Abstract
Objective
Levodopa is the first-line treatment for patients with Parkinson's disease (PD). However, only a few studies have focused on the tolerance of this drug in older patients with PD in the early and middle stages. Therefore, this study aimed to explore the effects of different levodopa doses on blood pressure (BP) in this subpopulation.
Methods
This cohort analysis enrolled 83 patients. The levodopa challenge test was used to evaluate drug responsiveness. After at least 12 h following anti-PD drug discontinuation, patients’ BPs were measured in a lying position, after 1 min standing, and after 3 min standing, in “off state” and best “on state.”
Results
BP in the 250 mg and 375 mg levodopa/benserazide groups decreased significantly in the lying and standing positions. The 3-min standing-position systolic BP was significantly influenced by the dose of levodopa/benserazide. However, no statistical change was observed in the 125 mg group. The postural-mediated systolic BP disparity was significant at 3 min in the upright position. Nineteen (incidence, 22.9%) and Twenty-five patients (incidence, 30.1%) developed complications of orthostatic hypotension (OH) in the “off state” and best “on state,” respectively. Mild cognitive impairment was a risk factor for OH occurrence in the “off state.” The OH occurrence in the best “on state” was associated with OH in the “off state” and urinary incontinence.
Conclusion
Our findings suggest that 250 mg or more of levodopa/benserazide could significantly reduce BP and orthostatic effect in older patients with PD in the early and middle stages. Therefore, they should routinely monitor their BP.
Trial registration number
ChiCTR2200055707.
Keywords: Older patient, Parkinson's disease, Levodopa/benserazide, Levodopa challenge test
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder among older adults. A population-based study showed that approximately 36 million Medicare beneficiaries were older than 65 years, suggesting that 1.6% of Americans receive PD treatment annually, similarly to the percentage of those with stroke [1]. China is encountering a silver wave because of an aging population, and the incidence of PD in China is similar to that of developed countries [2]. According to the Hoehn-Yahr classification, PD can be classified as early (stage 1–2), middle (stage 2.5–3), or late (stage 4–5) stage [3]. Autonomic dysfunction is a prominent PD symptom that progresses with disease severity. Among patients with PD in the late stage, non-motor symptoms could cause disability.
The occurrence of orthostatic hypotension (OH) is associated with PD progression. Symptoms of OH mainly result from cerebral and retinal hypoperfusion, including faintness and dizziness. They may even be accompanied by a transient loss of consciousness, leading to disability and reduced quality of life [4]. The estimated incidence of OH in patients with PD at all stages is approximately 20–50%, increasing to approximately 70% in patients with PD at the late stage [5]. Most recent studies focused on patients in the late stages, with only a few focusing on those in the early and middle stages. Furthermore, a few studies reported that OH could be in many de novo patients and deemed OH to be a highly frequent but undertreated complication in patients with early-stage PD [6,7].
OH in patients with PD is influenced by various factors, such as drug treatment [[8], [9], [10]]. Levodopa has a distinct tendency to cause hypotension, especially in older patients [11,12]. These studies revealed that patients with PD in the late stages were prone to experience OH caused by levodopa. However, the effect of levodopa on blood pressure (BP) in older patients with PD in the early and middle stages remains unclear. Additionally, whether its effect is dose-dependent in this subpopulation is seldom studied.
The levodopa challenge test (LCT) is a simple and safe method used by clinicians for diagnosing PD and reevaluating drug responsiveness [13]. The test is usually carried out in the morning, and patients must discontinue anti-PD drugs for more than 12 h, be on an empty stomach, and without antihypertensive drugs to objectively observe the effect of levodopa on BP in patients with PD. Hence, in this study, we aimed to explore the effect of levodopa on BP in older patients with PD in the early and middle stages in China and whether this effect is dose-dependent.
2. Material and methods
2.1. Patients
In this retrospective analysis, patients with idiopathic PD hospitalized at Xuanwu Hospital, Capital Medical University, from March 2022 to June 2022, were enrolled.
The inclusion criteria involved patients: 1) diagnosed with PD according to the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria [14], 2) with fluctuations in motor symptoms, and 3) aged 65 years.
The exclusion criteria included patients with: 1) late-stage PD; 2) secondary PD; 3) other neurodegenerative diseases, such as Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis, and spinocerebellar ataxias; 4) hypertensive emergency, malignant hypertension [15], or severe hypotension, which was defined as systolic BP (SBP) ≤ 90 mmHg with continuous symptoms such as dizziness, fatigue, and blurred vision; 5) serious organ disease complications, such as liver and kidney insufficiencies, cardiac insufficiency, and respiratory failure; and 6) malignant tumors.
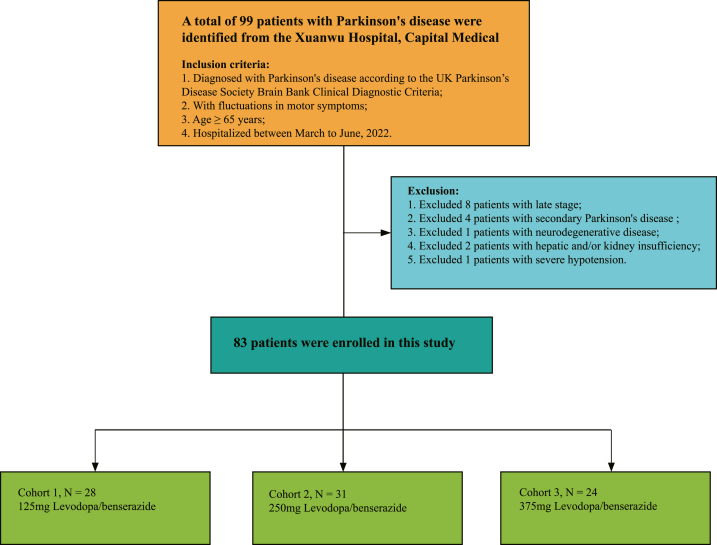
Finally, 83 patients were enrolled, and Fig. 1 illustrates the patient selection. The early, middle, and late stages classification refer to the Hoehn-Yahr classification [3]. The “early stage” refers to Hoehn-Yahr stages 1–2, the “middle stage” refers to Hoehn-Yahr stages 2.5–3, and the “late stage” refers to Hoehn-Yahr stages 4–5.
Fig. 1.
Flow diagram of the study sample.
2.2. Ethics statements
All patients provided written informed consent to participate in this study. The study was approved by the Ethics Committee of Xuan Wu Hospital, Capital Medical University, and registered with the Chinese Clinical Trial Registry (registration number: ChiCTR2200055707).
2.3. Clinical evaluation
A movement disorder specialist collected the medical history and physical examination data of all patients and recorded their demographics (sex, age, body mass index, past medical history), clinical features (disease course, Hoehn-Yahr classification, non-motor changes, and motor signs such as sleep, cognitive status, emotion, and dyskinesia), and drug use. Patients' motor subtypes and disease severity were evaluated using parts II, III, and V of the unified PD rating scale (UPDRS) (MDS-UPDRS II, III, and V, respectively) [16]. The Montreal cognitive assessment scale-Beijing version (MoCA-B) was used for screening cognitive impairment. MoCA-B scores less than 26 were classified as cognitive impairment, and 1 point was added to the total score for education ≤12 years to correct for educational level bias [17]. The Hamilton rating scale of depression-17 was used for screening depression, with a score of ≤7 indicating no depression and ≥8 indicating depression [18]. Patients’ rapid movement sleep behavior disorder (RBD) was evaluated using the RBD screen questionnaire; the questionnaire included 10 questions that summarized the clinical features of RBD, and the cut-off value was 6 [19].
2.4. LCT
Patients discontinued all anti-PD drugs for at least 12 h before the test to allow adequate elution of levodopa, resulting in a practically defined “discontinuation status.” Morning antihypertensive drugs were also discontinued. Patients were pretreated with 20 mg of domperidone 30 min before the test to minimize side effects. A movement disorder specialist performed the UPDRS III evaluation from 8:00 a.m. to 8:30 a.m., after which levodopa was administered under fasting conditions. The levodopa trial dose was 1.5 times more than the regular morning levodopa equivalent dose (LED) [16,20], and the calculated levodopa challenge dose was converted to levodopa/benserazide tablets (200 mg levodopa/50 mg benserazide, Shanghai Roche Pharmaceuticals Ltd, Shanghai, China). According to the calculated levodopa challenge dose, patients in the early and middle PD stages in this study received 125 mg, 250 mg, or 375 mg levodopa/benserazide. The levodopa equivalent daily dose (LEDD) was calculated following the recognized standard conversion [20]. The same researcher performed UPDRS III evaluation again at 30 min and 1, 2, and 3 h after administering the test dose to evaluate the improvement rate.
2.5. “Off state” and best “on state”
In this study, “off state” was defined as the period between discontinuing all anti-PD drugs for at least 12 h and the onset of the most severe motor symptoms in patients. The best “on state” was defined as the peak of levodopa benefit during the LCT.
2.6. BP measurement
BP, including SBP and diastolic blood pressure (DBP), were measured in the lying and standing positions during the “off state” and best “on state” using a proven BP monitoring device (OHEM-7051; Omron Corporation, Kyoto, Japan). First, we measured BP in the supine position after 10 min of rest in a comfortable environment. Second, we measured the standing BP at 1 and 3 min after participants stood up without external assistance. Additionally, hypotension symptoms, such as sleepiness, dizziness, fatigue, and blurred vision, within 3 min of upright posture were assessed. BP was measured in the “off state” and best “on state” during LCT. The consensus of the American Academy of Neurology and the American Autonomic Society defined OH as a decrease in the SBP ≥20 mmHg (1 mmHg = 0.133 kPa) with or without a decrease in the DBP ≥10 mmHg within 3 min during a standing or head-up tilt test [21].
2.7. Statistical analysis
First, a descriptive analysis of the baseline clinical features was performed. Next, clinical features among the three groups (125 mg, 250 mg, 375 mg levodopa/benserazide) were compared. Continuous variables with normal distribution were described as means ± standard deviations and compared using a one-way analysis of variance (ANOVA). Continuous variables with skewed distribution were described as median with interquartile range and the comparison was analyzed using the Kruskal–Wallis test. Categorical variables were represented as numbers (frequencies) and compared using the Chi-square test. Furthermore, BP values in lying and standing position among the three groups before and after the LCT were compared using repeated-measures ANOVA. Subsequently, the SBP and DBP differences among the three groups between “off state” and best “on state” were compared in lying position, after 1 min, and 3 min of standing. The statistical method used was paired sample t-test. In addition, the of levodopa (BP changes, △BP) was calculated and compared using Wilcoxon signed-rank test. Finally, the univariate and multivariate logistic regression analyses were performed to identify risk factors associated with OH occurrence. The odds ratio (OR) and 95% confidence intervals (CI) were reported. Statistical significance was set at a P-value <0.05. Data wrangling and analysis were performed using SPSS 23.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Clinical features of older patients with PD
Based on the Hoehn and Yahr classification, 63 of the 83 elderly patients with PD were in the early stage and 20 were in the middle stage. Table 1 presents the details of the demographics and clinical features of study participants. Three of the 74 patients who took levodopa also took carbidopa. Of 28 patients who took dopamine (DA) agonists, 20 and 8 took pramipexole and piribedil, respectively. Nine, seven, and seven patients took selegiline, amantadine, and entacapone, respectively. Another three patients discontinued all anti-PD drugs for 1 week upon admission.
Table 1.
Clinical features of older patients with Parkinson's disease.
| Characteristics | Total | Characteristics | Total |
|---|---|---|---|
| Sex | Anti-PD drugs | ||
| Male (n, %) | 49 (59.0) | Levodopa (n, %) | 74 (90.4) |
| Female (n, %) | 34 (41.0) | Dopamine agonists (n, %) | 28 (33.7) |
| Age (years) | 67.41 ± 2.02 | Amantadine (n, %) | 7 (8.4) |
| Disease duration (years) | 3 (1,5) | MAO-B inhibitors (n, %) | 9 (10.8) |
| Hoehn-Yahr stage | 2.17 ± 0.77 | COMT inhibitors (n, %) | 7 (8.4) |
| PIGD-dominant (n, %) | 37 (44.6) | Levodopa/benserazide dose (mg) | 269.07 ± 79.78 |
| BMI (kg/m2) | 22.43 ± 2.53 | Self-improvement Rate (%) | 33.59 ± 8.06 |
| Hyposmia (n, %) | 28 (33.7) | UPDRS-III scores (Off-state) | 32.15 ± 11.42 |
| Constipation (n, %) | 53 (63.9) | UPDRS-III scores (Best on-state) | 22.87 ± 10.11 |
| Pollakiuria (n, %) | 57 (68.7) | LEDD | 375 (300,575) |
| Uracratia (n, %) | 28 (33.7) | Lying position | |
| Cognitive impairment (n, %) | 29 (34.9) | SBP | 135.93 ± 20.17 |
| Depression (n, %) | 24 (28.9) | DBP | 76.22 ± 11.02 |
| Excessive sweating (n, %) | 14 (16.9) | 1 min standing | |
| Salivation (n, %) | 21 (25.3) | SBP | 131.86 ± 24.97 |
| RBD (n, %) | 34 (40.9) | DBP | 77.86 ± 12.03 |
| History of hypertension (n, %) | 41 (49.4) | 3 min standing | |
| Antihypertensive drugs (n, %) | 37 (44.6) | SBP | 132.27 ± 23.15 |
| History of stroke (n, %) | 17 (20.5) | DBP | 78.51 ± 11.32 |
Continuous variables with normal distribution were described as means ± standard deviations. Continuous variables with skewed distribution were described as median with interquartile range. Categorical variables were represented as numbers (frequencies).
Abbreviations: PIGD, postural instability/gait difficulty; BMI, body mass index; RBD, rapid eye movement (REM) sleep behavior disorder; MAO-B, monoamine oxidase B; COMT, catechol-O-methyltransferase; UPDRS-III, unified Parkinson's disease rating scale Part III; “off-state,” defined as the period when all anti-PD drugs were withdrawn for at least 12 h; Best “on-state,” defined as the peak of anti-PD drug benefits in the morning; LEDD, levodopa equivalent daily dosage; SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.2. Effects of different levodopa/benserazide doses on BP
Patients were divided into 125-, 250-, and 375-mg groups based on the different test doses of levodopa/benserazide. The three groups differed only in the disease course (P = 0.043), UPDRS III scores (P = 0.027), LEDD (P < 0.001), and type of drugs used (levodopa, P = 0.012; and amantadine, P = 0.005) (Table 2, Table 3). Of these patients, 19 out of 83 (22.9%) had OH in the “off state.” None of these patients had symptomatic hypotension. In the best “on state,” 25 patients had OH, with an incidence rate of 30.1%. No statistical difference was noted in OH incidence in the best “on state” and “off state” among the three groups (Table 2). Two patients in the 375-mg group developed significant hypotension symptoms, including dizziness, after standing for 3 min. The dizziness lasted about 5 min before subsiding gradually.
Table 2.
Comparison of clinical features of older patients with Parkinson's disease in different drug dose groups.
| 125 mg Levodopa/benserazide (n = 28) | 250 mg Levodopa/benserazide (n = 31) | 375 mg Levodopa/benserazide (n = 24) | Χ2 | P | 125 mg Levodopa/benserazide (n = 28) | 250 mg Levodopa/benserazide (n = 31) | 375 mg Levodopa/benserazide (n = 24) | Χ2 | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 1.077 | 0.584 | Anti-PD drugs | ||||||||
| Male (n, %) | 18 (64.3) | 17 (54.8) | 14 (58.3) | Levodopa (n, %) | 22 (78.6) | 28 (90.3) | 24 (100) | 8.814 | 0.012 | ||
| Female (n, %) | 10 (35.7) | 14 (45.2) | 10 (41.7) | Pramipexole (n, %) | 4 (14.3) | 8 (25.8) | 8 (33.3) | 2.643 | 0.267 | ||
| Piribedil (n, %) | 1 (3.6) | 3 (9.7) | 4 (16.7) | 2.545 | 0.280 | ||||||
| RBD (n, %) | 11 (39.3) | 13 (41.9) | 10 (41.7) | 0.058 | 0.972 | Amantadine (n, %) | 1 (3.5) | 1 (3.2) | 5 (20.8) | 10.653 | 0.005 |
| History of hypertension (n, %) | 12 (42.9) | 16 (51.6) | 13 (54.6) | 2.474 | 0.290 | MAO-B inhibitors (n, %) | 2 (7.1) | 3 (9.7) | 4 (16.7) | 1.027 | 0.598 |
| Antihypertensive drugs (n, %) | 12 (42.9) | 14 (45.2) | 11 (45.8) | 0.162 | 0.922 | COMT inhibitors (n, %) | 1 (3.5) | 3 (9.7) | 3 (12.5) | 0.859 | 0.651 |
| PIGD-dominant (n, %) | 12 (42.9) | 15 (48.4) | 10 (41.7) | 1.771 | 0.413 | Cognitive impairment (n, %) | 8 (28.6) | 11 (35.5) | 10 (41.7) | 2.002 | 0.368 |
| History of Stroke (n, %) | 6 (21.4) | 7 (22.6) | 4 (16.7) | 0.555 | 0.758 | Depression (n, %) | 8 (28.6) | 10 (32.3) | 6 (25) | 1.667 | 0.434 |
| Hyposmia (n, %) | 9 (32.1) | 11 (35.5) | 8 (33.3) | 0.276 | 0.118 | Excessive sweating (n, %) | 5 (17.9) | 6 (19.3) | 3 (12.5) | 0.496 | 0.780 |
| Constipation (n, %) | 18 (64.3) | 19 (61.3) | 16 (66.7) | 0.526 | 0.769 | Salivation (n, %) | 6 (21.4) | 9 (29.0) | 6 (25) | 3.548 | 0.170 |
| Pollakiuria (n, %) | 20 (71.4) | 21 (67.7) | 16 (66.7) | 0.296 | 0.813 | “Off state” OH | 5 (17.9) | 8 (23.5) | 6 (25) | 2.991 | 0.224 |
| Uracratia (n, %) | 9 (32.1) | 10 (32.3) | 9 (37.5) | 0.262 | 0.877 | Best “on state” OH | 6 (21.4) | 10 (32.3) | 9 (32.1) | 2.543 | 0.280 |
Data were represented as numbers (frequencies) and compared using Chi-square test. P-values with statistical significance (<0.05) are in bold.
RBD, rapid eye movement (REM) sleep behavior disorder; PIGD, postural instability/gait difficulty; MAO-B, monoamine oxidase B; COMT, catechol-O-methyltransferase; OH, orthostatic hypotension; “off-state,” defined as the period when all anti-PD drugs were withdrawn for at least 12 h; best “on-state,” defined as the peak of anti-PD drug benefit in the morning.
Table 3.
Comparison of clinical features of older patients with Parkinson's disease in different drug dose groups.
| 125 mg Levodopa/benserazide (n = 28) | 250 mg Levodopa/benserazide (n = 31) | 375 mg Levodopa/benserazide (n = 24) | F | P | 125 mg Levodopa/benserazide (n = 28) | 250 mg Levodopa/benserazide (n = 31) | 375 mg Levodopa/benserazide (n = 24) | H | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 66.37 ± 2.10 | 67.97 ± 2.72 | 66.95 ± 2.95 | 0.688 | 0.507 | Self-improvement Rate(%) | 35.50 ± 13.93 | 33.23 ± 7.51 | 33.40 ± 5.99 | 0.255 | 0.776 |
| Disease duration (years) | 2.80 ± 2.48 | 3.75 ± 3.55 | 5.07 ± 3.65 | 3.324 | 0.043 | UPDRS-III score (off state) | 24.75 ± 7.34 | 31.03 ± 9.11 | 36.85 ± 14.09 | 3.872 | 0.027 |
| Hoehn-Yahr stage | 1.88 ± 0.64 | 2.19 ± 0.88 | 2.25 ± 0.62 | 0.704 | 0.499 | UPDRS-III score (on state) | 14.53 ± 3.623 | 16.33 ± 9.25 | 16.96 ± 4.88 | 0.039 | 9.981 |
| BMI (kg/m2) | 23.12 ± 3.13 | 22.98 ± 3.37 | 22.12 ± 2.37 | 2.513 | 2.285 | LEDD (mg) | 150 (62.5187.5) | 300 (300,399) | 600 (566,675) | 7.677 | 0.000 |
Continuous variables with normal distribution were described as means ± standard deviations and compared using one-way ANOVA. Continuous variables with skewed distribution were described as median with interquartile range and the comparison was analyzed using the Kruskal–Wallis test. P-values with statistical significance (<0.05) are in bold.
BMI, body mass index; UPDRS-III, unified Parkinson's disease rating scale part III; LEDD, levodopa equivalent daily dosage.
Repeated-measures ANOVA testing was utilized to compare the differences in BP in the lying and standing positions among the different dose groups before and after LCT, and the specific statistical analysis results were as follows. During the best “on” state, SBP and DBP in the lying position did not differ significantly in the group effect, time effect, and the interaction. However, the 1-min standing SBP and DBP were significant for the time effect (F = 7.018, P = 0.01; F = 4.176, P = 0.024, respectively), but not the group effect (both F = 1.321, P = 0.275) or the interaction (both F = 1.098, P = 0.358). Moreover, there were significant differences in the 3-min standing SBP for the time effect (F = 4.276, P = 0.24), group effect (F = 4.155, P = 0.047), and the interaction (F = 4.095, P = 0.042). In contrast, the 3-min standing DBP was significant only for the time effect (F = 4.095, P = 0.042). These results indicate that the 3-min standing SBP rather than the lying position BP or 1-min standing BP was affected by the dose of levodopa [Table 4 and Fig. 2(A and B)].
Table 4.
Effects of different levodopa/benserazide doses on the blood pressure of older patients with Parkinson's disease.
| Value | Group | Time | Lying position | 1-min Standing | 3-min Standing |
|---|---|---|---|---|---|
| SBP | 125 mg | T0:“off state” | 138.88 ± 24.42 | 128.13 ± 15.62 | 124.13 ± 18.57 |
| T1:Best “on state” | 133.88 ± 17.02 | 124.88 ± 14.56 | 120.00 ± 12.74 | ||
| 250 mg | T0: “off state” | 134.77 ± 20.61 | 131.13 ± 25.13 | 136.26 ± 23.92 | |
| T1:Best “on state” | 127.39 ± 13.87# | 124.16 ± 20.73# | 120.23 ± 19.91# | ||
| 375 mg | T0: “off state” | 136.55 ± 18.6 | 131.45 ± 27.98 | 131.7 ± 23.41 | |
| T1:Best “on state” | 119.75 ± 18.81# | 116.30 ± 21.75# | 110.7 ± 13.88*# | ||
| Comparison among groups | F,P | 0.340,0.713 | 1.321,0.275 | 4.155,0.047 | |
| Comparison within groups | F,P | 0.290,0.743 | 7.018,0.010 | 4.276,0.024 | |
| Interaction (group × time) | F,P | 1.500,0.218 | 1.098,0.358 | 4.095,0.042 | |
| DBP | 125 mg | T0: “off state” | 78.25 ± 10.28 | 77.00 ± 6.82 | 75.63 ± 8.6 |
| T1:Best “on state” | 75.25 ± 12.26 | 74.63 ± 11.53 | 74.38 ± 10.62 | ||
| 250 mg | T0: “off state” | 74.94 ± 10.61 | 76.87 ± 12.88 | 77.71 ± 11.98 | |
| T1:Best “on state” | 67.65 ± 10.29# | 70.55 ± 13.08# | 71.71 ± 11.66# | ||
| 375 mg | T0: “off state” | 79.5 ± 10.73 | 79.75 ± 12.54 | 80.9 ± 11.26 | |
| T1:Best “on state” | 72.85 ± 11.68# | 72.15 ± 13.52# | 72.2 ± 13.43# | ||
| Comparison among groups | F,P | 0.660,0.550 | 1.321,0.275 | 0.212,0.810 | |
| Comparison within groups | F,P | 1.843,0.073 | 4.176,0.024 | 4.095,0.042 | |
| Interaction (group × time) | F,P | 0.187,0.832 | 1.098,0.358 | 0.660,0.550 |
Data are presented as means ± standard deviations and compared using repeated-measures ANOVA; statistically significant results (P < 0.05) are in bold.
The comparison between groups utilized the Least—Significant Difference test, * Comparison with the 250 mg group at the same time, P < 0.05; The intra-group comparison between two groups used the Bonferroni method, # Comparison with this group at T0, P < 0.05.
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Fig. 2.
Effects of different levodopa/benserazide doses on blood pressure. The differences were compared in lying position and after 1 min as well as 3 min of standing. The statistical method utilized was the repeated-measures ANOVA.
The results of the intra-group comparison showed that the SBP in the lying position, 1-min standing BP, and 3-min standing BP were significantly different between the “off state” and the best “on state” in the 250 mg and 375 mg groups (P < 0.05). Furthermore, the DBP in the lying position, 1-min standing BP, and 3-min standing BP was significantly different between the “off state” and the best “on state” in the 250 mg and 375 mg groups (P < 0.05) [Fig. 3(A-F)].
Fig. 3.
The differences in blood pressure between “off state” and best “on state” in different dose groups. The differences were compared in lying position, after 1 min, and 3 min of standing. The statistical method utilized was the paired sample t-test.
4. Orthostatic change of different dose of levodopa on BP
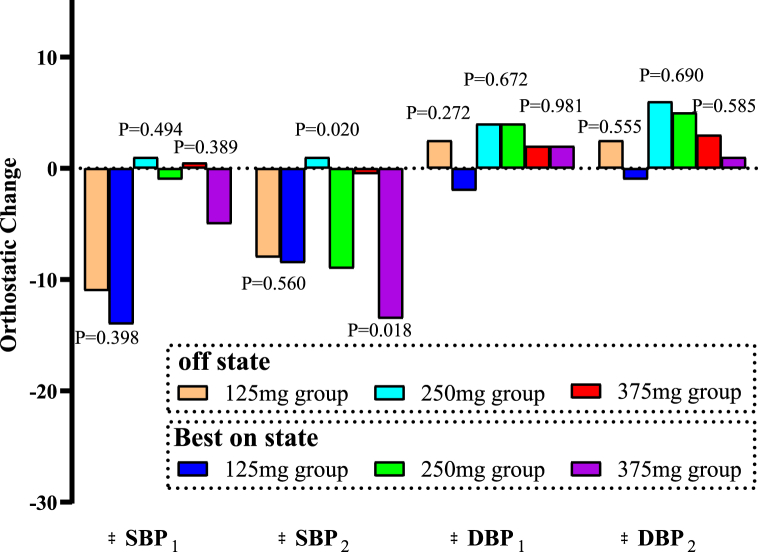
The Orthostatic effect of levodopa on BP was also analyzed. When patients in the 125-mg group changed from lying to standing for 1 min, the △SBP and △DBP were −14 mmHg and −2 mmHg, respectively. After standing for 3 min, the △SBP and △DBP were −8.5 mmHg and −1 mmHg, respectively. When patients in the 250-mg group changed posture for 1 and 3 min, the △SBP were −1 mmHg and −9 mmHg, respectively, whereas the △DBP were 4 and 5 mmHg, respectively. After patients in this group stood for 3 min, their SBP decreased significantly than when they were in the lying position (P = 0.020). When patients changed posture for 1 and 3 min in the 375-mg group, the △SBP were −5 mmHg and −13.5 mmHg, respectively, whereas the △DBP were 2 mmHg and 1 mmHg, respectively. When patients in this group changed from lying to standing for 3 min, the difference in the SBP decrease was significant (P = 0.018) (Table 5 and Fig. 4).
Table 5.
Standing effect of levodopa on the blood pressure of older patients with Parkinson's disease.
| “off state” | Best “on state” | P value (BP change by posture in “off state” vs. best “on state”) | |
|---|---|---|---|
| 125 mg Levodopa/benserazide (n = 28) | |||
| BP changes after 1-min standing vs. lying position | |||
| SBP | −11 (−34, 9.75) | −14 (−43.75, 5.25) | 0.398 |
| DBP | 2.5 (−11.5, 13.75) | −2 (−12, 7.5) | 0.272 |
| BP changes after 3-min standing vs. lying position | |||
| SBP | −8 (−38.75, 10.25) | −8.5 (−17, 2) | 0.560 |
| DBP | 2.5 (−14.25, 11.25) | −1 (−7.25, 9.5) | 0.555 |
| 250 mg Levodopa/benserazide (n = 31) | |||
| BP changes after 1-min standing vs. lying position | |||
| SBP | 1 (−11, 13) | −1 (−9, 8) | 0.494 |
| DBP | 4 (−5, 10) | 4 (−2, 7) | 0.672 |
| BP changes after 3-min standing vs. lying position | |||
| SBP | 1 (−3, 11) | −9 (−18, 0) | 0.020 |
| DBP | 6 (−1, 11) | 5 (0, 9) | 0.690 |
| 375 mg Levodopa/benserazide (n = 24) | |||
| BP changes after 1-min standing vs. lying position | |||
| SBP | 0.5 (−13.75, 6.75) | −5 (−19.25, 6.75) | 0.389 |
| DBP | 2 (−3.25, 7.5) | 2 (−4.5, 7.5) | 0.981 |
| BP changes after 3-min standing vs. lying position | |||
| SBP | −0.5 (−14, 8) | −13.5 (−25.25, −3.25) | 0.018 |
| DBP | 3 (−2.75, 5) | 1 (−5, 9.75) | 0.585 |
The standing effect of levodopa (BP changes, △BP) in the three groups was compared using Wilcoxon signed-rank test. SBP, systolic blood pressure; DBP, diastolic blood pressure. Statistical significance (<0.05) are in bold.
Fig. 4.
Orthostatic changes in BP associated with different dose of levodopa. The differences were compared in △SBP1/△DBP1 (BP changes after 1-min standing vs. lying position) and △SBP2/△DBP2 (BP changes after 3-min standing vs. lying position). The statistical method utilized was the Wilcoxon signed-rank test.
5. Analysis of OH influencing factors in the “off state” and best “on state”
Univariate logistic regression analysis revealed that cognitive impairment (OR = 10.64, 95% CI: 1.284–88.169, P = 0.028) and hypertension history (OR = 20.07, 95% CI: 0.955–421.766, P = 0.049) were associated with “off state” OH, while these associations only persisted for cognitive impairment (OR = 70.117, 95% CI: 3.2621507.273, P = 0.007) in multivariate logistic regression analysis. Based on univariate and multivariate logistic regression analysis, “off state” OH (OR = 0.087, 95% CI: 0.019–0.396, P = 0.002) and urinary incontinence (OR = 0.158,95% CI: 0.038–0.655, P = 0.011) were risk factors for best “on state” OH (Table 6).
Table 6.
Risk factors analysis of orthostatic hypotension and levodopa-induced orthostatic hypotension in the levodopa challenge test.
| Characteristics | “Off state” OH (n = 19) |
Best “on state” OH (n = 25) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value | Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value | |
| Cognitive impairment | 10.64 (1.284,88.169) | 0.028 | 70.117 (3.262,1507.273) | 0.007 | −0.964 (0.298,3.118) | 0.952 | – | – |
| “Off- state” OH | – | – | – | – | 64.579 (6.835,610.116) | 0.000 | 0.087 (0.019,0.396) | 0.002 |
| Best “on-state” OH | – | – | – | – | – | – | – | – |
| History of Hypertension | 20.07 (0.955,421.766) | 0.049 | 7.777 (0.833,72.569) | 0.072 | 0.762 (0.250,2.319) | 0.632 | – | – |
| Urinary incontinence | 0.284 (0.067,1.200) | 0.087 | – | – | 0.121 (0.035,0.422) | 0.001 | 0.158 (0.038, 0.655) | 0.011 |
Univariate and multivariate logistic regression analyses were performed to identify risk factors associated with OH occurrence. OR, odds ratio; CI, confidence interval; OH, orthostatic hypotension; “off-state,” defined as the period when all anti-PD drugs were withdrawn for at least 12 h; best “on-state,” defined as the peak of anti-PD drug benefit in the morning. P-values with statistical significance (<0.05) are in bold.
6. Discussion
In this study, we investigated the BP response in older patients with PD in the early and middle stages who received different doses of levodopa/benserazide during LCT. We found that 250 mg and 375 mg of levodopa/benserazide resulted in significant changes in BP before and after LCT. In addition, we found that the 3-min standing position SBP during the best “on state” was significantly affected by the dose of levodopa/benserazide. Meanwhile, we observed a drop in SBP (by 9 mmHg) from the supine position to the 3-min standing position when 250 mg levodopa/benserazide was administered during LCT. In contrast, the supine SBP was reduced by approximately 13.5 mmHg after 375 mg levodopa/benserazide uptake. These results provide evidence for the safety of 125 mg levodopa/benserazide administrations in this subpopulation. Our study revealed that the occurrence of OH in the best “on state” and “off state” were 30.1% and 22.9%, respectively, which was consistent with previous studies [22]. Subsequently, the risk factors associated with the best “on state” OH and “off state” OH were further discussed in our study. The risk factors revealed in our study might serve as an early warning sign to identify individuals with potential hypotension aggravation. Therefore, this study has important clinical implications.
Some studies have suggested that the BP-lowering effect of levodopa is dose-dependent, implying that 125-mg levodopa/benserazide does not affect patients' BPs, while higher doses lower BP [[23], [24], [25]]. However, a previous study showed that this phenomenon does not exist in older patients with PD [26]. Our study included patients with PD in the early and middle stages in China with a median disease course of 3 years and a mean age of 67.4 years. We discovered that 125 mg levodopa/benserazide is relatively safe. In contrast, 250 mg or 375 mg levodopa/benserazide could significantly decrease BP in this subpopulation. A previous study has reported impaired BP response and decreased heart rate response during an orthostatic test [27]. Another study of autonomic examination in patients with PD discovered that patients treated with levodopa over 2 years had significant reductions in SBP and mean arterial pressure response in standing tests and heart rate in tilting tests [28]. In this study, we discovered that patients’ SBP decreased continuously from the lying to the standing position in the 250- and 375-mg groups, and the postural-mediated changes in BP were outstanding. Levodopa acts as a neurotransmitter on the nerve nuclei of the cardiovascular center and induced a cardiac inhibitory response through the baroreceptor reflex, thereby reducing the heart rate variability and BP [[29], [30], [31]]. Therefore, BP monitoring is critical for older patients with PD who are tested or treated with 250 mg or more doses of levodopa/benserazide. Managing the older adult population with PD is more complicated when levodopa is used because PD, aging, and anti-PD drugs are all risk factors for OH. Levodopa is a DA precursor that plays a pharmacological role when converted into DA by DA decarboxylase. DA stimulation results in continuous norepinephrine release from sympathetic nerve endings, leading to norepinephrine deficiency, which may be one of the mechanisms of OH development after levodopa treatment in patients with PD [32]. Furthermore, DA can inhibit norepinephrine release from sympathetic fibers by activating peripheral presynaptic D2 receptors [33]. Meta-iodobenzylguanidine (MIBG) is a norepinephrine analog that can serve as an index of sympathetic denervation or dysfunction [34,35]. MIBG uptake is reduced in patients with early-stage PD [36], but its sensitivity and specificity in immediately diagnosed PD are comparable to those of LCT [37]. In this study, we did not use MIBG to confirm the levodopa effect on cardiac sympathetic denervation. However, based on previous studies [36,38], we speculated that the loss of cardiac accumulation of radiolabeled MIBG positively correlates with levodopa intake.
In our study, cognitive impairment was a risk factor for “off state” OH; cognitive impairment is associated with parasympathetic dysfunction in PD [39], which occurs in the early, middle, and late stages of PD [40], resulting in OH. Increased age and upright position were associated with worsening attention and executive dysfunction [[41], [42], [43]].
In addition, the relationship between urinary incontinence and OH is unclear. This is the first time urinary incontinence is proven as a risk factor for best “on state” OH in the early and middle stages of PD since previous studies have suggested that OH and urinary incontinence appear earlier in patients with multisystem atrophy than in those without. A correlation was observed between urinary incontinence and disease duration in the late stages of PD [44]. Urinary tract symptoms appeared late in all PD prodrome symptoms, mainly occurring 7–9 years before PD phenotypic transformation, with late-onset and mild symptoms [45]. This may be caused by denaturing and deposition of α-synuclein in the Lewy process, along with the bladder and the Lewy body of the sympathetic ganglion in early neurodegenerative disease involving the dorsal motor nucleus of the vagus nerve [43]. Therefore, autonomic nerve damage in elderly patients with PD might occur earlier.
In recent years, two new PD subtypes have emerged: “brain first” and “body first,” which follow the standard trajectory of Braak staging [46,47]. In the brain-first, pathogenic α-synuclein initially forms unilaterally in one hemisphere, leading to asymmetric nigrostriatal degeneration. Body-first with initial enteric pathology spreads through overlapping vagal innervation, leading to more symmetric brainstem involvement and more symmetric nigrostriatal degeneration [48]. Patients with body-first PD have more autonomic nervous system involvement and a larger burden of autonomic symptoms (including OH and constipation). Isolated rapid eye movement sleep behavior disorder has been identified as a strong marker of the body-first type. Some patients with RBD were included in our study, and the statistical results showed that RBD was not associated with OH in older patients with early and middle stages of PD. Perhaps the clinical situation in older people is more complicated.
This study had some limitations. First, this was a single-center study. Second, no difference in OH incidence was observed between different levodopa doses because the sample size was small. However, at the peak of levodopa efficacy, the number of patients who developed OH in the 375-mg group increased. In the future, cohort studies must be conducted in more centers, with more elderly adults and participants with multiple diseases and longer disease courses. Third, all participants were in the early-to-mid stage; more patients in the late stage must be enrolled in future studies. Fourth, patients in our study were divided into three groups based on their regular morning levodopa equivalent dose; the formulation meets the clinical efficacy evaluation, so randomized controlled trials will be required to confirm our conclusions in the future. Finally, this study could not distinguish between the effect of PD on orthostatic BP and the effect of levodopa, necessitating further investigation.
7. Conclusions
This study discovered that 250 mg or more doses of levodopa/benserazide could reduce BP and standing effects in elderly patients with PD in the early and middle stages in China. Older patients with urinary incontinence were more likely to develop OH during the LCT. Therefore, elderly patients with PD receiving 250 mg or more doses of levodopa/benserazide should routinely monitor their BP following the oral administration of anti-PD drugs at home or during an LCT at a hospital.
Funding
This study was supported by the National Key Research and Development Program of China (grant number: 2021YFC2501200).
Author contribution statement
Dan Su: Analyzed and interpreted the data; Wrote the paper.
Xiaojun Zhang, Yanling Su: Performed the experiments; Wrote the paper.
Piu Chan, Erhe Xu: Conceived and designed the experiments; Wrote the paper.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all participants for their participation in the study. This study was supported by the National Key Research and Development Program of China (grant number: 2021YFC2501200).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17876.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
figs1.
References
- 1.Clarke C.E. Mortality from Parkinson's disease. J Neurol Neurosurg Psychiatry. 2000;68:254–255. doi: 10.1136/jnnp.68.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z.X., Roman G.C., Hong Z., Wu C.B., Qu Q.M., Huang J.B., Zhou B., Geng Z.P., Wu J.X., Wen H.B., Zhao H., Zahner G.E. Parkinson's disease in China: prevalence in Beijing, Xian, Shanghai Met. Lancet. 2005;365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 4.Velseboer D.C., de Haan R.J., Wieling W., Goldstein D.S., de Bie R.M. Prevalence of orthostatic hypotension in Parkinson's disease: a systematic review and meta-analysis. Park. Relat. Disord. 2011;17:724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein D.S., Holmes C., Li S.T., Bruce S., Metman L.V., Cannon R.O. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 6.Hiorth Y.H., Pedersen K.F., Dalen I., Tysnes O.B., Alves G. Orthostatic hypotension in Parkinson disease: a 7-year prospective population-based study. Neurology. 2019;93:e1526–e1534. doi: 10.1212/WNL.0000000000008314. [DOI] [PubMed] [Google Scholar]
- 7.Jost W.H., Augustis S. Severity of orthostatic hypotension in the course of Parkinson's disease: no correlation with the duration of the disease. Park. Relat. Disord. 2015;21:314–316. doi: 10.1016/j.parkreldis.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Latt M.D., Lewis S., Zekry O., Fung V.S.C. Factors to consider in the selection of dopamine agonists for older persons with Parkinson's disease. Drugs Aging. 2019;36:189–202. doi: 10.1007/s40266-018-0629-0. [DOI] [PubMed] [Google Scholar]
- 9.Pursiainen V., Korpelainen T.J., Haapaniemi H.T., Sotaniemi A.K., Myllylä V.V. Selegiline and blood pressure in patients with Parkinson's disease. Acta Neurol Scand. 2007;115:104–108. doi: 10.1111/j.1600-0404.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Alves M., Caldeira D., Rato M.L., Duarte G.S., Ferreira A.N., Ferro J., Ferreira J.J. Cardiovascular Adverse Events Reported in placebo arm of randomized controlled trials in Parkinson's disease. J Parkinsons Dis. 2020;10:641–651. doi: 10.3233/JPD-191907. [DOI] [PubMed] [Google Scholar]
- 11.Fabbri M., Coelho M., Guedes L.C., Chendo I., Sousa C., Rosa M.M., Abreu D., Costa N., Godinho C., Antonini A., Ferreira J.J. Response of non-motor symptoms to levodopa in late-stage Parkinson's disease: results of a levodopa challenge test. Park. Relat. Disord. 2017;39:37–43. doi: 10.1016/j.parkreldis.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri M., Coelho M., Abreu D., Guedes L.C., Rosa M.M., Costa N., Antonini A., Ferreira J.J. Do patients with late-stage Parkinson's disease still respond to levodopa. Park. Relat. Disord. 2016;26:10–16. doi: 10.1016/j.parkreldis.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Saranza G., Lang A.E. Levodopa challenge test: indications, protocol, and guide. J Neurol. 2021;268:3135–3143. doi: 10.1007/s00415-020-09810-7. [DOI] [PubMed] [Google Scholar]
- 14.Daniel S.E., Lees A.J. Parkinson's Disease Society Brain Bank, London: Overview and Research. J Neural Transm Suppl. 1993;vol. 39:165–172. [PubMed] [Google Scholar]
- 15.Kincaid-Smith P.J. Malignant hypertension. Hypertens. 1991;9:893–899. [PubMed] [Google Scholar]
- 16.Stebbins G.T., Goetz C.G., Burn D.J., Jankovic J., Khoo T.K., Tilley B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov. Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 17.Gill D.J., Freshman A., Blender J.A., Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov. Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 18.Leentjens A.F., Verhey F.R., Lousberg R., Spitsbergen H., Wilmink F.W. The validity of the Hamilton and Montgomery-Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson's disease. Int. J. Geriatr. Psychiatr. 2000;15:644–649. doi: 10.1002/1099-1166(200007)15:7<644::aid-gps167>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Stiasny-Kolster K., Mayer G., Schafer S., Moller J.C., Heinzel-Gutenbrunner M., Oertel W.H. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov. Disord. 2007;22:2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 21.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 22.He X., Mo C., Zhang Y., Cai Y., Yang X., Qian Y., Xiao Q. Effect of Acute Levodopa Up-Titration on blood pressure in patients with early stage Parkinson's disease: results of a levodopa challenge test. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.778856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senard J.M., Verwaerde P., Rascol O., Montastruc J.L. Effects of acute levodopa administration on blood pressure and heart variability in never treated parkinsonians. Hypertens. Res. 1995;18(Suppl 1):S175–S177. doi: 10.1291/hypres.18.supplementi_s175. [DOI] [PubMed] [Google Scholar]
- 24.Noack C., Schroeder C., Heusser K., Lipp A. Cardiovascular effects of levodopa in Parkinson's disease. Park. Relat. Disord. 2014;20:815–818. doi: 10.1016/j.parkreldis.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig J., Remien P., Guballa C., Binder A., Binder S., Schattschneider J., Herzog J., Volkmann J., Deuschl G., Wasner G., Baron R. Psychiatry. Effects of subthalamic nucleus stimulation and levodopa on the autonomic nervous system in Parkinson's disease. J. Neurol. Neurosurg. 2007;78:742–745. doi: 10.1136/jnnp.2006.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grad B., Wener J., Rosenberg G., Wener S.W. Effects of levodopa therapy in Patients with Parkinson's disease: statistical evidence for reduced tolerance to levodopa in the elderly. J Am Geriatr Soc. 1974;22:489–494. doi: 10.1111/j.1532-5415.1974.tb05836.x. [DOI] [PubMed] [Google Scholar]
- 27.Mesec A., Sega S., Trost M., Pogacnik T. Acta neurol scand. The deterioration of cardiovascular reflexes in Parkinson's disease. 1999;100:296–299. doi: 10.1111/j.1600-0404.1999.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 28.Camerlingo M., Ferraro B., Gazzaniga G.C., Casto L., Cesana B.M., Mamoli A. Cardiovascular reflexes in Parkinson's disease: long-term effects of levodopa treatment on de novo patients. Acta Neurol. Scand. 1990;81:346–348. doi: 10.1111/j.1600-0404.1990.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 29.Sriranjini S.J., Ganesan M., Datta K., Pal P.K., Sathyaprabha T.N. Effect of a single dose of standard levodopa on cardiac autonomic function in Parkinson's disease. Neurol. India. 2011;59:659–663. doi: 10.4103/0028-3886.86536. [DOI] [PubMed] [Google Scholar]
- 30.Yue J.L., Okamura H., Goshima Y., Nakamura S., Geffard M., Misu Y. Baroreceptor-aortic nerve-mediated release of endogenous L-3,4-dihydroxyphenylalanine and its tonic depressor function in the nucleus tractus solitarii of rats. Neuroscience. 1994;62:145–161. doi: 10.1016/0306-4522(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 31.Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci. Lett. 1983;36:37–42. doi: 10.1016/0304-3940(83)90482-2. [DOI] [PubMed] [Google Scholar]
- 32.Spiers A.S., Calne D.B. Action of dopamine on the human iris. Br. Med. J. 1969;4:333–335. doi: 10.1136/bmj.4.5679.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannelli M., Ianni L., Lazzeri C., Castellani W., Pupilli C., La Villa G., Barletta G., Serio M., Franchi F. In vivo evidence that endogenous dopamine modulates sympathetic activity in man. Hypertension. 1999;34:398–402. doi: 10.1161/01.hyp.34.3.398. [DOI] [PubMed] [Google Scholar]
- 34.Wieland D.M., Brown L.E., Rogers W.L., Worthington K.C., Wu J.L., Clinthorne N.H., Otto C.A., Swanson D.P., Beierwaltes W.H. Myocardial imaging with a radioiodinated norepinephrine storage analog. J. Nucl. Med. 1981;22:22–31. [PubMed] [Google Scholar]
- 35.Takatsu H., Scheffel U., Fujiwara H. Sympathetic tone assessed by washout of iodine 125-labeled metaiodobenzylguanidine from the murine left ventricle--influence of immobilization stress and inhibition of the renin-angiotensin system. J. Nucl. Cardiol. 1995;2:507–512. doi: 10.1016/s1071-3581(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 36.Takatsu H., Nishida H., Matsuo H., Watanabe S., Nagashima K., Wada H., Noda T., Nishigaki K., Fujiwara H. Cardiac sympathetic denervation from the early stage of Parkinson's disease: clinical and experimental studies with radiolabeled MIBG. J. Nucl. Med. 2000;41:71–77. [PubMed] [Google Scholar]
- 37.Asayama S., Wate R., Kaneko S., Asayama T., Oki M., Tsuge A., Nagashima M., Morita J., Nakamura S., Nakamura M., Nishii M., Fujita K., Saito A., Nakano S., Ito H., Kusaka H. Levodopa challenge test and (123) I-metaiodobenzylguanidine scintigraphy for diagnosing Parkinson's disease. Acta Neurol Scand. 2013;128:160–165. doi: 10.1111/ane.12104. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg L.I., Whitsett T.L. Cardiovascular effects of levodopa. Clin Pharmacol Ther. 1971;12:376–382. doi: 10.1002/cpt1971122part2376. [DOI] [PubMed] [Google Scholar]
- 39.Cicero C.E., Raciti L., Monastero R., Mostile G., Donzuso G., Sciacca G., Luca A., Terravecchia C., Giuliano L., Baschi R., Davì M., Zappia M., Nicoletti A. Cardiovascular autonomic function and MCI in Parkinson's disease. Park. Relat. Disord. 2019;69:55–58. doi: 10.1016/j.parkreldis.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q., Chen L., Zheng Y., Zhang C., Huang L., Guo W., Fang Y., Zhou H., Liu Y., Chen J., Qian H., Xian W., Zeng J., Li J., Liu Z., Pei Z. Cognitive impairment is common in Parkinson's disease without dementia in the early and middle stages in a Han Chinese cohort. Park. Relat. Disord. 2012;18:161–165. doi: 10.1016/j.parkreldis.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Hohler A.D., Zuzuarregui J.R., Katz D.I., Depiero T.J., Hehl C.L., Leonard A., Allen V., Dentino J., Gardner M., Phenix H., Saint-Hilaire M., Ellis T. Differences in motor and cognitive function in patients with Parkinson's disease with and without orthostatic hypotension. Int. J. Neurosci. 2012;122:233–236. doi: 10.1080/00207454.2012.642038. [DOI] [PubMed] [Google Scholar]
- 42.Peralta C., Stampfer-Kountchev M., Karner E., Kollensperger M., Geser F., Wolf E., Seppi K., Benke T., Poewe W., Wenning G.K. Orthostatic hypotension and attention in Parkinson's disease with and without dementia. J. Neural. Transm. 2007;114:585–588. doi: 10.1007/s00702-006-0615-2. [DOI] [PubMed] [Google Scholar]
- 43.Ballard C., O'Brien J., Gray A., Cormack F., Ayre G., Rowan E., Thompson P., Bucks R., McKeith I., Walker M., Tovee M. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch. Neurol. 2001;58:977–982. doi: 10.1001/archneur.58.6.977. [DOI] [PubMed] [Google Scholar]
- 44.Wullner U., Schmitz-Hubsch T., Antony G., Fimmers R., Spottke A., Oertel W.H., Deuschl G., Klockgether T., Eggert K. Autonomic dysfunction in 3414 Parkinson's disease patients enrolled in the German Network on Parkinson's disease (KNP e.V.): the effect of ageing. Eur J Neurol. 2007;14:1405–1408. doi: 10.1111/j.1468-1331.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- 45.Fereshtehnejad S.M., Yao C., Pelletier A., Montplaisir J.Y., Gagnon J.F., Postuma R.B. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain. 2019;142:2051–2067. doi: 10.1093/brain/awz111. [DOI] [PubMed] [Google Scholar]
- 46.Horsager J., Knudsen K., Sommerauer M. Clinical and imaging evidence of brain-first and body-first Parkinson's disease. Neurobiol. Dis. 2022;164 doi: 10.1016/j.nbd.2022.105626. [DOI] [PubMed] [Google Scholar]
- 47.Horsager J., Andersen K.B., Knudsen K., Skjaerbaek C., Fedorova T.D., Okkels N., Schaeffer E., Bonkat S.K., Geday J., Otto M., Sommerauer M., Danielsen E.H., Bech E., Kraft J., Munk O.L., Hansen S.D., Pavese N., Goder R., Brooks D.J., Berg D., Borghammer P. Brain-first versus body-first Parkinson's disease: a multimodal imaging case-control study. Brain. 2020;143:3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 48.Knudsen K., Fedorova T.D., Horsager J., Andersen K.B., Skjaerbaek C., Berg D., Schaeffer E., Brooks D.J., Pavese N., Van Den Berge N., Borghammer P. Asymmetric Dopaminergic Dysfunction in Brain-First versus Body-First Parkinson's Disease Subtypes. J Parkinsons Dis. 2021;11:1677–1687. doi: 10.3233/JPD-212761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.