROS level in the brain compared to control saline-treated mice, while the troxerutin pre-treatment significantly reduced the ROS level (Fig. 5D).
3.5. Effect of troxerutin treatment on BBB permeability post-TBI
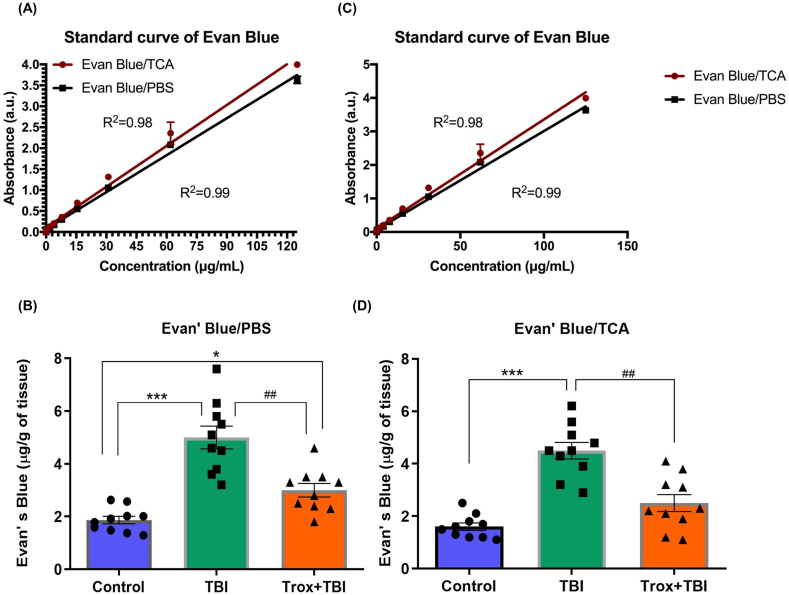
The BBB leakage is considered as an indicator of brain injury in both patients and animal models of TBI. The characterization of BBB damage and permeability was assessed by Evan's Blue dye (EB) and statistical analysis for all three groups indicate that troxerutin treatment reduced BBB permeability (F = 45.01, df = 2, p = 0.0002). The standard curves in two different solutions PBS and TCA were made for comparison and data analysis (Fig. 6A & B). The level of EB extravasation was significantly higher in the TBI group (5.00 μg/g of tissue, p < 0.001) compared with the control group (1.86 μg/g of tissue). Similarly, the troxerutin treated group (Trox + TBI) reduced the EB leakage (3.00 μg/g of tissue) compared to that of the TBI group. There was no significant difference observed after using two different solutions (Fig. 6C & D).
Fig. 6.
Troxerutin inhibits BBB disruption induced by TBI. (A) and (C) panels show the standard curves for Evans blue dye in trichloroacetic acid (TCA) and phosphate buffer saline (PBS) with different concentration of Evans blue dye in micrograms (μg). The bar graphs B & D show the quantification of Evans blue dye in the brain either in TCA or PBS. The data is presented as mean ± SEM (n = 10 animals/group); *P < 0.05, ***P < 0.001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by ##P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Troxerutin improved neurodegeneration induced by TBI
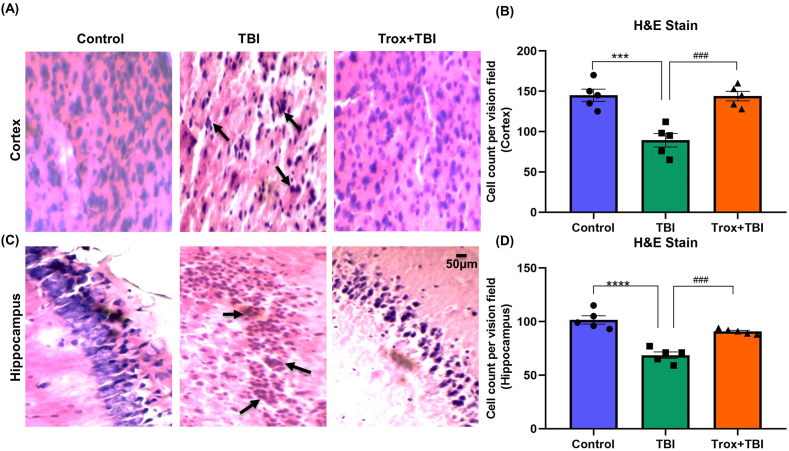
Next, we validated our results with H&E staining in the cortex and hippocampus of the mice brain in all experimental groups. The H&E stained sections of the cortex and hippocampus region in the saline or control group showed regular nuclei, uniform cellular morphology while stained sections of the TBI group showed significant morphological changes i.e. abnormal shrunken cytoplasm and irregular morphology. The H&E sections stained in (Trox + TBI group) improved cellular morphology in the cortex and CA1 region of the hippocampus compared with the TBI group (Fig. 7A and C). The data, both for cortex and hippocampus in all treated groups was statistically analyzed and quantified as cells count per vision field. The quantified number of cells in both regions of the brain among the groups showed a significant difference as shown in case of cortex (F = 18.91, df = 2, p = 0.0002) and hippocampus (F = 33.10, df = 2, p < 0.0001). The number of cells shrunken and reduced in the cortex region (Fig. 7B) in TBI group (89.20 ± 8.339) versus control i.e. (145.0 ± 7.583, ***p < 0.0005). However, the troxerutin treated group (Trox + TBI) had a statistically higher number of cells count (144.0 ± 5.891, ###p < 0.0005). In case of hippocampus the number of cell count is (101.6 ± 3.894) in control group and (68.60 ± 3.059, ****p < 0.0001) in TBI group (Fig. 7D). While the troxerutin treated group having (90.80 ± 1.068, ###p < 0.0005) compared with TBI.
Fig. 7.
Histology of stained H&E cortex and hippocampus sections of the mouse brain in different groups. The arrow indicates the abnormal cellular morphology in the cortex and hippocampus of TBI mouse brain. Panel-A represents the cortex morphology and (B) represents the quantification of cells in cortex (C) Hippocampus morphology and (D) quantification of cells in hippocampus. The data is represented as mean ± SEM (n = 5 animals/group); ***P < 0.001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by ###P < 0.0001.
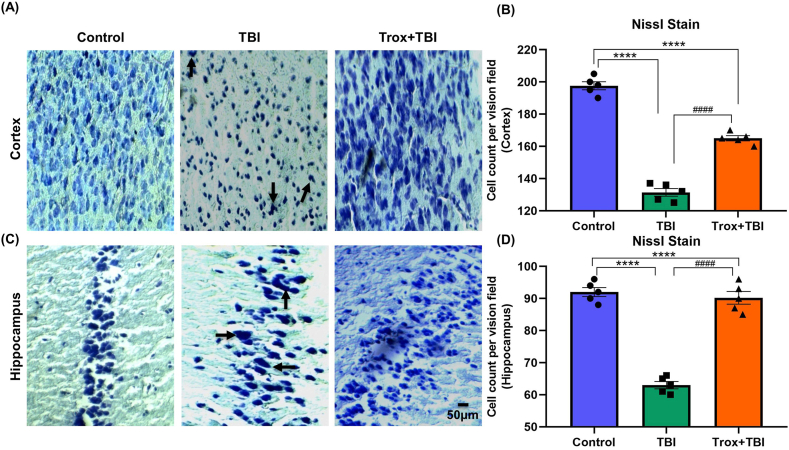
Furthermore, neuronal cell death and morphological changes induced by TBI were confirmed by cresyl violet staining. In the control group, the neuronal morphology was regular, intact, and without damage as well as a normal number of cells was in both regions of the brain with statistical significance among the groups in case of cortex (F = 226.4, df = 2, p < 0.0001) and hippocampus (F = 109.4, df = 2, p < 0.0001). Furthermore, in the TBI group, the number of neurons were reduced in both cortex and hippocampus regions. In the TBI group, the number of neuronal cells were reduced significantly in the cortex region (131.4 ± 2.3) compared with the control group (197.6 ± 2.50, ****p < 0.0001). Similarly troxerutin treatment (Trox + TBI group) showed a significant increase in cell count compared to that of the TBI group (131.4 ± 2.3 TBI vs Trox + TBI 165 ± 1.61, ####p < 0.0001). Similarly, in the CA1 region of the hippocampus TBI group showed a significant reduction in cell count compared with the control group (TBI 63.0 ± 1.14 vs Control 92.0 ± 1.41, ****p < 0.0001). However, treatment with troxerutin significantly increased neuronal cell count compared with the TBI group (TBI 63.0 ± 1.14 vs Trox + TBI 90.2 ± 1.98, ####p < 0.0001) in the CA1 region of the hippocampus. All the data of the cortex and hippocampus are presented in (Fig. 8A–D).
Fig. 8.
Troxerutin pre-treatment effects on TBI-induced neurodegeneration. The representative stained sections of cortex slices of cresyl violet-stained show the dead and damaged neurons with troxerutin treatment and without i.e. TBI only. (A) Cortical section and (C) Hippocampus sections. (B) Shows the histogram of Nissl stain in cortex and (D) shows histogram of Nissl stain in hippocampus and number of cells counted. The data is presented as mean ± SEM (n = 5 animals/group); ****P < 0.0001 control vs TBI and Trox + TBI while TBI vs Trox + TBI denoted by ####P < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The presently available treatment for TBI only temporarily rescues the brain damage and other neurological symptoms such as behavioral abnormalities [36]. The management available for TBI in the current scenario is the intracranial pressure control, use of hypertonic solutions, surgical and seizure interventions [37]. In the current study, we found that troxerutin supplementation in the TBI mouse model rescues the different pathological and deleterious effects. We found that treatment with troxerutin seven days before injury significantly improved the NSS score, body weight, food intake, glucose level, behavioral outcome, brain edema and neurodegeneration in a mouse model of mild-moderate closed head injury. Similarly, troxerutin pre-treatment also reduced the brain lesion volume, ROS and rescue the BBB leakage. The selected dose of troxerutin was derived from previous studies [20,38] and the pre-treatment paradigm is a new concept.
The motor and balance deficits following a single closed head injury weight drop model were significantly higher as depicted by the NSS score at different time intervals (1,4,24,72 and 168 h). The motor dysfunction gradually improved and the mice recovered partially with time. From the NSS score, it was clear that our experimental TBI apparatus using 400 g weight from a height of 1.6 cm induced mild-moderate injury. The data of our NSS score is consistent with other researchers performed with slight modifications in weight and height of the apparatus [23,24]. Furthermore, the troxerutin pre-treatment improved the NSS score and confers neuroprotection in the TBI animal model similar with the lipopolysaccharide and Parkinson animal models where troxerutin have shown its potential effects via reducing the oxidative stress and neuroinflammation [20,39]. The troxerutin pre-treatment in TBI group also showed improved metabolic aspects such as food intake, body weight and glucose level. Brain injury is linked with altered metabolism in different TBI animal models as various studies have elaborated these changes. The average body weight reduced significantly in the TBI group 24 h post-injury and a similar pattern was observed till day seven of the injury. The reason for weight loss in the TBI group might be due to the moderate level of TBI which is further linked with food intake. The control group and troxerutin pre-treated mice sustained the body weight for the observed period. The mechanism of reduced body weight in an animal model is a bio-indicator of animal health and is linked with motor dysfunction, loss of consciousness, seizures in the acute phase of TBI [[40], [41], [42]]. Furthermore, there is a need to observe the body weight for a longer period post-TBI as that is one of the limitations of our research we observed just for the acute phase. A close association of lower body weights and average food intake was observed in our TBI model. In the TBI group, there was a significant decrease in average food intake from day 1 post-injury till day 7 while troxerutin pre-treatment significantly improves the average food intake. Our studies also indicated that TBI induced hypoglycemia and is linked with the post-TBI energy crisis that may lead to cognitive impairment. A similar pattern of low blood glucose levels induced by TBI and treatment with glucose overcome the metabolic crisis has been reported in TBI in human and animal models [43,44].
Further, in this study we tried to link metabolic anomalies with anxiety and memory impairment in the weight drop TBI model. Therefore, we used common behavioral test such as open field, Y-Maze and MWM to evaluate the cognitive abnormalities in our mice model. The results of our behavior studies showed that pre-treatment with troxerutin significantly improved the total rearing, total movement, and total distance traveled in the open field compared with the TBI group. The animals in TBI group spent more time in the peripheral area compared to troxerutin treated group. The elevated anxiety level or behaviors were more prominent during the open field task in TBI group compared to the Trox + TBI group. The same pattern of improved behavior was observed for MWM and Y-maze results. These results are consistent with previous findings where troxerutin has shown neuroprotective capabilities in some other models such as cerebral ischemia, behavior-induced chronic stress and high-fat diet disturbed spatial memory [[45], [46], [47]]. The memory impairment is further linked with brain edema and lesion volume in different brain regions in our TBI model. The brain water contents significantly increased post-TBI and troxerutin treatment attenuated these changes. Similarly, our ROS results are also consistent with previous findings that troxerutin treatment reduced ROS levels as shown in the type 1 diabetes animal model [48]. The neuroprotective mechanism of troxerutin is based on activation of adenosine monophosphate-activated protein kinase (AMPK) and silent mating type information regulation 2 homolog 1 (SIRT1) that further inhibit the inflammatory cytokines. The second known mechanism is the activation of phosphatidylinositol 3-kinase (PI3K) that inhibits reactive oxygen species (ROS), MDA level and apoptosis [49]. The mice post-TBI showed disruption of BBB as evident from Evans blue dye presence in brain homogenate. These results are consistent with previous research reports that highlighted the disruption of BBB in the weight drop model of diffuse injury [50,51]. Our results showed that troxerutin treatment ameliorates BBB disruption after TBI and that plays a major role in secondary brain injury and pathology of TBI [46]. Finally, the brain histology results indicated that TBI induced abnormal neuronal morphology and irregular nuclei and cell death in the cortex and hippocampus region of the brain compared with the control group. The abnormal morphology and cellular damage in the CA1 region of the hippocampus is involved in such kind of memory impairments and as depicted by our behavior results [52]. The troxerutin-treated groups showed normal cellular morphology compared with the TBI group. The morphological features of our data supported our hypothesis that troxerutin is anti-inflammatory, neuroprotective, antioxidant and strong anti-apoptotic in nature. In several other models of neurological diseases, troxerutin is declared neuroprotective but so far it has not been studied in the TBI model of animals [53,54].
In summary, our results demonstrate that closed head injury induced metabolic stress in terms of body weight, glucose level, and food intake. The troxerutin overcomes these metabolic changes. Further, TBI specifically induced behavior deficits in mice, brain edema, increased ROS level in the brain, increased lesion volume, BBB interruption and morphological disruption in the brain. The neuroprotective activity of troxerutin may be due to its strong antioxidant nature. Hence, troxerutin can be a new target molecule for the structural and functional disabilities associated with TBI. Although, pretreatment has limited translational significance but the dietary supplementation with troxerutin enhances neurological function which might protect against subsequent injury. Furthermore, it is suggested that investigation is needed in the chronic TBI model to understand the modulatory and neuroprotective properties of troxerutin and extend this therapeutic potential in human TBI.
Author contribution statement
Ashfaq Ahmed Khan Malik: Performed the experiments; Wrote the paper.
Waqas Ahmad: Performed the experiments.
Farhan Younas: Conceived and designed the experiments.
Haroon Badshah; Shafiq Ur Rehman: Analyzed and interpreted the data.
Shatha Alharazay; Muhammad Imran Naseer; Osama Muthaffar; Rehmatullah Achakzai: Contributed reagents, materials, analysis tools or data.
Ikram Ullah: Conceived and designed the experiments; Wrote the paper.
Funding
King Abdulaziz University, DSR, Jeddah, Saudi Arabia; Institutional Fund Projects, Grant/ Award Number: IFPIP-177-117-1443
Institutional review board statement
The international guidelines for the use of laboratory animals by National Institute of Health, USA were followed in true spirit. The experimental procedures and protocols were approved in advance from the Ethical Committee with approval number (IIUI-SA-CIRBS/FBAS-EC 2020/1) of International Islamic University, Islamabad.
Informed consent statement
Not Applicable.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Sample availability
Not Applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are thankful for DSR, King Abdulaziz University for their support.The research work was funded by Institutional Fund Projects under grant no (IFPIP-177-117-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Contributor Information
Ashfaq Ahmed Khan Malik, Email: ashfaqkhanqau@gmail.com.
Waqas Ahmad, Email: waqas.phdns31@iiu.edu.pk.
Farhan Younas, Email: farhan.younas@iiu.edu.pk.
Haroon Badshah, Email: hbadshah@awkum.edu.pk.
Shatha Alharazy, Email: smalharazy@kau.edu.sa.
Shafiq Ur Rehman, Email: shafiq.qau.edu@gmail.com.
Muhammad Imran Naseer, Email: minaseer@kau.edu.sa.
Osama Yousef Muthaffar, Email: oymuthaffar@kau.edu.sa.
Rehmatullah Achakzai, Email: drn08@hotmail.com.
Ikram Ullah, Email: ikram.ullah@iiu.edu.pk.
References
- 1.Maas A.I.R., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 2.Roozenbeek B., Maas A.I.R., Menon D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Büki A., Chesnut R.M., Citerio G., Coburn M., Cooper D.J., Crowder A.T., Czeiter E., Czosnyka M., Diaz-Arrastia R., Dreier J.P., Duhaime A.-C., Ercole A., van Essen T.A., Feigin V.L., Gao G., Giacino J., Gonzalez-Lara L.E., Gruen R.L., Gupta D., Hartings J.A., Hill S., Jiang J.-Y., Ketharanathan N., Kompanje E.J.O., Lanyon L., Laureys S., Lecky F., Levin H., Lingsma H.F., Maegele M., Majdan M., Manley G., Marsteller J., Mascia L., McFadyen C., Mondello S., Newcombe V., Palotie A., Parizel P.M., Peul W., Piercy J., Polinder S., Puybasset L., Rasmussen T.E., Rossaint R., Smielewski P., Söderberg J., Stanworth S.J., Stein M.B., von Steinbüchel N., Stewart W., Steyerberg E.W., Stocchetti N., Synnot A., Te Ao B., Tenovuo O., Theadom A., Tibboel D., Videtta W., Wang K.K.W., Williams W.H., Wilson L., Yaffe K. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy J.D., Carroll L.J., Peloso P.M., Borg J., von Holst H., Holm L., Kraus J., Coronado V.G. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 5.Feigin V.L., Theadom A., Barker-Collo S., Starkey N.J., McPherson K., Kahan M., Dowell A., Brown P., Parag V., Kydd R., Jones K., Jones A., Ameratunga S. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013;12:53–64. doi: 10.1016/S1474-4422(12)70262-4. [DOI] [PubMed] [Google Scholar]
- 6.Peeters W., van den Brande R., Polinder S., Brazinova A., Steyerberg E.W., Lingsma H.F., Maas A.I.R. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015;157:1683–1696. doi: 10.1007/s00701-015-2512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindal A., Mahesh R., Bhatt S., Pandey D. Molecular modifications by regulating cAMP signaling and oxidant-antioxidant defence mechanisms, produce antidepressant-like effect: a possible mechanism of etazolate aftermaths of impact accelerated traumatic brain injury in rat model. Neurochem. Int. 2017;111:3–11. doi: 10.1016/j.neuint.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Ponsford J., Draper K., Schönberger M. Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J. Int. Neuropsychol. Soc. 2008;14:233–242. doi: 10.1017/S1355617708080272. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., He W., Wang Z., Zhang D., Sun J., Zhou J., Li Y., Su X. A comparison of molecular biology mechanism of shewanella putrefaciens between fresh and terrestrial sewage wastewater. Front. Bioeng. Biotechnol. 2016;4:86. doi: 10.3389/fbioe.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montenigro P.H., Alosco M.L., Martin B.M., Daneshvar D.H., Mez J., Chaisson C.E., Nowinski C.J., Au R., McKee A.C., Cantu R.C., McClean M.D., Stern R.A., Tripodis Y. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma. 2017;34:328–340. doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begum G., Yan H.Q., Li L., Singh A., Dixon C.E., Sun D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J. Neurosci. 2014;34:3743–3755. doi: 10.1523/JNEUROSCI.2872-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebold L., Obenaus A., Goyal R. Criteria to define mild, moderate, and severe traumatic brain injury in the mouse controlled cortical impact model. Exp. Neurol. 2018;310:48–57. doi: 10.1016/j.expneurol.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Leung L.Y., VandeVord P.J., Dal Cengio A.L., Bir C., Yang K.H., King A.I. Blast related neurotrauma: a review of cellular injury. Mol. Cell. BioMech. 2008;5:155–168. [PubMed] [Google Scholar]
- 14.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 16.Kessler M., Ubeaud G., Walter T., Sturm F., Jung L. Free radical scavenging and skin penetration of troxerutin and vitamin derivatives. J. Dermatol. Treat. 2002;13:133–141. doi: 10.1080/09546630260199505. [DOI] [PubMed] [Google Scholar]
- 17.Masood M.I., Schäfer K.H., Naseem M., Weyland M., Meiser P. Troxerutin flavonoid has neuroprotective properties and increases neurite outgrowth and migration of neural stem cells from the subventricular zone. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z.-F., Zhang Y.-Q., Fan S.-H., Zhuang J., Zheng Y.-L., Lu J., Wu D.-M., Shan Q., Hu B. Troxerutin protects against 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD+-depletion. J. Hazard Mater. 2015;283:98–109. doi: 10.1016/j.jhazmat.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Panat N.A., Singh B.G., Maurya D.K., Sandur S.K., Ghaskadbi S.S. Troxerutin, a natural flavonoid binds to DNA minor groove and enhances cancer cell killing in response to radiation. Chem. Biol. Interact. 2016;251:34–44. doi: 10.1016/j.cbi.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Jamali-Raeufy N., Kardgar S., Baluchnejadmojarad T., Roghani M., Goudarzi M. Troxerutin exerts neuroprotection against lipopolysaccharide (LPS) induced oxidative stress and neuroinflammation through targeting SIRT1/SIRT3 signaling pathway. Metab. Brain Dis. 2019;34:1505–1513. doi: 10.1007/s11011-019-00454-9. [DOI] [PubMed] [Google Scholar]
- 21.Babri S., Mohaddes G., Feizi I., Mohammadnia A., Niapour A., Alihemmati A., Amani M. Effect of troxerutin on synaptic plasticity of hippocampal dentate gyrus neurons in a β-amyloid model of Alzheimer׳s disease: an electrophysiological study. Eur. J. Pharmacol. 2014;732:19–25. doi: 10.1016/j.ejphar.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Yang L., Zhan L., Han H., Gao H., Guo Z., Qin C., Yang R., Liu X., Zhou D. The low-salt stimulon in Vibrio parahaemolyticus. Int. J. Food Microbiol. 2010;137:49–54. doi: 10.1016/j.ijfoodmicro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Khalin I., Jamari N.L.A., Razak N.B.A., Hasain Z.B., Nor M.A.B.M., Zainudin M.H.B.A., Omar A.B., Alyautdin R. A mouse model of weight-drop closed head injury: emphasis on cognitive and neurological deficiency. Neural Regen. Res. 2016;11:630–635. doi: 10.4103/1673-5374.180749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flierl M.A., Stahel P.F., Beauchamp K.M., Morgan S.J., Smith W.R., Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Gao W., Cheng S., Yin D., Li F., Wu Y., Sun D., Zhou S., Wang D., Zhang Y., Jiang R., Zhang J. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J. Neuroinflammation. 2017;14:167. doi: 10.1186/s12974-017-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong J., Gao Y., Li X., Li K., Li Q., Shen J., Han Z., Zhang J. Losartan treatment could improve the outcome of TBI mice. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00992. https://www.frontiersin.org/articles/10.3389/fneur.2020.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deacon R.M.J. Shallow water (paddling) variants of water maze tests in mice. J. Vis. Exp. 2013 doi: 10.3791/2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker L.B., Fu A.H., McCabe J.T. Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma. 2016;33:880–894. doi: 10.1089/neu.2015.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perri B.R., Smith D.H., Murai H., Sinson G., Saatman K.E., Raghupathi R., Bartus R.T., McIntosh T.K. Metabolic quantification of lesion volume following experimental traumatic brain injury in the rat. J. Neurotrauma. 1997;14:15–22. doi: 10.1089/neu.1997.14.15. [DOI] [PubMed] [Google Scholar]
- 30.Frank D., Gruenbaum B.F., Shelef I., Zvenigorodsky V., Benjamin Y., Shapoval O., Gal R., Zlotnik A., Melamed I., Boyko M. A novel histological technique to assess severity of traumatic brain injury in rodents: comparisons to neuroimaging and neurological outcomes. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.733115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi X.-F., Ai H., Lu W., Cai F. SAT: free software for the semi-automated analysis of rodent brain sections with 2,3,5-triphenyltetrazolium chloride staining. Front. Neurosci. 2019;13:102. doi: 10.3389/fnins.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao S., Zhu P., Yu X., Chen J., Li J., Yan F., Wang L., Yu J., Chen G. Hydrogen sulfide attenuates brain edema in early brain injury after subarachnoid hemorrhage in rats: possible involvement of MMP-9 induced blood-brain barrier disruption and AQP4 expression. Neurosci. Lett. 2016;621:88–97. doi: 10.1016/j.neulet.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Shinomol G.K. Muralidhara, Differential induction of oxidative impairments in brain regions of male mice following subchronic consumption of Khesari dhal (Lathyrus sativus) and detoxified Khesari dhal. Neurotoxicology. 2007;28:798–806. doi: 10.1016/j.neuro.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Rehman S.U., Ikram M., Ullah N., Alam S.I., Park H.Y., Badshah H., Choe K., Kim M.O. Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells. 2019;8 doi: 10.3390/cells8070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehman S.U., Ahmad A., Yoon G.-H., Khan M., Abid M.N., Kim M.O. Inhibition of c-jun N-terminal kinase protects against brain damage and improves learning and memory after traumatic brain injury in adult mice. Cerebr. Cortex. 2018;28:2854–2872. doi: 10.1093/cercor/bhx164. [DOI] [PubMed] [Google Scholar]
- 36.Arciniegas D.B., Anderson C.A., Topkoff J., McAllister T.W. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatric Dis. Treat. 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers N.J. Animal communication: when I'm calling you, will you answer too? Curr. Biol. 2017;27:R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 38.Lu J., Wu D., Zheng Z., Zheng Y., Hu B., Zhang Z. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain. 2011;134:783–797. doi: 10.1093/brain/awq376. [DOI] [PubMed] [Google Scholar]
- 39.Baluchnejadmojarad T., Jamali-Raeufy N., Zabihnejad S., Rabiee N., Roghani M. Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson's disease: possible involvement of PI3K/ERβ signaling. Eur. J. Pharmacol. 2017;801:72–78. doi: 10.1016/j.ejphar.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Andrade P., Banuelos-Cabrera I., Lapinlampi N., Paananen T., Ciszek R., Ndode-Ekane X.E., Pitkänen A. Acute non-convulsive status epilepticus after experimental traumatic brain injury in rats. J. Neurotrauma. 2019;36:1890–1907. doi: 10.1089/neu.2018.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitkänen A., Ekolle Ndode-Ekane X., Lapinlampi N., Puhakka N. Epilepsy biomarkers - toward etiology and pathology specificity. Neurobiol. Dis. 2019;123:42–58. doi: 10.1016/j.nbd.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapinlampi N., Andrade P., Paananen T., Hämäläinen E., Ekolle Ndode-Ekane X., Puhakka N., Pitkänen A. Postinjury weight rather than cognitive or behavioral impairment predicts development of posttraumatic epilepsy after lateral fluid-percussion injury in rats. Epilepsia. 2020;61:2035–2052. doi: 10.1111/epi.16632. [DOI] [PubMed] [Google Scholar]
- 43.Marion D.W. Optimum serum glucose levels for patients with severe traumatic brain injury. F1000 Med. Rep. 2009;1 doi: 10.3410/M1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moro N., Ghavim S., Harris N.G., Hovda D.A., Sutton R.L. Glucose administration after traumatic brain injury improves cerebral metabolism and reduces secondary neuronal injury. Brain Res. 2013;1535:124–136. doi: 10.1016/j.brainres.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diba R., Mohaddes G., Mirzaie Bavil F., Farajdokht F., Bayandor P., Hosseindoost M., Mehri K., Zavvari Oskuye Z., Babri S. Protective effects of troxerutin on maternal high-fat diet-induced impairments of spatial memory and apelin in the male offspring. Iran J. Basic Med. Sci. 2018;21:682–687. doi: 10.22038/IJBMS.2018.28170.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhào H., Liu Y., Zeng J., Li D., Huang Y. Troxerutin cerebroprotein hydrolysate injection ameliorates neurovascular injury induced by traumatic brain injury - via endothelial nitric oxide synthase pathway regulation. Int. J. Neurosci. 2018;128:1118–1127. doi: 10.1080/00207454.2018.1486828. [DOI] [PubMed] [Google Scholar]
- 47.Zamanian M., Bazmandegan G., Sureda A., Sobarzo-Sanchez E., Yousefi-Manesh H., Shirooie S. The protective roles and molecular mechanisms of troxerutin (vitamin P4) for the treatment of chronic diseases: a mechanistic review. Curr. Neuropharmacol. 2021;19:97–110. doi: 10.2174/1570159X18666200510020744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S., Li H., Zhang L., Li J., Wang R., Wang M. Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 2017;1657:355–360. doi: 10.1016/j.brainres.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Ahmadi Z., Mohammadinejad R., Roomiani S., Afshar E.G., Ashrafizadeh M. Biological and therapeutic effects of troxerutin: molecular signaling pathways come into view. J. Pharmacopuncture. 2021;24(1):1–13. doi: 10.3831/KPI.2021.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., Sun J., Wang F., Ding G., Chen W., Fang R., Yao Y., Pang M., Lu Z.-Q., Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–78. doi: 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 51.Tagge C.A., Fisher A.M., V Minaeva O., Gaudreau-Balderrama A., Moncaster J.A., Zhang X.-L., Wojnarowicz M.W., Casey N., Lu H., Kokiko-Cochran O.N., Saman S., Ericsson M., Onos K.D., Veksler R., Senatorov V.V.J., Kondo A., Zhou X.Z., Miry O., Vose L.R., Gopaul K.R., Upreti C., Nowinski C.J., Cantu R.C., Alvarez V.E., Hildebrandt A.M., Franz E.S., Konrad J., Hamilton J.A., Hua N., Tripodis Y., Anderson A.T., Howell G.R., Kaufer D., Hall G.F., Lu K.P., Ransohoff R.M., Cleveland R.O., Kowall N.W., Stein T.D., Lamb B.T., Huber B.R., Moss W.C., Friedman A., Stanton P.K., McKee A.C., Goldstein L.E. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141:422–458. doi: 10.1093/brain/awx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ezaki J., Shimada R., Shibuya M., Kibayashi K. Hippocampal neuronal degeneration in the traumatic brain injury mouse: non-trivial effect of scalp incision. Neurol. Res. 2016;38:994–1002. doi: 10.1080/01616412.2016.1228746. [DOI] [PubMed] [Google Scholar]
- 53.Mokhtari B., Badalzadeh R., Alihemmati A., Mohammadi M. Phosphorylation of GSK-3β and reduction of apoptosis as targets of troxerutin effect on reperfusion injury of diabetic myocardium. Eur. J. Pharmacol. 2015;765:316–321. doi: 10.1016/j.ejphar.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 54.Geetha R., Sathiya Priya C., Anuradha C.V. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem. Biol. Interact. 2017;278:74–83. doi: 10.1016/j.cbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.