Abstract
P-glycoprotein (Pgp) overexpressed in blood brain barrier (BBB) is hypothesized to lower brain drug concentrations and thus inhibit anticonvulsant effects in drug-resistant epilepsy. Pluronic P85 (P85) was proved to enhance the delivery of drugs into the brain by inhibition of Pgp. To determine whether the surfactant P85 [versus Pgp inhibitor tariquidar (TQD)] enhance phenytoin (PHT) into the brain in drug-resistant rats with chronic mesial temporal lobe epilepsy (MTLE) induced by lithium-pilocarpine, in brain of which Pgp were overexpressed, then direct verification of PHT transport via measurement of PHT concentration in brain using microdialysis. The drug-resistant model rats were randomly divided into three groups, which were treated with PHT, 1%P85 + PHT, or PHT+TQD, respectively. 1%P85 + PHT treatment displayed a lower ratio of the area under the curve (AUC) of the PHT concentration in the brain/plasma even than that of the PHT treatment in model rats (p < 0.05), while PHT+TQD showed the highest ratio of the AUC of all treatments. However, the ratio of the PHT concentration in the liver/plasma was similar in three model groups (p > 0.05). For the ratio of the kidney/plasma, PHT+TQD treatment model group had the highest ratio of the other treatments in model rats. Thus, P85 oppositely decreased PHT concentration in brain in drug-resistant model rats with Pgp overexpressed MTLE while TQD could increase PHT distribution in brain.
Keywords: Pluronic P85, Tariquidar, P-glycoprotein, Phenytoin, Microdialysis
1. Introduction
Drug resistance in epilepsy with uncontrolled seizures occurs in approximately 30% of epileptic patients and remains a major clinical problem (Kwan and Brodie, 2010, Golyala and Kwan, 2017), although advances have been achieved in antiepileptic drugs (AEDs) treatment over the past several decades. However, the pathogenic mechanism underlying drug-resistant epilepsy remains unknown.
An important characteristic of drug-resistant epilepsy is that most patients with medically refractory epilepsy are resistant to several AEDs, even though the AEDs to which they are resistant often act by different mechanisms (Loscher and Potschka, 2002). This phenomenon of multidrug resistance is prone to suggest the non-specific mechanism of multidrug transporters (MDTs), such as decreased drug uptake into the brain by overexpression of Pgp (Sisodiya, 2003). Pgp in the capillary endothelial cells of the BBB is thought to act as an active defense mechanism to restrict penetration of lipophilic substances into the brain (Loscher and Potschka, 2005a). In addition, several AEDs are lipophilic drugs which are substrates of Pgp (Loscher and Potschka, 2002, Loscher and Potschka, 2005b, Luna-Tortos et al., 2008, Schinkel and Jonker, 2003, Luna-Tortos et al., 2009). Overexpression of Pgp in epileptogenic tissue likely reduces penetration of AEDs into the epileptic lesion, thereby producing resistance to drug treatment (Loscher and Potschka, 2002, Sisodiya, 2003), which would be a possible explanation for multidrug resistance in epileptic patients. Studies have proven that Pgp are overexpressed in the BBB of epileptogenic brain tissue from drug-resistant patients with MTLE (Sisodiya et al., 2002, Tishler et al., 1995). In addition, reduced penetration of AEDs into epileptogenic brain tissue as a consequence of localized overexpression of Pgp in the BBB (Loscher and Potschka, 2002, Sisodiya, 2003) is a potential explanation for the observed reduction in the anticonvulsant activity of AEDs against focal seizures in kindled rats. Hence, animal models of chronic MTLE with Pgp overexpression allow researchers to determine whether Pgp inhibitors (surfactant P85 with the Pgp inhibition function or TQD) could act on the overexpressed Pgp to enhance the distribution of AEDs in brain.
PHT is ever a traditional first-line antiepileptic drug for its curative treatment for partial seizures. In view of its unbearable side effects in epilepsy treatment, PHT has become an alternative drug though it has good quality of therapeutic effect in part of patients with drug-resistant epilepsy. When faced with this dilemma, clinicians, especially those who major in epilepsy, are searching for a feasible solution to target the PHT into the epileptogenic lesion in the brain to control the seizures and in the same time to decrease the drug in plasma to avoid the peripheral side effects.
New anticonvulsants that are not susceptible to Pgp induced drug resistance could provide rapid relief of drug-resistant epilepsy. Poloxamer, with pluronic as its product name, is three block copolymer [polyoxyethylene-poly propylene-oxygen polyoxyethylene (PEO-PPO-PEO)]. Poloxamer is divided into four kinds according to its different characteristics. Among them, poloxamer 235 (Pluronic P85), as the second kind, can inhibit the function of Pgp with greatest extent for depletion of ATP and decreasing the micro viscosity of cell membrane. As to the first kind of poloxamer, poloxamer 188 (Pluronic F68) cannot inhibit the Pgp and is weak in cell osmosis though it increases the micro viscosity of cell membrane. In addition, poloxamer has the ability to repair the disruption of BBB (Bao et al., 2012, Wang et al., 2015). And P85 has been proved to enhance the delivery of digoxin across the BBB by inhibiting Pgp-mediated efflux (Batrakova et al., 1999, Batrakova et al., 2001). Moreover, our previous research has confirmed that P85 enhances delivery of PHT into the brain in normal rat (Fang et al., 2014). However, the performance of P85 as a delivery medium for AEDs in a drug-resistant rat model of chronic MTLE with overexpressed Pgp in brain has not been assessed. Tariquidar (TQD) inhibits Pgp with good selectively and high potency (Szakacs et al., 2006). A preclinical study showed that TQD potently inhibited Pgp function at the BBB, as reflected by markedly increased brain distribution of Pgp substrates (Bankstahl et al., 2008, Choo et al., 2006, Cutler et al., 2006). In addition, AEDs treatment combined with TQD has shown some promise as a method of overcoming Pgp-mediated drug resistance (Loscher and Potschka, 2005c). Therefore, TQD was used as a positive control in the present study, in which we assessed whether P85 plus PHT to increase drug to the hippocampus of drug-resistant rats with Pgp overexpression.
2. Materials and methods
2.1. Drugs and chemicals
PHT and carbamazepine were purchased from Sigma-Aldrich, Inc. (St. Louis, Missouri, USA). Tariquidar (XR9576, TQD) was obtained from MedKoo Biosciences, Inc. The P85 block copolymer was synthesized by Well Chemical Industry Co. Ltd (Nanjing, China). P85 was prepared in assay buffer (122 mM sodium chloride, 25 mM sodium bicarbonate, 10 mM glucose, 10 mM HEPES, 3 mM potassium chloride, 1.2 mM magnesium sulfate, 1.4 mM calcium chloride, and 0.4 mM potassium phosphate dibasic) (Batrakova et al., 1999) (Fang et al., 2016), while other drugs were prepared in phosphate buffered solution or ultrapure water. All of the solutions were injected in a volume of 2 ml/kg and were incubated at 37 °C for at least 1 h before using in the experiments.
2.2. Animals
Healthy Sprague-Dawley rats were purchased from the Experimental Animal Center of Guangdong Province (SCXK2008–0002) (6–8 week, female, weight 160–180 g) and housed under controlled environmental conditions (ambient temperature, 24–25 °C; humidity, 50–60%; lights on from 7:00 am to 7:00 pm) with free access to food and water. The rats were allowed to adapt to the new conditions for at least one week before they were used in the experiments. All procedures were performed in accordance with the Regulations of the Experimental Animal Administration issued by the Ministry of Science and Technology of the People’s Republic of China (http://www.most.gov.cn).
2.3. Induction of MTLE rats and screening of PHT non-responders as model rats
The onset of status epilepticus in normal rats were induced by li-pilocarpine according to the procedure mentioned in our published articles (Fang et al., 2014, Fang et al., 2016). The frequency of spontaneous recurrent seizures was recorded by continuous video-EEG monitoring (Fang et al., 2014, Fang et al., 2016). Then the successfully constructed MTLE rats were screened by the PHT treatment to select out the PHT non-responders as the model rats for this study (Fang et al., 2014, Fang et al., 2016), which were divided into three groups in the later experiments. The PHT non-responders were defined as having their seizure frequency suppressed less than 50% during the treatment period in comparison with their seizure frequency in the pre-drug control period.
2.4. Microdialysis procedure
The model rats were anaesthetized with chloral hydrate (380 mg/kg, i.p.) during the entire microdialysis experiment according to the procedure mentioned in our published articles (Fang et al., 2014, Fang et al., 2016). After an equilibration period of 2 h, the four groups of model rats were intravenously injected with a bolus dose of PHT (35 mg/kg), 1%P85 + PHT (PHT was dissolved in the 1%P85 solution), or PHT+TQD (TQD 15 mg/kg, injected 30 min before PHT administration), respectively.
2.5. High-performance liquid chromatography
High performance liquid chromatography (HPLC) with ultraviolet detection was used to analyze the PHT concentration in the plasma and dialysate samples based on our recently described articles (Fang et al., 2014, Fang et al., 2016).
2.6. Distribution of PHT in the organs (brain, liver and kidney)
The PHT concentration for brain, and liver and kidney distribution were conducted as the previous articles (Fang et al., 2014, Fang et al., 2016) in order to observe the distribution of PHT in different organs.
2.7. Tissue preparation and immunohistochemistry
Immunohistochemical experiment was performed as previous method (Fang et al., 2016). Co-labeling with Pgp and Glut1 (mouse monoclonal antibody against the glucose transporter, ab40084, Abcam, USA) was performed to verify Pgp expression in the BBB.
2.8. Statistics
The data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed in SPSS 20 (IBM, Armonk, NY, USA) using one-way ANOVA, and the Kruskal-Wallis test. All tests were two-tailed, and differences with P < 0.05 were considered to be significant.
3. Results
3.1. PHT distribution after application of 1%P85 and TQD
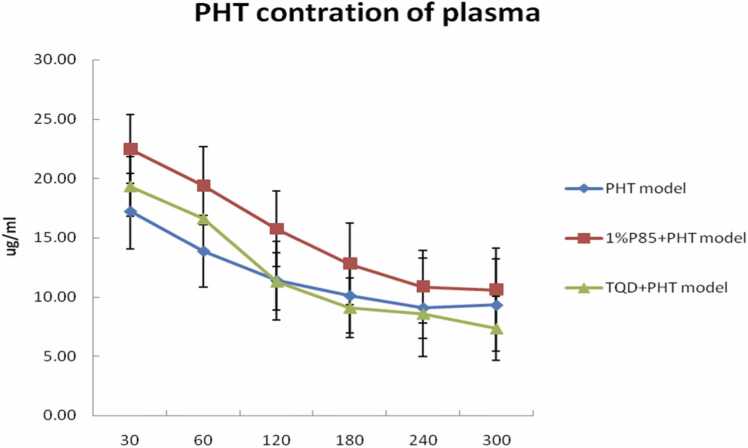
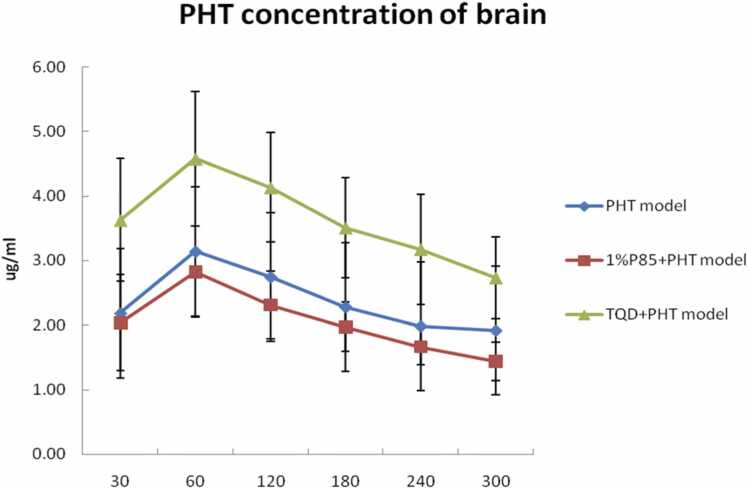
The maximal PHT concentration in the plasma was determined 30 min after the administration of a bolus dose of PHT, 1%P85 + PHT, or PHT+TQD for three model groups each, after 30 min the PHT concentration decreased (Fig. 1). The maximal PHT concentration in the extracellular fluid (ECF) of the hippocampus was achieved 60 min after drug administration in the three model groups, after 60 min the PHT concentration decreased (Fig. 2).
Fig. 1.
Effect of P85 (n = 7) and TQD (n = 5) on PHT concentration in plasma after a bolus dose of drugs in model rats. There were no obviously differences of PHT concentration in plasma (except at the 60 and 120 min) among PHT, 1%P85 + PHT, and TQD+PHT treatments in model rats (p > 0.05).
Fig. 2.
Effect of P85 (n = 7) and TQD (n = 5) on PHT concentration in brain dialysate after a bolus dose of drugs in model rats. The PHT concentration in TQD+PHT model group was significant higher than that of other two model groups (PHT and 1%P85 + PHT) (p < 0.05) while there were no significant differences of PHT concentration between PHT and 1%P85 + PHT model group.
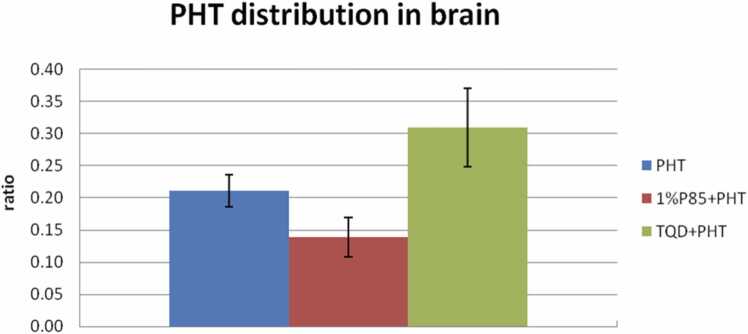
P85 concentration was only tested at 1% in model rats. Plasma PHT level seemed slightly higher than that of the PHT treatment in model rats, but there were no significant differences between them except at the 60 and 120 min (p > 0.05) (Fig. 1). While the PHT level of ECF seemed slightly lower than that of the PHT treatment in model rats. However, there were no significant differences between them (p > 0.05) (Fig. 2). In addition, P85 treatment obviously decreased the AUC ratio of PHT level in ECF/plasma up to 33.3% lower than that of the PHT treatment in model rats (p < 0.05) (Fig. 3).
Fig. 3.
Effect of P85 (n = 7) and TQD (n = 5) on brain PHT distribution after a bolus dose of drugs in model rats. P85 decreased the AUC ratio up to 33.3% relative to PHT treatment (p < 0.05), while TQD increased the AUC ratio up to almost 47.6% relative to PHT treatment (p < 0.05).
TQD was tested at a dose of 15 mg/kg, which injected 30 min before PHT administration. The plasma level of TQD treatment was similar to the PHT treatment in model rats. The ECF concentration of PHT showed a remarkable increase of TQD treatment when compared to that of the PHT treatment in model rats (p < 0.05). In addition, TQD enhanced the AUC ratio of PHT level in ECF/plasma up to almost 47.6% more than that of the PHT treatment in model rats (p < 0.05) (Fig. 3).
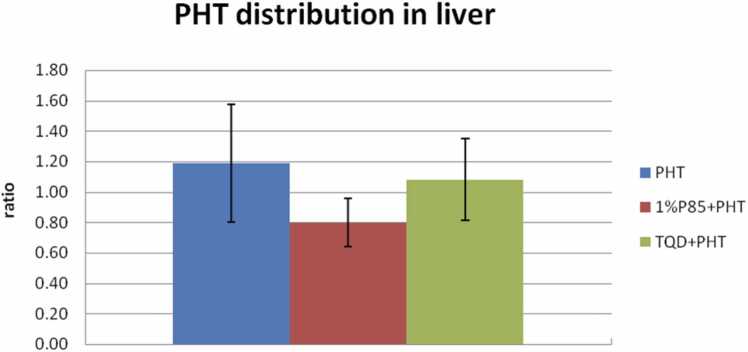
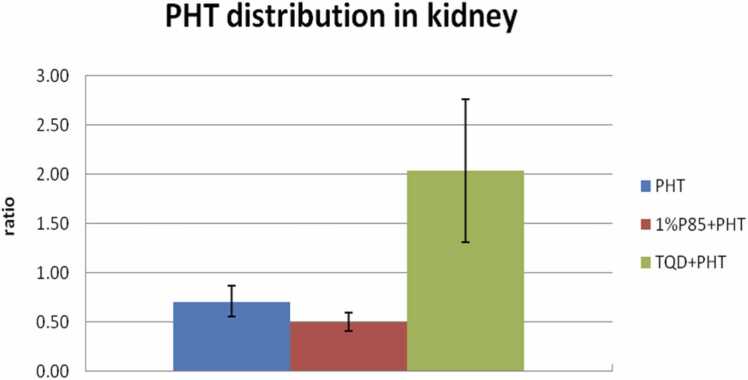
To evaluate the PHT distribution in other organs, PHT concentration in liver and kidney were detected after termination of the experiment at 300 min, and for PHT treatment in model rats, the ratio of liver/plasma ranged between 0.95 and 1.76 (mean, 1.19 ± 0.38). But for P85 treatment, the ratio of liver/plasma was 0.80 ± 0.16 (0.63–1.09), with no significant differences to that of PHT treatment (p > 0.05) (Fig. 4). There was also no obvious increase of drug accumulation in liver of TQD pretreated animals (PHT liver/plasma ratio, 1.08 ± 0.27) (0.73–1.42) than that of PHT treatment in model rats (p > 0.05). As for PHT distribution in kidney, the ratio of PHT level in kidney/plasma of PHT treatment ranged between 0.54 and 0.91 (mean, 0.71 ± 0.16). The ratios of kidney/plasma were 0.50 ± 0.09 (0.37–0.64) of P85 treatment, while 2.04 ± 0.73 (1.44–2.89) of TQD treatment in model rats (Fig. 5). There was a remarkable increase of PHT distribution in kidney of TQD treatment than that of PHT treatment group (p < 0.05).
Fig. 4.
Effect of P85 (n = 7) and TQD (n = 5) on liver PHT distribution after a bolus dose of drugs in model rats. There were no significant differences in liver distribution among three treatments model rats (p > 0.05). Though, P85 decreased the accumulation of PHT in liver, with a decrease rate of 32.8% relative to PHT treatment (p > 0.05).
Fig. 5.
Effect of P85 (n = 7) and TQD (n = 5) on kidney PHT distribution after a bolus dose of drugs in model rats. P85 (29.6%) slightly decreased the accumulation of PHT in kidney than that of PHT treatment (p > 0.05). While TQD (187.3%) evidently increased the accumulation of PHT in kidney than that of PHT treatment in model rats (p < 0.05).
3.2. Pgp expression in the hippocampus of the rats
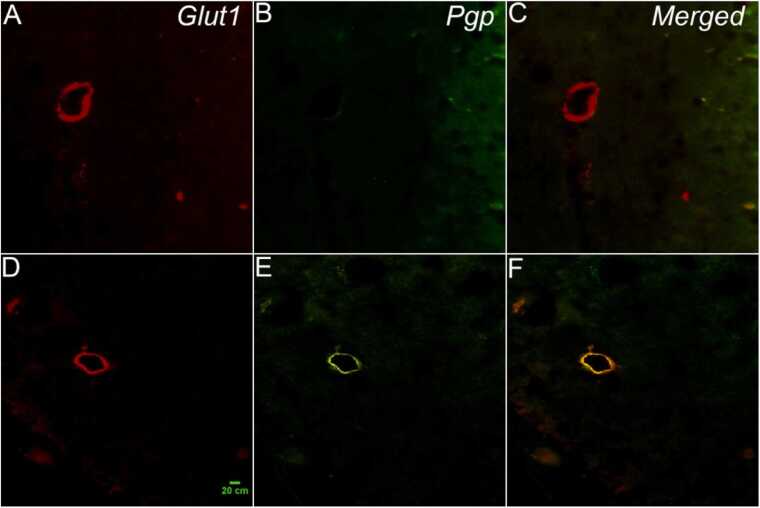
In the double Glut1/Pgp immunolabeled samples, high magnification images (400 ×) confirmed that the expression of Pgp corresponded to the endothelial cells, demonstrating that Pgp was localized on the luminal compartment of endothelial cells in model rats (Fig. 6).
Fig. 6.
Pgp-positive labeling in the hippocampus of the model rat is shown (800 ×magnification). A double-immunofluorescence assay for Pgp (green, B and E) and Glut1 (red, A and D) in the hippocampus confirms that Pgp is expressed in brain capillary endothelial cells (orange, C and F) because Glut1 exclusively labels capillary endothelial cells. A, B, and C are images of the normal rat, while D, E, and F are images of the model rat. The scale is 20 µm.
4. Discussion
We described the usage of the P85 with a potential function of Pgp inhibition in drug-resistant epileptic rats with overexpressed Pgp in brain. In this model, the P85 treatment appeared to exhibit less brain drug distribution when compared with the PHT treatment in model rats. There was also a slight reduction of drug distribution in organs of the P85 treatment in model rats. Thus, many probable explanations for the observed activity of the P85 molecule were proposed, although most were related to the specific biochemical properties of the molecule relative to membrane intercalation.
In cases involving pathological membrane fluidity or permeability, however, surfactants could restore and maintain ionic gradients by intercalating into the plasma membrane (Lee et al., 1992, Merchant et al., 1998, Marks et al., 2001). Besides, studies in vitro had proved that poloxamer 188 could be directly embedded in the cell membrane lipid layer and restore plasma membrane integrity in multiple cells (Serbest et al., 2006, Greenebaum et al., 2004, Baars et al., 2006, Hunter et al., 2010). By resealing plasma membranes, poloxamer 188 could prevent necrosis that affected lipid peroxidation to reduce cell loss in an excitotoxicity model in vitro (Marks et al., 2001). In addition, poloxamer 188 had been demonstrated to seal electroperrmeabilized muscle tissue when injected intravenously, as well as to reduce inflammation (Lee et al., 1992). Hence, we could presume that poloxamers could intercalate into the lipid bilayer of the plasma membrane, affecting the membrane fluidity, making the membrane more rigid, and giving intramembranous molecules less freedom of movement (Curry et al., 2004). P85 was also included as the poloxamer, which was proved to enhance the delivery of Pgp substrate drugs to the brain by inhibition of Pgp-mediated efflux, which was associated with membrane fluidization (inhibiting Pgp ATPase activity) and energy depletion (decreasing the ATP pool available for Pgp) (Batrakova et al., 1999, Neudeck et al., 2008). This ability of drug targeting through BBB was certified in normal rat in our previous study (Fang et al., 2014).
According to our previous studies, it could be found that there was remarkable increase of PHT concentration in ECF of PHT treatment in model rats when compared to that of PHT treatment in normal rats (p < 0.05) (Fang et al., 2014), while the PHT concentration in plasma was similar between PHT treatment in model and normal rats (p > 0.05). In addition, the PHT distribution in brain was much higher in model rats than that of PHT treatment in normal rats, which indicated that the conventional PHT could go through the BBB easier in drug-resistant model rats than in normal rats because there were disruption of BBB exist. The evidence of this study was identified with the conclusion that the AEDs, PHT could penetrate into the brain through the disruption of BBB (Marchi et al., 2009).
Poloxamers directly acted on drugs through connecting of chemical coupling or some functional groups, and turned out to be the micelle mixed with the unmodified poloxamer, which was transported into the brain mediated by carriers or receptor (Kabanov et al., 2002). In addition, the mechanism of P85 micelle targeted with drug was testified to increase the cell endocytosis. The poloxamers could target the drugs into the brain across the BBB by connecting guidance molecules (Kabanov et al., 1989, Batrakova et al., 1998). When P85 connected with the AEDs—PHT, the PHT micelle was formatted and proved to enhance the PHT concentration in the brain on normal rat (Fang et al., 2014). However, obvious BBB disruption had been proved in animal models with drug-resistant epilepsy, the conventional PHT could leakage into the brain through the damaged BBB and the PHT distribution was increased in these models (Marchi et al., 2009). Instead of enhancing the drug concentration in brain, the P85 decreased the PHT level oppositely in rat model with MTLE that had been certified with obvious BBB disruption. Hence, that the surfactant poloxamer was proved to be capable of restoring impaired BBB by sealing cell membrane could be regarded as the explanation for why P85 could decrease the PHT concentration in drug-resistant epileptic model rats in our study. Hence, we hypothesized that when P85 connected with the PHT to be the micelle, the endocytosis of capillary endothelial cell was increased as well as the increasing leakage of PHT in damaged BBB. However, poloxamer had the function to immediate repair the damaged BBB by inserting the molecular into the damaged endothelial cell membranes of BBB, which thus decreased the leakage of PHT. Therefore, the conventional PHT plus the P85 could not increase the drug distribution in brain of rats with damaged BBB of drug-resistant epilepsy, which was opposite to the primary design. Hence, the nanoparticle drug delivery system was essential to target the AEDs into the brain in drug-resistant epileptic rat with obvious BBB disruption, the P85 could be added as the surfact (Fang et al., 2016). As researchers demonstrated that the mechanisms of P85 coated nanoparticles across BBB was that after intravenous injection, poloxamer adsorbed the apolipoprotein E or B in plasma to possess the characteristics of low density lipoprotein to interact with the low density lipoprotein receptor in vascular endothelial cell of BBB in brain which was absorbed by endothelial cell of BBB into the brain (Liow et al., 2016).
Poloxamers prepared as micelles were proved as an effective method for the drug delivery system to target drugs into the central nervous system. However, we recommended that when poloxamers were prepared as micelles for brain target, limitation existed when there was the pathological basis of disruption of BBB in the experimental objects. For the function of resealing plasma membranes instantly, poloxamers (or pluronic) might decrease the concentration of drugs into the brain with the opposite intention, especially for the drugs which would be with obvious leakage into the brain depending on the damaged BBB, such as the lipophilic and hydrophilic compounds of AEDs (Marchi et al., 2009). Thus, in the treatment of drug-resistant epilepsy, poloxamers simply prepared as micelles were not suitable for targeting AEDs into the brain. Except for the drug-resistant epilepsy with disruption of BBB, other kinds of diseases with the damaged BBB were also avoided to take the micelles of poloxamers for drug brain delivery.
5. Conclusions
Instead of enhancing the PHT brain distribution as proved in normal rats, P85 decreased the PHT brain distribution which was opposite to what we expected first. But TQD enhanced the PHT brain distribution obviously while also increased PHT concentration in kidney in drug-resistant rats with Pgp overexpressed MTLE.
Ethics approval and consent to participate
All procedures involving animals were approved by the Animal Experimentation Ethics Committee of Guangzhou Medical University.
Funding
This work was supported by the National Natural Science Foundation of China [grant number: 81901328]; the Natural Science Foundation of Guangdong Province [grant number: 2018A030313821]; the Health Science and Technology Program of Guangzhou [grant number: 20221A010030]; the Science and Technology Plan Project of Guangdong Province [grant number: 2019B030316001]; and the Guangzhou Municipal Key Discipline in Medicine [grant number: 2021-2023].
CRediT authorship contribution statement
Ziyan Fang was the major contributor to manuscript writing, and contributed to the study design, revised the manuscript, and supervised the study. Penghui Cao made substantial contributions to the statistical conception and analyzed the data. Nannan Pan completed and collected all experimental data. Haoyang Lu completed and collected all experimental data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Consent for publication
All authors have agreed to the published version of this manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Baars D.C., Rundell S.A., Haut R.C. Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech. Model Mechanobiol. 2006;5:133–139. doi: 10.1007/s10237-006-0024-3. [DOI] [PubMed] [Google Scholar]
- Bankstahl J.P., Kuntner C., Abrahim A., Karch R., Stanek J., Wanek T., Wadsak W., Kletter K., Muller M., Loscher W., Langer O. Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J. Nucl. Med. 2008;49:1328–1335. doi: 10.2967/jnumed.108.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H.J., Wang T., Zhang M.Y., Liu R., Dai D.K., Wang Y.Q., Wang L., Zhang L., Gao Y.Z., Qin Z.H., Chen X.P., Tao L.Y. Poloxamer-188 attenuates TBI-induced blood-brain barrier damage leading to decreased brain edema and reduced cellular death. Neurochem Res. 2012;37:2856–2867. doi: 10.1007/s11064-012-0880-4. [DOI] [PubMed] [Google Scholar]
- Batrakova E.V., Han H.Y., Miller D.W., Kabanov A.V. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm. Res. 1998;15:1525–1532. doi: 10.1023/a:1011942814300. [DOI] [PubMed] [Google Scholar]
- Batrakova E.V., Li S., Miller D.W., Kabanov A.V. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm. Res. 1999;16:1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- Batrakova E.V., Li S., Vinogradov S.V., Alakhov V.Y., Miller D.W., Kabanov A.V. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J. Pharm. Exp. Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- Choo E.F., Kurnik D., Muszkat M., Ohkubo T., Shay S.D., Higginbotham J.N., Glaeser H., Kim R.B., Wood A.J., Wilkinson G.R. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J. Pharm. Exp. Ther. 2006;317:1012–1018. doi: 10.1124/jpet.105.099648. [DOI] [PubMed] [Google Scholar]
- Curry D.J., Wright D.A., Lee R.C., Kang U.J., Frim D.M. Surfactant poloxamer 188-related decreases in inflammation and tissue damage after experimental brain injury in rats. J. Neurosurg. 2004;101:91–96. doi: 10.3171/ped.2004.101.2.0091. [DOI] [PubMed] [Google Scholar]
- Cutler L., Howes C., Deeks N.J., Buck T.L., Jeffrey P. Development of a P-glycoprotein knockout model in rodents to define species differences in its functional effect at the blood-brain barrier. J. Pharm. Sci. 2006;95:1944–1953. doi: 10.1002/jps.20658. [DOI] [PubMed] [Google Scholar]
- Fang Z.Y., Chen S.D., Chen Y.S., Ni G.Z., Qin J.M., Yang L.B., Chen Z.Y., Zhou J.Q., Huang M., Wu C.B., Li Z., Zhou L.M. Pluronic P85 enhances the delivery of phenytoin to the brain versus verapamil in vivo. Lat. Am. J. Pharm. 2014;33:812–818. [Google Scholar]
- Fang Z., Chen S., Qin J., Chen B., Ni G., Chen Z., Zhou J., Li Z., Ning Y., Wu C., Zhou L. Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials. 2016;97:110–121. doi: 10.1016/j.biomaterials.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Golyala A., Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–156. doi: 10.1016/j.seizure.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Greenebaum B., Blossfield K., Hannig J., Carrillo C.S., Beckett M.A., Weichselbaum R.R., Lee R.C. Poloxamer 188 prevents acute necrosis of adult skeletal muscle cells following high-dose irradiation. Burns. 2004;30:539–547. doi: 10.1016/j.burns.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Hunter R.L., Luo A.Z., Zhang R., Kozar R.A., Moore F.A. Poloxamer 188 inhibition of ischemia/reperfusion injury: evidence for a novel anti-adhesive mechanism. Ann. Clin. Lab Sci. 2010;40:115–125. [PubMed] [Google Scholar]
- Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kabanov A.V., Chekhonin V.P., Alakhov V., Batrakova E.V., Lebedev A.S., Melik-Nubarov N.S., Arzhakov S.A., Levashov A.V., Morozov G.V., Severin E.S., et al. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. Micelles as microcontainers for drug targeting. FEBS Lett. 1989;258:343–345. doi: 10.1016/0014-5793(89)81689-8. [DOI] [PubMed] [Google Scholar]
- Kwan P., Brodie M.J. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9:27–29. doi: 10.1016/S1474-4422(09)70304-7. [DOI] [PubMed] [Google Scholar]
- Lee R.C., River L.P., Pan F.S., Ji L., Wollmann R.L. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liow J.S., Zoghbi S.S., Hu S., Hall M.D., Hines C.S., Shetty H.U., Araneta M.D., Page E.M., Pike V.W., Kreisl W.C., Herscovitch P., Gottesman M.M., Theodore W.H., Innis R.B. 18)F-FCWAY, a serotonin 1A receptor radioligand, is a substrate for efflux transport at the human blood-brain barrier. Neuroimage. 2016;138:134–140. doi: 10.1016/j.neuroimage.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W., Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J. Pharm. Exp. Ther. 2002;301:7–14. doi: 10.1124/jpet.301.1.7. [DOI] [PubMed] [Google Scholar]
- Loscher W., Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W., Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Loscher W., Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- Luna-Tortos C., Fedrowitz M., Loscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;55:1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Luna-Tortos C., Rambeck B., Jurgens U.H., Loscher W. The antiepileptic drug topiramate is a substrate for human P-glycoprotein but not multidrug resistance proteins. Pharm. Res. 2009;26:2464–2470. doi: 10.1007/s11095-009-9961-8. [DOI] [PubMed] [Google Scholar]
- Marchi N., Betto G., Fazio V., Fan Q., Ghosh C., Machado A., Janigro D. Blood-brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia. 2009;50:664–677. doi: 10.1111/j.1528-1167.2008.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J.D., Pan C.Y., Bushell T., Cromie W., Lee R.C. Amphiphilic, tri-block copolymers provide potent membrane-targeted neuroprotection. FASEB J. 2001;15:1107–1109. doi: 10.1096/fj.00-0547fje. [DOI] [PubMed] [Google Scholar]
- Merchant F.A., Holmes W.H., Capelli-Schellpfeffer M., Lee R.C., Toner M. Poloxamer 188 enhances functional recovery of lethally heat-shocked fibroblasts. J. Surg. Res. 1998;74:131–140. doi: 10.1006/jsre.1997.5252. [DOI] [PubMed] [Google Scholar]
- Neudeck B.L., Alford T.D., Faith N.G., Czuprynski C.J. The poloxamer P85 is protective against Listeria monocytogenes invasion. Foodborne Pathog. Dis. 2008;5:859–865. doi: 10.1089/fpd.2008.0125. [DOI] [PubMed] [Google Scholar]
- Schinkel A.H., Jonker J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Serbest G., Horwitz J., Jost M., Barbee K. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. FASEB J. 2006;20:308–310. doi: 10.1096/fj.05-4024fje. [DOI] [PubMed] [Google Scholar]
- Sisodiya S.M. Mechanisms of antiepileptic drug resistance. Curr. Opin. Neurol. 2003;16:197–201. doi: 10.1097/01.wco.0000063771.81810.6c. [DOI] [PubMed] [Google Scholar]
- Sisodiya S.M., Lin W.R., Harding B.N., Squier M.V., Thom M. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain. 2002;125:22–31. doi: 10.1093/brain/awf002. [DOI] [PubMed] [Google Scholar]
- Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Tishler D.M., Weinberg K.I., Hinton D.R., Barbaro N., Annett G.M., Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36:1–6. doi: 10.1111/j.1528-1157.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Wang T., Chen X., Wang Z., Zhang M., Meng H., Gao Y., Luo B., Tao L., Chen Y. Poloxamer-188 can attenuate blood-brain barrier damage to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2015;55:240–250. doi: 10.1007/s12031-014-0313-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.