Abstract
In the current COVID-19 pandemic scenario, it is still necessary to understand if differences exist between genders in terms of patients’ characteristics and clinical outcomes. For this reason, we retrospectively analyzed data obtained from a local register-based dataset of all SARS-CoV-2 positive patients diagnosed in the province of Catania (Italy). The main aim of this analysis was to understand any differences between genders in the distribution of previous medical conditions, and to evaluate which of them posed individuals at higher risk of death.
With this purpose, we analyzed data from 1424 patients with at least one underlying medical condition, who were tested positive for SARS-CoV-2 infection from February 2020 to December 2021. Overall, males were 59.5% of the total population and significantly younger than females (median ages: 68 years vs. 72 years; p = 0.011). The age distribution of cases by gender confirms that individuals from 70 to 79 years were the most affected in both genders. The comparison of underlying comorbidities by gender shows significant differences for diabetes (p < 0.001), other metabolic diseases (p = 0.006), and obesity (p = 0.019). Accordingly, multivariable logistic regression analysis confirmed that diabetes was more likely to be present in males than in females (p = 0.001), while other metabolic diseases and obesity were less likely to be present (p = 0.003 and p = 0.005, respectively). Although no difference in mortality was evident between genders (p = 0.141), both male and female COVID-19 patients had a significantly higher risk of death if they had comorbidities such as CVDs, kidney diseases, or chronic neurological diseases. Moreover, diabetes and chronic respiratory diseases were significant risk factors for COVID-19 mortality among men, whereas cancer was a significant contributor among women. Our findings confirm gender-differences in pre-existing medical conditions of COVID-19 patients, which may influence the risk of death. Further studies, however, are needed to understand physiological and pathological mechanisms underpinning these differences.
Keywords: Covid-19, SARS-CoV-2, Pandemic, Gender, Sex, Comorbidities, Mortality
1. Introduction
At the end of 2019, an outbreak of the Novel Coronavirus Disease 2019 (COVID-19) quickly spread throughout China, and suddenly evolved in a global pandemic [1]. Italy was one of the hardest-hit countries in the early stages of the pandemic, and more than 25 million cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection have been recorded by the end 2022.
Despite the relatively low lethality rates associated with COVID-19 (nearly 0.7% in Italy) [2], identifying personal and clinical characteristics that increase the risk of infection and adverse outcomes from SARS-CoV-2 infection remains a priority to combat the pandemic and to improve prevention strategies already in place [[3], [4], [5]]. Older age and pre-existing medical conditions (e.g., obesity, diabetes, hypertension, chronic respiratory and cardiovascular diseases [CVDs]) are now viewed as the most important factors contributing to the severity of COVID-19 [1,[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]].
There are, however, some differences between genders, which modulate a wide range of clinical manifestations, the course of disease, and the likelihood of adverse outcomes, such as hospitalization and death [14,[18], [19], [20], [21], [22]]. While being male is not considered a risk factor for SARS-CoV-2 infection per se [2], multiple studies have demonstrated that males tend to experience more severe disease and higher fatality rates from COVID-19 than females [[23], [24], [25], [26]]. An investigation conducted by Jin and colleagues focused on gender differences in COVID-19 patients, specifically exploring differences in disease severity and mortality rates [23]. Their results showed that, although age and prevalence of infection were comparable between the two groups, men had more severe disease and a higher risk of dying than women. From a biological perspective, certain biological differences between sexes (e.g., genetic, reproductive, hormonal, and other factors) could influence the susceptibility to COVID-19 and its progression [14,[27], [28], [29]]. For example, Mukherjee and colleagues suggested that sex-based differences in the expression of ACE2 receptor and TMPRSS2 protease, which facilitate SARS-CoV-2 entry into host cells, could explain the disparities observed in COVID-19 severity and fatality rates between males and females [30]. Additionally, the differences in clinical outcomes of COVID-19 infections between men and women may be due to gendered differences in behaviors, such as smoking, and prevalence of comorbidities [5,16,28,[31], [32], [33], [34], [35], [36]]. Sex and gender disparities in health have been observed in various conditions, including CVDs [[37], [38], [39]], diabetes [40], kidney diseases [41], cancer [[42], [43], [44]], neurological diseases [45,46], respiratory diseases [43], and others.
While differences in the prevalence of underlying health conditions may be a contributing factor to COVID-19 severity, there is ongoing debate regarding the extent to which gender plays a role in determining the risk of experiencing COVID-19 adverse outcomes [47]. Given the potential impact of gender on COVID-19 risk and outcomes, additional research is needed to develop personalized screening programs, preventive strategies, and treatments that take into account potential gender differences [35,48]. To address this need, we retrospectively analyzed data obtained from a local register-based dataset of all SARS-CoV-2 positive patients diagnosed in the province of Catania (Sicily, Italy). The main aim of this analysis was to understand any differences between genders in the distribution of previous medical conditions, and to evaluate which of them posed individuals at higher risk of death.
2. Materials and methods
2.1. Study population and design
The present analysis was conducted in the framework of the “Modelli innovativi per l’Analisi dati della Sorveglianza Sanitaria Integrata COVID-19” (MASSI) study, which aims to analyze the distribution and characteristics of SARS-CoV-2 positive patients diagnosed in the province of Catania (Catania, Italy) between February 2020 and November 2021. This is a retrospective epidemiological study, approved by the local Ethics Committee “Catania 2” (Catania, Italy) with the following protocol number: 31/C.E. del January 14, 2022. The study includes surveillance data obtained from the Provincial Health Authority (Azienda Sanitaria Provinciale, ASP) of Catania (Catania, Italy). The local surveillance system was developed and implemented on the basis of the national surveillance system, coordinated by the Department of Infectious Diseases of the Italian National Institute of Health (Istituto Superiore di Sanità, ISS). In general, the ISS is gathering and integrating microbiological and epidemiological data on all SARS-CoV-2 cases in Italy from 2020 onwards. At local level, each Italian region and province daily sent data on new cases of laboratory-confirmed SARS-CoV-2 infections to the ISS, through a dedicated web-based platform.
In the MASSI study, we included all SARS-CoV-2 positive patients detected by regional reference laboratories in the province of Catania (Italy), after performing a nasal or nasopharyngeal swab. All patients were followed from the date of first diagnosis until they received a negative swab test. Data included in the MASSI dataset are related to personal, clinical, and socio-demographic characteristics, underlying medical conditions, vaccination status, epidemiological history, hospital care at home or hospital admission and laboratory results. In the current analysis, we used data from a subgroup of patients with at least one previous medical condition (e.g., cancer, obesity, diabetes, other metabolic diseases, CVDs, HIV infection, chronic respiratory diseases, kidney diseases, liver diseases, and chronic neurological diseases). Patients lost to follow-up were excluded from the analyses. Since information on the date and place of death was not complete, the primary outcome was overall mortality during the entire follow-up period (i.e., from diagnosis to the negative conversion of each patient). It is necessary to highlight that the Italian surveillance system has adopted the World Health Organization (WHO) definition regarding overall mortality, which is defined as follows: a death attributed to a clinically compatible illness in a probable or confirmed COVID-19 case, unless there is an obvious unrelated cause of death, with no period of complete recovery between the illness and death [49]. Secondary outcomes were 30-day mortality, hospitalization, being intubated, and receiving drug therapy.
2.2. Statistical analysis
Summary statistics were used to describe personal and clinical characteristics of SARS-CoV-2 positive patients. Continuous variables were reported as mean and standard deviation (SD), or as median and interquartile range (IQR), according to their parametric or non-parametric distribution. Categorical variables were expressed as frequencies and percentages. We compared these variables between male and female patients using the Mann–Whitney U test for continuous variables, and the Chi-Squared test for those categorical. Next, a logistic regression analysis was applied to evaluate the relationship between comorbidities and gender-differences, adjusting for age. Multivariable logistic regression analysis was also applied to evaluate the odds of death associated with comorbidities and adjusted for age. Results were reported for the overall population and stratified by gender. Logistic regression outputs were expressed as odds ratios (ORs) with their 95% confidence intervals (95%CIs). All the analyses were performed using the SPSS software (version 26.0, SPSS, Chicago, IL, USA), and a significance level of 0.05 was considered significant.
3. Results
The present analysis was conducted on 1424 patients with at least one underlying medical condition, who were tested positive for SARS-CoV-2 infection from February 26, 2020 to December 31, 2021 in the Catania province (Italy). All patients resided in the province of Catania, and the majority of them (97.5%) were of Italian nationality. In particular, 2.4% of patients were already hospitalized or in a residential facility prior to receiving the diagnosis of COVID-19. The median time from diagnosis to the first negative swab, and therefore the duration of follow-up for the included patients, was 23 days (IQR = 15–33). No difference in the follow-up period was evident between genders (p = 0.058).
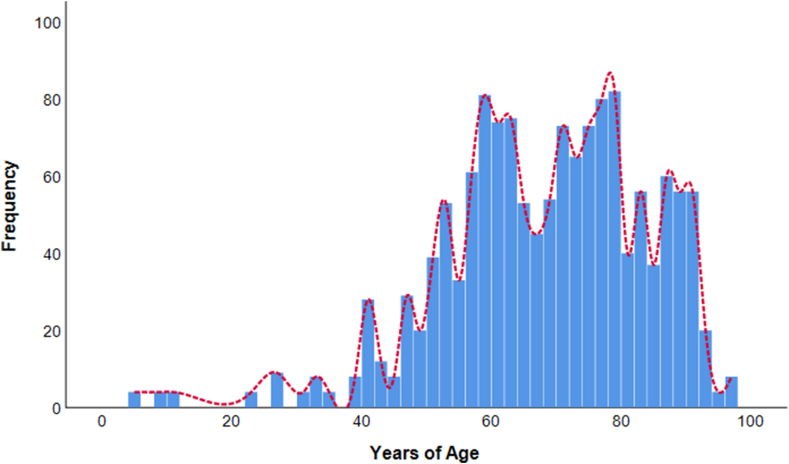
Overall, the study population had a mean age of 67.9 years (SD = 15.5) and a median of 69 years (IQR = 58–79). Based on the age distribution of identified cases (Fig. 1), the most affected were those between 70 and 79 years (26.2%) and those between 60 and 69 years (21.1%). In terms of vaccination, 43.9% of patients received the COVID-19 vaccine, while 56.1% were not vaccinated. Overall, 38.3% of patients were hospitalized while the remaining portions were treated at home (52.8%) or in residential facilities (8.9%). Additionally, 5.0% of patients were intubated and 9.5% received drug therapy against COVID-19.
Fig. 1.
Distribution of SARS-CoV-2 positive patients by age. The histogram shows the frequency of SARS-CoV-2 positive patients by age; the trend line is colored in red. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
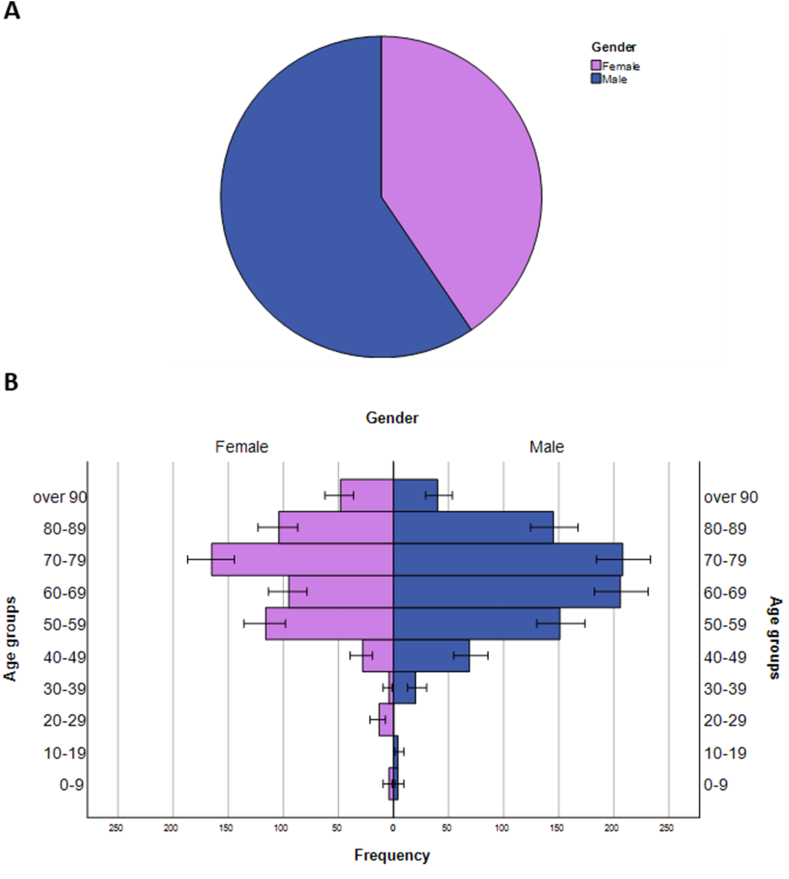
The comparison of characteristics by gender is reported in Table 1. In particular, males were 59.5% of the total population (Fig. 2A) and significantly younger than females (median ages: 68 years vs. 72 years; p = 0.011). The age distribution of cases by gender (Fig. 2B) confirms that individuals from 70 to 79 years were the most affected in both males and females (24.6% and 28.6%, respectively). However, the second most affected group was 60–69 years for males (24.3%) and 50–59 years for females (20.1%), respectively. Although there were no differences by gender in terms of vaccination and hospitalization status, a higher proportion of males were intubated (6.3%) or treated with drugs (11.3%) than females (3.5% and 7.1%, respectively) (Table 1).
Table 1.
Characteristics of the study population by gender.
| Characteristics | Male (n = 847) | Female (n = 577) | p-valuea |
|---|---|---|---|
| Age, median (IQR) | 68 (19) | 72 (23) | 0.011 |
| COVID-19 vaccination | 44.3% | 43.5% | 0.735 |
| Prevalence of Comorbidities | |||
| Cancer | 4.7% | 4.9% | 0.910 |
| Diabetes | 26.3% | 18.0% | <0.001 |
| CVD | 64.6% | 67.1% | 0.332 |
| HIV infection | 1.4% | 1.4% | 0.962 |
| Chronic Respiratory Disease | 18.5% | 16.8% | 0.404 |
| Kidney Disease | 8.0% | 7.1% | 0.520 |
| Other Metabolic Disease | 9.9% | 14.7% | 0.006 |
| Liver Disease | 0.7% | 0.2% | 0.156 |
| Chronic Neurological Disease | 15.2% | 16.8% | 0.423 |
| Obesity | 11.4% | 14.9% | 0.062 |
| Outcomes | |||
| Hospitalization | 38.8% | 37.6% | 0.436 |
| Residential facility | 8.4% | 9.4% | |
| Treatment at home | 52.6% | 53.1% | |
| Drug therapy | 11.3% | 7.1% | 0.004 |
| Intubation | 6.3% | 3.5% | 0.011 |
| Death | 36.5% | 32.8% | 0.141 |
Abbreviations: CVD, cardiovascular diseases; HIV, Human Immunodeficiency Virus.
Results are based on the Mann–Whitney U test for continuous variables, and the Chi-Squared test for those categorical.
Fig. 2.
Distribution of SARS-CoV-2 positive patients by gender overall and stratified by age groups. (A) The pie chart shows the proportion of male and female patients in the overall population; (B) The age pyramid shows the frequency of SARS-CoV-2 positive patients by gender and age group.
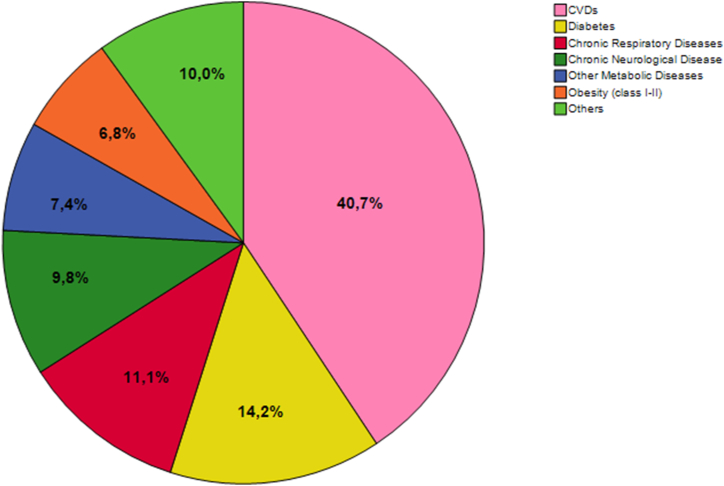
Without distinguishing by gender, a total of 2295 underlying comorbidities were recorded, corresponding to an average of 1.6 per patient. As shown in Fig. 3, the most common conditions were CVDs (40.7%), diabetes (14.2%), and chronic respiratory diseases (11.1%). Although not statistically significant, men showed a higher number of comorbidities compared to women (median = 2 per patient; IQR = 1 for males vs. median = 1 per patient; IQR = 1 for females; p = 0.340). The comparison of underlying comorbidities by gender (Table 1 and Fig. 4) shows significant differences for diabetes (26.3% in males vs. 18.0% in females; p < 0.001), other metabolic diseases (9.9% vs. 14.7%; p = 0.006), and obesity (10.7% vs. 14.9%; 0.019). Accordingly, multivariable logistic regression analysis (Table 2) confirmed that diabetes was more likely to be present in males than in females (OR = 1.786; 95%CI = 1.362–2.343; p = 0.001), while other metabolic diseases and obesity were less likely to be present in males (OR = 0.607; 95%CI = 0.436–0.846; p = 0.003 and OR = 0.626; 95%CI = 0.451–0.868; p = 0.005, respectively). Logistic regression analysis also confirmed a significant difference in terms of age between males and females (p = 0.001), as showed by bivariate analysis. No differences were evident for cancer, CVDs, HIV infection, chronic respiratory diseases, kidney diseases, liver diseases, and chronic neurological diseases (p-values >0.05).
Fig. 3.
Proportions of comorbidities of patients included in the study. The pie chart shows the proportion of each comorbidity among the total of 2295 comorbidities recorded in the study population.
Fig. 4.
Distribution of comorbidities by gender. The bar graphs show the relative frequency of comorbidities (%) among female and male patients.
Table 2.
Logistic regression of the association between comorbidities and gender.
| Characteristics | ORa | 95% CI |
p-value | |
|---|---|---|---|---|
| LL | UL | |||
| Age | 0.986 | 0.978 | 0.995 | 0.001 |
| Cancer | 1.059 | 0.634 | 1.770 | 0.825 |
| Diabetes | 1.786 | 1.362 | 2.343 | <0.001 |
| CVD | 1.009 | 0.790 | 1.290 | 0.941 |
| HIV infection | 0.798 | 0.315 | 2.026 | 0.635 |
| Chronic Respiratory Disease | 1.190 | 0.894 | 1.584 | 0.234 |
| Kidney Disease | 1.177 | 0.772 | 1.794 | 0.448 |
| Other Metabolic Disease | 0.607 | 0.436 | 0.846 | 0.003 |
| Liver Disease | 3.692 | 0.436 | 31.248 | 0.231 |
| Chronic Neurological Disease | 1.054 | 0.773 | 1.438 | 0.739 |
| Obesity | 0.626 | 0.451 | 0.868 | 0.005 |
Odds ratios, 95% Confidence Intervals, and p-values are based on a logistic regression model including all factors reported in the table (presence vs. absence of each comorbidity). For age, Odds ratios and 95% Confidence Intervals referred to the one-unit increase in the years of age.
Abbreviations: OR, odds ratio; CI, confidence interval; LL, lower limit; UL, upper limit; CVD, cardiovascular diseases; HIV, Human Immunodeficiency Virus.
Out of 1424 patients under investigation, 35.0% died but no difference in mortality was evident between genders (36.5% in males and 32.8% in females; p = 0.141). In terms of 30-day mortality, the percentage was 25.3% with no statistically significant difference between genders (26.1% in males and 24.2% in females; p = 0.388). Although there were no differences in mortality, a higher proportion of male patients were intubated (6.3% vs. 3.5%; p = 0.011) or treated with drugs (11.3% vs. 3.5%; p = 0.004) compared to female patients (Table 1). The bivariate analysis of the association between underlying comorbidities and mortality showed that diabetes, CVDs, chronic respiratory diseases, kidney diseases, and chronic neurological diseases were more common among patients who died compared to those who survived (Table 3). The age-adjusted logistic regression model pointed out that kidney diseases, chronic neurological diseases, CVDs, cancer, chronic respiratory diseases, and diabetes significantly contributed to the odds of dying in the overall population (Table 3).
Table 3.
Bivariate and age-adjusted logistic regression analyses of comorbidities associated with the odds of dying in the overall population.
| Characteristics | Bivariate analysisa |
Logistic regressionb |
|||||
|---|---|---|---|---|---|---|---|
| Alive (n = 926) | Dead (n = 498) | p-value | OR | LL | UL | p-value | |
| Cancer | 8.7% | 10.8% | 0.181 | 1.702 | 1.182 | 2.451 | 0.004 |
| Diabetes | 23.3% | 28.9% | 0.013 | 1.398 | 1.102 | 1.775 | 0.006 |
| CVD | 57.6% | 69.0% | <0.001 | 1.724 | 1.368 | 2.173 | <0.001 |
| HIV infection | 3.0% | 2.8% | 0.798 | 0.672 | 0.343 | 1.317 | 0.247 |
| Chronic Respiratory Disease | 17.4% | 24.0% | 0.001 | 1.672 | 1.295 | 2.160 | <0.001 |
| Kidney Disease | 20.3% | 5.7% | <0.001 | 4.179 | 3.010 | 5.804 | <0.001 |
| Other Metabolic Disease | 11.0% | 9.6% | 0.381 | 0.693 | 0.484 | 0.992 | 0.045 |
| Liver Disease | 1.6% | 1.4% | 0.799 | 1.209 | 0.500 | 2.919 | 0.674 |
| Chronic Neurological Disease | 6.5% | 15.3% | <0.001 | 3.385 | 2.413 | 4.749 | <0.001 |
| Obesity | 14.9% | 13.7% | 0.759 | 1.036 | 0.743 | 1.444 | 0.836 |
Abbreviations: OR, odds ratio; LL, lower limit; UL, upper limit; CVD, cardiovascular diseases; HIV, Human Immunodeficiency Virus.
Results are based on the Chi-Squared test.
Odds ratios, 95% Confidence Intervals, and p-values are based on an age-adjusted logistic regression model including all factors reported in the table (presence vs. absence of each comorbidity).
However, stratified analyses underlined some differences between genders. Among males, diabetes, CVDs, chronic respiratory diseases, kidney diseases, and chronic neurological diseases were more common among patients who died compared to those who survived (Table 4). The age-adjusted logistic regression analysis confirmed that kidney diseases, chronic neurological diseases, chronic respiratory diseases, CVDs, and diabetes significantly contributed to the odds of death among male patients (Table 4). Among females, CVDs, kidney diseases, and chronic neurological diseases were more common among patients who died compared to those who survived (Table 5). By contrast, other metabolic diseases were more common among those who survived. The age-adjusted logistic regression analysis confirmed that kidney diseases, chronic neurological diseases, cancers, and CVDs significantly contributed to the odds of death among female patients (Table 5).
Table 4.
Bivariate and age-adjusted logistic regression analyses of the comorbidities associated with the odds of dying in the male group.
| Characteristics | Bivariate analysisa |
Logistic regressionb |
|||||
|---|---|---|---|---|---|---|---|
| Alive (n = 538) | Dead (n = 309) | p-value | OR | LL | UL | p-value | |
| Cancer | 8.5% | 10.4% | 0.347 | 1.593 | 0.971 | 2.615 | 0.065 |
| Diabetes | 24.7% | 33.6% | 0.004 | 1.475 | 1.087 | 2.001 | 0.013 |
| CVD | 60.3% | 72.9% | <0.001 | 1.777 | 1.299 | 2.432 | <0.001 |
| HIV infection | 2.1% | 2.7% | 0.566 | 0.870 | 0.330 | 2.294 | 0.779 |
| Chronic Respiratory Disease | 19.0% | 28.3% | 0.001 | 1.853 | 1.343 | 2.558 | <0.001 |
| Kidney Disease | 5.9% | 19.9% | <0.001 | 3.946 | 2.547 | 6.112 | <0.001 |
| Other Metabolic Disease | 9.4% | 10.7% | 0.596 | 0.913 | 0.568 | 1.469 | 0.708 |
| Liver Disease | 2.1% | 1.2% | 0.320 | 0.804 | 0.257 | 2.512 | 0.708 |
| Chronic Neurological Disease | 5.2% | 13.1% | <0.001 | 3.440 | 2.124 | 5.573 | <0.001 |
| Obesity | 14.0% | 13.1% | 0.555 | 1.093 | 0.703 | 1.700 | 0.692 |
Abbreviations: OR, odds ratio; LL, lower limit; UL, upper limit; CVD, cardiovascular diseases; HIV, Human Immunodeficiency Virus.
Results are based on the Chi-Squared test.
Odds ratios, 95% Confidence Intervals, and p-values are based on an age-adjusted logistic regression model including all factors reported in the table (presence vs. absence of each comorbidity).
Table 5.
Bivariate and age-adjusted logistic regression analyses of comorbidities associated with the odds of dying in the female group.
| Characteristics | Bivariate analysisa |
Logistic regressionb |
|||||
|---|---|---|---|---|---|---|---|
| Alive (n = 388) | Dead (n = 189) | p-value | OR | LL | UL | p-value | |
| Cancer | 9.0% | 11.3% | 0.331 | 1.741 | 1.008 | 3.005 | 0.047 |
| Diabetes | 21.5% | 22.2% | 0.835 | 1.208 | 0.816 | 1.787 | 0.345 |
| CVD | 54.2% | 63.5% | 0.017 | 1.624 | 1.147 | 2.298 | 0.006 |
| HIV infection | 4.2% | 2.9% | 0.414 | 0.504 | 0.189 | 1.341 | 0.170 |
| Chronic Respiratory Disease | 15.4% | 18.0% | 0.370 | 1.400 | 0.909 | 2.154 | 0.127 |
| Kidney Disease | 5.5% | 20.9% | <0.001 | 4.654 | 2.810 | 7.709 | <0.001 |
| Other Metabolic Disease | 12.9% | 7.9% | 0.048 | 0.483 | 0.273 | 1.356 | 0.130 |
| Liver Disease | 0.9% | 1.7% | 0.350 | 2.423 | 0.542 | 10.830 | 0.247 |
| Chronic Neurological Disease | 8.1% | 18.4% | <0.001 | 3.326 | 2.059 | 5.372 | <0.001 |
| Obesity | 16.2% | 14.7% | 0.565 | 0.934 | 0.559 | 1.562 | 0.796 |
Abbreviations: OR, odds ratio; LL, lower limit; UL, upper limit; CVD, cardiovascular diseases; HIV, Human Immunodeficiency Virus.
Results are based on the Chi-Squared test.
Odds ratios, 95% Confidence Intervals, and p-values are based on an age-adjusted logistic regression model including all factors reported in the table (presence vs. absence of each comorbidity).
4. Discussion
Over the years, there has been a growing interest in exploring gender differences in relation to medical conditions and health status. Gender differences, in fact, can have a significant impact on behavior, responses, and outcomes, and neglecting these differences by not providing personalized and gender-sensitive care may exacerbate the situation. Recently, Machluf and colleagues conducted a review of the literature to examine the progress of gender-specific medicine research over time. However, their review highlighted a lack of gender-sensitive awareness in the translation of clinical research findings into gender-specific treatment regimens, guidelines, and preventive strategies, as well as in the development of gender-oriented health policies. Similarly to other diseases, COVID-19 exhibits a high degree of heterogeneity in its clinical course and disease severity [50,51]. Many factors can trigger the differences between individuals, including their clinical condition, access to healthcare services and preventive programs, and the local epidemiological context [14,23,25,27,48]. Despite the importance of developing personalized and gender-sensitive approaches to prevention and treatment, the current evidence does not provide a clear indication of how gender may affect COVID-19 manifestations and outcomes.
Existing evidence suggests that while the incidence of COVID-19 cases is similar between men and women [2,20,35], there are also indications of a higher proportion of men who have been hospitalized or required admission to intensive care compared to women [14,35,52]. Despite previous studies indicating that gender may impact different biological pathways leading to severe forms of the disease and adverse outcomes [20,30,50], the influence of gender on COVID-19 remains a matter of controversy. In this scenario, we hypothesized that the observed gender differences in COVID-19 outcomes may be attributed to the varying prevalence of pre-existing medical conditions. Accordingly, we used data from the “MASSI” study to explore differences between genders in the distribution of underlying medical comorbidities. Our study included only SARS-CoV-2 positive patients presenting with at least one previous medical condition. The majority of patients were male, and a significant age disparity between genders was observed, with males being significantly younger than females. In contrast, there were no disparities in the proportion of vaccinated patients between the two genders, suggesting a similar inclination toward vaccination. Despite their younger age, men displayed a greater number of comorbidities compared to women, albeit not significantly. This was in line with previous studies showing that men are usually more likely to present previous medical conditions (e.g., hypertension, cardiovascular disease, and respiratory conditions) than females [20,24,26,35,52]. This finding could be partly attributed to the higher prevalence of high-risk behaviors and lifestyles that are more prevalent among men than women, as previously reported in studies [8,20,26,48]. The increased exposure to high-risk behaviors could contribute to a higher risk of comorbidities, and hence a higher risk of adverse outcomes related to COVID-19 in men [14,23,25,26,48,50]. Our study found that diabetes was more prevalent in males, whereas obesity and other metabolic diseases were more common among females. These findings partially align with the study by Fortunato and colleagues, who reported gender disparities in the prevalence of diabetes but not in other medical conditions [14]. Several studies have demonstrated that metabolic disorders, such as obesity and diabetes, are significant risk factors for severe COVID-19 illness [[53], [54], [55], [56], [57], [58], [59]]. For example, obese patients often exhibit chronic low-grade inflammation, impaired immune response, and metabolic dysfunction, which could potentially explain their heightened susceptibility to severe COVID-1958. Similarly, diabetes has emerged as a commonly contributing factor not only to the severity and mortality of COVID-19 patients, but also to the associated complications [[53], [54], [55], [56], [57]]. A meta-analysis demonstrated that diabetes in patients with COVID-19 was associated with a two-fold increase in mortality as well as severity of COVID-1953. Another study reported that COVID-19 patients with diabetes were at an increased risk of severe complications, such as acute respiratory distress syndrome, multi-organ failure, and death [54].
Despite the different prevalence of comorbidities, in our study there was no gender difference in terms of mortality. However, our findings also indicated that men were more likely to require intubation and receive drug therapy for COVID-19. These results only partially align with the current consensus that men are at higher risk of severe forms of COVID-19 and death [14,25,36,60]. In particular, a recent meta-analysis - conducted by Peckham and colleagues on 3 million of COVID-19 cases - found that being male was associated with an increased risk of death [35]. Similar findings were obtained by Italian studies showing a higher number of deaths [61] and hospitalizations in intensive care unit [62,63] among males than females. Therefore, a comprehensive gender analysis of the COVID-19 outbreak should inform policies and public health efforts to address its gender-specific impacts more effectively [64]. Thus, our study aimed to investigate how comorbidities contribute to COVID-19 mortality across genders. Both male and female COVID-19 patients had a significantly higher risk of death if they had comorbidities such as CVDs, kidney diseases, or chronic neurological diseases. Furthermore, our study found that diabetes and chronic respiratory diseases were significant risk factors for COVID-19 mortality among men, whereas cancer was a significant contributor among women. This underscores the importance of considering the distribution and impact of comorbidities when assessing the risk of COVID-19 and its complications. However, further studies should be conducted to confirm our findings.
There are several limitations to our study that should be discussed. Firstly, it was a local registry analysis of clinical and epidemiological data collected over a relatively long period of time since the onset of the COVID-19 outbreak in Italy. Thus, data may suffer from some inaccuracies, especially in those periods characterized by overwhelming pressure on the healthcare system and on tracking activity. Secondly, socio-demographic, behavioral, and occupational information was not available or missing for the majority of cases. While gender is an important risk factor to consider, other factors - such as socio-demographic variables, minority status, residence, education, lifestyle habits, etc. - should be incorporated to more fully characterize the needs of sub-populations and properly address their unique needs. Thirdly, missing or inaccurate information on the date and place of death did not allow us to evaluate the risk of dying in the hospital or after a specific interval of time. However, we also calculated the 30-day mortality, which was 10% points lower than the overall mortality, with no gender differences. It is necessary to highlight that the follow-up period of our study extended from the time of diagnosis to the negative conversion of each patient. However, both dates may be influenced by possible delays in diagnosis and re-testing strategies for positive subjects, not necessarily corresponding to the period of positivity. Therefore, we preferred to consider overall mortality as the primary endpoint, in line with the WHO definitio. Furthermore, there was no information available on the severity of the disease, COVID-19-related respiratory failure, or admission to the intensive care unit, which limited our ability to assess these factors. As a result, we had to rely solely on intubation and pharmacological therapy against COVID-19 as proxy measures.
Despite the limitations mentioned above, our study revealed that males, even at a younger age, had a higher prevalence of medical conditions associated with the risk of death. While some risk factors were shared by both genders (e.g., CVDs, kidney diseases, or chronic neurological diseases), there were others that were gender-specific. In particular, diabetes and chronic respiratory diseases were associated with higher mortality risk in men, while cancer was a significant risk factor for mortality in women. By confirming these findings and increasing awareness of gender-specific medicine in clinical practice, we can potentially reduce disparities, develop tailored guidelines, and effectively address the unique characteristics and needs of specific subpopulations within each gender in the future.
Ethics statement
The study received approval from the local Ethics Committee “Catania 2" (Catania, Italy) under protocol number 31/C.E. dated January 14, 2022. Informed consent was waived as the study involved a secondary retrospective analysis of surveillance data obtained from the Provincial Health Authority (Azienda Sanitaria Provinciale, ASP) of Catania.
Author contribution statement
Antonella Agodi, Martina Barchitta: Conceived and designed the experiments; Analyzed and interpreted the data.
Andrea Maugeri: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Giuliana Favara, Roberta Magnano San Lio: Analyzed and interpreted the data; Wrote the paper.
Martina Puglisi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dario Sinatra, Giuseppe Liberti: Contributed reagents, materials, analysis tools or data; Performed the experiments; Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Additional information
[item-group: IG000001].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Italian National Institute of Health. COVID-19 Dashboard https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard.
- 3.Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D., Gan H., Sun Y.L., Fu W., Li W., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 4.Sokolowska M., Lukasik Z.M., Agache I., Akdis C.A., Akdis D., Akdis M., Barcik W., Brough H.A., Eiwegger T., Eljaszewicz A., et al. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives-A report of the European academy of allergy and clinical immunology (EAACI) Allergy. 2020;75:2445–2476. doi: 10.1111/all.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anca P.S., Toth P.P., Kempler P., Rizzo M. Gender differences in the battle against COVID-19: impact of genetics, comorbidities, inflammation and lifestyle on differences in outcomes. Int. J. Clin. Pract. 2021;75 doi: 10.1111/ijcp.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzo M., Foresti L., Montano N. Comparison of reported deaths from COVID-19 and increase in total mortality in Italy. JAMA Intern. Med. 2020;180:1250–1252. doi: 10.1001/jamainternmed.2020.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bwire G.M. Coronavirus: why men are more vulnerable to covid-19 than women? SN Compr. Clin. Med. 2020;2:874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Xu W., Zhang C., Yu J., Jiang B., et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in jiangsu province: a multicenter descriptive study. Clin. Infect. Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunato F., Martinelli D., Lo Caputo S., Santantonio T., Dattoli V., Lopalco P.L., Prato R. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barek M.A., Aziz M.A., Islam M.S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 17.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Du C., Zhang Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falahi S., Kenarkoohi A. Sex and gender differences in the outcome of patients with COVID-19. J. Med. Virol. 2021;93:151–152. doi: 10.1002/jmv.26243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Bai H., Liu J., Chen G., Liao Q., Yang J., Wu P., Wei J., Ma D., Ai J., Li K. Distinct clinical characteristics and risk factors for mortality in female inpatients with coronavirus disease 2019 (COVID-19): a sex-stratified, large-scale cohort study in wuhan, China. Clin. Infect. Dis. 2020;71:3188–3195. doi: 10.1093/cid/ciaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavin W., Campbell E., Zaidi S.A., Gavin N., Dbeibo L., Beeler C., Kuebler K., Abdel-Rahman A., Luetkemeyer M., Kara A. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am. J. Infect. Control. 2021;49:158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Y., Wu P., Lu W., Liu K., Ma K., Huang L., Cai J., Zhang H., Qin Y., Sun H., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin L., Li X., Shi J., Yu M., Wang K., Tao Y., Zhou Y., Zhou M., Xu S., Wu B., et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J. Med. Virol. 2020;92:2684–2692. doi: 10.1002/jmv.26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bienvenu L.A., Noonan J., Wang X., Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maleki Dana P., Sadoughi F., Hallajzadeh J., Asemi Z., Mansournia M.A., Yousefi B., Momen-Heravi M. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehospital Disaster Med. 2020;35:438–441. doi: 10.1017/S1049023X20000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein S.L., Dhakal S., Ursin R.L., Deshpande S., Sandberg K., Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee S., Pahan K. Is COVID-19 gender-sensitive? J. Neuroimmune Pharmacol. 2021;16:38–47. doi: 10.1007/s11481-020-09974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cataldo C., Masella R. Gender-related sociocultural differences and COVID-19: what influence on the effects of the pandemic? Epidemiol. Prev. 2020;44:398–399. doi: 10.19191/EP20.5-6.S2.144. [DOI] [PubMed] [Google Scholar]
- 34.Griffith D.M., Sharma G., Holliday C.S., Enyia O.K., Valliere M., Semlow A.R., Stewart E.C., Blumenthal R.S. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev. Chronic Dis. 2020;17:E63. doi: 10.5888/pcd17.200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., Rosser E.C., Webb K., Deakin C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kragholm K., Andersen M.P., Gerds T.A., Butt J.H., Østergaard L., Polcwiartek C., Phelps M., Andersson C., Gislason G.H., Torp-Pedersen C., et al. Association between male sex and outcomes of coronavirus disease 2019 (COVID-19)-A Danish nationwide, register-based study. Clin. Infect. Dis. 2021;73:e4025–e4030. doi: 10.1093/cid/ciaa924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sciomer S., Moscucci F., Dessalvi C.C., Deidda M., Mercuro G. Gender differences in cardiology: is it time for new guidelines? J. Cardiovasc. Med. 2018;19:685–688. doi: 10.2459/jcm.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 38.Regitz-Zagrosek V., Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017;97:1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 39.Doumas M., Papademetriou V., Faselis C., Kokkinos P. Gender differences in hypertension: myths and reality. Curr. Hypertens. Rep. 2013;15:321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 40.Raparelli V., Morano S., Franconi F., Lenzi A., Basili S. Sex differences in type-2 diabetes: implications for cardiovascular risk management. Curr. Pharmaceut. Des. 2017;23:1471–1476. doi: 10.2174/1381612823666170130153704. [DOI] [PubMed] [Google Scholar]
- 41.Bairey Merz C.N., Dember L.M., Ingelfinger J.R., Vinson A., Neugarten J., Sandberg K.L., Sullivan J.C., Maric-Bilkan C., Rankin T.L., Kimmel P.L., Star R.A. Sex and the kidneys: current understanding and research opportunities. Nat. Rev. Nephrol. 2019;15:776–783. doi: 10.1038/s41581-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali I., Högberg J., Hsieh J.H., Auerbach S., Korhonen A., Stenius U., Silins I. Gender differences in cancer susceptibility: role of oxidative stress. Carcinogenesis. 2016;37:985–992. doi: 10.1093/carcin/bgw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olak J., Colson Y. Gender differences in lung cancer: have we really come a long way, baby? J. Thorac. Cardiovasc. Surg. 2004:346–351. doi: 10.1016/j.jtcvs.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Wang G., He J., Ren S., Wu F., Zhang J., Wang F. Gender differences in colorectal cancer survival: a meta-analysis. Int. J. Cancer. 2017;141:1942–1949. doi: 10.1002/ijc.30827. [DOI] [PubMed] [Google Scholar]
- 45.Nebel R.A., Aggarwal N.T., Barnes L.L., Gallagher A., Goldstein J.M., Kantarci K., Mallampalli M.P., Mormino E.C., Scott L., Yu W.H., et al. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheim. Dement. 2018;14:1171–1183. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canevelli M., Quarata F., Remiddi F., Lucchini F., Lacorte E., Vanacore N., Bruno G., Cesari M. Sex and gender differences in the treatment of Alzheimer's disease: a systematic review of randomized controlled trials. Pharmacol. Res. 2017;115:218–223. doi: 10.1016/j.phrs.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 47.Raimondi F., Novelli L., Ghirardi A., Russo F.M., Pellegrini D., Biza R., Trapasso R., Giuliani L., Anelli M., Amoroso M., et al. Covid-19 and gender: lower rate but same mortality of severe disease in women-an observational study. BMC Pulm. Med. 2021;21:96. doi: 10.1186/s12890-021-01455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haitao T., Vermunt J.V., Abeykoon J., Ghamrawi R., Gunaratne M., Jayachandran M., Narang K., Parashuram S., Suvakov S., Garovic V.D. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin. Proc. 2020;95:2189–2203. doi: 10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Who . 2020. Estimating Mortality from COVID-19.https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 [Google Scholar]
- 50.Meijs D.A.M., van Bussel B.C.T., Stessel B., Mehagnoul-Schipper J., Hana A., Scheeren C.I.E., Peters S.A.E., van Mook W.N.K.A., van der Horst I.C.C., Marx G., et al. Better COVID-19 Intensive Care Unit survival in females, independent of age, disease severity, comorbidities, and treatment. Sci. Rep. 2022;12:734. doi: 10.1038/s41598-021-04531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 52.Jun T., Nirenberg S., Weinberger T., Sharma N., Pujadas E., Cordon-Cardo C., Kovatch P., Huang K.L. Analysis of sex-specific risk factors and clinical outcomes in COVID-19. Commun. Med. 2021;1:3. doi: 10.1038/s43856-021-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metabol. Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajpal A., Rahimi L., Ismail-Beigi F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J. Diabetes. 2020;12:895–908. doi: 10.1111/1753-0407.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dallavalasa S., Tulimilli S.V., Prakash J., Ramachandra R., Madhunapantula S.V., Veeranna R.P. COVID-19: diabetes perspective-pathophysiology and management. Pathogens. 2023;12 doi: 10.3390/pathogens12020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buscemi S., Corleo D., Randazzo C. Risk factors for COVID-19: diabetes, hypertension, and obesity. Adv. Exp. Med. Biol. 2021;1353:115–129. doi: 10.1007/978-3-030-85113-2_7. [DOI] [PubMed] [Google Scholar]
- 57.Singh A.K., Khunti K. COVID-19 and diabetes. Annu. Rev. Med. 2022;73:129–147. doi: 10.1146/annurev-med-042220-011857. [DOI] [PubMed] [Google Scholar]
- 58.Zhu X., Yang L., Huang K. COVID-19 and obesity: epidemiology, pathogenesis and treatment. Diabetes Metab. Syndr. Obes. 2020;13:4953–4959. doi: 10.2147/dmso.s285197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Lorenzo A., Estato V., Castro-Faria-Neto H.C., Tibirica E. Obesity-related inflammation and endothelial dysfunction in COVID-19: impact on disease severity. J. Inflamm. Res. 2021;14:2267–2276. doi: 10.2147/jir.s282710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakbar I., Luque-Paz D., Mege J.L., Einav S., Leone M. COVID-19 gender susceptibility and outcomes: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minnai F., De Bellis G., Dragani T.A., Colombo F. COVID-19 mortality in Italy varies by patient age, sex and pandemic wave. Sci. Rep. 2022;12:4604. doi: 10.1038/s41598-022-08573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern. Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machluf Y., Chaiter Y., Tal O. Gender medicine: lessons from COVID-19 and other medical conditions for designing health policy. World J. Clin. Cases. 2020;8:3645–3668. doi: 10.12998/wjcc.v8.i17.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.