Abstract
Thousands of tons of residual lignocellulosic biomass are produced and discarded by agroindustries in the Amazon. These biomasses could be harnessed and used in the preparation of activated carbon, in view of the growing demand for this product with high added value, however, little is known about their characteristics, in addition to their potential as precursors of activated carbon. Therefore, the aim of this work was to evaluate the potential of four different biomasses in the preparation and quality of activated carbon. Residues from the processing of the fruits of acai, babassu, Brazil nut, and oil palm were collected, characterized, carbonized, physically activated with CO2, and characterized. The contents of the total extractives, insoluble lignin, minerals, holocellulose, and elemental (CHNS–O) were analyzed. The surface area and surface morphology were determined from the AC produced, and adsorption tests for methylene blue and phenol were performed. The four biomasses showed potential for use in the preparation of CA; the residues presented high contents of lignin (21.83–55.76%) and carbon (46.49–53.79%). AC were predominantly microporous, although small mesopores could be observed. The AC had a surface area of 569.65–1101.26 m2 g−1, a high methylene blue (93–390 mg g−1), and phenol (159–595 mg g−1) adsorption capacities. Babassu-AC stood out compared to the AC of the other analyzed biomasses, reaching the best results.
Keywords: Biomass residues, Activated carbon, Physical activation and dye adsorption, Amazon
1. Introduction
Environmental concerns are recurrent today [1]. Technologies that aim to minimize the generation of waste, reuse and recycle waste and recover the aggregated matter and energy are required, especially strategies that use these residues in the production of products with greater added value [2,3]. These initiatives have social, economic, and environmental benefits in pursuing sustainable development [4]. In addition to adding value to waste, the application of these materials in new products can help solve local and global environmental problems that are of great concern to us today [5].
Utilization and valorization of these residues can be achieved via the production of activated carbon (AC), which has a high adsorption capacity [1,6,7]. AC can be used to remove organic compounds and metals and remove contaminants from aqueous solutions, in CO2 capture processes, and support of catalysts and capacitors [[8], [9], [10], [11]]. Any carbonaceous raw material can produce activated carbon, but not all AC are economically viable [12]. In Brazil, most activated carbon is produced from bovine bones, and other AC comes from precursors of vegetable origin, of which that from coconut shell is the most used [13]. In addition, Brazilian AC production is insufficient given the country's needs, and Brazil imports a large quantity of these products. In 2019, Brazil was the 34th largest importer of activated carbon globally, representing approximately US $ 12.4 million in imports. However, regarding exports, the value of AC export in Brazil was much lower, equivalent to $ 1.2 million, which indicates a significant financial deficit in using this product [14]. Given this scenario, the search for new material sources to produce AC is necessary, especially lignocellulosic raw material.
In the Amazon, several agribusinesses generate tons of waste daily [15], such as acai seeds, coconut kernel, and oil palm shells, by processing various types of fruit. An estimate of Brazilian production indicates that in 2018, 1.6 million tons (Mt), 221 thousand tons (kt), 50 kt, and 34 kt of oil palm, acai, Brazil nut, and babassu were produced, respectively; the state of Pará is the first in production and export center for the first three listed fruits [[16], [17], [18]]. As a result, the use of agroindustrial residues for the production of activated carbon can be of great importance in the region since this product can add value to some of the material that is usually underused/discarded, in addition to indicating a new production process, demonstrating parameters of optimization in improving product characteristics [[19], [20], [21]]. This use of activated carbon is even more useful for the Amazon region, which has shown a high potential for contaminated areas due to the exploitation of the region's natural resources [22].
The use of residual biomass found on a large scale in the region itself can contribute to the reduction of production costs, which can facilitate access to the product. Some studies already point to the use of some of these biomasses through the production of activated carbon, however, they are carried out via chemical activation processes [[23], [24], [25], [26], [27], [28], [29], [30]]. Therefore, this work has as a novelty the use of these residual biomasses through a simpler production process, which requires basically to heat produce AC, physical activation. In addition, it can serve as a basis for accelerating the production and scale-up tests for AC from these biomasses in the Amazon region, as can already be seen for waste wood [31]. Knowing the difficulties that the region faces with the difficult access to inputs used in the remediation of environmental problems, the creation of local products that can act in this segment is of great importance, both for researchers and for decision makers who act in the mitigation of environmental impacts.
Thus, the hypothesis of this work is that the agro-industrial residues generated in the Amazon region can be used to produce activated carbon that can be used in the adsorption of different types of pollutants, generating environmental, social and financial benefits, through the valorization of these residues. Therefore, the aim of this work is to evaluate the potential of four different biomasses, which are residues from Amazon agribusinesses, in the preparation and quality of activated carbon.
2. Experimental
2.1. Material
In the municipalities of Castanhal and Igarapé Acú residues of Euterpe oleracea Mart. (acai seed), Bertholletia excelsa H.B.K (Brazil nut) and Elaeis guineensis Jacq (oil palm shell) were obtained. Orbignya speciosa (babassu) waste was obtained in the city of Bragança. Both municipalities are in the northeast of the State of Pará in the Amazon region, Brazil. Fig. 1 shows an image of the “in nature” materials. Some of the materials were crushed and ground in a mill and passed through 40, 60,100, 200 and 270 mesh screens for characterization. The classified materials were stored in an air-conditioned room until they reached a constant mass at a temperature of 20 ± 2 °C and moisture of 65 ± 3%. The remainder of the material was reserved for later carbonization and activation.
Fig. 1.
Agro-industrial waste from the Amazon Brazil nut (A), Oil palm shell (B), Acai seed (C), Babassu Kernel (D) used to produce activated carbons.
2.2. Chemical analysis of biomass
2.2.1. Molecular, elemental and proximate analysis
For chemical characterization, quantification of insoluble lignin (NBR 7989/2010), total extractives (NBR 14853/2010) and minerals (NBR 13999/2003) was performed in the biomasses [32,33]. The milled material that passed through a 40-mesh sieve and was retained on a 60-mesh screen was used in this analysis.
Elemental analysis was performed to determine the amounts of carbon, hydrogen, nitrogen, and sulfur (CHNS) in the material using a Vario Micro Cube elemental analyzer (Elemental Excellence in Elements, Germany). The ground material that passed through a 200-mesh sieve and was retained on a 270-mesh sieve was used for this analysis. Proximate analysis was performed according to the standard of ASTM-D1762-84 [34]. All analyses were performed in triplicates.
2.3. Activated carbon (AC) production – pyrolysis and activation
All materials were pyrolyzed separately in a laboratory electric muffle furnace (Quimis, model Q318524) fitted with a gas condenser and a pyroligneous liquid collector. The biomasses were inserted inside the reactor and heated at a heating rate of 100 °C h−1 until reaching a final temperature of 450 °C, which was maintained for 1 h. These carbonization conditions are commonly used in several researches, due to obtaining higher gravimetric yields and desirable fixed carbon contents. Then, the carbons were crushed and screened using the fraction retained on a 12-mesh sieve to obtain the granular form according to IUPAC recommendations. Physical activation was performed separately, specie by specie, in an electric furnace of the cylindrical type with CO2 gas. The carbons were deposited in navicles-type porcelain crucibles and heated at temperature was 850 °C (with a heating rate of 10 °C min−1), which was maintained for 60 min (residence time at final temperature) using a flow of 150 mL min−1 of CO2 gas for all species. Furnace cooling was carried out naturally and gradually.
2.4. Surface morphology and textural analysis
Images were captured using a LEO EVO 40 XVP scanning electron microscope (Carl Zeiss, Germany). The surface area, volume and pore size of the AC were determined according to the adsorption and desorption isotherms of N2 at −196,15 °C using Micromeritic Tristar II 3020 equipment (Micromeritics, United States). Before the analysis, the samples were treated at 150 °C in vacuum for 2 h. The relative pressure range (P/P0) used for surface area analysis by the BET method and total pores (Vt) was 0.98. Already the size of the pores was calculation in the desorption.
2.5. Adsorption tests and model adjustment
2.5.1. Kinetics
Approximately 10 mg of AC and 100 mL of a methylene blue (MB) solution at a concentration of 10 mg. L−1 were used to determine the adsorption kinetics of the MB dye. The solutions were maintained under stirring, and aliquots were taken at time intervals of 10, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220 and 240 min. For the phenol adsorption kinetics, approximately 20 mg of AC and 50 mL of the phenol solution at a concentration of 100 mg L−1 were used. The solutions were maintained under stirring, and aliquots were removed at intervals of 10, 20, 30, 40, 50, 60, 80, 100, 120, 140, 160 and 180 min. The concentration the solutions was determined using a UV–visible spectrophotometer (AJ MICRONAL, model PEX AJX-3000 PC, Belo Horizonte -Brazil) at wavelengths of 665 nm and 270 nm for methylene blue and phenol, respectively.
The adsorption amount (qe or qt) of rhodamine B or malachite green on MSPA in the adsorption isotherm and kinetic experiment is calculated as follows:
| (1) |
| (2) |
where Co is the initial concentration and Ce is the equilibrium concentration. Ct is the rhodamine B or malachite green concentration over time. M is the quality of MSPA [35]. V is volume of solution.
2.5.2. Isotherms
The AC were tested for the adsorption of methylene blue and phenol. The solutions with the activated carbons were maintained under stirring at 100 rpm for 24 h at room temperature (25 ± 2 °C). The methylene blue isotherms were obtained using 10 mg of adsorbent and 10 mL of the solution at different concentrations (10, 25, 50, 100, 200, 400 and 800 mg L−1) of adsorbate. The isotherms for phenol were obtained using 10 mg of adsorbent and 10 mL of the solution at different concentrations (25, 50, 100, 250, 500 and 1000 mg L−1) of adsorbate. The equilibrium concentration was performed using a UV–visible spectrophotometer (AJ MICRONAL, model AJX-3000 PC) at wavelengths of 665 nm and 270 nm for methylene blue and phenol, respectively.
2.5.3. Model adjustments
The data were analyzed using Langmuir and Freundlich isotherm models.
3. Results and discussion
3.1. Molecular composition chemistry
The chemical composition of the biomasses is shown in Table 1. The lignin content of the studied biomasses was from 21.83 to 56.40%, and Brazil nut presented the highest percentage of lignin. High levels of lignin are related to higher levels of fixed carbon and, consequently, higher yields of carbon [36]. Because it has a larger amount of lignin than the other biomasses, Brazil nut also has the highest fixed carbon content among all the biomasses, i.e., 32.32%. A high lignin content in biomass favors the production of AC, as this substance is more resistant to thermal degradation than holocelluloses. Because it is a carbon-rich component and has a similar structure to bituminous carbon, a large amount of lignin in a precursor material is ideal for the production of AC [31,37].
Table 1.
Average values of the chemical composition, standard deviation (SD) and coefficient of variation (CV) of the residues of the four biomasses.
| Sample names | Chemical composition (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lignin | Extractives | Minerals | Holocellulosea | V.Mb | Ash | F.Ca,c | ||
| Acai | Mean SD CV |
21.83 0.37 1.71 |
20.33 1.35 6.65 |
2.19 0.09 4.27 |
55.65 1.54 2.79 |
75.65 0.82 0.01 |
1.87 0.10 0.06 |
22.48 0.91 0.04 |
| Babassu | Mean SD CV |
27.77 0.78 2.81 |
5.01 0.41 8.28 |
1.76 0.06 3.77 |
65.46 0.29 0.14 |
79.61 0.83 0.01 |
1.59 0.06 0.04 |
18.78 0.83 0.04 |
| Brazil nut | Mean SD CV |
56.40 5.35 0,09 |
4.45 0.24 5.42 |
2.71 0.02 0.83 |
36.44 1.02 0.78 |
65.61 0.29 0.00 |
2.07 0.05 0.02 |
32.32 0.27 0.01 |
| Oil palm shell | Mean SD CV |
32.91 0.30 0.01 |
24.79 0.12 0.49 |
2.67 0.01 0.68 |
39.63 1.07 1.94 |
79.62 0.41 0.01 |
2.46 0.14 0.06 |
17.92 0.46 0.03 |
Where = a: Values obtained by difference; b: Volatile matter; c: Fixed Carbon.
In the extractives, the variation was from 4.45 to 24.79% due to differences among the characteristics of the biomasses and due to the different processing conditions used. In general, acai and oil palm shell had higher extractive values of 20.33 and 24.79%, respectively. These values are approximately five times greater than the values of babassu and Brazil nuts (5.01 and 4.45%, respectively). The extractive content of these materials may have been affected by the remaining almond on the acai core and the oil in the pie after palm oil processing.
The biomasses showed values of 1.76–2.71% for minerals, especially babassu, which had the lowest ash content. Low values of this property are a positive factor for the production of AC since mineral components have an unfavorable effect on the adsorption process, preferably adsorbing water due to its hydrophilic character [38].
The level of holocellulose in the biomass was from 36.44 to 65.46%. Holocellulose can affect the porosity characteristics of AC since cellulose predominantly promotes the production of microporous materials [[39], [40], [41]].
3.2. Elemental composition
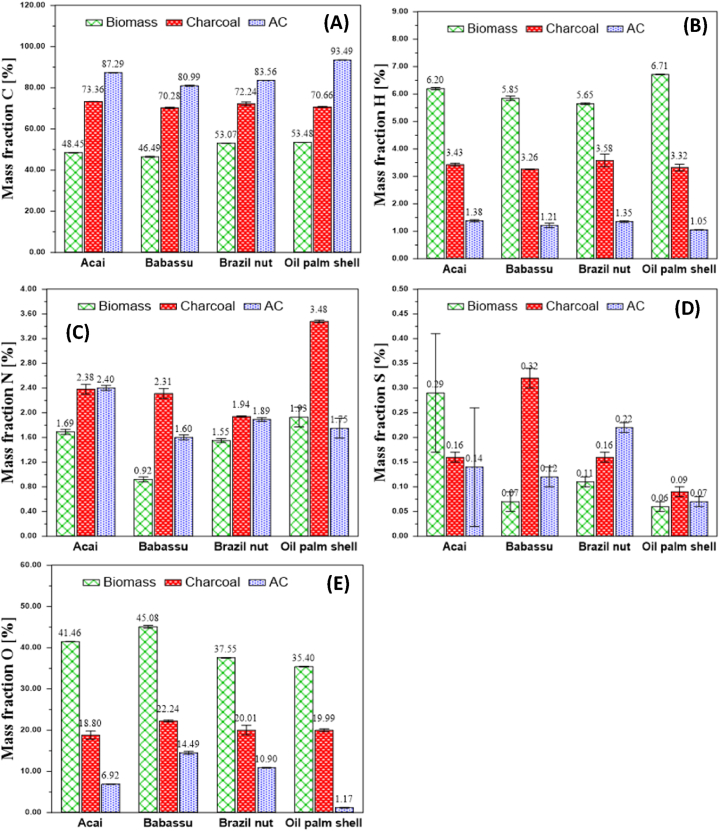
Fig. 2 shows the average values of the levels of carbon, hydrogen, nitrogen, sulfur and oxygen in the biomasses, charcoal and activated carbon. In the biomasses, the percentage of carbon was from 46.49 to 53.48%. The amount of carbon indicates the quality of the precursor material for the preparation of activated carbons since this is the main constituent of the structure of these products and affects their properties, such as porosity and surface area [42]. The levels of hydrogen, nitrogen and sulfur did not differ among the four biomasses. Regarding the oxygen content, the higher values in the biomasses, particularly babassu and acai, may indicate that they may have more functional groups on their surface carbons than charcoal and activated carbon. These functional groups act decisively in adsorption, as they affect the physical properties, wettability, polarity and acidity of the surface [[43], [44], [45]] Similar results were observed by other authors who studied residual biomass [31,46].
Fig. 2.
Average values of the elementary analysis and standard deviation (SD) for the four biomasses, charcoal and activated carbon. Carbon (A), Hydrogen (B), Nitrogen (C) Sulfur (D), Oxygen (O).
In general, in charcoal and AC the level of carbon was higher, and the levels of hydrogen and oxygen were lower than biomasses in nature. Therefore, there was a tendency of increasing carbon levels and decreasing hydrogen and oxygen levels during carbonization and activation. The levels of nitrogen and sulfur did not show a clear trend. Pyrolysis enabled us to obtain a product with a high carbon content due to the volatilization of compounds containing hydrogen and oxygen via thermal decomposition of its components [47]. Activation has similar results as carbonization since it works at high temperatures but uses an activator gas.
Thus, higher mass yields can be observed due to the trends of increasing carbon and decreasing oxygen, which cause a low oxygen/carbon ratio in the product. Increases in the carbonization-activation temperature are expected to cause an increase in the carbon content and a decrease in the hydrogen and oxygen contents since volatile compounds are lost with increasing temperature [48]. In all species, this behavior was observed. AC have a high carbon content (80.99–93.49%), which indicates that these materials may have a large surface area and pore volume. Sugarcane bagasse was used in the preparation of physically activated carbon and obtained values of 75.5–88.4% carbon [49]. Carbon content between 58.7 and 75.7% were obtained for activated carbon with CO2 that was prepared from black wattle bark [50]. Piassava fiber was used for the preparation of activated carbon with CO2, the authors found a carbon content between 82.03 and 13.53% for oxygen [51].
3.3. Morphological characteristics
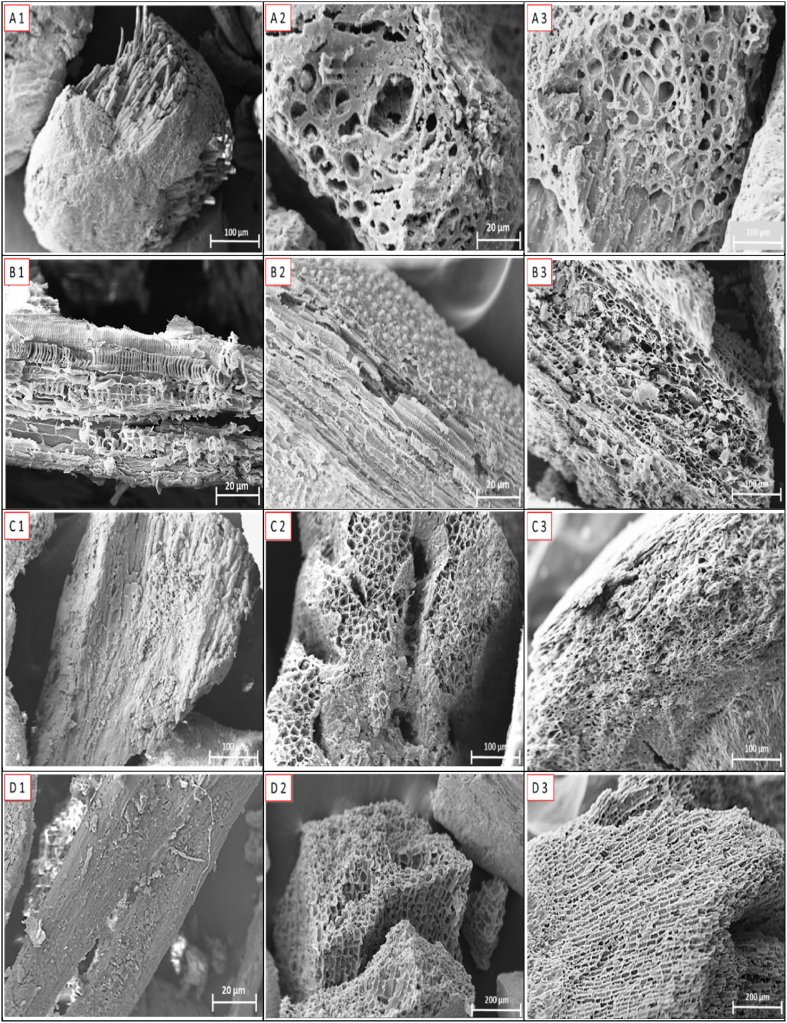
Fig. 3 shows SEM micrographs of the studied materials (A, B, C and D) in three manners: “in natura” (1), charcoal (2) and activated carbon (3). The biomass micrographs show a heterogeneous and amorphous surface, with different morphologies observed for all biomasses. The carbonization and activation process caused significant differences in their structures. The biomasses appeared to have a structure containing few pores with high heterogeneity. Some fibers were exposed, such as in acai and Brazil nut Fig. 3 (A1) and (C1). Babassu had a structure with greater porosity than the other biomasses Fig. 3(B1), with a porous structure in the shape of a honeycomb. The oil palm shell had a smoother and more homogeneous surface than the other biomasses Fig. 3 (D1), indicating this biomass had low porosity.
Fig. 3.
Micrographs of acai (A), babassu (B), Brazil nut (C) and oil palm shell (D) in three forms. A1, B1, C1 and D1 correspond to the biomasses. A2, B2, C2 and D2 correspond to charcoal; A3, B3, C3 and D3 correspond to activated carbon of different materials.
Significant changes occurred in the charcoals Fig. 3 (A2), (B2), (C2) and (D2) after the carbonization process. As a result, the charcoals developed heterogeneous surfaces, with globular fractions for babassu and macroporous structures for all the charcoals. The charcoals had a higher porosity than the biomasses. During carbonization, carbon atoms are rearranged randomly and irregularly in the form of cells (aromatic rings), leaving empty spaces [52].
A modification of the surface morphology after the activation process was observed Fig. 3 (A3), (B3), (C3) and (D3). A highly porous surface formed due to the intense elimination of volatile compounds during secondary reactions in the structure of the biomasses and charcoal due to the high activation temperature. The structure of activated carbon had a homogeneous structure and better formed pores than the biomasses. Activation tends to increase the porosity of charcoal since unstable carbons and byproducts of carbonization are removed, which can block the pores [52,53]. Apparently, the babassu and Brazil nut AC had a much more developed porous structure than the other AC Figure (A3), (B3) and (C3), following the trend observed in the biomasses and charcoal, which can be confirmed with the values presented in Table 2. An increase in porosity and changes in the morphology of the precursor material after the activation process also were observed by Refs. [54,55], which are mainly related to the shape and size of pores.
Table 2.
Textural properties of the activated carbons.
| Sample names | SBET (m2.g−1) | Vt (cm³.g−1) | Dav (nm) | References |
|---|---|---|---|---|
| Acai | 703 | 0.35 | 2.04 2.05 1.94 |
This work |
| Brazil nut | 911 | 0.45 | This work This work This work |
|
| Babassu | 1101 | 0.51 | ||
| Oil palm shell | 569 | 0.27 | 2.00 | |
| Piassava of Amazon | 492 | 0.82 | – | [12]Castro et al., 2019) |
| Charcoal waste | 736 | 0.58 | 1.24 | (Maneerung et al., 2016) |
| Buckwheat husk | 578 | 0.261 | – | (Pena et al., 2020) |
| Sawdust massaranduba | 697 | – | – | (Nobre et al., 2015) |
Where = SBET: surface area; Vt: total pore volume; Dav: average pore diameter.
3.4. N2 adsorption-desorption isotherms, surface area and pore structure
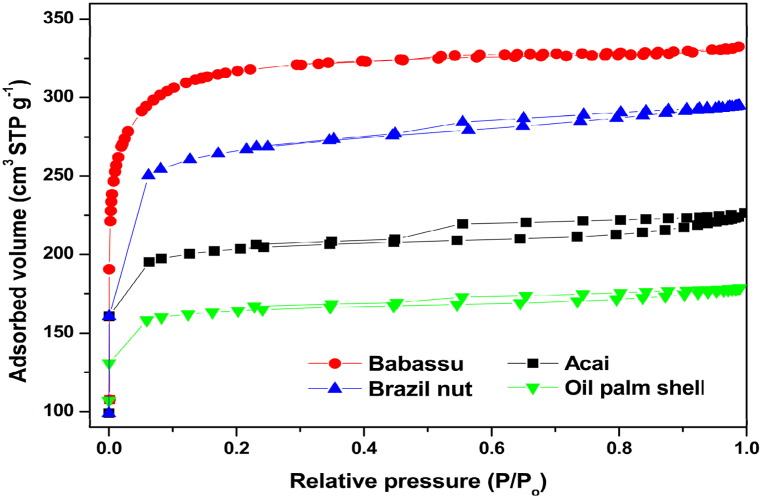
Fig. 4 shows the N2 adsorption isotherms and desorption from activated carbon. According to the IUPAC classification, the adsorption and desorption isotherms for Babassu were classified as type I, which are characteristic of microporous materials. For acai, Brazil nuts and oil palm shell, the isotherms had type I (b) characteristics of materials with narrow mesopores and a small hysteresis of type H4, which is typical of micro mesoporous materials. According to Table 2, the activated carbon with the greatest surface area was produced from babassu 1101.26 m2 g−1, followed by that produced from Brazil nut AC 911.75 m2 g−1, acai 703.14 m2 g−1 and oil palm 569.65 m2 g−1. The high surface area in babassu activated carbon can be attributed to the fibrous form of babassu coconut.
Fig. 4.
Nitrogen adsorption-desorption isotherms at 77 K for the activated ACs of the four biomasses.
The produced AC had a satisfactory surface area, comparable to the average of commercial activated carbon of 600–1.200 m2 g−1. Table 2 presents the values of the surface area from studies that used other types of precursors and the same type of activation. The studied AC had a larger surface area than those reported in the literature, which enabled us to infer that the carbons produced are likely to have a high adsorption capacity. Some studies show that the surface area of charcoal tends to increase significantly at lower pyrolysis temperatures (between 400 and 600 °C) and tends to decrease at higher temperatures (between 700 and 800 °C) [56]. However, it is worth noting that in the present study, activating chemistry agents were used, which ended up contributing efficiently to increasing the surface area of the carbon, demonstrating the importance of activation at correct temperatures and with the good use of activating agents.
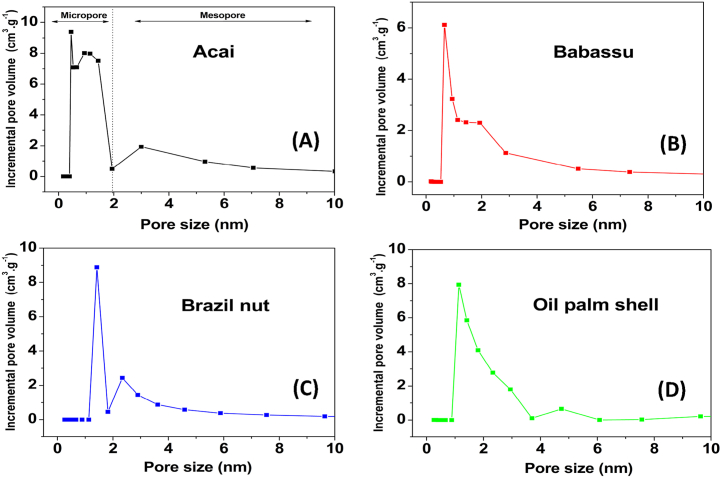
The distribution of the pore size for the AC when the Functional Density Theory (DFT) method was applied are shown in Fig. 5. We observed a typical distribution of pore size of the AC prepared from the four biomasses, which indicates that the main peak was below 2 nm. Thus, the predominantly microporous nature of the material was demonstrated. Mesopores were found in all the AC. The development of these mesopores may be associated with the burning of the internal walls of the existing micropores, which makes them have a uniform large size.
Fig. 5.
Pore size distribution of the ACs obtained from the four biomasses. Acai (A), Babassu (B), Brazil nut (C), Oil palm shell (D).
According to the authors, condensation may be responsible for the microporosity and large surface area in AC. The suggested mechanism was a condensation reaction, such as R, - OH + R, H + R, R, + H, O, followed by an attack on carbon by the steam generated. It has also been suggested that this type of oxygen action would affect the atomic configurations. Therefore, the microporosity developed may be related to the combination of oxygen and carbon in the raw material during the pyrolysis process, especially carbon monoxide. By observing the oxygen content of the biomasses and respective AC (Fig. 2), we can associate the characteristics of porosity and surface area with these contents. Thus, the results that stand out are for babassu, Brazil nut, acai and oil palm shell in order.
The same order is also followed for the microporosity and surface area results of the carbons. Microporous AC provide a greater capacity for the adsorption of small molecules, such as gases, and solvents of reduced dimensions and molecular masses [57]. In contrast, the development of mesoporosity is more desirable than microporosity when the AC are intended to be used in liquid phase applications, such as in the adsorption of organic acids [58].
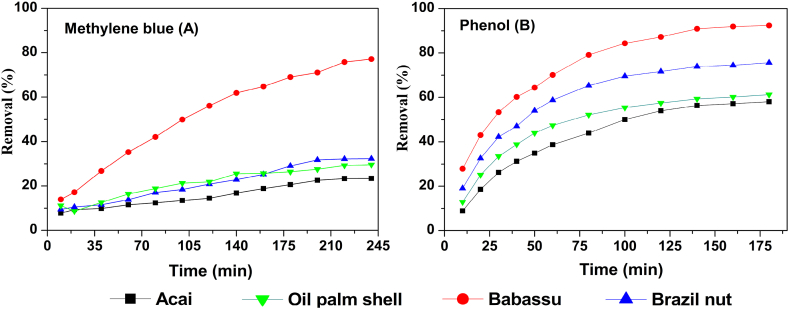
3.5. Adsorption kinetics
Fig. 6A and (B) shows the adsorption kinetics of AC for the removal of methylene blue and phenol compounds. There was rapid adsorption of methylene blue dye by babassu AC in the first 30 min (Fig. 6A). For the other AC, the adsorption was slower. This result may be related to the characteristics of these AC, such as their high surface area and porosity in addition to the filling of the active sites in the adsorbents. Even after 80 min, equilibrium was not achieved (which implies that the curve continued to increase with adsorption). The babassu AC showed a removal of approximately 80% for methylene blue at 245 min. The adsorption speed may be related to the number of adsorption sites and free functional groups available on the surface of the carbons in addition to the minor/major stereochemical impediment for dye molecules. The rapid filling of the adsorption sites and the subsequent adsorption process may become slower [59].
Fig. 6.
Adsorption kinetics for methylene blue and phenol (25 °C) on activated carbon. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The dye removal by babassu activated carbon was higher than that by the other AC produced. This difference can be attributed to the amount of oxygen in this biomass and its surface chemistry [60]. Babassu AC had the highest oxygen content or the AC produced (14.49%), and the presence of oxygen in the carbons of babassu AC is substantially linked to the surface, which is responsible for binding to the functional groups. These groups are very important in the adsorption process since they are on the surface and can be physically chemically bound to the adsorbed substances [9]. Thus, the surfaces can be modified in terms of acidity, polarity and wettability depending on the oxygen content and functional groups that form. Another factor that can explain this result is the surface area of these carbons.
Larger surface areas have more sites and a higher adsorption capacity than smaller surface areas. The babassu AC had the largest surface area of the AC produced, followed by the Brazil nut, acai and oil palm shell AC, as shown in Table 2. Porosity also affects the adsorption capacity. As observed in the electron micrographs (Fig. 3), the babassu AC had a higher porosity and better formed pores than the other AC produced. This factor especially contributes to physical adsorption. The aforementioned factors affect adsorption, but it is difficult to specify the impact of each factor. Therefore, the performance of an activated carbon in adsorption can be explained by these factors, but the characteristics of the adsorbate and adsorption conditions must also be determined.
Phenol removal (Fig. 6B) occurred most efficiently in the first 40 min of adsorption for all the AC and gradually decreased until equilibrium was reached.
The babassu AC again was notable in relation to the others, with almost 100% removal of phenol. The high removal of phenol by the babassu AC than the other AC produced may be related to its surface area, pore size and/or surface chemistry, as previously described. For all AC, equilibrium was reached in approximately 140 min. The removal of these dyes is related to the surface area. The lower removal of methylene blue and phenol by the Brazil nut, oil palm shell and acai AC can be attributed to the smaller surface area of both carbons. In addition, other factors, such as the adsorbate characteristics, surface chemistry, porosity, and adsorption conditions, may have affected the adsorption kinetics.
The adsorption efficiency by different activated carbons is reported in literature and compared with the activated carbons in this study. The AC prepared from the four biomasses showed high efficiency in removing these dyes, especially the babassu AC, even when comparable to chemically activated and commercial carbons. Ramírez Muñoz et al. [61] observed removal of 50% of MB in AC of oil palm waste chemically activated with ZnCl2 and achieving equilibrium in 120 min. Already Ghasemi and Asadpour [62] observed adsorption equilibrium curve in the removal of MB in 170 min commercial AC chemically activated with H2SO4. For phenol adsorption, Qu et al. [63], observed efficiency of 87.3% and adsorption equilibrium of 60 min for commercial activated carbons. El Hannafi et al. [64], obtained a 54% efficiency in removing phenol and adsorption equilibrium at 60 min in AC of Peach stone chemically activated with HCl.
3.6. Adsorption and adjustment of the models
3.6.1. Methylene blue
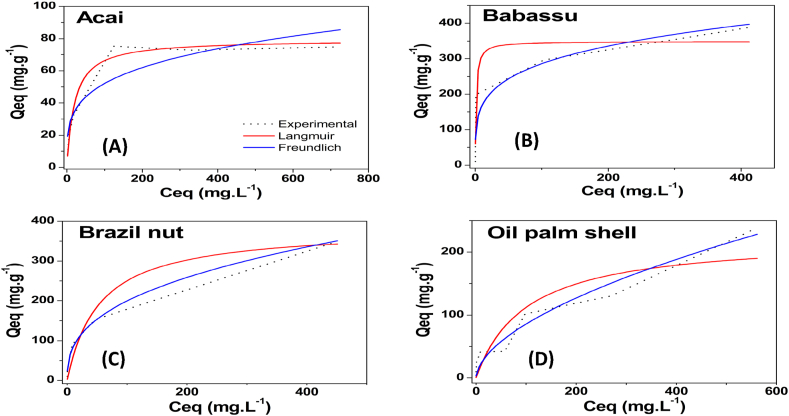
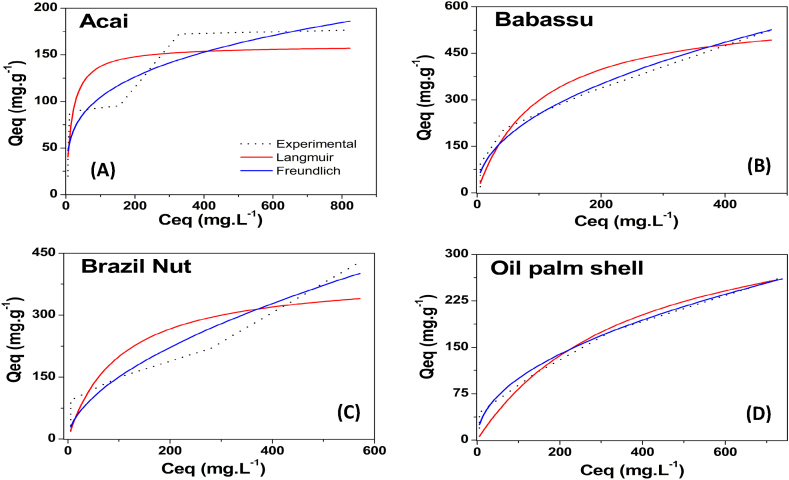
The methylene blue adsorption isotherms for the AC studied are shown in Fig. 7. AC are efficient for the adsorption of methylene blue, with values of 93–390 mg g−1. This efficiency may be related to the presence of mesopores since they have been reported to be a determining factor in the adsorption of large molecules, such as the methylene blue molecule [65]. Another possibly related factor is the surface area of the AC. The highest values were found for babassu and Brazil nut AC, which also had larger surface areas than the other AC produced.
Fig. 7.
Adsorption isotherms for methylene blue by the ACs produced from the four biomasses. Acai (A), Babassu (B), Brazil nut (C), Oil palm shell (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The activated carbons produced in this work showed satisfactory performance for removing methylene blue and were compared to other AC produced from different lignocellulosic biomasses and activation processes reported in the literature. The babassu and Brazil nut AC presented higher qm values than all the other AC. However, these values are approximately half of those of chemically activated AC, which shows that chemical activation is more efficient for producing materials with a high capacity to remove methylene blue. Castro et al. [31], and Albadarin et al. [66], working with activated carbon of different biomasses and physical activation obtained removal (qm) of 341.4 and 36.25 mg g−1 respectively. Small et al. [67], using physical activation in coke sand obtained removal of 124 mg g−1 for MB. Rodriguez Correa et al. [68], and Ramírez Muñoz et al. [61],observed removal of 747 and 763.4 mg g−1 for chemically activated carbons from different biomasses and Palm fiber respectively.
Langmuir and Freundlich models for the adsorption of methylene blue (MB) on the different produced AC are described in Table 3. Comparing the values of R2 (Qe vs. Ce), the Langmuir model presented a better fit for the isotherm data of methylene blue on the acai and babassu AC than the other AC produced. Thus, the occurrence of adsorption in the monolayer or a homogeneous surface indicates adsorption by chemisorption [66]. In the Brazil nut and oil palm shell AC, the data were better adjusted in the Freundlich model, which indicates that adsorption probably occurs in multilayers.
Table 3.
Langmuir and Freundlich parameters for the adsorption of BM on the activated carbon produced form the different biomasses.
| Molecule | ACs | Langmuir parameters |
Freundlich parameters |
||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | ||
| Methylene blue | Acai | 79.37 | 0.050 | 0.975 | 16.49 | 0.249 | 0.818 |
| Babassu | 348.88 | 0.744 | 0.890 | 98.05 | 0.232 | 0.830 | |
| Brazil nut | 383.33 | 0.018 | 0.948 | 35.62 | 0.018 | 0.994 | |
| Oil palm shell | 223.98 | 0.010 | 0.840 | 6.24 | 0.568 | 0.937 | |
Where = qm: maximum adsorption capacity (mg.g−1); KL: Langmuir Constant (L.mg−1); R2: correlation coefficient; KF: Freundlich constant [(mg. L−1)(L. mg−1)1/n]; 1/n: Freundlich parameter.
3.6.2. Phenol
The phenol adsorption isotherms for the AC produced from the different biomasses are shown in Fig. 8. All the AC had a high capacity for phenol adsorption, with a maximum capacity greater than 159 mg g−1, especially the babassu AC (596, 69 mg g−1). The result of adsorption can be considered satisfactory, since the, in aqueous solutions H bonds decreases the adsorption of phenol [69]. This high adsorption can be attributed to the large surface area and microporosity of this carbon.
Fig. 8.
Adsorption isotherms for Phenol by the ACs produced from the four biomasses. Acai (A), Babassu (B), Brazil nut (C), Oil palm shell (D).
Table 4 presents the Langmuir and Freundlich parameters for phenol adsorption. It is observed that for all the activated carbons in this study, the Freundlich model obtained a better adjustment of the adsorption data than the Langmuir model, this tendency was observed in other works [69,70]. All the AC had a high adsorption capacity. The 1/n values of the AC were 0.274–0.563, which indicates a heterogeneous surface of these materials.
Table 4.
Langmuir and Freundlich parameters for the adsorption of phenol by the activated carbons produced from the different biomasses.
| Molecule | ACs | Langmuir parameters |
Freundlich parameters |
||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | ||
| Phenol | Acai | 159.95 | 0.060 | 0.738 | 29.55 | 0.274 | 0.807 |
| Babassu | 596.69 | 0.010 | 0.949 | 29.31 | 0.468 | 0.969 | |
| Brazil nut | 399.50 | 0.010 | 0.806 | 11.19 | 0.563 | 0.915 | |
| Oil palm shell | 391.00 | 0.002 | 0.910 | 10.82 | 0.524 | 0.982 | |
Where = qm: maximum adsorption capacity (mg.g−1); KL: Langmuir Constant (L.mg−1); R2: correlation coefficient; KF: Freundlich constant [(mg L−1)(L.mg−1)1/n]; 1/n: Freundlich parameter.
3.7. Practical applications and future perspectives
The evaluated materials showed promising results for use to produce activated carbon. With the use of residual biomass available in the Amazon region itself, the costs to produce activated carbon can be significantly reduced. As a result, water treatment costs in the region can follow the same cost reduction trend. It is important to remember the importance of tropical forests for climate control, and it is worth highlighting the importance of the Amazon for local and global biodiversity. Thus, actions aimed at its preservation and the remediation of environmental problems found in this region should be valued. Especially when adding value to waste available on a large scale in the region. The study of new adsorbent materials that can remedy pollution in aquatic and terrestrial environments is extremely important to ensure the maintenance of a possible production of activated carbon, guaranteeing good results and a reduction in costs. With this, it is possible to directly achieve goals 6 (Clean water and sanitation), 14 (Life below water), and help indirectly achieve goal 13 (Climate action) of the United Nations Sustainable Development Goals [71].
Future studies may focus on evaluating the adsorption of other organic and inorganic pollutants. In addition, other types and conditions of physical and chemical adsorption can be evaluated, to increase the adsorption capacity of the biomass investigated in this study. Studies that evaluate the effect of pH, as well as studies that evaluate desorption, to establish the life cycle of the adsorbents produced must be carried out, in order to generate more knowledge about the materials. In addition, studies that investigate the adsorption mechanism of the adsorbents characterized in this study are of great importance to guarantee the commercial and industrial application in an appropriate way, guaranteeing improvements in the adsorption of pollutants.
Other residual biomass found in the Amazon region should also be evaluated in the production of activated carbon, with the aim of increasing the availability of precursor materials that can be used in this production. Finally, we suggest that studies that evaluate the economic feasibility of producing activated carbon with these residual biomasses should be carried out, in this way, more accurate information about the costs of production and application of the material can be obtained.
4. Conclusion
The residues produced from four biomasses have high potential to be used as precursors in the preparation of physically activated carbon. The prepared activated carbons had high microporosity with a surface area of 569.65 m2 g−1 for oil palm shell and 1101.26 m2 g−1 for Babassu. All the activated carbons had a high adsorption capacity for absorption of methylene blue (79.37–383.88 mg g−1) and phenol (159.95–596.69 mg g−1). Among the activated carbons produced from the four biomasses, the Babassu and Brazil nut activated carbons were superior because in addition to having a large surface area, they had a greater adsorption capacity. Babassu AC had a higher oxygen content and surface area than the other AC produced and an excellent porosity adsorption capacity for methylene blue and phenol. With the findings of this study, it is possible to devise strategies for the reuse of waste in a higher value-added production, bringing different benefits to the Amazon region, ensuring the maintenance of its sustainability in different aspects.
New studies can evaluate other conditions of physical and chemical activation, aiming at increasing the adsorption capacity of biomass. In addition, the study of other residual biomass available in the region and research that assess the economic viability of AC production are of great importance for the advancement of the sector in the Amazon region. Studies that evaluate the adsorption mechanism of these studied materials should also be developed, to facilitate the understanding of the factors involved in the adsorption process.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors have no conflict of interest to declare. For their financial support, Coordination of Superior Level Staff Improvement (CAPES), National Council for Scientific and Technological Development (CNPq), and Graduate Program in Wood Science and Technology (UFLA), Brazil. PROPESP/UFPA (PAPQ), The State University of Pará, Brazil, The Laboratory of Oils of the Amazon (LOA), Banco da Amazônia (233/2022) and the Science and Technology Park (PCT) -GUAMÁ, Brazil are also acknowledged for their effort.
Contributor Information
João Rodrigo Coimbra Nobre, Email: rodrigonobre@uepa.br, rodrigonobre@hotmail.com.br.
Elias Costa de Souza, Email: eliasrem@usp.br, eliasrem@hotmail.com.
References
- 1.Reza M.S., Yun C.S., Afroze S., Radenahmad N., Bakar M.S.A., Saidur R., Taweekun J., Azad A.K. Preparation of activated carbon from biomass and its' applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020;27:208–238. doi: 10.1080/25765299.2020.1766799. [DOI] [Google Scholar]
- 2.Fito J., Abrham S., Angassa K. Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. Int. J. Environ. Res. 2020;14:501–511. doi: 10.1007/s41742-020-00273-2. [DOI] [Google Scholar]
- 3.El-Bery H.M., Saleh M., El-Gendy R.A., Saleh M.R., Thabet S.M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-09475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho E.J., Trinh L.T.P., Song Y., Lee Y.G., Bae H.J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020;298 doi: 10.1016/j.biortech.2019.122386. [DOI] [PubMed] [Google Scholar]
- 5.Igwegbe C.A., Aniagor C.O., Oba S.N., Yap P.S., Iwuchukwu F.U., Liu T., de Souza E.C., Ighalo J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: a comprehensive review. J. Ind. Eng. Chem. 2021;104:117–135. doi: 10.1016/J.JIEC.2021.08.015. [DOI] [Google Scholar]
- 6.Ramutshatsha-Makhwedzha D., Mavhungu A., Moropeng M.L., Mbaya R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oladimeji T.E., Odunoye B.O., Elehinafe F.B., Obanla O.R., Odunlami O.A. Production of activated carbon from sawdust and its efficiency in the treatment of sewage water. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e05960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Souza L.K.C., Gonçalves A.A.S., Queiroz L.S., Chaar J.S., Geraldo N., Filho R., Carlos E.F. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO 2 capture. Sustain. Mater. Tech. 2020;25 doi: 10.1016/j.susmat.2020.e00168. [DOI] [Google Scholar]
- 9.Souza E.C., Pimenta A.S., Silva A.J.F., Nascimento P.F.P., Ighalo J.O. Biomass Convers Biorefin; 2021. Oxidized eucalyptus Charcoal: a Renewable Biosorbent for Removing Heavy Metals from Aqueous Solutions. [DOI] [Google Scholar]
- 10.Lam E., Luong J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014;4:3393–3410. doi: 10.1021/cs5008393. [DOI] [Google Scholar]
- 11.Igwegbe C.A., Aniagor C.O., Oba S.N., Yap P.-S., Iwuchukwu F.U., Liu T., Souza E.C., Ighalo J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: a comprehensive review. J. Ind. Eng. Chem. 2021;104:117–135. doi: 10.1016/j.jiec.2021.08.015. [DOI] [Google Scholar]
- 12.Ukanwa K.S., Patchigolla K., Sakrabani R., Anthony E., Mandavgane S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability. 2019;11:1–35. doi: 10.3390/su11226204. [DOI] [Google Scholar]
- 13.Bispo M.D., Schneider J.K., Da Silva Oliveira D., Tomasini D., Da Silva MacIel G.P., Schena T., Onorevoli B., Bjerk T.R., Jacques R.A., Krause L.C., Caramão E.B. Production of activated biochar from coconut fiber for the removal of organic compounds from phenolic. J. Environ. Chem. Eng. 2018;6:2743–2750. doi: 10.1016/j.jece.2018.04.029. [DOI] [Google Scholar]
- 14.TrendEconomy . 2018. World Merchandise Exports and Imports by Commodity. [Google Scholar]
- 15.Reis J.S., Araujo R.O., Lima V.M.R., Queiroz L.S., da Costa C.E.F., Pardauil J.J.R., Chaar J.S., Rocha Filho G.N., de Souza L.K.C. Combustion properties of potential Amazon biomass waste for use as fuel. J. Therm. Anal. Calorim. 2019;138:3535–3539. doi: 10.1007/s10973-019-08457-5. [DOI] [Google Scholar]
- 16.Araujo R.O., Chaar J. da S., Queiroz L.S., da Rocha Filho G.N., da Costa C.E.F., da Silva G.C.T., Landers R., Costa M.J.F., Gonçalves A.A.S., de Souza L.K.C. Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions. Energy Convers. Manag. 2019;196:821–830. doi: 10.1016/j.enconman.2019.06.059. [DOI] [Google Scholar]
- 17.SIDRA . 2018. Produção da Extração Vegetal e da Silvicultura. [Google Scholar]
- 18.Reis J.S., Araujo R.O., Lima V.M.R., Queiroz L.S., da Costa C.E.F., Pardauil J.J.R., Chaar J.S., Rocha Filho G.N., de Souza L.K.C. Combustion properties of potential Amazon biomass waste for use as fuel. J. Therm. Anal. Calorim. 2019;138:3535–3539. doi: 10.1007/s10973-019-08457-5. [DOI] [Google Scholar]
- 19.Castro J.P., Nobre J.R.C., Bianchi M.L., Trugilho P.F., Napoli A., Sen Chiou B., Williams T.G., Wood D.F., Avena-Bustillos R.J., Orts W.J., Tonoli G.H.D. Activated carbons prepared by physical activation from different pretreatments of amazon piassava fibers. J. Nat. Fibers. 2019;16:961–976. doi: 10.1080/15440478.2018.1442280. [DOI] [Google Scholar]
- 20.Rodriguez Correa C., Otto T., Kruse A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy. 2017;97:53–64. doi: 10.1016/j.biombioe.2016.12.017. [DOI] [Google Scholar]
- 21.Tounsadi H., Khalidi A., Machrouhi A., Farnane M., Rachid E., Elhalil A., Sadiq M., Barka N. Highly efficient activated carbon from Glebionis coronaria L. biomass: optimization of preparation conditions and heavy metals removal using experimental design approach. J. Environ. Chem. Eng. 2016;4 doi: 10.1016/j.jece.2016.10.020. [DOI] [Google Scholar]
- 22.Pereira W.V. da S., Teixeira R.A., de Souza E.S., de Moraes A.L.F., Campos W.E.O., do Amarante C.B., Martins G.C., Fernandes A.R. Chemical fractionation and bioaccessibility of potentially toxic elements in area of artisanal gold mining in the Amazon. J. Environ. Manag. 2020;267 doi: 10.1016/j.jenvman.2020.110644. [DOI] [PubMed] [Google Scholar]
- 23.Catharin C.W., Chaves A.R., de Souza P.S., Pérez C.N. Babassu activated carbon as catalyst for chalcone production by Claisen–Schmidt reaction: kinetic study, mechanism proposal and continuous flow bed reactor. Braz. J. Chem. Eng. 2020;37:415–423. doi: 10.1007/s43153-020-00034-w. [DOI] [Google Scholar]
- 24.Queiroz L.S., de Souza L.K.C., Thomaz K.T.C., Leite Lima E.T., da Rocha Filho G.N., do Nascimento L.A.S., de Oliveira Pires L.H., Faial K. do C.F., da Costa C.E.F. Activated carbon obtained from amazonian biomass tailings (acai seed): modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020;270 doi: 10.1016/j.jenvman.2020.110868. [DOI] [PubMed] [Google Scholar]
- 25.Pang Y.L., Lim S., Lee R.K.L. Enhancement of sonocatalytic degradation of organic dye by using titanium dioxide (TiO2)/activated carbon (AC) derived from oil palm empty fruit bunch. Environ. Sci. Pollut. Control Ser. 2020;27:34638–34652. doi: 10.1007/s11356-019-05373-x. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A., Razzino C. do A., Dasgupta A., Fujisawa K., Vieira L.H.S., Subramanian S., Costa R.S., Lobo A.O., Ferreira O.P., Robinson J., Terrones M., Terrones H., Viana B.C. Structural and electrochemical properties of babassu coconut mesocarp-generated activated carbon and few-layer graphene. Carbon N Y. 2019;145:175–186. doi: 10.1016/j.carbon.2018.12.114. [DOI] [Google Scholar]
- 27.De Souza L.K.C., Gonçalves A.A.S., Queiroz L.S., Chaar J.S., Geraldo N., Filho R., Carlos E.F. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO 2 capture. Sustain. Mater. Tech. 2020;25 doi: 10.1016/j.susmat.2020.e00168. [DOI] [Google Scholar]
- 28.de Sousa Ribeiro L.A., Thim G.P., Alvarez-Mendez M.O., dos Reis Coutinho A., de Moraes N.P., Rodrigues L.A. Preparation, characterization, and application of low-cost açaí seed-based activated carbon for phenol adsorption. Int. J. Environ. Res. 2018;12:755–764. doi: 10.1007/s41742-018-0128-5. [DOI] [Google Scholar]
- 29.dos Melo S. dos S., de Diniz J.E.M., Guimarães J.H., da Costa J.S., Brasil D.S.B., dos S.S., de Morais S., Brito D.C., Carvalho J.C.T., dos Santos C.B.R., da Silva D.L. Production and characterization of absorbent heat from the bark of residual Brazil nut bark (Bertholletia Excelsa l.) Chem. Cent. J. 2015;9:1–9. doi: 10.1186/s13065-015-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad N., Ibrahim N., Yun Fu P., Ahmad R. Influence of carbonisation temperature on the surface pore characteristics of acid-treated oil palm empty fruit bunch activated carbon. J. Teknol. 2020;82:127–133. doi: 10.11113/jt.v82.14598. [DOI] [Google Scholar]
- 31.Castro J.P., Nobre J.R.C., Bianchi M.L., Trugilho P.F., Napoli A., Sen Chiou B., Williams T.G., Wood D.F., Avena-Bustillos R.J., Orts W.J., Tonoli G.H.D. Activated carbons prepared by physical activation from different pretreatments of amazon piassava fibers. J. Nat. Fibers. 2019;16:961–976. doi: 10.1080/15440478.2018.1442280. [DOI] [Google Scholar]
- 32.de N.T A.B. 2010. ABNT, NBR 14853 Wood - Determination of soluble matter in ethanol-toluene and in dichloromethane and in acetone, Associação Brasileira de Normas Técnicas. [Google Scholar]
- 33.de N.T A.B. vols. 1–6. 2010. ABNT, (NBR 7989:2010 - Pulp and Wood - Determination of Acid-Insoluble Lignin). [Google Scholar]
- 34.ASTM, D 1762-84: Standard Test Method for Chemical Analysis of Wood Charcoal, vol. 84 (2007) 1–2. 10.1520/D1762-84R07.2. [DOI]
- 35.Cheng S., Zhao S., Xing B., Shi C., Meng W., Zhang C., Bo Z. Facile one-pot green synthesis of magnetic separation photocatalyst-adsorbent and its application. J. Water Proc. Eng. 2022;47 doi: 10.1016/J.JWPE.2022.102802. [DOI] [Google Scholar]
- 36.Cagnon B., Py X., Guillot A., Stoeckli F., Chambat G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009;100:292–298. doi: 10.1016/j.biortech.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Suhas P.J.M., Carrott M.M.L., Ribeiro Carrott. Lignin – from natural adsorbent to activated carbon: a review. Bioresour. Technol. 2007;98:2301–2312. doi: 10.1016/j.biortech.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Moreno-Castilla C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N Y. 2004;42:83–94. doi: 10.1016/j.carbon.2003.09.022. [DOI] [Google Scholar]
- 39.Cagnon B., Py X., Guillot A., Stoeckli F., Chambat G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009;100:292–298. doi: 10.1016/j.biortech.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Yahya M.A., Al-Qodah Z., Ngah C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew. Sustain. Energy Rev. 2015;46:218–235. doi: 10.1016/j.rser.2015.02.051. [DOI] [Google Scholar]
- 41.Hassan M.F., Sabri M.A., Fazal H., Hafeez A., Shezad N., Hussain M. Recent trends in activated carbon fibers production from various precursors and applications—a comparative review. J. Anal. Appl. Pyrolysis. 2020;145 doi: 10.1016/j.jaap.2019.104715. [DOI] [Google Scholar]
- 42.Bansal R.C., Goyal M. CRC Press; 2005. Activated Carbon Adsorption. [DOI] [Google Scholar]
- 43.Gonçalves M., Sánchez-García L., De Oliveira Jardim E., Silvestre-Albero J., Rodríguez-Reinoso F. Ammonia removal using activated carbons: effect of the surface chemistry in dry and moist conditions. Environ. Sci. Technol. 2011;45:10605–10610. doi: 10.1021/es203093v. [DOI] [PubMed] [Google Scholar]
- 44.Yao S., Zhang J., Shen D., Xiao R., Gu S., Zhao M., Liang J. vol. 463. 2016. pp. 118–127. (Journal of Colloid and Interface Science Removal of Pb (II) from Water by the Activated Carbon Modified by Nitric Acid under Microwave Heating). [DOI] [PubMed] [Google Scholar]
- 45.Queiroz L.S., de Souza L.K.C., Thomaz K.T.C., Leite Lima E.T., da Rocha Filho G.N., do Nascimento L.A.S., de Oliveira Pires L.H., Faial K. do C.F., da Costa C.E.F. Activated carbon obtained from amazonian biomass tailings (acai seed): modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020;270 doi: 10.1016/j.jenvman.2020.110868. [DOI] [PubMed] [Google Scholar]
- 46.Demiral H., Demiral İ., Karabacakoğlu B., Tümsek F. Production of activated carbon from olive bagasse by physical activation. Chem. Eng. Res. Des. 2011;89:206–213. doi: 10.1016/j.cherd.2010.05.005. [DOI] [Google Scholar]
- 47.Wang D., Jiang P., Zhang H., Yuan W. Biochar production and applications in agro and forestry systems: a review. Sci. Total Environ. 2020;723 doi: 10.1016/j.scitotenv.2020.137775. [DOI] [PubMed] [Google Scholar]
- 48.Jin X.-J., Zhang M.-Y., Wu Y., Zhang J., Mu J. Nitrogen-enriched waste medium density fiberboard-based activated carbons as materials for supercapacitors. Ind. Crop. Prod. 2013;43:617–622. doi: 10.1016/j.indcrop.2012.08.006. [DOI] [Google Scholar]
- 49.Giusto L.A.R., Pissetti F.L., Castro T.S., Magalhães F. Preparation of activated carbon from sugarcane bagasse soot and methylene blue adsorption. Water Air Soil Pollut. 2017;228 doi: 10.1007/s11270-017-3422-5. [DOI] [Google Scholar]
- 50.de Aguiar Linhares F., Romeu Marcílio N., Juarez Melo P. Estudo da produção de carvão ativado a partir do resíduo de casca da acácia negra com e sem ativação química. Scientia Cum Industria. 2016;4:74–79. doi: 10.18226/23185279.v4iss2p74. [DOI] [Google Scholar]
- 51.Castro J.P., Nobre J.R.C., Napoli A., Trugilho P.F., Tonoli G.H.D., Wood D.F., Bianchi M.L. Pretreatment affects activated carbon from piassava. Polymers. 2020;12:1483. doi: 10.3390/polym12071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bansal R.C., Goyal M. CRC Press; 2005. Activated Carbon Adsorption. [DOI] [Google Scholar]
- 53.García P.G. Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018;82:1393–1414. doi: 10.1016/j.rser.2017.04.117. [DOI] [Google Scholar]
- 54.Aguayo-Villarreal I.A., Bonilla-Petriciolet A., Muñiz-Valencia R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017;230:686–695. doi: 10.1016/j.molliq.2017.01.039. [DOI] [Google Scholar]
- 55.Couto G.M., Dessimoni A.L. de A., Bianchi M.L., Perígolo D.M., Trugilho P.F. Use of sawdust Eucalyptus sp. in the preparation of activated carbons. Cienc. E Agrotecnol. 2012;36:69–77. doi: 10.1590/S1413-70542012000100009. [DOI] [Google Scholar]
- 56.Cheng S., Meng M., Xing B., Shi C., Nie Y., Xia D., Yi G., Zhang C., Xia H. Preparation of valuable pyrolysis products from poplar waste under different temperatures by pyrolysis: evaluation of pyrolysis products. Bioresour. Technol. 2022;364 doi: 10.1016/J.BIORTECH.2022.128011. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Almodovar-Arbelo N.E., Weidman J.L., Corti D.S., Boudouris B.W., Phillip W.A. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. NPJ Clean Water. 2018;1:1–14. doi: 10.1038/s41545-018-0002-1. [DOI] [Google Scholar]
- 58.Yang R.T. John Wiley & Sons; New Jersey: 2003. Adsorbents: Fundamentals and Applications. [Google Scholar]
- 59.Albadarin A.B., Collins M.N., Naushad M., Shirazian S., Walker G., Mangwandi C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017;307:264–272. doi: 10.1016/j.cej.2016.08.089. [DOI] [Google Scholar]
- 60.Sun Y., Yu I.K.M., Tsang D.C.W., Fan J., Clark J.H., Luo G., Zhang S., Khan E., Graham N.J.D. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020;398 doi: 10.1016/j.cej.2020.125505. [DOI] [Google Scholar]
- 61.Ramírez Muñoz A.P., Giraldo S., Flórez Yepes E., Acelas Soto N.Y. Preparación de carbón activado a partir de residuos de palma de aceite y su aplicación para la remoción de colorantes. Rev. Colomb. Quím. 2017;46:33. doi: 10.15446/rev.colomb.quim.v46n1.62851. [DOI] [Google Scholar]
- 62.Ghasemi J., Asadpour S. Thermodynamics' study of the adsorption process of methylene blue on activated carbon at different ionic strengths. J. Chem. Thermodyn. 2007;39:967–971. doi: 10.1016/j.jct.2006.10.018. [DOI] [Google Scholar]
- 63.Qu G., Liang D., Qu D., Huang Y., Liu T., Mao H., Ji P., Huang D. Simultaneous removal of cadmium ions and phenol from water solution by pulsed corona discharge plasma combined with activated carbon. Chem. Eng. J. 2013;228:28–35. doi: 10.1016/j.cej.2013.04.114. [DOI] [Google Scholar]
- 64.El Hannafi N., Boumakhla M.A., Berrama T., Bendjama Z. Elimination of phenol by adsorption on activated carbon prepared from the peach cores: modelling and optimisation. Desalination. 2008;223:264–268. doi: 10.1016/j.desal.2007.01.229. [DOI] [Google Scholar]
- 65.Bestani B., Benderdouche N., Benstaali B., Belhakem M., Addou A. Methylene blue and iodine adsorption onto an activated desert plant. Bioresour. Technol. 2008;99:8441–8444. doi: 10.1016/j.biortech.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 66.Albadarin A.B., Collins M.N., Naushad M., Shirazian S., Walker G., Mangwandi C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017;307:264–272. doi: 10.1016/j.cej.2016.08.089. [DOI] [Google Scholar]
- 67.Small C.C., Hashisho Z., Ulrich A.C. Preparation and characterization of activated carbon from oil sands coke. Fuel. 2012;92:69–76. doi: 10.1016/j.fuel.2011.07.017. [DOI] [Google Scholar]
- 68.Rodriguez Correa C., Otto T., Kruse A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy. 2017;97:53–64. doi: 10.1016/j.biombioe.2016.12.017. [DOI] [Google Scholar]
- 69.Derylo-Marczewska A., Sternik D., Swiatkowski A., Kusmierek K., Gac W., Buczek B. Adsorption of phenol from aqueous and cyclohexane solutions on activated carbons with differentiated surface chemistry. Thermochim. Acta. 2022;715 doi: 10.1016/j.tca.2022.179299. [DOI] [Google Scholar]
- 70.Wei X., Huang S., Yang J., Liu P., Li X., Wu Y., Wu S. Adsorption of phenol from aqueous solution on activated carbons prepared from antibiotic mycelial residues and traditional biomass. Fuel Process. Technol. 2023:242. doi: 10.1016/j.fuproc.2023.107663. [DOI] [Google Scholar]
- 71.United Nations . 2015. Agenda of Sustainable Development Goals 2030.https://sdgs.un.org/goals [Google Scholar]