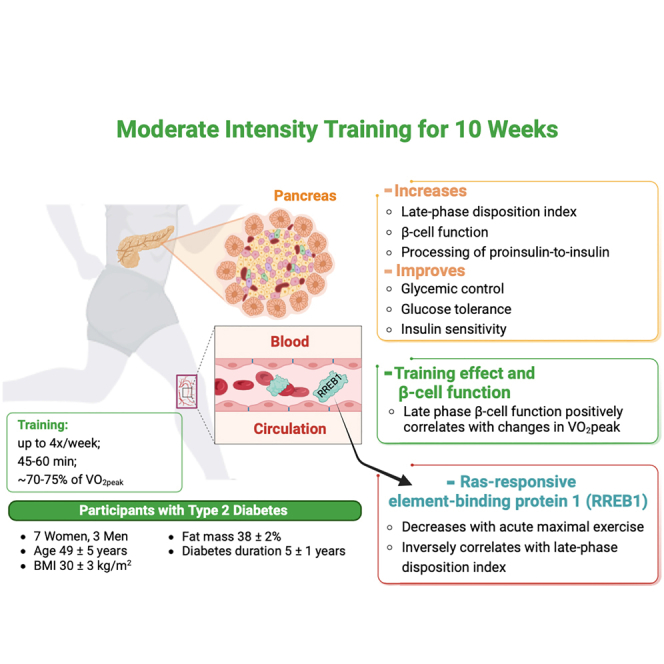
Summary
Physical activity is important for type 2 diabetes treatment, yet the underlying mechanisms for these beneficial effects of exercise are not fully understood. Here, we investigated the effects of exercise training on biphasic β-cell insulin secretory function, a key factor regulating blood glucose. Adults with type 2 diabetes (7F/3M, age 49 ± 5 years, BMI 30 ± 3 kg/m2) completed a 10-week moderate-intensity exercise program and multiple components of glucose homeostasis were measured. Training improved glycemic control, insulin sensitivity, and processing of proinsulin-to-insulin. Training increased late phase β-cell function by 38% (p = 0.01), which was correlated with changes in VO2peak suggesting training response-dependent effects. Ras-Responsive Element Binding Protein 1 (RREB1) concentrations, a protein postulated to increase type 2 diabetes risk, were inversely correlated with increases in training-induced late-phase disposition index, consistent with an inhibitory role of RREB1 on insulin secretion. Moderate-intensity exercise training improves late-phase β-cell function and glycemic control in adults with type 2 diabetes.
Subject areas: Kinesiology, Human metabolism
Graphical abstract
Highlights
-
•
Exercise training in type 2 diabetes improves with late phase β-cell function
-
•
Exercise training effects on VO2peak correlate with late phase β-cell function
-
•
Acute exercise decreases RREB1 and RREB1 inversely correlates with β-cell function
Kinesiology; Human metabolism
Introduction
Type 2 diabetes is a progressive, multifactorial disease which has become a worldwide health burden with enormous costs to the healthcare system. In addition to peripheral insulin resistance, impaired insulin secretion by the β-cell is one of the hallmarks of type 2 diabetes.1 In healthy individuals, a glucose challenge results in insulin being secreted and released from the β-cell in a biphasic manner. In contrast, people with type 2 diabetes are typically characterized by β-cell dysfunction where there is a reduced first phase insulin release from pre-stored granules, defective conversion of proinsulin to insulin, and elevated proinsulin to insulin ratio.2 During the second phase of insulin release, there is insufficient insulin protein synthesis, all leading to hyperglycemia and contributing to type 2 diabetes.2 Thus, improving insulin secretion and identifying new therapeutic targets for reversing β-cell dysfunction is critical for the prevention and treatment of type 2 diabetes.
Exercise training is important in the treatment of type 2 diabetes because this can improve glycemic control. It is thought to be primarily related to improvements in peripheral insulin resistance, but exercise training can also affect β-cell function. Previous studies have demonstrated the beneficial effects of moderate-intensity exercise training on β-cell function in adults with type 2 diabetes.3,4,5,6 However, whether moderate-intensity training affects biphasic insulin secretion is not known. Moderate-intensity exercise training can increase cardiovascular fitness.7,8,9,10 However, it is now well documented that there is significant variability among people in their responses to VO2peak following exercise training. Several studies have shown inter-individual differences with changes in cardiovascular fitness, measured as VO2peak, in response to exercise training in people without diabetes.11,12,13,14,15 Less is known about the heterogeneous responses in VO2peak, in response to moderate-intensity exercise training in people with type 2 diabetes. Exercise training improves glycemic control in type 2 diabetes, which may be partially explained by improved insulin secretory function. We hypothesize that in adults with type 2 diabetes, improved cardiorespiratory fitness after exercise training associates with improved β-cell insulin secretory function.
Exercise induces the secretion of different molecules promoting tissue cross-talk.16 Some of these exercise-induced circulating factors can regulate glucose metabolism.16,17 Changes in β-cell function during exercise are also linked to numerous factors that are released into the circulation during activity.18 It is therefore important to identify potential circulating factors induced by exercise training that affect insulin secretion, and determine the responses to exercise training in people with type 2 diabetes. Ras-Responsive Element Binding Protein 1 (RREB1) was identified as a risk factor for type 2 diabetes by genome-wide association studies.19 RREB1 is a zinc finger transcriptional factor that binds to the calcitonin gene promoter and increases the expression of calcitonin in medullary thyroid carcinoma.20 Calcitonin inhibits glucose-induced insulin responses in humans in a dose-dependent manner during systemic infusion.21 Whether there is an association between circulating RREB1 concentrations and β-cell insulin secretory function in adults with type 2 diabetes is not known.
The aims of this study were to determine the effects of moderate-intensity endurance exercise training on the biphasic aspects of β-cell function, and to determine if exercise training in adults with type 2 diabetes results in heterogeneous responses in VO2peak. We also determined whether RREB1 associates with biphasic insulin secretion in adults with type 2 diabetes. Although exercise is known to reduce peripheral insulin resistance through adaptions in skeletal muscle, adipose tissue, and other organs, understanding the effects of exercise training on β-cell insulin secretory function in people with type 2 diabetes may ultimately lead to the identification of factors that regulate insulin secretion.
Results
Moderate-intensity exercise training improves glucose tolerance and late phase β-cell function
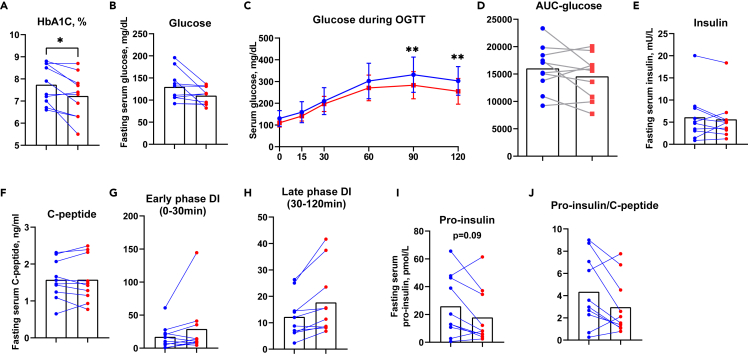
Ten overweight or obese adults with type 2 diabetes (7 women and 3 men) completed a 10-week moderate-intensity endurance exercise training program, exercising for 4 sessions/week for 45–60 min at ∼70–75% of VO2peak by week 4 (Table 1). The exercise training intervention did not alter body weight, body mass index (BMI), fat mass, or lean mass. Exercise training improved glycemic control, as HbA1C decreased from 7.7 ± 0.9% to 7.2 ± 1% (Figure 1A). Fasting glucose showed a trend toward a decrease (p = 0.07, Figure 1B). All participants underwent an oral glucose tolerance test (OGTT) and glucose levels decreased by 18% and 15% at 90 min and 120 min, respectively (Figure 1C). The area under the curve for the OGTT did not change significantly following exercise training (Figure 1D), and fasting insulin and C-peptide were similar pre- and post-training (Figures 1E and 1F). The Stumvoll index,22 a marker of insulin sensitivity that utilizes demographic data including age, sex, and BMI, along with glucose and insulin during OGTT, improved by 25% (Table 1). Exercise training did not lead to any significant changes in other glucose tolerance indices such as insulin resistance by HOMA-IR, insulin sensitivity index by QUICKI index, and Matsuda index (Table 2). Taken together, these data demonstrate that 10 weeks of moderate-intensity endurance exercise training improves glycemic control and insulin sensitivity as measured by the Stumvoll index in adults with type 2 diabetes.
Table 1.
Characteristics pre- and post-training intervention for type 2 diabetes
| Pre-training | Post-training | p | |||||
|---|---|---|---|---|---|---|---|
| Gender | 7 Female, 3 male | ||||||
| Age (years) | 49.00 | ± | 5.00 | ||||
| Body weight (kg) | 82.50 | ± | 8.60 | 81.90 | ± | 8.97 | 0.59 |
| BMI (kg/m2) | 29.88 | ± | 3.39 | 29.49 | ± | 3.41 | 0.33 |
| Fat mass (kg) | 31.47 | ± | 6.85 | 30.61 | ± | 7.23 | 0.47 |
| Lean mass (kg) | 51.00 | ± | 7.06 | 51.59 | ± | 6.77 | 0.32 |
| VO2peak (mL/kg/min) | 19.24 | ± | 5.07 | 19.29 | ± | 4.98 | 0.94 |
Figure 1.
Moderate-intensity exercise training improves glucose tolerance and late phase β-cell function in adults with type 2 diabetes
HbA1C(A), fasting glucose(B), glucose during OGTT (C) and area under the curve (D) pre- (blue) and post- (red) training. Fasting insulin (E) and fasting C-peptide (F) pre- (blue) and post- (red) training. Early phase DI (G), late phase DI (H), pro-insulin (I), and pro-insulin to C-peptide ratio (J) pre- (blue) and post- (red) training. ∗p < 0.05, ∗∗p < 0.01, post-versus pre-training. Data was expressed as average ±SD (C).
Table 2.
Insulin sensitivity pre- and post-training intervention
| Pre-training | Post-training | p | |||||
|---|---|---|---|---|---|---|---|
| HOMA-IR | 2.18 | ± | 2.75 | 1.64 | ± | 1.69 | 0.21 |
| QUICKI Index | 0.38 | ± | 0.06 | 0.38 | ± | 0.05 | 0.35 |
| Matsuda Index | 7.77 | ± | 7.04 | 7.28 | ± | 4.09 | 0.48 |
| Stumvoll Index | 0.04 | ± | 0.02 | 0.05 | ± | 0.02 | 0.01 |
| HOMA-β | 37.17 | ± | 29.21 | 45.84 | ± | 29.72 | 0.34 |
| Oral disposition index | 0.04 | ± | 0.04 | 0.06 | ± | 0.08 | 0.29 |
To determine whether exercise training affects the different phases of β-cell function in adults with type 2 diabetes, the disposition index (DI) was subdivided into early phase DI (0-30min) and late phase DI (30-120min of the OGTT). Early phase DI is linked to the immediate release of pre-stored insulin from granules, whereas late phase DI is characterized by insulin synthesis and replenishment of the insulin storage pool in the β-cell.2 We found that 6 out of 10 subjects demonstrated increased early DI, although this was not statistically significant (p = 0.1, Figure 1G). Remarkably, the late phase DI increased in all subjects, with an average 38% improvement (p = 0.01, Figure 1H), suggesting moderate-intensity exercise training significantly improves late phase β-cell function in adults with type 2 diabetes.
Pro-insulin is a precursor of insulin. Since late phase β-cell function is associated with the insulin synthesis pathway, we evaluated the processing of proinsulin to mature insulin. In β-cells, pro-insulin is cleaved by proteases to form insulin and C-peptide in a 1:1 ratio, and cleavage predominantly takes place in the endoplasmic reticulum (ER). Thus, pro-insulin to C-peptide ratio is commonly used as a measure of insulin processing efficiency, and a biomarker of β-cell ER stress.23,24,25,26 Following exercise training, fasting serum pro-insulin tended to decrease by 18% (p = 0.09, Figure 1I). Exercise training decreased the pro-insulin to C-peptide ratio by 8% (Figure 1J), suggesting that exercise training decreases the accumulation of pro-insulin, promotes the processing of pro-insulin to mature insulin, and enhances late phase β-cell function in adults with type 2 diabetes.
Subgroup analysis based on changes of VO2peak pre-to post-training
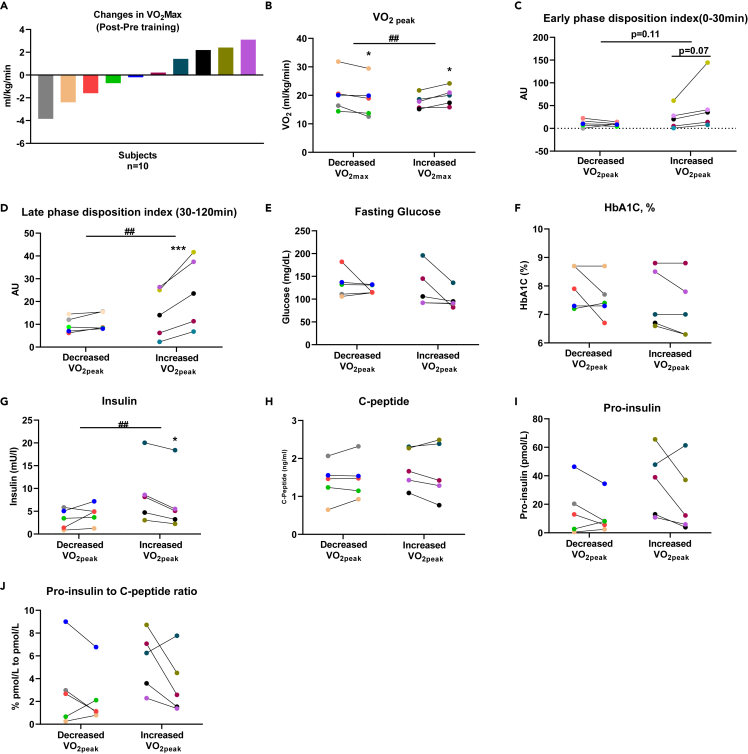
To determine if there was a relationship between the response to exercise training as determined by cardiorespiratory fitness and biphasic insulin secretion, we evaluated and identified a strong association between increases in VO2peak in response to endurance training with increases in late phase DI. Next, we examined the response in β-cell function by subgroup analysis based on changes in VO2peak (Figure 2A). Exercise training led to variable changes in VO2peak and we therefore divided our subjects in two subgroups: subjects in group 1 (n = 5) showed absolute increases in VO2peak while in subjects in group 2 (n = 5) showed decreases in VO2peak with exercise training (Figure 2B). Across subgroups, both the baseline and pre-to post-training subject characteristics, including age, BMI, body and composition, HbA1C, and C-peptide were not statistically different (Table 3). Subjects in group 1 whose VO2peak increased with exercise training, showed increased early (trend, p = 0.07) (Figure 2C) and late phase DI (p=<0.001) (Figure 2D). Serum glucose and HbA1C were not statistically different (Figures 2E and 2F), but fasting insulin decreased in subjects with increased VO2peak (Figure 2G). There was no change in C-peptide in either group (Figure 2H). Albeit not statistically significant, pro-insulin levels and pro-insulin/C-peptide ratio decreased in four out of five subjects with increased VO2peak (Figures 2I and 2J). Together, these data demonstrate that the heterogeneous responses to exercise training correlate with responses in late phase β-cell function and insulin secretion in adults with type 2 diabetes.
Figure 2.
Subgroup analysis based on changes of VO2peak pre- to post- training
(A) changes in VO2peak, each individual is coded with one color.
(B) VO2peak profile pre-to post-training by subgroup. Subgroup analysis of early phase DI (C), late phase DI (D), fasting serum glucose (E), HbA1C (F), fasting serum insulin (G), fasting serum c-peptide (H), fasting serum pro-insulin (I), proinsulin to C-peptide ratio (J). two-way ANOVA was used for sub-group analysis. ##p < 0.01 for interaction effect, ∗p < 0.05 and ∗∗∗p < 0.001 for differences pre-training and post-training.
Table 3.
Sub-group analysis for pre- and post-training intervention by change in VO2peak
| Subjects with decreased VO2peak |
Subjects with increased VO2peak |
p |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | (two-way ANOVA) | |||||||||
| Gender | 3 Female, 2 male | 4 Female, 1 male | |||||||||||
| Age | 51.0 | ± | 6.0 | 47.0 | ± | 4.0 | |||||||
| Body weight (kg) | 79.1 | ± | 5.7 | 79.6 | ± | 5.7 | 85.9 | ± | 10.3 | 84.2 | ± | 11.6 | 0.4 |
| BMI | 28.5 | ± | 3.5 | 28.6 | ± | 3.7 | 31.3 | ± | 3.0 | 30.4 | ± | 3.2 | 0.2 |
| Fat mass (kg) | 28.6 | ± | 8.3 | 28.8 | ± | 9.3 | 34.3 | ± | 4.0 | 32.5 | ± | 4.8 | 0.4 |
| Lean mass (kg) | 50.3 | ± | 7.4 | 51.4 | ± | 7.2 | 51.7 | ± | 7.5 | 51.8 | ± | 7.2 | 0.4 |
| VO2 peak (mL/kg/min) | 20.7 | ± | 6.8 | 18.9 | ± | 6.7∗ | 17.8 | ± | 2.6 | 19.7 | ± | 3.2∗ | <0.01 |
Moderate-intensity exercise training does not change glucagon and amylin in adults with type 2 diabetes
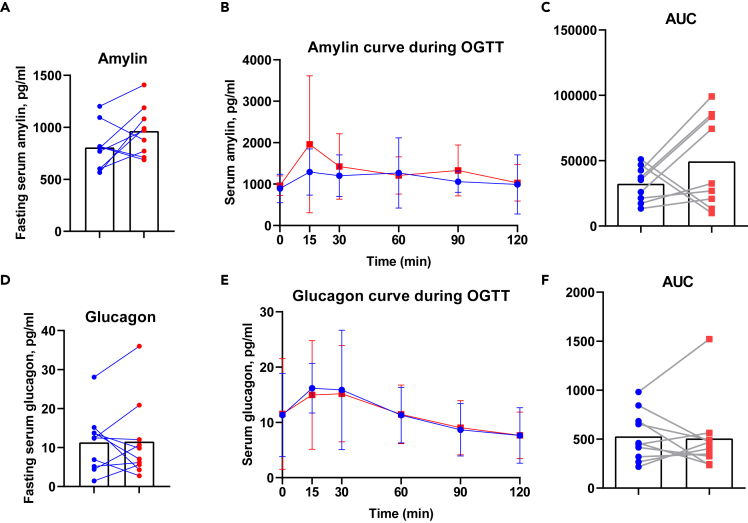
Because one of the key findings in our study is the effect of exercise training on late phase DI in adults with type 2 diabetes, we next investigated how exercise training may improve insulin synthesis. Late phase DI is associated with insulin synthesis in the β-cell of the pancreas and exercise training may also affect other aspects of pancreatic function. Here, we assessed other pancreatic hormones involved in the regulation of insulin synthesis in response to OGTT. Amylin, a 37-residue peptide hormone that is co-secreted from the pancreatic β-cells with insulin, and glucagon, a 29-amino acid peptide hormone produced by the pancreatic α-cells and glucose counter-regulatory hormone, were measured. Amylin and glucagon concentrations under fasting and during OGTT were similar pre- and post-exercise training and did not change with training (Figure 3) suggesting that the effects of exercise training specifically relate to the insulin-producing function of β-cells, and not to endocrine factors from α-cells and β-cells.
Figure 3.
Moderate-intensity exercise training does not change glucagon and amylin in adults with type 2 diabetes
Fasting amylin (A), amylin during OGTT (B), and area under the curve (C) pre- (blue) and post- (red) training. Fasting glucagon (D), glucagon during OGTT (E), and area under the curve (F) pre- (blue) and post- (red) training. Data was expressed as average ± SD (B and E).
Changes in VO2peak positively correlate with exercise-induced improvements in β-cell function
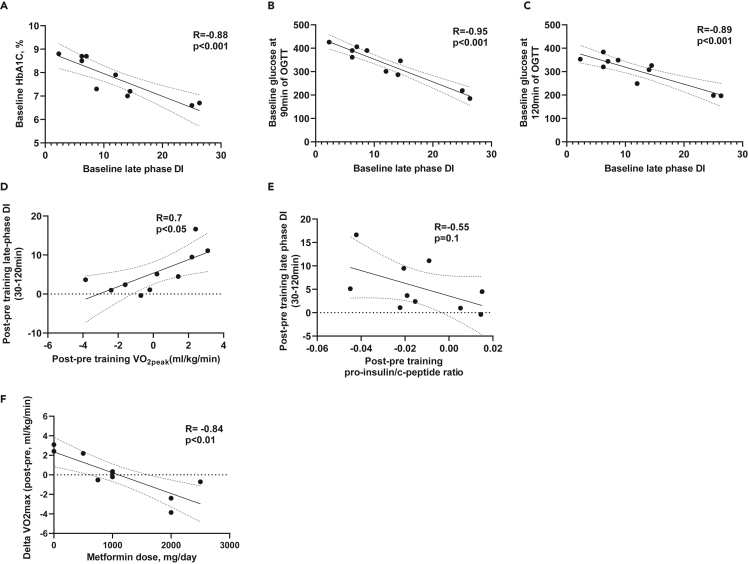
To better understand exercise-induced improvements in late phase DI index and the relationship with other metabolic parameters, we next evaluated and identified significant associations between late phase DI and all of the measured parameters, including anthropometrics, peak exercise capacity, glucose parameters, and pancreatic hormones at baseline and post-training intervention. Baseline late phase DI negatively correlated with baseline HbA1C (Figure 4A), and serum glucose concentrations at 90min and 120min during OGTT (Figures 4B and 4C). Changes in late phase DI post training positively correlated with changes in VO2peak (Figure 4D). The decrease in pro-insulin to C-peptide ratio tended to negatively correlate with increased late phase DI (R = −0.55, p = 0.1, Figure 4E), indicating a potential link between exercise-induced improvements in proinsulin to mature insulin processing and improved late phase DI. Of interest, we also observed a correlation between higher metformin dose and lower change in VO2peak (Figure 4F). Together, these data show an inverse relationship between late phase DI and glucose concentrations at baseline, and a positive association between changes in DI and change in VO2peak after exercise training.
Figure 4.
Changes in VO2peak positively correlate with exercise-induced improvements in β-cell function
Correlation between baseline (pre-training) late phase DI and baseline HbA1C (A), glucose at 90min of OGTT (B) and 120min of OGTT (C). Correlation between changes in late phase DI and changes in VO2peak (D) and pro-insulin/C-peptide ratio (E). Correlation between changes in VO2peak and metformin use (F).
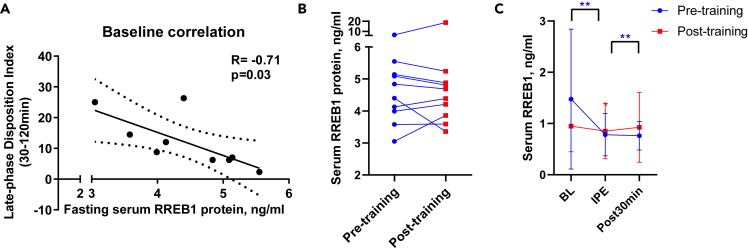
Fasting serum RREB1 is negatively associated with late phase β-cell function
RREB1 is a transcription factor that is ubiquitously expressed in all tissues throughout the body. Mutations in RREB1, identified by genome-wide studies, are linked to increased risk for type 2 diabetes.19 Therefore, RREB1 concentrations may play a role in regulating β-cell function.27 To examine whether RREB1 plays a role in exercise-induced improvements in late phase DI, we first measured serum RREB1 pre- and post-training. Of interest, baseline serum RREB1 protein concentrations were negatively associated with baseline late phase DI (Figure 5A) and the exercise training intervention tended to decrease serum RREB1 concentrations (Figure 5B). Based on these findings, we investigated whether acute exercise regulates RREB1 and measured RREB1 in the same subjects in response to an acute bout of maximal exercise during the cardiorespiratory fitness test. We found that a bout of maximal exercise decreased RREB1 concentrations by 51% (p = 0.0039) (Figure 5C). Together, these data indicate RREB1 as an exercise-regulated circulating protein, which relates to improved β-cell function with exercise training.
Figure 5.
Fasting serum RREB1 is negatively associated with late phase β-cell function
(A) Pearson’s correlation analysis between baseline fasting serum RREB1 protein and late phase disposition index.
(B) Fasting serum RREB1 pre- and post-training.
(C) Serum RREB1 pre-, immediately post-, and 30 min post-acute bout of maximal exercise pre- and post-training. ∗p < 0.05 and ∗∗p < 0.01 for differences between pre- and post-training. Data was expressed as average ± SD (C).
Discussion
Exercise training is critical in the prevention and treatment of type 2 diabetes, but the effects of exercise training on β-cell function and insulin secretion are not fully understood. Here, we make the novel observation that 10 weeks of moderate-intensity endurance training improves late phase β-cell insulin secretory function in adults with type 2 diabetes. We find that exercise training improves glycemic control, which may be partially explained by improvement in insulin secretory function. We also observe heterogeneous responses in VO2peak among adults with type 2 diabetes, and find that the improvement in late phase β-cell function is associated with a change in VO2peak, and pre-training circulating RREB1 protein concentrations.
Type 2 diabetes is characterized by peripheral insulin resistance and β-cell dysfunction. Type 2 diabetes develops when β-cells fail to compensate for insulin resistance, and β-cell dysfunction progressively worsens with disease duration.28 In our study, we determined late phase β-cell function by disposition index derived from 30 min to 120 min of OGTT, which is an integrated and sensitive measure of β-cell function adjusted for insulin sensitivity.29,30 Previous endurance training studies have demonstrated that in adults with type 2 diabetes, 12 weeks of cycling (up to 75% VO2peak) improved overall β-cell insulin secretory function as measured by a hyperglycemic clamp.4 However, this improvement was only observed in adults with moderate β-cell secreting capacity at baseline, but not low β-cell secreting capacity.4 This is in line with our study, as low β-cell secretion is generally seen with a longer duration of diabetes and insulin dependence, which was not observed in our subjects. In contrast to our finding of improved β-cell function, two other studies that investigated endurance exercise training in adults with type 2 diabetes showed no change in overall insulin secretion rate during OGTT.31,32 The methods used to measure β-cell function, pre-training insulin secretory capacity, stages of type 2 diabetes, and the types of exercise training could all be potential factors for differences in exercise training effects on β-cell function. Although GLP-1 has previously been investigated in relation to exercise training in various studies, it was not the primary focus of our study to measure GLP-1. We did not determine changes in GLP-1 concentrations and cannot exclude a role for GLP-1 as potential contributor. Our study has dissected the biphasic pancreatic β-cell insulin secretory function in response to endurance exercise training and supports the beneficial effects of endurance exercise training in adults with type 2 diabetes.
We found that endurance training resulted in heterogeneous changes in VO2peak among our subjects with type 2 diabetes with five subjects increasing and five subjects decreasing VO2peak. Lower VO2peak is associated with poor glycemic control,13 making it important to understand why some subjects fail to increase VO2peak. Previous endurance exercise training studies in adults with type 2 diabetes have also shown a heterogeneous response in VO2peak.33,34,35 Here, we identify a positive association between VO2peak and exercise-induced improvements in late phase β-cell insulin secretory function and also find that while late phase β-cell insulin secretory function improved in all participants, this beneficial adaptation was more evident in the subgroup with improved VO2peak. Whether there is a connection between these changes in insulin secretory function and VO2peak is not known, but will be important to study in subjects with both pre-diabetes and type 2 diabetes of longer duration and with different training regimens such as high-intensity interval training.
Factors including, but not limited to medications could affect the changes in VO2peak in response to exercise training.36,37 On analyzing all parameters, we observed that the heterogeneous response of VO2peak is linked to doses of metformin intake in these participants. Metformin has been reported to blunt the improvements in VO2peak, insulin sensitivity, and hyperinsulinemia by exercise training in adults with prediabetes.38,39,40 Given the number of people that are treated with metformin, future studies are needed to determine the specific mechanisms by which metformin may negatively affect cardiorespiratory fitness in response to exercise training.
We determined that RREB1 is an exercise-regulated circulating factor. RREB1 is present in exosomes.41 Little is known about RREB1 but, based on the RREB1 reductions after 12–14 min of maximal exercise, we speculate the individual exercise sessions that occur during the weeks of exercise training lead to recurring rapid RREB1 clearance from the circulation and reduced RREB1 concentrations, potentially impacting late phase DI. In line with this, GWAS studies have determined an association between the RREB1 locus and type 2 diabetes. Moreover, carriers of a minor allele p.Ans1171-RREB1, predicted to reduce RREB1 function, tend to have reduced risk of type 2 diabetes.27 Our findings show that exercise regulates circulating RREB1, and, as RREB1 is a secreted protein as well as a transcriptional factor in the cell nucleus, it will be important in future studies to determine whether RREB1 regulates pancreatic β-cell insulin secretion in response to exercise training.
In summary, we find that in adults with type 2 diabetes, moderate-intensity endurance training improves glycemic control as well as late phase β-cell function. This effect of exercise training on late phase β-cell function is more pronounced in subjects with improved VO2peak. In addition, we demonstrate that RREB1 is an exercise-induced circulating factor and that RREB1 concentrations are negatively associated with late phase β-cell function. Whether and how RREB1 affects β-cell function remains to be explored. We anticipate further investigations in this field to elucidate the underlying mechanisms, and potentially lead to the development of targeted exercise interventions to enhance β-cell function and improve glycemic control in individuals with type 2 diabetes.
Together, our study presents novel findings by evaluating distinct phases of beta cell function, stratifying late-phase function based on VO2peak, and identifying a new link between RREB1 and β-cell function. These insights deepen our understanding of beta cell function dynamics, the relationship between function and aerobic capacity, and potential molecular mechanisms for regulating beta cell performance, with implications for future therapeutic interventions. Understanding the molecular underpinnings regulating insulin secretion in response to exercise training is critically important in identifying novel targets for new therapies for type 2 diabetes.
Limitations of the study
Some of the limitations of the study include a lower number of male participants, hence, we cannot definitively exclude sex-differences in the effects of exercise training on late phase insulin secretion. Determining whether exercise-induced improvements in late phase β-cell insulin secretory function are directly affected by metformin requires a larger sample size. We show a correlation between circulating RREB1 protein concentrations and exercise-induced improvements in late phase β-cell function, but causality cannot be determined in this study. It will be important to carry out future mechanistic studies to determine the role of RREB1 in regulating insulin secretion in response to exercise training.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human serum samples | This study | N/A |
| Critical commercial assays | ||
| Human insulin ELISA | Mercodia | Cat#10-1113-01 |
| Human c-peptide ELISA | Crystal Chem | Cat#80954 |
| Human amylin ELISA | Novus | Cat#NBP2-76733 |
| Human glucagon ELISA | Crystal Chem | Cat#81520 |
| Human pro-insulin ELISA | Crystal Chem | Cat#90110 |
| RREB1 ELISA | Mybiosource | Cat#MBS7248141 |
| Software and algorithms | ||
| GraphPad Prism v9 | GraphPad | https://www.graphpad.com |
| Other | ||
| Bio impedance body composition analyzer | Tanita | Cat#TBF-215 |
| OneTouch Verio Glucometer | OneTouch | Cat#XBHNGBW4 |
| DCA Vantage Analyzer | Siemens | Cat#06489205 |
| H1 Heart rate monitor | Polar | Cat#92053169 |
| RT 6000D Centrifuge | Sorvall | Cat#323710 |
| VO2peakmetabolic system | ParvoMedics MMS-2400 | Cat#24-4150HCU |
| 75g oral glucose solution | Trutol | Cat#401223P |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact: Roeland J. W. Middelbeek, MD (roeland.middelbeek@joslin.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Ten adults with type 2 diabetes (7 females, 3 males) completed a supervised 10-week exercise training program. Inclusion criteria included: HbA1c 6.5-9.0%, BMI 25-37kg/m2, age 25-55 years. All subjects were previously sedentary (≤150mins of moderate-to-intense activity/week in the last 3 months). Exclusion criteria consisted of: type 1 diabetes, severe complications of diabetes, heart or lung disease, current dieting or weight loss efforts, cancer, renal or hepatic dysfunction, neurological disease, clinical history of stroke, uncontrolled hypertension, and inability to exercise at 50% of predicted heart rate reserve, among others. Subjects taking beta-blockers were excluded. Subjects were asked to maintain a constant diet, avoid weight-loss efforts, and maintain all extracurricular activities during the study. During the training program, subjects continued their current medication regimen. Anti-hyperglycemic medications used by participants included: Biguanides (8/10), GLP-1 receptor agonists (5/10), SGLT2-inhibitors (3/10), Meglitinides (1/10), Sulfonylureas (2/10), and Glargine insulin (2/10). The study was approved by the Joslin Diabetes Center’s Institutional Review Board and registered at Clinicaltrials.gov (#NCT03133156).
Method details
VO2peak test and exercise training intervention
To determine peak aerobic capacity, a standard VO2peak test was conducted by the exercise physiologist. Subjects were not required to be fasted. For the 10-week supervised exercise training program, training intensity was based on a target heart rate range (∼70-75% of VO2peak). Initially, subjects exercised 20-30min per day at ∼40-50% VO2 peak, 3 days per week. The duration, intensity and frequency of exercise were gradually increased to 45-60 min of exercise per day, 4 times per week by week 4, at ∼70-75% of VO2 peak. Subjects wore activity trackers and heart rate monitors during activities to assure compliance with the training regimen.
Body composition and metabolic measurements
Body composition was obtained by bioimpedance (Tanita, Arlington Heights, Illinois). An OGTT was performed pre- and post-training. For the OGTT, subjects were given a 75g-oral glucose solution and blood was drawn through an IV catheter at 0, 15, 30, 60, 90, and 120 min. Serum was obtained by centrifuging the blood at 2800rpm, 15 minutes, at 4°C in the Sorvall RT 6000D centrifuge. Capillary blood glucose was measured by glucometer (Onetouch), serum insulin was measured by ELISA (Mercodia). HbA1C was measured by Siemens DCA Vantage Analyzer. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated as the product of fasting glucose and insulin (Matthews et al., 1985). HOMA-β was calculated as ((20 X fasting insulin)/ (Fasting glucose-3.5))∗100%.42,43 QUICKI index was calculated as 1/(log (fasting insulin) + log (fasting glucose)).44 Matsuda index was calculated as 10,000/(fasting glucose × fasting insulin × mean of glucose from 0min to 120min of OGTT × mean of insulin from 0min to 120min of OGTT)1/2.45 Stumvoll index was calculated as 0.226-(0.0032× BMI- (0.0000645 × insulin at 2-hr OGTT)-0.0037×glucose at 1.5-hr OGTT).22 Oral disposition index was calculated as ΔInsulin0–30/ΔGlucose0–30 × (1/fasting insulin).46 Early phase DI was calculated as the product of Matsuda index and early phase insulinogenic index (Insulin30 −0/Glucose30 −0).47,48,49 Late phase DI was calculated as the product of Matsuda index and late phase insulinogenic index (Insulin tAUC (30-120min) / Glucose tAUC (30-120min)).47,48,49
Hormonal and cytokine measurements
Fasting serum samples collected during OGTT, pre-training and at least 48 hours after the last exercise bout of the training program, were analyzed for insulin (Mercodia), C-peptide (Crystal Chem), amylin (Novus), glucagon (Crystal Chem), and pro-insulin (Crystal Chem) only at 0 min of OGTT, according to manufacturer’s instructions. RREB1 was measured in fasting serum samples before, immediately after, and 30minutes of rest post VO2peak using a commercially available ELISA (Mybiosource). All the assays were run in duplicates.
Quantification and statistical analysis
Prism v9 (GraphPad) was used for statistical analyses. Data are described as mean ± SD. Differences in pre- and post-exercise training intervention were compared by two-tailed paired t-test. Sub-group analyses were performed by two-way ANOVA, with Bonferroni's multiple comparisons test as post hoc analyses when there was significant interaction. The post to pre training study outcomes were calculated as post (raw value) minus pre (raw value). Normality of the data was assessed by a Shapiro-Wilk test and a D'Agostino & Pearson omnibus test. Pearson’s correlation was used to examine associations for parametric data. Spearman’s rank correlation was for nonparametric data. Significance was accepted as p ≤ 0.05.
Acknowledgments
This study was supported by NIH NIDDK grants: K23-DK114550 and BADERC P&F funding (to R.W.J.M), R01-DK112283 and R01-DK099511 (to L.J.G.), F32-DK126432 and Joslin Diabetes Center P&F (to M.V), and P30-DK36836 (to Joslin Diabetes Center), P30-DK057521 (to BADERC). We like to thank Jeffrey Richard, Taylor Pierce, Julianne O’Connell, and the CRC staff for their assistance. We thank Dr. Vasileios-Arsenios Lioutas for his valuable assistance in performing statistical analysis of our data. We also thank the study participants for their participation. The data have been previously presented at the American Diabetes Association’s Scientific Sessions 2022 in New Orleans.
Author contributions
Conceptualization, H.Z., L.J.G., and R.J.W.M.; Methodology, H.Z., L.J.G., and R.J.W.M.; Investigation, H.Z., L.S., N.P.C., M.F.H., P.N., M.V., and R.J.W.M.; Writing – Original Draft, H.Z.; Writing – Review and Editing, L.J.G. and R.J.W.M.; Funding Acquisition, L.J.G. and R.J.W.M.; Supervision, L.J.G. and R.J.W.M.
Declaration of interests
The authors declare no competing interests. R.J.W.M. and L.J.G. have received research support from Novo Nordisk, which is unrelated to this work.
Published: June 28, 2023
Contributor Information
Laurie J. Goodyear, Email: laurie.goodyear@joslin.harvard.edu.
Roeland J.W. Middelbeek, Email: roeland.middelbeek@joslin.harvard.edu.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu Z., Gilbert E.R., Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013;9:25–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Bloem C.J., Chang A.M. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J. Clin. Endocrinol. Metab. 2008;93:387–392. doi: 10.1210/jc.2007-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dela F., von Linstow M.E., Mikines K.J., Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1024–E1031. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- 5.Hordern M.D., Cooney L.M., Beller E.M., Prins J.B., Marwick T.H., Coombes J.S. Determinants of changes in blood glucose response to short-term exercise training in patients with Type 2 diabetes. Clin. Sci. 2008;115:273–281. doi: 10.1042/CS20070422. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Zheng Q., Miller J.D., Zuo P., Yuan X., Feng J., Liu C., Bao S., Lou Q. Aerobic training reduces pancreatic fat content and improves beta-cell function: a randomized controlled trial using IDEAL-IQ magnetic resonance imaging. Diabetes. Metab. Res. Rev. 2022;38:e3516. doi: 10.1002/dmrr.3516. [DOI] [PubMed] [Google Scholar]
- 7.Boulé N.G., Kenny G.P., Haddad E., Wells G.A., Sigal R.J. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46:1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 8.Branch J.D., Pate R.R., Bourque S.P. Moderate intensity exercise training improves cardiorespiratory fitness in women. J. Womens Health Gend. Based. Med. 2000;9:65–73. doi: 10.1089/152460900318984. [DOI] [PubMed] [Google Scholar]
- 9.Lin X., Zhang X., Guo J., Roberts C.K., McKenzie S., Wu W.C., Liu S., Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015;4:e002014. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nystoriak M.A., Bhatnagar A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018;5:135. doi: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips B.E., Kelly B.M., Lilja M., Ponce-González J.G., Brogan R.J., Morris D.L., Gustafsson T., Kraus W.E., Atherton P.J., Vollaard N.B.J., et al. A practical and time-efficient high-intensity interval training program modifies cardio-metabolic risk factors in adults with risk factors for type II diabetes. Front. Endocrinol. 2017;8:229. doi: 10.3389/fendo.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R., de Lannoy L., Stotz P.J. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin. Proc. 2015;90:1506–1514. doi: 10.1016/j.mayocp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Solomon T.P.J., Malin S.K., Karstoft K., Knudsen S.H., Haus J.M., Laye M.J., Kirwan J.P. Association between cardiorespiratory fitness and the determinants of glycemic control across the entire glucose tolerance continuum. Diabetes Care. 2015;38:921–929. doi: 10.2337/dc14-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seward S., Ramos J., Drummond C., Dalleck A., Byrd B., Kehmeier M., Dalleck L. Inter-individual variability in metabolic syndrome severity score and VO(2)max changes following personalized, community-based exercise programming. Int. J. Environ. Res. Public Health. 2019;16:4855. doi: 10.3390/ijerph16234855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson P.J., Atkinson G., Batterham A.M. Inter-individual responses of maximal oxygen uptake to exercise training: a critical review. Sports Med. 2017;47:1501–1513. doi: 10.1007/s40279-017-0680-8. [DOI] [PubMed] [Google Scholar]
- 16.Chow L.S., Gerszten R.E., Taylor J.M., Pedersen B.K., van Praag H., Trappe S., Febbraio M.A., Galis Z.S., Gao Y., Haus J.M., et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022;18:273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H., Alves C.R.R., Stanford K.I., Middelbeek R.J.W., Nigro P., Ryan R.E., Xue R., Sakaguchi M., Lynes M.D., So K., et al. TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019;1:291–303. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curran M., Drayson M.T., Andrews R.C., Zoppi C., Barlow J.P., Solomon T.P.J., Narendran P. The benefits of physical exercise for the health of the pancreatic beta-cell: a review of the evidence. Exp. Physiol. 2020;105:579–589. doi: 10.1113/EP088220. [DOI] [PubMed] [Google Scholar]
- 19.Deng Y.N., Xia Z., Zhang P., Ejaz S., Liang S. Transcription factor RREB1: from target genes towards biological functions. Int. J. Biol. Sci. 2020;16:1463–1473. doi: 10.7150/ijbs.40834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiagalingam A., De Bustros A., Borges M., Jasti R., Compton D., Diamond L., Mabry M., Ball D.W., Baylin S.B., Nelkin B.D. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol. Cell Biol. 1996;16:5335–5345. doi: 10.1128/MCB.16.10.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giugliano D., Passariello N., Sgambato S., Torella R., D'Onofrio F. Calcitonin modulation of insulin and glucagon secretion in man. Am. J. Physiol. 1982;242:E206–E213. doi: 10.1152/ajpendo.1982.242.3.E206. [DOI] [PubMed] [Google Scholar]
- 22.Stumvoll M., Van Haeften T., Fritsche A., Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001;24:796–797. doi: 10.2337/diacare.24.4.796. [DOI] [PubMed] [Google Scholar]
- 23.Brusco N., Sebastiani G., Di Giuseppe G., Licata G., Grieco G.E., Fignani D., Nigi L., Formichi C., Aiello E., Auddino S., et al. Intra-islet insulin synthesis defects are associated with endoplasmic reticulum stress and loss of beta cell identity in human diabetes. Diabetologia. 2023;66:354–366. doi: 10.1007/s00125-022-05814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loopstra-Masters R.C., Haffner S.M., Lorenzo C., Wagenknecht L.E., Hanley A.J. Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetologia. 2011;54:3047–3054. doi: 10.1007/s00125-011-2322-2. [DOI] [PubMed] [Google Scholar]
- 25.Tersey S.A., Nishiki Y., Templin A.T., Cabrera S.M., Stull N.D., Colvin S.C., Evans-Molina C., Rickus J.L., Maier B., Mirmira R.G. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Then C., Gar C., Thorand B., Huth C., Then H., Meisinger C., Heier M., Peters A., Koenig W., Rathmann W., et al. Proinsulin to insulin ratio is associated with incident type 2 diabetes but not with vascular complications in the KORA F4/FF4 study. BMJ Open Diabetes Res. Care. 2020;8:e001425. doi: 10.1136/bmjdrc-2020-001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattis K.K., Krentz N.A.J., Metzendorf C., Abaitua F., Spigelman A.F., Sun H., Ikle J.M., Thaman S., Rottner A.K., Bautista A., et al. Loss of RREB1 in pancreatic beta cells reduces cellular insulin content and affects endocrine cell gene expression. Diabetologia. 2023;66:674–694. doi: 10.1007/s00125-022-05856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitabchi A.E., Temprosa M., Knowler W.C., Kahn S.E., Fowler S.E., Haffner S.M., Andres R., Saudek C., Edelstein S.L., Arakaki R., et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzo C., Wagenknecht L.E., Rewers M.J., Karter A.J., Bergman R.N., Hanley A.J.G., Haffner S.M. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwoudt S., Fealy C.E., Foucher J.A., Scelsi A.R., Malin S.K., Pagadala M., Rocco M., Burguera B., Kirwan J.P. Functional high-intensity training improves pancreatic beta-cell function in adults with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017;313:E314–E320. doi: 10.1152/ajpendo.00407.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacchi E., Negri C., Zanolin M.E., Milanese C., Faccioli N., Trombetta M., Zoppini G., Cevese A., Bonadonna R.C., Schena F., et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns N., Finucane F.M., Hatunic M., Gilman M., Murphy M., Gasparro D., Mari A., Gastaldelli A., Nolan J.J. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia. 2007;50:1500–1508. doi: 10.1007/s00125-007-0655-7. [DOI] [PubMed] [Google Scholar]
- 33.Caron J., duManoir G.R., Labrecque L., Chouinard A., Ferland A., Poirier P., Legault S., Brassard P. Impact of type 2 diabetes on cardiorespiratory function and exercise performance. Physiol. Rep. 2017;5:e13145. doi: 10.14814/phy2.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirwan J.P., Sacks J., Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve. Clin. J. Med. 2017;84:S15–S21. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najafipour F., Mobasseri M., Yavari A., Nadrian H., Aliasgarzadeh A., Mashinchi Abbasi N., Niafar M., Houshyar Gharamaleki J., Sadra V. Effect of regular exercise training on changes in HbA1c, BMI and VO(2)max among patients with type 2 diabetes mellitus: an 8-year trial. BMJ Open Diabetes Res. Care. 2017;5:e000414. doi: 10.1136/bmjdrc-2017-000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Cabañas A., Morales-Palomo F., Alvarez-Jimenez L., Ortega J.F., Mora-Rodriguez R. Effects of chronic metformin treatment on training adaptations in men and women with hyperglycemia: a prospective study. Obesity. 2022;30:1219–1230. doi: 10.1002/oby.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun B., Eze P., Stephens B.R., Hagobian T.A., Sharoff C.G., Chipkin S.R., Goldstein B. Impact of metformin on peak aerobic capacity. Appl. Physiol. Nutr. Metab. 2008;33:61–67. doi: 10.1139/H07-144. [DOI] [PubMed] [Google Scholar]
- 38.Malin S.K., Gerber R., Chipkin S.R., Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131–136. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malin S.K., Stewart N.R. Metformin may contribute to inter-individual variability for glycemic responses to exercise. Front. Endocrinol. 2020;11:519. doi: 10.3389/fendo.2020.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharoff C.G., Hagobian T.A., Malin S.K., Chipkin S.R., Yu H., Hirshman M.F., Goodyear L.J., Braun B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am. J. Physiol. Endocrinol. Metab. 2010;298:E815–E823. doi: 10.1152/ajpendo.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ung T.H., Madsen H.J., Hellwinkel J.E., Lencioni A.M., Graner M.W. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105:1384–1392. doi: 10.1111/cas.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 43.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 44.Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G., Quon M.J. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 46.Utzschneider K.M., Prigeon R.L., Faulenbach M.V., Tong J., Carr D.B., Boyko E.J., Leonetti D.L., McNeely M.J., Fujimoto W.Y., Kahn S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldfine A.B., Bouche C., Parker R.A., Kim C., Kerivan A., Soeldner J.S., Martin B.C., Warram J.H., Kahn C.R. Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc. Natl. Acad. Sci. USA. 2003;100:2724–2729. doi: 10.1073/pnas.0438009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin B.C., Warram J.H., Krolewski A.S., Bergman R.N., Soeldner J.S., Kahn C.R. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 49.Stumvoll M., Tataranni P.A., Stefan N., Vozarova B., Bogardus C. Glucose allostasis. Diabetes. 2003;52:903–909. doi: 10.2337/diabetes.52.4.903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.