Abstract
Background
Pulmonary alveolar proteinosis (PAP) is a rare lung disease that mainly presents with dyspnea. PAP diagnosis can be easily missed in the background of a coronavirus disease 2019 (COVID-19) infection, due to the similarity of their presentation and radiological findings. We present a case report of a post-COVID-19 patient, who later developed severe PAP.
Case presentation
A 55-year-old male patient presented to the emergency department with progressive exertional dyspnea and hypoxia following a COVID-19 infection. Chest X-ray showed severe bilateral infiltrates. Patient received multiple courses of broad-spectrum antibiotics and prolonged course of corticosteroids without improvement. “Crazy paving” appearance in a follow up chest computed tomography raised the suspicion of PAP of what was initially thought to be a post-COVID-19 syndrome presentation. A diagnostic segmental bronchioalveolar lavage with a lung biopsy revealed a proteinaceous material filling the alveoli, with a positive periodic acid–Schiff (PAS) stain. Due to severe hypoxia, therapeutic segmental followed by whole lung lavage was performed with significant improvement.
Conclusion
Diagnosing PAP is challenging due to the rarity of the disease. An accurate diagnosis of PAP requires a combination of medical history, imaging, and bronchoalveolar lavage staining positive for PAS. Decision whether to treat with a segmental or whole lung lavage is individualized to each patient. Further studies are needed to confirm whether COVID-19 or long-term use of steroids might be contributing to PAP.
Keywords: Pulmonary alveolar proteinosis, COVID-19, Bronchioalveolar lavage
1. Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by the accumulation of surfactant proteins within the alveoli due to either increased surfactant production or decreased clearance. When PAP is clinically suspected, a bronchoalveolar lavage (BAL) in addition to computed tomography (CT) scan typical presentation is often sufficient for the diagnosis of PAP [[1], [2], [3], [4]]. Total or whole lung lavage (WLL) is considered the mainstay treatment in symptomatic patients. Trials of systemic corticosteroids were ineffective in PAP cases and may increase mortality [5]. Rituximab and plasmapheresis were suggested as alternative therapy in certain cases [1,6]. PAP is mostly autoimmune, and is treated by inhaled granulocyte-macrophage colony stimulating factor (GM-CSF) replacement therapy. Other types include secondary PAP, which lacks anti-GM-CSF antibodies, and congenital PAP, which is the least common and it is caused by genetic mutations in GM-CSF receptor proteins or surfactant proteins [6,7]. The coronavirus disease 2019 (COVID-19) pandemic has brought attention to the potential impacts of viral infections on respiratory diseases including PAP. Some case reports have suggested that COVID-19 may exacerbate PAP or even lead to the development of PAP in previously healthy individuals [8]. In this article, we present a case of a post-COVID-19 patient who developed PAP.
2. Case presentation
A 55-year-old white male was admitted with progressive exertional dyspnea and hypoxia for 3 months, associated with generalized weakness and productive cough of brown sputum. He was evaluated in another facility a month earlier and was discharged on home oxygen after receiving a course of levofloxacin and prednisolone without improvement. He has diabetes mellitus for 5 years, hypertension for 6 years, and he is a 40-pack-years smoker. He works as a car dealer with no history of occupational inhalational injury. His environmental history implies working in a car dealership that is in an open area in the United States. He lives in a well-ventilated two-bedroom apartment.He was diagnosed with COVID-19 infection 2 months prior to his presentation, that was manifested as dry cough and dyspnea. He was afebrile and his oxygen saturation (SpO2) was around 80% on room air. COVID-19 PCR was negative. Chest imaging revealed diffuse bilateral infiltrates with diffuse ground glass opacities (GGO) and a crazy-paving appearance predominantly involving the lower lobes. He was treated as a post-COVID fibrosis patient with a superimposed infection. The patient was discharged against medical advice after one day on home oxygen at 5 L/minute, empirical antibiotics, and prednisolone.
Two weeks later, he returned to our ED with significantly increased dyspnea, occasional dry cough, general weakness, and fever with a temperature of 38.6 °C. The patient had severe respiratory insufficiency, which prevented him from performing a pulmonary function test (PFT). Chest examination yielded a decreased air entry at both lung bases with minimal crackles. His SpO2 on room air was in the 70s. White blood cell count was 14.7 × 103/mm3 (neutrophiles = 86%, lymphocytes = 10%, monocytes = 2% and eosinophils = 0.1%), Hemoglobin level of 13.9 g/dl and platelets count of 393 × 103/mm3, C-reactive protein = 78 mg/L and an erythrocyte sedimentation rate = 60 mm/hour. Arterial blood gases, on a 100% FiO2 via a non-rebreather mask at rest, showed pH 7.41, PaCO2 37 mmHg, PaO2 58 mmHg and SpO2 86.6%. A repeat Chest X-ray (CXR) and CT scan indicated worsening of the extensive GGO and crazy paving infiltrates with no evidence of pulmonary embolism (Fig. 1A, Fig. 2A and B). The patient was admitted to medical ICU and started on broad spectrum antibiotics. Blood culture, sputum culture and COVID-19 nasopharyngeal swab PCR were negative. The patient initially refused bronchoscopy due to fear of intubation and becoming ventilator dependent. His clinical course and oxygen requirement deteriorated over the next 5 weeks and became dependent on high-flow oxygen with oxygen saturation of 88–92% on 100% FiO2. He eventually agreed to proceed with a diagnostic flexible bronchoscopy procedure. BAL of the right upper lobe revealed thick milky white fluids (Fig. 3) and a transbronchial lung biopsy revealed a preserved lung parenchyma with alveoli filled with pink proteinaceous material positive for periodic acid-Schiff (PAS) stain confirming PAP diagnosis (Fig. 4A–C). A decision was made to perform a therapeutic lung lavage procedure.
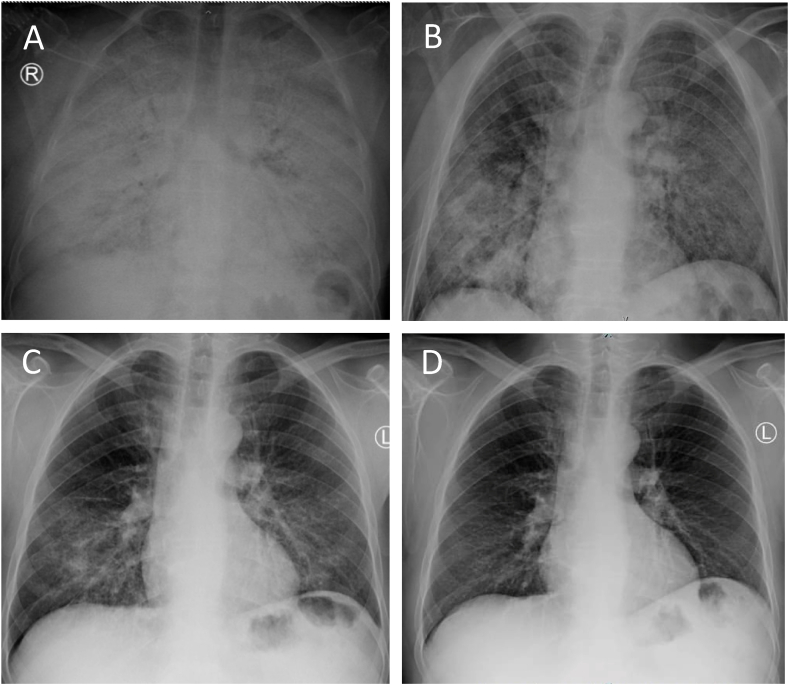
Fig. 1.
(A) A Chest X-ray (CXR) prior to diagnostic bronchoscopy. (B) CXR upon discharge. (C) CXR 6 weeks post lung lavage. (D) A CXR 3 months post lung lavage.D.
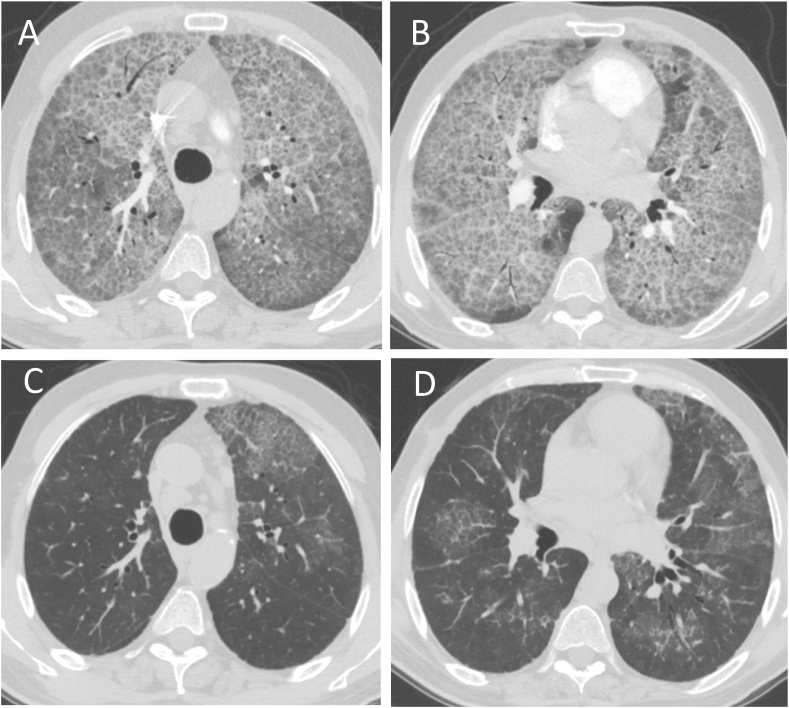
Fig. 2.
(A, B) Chest CT scan showing diffuse ground glass opacities and a crazy-paving appearance. (C, D) Chest CT scans 6 weeks post lavage.
Fig. 3.
Milky white thick fluids from bronchoalveolar lavage of the right middle lobe.
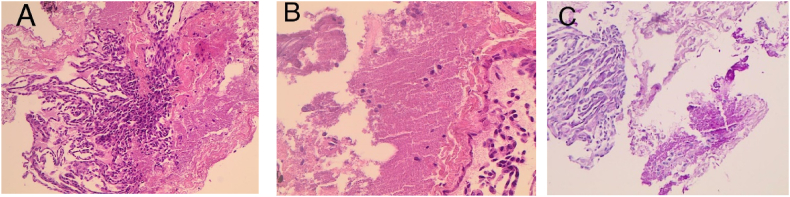
Fig. 4.
(A) Microscopic examination shows fragments of lung tissue with alveolar spaces filled with amorphous eosinophilic material. (B) High power magnification shows alveolar spaces containing amorphous eosinophilic material. (C) PAS special stain highlights eosinophilic material.
A segmental lung lavage (SLL) using a single lumen endotracheal tube (ETT) was chosen rather than a whole lung lavage (WLL), to evade the possible risk of worsening oxygenation during high volume lavage. The flexible bronchoscope was wedged into each segment of the right upper, then middle and lower lobes, with good hemodynamic tolerance. After 2 hours of copious irrigation using the lobar washing technique; the patient’s oxygenation started to improve significantly, and procedure plan was changed to attempt a WLL of the left lung. A double lumen ETT was placed and partial WLL of the left lung was performed. At the end of the right SLL and left WLL, a total of 14 L of warm normal saline was irrigated with a yield of about 12 L of thick milky secretions. The procedure was concluded after 6 hours without achieving the goal of clear lavage fluid return. The patient was extubated in the recovery room without complications. Although oxygenation requirement post lavage improved significantly; FiO2 of 40–50%, chest X-ray showed only modest improvement. A week later, WLL of the right lung was performed using a total of 25 L of warm saline with clear fluid return at the end of the procedure. The patient continued to improve and was discharged on home oxygen at 3 L/min per nasal cannula 3 days after the second lavage. His ABGs on room air upon discharge showed a pH of 7.37, a PaCO2 of 44 mmHg, a PaO2 of 58 mmHg, an HCO3 of 37 mmol/L and a SpO2 of 83%. A month later, the patient was weaned off oxygen supplements and his dyspnea resolved. In comparison to CXR from the time of diagnostic bronchoscopy (Fig. 1A) and discharge (Fig. 1B), a dramatic radiologic improvement was noted in a follow up imaging done 6 weeks (Fig. 1C) and 3 months and after discharge (Fig. 1D). The improvement was also noted in CT images 6 weeks post-lavage (Fig. 2C and D).
Upon follow up in the clinic after 3 months, the patient was off oxygen supplementation, able to carry out his daily life activities with ease and had no active respiratory complaints. His ABGs on room air were the following: pH of 7.41, a PaCO2 of 41 mmHg, a PaO2 of 81 mmHg, an HCO3 of 21 mmol/L and a SpO2 of 94%.
Informed consent to the publishing of all clinical data and images was explained and obtained from the patient.
3. Discussion
Pulmonary alveolar proteinosis (PAP) is a rare syndrome with an incidence of 0.2 cases per million that causes an excessively slow accumulation of surfactant proteins throughout terminal bronchioles and alveoli [9,10]. Previous reports estimate a clinically silent disease in almost a third of all cases [11].
Few previous reports have found that PAP was associated with or mistaken for a recent COVID-19 infection [12,13]. Compared to previously reported PAP cases, our case seems to have a more severe presentation of PAP with extensive diffuse parenchymal lung involvement associated with severe hypoxia. PAP diagnosis may be missed initially in the pandemic era given that its presentation of dyspnea and its characteristic radiological findings are like those of COVID-19 infection, which are typically described as bilateral GGO appearance with a crazy paving pattern on CT scan [14]. Moreover, similar cases have reported a suspicion that COVID-19 infection might be a direct culprit in exacerbating an underlying PAP syndrome [13,15]. PAP can be diagnosed through a combination of medical history, imaging, and bronchoalveolar lavage [16]. Given our patient’s initial refusal for a diagnostic bronchoscopy/BAL; history of a prior COVID-19 infection, and that PAP is a rare entity on its own; a post-COVID-19 syndrome, cryptogenic organizing pneumonia, interstitial pneumonia/interstitial lung disease, atypical infection such as pneumocystis jirovecii pneumonia or superimposed infection were higher up in the list of our differential diagnosis [17]. From the clinical course of our case, we hypothesize that PAP can be induced by a previous COVID-19 infection or aggravated by the chronic use of corticosteroids, which can inhibit the clearing process of macrophages [13,18]. The use of corticosteroids not only can induce PAP, but it can also inhibit phagocytosis and catabolism of alveolar macrophages, it can increase the susceptibility of infections by inhibiting bactericidal functions of granulocytes and macrophages, and it can worsen the disease severity by accelerating surfactant production in type II pneumocytes, specifically in autoimmune PAP [5]. Due to the unavailability of anti-GM-CSF antibody, primary autoimmune PAP could not be entirely ruled out. There is also another possibility that the patient had PAP for several years and the COVID-19 infection and its interventions exacerbated and led to the diagnosis of PAP.
Most PAP cases previously reported through the literature, received a therapeutic lavage which played a role in lessening the severity of the disease, and resulting in a more favorable clinical outcome [3]. Up to this time, WLL under general anesthesia (GA) to aspirate lipoproteinous accumulations, is considered the golden standard of care [19]. However, an SLL can be used in high-risk cases that cannot tolerate GA [20]. To date, our case was the first to start with SLL to then proceed with WLL. It is also worth mentioning that even though the left lung WLL did not achieve the goal of clear lavage fluid return; progressive clearance of the left lung was indicated on follow-up chest imaging, and subsequently a planned WLL of the left lung was canceled. This phenomenon supports an “overfeeding” theory that macrophage become relatively immobile and macrophage clearance might be reactivated by the partial removal of the lipoproteinous material filling the alveoli [21]. Our study possesses some limitations including the inability to perform PFTs or exercise tolerance on the patient and the inability to rule out autoimmune PAP.
In conclusion, PAP is a rare condition characterized by the accumulation of surfactant proteins in the bronchioles and alveoli. An accurate diagnosis of PAP requires a combination of high clinical suspicion, imaging, and bronchoalveolar lavage staining positive for PAS. In the COVID-19 pandemic, diagnosing and treating PAP can be challenging.
Funding information
The authors have not received any funding for this research paper.
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Borie R., Danel C., Debray M.P., Taille C., Dombret M.C., Aubier M., et al. Pulmonary alveolar proteinosis. Eur. Respir. Rev. [Internet] 2011;20(120):98–107. doi: 10.1183/09059180.00001311. https://err.ersjournals.com/content/20/120/98 [cited 2023 Feb 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamboj A., Lause M., Duggirala V. Severe pulmonary alveolar proteinosis in a young adult. Am. J. Med. [Internet] 2018;131(5):e199–e200. doi: 10.1016/j.amjmed.2017.12.019. http://www.amjmed.com/article/S000293431830007X/fulltext [cited 2023 Feb 19] Available from: [DOI] [PubMed] [Google Scholar]
- 3.Trapnell B.C., Whitsett J.A., Nakata K. Pulmonary alveolar proteinosis. StatPearls [Internet] 2003;349(26):2527–2539. doi: 10.1056/NEJMra023226. https://www.nejm.org/doi/full/10.1056/NEJMra023226 [cited 2023 Feb 19] Available from: [DOI] [PubMed] [Google Scholar]
- 4.McElvaney O.J., Horan D., Franciosi A.N., Gunaratnam C., McElvaney N.G. Pulmonary alveolar proteinosis. QJM: Int. J. Med. [Internet] 2018;111(3):185–186. doi: 10.1093/qjmed/hcx235. https://academic.oup.com/qjmed/article/111/3/185/4732158 [cited 2023 Feb 19] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Akasaka K., Tanaka T., Kitamura N., Ohkouchi S., Tazawa R., Takada T., et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: a retrospective cohort study. BMC Pulm Med. [Internet] 2015;15(1) doi: 10.1186/s12890-015-0085-0. https://pubmed.ncbi.nlm.nih.gov/26264717/ [cited 2023 Jan 16] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvaterra E., Campo I. Pulmonary alveolar proteinosis: from classification to therapy. Breathe [Internet] 2020;16(2):1–12. doi: 10.1183/20734735.0018-2020. https://breathe.ersjournals.com/content/16/2/200018 [cited 2023 Jul 3] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borie R., Danel C., Debray M.P., Taille C., Dombret M.C., Aubier M., et al. Pulmonary alveolar proteinosis. Eur. Respir. Rev. [Internet] 2011;20(120):98–107. doi: 10.1183/09059180.00001311. https://err.ersjournals.com/content/20/120/98 [cited 2023 Jul 3] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papiris S.A., Campo I., Mariani F., Kallieri M., Kolilekas L., Papaioannou A.I., et al. COVID-19 in patients with Pulmonary Alveolar Proteinosis A European multicenter study. ERJ Open Res. [Internet] 2023;9(1) doi: 10.1183/23120541.00199-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A., Abdelmalak B., Inoue Y., Culver D.A. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir. Med. [Internet] 2018;6(7):554–565. doi: 10.1016/S2213-2600(18)30043-2. https://pubmed.ncbi.nlm.nih.gov/29397349/ [cited 2022 Dec 17] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Carrington J.M., Hershberger D.M. Pulmonary alveolar proteinosis. StatPearls [Internet] 2022 https://www.ncbi.nlm.nih.gov/books/NBK482308/ [cited 2022 Dec 17]; Available from: [PubMed] [Google Scholar]
- 11.Inoue Y., Trapnell B.C., Tazawa R., Arai T., Takada T., Hizawa N., et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. [Internet] 2008;177(7):752–762. doi: 10.1164/rccm.200708-1271OC. https://pubmed.ncbi.nlm.nih.gov/18202348/ [cited 2022 Dec 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surbhi S., Singh Y., Soni K.D., Trikha A. Proteinaceous lung with COVID-19: the mimicker. Cureus [Internet] 2021;13(9) doi: 10.7759/cureus.18144. https://www.cureus.com/articles/70817-proteinaceous-lung-with-covid-19-the-mimicker [cited 2022 Dec 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bote S.M., Morueco M.A.H., Arcos B.A., Lopez J.G., Ballesteros M.B.L.M. Alveolar proteinosis in COVID-19: clinical case. Case Rep. Pulmonol. [Internet] 2022;2022:1–4. doi: 10.1155/2022/1842566. [cited 2022 Dec 17] Available from:/pmc/articles/PMC9617725/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duzgun S.A., Durhan G., Demirkazik F.B., Akpinar M.G., Ariyurek O.M. COVID-19 pneumonia: the great radiological mimicker. Insights Imaging [Internet] 2020;11(1):1–15. doi: 10.1186/s13244-020-00933-z. https://insightsimaging.springeropen.com/articles/10.1186/s13244-020-00933-z [cited 2022 Dec 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sisman M., Karapolat S., Topaloglu O., Akdogan A., Turkyilmaz A. A case of pulmonary alveolar proteinosis misdiagnosed as COVID-19 pneumonia. Cir. Cir. 2022;90(3) doi: 10.24875/CIRU.21000746. [DOI] [PubMed] [Google Scholar]
- 16.Jouneau S., Ménard C., Lederlin M. Pulmonary alveolar proteinosis. Respirology [Internet] 2020;25(8):816–826. doi: 10.1111/resp.13831. https://pubmed.ncbi.nlm.nih.gov/32363736/ [cited 2022 Dec 17] Available from: [DOI] [PubMed] [Google Scholar]
- 17.Hanfi S.H., Lalani T.K., Saghir A., McIntosh L.J., Lo H.S., Kotecha H.M. COVID-19 and its mimics: what the radiologist needs to know. J. Thorac. Imaging [Internet] 2021;36(1):W1–W10. doi: 10.1097/RTI.0000000000000554. https://pubmed.ncbi.nlm.nih.gov/32852419/ [cited 2022 Dec 17] Available from: [DOI] [PubMed] [Google Scholar]
- 18.Hosoda C., Saito K., Fujimoto S., Yamanaka Y., Watanabe N., Miyagawa H., et al. Pulmonary alveolar proteinosis developing during steroid treatment in a patient with organizing pneumonia in association with atypical chronic myeloid leukemia. Clin. Case Rep. [Internet] 2019;7(3):477–481. doi: 10.1002/ccr3.2014. https://onlinelibrary.wiley.com/doi/full/10.1002/ccr3.2014 [cited 2022 Dec 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers K.J., Avadhanula V., Patel P.R., Sarkar P.K., Piedra P., Zarrin-Khameh N. Whole lung lavage: treating pulmonary alveolar proteinosis at the time of COVID pandemic. Respir. Med. Case Rep. 2022;39 doi: 10.1016/j.rmcr.2022.101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadeghi H.A. Segmental lung lavage with fiberoptic bronchoscopy in a patient with special presentation of pulmonary alveolar proteinosis. Tanaffos [Internet] 2013;12(4):48. [cited 2022 Dec 17] Available from:/pmc/articles/PMC4153261/ [PMC free article] [PubMed] [Google Scholar]
- 21.Golde D.W., Territo M., Finley T.N., Cline M.J. Defective lung macrophages in pulmonary alveolar proteinosis. Ann. Intern. Med. 1976;85(3):304–309. doi: 10.7326/0003-4819-85-3-304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.