Summary
The association between gut microbiota and development of Graves’ disease (GD) remains unclear. This study aimed to profile the gut microbiota of 65 patients newly diagnosed with GD before and after treatment and 33 physical examination personnel via 16S rRNA sequencing. Significant differences in the gut microbiota composition were observed between the two groups, showing relative bacterial abundances of 1 class, 1 order, 5 families, and 14 genera. After treatment, the abundance of the significantly enriched biota in the GD group decreased considerably, whereas that of the previously decreased biota increased considerably. Further, interleukin-17 levels decreased significantly. The random forest method was used to identify 12 genera that can distinguish patients with GD from healthy controls. Our study revealed that the gut microbiota of patients with GD exhibit unique characteristics compared with that of healthy individuals, which may be related to an imbalance in the immune system and gut microbiota.
Subject areas: Human metabolism, Microbial physiology, Microbiome, Pathology
Graphical abstract

Highlights
-
•
Gut microbiota (GM) of GD exhibits unique characteristics compared to that of healthy individuals
-
•
GD patients are associated with GM and immune dysregulation
-
•
Twelve bacterial genera can distinguish patients with GD far more accurately
-
•
After treatment with ATD, GM of GD patients gradually recovered
Human metabolism, Microbial physiology, Microbiome, Pathology
Introduction
Graves’ disease (GD) is an autoimmune disease caused by a combination of genetic and environmental factors, whose symptoms include palpitations, increased appetite, weight loss, irritability, and other symptoms of enhanced metabolic functions in various systems of the body. This disease also causes pretibial myxedema; Graves’ ophthalmopathy, which may cause blindness in some severe cases; and hyperthyroid crisis with high mortality rates.1,2 A study showed that the prevalence of GD is approximately 0.5% worldwide, and its incidence has increased significantly in recent years.3 The gastrointestinal tract has the largest and most complex microecosystem in the human body and plays an important role in metabolism, digestion and absorption, immune function, and defense mechanisms against pathogens.4,5
Under normal circumstances, the composition and function of the gut microbiota (GM) in the body are maintained in a relatively stable state6; however, increasing evidence has demonstrated that the occurrence of autoimmune diseases is related to an imbalance in the GM. Some studies have proven that the structure of the GM is altered in patients with autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes mellitus, and asthma.7,8 It is speculated that the mechanism of interaction between GM and autoimmune diseases may be molecular simulation, bystander activation, or epitope diffusion.9,10,11 Recently, the concept of the thyroid–gut axis has attracted increasing attention by researchers.12 Studies have shown that the GM in patients with Hashimoto’s thyroiditis is imbalanced, and the reduction in the number of some anti-inflammatory bacteria may cause Th17/Treg imbalance, thereby promoting the occurrence and development of the disease.13,14
Furthermore, the occurrence of GD may be related to chronic enterovirus infection. For instance, Yersinia enterocolitica, Helicobacter pylori, and hepatitis C virus infections have been reported to be involved in the pathogenesis of GD; however, the specific mechanisms remain unclear.15,16 Some studies have shown that the structure and diversity of the GM in patients with GD are altered; however, these results are inconsistent,17,18,19,20 which may be related to individual differences in samples, number of samples, course of patients with GD, and sequencing platforms and sequencing depths in each study. On this basis, this study recruited patients newly diagnosed with GD and analyzed differences in the GM between the GD and Control (Con) groups. Moreover, patients with GD were treated with antithyroid drugs, and alterations in their GM were longitudinally assessed.
Results
Baseline analysis
Clinical features
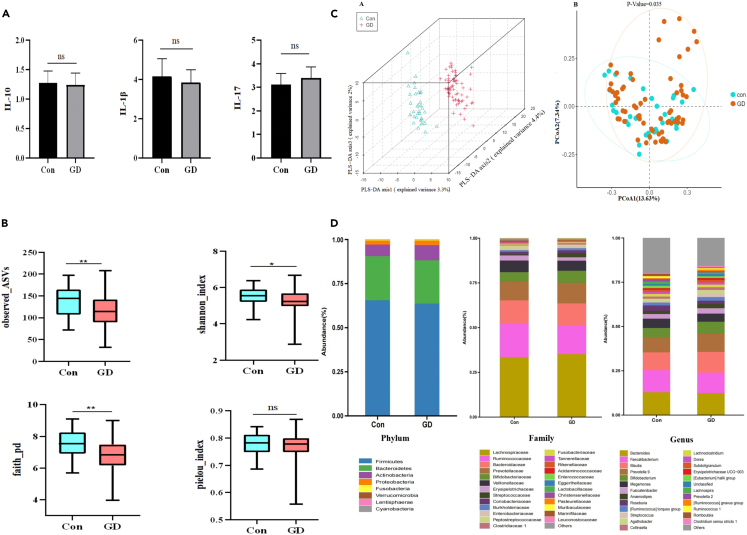
We analyzed the clinical characteristics of 98 participants at baseline, and no significant differences in age, sex, and body mass index (BMI) were observed between the two groups. In contrast, free triiodothyronine (FT3), free thyroxine (FT4), thyroperoxidase antibody (TPOAb), thyroglobulin antibody (TgAb), and thyrotrophin receptor antibody (TRAb) levels were significantly higher and thyroid-stimulating hormone (TSH) levels were significantly lower in the GD group than in the Con group (Table 1). Furthermore, we measured the levels of cytokines secreted by Th17 and Treg lymphocytes and found no significant difference in IL-10 and IL-1β levels between the two groups. Although IL-17 levels increased in the GD group compared with those in the Con group, the difference was insignificant (Figure 1A).
Table 1.
Baseline characteristics of control and GD groups
| Con group (n = 33) | GD group (n = 65) | p value | |
|---|---|---|---|
| Age (years) | 27.00 (26.00–29.00) | 30.00 (25.00–40.50) | 0.0547 |
| Sex(male/female) | 10/23 | 18/47 | 0.8159 |
| BMI (kg/m2) | 20.03 (19.29–21.96) | 20.81 (18.93–22.76) | 0.4177 |
| FT3 (pg/mL) | 3.04 (2.77–3.34) | 18.85(12.60–28.88) | <0.0001∗∗∗∗ |
| FT4 (ng/dL) | 1.14 (1.01–1.28) | 6.53 (4.19–11.66) | <0.0001∗∗∗∗ |
| TSH (μIU/mL) | 2.43 (1.80–3.29) | 0.0025 (0.0025–0.005) | <0.0001∗∗∗∗ |
| TRAb (U/L) | 1.37 (0.35–1.76) | 5.10 (3.10–19.00) | <0.0001∗∗∗∗ |
| TPO-Ab (IU/mL) | 23.62 (20.13–26.90) | 133.70 (24.25–320.90) | <0.0001∗∗∗∗ |
| TG-Ab (IU/mL) | 5.00 (5.00–7.82) | 371.2 (56.72–791.00) | <0.0001∗∗∗∗ |
GD, Graves' disease; BMI, body mass index; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TPO-Ab, thyroperoxidase antibody; TG-Ab, thyroglobulin antibody; TRAb, thyrotrophin receptor antibody. Data are median (25th–75th percentile) unless otherwise indicated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Figure 1.
Baseline Clinical and gut microbiota characteristics of control and GD group
(A) Comparison of Cytokines in GD patients and Con.ns:p > 0.05.
(B) Comparison of alpha diversity in GD patients and Con at the ASV level. The observed_ASVs indices reflect the abundance of microbiota. The shannon_index and faith_pd indices reflect the alpha diversity, and the pielou_index reflects the evenness.ns:p > 0.05; ∗p < 0.05; ∗∗p < 0.01. GD, Graves’ disease; Con, Control group; ASV,Amplicon Sequence Varia.
(C) A The PLS-DA (Partial Least Squares Discriminant Analysis) showed a significant separation between GD and Con. B.Principal coordinates analysis (PCOA) based on the distance matrix of bray-curtis distance at the ASVs showed that the gut microbiota of GD was separated clearly from those of Con.
(D) Composition of the gut microbiota at the phylum, family and genus level.
Characteristics of the gut microbiota
In total, 937 amplicon sequence variants (ASVs) were obtained via fecal sample sequencing. Alpha diversity analysis based on the ASV level showed that the observed_ASVs, faith_pd, and shannon_index were significantly lower in the GD group than in the Con group. In contrast, no significant difference was observed in the pielou_index (Figure 1B), indicating that the richness and diversity of the intestinal flora in patients with GD reduced significantly, whereas evenness did not change significantly. Then, we evaluated the beta diversity of the two groups. Based on bray_curtis dissimilarity PCoA analysis (p < 0.05 in permutational MANOVA) and partial least squares discriminant analysis (PLS-DA) plot, the GM composition of patients with GD was significantly different from that of healthy controls (Figure 1C).
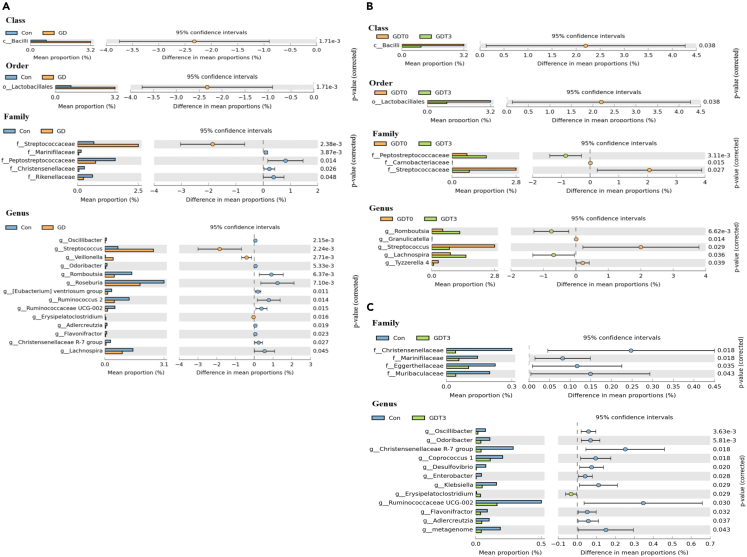
Furthermore, we analyzed the taxonomic units of the two groups, and the stacked graph showed certain differences between the GD and Con groups at the phylum, family, and genus levels (Figure 1D). To explore the specific GM structure of patients with GD, we further analyzed the differences in the GM between the two groups. The results revealed that Firmicutes and Bacteroidetes were the dominant phyla in both groups at the phylum level; however, no significant difference in the Firmicutes/Bacteroidetes ratio (F/B) was observed between the two groups. In the GD group, the abundances of Bacilli at the class level; Lactobacillales at the order level; Streptococcaceae at the family level; and Streptococcus, Veillonella, and Erysipelatoclostridium at the genus level were significantly enriched. However, the abundance of Peptostreptococcaceae, Christensenellaceae, Marinifilaceae, and Rikenellaceae at the family level and the abundance of 10 genera, such as Roseburia, Romboutsia, Lachnospira, and Eubacterium ventriosum, at the genus level were significantly lower in the GD group than in the Con group (Figure 2A). Our findings suggest that patients with GD have distinct GM compared with healthy controls.
Figure 2.
Abundance of gut microbiota in Con,GDT0 and GDT3 groups
(A) Differentially abundant taxa from the phylum to genus level were further analyzed between Con and GD groups by STAMP analysis using Welch’s t test (p < 0.05, q < 0.05).
(B) Differentially abundant taxa from the phylum to genus level were further analyzed between GDT0and GDT3 groups by STAMP analysis using Welch’s t test.
(C) Differentially abundant taxa from the phylum to genus level were further analyzed between Con and GDT3 groups by STAMP analysis using Welch’s t test.
To further understand the biomarkers of the GM in patients with GD, we performed random forest analysis and cross-validation of the genera obtained from participant samples and finally identified 12 genera (Figure 3A). Species clustering analysis revealed that the GD group was significantly different from the Con group, and receiver–operating characteristic (ROC) curve analysis revealed that the area under the ROC curve value was 0.9021 (95% confidence interval: 0.8451–0.9591) (Figure 3B), indicating that the above-mentioned microorganisms could be used as potential biomarkers to distinguish the GD group from the Con group.
Figure 3.
Potential biomarkers of gut microbiota in GD patients
(A) Top 12 biomarker genus categories identified using random forests. Biomarker taxa are ranked in descending order of importance to the accuracy of the model. The insert represents 10-fold cross-validation error as a function of the number of input classes used to differentiate GD patients in order of variable importance.
(B) The ROC curve was used to assess the diagnostic accuracy of the 12 genera based on random forest results.Heatmap showing abundance of 12 genera based on random forest results. Species cluster shows that two groups can be clearly separated. The color of the spot corresponds to the Log10transformed relative abundance of each genera.
(C) The relationships among 10 clinical parameters and the relative abundance of the 12 genera in the GD and Con groups were estimated using Spearman correlation analysis. Heatmaps showing correlations between clinical factors and gut microbiota at genus level. Color intensity represent magnitude of correlation. Red = positive correlations; blue = negative correlations. ‘∗’ denotes adjusted p < 0.05; ‘∗∗’ denotes adjusted p < 0.01.
To explore the relationship between biomarkers and clinical parameters, we performed Spearman’s correlation analysis of biomarkers and clinical indexes. Our findings revealed that the abundance of Veillonella was significantly positively correlated with FT3, FT4, and TRAb, that of 10 genera was significantly negatively correlated with FT3 and FT4, and that of 8 genera was significantly negatively correlated with TRAb. However, TSH was significantly negatively correlated with the abundance of Veillonella and significantly positively correlated with the abundance of 10 genera. BMI was significantly negatively correlated with Turicibacter abundance. Regarding cytokines, IL-17 levels were also significantly negatively correlated with Eubacteriumhallii group abundance (Figure 3C).
Subgroup analysis
We further analyzed the characteristics of 18 patients with confirmed GD and impaired liver function before treatment (GDH). Regarding clinical parameters, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels increased significantly in the GDH group, whereas TgAb levels decreased significantly; the other parameters in the GDH group were not significantly different from those in patients with GD without hepatic impairment (GDN) (Table S1).
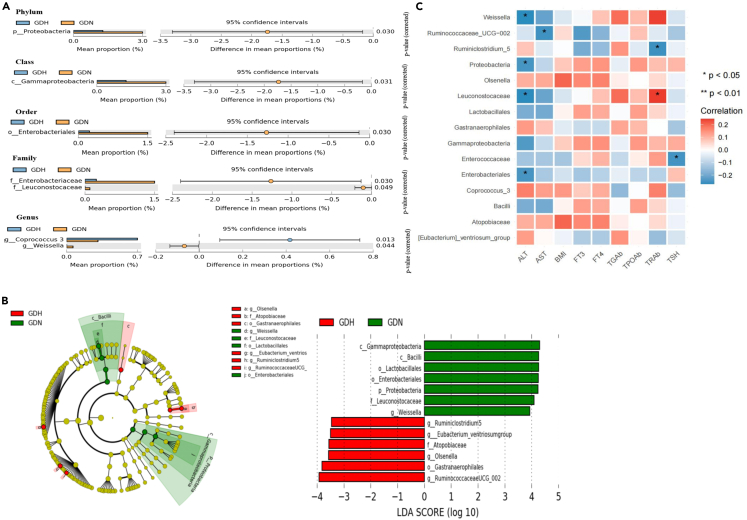
Gut microbial specificity analysis revealed that the abundances of Proteobacteria at the phylum level, Gammaproteobacteria at the class level, Enterobacteriales at the order level, Enterobacteriaceae and Leuconostocaceae at the family level, and Weissella at the genus level were significantly lower in the GDH group, whereas the abundance of Coprococcus 3 was significantly enriched (Figure 4A). Moreover, we explored the microbiota that showed significant differences between the two groups via linear discriminant analysis effect size (LEfSe) analysis. As a result, 13 discriminative features were identified, including one phylum, two classes, three orders, two families, and five genera (Figure 4B). Then, Spearman’s correlation analysis of the 15 bacterial taxa and clinical indicators was performed. The results revealed that ALT was significantly negatively correlated with the abundances of Weissella, Enterobacteriales, Leuconostocaceae, and Proteobacteria and that AST was significantly negatively correlated with Ruminococcaceae UCG-002 abundance (Figure 4C).
Figure 4.
Gut microbiota characteristics of GDH and GDN group
(A) Subgroup analysis:Differentially abundant taxa from the phylum to genus level were further analyzed between GDH and GDN subgroups by STAMP analysis using Welch’s ttest (p < 0.05, q < 0.05).
(B) LEfSe analysis shows bacterial taxa significantly enriched in the GDH (red) or GDN(green) groups. Taxonomic cladogram and linear discriminant analysis (LDA) scores show differences among taxa between GDH and GDN. Only taxa meeting a significant LDA threshold value of >3 are shown.
(C) The relationships among 9 clinical parameters and the relative abundance of the 15 gut microbiota in the GDN and GDH subgroups were estimated using Spearman correlation analysis.Heatmaps showing correlations between clinical factors and gut microbiota. Color intensity represent magnitude of correlation. Red = positive correlations; blue = negative correlations. ‘∗’ denotes adjusted p < 0.05.
Longitudinal follow-up analysis
We analyzed the clinical parameters and GM of patients with GD before (GDT0) and after drug treatment (GDT3). Our findings revealed that FT3, FT4, and TgAb levels decreased significantly, whereas TSH levels increased significantly after 3 months of treatment. TPOAb and TRAb levels showed a downward trend, with no significant difference between the two groups (Table 2). Furthermore, IL-17 levels decreased significantly, whereas IL-10 levels showed an upward trend; however, the difference was insignificant (Figure 5A). No significant difference in FT3, FT4, and cytokine levels was observed between the Con and GDT3 groups; however, the TSH level in the GDT3 group was significantly lower than that in the Con group. TgAb, TPOAb, and TRAb levels in the GDT3 group were significantly higher than those in the Con group, indicating that thyroid function indexes improved after 3 months of drug treatment; however, the patients were still in the state of hyperthyroidism (Table S2).
Table 2.
Changes of clinical parameters in GD group before and after treatment (n = 37)
| GDT0 | GDT3 | p value | |
|---|---|---|---|
| BMI (kg/m2) | 21.09(19.36–23.15) | 22.76(20.73–24.73) | <0.0001∗∗∗∗ |
| FT3 (pg/mL) | 15.65(9.97–48.83) | 2.94(2.64–11.56) | <0.0001∗∗∗∗ |
| FT4 (ng/dL) | 4.52(3.38–11.66) | 1.06(0.84–3.65) | <0.0001∗∗∗∗ |
| TSH (μIU/mL) | 0.0025(0.0025–0.0060) | 0.987(0.0025–2.930) | 0.0017∗∗ |
| TRAb (U/L) | 4.90(3.05–8.7) | 3.00(1.15–8.15) | 0.9101 |
| TPO-Ab (IU/mL) | 128.3(21.87–299.50) | 117.2(16.25–250.60) | 0.1071 |
| Tg-Ab (IU/mL) | 246.3(65.38–725.1) | 90.20(17.42–488.10) | 0.0229∗ |
GDT0,GD group before drug treatment; GDT3,GD group after 3 months of drug treatment.
Figure 5.
Clinical and gut microbiota characteristics of each group after treatment
(A) Comparison of Cytokines in GD patients before treatment(GDT0) and after 3 months of treatment(GDT3).ns:p > 0.05; ‘∗∗’:p < 0.01.
(B) Comparison of alpha diversity in GDT0 patients,GDT3 patients and Con at the ASV level.ns:p > 0.05; ∗p < 0.05; ∗∗p < 0.01.
(C) LEfSe analysis shows bacterial taxa significantly enriched in the GDT0 (red) or GDT3(green) groups. Taxonomic cladogram and linear discriminant analysis (LDA) scores show differences among taxa between GDT0 and GDT3. Only taxa meeting a significant LDA threshold value of >3 are shown.
Alpha diversity analysis based on the ASV level revealed no significant difference in richness or diversity between the two groups (Figure 5B). However, specific analysis of the GM revealed that the abundances of Bacilli at the class level, Lactobacillales at the order level, Streptococcaceae at the family level, and Streptococcus at the genus level decreased significantly in the GDT3 group. In contrast, the abundances of Peptostreptococcaceae at the family level and Romboutsia and Lachnospira at the genus level increased significantly (Figure 2B). The abundance of E. ventriosum showed an upward trend after treatment. Based on the analysis of different bacterial genera between the GD and Con groups (Figure 2A), changes in the GDT3 group were similar to those of the Con group, indicating that the intestinal flora of patients with GD after treatment had been gradually reconstructed and recovered. LEfSe analysis also identified one class, one order, two families, and three genera that could clearly distinguish the GM of the GD groups before and after treatment(Figure 5C). Compared with the Con group, the alpha diversity (richness and diversity) of the GDT3 group decreased significantly. Although no significant differences in the abundance of microbiota at the class and order levels were observed between the two groups, the abundances of Christensenellaceae and Marinifilaceae were significantly lower in the GDT3 group than in the control group at the family level. At the genus level, the abundance of Erysipelatoclostridium was significantly enriched, whereas that of Oscillibacter, Odoribacter, and Christensenellaceae R-7 was significantly lower in the GDT3 group than in the control group(Figure 2C), indicating that although the GM is recovering after treatment, there is still some level of disturbance.
Discussion
Our results showed that the GM of patients with GD was significantly different from that of healthy controls. The diversity and richness of the GM in patients with GD reduced significantly, and different degrees of disorder were observed at each level; in other words, the abundance of flora that produced short-chain fatty acids (SCFAs) and had anti-inflammatory properties decreased. After 3 months of drug treatment, the thyroid function and GM of patients with GD at each level recovered to a certain extent; moreover, IL-17 levels decreased significantly. These results suggest that the occurrence of GD is related to the imbalance in immune and GM. Hence, appropriate drug treatment allowed the immune and gut microbiota of patients with GD to gradually recover. Using random forests, we identified 12 genera that could distinguish patients with GD from healthy controls far more accurately (AUC = 0.9021), and Spearman correlation analysis confirmed that most of these genera were significantly associated with clinical indicators. Finally, subgroup analysis revealed that Weissella reduction may mediate liver damage in patients with GD.
The GM is a complex microecosystem and is a recent research hotspot. There are limited studies on GD and GM, with inconsistent findings. Studies have shown that there is no significant change in the richness and diversity of the GM in patients with GD20,21,22,23 or that the diversity is reduced, with no significant difference in richness.24 However, Su et al.,17 Wen et al.,18 Ishaq et al.,19 and Chen et al.24 showed that both the richness and diversity of the GM reduced in patients with GD; these findings are consistent with our results. Firmicutes and Bacteroidetes are the dominant phyla in the human GM. Studies have shown that the F/B value represents the health status of the body. In some individuals with autoimmune diseases or obesity, the abundance of Firmicutes decreases, whereas that of Bacteroidetes increases; thus, the F/B value decreases.25,26 Wen et al.18 and Su et al.17 revealed a decrease in the F/B value in patients with GD. Consistent with the findings of Cornejo-Pareja21 and Yang22 et al., our study showed that although the abundances of Firmicutes and Bacteroidetes in the intestinal flora of patients with GD had altered considerably, these changes were not significantly different from those in the Con group. Furthermore, the F/B value did not change significantly, which may be related to the different geographical areas of the patients with GD and different courses of the disease.
SCFAs in the gut are produced via microbial fermentation of dietary fibers. They are a source of energy for intestinal epithelial cells, which can maintain the integrity of the intestinal epithelial barrier and reduce intestinal permeability and levels of circulating lipopolysaccharides, thereby promoting the body to achieve a healthy state.27,28 Studies have shown that Peptostreptococcaceae, Christensenellaceae, Rikenellaceae, Roseburia, Romboutsia, Lachnospira, Odoribacter, E. ventriosum, Oscillibacter, and other biological flora can produce SCFAs and maintain a healthy intestinal microecological environment.29,30,31,32,33,34 In the present study, the abundance of the above-mentioned microbiota decreased significantly in patients with GD. Teofani et al.30 showed that the abundances of Christensenellaceae and Rikenellaceae reduced significantly in patients with inflammatory bowel disease and were correlated with their disease severity. Furthermore, He et al.35 demonstrated that the abundances of Oscillibacter and Romboutsia reduced significantly in patients with systemic lupus erythematosus. The abundance of Lachnospira has also been reported to be significantly reduced in patients with ankylosing spondylitis,36 type 1 diabetes mellitus,37 IgE-related allergic disease,38 and systemic lupus erythematosus.39 The disorders associated with the above-mentioned biota are related to the occurrence and development of autoimmune diseases. Su et al.17 performed a metabolic analysis of the intestinal flora in patients with GD and showed that the levels of propionate and butyrate reduced significantly. In our study, the abundance of the above-mentioned biota in patients with GD also increased significantly after 3 months of drug treatment, with a concurrent improvement in clinical symptoms and indicators of hyperthyroidism. Therefore, we speculate that a reduction in the abundance of SCFA-producing biota in patients with GD promotes the occurrence and development of GD.
Studies have shown that Th17/Treg imbalance is related to the occurrence and development of autoimmune thyroid disease40; this phenomenon also exists in patients with primary and recurrent GD.41,42 In this study, an increasing trend of IL-17 was observed in patients newly diagnosed with GD; however, the difference was insignificant, which may be because all patients with GD were in the initial stage or the sample size was relatively small. Recent studies have clarified that some GM can affect the Th17/Treg balance by secreting inflammatory cytokines or small molecules, such as polysaccharide A.43,44 Clostridium IV and XIVa clusters have been reported to produce butyrate and regulate Th17/Treg balance, thereby promoting healthy homeostasis.45 In general, Roseburia, Ruminococcus, Dorea, E. ventriosum, and Blautia belong to the Clostridium XIVa cluster, whereas the genera Flavonifractor, Faecalibacterium, and Subdoligranulum belong to the Clostridium IV cluster.46 In this study, the abundances of Roseburia, Ruminococcus, E. ventriosum, Flavonifractor, and Lachnospira significantly decreased in patients with GD, and IL-17 levels tended to increase. After 3 months of drug treatment, the abundances of the above-mentioned bacteria increased in the GD group, and IL-17 levels significantly decreased. Correlation analysis also showed that IL-17 levels were significantly negatively correlated with the abundance of E. ventriosum. Therefore, the instability of the Th17/Treg balance was speculated in patients with GD.
Veillonella has been reported to be associated with inflammatory diseases, such as periodontitis, bacteremia, and pneumonia.47,48,49 It has been shown that the abundance of Streptococcaceae increased significantly in mice with chronic inflammation fed with chronic high-fat diet,50 and Streptococcus has been reported to cause meningitis, bacterial pneumonia, endocarditis, and erysipelas.51 Studies have also shown that Erysipelatoclostridium abundance is positively correlated with inflammation.52 Adlercreutzia can convert phytoestrogens into monomers via β-glucosidases, thereby reducing oxidative stress and inflammatory cytokine production (chemoattracting proteins-1 and IL-6).53 Furthermore, Roseburia attenuates the expression of inflammatory cytokines, such as TNF-α and IL-1β.54 The current study results showed that the abundances of Streptococcaceae, Streptococcus, Veillonella, and Erysipelatoclostridium in patients with GD increased significantly, in contrast to the abundances of Adlercreutzia and Roseburia, which reduced significantly. Moreover, the abundances of Streptococcaceae and Streptococcus decreased significantly after 3 months of drug treatment, indicating that patients with GD are in a state of excessive inflammation because of an imbalance in anti-inflammatory and inflammatory flora. Combined with the specificity of the GM and clinical features of the patients newly diagnosed with GD in this study, which were consistent with those of Su et al.,17 the current study believed that disorders associated with the intestinal flora promoted immunity imbalances and GD occurrence.
GD itself can also cause impaired liver function. Mayneris-Perxachs et al. suggested that the abundance of Leuconostocaceae reduced significantly in patients with obesity and fatty liver55 and that its supplementation could improve liver metabolism and reduce liver damage.56 Some lactic acid bacteria (LAB) strains have been shown to improve intestinal barrier function and are considered probiotics.57 Kanmani et al.58 showed that LAB can attenuate the TGF-β signaling pathway associated with liver fibrosis and reduce the production of inflammatory factors and hepatic steatosis in patients with obesity.59 Weissella belongs to the Leuconostocaceae family of the Lactobacillales order, which has also been reported to inhibit the production of proinflammatory cytokines and induce the production of anti-inflammatory mediators to modulate immune cell responses and protect the liver.60,61 Using LEfSe analysis, we observed that the abundances of Leuconostocaceae and Weissella in the GDH group were significantly lower than those in the GDN group. Spearman correlation analysis also revealed that the abundances of Weissella and Leuconostocaceae were significantly negatively correlated with hepatic impairment. Accordingly, the reduction in the abundance of Weissella may mediate hepatic impairment in patients with GD.
The incidence of GD is increasing every year3; however, patients are generally diagnosed with GD only when they have obvious clinical symptoms. Our study identified 12 bacterial genera that can distinguish patients with GD from healthy controls far more accurately, which may help identify patients with GD in the preclinical stage and implement corresponding interventions. In the follow-up study, the fecal bacteria transplantation technology can be used to further verify the effect of each genus on GD occurrence and provide a certain reference value for the clinical treatment of GD.
In conclusion, this study reveals that the occurrence of GD may be related to the disturbed immune system and GM. Following appropriate drug therapy, the abundances of the immune and GM gradually recovered in patients with GD. Furthermore, our study identified 12 bacterial genera that can distinguish patients with GD from healthy controls far more accurately, which may help identify patients with GD in the preclinical stage.
Limitations of the study
However, our study has several limitations. First, the sample size was relatively small, and future studies with a larger sample size are warranted for further verification. Second, this study employed a 3-month follow-up, which is relatively short; thus, extending the follow-up time is necessary to further observe the changes in clinical indicators and the intestinal microbiota in patients with GD more dynamically. Finally, this study sequenced the V3–V4 region of the 16S rRNA gene, which may not be accurate for representing the true proportion of microorganisms in each sample.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Stool samples | Healthy and GD volunteers | NA |
| Critical commercial assays | ||
| QIAamp PowerFecal Pro DNA Kit | QIAGEN company in USA | NA |
| Oligonucleotides | ||
| 16S rRNA gene V3-V4 region primer(F 5′-CCTACGGGNGGCWGCAG-3′ and R 5′-GACTACHVGGGTATCTAATCC-3′) | Klindworth et al.62 | NA |
| Deposited data | ||
| Raw and analyzed data | This paper | SRA:PRJNA850658 |
| Software and algorithms | ||
| GraphPad Prism 8 | GraphPad Software Inc. | https://www.graphpad.com/ |
| QIIME v2020.2 | GitHub | http://qiime.org |
| R software | RStudio | https://www.r-project.org/ |
| LEfSe | Galaxy | http://huttenhower.sph.harvard.edu/galaxy |
| STAMP | GNU GPL | http://kiwi.cs.dal.ca/Software/STAMP |
| DADA2 | BiocManager | https://benjjneb.github.io/dada2/dada |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jixiong Xu (Jixiong.Xu@ncu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Patients and samples
This prospective observational study enrolled 65 patients newly diagnosed with GD (18 men and 47 women; aged 25.00–40.50 years; median age, 30.00 years) of Chinese Han ethnicity who visited the Endocrinology Department of the First Affiliated Hospital of Nanchang University, Nanchang City, Jiangxi Province between October 2018 and September 2019. Of the 65 patients, 37 patients completed the 3-month follow-up. Methimazole was administered once daily at a dose of 20–30 mg/day for the first month in each patient; this dose was tapered or increased at each subsequent visit if the patient became euthyroid or remained hyperthyroid, respectively. Overall, 33 volunteers (10 men and 23 women; aged 26.00–29.00 years; median age, 27.00 years) who underwent physical examinations at our hospital’s health clinic without any confirmed disease were selected as the healthy control group. The inclusion criteria for the GD group were consistent with the American Thyroid Association guidelines63 as follows: 1. hypermetabolic symptoms and signs caused by thyrotoxicemia; 2. diffuse thyroid enlargement (confirmed via palpation or ultrasonography); 3. increased serum FT3 and FT4 levels and decreased TSH levels; and 4. serum TRAb positivity. Further, the exclusion criteria were as follows: 1. patients who had administered antibiotics within 3 months (for a period of >3 days) before enrollment; 2. patients who had administered prebiotics, probiotics, or lactic acid products continuously within 4 weeks before enrollment; 3. patients who had been prescribed with antithyroid drugs for past treatment; 4. patients with malignant tumors, gastrointestinal disease, history of gastrointestinal disease surgery, or recent history of other types of surgery; 5. patients with other autoimmune diseases, liver diseases, or endocrine system diseases; or 6. pregnant or breastfeeding patients. Peripheral venous blood and fecal samples were collected on an empty stomach after enrollment and stored at −80°C until analysis; further, demographic and clinical data were collected from all volunteers.
Ethics statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (ethics no. 2018-063), and written informed consent was obtained from all participants. This study was conducted according to the guidelines provided by the World Medical Association and Declaration of Helsinki.
Method details
Laboratory investigation methods
Electrochemiluminescence immunoassays were used to determine serum FT3, FT4, TSH, TPOAb, TgAb, and TRAb levels. The reference ranges are as follows: FT3, 2.0–4.4 pg/mL; FT4, 0.93–1.70 ng/dL; TSH, 0.27–4.2 uIU/mL; TgAb, 0–115 IU/mL; TPOAb, 0–34 IU/mL; and TRAb, 0–1.5 U/L. Biochemical experiments were performed to evaluate liver function using the following standard values: ALT (7–40 U/L) and AST (13–35 U/L). The Luminex detection technology was used to determine serum cytokine levels (IL-1β, IL-10, and IL-17).
Fecal DNA extraction and 16S rRNA gene sequencing
Microbial DNA extraction from naturally lysed fecal samples was performed using magnetic bead beating and QIAamp PowerFecalPro DNA extraction kit. DNA concentration of the extracted samples was determined using a Biodrop ultra-micro protein accounting analyzer(UK), and the extracted DNA samples were stored at −20°C for later use. Targeted polymerase chain reaction based on the V3–V4 region of the 16S rRNA gene was performed using62 primers (F 5′-CCTACGGGNGGCWGCAG-3′ and R 5′-GACTACHVGGGTATCTAATCC-3′), followed by amplicon sequencing on the Illumina Miseq platform. DNA from all fecal samples in this study was included in the same sequencing run.
Quantification and statistical analysis
Clinical data analysis
Data were analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) software system. Data normality was examined using the Kolmogorov–Smirnov test. Normally distributed continuous data were expressed as means ± standard deviations, whereas skewed continuous data were expressed as medians and interquartile ranges. Clinical data in this study did not show a normal distribution; thus, Mann–Whitney U test was used. Enumeration data were compared between groups using chi-square test. Data before and after treatment were analyzed using paired t-test, and P-values of <0.05 were considered to indicate statistical significance.
Sequencing data analysis
We used QIIME2 (2020.11) platform64 to visually assess the sequencing data volume and sample quality. After filtering, denoising, and removing untrusted sequences and chimeras, high-quality sequences were obtained. In total, 2,565,000 high-quality sequences were obtained in this study, with an average of 19,000 sequences per sample. Overall, 3,457 ASVs were identified in this study using DADA2 plugin.65 A phylogenetic tree was constructed based on the processed alignment files using FastTree, and ASVs were annotated based on SILVA 132 release database.66 To reduce the impact of false sequences on our results, ASVs with a total number of sequences of <0.005% were removed from microbial data analysis in this study.67 Alpha and beta diversity analyses were performed using QIIME2 and were visualized using GraphPad Prism 8 (GraphPad Software) and R (version 4.1.3). PLS-DA was performed using the mixOmics package in R (version 3.6.1)68 and was visualized using Wekemo Bioincloud platform (https://www.bioincloud.tech/#/). Using Statistical Analysis of Metagenome Profiles (v2.1.3), Welch’s t-test (P < 0.05), and Benjamini–Hochberg false discovery rate method (q < 0.05) as multiple testing corrections, differences in microbial abundance were determined between the two groups.69 The correlation between the microbiota and clinical data was assessed using Spearman correlation analysis. A random forest model was constructed using the randomForest package in R (version 4.1.3) to identify the genera that could distinguish the GD group from the Con group. LEfSe analysis was used to investigate the differentiation of microbiota with loss of liver function and GD group before and after treatment (https://huttenhower.sph.harvard.edu/galaxy/). Part of the graphic summary is from the biorender platform.
Acknowledgments
The authors would like to thank State Key Laboratory of Microbial Metabolism, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University for performing the fecal microbiota 16S rRNA sequencing. The manuscript was supported by grants from The Key Research and development project of Jiangxi Province [grant numbers. 20201BBG71006].
Author contributions
Y.D., J.W., and G.X. conceptualized and designed the study. G.Z., S.L., and J.Z. contributed to the collection of data and specimens. Y.D., J.W., and G.X. analyzed the data and drafted the manuscript. Y.D., W.C., and J.X. reviewed and edited the manuscript. All authors contributed to the article and approved the final mauscript.
Declaration of interests
The authors declare no competing interests.
Published: June 28, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107188.
Supplemental information
Data and code availability
The 16S rRNA sequencing set of this article has been deposited in the Genome Sequence Archive (GSA) under the BioProject accession code PRJNA850658.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Smith T.J., Hegedüs L. Graves' disease. N. Engl. J. Med. 2016;375:1552–1565. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 2.Draman M.S., Ludgate M. Thyroid eye disease- an update. Expet Rev. Ophthalmol. 2016;11:273–284. [Google Scholar]
- 3.Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat. Rev. Endocrinol. 2013;9:724–734. doi: 10.1038/nrendo.2013.193. [DOI] [PubMed] [Google Scholar]
- 4.Walsh C.J., Guinane C.M., O'Toole P.W., Cotter P.D. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588:4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Kamada N., Chen G.Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power S.E., O'Toole P.W., Stanton C., Ross R.P., Fitzgerald G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014;111:387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira G.L.V., Leite A.Z., Higuchi B.S., Gonzaga M.I., Mariano V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152:1–12. doi: 10.1111/imm.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishaq H.M., Shahzad M., Wu X., Ma C., Xu J. Gut microbe analysis between Asthma patients and healthy volunteers in Shaanxi province, Xian, China. Pak. J. Zool. 2018;50:165–173. [Google Scholar]
- 9.Benvenga S., Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev. Endocr. Metab. Disord. 2016;17:485–498. doi: 10.1007/s11154-016-9363-2. [DOI] [PubMed] [Google Scholar]
- 10.Arata N., Ando T., Unger P., Davies T.F. By-stander activation in autoimmune thyroiditis: studies on experimental autoimmune thyroiditisin the GFP+ fluorescent mouse. Clin. Immunol. 2006;121:108–117. doi: 10.1016/j.clim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Thrasyvoulides A., Lymberi P. Evidence for intramolecular B-cell epitope spreading during experimental immunization with an immunogenic thyroglobulin peptide. Clin. Exp. Immunol. 2003;132:401–407. doi: 10.1046/j.1365-2249.2003.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knezevic J., Starchl C., Tmava Berisha A., Amrein K. Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishaq H.M., Mohammad I.S., Guo H., Shahzad M., Hou Y.J., Ma C., Naseem Z., Wu X., Shi P., Xu J. Molecular estimation of alteration in intestinal microbial com-position in Hashimoto's thyroiditis patients. Biomed. Pharmacother. 2017;95:865–874. doi: 10.1016/j.biopha.2017.08.101. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F., Feng J., Li J., Zhao L., Liu Y., Chen H., Jin Y., Zhu B., Wei Y. Alterations of the gut microbiota in Hashimoto's thyroiditis patients. Thyroid. 2018;28:175–186. doi: 10.1089/thy.2017.0395. [DOI] [PubMed] [Google Scholar]
- 15.Virili C., Stramazzo I., Centanni M. Gut microbiome and thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2021;35:101506. doi: 10.1016/j.beem.2021.101506. [DOI] [PubMed] [Google Scholar]
- 16.Hammerstad S.S., Tauriainen S., Hyöty H., Paulsen T., Norheim I., Dahl-Jørgensen K. Detection of entero-virus in the thyroid tissue of patients with graves’ disease. J. Med. Virol. 2013;85:512–518. doi: 10.1002/jmv.23476. [DOI] [PubMed] [Google Scholar]
- 17.Su X., Yin X., Liu Y., Yan X., Zhang S., Wang X., Lin Z., Zhou X., Gao J., Wang Z., Zhang Q. Gut dysbiosis contributes to the imbalance of Treg and Th17 cells in Graves' disease patients by propionic acid. J. Clin. Endocrinol. Metab. 2020;105:3526–3547. doi: 10.1210/clinem/dgaa511. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W., Yu X., Kosik R.O., Song Y., Qiao T., Tong J., Liu S., Fan S., Luo Q., Chai L., et al. Gut microbiota may play a significant role in the pathogenesis of Graves' disease. Thyroid. 2021;31:810–820. doi: 10.1089/thy.2020.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishaq H.M., Mohammad I.S., Shahzad M., Ma C., Raza M.A., Wu X., Guo H., Shi P., Xu J. Molecular alteration analysis of human gut microbial composition in Graves' disease patients. Int. J. Biol. Sci. 2018;14:1558–1570. doi: 10.7150/ijbs.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S.C., Lin S.F., Chen S.T., Chang P.Y., Yeh Y.M., Lo F.S., Lu J.J. Alterations of gut microbiota in patients with Graves' disease. Front. Cell. Infect. Microbiol. 2021;11:663131. doi: 10.3389/fcimb.2021.663131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornejo-Pareja I., Ruiz-Limón P., Gómez-Pérez A.M., Molina-Vega M., Moreno-Indias I., Tinahones F.J. Differential microbial pattern description in subjects with Autoimmune-based thyroid diseases: a pilot study. J. Pers. Med. 2020;10:192. doi: 10.3390/jpm10040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M., Sun B., Li J., Yang B., Xu J., Zhou X., Yu J., Zhang X., Zhang Q., Zhou S., et al. Alteration of the intestinal flora may participate in the development of Graves' disease: a study conducted among the Han population in southwest China. Endocr. Connect. 2019;8:822–828. doi: 10.1530/EC-19-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M., Li F., Zhang R., Wu Y., Yang Q., Wang F., Yu Z., Liu J., Cha B., Gong Q., et al. Alteration of the intestinal microbial flora and the serum IL-17 level in patients with Graves' disease complicated with vitamin D deficiency. Int. Arch. Allergy Immunol. 2022;183:225–234. doi: 10.1159/000518949. [DOI] [PubMed] [Google Scholar]
- 24.Chen J., Wang W., Guo Z., Huang S., Lei H., Zang P., Lu B., Shao J., Gu P. Associations between gut microbiota and thyroidal function status in Chinese patients with Graves' disease. J. Endocrinol. Invest. 2021;44:1913–1926. doi: 10.1007/s40618-021-01507-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhi C., Huang J., Wang J., Cao H., Bai Y., Guo J., Su Z. Connection between gut microbiome and the development of obesity. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1987–1998. doi: 10.1007/s10096-019-03623-x. [DOI] [PubMed] [Google Scholar]
- 26.El-Zawawy H.T., Ahmed S.M., El-Attar E.A., Ahmed A.A., Roshdy Y.S., Header D.A. Study of gut microbiome in Egyptian patients with autoimmune thyroid diseases. Int. J. Clin. Pract. 2021;75:e14038. doi: 10.1111/ijcp.14038. [DOI] [PubMed] [Google Scholar]
- 27.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Jiang Q., Liu Z., Shen S., Ai J., Zhu Y., Zhou L. Alteration of gut microbiota relates to metabolic disorders in primary Aldosteronism patients. Front. Endocrinol. 2021;12:667951. doi: 10.3389/fendo.2021.667951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernad-Roche M., Bellés A., Grasa L., Casanova-Higes A., Mainar-Jaime R.C. Effects of dietary supplementation with protected sodium butyrate on gut microbiota in growing-finishing pigs. Animals. 2021;11:2137. doi: 10.3390/ani11072137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y., Xie L., Zhang Z., Zhang W., Tang J., He X., Zhou J., Peng W. Tremella fuciformis polysaccharides inhibited colonic inflammation in dextran sulfate sodium-treated mice via Foxp3+ T cells, gut microbiota, and bacterial metabolites. Front. Immunol. 2021;12:648162. doi: 10.3389/fimmu.2021.648162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teofani A., Marafini I., Laudisi F., Pietrucci D., Salvatori S., Unida V., Biocca S., Monteleone G., Desideri A. Intestinal taxa abundance and diversity in inflammatory Bowel disease patients: an analysis including covariates and confounders. Nutrients. 2022;14:260. doi: 10.3390/nu14020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q., Liu J., Wang X., Robinson K., Whitmore M.A., Stewart S.N., Zhao J., Zhang G. Identification of an intestinal microbiota signature associated with the severity of necrotic enteritis. Front. Microbiol. 2021;12:703693. doi: 10.3389/fmicb.2021.703693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Y., Gong Z., Sun G., Xu J., Qi C., Sun W., Jiang H., Cao P., Ju H. Dysbiosis of gut microbiota in patients with acute myocardial infarction. Front. Microbiol. 2021;12:680101. doi: 10.3389/fmicb.2021.680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H.M., Huang H.L., Liu Y.D., Zhu J.Q., Zhou Y.L., Chen H.T., Xu J., Zhao H.L., Guo X., Shi W., et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021;21:279. doi: 10.1186/s12866-021-02342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He H., Xu H., Xu J., Zhao H., Lin Q., Zhou Y., Nie Y. Sodium butyrate ameliorates gut microbiota dysbiosis in lupus-like mice. Front. Nutr. 2020;7:604283. doi: 10.3389/fnut.2020.604283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L., Han R., Zhang X., Fang G., Chen J., Li J., Xu S., Qian L., Chen W., Pan F. Fecal microbiota in patients with ankylosing spondylitis: Correlation with dietary factors and disease activity. Clin. Chim. Acta. 2019;497:189–196. doi: 10.1016/j.cca.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Leiva-Gea I., Sánchez-Alcoholado L., Martín-Tejedor B., Castellano-Castillo D., Moreno-Indias I., Urda-Cardona A., Tinahones F.J., Fernández-García J.C., Queipo-Ortuño M.I. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care. 2018;41:2385–2395. doi: 10.2337/dc18-0253. [DOI] [PubMed] [Google Scholar]
- 38.Simonyté Sjödin K., Hammarström M.L., Rydén P., Sjödin A., Hernell O., Engstrand L., West C.E. Temporal and long-term gut microbiota variation in allergic disease: a prospective study from infancy to school age. Allergy. 2019;74:176–185. doi: 10.1111/all.13485. [DOI] [PubMed] [Google Scholar]
- 39.Xiang K., Wang P., Xu Z., Hu Y.Q., He Y.S., Chen Y., Feng Y.T., Yin K.J., Huang J.X., Wang J., et al. Causal effects of gut microbiome on systemic Lupus erythematosus: a two-sample mendelian randomization study. Front. Immunol. 2021;12:667097. doi: 10.3389/fimmu.2021.667097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Amaro R., Marazuela M. T regulatory (Treg) and T helper 17 (Th17) lymphocytes in thyroid autoimmunity. Endocrine. 2016;52:30–38. doi: 10.1007/s12020-015-0759-7. [DOI] [PubMed] [Google Scholar]
- 41.Li J.R., Hong F.Y., Zeng J.Y., Huang G.L. Functional interleukin-17 receptor A are present in the thyroid gland in intractable Graves disease. Cell. Immunol. 2013;281:85–90. doi: 10.1016/j.cellimm.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Nakano A., Watanabe M., Iida T., Kuroda S., Matsuzuka F., Miyauchi A., Iwatani Y. Apoptosis-induced decrease of intrathyroidal CD4(+)CD25(+) regulatory T cells in autoimmune thyroid diseases. Thyroid. 2007;17:25–31. doi: 10.1089/thy.2006.0231. [DOI] [PubMed] [Google Scholar]
- 43.Omenetti S., Pizarro T.T. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front. Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou J., Tang Y., Chen Y., Chen D. The role of the microbiota in Graves' disease and Graves' orbitopathy. Front. Cell. Infect. Microbiol. 2021;11:739707. doi: 10.3389/fcimb.2021.739707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K., et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 47.Mashima I., Theodorea C.F., Thaweboon B., Thaweboon S., Nakazawa F. Identification of Veillonella species in the tongue biofilm by using a Novel one-step polymerase chain reaction method. PLoS One. 2016;11:e0157516. doi: 10.1371/journal.pone.0157516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marriott D., Stark D., Harkness J. Veillonella parvula discitis and secondary bacteremia: a rare infection complicating endoscopy and colonoscopy? J. Clin. Microbiol. 2007;45:672–674. doi: 10.1128/JCM.01633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah A., Panjabi C., Nair V., Chaudhry R., Thukral S.S. Veillonella as a cause of chronic anaerobic pneumonitis. Int. J. Infect. Dis. 2008;12:e115–e117. doi: 10.1016/j.ijid.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Zeng H., Ishaq S.L., Zhao F.Q., Wright A.D.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016;35:30–36. doi: 10.1016/j.jnutbio.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Ge R., Sun X. Iron acquisition and regulation systems in Streptococcus species. Metallomics. 2014;6:996–1003. doi: 10.1039/c4mt00011k. [DOI] [PubMed] [Google Scholar]
- 52.Xiao C., Fedirko V., Beitler J., Bai J., Peng G., Zhou C., Gu J., Zhao H., Lin I.H., Chico C.E., et al. The role of the gut microbiome in cancer-related fatigue: pilot study on epigenetic mechanisms. Support. Care Cancer. 2021;29:3173–3182. doi: 10.1007/s00520-020-05820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schepici G., Silvestro S., Bramanti P., Mazzon E. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant. 2019;28:1507–1527. doi: 10.1177/0963689719873890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo B., Jeon K., Moon S., Lee K., Kim W.K., Jeong H., Cha K.H., Lim M.Y., Kang W., Kweon M.N., et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell Host Microbe. 2020;27:25–40.e6. doi: 10.1016/j.chom.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Mayneris-Perxachs J., Cardellini M., Hoyles L., Latorre J., Davato F., Moreno-Navarrete J.M., Arnoriaga-Rodríguez M., Serino M., Abbott J., Barton R.H., et al. Iron status influences non-alcoholic fatty liver disease in obesity through the gut microbiome. Microbiome. 2021;9:104. doi: 10.1186/s40168-021-01052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun M., Wang Q., Zhang M., Zhang G., Wu T., Liu R., Sui W., Zhang J., Yin J., Zhang M. Leuconostoc pseudomesenteroides improves microbiota dysbiosis and liver metabolism imbalance and ameliorates the correlation between dihydroceramide and strains of Firmicutes and Proteobacteria in high fat diet obese mice. Food Funct. 2020;11:6855–6865. doi: 10.1039/d0fo01009j. [DOI] [PubMed] [Google Scholar]
- 57.Reuben R.C., Roy P.C., Sarkar S.L., Rubayet Ul Alam A.S.M., Jahid I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020;103:1223–1237. doi: 10.3168/jds.2019-17092. [DOI] [PubMed] [Google Scholar]
- 58.Kanmani P., Kim H. Probiotics counteract the expression of hepatic profibrotic genes via the attenuation of TGF-β/SMAD signaling and autophagy in hepatic stellate cells. PLoS One. 2022;17:e0262767. doi: 10.1371/journal.pone.0262767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi S.I., You S., Kim S., Won G., Kang C.H., Kim G.H. Weissella cibaria MG5285 and Lactobacillus reuteri MG5149 attenuated fat accumulation in adipose and hepatic steatosis in high-fat diet-induced C57BL/6J obese mice. Food Nutr. Res. 2021;65 doi: 10.29219/fnr.v65.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvim L.B., Sandes S.H.C., Silva B.C., Steinberg R.S., Campos M.H.A., Acurcio L.B., Arantes R.M.E., Nicoli J.R., Neumann E., Nunes Á.C. Weissella paramesenteroides WpK4 reduces gene expression of intestinal cytokines, and hepatic and splenic injuries in a murine model of typhoid fever. Benef. Microbes. 2016;7:61–73. doi: 10.3920/BM2015.0093. [DOI] [PubMed] [Google Scholar]
- 61.Yan Y., Chen H., Sun L., Zhang W., Lu X., Li Z., Xu J., Ren Q. The changes of microbial diversity and flavor compounds during the fermentation of millet Huangjiu, a traditional Chinese beverage. PLoS One. 2022;17:e0262353. doi: 10.1371/journal.pone.0262353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L., Rivkees S.A., Samuels M., Sosa J.A., Stan M.N., et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of Thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 64.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pruesse E., Peplies J., Glöckner F.O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rohart F., Gautier B., Singh A., Lê Cao K.A. mixOmics: An R package for 'omics feature selection and multiple data integration. PLoS Comput. Biol. 2017;13:e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA sequencing set of this article has been deposited in the Genome Sequence Archive (GSA) under the BioProject accession code PRJNA850658.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.