Abstract
Background
Bone grafting is commonly used in spine surgery to supplement or replace the need for autografts. This is harvested, prepared, and utilized predominantly for osteoconductive properties. Anterior cervical discectomy and fusion, a procedure to decompress and fuse the spine which treats herniated discs and compressed nerves, commonly uses Polyetheretherketone (PEEK) interbody filled with allograft bone matrices to reconstruct the disc space after a discectomy is performed.
Case Description
The presented case is one of a 57-year-old male patient who underwent an uneventful cervical 5–6 and cervical 6–7 discectomy and fusion using a PEEK interbody and bone allograft. The allograft had been prepared using cancellous bone particles with preserved living cells and demineralized cortical bone fibers to facilitate bone repair and healing, which is a common technique. The allograft was aseptically processed to preserve native factors that can support bone repair and prevent contamination and cross-contamination of the product. Additionally, the product was sterilized using gamma irradiation to further prevent contamination.
Outcome
Unfortunately, with the presented case, the State's Department of Health and The Center for Diseases Control and Prevention identified that the graft was from a source contaminated with tuberculosis. The patient being reported went on to develop disseminated tuberculosis, including lung abscesses and osteomyelitis.
Conclusions
The current case highlights that there was contamination of the donor bone sources. Tuberculosis was not screened in the tissue donor even though he had risk factors, symptoms, and signs consistent with tuberculosis. Although there are methods to screen potential organ donors for tuberculosis, there is currently no approved standard laboratory tuberculosis screening tool for bone grafts. Thus, this emphasizes the importance of proper screening among individual institutions for even the most uncommon diseases in all donated bone grafts.
Keywords: Mycobacterium tuberculosis, Bone graft, Allograft, Anterior cervical discectomy and fusion, Screening protocol, Infectious disease
Introduction
Bone grafting is commonly used in spine surgery to supplement or replace the need for autografts. This is harvested, prepared, and utilized predominantly for osteoconductive properties. Anterior cervical discectomy and fusion (ACDF) commonly use structural allografts to reconstruct the disc space after discectomy is performed and the preparation and processing of such grafts is of critical importance.
The use of allografts has significantly changed how spine surgeries, such as ACDFs, are performed. In a study published in Cureus, 21,000 patients participated in a study comparing the use of autografts and allografts, and their effects on postoperative complications. Compared to allografts, patients who received an autograft had a significantly greater incidence of extended length of hospital stay and increased overall postoperative complications, which increased the overall cost of care [1]. Thus, there began a push for allograft usage in surgical spinal procedures.
Sterilization of allografts is necessary to reduce and prevent the risk of transmission of infectious agents, and this definitive method helps eliminate microorganisms from being transferred to the hosts. Allografts can be sterilized in several ways, but one of the most widespread and successful methods of sterilization is radiation. Gamma irradiation with cobalt-60 at doses of 15 to 30 kGy has been adopted by various tissue banks to not only sterilize the tissue grafts but also to keep intact the biochemical properties of the tissue for successful allograft implantation [2].
Screening bone grafts for diseases is also of utmost importance and varies greatly between regions. According to the Clinical Infectious Diseases Journal, infections for which donors are routinely screened include treponema pallidum, mycobacterium tuberculosis, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, HIV, HBV and HCV [3]. In certain regions that are more exposed to specific fungi, parasites, and viruses, additional screening may be performed. Donors may also be screened for infectious agents in the transplantation of specific organs (Toxoplasma gondii in cardiac recipients) [3].
The FiberCel bone graft was prepared using cryopreserved cancellous and corticocancellous bone matrices with preserved living cells and demineralized cortical bone fibers that were aseptically processed. The products were then stored at -75 degrees Celsius to ensure that their properties were maintained and sterility was achieved[4]. The following case is an example of a contaminated bone graft and its sequelae.
The case report was IRB-approved and ethical guidelines were taken into consideration.
Case presentation
Initial patient presentation
On March 1, 2021, a 57-year-old white male in the United States presented with neck pain and cervical radiculopathy that had persisted despite conservative measures, medications, and cervical injections. The patient denied drug and alcohol use, and the patient had a history of tobacco use consisting of cigarettes. The patient denied any recent immigration, travel, or incarceration history.
Radiographs demonstrated severe degenerative changes at C5–C6 and C6–7, and MRI showed multilevel moderate degenerative disc disease and straightening of the normal cervical lordosis (Fig. 1).
Fig. 1.
X-ray results dated December 14, 2020 showing 2 views of the cervical spine. MRI results dated December 17, 2020 showing axial and sagittal images obtained through the cervical spine without contrast.
Therapeutic intervention
On March 16, 2021, the patient underwent a two-level ACDF at C5–6 and C6–7.
The graft used was a Polyetheretherketone (PEEK) interbody spacer filled with FiberCel Fiber Viable Bone Matrix. The graft was placed in a PEEK material Talos cage. The graft brand and manufacturer were Aziyo Biologics, distributed by Medtronic SpinalGraft Technologies LLC. The lot number which was recalled was NMDS210011.
Postoperative images were taken on March 31, 2021 and showed the postoperative construct (Fig. 2).
Fig. 2.
X-ray results dated March 31, 2021 showing 2 views of the cervical spine.
Postoperative course and pertinent aspects of the case presentation
Following the surgical ACDF in April 2021, the patient only had minor complaints of residual neck pain and stiffness. However, the patient stated that he had significant improvement in tingling and weakness.
On May 28, 2021, the patient presented with neck pain after falling and hitting the wall and subsequently developed shooting pain from his neck into his shoulders. Pertinent positives included diaphoresis, cough, back pain, falls, joint pain, myalgias, tingling, tremors, weakness, and easy bruising and bleeding.
On June 4, 2021, the State's Department of Health and The Centers for Disease Control and Prevention (CDC) received notice about the tuberculosis-infected bone graft following a customer complaint from one hospital that initially reported postsurgical infection in 7 of their 23 patients who received FiberCel from the Donor Lot. After the notification to the State Department and CDC, hospitals across the United States who received the same product were notified and subsequently, the surgeons were notified by the hospitals.
On June 7, 2021, the surgeon who performed the spinal procedures personally called each patient to make them aware of the possible contamination with the bone graft and advised them to come to the hospital for testing and further management. Although the patient described in this case report had neck pain prior to the recall, his symptoms were nonspecific and because of his fall, there were no signs or symptoms directly pointing to an infected bone graft. The patient presented to the orthopedic office with neck pain and reported fever, weight loss, chills, and night sweats. His physical exam included significant tenderness in the cervical region. The patient also received a chest X-ray showing findings suspicious of tuberculosis. Due to the allograft recall and the patient's clinical findings, the infectious disease physician carried out a blood test that confirmed exposure to tuberculosis as the patient tested positive with QuantiFERON Gold.
The donor of the FiberCel bone graft was screened for a series of viruses, including HIV and hepatitis B and C, but tuberculosis was not listed in the screening tests [4]. The FiberCel product label contained other information on sterility control and precautions, but the company failed to screen for even uncommon bacteria, viruses, and fungi. Due to this failure, hundreds of patients suffered the consequences and long-term health effects associated with a contaminated graft.
Hospitals also must take responsibility for ensuring that all bacteria, fungi, and viruses, whether common or uncommon, should be screened for and ruled out prior to accepting bone grafts from any company. Additionally, hospitals must also ensure that proper measures are taken to decontaminate and sterilize all the bone grafts and that proper documentation is noted on these procedures. This ultimately changed the informed consent protocol for surgical procedures at the institution, as possible infections with bacteria, parasites, fungi, and viruses are included as risk factors when undergoing any transplantation. Additionally, the hospital no longer partners with FiberCel.
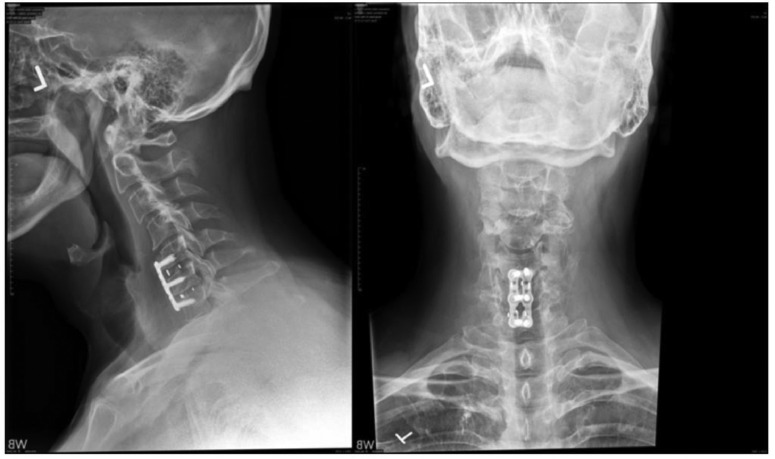
Prerevision X-rays showed that the upper level appeared to be collapsing and the graft pulling away from C5 when compared with previous images (Fig. 3).
Fig. 3.
Prerevision X-rays dated June 7, 2021 showing 2 views of the cervical spine.
The chest X-rays showed at least 3 areas of cavitation within the lower left hemithorax with associated air-fluid levels. The largest air-fluid level measures at least 12 × 8.4 × 8.1 cm in greatest dimension. Mild degenerative changes were also present within the thoracic spine (Fig. 4).
Fig. 4.
Chest X-rays dated June 7, 2021 showing left lower lobe cavitary lesions.
On June 8, 2021, the patient started on outpatient RIPE (rifampin, isoniazid, pyrazinamide, ethambutol) therapy to treat TB per the CDC guidelines.
The MRI images showed an epidural fluid collection posterior to C5, C6, and C7 with fluid collection measuring 10 mm in the anterior to posterior dimension resulting in severe spinal canal stenosis and compression of the cervical spinal cord. Additionally, compression of the spinal cord appeared at the C5 and C6 levels, and there was a mild anterior extension of the fluid collection into the prevertebral soft tissues at the C6 and C7 levels. There was also marrow edema at C5–C7 consistent with osteomyelitis (Fig. 5).
Fig. 5.
MRI images dated June 8, 2021.
Revision surgery
On June 9, 2021, surgery for the removal of hardware was performed. The procedure included an anterior cervical debridement and decompression at C4–C7 with C5 and C6 corpectomies and debridement of infected intraspinal granulation tissue and pus, removal of infected loose spinal instrumentation at C5–C7, anterior cervical fusion with spinal reconstruction at C4–C7, insertion of Medtronic titanium expandible stratosphere cervical reconstruction cage at C4-C7, anterior cervical plating with Medtronic Atlantis static cervical plate at C4–C7, use of osteocell cellular bone graft, use of operating microscope for lysis of neurovascular adhesions, and evacuation of the epidural abscess of the cervical spine.
Procedural findings include extensive granulation tissue and creamy yellow pus ventral to the spine and in the spinal canal from C5–C7. Multiple specimens were sent for microbiology.
The patient was discharged on the same day as his revision procedure.
Postrevision X-rays showed long construct cervical fusion bridging C4–C7 with anterior plate and 4 screws placed. Additionally, images showed corpectomy hardware taking the place of the C5 and C6 vertebral bodies (Fig. 6).
Fig. 6.
Postrevision X-rays dated August 4, 2021 showing 4 views of the cervical spine.
Postrevision chest X-rays showed that previous fluid-filled cavities in the left lung base had decreased. There was also increased infiltration in the left base in the anterior aspect of the chest (Fig. 7).
Fig. 7.
Postrevision chest X-rays dated August 5, 2021 showing 2 views.
On August 9, 2021, the patient completed the four-drug TB regimen and was de-escalated to 2 drugs with rifampin and isoniazid per the CDC recommendations.
Postrevision MRI images showed the previously seen epidural abscess resolution at the C5, C6, and C7 vertebral levels. The prevertebral abscess anterior to C5, C6, and C7 vertebral bodies had also resolved. However, a small amount of fluid was present anterior to the C3 vertebral body (Fig. 8).
Fig. 8.
Postrevision MRI dated August 31, 202.
On June 13, 2022, the patient stopped the TB treatment with rifampin and isoniazid which is 1 year after initiation. Postrevision chest X-ray images showed chronic interstitial markings present in the left lung base, postoperative changes in the left apex, cervical spinal fusion, and chronic changes in the lower cervical spine (Fig. 9).
Fig. 9.
Postrevision chest X-ray dated January 4, 2023.
Long-term consequences
The patient ultimately suffered long-term side effects from the failure of proper screening by a bone graft company that had produced thousands of allografts. Some of these long-term side effects included worsened major depressive disorder after the revision surgery for his TB-infected bone graft. Additionally, the patient still suffered constant joint and neck pain along with neurologic weakness. The patient's neurologist stated that the patient continued to experience numbness that extended down the ventral surface of his forearm and across the dorsal surface of his hand. The patient also stated that he struggled with handwriting, constantly dropped items, and had pain and pruritus in his lateral forearms and palms.
Discussion
The presented case highlights the issues surrounding the contamination of a bone graft product that led to the transfer of an infectious disease to hundreds of bone graft recipients and the significant sequelae of tuberculosis.
On June 2, 2021, FiberCel, a product manufacturer sent a voluntary recall notice for a bone repair product under Lot # NMDS210011 [5]. The Lancet Infectious Diseases Journal found that the tissue donor had unrecognized risk factors and symptoms consistent with Mycobacterium Tuberculosis (MTB), leading to the processing and distribution of 154 units of bone allograft product containing live cells to 37 hospitals and ambulatory centers across the nation [6]. Unfortunately, 136 units were implanted into 113 patients, and 87 of the 113 product recipients developed microbiological or imaging evidence of TB. Furthermore, 8 product recipients died 8 to 99 days after product implantation. Many of these affected patients suffered from short- and long-term health consequences, including the development of abscesses, osteomyelitis, and discitis [7].
Allograft bone grafts undergo rigorous processing and sterilization procedures to minimize the risk of disease transmission and promote biocompatibility. Donors are screened for various infectious diseases, including HIV, hepatitis, and others, to minimize the risk of transmitting infections. FiberCel was prepared from a donor who was screened and tested for HIV-1 and HIV-2, HBV and HCV, syphilis, and HTLV, but was not screened for TB.
Under standard practice, the bone graft is retrieved from the donor through a surgical procedure following appropriate aseptic techniques. The harvested bone is subjected to thorough cleaning and decontamination processes to remove residual tissue and cellular debris. Sterilization methods can include irradiation (gamma or electron beam) or ethylene oxide gas sterilization. These methods effectively kill microorganisms while maintaining the structural integrity of the graft. Quality control measures, such as testing for sterility, residual DNA, and biomechanical properties, are performed to ensure the safety and efficacy of the allograft [2].
The allograft from FiberCel was preserved in 5% Dimethyl Sulfoxide in a 0.9% Sodium Chloride solution. It was packaged in a sterile polycarbonate jar. Destructive microbiological testing per USP 71 sterility tests was performed on samples from each lot and showed “No Growth” after a 14-day incubation period [4].
This case is one of many patients affected by MTB after undergoing spinal surgery since the 2021 outbreak linked to the contaminated bone allograft. Patient X came in for a standard procedure of a cervical discectomy and fusion for cervical spine pain and expected a standard recovery. However, he contracted TB from a contaminated bone graft not adequately screened for by Aziyo Biologics, the creators of FiberCel. The tuberculosis cases reported in 2021 are not the only known graft infections in health care. In 2011, the International Orthopaedics Journal documented allograft cases that transmitted human immunodeficiency virus (HIV), hepatitis C virus (HCV), human T-lymphotropic virus (HTLV), and unspecified hepatitis [8].
The most important lesson learned from experiences such as the TB outbreak from the infected bone allograft includes thoroughly understanding how to prevent future occurrences which can threaten the health and safety of our patients. Hospitals and physicians trust their manufacturers to provide them with the highest quality and safest products possible. Not having proper protocols in place to prevent these problems from reoccurring will ultimately cost the lives of the patients. Ensuring that this trust and mutual understanding exists is important and taking the necessary precautions in the future is essential. Having stringent donor requirements and testing prior to donation is the most effective way to properly select healthy donors. The CDC and other government agencies must also act swiftly in such events to minimize negative consequences and to prevent the further spread of these deadly infectious diseases.
Patient Informed Consent Statement
The authors have received informed patient consent for this case report.
Declaration of Competing Interest
The authors of this case report declare no conflicts of interest.
Acknowledgments
Ethical Approval
This research was approved by the Reid Health Institutional Review Board.
Acknowledgment
Not applicable.
Funding
No funding was provided for the design, development, or conduction of this case report.
Footnotes
FDA device/drug status: Not applicable
Author disclosures: TR: Nothing to disclose. MN: Nothing to disclose. HV: Nothing to disclose.
References
- 1.Ouro-Rodrigues E, Gowd AK, Ramos Williams O, et al. Allograft versus autograft in anterior cervical discectomy and fusion: A propensity-matched analysis. Cureus. Published online. 2022 doi: 10.7759/cureus.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R, Singh D, Singh A. Radiation sterilization of tissue allografts: a review. World J Radiol. 2016;8(4):355. doi: 10.4329/wjr.v8.i4.355/wjr.v8.i4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman JA, Greenwald MA, Grossi PA. Transmission of infection with human allografts: essential considerations in donor screening. Clin Infect Dis. 2012;55(5):720–727. doi: 10.1093/cid/cis519. [DOI] [PubMed] [Google Scholar]
- 4.Ifu-0021 rev03 fibercel package insert.docxjul022020081809 - Aziyo. FiberCel. Accessed June 5, 2023. https://www.aziyo.com/wp-content/uploads/2020/07/IFU-0021-Rev03-FiberCel.pdf. Accessed June 5, 2023.
- 5.Multistate outbreak of tuberculosis (TB) associated with a suspected contaminated bone allograft material used in surgical procedures. Centers for Disease Control and Prevention. https://www.cdc.gov/hai/outbreaks/TB-bone-allograft.html. Published June 14, 2021. Accessed January 20, 2023.
- 6.Schwartz NG, Hernandez-Romieu AC, Annambhotla P, et al. Nationwide tuberculosis outbreak in the USA linked to a bone graft product: An outbreak report. Lancet Infect Dis. 2022;22(11):1617–1625. doi: 10.1016/s1473-3099(22)00425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Wilson WW, Schwartz NG, et al. Notes from the field: tuberculosis outbreak linked to a contaminated bone graft product used in spinal surgery — Delaware, March-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1261–1263. doi: 10.15585/mmwr.mm7036a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project Notify. Int Orthop. 2011;36(3):633–641. doi: 10.1007/s00264-011-1391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]